User login
'Vogl, New York' offers San Antonio perspectives
In an interview with Frontline Medical News, New York oncologist and San Antonio Breast Cancer Symposium star gadfly Dr. Steven Vogl gives his quick take on several of the studies making news at this year's meeting.
In an interview with Frontline Medical News, New York oncologist and San Antonio Breast Cancer Symposium star gadfly Dr. Steven Vogl gives his quick take on several of the studies making news at this year's meeting.
In an interview with Frontline Medical News, New York oncologist and San Antonio Breast Cancer Symposium star gadfly Dr. Steven Vogl gives his quick take on several of the studies making news at this year's meeting.
New mega-review underscores mammography’s benefits
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
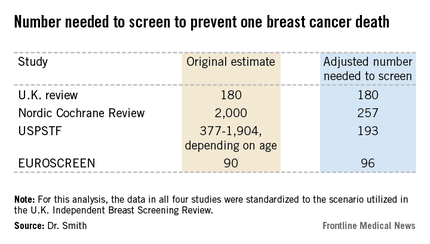
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.
But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
EXPERT OPINION FROM SABCS 2013
No survival benefit to bisphosphonate in chemoresistant breast cancer
The bisphosphonate zolendronate didn't improve survival in patients with chemoresistant breast cancer, according to results from the phase III NATAN trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Gunter von Minckwitz discusses the trial's results and clinical implications, and whether a role remains for bisphosphonates in postmenopausal patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The bisphosphonate zolendronate didn't improve survival in patients with chemoresistant breast cancer, according to results from the phase III NATAN trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Gunter von Minckwitz discusses the trial's results and clinical implications, and whether a role remains for bisphosphonates in postmenopausal patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The bisphosphonate zolendronate didn't improve survival in patients with chemoresistant breast cancer, according to results from the phase III NATAN trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Gunter von Minckwitz discusses the trial's results and clinical implications, and whether a role remains for bisphosphonates in postmenopausal patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Esophageal ultrasound unreliable for tumor staging
Endoscopic esophageal ultrasound understages many early-stage esophageal cancers, possibly leading to undertreatment for many patients, a study has shown.
A review of more than 100 patients with esophageal cancer found that the procedure correctly staged tumor depth in just 39% of pT1a tumors and 51% of pT1b tumors, Dr. Edward Bergeron and his colleagues reported online Dec. 5 in the Journal of Thoracic and Cardiovascular Surgery (2013 [doi:10.1016/j.jtcvs.2013.10.003]).
"Esophageal ultrasound has [been] shown to be inadequate for the definitive evaluation of lymph nodes in early-stage esophageal cancers," wrote Dr. Bergeron and his coauthors. "As a result, [it] should have a limited role beyond the initial staging examination to rule out more advanced lesions that would require neoadjuvant therapy before surgery."
Dr. Bergeron of the University of Michigan, Ann Arbor, and his team conducted a retrospective study of 107 patients who underwent esophagectomy for early esophageal cancers. The patients’ mean age was 66 years. The majority of the tumors (91%) were in the distal esophagus. Most (83%) were adenocarcinomas, and 63% showed signs of Barrett’s metaplasia.
For those with visible lesions, the average tumor size was 9 mm. More than half of the tumors (55%) were confined to the mucosa; 33% were pT1b with submucosal involvement.
Esophageal ultrasound understaged tumor depth in 32% of the pT1a tumors and 49% of the pT1b tumors. Ultrasound overstaged 29% of the pT1a and 51% of the pT1b tumors.
"In particular, three tumors on esophageal ultrasound invaded into but not through the muscularis mucosa," the authors wrote. "Two of these tumors were staged as pT2 [adenocarcinomas], and one of these tumors was staged as a pT3 squamous cell carcinoma on histology."
The sensitivity of esophageal ultrasound for detecting cT1a tumor invasion was 42%; the specificity was 81%.
Of the 1,083 lymph nodes harvested, 14 from nine patients showed metastatic disease. All of these patients had adenocarcinomas that were understaged by esophageal ultrasound, with no evidence of lymph node involvement.
Among these, disease had spread to the muscularis mucosa in one, to the submucosa in six, and to the muscularis propria in two.
"Thus, eight of the nine patients with positive pathologic lymph nodes had tumor invasion into the submucosal level or further," the authors wrote.
In addition, they noted, two of the nine patients also had multiple metastatic nodes, which led to reclassification as pN2 disease. One of these had tumor invasion into the submucosa and one into the muscularis propria.
"It is notable that 48 patients with pathologic T1a-1p disease had no evidence of lymph node spread," the authors wrote.
Esophageal ultrasound understaged 10% (9/90) of patients with lymph node metastases who had been preoperatively staged as cN0. In addition, 17 patients proved to be node negative after ultrasound staged them as having nodes suspicious of metastasis.
All patients underwent esophagectomy. The median blood loss was 250 mL. One patient needed a splenectomy after an intraoperative injury. The median length of stay was 8 days. One patient died after an unexplained respiratory arrest on postoperative day 2.
None of the authors declared any relevant financial conflicts.
Endoscopic esophageal ultrasound understages many early-stage esophageal cancers, possibly leading to undertreatment for many patients, a study has shown.
A review of more than 100 patients with esophageal cancer found that the procedure correctly staged tumor depth in just 39% of pT1a tumors and 51% of pT1b tumors, Dr. Edward Bergeron and his colleagues reported online Dec. 5 in the Journal of Thoracic and Cardiovascular Surgery (2013 [doi:10.1016/j.jtcvs.2013.10.003]).
"Esophageal ultrasound has [been] shown to be inadequate for the definitive evaluation of lymph nodes in early-stage esophageal cancers," wrote Dr. Bergeron and his coauthors. "As a result, [it] should have a limited role beyond the initial staging examination to rule out more advanced lesions that would require neoadjuvant therapy before surgery."
Dr. Bergeron of the University of Michigan, Ann Arbor, and his team conducted a retrospective study of 107 patients who underwent esophagectomy for early esophageal cancers. The patients’ mean age was 66 years. The majority of the tumors (91%) were in the distal esophagus. Most (83%) were adenocarcinomas, and 63% showed signs of Barrett’s metaplasia.
For those with visible lesions, the average tumor size was 9 mm. More than half of the tumors (55%) were confined to the mucosa; 33% were pT1b with submucosal involvement.
Esophageal ultrasound understaged tumor depth in 32% of the pT1a tumors and 49% of the pT1b tumors. Ultrasound overstaged 29% of the pT1a and 51% of the pT1b tumors.
"In particular, three tumors on esophageal ultrasound invaded into but not through the muscularis mucosa," the authors wrote. "Two of these tumors were staged as pT2 [adenocarcinomas], and one of these tumors was staged as a pT3 squamous cell carcinoma on histology."
The sensitivity of esophageal ultrasound for detecting cT1a tumor invasion was 42%; the specificity was 81%.
Of the 1,083 lymph nodes harvested, 14 from nine patients showed metastatic disease. All of these patients had adenocarcinomas that were understaged by esophageal ultrasound, with no evidence of lymph node involvement.
Among these, disease had spread to the muscularis mucosa in one, to the submucosa in six, and to the muscularis propria in two.
"Thus, eight of the nine patients with positive pathologic lymph nodes had tumor invasion into the submucosal level or further," the authors wrote.
In addition, they noted, two of the nine patients also had multiple metastatic nodes, which led to reclassification as pN2 disease. One of these had tumor invasion into the submucosa and one into the muscularis propria.
"It is notable that 48 patients with pathologic T1a-1p disease had no evidence of lymph node spread," the authors wrote.
Esophageal ultrasound understaged 10% (9/90) of patients with lymph node metastases who had been preoperatively staged as cN0. In addition, 17 patients proved to be node negative after ultrasound staged them as having nodes suspicious of metastasis.
All patients underwent esophagectomy. The median blood loss was 250 mL. One patient needed a splenectomy after an intraoperative injury. The median length of stay was 8 days. One patient died after an unexplained respiratory arrest on postoperative day 2.
None of the authors declared any relevant financial conflicts.
Endoscopic esophageal ultrasound understages many early-stage esophageal cancers, possibly leading to undertreatment for many patients, a study has shown.
A review of more than 100 patients with esophageal cancer found that the procedure correctly staged tumor depth in just 39% of pT1a tumors and 51% of pT1b tumors, Dr. Edward Bergeron and his colleagues reported online Dec. 5 in the Journal of Thoracic and Cardiovascular Surgery (2013 [doi:10.1016/j.jtcvs.2013.10.003]).
"Esophageal ultrasound has [been] shown to be inadequate for the definitive evaluation of lymph nodes in early-stage esophageal cancers," wrote Dr. Bergeron and his coauthors. "As a result, [it] should have a limited role beyond the initial staging examination to rule out more advanced lesions that would require neoadjuvant therapy before surgery."
Dr. Bergeron of the University of Michigan, Ann Arbor, and his team conducted a retrospective study of 107 patients who underwent esophagectomy for early esophageal cancers. The patients’ mean age was 66 years. The majority of the tumors (91%) were in the distal esophagus. Most (83%) were adenocarcinomas, and 63% showed signs of Barrett’s metaplasia.
For those with visible lesions, the average tumor size was 9 mm. More than half of the tumors (55%) were confined to the mucosa; 33% were pT1b with submucosal involvement.
Esophageal ultrasound understaged tumor depth in 32% of the pT1a tumors and 49% of the pT1b tumors. Ultrasound overstaged 29% of the pT1a and 51% of the pT1b tumors.
"In particular, three tumors on esophageal ultrasound invaded into but not through the muscularis mucosa," the authors wrote. "Two of these tumors were staged as pT2 [adenocarcinomas], and one of these tumors was staged as a pT3 squamous cell carcinoma on histology."
The sensitivity of esophageal ultrasound for detecting cT1a tumor invasion was 42%; the specificity was 81%.
Of the 1,083 lymph nodes harvested, 14 from nine patients showed metastatic disease. All of these patients had adenocarcinomas that were understaged by esophageal ultrasound, with no evidence of lymph node involvement.
Among these, disease had spread to the muscularis mucosa in one, to the submucosa in six, and to the muscularis propria in two.
"Thus, eight of the nine patients with positive pathologic lymph nodes had tumor invasion into the submucosal level or further," the authors wrote.
In addition, they noted, two of the nine patients also had multiple metastatic nodes, which led to reclassification as pN2 disease. One of these had tumor invasion into the submucosa and one into the muscularis propria.
"It is notable that 48 patients with pathologic T1a-1p disease had no evidence of lymph node spread," the authors wrote.
Esophageal ultrasound understaged 10% (9/90) of patients with lymph node metastases who had been preoperatively staged as cN0. In addition, 17 patients proved to be node negative after ultrasound staged them as having nodes suspicious of metastasis.
All patients underwent esophagectomy. The median blood loss was 250 mL. One patient needed a splenectomy after an intraoperative injury. The median length of stay was 8 days. One patient died after an unexplained respiratory arrest on postoperative day 2.
None of the authors declared any relevant financial conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Major finding: Esophageal ultrasound correctly staged tumor depth in just 39% of pT1a esophageal tumors and 51% of pT1b esophageal tumors.
Data source: A retrospective study of 107 patients.
Disclosures: Dr. Bergeron said he had no relevant financial disclosures.
Radiotherapy can be omitted for many older breast cancer patients
SAN ANTONIO – Avoiding whole-breast radiation therapy is a reasonable – and even attractive – option for many older women with early-stage breast cancer, according to the results of the Postoperative Radiotherapy in Minimum-Risk Elderly (PRIME II) trial.
The patient population identified in PRIME II as being suitable for omission of postoperative radiotherapy on the basis of a relatively benign natural history consists of women aged 65 or older who are on adjuvant hormone therapy after undergoing lumpectomy with clear margins for estrogen receptor–rich, axillary node–negative breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PRIME II was a six-country trial in which 1,326 patients 65 or older with hormone receptor–positive early breast cancer were randomized to radiotherapy or no radiotherapy following breast-conserving surgery and endocrine therapy. The 5-year actuarial rate of ipsilateral breast cancer recurrence – the primary study endpoint – was 1.3% in those who received radiotherapy and 4.1% in those who did not, Dr. Ian H. Kunkler reported at the San Antonio Breast Cancer Symposium.
The 5-year actuarial rate of overall survival was 94.2% in patients randomized to radiotherapy and closely similar at 93.8% in the no-radiotherapy group, added Dr. Kunkler, professor of clinical oncology at the University of Edinburgh.
The relative benefit of radiotherapy was even smaller in the 91% of subjects who had estrogen-rich tumors as defined by an ER score of at least 7. They had a local recurrence rate of 3.2% with radiotherapy and 0.8% without. While that absolute 2.4% difference was statistically significant, it is arguably not clinically meaningful. For every 100 women who fit the description carefully defined in PRIME II and who undergo radiotherapy, three will have a recurrence prevented, one will have a recurrence anyway, and 96 will have had treatment that was not beneficial, he said.
"I think we’re really at the cusp of overtreatment here. I think it’s a matter for discussion between the physician and patient as to whether that very modest benefit is worth the potential complications of radiotherapy and the burdens of ongoing treatment, as well as the costs to the health service. Older patients find radiotherapy very burdensome, the relative benefits are very small, and there is no compromise in terms of overall survival with its omission," Dr. Kunkler said.
An important caveat: Among the 9% of patients with low estrogen receptor status, the local recurrence rate was 11.1% with no radiotherapy compared to zero with radiation.
"This is a group for whom radiotherapy should not be omitted," Dr. Kunkler declared.
More than one-half of all early breast cancers present in women aged 65 or older. While postoperative radiotherapy after lumpectomy has been the standard of care regardless of age and other risk factors, there has been only sparse high-quality supporting evidence for this practice in older patients.
Dr. Kunkler estimated that the PRIME II findings are generalizable to 60%-70% of all breast cancer patients over age 65. He predicted that the PRIME II study will "very likely" alter practice in the United Kingdom, and symposium codirector Dr. C. Kent Osborne predicted that the study will be practice changing in the United States as well.
"When I was in training, everybody thought that more was better: more drug treatment, more radiation, more surgery, high-dose chemotherapy, and bone marrow transplant. As we’ve evolved over the last 3 decades, that’s turning out not to be the case. I think we’re gradually doing less and less treatment, either with radiotherapy or with surgery, to control the local disease in appropriate patients. And I think more and more people will begin to accept it," said Dr. Osborne, director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston.
PRIME II was funded by the Chief Scientist Office for Scotland. Dr. Kunkler declared having no conflicts of interest.
SAN ANTONIO – Avoiding whole-breast radiation therapy is a reasonable – and even attractive – option for many older women with early-stage breast cancer, according to the results of the Postoperative Radiotherapy in Minimum-Risk Elderly (PRIME II) trial.
The patient population identified in PRIME II as being suitable for omission of postoperative radiotherapy on the basis of a relatively benign natural history consists of women aged 65 or older who are on adjuvant hormone therapy after undergoing lumpectomy with clear margins for estrogen receptor–rich, axillary node–negative breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PRIME II was a six-country trial in which 1,326 patients 65 or older with hormone receptor–positive early breast cancer were randomized to radiotherapy or no radiotherapy following breast-conserving surgery and endocrine therapy. The 5-year actuarial rate of ipsilateral breast cancer recurrence – the primary study endpoint – was 1.3% in those who received radiotherapy and 4.1% in those who did not, Dr. Ian H. Kunkler reported at the San Antonio Breast Cancer Symposium.
The 5-year actuarial rate of overall survival was 94.2% in patients randomized to radiotherapy and closely similar at 93.8% in the no-radiotherapy group, added Dr. Kunkler, professor of clinical oncology at the University of Edinburgh.
The relative benefit of radiotherapy was even smaller in the 91% of subjects who had estrogen-rich tumors as defined by an ER score of at least 7. They had a local recurrence rate of 3.2% with radiotherapy and 0.8% without. While that absolute 2.4% difference was statistically significant, it is arguably not clinically meaningful. For every 100 women who fit the description carefully defined in PRIME II and who undergo radiotherapy, three will have a recurrence prevented, one will have a recurrence anyway, and 96 will have had treatment that was not beneficial, he said.
"I think we’re really at the cusp of overtreatment here. I think it’s a matter for discussion between the physician and patient as to whether that very modest benefit is worth the potential complications of radiotherapy and the burdens of ongoing treatment, as well as the costs to the health service. Older patients find radiotherapy very burdensome, the relative benefits are very small, and there is no compromise in terms of overall survival with its omission," Dr. Kunkler said.
An important caveat: Among the 9% of patients with low estrogen receptor status, the local recurrence rate was 11.1% with no radiotherapy compared to zero with radiation.
"This is a group for whom radiotherapy should not be omitted," Dr. Kunkler declared.
More than one-half of all early breast cancers present in women aged 65 or older. While postoperative radiotherapy after lumpectomy has been the standard of care regardless of age and other risk factors, there has been only sparse high-quality supporting evidence for this practice in older patients.
Dr. Kunkler estimated that the PRIME II findings are generalizable to 60%-70% of all breast cancer patients over age 65. He predicted that the PRIME II study will "very likely" alter practice in the United Kingdom, and symposium codirector Dr. C. Kent Osborne predicted that the study will be practice changing in the United States as well.
"When I was in training, everybody thought that more was better: more drug treatment, more radiation, more surgery, high-dose chemotherapy, and bone marrow transplant. As we’ve evolved over the last 3 decades, that’s turning out not to be the case. I think we’re gradually doing less and less treatment, either with radiotherapy or with surgery, to control the local disease in appropriate patients. And I think more and more people will begin to accept it," said Dr. Osborne, director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston.
PRIME II was funded by the Chief Scientist Office for Scotland. Dr. Kunkler declared having no conflicts of interest.
SAN ANTONIO – Avoiding whole-breast radiation therapy is a reasonable – and even attractive – option for many older women with early-stage breast cancer, according to the results of the Postoperative Radiotherapy in Minimum-Risk Elderly (PRIME II) trial.
The patient population identified in PRIME II as being suitable for omission of postoperative radiotherapy on the basis of a relatively benign natural history consists of women aged 65 or older who are on adjuvant hormone therapy after undergoing lumpectomy with clear margins for estrogen receptor–rich, axillary node–negative breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PRIME II was a six-country trial in which 1,326 patients 65 or older with hormone receptor–positive early breast cancer were randomized to radiotherapy or no radiotherapy following breast-conserving surgery and endocrine therapy. The 5-year actuarial rate of ipsilateral breast cancer recurrence – the primary study endpoint – was 1.3% in those who received radiotherapy and 4.1% in those who did not, Dr. Ian H. Kunkler reported at the San Antonio Breast Cancer Symposium.
The 5-year actuarial rate of overall survival was 94.2% in patients randomized to radiotherapy and closely similar at 93.8% in the no-radiotherapy group, added Dr. Kunkler, professor of clinical oncology at the University of Edinburgh.
The relative benefit of radiotherapy was even smaller in the 91% of subjects who had estrogen-rich tumors as defined by an ER score of at least 7. They had a local recurrence rate of 3.2% with radiotherapy and 0.8% without. While that absolute 2.4% difference was statistically significant, it is arguably not clinically meaningful. For every 100 women who fit the description carefully defined in PRIME II and who undergo radiotherapy, three will have a recurrence prevented, one will have a recurrence anyway, and 96 will have had treatment that was not beneficial, he said.
"I think we’re really at the cusp of overtreatment here. I think it’s a matter for discussion between the physician and patient as to whether that very modest benefit is worth the potential complications of radiotherapy and the burdens of ongoing treatment, as well as the costs to the health service. Older patients find radiotherapy very burdensome, the relative benefits are very small, and there is no compromise in terms of overall survival with its omission," Dr. Kunkler said.
An important caveat: Among the 9% of patients with low estrogen receptor status, the local recurrence rate was 11.1% with no radiotherapy compared to zero with radiation.
"This is a group for whom radiotherapy should not be omitted," Dr. Kunkler declared.
More than one-half of all early breast cancers present in women aged 65 or older. While postoperative radiotherapy after lumpectomy has been the standard of care regardless of age and other risk factors, there has been only sparse high-quality supporting evidence for this practice in older patients.
Dr. Kunkler estimated that the PRIME II findings are generalizable to 60%-70% of all breast cancer patients over age 65. He predicted that the PRIME II study will "very likely" alter practice in the United Kingdom, and symposium codirector Dr. C. Kent Osborne predicted that the study will be practice changing in the United States as well.
"When I was in training, everybody thought that more was better: more drug treatment, more radiation, more surgery, high-dose chemotherapy, and bone marrow transplant. As we’ve evolved over the last 3 decades, that’s turning out not to be the case. I think we’re gradually doing less and less treatment, either with radiotherapy or with surgery, to control the local disease in appropriate patients. And I think more and more people will begin to accept it," said Dr. Osborne, director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston.
PRIME II was funded by the Chief Scientist Office for Scotland. Dr. Kunkler declared having no conflicts of interest.
AT SABCS 2013
Major finding: The 5-year ipsilateral breast cancer recurrence rate in a selected population of older women undergoing breast-conserving surgery and adjuvant hormone therapy was 1.3% with postoperative radiotherapy and 4.1% without it, a modest difference that did not impact overall survival.
Data source: A prospective randomized trial in six countries, involving 1,326 patients aged 65 or older who underwent lumpectomy with clear margins for hormone receptor–positive, axillary node–negative breast cancer and were on adjuvant endocrine therapy. They were randomized to postoperative radiotherapy or no radiotherapy.
Disclosures: The PRIME II study was funded by the Chief Scientist Office for Scotland. The presenter reported having no financial conflicts.
Laparoscopic radical trachelectomy preserves fertility potential
WASHINGTON – Laparoscopic radical trachelectomy can be performed safely in well-selected patients with early cervical cancer who wish to preserve their fertility.
In a small retrospective analysis of 10 women, potential fertility was preserved in 8 women, Dr. Rene Pareja said at the AAGL Global Congress.
By 12 months, no pregnancies had been achieved, reported Dr. Pareja of the Instituto de Cancerología–Clinica las Américas (IDC) in Medellin, Colombia. However, conception should be possible for those who desire to have a child, he said at the meeting sponsored by AAGL.
Patients in the series were treated from 2009 to 2013. All had stage IB1 disease. In nine women, the lesion was less than 2 cm; in one woman, it was 3 cm. Half of the cancers were squamous, and the other half were adenocarcinomas.
All 10 patients underwent a minimally invasive radical trachelectomy. The mean surgical time was 240 minutes, with an estimated blood loss of 100 cc. There were no transfusions and no conversions to open surgery. One cystotomy was repaired laparoscopically. The mean hospital stay was 2 days.
Surgeons recovered a mean of 16 nodes from each patient (range, 10-24); none of these were positive. Four women had no residual disease. One had a positive endocervical margin on pathology; she underwent a hysterectomy. None of the patients required either chemotherapy or radiation therapy.
In addition to the hysterectomy, there were four postoperative complications: one necrosis of the right uterine cornua, one ureterovaginal fistula, and two lymphocysts.
At a mean follow-up of 12 months, there have been no cancer recurrences. One patient is attempting to conceive, although she has not yet done so.
The extant literature supports Dr. Pareja’s experience of laparoscopic radical trachelectomy. Since 2003, the procedure has been reported in 150 patients. Among these, there have been 38 pregnancies, 13 miscarriages, and 20 live births. Dr. Pareja did not say what percentage of women in these studies were attempting to conceive, however.
Five patients reported in the literature have had recurrent cancer and three have died, although Dr. Pareja did not mention whether these deaths were related to the cancers.
So far, the safety and obstetrical outcomes of his patients compare well with those reported in other forms of cervical cancer surgery. Among the 150 reported cases of laparoscopic radical trachelectomy, the relapse rate was 3.3% and death rate 2.9%. The total pregnancy rate was 25% and the delivery rate 13%.
Among the 1,088 reported cases of vaginal radical trachelectomy, there was a 4% relapse rate and 2.9% death rate. The total pregnancy rate was 24% and the delivery rate 28%.
Among the 485 reported cases of abdominal radical trachelectomy, the relapse rate was 3.8% and the death rate 0.4%. The pregnancy rate was 16% and the delivery rate 11%.
Dr. Pareja had no financial disclosures.
WASHINGTON – Laparoscopic radical trachelectomy can be performed safely in well-selected patients with early cervical cancer who wish to preserve their fertility.
In a small retrospective analysis of 10 women, potential fertility was preserved in 8 women, Dr. Rene Pareja said at the AAGL Global Congress.
By 12 months, no pregnancies had been achieved, reported Dr. Pareja of the Instituto de Cancerología–Clinica las Américas (IDC) in Medellin, Colombia. However, conception should be possible for those who desire to have a child, he said at the meeting sponsored by AAGL.
Patients in the series were treated from 2009 to 2013. All had stage IB1 disease. In nine women, the lesion was less than 2 cm; in one woman, it was 3 cm. Half of the cancers were squamous, and the other half were adenocarcinomas.
All 10 patients underwent a minimally invasive radical trachelectomy. The mean surgical time was 240 minutes, with an estimated blood loss of 100 cc. There were no transfusions and no conversions to open surgery. One cystotomy was repaired laparoscopically. The mean hospital stay was 2 days.
Surgeons recovered a mean of 16 nodes from each patient (range, 10-24); none of these were positive. Four women had no residual disease. One had a positive endocervical margin on pathology; she underwent a hysterectomy. None of the patients required either chemotherapy or radiation therapy.
In addition to the hysterectomy, there were four postoperative complications: one necrosis of the right uterine cornua, one ureterovaginal fistula, and two lymphocysts.
At a mean follow-up of 12 months, there have been no cancer recurrences. One patient is attempting to conceive, although she has not yet done so.
The extant literature supports Dr. Pareja’s experience of laparoscopic radical trachelectomy. Since 2003, the procedure has been reported in 150 patients. Among these, there have been 38 pregnancies, 13 miscarriages, and 20 live births. Dr. Pareja did not say what percentage of women in these studies were attempting to conceive, however.
Five patients reported in the literature have had recurrent cancer and three have died, although Dr. Pareja did not mention whether these deaths were related to the cancers.
So far, the safety and obstetrical outcomes of his patients compare well with those reported in other forms of cervical cancer surgery. Among the 150 reported cases of laparoscopic radical trachelectomy, the relapse rate was 3.3% and death rate 2.9%. The total pregnancy rate was 25% and the delivery rate 13%.
Among the 1,088 reported cases of vaginal radical trachelectomy, there was a 4% relapse rate and 2.9% death rate. The total pregnancy rate was 24% and the delivery rate 28%.
Among the 485 reported cases of abdominal radical trachelectomy, the relapse rate was 3.8% and the death rate 0.4%. The pregnancy rate was 16% and the delivery rate 11%.
Dr. Pareja had no financial disclosures.
WASHINGTON – Laparoscopic radical trachelectomy can be performed safely in well-selected patients with early cervical cancer who wish to preserve their fertility.
In a small retrospective analysis of 10 women, potential fertility was preserved in 8 women, Dr. Rene Pareja said at the AAGL Global Congress.
By 12 months, no pregnancies had been achieved, reported Dr. Pareja of the Instituto de Cancerología–Clinica las Américas (IDC) in Medellin, Colombia. However, conception should be possible for those who desire to have a child, he said at the meeting sponsored by AAGL.
Patients in the series were treated from 2009 to 2013. All had stage IB1 disease. In nine women, the lesion was less than 2 cm; in one woman, it was 3 cm. Half of the cancers were squamous, and the other half were adenocarcinomas.
All 10 patients underwent a minimally invasive radical trachelectomy. The mean surgical time was 240 minutes, with an estimated blood loss of 100 cc. There were no transfusions and no conversions to open surgery. One cystotomy was repaired laparoscopically. The mean hospital stay was 2 days.
Surgeons recovered a mean of 16 nodes from each patient (range, 10-24); none of these were positive. Four women had no residual disease. One had a positive endocervical margin on pathology; she underwent a hysterectomy. None of the patients required either chemotherapy or radiation therapy.
In addition to the hysterectomy, there were four postoperative complications: one necrosis of the right uterine cornua, one ureterovaginal fistula, and two lymphocysts.
At a mean follow-up of 12 months, there have been no cancer recurrences. One patient is attempting to conceive, although she has not yet done so.
The extant literature supports Dr. Pareja’s experience of laparoscopic radical trachelectomy. Since 2003, the procedure has been reported in 150 patients. Among these, there have been 38 pregnancies, 13 miscarriages, and 20 live births. Dr. Pareja did not say what percentage of women in these studies were attempting to conceive, however.
Five patients reported in the literature have had recurrent cancer and three have died, although Dr. Pareja did not mention whether these deaths were related to the cancers.
So far, the safety and obstetrical outcomes of his patients compare well with those reported in other forms of cervical cancer surgery. Among the 150 reported cases of laparoscopic radical trachelectomy, the relapse rate was 3.3% and death rate 2.9%. The total pregnancy rate was 25% and the delivery rate 13%.
Among the 1,088 reported cases of vaginal radical trachelectomy, there was a 4% relapse rate and 2.9% death rate. The total pregnancy rate was 24% and the delivery rate 28%.
Among the 485 reported cases of abdominal radical trachelectomy, the relapse rate was 3.8% and the death rate 0.4%. The pregnancy rate was 16% and the delivery rate 11%.
Dr. Pareja had no financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Major finding: A laparoscopic radical trachelectomy preserved fertility in 8 of 10 women with early cervical cancer, with no recurrences at a mean follow-up of 12 months.
Data source: A retrospective study involving 10 women.
Disclosures: Dr. Pareja had no financial disclosures.
Simple intensity-modulated radiotherapy improves breast cancer cosmesis
Dose homogeneity with intensity-modulated radiotherapy reduced the risk of skin telangiectasia, resulting in superior overall cosmesis for early breast cancer patients in a 5-year single-center trial.
"This study should act as an evidence-based lever for change for radiotherapy centers that have yet to implement breast IMRT (intensity-modulated radiotherapy)," wrote Dr. Mukesh B. Mukesh of Cambridge (England) University Hospitals National Health Service Foundation Trust, and colleagues.
The Cambridge Breast IMRT Trial randomized 1,145 women with early breast cancer to receive either standard radiotherapy or simple intensity-modulated radiotherapy (IMRT), which uses additional irradiation fields to smooth out the dose to the target area.
In the multivariate analysis, significantly fewer patients in the IMRT arm had suboptimal overall cosmesis (OR, 0.65; 95% CI, 0.44 to 0.98; P = .038) and skin telangiectasia (OR, 0.57; 95% CI, 0.34 to 0.95; P = .031), according to data published online in the Journal of Clinical Oncology (doi:10.1200/JCO.2013.49.7842). However the two groups did not significantly differ based on photographically assessed breast shrinkage, clinically assessed edema, tumor bed induration or pigmentation, according to the researchers.
Also, there was no statistically significant difference in 5-year locoregional recurrence and overall survival rates.
Surgical cosmesis had a significant impact on outcomes, as patients with moderate to poor baseline surgical cosmesis were more likely to have suboptimal final cosmesis (OR, 8.15; 95% CI, 6.09 to 10.92; P < .001), tumor bed induration (OR, 1.80; 95% CI, 1.44 to 2.26; P < .001), and photographically assessed breast shrinkage (OR, 1.54; 95% CI, 1.21 to 1.96; P < .001).
Factors such as large breast volume and tumor bed boost were also associated with an increased risk of suboptimal overall cosmesis. Older patients, and those with postoperative breast infections, large breast volume, and tumor bed boost, were also more likely to develop skin telangiectasia.
The patients enrolled in the study all had operable unilateral, histologically confirmed invasive breast cancer or ductal carcinoma in situ requiring radiotherapy after breast-conservation surgery.
All patients were assigned a standard radiotherapy plan. Those with satisfactory dose homogeneity were not randomly assigned but treated with standard radiotherapy and followed up as if randomly assigned, while those whose plan had significant dose inhomogeneity (defined as > or = 2 cm3 volume receiving greater than 107% of the prescribed dose) were randomized between standard radiotherapy (control arm) and simple IMRT.
There were a significant number of patient withdrawals at the 5-year analysis due to factors such as travel difficulties, social issues, and personal choice, and cancer-related factors.
The researchers declared having no conflicts of interest.
Dose homogeneity with intensity-modulated radiotherapy reduced the risk of skin telangiectasia, resulting in superior overall cosmesis for early breast cancer patients in a 5-year single-center trial.
"This study should act as an evidence-based lever for change for radiotherapy centers that have yet to implement breast IMRT (intensity-modulated radiotherapy)," wrote Dr. Mukesh B. Mukesh of Cambridge (England) University Hospitals National Health Service Foundation Trust, and colleagues.
The Cambridge Breast IMRT Trial randomized 1,145 women with early breast cancer to receive either standard radiotherapy or simple intensity-modulated radiotherapy (IMRT), which uses additional irradiation fields to smooth out the dose to the target area.
In the multivariate analysis, significantly fewer patients in the IMRT arm had suboptimal overall cosmesis (OR, 0.65; 95% CI, 0.44 to 0.98; P = .038) and skin telangiectasia (OR, 0.57; 95% CI, 0.34 to 0.95; P = .031), according to data published online in the Journal of Clinical Oncology (doi:10.1200/JCO.2013.49.7842). However the two groups did not significantly differ based on photographically assessed breast shrinkage, clinically assessed edema, tumor bed induration or pigmentation, according to the researchers.
Also, there was no statistically significant difference in 5-year locoregional recurrence and overall survival rates.
Surgical cosmesis had a significant impact on outcomes, as patients with moderate to poor baseline surgical cosmesis were more likely to have suboptimal final cosmesis (OR, 8.15; 95% CI, 6.09 to 10.92; P < .001), tumor bed induration (OR, 1.80; 95% CI, 1.44 to 2.26; P < .001), and photographically assessed breast shrinkage (OR, 1.54; 95% CI, 1.21 to 1.96; P < .001).
Factors such as large breast volume and tumor bed boost were also associated with an increased risk of suboptimal overall cosmesis. Older patients, and those with postoperative breast infections, large breast volume, and tumor bed boost, were also more likely to develop skin telangiectasia.
The patients enrolled in the study all had operable unilateral, histologically confirmed invasive breast cancer or ductal carcinoma in situ requiring radiotherapy after breast-conservation surgery.
All patients were assigned a standard radiotherapy plan. Those with satisfactory dose homogeneity were not randomly assigned but treated with standard radiotherapy and followed up as if randomly assigned, while those whose plan had significant dose inhomogeneity (defined as > or = 2 cm3 volume receiving greater than 107% of the prescribed dose) were randomized between standard radiotherapy (control arm) and simple IMRT.
There were a significant number of patient withdrawals at the 5-year analysis due to factors such as travel difficulties, social issues, and personal choice, and cancer-related factors.
The researchers declared having no conflicts of interest.
Dose homogeneity with intensity-modulated radiotherapy reduced the risk of skin telangiectasia, resulting in superior overall cosmesis for early breast cancer patients in a 5-year single-center trial.
"This study should act as an evidence-based lever for change for radiotherapy centers that have yet to implement breast IMRT (intensity-modulated radiotherapy)," wrote Dr. Mukesh B. Mukesh of Cambridge (England) University Hospitals National Health Service Foundation Trust, and colleagues.
The Cambridge Breast IMRT Trial randomized 1,145 women with early breast cancer to receive either standard radiotherapy or simple intensity-modulated radiotherapy (IMRT), which uses additional irradiation fields to smooth out the dose to the target area.
In the multivariate analysis, significantly fewer patients in the IMRT arm had suboptimal overall cosmesis (OR, 0.65; 95% CI, 0.44 to 0.98; P = .038) and skin telangiectasia (OR, 0.57; 95% CI, 0.34 to 0.95; P = .031), according to data published online in the Journal of Clinical Oncology (doi:10.1200/JCO.2013.49.7842). However the two groups did not significantly differ based on photographically assessed breast shrinkage, clinically assessed edema, tumor bed induration or pigmentation, according to the researchers.
Also, there was no statistically significant difference in 5-year locoregional recurrence and overall survival rates.
Surgical cosmesis had a significant impact on outcomes, as patients with moderate to poor baseline surgical cosmesis were more likely to have suboptimal final cosmesis (OR, 8.15; 95% CI, 6.09 to 10.92; P < .001), tumor bed induration (OR, 1.80; 95% CI, 1.44 to 2.26; P < .001), and photographically assessed breast shrinkage (OR, 1.54; 95% CI, 1.21 to 1.96; P < .001).
Factors such as large breast volume and tumor bed boost were also associated with an increased risk of suboptimal overall cosmesis. Older patients, and those with postoperative breast infections, large breast volume, and tumor bed boost, were also more likely to develop skin telangiectasia.
The patients enrolled in the study all had operable unilateral, histologically confirmed invasive breast cancer or ductal carcinoma in situ requiring radiotherapy after breast-conservation surgery.
All patients were assigned a standard radiotherapy plan. Those with satisfactory dose homogeneity were not randomly assigned but treated with standard radiotherapy and followed up as if randomly assigned, while those whose plan had significant dose inhomogeneity (defined as > or = 2 cm3 volume receiving greater than 107% of the prescribed dose) were randomized between standard radiotherapy (control arm) and simple IMRT.
There were a significant number of patient withdrawals at the 5-year analysis due to factors such as travel difficulties, social issues, and personal choice, and cancer-related factors.
The researchers declared having no conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Significantly fewer patients in the IMRT arm had suboptimal overall cosmesis (OR, 0.65; 95%CI, 0.44 to 0.98; P = .038) and skin telangiectasia (OR, 0.57; 95% CI, 0.34 to 0.95; P = .031).
Data source: Single-center randomized controlled trial of 1,145 patients with early breast cancer.
Disclosures: Dr. Mukesh had no conflicts of interest to declare.
Electrocautery incision of lymph nodes improved biopsy yield
Endobronchial ultrasound–guided biopsies made after an electrocautery incision to the lymph node improved biopsy yields from 39% to 71% in 38 nodes, according to a small study presented at the annual meeting of the American College of Chest Physicians meeting.
"Because it is not always possible to pass biopsy forceps through defects in the lymph node – the literature indicates a failure rate of between 10% and 29% – we developed a novel technique," said presenter Dr. Kyle Bramley of Yale University, New Haven, Conn.
The technique employs EBUS, and involves passing an electrocautery knife activated at 40 W through the working channel of the scope in order to make an incision in the bronchial wall and enlarge the defect in the lymph node. This facilitates passage of the forceps into the node so that a larger biopsy sample can be obtained.
To test their technique, Dr. Bramley and his colleagues designed a prospective observational cohort study at a single tertiary academic medical center. Twenty patients (mean age, 68 years), including 11 women, who were undergoing EBUS were enrolled. An associated lung mass was present in 14 (70%) of the participants; 6 (30%) had isolated lymphadenopathy. One patient had prior lymphoma, and two others had prior lung cancer.
The researchers evaluated 68 nodes in all; 19 patients had nodes greater than 9 mm. Cautery was only used when initial attempts failed to biopsy nodes 9 mm or larger using EBUS-guided miniforceps of 1.2 mm.
The average node size biopsied using EBUS-transbronchial needle aspiration (EBUS-TBNA) was 5.7 mm. The average forceps-biopsied node was 15.8 mm.
In all, 23 nodes were biopsied successfully on the first pass using EBUS-TBNA only. The biopsies yielded diagnostic material such as lymphocytes, malignancy, or granulomas in 15 of these nodes.
Of the 15 nodes that required cautery, 12 yielded diagnostic material, and 3 had no diagnostic material.
The overall yield increased from 39% (15 out of 38) without cautery to 71% (27 out of 38) when cautery was used.
Notably, four patients had clinically relevant discrepancies between their cytologies and histopathologies. "In all four, TBNA provided a definitive diagnosis," said Dr. Bramley. "The forceps provided fibroconnective tissue or necrotic debris."
These results did not negate the efficacy of the cautery technique, according to Dr. Bramley. "We think we had a forceps issue ... the 1.2 mm are flexible, but they were unable to push all the way through a tough lymph node capsule."
Dr. Bramley also said that other factors, including the operator learning curve, the smaller size of the nodes the investigators attempted to biopsy, and the "nonideal" population they were studying, contributed to these results.
He and his colleagues have since adjusted the procedure to make cauterization routine and to include a 1.9-mm transbronchial biopsy forceps needle, "which, incidentally, is a lot less expensive than the larger forceps we’d been using," he said.
Although more study is needed, Dr. Bramley said he and his team believed that this technique would be appropriate for future use in isolated mediastinal lymphadenopathy, especially with a low suspicion of non–small cell lung carcinoma; evaluation of lymphoma; and clinical trials requiring core biopsy.
Dr. Bramley had no relevant disclosures.
Dr. Frank Podbielski, FCCP, comments: The authors have again proven that a larger pathology specimen obtained at the time of biopsy significantly improves diagnostic accuracy, especially in the setting of mediastinal nodes that are difficult to access and thus require an electrocautery incision through the airway in concert with EBUS guidance.
Dr. Francis J. Podbielski leads the Lung Cancer Program at Jordan Hospital in Plymouth, Mass.
Dr. Frank Podbielski, FCCP, comments: The authors have again proven that a larger pathology specimen obtained at the time of biopsy significantly improves diagnostic accuracy, especially in the setting of mediastinal nodes that are difficult to access and thus require an electrocautery incision through the airway in concert with EBUS guidance.
Dr. Francis J. Podbielski leads the Lung Cancer Program at Jordan Hospital in Plymouth, Mass.
Dr. Frank Podbielski, FCCP, comments: The authors have again proven that a larger pathology specimen obtained at the time of biopsy significantly improves diagnostic accuracy, especially in the setting of mediastinal nodes that are difficult to access and thus require an electrocautery incision through the airway in concert with EBUS guidance.
Dr. Francis J. Podbielski leads the Lung Cancer Program at Jordan Hospital in Plymouth, Mass.
Endobronchial ultrasound–guided biopsies made after an electrocautery incision to the lymph node improved biopsy yields from 39% to 71% in 38 nodes, according to a small study presented at the annual meeting of the American College of Chest Physicians meeting.
"Because it is not always possible to pass biopsy forceps through defects in the lymph node – the literature indicates a failure rate of between 10% and 29% – we developed a novel technique," said presenter Dr. Kyle Bramley of Yale University, New Haven, Conn.
The technique employs EBUS, and involves passing an electrocautery knife activated at 40 W through the working channel of the scope in order to make an incision in the bronchial wall and enlarge the defect in the lymph node. This facilitates passage of the forceps into the node so that a larger biopsy sample can be obtained.
To test their technique, Dr. Bramley and his colleagues designed a prospective observational cohort study at a single tertiary academic medical center. Twenty patients (mean age, 68 years), including 11 women, who were undergoing EBUS were enrolled. An associated lung mass was present in 14 (70%) of the participants; 6 (30%) had isolated lymphadenopathy. One patient had prior lymphoma, and two others had prior lung cancer.
The researchers evaluated 68 nodes in all; 19 patients had nodes greater than 9 mm. Cautery was only used when initial attempts failed to biopsy nodes 9 mm or larger using EBUS-guided miniforceps of 1.2 mm.
The average node size biopsied using EBUS-transbronchial needle aspiration (EBUS-TBNA) was 5.7 mm. The average forceps-biopsied node was 15.8 mm.
In all, 23 nodes were biopsied successfully on the first pass using EBUS-TBNA only. The biopsies yielded diagnostic material such as lymphocytes, malignancy, or granulomas in 15 of these nodes.
Of the 15 nodes that required cautery, 12 yielded diagnostic material, and 3 had no diagnostic material.
The overall yield increased from 39% (15 out of 38) without cautery to 71% (27 out of 38) when cautery was used.
Notably, four patients had clinically relevant discrepancies between their cytologies and histopathologies. "In all four, TBNA provided a definitive diagnosis," said Dr. Bramley. "The forceps provided fibroconnective tissue or necrotic debris."
These results did not negate the efficacy of the cautery technique, according to Dr. Bramley. "We think we had a forceps issue ... the 1.2 mm are flexible, but they were unable to push all the way through a tough lymph node capsule."
Dr. Bramley also said that other factors, including the operator learning curve, the smaller size of the nodes the investigators attempted to biopsy, and the "nonideal" population they were studying, contributed to these results.
He and his colleagues have since adjusted the procedure to make cauterization routine and to include a 1.9-mm transbronchial biopsy forceps needle, "which, incidentally, is a lot less expensive than the larger forceps we’d been using," he said.
Although more study is needed, Dr. Bramley said he and his team believed that this technique would be appropriate for future use in isolated mediastinal lymphadenopathy, especially with a low suspicion of non–small cell lung carcinoma; evaluation of lymphoma; and clinical trials requiring core biopsy.
Dr. Bramley had no relevant disclosures.
Endobronchial ultrasound–guided biopsies made after an electrocautery incision to the lymph node improved biopsy yields from 39% to 71% in 38 nodes, according to a small study presented at the annual meeting of the American College of Chest Physicians meeting.
"Because it is not always possible to pass biopsy forceps through defects in the lymph node – the literature indicates a failure rate of between 10% and 29% – we developed a novel technique," said presenter Dr. Kyle Bramley of Yale University, New Haven, Conn.
The technique employs EBUS, and involves passing an electrocautery knife activated at 40 W through the working channel of the scope in order to make an incision in the bronchial wall and enlarge the defect in the lymph node. This facilitates passage of the forceps into the node so that a larger biopsy sample can be obtained.
To test their technique, Dr. Bramley and his colleagues designed a prospective observational cohort study at a single tertiary academic medical center. Twenty patients (mean age, 68 years), including 11 women, who were undergoing EBUS were enrolled. An associated lung mass was present in 14 (70%) of the participants; 6 (30%) had isolated lymphadenopathy. One patient had prior lymphoma, and two others had prior lung cancer.
The researchers evaluated 68 nodes in all; 19 patients had nodes greater than 9 mm. Cautery was only used when initial attempts failed to biopsy nodes 9 mm or larger using EBUS-guided miniforceps of 1.2 mm.
The average node size biopsied using EBUS-transbronchial needle aspiration (EBUS-TBNA) was 5.7 mm. The average forceps-biopsied node was 15.8 mm.
In all, 23 nodes were biopsied successfully on the first pass using EBUS-TBNA only. The biopsies yielded diagnostic material such as lymphocytes, malignancy, or granulomas in 15 of these nodes.
Of the 15 nodes that required cautery, 12 yielded diagnostic material, and 3 had no diagnostic material.
The overall yield increased from 39% (15 out of 38) without cautery to 71% (27 out of 38) when cautery was used.
Notably, four patients had clinically relevant discrepancies between their cytologies and histopathologies. "In all four, TBNA provided a definitive diagnosis," said Dr. Bramley. "The forceps provided fibroconnective tissue or necrotic debris."
These results did not negate the efficacy of the cautery technique, according to Dr. Bramley. "We think we had a forceps issue ... the 1.2 mm are flexible, but they were unable to push all the way through a tough lymph node capsule."
Dr. Bramley also said that other factors, including the operator learning curve, the smaller size of the nodes the investigators attempted to biopsy, and the "nonideal" population they were studying, contributed to these results.
He and his colleagues have since adjusted the procedure to make cauterization routine and to include a 1.9-mm transbronchial biopsy forceps needle, "which, incidentally, is a lot less expensive than the larger forceps we’d been using," he said.
Although more study is needed, Dr. Bramley said he and his team believed that this technique would be appropriate for future use in isolated mediastinal lymphadenopathy, especially with a low suspicion of non–small cell lung carcinoma; evaluation of lymphoma; and clinical trials requiring core biopsy.
Dr. Bramley had no relevant disclosures.
Major finding: EBUS-guided lymph node biopsies made after electrocautery incision improved biopsy yields from 39% to 71% in 38 lymph nodes.
Data source: Prospective observational cohort study of 20 patients at a single tertiary academic medical center.
Disclosures: Dr. Bramley had no relevant disclosures.
Genetic profiling transforms cancer treatment trials
BRUSSELS – The ballooning list of genetic markers linked with various cancers is spawning a radical shift in the design of oncology treatment trials.
These days, the trend is to incorporate detailed genetic analysis into the trials, so that once the results are in, researchers can try to correlate treatment responses or failures with variations in each tumor’s genetic profile.
Leaders in the field say that, ideally, the tumor of every single patient now entering a cancer treatment trial should undergo a baseline genetic analysis, either using a large panel of targeted genetic markers or full-genome sequencing – although they acknowledge that, for the time being, complete sequencing provides much more information than can be used practically.
This changing paradigm of cancer treatment trial design comes with a major, built-in limitation that seems solvable only by dramatically increasing the scope of patients enrolled in trials: Each mutational cancer "driver" seems specific for just a few percent of patients. That means making statistically meaningful correlations among the responses of patients to various drugs and their tumors’ genetic profiles requires sifting through thousands of patients, far more than usually enroll in treatment trials today.
"The big challenge is to identify the mutations or genetic alterations that help inform the results of clinical trials. There are a lot of potential genetic markers, but very few have been validated. Tumors are being sequenced, and we find lots of mutations; but we don’t yet know what to do with most of this information," said Dr. Francisco J. Esteva, professor of medicine and director of the breast medical oncology program at New York University in an interview during a meeting on markers in cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"Finding ‘actionable’ mutations is very complicated because of next-generation sequencing," Dr. Esteva added. "We have some very good inhibitor drugs" aimed at certain mutations that can be highly effective in selected patients, "but when we give these drugs to larger populations of patients, the drugs may not work."
Even though a tumor may carry a genetic mutation identified as a cancer driver and thus an effective target for drug treatment in some patients, the mutation may not be the most important driver in other patients.
"Trying to find the mutations or other genetic changes that can be effective targets for treatment sounds simple, but it’s really not so simple," noted Dr. Esteva, an organizer of the meeting, sponsored by the American Society of Clinical Oncology, the European Organization for Research and Treatment of Cancer, and the National Cancer Institute.
"We’ve been in an era where we looked at one genetic marker at a time. Now it’s pretty clear that this approach will not move us forward fast enough," said Dr. Lisa A. Carey, professor and medical director of the breast center at the University of North Carolina in Chapel Hill, and another organizer of the meeting.
"Looking at a full genetic profile of the tumor has great appeal but is also more complicated," Dr. Carey explained. "Today, we have a limited portfolio of genetic markers with known clinical significance and a limited portfolio of drugs. We’ve had some huge success stories [with targeted therapy], but it’s not simple, and it is far from solved."
"Finding a target in a patient’s tumor and having an agent that inhibits the target does not imply clinical benefit," said Dr. Shivaani Kummar, head of early clinical trials development in the division of cancer treatment and diagnosis of the National Cancer Institute.
The enormous volume of genetic information now available from next-generation sequencing, which can supply data on hundreds or even potentially thousands of individual genes for a relatively affordable price, "is affecting trial design, leading to ‘umbrella’ designs that use a variety of genetic markers and panels of several drugs," said Dr. Robert L. Becker Jr., a chief medical officer at the Center for Devices and Radiological Health of the Food and Drug Administration.
"Is the current model for development of diagnostic markers and drugs sustainable?’" Dr. Becker asked. "Next-generation sequencing is different, because it can query an almost unlimited number of analytes in a single assay."
At the meeting, a series of researchers from the United States and Europe described and discussed several recently designed drug treatment trials prospectively structured to collect wide-ranging genetic data from the tumors of enrolled patients.
One example is the MATCH (Molecular Analysis for Therapy Choice) trial, expected to start in 2014 with a goal of enrolling about 1,000 patients with solid tumors that have progressed following initial treatment with at least one drug. The investigators will use targeted mutations or amplifications as well as whole exome sequencing to try to better assign patients to their next regimen, said Dr. Kummar.
"It’s a new paradigm of trial design for cancer," said Dr. Esteva. "Going forward, all major drug trials should be designed" to include genetic analyses.
But the MATCH trial, as well as several others outlined at the meeting, will require casting large nets to find adequate numbers of appropriate patients to enroll.
"It’s a small subset of patients with each type of mutation, so getting the sample size required to get information about treatments will require a community effort," said Dr. Esteva. "It’s very important to have a lot of patients to get meaningful results." Progressing from studies with relatively small numbers of patients at academic centers to the larger populations of patients treated by community oncologists "will be one of the biggest challenges," he said.
Incorporating routine genetic analyses into trials and into routine practice also raises other issues, Dr. Carey noted. "Does the tumor evolve during treatment, so that what was found with testing at baseline is no longer the disease being treated?"
Once genetic profiling of tumors becomes routine, another challenge will be efficiently and reliably alerting physicians and patients when a genetic marker that had no known clinical consequence when it was first found in a patient’s tumor is subsequently discovered to be effectively treated with a targeted drug, she said.
And no consensus has emerged on whether it’s best to do genetic assessments of tumors using specimens collected by direct biopsy or by collecting tumor cells or tumor DNA circulating in a patient’s blood, Dr. Carey cautioned.
For now, the key step is making genetic analysis a routine part of every cancer-treatment trial. "We need tumor analyses for every patient," she said. "We should get correlates for 100% of cancers."
Dr. Becker, Dr. Carey, Dr. Esteva, and Dr. Kummar all said that they had no disclosures.
On Twitter @mitchelzoler
BRUSSELS – The ballooning list of genetic markers linked with various cancers is spawning a radical shift in the design of oncology treatment trials.
These days, the trend is to incorporate detailed genetic analysis into the trials, so that once the results are in, researchers can try to correlate treatment responses or failures with variations in each tumor’s genetic profile.
Leaders in the field say that, ideally, the tumor of every single patient now entering a cancer treatment trial should undergo a baseline genetic analysis, either using a large panel of targeted genetic markers or full-genome sequencing – although they acknowledge that, for the time being, complete sequencing provides much more information than can be used practically.
This changing paradigm of cancer treatment trial design comes with a major, built-in limitation that seems solvable only by dramatically increasing the scope of patients enrolled in trials: Each mutational cancer "driver" seems specific for just a few percent of patients. That means making statistically meaningful correlations among the responses of patients to various drugs and their tumors’ genetic profiles requires sifting through thousands of patients, far more than usually enroll in treatment trials today.
"The big challenge is to identify the mutations or genetic alterations that help inform the results of clinical trials. There are a lot of potential genetic markers, but very few have been validated. Tumors are being sequenced, and we find lots of mutations; but we don’t yet know what to do with most of this information," said Dr. Francisco J. Esteva, professor of medicine and director of the breast medical oncology program at New York University in an interview during a meeting on markers in cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"Finding ‘actionable’ mutations is very complicated because of next-generation sequencing," Dr. Esteva added. "We have some very good inhibitor drugs" aimed at certain mutations that can be highly effective in selected patients, "but when we give these drugs to larger populations of patients, the drugs may not work."
Even though a tumor may carry a genetic mutation identified as a cancer driver and thus an effective target for drug treatment in some patients, the mutation may not be the most important driver in other patients.
"Trying to find the mutations or other genetic changes that can be effective targets for treatment sounds simple, but it’s really not so simple," noted Dr. Esteva, an organizer of the meeting, sponsored by the American Society of Clinical Oncology, the European Organization for Research and Treatment of Cancer, and the National Cancer Institute.
"We’ve been in an era where we looked at one genetic marker at a time. Now it’s pretty clear that this approach will not move us forward fast enough," said Dr. Lisa A. Carey, professor and medical director of the breast center at the University of North Carolina in Chapel Hill, and another organizer of the meeting.
"Looking at a full genetic profile of the tumor has great appeal but is also more complicated," Dr. Carey explained. "Today, we have a limited portfolio of genetic markers with known clinical significance and a limited portfolio of drugs. We’ve had some huge success stories [with targeted therapy], but it’s not simple, and it is far from solved."
"Finding a target in a patient’s tumor and having an agent that inhibits the target does not imply clinical benefit," said Dr. Shivaani Kummar, head of early clinical trials development in the division of cancer treatment and diagnosis of the National Cancer Institute.
The enormous volume of genetic information now available from next-generation sequencing, which can supply data on hundreds or even potentially thousands of individual genes for a relatively affordable price, "is affecting trial design, leading to ‘umbrella’ designs that use a variety of genetic markers and panels of several drugs," said Dr. Robert L. Becker Jr., a chief medical officer at the Center for Devices and Radiological Health of the Food and Drug Administration.
"Is the current model for development of diagnostic markers and drugs sustainable?’" Dr. Becker asked. "Next-generation sequencing is different, because it can query an almost unlimited number of analytes in a single assay."
At the meeting, a series of researchers from the United States and Europe described and discussed several recently designed drug treatment trials prospectively structured to collect wide-ranging genetic data from the tumors of enrolled patients.
One example is the MATCH (Molecular Analysis for Therapy Choice) trial, expected to start in 2014 with a goal of enrolling about 1,000 patients with solid tumors that have progressed following initial treatment with at least one drug. The investigators will use targeted mutations or amplifications as well as whole exome sequencing to try to better assign patients to their next regimen, said Dr. Kummar.
"It’s a new paradigm of trial design for cancer," said Dr. Esteva. "Going forward, all major drug trials should be designed" to include genetic analyses.
But the MATCH trial, as well as several others outlined at the meeting, will require casting large nets to find adequate numbers of appropriate patients to enroll.
"It’s a small subset of patients with each type of mutation, so getting the sample size required to get information about treatments will require a community effort," said Dr. Esteva. "It’s very important to have a lot of patients to get meaningful results." Progressing from studies with relatively small numbers of patients at academic centers to the larger populations of patients treated by community oncologists "will be one of the biggest challenges," he said.
Incorporating routine genetic analyses into trials and into routine practice also raises other issues, Dr. Carey noted. "Does the tumor evolve during treatment, so that what was found with testing at baseline is no longer the disease being treated?"
Once genetic profiling of tumors becomes routine, another challenge will be efficiently and reliably alerting physicians and patients when a genetic marker that had no known clinical consequence when it was first found in a patient’s tumor is subsequently discovered to be effectively treated with a targeted drug, she said.
And no consensus has emerged on whether it’s best to do genetic assessments of tumors using specimens collected by direct biopsy or by collecting tumor cells or tumor DNA circulating in a patient’s blood, Dr. Carey cautioned.
For now, the key step is making genetic analysis a routine part of every cancer-treatment trial. "We need tumor analyses for every patient," she said. "We should get correlates for 100% of cancers."
Dr. Becker, Dr. Carey, Dr. Esteva, and Dr. Kummar all said that they had no disclosures.
On Twitter @mitchelzoler
BRUSSELS – The ballooning list of genetic markers linked with various cancers is spawning a radical shift in the design of oncology treatment trials.
These days, the trend is to incorporate detailed genetic analysis into the trials, so that once the results are in, researchers can try to correlate treatment responses or failures with variations in each tumor’s genetic profile.
Leaders in the field say that, ideally, the tumor of every single patient now entering a cancer treatment trial should undergo a baseline genetic analysis, either using a large panel of targeted genetic markers or full-genome sequencing – although they acknowledge that, for the time being, complete sequencing provides much more information than can be used practically.
This changing paradigm of cancer treatment trial design comes with a major, built-in limitation that seems solvable only by dramatically increasing the scope of patients enrolled in trials: Each mutational cancer "driver" seems specific for just a few percent of patients. That means making statistically meaningful correlations among the responses of patients to various drugs and their tumors’ genetic profiles requires sifting through thousands of patients, far more than usually enroll in treatment trials today.
"The big challenge is to identify the mutations or genetic alterations that help inform the results of clinical trials. There are a lot of potential genetic markers, but very few have been validated. Tumors are being sequenced, and we find lots of mutations; but we don’t yet know what to do with most of this information," said Dr. Francisco J. Esteva, professor of medicine and director of the breast medical oncology program at New York University in an interview during a meeting on markers in cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"Finding ‘actionable’ mutations is very complicated because of next-generation sequencing," Dr. Esteva added. "We have some very good inhibitor drugs" aimed at certain mutations that can be highly effective in selected patients, "but when we give these drugs to larger populations of patients, the drugs may not work."
Even though a tumor may carry a genetic mutation identified as a cancer driver and thus an effective target for drug treatment in some patients, the mutation may not be the most important driver in other patients.
"Trying to find the mutations or other genetic changes that can be effective targets for treatment sounds simple, but it’s really not so simple," noted Dr. Esteva, an organizer of the meeting, sponsored by the American Society of Clinical Oncology, the European Organization for Research and Treatment of Cancer, and the National Cancer Institute.
"We’ve been in an era where we looked at one genetic marker at a time. Now it’s pretty clear that this approach will not move us forward fast enough," said Dr. Lisa A. Carey, professor and medical director of the breast center at the University of North Carolina in Chapel Hill, and another organizer of the meeting.
"Looking at a full genetic profile of the tumor has great appeal but is also more complicated," Dr. Carey explained. "Today, we have a limited portfolio of genetic markers with known clinical significance and a limited portfolio of drugs. We’ve had some huge success stories [with targeted therapy], but it’s not simple, and it is far from solved."
"Finding a target in a patient’s tumor and having an agent that inhibits the target does not imply clinical benefit," said Dr. Shivaani Kummar, head of early clinical trials development in the division of cancer treatment and diagnosis of the National Cancer Institute.
The enormous volume of genetic information now available from next-generation sequencing, which can supply data on hundreds or even potentially thousands of individual genes for a relatively affordable price, "is affecting trial design, leading to ‘umbrella’ designs that use a variety of genetic markers and panels of several drugs," said Dr. Robert L. Becker Jr., a chief medical officer at the Center for Devices and Radiological Health of the Food and Drug Administration.
"Is the current model for development of diagnostic markers and drugs sustainable?’" Dr. Becker asked. "Next-generation sequencing is different, because it can query an almost unlimited number of analytes in a single assay."
At the meeting, a series of researchers from the United States and Europe described and discussed several recently designed drug treatment trials prospectively structured to collect wide-ranging genetic data from the tumors of enrolled patients.
One example is the MATCH (Molecular Analysis for Therapy Choice) trial, expected to start in 2014 with a goal of enrolling about 1,000 patients with solid tumors that have progressed following initial treatment with at least one drug. The investigators will use targeted mutations or amplifications as well as whole exome sequencing to try to better assign patients to their next regimen, said Dr. Kummar.
"It’s a new paradigm of trial design for cancer," said Dr. Esteva. "Going forward, all major drug trials should be designed" to include genetic analyses.
But the MATCH trial, as well as several others outlined at the meeting, will require casting large nets to find adequate numbers of appropriate patients to enroll.
"It’s a small subset of patients with each type of mutation, so getting the sample size required to get information about treatments will require a community effort," said Dr. Esteva. "It’s very important to have a lot of patients to get meaningful results." Progressing from studies with relatively small numbers of patients at academic centers to the larger populations of patients treated by community oncologists "will be one of the biggest challenges," he said.
Incorporating routine genetic analyses into trials and into routine practice also raises other issues, Dr. Carey noted. "Does the tumor evolve during treatment, so that what was found with testing at baseline is no longer the disease being treated?"
Once genetic profiling of tumors becomes routine, another challenge will be efficiently and reliably alerting physicians and patients when a genetic marker that had no known clinical consequence when it was first found in a patient’s tumor is subsequently discovered to be effectively treated with a targeted drug, she said.
And no consensus has emerged on whether it’s best to do genetic assessments of tumors using specimens collected by direct biopsy or by collecting tumor cells or tumor DNA circulating in a patient’s blood, Dr. Carey cautioned.
For now, the key step is making genetic analysis a routine part of every cancer-treatment trial. "We need tumor analyses for every patient," she said. "We should get correlates for 100% of cancers."
Dr. Becker, Dr. Carey, Dr. Esteva, and Dr. Kummar all said that they had no disclosures.
On Twitter @mitchelzoler
EXPERT ANALYSIS AT THE MARKERS IN CANCER MEETING
Axillary treatment unnecessary after some total mastectomies
SAN FRANCISCO – Regional recurrence and recurrence-free survival rates were statistically similar in subgroups of 730 patients who had sentinel lymph node–positive, invasive breast cancer and underwent total mastectomy.
The outcomes were comparable regardless of whether or not patients had a subsequent completion axillary lymph node dissection and radiation therapy. A marginally higher 10-year regional recurrence rate of 4.9% in patients who did not have completion lymphadenectomy was not significantly different from rates seen in patients who had completion lymphadenectomy but not radiation therapy (1.4% of whom had a regional recurrence) or in patients who had completion lymphadenectomy plus radiation therapy (3.1% had a regional recurrence), in the retrospective institutional study.
Recurrence-free survival rates also did not differ between groups, reported Dr. Elizabeth FitzSullivan and her associates.
Using the M.D. Anderson Cancer Center nomogram to predict whether lymph nodes other than the sentinel node will have cancer, the investigators predicted a significantly lower probability of additional lymph node positivity in the 98 patients who did not undergo completion lymphadenectomy (a median 10% probability), compared with the 632 patients who had a completion lymphadenectomy (23% probability), reported Dr. FitzSullivan of the University of Texas M.D. Anderson Cancer Center, Houston.
"In select patients with early-stage breast cancer treated with mastectomy with a positive sentinel lymph node biopsy, completion lymphadenectomy may be avoided without adversely affecting recurrence or recurrence-free survival," the investigators concluded in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology. The study won a Merit Award at the meeting.
The retrospective analysis of data from patients treated at the M.D. Anderson Cancer Center during 1994-2010 defined completion lymphadenectomy as removal of 10 or more lymph nodes. Median follow-up was 66 months.
In general, patients with early-stage breast cancer who are treated with conserving therapy and who have minimal axillary disease on sentinel lymph node biopsy often don’t undergo completion lymphadenectomy. The increasing rate of total mastectomies heightens the need to identify patients who undergo total mastectomy who may not benefit from completion lymphadenectomy and axillary radiation therapy, the researchers noted.
There were several baseline characteristics that differed significantly between patients who did or did not undergo completion lymphadenectomy in the study. Compared with patients who did have completion lymphadenectomy, those who did not undergo the additional axillary surgery were older (57 years vs. 53 years, respectively); had smaller tumors (a median of 2 cm vs. 2.3 cm); and were less likely to have stage T3 disease (8% vs. 17%), lymphovascular invasion (25% vs. 41%), or extranodal extension of disease (4% vs. 24%). The median size of sentinel node metastasis was smaller in patients who did not have completion lymphadenectomy (1.1 mm) compared with those who did (4 mm).
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. FitzSullivan reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Regional recurrence and recurrence-free survival rates were statistically similar in subgroups of 730 patients who had sentinel lymph node–positive, invasive breast cancer and underwent total mastectomy.
The outcomes were comparable regardless of whether or not patients had a subsequent completion axillary lymph node dissection and radiation therapy. A marginally higher 10-year regional recurrence rate of 4.9% in patients who did not have completion lymphadenectomy was not significantly different from rates seen in patients who had completion lymphadenectomy but not radiation therapy (1.4% of whom had a regional recurrence) or in patients who had completion lymphadenectomy plus radiation therapy (3.1% had a regional recurrence), in the retrospective institutional study.
Recurrence-free survival rates also did not differ between groups, reported Dr. Elizabeth FitzSullivan and her associates.
Using the M.D. Anderson Cancer Center nomogram to predict whether lymph nodes other than the sentinel node will have cancer, the investigators predicted a significantly lower probability of additional lymph node positivity in the 98 patients who did not undergo completion lymphadenectomy (a median 10% probability), compared with the 632 patients who had a completion lymphadenectomy (23% probability), reported Dr. FitzSullivan of the University of Texas M.D. Anderson Cancer Center, Houston.
"In select patients with early-stage breast cancer treated with mastectomy with a positive sentinel lymph node biopsy, completion lymphadenectomy may be avoided without adversely affecting recurrence or recurrence-free survival," the investigators concluded in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology. The study won a Merit Award at the meeting.
The retrospective analysis of data from patients treated at the M.D. Anderson Cancer Center during 1994-2010 defined completion lymphadenectomy as removal of 10 or more lymph nodes. Median follow-up was 66 months.
In general, patients with early-stage breast cancer who are treated with conserving therapy and who have minimal axillary disease on sentinel lymph node biopsy often don’t undergo completion lymphadenectomy. The increasing rate of total mastectomies heightens the need to identify patients who undergo total mastectomy who may not benefit from completion lymphadenectomy and axillary radiation therapy, the researchers noted.
There were several baseline characteristics that differed significantly between patients who did or did not undergo completion lymphadenectomy in the study. Compared with patients who did have completion lymphadenectomy, those who did not undergo the additional axillary surgery were older (57 years vs. 53 years, respectively); had smaller tumors (a median of 2 cm vs. 2.3 cm); and were less likely to have stage T3 disease (8% vs. 17%), lymphovascular invasion (25% vs. 41%), or extranodal extension of disease (4% vs. 24%). The median size of sentinel node metastasis was smaller in patients who did not have completion lymphadenectomy (1.1 mm) compared with those who did (4 mm).
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. FitzSullivan reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Regional recurrence and recurrence-free survival rates were statistically similar in subgroups of 730 patients who had sentinel lymph node–positive, invasive breast cancer and underwent total mastectomy.
The outcomes were comparable regardless of whether or not patients had a subsequent completion axillary lymph node dissection and radiation therapy. A marginally higher 10-year regional recurrence rate of 4.9% in patients who did not have completion lymphadenectomy was not significantly different from rates seen in patients who had completion lymphadenectomy but not radiation therapy (1.4% of whom had a regional recurrence) or in patients who had completion lymphadenectomy plus radiation therapy (3.1% had a regional recurrence), in the retrospective institutional study.
Recurrence-free survival rates also did not differ between groups, reported Dr. Elizabeth FitzSullivan and her associates.
Using the M.D. Anderson Cancer Center nomogram to predict whether lymph nodes other than the sentinel node will have cancer, the investigators predicted a significantly lower probability of additional lymph node positivity in the 98 patients who did not undergo completion lymphadenectomy (a median 10% probability), compared with the 632 patients who had a completion lymphadenectomy (23% probability), reported Dr. FitzSullivan of the University of Texas M.D. Anderson Cancer Center, Houston.
"In select patients with early-stage breast cancer treated with mastectomy with a positive sentinel lymph node biopsy, completion lymphadenectomy may be avoided without adversely affecting recurrence or recurrence-free survival," the investigators concluded in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology. The study won a Merit Award at the meeting.
The retrospective analysis of data from patients treated at the M.D. Anderson Cancer Center during 1994-2010 defined completion lymphadenectomy as removal of 10 or more lymph nodes. Median follow-up was 66 months.
In general, patients with early-stage breast cancer who are treated with conserving therapy and who have minimal axillary disease on sentinel lymph node biopsy often don’t undergo completion lymphadenectomy. The increasing rate of total mastectomies heightens the need to identify patients who undergo total mastectomy who may not benefit from completion lymphadenectomy and axillary radiation therapy, the researchers noted.
There were several baseline characteristics that differed significantly between patients who did or did not undergo completion lymphadenectomy in the study. Compared with patients who did have completion lymphadenectomy, those who did not undergo the additional axillary surgery were older (57 years vs. 53 years, respectively); had smaller tumors (a median of 2 cm vs. 2.3 cm); and were less likely to have stage T3 disease (8% vs. 17%), lymphovascular invasion (25% vs. 41%), or extranodal extension of disease (4% vs. 24%). The median size of sentinel node metastasis was smaller in patients who did not have completion lymphadenectomy (1.1 mm) compared with those who did (4 mm).
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. FitzSullivan reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Median 10-year regional recurrence rates were 4.9% in patients who did not undergo completion lymphadenectomy, 3.1% for patients who had completion lymphadenectomy plus radiation therapy, and 1.4% for patients with completion lymphadenectomy but not radiation therapy.
Data source: A retrospective study of 730 patients with invasive breast cancer and a positive sentinel lymph node biopsy, treated with total mastectomy, between 1994 and 2010 at the University of Texas M.D. Anderson Cancer Center, Houston.
Disclosures: Dr. FitzSullivan reported having no financial disclosures.











