User login
Should patients undergoing surgical treatment for cervical lesions also receive an HPV vaccination?
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Researcher revisits ‘03 guidance on monkeypox in pregnant women
In creating a guide about monkeypox for ob.gyns., Denise J. Jamieson, MD, MPH, turned to research she relied on during another outbreak of the disease nearly 20 years ago.
Dr. Jamieson, the James Robert McCord Professor and chair of the department of gynecology and obstetrics at Emory Healthcare, Atlanta, had been working for the Centers for Disease Control and Prevention in 2003 when doctors diagnosed monkeypox in several states.
That year, the virus was mainly transmitted by contact with pet prairie dogs, including in childcare and school settings. Of the approximately 70 suspected and confirmed cases, 55% occurred in female patients, according to one study .
Dr. Jamieson, an obstetrician with a focus on emerging infectious diseases, and colleagues at the agency published a commentary in Obstetrics & Gynecology highlighting the need for physicians to stay up to date with relevant information about the virus.
Fast forward to 2022: Dr. Jamieson – again with coauthors from the CDC – is delivering a similar message in the same journal about the need for clinicians to be prepared for this virus.
“Most ob.gyns. have never seen a case of monkeypox virus infection and may not be aware of testing, treatment, or pre-exposure or postexposure vaccine options,” she and her coauthors wrote in a primer published online.
But if a woman were to contract the virus, her ob.gyn. might well be the first clinician she called. “We are often the first people, the first physicians to see and evaluate women with various symptoms,” Dr. Jamieson said.
To promptly diagnose, treat, and prevent further spread of monkeypox, ob.gyns. need up-to-date information, Dr. Jamieson and colleagues said.
Based on data from related viruses like smallpox, monkeypox may be more severe in pregnant women and entail risk for adverse pregnancy outcomes, Dr. Jamieson said.
Outliers
So far this year, monkeypox has predominantly spread among men who have sex with men. Cases have occurred in women, however, some of whom have required hospitalization.
According to the CDC, as of July 25, 1,373 cases of monkeypox in the United States were in men and 13 in women. The total confirmed case count exceeded 5,800 as of Aug. 1. The agency recently announced that it planned to make the disease a reportable condition.
In the United Kingdom, which has been hit hard by the outbreak, researchers are keeping a close eye on the number of cases in women to assess how the disease is spreading.
At least one case of monkeypox in the United States has occurred in a pregnant woman who delivered. The mother and baby, who received immune globulin as a preventive measure, are doing well, according to health officials.
“We know that infection can occur through placental transfer. In the case that we are aware of presently, it does not appear that the virus was transmitted,” said John T. Brooks, MD, the CDC’s chief medical officer in the division of HIV prevention, on a July 23 call with clinicians.
While monkeypox can be transmitted in utero and during sexual activity, it also can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC.
A preferred vaccine and antiviral in pregnancy
One monkeypox vaccine, Jynneos, is preferred for use during pregnancy, while another, ACAM2000, is contraindicated, the CDC advises.
Jynneos can be offered to people who are pregnant or breastfeeding who are eligible for vaccination based on confirmed or likely contact with cases, ideally within 4 days of exposure. People at high risk for exposure, such as laboratory workers, may receive the vaccine in advance.
Developmental toxicity studies in animals showed no evidence of harm with the Jynneos vaccine, Dr. Jamieson said.
ACAM2000, however, can cause fetal vaccinia and should not be used in people who are pregnant or breastfeeding, according to the CDC.
The Society for Maternal-Fetal Medicine notes that, if treatment for monkeypox is warranted, tecovirimat should be considered the first-line antiviral for pregnant, recently pregnant, and breastfeeding people, in line with CDC guidance.
Current outbreak ‘very different,’ but lessons apply
In 2003, some women exposed to monkeypox through contact with infected prairie dogs were pregnant – which is how Dr. Jamieson came to be involved in responding to the outbreak and studying the effects of the virus in pregnancy.
“When this resurfaced this year, of course it caught my attention,” Dr. Jamieson said. The extensive person-to-person transmission and far greater number of cases today make the current outbreak “very different” from the prior one, she said.
But key principles in managing the disease and understanding its potential risks in pregnancy – despite relatively limited information – remain the same.
“Whenever you are looking at an infectious disease, you want to think about, are pregnant persons more susceptible or more likely to have severe disease,” Dr. Jamieson said. Smallpox, a similar orthopoxvirus, “is more severe during pregnancy with a higher case fatality rate,” which is one reason for concern with monkeypox in this population.
In terms of pregnancy outcomes, researchers have data from only a handful of confirmed cases of monkeypox, which makes it difficult to draw conclusions, Dr. Jamieson said. A review of five cases from outside the United States in prior years found that three resulted in loss of the pregnancy. One resulted in preterm delivery of an infant who subsequently died. One child was apparently healthy and born at term.
Addition to the differential diagnosis
A separate team of researchers has proposed a clinical management algorithm for pregnant women with suspected exposure to monkeypox.
“Clinicians must maintain a high index of suspicion for monkeypox virus in any pregnant woman presenting with lymphadenopathy and vesiculopustular rash – including rash localized to the genital or perianal region – even if there are no apparent epidemiological links,” Pradip Dashraath, MBBS, National University Hospital, Singapore, and coauthors wrote in The Lancet.
Jamieson echoed the call for increased vigilance.
“As ob.gyns., people may present to us with genital lesions concerning for sexually transmitted infection. And it is important to include monkeypox in our differential,” Dr. Jamieson said. “We are trying to get the word out that it needs to be part of what you think about when you see a patient with genital ulcers.”
Health care professionals have acquired monkeypox through contact with patients or fomites, so clinicians should be sure to use appropriate precautions when evaluating patients who might have monkeypox, Dr. Jamieson added. Appropriate protective measures include wearing a gown, gloves, eye protection, and an N95.
A version of this article first appeared on Medscape.com.
In creating a guide about monkeypox for ob.gyns., Denise J. Jamieson, MD, MPH, turned to research she relied on during another outbreak of the disease nearly 20 years ago.
Dr. Jamieson, the James Robert McCord Professor and chair of the department of gynecology and obstetrics at Emory Healthcare, Atlanta, had been working for the Centers for Disease Control and Prevention in 2003 when doctors diagnosed monkeypox in several states.
That year, the virus was mainly transmitted by contact with pet prairie dogs, including in childcare and school settings. Of the approximately 70 suspected and confirmed cases, 55% occurred in female patients, according to one study .
Dr. Jamieson, an obstetrician with a focus on emerging infectious diseases, and colleagues at the agency published a commentary in Obstetrics & Gynecology highlighting the need for physicians to stay up to date with relevant information about the virus.
Fast forward to 2022: Dr. Jamieson – again with coauthors from the CDC – is delivering a similar message in the same journal about the need for clinicians to be prepared for this virus.
“Most ob.gyns. have never seen a case of monkeypox virus infection and may not be aware of testing, treatment, or pre-exposure or postexposure vaccine options,” she and her coauthors wrote in a primer published online.
But if a woman were to contract the virus, her ob.gyn. might well be the first clinician she called. “We are often the first people, the first physicians to see and evaluate women with various symptoms,” Dr. Jamieson said.
To promptly diagnose, treat, and prevent further spread of monkeypox, ob.gyns. need up-to-date information, Dr. Jamieson and colleagues said.
Based on data from related viruses like smallpox, monkeypox may be more severe in pregnant women and entail risk for adverse pregnancy outcomes, Dr. Jamieson said.
Outliers
So far this year, monkeypox has predominantly spread among men who have sex with men. Cases have occurred in women, however, some of whom have required hospitalization.
According to the CDC, as of July 25, 1,373 cases of monkeypox in the United States were in men and 13 in women. The total confirmed case count exceeded 5,800 as of Aug. 1. The agency recently announced that it planned to make the disease a reportable condition.
In the United Kingdom, which has been hit hard by the outbreak, researchers are keeping a close eye on the number of cases in women to assess how the disease is spreading.
At least one case of monkeypox in the United States has occurred in a pregnant woman who delivered. The mother and baby, who received immune globulin as a preventive measure, are doing well, according to health officials.
“We know that infection can occur through placental transfer. In the case that we are aware of presently, it does not appear that the virus was transmitted,” said John T. Brooks, MD, the CDC’s chief medical officer in the division of HIV prevention, on a July 23 call with clinicians.
While monkeypox can be transmitted in utero and during sexual activity, it also can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC.
A preferred vaccine and antiviral in pregnancy
One monkeypox vaccine, Jynneos, is preferred for use during pregnancy, while another, ACAM2000, is contraindicated, the CDC advises.
Jynneos can be offered to people who are pregnant or breastfeeding who are eligible for vaccination based on confirmed or likely contact with cases, ideally within 4 days of exposure. People at high risk for exposure, such as laboratory workers, may receive the vaccine in advance.
Developmental toxicity studies in animals showed no evidence of harm with the Jynneos vaccine, Dr. Jamieson said.
ACAM2000, however, can cause fetal vaccinia and should not be used in people who are pregnant or breastfeeding, according to the CDC.
The Society for Maternal-Fetal Medicine notes that, if treatment for monkeypox is warranted, tecovirimat should be considered the first-line antiviral for pregnant, recently pregnant, and breastfeeding people, in line with CDC guidance.
Current outbreak ‘very different,’ but lessons apply
In 2003, some women exposed to monkeypox through contact with infected prairie dogs were pregnant – which is how Dr. Jamieson came to be involved in responding to the outbreak and studying the effects of the virus in pregnancy.
“When this resurfaced this year, of course it caught my attention,” Dr. Jamieson said. The extensive person-to-person transmission and far greater number of cases today make the current outbreak “very different” from the prior one, she said.
But key principles in managing the disease and understanding its potential risks in pregnancy – despite relatively limited information – remain the same.
“Whenever you are looking at an infectious disease, you want to think about, are pregnant persons more susceptible or more likely to have severe disease,” Dr. Jamieson said. Smallpox, a similar orthopoxvirus, “is more severe during pregnancy with a higher case fatality rate,” which is one reason for concern with monkeypox in this population.
In terms of pregnancy outcomes, researchers have data from only a handful of confirmed cases of monkeypox, which makes it difficult to draw conclusions, Dr. Jamieson said. A review of five cases from outside the United States in prior years found that three resulted in loss of the pregnancy. One resulted in preterm delivery of an infant who subsequently died. One child was apparently healthy and born at term.
Addition to the differential diagnosis
A separate team of researchers has proposed a clinical management algorithm for pregnant women with suspected exposure to monkeypox.
“Clinicians must maintain a high index of suspicion for monkeypox virus in any pregnant woman presenting with lymphadenopathy and vesiculopustular rash – including rash localized to the genital or perianal region – even if there are no apparent epidemiological links,” Pradip Dashraath, MBBS, National University Hospital, Singapore, and coauthors wrote in The Lancet.
Jamieson echoed the call for increased vigilance.
“As ob.gyns., people may present to us with genital lesions concerning for sexually transmitted infection. And it is important to include monkeypox in our differential,” Dr. Jamieson said. “We are trying to get the word out that it needs to be part of what you think about when you see a patient with genital ulcers.”
Health care professionals have acquired monkeypox through contact with patients or fomites, so clinicians should be sure to use appropriate precautions when evaluating patients who might have monkeypox, Dr. Jamieson added. Appropriate protective measures include wearing a gown, gloves, eye protection, and an N95.
A version of this article first appeared on Medscape.com.
In creating a guide about monkeypox for ob.gyns., Denise J. Jamieson, MD, MPH, turned to research she relied on during another outbreak of the disease nearly 20 years ago.
Dr. Jamieson, the James Robert McCord Professor and chair of the department of gynecology and obstetrics at Emory Healthcare, Atlanta, had been working for the Centers for Disease Control and Prevention in 2003 when doctors diagnosed monkeypox in several states.
That year, the virus was mainly transmitted by contact with pet prairie dogs, including in childcare and school settings. Of the approximately 70 suspected and confirmed cases, 55% occurred in female patients, according to one study .
Dr. Jamieson, an obstetrician with a focus on emerging infectious diseases, and colleagues at the agency published a commentary in Obstetrics & Gynecology highlighting the need for physicians to stay up to date with relevant information about the virus.
Fast forward to 2022: Dr. Jamieson – again with coauthors from the CDC – is delivering a similar message in the same journal about the need for clinicians to be prepared for this virus.
“Most ob.gyns. have never seen a case of monkeypox virus infection and may not be aware of testing, treatment, or pre-exposure or postexposure vaccine options,” she and her coauthors wrote in a primer published online.
But if a woman were to contract the virus, her ob.gyn. might well be the first clinician she called. “We are often the first people, the first physicians to see and evaluate women with various symptoms,” Dr. Jamieson said.
To promptly diagnose, treat, and prevent further spread of monkeypox, ob.gyns. need up-to-date information, Dr. Jamieson and colleagues said.
Based on data from related viruses like smallpox, monkeypox may be more severe in pregnant women and entail risk for adverse pregnancy outcomes, Dr. Jamieson said.
Outliers
So far this year, monkeypox has predominantly spread among men who have sex with men. Cases have occurred in women, however, some of whom have required hospitalization.
According to the CDC, as of July 25, 1,373 cases of monkeypox in the United States were in men and 13 in women. The total confirmed case count exceeded 5,800 as of Aug. 1. The agency recently announced that it planned to make the disease a reportable condition.
In the United Kingdom, which has been hit hard by the outbreak, researchers are keeping a close eye on the number of cases in women to assess how the disease is spreading.
At least one case of monkeypox in the United States has occurred in a pregnant woman who delivered. The mother and baby, who received immune globulin as a preventive measure, are doing well, according to health officials.
“We know that infection can occur through placental transfer. In the case that we are aware of presently, it does not appear that the virus was transmitted,” said John T. Brooks, MD, the CDC’s chief medical officer in the division of HIV prevention, on a July 23 call with clinicians.
While monkeypox can be transmitted in utero and during sexual activity, it also can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC.
A preferred vaccine and antiviral in pregnancy
One monkeypox vaccine, Jynneos, is preferred for use during pregnancy, while another, ACAM2000, is contraindicated, the CDC advises.
Jynneos can be offered to people who are pregnant or breastfeeding who are eligible for vaccination based on confirmed or likely contact with cases, ideally within 4 days of exposure. People at high risk for exposure, such as laboratory workers, may receive the vaccine in advance.
Developmental toxicity studies in animals showed no evidence of harm with the Jynneos vaccine, Dr. Jamieson said.
ACAM2000, however, can cause fetal vaccinia and should not be used in people who are pregnant or breastfeeding, according to the CDC.
The Society for Maternal-Fetal Medicine notes that, if treatment for monkeypox is warranted, tecovirimat should be considered the first-line antiviral for pregnant, recently pregnant, and breastfeeding people, in line with CDC guidance.
Current outbreak ‘very different,’ but lessons apply
In 2003, some women exposed to monkeypox through contact with infected prairie dogs were pregnant – which is how Dr. Jamieson came to be involved in responding to the outbreak and studying the effects of the virus in pregnancy.
“When this resurfaced this year, of course it caught my attention,” Dr. Jamieson said. The extensive person-to-person transmission and far greater number of cases today make the current outbreak “very different” from the prior one, she said.
But key principles in managing the disease and understanding its potential risks in pregnancy – despite relatively limited information – remain the same.
“Whenever you are looking at an infectious disease, you want to think about, are pregnant persons more susceptible or more likely to have severe disease,” Dr. Jamieson said. Smallpox, a similar orthopoxvirus, “is more severe during pregnancy with a higher case fatality rate,” which is one reason for concern with monkeypox in this population.
In terms of pregnancy outcomes, researchers have data from only a handful of confirmed cases of monkeypox, which makes it difficult to draw conclusions, Dr. Jamieson said. A review of five cases from outside the United States in prior years found that three resulted in loss of the pregnancy. One resulted in preterm delivery of an infant who subsequently died. One child was apparently healthy and born at term.
Addition to the differential diagnosis
A separate team of researchers has proposed a clinical management algorithm for pregnant women with suspected exposure to monkeypox.
“Clinicians must maintain a high index of suspicion for monkeypox virus in any pregnant woman presenting with lymphadenopathy and vesiculopustular rash – including rash localized to the genital or perianal region – even if there are no apparent epidemiological links,” Pradip Dashraath, MBBS, National University Hospital, Singapore, and coauthors wrote in The Lancet.
Jamieson echoed the call for increased vigilance.
“As ob.gyns., people may present to us with genital lesions concerning for sexually transmitted infection. And it is important to include monkeypox in our differential,” Dr. Jamieson said. “We are trying to get the word out that it needs to be part of what you think about when you see a patient with genital ulcers.”
Health care professionals have acquired monkeypox through contact with patients or fomites, so clinicians should be sure to use appropriate precautions when evaluating patients who might have monkeypox, Dr. Jamieson added. Appropriate protective measures include wearing a gown, gloves, eye protection, and an N95.
A version of this article first appeared on Medscape.com.
FROM OBSTETRICS AND GYNECOLOGY
U.S. clears 786,000 monkeypox vaccine doses for distribution
More than 780,000 doses of the JYNNEOS monkeypox vaccine will be available in the United States beginning July 29, the Department of Health & Human Services announced on July 28 in a press call.
HHS Secretary Xavier Becerra urged local and state public health departments to use these doses for preventive vaccination efforts to stay ahead of the virus and end the outbreak, noting that the HHS and Centers for Disease Control and Prevention do not control how vaccines are distributed at state and local levels. “We don’t have the authority to tell them what to do,” he said during the call. “We need them to work with us.”
As of July 28, there were 4,907 reported cases of monkeypox in the United States and officials expect cases will continue to rise in the coming weeks.
The vaccine is manufactured by the small Danish company Bavarian Nordic. These additional 786,000 doses were previously stored at a plant in Denmark, awaiting the completion of an inspection and authorization of the vaccine plant by the Food and Drug Administration. The agency announced on July 27 that both the vaccine doses and the manufacturing plant met standards.
With the announcement of these additional doses, the vaccine allocation plan is also being updated to take into account two important factors: the number of people at high risk in a jurisdiction and the number of new cases reported since the last vaccine allocation.
“This update gives greater weight to prioritizing vaccines to areas with the greatest number of people at risk, which includes men who have sex with men who have HIV or who are eligible for HIV pre-exposure prophylaxis, while still considering where we are seeing cases increase,” said Capt. Jennifer McQuiston, DVM, deputy director of the division of high consequence pathogens and pathology at the CDC.
Capt.McQuiston also provided additional demographic information on the U.S. outbreak. The median age of people with confirmed cases is 35 years old, with a range from 17 to 76. (This does not include the two cases in children reported on July 22.) Of the cases where sex at birth was provided, 99% were individuals assigned male sex at birth. In cases with reported ethnicity and race, 37% were non-Hispanic White people, 31% were Hispanic/Latino, 27% were Black or African American, and 4% were of Asian descent. The most common symptoms were rash – present in 99% of cases – malaise, fever, and swollen lymph nodes.
HHS and CDC did not have data on how many people have received at least one dose of the monkeypox vaccine. When asked how many people need to be fully vaccinated against monkeypox to contain the outbreak, Mr. Becerra did not provide an estimate but implied that preventive vaccination could help limit the number of vaccines needed and expressed optimism about quelling the outbreak in the United States. “We believe that we have done everything we can at the federal level to work with our state and local partners and communities affected to make sure we can stay ahead of this and end this outbreak,” he said, “but everybody’s got to do their part.”
A version of this article first appeared on Medscape.com.
More than 780,000 doses of the JYNNEOS monkeypox vaccine will be available in the United States beginning July 29, the Department of Health & Human Services announced on July 28 in a press call.
HHS Secretary Xavier Becerra urged local and state public health departments to use these doses for preventive vaccination efforts to stay ahead of the virus and end the outbreak, noting that the HHS and Centers for Disease Control and Prevention do not control how vaccines are distributed at state and local levels. “We don’t have the authority to tell them what to do,” he said during the call. “We need them to work with us.”
As of July 28, there were 4,907 reported cases of monkeypox in the United States and officials expect cases will continue to rise in the coming weeks.
The vaccine is manufactured by the small Danish company Bavarian Nordic. These additional 786,000 doses were previously stored at a plant in Denmark, awaiting the completion of an inspection and authorization of the vaccine plant by the Food and Drug Administration. The agency announced on July 27 that both the vaccine doses and the manufacturing plant met standards.
With the announcement of these additional doses, the vaccine allocation plan is also being updated to take into account two important factors: the number of people at high risk in a jurisdiction and the number of new cases reported since the last vaccine allocation.
“This update gives greater weight to prioritizing vaccines to areas with the greatest number of people at risk, which includes men who have sex with men who have HIV or who are eligible for HIV pre-exposure prophylaxis, while still considering where we are seeing cases increase,” said Capt. Jennifer McQuiston, DVM, deputy director of the division of high consequence pathogens and pathology at the CDC.
Capt.McQuiston also provided additional demographic information on the U.S. outbreak. The median age of people with confirmed cases is 35 years old, with a range from 17 to 76. (This does not include the two cases in children reported on July 22.) Of the cases where sex at birth was provided, 99% were individuals assigned male sex at birth. In cases with reported ethnicity and race, 37% were non-Hispanic White people, 31% were Hispanic/Latino, 27% were Black or African American, and 4% were of Asian descent. The most common symptoms were rash – present in 99% of cases – malaise, fever, and swollen lymph nodes.
HHS and CDC did not have data on how many people have received at least one dose of the monkeypox vaccine. When asked how many people need to be fully vaccinated against monkeypox to contain the outbreak, Mr. Becerra did not provide an estimate but implied that preventive vaccination could help limit the number of vaccines needed and expressed optimism about quelling the outbreak in the United States. “We believe that we have done everything we can at the federal level to work with our state and local partners and communities affected to make sure we can stay ahead of this and end this outbreak,” he said, “but everybody’s got to do their part.”
A version of this article first appeared on Medscape.com.
More than 780,000 doses of the JYNNEOS monkeypox vaccine will be available in the United States beginning July 29, the Department of Health & Human Services announced on July 28 in a press call.
HHS Secretary Xavier Becerra urged local and state public health departments to use these doses for preventive vaccination efforts to stay ahead of the virus and end the outbreak, noting that the HHS and Centers for Disease Control and Prevention do not control how vaccines are distributed at state and local levels. “We don’t have the authority to tell them what to do,” he said during the call. “We need them to work with us.”
As of July 28, there were 4,907 reported cases of monkeypox in the United States and officials expect cases will continue to rise in the coming weeks.
The vaccine is manufactured by the small Danish company Bavarian Nordic. These additional 786,000 doses were previously stored at a plant in Denmark, awaiting the completion of an inspection and authorization of the vaccine plant by the Food and Drug Administration. The agency announced on July 27 that both the vaccine doses and the manufacturing plant met standards.
With the announcement of these additional doses, the vaccine allocation plan is also being updated to take into account two important factors: the number of people at high risk in a jurisdiction and the number of new cases reported since the last vaccine allocation.
“This update gives greater weight to prioritizing vaccines to areas with the greatest number of people at risk, which includes men who have sex with men who have HIV or who are eligible for HIV pre-exposure prophylaxis, while still considering where we are seeing cases increase,” said Capt. Jennifer McQuiston, DVM, deputy director of the division of high consequence pathogens and pathology at the CDC.
Capt.McQuiston also provided additional demographic information on the U.S. outbreak. The median age of people with confirmed cases is 35 years old, with a range from 17 to 76. (This does not include the two cases in children reported on July 22.) Of the cases where sex at birth was provided, 99% were individuals assigned male sex at birth. In cases with reported ethnicity and race, 37% were non-Hispanic White people, 31% were Hispanic/Latino, 27% were Black or African American, and 4% were of Asian descent. The most common symptoms were rash – present in 99% of cases – malaise, fever, and swollen lymph nodes.
HHS and CDC did not have data on how many people have received at least one dose of the monkeypox vaccine. When asked how many people need to be fully vaccinated against monkeypox to contain the outbreak, Mr. Becerra did not provide an estimate but implied that preventive vaccination could help limit the number of vaccines needed and expressed optimism about quelling the outbreak in the United States. “We believe that we have done everything we can at the federal level to work with our state and local partners and communities affected to make sure we can stay ahead of this and end this outbreak,” he said, “but everybody’s got to do their part.”
A version of this article first appeared on Medscape.com.
Single dose of HPV vaccine is ‘game changer,’ says WHO
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Methotrexate’s impact on COVID-19 vaccination: New insights made
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How to overcome hesitancy for COVID-19 and other vaccines
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.
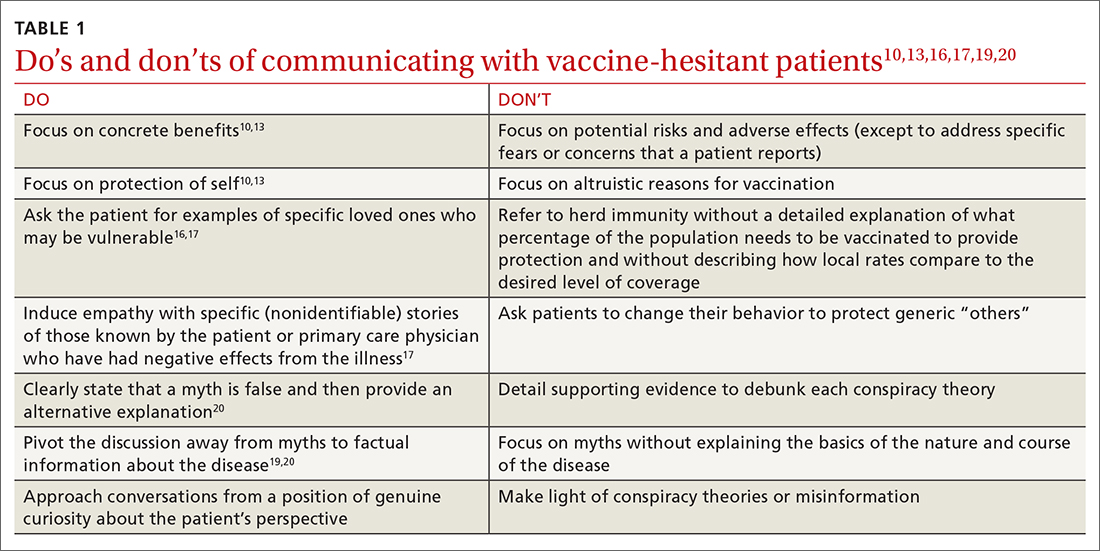
Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.

Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.

Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
PRACTICE RECOMMENDATIONS
› Focus on personal benefits of vaccination with patients who express strong hesitancy and endorse vaccine myths; refocus the conversation away from myths and back to disease facts. C
› Emphasize personal and collective benefit to patients who are uncertain about vaccination; provide education about herd immunity and local vaccine coverage. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Amazon involved with new cancer vaccine clinical trial
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
Some have heavier periods after COVID vaccine
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
FROM SCIENCE ADVANCES
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.








