User login
Discussing immunization with vaccine-hesitant parents requires caring, individualized approach
ORLANDO – Putting parents at ease, making vaccination the default option during discussions, appealing to their identity as a good parent, and focusing on positive word choice during discussions are the techniques two pediatricians have recommended using to get vaccine-hesitant parents to immunize their children.
“Your goal is to get parents to immunize their kids,” Katrina Saba, MD, of the Permanente Medical Group in Oakland, Calif., said during an interactive group panel at the annual meeting of the American Academy of Pediatrics. “Our goal is not to win a debate. You don’t have to correct every mistaken idea.”
“And really importantly, as we know, belief trumps science,” she added. “Their belief is so much stronger than our proof, and their belief will not be changed by evidence.”
Many parents who are vaccine- hesitant also belong to a social network that forms or reinforces their beliefs, and attacking those beliefs is the same as attacking their identity, Dr. Saba noted. “When you attack someone’s identity, you are immediately seen as not like them, and if you’re not like them, you’ve lost your strength in persuading them.”
Dr. Saba; Kenneth Hempstead, MD; and other pediatricians and educators in the Permanente Medical Group developed a framework for pediatricians and educators to talk with their patients about immunization at their center after California passed a law in 2013 that required health care professionals to discuss vaccines with patients and sign off that they had that discussion.
“We felt that, if we were going to be by law required to have that discussion, maybe we needed some new tools to have [the discussion] more effectively,” Dr. Saba said. “Because clearly, [what we were doing ] wasn’t working or there wouldn’t have been a need for that law.”
Dr. Hempstead explained the concerns of three major categories of vaccine-hesitant parents: those patients who are unsure of whether they should vaccinate, parents who wish to delay vaccination, and parents who refuse vaccination of their children.
Each parent requires a different approach for discussing the importance of vaccination based on their level of vaccine hesitancy, he said. For parents who are unsure, they may require general information about the safety and importance of vaccines.
Parents who delay immunization may have less trust in vaccines, have done research in their own social networks, and may present alternatives to a standard immunization schedule or want to omit certain vaccines from their child’s immunization schedule, he noted. Using the analogy of a car seat is one approach to identify the importance of vaccination to these parents: “Waiting to give the shots is like putting your baby in the car seat after you’ve already arrived at the store – the protection isn’t there for the most important part of the journey!”
In cases where parents refuse vaccination, you should not expect to change a parent’s mind in a single visit, but instead focus on building the patient-provider bond. However, presenting information the parent may have already seen, such as vaccination data from the Food and Drug Administration or Centers for Disease Control and Prevention, may alienate parents who identify with groups that share vaccine-hesitant viewpoints and erode your ability to persuade a parent’s intent to vaccinate.
A study by Nyhan et al. randomized parents to receive one of four pieces of interventions about the MMR vaccine: information from the CDC explaining the lack of evidence linking autism and the vaccine, information about the dangers of the diseases prevented by the vaccine, images of children who have had diseases prevented by the vaccine, and a “dramatic narrative” from a CDC fact sheet about a child who nearly died of measles. The researchers found no informational intervention helped in persuading to vaccinate in parents who had the “least favorable” attitudes toward the vaccine. And in the case of the dramatic narrative, there was an increased misperception about the MMR vaccine (Pediatrics. 2014;133[4]:e835-e842).
Dr. Hempstead and Dr. Saba outlined four rhetorical devices to include in conversations with patients about vaccination: cognitive ease, natural assumption, appeal to identity, and using advantageous terms.
Cognitive ease
Cognitive ease means creating an environment in which the patient is relaxed, comfortable, and more likely to be agreeable. Recognize when the tone shifts, and strive to maintain this calm and comfortable environment throughout the discussion. “If your blood pressure is coming up, that means theirs is, too,” Dr. Hempstead said.
Natural assumption
How you are offering the vaccination also matters, he added. Rather than asking whether a patient wants to vaccinate (“Have you thought about your flu vaccine this year?”), instead frame the discussion with vaccination as the default option (“Is your child due for a flu vaccination this year? Yes, he is. Let’s get that taken care of today”). Equating inaction with vaccination prevents the risk fallacy phenomenon from occurring in which, when given multiple options, people give equal weight to each option and may choose not to vaccinate, Dr. Hempstead noted.
Dr. Saba cited research that backed this approach. In a study by Opel et al., using a “presumptive” approach instead of a “participatory” approach when discussing a provider’s recommendation to vaccinate helped: The presumptive conversations had an odds ratio of 17.5, compared with the participatory approach. In cases in which parents resisted the provider’s recommendations, 50% of providers persisted with their original recommendations, and 47% of parents who initially resisted the recommendations agreed to vaccinate (Pediatrics. 2013;132[6]:1037-46).
Appeal to identity
Another strategy to use is appealing to the patient’s identity as a good parent and link the concept of vaccination with the good parent identity. Forging a new common identity with the parents through common beliefs – such as recognizing that networks to which parents belong are an important part of his or her identify – and reemphasizing the mutual desire to protect the child is another strategy.
Using advantageous terms
Positive terms, such as “protection,” “health,” “safety,” and “what’s best,” are much better words to use in conversation with parents and have more staying power than negative terms, like “autism” and “side effects,” Dr. Hempstead said.
“Stay with positive messaging,” he said. “Immediately coming back to the positive impact of this vaccine, why we care so much, why we’re doing this vaccine, is absolutely critical.”
Dr. Hempstead and Dr. Saba reported no relevant conflicts of interest.
ORLANDO – Putting parents at ease, making vaccination the default option during discussions, appealing to their identity as a good parent, and focusing on positive word choice during discussions are the techniques two pediatricians have recommended using to get vaccine-hesitant parents to immunize their children.
“Your goal is to get parents to immunize their kids,” Katrina Saba, MD, of the Permanente Medical Group in Oakland, Calif., said during an interactive group panel at the annual meeting of the American Academy of Pediatrics. “Our goal is not to win a debate. You don’t have to correct every mistaken idea.”
“And really importantly, as we know, belief trumps science,” she added. “Their belief is so much stronger than our proof, and their belief will not be changed by evidence.”
Many parents who are vaccine- hesitant also belong to a social network that forms or reinforces their beliefs, and attacking those beliefs is the same as attacking their identity, Dr. Saba noted. “When you attack someone’s identity, you are immediately seen as not like them, and if you’re not like them, you’ve lost your strength in persuading them.”
Dr. Saba; Kenneth Hempstead, MD; and other pediatricians and educators in the Permanente Medical Group developed a framework for pediatricians and educators to talk with their patients about immunization at their center after California passed a law in 2013 that required health care professionals to discuss vaccines with patients and sign off that they had that discussion.
“We felt that, if we were going to be by law required to have that discussion, maybe we needed some new tools to have [the discussion] more effectively,” Dr. Saba said. “Because clearly, [what we were doing ] wasn’t working or there wouldn’t have been a need for that law.”
Dr. Hempstead explained the concerns of three major categories of vaccine-hesitant parents: those patients who are unsure of whether they should vaccinate, parents who wish to delay vaccination, and parents who refuse vaccination of their children.
Each parent requires a different approach for discussing the importance of vaccination based on their level of vaccine hesitancy, he said. For parents who are unsure, they may require general information about the safety and importance of vaccines.
Parents who delay immunization may have less trust in vaccines, have done research in their own social networks, and may present alternatives to a standard immunization schedule or want to omit certain vaccines from their child’s immunization schedule, he noted. Using the analogy of a car seat is one approach to identify the importance of vaccination to these parents: “Waiting to give the shots is like putting your baby in the car seat after you’ve already arrived at the store – the protection isn’t there for the most important part of the journey!”
In cases where parents refuse vaccination, you should not expect to change a parent’s mind in a single visit, but instead focus on building the patient-provider bond. However, presenting information the parent may have already seen, such as vaccination data from the Food and Drug Administration or Centers for Disease Control and Prevention, may alienate parents who identify with groups that share vaccine-hesitant viewpoints and erode your ability to persuade a parent’s intent to vaccinate.
A study by Nyhan et al. randomized parents to receive one of four pieces of interventions about the MMR vaccine: information from the CDC explaining the lack of evidence linking autism and the vaccine, information about the dangers of the diseases prevented by the vaccine, images of children who have had diseases prevented by the vaccine, and a “dramatic narrative” from a CDC fact sheet about a child who nearly died of measles. The researchers found no informational intervention helped in persuading to vaccinate in parents who had the “least favorable” attitudes toward the vaccine. And in the case of the dramatic narrative, there was an increased misperception about the MMR vaccine (Pediatrics. 2014;133[4]:e835-e842).
Dr. Hempstead and Dr. Saba outlined four rhetorical devices to include in conversations with patients about vaccination: cognitive ease, natural assumption, appeal to identity, and using advantageous terms.
Cognitive ease
Cognitive ease means creating an environment in which the patient is relaxed, comfortable, and more likely to be agreeable. Recognize when the tone shifts, and strive to maintain this calm and comfortable environment throughout the discussion. “If your blood pressure is coming up, that means theirs is, too,” Dr. Hempstead said.
Natural assumption
How you are offering the vaccination also matters, he added. Rather than asking whether a patient wants to vaccinate (“Have you thought about your flu vaccine this year?”), instead frame the discussion with vaccination as the default option (“Is your child due for a flu vaccination this year? Yes, he is. Let’s get that taken care of today”). Equating inaction with vaccination prevents the risk fallacy phenomenon from occurring in which, when given multiple options, people give equal weight to each option and may choose not to vaccinate, Dr. Hempstead noted.
Dr. Saba cited research that backed this approach. In a study by Opel et al., using a “presumptive” approach instead of a “participatory” approach when discussing a provider’s recommendation to vaccinate helped: The presumptive conversations had an odds ratio of 17.5, compared with the participatory approach. In cases in which parents resisted the provider’s recommendations, 50% of providers persisted with their original recommendations, and 47% of parents who initially resisted the recommendations agreed to vaccinate (Pediatrics. 2013;132[6]:1037-46).
Appeal to identity
Another strategy to use is appealing to the patient’s identity as a good parent and link the concept of vaccination with the good parent identity. Forging a new common identity with the parents through common beliefs – such as recognizing that networks to which parents belong are an important part of his or her identify – and reemphasizing the mutual desire to protect the child is another strategy.
Using advantageous terms
Positive terms, such as “protection,” “health,” “safety,” and “what’s best,” are much better words to use in conversation with parents and have more staying power than negative terms, like “autism” and “side effects,” Dr. Hempstead said.
“Stay with positive messaging,” he said. “Immediately coming back to the positive impact of this vaccine, why we care so much, why we’re doing this vaccine, is absolutely critical.”
Dr. Hempstead and Dr. Saba reported no relevant conflicts of interest.
ORLANDO – Putting parents at ease, making vaccination the default option during discussions, appealing to their identity as a good parent, and focusing on positive word choice during discussions are the techniques two pediatricians have recommended using to get vaccine-hesitant parents to immunize their children.
“Your goal is to get parents to immunize their kids,” Katrina Saba, MD, of the Permanente Medical Group in Oakland, Calif., said during an interactive group panel at the annual meeting of the American Academy of Pediatrics. “Our goal is not to win a debate. You don’t have to correct every mistaken idea.”
“And really importantly, as we know, belief trumps science,” she added. “Their belief is so much stronger than our proof, and their belief will not be changed by evidence.”
Many parents who are vaccine- hesitant also belong to a social network that forms or reinforces their beliefs, and attacking those beliefs is the same as attacking their identity, Dr. Saba noted. “When you attack someone’s identity, you are immediately seen as not like them, and if you’re not like them, you’ve lost your strength in persuading them.”
Dr. Saba; Kenneth Hempstead, MD; and other pediatricians and educators in the Permanente Medical Group developed a framework for pediatricians and educators to talk with their patients about immunization at their center after California passed a law in 2013 that required health care professionals to discuss vaccines with patients and sign off that they had that discussion.
“We felt that, if we were going to be by law required to have that discussion, maybe we needed some new tools to have [the discussion] more effectively,” Dr. Saba said. “Because clearly, [what we were doing ] wasn’t working or there wouldn’t have been a need for that law.”
Dr. Hempstead explained the concerns of three major categories of vaccine-hesitant parents: those patients who are unsure of whether they should vaccinate, parents who wish to delay vaccination, and parents who refuse vaccination of their children.
Each parent requires a different approach for discussing the importance of vaccination based on their level of vaccine hesitancy, he said. For parents who are unsure, they may require general information about the safety and importance of vaccines.
Parents who delay immunization may have less trust in vaccines, have done research in their own social networks, and may present alternatives to a standard immunization schedule or want to omit certain vaccines from their child’s immunization schedule, he noted. Using the analogy of a car seat is one approach to identify the importance of vaccination to these parents: “Waiting to give the shots is like putting your baby in the car seat after you’ve already arrived at the store – the protection isn’t there for the most important part of the journey!”
In cases where parents refuse vaccination, you should not expect to change a parent’s mind in a single visit, but instead focus on building the patient-provider bond. However, presenting information the parent may have already seen, such as vaccination data from the Food and Drug Administration or Centers for Disease Control and Prevention, may alienate parents who identify with groups that share vaccine-hesitant viewpoints and erode your ability to persuade a parent’s intent to vaccinate.
A study by Nyhan et al. randomized parents to receive one of four pieces of interventions about the MMR vaccine: information from the CDC explaining the lack of evidence linking autism and the vaccine, information about the dangers of the diseases prevented by the vaccine, images of children who have had diseases prevented by the vaccine, and a “dramatic narrative” from a CDC fact sheet about a child who nearly died of measles. The researchers found no informational intervention helped in persuading to vaccinate in parents who had the “least favorable” attitudes toward the vaccine. And in the case of the dramatic narrative, there was an increased misperception about the MMR vaccine (Pediatrics. 2014;133[4]:e835-e842).
Dr. Hempstead and Dr. Saba outlined four rhetorical devices to include in conversations with patients about vaccination: cognitive ease, natural assumption, appeal to identity, and using advantageous terms.
Cognitive ease
Cognitive ease means creating an environment in which the patient is relaxed, comfortable, and more likely to be agreeable. Recognize when the tone shifts, and strive to maintain this calm and comfortable environment throughout the discussion. “If your blood pressure is coming up, that means theirs is, too,” Dr. Hempstead said.
Natural assumption
How you are offering the vaccination also matters, he added. Rather than asking whether a patient wants to vaccinate (“Have you thought about your flu vaccine this year?”), instead frame the discussion with vaccination as the default option (“Is your child due for a flu vaccination this year? Yes, he is. Let’s get that taken care of today”). Equating inaction with vaccination prevents the risk fallacy phenomenon from occurring in which, when given multiple options, people give equal weight to each option and may choose not to vaccinate, Dr. Hempstead noted.
Dr. Saba cited research that backed this approach. In a study by Opel et al., using a “presumptive” approach instead of a “participatory” approach when discussing a provider’s recommendation to vaccinate helped: The presumptive conversations had an odds ratio of 17.5, compared with the participatory approach. In cases in which parents resisted the provider’s recommendations, 50% of providers persisted with their original recommendations, and 47% of parents who initially resisted the recommendations agreed to vaccinate (Pediatrics. 2013;132[6]:1037-46).
Appeal to identity
Another strategy to use is appealing to the patient’s identity as a good parent and link the concept of vaccination with the good parent identity. Forging a new common identity with the parents through common beliefs – such as recognizing that networks to which parents belong are an important part of his or her identify – and reemphasizing the mutual desire to protect the child is another strategy.
Using advantageous terms
Positive terms, such as “protection,” “health,” “safety,” and “what’s best,” are much better words to use in conversation with parents and have more staying power than negative terms, like “autism” and “side effects,” Dr. Hempstead said.
“Stay with positive messaging,” he said. “Immediately coming back to the positive impact of this vaccine, why we care so much, why we’re doing this vaccine, is absolutely critical.”
Dr. Hempstead and Dr. Saba reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Study confirms advice to halt methotrexate when giving flu vaccine to RA patients
CHICAGO – Discontinuing methotrexate for 2 weeks in patients with RA starting the day they receive the seasonal influenza vaccine significantly improves the vaccine’s immunogenicity without aggravating disease activity, Kevin L. Winthrop, MD, reported at the annual meeting of the American College of Rheumatology.
“I think this is potentially clinically practice changing because now there are two studies showing the same thing,” said Dr. Winthrop, a professor of public health and preventive medicine at Oregon Health & Science University, Portland.
Based upon these prospective randomized studies, which he conducted together with investigators at Seoul National University in South Korea, initiating a 2-week halt of methotrexate on the day the influenza vaccine is given to patients with RA is now his routine practice, and he recommends other physicians do the same.
The first prospective, randomized trial included 199 RA patients on stable doses of methotrexate who were assigned to one of four groups in conjunction with seasonal influenza vaccination. One group continued their methotrexate as usual, the second stopped the drug for 1 month prior to vaccination and then restarted it at the time of vaccination, the third group halted methotrexate for 2 weeks before and 2 weeks after vaccination, and the fourth suspended methotrexate for 4 weeks starting on the day they got their flu shot. Everyone received trivalent influenza vaccine containing H1N1, H3N2, and B/Yamagata.
The lowest rate of satisfactory vaccine response as defined by at least a 300% titer increase 1 month after vaccination occurred in the group that continued their methotrexate as usual. The group that halted the drug for 2 weeks before and 2 weeks after influenza vaccination had a 51% satisfactory vaccine response against all three antigens, compared with a 31.5% rate in the methotrexate-as-usual group. RA flare rates ranged from 21% to 39% across the four study arms, differences that weren’t statistically significant (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
Next Dr. Winthrop and his colleagues conducted a confirmatory prospective, multicenter, randomized trial in which they sought to nail down the optimal duration and timing of methotrexate discontinuation. A total of 320 RA patients on stable doses of methotrexate were assigned to halt the drug for 2 weeks starting at the time they received a quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains, or to continue their methotrexate throughout.
A satisfactory vaccine response was achieved in 75.5% of the group that discontinued the drug, significantly better than the 54.5% rate in the methotrexate continuers. The absolute difference in seroprotection was greater in patients who halted their methotrexate for 2 weeks after vaccination for all four antigens: an absolute 11% difference for H1N1, 16% for H3N2, 12% for B/Yamagata, and 15% for B/Victoria (Ann Rheum Dis. 2018 Jun;77[6]:898-904).
“It does seem to be a nice strategy. The percentage of people who flared their RA during their 2 weeks off methotrexate was very low, so there seems to be a good reason to do this,” according to Dr. Winthrop.
Some rheumatologists he has spoken with initially balked at the plausibility of the results.
“I had the same thought about these studies: It doesn’t make sense to me in terms of how methotrexate works, and why we would see this effect acutely by stopping methotrexate for just 2 weeks?” he said.
But then a coinvestigator drilled down deeper into the data and came up with the explanation: The benefit in terms of enhanced flu vaccine immunogenicity through temporary withholding of methotrexate was confined to the subgroup of RA patients with high baseline levels of B-cell activation factor (BAFF). In contrast, withholding methotrexate didn’t affect the vaccine response in patients with low or normal baseline BAFF (Ann Rheum Dis. 2018 Oct 8. doi: 10.1136/annrheumdis-2018-214025).
“I don’t know how to check anyone’s BAFF levels. I don’t think there’s a commercial test out there. But this does help explain why we saw this observation. So I think I would still hold everyone’s methotrexate for 2 weeks. That’s how I approach it. And they may get benefit from it, and they may not,” he said.
Dr. Winthrop reported having no financial conflicts regarding the study, which was funded by GC Pharma.
CHICAGO – Discontinuing methotrexate for 2 weeks in patients with RA starting the day they receive the seasonal influenza vaccine significantly improves the vaccine’s immunogenicity without aggravating disease activity, Kevin L. Winthrop, MD, reported at the annual meeting of the American College of Rheumatology.
“I think this is potentially clinically practice changing because now there are two studies showing the same thing,” said Dr. Winthrop, a professor of public health and preventive medicine at Oregon Health & Science University, Portland.
Based upon these prospective randomized studies, which he conducted together with investigators at Seoul National University in South Korea, initiating a 2-week halt of methotrexate on the day the influenza vaccine is given to patients with RA is now his routine practice, and he recommends other physicians do the same.
The first prospective, randomized trial included 199 RA patients on stable doses of methotrexate who were assigned to one of four groups in conjunction with seasonal influenza vaccination. One group continued their methotrexate as usual, the second stopped the drug for 1 month prior to vaccination and then restarted it at the time of vaccination, the third group halted methotrexate for 2 weeks before and 2 weeks after vaccination, and the fourth suspended methotrexate for 4 weeks starting on the day they got their flu shot. Everyone received trivalent influenza vaccine containing H1N1, H3N2, and B/Yamagata.
The lowest rate of satisfactory vaccine response as defined by at least a 300% titer increase 1 month after vaccination occurred in the group that continued their methotrexate as usual. The group that halted the drug for 2 weeks before and 2 weeks after influenza vaccination had a 51% satisfactory vaccine response against all three antigens, compared with a 31.5% rate in the methotrexate-as-usual group. RA flare rates ranged from 21% to 39% across the four study arms, differences that weren’t statistically significant (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
Next Dr. Winthrop and his colleagues conducted a confirmatory prospective, multicenter, randomized trial in which they sought to nail down the optimal duration and timing of methotrexate discontinuation. A total of 320 RA patients on stable doses of methotrexate were assigned to halt the drug for 2 weeks starting at the time they received a quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains, or to continue their methotrexate throughout.
A satisfactory vaccine response was achieved in 75.5% of the group that discontinued the drug, significantly better than the 54.5% rate in the methotrexate continuers. The absolute difference in seroprotection was greater in patients who halted their methotrexate for 2 weeks after vaccination for all four antigens: an absolute 11% difference for H1N1, 16% for H3N2, 12% for B/Yamagata, and 15% for B/Victoria (Ann Rheum Dis. 2018 Jun;77[6]:898-904).
“It does seem to be a nice strategy. The percentage of people who flared their RA during their 2 weeks off methotrexate was very low, so there seems to be a good reason to do this,” according to Dr. Winthrop.
Some rheumatologists he has spoken with initially balked at the plausibility of the results.
“I had the same thought about these studies: It doesn’t make sense to me in terms of how methotrexate works, and why we would see this effect acutely by stopping methotrexate for just 2 weeks?” he said.
But then a coinvestigator drilled down deeper into the data and came up with the explanation: The benefit in terms of enhanced flu vaccine immunogenicity through temporary withholding of methotrexate was confined to the subgroup of RA patients with high baseline levels of B-cell activation factor (BAFF). In contrast, withholding methotrexate didn’t affect the vaccine response in patients with low or normal baseline BAFF (Ann Rheum Dis. 2018 Oct 8. doi: 10.1136/annrheumdis-2018-214025).
“I don’t know how to check anyone’s BAFF levels. I don’t think there’s a commercial test out there. But this does help explain why we saw this observation. So I think I would still hold everyone’s methotrexate for 2 weeks. That’s how I approach it. And they may get benefit from it, and they may not,” he said.
Dr. Winthrop reported having no financial conflicts regarding the study, which was funded by GC Pharma.
CHICAGO – Discontinuing methotrexate for 2 weeks in patients with RA starting the day they receive the seasonal influenza vaccine significantly improves the vaccine’s immunogenicity without aggravating disease activity, Kevin L. Winthrop, MD, reported at the annual meeting of the American College of Rheumatology.
“I think this is potentially clinically practice changing because now there are two studies showing the same thing,” said Dr. Winthrop, a professor of public health and preventive medicine at Oregon Health & Science University, Portland.
Based upon these prospective randomized studies, which he conducted together with investigators at Seoul National University in South Korea, initiating a 2-week halt of methotrexate on the day the influenza vaccine is given to patients with RA is now his routine practice, and he recommends other physicians do the same.
The first prospective, randomized trial included 199 RA patients on stable doses of methotrexate who were assigned to one of four groups in conjunction with seasonal influenza vaccination. One group continued their methotrexate as usual, the second stopped the drug for 1 month prior to vaccination and then restarted it at the time of vaccination, the third group halted methotrexate for 2 weeks before and 2 weeks after vaccination, and the fourth suspended methotrexate for 4 weeks starting on the day they got their flu shot. Everyone received trivalent influenza vaccine containing H1N1, H3N2, and B/Yamagata.
The lowest rate of satisfactory vaccine response as defined by at least a 300% titer increase 1 month after vaccination occurred in the group that continued their methotrexate as usual. The group that halted the drug for 2 weeks before and 2 weeks after influenza vaccination had a 51% satisfactory vaccine response against all three antigens, compared with a 31.5% rate in the methotrexate-as-usual group. RA flare rates ranged from 21% to 39% across the four study arms, differences that weren’t statistically significant (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
Next Dr. Winthrop and his colleagues conducted a confirmatory prospective, multicenter, randomized trial in which they sought to nail down the optimal duration and timing of methotrexate discontinuation. A total of 320 RA patients on stable doses of methotrexate were assigned to halt the drug for 2 weeks starting at the time they received a quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains, or to continue their methotrexate throughout.
A satisfactory vaccine response was achieved in 75.5% of the group that discontinued the drug, significantly better than the 54.5% rate in the methotrexate continuers. The absolute difference in seroprotection was greater in patients who halted their methotrexate for 2 weeks after vaccination for all four antigens: an absolute 11% difference for H1N1, 16% for H3N2, 12% for B/Yamagata, and 15% for B/Victoria (Ann Rheum Dis. 2018 Jun;77[6]:898-904).
“It does seem to be a nice strategy. The percentage of people who flared their RA during their 2 weeks off methotrexate was very low, so there seems to be a good reason to do this,” according to Dr. Winthrop.
Some rheumatologists he has spoken with initially balked at the plausibility of the results.
“I had the same thought about these studies: It doesn’t make sense to me in terms of how methotrexate works, and why we would see this effect acutely by stopping methotrexate for just 2 weeks?” he said.
But then a coinvestigator drilled down deeper into the data and came up with the explanation: The benefit in terms of enhanced flu vaccine immunogenicity through temporary withholding of methotrexate was confined to the subgroup of RA patients with high baseline levels of B-cell activation factor (BAFF). In contrast, withholding methotrexate didn’t affect the vaccine response in patients with low or normal baseline BAFF (Ann Rheum Dis. 2018 Oct 8. doi: 10.1136/annrheumdis-2018-214025).
“I don’t know how to check anyone’s BAFF levels. I don’t think there’s a commercial test out there. But this does help explain why we saw this observation. So I think I would still hold everyone’s methotrexate for 2 weeks. That’s how I approach it. And they may get benefit from it, and they may not,” he said.
Dr. Winthrop reported having no financial conflicts regarding the study, which was funded by GC Pharma.
REPORTING FROM THE ACR ANNUAL MEETING
Struggling to reach an HCV vaccine
Currently, there is no effective hepatitis C virus (HCV) vaccine available despite numerous ongoing studies, according to the results of a review published in Gastroenterology.
In their article, Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues reviewed the limited feasibility of applying traditional vaccine design to HCV and the problem of genetic diversity in the virus, as well as trials of vaccines designed to elicit T-cell responses.
One profound difficulty in the development and testing of an HCV vaccine is that the cohort most predictably at risk for high infection levels, people who inject drugs, are notoriously difficult to recruit, maintain consistent treatment, and follow up on – all necessary aspects of an appropriate vaccine trial.
Thus, at present, adjuvant envelope or core protein and virus-vectored nonstructural antigen vaccines have been tested only in healthy volunteers who are not at risk for HCV infection; viral vectors encoding nonstructural proteins remain the only vaccine strategy tested in truly at-risk individuals, according to Dr. Bailey and his colleagues.
“Although pharmaceutical companies invest in drug development, vaccine development requires investment from sources beyond government and charitable foundations. A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” Dr. Bailey and his colleagues concluded.
The authors reported having no conflicts.
SOURCE: Bailey JR et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.08.060.
Currently, there is no effective hepatitis C virus (HCV) vaccine available despite numerous ongoing studies, according to the results of a review published in Gastroenterology.
In their article, Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues reviewed the limited feasibility of applying traditional vaccine design to HCV and the problem of genetic diversity in the virus, as well as trials of vaccines designed to elicit T-cell responses.
One profound difficulty in the development and testing of an HCV vaccine is that the cohort most predictably at risk for high infection levels, people who inject drugs, are notoriously difficult to recruit, maintain consistent treatment, and follow up on – all necessary aspects of an appropriate vaccine trial.
Thus, at present, adjuvant envelope or core protein and virus-vectored nonstructural antigen vaccines have been tested only in healthy volunteers who are not at risk for HCV infection; viral vectors encoding nonstructural proteins remain the only vaccine strategy tested in truly at-risk individuals, according to Dr. Bailey and his colleagues.
“Although pharmaceutical companies invest in drug development, vaccine development requires investment from sources beyond government and charitable foundations. A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” Dr. Bailey and his colleagues concluded.
The authors reported having no conflicts.
SOURCE: Bailey JR et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.08.060.
Currently, there is no effective hepatitis C virus (HCV) vaccine available despite numerous ongoing studies, according to the results of a review published in Gastroenterology.
In their article, Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues reviewed the limited feasibility of applying traditional vaccine design to HCV and the problem of genetic diversity in the virus, as well as trials of vaccines designed to elicit T-cell responses.
One profound difficulty in the development and testing of an HCV vaccine is that the cohort most predictably at risk for high infection levels, people who inject drugs, are notoriously difficult to recruit, maintain consistent treatment, and follow up on – all necessary aspects of an appropriate vaccine trial.
Thus, at present, adjuvant envelope or core protein and virus-vectored nonstructural antigen vaccines have been tested only in healthy volunteers who are not at risk for HCV infection; viral vectors encoding nonstructural proteins remain the only vaccine strategy tested in truly at-risk individuals, according to Dr. Bailey and his colleagues.
“Although pharmaceutical companies invest in drug development, vaccine development requires investment from sources beyond government and charitable foundations. A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” Dr. Bailey and his colleagues concluded.
The authors reported having no conflicts.
SOURCE: Bailey JR et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.08.060.
FROM GASTROENTEROLOGY
Influenza update 2018–2019: 100 years after the great pandemic
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
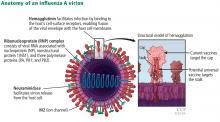
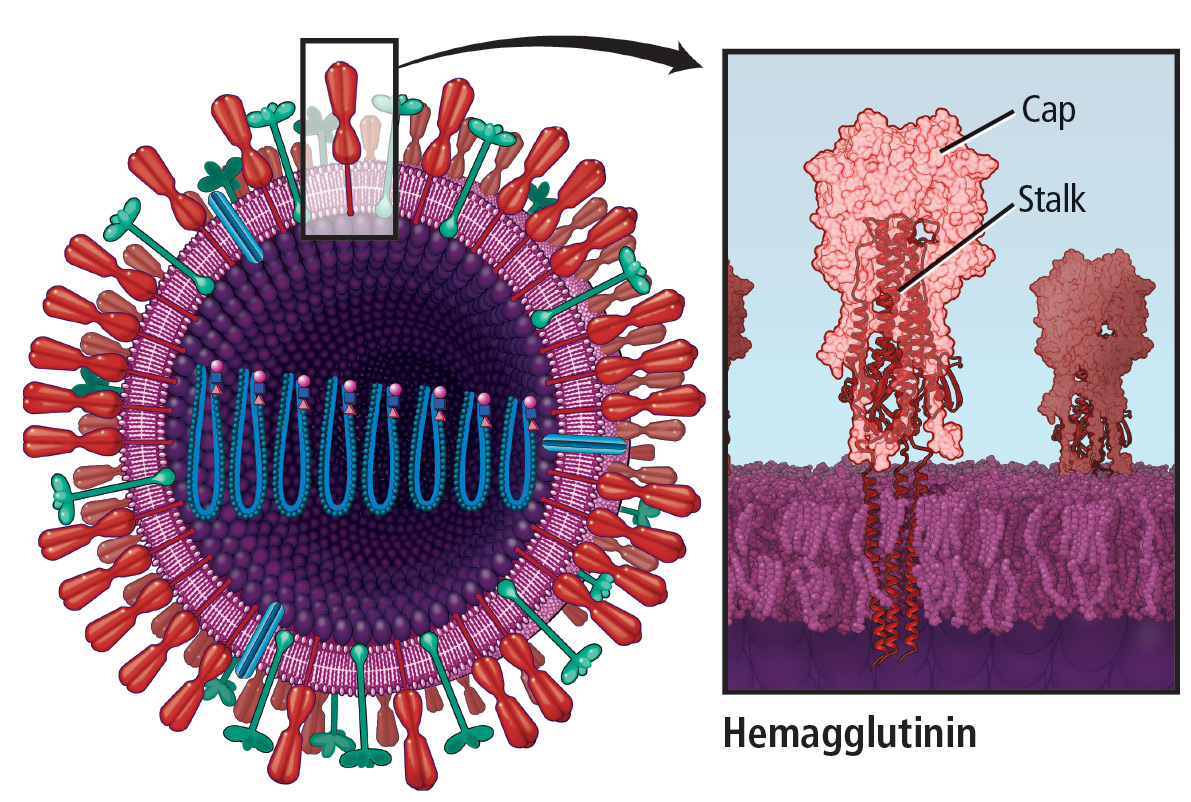
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
KEY POINTS
- Influenza A(H7N9) is a prime candidate to cause the next influenza pandemic.
- Influenza vaccine prevents 300 to 4,000 deaths in the United States every year.
- The 2018–2019 quadrivalent influenza vaccine contains updated A(H3N2) and B/Victoria lineage components different from those in the 2017–2018 Northern Hemisphere vaccine.
- The live-attenuated influenza vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, is recommended for the 2018–2019 influenza season.
- Influenza vaccine is recommended any time during pregnancy and is associated with lower infant mortality rates.
- Overall influenza vaccination rates remain below the 80% target for all Americans and 90% for at-risk populations.
Medical exemptions spike after vaccine policy change
according to data from interviews with health officials and immunization staff after implementation of the policy.
In a study published in Pediatrics, Salini Mohanty, DrPH, of the University of Pennsylvania School of Nursing, Philadelphia, and her colleagues conducted semistructured phone interviews with 40 health officers and immunization staff who represented 35 of 61 California heath jurisdictions. The interviews occurred between August 2017 and September 2017, and participants discussed their experiences with medical exemption requests after the policy change.
Although the percentage of fully vaccinated kindergarten students in California increased from 93% in 2015-2016 to 95% in 2017-2018, and the rate of personal belief exemptions declined, overall medical exemption requests rose 250% from 0.2% in 2015-2016 to 0.7% 2017-2018, the researchers noted.
They identified four main issues based on participant responses: the role of stakeholders, the review of medical exemptions received by schools, the medical exemptions perceived as problematic, and the general frustration and concern over medical exemptions.
Based on the interviews, one concerning subtheme involved reports that some physicians wrote medical exemptions for vaccine-hesitant parents based on conditions such as allergies and autoimmune diseases.
“The Internet provides access to physicians who are willing to sign off on exemptions and to websites used to instruct parents on how to get physicians to approve medical exemptions,” the researchers said.
“Understanding how physicians interpret the law is important because they are writing the medical exemptions,” Dr. Mohanty and her associates noted, and they proposed increased outreach and education of physicians about the law to reduce problematic medical exemptions.
Many health officials expressed frustration with their inability to review medical exemptions submitted directly to schools. In fact, interviewees cited one California jurisdiction that was named in a lawsuit for attempting to track medical exemptions, “which had an impact on other jurisdictions decision to track,” they said.
Officials also expressed concern that parents’ use of medical exemptions to replace personal belief exemptions would reduce herd immunity. Overall, regions with high levels of personal belief exemptions showed the largest increases in medical exemptions after SB277, which could put these regions at increased risk for vaccine-preventable outbreaks, the researchers noted.
There also were reports of physicians “who advertised medical exemptions online for a fee.” Officials also reported “receiving medical exemptions signed by physicians who do not typically treat children (cardiologists, dermatologists, surgeons, and physicians at medical marijuana dispensaries) and by unauthorized nonphysician providers, including nurse practitioners,” Dr. Mohanty and her associates said.
The study findings were limited by several factors including small sample size and potential recall bias, the researchers noted. However, the study is the first to include perspectives of local health officials after a change in vaccine exemption policy.
The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
SOURCE: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
Passage of SB277 has had a positive impact on the proportion of California kindergarteners who are fully vaccinated, Richard J. Pan, MD, MPH, and Dorit Rubinstein Reiss, LLB, PhD, wrote in an editorial.
“Vaccines are one of the greatest public health successes in history. Mandating vaccination for school is an effective strategy to prevent outbreaks,” they said. However, “this protection is undermined when unscrupulous physicians monetize their license and abuse the authority delegated to them from the state by granting unwarranted [medical exemptions (MEs)],” they said.
The editorialists emphasized that states have the authority to mandate vaccinations in the interest of public health, and that allowing physicians to grant medical exemptions is appropriate because doctors know their patients and know whether exemptions are needed.
“However, the lack of cooperation by patients’ families who desire unwarranted MEs makes disciplining physicians who are engaged in this unprofessional behavior difficult and costly because licensing boards need to subpoena patient records over families’ objections to obtain evidence. Similarly, professional standard-setting organizations, including professional associations and certification boards, have been reluctant to withdraw credentials or expel members who promote vaccine misinformation and grant unwarranted MEs,” they said. They proposed strategies including establishing a searchable database for MEs, allowing public health officials the option to review and invalidate MEs, and requiring parents to submit MEs to public health departments as well as to schools.
“Pediatricians can partner with public health advocates and proscience parents to pass laws that empower public health officers to protect our children and community. Every child needs community immunity,” they said.
Dr. Pan is a California State Senator, Sacramento, and Dr. Reiss is at the Hastings College of the Law, University of California, San Francisco. Their comments on the article by Mohanty et al. were published in Pediatrics (2018;142[5]:e20182009). Dr. Pan authored legislation (Senate Bill 277) to abolish nonmedical exemption. Dr. Reiss’s family owns regular stock in GlaxoSmithKline.
Passage of SB277 has had a positive impact on the proportion of California kindergarteners who are fully vaccinated, Richard J. Pan, MD, MPH, and Dorit Rubinstein Reiss, LLB, PhD, wrote in an editorial.
“Vaccines are one of the greatest public health successes in history. Mandating vaccination for school is an effective strategy to prevent outbreaks,” they said. However, “this protection is undermined when unscrupulous physicians monetize their license and abuse the authority delegated to them from the state by granting unwarranted [medical exemptions (MEs)],” they said.
The editorialists emphasized that states have the authority to mandate vaccinations in the interest of public health, and that allowing physicians to grant medical exemptions is appropriate because doctors know their patients and know whether exemptions are needed.
“However, the lack of cooperation by patients’ families who desire unwarranted MEs makes disciplining physicians who are engaged in this unprofessional behavior difficult and costly because licensing boards need to subpoena patient records over families’ objections to obtain evidence. Similarly, professional standard-setting organizations, including professional associations and certification boards, have been reluctant to withdraw credentials or expel members who promote vaccine misinformation and grant unwarranted MEs,” they said. They proposed strategies including establishing a searchable database for MEs, allowing public health officials the option to review and invalidate MEs, and requiring parents to submit MEs to public health departments as well as to schools.
“Pediatricians can partner with public health advocates and proscience parents to pass laws that empower public health officers to protect our children and community. Every child needs community immunity,” they said.
Dr. Pan is a California State Senator, Sacramento, and Dr. Reiss is at the Hastings College of the Law, University of California, San Francisco. Their comments on the article by Mohanty et al. were published in Pediatrics (2018;142[5]:e20182009). Dr. Pan authored legislation (Senate Bill 277) to abolish nonmedical exemption. Dr. Reiss’s family owns regular stock in GlaxoSmithKline.
Passage of SB277 has had a positive impact on the proportion of California kindergarteners who are fully vaccinated, Richard J. Pan, MD, MPH, and Dorit Rubinstein Reiss, LLB, PhD, wrote in an editorial.
“Vaccines are one of the greatest public health successes in history. Mandating vaccination for school is an effective strategy to prevent outbreaks,” they said. However, “this protection is undermined when unscrupulous physicians monetize their license and abuse the authority delegated to them from the state by granting unwarranted [medical exemptions (MEs)],” they said.
The editorialists emphasized that states have the authority to mandate vaccinations in the interest of public health, and that allowing physicians to grant medical exemptions is appropriate because doctors know their patients and know whether exemptions are needed.
“However, the lack of cooperation by patients’ families who desire unwarranted MEs makes disciplining physicians who are engaged in this unprofessional behavior difficult and costly because licensing boards need to subpoena patient records over families’ objections to obtain evidence. Similarly, professional standard-setting organizations, including professional associations and certification boards, have been reluctant to withdraw credentials or expel members who promote vaccine misinformation and grant unwarranted MEs,” they said. They proposed strategies including establishing a searchable database for MEs, allowing public health officials the option to review and invalidate MEs, and requiring parents to submit MEs to public health departments as well as to schools.
“Pediatricians can partner with public health advocates and proscience parents to pass laws that empower public health officers to protect our children and community. Every child needs community immunity,” they said.
Dr. Pan is a California State Senator, Sacramento, and Dr. Reiss is at the Hastings College of the Law, University of California, San Francisco. Their comments on the article by Mohanty et al. were published in Pediatrics (2018;142[5]:e20182009). Dr. Pan authored legislation (Senate Bill 277) to abolish nonmedical exemption. Dr. Reiss’s family owns regular stock in GlaxoSmithKline.
according to data from interviews with health officials and immunization staff after implementation of the policy.
In a study published in Pediatrics, Salini Mohanty, DrPH, of the University of Pennsylvania School of Nursing, Philadelphia, and her colleagues conducted semistructured phone interviews with 40 health officers and immunization staff who represented 35 of 61 California heath jurisdictions. The interviews occurred between August 2017 and September 2017, and participants discussed their experiences with medical exemption requests after the policy change.
Although the percentage of fully vaccinated kindergarten students in California increased from 93% in 2015-2016 to 95% in 2017-2018, and the rate of personal belief exemptions declined, overall medical exemption requests rose 250% from 0.2% in 2015-2016 to 0.7% 2017-2018, the researchers noted.
They identified four main issues based on participant responses: the role of stakeholders, the review of medical exemptions received by schools, the medical exemptions perceived as problematic, and the general frustration and concern over medical exemptions.
Based on the interviews, one concerning subtheme involved reports that some physicians wrote medical exemptions for vaccine-hesitant parents based on conditions such as allergies and autoimmune diseases.
“The Internet provides access to physicians who are willing to sign off on exemptions and to websites used to instruct parents on how to get physicians to approve medical exemptions,” the researchers said.
“Understanding how physicians interpret the law is important because they are writing the medical exemptions,” Dr. Mohanty and her associates noted, and they proposed increased outreach and education of physicians about the law to reduce problematic medical exemptions.
Many health officials expressed frustration with their inability to review medical exemptions submitted directly to schools. In fact, interviewees cited one California jurisdiction that was named in a lawsuit for attempting to track medical exemptions, “which had an impact on other jurisdictions decision to track,” they said.
Officials also expressed concern that parents’ use of medical exemptions to replace personal belief exemptions would reduce herd immunity. Overall, regions with high levels of personal belief exemptions showed the largest increases in medical exemptions after SB277, which could put these regions at increased risk for vaccine-preventable outbreaks, the researchers noted.
There also were reports of physicians “who advertised medical exemptions online for a fee.” Officials also reported “receiving medical exemptions signed by physicians who do not typically treat children (cardiologists, dermatologists, surgeons, and physicians at medical marijuana dispensaries) and by unauthorized nonphysician providers, including nurse practitioners,” Dr. Mohanty and her associates said.
The study findings were limited by several factors including small sample size and potential recall bias, the researchers noted. However, the study is the first to include perspectives of local health officials after a change in vaccine exemption policy.
The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
SOURCE: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
according to data from interviews with health officials and immunization staff after implementation of the policy.
In a study published in Pediatrics, Salini Mohanty, DrPH, of the University of Pennsylvania School of Nursing, Philadelphia, and her colleagues conducted semistructured phone interviews with 40 health officers and immunization staff who represented 35 of 61 California heath jurisdictions. The interviews occurred between August 2017 and September 2017, and participants discussed their experiences with medical exemption requests after the policy change.
Although the percentage of fully vaccinated kindergarten students in California increased from 93% in 2015-2016 to 95% in 2017-2018, and the rate of personal belief exemptions declined, overall medical exemption requests rose 250% from 0.2% in 2015-2016 to 0.7% 2017-2018, the researchers noted.
They identified four main issues based on participant responses: the role of stakeholders, the review of medical exemptions received by schools, the medical exemptions perceived as problematic, and the general frustration and concern over medical exemptions.
Based on the interviews, one concerning subtheme involved reports that some physicians wrote medical exemptions for vaccine-hesitant parents based on conditions such as allergies and autoimmune diseases.
“The Internet provides access to physicians who are willing to sign off on exemptions and to websites used to instruct parents on how to get physicians to approve medical exemptions,” the researchers said.
“Understanding how physicians interpret the law is important because they are writing the medical exemptions,” Dr. Mohanty and her associates noted, and they proposed increased outreach and education of physicians about the law to reduce problematic medical exemptions.
Many health officials expressed frustration with their inability to review medical exemptions submitted directly to schools. In fact, interviewees cited one California jurisdiction that was named in a lawsuit for attempting to track medical exemptions, “which had an impact on other jurisdictions decision to track,” they said.
Officials also expressed concern that parents’ use of medical exemptions to replace personal belief exemptions would reduce herd immunity. Overall, regions with high levels of personal belief exemptions showed the largest increases in medical exemptions after SB277, which could put these regions at increased risk for vaccine-preventable outbreaks, the researchers noted.
There also were reports of physicians “who advertised medical exemptions online for a fee.” Officials also reported “receiving medical exemptions signed by physicians who do not typically treat children (cardiologists, dermatologists, surgeons, and physicians at medical marijuana dispensaries) and by unauthorized nonphysician providers, including nurse practitioners,” Dr. Mohanty and her associates said.
The study findings were limited by several factors including small sample size and potential recall bias, the researchers noted. However, the study is the first to include perspectives of local health officials after a change in vaccine exemption policy.
The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
SOURCE: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
FROM PEDIATRICS
Key clinical point: Medical exemptions for childhood vaccinations in California increased after the implementation of Senate Bill 277 (SB277) eliminating nonmedical exemptions.
Major finding: Medical exemptions in California increased by 250% after the SB277 took effect.
Study details: The data come from 34 interviews with 40 health officers and immunization staff about their experiences with medical exemptions before and after the passage of SB277.
Disclosures: The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
Source: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
Full-dose quadrivalent flu vaccine shows increased efficacy in children
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
REPORTING FROM AN ACIP MEETING
Vaccine protects against flu-related hospitalizations in pregnancy
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
FROM AN ACIP MEETING
ACIP resuscitates pertussis working group
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
AT AN ACIP MEETING
ACIP votes unanimously in favor of immunization schedule update and redesign
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
AT AN ACIP MEETING
ACIP supports hepatitis A vaccine for homeless individuals
Homeless individuals aged 1 year and older should be vaccinated against hepatitis A, based on a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“It is important that we take a national approach to vaccinating homeless” people, Noele Nelson, MD, PhD, MPH, of the CDC’s Division of Viral Hepatitis, said in a presentation prior to the vote, in which all 11 committee members voted in favor of hepatitis A vaccination for the homeless population.
Even limited vaccination will increase the herd immunity of the homeless population over time, she said.
Dr. Nelson presented data on the pros and cons of routine hepatitis A vaccination for homeless individuals aged 1 year and older. The Hepatitis Vaccines Work Group convened four meetings in advance of the October ACIP meeting and reached a consensus that homelessness is an independent indication for hepatitis A vaccination, she said.
If the hepatitis A vaccine is included as an ACIP recommendation, “it is more likely to be considered by homeless service providers,” noted Dr. Nelson. She also cited a low quality of evidence for adverse events associated with hepatitis A vaccination.
The work group considerations in the wake of a nationwide hepatitis A outbreak earlier in 2018 included the challenges of controlling outbreaks, which can spread quickly among the homeless population because of poor personal hygiene, limited sanitation, and tight living quarters. These factors make the homeless population more reliant on a vaccine for protection. An outbreak in San Diego, Calif., in particular, occurred largely in the homeless population.
“Routine vaccination is a more feasible approach to reach the homeless over time through regular homeless care providers,” Dr. Nelson said. As for costs, integrating vaccination into routine care for the homeless is cheaper and much less disruptive than the cost of responding to an outbreak.
The “cons” of recommending routine hepatitis A vaccination for the homeless population included the challenges of administrative record keeping. However, during the public comment period, Mae Morgan, MD, an internist who is medical director of Mercy Care Decatur Street & City of Refuge in Atlanta, emphasized that local homeless care organizations have procedures to manage routine vaccination. “If anyone is concerned that there is not a network in place, there are health centers to do this [that] would implement the vaccine.”
The ACIP committee members had no financial conflicts to disclose.
Homeless individuals aged 1 year and older should be vaccinated against hepatitis A, based on a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“It is important that we take a national approach to vaccinating homeless” people, Noele Nelson, MD, PhD, MPH, of the CDC’s Division of Viral Hepatitis, said in a presentation prior to the vote, in which all 11 committee members voted in favor of hepatitis A vaccination for the homeless population.
Even limited vaccination will increase the herd immunity of the homeless population over time, she said.
Dr. Nelson presented data on the pros and cons of routine hepatitis A vaccination for homeless individuals aged 1 year and older. The Hepatitis Vaccines Work Group convened four meetings in advance of the October ACIP meeting and reached a consensus that homelessness is an independent indication for hepatitis A vaccination, she said.
If the hepatitis A vaccine is included as an ACIP recommendation, “it is more likely to be considered by homeless service providers,” noted Dr. Nelson. She also cited a low quality of evidence for adverse events associated with hepatitis A vaccination.
The work group considerations in the wake of a nationwide hepatitis A outbreak earlier in 2018 included the challenges of controlling outbreaks, which can spread quickly among the homeless population because of poor personal hygiene, limited sanitation, and tight living quarters. These factors make the homeless population more reliant on a vaccine for protection. An outbreak in San Diego, Calif., in particular, occurred largely in the homeless population.
“Routine vaccination is a more feasible approach to reach the homeless over time through regular homeless care providers,” Dr. Nelson said. As for costs, integrating vaccination into routine care for the homeless is cheaper and much less disruptive than the cost of responding to an outbreak.
The “cons” of recommending routine hepatitis A vaccination for the homeless population included the challenges of administrative record keeping. However, during the public comment period, Mae Morgan, MD, an internist who is medical director of Mercy Care Decatur Street & City of Refuge in Atlanta, emphasized that local homeless care organizations have procedures to manage routine vaccination. “If anyone is concerned that there is not a network in place, there are health centers to do this [that] would implement the vaccine.”
The ACIP committee members had no financial conflicts to disclose.
Homeless individuals aged 1 year and older should be vaccinated against hepatitis A, based on a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“It is important that we take a national approach to vaccinating homeless” people, Noele Nelson, MD, PhD, MPH, of the CDC’s Division of Viral Hepatitis, said in a presentation prior to the vote, in which all 11 committee members voted in favor of hepatitis A vaccination for the homeless population.
Even limited vaccination will increase the herd immunity of the homeless population over time, she said.
Dr. Nelson presented data on the pros and cons of routine hepatitis A vaccination for homeless individuals aged 1 year and older. The Hepatitis Vaccines Work Group convened four meetings in advance of the October ACIP meeting and reached a consensus that homelessness is an independent indication for hepatitis A vaccination, she said.
If the hepatitis A vaccine is included as an ACIP recommendation, “it is more likely to be considered by homeless service providers,” noted Dr. Nelson. She also cited a low quality of evidence for adverse events associated with hepatitis A vaccination.
The work group considerations in the wake of a nationwide hepatitis A outbreak earlier in 2018 included the challenges of controlling outbreaks, which can spread quickly among the homeless population because of poor personal hygiene, limited sanitation, and tight living quarters. These factors make the homeless population more reliant on a vaccine for protection. An outbreak in San Diego, Calif., in particular, occurred largely in the homeless population.
“Routine vaccination is a more feasible approach to reach the homeless over time through regular homeless care providers,” Dr. Nelson said. As for costs, integrating vaccination into routine care for the homeless is cheaper and much less disruptive than the cost of responding to an outbreak.
The “cons” of recommending routine hepatitis A vaccination for the homeless population included the challenges of administrative record keeping. However, during the public comment period, Mae Morgan, MD, an internist who is medical director of Mercy Care Decatur Street & City of Refuge in Atlanta, emphasized that local homeless care organizations have procedures to manage routine vaccination. “If anyone is concerned that there is not a network in place, there are health centers to do this [that] would implement the vaccine.”
The ACIP committee members had no financial conflicts to disclose.
FROM AN ACIP MEETING