User login
Anticoagulant choice, PPI cotherapy impact risk of upper GI bleeding
Patients receiving oral anticoagulant treatment had the lowest risk of gastrointestinal bleeding when taking apixaban, compared with rivaroxaban, dabigatran, and warfarin, according to a recent study.
Further, patients who received proton pump inhibitor (PPI) cotherapy had a lower overall risk of gastrointestinal bleeding, according to Wayne A. Ray, PhD, from the department of health policy at Vanderbilt University, Nashville, Tenn., and his colleagues.
“These findings indicate the potential benefits of a gastrointestinal bleeding risk assessment before initiating anticoagulant treatment,” Dr. Ray and his colleagues wrote in their study, which was published in JAMA.
Dr. Ray and his colleagues performed a retrospective, population-based study of 1,643,123 Medicare beneficiaries (mean age, 76.4 years) who received 1,713,183 new episodes of oral anticoagulant treatment between January 2011 and September 2015. They analyzed how patients reacted to apixaban, dabigatran, rivaroxaban, or warfarin both with and without PPI cotherapy.
Overall, the risk of gastrointestinal bleeding across 754,389 person-years without PPI therapy was 115 per 10,000 person-years (95% confidence interval, 112-118) in 7,119 patients. The researchers found the risk of gastrointestinal bleeding was highest in patients taking rivaroxaban (1,278 patients; 144 per 10,000 person-years; 95% CI, 136-152) and lowest when taking apixaban (279 patients; 120 per 10,000 person-years; incidence rate ratio, 1,97; 95% CI, 1.73-2.25), compared with dabigatran (629 patients; 120 per 10,000 person-years; IRR, 1.19; 95% CI, 1.08-1.32) and warfarin (4,933 patients; 113 per 10,000 person-years; IRR, 1.27; 95% CI, 1.19-1.35). There was a significantly lower incidence of gastrointestinal bleeding for apixaban, compared with warfarin (IRR, 0.64; 95% CI, 0.57-0.73) and dabigatran (IRR, 0.61; 95% CI, 0.52-0.70).
There was a lower overall incidence of gastrointestinal bleeding when receiving PPI cotherapy (264,447 person-years; 76 per 10,000 person-years), compared with patients who received anticoagulant treatment without PPI cotherapy (IRR, 0.66; 95% CI, 0.62-0.69). This reduced incidence of gastrointestinal bleeding was also seen in patients receiving PPI cotherapy and taking apixaban (IRR, 0.66; 95% CI, 0.52-0.85), dabigatran (IRR, 0.49; 95% CI, 0.41-0.59), rivaroxaban (IRR, 0.75; 95% CI, 0.68-0.84), and warfarin (IRR, 0.65; 95% CI, 0.62-0.69).
The researchers noted that limitations in this study included potential misclassification of anticoagulant treatment, PPI cotherapy, and NSAIDs because of a reliance on filled prescription data; confounding by unmeasured factors such as aspirin exposure or Helicobacter pylori infection; and gastrointestinal bleeding being measured using a disease risk score.
This study was supported by a grant from the National Heart, Lung, and Blood Institute. The authors reported no relevant conflicts of interest.
SOURCE: Ray WA et al. JAMA. 2018 Dec 4. doi: 10.1001/jama.2018.17242.
Patients receiving oral anticoagulant treatment had the lowest risk of gastrointestinal bleeding when taking apixaban, compared with rivaroxaban, dabigatran, and warfarin, according to a recent study.
Further, patients who received proton pump inhibitor (PPI) cotherapy had a lower overall risk of gastrointestinal bleeding, according to Wayne A. Ray, PhD, from the department of health policy at Vanderbilt University, Nashville, Tenn., and his colleagues.
“These findings indicate the potential benefits of a gastrointestinal bleeding risk assessment before initiating anticoagulant treatment,” Dr. Ray and his colleagues wrote in their study, which was published in JAMA.
Dr. Ray and his colleagues performed a retrospective, population-based study of 1,643,123 Medicare beneficiaries (mean age, 76.4 years) who received 1,713,183 new episodes of oral anticoagulant treatment between January 2011 and September 2015. They analyzed how patients reacted to apixaban, dabigatran, rivaroxaban, or warfarin both with and without PPI cotherapy.
Overall, the risk of gastrointestinal bleeding across 754,389 person-years without PPI therapy was 115 per 10,000 person-years (95% confidence interval, 112-118) in 7,119 patients. The researchers found the risk of gastrointestinal bleeding was highest in patients taking rivaroxaban (1,278 patients; 144 per 10,000 person-years; 95% CI, 136-152) and lowest when taking apixaban (279 patients; 120 per 10,000 person-years; incidence rate ratio, 1,97; 95% CI, 1.73-2.25), compared with dabigatran (629 patients; 120 per 10,000 person-years; IRR, 1.19; 95% CI, 1.08-1.32) and warfarin (4,933 patients; 113 per 10,000 person-years; IRR, 1.27; 95% CI, 1.19-1.35). There was a significantly lower incidence of gastrointestinal bleeding for apixaban, compared with warfarin (IRR, 0.64; 95% CI, 0.57-0.73) and dabigatran (IRR, 0.61; 95% CI, 0.52-0.70).
There was a lower overall incidence of gastrointestinal bleeding when receiving PPI cotherapy (264,447 person-years; 76 per 10,000 person-years), compared with patients who received anticoagulant treatment without PPI cotherapy (IRR, 0.66; 95% CI, 0.62-0.69). This reduced incidence of gastrointestinal bleeding was also seen in patients receiving PPI cotherapy and taking apixaban (IRR, 0.66; 95% CI, 0.52-0.85), dabigatran (IRR, 0.49; 95% CI, 0.41-0.59), rivaroxaban (IRR, 0.75; 95% CI, 0.68-0.84), and warfarin (IRR, 0.65; 95% CI, 0.62-0.69).
The researchers noted that limitations in this study included potential misclassification of anticoagulant treatment, PPI cotherapy, and NSAIDs because of a reliance on filled prescription data; confounding by unmeasured factors such as aspirin exposure or Helicobacter pylori infection; and gastrointestinal bleeding being measured using a disease risk score.
This study was supported by a grant from the National Heart, Lung, and Blood Institute. The authors reported no relevant conflicts of interest.
SOURCE: Ray WA et al. JAMA. 2018 Dec 4. doi: 10.1001/jama.2018.17242.
Patients receiving oral anticoagulant treatment had the lowest risk of gastrointestinal bleeding when taking apixaban, compared with rivaroxaban, dabigatran, and warfarin, according to a recent study.
Further, patients who received proton pump inhibitor (PPI) cotherapy had a lower overall risk of gastrointestinal bleeding, according to Wayne A. Ray, PhD, from the department of health policy at Vanderbilt University, Nashville, Tenn., and his colleagues.
“These findings indicate the potential benefits of a gastrointestinal bleeding risk assessment before initiating anticoagulant treatment,” Dr. Ray and his colleagues wrote in their study, which was published in JAMA.
Dr. Ray and his colleagues performed a retrospective, population-based study of 1,643,123 Medicare beneficiaries (mean age, 76.4 years) who received 1,713,183 new episodes of oral anticoagulant treatment between January 2011 and September 2015. They analyzed how patients reacted to apixaban, dabigatran, rivaroxaban, or warfarin both with and without PPI cotherapy.
Overall, the risk of gastrointestinal bleeding across 754,389 person-years without PPI therapy was 115 per 10,000 person-years (95% confidence interval, 112-118) in 7,119 patients. The researchers found the risk of gastrointestinal bleeding was highest in patients taking rivaroxaban (1,278 patients; 144 per 10,000 person-years; 95% CI, 136-152) and lowest when taking apixaban (279 patients; 120 per 10,000 person-years; incidence rate ratio, 1,97; 95% CI, 1.73-2.25), compared with dabigatran (629 patients; 120 per 10,000 person-years; IRR, 1.19; 95% CI, 1.08-1.32) and warfarin (4,933 patients; 113 per 10,000 person-years; IRR, 1.27; 95% CI, 1.19-1.35). There was a significantly lower incidence of gastrointestinal bleeding for apixaban, compared with warfarin (IRR, 0.64; 95% CI, 0.57-0.73) and dabigatran (IRR, 0.61; 95% CI, 0.52-0.70).
There was a lower overall incidence of gastrointestinal bleeding when receiving PPI cotherapy (264,447 person-years; 76 per 10,000 person-years), compared with patients who received anticoagulant treatment without PPI cotherapy (IRR, 0.66; 95% CI, 0.62-0.69). This reduced incidence of gastrointestinal bleeding was also seen in patients receiving PPI cotherapy and taking apixaban (IRR, 0.66; 95% CI, 0.52-0.85), dabigatran (IRR, 0.49; 95% CI, 0.41-0.59), rivaroxaban (IRR, 0.75; 95% CI, 0.68-0.84), and warfarin (IRR, 0.65; 95% CI, 0.62-0.69).
The researchers noted that limitations in this study included potential misclassification of anticoagulant treatment, PPI cotherapy, and NSAIDs because of a reliance on filled prescription data; confounding by unmeasured factors such as aspirin exposure or Helicobacter pylori infection; and gastrointestinal bleeding being measured using a disease risk score.
This study was supported by a grant from the National Heart, Lung, and Blood Institute. The authors reported no relevant conflicts of interest.
SOURCE: Ray WA et al. JAMA. 2018 Dec 4. doi: 10.1001/jama.2018.17242.
FROM JAMA
Key clinical point: In patients receiving oral anticoagulant treatment, risk of gastrointestinal bleeding was highest in patients taking rivaroxaban, lowest when taking apixaban, and there was a lower overall incidence of gastrointestinal bleeding when receiving proton pump inhibitor cotherapy.
Major finding: Per 10,000 person-years, the incidence rate of gastrointestinal bleeding was 144 for rivaroxaban, 73 for apixaban, 120 for dabigatran, and 113 for warfarin; there was a gastrointestinal bleeding incidence rate ratio of 0.66 for patients using protein pump inhibitor cotherapy.
Study details: A retrospective, population-based study of 1,643,123 Medicare beneficiaries who received oral anticoagulant treatment between January 2011 and September 2015.
Disclosures: This study was supported by a grant from the National Heart, Lung, and Blood Institute. The authors reported no relevant conflicts of interest.
Source: Ray WA et al. JAMA. 2018 Dec 4. doi: 10.1001/jama.2018.17242.
Surgical model to study reflux esophagitis after esophagojejunostomy
During a wound repair process in rats, metaplastic columnar-lined esophagus was produced and increased in length following esophagojejunostomy, which may be independent of stem cell reprogramming, according to results from an anastomosed rodent study.
The investigators studied esophageal and tissue sections of 52 rats at different time points after esophagojejunostomy and samples were analyzed for length, type, and location of columnar lining. In addition, the sections were examined immunophenotypically to elucidate the molecular changes that occur during ulceration. Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital and the department of pathology at Harvard Medical School, Boston, and colleagues reported the findings in Cellular and Molecular Gastroenterology and Hepatology.
“This rodent columnar-lined esophagus has been proposed to develop from cellular reprogramming of progenitor cells, but studies on early columnar-lined esophagus development are lacking,” the researchers wrote.
In the model, ulceration was seen 2 weeks after surgery, which began distally at the esophagojejunal anastomosis. Representative of wound healing, reepithelialization of the ulcer region took place through formation of immature glands, which were found to bud directly from jejunal crypts.
After immunophenotypic analysis, the researchers reported that “immunohistochemical characterization of neoglandular epithelium located immediately proximal to the anastomosis showed features similar to those of the native nonproliferating jejunal epithelium located immediately distal to the anastomosis.” They further reported that “the columnar-lined esophagus’s immunoprofile was similar to jejunal crypt epithelium.”
Upon further examination of the ulcer segment, Dr. Agoston and colleagues found that columnar-lined esophagus elongated from 0.15 mm (standard error of the mean, ± 0.1) to 5.22 mm (SEM, ± 0.37) at 2 and 32 weeks post esophagojejunostomy, respectively.
“There was a highly significant linear relationship between the length of the neoglandular epithelium in the distal esophagus and the number of weeks after surgery (correlation coefficient, 0.94; P less than .0001),” the investigators stated.
Locational analysis revealed epithelial-mesenchymal transition markers being expressed by spindle-shaped cells at the leading edge of the columnar-lined esophagus. In addition, neoglands were identified within esophageal ulcer beds and actively dividing squamous epithelium was seen exclusively at the proximal ulcer border.
Following the systematic analysis, the authors noted that the columnar-lined esophagus was most likely the result of jejunal cell migration into the esophagus. They suggested that if compared, jejunal cells may competitively dominate squamous cells in the context of chronic gastroesophageal reflux disease. Furthermore, they observed that the region of ulceration following esophagojejunostomy in their model was more expansive than that reported in other comparable rodent models of reflux esophagitis.
“The reason for this difference is not clear, but we speculate that it is the result of technical aspects of our reflux-inducing surgery,” the researchers wrote. They further explained that “we intentionally fashioned a large anastomotic orifice between the esophagus and jejunum, perhaps larger than that fashioned by other investigators.” And they concluded, “we suspect that this larger orifice resulted in esophageal exposure to larger volumes of refluxate and, consequently, larger areas of ulceration.”
The authors acknowledged their results may not be fully applicable in the context of human Barrett’s esophagus, given the rodent model. However, they do believe the findings may provide a basis to help understand the wound repair process, particularly the distal edge of ulcers that border the columnar epithelium.
“Using a rat model of reflux esophagitis via surgical esophagojejunostomy, we have shown that a metaplastic, columnar-lined esophagus develops via a wound healing process, and not via genetic reprogramming of progenitor cells,” the researchers concluded.
The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
SOURCE: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
Agoston et al. reported that metaplastic columnar-lined esophagus develops in a wound-healing process on the distal edge of the ulcer, starting distally at the esophagojejunal anastomosis in the esophagojejunal anastomosed rat model. They also concluded that the columnar-lined esophagus was caused through migration of jejunal cells into the esophagus.
These new findings bring up a couple of issues. One is that metaplastic columnar-lined esophagus originates from jejunal crypt budding over the anastomosis. Some researchers may think this is not metaplasia, as there is no reprogramming of the stem cells. However, the definition of metaplasia is an endpoint such that a normal lineage is placed in an abnormal position, and it can be called metaplasia even it is from budding of jejunal crypt. This new finding is not denying metaplasia.
The second issue is whether these rodent models are really mimicking human metaplastic columnar-lined esophagus or not. In humans, metaplastic columnar-lined esophagus usually accompanies gastroesophageal reflux, but jejunum is not next to esophagus, and jejunal crypt budding is less likely. However, it is common to observe ulcerated lesions in the proximal front of long-segment Barrett’s esophagus in humans. In this process, the model of Agosto et al. is describing the human metaplastic columnar-lined esophagus elongation.
There would be more reprogramming happening in the body of animals under the effect of microenvironment. This is a kind of adaptation, and analyzing key factors for this reprogramming would be the path to clarifying carcinogenesis in the metaplastic field and also a way to advance regenerative medicine.
Sachiyo Nomura MD, PhD, AGAF, FACS, is an investigator in gastrointestinal carcinogenesis and epithelial biology, and a gastrointestinal surgeon, at University of Tokyo Hospital, department of stomach and esophageal surgery, as well as an associate professor, department of gastrointestinal surgery, graduate school of medicine, at the university. She has no conflicts.
Agoston et al. reported that metaplastic columnar-lined esophagus develops in a wound-healing process on the distal edge of the ulcer, starting distally at the esophagojejunal anastomosis in the esophagojejunal anastomosed rat model. They also concluded that the columnar-lined esophagus was caused through migration of jejunal cells into the esophagus.
These new findings bring up a couple of issues. One is that metaplastic columnar-lined esophagus originates from jejunal crypt budding over the anastomosis. Some researchers may think this is not metaplasia, as there is no reprogramming of the stem cells. However, the definition of metaplasia is an endpoint such that a normal lineage is placed in an abnormal position, and it can be called metaplasia even it is from budding of jejunal crypt. This new finding is not denying metaplasia.
The second issue is whether these rodent models are really mimicking human metaplastic columnar-lined esophagus or not. In humans, metaplastic columnar-lined esophagus usually accompanies gastroesophageal reflux, but jejunum is not next to esophagus, and jejunal crypt budding is less likely. However, it is common to observe ulcerated lesions in the proximal front of long-segment Barrett’s esophagus in humans. In this process, the model of Agosto et al. is describing the human metaplastic columnar-lined esophagus elongation.
There would be more reprogramming happening in the body of animals under the effect of microenvironment. This is a kind of adaptation, and analyzing key factors for this reprogramming would be the path to clarifying carcinogenesis in the metaplastic field and also a way to advance regenerative medicine.
Sachiyo Nomura MD, PhD, AGAF, FACS, is an investigator in gastrointestinal carcinogenesis and epithelial biology, and a gastrointestinal surgeon, at University of Tokyo Hospital, department of stomach and esophageal surgery, as well as an associate professor, department of gastrointestinal surgery, graduate school of medicine, at the university. She has no conflicts.
Agoston et al. reported that metaplastic columnar-lined esophagus develops in a wound-healing process on the distal edge of the ulcer, starting distally at the esophagojejunal anastomosis in the esophagojejunal anastomosed rat model. They also concluded that the columnar-lined esophagus was caused through migration of jejunal cells into the esophagus.
These new findings bring up a couple of issues. One is that metaplastic columnar-lined esophagus originates from jejunal crypt budding over the anastomosis. Some researchers may think this is not metaplasia, as there is no reprogramming of the stem cells. However, the definition of metaplasia is an endpoint such that a normal lineage is placed in an abnormal position, and it can be called metaplasia even it is from budding of jejunal crypt. This new finding is not denying metaplasia.
The second issue is whether these rodent models are really mimicking human metaplastic columnar-lined esophagus or not. In humans, metaplastic columnar-lined esophagus usually accompanies gastroesophageal reflux, but jejunum is not next to esophagus, and jejunal crypt budding is less likely. However, it is common to observe ulcerated lesions in the proximal front of long-segment Barrett’s esophagus in humans. In this process, the model of Agosto et al. is describing the human metaplastic columnar-lined esophagus elongation.
There would be more reprogramming happening in the body of animals under the effect of microenvironment. This is a kind of adaptation, and analyzing key factors for this reprogramming would be the path to clarifying carcinogenesis in the metaplastic field and also a way to advance regenerative medicine.
Sachiyo Nomura MD, PhD, AGAF, FACS, is an investigator in gastrointestinal carcinogenesis and epithelial biology, and a gastrointestinal surgeon, at University of Tokyo Hospital, department of stomach and esophageal surgery, as well as an associate professor, department of gastrointestinal surgery, graduate school of medicine, at the university. She has no conflicts.
During a wound repair process in rats, metaplastic columnar-lined esophagus was produced and increased in length following esophagojejunostomy, which may be independent of stem cell reprogramming, according to results from an anastomosed rodent study.
The investigators studied esophageal and tissue sections of 52 rats at different time points after esophagojejunostomy and samples were analyzed for length, type, and location of columnar lining. In addition, the sections were examined immunophenotypically to elucidate the molecular changes that occur during ulceration. Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital and the department of pathology at Harvard Medical School, Boston, and colleagues reported the findings in Cellular and Molecular Gastroenterology and Hepatology.
“This rodent columnar-lined esophagus has been proposed to develop from cellular reprogramming of progenitor cells, but studies on early columnar-lined esophagus development are lacking,” the researchers wrote.
In the model, ulceration was seen 2 weeks after surgery, which began distally at the esophagojejunal anastomosis. Representative of wound healing, reepithelialization of the ulcer region took place through formation of immature glands, which were found to bud directly from jejunal crypts.
After immunophenotypic analysis, the researchers reported that “immunohistochemical characterization of neoglandular epithelium located immediately proximal to the anastomosis showed features similar to those of the native nonproliferating jejunal epithelium located immediately distal to the anastomosis.” They further reported that “the columnar-lined esophagus’s immunoprofile was similar to jejunal crypt epithelium.”
Upon further examination of the ulcer segment, Dr. Agoston and colleagues found that columnar-lined esophagus elongated from 0.15 mm (standard error of the mean, ± 0.1) to 5.22 mm (SEM, ± 0.37) at 2 and 32 weeks post esophagojejunostomy, respectively.
“There was a highly significant linear relationship between the length of the neoglandular epithelium in the distal esophagus and the number of weeks after surgery (correlation coefficient, 0.94; P less than .0001),” the investigators stated.
Locational analysis revealed epithelial-mesenchymal transition markers being expressed by spindle-shaped cells at the leading edge of the columnar-lined esophagus. In addition, neoglands were identified within esophageal ulcer beds and actively dividing squamous epithelium was seen exclusively at the proximal ulcer border.
Following the systematic analysis, the authors noted that the columnar-lined esophagus was most likely the result of jejunal cell migration into the esophagus. They suggested that if compared, jejunal cells may competitively dominate squamous cells in the context of chronic gastroesophageal reflux disease. Furthermore, they observed that the region of ulceration following esophagojejunostomy in their model was more expansive than that reported in other comparable rodent models of reflux esophagitis.
“The reason for this difference is not clear, but we speculate that it is the result of technical aspects of our reflux-inducing surgery,” the researchers wrote. They further explained that “we intentionally fashioned a large anastomotic orifice between the esophagus and jejunum, perhaps larger than that fashioned by other investigators.” And they concluded, “we suspect that this larger orifice resulted in esophageal exposure to larger volumes of refluxate and, consequently, larger areas of ulceration.”
The authors acknowledged their results may not be fully applicable in the context of human Barrett’s esophagus, given the rodent model. However, they do believe the findings may provide a basis to help understand the wound repair process, particularly the distal edge of ulcers that border the columnar epithelium.
“Using a rat model of reflux esophagitis via surgical esophagojejunostomy, we have shown that a metaplastic, columnar-lined esophagus develops via a wound healing process, and not via genetic reprogramming of progenitor cells,” the researchers concluded.
The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
SOURCE: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
During a wound repair process in rats, metaplastic columnar-lined esophagus was produced and increased in length following esophagojejunostomy, which may be independent of stem cell reprogramming, according to results from an anastomosed rodent study.
The investigators studied esophageal and tissue sections of 52 rats at different time points after esophagojejunostomy and samples were analyzed for length, type, and location of columnar lining. In addition, the sections were examined immunophenotypically to elucidate the molecular changes that occur during ulceration. Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital and the department of pathology at Harvard Medical School, Boston, and colleagues reported the findings in Cellular and Molecular Gastroenterology and Hepatology.
“This rodent columnar-lined esophagus has been proposed to develop from cellular reprogramming of progenitor cells, but studies on early columnar-lined esophagus development are lacking,” the researchers wrote.
In the model, ulceration was seen 2 weeks after surgery, which began distally at the esophagojejunal anastomosis. Representative of wound healing, reepithelialization of the ulcer region took place through formation of immature glands, which were found to bud directly from jejunal crypts.
After immunophenotypic analysis, the researchers reported that “immunohistochemical characterization of neoglandular epithelium located immediately proximal to the anastomosis showed features similar to those of the native nonproliferating jejunal epithelium located immediately distal to the anastomosis.” They further reported that “the columnar-lined esophagus’s immunoprofile was similar to jejunal crypt epithelium.”
Upon further examination of the ulcer segment, Dr. Agoston and colleagues found that columnar-lined esophagus elongated from 0.15 mm (standard error of the mean, ± 0.1) to 5.22 mm (SEM, ± 0.37) at 2 and 32 weeks post esophagojejunostomy, respectively.
“There was a highly significant linear relationship between the length of the neoglandular epithelium in the distal esophagus and the number of weeks after surgery (correlation coefficient, 0.94; P less than .0001),” the investigators stated.
Locational analysis revealed epithelial-mesenchymal transition markers being expressed by spindle-shaped cells at the leading edge of the columnar-lined esophagus. In addition, neoglands were identified within esophageal ulcer beds and actively dividing squamous epithelium was seen exclusively at the proximal ulcer border.
Following the systematic analysis, the authors noted that the columnar-lined esophagus was most likely the result of jejunal cell migration into the esophagus. They suggested that if compared, jejunal cells may competitively dominate squamous cells in the context of chronic gastroesophageal reflux disease. Furthermore, they observed that the region of ulceration following esophagojejunostomy in their model was more expansive than that reported in other comparable rodent models of reflux esophagitis.
“The reason for this difference is not clear, but we speculate that it is the result of technical aspects of our reflux-inducing surgery,” the researchers wrote. They further explained that “we intentionally fashioned a large anastomotic orifice between the esophagus and jejunum, perhaps larger than that fashioned by other investigators.” And they concluded, “we suspect that this larger orifice resulted in esophageal exposure to larger volumes of refluxate and, consequently, larger areas of ulceration.”
The authors acknowledged their results may not be fully applicable in the context of human Barrett’s esophagus, given the rodent model. However, they do believe the findings may provide a basis to help understand the wound repair process, particularly the distal edge of ulcers that border the columnar epithelium.
“Using a rat model of reflux esophagitis via surgical esophagojejunostomy, we have shown that a metaplastic, columnar-lined esophagus develops via a wound healing process, and not via genetic reprogramming of progenitor cells,” the researchers concluded.
The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
SOURCE: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Post esophagojejunostomy in rats, a wound repair process produces metaplastic columnar-lined esophagus likely independent of progenitor cell reprogramming.
Major finding: In an esophageal tissue section, columnar-lined esophagus length was significantly elongated from 0.15 (±0.1) mm to 5.22 (±0.37) mm at 2 and 32 weeks, respectively, following esophagojejunostomy.
Study details: Histologic and immunophenotypic analysis of 52 rats investigating the molecular characteristics of columnar-lined esophagus, specific to ulceration and wound healing, following surgery in an anastomosed rodent model.
Disclosures: The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
Source: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
AGA Clinical Practice Update: Diagnosis of rumination syndrome
Additionally, promote diaphragmatic breathing to help manage the condition, advised authors of an expert review of clinical practice updates for rumination syndrome published in Clinical Gastroenterology and Hepatology.
“Patients, not unsurprisingly, typically use the word ‘vomiting’ to describe rumination events, and many patients are misdiagnosed as having refractory vomiting, gastroesophageal reflux disease, or gastroparesis,” Magnus Halland, MD, of the Mayo Clinic in Rochester, Minn., and colleagues wrote in the review. “A long delay in receiving a diagnosis is common and can lead to unnecessary testing, reduced quality of life, and even invasive procedures such as surgery or feeding tubes.”
Rumination syndrome differs from vomiting, the authors noted, because the retrograde flow of ingested gastric content does not have an acidic taste and may in fact taste like food or drink recently ingested. Rumination can occur without any preceding events, after a reflux episode or by the swallowing of air that causes gastric straining but typically happens within 1 hour to 2 hours after a meal. Patients can experience weight loss, dental erosions and caries, heartburn, nausea, bloating, diarrhea, abdominal pain, abdominal discomfort, and belching, among other symptoms, in the presence of rumination syndrome, the authors said.
Dr. Halland and his colleagues provided seven best practice recommendations for rumination syndrome in their updates, which include:
- Patients who show symptoms of consistent postprandial regurgitation, often misdiagnosed with refractory gastroesophageal reflux or vomiting, should be considered for rumination syndrome.
- Patients who have dysphagia, nausea, nocturnal regurgitation, or gastric symptoms outside of meals are less likely to have rumination syndrome, but those symptoms do not exclude the condition.
- Rome IV criteria are advised to diagnose rumination syndrome after medical work-up, which includes “persistent or recurrent regurgitation of recently ingested food into the mouth with subsequent spitting or remastication and swallowing” not preceded by retching where patients fulfill these symptom criteria for 3 months with a minimum of 6 months of symptoms before diagnosis.
- Patients should receive first-line therapy for rumination syndrome consisting of diaphragmatic breathing with or without biofeedback.
- Patients should be referred to a speech therapist, gastroenterologist, psychologist, or other knowledgeable health practitioners to learn effective diaphragmatic breathing.
- Current limitations in the diagnosis of rumination syndrome include need for expertise and lack of standardized protocols, but “testing for rumination syndrome with postprandial high-resolution esophageal impedance manometry can be used to support the diagnosis.”
- Bacloflen (10 mg) taken three times daily is a “reasonable next step” for patients who do not respond to treatment.
The authors acknowledged that many questions, such as the pathophysiology and initiating factors of rumination syndrome, are unknown and noted future studies are needed to address epidemiology, develop validated tools for measuring symptoms, and study diaphragmatic breathing’s effect on reducing symptoms of rumination syndrome as well as the condition’s impact on quality of life.
“Indeed, the basic question of how subconsciously one can learn to regurgitate still needs to be answered,” Dr. Halland and his colleagues wrote.
The authors report no relevant conflicts of interest.
SOURCE: Halland M et al. Clin Gastroenterol Hepatol. 2018 Jun 11. doi: 10.1016/j.cgh.2018.05.049.
Additionally, promote diaphragmatic breathing to help manage the condition, advised authors of an expert review of clinical practice updates for rumination syndrome published in Clinical Gastroenterology and Hepatology.
“Patients, not unsurprisingly, typically use the word ‘vomiting’ to describe rumination events, and many patients are misdiagnosed as having refractory vomiting, gastroesophageal reflux disease, or gastroparesis,” Magnus Halland, MD, of the Mayo Clinic in Rochester, Minn., and colleagues wrote in the review. “A long delay in receiving a diagnosis is common and can lead to unnecessary testing, reduced quality of life, and even invasive procedures such as surgery or feeding tubes.”
Rumination syndrome differs from vomiting, the authors noted, because the retrograde flow of ingested gastric content does not have an acidic taste and may in fact taste like food or drink recently ingested. Rumination can occur without any preceding events, after a reflux episode or by the swallowing of air that causes gastric straining but typically happens within 1 hour to 2 hours after a meal. Patients can experience weight loss, dental erosions and caries, heartburn, nausea, bloating, diarrhea, abdominal pain, abdominal discomfort, and belching, among other symptoms, in the presence of rumination syndrome, the authors said.
Dr. Halland and his colleagues provided seven best practice recommendations for rumination syndrome in their updates, which include:
- Patients who show symptoms of consistent postprandial regurgitation, often misdiagnosed with refractory gastroesophageal reflux or vomiting, should be considered for rumination syndrome.
- Patients who have dysphagia, nausea, nocturnal regurgitation, or gastric symptoms outside of meals are less likely to have rumination syndrome, but those symptoms do not exclude the condition.
- Rome IV criteria are advised to diagnose rumination syndrome after medical work-up, which includes “persistent or recurrent regurgitation of recently ingested food into the mouth with subsequent spitting or remastication and swallowing” not preceded by retching where patients fulfill these symptom criteria for 3 months with a minimum of 6 months of symptoms before diagnosis.
- Patients should receive first-line therapy for rumination syndrome consisting of diaphragmatic breathing with or without biofeedback.
- Patients should be referred to a speech therapist, gastroenterologist, psychologist, or other knowledgeable health practitioners to learn effective diaphragmatic breathing.
- Current limitations in the diagnosis of rumination syndrome include need for expertise and lack of standardized protocols, but “testing for rumination syndrome with postprandial high-resolution esophageal impedance manometry can be used to support the diagnosis.”
- Bacloflen (10 mg) taken three times daily is a “reasonable next step” for patients who do not respond to treatment.
The authors acknowledged that many questions, such as the pathophysiology and initiating factors of rumination syndrome, are unknown and noted future studies are needed to address epidemiology, develop validated tools for measuring symptoms, and study diaphragmatic breathing’s effect on reducing symptoms of rumination syndrome as well as the condition’s impact on quality of life.
“Indeed, the basic question of how subconsciously one can learn to regurgitate still needs to be answered,” Dr. Halland and his colleagues wrote.
The authors report no relevant conflicts of interest.
SOURCE: Halland M et al. Clin Gastroenterol Hepatol. 2018 Jun 11. doi: 10.1016/j.cgh.2018.05.049.
Additionally, promote diaphragmatic breathing to help manage the condition, advised authors of an expert review of clinical practice updates for rumination syndrome published in Clinical Gastroenterology and Hepatology.
“Patients, not unsurprisingly, typically use the word ‘vomiting’ to describe rumination events, and many patients are misdiagnosed as having refractory vomiting, gastroesophageal reflux disease, or gastroparesis,” Magnus Halland, MD, of the Mayo Clinic in Rochester, Minn., and colleagues wrote in the review. “A long delay in receiving a diagnosis is common and can lead to unnecessary testing, reduced quality of life, and even invasive procedures such as surgery or feeding tubes.”
Rumination syndrome differs from vomiting, the authors noted, because the retrograde flow of ingested gastric content does not have an acidic taste and may in fact taste like food or drink recently ingested. Rumination can occur without any preceding events, after a reflux episode or by the swallowing of air that causes gastric straining but typically happens within 1 hour to 2 hours after a meal. Patients can experience weight loss, dental erosions and caries, heartburn, nausea, bloating, diarrhea, abdominal pain, abdominal discomfort, and belching, among other symptoms, in the presence of rumination syndrome, the authors said.
Dr. Halland and his colleagues provided seven best practice recommendations for rumination syndrome in their updates, which include:
- Patients who show symptoms of consistent postprandial regurgitation, often misdiagnosed with refractory gastroesophageal reflux or vomiting, should be considered for rumination syndrome.
- Patients who have dysphagia, nausea, nocturnal regurgitation, or gastric symptoms outside of meals are less likely to have rumination syndrome, but those symptoms do not exclude the condition.
- Rome IV criteria are advised to diagnose rumination syndrome after medical work-up, which includes “persistent or recurrent regurgitation of recently ingested food into the mouth with subsequent spitting or remastication and swallowing” not preceded by retching where patients fulfill these symptom criteria for 3 months with a minimum of 6 months of symptoms before diagnosis.
- Patients should receive first-line therapy for rumination syndrome consisting of diaphragmatic breathing with or without biofeedback.
- Patients should be referred to a speech therapist, gastroenterologist, psychologist, or other knowledgeable health practitioners to learn effective diaphragmatic breathing.
- Current limitations in the diagnosis of rumination syndrome include need for expertise and lack of standardized protocols, but “testing for rumination syndrome with postprandial high-resolution esophageal impedance manometry can be used to support the diagnosis.”
- Bacloflen (10 mg) taken three times daily is a “reasonable next step” for patients who do not respond to treatment.
The authors acknowledged that many questions, such as the pathophysiology and initiating factors of rumination syndrome, are unknown and noted future studies are needed to address epidemiology, develop validated tools for measuring symptoms, and study diaphragmatic breathing’s effect on reducing symptoms of rumination syndrome as well as the condition’s impact on quality of life.
“Indeed, the basic question of how subconsciously one can learn to regurgitate still needs to be answered,” Dr. Halland and his colleagues wrote.
The authors report no relevant conflicts of interest.
SOURCE: Halland M et al. Clin Gastroenterol Hepatol. 2018 Jun 11. doi: 10.1016/j.cgh.2018.05.049.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Experts update diagnostic guidelines for eosinophilic esophagitis
The diagnosis of eosinophilic esophagitis no longer needs to include a trial of proton pump inhibitor (PPI) therapy, according to an updated international consensus statement published in the October issue of Gastroenterology.
“An initial rationale for the PPI trial was to distinguish eosinophilic esophagitis from gastroesophageal reflux disease, but it is now known that these conditions have a complex relationship and are not necessarily mutually exclusive,” wrote Evan S. Dellon, MD, of the University of North Carolina at Chapel Hill, and his associates. According to current evidence, “PPIs are better classified as a treatment for esophageal eosinophilia that may be due to eosinophilic esophagitis than as a diagnostic criterion,” they said.
Diagnostic guidelines for eosinophilic esophagitis were published first in 2007 and were updated in 2011. The guideline authors recommended either pH monitoring or an 8-week trial of high-dose PPI therapy to rule out inflammation from gastroesophageal reflux disease (GERD).
But subsequent publications described patients with symptomatic esophageal eosinophilia who responded to PPIs and lacked classic GERD symptoms. Guidelines called this condition “PPI-responsive esophageal eosinophilia” and considered it a separate entity from GERD.
However, an “evolving body of research” shows that eosinophilic esophagitis can overlap with GERD, Dr. Dellon and his associates wrote. Furthermore, each of these conditions can trigger the other. Eosinophilic esophagitis can decrease esophageal compliance, leading to secondary reflux, while gastroesophageal reflux can erode the esophageal epithelium, triggering antigen exposure and eosinophilia.
Therefore, Dr. Dellon and his associates recommended defining eosinophilic esophagitis as signs and symptoms of esophageal dysfunction and an esophageal biopsy showing at least 15 eosinophils per high-power field, or approximately 60 eosinophils per millimeter, with infiltration limited to the esophagus. They stressed the importance of esophageal biopsy even if endoscopy shows normal mucosa. “As per prior guidelines, multiple biopsies from two or more esophageal levels, targeting areas of apparent inflammation, are recommended to increase the diagnostic yield,” they added. “Gastric and duodenal biopsies should be obtained as clinically indicated by symptoms, endoscopic findings in the stomach or duodenum, or high index of suspicion for a mucosal process.”
Physicians should increase their suspicion of eosinophilic esophagitis if patients have other types of atopy or endoscopic findings of “rings, furrows, exudates, edema, stricture, narrowing, and crepe-paper mucosa,” they added. In addition to GERD, they recommended looking carefully for other conditions that can trigger esophageal eosinophilia, such as pemphigus, drug hypersensitivity reactions, achalasia, and Crohn’s disease with esophageal involvement.
To create the guideline, Dr. Dellon and his associates searched PubMed for studies of all designs and sizes published from 1966 through December 2016. Teams of experts on specific topics then reviewed and discussed relevant literature. In May 2017, 43 reviewers met for 8 hours to present and discuss conclusions. There was 100% agreement to remove the PPI trial from the diagnostic criteria, the experts noted.
The authors disclosed financial support from the International Gastrointestinal Eosinophilic Diseases Researchers (TIGERS), The David and Denise Bunning Family, and the Rare Disease Clinical Research Network. Dr. Dellon disclosed consulting relationships and receiving research funding from Adare, Celgene/Receptos, Regeneron, and Shire among others. The majority of his coauthors also disclosed relationships with numerous medical companies.
SOURCE: Dellon ES et al. Gastroenterology. 2018 Jul 12. doi: 10.1053/j.gastro.2018.07.009.
Studies in the 1980s linked the presence of esophageal mucosal eosinophils with increased acid exposure on pH monitoring. For the next 2 decades, clinicians viewed eosinophils on esophageal biopsies as diagnostic for GERD such that the initial description of EoE by Attwood in 1993 distinguished EoE from GERD by the presence of esophageal eosinophilia in the absence of either reflux esophagitis or abnormal acid exposure on pH testing. Consequently, the initial diagnostic criteria for EoE in 2007 included a lack of response to PPI and/or normal pH testing to establish the diagnosis of EoE. Reflecting growing uncertainty regarding the ability of PPI therapy to differentiate acid-induced from allergic inflammatory mechanisms, an updated consensus in 2011 introduced the terminology “PPI responsive esophageal eosinophilia (PPIREE)” to describe an increasingly recognized subset of patients with suspected EoE that resolved with PPI. Now, supported by scientific evidence accumulated over the past decade, AGREE has taken a step back by removing the PPI trial from the diagnosis of EoE, thereby abandoning the PPIREE terminology. This step simplifies the diagnosis of EoE and acknowledges that a histologic response to PPI does not “rule in” GERD or “rule out” EoE. It is important to emphasize that the updated criteria still advocate careful consideration of secondary causes of esophageal eosinophilia prior to the diagnosis of EoE.
Ramifications of the updated diagnostic criteria include the opportunities for clinicians to consider use of topical corticosteroids and diet therapies, rather than mandate an up-front PPI trial, in patients with EoE. On a practical level, based on their effectiveness, safety, and ease of administration, PPIs remain positioned as a favorable initial intervention for EoE. Conceptually, however, the paradigm shift highlights the ability of research to improve our understanding of disease pathogenesis and thereby impact clinical management.
Ikuo Hirano, MD, AGAF, is in the division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804), which is part of the Rare Disease Clinical Research Network. He has received research funding and consulting fees from Celgene, Regeneron, Shire, and others.
Studies in the 1980s linked the presence of esophageal mucosal eosinophils with increased acid exposure on pH monitoring. For the next 2 decades, clinicians viewed eosinophils on esophageal biopsies as diagnostic for GERD such that the initial description of EoE by Attwood in 1993 distinguished EoE from GERD by the presence of esophageal eosinophilia in the absence of either reflux esophagitis or abnormal acid exposure on pH testing. Consequently, the initial diagnostic criteria for EoE in 2007 included a lack of response to PPI and/or normal pH testing to establish the diagnosis of EoE. Reflecting growing uncertainty regarding the ability of PPI therapy to differentiate acid-induced from allergic inflammatory mechanisms, an updated consensus in 2011 introduced the terminology “PPI responsive esophageal eosinophilia (PPIREE)” to describe an increasingly recognized subset of patients with suspected EoE that resolved with PPI. Now, supported by scientific evidence accumulated over the past decade, AGREE has taken a step back by removing the PPI trial from the diagnosis of EoE, thereby abandoning the PPIREE terminology. This step simplifies the diagnosis of EoE and acknowledges that a histologic response to PPI does not “rule in” GERD or “rule out” EoE. It is important to emphasize that the updated criteria still advocate careful consideration of secondary causes of esophageal eosinophilia prior to the diagnosis of EoE.
Ramifications of the updated diagnostic criteria include the opportunities for clinicians to consider use of topical corticosteroids and diet therapies, rather than mandate an up-front PPI trial, in patients with EoE. On a practical level, based on their effectiveness, safety, and ease of administration, PPIs remain positioned as a favorable initial intervention for EoE. Conceptually, however, the paradigm shift highlights the ability of research to improve our understanding of disease pathogenesis and thereby impact clinical management.
Ikuo Hirano, MD, AGAF, is in the division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804), which is part of the Rare Disease Clinical Research Network. He has received research funding and consulting fees from Celgene, Regeneron, Shire, and others.
Studies in the 1980s linked the presence of esophageal mucosal eosinophils with increased acid exposure on pH monitoring. For the next 2 decades, clinicians viewed eosinophils on esophageal biopsies as diagnostic for GERD such that the initial description of EoE by Attwood in 1993 distinguished EoE from GERD by the presence of esophageal eosinophilia in the absence of either reflux esophagitis or abnormal acid exposure on pH testing. Consequently, the initial diagnostic criteria for EoE in 2007 included a lack of response to PPI and/or normal pH testing to establish the diagnosis of EoE. Reflecting growing uncertainty regarding the ability of PPI therapy to differentiate acid-induced from allergic inflammatory mechanisms, an updated consensus in 2011 introduced the terminology “PPI responsive esophageal eosinophilia (PPIREE)” to describe an increasingly recognized subset of patients with suspected EoE that resolved with PPI. Now, supported by scientific evidence accumulated over the past decade, AGREE has taken a step back by removing the PPI trial from the diagnosis of EoE, thereby abandoning the PPIREE terminology. This step simplifies the diagnosis of EoE and acknowledges that a histologic response to PPI does not “rule in” GERD or “rule out” EoE. It is important to emphasize that the updated criteria still advocate careful consideration of secondary causes of esophageal eosinophilia prior to the diagnosis of EoE.
Ramifications of the updated diagnostic criteria include the opportunities for clinicians to consider use of topical corticosteroids and diet therapies, rather than mandate an up-front PPI trial, in patients with EoE. On a practical level, based on their effectiveness, safety, and ease of administration, PPIs remain positioned as a favorable initial intervention for EoE. Conceptually, however, the paradigm shift highlights the ability of research to improve our understanding of disease pathogenesis and thereby impact clinical management.
Ikuo Hirano, MD, AGAF, is in the division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804), which is part of the Rare Disease Clinical Research Network. He has received research funding and consulting fees from Celgene, Regeneron, Shire, and others.
The diagnosis of eosinophilic esophagitis no longer needs to include a trial of proton pump inhibitor (PPI) therapy, according to an updated international consensus statement published in the October issue of Gastroenterology.
“An initial rationale for the PPI trial was to distinguish eosinophilic esophagitis from gastroesophageal reflux disease, but it is now known that these conditions have a complex relationship and are not necessarily mutually exclusive,” wrote Evan S. Dellon, MD, of the University of North Carolina at Chapel Hill, and his associates. According to current evidence, “PPIs are better classified as a treatment for esophageal eosinophilia that may be due to eosinophilic esophagitis than as a diagnostic criterion,” they said.
Diagnostic guidelines for eosinophilic esophagitis were published first in 2007 and were updated in 2011. The guideline authors recommended either pH monitoring or an 8-week trial of high-dose PPI therapy to rule out inflammation from gastroesophageal reflux disease (GERD).
But subsequent publications described patients with symptomatic esophageal eosinophilia who responded to PPIs and lacked classic GERD symptoms. Guidelines called this condition “PPI-responsive esophageal eosinophilia” and considered it a separate entity from GERD.
However, an “evolving body of research” shows that eosinophilic esophagitis can overlap with GERD, Dr. Dellon and his associates wrote. Furthermore, each of these conditions can trigger the other. Eosinophilic esophagitis can decrease esophageal compliance, leading to secondary reflux, while gastroesophageal reflux can erode the esophageal epithelium, triggering antigen exposure and eosinophilia.
Therefore, Dr. Dellon and his associates recommended defining eosinophilic esophagitis as signs and symptoms of esophageal dysfunction and an esophageal biopsy showing at least 15 eosinophils per high-power field, or approximately 60 eosinophils per millimeter, with infiltration limited to the esophagus. They stressed the importance of esophageal biopsy even if endoscopy shows normal mucosa. “As per prior guidelines, multiple biopsies from two or more esophageal levels, targeting areas of apparent inflammation, are recommended to increase the diagnostic yield,” they added. “Gastric and duodenal biopsies should be obtained as clinically indicated by symptoms, endoscopic findings in the stomach or duodenum, or high index of suspicion for a mucosal process.”
Physicians should increase their suspicion of eosinophilic esophagitis if patients have other types of atopy or endoscopic findings of “rings, furrows, exudates, edema, stricture, narrowing, and crepe-paper mucosa,” they added. In addition to GERD, they recommended looking carefully for other conditions that can trigger esophageal eosinophilia, such as pemphigus, drug hypersensitivity reactions, achalasia, and Crohn’s disease with esophageal involvement.
To create the guideline, Dr. Dellon and his associates searched PubMed for studies of all designs and sizes published from 1966 through December 2016. Teams of experts on specific topics then reviewed and discussed relevant literature. In May 2017, 43 reviewers met for 8 hours to present and discuss conclusions. There was 100% agreement to remove the PPI trial from the diagnostic criteria, the experts noted.
The authors disclosed financial support from the International Gastrointestinal Eosinophilic Diseases Researchers (TIGERS), The David and Denise Bunning Family, and the Rare Disease Clinical Research Network. Dr. Dellon disclosed consulting relationships and receiving research funding from Adare, Celgene/Receptos, Regeneron, and Shire among others. The majority of his coauthors also disclosed relationships with numerous medical companies.
SOURCE: Dellon ES et al. Gastroenterology. 2018 Jul 12. doi: 10.1053/j.gastro.2018.07.009.
The diagnosis of eosinophilic esophagitis no longer needs to include a trial of proton pump inhibitor (PPI) therapy, according to an updated international consensus statement published in the October issue of Gastroenterology.
“An initial rationale for the PPI trial was to distinguish eosinophilic esophagitis from gastroesophageal reflux disease, but it is now known that these conditions have a complex relationship and are not necessarily mutually exclusive,” wrote Evan S. Dellon, MD, of the University of North Carolina at Chapel Hill, and his associates. According to current evidence, “PPIs are better classified as a treatment for esophageal eosinophilia that may be due to eosinophilic esophagitis than as a diagnostic criterion,” they said.
Diagnostic guidelines for eosinophilic esophagitis were published first in 2007 and were updated in 2011. The guideline authors recommended either pH monitoring or an 8-week trial of high-dose PPI therapy to rule out inflammation from gastroesophageal reflux disease (GERD).
But subsequent publications described patients with symptomatic esophageal eosinophilia who responded to PPIs and lacked classic GERD symptoms. Guidelines called this condition “PPI-responsive esophageal eosinophilia” and considered it a separate entity from GERD.
However, an “evolving body of research” shows that eosinophilic esophagitis can overlap with GERD, Dr. Dellon and his associates wrote. Furthermore, each of these conditions can trigger the other. Eosinophilic esophagitis can decrease esophageal compliance, leading to secondary reflux, while gastroesophageal reflux can erode the esophageal epithelium, triggering antigen exposure and eosinophilia.
Therefore, Dr. Dellon and his associates recommended defining eosinophilic esophagitis as signs and symptoms of esophageal dysfunction and an esophageal biopsy showing at least 15 eosinophils per high-power field, or approximately 60 eosinophils per millimeter, with infiltration limited to the esophagus. They stressed the importance of esophageal biopsy even if endoscopy shows normal mucosa. “As per prior guidelines, multiple biopsies from two or more esophageal levels, targeting areas of apparent inflammation, are recommended to increase the diagnostic yield,” they added. “Gastric and duodenal biopsies should be obtained as clinically indicated by symptoms, endoscopic findings in the stomach or duodenum, or high index of suspicion for a mucosal process.”
Physicians should increase their suspicion of eosinophilic esophagitis if patients have other types of atopy or endoscopic findings of “rings, furrows, exudates, edema, stricture, narrowing, and crepe-paper mucosa,” they added. In addition to GERD, they recommended looking carefully for other conditions that can trigger esophageal eosinophilia, such as pemphigus, drug hypersensitivity reactions, achalasia, and Crohn’s disease with esophageal involvement.
To create the guideline, Dr. Dellon and his associates searched PubMed for studies of all designs and sizes published from 1966 through December 2016. Teams of experts on specific topics then reviewed and discussed relevant literature. In May 2017, 43 reviewers met for 8 hours to present and discuss conclusions. There was 100% agreement to remove the PPI trial from the diagnostic criteria, the experts noted.
The authors disclosed financial support from the International Gastrointestinal Eosinophilic Diseases Researchers (TIGERS), The David and Denise Bunning Family, and the Rare Disease Clinical Research Network. Dr. Dellon disclosed consulting relationships and receiving research funding from Adare, Celgene/Receptos, Regeneron, and Shire among others. The majority of his coauthors also disclosed relationships with numerous medical companies.
SOURCE: Dellon ES et al. Gastroenterology. 2018 Jul 12. doi: 10.1053/j.gastro.2018.07.009.
FROM GASTROENTEROLOGY
Key clinical point: The diagnosis of eosinophilic esophagitis no longer needs to include a trial of proton pump inhibitor therapy.
Major finding: Eosinophilic esophagitis and gastroesophageal reflux disease are not mutually exclusive.
Study details: Review by an international consensus panel of studies published between 1966 and 2016.
Disclosures: The authors disclosed financial support from the International Gastrointestinal Eosinophilic Diseases Researchers (TIGERS), The David and Denise Bunning Family, the Rare Disease Clinical Research Network. Dr. Dellon disclosed consulting relationships with Adare, Allakos, Alivio, Banner, Celgene/Receptos, Enumeral, GSK, Regeneron, and Shire. He also reported receiving research funding from Adare, Celgene/Receptos, Miraca, Meritage, Nutricia, Regeneron, and Shire and educational grants from Banner and Holoclara. The majority of his coauthors disclosed relationships with numerous medical companies.
Source: Dellon ES et al. Gastroenterology. 2018 Jul 12. doi: 10.1053/j.gastro.2018.07.009.
GERD patients who fail PPI often have functional heartburn or hypersensitivity
Abnormal pH results were similar in patients with gastroesophageal reflux disease (GERD) who improved or failed to improve on a once-daily dose of a proton pump inhibitor (PPI), but 75% of patients who failed treatment demonstrated either functional heartburn or reflux hypersensitivity, based on data from 29 adults.
Previous research on PPI failure in GERD patients has focused on twice-daily doses; “the purpose of the study was to compare impedance-pH parameters between patients who failed versus those who responded to PPI once daily,” wrote Jason Abdallah, MD, of Case Western Reserve University in Cleveland and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the investigators reviewed data from adults diagnosed with GERD who were treated with PPI therapy. The 16 who reported heartburn and/or regurgitation at least twice a week for 3 months while on a standard, once-daily PPI dose were classified as the failure group. The 13 patients who reported complete symptom resolution for at least 4 weeks while on the same standard dose were classified as the success group.
Most of the patients in the PPI-failure group (75%) were found to have either functional heartburn or reflux hypersensitivity with GERD. Impedance and pH parameters did not differ significantly between the PPI-failure and -success group, the researchers noted. Abnormal pH test results were similar between the groups, occurring in four of the patients who were successfully treated with PPI (31%) and four of the patients who failed PPI treatment (25%).
All patients completed the Short-Form 36 (SF-36) and GERD Health-Related Quality of Life (GERD-HRQL) questionnaires, and all underwent upper endoscopy and combined 24-hour esophageal impedance and pH monitoring within 2-4 weeks of study enrollment and while following their PPI treatment plans. There were no significant differences in demographic characteristics between the success and failure groups; the mean ages were 55 years and 47 years, respectively.
The patients in the success group averaged higher scores on the SF-36 than the failure group, but the difference was not significant. On the GERD-HRQL, treatment-failure patients reported that overall heartburn and either heartburn or bloating while lying down were the symptoms they found most annoying on a daily basis.
Among the treatment-failure patients, 10 (62%) had normal acid exposure and negative symptom-reflux association, 2 patients (13%) had normal acid exposure and positive symptom-reflux association, and 4 patients (25%) had abnormal esophageal acid exposure. Patients in the treatment failure group reported a total of 315 episodes of either heartburn or regurgitation.
Endoscopy findings were normal in most of the patients in both groups; 81% of the treatment-failure patients and 69% of the treatment-success patients had normal upper endoscopy findings. Abnormal findings in the treatment-success group included one case of erosive esophagitis, two cases of Barrett’s esophagus, three cases of nonobstructive Schatzki rings, and five cases of hiatal hernia. Abnormal findings in the treatment-failure group included two cases of Schatzki rings, one case of esophageal stricture, and three cases of hiatal hernia.
The total number of reflux events was similar between the groups; 1,279 in the treatment-failure group and 1,099 in the treatment-success group, with the number of reflux events per patient averaging 80 and 84, respectively.
“Our results support the hypothesis that PPI failure is primarily driven by esophageal hypersensitivity,” the researchers noted. The similarity in impedance and reflux “implies that the shift to nonacidic reflux is a general PPI phenomenon, as opposed to being unique to PPI-failure patients,” they said.
The study was limited by the small patient population, but the results provide some insight into refractory GERD and suggest that patients who fail to respond to once-daily PPI might benefit from a neuromodulator, as well as psychological interventions including cognitive-behavioral therapy, hypnotherapy, relaxation techniques, mindfulness, and biofeedback, the researchers concluded.
Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
SOURCE: Abdallah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
Abnormal pH results were similar in patients with gastroesophageal reflux disease (GERD) who improved or failed to improve on a once-daily dose of a proton pump inhibitor (PPI), but 75% of patients who failed treatment demonstrated either functional heartburn or reflux hypersensitivity, based on data from 29 adults.
Previous research on PPI failure in GERD patients has focused on twice-daily doses; “the purpose of the study was to compare impedance-pH parameters between patients who failed versus those who responded to PPI once daily,” wrote Jason Abdallah, MD, of Case Western Reserve University in Cleveland and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the investigators reviewed data from adults diagnosed with GERD who were treated with PPI therapy. The 16 who reported heartburn and/or regurgitation at least twice a week for 3 months while on a standard, once-daily PPI dose were classified as the failure group. The 13 patients who reported complete symptom resolution for at least 4 weeks while on the same standard dose were classified as the success group.
Most of the patients in the PPI-failure group (75%) were found to have either functional heartburn or reflux hypersensitivity with GERD. Impedance and pH parameters did not differ significantly between the PPI-failure and -success group, the researchers noted. Abnormal pH test results were similar between the groups, occurring in four of the patients who were successfully treated with PPI (31%) and four of the patients who failed PPI treatment (25%).
All patients completed the Short-Form 36 (SF-36) and GERD Health-Related Quality of Life (GERD-HRQL) questionnaires, and all underwent upper endoscopy and combined 24-hour esophageal impedance and pH monitoring within 2-4 weeks of study enrollment and while following their PPI treatment plans. There were no significant differences in demographic characteristics between the success and failure groups; the mean ages were 55 years and 47 years, respectively.
The patients in the success group averaged higher scores on the SF-36 than the failure group, but the difference was not significant. On the GERD-HRQL, treatment-failure patients reported that overall heartburn and either heartburn or bloating while lying down were the symptoms they found most annoying on a daily basis.
Among the treatment-failure patients, 10 (62%) had normal acid exposure and negative symptom-reflux association, 2 patients (13%) had normal acid exposure and positive symptom-reflux association, and 4 patients (25%) had abnormal esophageal acid exposure. Patients in the treatment failure group reported a total of 315 episodes of either heartburn or regurgitation.
Endoscopy findings were normal in most of the patients in both groups; 81% of the treatment-failure patients and 69% of the treatment-success patients had normal upper endoscopy findings. Abnormal findings in the treatment-success group included one case of erosive esophagitis, two cases of Barrett’s esophagus, three cases of nonobstructive Schatzki rings, and five cases of hiatal hernia. Abnormal findings in the treatment-failure group included two cases of Schatzki rings, one case of esophageal stricture, and three cases of hiatal hernia.
The total number of reflux events was similar between the groups; 1,279 in the treatment-failure group and 1,099 in the treatment-success group, with the number of reflux events per patient averaging 80 and 84, respectively.
“Our results support the hypothesis that PPI failure is primarily driven by esophageal hypersensitivity,” the researchers noted. The similarity in impedance and reflux “implies that the shift to nonacidic reflux is a general PPI phenomenon, as opposed to being unique to PPI-failure patients,” they said.
The study was limited by the small patient population, but the results provide some insight into refractory GERD and suggest that patients who fail to respond to once-daily PPI might benefit from a neuromodulator, as well as psychological interventions including cognitive-behavioral therapy, hypnotherapy, relaxation techniques, mindfulness, and biofeedback, the researchers concluded.
Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
SOURCE: Abdallah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
Abnormal pH results were similar in patients with gastroesophageal reflux disease (GERD) who improved or failed to improve on a once-daily dose of a proton pump inhibitor (PPI), but 75% of patients who failed treatment demonstrated either functional heartburn or reflux hypersensitivity, based on data from 29 adults.
Previous research on PPI failure in GERD patients has focused on twice-daily doses; “the purpose of the study was to compare impedance-pH parameters between patients who failed versus those who responded to PPI once daily,” wrote Jason Abdallah, MD, of Case Western Reserve University in Cleveland and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the investigators reviewed data from adults diagnosed with GERD who were treated with PPI therapy. The 16 who reported heartburn and/or regurgitation at least twice a week for 3 months while on a standard, once-daily PPI dose were classified as the failure group. The 13 patients who reported complete symptom resolution for at least 4 weeks while on the same standard dose were classified as the success group.
Most of the patients in the PPI-failure group (75%) were found to have either functional heartburn or reflux hypersensitivity with GERD. Impedance and pH parameters did not differ significantly between the PPI-failure and -success group, the researchers noted. Abnormal pH test results were similar between the groups, occurring in four of the patients who were successfully treated with PPI (31%) and four of the patients who failed PPI treatment (25%).
All patients completed the Short-Form 36 (SF-36) and GERD Health-Related Quality of Life (GERD-HRQL) questionnaires, and all underwent upper endoscopy and combined 24-hour esophageal impedance and pH monitoring within 2-4 weeks of study enrollment and while following their PPI treatment plans. There were no significant differences in demographic characteristics between the success and failure groups; the mean ages were 55 years and 47 years, respectively.
The patients in the success group averaged higher scores on the SF-36 than the failure group, but the difference was not significant. On the GERD-HRQL, treatment-failure patients reported that overall heartburn and either heartburn or bloating while lying down were the symptoms they found most annoying on a daily basis.
Among the treatment-failure patients, 10 (62%) had normal acid exposure and negative symptom-reflux association, 2 patients (13%) had normal acid exposure and positive symptom-reflux association, and 4 patients (25%) had abnormal esophageal acid exposure. Patients in the treatment failure group reported a total of 315 episodes of either heartburn or regurgitation.
Endoscopy findings were normal in most of the patients in both groups; 81% of the treatment-failure patients and 69% of the treatment-success patients had normal upper endoscopy findings. Abnormal findings in the treatment-success group included one case of erosive esophagitis, two cases of Barrett’s esophagus, three cases of nonobstructive Schatzki rings, and five cases of hiatal hernia. Abnormal findings in the treatment-failure group included two cases of Schatzki rings, one case of esophageal stricture, and three cases of hiatal hernia.
The total number of reflux events was similar between the groups; 1,279 in the treatment-failure group and 1,099 in the treatment-success group, with the number of reflux events per patient averaging 80 and 84, respectively.
“Our results support the hypothesis that PPI failure is primarily driven by esophageal hypersensitivity,” the researchers noted. The similarity in impedance and reflux “implies that the shift to nonacidic reflux is a general PPI phenomenon, as opposed to being unique to PPI-failure patients,” they said.
The study was limited by the small patient population, but the results provide some insight into refractory GERD and suggest that patients who fail to respond to once-daily PPI might benefit from a neuromodulator, as well as psychological interventions including cognitive-behavioral therapy, hypnotherapy, relaxation techniques, mindfulness, and biofeedback, the researchers concluded.
Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
SOURCE: Abdallah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: PPI failure in GERD patients appears to be driven by esophageal hypersensitivity, not significantly associated with reflux.
Major finding: Most (75%) of the patients who failed PPI treatment had heartburn or reflux hypersensitivity with GERD.
Study details: The data come from a prospective cohort study of 29 adults with GERD.
Disclosures: Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
Source: Abdullah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
POEM effective for more than achalasia
.
The procedure was clinically successful and relieved chest pain in most patients, reported Mouen A. Khashab, MD, director of therapeutic endoscopy at Johns Hopkins Hospital in Baltimore.
POEM was introduced in 2008 as a less invasive alternative to laparoscopic Heller myotomy. During the procedure, submucosal tunneling is performed through the lower esophageal sphincter to the gastric cardia, thereby weakening the lower esophageal sphincter to allow passage of food.
POEM is clinically successful in 80%-90% of patients with achalasia. Although the procedure is regarded as safe and effective for achalasia, it has not been thoroughly researched for treatment of other esophageal motility disorders, including junction outflow obstruction (EGJOO), jackhammer esophagus (JE), or esophagogastric distal esophageal spasm (DES). EGJOO is similar to achalasia, but with peristalsis and a mean integrated relaxation pressure (IRP) greater than 15 mm Hg. Both JE and DES are spastic esophageal disorders. Patients with JE exhibit extreme esophageal hypercontractility, whereas patients with DES have a normal mean IRP and at least 20% premature contractions.
“The role POEM plays in management of these disorders is not clear, mainly due to scarcity of studies on this topic,” the authors wrote in Endoscopy International Open. “A previous multicenter study investigated the role of POEM in 73 patients with spastic esophageal disorders. However, the vast majority of patients (n = 54) in that study had type III (spastic) achalasia.” Since therapies such as botulinum toxin injections and calcium channel blockers are ineffective for many patients with nonachalasia esophageal motility disorders, “POEM is potentially an ideal treatment.”
The international, multicenter study involved 11 treatment centers and 50 patients. Patients with JE (n = 18), EGJOO (n = 15), and DES (n = 17) were included, each diagnosed according to the Chicago classification of esophageal motility disorders. Patients with type III achalasia were excluded.
Outcomes included technical success (completion of myotomy) and clinical success (Eckardt score at least 3 and symptom improvement). Prior to the procedure, the mean Eckardt score was 6.9 and chest pain was reported by almost three-quarters of the patients (72%).
Technical success was achieved in all patients. Myotomy thickness varied between cases; approximately half had a selective inner circular myotomy (48%), slightly less had a full-thickness myotomy (44%), and several were undefined (8%). Mean esophageal myotomy length was 12.5 cm and mean gastric myotomy length was 2.5 cm. Mean procedure time was approximately 90 minutes. Median duration of hospital stay was 2 days.
Nine adverse events (AEs) occurred in 8 patients, including submucosal hematoma, aspiration pneumonia, inadvertent mucosotomy, postprocedure pain, esophageal leak, bleed, and symptomatic capno-thorax/peritoneum.
“Although AEs occurred in 18% of patients,” the authors noted, “55.6% were rated as mild and 44.4% as moderate with no severe events. Most AEs can be managed intraprocedurally.”
Median follow-up time was approximately 8 months, during which 42 patients (87.5%) achieved clinical success, with many dramatically improved; over half of the patients (52%) had Eckardt scores of 0 or 1. From the group of patients who had chest pain prior to the procedure, 87% had resolution of chest pain. Although reflux developed in almost a quarter of the patients (22.2%), this was successfully managed with proton pump inhibitors in all instances. Most patients (82.9%) who underwent postoperative manometry had resolution of preoperative abnormalities.
Subgroup analysis was also performed. Clinical success was achieved in 94.1% of patients with DES, 93.3% of patients with EGJOO, and 75.0% with JE. Collectively, the spastic disorders (DES/JE) had a lower numerical response than EGJOO. However, the authors noted that “the difference was not statistically significant (P = .41), likely a type II error due to the relatively small number of included patients.” In all subgroups, postprocedural mean Eckardt scores decreased to less than 2. Patients with EGJOO were most likely to achieve Eckardt scores of 0 or 1. AEs were similar between subgroups.
“Remarkably, chest pain improved in more than 85% of patients,” the authors wrote. “Chest pain is frequently the major presenting symptom in these disorders and is difficult to treat.”
“It is important to mention that a long esophageal myotomy is essential to ensure that proximal esophageal spasms are effectively covered and treated,” the authors wrote. “Mean length of esophageal myotomy in patients with DES and JE in the current study was about 14 cm, which is more than twice the length of a typical endoscopic or surgical myotomy performed in achalasia patients.”
Even with the need for an extended myotomy, “results from the current study along with published data suggest POEM as an effective technique” for nonachalasia esophageal motility disorders, the authors concluded.
Since retrospective studies are inherently limited by design, the authors encouraged randomized trials to clarify the primary role of POEM in the management of nonachalasia esophageal motility disorders.
The authors reported compensation from Olympus, Boston Scientific, and Cook Medical.
SOURCE: Khashab MA et al. Endosc Int Open. 2018 Aug 10. doi: 10.1055/a-0625-6288.
.
The procedure was clinically successful and relieved chest pain in most patients, reported Mouen A. Khashab, MD, director of therapeutic endoscopy at Johns Hopkins Hospital in Baltimore.
POEM was introduced in 2008 as a less invasive alternative to laparoscopic Heller myotomy. During the procedure, submucosal tunneling is performed through the lower esophageal sphincter to the gastric cardia, thereby weakening the lower esophageal sphincter to allow passage of food.
POEM is clinically successful in 80%-90% of patients with achalasia. Although the procedure is regarded as safe and effective for achalasia, it has not been thoroughly researched for treatment of other esophageal motility disorders, including junction outflow obstruction (EGJOO), jackhammer esophagus (JE), or esophagogastric distal esophageal spasm (DES). EGJOO is similar to achalasia, but with peristalsis and a mean integrated relaxation pressure (IRP) greater than 15 mm Hg. Both JE and DES are spastic esophageal disorders. Patients with JE exhibit extreme esophageal hypercontractility, whereas patients with DES have a normal mean IRP and at least 20% premature contractions.
“The role POEM plays in management of these disorders is not clear, mainly due to scarcity of studies on this topic,” the authors wrote in Endoscopy International Open. “A previous multicenter study investigated the role of POEM in 73 patients with spastic esophageal disorders. However, the vast majority of patients (n = 54) in that study had type III (spastic) achalasia.” Since therapies such as botulinum toxin injections and calcium channel blockers are ineffective for many patients with nonachalasia esophageal motility disorders, “POEM is potentially an ideal treatment.”
The international, multicenter study involved 11 treatment centers and 50 patients. Patients with JE (n = 18), EGJOO (n = 15), and DES (n = 17) were included, each diagnosed according to the Chicago classification of esophageal motility disorders. Patients with type III achalasia were excluded.
Outcomes included technical success (completion of myotomy) and clinical success (Eckardt score at least 3 and symptom improvement). Prior to the procedure, the mean Eckardt score was 6.9 and chest pain was reported by almost three-quarters of the patients (72%).
Technical success was achieved in all patients. Myotomy thickness varied between cases; approximately half had a selective inner circular myotomy (48%), slightly less had a full-thickness myotomy (44%), and several were undefined (8%). Mean esophageal myotomy length was 12.5 cm and mean gastric myotomy length was 2.5 cm. Mean procedure time was approximately 90 minutes. Median duration of hospital stay was 2 days.
Nine adverse events (AEs) occurred in 8 patients, including submucosal hematoma, aspiration pneumonia, inadvertent mucosotomy, postprocedure pain, esophageal leak, bleed, and symptomatic capno-thorax/peritoneum.
“Although AEs occurred in 18% of patients,” the authors noted, “55.6% were rated as mild and 44.4% as moderate with no severe events. Most AEs can be managed intraprocedurally.”
Median follow-up time was approximately 8 months, during which 42 patients (87.5%) achieved clinical success, with many dramatically improved; over half of the patients (52%) had Eckardt scores of 0 or 1. From the group of patients who had chest pain prior to the procedure, 87% had resolution of chest pain. Although reflux developed in almost a quarter of the patients (22.2%), this was successfully managed with proton pump inhibitors in all instances. Most patients (82.9%) who underwent postoperative manometry had resolution of preoperative abnormalities.
Subgroup analysis was also performed. Clinical success was achieved in 94.1% of patients with DES, 93.3% of patients with EGJOO, and 75.0% with JE. Collectively, the spastic disorders (DES/JE) had a lower numerical response than EGJOO. However, the authors noted that “the difference was not statistically significant (P = .41), likely a type II error due to the relatively small number of included patients.” In all subgroups, postprocedural mean Eckardt scores decreased to less than 2. Patients with EGJOO were most likely to achieve Eckardt scores of 0 or 1. AEs were similar between subgroups.
“Remarkably, chest pain improved in more than 85% of patients,” the authors wrote. “Chest pain is frequently the major presenting symptom in these disorders and is difficult to treat.”
“It is important to mention that a long esophageal myotomy is essential to ensure that proximal esophageal spasms are effectively covered and treated,” the authors wrote. “Mean length of esophageal myotomy in patients with DES and JE in the current study was about 14 cm, which is more than twice the length of a typical endoscopic or surgical myotomy performed in achalasia patients.”
Even with the need for an extended myotomy, “results from the current study along with published data suggest POEM as an effective technique” for nonachalasia esophageal motility disorders, the authors concluded.
Since retrospective studies are inherently limited by design, the authors encouraged randomized trials to clarify the primary role of POEM in the management of nonachalasia esophageal motility disorders.
The authors reported compensation from Olympus, Boston Scientific, and Cook Medical.
SOURCE: Khashab MA et al. Endosc Int Open. 2018 Aug 10. doi: 10.1055/a-0625-6288.
.
The procedure was clinically successful and relieved chest pain in most patients, reported Mouen A. Khashab, MD, director of therapeutic endoscopy at Johns Hopkins Hospital in Baltimore.
POEM was introduced in 2008 as a less invasive alternative to laparoscopic Heller myotomy. During the procedure, submucosal tunneling is performed through the lower esophageal sphincter to the gastric cardia, thereby weakening the lower esophageal sphincter to allow passage of food.
POEM is clinically successful in 80%-90% of patients with achalasia. Although the procedure is regarded as safe and effective for achalasia, it has not been thoroughly researched for treatment of other esophageal motility disorders, including junction outflow obstruction (EGJOO), jackhammer esophagus (JE), or esophagogastric distal esophageal spasm (DES). EGJOO is similar to achalasia, but with peristalsis and a mean integrated relaxation pressure (IRP) greater than 15 mm Hg. Both JE and DES are spastic esophageal disorders. Patients with JE exhibit extreme esophageal hypercontractility, whereas patients with DES have a normal mean IRP and at least 20% premature contractions.
“The role POEM plays in management of these disorders is not clear, mainly due to scarcity of studies on this topic,” the authors wrote in Endoscopy International Open. “A previous multicenter study investigated the role of POEM in 73 patients with spastic esophageal disorders. However, the vast majority of patients (n = 54) in that study had type III (spastic) achalasia.” Since therapies such as botulinum toxin injections and calcium channel blockers are ineffective for many patients with nonachalasia esophageal motility disorders, “POEM is potentially an ideal treatment.”
The international, multicenter study involved 11 treatment centers and 50 patients. Patients with JE (n = 18), EGJOO (n = 15), and DES (n = 17) were included, each diagnosed according to the Chicago classification of esophageal motility disorders. Patients with type III achalasia were excluded.
Outcomes included technical success (completion of myotomy) and clinical success (Eckardt score at least 3 and symptom improvement). Prior to the procedure, the mean Eckardt score was 6.9 and chest pain was reported by almost three-quarters of the patients (72%).
Technical success was achieved in all patients. Myotomy thickness varied between cases; approximately half had a selective inner circular myotomy (48%), slightly less had a full-thickness myotomy (44%), and several were undefined (8%). Mean esophageal myotomy length was 12.5 cm and mean gastric myotomy length was 2.5 cm. Mean procedure time was approximately 90 minutes. Median duration of hospital stay was 2 days.
Nine adverse events (AEs) occurred in 8 patients, including submucosal hematoma, aspiration pneumonia, inadvertent mucosotomy, postprocedure pain, esophageal leak, bleed, and symptomatic capno-thorax/peritoneum.
“Although AEs occurred in 18% of patients,” the authors noted, “55.6% were rated as mild and 44.4% as moderate with no severe events. Most AEs can be managed intraprocedurally.”
Median follow-up time was approximately 8 months, during which 42 patients (87.5%) achieved clinical success, with many dramatically improved; over half of the patients (52%) had Eckardt scores of 0 or 1. From the group of patients who had chest pain prior to the procedure, 87% had resolution of chest pain. Although reflux developed in almost a quarter of the patients (22.2%), this was successfully managed with proton pump inhibitors in all instances. Most patients (82.9%) who underwent postoperative manometry had resolution of preoperative abnormalities.
Subgroup analysis was also performed. Clinical success was achieved in 94.1% of patients with DES, 93.3% of patients with EGJOO, and 75.0% with JE. Collectively, the spastic disorders (DES/JE) had a lower numerical response than EGJOO. However, the authors noted that “the difference was not statistically significant (P = .41), likely a type II error due to the relatively small number of included patients.” In all subgroups, postprocedural mean Eckardt scores decreased to less than 2. Patients with EGJOO were most likely to achieve Eckardt scores of 0 or 1. AEs were similar between subgroups.
“Remarkably, chest pain improved in more than 85% of patients,” the authors wrote. “Chest pain is frequently the major presenting symptom in these disorders and is difficult to treat.”
“It is important to mention that a long esophageal myotomy is essential to ensure that proximal esophageal spasms are effectively covered and treated,” the authors wrote. “Mean length of esophageal myotomy in patients with DES and JE in the current study was about 14 cm, which is more than twice the length of a typical endoscopic or surgical myotomy performed in achalasia patients.”
Even with the need for an extended myotomy, “results from the current study along with published data suggest POEM as an effective technique” for nonachalasia esophageal motility disorders, the authors concluded.
Since retrospective studies are inherently limited by design, the authors encouraged randomized trials to clarify the primary role of POEM in the management of nonachalasia esophageal motility disorders.
The authors reported compensation from Olympus, Boston Scientific, and Cook Medical.
SOURCE: Khashab MA et al. Endosc Int Open. 2018 Aug 10. doi: 10.1055/a-0625-6288.
FROM ENDOSCOPY INTERNATIONAL OPEN
Key clinical point: Peroral endoscopic myotomy (POEM) is safe and effective for jackhammer esophagus (JE), esophagogastric junction outflow obstruction (EGJOO), and distal esophageal spasm (DES).
Major finding: POEM was clinically successful in approximately 90% of patients with nonachalasia esophageal motility disorders.
Study details: A retrospective, multicenter study involving 50 patients with nonachalasia esophageal motility disorders.
Disclosures: Authors reported compensation from Olympus, Boston Scientific, and Cook Medical.
Source: Khashab MA et al. Endosc Int Open. 2018 Aug 10. doi: 10.1055/a-0625-6288
Eosinophilic esophagitis: Faces and facets of a new disease
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Patient-reported outcomes in esophageal diseases
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).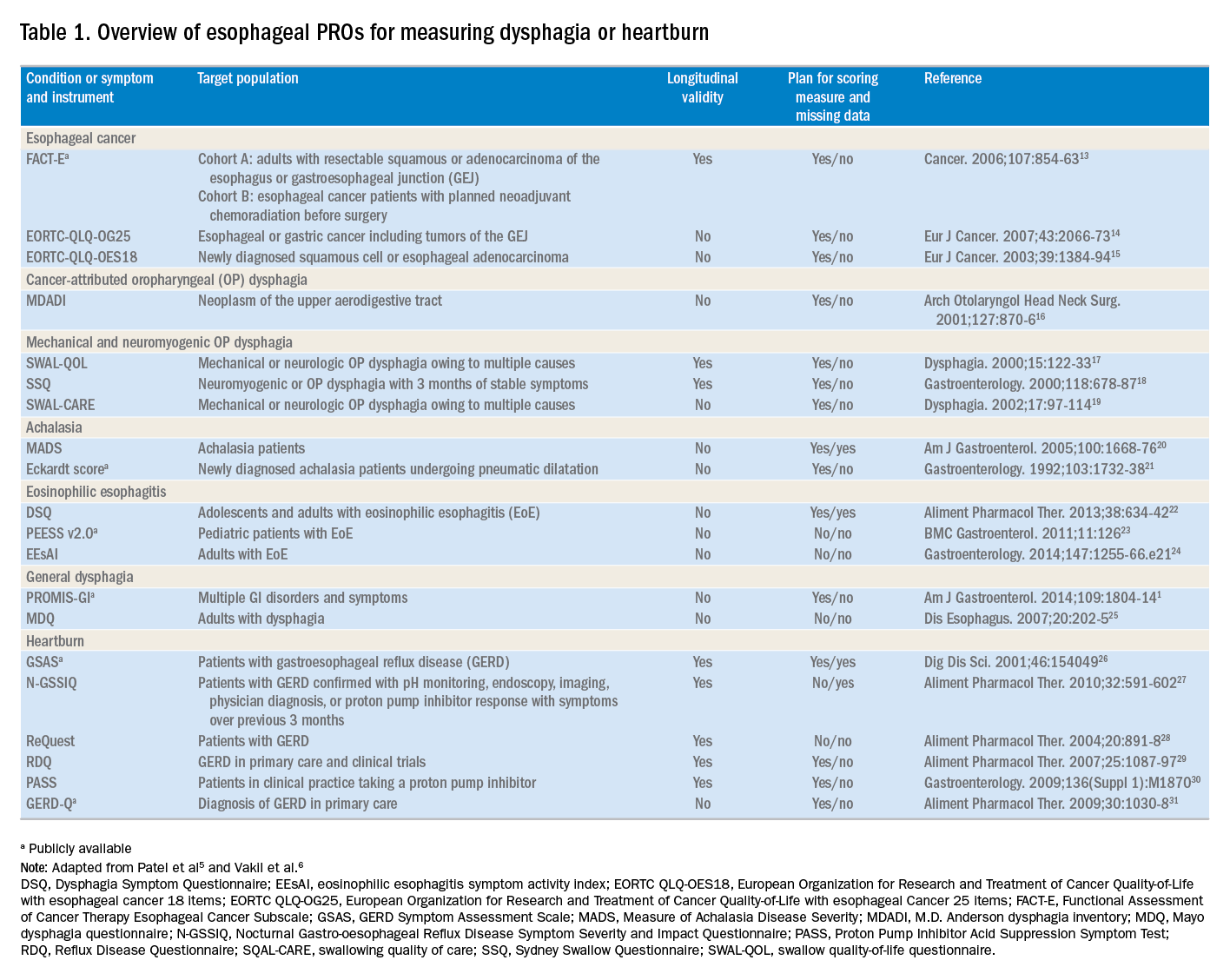
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
Study models surveillance interval after ablation of Barrett’s esophagus
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
FROM GASTROENTEROLOGY
Key clinical point: Baseline histologic grade was the most important predictor of recurrence after radiofrequency ablation of Barrett’s esophagus.
Major finding: The proposed surveillance intervals were 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually.
Study details: An analysis of data from the United States Radiofrequency Ablation Registry and the United Kingdom National Halo Registry.
Disclosures: The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
Source: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Upper GI tract
Rhonda Souza, MD, AGAF, started off this session with a review of eosinophilic esophagitis (EoE). She explained the challenges of the six-food elimination diet and described an alternative step-up elimination diet. In one study of the step-up elimination diet, food triggers were identified in 88% of patients during the reintroduction of food groups. Proton pump inhibitors are recommended for patients who do not respond to or refuse diet therapy; 30%-50% of patients with EoE will respond to the drugs. She explained that PPIs might have eosinophil-reducing effects independent of gastric acid–lowering effects, and said that PPIs should be stopped for 3-4 weeks before a diagnostic endoscopy is performed if EoE is suspected. Dr. Souza also spoke about topical steroids, biologic agents, and gradual esophageal dilation.
Ronnie Fass, MD, then addressed the management of patients with documented gastroesophageal reflux disease (GERD), or heartburn without documented GERD, who are unresponsive to PPIs. He referred to the management algorithm that he and Prakash Gyawali, MD, MRCP, published this year (Gastroenterology 2018;154:302-18), and described the optimization of PPI therapy before doubling the dose, as well as the testing that should be done if the dose increase does not relieve symptoms. Dr. Fass showed that there are various possible mechanisms for refractory GERD or heartburn, including weakly acidic or alkaline reflux, functional heartburn, and reflux hypersensitivity. He reviewed the Rome IV diagnostic criteria and treatment for the latter two conditions, and discussed the role of esophageal manometry to exclude esophageal motor disorders in patients with refractory GERD and heartburn.
Colin Howden, MD, AGAF, presented data on the risks and benefits of PPIs. He reviewed the Hill criteria to prove causation and methodically reviewed whether these criteria applied to various reported risks of PPIs, from C. difficile infection and bacterial gastroenteritis to kidney disease and interference with calcium absorption. He concluded that the absolute risks are low, that most data are retrospective and prone to bias, and that causality has generally not been demonstrated. Benefit usually outweighs risk if there is a valid indication for PPI use, he said, but the lowest effective dose should be used.
Jan Tack, MD, PhD, then reviewed functional dyspepsia (FD). He described the Rome IV criteria for FD and the two main subtypes of epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS), noting that some patients have both. Dr. Tack then discussed the pathophysiologic mechanisms and treatment approaches. For EPS, he recommended a trial of PPIs, then tricyclic antidepressants, and for refractory cases, behavioral therapy. For PDS, he recommended a prokinetic agent if available or acid suppression. If no response, then a 5HT1A agonist such as buspirone can be used for early satiety or mirtazapine for those with weight loss. In refractory cases, he said, a prokinetic agent such as prucalopride can be considered if there is delayed gastric emptying.
Baharak Moshiree, MD, AGAF, discussed the causes, diagnosis, and treatment of chronic nausea. Gastrointestinal causes include FD, gastroparesis, irritable bowel syndrome, celiac disease, and small intestinal bacterial overgrowth. Dr. Moshiree outlined the Rome IV criteria for chronic nausea and vomiting syndrome, cyclic vomiting syndrome, and cannabinoid hyperemesis syndrome. She also described the overlap between FD and gastroparesis and noted that nausea is a common symptom of both. Various tests can be used to rule out structural GI causes and to measure motility and gastric accommodation. In addition to treatment such as antiemetics, prokinetic agents, and neuromodulators, Dr. Moshiree examined the evidence of emerging therapies such as aprepitant, an NK1 antagonist, for gastroparesis or unexplained chronic nausea and vomiting.
Barham Abu Dayyeh, MD, MPH, addressed endoscopic management of patients after bariatric surgery. He showed that hemorrhage or marginal ulcers postsurgery can be treated with PPIs, hemoclips for bleeding ulcers, and endoscopic suturing or surgery for recalcitrant ulcers. He also discussed the management of a stenosis after Roux-en-Y gastric bypass, which now includes lumen-opposing stents, as well as the management of leaks from sleeve gastrectomy and gastric bypass and the management of biliary complications. Lastly, he reviewed the modifiable risk factors for weight regain after Roux-en-Y gastric bypass, such as gastrogastric fistula and gastrojejunal stoma dilation, and how they can be endoscopically managed.
Dr. Chang is vice-chief, Vatche and Tamar Manoukian division of digestive diseases, program director, UCLA GI fellowship program, codirector, G. Oppenheimer Center for Neurobiology of Stress and Resilience, and professor of medicine at the David Geffen School of Medicine at UCLA. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2018. She is on the advisory board for Synergy, IM HealthSciences, and Salix; an adviser for Metameconnect.com and ModifyHealth; and a speaker for Allergan and Takeda.
Rhonda Souza, MD, AGAF, started off this session with a review of eosinophilic esophagitis (EoE). She explained the challenges of the six-food elimination diet and described an alternative step-up elimination diet. In one study of the step-up elimination diet, food triggers were identified in 88% of patients during the reintroduction of food groups. Proton pump inhibitors are recommended for patients who do not respond to or refuse diet therapy; 30%-50% of patients with EoE will respond to the drugs. She explained that PPIs might have eosinophil-reducing effects independent of gastric acid–lowering effects, and said that PPIs should be stopped for 3-4 weeks before a diagnostic endoscopy is performed if EoE is suspected. Dr. Souza also spoke about topical steroids, biologic agents, and gradual esophageal dilation.
Ronnie Fass, MD, then addressed the management of patients with documented gastroesophageal reflux disease (GERD), or heartburn without documented GERD, who are unresponsive to PPIs. He referred to the management algorithm that he and Prakash Gyawali, MD, MRCP, published this year (Gastroenterology 2018;154:302-18), and described the optimization of PPI therapy before doubling the dose, as well as the testing that should be done if the dose increase does not relieve symptoms. Dr. Fass showed that there are various possible mechanisms for refractory GERD or heartburn, including weakly acidic or alkaline reflux, functional heartburn, and reflux hypersensitivity. He reviewed the Rome IV diagnostic criteria and treatment for the latter two conditions, and discussed the role of esophageal manometry to exclude esophageal motor disorders in patients with refractory GERD and heartburn.
Colin Howden, MD, AGAF, presented data on the risks and benefits of PPIs. He reviewed the Hill criteria to prove causation and methodically reviewed whether these criteria applied to various reported risks of PPIs, from C. difficile infection and bacterial gastroenteritis to kidney disease and interference with calcium absorption. He concluded that the absolute risks are low, that most data are retrospective and prone to bias, and that causality has generally not been demonstrated. Benefit usually outweighs risk if there is a valid indication for PPI use, he said, but the lowest effective dose should be used.
Jan Tack, MD, PhD, then reviewed functional dyspepsia (FD). He described the Rome IV criteria for FD and the two main subtypes of epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS), noting that some patients have both. Dr. Tack then discussed the pathophysiologic mechanisms and treatment approaches. For EPS, he recommended a trial of PPIs, then tricyclic antidepressants, and for refractory cases, behavioral therapy. For PDS, he recommended a prokinetic agent if available or acid suppression. If no response, then a 5HT1A agonist such as buspirone can be used for early satiety or mirtazapine for those with weight loss. In refractory cases, he said, a prokinetic agent such as prucalopride can be considered if there is delayed gastric emptying.
Baharak Moshiree, MD, AGAF, discussed the causes, diagnosis, and treatment of chronic nausea. Gastrointestinal causes include FD, gastroparesis, irritable bowel syndrome, celiac disease, and small intestinal bacterial overgrowth. Dr. Moshiree outlined the Rome IV criteria for chronic nausea and vomiting syndrome, cyclic vomiting syndrome, and cannabinoid hyperemesis syndrome. She also described the overlap between FD and gastroparesis and noted that nausea is a common symptom of both. Various tests can be used to rule out structural GI causes and to measure motility and gastric accommodation. In addition to treatment such as antiemetics, prokinetic agents, and neuromodulators, Dr. Moshiree examined the evidence of emerging therapies such as aprepitant, an NK1 antagonist, for gastroparesis or unexplained chronic nausea and vomiting.
Barham Abu Dayyeh, MD, MPH, addressed endoscopic management of patients after bariatric surgery. He showed that hemorrhage or marginal ulcers postsurgery can be treated with PPIs, hemoclips for bleeding ulcers, and endoscopic suturing or surgery for recalcitrant ulcers. He also discussed the management of a stenosis after Roux-en-Y gastric bypass, which now includes lumen-opposing stents, as well as the management of leaks from sleeve gastrectomy and gastric bypass and the management of biliary complications. Lastly, he reviewed the modifiable risk factors for weight regain after Roux-en-Y gastric bypass, such as gastrogastric fistula and gastrojejunal stoma dilation, and how they can be endoscopically managed.
Dr. Chang is vice-chief, Vatche and Tamar Manoukian division of digestive diseases, program director, UCLA GI fellowship program, codirector, G. Oppenheimer Center for Neurobiology of Stress and Resilience, and professor of medicine at the David Geffen School of Medicine at UCLA. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2018. She is on the advisory board for Synergy, IM HealthSciences, and Salix; an adviser for Metameconnect.com and ModifyHealth; and a speaker for Allergan and Takeda.
Rhonda Souza, MD, AGAF, started off this session with a review of eosinophilic esophagitis (EoE). She explained the challenges of the six-food elimination diet and described an alternative step-up elimination diet. In one study of the step-up elimination diet, food triggers were identified in 88% of patients during the reintroduction of food groups. Proton pump inhibitors are recommended for patients who do not respond to or refuse diet therapy; 30%-50% of patients with EoE will respond to the drugs. She explained that PPIs might have eosinophil-reducing effects independent of gastric acid–lowering effects, and said that PPIs should be stopped for 3-4 weeks before a diagnostic endoscopy is performed if EoE is suspected. Dr. Souza also spoke about topical steroids, biologic agents, and gradual esophageal dilation.
Ronnie Fass, MD, then addressed the management of patients with documented gastroesophageal reflux disease (GERD), or heartburn without documented GERD, who are unresponsive to PPIs. He referred to the management algorithm that he and Prakash Gyawali, MD, MRCP, published this year (Gastroenterology 2018;154:302-18), and described the optimization of PPI therapy before doubling the dose, as well as the testing that should be done if the dose increase does not relieve symptoms. Dr. Fass showed that there are various possible mechanisms for refractory GERD or heartburn, including weakly acidic or alkaline reflux, functional heartburn, and reflux hypersensitivity. He reviewed the Rome IV diagnostic criteria and treatment for the latter two conditions, and discussed the role of esophageal manometry to exclude esophageal motor disorders in patients with refractory GERD and heartburn.
Colin Howden, MD, AGAF, presented data on the risks and benefits of PPIs. He reviewed the Hill criteria to prove causation and methodically reviewed whether these criteria applied to various reported risks of PPIs, from C. difficile infection and bacterial gastroenteritis to kidney disease and interference with calcium absorption. He concluded that the absolute risks are low, that most data are retrospective and prone to bias, and that causality has generally not been demonstrated. Benefit usually outweighs risk if there is a valid indication for PPI use, he said, but the lowest effective dose should be used.
Jan Tack, MD, PhD, then reviewed functional dyspepsia (FD). He described the Rome IV criteria for FD and the two main subtypes of epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS), noting that some patients have both. Dr. Tack then discussed the pathophysiologic mechanisms and treatment approaches. For EPS, he recommended a trial of PPIs, then tricyclic antidepressants, and for refractory cases, behavioral therapy. For PDS, he recommended a prokinetic agent if available or acid suppression. If no response, then a 5HT1A agonist such as buspirone can be used for early satiety or mirtazapine for those with weight loss. In refractory cases, he said, a prokinetic agent such as prucalopride can be considered if there is delayed gastric emptying.
Baharak Moshiree, MD, AGAF, discussed the causes, diagnosis, and treatment of chronic nausea. Gastrointestinal causes include FD, gastroparesis, irritable bowel syndrome, celiac disease, and small intestinal bacterial overgrowth. Dr. Moshiree outlined the Rome IV criteria for chronic nausea and vomiting syndrome, cyclic vomiting syndrome, and cannabinoid hyperemesis syndrome. She also described the overlap between FD and gastroparesis and noted that nausea is a common symptom of both. Various tests can be used to rule out structural GI causes and to measure motility and gastric accommodation. In addition to treatment such as antiemetics, prokinetic agents, and neuromodulators, Dr. Moshiree examined the evidence of emerging therapies such as aprepitant, an NK1 antagonist, for gastroparesis or unexplained chronic nausea and vomiting.
Barham Abu Dayyeh, MD, MPH, addressed endoscopic management of patients after bariatric surgery. He showed that hemorrhage or marginal ulcers postsurgery can be treated with PPIs, hemoclips for bleeding ulcers, and endoscopic suturing or surgery for recalcitrant ulcers. He also discussed the management of a stenosis after Roux-en-Y gastric bypass, which now includes lumen-opposing stents, as well as the management of leaks from sleeve gastrectomy and gastric bypass and the management of biliary complications. Lastly, he reviewed the modifiable risk factors for weight regain after Roux-en-Y gastric bypass, such as gastrogastric fistula and gastrojejunal stoma dilation, and how they can be endoscopically managed.
Dr. Chang is vice-chief, Vatche and Tamar Manoukian division of digestive diseases, program director, UCLA GI fellowship program, codirector, G. Oppenheimer Center for Neurobiology of Stress and Resilience, and professor of medicine at the David Geffen School of Medicine at UCLA. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2018. She is on the advisory board for Synergy, IM HealthSciences, and Salix; an adviser for Metameconnect.com and ModifyHealth; and a speaker for Allergan and Takeda.