User login
NDMA found in samples of ranitidine, FDA says
According to the Food and Drug Administration,
“NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a prepared statement issued on Sept. 13, 2019. “The FDA has been investigating NDMA and other nitrosamine impurities in blood pressure and heart failure medicines called Angiotensin II Receptor Blockers (ARBs) since last year. In the case of ARBs, the FDA has recommended numerous recalls as it discovered unacceptable levels of nitrosamines.”
Dr. Woodcock said that the agency is working with industry partners to determine whether the low levels of NDMA in ranitidine pose a risk to patients, and it plans to post that information when it becomes available. For now, “patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health,” she said. “Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
Dr. Woodcock emphasized that the FDA is not suggesting that individuals stop taking ranitidine at this time. “However, patients taking prescription ranitidine who wish to discontinue use should talk to their health care professional about other treatment options,” she said. “People taking OTC ranitidine could consider using other OTC medicines approved for their condition. There are multiple drugs on the market that are approved for the same or similar uses as ranitidine.”
She advised consumers and health care professionals to report any adverse reactions with ranitidine to the FDA’s MedWatch program to help the agency better understand the problem.
Visit the AGA GI Patient Center for education to share with your patients about GERD, including symptoms, testing, lifestyle modifications and drug treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
According to the Food and Drug Administration,
“NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a prepared statement issued on Sept. 13, 2019. “The FDA has been investigating NDMA and other nitrosamine impurities in blood pressure and heart failure medicines called Angiotensin II Receptor Blockers (ARBs) since last year. In the case of ARBs, the FDA has recommended numerous recalls as it discovered unacceptable levels of nitrosamines.”
Dr. Woodcock said that the agency is working with industry partners to determine whether the low levels of NDMA in ranitidine pose a risk to patients, and it plans to post that information when it becomes available. For now, “patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health,” she said. “Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
Dr. Woodcock emphasized that the FDA is not suggesting that individuals stop taking ranitidine at this time. “However, patients taking prescription ranitidine who wish to discontinue use should talk to their health care professional about other treatment options,” she said. “People taking OTC ranitidine could consider using other OTC medicines approved for their condition. There are multiple drugs on the market that are approved for the same or similar uses as ranitidine.”
She advised consumers and health care professionals to report any adverse reactions with ranitidine to the FDA’s MedWatch program to help the agency better understand the problem.
Visit the AGA GI Patient Center for education to share with your patients about GERD, including symptoms, testing, lifestyle modifications and drug treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
According to the Food and Drug Administration,
“NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a prepared statement issued on Sept. 13, 2019. “The FDA has been investigating NDMA and other nitrosamine impurities in blood pressure and heart failure medicines called Angiotensin II Receptor Blockers (ARBs) since last year. In the case of ARBs, the FDA has recommended numerous recalls as it discovered unacceptable levels of nitrosamines.”
Dr. Woodcock said that the agency is working with industry partners to determine whether the low levels of NDMA in ranitidine pose a risk to patients, and it plans to post that information when it becomes available. For now, “patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health,” she said. “Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
Dr. Woodcock emphasized that the FDA is not suggesting that individuals stop taking ranitidine at this time. “However, patients taking prescription ranitidine who wish to discontinue use should talk to their health care professional about other treatment options,” she said. “People taking OTC ranitidine could consider using other OTC medicines approved for their condition. There are multiple drugs on the market that are approved for the same or similar uses as ranitidine.”
She advised consumers and health care professionals to report any adverse reactions with ranitidine to the FDA’s MedWatch program to help the agency better understand the problem.
Visit the AGA GI Patient Center for education to share with your patients about GERD, including symptoms, testing, lifestyle modifications and drug treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Diagnosis and management of gastroparesis and functional dyspepsia pose challenges
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
EXPERT ANALYSIS FROM FRESTON CONFERENCE 2019
Endoscopic therapy decreases recurrence of intestinal metaplasia, dysplasia in patients with Barrett’s esophagus
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Can dietary therapies treat GERD effectively?
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
REPORTING FROM FRESTON CONFERENCE 2019
Developments in gastric cancer
In the United States, gastric cancer accounts for 1.6% of all cancers with an estimated 27,510 cases in 2019 per the SEER database. Although the incidence of gastric cancer has been decreasing in the United States, there have been alarming trends, suggesting an increased rate in select populations, especially in the young Hispanic population in the age group of 20-49 years (SEER Cancer Statistics Review [CSR] 1975-2015).
Risk factors for gastric cancer include increasing age, male sex, presence of intestinal metaplasia, and varying degrees of dysplasia (Endoscopy. 2019;51[4]:365-88). Gastric cancer is primarily characterized into two subtypes: intestinal type, which is the more common type associated with gastric intestinal metaplasia (GIM), and the diffuse type, which is genetically determined.
GIM, a precancerous lesion, is defined as the replacement of the normal gastric mucosa by intestinal epithelium and can be limited (confined to one region of the stomach) or extensive (involving more than two regions of the stomach). Risk factors for GIM include Helicobacter pylori infection, age, smoking status, and presence of a first-degree relative with gastric cancer. Histologically, GIM is characterized as either complete – defined as the presence of small intestinal-type mucosa with mature absorptive cells, goblet cells, and a brush border – or incomplete – with columnar “intermediate” cells in various stages of differentiation, irregular mucin droplets, without a brush border. Extensive and incomplete type of GIM is associated with a higher risk of gastric cancer (Endoscopy. 2019;51[4]:365-88).
Gastric cancer screening has been shown to be effective in countries with a high incidence of gastric cancer. However, in low-incidence countries, at-risk patients can be identified based on epidemiology, genetics, and environmental risk factors as well as incidence of H. pylori, and serologic markers of chronic inflammation such as pepsinogen, and gastrin (Am J Gastroenterol. 2017;112[5]:704-15). H. pylori eradication has been shown to reduce the risk of developing gastric adenocarcinoma in patients with H. pylori-associated GIM. For detection of dysplasia and early gastric cancer, patients with GIM should undergo a full systematic endoscopy protocol of the stomach with clear photographic documentation of gastric regions and pathology.
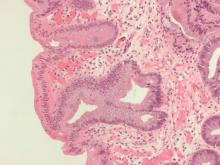
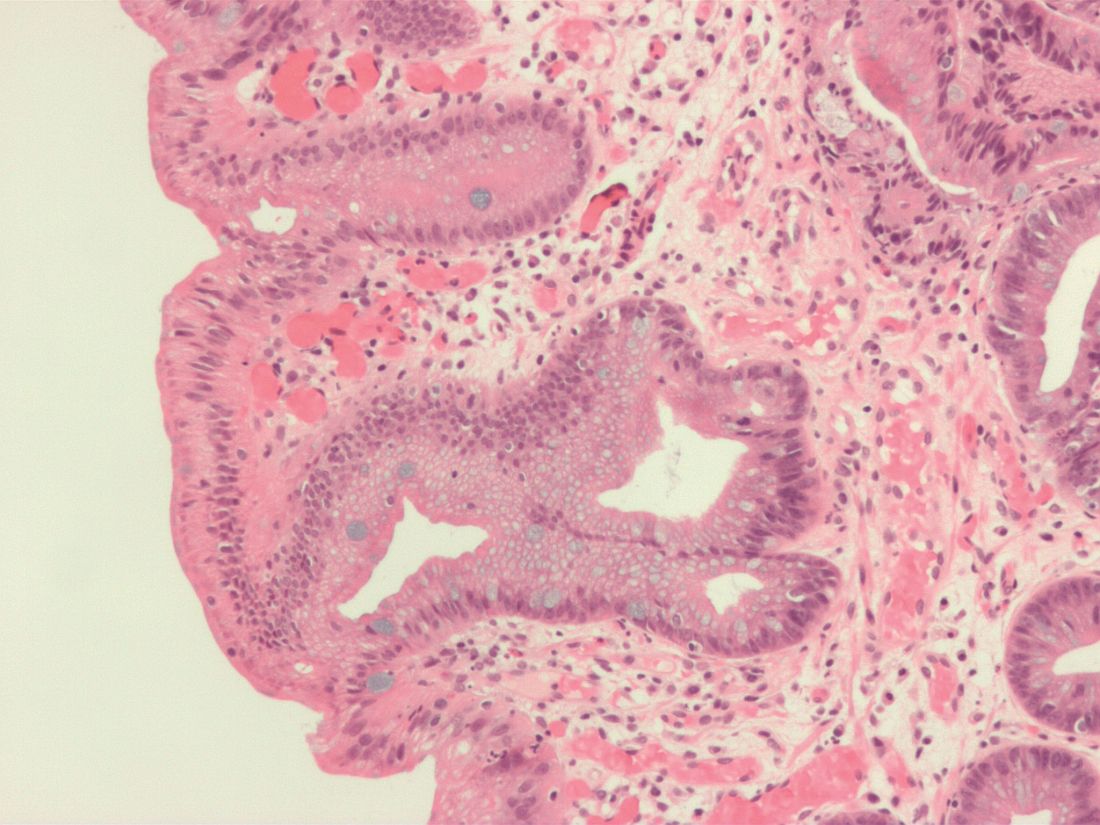
On standard white-light endoscopy, GIM appears as small gray-white, slightly elevated plaques surrounded by mixed patchy pink and pale areas of mucosa causing an irregular uneven surface. Sometimes GIM can present as patchy erythema with mottling.
On the other hand, presence of features such as differences in color, loss of vascularity, elevation or depression, nodularity or thickening, and abnormal convergence or flattening of folds should raise suspicion for gastric dysplasia or early gastric cancer. Presence of GIM on endoscopy should be documented in detail with photographic evidence including the location and extent of GIM, and obtaining mapping biopsies that include at least two biopsies from the antrum (from lesser and greater curve) and from the body (lesser and greater curve). Endoscopic surveillance is recommended every 3 years in patients with extensive GIM affecting the antrum and body, incomplete GIM, and a family history of gastric cancer.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Sharma is professor of medicine and director of fellowship training, division of gastroenterology and hepatology, University of Kansas, Kansas City.
In the United States, gastric cancer accounts for 1.6% of all cancers with an estimated 27,510 cases in 2019 per the SEER database. Although the incidence of gastric cancer has been decreasing in the United States, there have been alarming trends, suggesting an increased rate in select populations, especially in the young Hispanic population in the age group of 20-49 years (SEER Cancer Statistics Review [CSR] 1975-2015).
Risk factors for gastric cancer include increasing age, male sex, presence of intestinal metaplasia, and varying degrees of dysplasia (Endoscopy. 2019;51[4]:365-88). Gastric cancer is primarily characterized into two subtypes: intestinal type, which is the more common type associated with gastric intestinal metaplasia (GIM), and the diffuse type, which is genetically determined.
GIM, a precancerous lesion, is defined as the replacement of the normal gastric mucosa by intestinal epithelium and can be limited (confined to one region of the stomach) or extensive (involving more than two regions of the stomach). Risk factors for GIM include Helicobacter pylori infection, age, smoking status, and presence of a first-degree relative with gastric cancer. Histologically, GIM is characterized as either complete – defined as the presence of small intestinal-type mucosa with mature absorptive cells, goblet cells, and a brush border – or incomplete – with columnar “intermediate” cells in various stages of differentiation, irregular mucin droplets, without a brush border. Extensive and incomplete type of GIM is associated with a higher risk of gastric cancer (Endoscopy. 2019;51[4]:365-88).
Gastric cancer screening has been shown to be effective in countries with a high incidence of gastric cancer. However, in low-incidence countries, at-risk patients can be identified based on epidemiology, genetics, and environmental risk factors as well as incidence of H. pylori, and serologic markers of chronic inflammation such as pepsinogen, and gastrin (Am J Gastroenterol. 2017;112[5]:704-15). H. pylori eradication has been shown to reduce the risk of developing gastric adenocarcinoma in patients with H. pylori-associated GIM. For detection of dysplasia and early gastric cancer, patients with GIM should undergo a full systematic endoscopy protocol of the stomach with clear photographic documentation of gastric regions and pathology.
On standard white-light endoscopy, GIM appears as small gray-white, slightly elevated plaques surrounded by mixed patchy pink and pale areas of mucosa causing an irregular uneven surface. Sometimes GIM can present as patchy erythema with mottling.
On the other hand, presence of features such as differences in color, loss of vascularity, elevation or depression, nodularity or thickening, and abnormal convergence or flattening of folds should raise suspicion for gastric dysplasia or early gastric cancer. Presence of GIM on endoscopy should be documented in detail with photographic evidence including the location and extent of GIM, and obtaining mapping biopsies that include at least two biopsies from the antrum (from lesser and greater curve) and from the body (lesser and greater curve). Endoscopic surveillance is recommended every 3 years in patients with extensive GIM affecting the antrum and body, incomplete GIM, and a family history of gastric cancer.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Sharma is professor of medicine and director of fellowship training, division of gastroenterology and hepatology, University of Kansas, Kansas City.
In the United States, gastric cancer accounts for 1.6% of all cancers with an estimated 27,510 cases in 2019 per the SEER database. Although the incidence of gastric cancer has been decreasing in the United States, there have been alarming trends, suggesting an increased rate in select populations, especially in the young Hispanic population in the age group of 20-49 years (SEER Cancer Statistics Review [CSR] 1975-2015).
Risk factors for gastric cancer include increasing age, male sex, presence of intestinal metaplasia, and varying degrees of dysplasia (Endoscopy. 2019;51[4]:365-88). Gastric cancer is primarily characterized into two subtypes: intestinal type, which is the more common type associated with gastric intestinal metaplasia (GIM), and the diffuse type, which is genetically determined.
GIM, a precancerous lesion, is defined as the replacement of the normal gastric mucosa by intestinal epithelium and can be limited (confined to one region of the stomach) or extensive (involving more than two regions of the stomach). Risk factors for GIM include Helicobacter pylori infection, age, smoking status, and presence of a first-degree relative with gastric cancer. Histologically, GIM is characterized as either complete – defined as the presence of small intestinal-type mucosa with mature absorptive cells, goblet cells, and a brush border – or incomplete – with columnar “intermediate” cells in various stages of differentiation, irregular mucin droplets, without a brush border. Extensive and incomplete type of GIM is associated with a higher risk of gastric cancer (Endoscopy. 2019;51[4]:365-88).
Gastric cancer screening has been shown to be effective in countries with a high incidence of gastric cancer. However, in low-incidence countries, at-risk patients can be identified based on epidemiology, genetics, and environmental risk factors as well as incidence of H. pylori, and serologic markers of chronic inflammation such as pepsinogen, and gastrin (Am J Gastroenterol. 2017;112[5]:704-15). H. pylori eradication has been shown to reduce the risk of developing gastric adenocarcinoma in patients with H. pylori-associated GIM. For detection of dysplasia and early gastric cancer, patients with GIM should undergo a full systematic endoscopy protocol of the stomach with clear photographic documentation of gastric regions and pathology.
On standard white-light endoscopy, GIM appears as small gray-white, slightly elevated plaques surrounded by mixed patchy pink and pale areas of mucosa causing an irregular uneven surface. Sometimes GIM can present as patchy erythema with mottling.
On the other hand, presence of features such as differences in color, loss of vascularity, elevation or depression, nodularity or thickening, and abnormal convergence or flattening of folds should raise suspicion for gastric dysplasia or early gastric cancer. Presence of GIM on endoscopy should be documented in detail with photographic evidence including the location and extent of GIM, and obtaining mapping biopsies that include at least two biopsies from the antrum (from lesser and greater curve) and from the body (lesser and greater curve). Endoscopic surveillance is recommended every 3 years in patients with extensive GIM affecting the antrum and body, incomplete GIM, and a family history of gastric cancer.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Sharma is professor of medicine and director of fellowship training, division of gastroenterology and hepatology, University of Kansas, Kansas City.
Upper and lower gastroenterology – the state of the art
In the upper GI section of the Postgraduate course program, Ikuo Hirano, MD, educated us on the refractory patient with eosinophilic esophagitis, reinforcing the need for chronic maintenance treatment and the complementary role of dilation. Gregory Ginsberg, MD, elucidated the specific strategies needed for gastric polyps with advice on which to leave and which to resect. Sachin Wani, MD, carefully outlined the changing landscape of Barrett’s esophagus with emphasis on our move to ablate rather than observe low-grade dysplasia. In the difficult area of treating gastroparesis, Linda Nguyen, MD, acquainted us with some of the newer medications for this disorder and discussed the emerging role of endoscopic pyloromyotomy. Michael Camilleri, MD, delivered a thorough analysis on the concept of leaky gut with data-driven recommendations on testing and the lack of adequate treatment. Finally, William Chey, MD, gave perspective to diagnosis and treatment of small-bowel bacterial overgrowth, particularly with its role in irritable bowel syndrome.
In the lower GI section of the course, Sunanda Kane, MD, gave a wonderful overview the present and emerging biologics for treatment of inflammatory bowel disease (IBD). David Rubin, MD, shared his expertise and vast experience for best management of ulcerative colitis while Edward Loftus Jr., MD, discussed the fact and fiction of diet-based therapy in IBD. This was followed by a timely lecture by Christina Ha, MD, on the need to think well outside the GI tract in IBD, discussing infections, cancers, and vaccinations in patients with IBD. The IBD section finished with an erudite and timely lecture by Marla Dubinsky, MD, evaluating the controversy over use of biosimilars in our clinical practice. The remainder of the lower GI section started with AGA President David Lieberman, MD, analyzing recent data on the need to move the colonic cancer screening age to 45 years, particularly in African Americans. Following this was a timely talk by Xavie Llor, MD, PhD, on when to suspect and how to test for the expanding definition of Lynch syndrome. Lin Chang, MD, delivered the penultimate clinical lecture on management of irritable bowel syndrome based on her many years of clinical expertise in this area. Finally, Gail Hecht, MD, AGAF, a former AGA president, summarized the exciting world of microbiome research from the recent annual Gut Microbiota for Health World Summit. All in all it was considered one of the best AGA Postgraduate courses by many and we look forward to even greater improvements for 2020.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Katzka is professor of medicine and head of the Esophageal Interest Group at the Mayo Clinic in Rochester, Minn. He is on the advisory boards for Shire and Celgene.
In the upper GI section of the Postgraduate course program, Ikuo Hirano, MD, educated us on the refractory patient with eosinophilic esophagitis, reinforcing the need for chronic maintenance treatment and the complementary role of dilation. Gregory Ginsberg, MD, elucidated the specific strategies needed for gastric polyps with advice on which to leave and which to resect. Sachin Wani, MD, carefully outlined the changing landscape of Barrett’s esophagus with emphasis on our move to ablate rather than observe low-grade dysplasia. In the difficult area of treating gastroparesis, Linda Nguyen, MD, acquainted us with some of the newer medications for this disorder and discussed the emerging role of endoscopic pyloromyotomy. Michael Camilleri, MD, delivered a thorough analysis on the concept of leaky gut with data-driven recommendations on testing and the lack of adequate treatment. Finally, William Chey, MD, gave perspective to diagnosis and treatment of small-bowel bacterial overgrowth, particularly with its role in irritable bowel syndrome.
In the lower GI section of the course, Sunanda Kane, MD, gave a wonderful overview the present and emerging biologics for treatment of inflammatory bowel disease (IBD). David Rubin, MD, shared his expertise and vast experience for best management of ulcerative colitis while Edward Loftus Jr., MD, discussed the fact and fiction of diet-based therapy in IBD. This was followed by a timely lecture by Christina Ha, MD, on the need to think well outside the GI tract in IBD, discussing infections, cancers, and vaccinations in patients with IBD. The IBD section finished with an erudite and timely lecture by Marla Dubinsky, MD, evaluating the controversy over use of biosimilars in our clinical practice. The remainder of the lower GI section started with AGA President David Lieberman, MD, analyzing recent data on the need to move the colonic cancer screening age to 45 years, particularly in African Americans. Following this was a timely talk by Xavie Llor, MD, PhD, on when to suspect and how to test for the expanding definition of Lynch syndrome. Lin Chang, MD, delivered the penultimate clinical lecture on management of irritable bowel syndrome based on her many years of clinical expertise in this area. Finally, Gail Hecht, MD, AGAF, a former AGA president, summarized the exciting world of microbiome research from the recent annual Gut Microbiota for Health World Summit. All in all it was considered one of the best AGA Postgraduate courses by many and we look forward to even greater improvements for 2020.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Katzka is professor of medicine and head of the Esophageal Interest Group at the Mayo Clinic in Rochester, Minn. He is on the advisory boards for Shire and Celgene.
In the upper GI section of the Postgraduate course program, Ikuo Hirano, MD, educated us on the refractory patient with eosinophilic esophagitis, reinforcing the need for chronic maintenance treatment and the complementary role of dilation. Gregory Ginsberg, MD, elucidated the specific strategies needed for gastric polyps with advice on which to leave and which to resect. Sachin Wani, MD, carefully outlined the changing landscape of Barrett’s esophagus with emphasis on our move to ablate rather than observe low-grade dysplasia. In the difficult area of treating gastroparesis, Linda Nguyen, MD, acquainted us with some of the newer medications for this disorder and discussed the emerging role of endoscopic pyloromyotomy. Michael Camilleri, MD, delivered a thorough analysis on the concept of leaky gut with data-driven recommendations on testing and the lack of adequate treatment. Finally, William Chey, MD, gave perspective to diagnosis and treatment of small-bowel bacterial overgrowth, particularly with its role in irritable bowel syndrome.
In the lower GI section of the course, Sunanda Kane, MD, gave a wonderful overview the present and emerging biologics for treatment of inflammatory bowel disease (IBD). David Rubin, MD, shared his expertise and vast experience for best management of ulcerative colitis while Edward Loftus Jr., MD, discussed the fact and fiction of diet-based therapy in IBD. This was followed by a timely lecture by Christina Ha, MD, on the need to think well outside the GI tract in IBD, discussing infections, cancers, and vaccinations in patients with IBD. The IBD section finished with an erudite and timely lecture by Marla Dubinsky, MD, evaluating the controversy over use of biosimilars in our clinical practice. The remainder of the lower GI section started with AGA President David Lieberman, MD, analyzing recent data on the need to move the colonic cancer screening age to 45 years, particularly in African Americans. Following this was a timely talk by Xavie Llor, MD, PhD, on when to suspect and how to test for the expanding definition of Lynch syndrome. Lin Chang, MD, delivered the penultimate clinical lecture on management of irritable bowel syndrome based on her many years of clinical expertise in this area. Finally, Gail Hecht, MD, AGAF, a former AGA president, summarized the exciting world of microbiome research from the recent annual Gut Microbiota for Health World Summit. All in all it was considered one of the best AGA Postgraduate courses by many and we look forward to even greater improvements for 2020.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Katzka is professor of medicine and head of the Esophageal Interest Group at the Mayo Clinic in Rochester, Minn. He is on the advisory boards for Shire and Celgene.
Large prospective trial offers reassurance for long-term PPI use
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
FROM GASTROENTEROLOGY
Key clinical point: Aside from a possible increased risk of enteric infections, long-term use of pantoprazole is safe in patients with stable peripheral artery and cardiovascular disease.
Major finding: Enteric infections were 33% more common in the pantoprazole group than in the placebo group.
Study details: A placebo-controlled, double-blind, randomized trial involving 17,598 patients with stable peripheral artery disease and cardiovascular disease.
Disclosures: The COMPASS trial was funded by Bayer AG. The investigators disclosed relationships with Bayer, Allergan, Takeda, Janssen, and others.
Source: Moayyedi P et al. Gastroenterology. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
Pantoprazole not needed for most patients on anticoagulant/antiplatelet therapies
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
FROM GASTROENTEROLOGY
POEM outperforms pneumatic dilation in randomized achalasia trial
Peroral endoscopic myotomy (POEM) had a success rate exceeding 90%, versus just about 50% for standard balloon dilation in what investigators say is, to their knowledge, the first-ever randomized trial to evaluate POEM as a first-line modality for this esophageal motility disorder.
Reflux esophagitis was the major downside of POEM, according to investigators, who reported the complication in 41% of patients at a 2-year follow-up, as compared to just 7% of patients undergoing the standard balloon dilation.
Nevertheless, there were no serious adverse events among 63 POEM-treated patients, while one patient out of 63 undergoing pneumatic dilation had a perforation that required endoscopic closure and hospitalization, according to senior study author Albert J. Bredenoord, MD, PhD, of Amsterdam University Medical Center.
“These findings support consideration of POEM as an initial treatment option for patients with achalasia,” Dr. Bredenoord and coinvestigators said in a report on the study appearing in JAMA.
While endoscopic pneumatic dilation is the usual treatment for achalasia, POEM has become more commonly used following case series showing high rates of efficacy, according to the authors.
The POEM procedure also offers advantages over laparoscopic Heller myotomy, which is invasive and associated with severe complications, including a transmural perforation rate of 4%-10%, they said in their report.
Their randomized trial included 133 adults with newly diagnosed achalasia enrolled at one of six hospitals in Germany, Hong Kong, Italy, Netherlands, and the United States.
Patients were randomly assigned to undergo 1-2 pneumatic dilations performed by an endoscopist who had performed at least 20 such procedures in the past, or to a POEM procedure likewise performed by an expert who had already done more than 20 such procedures.
At baseline, patients’ Eckardt symptom scores ranged from 6 to 9 on a scale with 0 indicating the lowest severity, to 12 indicating the highest. The median Eckardt scores were 8 in the POEM group and 7 in the pneumatic dilation group.
Treatment success, defined as an Eckardt score under 3 and no severe complications or retreatment at 2 years, was achieved by 58 of 63 patients (92%) in the POEM group, compared with 34 of 63 patients (54%) in the pneumatic dilation group (P less than .001), investigators reported.
Reflux esophagitis was observed in 22 of 54 POEM-treated patients (41%) who underwent endoscopy at a 2-year evaluation, compared with only 2 of 29 patients (7%) who had received the balloon dilation procedure (P = .002). In line with that finding, both reflux symptoms and daily proton pump inhibitor use were more common in the POEM group, investigators said.
However, there were no differences between POEM and pneumatic dilation groups in quality of life and other secondary endpoints, including median barium column height and median integrated relaxation pressure, they reported.
Two serious adverse events related to treatment were seen, according to investigators, including one perforation requiring an endoscopic closure plus antibiotics and hospitalization for 13 days, and one hospital admission for a night because of severe chest pain with no signs of perforation.
“Although POEM is more invasive and requires more technical endoscopic skills, the risk of severe complications was not higher than with pneumatic dilation, especially when performed by experienced endoscopists,” Dr. Bredenoord and coauthors said in their report.
However, these results do not imply that the traditional dilation procedure should be abandoned, they said, as POEM is more invasive, more involved, and more likely to result in reflux esophagitis.
“It seems reasonable to offer both options to treatment-naive patients with achalasia and counsel them to select treatment based on the patient’s characteristics, personal preference, comorbidity, and disease subtype,” they said.
Funding for the study came from Fonds NutsOhra and the European Society of Gastrointestinal Endoscopy. Dr. Bredenoord reported disclosures related to Norgine, Laborie, Medtronic, Diversatek, Nutricia, Regeneron, Celgene, Bayer, and Dr. Falk Pharma.
SOURCE: Ponds FA et al. JAMA. 2019;322(2):134-44. doi: 10.1001/jama.2019.8859.
Peroral endoscopic myotomy (POEM) had a success rate exceeding 90%, versus just about 50% for standard balloon dilation in what investigators say is, to their knowledge, the first-ever randomized trial to evaluate POEM as a first-line modality for this esophageal motility disorder.
Reflux esophagitis was the major downside of POEM, according to investigators, who reported the complication in 41% of patients at a 2-year follow-up, as compared to just 7% of patients undergoing the standard balloon dilation.
Nevertheless, there were no serious adverse events among 63 POEM-treated patients, while one patient out of 63 undergoing pneumatic dilation had a perforation that required endoscopic closure and hospitalization, according to senior study author Albert J. Bredenoord, MD, PhD, of Amsterdam University Medical Center.
“These findings support consideration of POEM as an initial treatment option for patients with achalasia,” Dr. Bredenoord and coinvestigators said in a report on the study appearing in JAMA.
While endoscopic pneumatic dilation is the usual treatment for achalasia, POEM has become more commonly used following case series showing high rates of efficacy, according to the authors.
The POEM procedure also offers advantages over laparoscopic Heller myotomy, which is invasive and associated with severe complications, including a transmural perforation rate of 4%-10%, they said in their report.
Their randomized trial included 133 adults with newly diagnosed achalasia enrolled at one of six hospitals in Germany, Hong Kong, Italy, Netherlands, and the United States.
Patients were randomly assigned to undergo 1-2 pneumatic dilations performed by an endoscopist who had performed at least 20 such procedures in the past, or to a POEM procedure likewise performed by an expert who had already done more than 20 such procedures.
At baseline, patients’ Eckardt symptom scores ranged from 6 to 9 on a scale with 0 indicating the lowest severity, to 12 indicating the highest. The median Eckardt scores were 8 in the POEM group and 7 in the pneumatic dilation group.
Treatment success, defined as an Eckardt score under 3 and no severe complications or retreatment at 2 years, was achieved by 58 of 63 patients (92%) in the POEM group, compared with 34 of 63 patients (54%) in the pneumatic dilation group (P less than .001), investigators reported.
Reflux esophagitis was observed in 22 of 54 POEM-treated patients (41%) who underwent endoscopy at a 2-year evaluation, compared with only 2 of 29 patients (7%) who had received the balloon dilation procedure (P = .002). In line with that finding, both reflux symptoms and daily proton pump inhibitor use were more common in the POEM group, investigators said.
However, there were no differences between POEM and pneumatic dilation groups in quality of life and other secondary endpoints, including median barium column height and median integrated relaxation pressure, they reported.
Two serious adverse events related to treatment were seen, according to investigators, including one perforation requiring an endoscopic closure plus antibiotics and hospitalization for 13 days, and one hospital admission for a night because of severe chest pain with no signs of perforation.
“Although POEM is more invasive and requires more technical endoscopic skills, the risk of severe complications was not higher than with pneumatic dilation, especially when performed by experienced endoscopists,” Dr. Bredenoord and coauthors said in their report.
However, these results do not imply that the traditional dilation procedure should be abandoned, they said, as POEM is more invasive, more involved, and more likely to result in reflux esophagitis.
“It seems reasonable to offer both options to treatment-naive patients with achalasia and counsel them to select treatment based on the patient’s characteristics, personal preference, comorbidity, and disease subtype,” they said.
Funding for the study came from Fonds NutsOhra and the European Society of Gastrointestinal Endoscopy. Dr. Bredenoord reported disclosures related to Norgine, Laborie, Medtronic, Diversatek, Nutricia, Regeneron, Celgene, Bayer, and Dr. Falk Pharma.
SOURCE: Ponds FA et al. JAMA. 2019;322(2):134-44. doi: 10.1001/jama.2019.8859.
Peroral endoscopic myotomy (POEM) had a success rate exceeding 90%, versus just about 50% for standard balloon dilation in what investigators say is, to their knowledge, the first-ever randomized trial to evaluate POEM as a first-line modality for this esophageal motility disorder.
Reflux esophagitis was the major downside of POEM, according to investigators, who reported the complication in 41% of patients at a 2-year follow-up, as compared to just 7% of patients undergoing the standard balloon dilation.
Nevertheless, there were no serious adverse events among 63 POEM-treated patients, while one patient out of 63 undergoing pneumatic dilation had a perforation that required endoscopic closure and hospitalization, according to senior study author Albert J. Bredenoord, MD, PhD, of Amsterdam University Medical Center.
“These findings support consideration of POEM as an initial treatment option for patients with achalasia,” Dr. Bredenoord and coinvestigators said in a report on the study appearing in JAMA.
While endoscopic pneumatic dilation is the usual treatment for achalasia, POEM has become more commonly used following case series showing high rates of efficacy, according to the authors.
The POEM procedure also offers advantages over laparoscopic Heller myotomy, which is invasive and associated with severe complications, including a transmural perforation rate of 4%-10%, they said in their report.
Their randomized trial included 133 adults with newly diagnosed achalasia enrolled at one of six hospitals in Germany, Hong Kong, Italy, Netherlands, and the United States.
Patients were randomly assigned to undergo 1-2 pneumatic dilations performed by an endoscopist who had performed at least 20 such procedures in the past, or to a POEM procedure likewise performed by an expert who had already done more than 20 such procedures.
At baseline, patients’ Eckardt symptom scores ranged from 6 to 9 on a scale with 0 indicating the lowest severity, to 12 indicating the highest. The median Eckardt scores were 8 in the POEM group and 7 in the pneumatic dilation group.
Treatment success, defined as an Eckardt score under 3 and no severe complications or retreatment at 2 years, was achieved by 58 of 63 patients (92%) in the POEM group, compared with 34 of 63 patients (54%) in the pneumatic dilation group (P less than .001), investigators reported.
Reflux esophagitis was observed in 22 of 54 POEM-treated patients (41%) who underwent endoscopy at a 2-year evaluation, compared with only 2 of 29 patients (7%) who had received the balloon dilation procedure (P = .002). In line with that finding, both reflux symptoms and daily proton pump inhibitor use were more common in the POEM group, investigators said.
However, there were no differences between POEM and pneumatic dilation groups in quality of life and other secondary endpoints, including median barium column height and median integrated relaxation pressure, they reported.
Two serious adverse events related to treatment were seen, according to investigators, including one perforation requiring an endoscopic closure plus antibiotics and hospitalization for 13 days, and one hospital admission for a night because of severe chest pain with no signs of perforation.
“Although POEM is more invasive and requires more technical endoscopic skills, the risk of severe complications was not higher than with pneumatic dilation, especially when performed by experienced endoscopists,” Dr. Bredenoord and coauthors said in their report.
However, these results do not imply that the traditional dilation procedure should be abandoned, they said, as POEM is more invasive, more involved, and more likely to result in reflux esophagitis.
“It seems reasonable to offer both options to treatment-naive patients with achalasia and counsel them to select treatment based on the patient’s characteristics, personal preference, comorbidity, and disease subtype,” they said.
Funding for the study came from Fonds NutsOhra and the European Society of Gastrointestinal Endoscopy. Dr. Bredenoord reported disclosures related to Norgine, Laborie, Medtronic, Diversatek, Nutricia, Regeneron, Celgene, Bayer, and Dr. Falk Pharma.
SOURCE: Ponds FA et al. JAMA. 2019;322(2):134-44. doi: 10.1001/jama.2019.8859.
FROM JAMA
Endoscopic treatment effective in T1b esophageal cancer
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates would not be significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment, in the absence of lymph node metastasis. The risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC), and in those with deeper submucosal invasion (greater than 200 microm for ESCC or greater than 500 microm for EAC). Patients also have a higher risk of lymph node metastasis if they have poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion.
In the studies cited in the review, overall 5-year survival for esophageal squamous cell CA (ESCC) ranged from 68.6% (chemoradiation therapy alone) to 75% (endoscopic resection followed by chemoradiation), compared with 77.7% for surgery. For esophageal adenocarcinoma (EAC), there is less data but greater efficacy, with one study demonstrating 5-year overall survival of 93.9% in the ESD group compared with 97.3% in the surgery group. However, only approximately 15 patients in this study were diagnosed T1b.
The authors concluded that “ESD or EMR can be successfully applied to submucosal invasive cancers that have a low risk of metastatic potential....Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer.”
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates would not be significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment, in the absence of lymph node metastasis. The risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC), and in those with deeper submucosal invasion (greater than 200 microm for ESCC or greater than 500 microm for EAC). Patients also have a higher risk of lymph node metastasis if they have poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion.
In the studies cited in the review, overall 5-year survival for esophageal squamous cell CA (ESCC) ranged from 68.6% (chemoradiation therapy alone) to 75% (endoscopic resection followed by chemoradiation), compared with 77.7% for surgery. For esophageal adenocarcinoma (EAC), there is less data but greater efficacy, with one study demonstrating 5-year overall survival of 93.9% in the ESD group compared with 97.3% in the surgery group. However, only approximately 15 patients in this study were diagnosed T1b.
The authors concluded that “ESD or EMR can be successfully applied to submucosal invasive cancers that have a low risk of metastatic potential....Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer.”
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
Findings from a systematic review may help guide treatment of patients with T1b esophageal cancer.
The review suggests that endoscopic submucosal dissection and endoscopic mucosal resection are appropriate for T1b esophageal cancers with a low risk of metastasis. The authors identified several factors associated with a higher risk of lymph node metastasis and said patients with these risk factors may benefit from adjuvant chemotherapy and radiation.
Mohamed O. Othman, MD, of Baylor College of Medicine, Houston, and colleagues conducted the review. Their report is in Clinical Gastroenterology and Hepatology.
The authors cited studies suggesting that survival rates would not be significantly different among early-stage esophageal cancer patients who undergo esophagectomy and those who receive endoscopic treatment, in the absence of lymph node metastasis. The risk of lymph node metastasis is higher in esophageal squamous cell carcinoma (ESCC) than in esophageal adenocarcinoma (EAC), and in those with deeper submucosal invasion (greater than 200 microm for ESCC or greater than 500 microm for EAC). Patients also have a higher risk of lymph node metastasis if they have poorly differentiated tumors, tumors larger than 2 cm, or lymphovascular invasion.
In the studies cited in the review, overall 5-year survival for esophageal squamous cell CA (ESCC) ranged from 68.6% (chemoradiation therapy alone) to 75% (endoscopic resection followed by chemoradiation), compared with 77.7% for surgery. For esophageal adenocarcinoma (EAC), there is less data but greater efficacy, with one study demonstrating 5-year overall survival of 93.9% in the ESD group compared with 97.3% in the surgery group. However, only approximately 15 patients in this study were diagnosed T1b.
The authors concluded that “ESD or EMR can be successfully applied to submucosal invasive cancers that have a low risk of metastatic potential....Future research should focus on novel biological and immunohistochemistry markers which can aid in the prediction of tumor behavior and lymph node metastasis status in T1b esophageal cancer.”
The authors disclosed relationships with Olympus, Boston Scientific, Lumendi, Aries Pharmaceutical, and Fujinon.
SOURCE: Othman MO et al. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.05.045.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY