User login
VIDEO: Elective neck dissection during primary surgery improves oral cancer survival
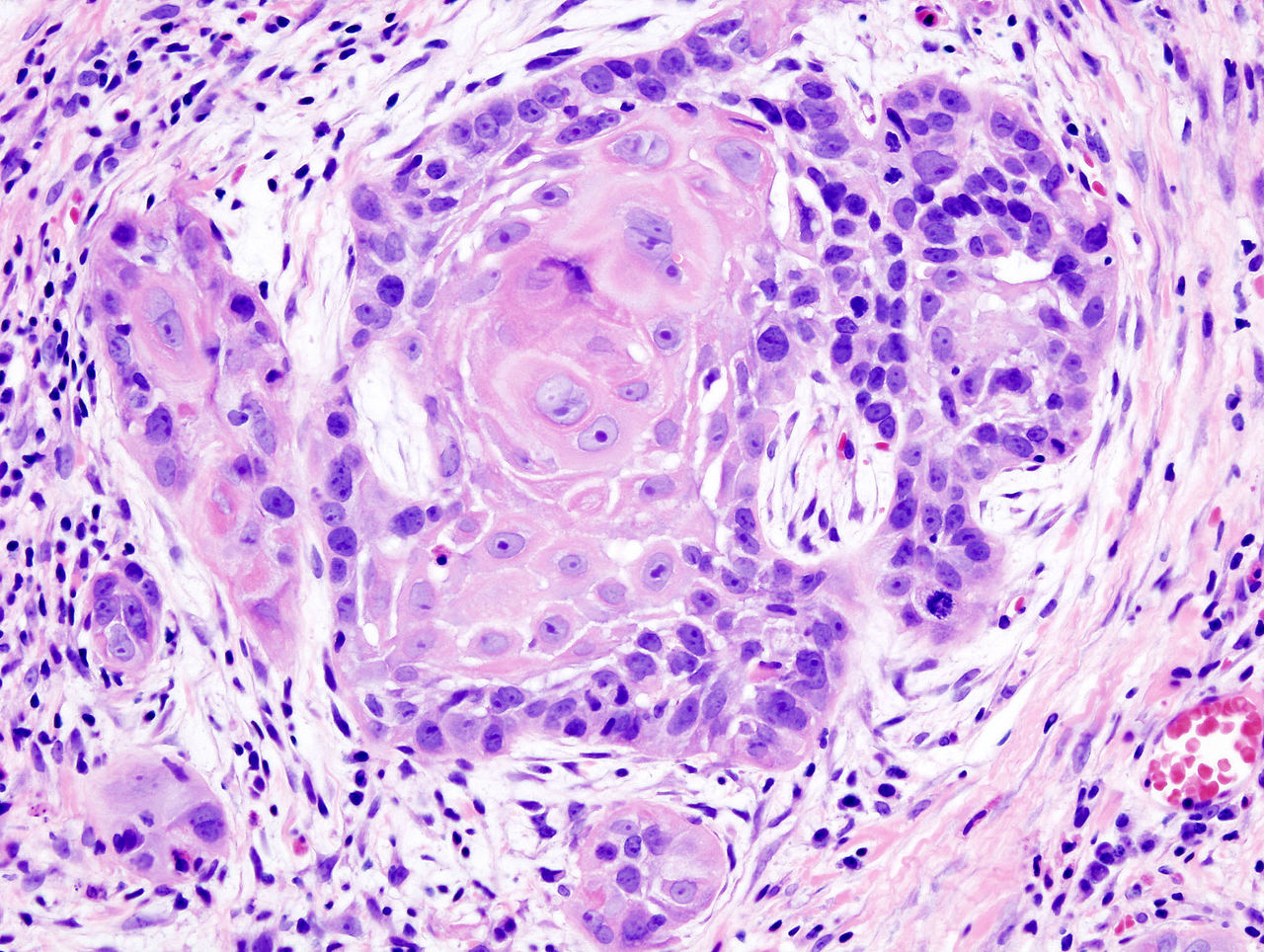
CHICAGO – Patients who had elective neck dissection at the time of primary surgery for oral cancers had a 12.5% better overall survival rate than did patients who had therapeutic neck dissections at the time of recurrence.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil D’Cruz of the head and neck service of Tata Memorial Centre, Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study,” he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Patients who had elective neck dissection at the time of primary surgery for oral cancers had a 12.5% better overall survival rate than did patients who had therapeutic neck dissections at the time of recurrence.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil D’Cruz of the head and neck service of Tata Memorial Centre, Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study,” he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Patients who had elective neck dissection at the time of primary surgery for oral cancers had a 12.5% better overall survival rate than did patients who had therapeutic neck dissections at the time of recurrence.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil D’Cruz of the head and neck service of Tata Memorial Centre, Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study,” he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE 2015 ASCO ANNUAL MEETING
AATS: No postpneumonectomy survival benefit in adding radiation to chemotherapy for NSCLC
SEATTLE – Adding radiotherapy to neoadjuvant chemotherapy does not improve long-term survival after pneumonectomy for non–small cell lung cancer, according to a Turkish investigation of 140 patients.
In the study, 100 (71.4%) patients had two to six cycles of platinum-based chemotherapy at least 3 weeks before surgery; 40 (28.6%) others underwent the same regimen with the addition of radiotherapy dosed at 45-66 Gy 6-8 weeks before surgery.
Five-year survival was 48% in the chemotherapy group and 50% in the chemoradiation group, an insignificant difference (P = .7).
“Chemotherapy before surgery is definitely beneficial, but I think we will get rid of the radiotherapy” in patients with operable tumors, said lead investigator Dr. Cengiz Gebitekin, professor and head of thoracic surgery at Uludag University in Bursa, Turkey.
“It does not provide any survival benefit,” and it might cause harm, he noted, adding that “the benefit of neoadjuvant treatment comes from the chemotherapy.”
The chemoradiation group showed a tendency toward tumor down-staging and higher complete response rates, but also a trend toward more radiation-induced tissue damage. The rate of bronchopleural fistula was 3% in the chemotherapy group and 5% in the chemoradiation group, although the difference was not significant. Even so, “some of these patients had pneumonectomies because of lung damage from the radiotherapy,” Dr. Gebitekin said at the annual meeting of the American Association for Thoracic Surgery.
It’s uncertain why radiation didn’t improve survival. The investigators excluded patients with known metastases or other malignancies, but it’s possible that some patients had occult metastases that had spread beyond the field of their localized neoadjuvant radiation, he said.
The patients were treated between 2000 and 2013 at Uludag University, Istanbul University, and Zurich University Hospital. They were 55 years old on average, and 84% were men.
About 40% of patients in both the chemotherapy and chemoradiation groups had right pneumonectomies; the rest had left pneumonectomies. Bronchopleural fistulas and other comorbidities were more common after right pneumonectomies, but not significantly so.
Seven patients (5%) in the chemotherapy group but none in the chemoradiation group died within 90 days of surgery.
About 32% of chemotherapy patients and 28% of chemoradiation patients (P = .6) developed major morbidities following surgery, including arrhythmias, pneumonia, empyema, and other problems.
Staples were used to close the bronchus in almost all patients, with the stump covered with live tissue in about 70%.
The majority of patients had stage IIb or IIIa disease on postop staging; postop staging was the only factor predictive of long-term survival, with higher-stage patients doing worse.
Dr. Gebitekin said that he had no relevant disclosures.
SEATTLE – Adding radiotherapy to neoadjuvant chemotherapy does not improve long-term survival after pneumonectomy for non–small cell lung cancer, according to a Turkish investigation of 140 patients.
In the study, 100 (71.4%) patients had two to six cycles of platinum-based chemotherapy at least 3 weeks before surgery; 40 (28.6%) others underwent the same regimen with the addition of radiotherapy dosed at 45-66 Gy 6-8 weeks before surgery.
Five-year survival was 48% in the chemotherapy group and 50% in the chemoradiation group, an insignificant difference (P = .7).
“Chemotherapy before surgery is definitely beneficial, but I think we will get rid of the radiotherapy” in patients with operable tumors, said lead investigator Dr. Cengiz Gebitekin, professor and head of thoracic surgery at Uludag University in Bursa, Turkey.
“It does not provide any survival benefit,” and it might cause harm, he noted, adding that “the benefit of neoadjuvant treatment comes from the chemotherapy.”
The chemoradiation group showed a tendency toward tumor down-staging and higher complete response rates, but also a trend toward more radiation-induced tissue damage. The rate of bronchopleural fistula was 3% in the chemotherapy group and 5% in the chemoradiation group, although the difference was not significant. Even so, “some of these patients had pneumonectomies because of lung damage from the radiotherapy,” Dr. Gebitekin said at the annual meeting of the American Association for Thoracic Surgery.
It’s uncertain why radiation didn’t improve survival. The investigators excluded patients with known metastases or other malignancies, but it’s possible that some patients had occult metastases that had spread beyond the field of their localized neoadjuvant radiation, he said.
The patients were treated between 2000 and 2013 at Uludag University, Istanbul University, and Zurich University Hospital. They were 55 years old on average, and 84% were men.
About 40% of patients in both the chemotherapy and chemoradiation groups had right pneumonectomies; the rest had left pneumonectomies. Bronchopleural fistulas and other comorbidities were more common after right pneumonectomies, but not significantly so.
Seven patients (5%) in the chemotherapy group but none in the chemoradiation group died within 90 days of surgery.
About 32% of chemotherapy patients and 28% of chemoradiation patients (P = .6) developed major morbidities following surgery, including arrhythmias, pneumonia, empyema, and other problems.
Staples were used to close the bronchus in almost all patients, with the stump covered with live tissue in about 70%.
The majority of patients had stage IIb or IIIa disease on postop staging; postop staging was the only factor predictive of long-term survival, with higher-stage patients doing worse.
Dr. Gebitekin said that he had no relevant disclosures.
SEATTLE – Adding radiotherapy to neoadjuvant chemotherapy does not improve long-term survival after pneumonectomy for non–small cell lung cancer, according to a Turkish investigation of 140 patients.
In the study, 100 (71.4%) patients had two to six cycles of platinum-based chemotherapy at least 3 weeks before surgery; 40 (28.6%) others underwent the same regimen with the addition of radiotherapy dosed at 45-66 Gy 6-8 weeks before surgery.
Five-year survival was 48% in the chemotherapy group and 50% in the chemoradiation group, an insignificant difference (P = .7).
“Chemotherapy before surgery is definitely beneficial, but I think we will get rid of the radiotherapy” in patients with operable tumors, said lead investigator Dr. Cengiz Gebitekin, professor and head of thoracic surgery at Uludag University in Bursa, Turkey.
“It does not provide any survival benefit,” and it might cause harm, he noted, adding that “the benefit of neoadjuvant treatment comes from the chemotherapy.”
The chemoradiation group showed a tendency toward tumor down-staging and higher complete response rates, but also a trend toward more radiation-induced tissue damage. The rate of bronchopleural fistula was 3% in the chemotherapy group and 5% in the chemoradiation group, although the difference was not significant. Even so, “some of these patients had pneumonectomies because of lung damage from the radiotherapy,” Dr. Gebitekin said at the annual meeting of the American Association for Thoracic Surgery.
It’s uncertain why radiation didn’t improve survival. The investigators excluded patients with known metastases or other malignancies, but it’s possible that some patients had occult metastases that had spread beyond the field of their localized neoadjuvant radiation, he said.
The patients were treated between 2000 and 2013 at Uludag University, Istanbul University, and Zurich University Hospital. They were 55 years old on average, and 84% were men.
About 40% of patients in both the chemotherapy and chemoradiation groups had right pneumonectomies; the rest had left pneumonectomies. Bronchopleural fistulas and other comorbidities were more common after right pneumonectomies, but not significantly so.
Seven patients (5%) in the chemotherapy group but none in the chemoradiation group died within 90 days of surgery.
About 32% of chemotherapy patients and 28% of chemoradiation patients (P = .6) developed major morbidities following surgery, including arrhythmias, pneumonia, empyema, and other problems.
Staples were used to close the bronchus in almost all patients, with the stump covered with live tissue in about 70%.
The majority of patients had stage IIb or IIIa disease on postop staging; postop staging was the only factor predictive of long-term survival, with higher-stage patients doing worse.
Dr. Gebitekin said that he had no relevant disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Limit neoadjuvant treatment to chemotherapy for non–small cell lung cancer patients.
Major finding: About half of patients were alive 5 years after pneumonectomies for lung cancer, whether they had neoadjuvant chemotherapy or chemoradiation.
Data source: Retrospective study of 140 pneumonectomy patients.
Disclosures: Dr. Gebitekin had no disclosures.
ASCO: German trial argues against complete nodal dissection for SLN-positive melanoma
CHICAGO – The case against performing a complete lymph node dissection in patients with melanoma who have micrometastases in their sentinel lymph node just got stronger in light of findings from a randomized, phase III clinical trial conducted by the German Dermatologic Cooperative Oncology Group (DeCOG).
With 483 patients studied and a median follow-up approaching 3 years, the rate of distant metastasis–free survival did not differ significantly between those who had a complete lymph node dissection and those who had simple watchful waiting, with just 0.3% separating the groups, the investigators reported at the annual meeting of the American Society of Clinical Oncology. Most other key outcomes were likewise statistically indistinguishable between groups.
“This is the first study which tested the general recommendation of complete lymphadenectomy in patients with positive nodes,” senior investigator Dr. Claus Garbe, a professor of dermatology at the University of Tübingen (Germany), said in a press briefing. “We cannot confirm this recommendation, and we expect that the surgical [practice] will change.”
However, Dr. Lynn Schuchter, an ASCO expert, as well as chief of hematology oncology and the C. Willard Robinson Professor of Hematology-Oncology at Penn Medicine in Philadelphia, took a more cautious view, saying that the findings require confirmation before being widely adopted into clinical practice.
“I would say this is a really important study. However, it’s a relatively small study, and I don’t think we would make a complete change in our recommendations yet based upon this study,” she commented.
The ongoing international Multicenter Selective Lymphadenectomy Trial II (MSLT-II), which has a target enrollment of about 1,900 patients and is designed to detect a smaller difference between groups, will provide further information on this issue, according to Dr. Schuchter. “So I think we’ll wait in terms of making definitive changes in our management, for the results of another, larger study with longer follow-up,” she said. “But [the German study] gives us, in that patient who is very concerned about lymphedema ... pertinent information to feel comfortable considering more of a watch-and-wait approach, in terms of monitoring somebody and not doing that surgery.”
Dr. Garbe and colleagues enrolled in their trial patients who underwent resection of a primary cutaneous melanoma of the trunk or extremities at least 1.00 mm in thickness and were determined to have stage III disease with a positive sentinel node containing individual tumor cells or micrometastases. They were randomly assigned to observation only or complete lymph node dissection. Both groups had a lymph node ultrasound exam every 3 months and CT/MRI or PET scans every 6 months.
With a median follow-up of 35 months, patients who had the complete lymph node dissection were about half as likely to develop regional metastases as peers who had watchful waiting (8.3% vs. 14.6%). But the groups did not differ significantly with respect to 3- and 5-year rates of recurrence-free survival, distant metastasis–free survival (the trial’s primary endpoint), and melanoma-specific survival.
The investigators plan to repeat their analysis in 3 years but do not expect the findings will change, according to Dr. Garbe, as the large majority of melanoma recurrences happen in the first 3 years after initial diagnosis.
Dr. Garbe disclosed ties to Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche Pharma AG. The trial was funded by German Cancer Aid.
CHICAGO – The case against performing a complete lymph node dissection in patients with melanoma who have micrometastases in their sentinel lymph node just got stronger in light of findings from a randomized, phase III clinical trial conducted by the German Dermatologic Cooperative Oncology Group (DeCOG).
With 483 patients studied and a median follow-up approaching 3 years, the rate of distant metastasis–free survival did not differ significantly between those who had a complete lymph node dissection and those who had simple watchful waiting, with just 0.3% separating the groups, the investigators reported at the annual meeting of the American Society of Clinical Oncology. Most other key outcomes were likewise statistically indistinguishable between groups.
“This is the first study which tested the general recommendation of complete lymphadenectomy in patients with positive nodes,” senior investigator Dr. Claus Garbe, a professor of dermatology at the University of Tübingen (Germany), said in a press briefing. “We cannot confirm this recommendation, and we expect that the surgical [practice] will change.”
However, Dr. Lynn Schuchter, an ASCO expert, as well as chief of hematology oncology and the C. Willard Robinson Professor of Hematology-Oncology at Penn Medicine in Philadelphia, took a more cautious view, saying that the findings require confirmation before being widely adopted into clinical practice.
“I would say this is a really important study. However, it’s a relatively small study, and I don’t think we would make a complete change in our recommendations yet based upon this study,” she commented.
The ongoing international Multicenter Selective Lymphadenectomy Trial II (MSLT-II), which has a target enrollment of about 1,900 patients and is designed to detect a smaller difference between groups, will provide further information on this issue, according to Dr. Schuchter. “So I think we’ll wait in terms of making definitive changes in our management, for the results of another, larger study with longer follow-up,” she said. “But [the German study] gives us, in that patient who is very concerned about lymphedema ... pertinent information to feel comfortable considering more of a watch-and-wait approach, in terms of monitoring somebody and not doing that surgery.”
Dr. Garbe and colleagues enrolled in their trial patients who underwent resection of a primary cutaneous melanoma of the trunk or extremities at least 1.00 mm in thickness and were determined to have stage III disease with a positive sentinel node containing individual tumor cells or micrometastases. They were randomly assigned to observation only or complete lymph node dissection. Both groups had a lymph node ultrasound exam every 3 months and CT/MRI or PET scans every 6 months.
With a median follow-up of 35 months, patients who had the complete lymph node dissection were about half as likely to develop regional metastases as peers who had watchful waiting (8.3% vs. 14.6%). But the groups did not differ significantly with respect to 3- and 5-year rates of recurrence-free survival, distant metastasis–free survival (the trial’s primary endpoint), and melanoma-specific survival.
The investigators plan to repeat their analysis in 3 years but do not expect the findings will change, according to Dr. Garbe, as the large majority of melanoma recurrences happen in the first 3 years after initial diagnosis.
Dr. Garbe disclosed ties to Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche Pharma AG. The trial was funded by German Cancer Aid.
CHICAGO – The case against performing a complete lymph node dissection in patients with melanoma who have micrometastases in their sentinel lymph node just got stronger in light of findings from a randomized, phase III clinical trial conducted by the German Dermatologic Cooperative Oncology Group (DeCOG).
With 483 patients studied and a median follow-up approaching 3 years, the rate of distant metastasis–free survival did not differ significantly between those who had a complete lymph node dissection and those who had simple watchful waiting, with just 0.3% separating the groups, the investigators reported at the annual meeting of the American Society of Clinical Oncology. Most other key outcomes were likewise statistically indistinguishable between groups.
“This is the first study which tested the general recommendation of complete lymphadenectomy in patients with positive nodes,” senior investigator Dr. Claus Garbe, a professor of dermatology at the University of Tübingen (Germany), said in a press briefing. “We cannot confirm this recommendation, and we expect that the surgical [practice] will change.”
However, Dr. Lynn Schuchter, an ASCO expert, as well as chief of hematology oncology and the C. Willard Robinson Professor of Hematology-Oncology at Penn Medicine in Philadelphia, took a more cautious view, saying that the findings require confirmation before being widely adopted into clinical practice.
“I would say this is a really important study. However, it’s a relatively small study, and I don’t think we would make a complete change in our recommendations yet based upon this study,” she commented.
The ongoing international Multicenter Selective Lymphadenectomy Trial II (MSLT-II), which has a target enrollment of about 1,900 patients and is designed to detect a smaller difference between groups, will provide further information on this issue, according to Dr. Schuchter. “So I think we’ll wait in terms of making definitive changes in our management, for the results of another, larger study with longer follow-up,” she said. “But [the German study] gives us, in that patient who is very concerned about lymphedema ... pertinent information to feel comfortable considering more of a watch-and-wait approach, in terms of monitoring somebody and not doing that surgery.”
Dr. Garbe and colleagues enrolled in their trial patients who underwent resection of a primary cutaneous melanoma of the trunk or extremities at least 1.00 mm in thickness and were determined to have stage III disease with a positive sentinel node containing individual tumor cells or micrometastases. They were randomly assigned to observation only or complete lymph node dissection. Both groups had a lymph node ultrasound exam every 3 months and CT/MRI or PET scans every 6 months.
With a median follow-up of 35 months, patients who had the complete lymph node dissection were about half as likely to develop regional metastases as peers who had watchful waiting (8.3% vs. 14.6%). But the groups did not differ significantly with respect to 3- and 5-year rates of recurrence-free survival, distant metastasis–free survival (the trial’s primary endpoint), and melanoma-specific survival.
The investigators plan to repeat their analysis in 3 years but do not expect the findings will change, according to Dr. Garbe, as the large majority of melanoma recurrences happen in the first 3 years after initial diagnosis.
Dr. Garbe disclosed ties to Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche Pharma AG. The trial was funded by German Cancer Aid.
AT ASCO 2015
Key clinical point: Complete nodal dissection fails to reduce distant metastasis in patients with melanoma who have micrometastases in sentinel nodes.
Major finding: Patients who did and did not undergo complete lymph node dissection were statistically indistinguishable with respect to distant metastases–free survival, recurrence-free survival, and melanoma-specific survival.
Data source: A randomized, phase III trial in 483 patients with stage III melanoma and micrometastases in their sentinel nodes.
Disclosures: Dr. Garbe disclosed ties to Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche Pharma AG. The trial was funded by German Cancer Aid.
Consider laser ablation therapy for treatment of benign thyroid nodules
NASHVILLE – Ultrasound-guided laser ablation therapy was found to be a clinically safe, effective, and well-tolerated option for the treatment of benign thyroid nodules, both solid and mixed, in a retrospective, multicenter study presented at the annual meeting of the American Association of Clinical Endocrinologists.
“We know that image-guided laser ablation of solid thyroid nodules has demonstrated favorable results in several prospective randomized trials,” said Dr. Enrico Papini of Regina Apostolorum Hospital in Rome. “However, these results were obtained in selected patients, with single treatments and fixed modalities of treatment; so the question is, what happens in a real. clinical practice?”
Dr. Papini explained that the aim of the study was to assess clinical efficacy and side effects of laser ablation therapy (LAT) in a large series of unselected benign thyroid nodules of variable structure and size, using data from centers who use LAT as a standard operating technique. Patients with solid or mixed nodules with up to 40% fluid composition, benign cytological findings, and normal thyroid function were included.
Clinical records of 1,534 thyroid nodules in 1,531 patients, all of whom were treated in the last 10 years, was collected from eight Italian thyroid referral centers. A total of 1,837 LAT procedures were performed on these nodules, of which 1,280 (83% of 1,534) were treated in a single session. All nodules were treated in no more than three consecutive sessions, with a fixed output power of 3 watts. According to Dr. Papini, the laser is only fired for up to 10 minutes.
Mean nodule volume significantly decreased following LAT from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11%, with an overall range of 48%-100%. Mixed nodules experienced significantly larger decreases than solid ones. On average, mixed nodule volume decreased 79% ± 7%, versus 72% ± 11% for solid nodules (P < .001) because of fluid components being drained prior to LAT.
Symptoms decreased from 49% at baseline to 10% at 12 months post-treatment. Similarly robust reductions were also seen in cosmetic signs, which decreased 86% to 8% over 12 months. Only 17 patients experienced a complication, including 8 with a “major” complication of dysphonia, which resolved within 2-84 days, and 9 with “minor” complications, such as skin burn and hematoma.
“Laser ablation was performed in outpatient setting, with no hospital admission after treatment,” said Dr. Papini. “It was well-tolerated, and severe pain – requiring more than 3 days of analgesics – occurred in less than 2% of patients.”
Dr. Papini did not report any relevant financial disclosures.
NASHVILLE – Ultrasound-guided laser ablation therapy was found to be a clinically safe, effective, and well-tolerated option for the treatment of benign thyroid nodules, both solid and mixed, in a retrospective, multicenter study presented at the annual meeting of the American Association of Clinical Endocrinologists.
“We know that image-guided laser ablation of solid thyroid nodules has demonstrated favorable results in several prospective randomized trials,” said Dr. Enrico Papini of Regina Apostolorum Hospital in Rome. “However, these results were obtained in selected patients, with single treatments and fixed modalities of treatment; so the question is, what happens in a real. clinical practice?”
Dr. Papini explained that the aim of the study was to assess clinical efficacy and side effects of laser ablation therapy (LAT) in a large series of unselected benign thyroid nodules of variable structure and size, using data from centers who use LAT as a standard operating technique. Patients with solid or mixed nodules with up to 40% fluid composition, benign cytological findings, and normal thyroid function were included.
Clinical records of 1,534 thyroid nodules in 1,531 patients, all of whom were treated in the last 10 years, was collected from eight Italian thyroid referral centers. A total of 1,837 LAT procedures were performed on these nodules, of which 1,280 (83% of 1,534) were treated in a single session. All nodules were treated in no more than three consecutive sessions, with a fixed output power of 3 watts. According to Dr. Papini, the laser is only fired for up to 10 minutes.
Mean nodule volume significantly decreased following LAT from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11%, with an overall range of 48%-100%. Mixed nodules experienced significantly larger decreases than solid ones. On average, mixed nodule volume decreased 79% ± 7%, versus 72% ± 11% for solid nodules (P < .001) because of fluid components being drained prior to LAT.
Symptoms decreased from 49% at baseline to 10% at 12 months post-treatment. Similarly robust reductions were also seen in cosmetic signs, which decreased 86% to 8% over 12 months. Only 17 patients experienced a complication, including 8 with a “major” complication of dysphonia, which resolved within 2-84 days, and 9 with “minor” complications, such as skin burn and hematoma.
“Laser ablation was performed in outpatient setting, with no hospital admission after treatment,” said Dr. Papini. “It was well-tolerated, and severe pain – requiring more than 3 days of analgesics – occurred in less than 2% of patients.”
Dr. Papini did not report any relevant financial disclosures.
NASHVILLE – Ultrasound-guided laser ablation therapy was found to be a clinically safe, effective, and well-tolerated option for the treatment of benign thyroid nodules, both solid and mixed, in a retrospective, multicenter study presented at the annual meeting of the American Association of Clinical Endocrinologists.
“We know that image-guided laser ablation of solid thyroid nodules has demonstrated favorable results in several prospective randomized trials,” said Dr. Enrico Papini of Regina Apostolorum Hospital in Rome. “However, these results were obtained in selected patients, with single treatments and fixed modalities of treatment; so the question is, what happens in a real. clinical practice?”
Dr. Papini explained that the aim of the study was to assess clinical efficacy and side effects of laser ablation therapy (LAT) in a large series of unselected benign thyroid nodules of variable structure and size, using data from centers who use LAT as a standard operating technique. Patients with solid or mixed nodules with up to 40% fluid composition, benign cytological findings, and normal thyroid function were included.
Clinical records of 1,534 thyroid nodules in 1,531 patients, all of whom were treated in the last 10 years, was collected from eight Italian thyroid referral centers. A total of 1,837 LAT procedures were performed on these nodules, of which 1,280 (83% of 1,534) were treated in a single session. All nodules were treated in no more than three consecutive sessions, with a fixed output power of 3 watts. According to Dr. Papini, the laser is only fired for up to 10 minutes.
Mean nodule volume significantly decreased following LAT from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11%, with an overall range of 48%-100%. Mixed nodules experienced significantly larger decreases than solid ones. On average, mixed nodule volume decreased 79% ± 7%, versus 72% ± 11% for solid nodules (P < .001) because of fluid components being drained prior to LAT.
Symptoms decreased from 49% at baseline to 10% at 12 months post-treatment. Similarly robust reductions were also seen in cosmetic signs, which decreased 86% to 8% over 12 months. Only 17 patients experienced a complication, including 8 with a “major” complication of dysphonia, which resolved within 2-84 days, and 9 with “minor” complications, such as skin burn and hematoma.
“Laser ablation was performed in outpatient setting, with no hospital admission after treatment,” said Dr. Papini. “It was well-tolerated, and severe pain – requiring more than 3 days of analgesics – occurred in less than 2% of patients.”
Dr. Papini did not report any relevant financial disclosures.
AT AACE 2015
Key clinical point: Ultrasound-guided laser ablation therapy is a clinically effective and well-tolerated tool for treating benign solid and mixed thyroid nodules.
Major finding: In 1,837 treatments for 1,534 nodules, mean nodule volume decreased from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11% (range 48%-100%).
Data source: Retrospective, multicenter study of 1,534 benign solid and mixed thyroid nodules.
Disclosures: Dr. Papini did not report any relevant financial disclosures.
Oral cancer survival lower with positive margins, public insurance
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Key clinical point: Treatment factors such as neck dissection, tumor margins, type of insurance, and health care facility impact 5-year survival after surgery for early stage oral cavity cancer.
Major finding: Radiation, chemotherapy, positive tumor margin, nonacademic facility, and nonprivate insurance were significantly associated with lower 5-year survival.
Data source: Retrospective study of 6,830 patients in National Cancer Data Base who underwent surgery to treat stage I or II oral cavity squamous cell cancer.
Disclosures: The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
Neoadjuvant chemotherapy for triple negative breast cancer improves conservation
SAN DIEGO – More women with triple negative breast cancer are able to have breast-conserving therapy (BCT) when they receive neoadjuvant systemic chemotherapy.
In a recent trial, 42% of women who were considered ineligible for BCT became eligible for the tissue-sparing surgery after their course of neoadjuvant chemotherapy (NACT) was administered. However, surgeons and patients must work together to decide on the best course of action, according to Dr. David Ollila of the University of North Carolina, Chapel Hill.
The relationship between NACT and the option to have breast-sparing surgery had not been well understood previously, especially for women with aggressive triple negative breast cancer, Dr. Ollila said at the annual meeting of the American Surgical Association.
What has been known is that women with breast cancer who receive NACT show overall improved pathological complete response (Lancet 2014;384:164-72), which is associated with better event-free and overall survival.
The current study, conducted within the larger CALGB 40603 randomized phase II clinical trial, captured the number of patients who converted from BCT-ineligible to BCT-eligible after NACT and before surgery. Investigators also tracked pathological complete response, defined as tumor-free margins on pathologic examination, for those receiving each type of surgery. Dr. Ollila presented his findings on behalf of his coinvestigators in the Alliance for Clinical Trials in Oncology.
The study used a 2x2 factorial design to compare paclitaxel with and without carboplatin followed by doxorubicin plus cyclophosphamide with and without bevacizumab. Before NACT, the surgeon determined whether or not the patient was a candidate for BCT and if not, why not. The process was repeated after NACT, with the surgeon again documenting his or her reasoning for the choice.
Complete data were available for 404 women, distributed evenly across chemotherapy treatment arms. Before NACT, 219 (54%) were judged by their surgeons to be BCT candidates, and 197 (90%) were still deemed eligible for BCT after NACT. Of the 185 (46%) judged ineligible for BCT before chemotherapy, 72 (42%) became eligible for tissue sparing surgery after NACT. “We achieved a very high conversion rate ... from BCT ineligible to eligible,” Dr. Ollila noted.
Overall, just over two-thirds of the 404 women (n = 275, 68%) became candidates for BCT before surgery, and of those, about two-thirds (n = 191, 69%) went on to have an attempted BCT surgery. Surgery was successful for 178 of these women. “Neoadjuvant chemotherapy led to BCT in 93% of selected triple negative patients,”said Dr. Ollila.
Pathological complete response did not differ significantly among the women who received BCT or mastectomy, whether the decision was made before or after chemotherapy.
Discussant Dr. Lisa Newman of the University of Michigan observed that NACT allows some breathing space for the patient and her surgeon to weigh choices, and when indicated, to receive genetic testing.
“We know from numerous population-based and institutional studies that many are opting for bilateral mastectomy, even if a lumpectomy would be optimal,”said Dr. Newman.
She asked whether patient decision making was tracked for this study. Dr. Ollila replied, “We did not include patient factors. We do not have prospective data on what the patient was thinking because we focused on the surgeon.” However, he said, plans are underway to quantify the patient perspective during the decision-making process.
The takeaway message, said Dr. Ollila, is that “We are letting people think that mastectomy is the best option; I think that sequential surgery is all right. I think we just need to try breast conserving therapy more often than we are.”
The study was partially sponsored by the Breast Cancer Research Foundation. The authors reported no conflicts of interest.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
SAN DIEGO – More women with triple negative breast cancer are able to have breast-conserving therapy (BCT) when they receive neoadjuvant systemic chemotherapy.
In a recent trial, 42% of women who were considered ineligible for BCT became eligible for the tissue-sparing surgery after their course of neoadjuvant chemotherapy (NACT) was administered. However, surgeons and patients must work together to decide on the best course of action, according to Dr. David Ollila of the University of North Carolina, Chapel Hill.
The relationship between NACT and the option to have breast-sparing surgery had not been well understood previously, especially for women with aggressive triple negative breast cancer, Dr. Ollila said at the annual meeting of the American Surgical Association.
What has been known is that women with breast cancer who receive NACT show overall improved pathological complete response (Lancet 2014;384:164-72), which is associated with better event-free and overall survival.
The current study, conducted within the larger CALGB 40603 randomized phase II clinical trial, captured the number of patients who converted from BCT-ineligible to BCT-eligible after NACT and before surgery. Investigators also tracked pathological complete response, defined as tumor-free margins on pathologic examination, for those receiving each type of surgery. Dr. Ollila presented his findings on behalf of his coinvestigators in the Alliance for Clinical Trials in Oncology.
The study used a 2x2 factorial design to compare paclitaxel with and without carboplatin followed by doxorubicin plus cyclophosphamide with and without bevacizumab. Before NACT, the surgeon determined whether or not the patient was a candidate for BCT and if not, why not. The process was repeated after NACT, with the surgeon again documenting his or her reasoning for the choice.
Complete data were available for 404 women, distributed evenly across chemotherapy treatment arms. Before NACT, 219 (54%) were judged by their surgeons to be BCT candidates, and 197 (90%) were still deemed eligible for BCT after NACT. Of the 185 (46%) judged ineligible for BCT before chemotherapy, 72 (42%) became eligible for tissue sparing surgery after NACT. “We achieved a very high conversion rate ... from BCT ineligible to eligible,” Dr. Ollila noted.
Overall, just over two-thirds of the 404 women (n = 275, 68%) became candidates for BCT before surgery, and of those, about two-thirds (n = 191, 69%) went on to have an attempted BCT surgery. Surgery was successful for 178 of these women. “Neoadjuvant chemotherapy led to BCT in 93% of selected triple negative patients,”said Dr. Ollila.
Pathological complete response did not differ significantly among the women who received BCT or mastectomy, whether the decision was made before or after chemotherapy.
Discussant Dr. Lisa Newman of the University of Michigan observed that NACT allows some breathing space for the patient and her surgeon to weigh choices, and when indicated, to receive genetic testing.
“We know from numerous population-based and institutional studies that many are opting for bilateral mastectomy, even if a lumpectomy would be optimal,”said Dr. Newman.
She asked whether patient decision making was tracked for this study. Dr. Ollila replied, “We did not include patient factors. We do not have prospective data on what the patient was thinking because we focused on the surgeon.” However, he said, plans are underway to quantify the patient perspective during the decision-making process.
The takeaway message, said Dr. Ollila, is that “We are letting people think that mastectomy is the best option; I think that sequential surgery is all right. I think we just need to try breast conserving therapy more often than we are.”
The study was partially sponsored by the Breast Cancer Research Foundation. The authors reported no conflicts of interest.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
SAN DIEGO – More women with triple negative breast cancer are able to have breast-conserving therapy (BCT) when they receive neoadjuvant systemic chemotherapy.
In a recent trial, 42% of women who were considered ineligible for BCT became eligible for the tissue-sparing surgery after their course of neoadjuvant chemotherapy (NACT) was administered. However, surgeons and patients must work together to decide on the best course of action, according to Dr. David Ollila of the University of North Carolina, Chapel Hill.
The relationship between NACT and the option to have breast-sparing surgery had not been well understood previously, especially for women with aggressive triple negative breast cancer, Dr. Ollila said at the annual meeting of the American Surgical Association.
What has been known is that women with breast cancer who receive NACT show overall improved pathological complete response (Lancet 2014;384:164-72), which is associated with better event-free and overall survival.
The current study, conducted within the larger CALGB 40603 randomized phase II clinical trial, captured the number of patients who converted from BCT-ineligible to BCT-eligible after NACT and before surgery. Investigators also tracked pathological complete response, defined as tumor-free margins on pathologic examination, for those receiving each type of surgery. Dr. Ollila presented his findings on behalf of his coinvestigators in the Alliance for Clinical Trials in Oncology.
The study used a 2x2 factorial design to compare paclitaxel with and without carboplatin followed by doxorubicin plus cyclophosphamide with and without bevacizumab. Before NACT, the surgeon determined whether or not the patient was a candidate for BCT and if not, why not. The process was repeated after NACT, with the surgeon again documenting his or her reasoning for the choice.
Complete data were available for 404 women, distributed evenly across chemotherapy treatment arms. Before NACT, 219 (54%) were judged by their surgeons to be BCT candidates, and 197 (90%) were still deemed eligible for BCT after NACT. Of the 185 (46%) judged ineligible for BCT before chemotherapy, 72 (42%) became eligible for tissue sparing surgery after NACT. “We achieved a very high conversion rate ... from BCT ineligible to eligible,” Dr. Ollila noted.
Overall, just over two-thirds of the 404 women (n = 275, 68%) became candidates for BCT before surgery, and of those, about two-thirds (n = 191, 69%) went on to have an attempted BCT surgery. Surgery was successful for 178 of these women. “Neoadjuvant chemotherapy led to BCT in 93% of selected triple negative patients,”said Dr. Ollila.
Pathological complete response did not differ significantly among the women who received BCT or mastectomy, whether the decision was made before or after chemotherapy.
Discussant Dr. Lisa Newman of the University of Michigan observed that NACT allows some breathing space for the patient and her surgeon to weigh choices, and when indicated, to receive genetic testing.
“We know from numerous population-based and institutional studies that many are opting for bilateral mastectomy, even if a lumpectomy would be optimal,”said Dr. Newman.
She asked whether patient decision making was tracked for this study. Dr. Ollila replied, “We did not include patient factors. We do not have prospective data on what the patient was thinking because we focused on the surgeon.” However, he said, plans are underway to quantify the patient perspective during the decision-making process.
The takeaway message, said Dr. Ollila, is that “We are letting people think that mastectomy is the best option; I think that sequential surgery is all right. I think we just need to try breast conserving therapy more often than we are.”
The study was partially sponsored by the Breast Cancer Research Foundation. The authors reported no conflicts of interest.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Neoadjuvant chemotherapy (NACT) for triple negative breast cancer resulted in increased eligibility for breast-conserving therapy (BCT).
Major finding: NACT allowed 42% of women with triple negative breast cancer previously ineligible for BCT to become BCT candidates.
Data source: CALGB 40603, a prospective, randomized controlled trial for women with stage II-III triple negative breast cancer.
Disclosures: The study was partially sponsored by the Breast Cancer Research Foundation. The authors reported no conflicts of interest.
ASA: Electroporation shows promise for locally advanced pancreatic cancer
SAN DIEGO – A surgical technique for ablating pancreatic tumors improved overall survival for individuals with locally advanced pancreatic cancer when combined with optimized chemotherapy and radiation therapy.
Pancreatic cancer is the fourth-leading cause of cancer death in the United States. It often is not detected until disease is advanced, with a 5-year survival rate of less than 6%. Further, the pancreas has tight anatomic association with such major vessels as the superior mesenteric artery and the celiac trunk, meaning that the primary tumor of advanced pancreatic cancer is difficult to resect with conventional surgical techniques.
Irreversible electroporation (IRE) is an Food and Drug Administration–approved surgical technique that delivers multiple targeted high-voltage charges of short duration – 70-90 microseconds – to tumor tissue. Cell death occurs slowly by apoptosis over 6-8 weeks, thus avoiding a large inflammatory response and allowing macrophages to help clear cell debris, Dr. Robert Martin of the University of Louisville (Ky.) said at the annual meeting of the American Surgical Association.
Previous work by Dr. Martin and others showed IRE to be safe for appropriately selected patients with locally advanced pancreatic cancers. For this study, Dr. Martin and his colleagues maintained an approved, prospective soft-tissue ablation registry for patients (n = 200; median age, 62 years) with locally advanced pancreatic cancer treated in participating centers from 2010 to 2014.
Participating patients received chemotherapy, radiation, or both as initial treatment according to the protocol of individual institutions. At 4-6 weeks after completing initial treatment, patients were assessed using a triple-phase CT scan and tumor markers. Those who showed no metastatic disease and whose tumors were stable were considered for IRE. Patients were generally well nourished and without multiple comorbidities.
The IRE procedure was performed with (n = 50) or without (n = 150) conventional tumor resection, depending on surgeon judgment. Complete tumor ablation occurred in all 50 patients who received resection and marginal IRE and in 148 of 150 who received IRE alone. Of the patients who had surgery, 20 experienced a total of 49 complications; of those who had IRE alone, 54 had a total of 100 complications. The overall grade of complications was 2, and mean length of stay was 6 days.
The disease progressed in 29% of the 200 patients, with a median overall survival of 28.3 months for those who had resection and IRE and 23.2 months for those receiving IRE alone. Patients receiving standard care survived a median of 13 months (P = .01).
IRE had a reasonable level of safety and demonstrated excellent local control of tumor growth, noted discussant Dr. Jeffrey Drebin, chair of the department of surgery at Penn Medicine, Philadelphia. He asked, however, whether the study would have benefited from an intention-to-treat analysis, since not all patients with locally advanced disease will be candidates for optimized chemotherapy.
Dr. Martin noted that, as better neoadjuvant treatments have come along, “we are taking far more patients to the operating room … because we are rewarded by being able to resect their tumors.”
He called for “cautious optimism” regarding the role of IRE. The next step should include validation of the study results in the United States, first through a single-arm study and then in a randomized controlled trial comparing IRE to radiation therapy. Full assessment of efficacy also will hinge on identifying appropriate imaging techniques for precise documentation of tumor response.
An attitude of “persistent nihilism” in treating pancreatic cancer can pose a barrier to the willingness of patients and providers to try multimodality, aggressive treatment, he said. “Precise management of locally advanced pancreatic cancer with trimodality treatment can lead to improvement.”
Dr. Martin reported compensation from Angiodynamics. The other authors reported no disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
SAN DIEGO – A surgical technique for ablating pancreatic tumors improved overall survival for individuals with locally advanced pancreatic cancer when combined with optimized chemotherapy and radiation therapy.
Pancreatic cancer is the fourth-leading cause of cancer death in the United States. It often is not detected until disease is advanced, with a 5-year survival rate of less than 6%. Further, the pancreas has tight anatomic association with such major vessels as the superior mesenteric artery and the celiac trunk, meaning that the primary tumor of advanced pancreatic cancer is difficult to resect with conventional surgical techniques.
Irreversible electroporation (IRE) is an Food and Drug Administration–approved surgical technique that delivers multiple targeted high-voltage charges of short duration – 70-90 microseconds – to tumor tissue. Cell death occurs slowly by apoptosis over 6-8 weeks, thus avoiding a large inflammatory response and allowing macrophages to help clear cell debris, Dr. Robert Martin of the University of Louisville (Ky.) said at the annual meeting of the American Surgical Association.
Previous work by Dr. Martin and others showed IRE to be safe for appropriately selected patients with locally advanced pancreatic cancers. For this study, Dr. Martin and his colleagues maintained an approved, prospective soft-tissue ablation registry for patients (n = 200; median age, 62 years) with locally advanced pancreatic cancer treated in participating centers from 2010 to 2014.
Participating patients received chemotherapy, radiation, or both as initial treatment according to the protocol of individual institutions. At 4-6 weeks after completing initial treatment, patients were assessed using a triple-phase CT scan and tumor markers. Those who showed no metastatic disease and whose tumors were stable were considered for IRE. Patients were generally well nourished and without multiple comorbidities.
The IRE procedure was performed with (n = 50) or without (n = 150) conventional tumor resection, depending on surgeon judgment. Complete tumor ablation occurred in all 50 patients who received resection and marginal IRE and in 148 of 150 who received IRE alone. Of the patients who had surgery, 20 experienced a total of 49 complications; of those who had IRE alone, 54 had a total of 100 complications. The overall grade of complications was 2, and mean length of stay was 6 days.
The disease progressed in 29% of the 200 patients, with a median overall survival of 28.3 months for those who had resection and IRE and 23.2 months for those receiving IRE alone. Patients receiving standard care survived a median of 13 months (P = .01).
IRE had a reasonable level of safety and demonstrated excellent local control of tumor growth, noted discussant Dr. Jeffrey Drebin, chair of the department of surgery at Penn Medicine, Philadelphia. He asked, however, whether the study would have benefited from an intention-to-treat analysis, since not all patients with locally advanced disease will be candidates for optimized chemotherapy.
Dr. Martin noted that, as better neoadjuvant treatments have come along, “we are taking far more patients to the operating room … because we are rewarded by being able to resect their tumors.”
He called for “cautious optimism” regarding the role of IRE. The next step should include validation of the study results in the United States, first through a single-arm study and then in a randomized controlled trial comparing IRE to radiation therapy. Full assessment of efficacy also will hinge on identifying appropriate imaging techniques for precise documentation of tumor response.
An attitude of “persistent nihilism” in treating pancreatic cancer can pose a barrier to the willingness of patients and providers to try multimodality, aggressive treatment, he said. “Precise management of locally advanced pancreatic cancer with trimodality treatment can lead to improvement.”
Dr. Martin reported compensation from Angiodynamics. The other authors reported no disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
SAN DIEGO – A surgical technique for ablating pancreatic tumors improved overall survival for individuals with locally advanced pancreatic cancer when combined with optimized chemotherapy and radiation therapy.
Pancreatic cancer is the fourth-leading cause of cancer death in the United States. It often is not detected until disease is advanced, with a 5-year survival rate of less than 6%. Further, the pancreas has tight anatomic association with such major vessels as the superior mesenteric artery and the celiac trunk, meaning that the primary tumor of advanced pancreatic cancer is difficult to resect with conventional surgical techniques.
Irreversible electroporation (IRE) is an Food and Drug Administration–approved surgical technique that delivers multiple targeted high-voltage charges of short duration – 70-90 microseconds – to tumor tissue. Cell death occurs slowly by apoptosis over 6-8 weeks, thus avoiding a large inflammatory response and allowing macrophages to help clear cell debris, Dr. Robert Martin of the University of Louisville (Ky.) said at the annual meeting of the American Surgical Association.
Previous work by Dr. Martin and others showed IRE to be safe for appropriately selected patients with locally advanced pancreatic cancers. For this study, Dr. Martin and his colleagues maintained an approved, prospective soft-tissue ablation registry for patients (n = 200; median age, 62 years) with locally advanced pancreatic cancer treated in participating centers from 2010 to 2014.
Participating patients received chemotherapy, radiation, or both as initial treatment according to the protocol of individual institutions. At 4-6 weeks after completing initial treatment, patients were assessed using a triple-phase CT scan and tumor markers. Those who showed no metastatic disease and whose tumors were stable were considered for IRE. Patients were generally well nourished and without multiple comorbidities.
The IRE procedure was performed with (n = 50) or without (n = 150) conventional tumor resection, depending on surgeon judgment. Complete tumor ablation occurred in all 50 patients who received resection and marginal IRE and in 148 of 150 who received IRE alone. Of the patients who had surgery, 20 experienced a total of 49 complications; of those who had IRE alone, 54 had a total of 100 complications. The overall grade of complications was 2, and mean length of stay was 6 days.
The disease progressed in 29% of the 200 patients, with a median overall survival of 28.3 months for those who had resection and IRE and 23.2 months for those receiving IRE alone. Patients receiving standard care survived a median of 13 months (P = .01).
IRE had a reasonable level of safety and demonstrated excellent local control of tumor growth, noted discussant Dr. Jeffrey Drebin, chair of the department of surgery at Penn Medicine, Philadelphia. He asked, however, whether the study would have benefited from an intention-to-treat analysis, since not all patients with locally advanced disease will be candidates for optimized chemotherapy.
Dr. Martin noted that, as better neoadjuvant treatments have come along, “we are taking far more patients to the operating room … because we are rewarded by being able to resect their tumors.”
He called for “cautious optimism” regarding the role of IRE. The next step should include validation of the study results in the United States, first through a single-arm study and then in a randomized controlled trial comparing IRE to radiation therapy. Full assessment of efficacy also will hinge on identifying appropriate imaging techniques for precise documentation of tumor response.
An attitude of “persistent nihilism” in treating pancreatic cancer can pose a barrier to the willingness of patients and providers to try multimodality, aggressive treatment, he said. “Precise management of locally advanced pancreatic cancer with trimodality treatment can lead to improvement.”
Dr. Martin reported compensation from Angiodynamics. The other authors reported no disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Irreversible electroporation was associated with a survival advantage, compared with standard care, for those with stage III pancreatic cancer.
Major findings: Median overall survival was 28.3 months for those who had resection and IRE and 23.2 months for those receiving IRE alone. Patients receiving standard care survived a median of 13 months (P = .01).
Data source: Prospective multicenter registry of 200 patients with stage III locally advanced pancreatic adenocarcinoma.
Disclosures: Dr. Martin reported compensation from Angiodynamics. The other authors reported no disclosures.
ASA: Radiation lowers local recurrence risk for DCIS patients with close or positive margins
SAN DIEGO – Radiation may benefit women with ductal carcinoma in situ (DCIS) who have breast-conserving surgery if their tumor margins are close or positive; however, wide tumor margins alone also may convey the same protection from local recurrence, Dr. Kimberly Van Zee reported at the annual meeting of the American Surgical Association.
Dr. Van Zee and her colleagues on the breast surgery service at New York’s Sloan Kettering Cancer Center examined the data from a large institutional database of DCIS patients to assess the relative benefit of radiation for various margin widths. They discovered that after adjusting for the other variables, patients with the widest tumor margins saw very little reduction in risk of 10-year recurrence when radiation was added – only 6%. However, this was still a significant difference and represented a hazard ratio of 0.54. Radiation gave patients with positive margins an absolute 18% risk reduction, for a hazard ratio of 0.10.
“We know that radiation reduces risk in all subsets of women with DCIS undergoing breast-conserving surgery,” she said. “But we really wanted to evaluate the relationship between margin width and recurrence and find the best margin width for DCIS with breast-conserving surgery.”
Over 20% of breast cancers are DCIS, and though overall mortality is low, as many as one in three patients will have local recurrence of their cancer. Radiation reduces the risk of local recurrence by about 50%, but it does not reduce the already low mortality associated with DCIS, she said.
Since radiation for DCIS may be associated with an increased risk for cardiovascular disease and certain rare malignancies, Dr. Van Zee said she and her colleagues were interested in identifying those women who were already at low risk for recurrence and would see little increased benefit from radiation.
Dr. Van Zee and her associates conducted a retrospective review of a prospectively collected database of women with DCIS who received treatment at Sloan Kettering Cancer Center between 1978 and 2010. The database contained multiple patient- and procedure-specific variables that were also factored into multivariable analysis in order to evaluate the relationships between margin width and recurrence, and to account for the contribution of radiation to reducing the risk of recurrence in women who received breast-conserving surgery for DCIS.
Overall, the database contained data for nearly 3,000 patients. Of the 2,996 studied, 72% were over the age of 50 and about 67% were postmenopausal. In 87% of cases, the diagnosis was made radiologically rather than clinically, and 60% of the patients had low or intermediate nuclear grade disease.
Dr. Van Zee and her colleagues assessed the 10-year recurrence rate for the 2,788 women whose excision margin width was known. Only 3% of these women had positive margins, and 75% had margin widths greater than 2 mm.
On multivariable analysis, wider margin width was associated with a significantly decreased 10-year risk of recurrence, but only for individuals who had not received radiation (P less than .0001). The hazard ratios for recurrence became progressively lower as margins widened, dropping to 0.31 for a margin of 10 mm or more.
Dr. Van Zee noted that the study was limited by its retrospective nature and the relatively small number of cases with positive margins. Also, cases with positive or close margins usually had more limited or focal disease at the margins, so recurrence rate estimates for this group may have underestimated risk of recurrence for those who had more significant disease.
During the discussion following her presentation, Dr. Van Zee noted that multiple factors are related to the risk of local recurrence, and that nomograms exist to help calculate risk and guide the decision to recommend radiation in women with close margins.
Dr. Patrick Borgen, chairman of the department of surgery at Maimonides Medical Center, New York, remarked that “biology beats technique. A growing wealth of information exists that there is a reservoir of DCIS that will progress so slowly as not to be significant. Is the next step in refining our approach better class prediction using genomic profiling?”
Dr. Van Zee agreed that genomic profiling will play a role, but noted that a study comparing DCIS score and multiple clinical variables would be expensive and archival pathology specimens would be difficult to obtain. Studies will mostly have to be prospective, she said.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
This article was updated May 11, 2015.
SAN DIEGO – Radiation may benefit women with ductal carcinoma in situ (DCIS) who have breast-conserving surgery if their tumor margins are close or positive; however, wide tumor margins alone also may convey the same protection from local recurrence, Dr. Kimberly Van Zee reported at the annual meeting of the American Surgical Association.
Dr. Van Zee and her colleagues on the breast surgery service at New York’s Sloan Kettering Cancer Center examined the data from a large institutional database of DCIS patients to assess the relative benefit of radiation for various margin widths. They discovered that after adjusting for the other variables, patients with the widest tumor margins saw very little reduction in risk of 10-year recurrence when radiation was added – only 6%. However, this was still a significant difference and represented a hazard ratio of 0.54. Radiation gave patients with positive margins an absolute 18% risk reduction, for a hazard ratio of 0.10.
“We know that radiation reduces risk in all subsets of women with DCIS undergoing breast-conserving surgery,” she said. “But we really wanted to evaluate the relationship between margin width and recurrence and find the best margin width for DCIS with breast-conserving surgery.”
Over 20% of breast cancers are DCIS, and though overall mortality is low, as many as one in three patients will have local recurrence of their cancer. Radiation reduces the risk of local recurrence by about 50%, but it does not reduce the already low mortality associated with DCIS, she said.
Since radiation for DCIS may be associated with an increased risk for cardiovascular disease and certain rare malignancies, Dr. Van Zee said she and her colleagues were interested in identifying those women who were already at low risk for recurrence and would see little increased benefit from radiation.
Dr. Van Zee and her associates conducted a retrospective review of a prospectively collected database of women with DCIS who received treatment at Sloan Kettering Cancer Center between 1978 and 2010. The database contained multiple patient- and procedure-specific variables that were also factored into multivariable analysis in order to evaluate the relationships between margin width and recurrence, and to account for the contribution of radiation to reducing the risk of recurrence in women who received breast-conserving surgery for DCIS.
Overall, the database contained data for nearly 3,000 patients. Of the 2,996 studied, 72% were over the age of 50 and about 67% were postmenopausal. In 87% of cases, the diagnosis was made radiologically rather than clinically, and 60% of the patients had low or intermediate nuclear grade disease.
Dr. Van Zee and her colleagues assessed the 10-year recurrence rate for the 2,788 women whose excision margin width was known. Only 3% of these women had positive margins, and 75% had margin widths greater than 2 mm.
On multivariable analysis, wider margin width was associated with a significantly decreased 10-year risk of recurrence, but only for individuals who had not received radiation (P less than .0001). The hazard ratios for recurrence became progressively lower as margins widened, dropping to 0.31 for a margin of 10 mm or more.
Dr. Van Zee noted that the study was limited by its retrospective nature and the relatively small number of cases with positive margins. Also, cases with positive or close margins usually had more limited or focal disease at the margins, so recurrence rate estimates for this group may have underestimated risk of recurrence for those who had more significant disease.
During the discussion following her presentation, Dr. Van Zee noted that multiple factors are related to the risk of local recurrence, and that nomograms exist to help calculate risk and guide the decision to recommend radiation in women with close margins.
Dr. Patrick Borgen, chairman of the department of surgery at Maimonides Medical Center, New York, remarked that “biology beats technique. A growing wealth of information exists that there is a reservoir of DCIS that will progress so slowly as not to be significant. Is the next step in refining our approach better class prediction using genomic profiling?”
Dr. Van Zee agreed that genomic profiling will play a role, but noted that a study comparing DCIS score and multiple clinical variables would be expensive and archival pathology specimens would be difficult to obtain. Studies will mostly have to be prospective, she said.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
This article was updated May 11, 2015.
SAN DIEGO – Radiation may benefit women with ductal carcinoma in situ (DCIS) who have breast-conserving surgery if their tumor margins are close or positive; however, wide tumor margins alone also may convey the same protection from local recurrence, Dr. Kimberly Van Zee reported at the annual meeting of the American Surgical Association.
Dr. Van Zee and her colleagues on the breast surgery service at New York’s Sloan Kettering Cancer Center examined the data from a large institutional database of DCIS patients to assess the relative benefit of radiation for various margin widths. They discovered that after adjusting for the other variables, patients with the widest tumor margins saw very little reduction in risk of 10-year recurrence when radiation was added – only 6%. However, this was still a significant difference and represented a hazard ratio of 0.54. Radiation gave patients with positive margins an absolute 18% risk reduction, for a hazard ratio of 0.10.
“We know that radiation reduces risk in all subsets of women with DCIS undergoing breast-conserving surgery,” she said. “But we really wanted to evaluate the relationship between margin width and recurrence and find the best margin width for DCIS with breast-conserving surgery.”
Over 20% of breast cancers are DCIS, and though overall mortality is low, as many as one in three patients will have local recurrence of their cancer. Radiation reduces the risk of local recurrence by about 50%, but it does not reduce the already low mortality associated with DCIS, she said.
Since radiation for DCIS may be associated with an increased risk for cardiovascular disease and certain rare malignancies, Dr. Van Zee said she and her colleagues were interested in identifying those women who were already at low risk for recurrence and would see little increased benefit from radiation.
Dr. Van Zee and her associates conducted a retrospective review of a prospectively collected database of women with DCIS who received treatment at Sloan Kettering Cancer Center between 1978 and 2010. The database contained multiple patient- and procedure-specific variables that were also factored into multivariable analysis in order to evaluate the relationships between margin width and recurrence, and to account for the contribution of radiation to reducing the risk of recurrence in women who received breast-conserving surgery for DCIS.
Overall, the database contained data for nearly 3,000 patients. Of the 2,996 studied, 72% were over the age of 50 and about 67% were postmenopausal. In 87% of cases, the diagnosis was made radiologically rather than clinically, and 60% of the patients had low or intermediate nuclear grade disease.
Dr. Van Zee and her colleagues assessed the 10-year recurrence rate for the 2,788 women whose excision margin width was known. Only 3% of these women had positive margins, and 75% had margin widths greater than 2 mm.
On multivariable analysis, wider margin width was associated with a significantly decreased 10-year risk of recurrence, but only for individuals who had not received radiation (P less than .0001). The hazard ratios for recurrence became progressively lower as margins widened, dropping to 0.31 for a margin of 10 mm or more.
Dr. Van Zee noted that the study was limited by its retrospective nature and the relatively small number of cases with positive margins. Also, cases with positive or close margins usually had more limited or focal disease at the margins, so recurrence rate estimates for this group may have underestimated risk of recurrence for those who had more significant disease.
During the discussion following her presentation, Dr. Van Zee noted that multiple factors are related to the risk of local recurrence, and that nomograms exist to help calculate risk and guide the decision to recommend radiation in women with close margins.
Dr. Patrick Borgen, chairman of the department of surgery at Maimonides Medical Center, New York, remarked that “biology beats technique. A growing wealth of information exists that there is a reservoir of DCIS that will progress so slowly as not to be significant. Is the next step in refining our approach better class prediction using genomic profiling?”
Dr. Van Zee agreed that genomic profiling will play a role, but noted that a study comparing DCIS score and multiple clinical variables would be expensive and archival pathology specimens would be difficult to obtain. Studies will mostly have to be prospective, she said.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
This article was updated May 11, 2015.
AT THE ASA ANNUAL MEETING
Key clinical point: Ductal carcinoma in situ patients with close or positive tumor margins may benefit more from radiation.
Major finding: Radiation gave patients with positive margins an absolute 18% risk reduction, for a hazard ratio of 0.10.
Data source: Retrospective review of a prospective database of DCIS patients undergoing breast-conserving surgery from 1978 to 2010.
Disclosures: The authors reported no disclosures.
ELCC: Urine tumor DNA shows high testing promise
GENEVA – Blood sampling provides a less invasive alternative to tumor biopsy for collecting cancer DNA for mutation testing, but retrieving tumor DNA from a patient’s urine is least invasive of all.
In an early phase of clinical investigation, researchers assessed the feasibility of using urine to collect cell-free tumor DNA to detect mutations in 34 patients with advanced non–small cell lung cancer. The results suggested that testing circulating tumor (ct) DNA isolated from patients’ urine was sensitive compared with testing DNA from biopsied specimens of the primary tumor, and it was able to flag tumor changes early, before clinically-identifiable effects appeared, Dr. Hatim Husain said at the European Lung Cancer Conference.
He used ctDNA isolated from patients’ urine to test for the presence of three different resistance mutations within the epidermal growth factor receptor (EGFR) gene. Genetic testing of biopsy specimens showed 10 patients carried the T790M mutation, 18 carried an exon 19 deletion, and eight carried an exon 21 L858R mutation. Of these 36 mutations in 34 patients, testing ctDNA isolated from urine identified 35 as positive, a 97% overall concordance rate.
In addition, testing with ctDNA from urine also picked up three additional T790M mutations not seen in the three corresponding tumor-biopsy specimens, but in patients with high clinical suspicion for carrying an EGFR mutation, Dr. Husain reported.
Further evidence for the utility of urinary ctDNA came from following 22 patients on treatment with erlotinib (Tarceva) and monitored for their acquisition of an EGFR-gene mutation making the tumor erlotinib resistant. Dr. Husain and his associates ran a DNA test every 3-6 weeks and tracked the time until patients developed radiographic progression. Using urinary ctDNA, they found four patients who developed EGFR mutations 29-111 days before clinical progression of the tumor became radiographically apparent.
The ctDNA that ends up in a patient’s urine starts out circulating in the blood; urine works as the main elimination route. Urine ctDNA is more concentrated than in blood, and ctDNA remains stable in urine at ambient temperature for 2 weeks, said Dr. Husain, an oncology researcher at the University of California San Diego, La Jolla.
“These interim results suggest that use of urinary ctDNA has potential to detect EGFR T790M status in a higher number of study subjects and may make some patients eligible for therapy who would by tissue biopsy be falsely classified as negative,” Dr. Husain said in a written statement. “Detecting the emergence of EGFR T790M mutations before progression has the potential to enable physicians to better align therapeutic selection and inform early therapeutic decision making,” he said.
Testing ctDNA in patients’ urine is a “novel way to do noninvasive testing,” said Dr. Egbert F. Smit, professor of pulmonary medicine at VU University Medical Center in Amsterdam and the meeting’s designated discussant for Dr. Husain’s report. “It’s attractive for collecting ctDNA because you get a high concentration, and it has potential for a high level of sensitivity. It may have potential for showing how a tumor reacts to treatment. The method seems robust, but we still need data on reproducibility and cost effectiveness,” Dr. Smit cautioned.
On Twitter @mitchelzoler
GENEVA – Blood sampling provides a less invasive alternative to tumor biopsy for collecting cancer DNA for mutation testing, but retrieving tumor DNA from a patient’s urine is least invasive of all.
In an early phase of clinical investigation, researchers assessed the feasibility of using urine to collect cell-free tumor DNA to detect mutations in 34 patients with advanced non–small cell lung cancer. The results suggested that testing circulating tumor (ct) DNA isolated from patients’ urine was sensitive compared with testing DNA from biopsied specimens of the primary tumor, and it was able to flag tumor changes early, before clinically-identifiable effects appeared, Dr. Hatim Husain said at the European Lung Cancer Conference.
He used ctDNA isolated from patients’ urine to test for the presence of three different resistance mutations within the epidermal growth factor receptor (EGFR) gene. Genetic testing of biopsy specimens showed 10 patients carried the T790M mutation, 18 carried an exon 19 deletion, and eight carried an exon 21 L858R mutation. Of these 36 mutations in 34 patients, testing ctDNA isolated from urine identified 35 as positive, a 97% overall concordance rate.
In addition, testing with ctDNA from urine also picked up three additional T790M mutations not seen in the three corresponding tumor-biopsy specimens, but in patients with high clinical suspicion for carrying an EGFR mutation, Dr. Husain reported.
Further evidence for the utility of urinary ctDNA came from following 22 patients on treatment with erlotinib (Tarceva) and monitored for their acquisition of an EGFR-gene mutation making the tumor erlotinib resistant. Dr. Husain and his associates ran a DNA test every 3-6 weeks and tracked the time until patients developed radiographic progression. Using urinary ctDNA, they found four patients who developed EGFR mutations 29-111 days before clinical progression of the tumor became radiographically apparent.
The ctDNA that ends up in a patient’s urine starts out circulating in the blood; urine works as the main elimination route. Urine ctDNA is more concentrated than in blood, and ctDNA remains stable in urine at ambient temperature for 2 weeks, said Dr. Husain, an oncology researcher at the University of California San Diego, La Jolla.
“These interim results suggest that use of urinary ctDNA has potential to detect EGFR T790M status in a higher number of study subjects and may make some patients eligible for therapy who would by tissue biopsy be falsely classified as negative,” Dr. Husain said in a written statement. “Detecting the emergence of EGFR T790M mutations before progression has the potential to enable physicians to better align therapeutic selection and inform early therapeutic decision making,” he said.
Testing ctDNA in patients’ urine is a “novel way to do noninvasive testing,” said Dr. Egbert F. Smit, professor of pulmonary medicine at VU University Medical Center in Amsterdam and the meeting’s designated discussant for Dr. Husain’s report. “It’s attractive for collecting ctDNA because you get a high concentration, and it has potential for a high level of sensitivity. It may have potential for showing how a tumor reacts to treatment. The method seems robust, but we still need data on reproducibility and cost effectiveness,” Dr. Smit cautioned.
On Twitter @mitchelzoler
GENEVA – Blood sampling provides a less invasive alternative to tumor biopsy for collecting cancer DNA for mutation testing, but retrieving tumor DNA from a patient’s urine is least invasive of all.
In an early phase of clinical investigation, researchers assessed the feasibility of using urine to collect cell-free tumor DNA to detect mutations in 34 patients with advanced non–small cell lung cancer. The results suggested that testing circulating tumor (ct) DNA isolated from patients’ urine was sensitive compared with testing DNA from biopsied specimens of the primary tumor, and it was able to flag tumor changes early, before clinically-identifiable effects appeared, Dr. Hatim Husain said at the European Lung Cancer Conference.
He used ctDNA isolated from patients’ urine to test for the presence of three different resistance mutations within the epidermal growth factor receptor (EGFR) gene. Genetic testing of biopsy specimens showed 10 patients carried the T790M mutation, 18 carried an exon 19 deletion, and eight carried an exon 21 L858R mutation. Of these 36 mutations in 34 patients, testing ctDNA isolated from urine identified 35 as positive, a 97% overall concordance rate.
In addition, testing with ctDNA from urine also picked up three additional T790M mutations not seen in the three corresponding tumor-biopsy specimens, but in patients with high clinical suspicion for carrying an EGFR mutation, Dr. Husain reported.
Further evidence for the utility of urinary ctDNA came from following 22 patients on treatment with erlotinib (Tarceva) and monitored for their acquisition of an EGFR-gene mutation making the tumor erlotinib resistant. Dr. Husain and his associates ran a DNA test every 3-6 weeks and tracked the time until patients developed radiographic progression. Using urinary ctDNA, they found four patients who developed EGFR mutations 29-111 days before clinical progression of the tumor became radiographically apparent.
The ctDNA that ends up in a patient’s urine starts out circulating in the blood; urine works as the main elimination route. Urine ctDNA is more concentrated than in blood, and ctDNA remains stable in urine at ambient temperature for 2 weeks, said Dr. Husain, an oncology researcher at the University of California San Diego, La Jolla.
“These interim results suggest that use of urinary ctDNA has potential to detect EGFR T790M status in a higher number of study subjects and may make some patients eligible for therapy who would by tissue biopsy be falsely classified as negative,” Dr. Husain said in a written statement. “Detecting the emergence of EGFR T790M mutations before progression has the potential to enable physicians to better align therapeutic selection and inform early therapeutic decision making,” he said.
Testing ctDNA in patients’ urine is a “novel way to do noninvasive testing,” said Dr. Egbert F. Smit, professor of pulmonary medicine at VU University Medical Center in Amsterdam and the meeting’s designated discussant for Dr. Husain’s report. “It’s attractive for collecting ctDNA because you get a high concentration, and it has potential for a high level of sensitivity. It may have potential for showing how a tumor reacts to treatment. The method seems robust, but we still need data on reproducibility and cost effectiveness,” Dr. Smit cautioned.
On Twitter @mitchelzoler
AT ELCC 2015
Key clinical point: Urine showed potential as a fully noninvasive source for circulating tumor DNA with high testing concordance compared with tumor-biopsy DNA in initial clinical experience with 34 lung cancer patients.
Major finding: Urine circulating tumor DNA had overall concordance of 97% compared with tumor-biopsy DNA for detecting treatment-altering mutations.
Data source: Single-center study of 34 patients with advanced non-small cell lung cancer.
Disclosures: The study was sponsored by Trovagene, the company developing the tests used in the study. Several coauthors on the study are Trovagene employees. Dr. Husain had no personal disclosures.
Biomarker correlates with pancreatic cancer severity
HOUSTON – A circulating biomarker has the potential to identify metastatic pancreatic cancer and may be able to predict prognosis, investigators said.
Levels of a gene encoding for the gap-junction beta 3 (GJB3) protein were highly elevated in both pancreatic cancer cell lines and in blood samples from patients with pancreatic ductal adenocarcinoma (PDAC), but the gene was undetectable in blood samples from patients without cancer, reported Dr. Raoud Marayati from the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
An analysis of gene expression in tumors from patients with PDAC also showed that patients with tumors expressing higher levels of GJB3 had significantly worse overall survival rates.
“GJB3 is highly expressed in blood samples from patients with metastatic versus local pancreatic cancer. GJB3 is a potential circulating biomarker for metastatic pancreatic cancer,” Dr. Marayati said at the annual Society of Surgical Oncology Cancer Symposium.
Markers lacking
PDAC is a highly aggressive cancer with a propensity for early invasion and metastasis. More than half of all patients (53%) have metastatic disease at the time of diagnosis, and 5-year survival for these patients is only 2.3%, according to the Surveillance, Epidemiology, and End Results (SEER) cancer statistics review for 2014, she noted.
The only currently available clinical biomarker for PDAC, carbohydrate antigen 19-9 (CA 19-9), generally correlates with treatment response and disease recurrence but has low sensitivity and specificity, Dr. Marayati said.
Circulating tumor cells (CTCs) – cells shed from the primary tumor into circulation – hold promise for better detection of cancer, but CTCs from pancreatic tumors have proven to be extremely difficult to isolate and count, she added.
To see whether they could identify circulating biomarkers of metastatic PDAC, Dr. Marayati and colleagues working in the laboratory of Dr. Jen Jen Yeh at the university first identified 76 genes that are differentially overexpressed in metastatic PDAC tumors, compared with localized primary tumors and with normal tissues.
The investigators looked for expression of the genes in 11 pancreatic cancer cell lines and in blood samples from 20 patients with pancreatic cancer and four without cancer. As noted, one gene, GJB3, was highly expressed in all of the cell-line samples but could not be detected in white blood cells from patients without PDAC.
To validate GJB3 as a potential cancer marker, the authors looked at expression levels in circulating tumor cells and found that, among the patients with cancer, expression levels of GBJ3 were significantly higher in those with metastatic disease, compared with localized disease (P = .016).
“That would suggest that GJB3 is a potential circulating biomarker specifically for metastatic pancreatic cancer patients,” Dr. Marayati said.
The investigators hypothesized that GJB3 may play a role in the biology of pancreatic cancer metastasis. To test this idea, they looked at resected tumors from 131 patients with primary pancreatic cancer and found that high tumor expression of GJB3 was associated with worse survival. Median overall survival among 32 patients with low levels of gene expression in their tumors was 24 months, compared with 15 months for 99 patients with high levels of GJB3 expression (P = .031).
The investigators plan to further validate the marker by testing it with larger samples from both patients with pancreatic cancer and controls.
The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
HOUSTON – A circulating biomarker has the potential to identify metastatic pancreatic cancer and may be able to predict prognosis, investigators said.
Levels of a gene encoding for the gap-junction beta 3 (GJB3) protein were highly elevated in both pancreatic cancer cell lines and in blood samples from patients with pancreatic ductal adenocarcinoma (PDAC), but the gene was undetectable in blood samples from patients without cancer, reported Dr. Raoud Marayati from the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
An analysis of gene expression in tumors from patients with PDAC also showed that patients with tumors expressing higher levels of GJB3 had significantly worse overall survival rates.
“GJB3 is highly expressed in blood samples from patients with metastatic versus local pancreatic cancer. GJB3 is a potential circulating biomarker for metastatic pancreatic cancer,” Dr. Marayati said at the annual Society of Surgical Oncology Cancer Symposium.
Markers lacking
PDAC is a highly aggressive cancer with a propensity for early invasion and metastasis. More than half of all patients (53%) have metastatic disease at the time of diagnosis, and 5-year survival for these patients is only 2.3%, according to the Surveillance, Epidemiology, and End Results (SEER) cancer statistics review for 2014, she noted.
The only currently available clinical biomarker for PDAC, carbohydrate antigen 19-9 (CA 19-9), generally correlates with treatment response and disease recurrence but has low sensitivity and specificity, Dr. Marayati said.
Circulating tumor cells (CTCs) – cells shed from the primary tumor into circulation – hold promise for better detection of cancer, but CTCs from pancreatic tumors have proven to be extremely difficult to isolate and count, she added.
To see whether they could identify circulating biomarkers of metastatic PDAC, Dr. Marayati and colleagues working in the laboratory of Dr. Jen Jen Yeh at the university first identified 76 genes that are differentially overexpressed in metastatic PDAC tumors, compared with localized primary tumors and with normal tissues.
The investigators looked for expression of the genes in 11 pancreatic cancer cell lines and in blood samples from 20 patients with pancreatic cancer and four without cancer. As noted, one gene, GJB3, was highly expressed in all of the cell-line samples but could not be detected in white blood cells from patients without PDAC.
To validate GJB3 as a potential cancer marker, the authors looked at expression levels in circulating tumor cells and found that, among the patients with cancer, expression levels of GBJ3 were significantly higher in those with metastatic disease, compared with localized disease (P = .016).
“That would suggest that GJB3 is a potential circulating biomarker specifically for metastatic pancreatic cancer patients,” Dr. Marayati said.
The investigators hypothesized that GJB3 may play a role in the biology of pancreatic cancer metastasis. To test this idea, they looked at resected tumors from 131 patients with primary pancreatic cancer and found that high tumor expression of GJB3 was associated with worse survival. Median overall survival among 32 patients with low levels of gene expression in their tumors was 24 months, compared with 15 months for 99 patients with high levels of GJB3 expression (P = .031).
The investigators plan to further validate the marker by testing it with larger samples from both patients with pancreatic cancer and controls.
The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
HOUSTON – A circulating biomarker has the potential to identify metastatic pancreatic cancer and may be able to predict prognosis, investigators said.
Levels of a gene encoding for the gap-junction beta 3 (GJB3) protein were highly elevated in both pancreatic cancer cell lines and in blood samples from patients with pancreatic ductal adenocarcinoma (PDAC), but the gene was undetectable in blood samples from patients without cancer, reported Dr. Raoud Marayati from the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
An analysis of gene expression in tumors from patients with PDAC also showed that patients with tumors expressing higher levels of GJB3 had significantly worse overall survival rates.
“GJB3 is highly expressed in blood samples from patients with metastatic versus local pancreatic cancer. GJB3 is a potential circulating biomarker for metastatic pancreatic cancer,” Dr. Marayati said at the annual Society of Surgical Oncology Cancer Symposium.
Markers lacking
PDAC is a highly aggressive cancer with a propensity for early invasion and metastasis. More than half of all patients (53%) have metastatic disease at the time of diagnosis, and 5-year survival for these patients is only 2.3%, according to the Surveillance, Epidemiology, and End Results (SEER) cancer statistics review for 2014, she noted.
The only currently available clinical biomarker for PDAC, carbohydrate antigen 19-9 (CA 19-9), generally correlates with treatment response and disease recurrence but has low sensitivity and specificity, Dr. Marayati said.
Circulating tumor cells (CTCs) – cells shed from the primary tumor into circulation – hold promise for better detection of cancer, but CTCs from pancreatic tumors have proven to be extremely difficult to isolate and count, she added.
To see whether they could identify circulating biomarkers of metastatic PDAC, Dr. Marayati and colleagues working in the laboratory of Dr. Jen Jen Yeh at the university first identified 76 genes that are differentially overexpressed in metastatic PDAC tumors, compared with localized primary tumors and with normal tissues.
The investigators looked for expression of the genes in 11 pancreatic cancer cell lines and in blood samples from 20 patients with pancreatic cancer and four without cancer. As noted, one gene, GJB3, was highly expressed in all of the cell-line samples but could not be detected in white blood cells from patients without PDAC.
To validate GJB3 as a potential cancer marker, the authors looked at expression levels in circulating tumor cells and found that, among the patients with cancer, expression levels of GBJ3 were significantly higher in those with metastatic disease, compared with localized disease (P = .016).
“That would suggest that GJB3 is a potential circulating biomarker specifically for metastatic pancreatic cancer patients,” Dr. Marayati said.
The investigators hypothesized that GJB3 may play a role in the biology of pancreatic cancer metastasis. To test this idea, they looked at resected tumors from 131 patients with primary pancreatic cancer and found that high tumor expression of GJB3 was associated with worse survival. Median overall survival among 32 patients with low levels of gene expression in their tumors was 24 months, compared with 15 months for 99 patients with high levels of GJB3 expression (P = .031).
The investigators plan to further validate the marker by testing it with larger samples from both patients with pancreatic cancer and controls.
The study funding source was not disclosed. Dr. Marayati reported having no disclosures.
AT SSO 2015
Key clinical point: GJB3 may be a serum marker for metastatic pancreatic ductal adenocarcinoma.
Major finding: GJB3 was expressed at significantly higher levels in patients with metastatic vs. localized PDAC.
Data source: Gene expression analyses involving patient tumor samples and 11 pancreatic cancer cell lines.
Disclosures: The study funding source was not disclosed. Dr. Marayati reported having no disclosures.