User login
Transfusion Medicine
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
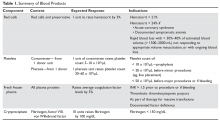
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
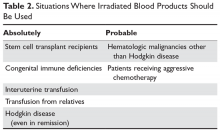
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
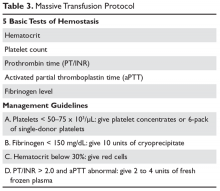
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
Restrictive transfusions do not increase long-term CV surgery risk
MUNICH – in a large, randomized trial presented at the annual congress of the European Society of Cardiology, and published simultaneously in the New England Journal of Medicine.
The investigators previously reported that 28 day outcomes were non-inferior with the restrictive approach, but they wanted to look into 6 month results to rule out latent problems, such as sequelae from perioperative organ hypoxia.
The new outcomes from the study, dubbed the Transfusion Requirements in Cardiac Surgery (TRICS) III trial, offer some reassurance at a time when cardiac surgeons are shifting towards more restrictive transfusion policies (N Engl J Med. 2018 Aug 26.doi: 10.1056/NEJMoa1808561).
The trial randomized 2,317 patients to red cell transfusions if their hemoglobin concentrations fell below 7.5 g/dL intraoperatively or postoperatively. Another 2,347 were randomized to the liberal approach, with transfusions below 9.5 g/dL in the operating room and ICU, and below 8.5 g/dL outside of the ICU. The arms were well matched, with a mean score of 8 in both groups on the 47-point European System for Cardiac Operative Risk Evaluation I score.
At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive threshold); 6.2% of patients in the restrictive-threshold group, versus 6.4% in the liberal-arm, had died at that point, a statistically insignificant difference.
Also at 6 months, 43.8% of patients in the restrictive-threshold group, versus 42.8% in the liberal arm, met the study’s secondary composite outcome, which included the components of the primary outcome plus hospital readmissions, ED visits, and coronary revascularization. The difference was again statistically insignificant.
The restrictive strategy saved a lot of blood. Just over half of the patients, versus almost three-quarters in the liberal arm, were transfused during their index admissions. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units.
Unexpectedly, patients 75 years and older had a lower risk of poor outcomes with the restrictive strategy, while the liberal strategy was associated with lower risk in younger subjects. The findings “appear to contradict” current practice, “in which a liberal transfusion strategy is used in older patients undergoing cardiac or noncardiac surgery,” said investigators led by C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
“One could hypothesize that older patients may have unacceptable adverse effects related to transfusion (e.g., volume overload and inflammatory and infectious complications) or that there may be age-related differences in the adverse-effect profile of transfusion or anemia. ... Whether transfusion thresholds should differ according to age” needs to be determined, they said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded. The trial was conducted in 19 countries, including China and India; results were consistent across study sites.
The work was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer had no relevant disclosures.
SOURCE: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
MUNICH – in a large, randomized trial presented at the annual congress of the European Society of Cardiology, and published simultaneously in the New England Journal of Medicine.
The investigators previously reported that 28 day outcomes were non-inferior with the restrictive approach, but they wanted to look into 6 month results to rule out latent problems, such as sequelae from perioperative organ hypoxia.
The new outcomes from the study, dubbed the Transfusion Requirements in Cardiac Surgery (TRICS) III trial, offer some reassurance at a time when cardiac surgeons are shifting towards more restrictive transfusion policies (N Engl J Med. 2018 Aug 26.doi: 10.1056/NEJMoa1808561).
The trial randomized 2,317 patients to red cell transfusions if their hemoglobin concentrations fell below 7.5 g/dL intraoperatively or postoperatively. Another 2,347 were randomized to the liberal approach, with transfusions below 9.5 g/dL in the operating room and ICU, and below 8.5 g/dL outside of the ICU. The arms were well matched, with a mean score of 8 in both groups on the 47-point European System for Cardiac Operative Risk Evaluation I score.
At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive threshold); 6.2% of patients in the restrictive-threshold group, versus 6.4% in the liberal-arm, had died at that point, a statistically insignificant difference.
Also at 6 months, 43.8% of patients in the restrictive-threshold group, versus 42.8% in the liberal arm, met the study’s secondary composite outcome, which included the components of the primary outcome plus hospital readmissions, ED visits, and coronary revascularization. The difference was again statistically insignificant.
The restrictive strategy saved a lot of blood. Just over half of the patients, versus almost three-quarters in the liberal arm, were transfused during their index admissions. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units.
Unexpectedly, patients 75 years and older had a lower risk of poor outcomes with the restrictive strategy, while the liberal strategy was associated with lower risk in younger subjects. The findings “appear to contradict” current practice, “in which a liberal transfusion strategy is used in older patients undergoing cardiac or noncardiac surgery,” said investigators led by C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
“One could hypothesize that older patients may have unacceptable adverse effects related to transfusion (e.g., volume overload and inflammatory and infectious complications) or that there may be age-related differences in the adverse-effect profile of transfusion or anemia. ... Whether transfusion thresholds should differ according to age” needs to be determined, they said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded. The trial was conducted in 19 countries, including China and India; results were consistent across study sites.
The work was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer had no relevant disclosures.
SOURCE: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
MUNICH – in a large, randomized trial presented at the annual congress of the European Society of Cardiology, and published simultaneously in the New England Journal of Medicine.
The investigators previously reported that 28 day outcomes were non-inferior with the restrictive approach, but they wanted to look into 6 month results to rule out latent problems, such as sequelae from perioperative organ hypoxia.
The new outcomes from the study, dubbed the Transfusion Requirements in Cardiac Surgery (TRICS) III trial, offer some reassurance at a time when cardiac surgeons are shifting towards more restrictive transfusion policies (N Engl J Med. 2018 Aug 26.doi: 10.1056/NEJMoa1808561).
The trial randomized 2,317 patients to red cell transfusions if their hemoglobin concentrations fell below 7.5 g/dL intraoperatively or postoperatively. Another 2,347 were randomized to the liberal approach, with transfusions below 9.5 g/dL in the operating room and ICU, and below 8.5 g/dL outside of the ICU. The arms were well matched, with a mean score of 8 in both groups on the 47-point European System for Cardiac Operative Risk Evaluation I score.
At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive threshold); 6.2% of patients in the restrictive-threshold group, versus 6.4% in the liberal-arm, had died at that point, a statistically insignificant difference.
Also at 6 months, 43.8% of patients in the restrictive-threshold group, versus 42.8% in the liberal arm, met the study’s secondary composite outcome, which included the components of the primary outcome plus hospital readmissions, ED visits, and coronary revascularization. The difference was again statistically insignificant.
The restrictive strategy saved a lot of blood. Just over half of the patients, versus almost three-quarters in the liberal arm, were transfused during their index admissions. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units.
Unexpectedly, patients 75 years and older had a lower risk of poor outcomes with the restrictive strategy, while the liberal strategy was associated with lower risk in younger subjects. The findings “appear to contradict” current practice, “in which a liberal transfusion strategy is used in older patients undergoing cardiac or noncardiac surgery,” said investigators led by C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
“One could hypothesize that older patients may have unacceptable adverse effects related to transfusion (e.g., volume overload and inflammatory and infectious complications) or that there may be age-related differences in the adverse-effect profile of transfusion or anemia. ... Whether transfusion thresholds should differ according to age” needs to be determined, they said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded. The trial was conducted in 19 countries, including China and India; results were consistent across study sites.
The work was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer had no relevant disclosures.
SOURCE: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: A restrictive transfusion strategy during cardiovascular surgery, versus a more traditional liberal approach, did not increase the risk of poor outcomes at 6 months.
Major finding: At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive approach).
Study details: Randomized, multicenter trial with over 5,000 surgery patients
Disclosures: The work was funded by the Canadian Institutes of Health Research, among others. The lead investigator had no relevant disclosures.
Source: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
Prehospital plasma outperforms standard care
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
The Prehospital Air Medical Plasma (PAMPer) trial shows that prehospital plasma improves survival rates in trauma patients at risk for hemorrhagic shock, and its implementation should therefore be considered, according to Jeremy W. Cannon, MD.
“Severe hemorrhage from injury claims the lives of nearly 50,000 Americans every year,” Dr. Cannon wrote in an accompanying editorial. “Because many of these deaths occur in young, vital people, this number translates to an astounding loss of almost 2,000,000 years of productive life.”
Since most deaths occur in less than 2 hours, severe hemorrhage requires rapid intervention; however, the best methods of intervention are unclear. In 1994, Bickell et al. demonstrated that administration of crystalloid-based therapy was more beneficial in a goal-directed, operative setting than it was in transit to a trauma center. Other studies have shown that rapid transport and tourniquet application, when appropriate, are essential.
“But what about patients who have blunt injuries and longer transport times?” asked Dr. Cannon. Recently, damage-control resuscitation has proven effective in the trauma center – a combination of red cells, plasma, and platelets in approximately equal proportions, with minimal nonhemostatic crystalloid solution. Researchers now ask whether this intervention has a place in the prehospital setting.
The PAMPer trial suggests that the number needed to treat is 10 to save one life. These results “should motivate trauma center personnel and air medical crews across the country to consider implementing this lifesaving approach,” Dr. Cannon wrote.
Several blood products are candidates for prehospital resuscitation; their selection depends on both logistical and medical factors. Although fresh-frozen plasma was used in the PAMPer trial, it has a shelf life of only 5 days once thawed, leading to more frequent replenishment. Never-frozen plasma lasts 26 days, which could be easier to keep in stock. Beyond plasma, “refrigerated whole blood has a shelf life of 21 days and offers the benefit of both platelets and oxygen delivery, making it perhaps the ideal product for prehospital resuscitation,” Dr. Cannon wrote.
Jeremy W. Cannon, MD, is at the University of Pennsylvania in Philadelphia and the Uniformed Services University in Bethesda, Md. These comments are adapted from an accompanying editorial (N Engl J Med. 2018 Jul 26;379[4]:387-8). Dr. Cannon reported nonfinancial support from Prytime Medical Devices outside the submitted work and institutional participation in a research network funded by the Department of Defense.
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
Prehospital administration of plasma to trauma patients at risk for hemorrhagic shock improves odds of survival, according to the Prehospital Air Medical Plasma (PAMPer) trial.
Patients who received plasma also had a decreased median prothrombin to time ratio, compared with those who received standard-care resuscitation alone.
Modern trauma patients benefit “from receiving less crystalloid-based therapy and early balanced blood component–based therapy once they arrive at a facility for definitive care,” Jason L. Sperry, MD, of the University of Pittsburgh and his coauthors wrote in the New England Journal of Medicine. These newer strategies aim to mitigate coagulopathy and its associated downstream complications.
Still, “a majority of deaths from traumatic hemorrhage continue to occur in the first hours after arrival at the trauma center, which underscores the importance of the prehospital environment for early interventions that provide benefit,” the investigators noted.
The PAMPer trial (NCT01818427) examined the efficacy and safety of prehospital plasma resuscitation, comparing it with standard-care resuscitation, in severely injured patients. The phase 3, randomized, superiority trial involved 501 trauma patients at risk for hemorrhagic shock who were transported from 27 air medical bases to nine trauma centers. In total, 230 patients received prehospital plasma and standard-care resuscitation, and 271 patients received standard-care alone. Standard-care included infusion of crystalloid fluids in a goal-directed manner. Air medical bases delivered each type of care in 1-month intervals.
Patients were eligible if they exhibited severe hypotension (systolic blood pressure less than 70 mm Hg) or both hypotension (systolic blood pressure less than 90 mm Hg) and tachycardia (greater than 108 beats per minute).
Eligible patients received two units of thawed plasma, which was completely delivered before administration of any other fluids. If infusion of plasma was not complete upon arrival at the trauma center, infusion was completed before any in-hospital fluids were administered. Following preexisting local protocols, 13 of 27 air transport teams carried 2U of red blood cells. If red blood cells were delivered, then this was performed after plasma administration if the patient was still hypotensive or obviously bleeding.
The 30-day mortality rate among all patients was 29.6%. Approximately one in three patients received a prehospital red blood cell transfusion, and nearly 60% underwent surgery within 24 hours of hospital admission.
A greater percentage of patients in the standard-care group were given red blood cell transfusions, compared with those in the plasma group. The standard-care patients also received greater volumes of crystalloid solution than did those receiving plasma.
The primary outcome, 30-day mortality, was 9.8% lower for patients who received plasma, compared with patients who received standard-care alone (23.2% vs. 33.0%; P = .03). Multivariate regression analysis revealed that plasma delivery accounted for a 39% lower risk of death within 30 days, compared with standard care (P = .02). Within 3 hours of randomization, Kaplan-Meier curves revealed a separation between the two treatment groups that persisted until 30 days.
Median prothrombin to time ratio, the only statistically significant secondary outcome, was lower in the plasma group than it was in the standard-care group (1.2 vs. 1.3; P less than .001).
Other measured parameters were similar between the two main treatment groups, including rates of nosocomial infections, allergic or transfusion-related reactions, acute lung injury/acute respiratory distress syndrome, and multiorgan failure.
“Although we cannot determine the independent or additive effects of prehospital administration of plasma and packed red cells, the survival benefits attributable to plasma administration persisted after adjustment for prehospital red-cell administration, and a subgroup analysis showed no heterogeneity of the treatment effect,” the investigators wrote.
The PAMPer trial was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
SOURCE: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Thirty-day mortality was 9.8 percentage points lower for patients receiving plasma, compared with patients receiving standard care (23.2% vs. 33.0%; P = .03).
Study details: A randomized, controlled superiority trial involving 501 trauma patients at risk for hemorrhagic shock, with 230 patients receiving plasma and 271 receiving standard-care during air medical transport.
Disclosures: The study was supported by a grant from the U.S. Army Medical Research and Materiel Command. One author reported funding from Janssen Pharmaceuticals, CSL Behring, Haemonetics, and Accriva Diagnostics, as well as having been named on a patent on TLR4 inhibitors for the treatment of inflammatory and infectious disorders.
Source: Sperry JL et al. N Engl J Med. 2018 Jul 26;379(4):315-26.
FDA gives green light to freeze-dried plasma in combat
The Department of Defense has received emergency use authorization from the Food and Drug Administration to use pathogen-reduced, leukocyte-depleted, freeze-dried plasma for the emergency treatment of hemorrhage and coagulopathy in combat situations.
Hemorrhage and coagulopathy are the leading causes of preventable deaths among combat trauma casualties. While plasma contains proteins that help clot blood and thus can treat these conditions, it isn’t feasible to keep it on hand for combat emergencies in the field because of logistical and operational requirements, such as refrigeration or thawing periods. This freeze-dried plasma product, on the other hand, can be easily reconstituted in situations in which refrigeration isn’t possible.
The FDA authorization allows for the use of a French-made, powdered, freeze-dried product. This emergency use authorization came about in part because of a joint program established between the FDA and the Department of Defense in January 2018.
“Earlier this year, we reaffirmed our commitment to the Department of Defense and to the dedicated men and women protecting our country, by expediting the development and availability of safe and effective, priority medical products that are essential to the health of our military service members,” said FDA commissioner Scott Gottlieb, MD. “This is especially true when it comes to products used to treat injuries in a potential battlefield setting.”
More information about this emergency use authorization can be found in the FDA’s full press announcement.
The Department of Defense has received emergency use authorization from the Food and Drug Administration to use pathogen-reduced, leukocyte-depleted, freeze-dried plasma for the emergency treatment of hemorrhage and coagulopathy in combat situations.
Hemorrhage and coagulopathy are the leading causes of preventable deaths among combat trauma casualties. While plasma contains proteins that help clot blood and thus can treat these conditions, it isn’t feasible to keep it on hand for combat emergencies in the field because of logistical and operational requirements, such as refrigeration or thawing periods. This freeze-dried plasma product, on the other hand, can be easily reconstituted in situations in which refrigeration isn’t possible.
The FDA authorization allows for the use of a French-made, powdered, freeze-dried product. This emergency use authorization came about in part because of a joint program established between the FDA and the Department of Defense in January 2018.
“Earlier this year, we reaffirmed our commitment to the Department of Defense and to the dedicated men and women protecting our country, by expediting the development and availability of safe and effective, priority medical products that are essential to the health of our military service members,” said FDA commissioner Scott Gottlieb, MD. “This is especially true when it comes to products used to treat injuries in a potential battlefield setting.”
More information about this emergency use authorization can be found in the FDA’s full press announcement.
The Department of Defense has received emergency use authorization from the Food and Drug Administration to use pathogen-reduced, leukocyte-depleted, freeze-dried plasma for the emergency treatment of hemorrhage and coagulopathy in combat situations.
Hemorrhage and coagulopathy are the leading causes of preventable deaths among combat trauma casualties. While plasma contains proteins that help clot blood and thus can treat these conditions, it isn’t feasible to keep it on hand for combat emergencies in the field because of logistical and operational requirements, such as refrigeration or thawing periods. This freeze-dried plasma product, on the other hand, can be easily reconstituted in situations in which refrigeration isn’t possible.
The FDA authorization allows for the use of a French-made, powdered, freeze-dried product. This emergency use authorization came about in part because of a joint program established between the FDA and the Department of Defense in January 2018.
“Earlier this year, we reaffirmed our commitment to the Department of Defense and to the dedicated men and women protecting our country, by expediting the development and availability of safe and effective, priority medical products that are essential to the health of our military service members,” said FDA commissioner Scott Gottlieb, MD. “This is especially true when it comes to products used to treat injuries in a potential battlefield setting.”
More information about this emergency use authorization can be found in the FDA’s full press announcement.
FDA recommends pooled Zika testing of blood donations
The moving away from individual testing of donations and toward pooled testing.
“This [practice of pooled testing] is usually more cost effective and less burdensome for blood establishments,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement. “However, the FDA will continue to monitor the situation closely and, as appropriate, reconsider what measures are needed to maintain the safety of the blood supply.”
The new testing recommendations reflect the decreasing number of cases of Zika virus infection in the U.S. and its territories, as well as advice from the agency’s Blood Products Advisory Committee.
The guidance makes an exception to its pooled testing recommendations: Donations from areas where a positive donation has been detected or in which the risk of mosquito-borne transmission of Zika virus is high should be tested individually. The guidance also allows the use of an FDA-approved pathogen-reduction device for plasma and certain platelet products.
The moving away from individual testing of donations and toward pooled testing.
“This [practice of pooled testing] is usually more cost effective and less burdensome for blood establishments,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement. “However, the FDA will continue to monitor the situation closely and, as appropriate, reconsider what measures are needed to maintain the safety of the blood supply.”
The new testing recommendations reflect the decreasing number of cases of Zika virus infection in the U.S. and its territories, as well as advice from the agency’s Blood Products Advisory Committee.
The guidance makes an exception to its pooled testing recommendations: Donations from areas where a positive donation has been detected or in which the risk of mosquito-borne transmission of Zika virus is high should be tested individually. The guidance also allows the use of an FDA-approved pathogen-reduction device for plasma and certain platelet products.
The moving away from individual testing of donations and toward pooled testing.
“This [practice of pooled testing] is usually more cost effective and less burdensome for blood establishments,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the statement. “However, the FDA will continue to monitor the situation closely and, as appropriate, reconsider what measures are needed to maintain the safety of the blood supply.”
The new testing recommendations reflect the decreasing number of cases of Zika virus infection in the U.S. and its territories, as well as advice from the agency’s Blood Products Advisory Committee.
The guidance makes an exception to its pooled testing recommendations: Donations from areas where a positive donation has been detected or in which the risk of mosquito-borne transmission of Zika virus is high should be tested individually. The guidance also allows the use of an FDA-approved pathogen-reduction device for plasma and certain platelet products.
RBC transfusions with surgery may increase VTE risk
In patients undergoing surgery, RBC transfusions may be associated with the development of new or progressive venous thromboembolism within 30 days of the procedure, results of a recent registry study suggest.
Patients who received perioperative RBC transfusions had significantly higher odds of developing postoperative venous thromboembolism (VTE) overall, as well as higher odds specifically for deep venous thrombosis (DVT) and pulmonary embolism (PE), according to results published in JAMA Surgery.
“In a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist,” Dr. Goel and her coauthors wrote.
The analysis was based on prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014, of which 47,410 (6.3%) received one or more perioperative RBC transfusions. VTE occurred in 6,309 patients (0.8%), of which 4,336 cases were DVT (0.6%) and 2,514 were PE (0.3%).
The patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3) versus those who had no transfusions, according to results of a multivariable analysis adjusting for age, sex, length of hospital stay, use of mechanical ventilation, and other potentially confounding factors.
Similarly, researchers found transfused patients had higher odds of both DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1).
Odds of VTE increased significantly along with increasing number of perioperative RBC transfusions from an aOR of 2.1 for those with just one transfusion to 4.5 for those who had three or more transfusion events (P less than .001 for trend), results of a dose-response analysis showed.
The association between RBC transfusions perioperatively and VTE postoperatively remained robust after propensity score matching and was statistically significant in all surgical subspecialties, the researchers reported.
However, they also noted that these results will require validation in prospective cohort studies and randomized clinical trials. “If proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.”
The study was funded in part by grants from the National Institutes of Health and Cornell University. The researchers reported having no conflicts of interest.
SOURCE: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
In patients undergoing surgery, RBC transfusions may be associated with the development of new or progressive venous thromboembolism within 30 days of the procedure, results of a recent registry study suggest.
Patients who received perioperative RBC transfusions had significantly higher odds of developing postoperative venous thromboembolism (VTE) overall, as well as higher odds specifically for deep venous thrombosis (DVT) and pulmonary embolism (PE), according to results published in JAMA Surgery.
“In a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist,” Dr. Goel and her coauthors wrote.
The analysis was based on prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014, of which 47,410 (6.3%) received one or more perioperative RBC transfusions. VTE occurred in 6,309 patients (0.8%), of which 4,336 cases were DVT (0.6%) and 2,514 were PE (0.3%).
The patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3) versus those who had no transfusions, according to results of a multivariable analysis adjusting for age, sex, length of hospital stay, use of mechanical ventilation, and other potentially confounding factors.
Similarly, researchers found transfused patients had higher odds of both DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1).
Odds of VTE increased significantly along with increasing number of perioperative RBC transfusions from an aOR of 2.1 for those with just one transfusion to 4.5 for those who had three or more transfusion events (P less than .001 for trend), results of a dose-response analysis showed.
The association between RBC transfusions perioperatively and VTE postoperatively remained robust after propensity score matching and was statistically significant in all surgical subspecialties, the researchers reported.
However, they also noted that these results will require validation in prospective cohort studies and randomized clinical trials. “If proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.”
The study was funded in part by grants from the National Institutes of Health and Cornell University. The researchers reported having no conflicts of interest.
SOURCE: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
In patients undergoing surgery, RBC transfusions may be associated with the development of new or progressive venous thromboembolism within 30 days of the procedure, results of a recent registry study suggest.
Patients who received perioperative RBC transfusions had significantly higher odds of developing postoperative venous thromboembolism (VTE) overall, as well as higher odds specifically for deep venous thrombosis (DVT) and pulmonary embolism (PE), according to results published in JAMA Surgery.
“In a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist,” Dr. Goel and her coauthors wrote.
The analysis was based on prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014, of which 47,410 (6.3%) received one or more perioperative RBC transfusions. VTE occurred in 6,309 patients (0.8%), of which 4,336 cases were DVT (0.6%) and 2,514 were PE (0.3%).
The patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3) versus those who had no transfusions, according to results of a multivariable analysis adjusting for age, sex, length of hospital stay, use of mechanical ventilation, and other potentially confounding factors.
Similarly, researchers found transfused patients had higher odds of both DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1).
Odds of VTE increased significantly along with increasing number of perioperative RBC transfusions from an aOR of 2.1 for those with just one transfusion to 4.5 for those who had three or more transfusion events (P less than .001 for trend), results of a dose-response analysis showed.
The association between RBC transfusions perioperatively and VTE postoperatively remained robust after propensity score matching and was statistically significant in all surgical subspecialties, the researchers reported.
However, they also noted that these results will require validation in prospective cohort studies and randomized clinical trials. “If proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.”
The study was funded in part by grants from the National Institutes of Health and Cornell University. The researchers reported having no conflicts of interest.
SOURCE: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
FROM JAMA SURGERY
Key clinical point:
Major finding: Patients who received perioperative RBC transfusions had significantly increased odds of developing VTE in the 30-day postoperative period (adjusted odds ratio, 2.1; 95% confidence interval, 2.0-2.3), compared with patients who did not receive transfusions.
Study details: An analysis of prospectively collected North American registry data including 750,937 patients who underwent a surgical procedure in 2014.
Disclosures: The study was funded in part by grants from the National Institutes of Health and Cornell University, New York. The researchers reported having no conflicts of interest.
Source: Goel R et al. JAMA Surg. 2018 Jun 13. doi: 10.1001/jamasurg.2018.1565.
In abdominal myomectomy, cell salvage may reduce transfusions
ORLANDO – Cell salvage may help reduce the need for allogeneic blood transfusion in patients undergoing abdominal myomectomy, according to findings from a retrospective cohort study.
Of 138 women who underwent abdominal myomectomy, 52 had no cell salvage and 86 had cell salvage ordered. Of those who had cell salvage ordered, 60 had salvage fully set up and 26 had salvage on standby; 46 of the 60 with full set-up had autologous blood returned, and of those, 14 required subsequent allogeneic transfusion of more than 20 U of blood, Julian A. Gingold, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Notable differences between those who did and those who did not have cell salvage ordered included the number of patients with fibroids weighing more than 250 g (52% vs. 13%) and with five or more total fibroids (83% vs. 56%), he said.
“And interestingly, reproductive surgeons were less likely (than general surgeons) to order cell salvage,” he said.
Surgery was performed by a reproductive surgeon in 25% of cases in the cell salvage group vs. in 67% of cases in the non–cell salvage group.
The finding of comparable allogeneic transfusion requirement between the two groups despite differences in blood loss and “arguably less complex surgeries [in those] without cell salvage” was striking, Dr. Gingold said.
“If you take these 86 patients who had cell salvage intraoperatively, 14 of them ultimately required donor blood, and that amounted to 23 units, giving kind of a lower limit for the potential benefit of cell salvage in this patient cohort,” he said.
Abdominal myomectomy often has a high rate of blood loss, with about a 10%-20% rate of transfusion. While the technique of cell salvage is widely used in other fields, it hasn’t been fully investigated in the context of gynecologic surgery, he said, explaining the rationale for the study.
Subjects included were women aged 18-60 years who underwent abdominal myomectomy for benign indications at the Cleveland Clinic during 2011-2016. Those with current malignancy or with surgery performed by a gynecologic oncologist were excluded.
The non–cell salvage and cell salvage groups were comparable with respect to age, body mass index, ethnicity, and preoperative and postoperative hemoglobin.
Dr. Gingold noted that “prospective study with randomization will be required to better define the role of cell salvage in abdominal myomectomies.”
During a question and answer session following his presentation, Charles Ascher-Walsh, MD, of Mount Sinai Health System, N.Y. noted that the transfusion rates and blood loss were high in the study, and that solid data support the use of tourniquets for patients undergoing abdominal myomectomy, with studies showing only a 1%-2% transfusion rate with continuous tourniquet use. Dr. Gingold said the use of tourniquets in the study was low and was dictated by surgeon preference. He agreed that tourniquet use is “certainly an option that should be adjunctive,” and that it would be useful to look at the outcomes in the cases with and without tourniquet use.
Dr. Gingold reported having no disclosures.
SOURCE: Gingold J et al. SGS 2018 Oral Poster 18.
ORLANDO – Cell salvage may help reduce the need for allogeneic blood transfusion in patients undergoing abdominal myomectomy, according to findings from a retrospective cohort study.
Of 138 women who underwent abdominal myomectomy, 52 had no cell salvage and 86 had cell salvage ordered. Of those who had cell salvage ordered, 60 had salvage fully set up and 26 had salvage on standby; 46 of the 60 with full set-up had autologous blood returned, and of those, 14 required subsequent allogeneic transfusion of more than 20 U of blood, Julian A. Gingold, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Notable differences between those who did and those who did not have cell salvage ordered included the number of patients with fibroids weighing more than 250 g (52% vs. 13%) and with five or more total fibroids (83% vs. 56%), he said.
“And interestingly, reproductive surgeons were less likely (than general surgeons) to order cell salvage,” he said.
Surgery was performed by a reproductive surgeon in 25% of cases in the cell salvage group vs. in 67% of cases in the non–cell salvage group.
The finding of comparable allogeneic transfusion requirement between the two groups despite differences in blood loss and “arguably less complex surgeries [in those] without cell salvage” was striking, Dr. Gingold said.
“If you take these 86 patients who had cell salvage intraoperatively, 14 of them ultimately required donor blood, and that amounted to 23 units, giving kind of a lower limit for the potential benefit of cell salvage in this patient cohort,” he said.
Abdominal myomectomy often has a high rate of blood loss, with about a 10%-20% rate of transfusion. While the technique of cell salvage is widely used in other fields, it hasn’t been fully investigated in the context of gynecologic surgery, he said, explaining the rationale for the study.
Subjects included were women aged 18-60 years who underwent abdominal myomectomy for benign indications at the Cleveland Clinic during 2011-2016. Those with current malignancy or with surgery performed by a gynecologic oncologist were excluded.
The non–cell salvage and cell salvage groups were comparable with respect to age, body mass index, ethnicity, and preoperative and postoperative hemoglobin.
Dr. Gingold noted that “prospective study with randomization will be required to better define the role of cell salvage in abdominal myomectomies.”
During a question and answer session following his presentation, Charles Ascher-Walsh, MD, of Mount Sinai Health System, N.Y. noted that the transfusion rates and blood loss were high in the study, and that solid data support the use of tourniquets for patients undergoing abdominal myomectomy, with studies showing only a 1%-2% transfusion rate with continuous tourniquet use. Dr. Gingold said the use of tourniquets in the study was low and was dictated by surgeon preference. He agreed that tourniquet use is “certainly an option that should be adjunctive,” and that it would be useful to look at the outcomes in the cases with and without tourniquet use.
Dr. Gingold reported having no disclosures.
SOURCE: Gingold J et al. SGS 2018 Oral Poster 18.
ORLANDO – Cell salvage may help reduce the need for allogeneic blood transfusion in patients undergoing abdominal myomectomy, according to findings from a retrospective cohort study.
Of 138 women who underwent abdominal myomectomy, 52 had no cell salvage and 86 had cell salvage ordered. Of those who had cell salvage ordered, 60 had salvage fully set up and 26 had salvage on standby; 46 of the 60 with full set-up had autologous blood returned, and of those, 14 required subsequent allogeneic transfusion of more than 20 U of blood, Julian A. Gingold, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Notable differences between those who did and those who did not have cell salvage ordered included the number of patients with fibroids weighing more than 250 g (52% vs. 13%) and with five or more total fibroids (83% vs. 56%), he said.
“And interestingly, reproductive surgeons were less likely (than general surgeons) to order cell salvage,” he said.
Surgery was performed by a reproductive surgeon in 25% of cases in the cell salvage group vs. in 67% of cases in the non–cell salvage group.
The finding of comparable allogeneic transfusion requirement between the two groups despite differences in blood loss and “arguably less complex surgeries [in those] without cell salvage” was striking, Dr. Gingold said.
“If you take these 86 patients who had cell salvage intraoperatively, 14 of them ultimately required donor blood, and that amounted to 23 units, giving kind of a lower limit for the potential benefit of cell salvage in this patient cohort,” he said.
Abdominal myomectomy often has a high rate of blood loss, with about a 10%-20% rate of transfusion. While the technique of cell salvage is widely used in other fields, it hasn’t been fully investigated in the context of gynecologic surgery, he said, explaining the rationale for the study.
Subjects included were women aged 18-60 years who underwent abdominal myomectomy for benign indications at the Cleveland Clinic during 2011-2016. Those with current malignancy or with surgery performed by a gynecologic oncologist were excluded.
The non–cell salvage and cell salvage groups were comparable with respect to age, body mass index, ethnicity, and preoperative and postoperative hemoglobin.
Dr. Gingold noted that “prospective study with randomization will be required to better define the role of cell salvage in abdominal myomectomies.”
During a question and answer session following his presentation, Charles Ascher-Walsh, MD, of Mount Sinai Health System, N.Y. noted that the transfusion rates and blood loss were high in the study, and that solid data support the use of tourniquets for patients undergoing abdominal myomectomy, with studies showing only a 1%-2% transfusion rate with continuous tourniquet use. Dr. Gingold said the use of tourniquets in the study was low and was dictated by surgeon preference. He agreed that tourniquet use is “certainly an option that should be adjunctive,” and that it would be useful to look at the outcomes in the cases with and without tourniquet use.
Dr. Gingold reported having no disclosures.
SOURCE: Gingold J et al. SGS 2018 Oral Poster 18.
REPORTING FROM SGS 2018
Key clinical point: Cell salvage may help reduce the need for allogeneic blood transfusion in patients undergoing abdominal myomectomy.
Major finding: Transfusion rates were similar (23% and 17%) despite greater blood loss in the cell salvage group.
Study details: A retrospective review of 138 patients’ charts.
Disclosures: Dr. Gingold reported having no disclosures.
Source: Gingold J et al. SGS 2018 Oral poster 18.
Blood type A linked to more-severe diarrhea
Diarrhea associated with enterotoxigenic Escherichia coli (ETEC) infection is more severe among individuals with blood type A, based on data from 106 adults exposed to the agent.
The reseachers speculate that the increased severity is related, in part, to the presence of an adhesin molecule known as EtpA, which binds to the surface of the cells and combines with blood group A glycans to promote a more severe infection.
“Our studies suggest that the many ETEC strains that produce the EtpA adhesin may be particularly well equipped to preferentially infect hosts who express A blood group antigen on mucosal surfaces,” wrote Pardeep Kumar, MD, of Washington University, Saint Louis, and his colleagues.
ETEC, associated with choleralike illness, remains a major cause of infectious diarrhea in developing countries. No current vaccine offers significant protection against it, but EtpA may be useful in vaccine development. “More recently discovered virulence factors and an improved understanding of ETEC microbial pathogenesis could offer additional avenues to protect those at highest risk for severe disease and uncover novel molecular targets for future vaccine development,” the researchers said.
In a study published in the Journal of Clinical Investigation, the researchers examined blood samples from 106 adults who had participated in previous human infection model studies. The volunteers were challenged with an ETEC strain known as H10407 that was originally isolated from a case of choleralike illness.
Diarrhea was more frequent among A blood type individuals, compared with other types. Individuals with B or O blood types were significantly more likely to have no diarrhea or mild diarrhea after the E. coli challenge, compared with type A individuals (44% vs. 19%).
Overall, significantly more individuals with A or AB blood types developed moderate to severe diarrhea, compared with those with B or O blood types (81% vs. 56%). In addition, significantly more individuals with blood type A needed early initiation of antibiotic therapy, compared with those with B or O blood types (72% vs. 43%).
The study findings were limited by several factors, including the use of higher levels of bacteria to cause the infection than a typical exposure in nature. Also, the volunteers were treated with antibiotics once they met the criteria for severe diarrhea.
The researchers also noted that other blood group A lectins in addition to EtpA may not have been identified.
“While our recent studies have potentially important clinical and [vaccinological] implications, further study of the relationship between blood group, disease severity, and antigen expression could guide and inform use of these antigens in vaccines,” they wrote.
The study was supported by funds from the Enteric Vaccine Initiative of PATH, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
SOURCE: Kumar P et al. J Clin Invest. 2018 May 17. doi: 10.1172/JCI97659.
Diarrhea associated with enterotoxigenic Escherichia coli (ETEC) infection is more severe among individuals with blood type A, based on data from 106 adults exposed to the agent.
The reseachers speculate that the increased severity is related, in part, to the presence of an adhesin molecule known as EtpA, which binds to the surface of the cells and combines with blood group A glycans to promote a more severe infection.
“Our studies suggest that the many ETEC strains that produce the EtpA adhesin may be particularly well equipped to preferentially infect hosts who express A blood group antigen on mucosal surfaces,” wrote Pardeep Kumar, MD, of Washington University, Saint Louis, and his colleagues.
ETEC, associated with choleralike illness, remains a major cause of infectious diarrhea in developing countries. No current vaccine offers significant protection against it, but EtpA may be useful in vaccine development. “More recently discovered virulence factors and an improved understanding of ETEC microbial pathogenesis could offer additional avenues to protect those at highest risk for severe disease and uncover novel molecular targets for future vaccine development,” the researchers said.
In a study published in the Journal of Clinical Investigation, the researchers examined blood samples from 106 adults who had participated in previous human infection model studies. The volunteers were challenged with an ETEC strain known as H10407 that was originally isolated from a case of choleralike illness.
Diarrhea was more frequent among A blood type individuals, compared with other types. Individuals with B or O blood types were significantly more likely to have no diarrhea or mild diarrhea after the E. coli challenge, compared with type A individuals (44% vs. 19%).
Overall, significantly more individuals with A or AB blood types developed moderate to severe diarrhea, compared with those with B or O blood types (81% vs. 56%). In addition, significantly more individuals with blood type A needed early initiation of antibiotic therapy, compared with those with B or O blood types (72% vs. 43%).
The study findings were limited by several factors, including the use of higher levels of bacteria to cause the infection than a typical exposure in nature. Also, the volunteers were treated with antibiotics once they met the criteria for severe diarrhea.
The researchers also noted that other blood group A lectins in addition to EtpA may not have been identified.
“While our recent studies have potentially important clinical and [vaccinological] implications, further study of the relationship between blood group, disease severity, and antigen expression could guide and inform use of these antigens in vaccines,” they wrote.
The study was supported by funds from the Enteric Vaccine Initiative of PATH, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
SOURCE: Kumar P et al. J Clin Invest. 2018 May 17. doi: 10.1172/JCI97659.
Diarrhea associated with enterotoxigenic Escherichia coli (ETEC) infection is more severe among individuals with blood type A, based on data from 106 adults exposed to the agent.
The reseachers speculate that the increased severity is related, in part, to the presence of an adhesin molecule known as EtpA, which binds to the surface of the cells and combines with blood group A glycans to promote a more severe infection.
“Our studies suggest that the many ETEC strains that produce the EtpA adhesin may be particularly well equipped to preferentially infect hosts who express A blood group antigen on mucosal surfaces,” wrote Pardeep Kumar, MD, of Washington University, Saint Louis, and his colleagues.
ETEC, associated with choleralike illness, remains a major cause of infectious diarrhea in developing countries. No current vaccine offers significant protection against it, but EtpA may be useful in vaccine development. “More recently discovered virulence factors and an improved understanding of ETEC microbial pathogenesis could offer additional avenues to protect those at highest risk for severe disease and uncover novel molecular targets for future vaccine development,” the researchers said.
In a study published in the Journal of Clinical Investigation, the researchers examined blood samples from 106 adults who had participated in previous human infection model studies. The volunteers were challenged with an ETEC strain known as H10407 that was originally isolated from a case of choleralike illness.
Diarrhea was more frequent among A blood type individuals, compared with other types. Individuals with B or O blood types were significantly more likely to have no diarrhea or mild diarrhea after the E. coli challenge, compared with type A individuals (44% vs. 19%).
Overall, significantly more individuals with A or AB blood types developed moderate to severe diarrhea, compared with those with B or O blood types (81% vs. 56%). In addition, significantly more individuals with blood type A needed early initiation of antibiotic therapy, compared with those with B or O blood types (72% vs. 43%).
The study findings were limited by several factors, including the use of higher levels of bacteria to cause the infection than a typical exposure in nature. Also, the volunteers were treated with antibiotics once they met the criteria for severe diarrhea.
The researchers also noted that other blood group A lectins in addition to EtpA may not have been identified.
“While our recent studies have potentially important clinical and [vaccinological] implications, further study of the relationship between blood group, disease severity, and antigen expression could guide and inform use of these antigens in vaccines,” they wrote.
The study was supported by funds from the Enteric Vaccine Initiative of PATH, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
SOURCE: Kumar P et al. J Clin Invest. 2018 May 17. doi: 10.1172/JCI97659.
FROM THE JOURNAL OF CLINICAL INVESTIGATION
Key clinical point: Severe diarrhea was significantly more frequent in adults with an A blood type, compared with B or O types.
Major finding: The overall relative risk of moderate to severe diarrhea was 1.44 for A blood groups, compared with non-A blood groups.
Study details: The data come from 106 adult volunteers who had participated in previous human infection model studies.
Disclosures: The study was supported by funds from the PATH Enteric Vaccine Initiative, the Department of Veterans Affairs, the National Institutes of Health, and the Digestive Diseases Research Core Center at Washington University School of Medicine, St. Louis. The researchers had no financial conflicts to disclose.
Source: Kumar P et al. J Clin Invest. 2018 May 17; doi: 10.1172/JCI97659.
Screening blood donations for Zika proved costly, low yield
Testing donated blood for Zika virus in the United States confirmed just 8 authentic cases and cost nearly $42 million over a period of 15 months, investigators reported online May 10 in the New England Journal of Medicine.
That price did not reflect a commercial price hike, said Paula Saá, PhD, of the American Red Cross in Gaithersburg, MD, and her associates. Furthermore, more than half of the Zika cases had a low viral level that might not be infectious.
Among more than 4 million screened donations, 9% were tested in pools. All pooled tests were negative. The other 3.9 million donations were screened individually. Only 160 were positive, and just 6 were confirmed positive on repeat TMA. Two of these six confirmed positives also were RNA-positive on reverse transcription polymerase chain reaction, while two were equivocal and two were negative. The positive and equivocal donations were negative for Zika immunoglobulin M on enzyme-linked immunosorbent assay, which indicated acute infections. The RNA-negative infections were IgM-positive, indicating prior infections.
Three more donations were initially positive on TMA, were negative on repeat TMA, and were IgM-positive, bringing the total case count to nine. Among these, two donors had been infected in Florida, six had traveled to Zika-endemic areas, and one had received an experimental Zika vaccine, according to the researchers.
For each detection of authentic Zika virus RNA infection in U.S.-donated blood, testing had cost $5.3 million, they concluded. They called the current FDA recommendation to individually screen all U.S. blood donations for Zika virus “low-yield” and “high cost.” Of three acute infections with enough sample left for pooled testing, all were positive, they noted.
The American Red Cross and Grifols Diagnostic Solutions provided funding. Dr. Saá disclosed research support from Grifols, which makes the TMA test used in the study. She had no other conflicts of interest.
SOURCE: Saá P et al. New Engl J Med. 2018;378:1778-88.
Despite these findings, it would be premature to stop testing U.S. blood donations for Zika virus, wrote Evan M. Bloch, MBChB; Paul M. Ness, MD; Aaron A.R. Tobian, MD, PhD; and Jeremy Sugarman, MD, MPH, in an editorial accompanying the study.
Nonetheless, “actual and perceived risks to the blood supply seem to be conflated,” the experts wrote. They noted that the United States currently has no active areas of Zika virus transmission and that confirmed mosquito-borne, locally acquired infections fell from 226 in 2016 to two the following year.
There is no historical precedent for ending a policy of testing blood donations for pathogens, they added. Consequently, ending widespread screening “may actually prove to be far more challenging than the decision to start.” For now, a precautionary, risk-based approach should entail “continuous review” of screening policies and “reassessment as new data emerge.”
The editorialists are with Johns Hopkins University and Johns Hopkins University’s Bergman Institute of Bioethics, both in Baltimore. They reported having no relevant conflicts of interest. These comments are from their editorial (New Engl J Med. 2018;378:19:1837-41).
Despite these findings, it would be premature to stop testing U.S. blood donations for Zika virus, wrote Evan M. Bloch, MBChB; Paul M. Ness, MD; Aaron A.R. Tobian, MD, PhD; and Jeremy Sugarman, MD, MPH, in an editorial accompanying the study.
Nonetheless, “actual and perceived risks to the blood supply seem to be conflated,” the experts wrote. They noted that the United States currently has no active areas of Zika virus transmission and that confirmed mosquito-borne, locally acquired infections fell from 226 in 2016 to two the following year.
There is no historical precedent for ending a policy of testing blood donations for pathogens, they added. Consequently, ending widespread screening “may actually prove to be far more challenging than the decision to start.” For now, a precautionary, risk-based approach should entail “continuous review” of screening policies and “reassessment as new data emerge.”
The editorialists are with Johns Hopkins University and Johns Hopkins University’s Bergman Institute of Bioethics, both in Baltimore. They reported having no relevant conflicts of interest. These comments are from their editorial (New Engl J Med. 2018;378:19:1837-41).
Despite these findings, it would be premature to stop testing U.S. blood donations for Zika virus, wrote Evan M. Bloch, MBChB; Paul M. Ness, MD; Aaron A.R. Tobian, MD, PhD; and Jeremy Sugarman, MD, MPH, in an editorial accompanying the study.
Nonetheless, “actual and perceived risks to the blood supply seem to be conflated,” the experts wrote. They noted that the United States currently has no active areas of Zika virus transmission and that confirmed mosquito-borne, locally acquired infections fell from 226 in 2016 to two the following year.
There is no historical precedent for ending a policy of testing blood donations for pathogens, they added. Consequently, ending widespread screening “may actually prove to be far more challenging than the decision to start.” For now, a precautionary, risk-based approach should entail “continuous review” of screening policies and “reassessment as new data emerge.”
The editorialists are with Johns Hopkins University and Johns Hopkins University’s Bergman Institute of Bioethics, both in Baltimore. They reported having no relevant conflicts of interest. These comments are from their editorial (New Engl J Med. 2018;378:19:1837-41).
Testing donated blood for Zika virus in the United States confirmed just 8 authentic cases and cost nearly $42 million over a period of 15 months, investigators reported online May 10 in the New England Journal of Medicine.
That price did not reflect a commercial price hike, said Paula Saá, PhD, of the American Red Cross in Gaithersburg, MD, and her associates. Furthermore, more than half of the Zika cases had a low viral level that might not be infectious.
Among more than 4 million screened donations, 9% were tested in pools. All pooled tests were negative. The other 3.9 million donations were screened individually. Only 160 were positive, and just 6 were confirmed positive on repeat TMA. Two of these six confirmed positives also were RNA-positive on reverse transcription polymerase chain reaction, while two were equivocal and two were negative. The positive and equivocal donations were negative for Zika immunoglobulin M on enzyme-linked immunosorbent assay, which indicated acute infections. The RNA-negative infections were IgM-positive, indicating prior infections.
Three more donations were initially positive on TMA, were negative on repeat TMA, and were IgM-positive, bringing the total case count to nine. Among these, two donors had been infected in Florida, six had traveled to Zika-endemic areas, and one had received an experimental Zika vaccine, according to the researchers.
For each detection of authentic Zika virus RNA infection in U.S.-donated blood, testing had cost $5.3 million, they concluded. They called the current FDA recommendation to individually screen all U.S. blood donations for Zika virus “low-yield” and “high cost.” Of three acute infections with enough sample left for pooled testing, all were positive, they noted.
The American Red Cross and Grifols Diagnostic Solutions provided funding. Dr. Saá disclosed research support from Grifols, which makes the TMA test used in the study. She had no other conflicts of interest.
SOURCE: Saá P et al. New Engl J Med. 2018;378:1778-88.
Testing donated blood for Zika virus in the United States confirmed just 8 authentic cases and cost nearly $42 million over a period of 15 months, investigators reported online May 10 in the New England Journal of Medicine.
That price did not reflect a commercial price hike, said Paula Saá, PhD, of the American Red Cross in Gaithersburg, MD, and her associates. Furthermore, more than half of the Zika cases had a low viral level that might not be infectious.
Among more than 4 million screened donations, 9% were tested in pools. All pooled tests were negative. The other 3.9 million donations were screened individually. Only 160 were positive, and just 6 were confirmed positive on repeat TMA. Two of these six confirmed positives also were RNA-positive on reverse transcription polymerase chain reaction, while two were equivocal and two were negative. The positive and equivocal donations were negative for Zika immunoglobulin M on enzyme-linked immunosorbent assay, which indicated acute infections. The RNA-negative infections were IgM-positive, indicating prior infections.
Three more donations were initially positive on TMA, were negative on repeat TMA, and were IgM-positive, bringing the total case count to nine. Among these, two donors had been infected in Florida, six had traveled to Zika-endemic areas, and one had received an experimental Zika vaccine, according to the researchers.
For each detection of authentic Zika virus RNA infection in U.S.-donated blood, testing had cost $5.3 million, they concluded. They called the current FDA recommendation to individually screen all U.S. blood donations for Zika virus “low-yield” and “high cost.” Of three acute infections with enough sample left for pooled testing, all were positive, they noted.
The American Red Cross and Grifols Diagnostic Solutions provided funding. Dr. Saá disclosed research support from Grifols, which makes the TMA test used in the study. She had no other conflicts of interest.
SOURCE: Saá P et al. New Engl J Med. 2018;378:1778-88.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Individually screening all blood donations for Zika virus was low yield and costly.
Major finding: Testing confirmed only 8 authentic cases and cost nearly $42 million over 15 months.
Study details: Screening and confirmatory testing of 4,325,889 donations of blood in the United States during 2016-2017.
Disclosures: American Red Cross and Grifols Diagnostic Solutions provided funding. Grifols makes a test used in the study. Dr. Saá disclosed research support from Grifols but had no other conflicts of interest.
Source: Saá P et al. New Engl J Med. 2018;378:1778-88. doi: 10.1056/NEJMoa1714977
FDA approves tests to screen for tickborne parasite in blood supply
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.