User login
Split-dose oxycodone protocol reduces opioid use after cesarean
according to a study published in Obstetrics & Gynecology.
A retrospective study reviewed medical records of 1,050 women undergoing cesarean delivery, 508 of whom were treated after a change in protocol for postdelivery oxycodone orders. Instead of a 5-mg oral dose given for a verbal pain score of 4/10 or below and 10 mg for a pain score of 5-10/10, patients were given 2.5-mg or 5-mg dose respectively, with a nurse check after 1 hour to see if more of the same dosage was needed.
The split-dose approach was associated with a 56% reduction in median opioid consumption in the first 48 hours after cesarean delivery; 10 mg before the change in practice to 4.4 mg after it. There was also a 6.9-percentage-point decrease in the number of patients needing any postoperative opioids.
While the study did show a slight increase in average verbal pain scores in the first 58 hours after surgery – from a mean of 1.8 before the split-dose protocol was introduced to 2 after it was introduced – there was no increase in the use of nonsteroidal anti-inflammatory drugs, acetaminophen, or gabapentin, and no difference in peak verbal pain scores.
“Our goal with the introduction of this new order set was to use a patient-centered, response-feedback approach to postcesarean delivery analgesia in the form of split doses of oxycodone rather than the traditional standard dose model,” wrote Jalal A. Nanji, MD, of the department of anesthesiology and pain medicine at the University of Alberta, Edmonton, and coauthors. “Involving patients in the decision for how much postcesarean delivery analgesia they will receive has been found to reduce opioid use and improve maternal satisfaction.”
The number of patients reporting postoperative nausea or vomiting was halved in those treated with the split-dose regimen, with no difference in mean overall patient satisfaction score.
Dr. Nanji and associates wrote that women viewed avoiding nausea or vomiting after a cesarean as a high priority, and targeting the root cause – excessive opioid use – was preferable to treating nausea and vomiting with antiemetics.
They also noted that input from nursing staff was vital in developing the new split-order set, not only because it directly affected nursing work flow but also to optimize the process.
“With the opioid epidemic on the rise and the increase in efforts by physicians to decrease outpatient opioid prescriptions, this study is extremely relevant and timely,” commented Marissa Platner, MD, an assistant professor in maternal-fetal medicine at Emory University, Atlanta.
“Although this study is retrospective and, therefore, there are inherent biases and an inability to control all contributing factors, it clearly demonstrates that, overall, there seem to be improved outcomes with split-dose protocol of opioid administration during the postoperative period in terms of overall patient satisfaction, opioid consumption, and postoperative nausea and vomiting. The patient-centered nature and response-feedback design of this study also contributes to its strength and improves its generalizability. In order to encourage others to considering adapting protocol in other institutions, it should be evaluated via a randomized controlled trial," Dr. Platner said in an interview.*
"The premise and execution of this study were novel and interesting," commented Katrina Mark, MD, associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. "The authors found that by decreasing the standard doses of oxycodone ordered after a cesarean section and asking women if they desired better pain control, rather than reacting only to a pain score, patients’ overall postoperative usage of opiates also decreased. In decreasing the amount of opiates used, the authors also observed a decrease some of the side effects associated with opiate use, which is promising.
"This study, among other recent studies, highlights the fact that postoperative prescribing standards are not evidence-based and may lead to overprescribing of opiates. Improving prescribing practices is a noble and important goal. In this study, a change in clinical practice among both nurses and prescribers is likely what caused the greatest change. The use of a protocol which prescribed oxycodone based on asking if a woman desired improved pain control, rather than prescribing only based on her pain score response, makes a lot of intuitive sense. Decreasing opioid consumption requires education of healthcare providers and patients, and protocols like this one will help to encourage that conversation," she noted in an interview.
"Before the findings of this study can be widely adopted, however, there are two major points that will need to be addressed," Dr. Mark emphasized. "The first is patient satisfaction. The peak pain scores were not different between the groups, but the mean pain scores were. The authors deemed this clinically insignificant, which it may be. However, without the patients’ perspective on this new protocol, it is difficult to tell if the opioid usage decreased because women actually needed less opiates or if it decreased because the system discouraged opioid use and made it more challenging for them to obtain the medicine they needed to achieve adequate pain control. The desire to decrease opioid prescribing is warranted, and likely completely appropriate, but there is certainly a role for opioids in pain management. We should not be so motivated to decrease use that we cause unnecessary suffering. The second point that will need to be addressed is the effect on nursing practice. There was no standardized evaluation of the impact that this protocol had on the nursing staff, and it is unclear if this protocol would require greater resources than may be readily available at all hospitals."**
The study was supported by the department of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. One author declared travel funding from a university. No other conflicts of interest were declared. Dr. Platner and Dr. Mark also had no relevant financial disclosures.*
SOURCE: Nanji J et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003305.
*This article was updated on 7/15/2019.
**It was updated again on 7/17/2019.
according to a study published in Obstetrics & Gynecology.
A retrospective study reviewed medical records of 1,050 women undergoing cesarean delivery, 508 of whom were treated after a change in protocol for postdelivery oxycodone orders. Instead of a 5-mg oral dose given for a verbal pain score of 4/10 or below and 10 mg for a pain score of 5-10/10, patients were given 2.5-mg or 5-mg dose respectively, with a nurse check after 1 hour to see if more of the same dosage was needed.
The split-dose approach was associated with a 56% reduction in median opioid consumption in the first 48 hours after cesarean delivery; 10 mg before the change in practice to 4.4 mg after it. There was also a 6.9-percentage-point decrease in the number of patients needing any postoperative opioids.
While the study did show a slight increase in average verbal pain scores in the first 58 hours after surgery – from a mean of 1.8 before the split-dose protocol was introduced to 2 after it was introduced – there was no increase in the use of nonsteroidal anti-inflammatory drugs, acetaminophen, or gabapentin, and no difference in peak verbal pain scores.
“Our goal with the introduction of this new order set was to use a patient-centered, response-feedback approach to postcesarean delivery analgesia in the form of split doses of oxycodone rather than the traditional standard dose model,” wrote Jalal A. Nanji, MD, of the department of anesthesiology and pain medicine at the University of Alberta, Edmonton, and coauthors. “Involving patients in the decision for how much postcesarean delivery analgesia they will receive has been found to reduce opioid use and improve maternal satisfaction.”
The number of patients reporting postoperative nausea or vomiting was halved in those treated with the split-dose regimen, with no difference in mean overall patient satisfaction score.
Dr. Nanji and associates wrote that women viewed avoiding nausea or vomiting after a cesarean as a high priority, and targeting the root cause – excessive opioid use – was preferable to treating nausea and vomiting with antiemetics.
They also noted that input from nursing staff was vital in developing the new split-order set, not only because it directly affected nursing work flow but also to optimize the process.
“With the opioid epidemic on the rise and the increase in efforts by physicians to decrease outpatient opioid prescriptions, this study is extremely relevant and timely,” commented Marissa Platner, MD, an assistant professor in maternal-fetal medicine at Emory University, Atlanta.
“Although this study is retrospective and, therefore, there are inherent biases and an inability to control all contributing factors, it clearly demonstrates that, overall, there seem to be improved outcomes with split-dose protocol of opioid administration during the postoperative period in terms of overall patient satisfaction, opioid consumption, and postoperative nausea and vomiting. The patient-centered nature and response-feedback design of this study also contributes to its strength and improves its generalizability. In order to encourage others to considering adapting protocol in other institutions, it should be evaluated via a randomized controlled trial," Dr. Platner said in an interview.*
"The premise and execution of this study were novel and interesting," commented Katrina Mark, MD, associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. "The authors found that by decreasing the standard doses of oxycodone ordered after a cesarean section and asking women if they desired better pain control, rather than reacting only to a pain score, patients’ overall postoperative usage of opiates also decreased. In decreasing the amount of opiates used, the authors also observed a decrease some of the side effects associated with opiate use, which is promising.
"This study, among other recent studies, highlights the fact that postoperative prescribing standards are not evidence-based and may lead to overprescribing of opiates. Improving prescribing practices is a noble and important goal. In this study, a change in clinical practice among both nurses and prescribers is likely what caused the greatest change. The use of a protocol which prescribed oxycodone based on asking if a woman desired improved pain control, rather than prescribing only based on her pain score response, makes a lot of intuitive sense. Decreasing opioid consumption requires education of healthcare providers and patients, and protocols like this one will help to encourage that conversation," she noted in an interview.
"Before the findings of this study can be widely adopted, however, there are two major points that will need to be addressed," Dr. Mark emphasized. "The first is patient satisfaction. The peak pain scores were not different between the groups, but the mean pain scores were. The authors deemed this clinically insignificant, which it may be. However, without the patients’ perspective on this new protocol, it is difficult to tell if the opioid usage decreased because women actually needed less opiates or if it decreased because the system discouraged opioid use and made it more challenging for them to obtain the medicine they needed to achieve adequate pain control. The desire to decrease opioid prescribing is warranted, and likely completely appropriate, but there is certainly a role for opioids in pain management. We should not be so motivated to decrease use that we cause unnecessary suffering. The second point that will need to be addressed is the effect on nursing practice. There was no standardized evaluation of the impact that this protocol had on the nursing staff, and it is unclear if this protocol would require greater resources than may be readily available at all hospitals."**
The study was supported by the department of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. One author declared travel funding from a university. No other conflicts of interest were declared. Dr. Platner and Dr. Mark also had no relevant financial disclosures.*
SOURCE: Nanji J et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003305.
*This article was updated on 7/15/2019.
**It was updated again on 7/17/2019.
according to a study published in Obstetrics & Gynecology.
A retrospective study reviewed medical records of 1,050 women undergoing cesarean delivery, 508 of whom were treated after a change in protocol for postdelivery oxycodone orders. Instead of a 5-mg oral dose given for a verbal pain score of 4/10 or below and 10 mg for a pain score of 5-10/10, patients were given 2.5-mg or 5-mg dose respectively, with a nurse check after 1 hour to see if more of the same dosage was needed.
The split-dose approach was associated with a 56% reduction in median opioid consumption in the first 48 hours after cesarean delivery; 10 mg before the change in practice to 4.4 mg after it. There was also a 6.9-percentage-point decrease in the number of patients needing any postoperative opioids.
While the study did show a slight increase in average verbal pain scores in the first 58 hours after surgery – from a mean of 1.8 before the split-dose protocol was introduced to 2 after it was introduced – there was no increase in the use of nonsteroidal anti-inflammatory drugs, acetaminophen, or gabapentin, and no difference in peak verbal pain scores.
“Our goal with the introduction of this new order set was to use a patient-centered, response-feedback approach to postcesarean delivery analgesia in the form of split doses of oxycodone rather than the traditional standard dose model,” wrote Jalal A. Nanji, MD, of the department of anesthesiology and pain medicine at the University of Alberta, Edmonton, and coauthors. “Involving patients in the decision for how much postcesarean delivery analgesia they will receive has been found to reduce opioid use and improve maternal satisfaction.”
The number of patients reporting postoperative nausea or vomiting was halved in those treated with the split-dose regimen, with no difference in mean overall patient satisfaction score.
Dr. Nanji and associates wrote that women viewed avoiding nausea or vomiting after a cesarean as a high priority, and targeting the root cause – excessive opioid use – was preferable to treating nausea and vomiting with antiemetics.
They also noted that input from nursing staff was vital in developing the new split-order set, not only because it directly affected nursing work flow but also to optimize the process.
“With the opioid epidemic on the rise and the increase in efforts by physicians to decrease outpatient opioid prescriptions, this study is extremely relevant and timely,” commented Marissa Platner, MD, an assistant professor in maternal-fetal medicine at Emory University, Atlanta.
“Although this study is retrospective and, therefore, there are inherent biases and an inability to control all contributing factors, it clearly demonstrates that, overall, there seem to be improved outcomes with split-dose protocol of opioid administration during the postoperative period in terms of overall patient satisfaction, opioid consumption, and postoperative nausea and vomiting. The patient-centered nature and response-feedback design of this study also contributes to its strength and improves its generalizability. In order to encourage others to considering adapting protocol in other institutions, it should be evaluated via a randomized controlled trial," Dr. Platner said in an interview.*
"The premise and execution of this study were novel and interesting," commented Katrina Mark, MD, associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. "The authors found that by decreasing the standard doses of oxycodone ordered after a cesarean section and asking women if they desired better pain control, rather than reacting only to a pain score, patients’ overall postoperative usage of opiates also decreased. In decreasing the amount of opiates used, the authors also observed a decrease some of the side effects associated with opiate use, which is promising.
"This study, among other recent studies, highlights the fact that postoperative prescribing standards are not evidence-based and may lead to overprescribing of opiates. Improving prescribing practices is a noble and important goal. In this study, a change in clinical practice among both nurses and prescribers is likely what caused the greatest change. The use of a protocol which prescribed oxycodone based on asking if a woman desired improved pain control, rather than prescribing only based on her pain score response, makes a lot of intuitive sense. Decreasing opioid consumption requires education of healthcare providers and patients, and protocols like this one will help to encourage that conversation," she noted in an interview.
"Before the findings of this study can be widely adopted, however, there are two major points that will need to be addressed," Dr. Mark emphasized. "The first is patient satisfaction. The peak pain scores were not different between the groups, but the mean pain scores were. The authors deemed this clinically insignificant, which it may be. However, without the patients’ perspective on this new protocol, it is difficult to tell if the opioid usage decreased because women actually needed less opiates or if it decreased because the system discouraged opioid use and made it more challenging for them to obtain the medicine they needed to achieve adequate pain control. The desire to decrease opioid prescribing is warranted, and likely completely appropriate, but there is certainly a role for opioids in pain management. We should not be so motivated to decrease use that we cause unnecessary suffering. The second point that will need to be addressed is the effect on nursing practice. There was no standardized evaluation of the impact that this protocol had on the nursing staff, and it is unclear if this protocol would require greater resources than may be readily available at all hospitals."**
The study was supported by the department of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. One author declared travel funding from a university. No other conflicts of interest were declared. Dr. Platner and Dr. Mark also had no relevant financial disclosures.*
SOURCE: Nanji J et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003305.
*This article was updated on 7/15/2019.
**It was updated again on 7/17/2019.
FROM OBSTETRICS & GYNECOLOGY
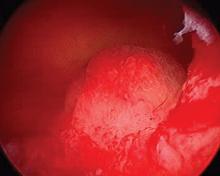
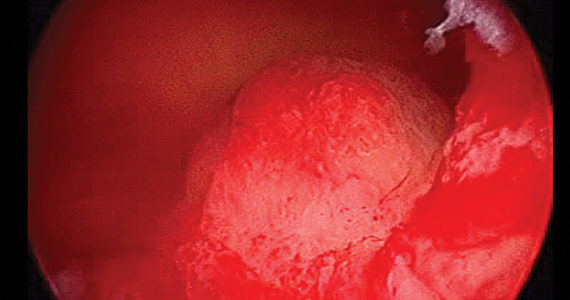
Consider cutaneous endometriosis in women with umbilical lesions
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
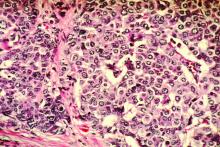
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
The FDA has revised its guidance on fish consumption
The revision touts the health benefits of fish and shellfish and promotes safer fish choices for those who should limit mercury exposure – including women who are or might become pregnant, women who are breastfeeding, and young children.
Those individuals should avoid commercial fish with the highest levels of mercury and should instead choose from “the many types of fish that are lower in mercury – including ones commonly found in grocery stores, such as salmon, shrimp, pollock, canned light tuna, tilapia, catfish, and cod,” according to an FDA press statement.
The potential health benefits of eating fish were highlighted in the U.S. Department of Health and Human Services/Department of Agriculture 2015-2020 Dietary Guidelines for Americans, and in 2017 the FDA and the Environmental Protection Agency released advice on fish consumption, including a user-friendly reference chart regarding mercury levels in various types of fish.
Although the information in the chart has not changed, the FDA revised its advice to expand on the “information about the benefits of fish as part of healthy eating patterns by promoting the science-based recommendations of the 2015-2020 Dietary Guidelines for Americans.”
The advice calls for consumption of at least 8 ounces of seafood per week for adults (less for children) based on a 2,000 calorie diet, and for women who are pregnant or breastfeeding, 8-12 ounces of a variety of seafood per week selected from choices lower in mercury.
“The FDA’s revised advice highlights the many nutrients found in fish, several of which have important roles in growth and development during pregnancy and early childhood. It also highlights the potential health benefits of eating fish as part of a healthy eating pattern, particularly for improving heart health and lowering the risk of obesity,” the press release states.
Despite these benefits – and the recommendations for intake – concerns about mercury in fish have led many pregnant women in the United States to consume far less than the recommended amount of seafood, according to Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition.
“Our goal is to make sure Americans are equipped with this knowledge so that they can reap the benefits of eating fish, while choosing types of fish that are safe for them and their families to eat,” Dr. Mayne said in the FDA statement.
In response to the revised guidance, John S. Cullen, MD, president of the American Academy of Family Physicians, said that all women should be counseled to eat a well-balanced and varied diet including meats, dairy products, fruits, vegetables, and grains, and pregnant women should limit their intake of fish and seafood products to 8-12 ounces, or about 2-3 fish meals, per week.
Pregnant women may eat salmon in moderation, but should avoid raw seafood of any type because of possible contamination with parasites and Norwalk-like viruses, he said, adding that seafood like shark, swordfish, king mackerel, tilefish, Bigeye (Ahi) tuna steaks, and other long-lived fish high on the food chain should be avoided completely because of high mercury levels.
“While the AAFP did not review the revised advice to the dietary guidelines, family physicians are on the front lines encouraging healthy nutrition for pregnant and breastfeeding women and young children. It’s an ongoing, important part of the patient-physician conversation that begins with the initial prenatal visit,” Dr. Cullen said in a statement.
Similarly, Christopher M. Zahn, MD, vice president of practice activities for the American College of Obstetricians and Gynecologists, said the FDA/EPA updated guidance is in line with ACOG recommendations.
“The guidance continues to underscore the value of eating seafood 2-3 times per week during pregnancy and the importance of avoiding fish products that are high in mercury. The additional emphasis on healthy eating patterns mirrors ACOG’s long-standing guidance on the importance of a well-balanced, varied, nutritional diet that is consistent with a woman’s access to food and food preferences,” he said in a statement, noting that “seafood is a nutrient-rich food that has proven beneficial to women and in aiding the development of a fetus throughout pregnancy.”
The revision touts the health benefits of fish and shellfish and promotes safer fish choices for those who should limit mercury exposure – including women who are or might become pregnant, women who are breastfeeding, and young children.
Those individuals should avoid commercial fish with the highest levels of mercury and should instead choose from “the many types of fish that are lower in mercury – including ones commonly found in grocery stores, such as salmon, shrimp, pollock, canned light tuna, tilapia, catfish, and cod,” according to an FDA press statement.
The potential health benefits of eating fish were highlighted in the U.S. Department of Health and Human Services/Department of Agriculture 2015-2020 Dietary Guidelines for Americans, and in 2017 the FDA and the Environmental Protection Agency released advice on fish consumption, including a user-friendly reference chart regarding mercury levels in various types of fish.
Although the information in the chart has not changed, the FDA revised its advice to expand on the “information about the benefits of fish as part of healthy eating patterns by promoting the science-based recommendations of the 2015-2020 Dietary Guidelines for Americans.”
The advice calls for consumption of at least 8 ounces of seafood per week for adults (less for children) based on a 2,000 calorie diet, and for women who are pregnant or breastfeeding, 8-12 ounces of a variety of seafood per week selected from choices lower in mercury.
“The FDA’s revised advice highlights the many nutrients found in fish, several of which have important roles in growth and development during pregnancy and early childhood. It also highlights the potential health benefits of eating fish as part of a healthy eating pattern, particularly for improving heart health and lowering the risk of obesity,” the press release states.
Despite these benefits – and the recommendations for intake – concerns about mercury in fish have led many pregnant women in the United States to consume far less than the recommended amount of seafood, according to Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition.
“Our goal is to make sure Americans are equipped with this knowledge so that they can reap the benefits of eating fish, while choosing types of fish that are safe for them and their families to eat,” Dr. Mayne said in the FDA statement.
In response to the revised guidance, John S. Cullen, MD, president of the American Academy of Family Physicians, said that all women should be counseled to eat a well-balanced and varied diet including meats, dairy products, fruits, vegetables, and grains, and pregnant women should limit their intake of fish and seafood products to 8-12 ounces, or about 2-3 fish meals, per week.
Pregnant women may eat salmon in moderation, but should avoid raw seafood of any type because of possible contamination with parasites and Norwalk-like viruses, he said, adding that seafood like shark, swordfish, king mackerel, tilefish, Bigeye (Ahi) tuna steaks, and other long-lived fish high on the food chain should be avoided completely because of high mercury levels.
“While the AAFP did not review the revised advice to the dietary guidelines, family physicians are on the front lines encouraging healthy nutrition for pregnant and breastfeeding women and young children. It’s an ongoing, important part of the patient-physician conversation that begins with the initial prenatal visit,” Dr. Cullen said in a statement.
Similarly, Christopher M. Zahn, MD, vice president of practice activities for the American College of Obstetricians and Gynecologists, said the FDA/EPA updated guidance is in line with ACOG recommendations.
“The guidance continues to underscore the value of eating seafood 2-3 times per week during pregnancy and the importance of avoiding fish products that are high in mercury. The additional emphasis on healthy eating patterns mirrors ACOG’s long-standing guidance on the importance of a well-balanced, varied, nutritional diet that is consistent with a woman’s access to food and food preferences,” he said in a statement, noting that “seafood is a nutrient-rich food that has proven beneficial to women and in aiding the development of a fetus throughout pregnancy.”
The revision touts the health benefits of fish and shellfish and promotes safer fish choices for those who should limit mercury exposure – including women who are or might become pregnant, women who are breastfeeding, and young children.
Those individuals should avoid commercial fish with the highest levels of mercury and should instead choose from “the many types of fish that are lower in mercury – including ones commonly found in grocery stores, such as salmon, shrimp, pollock, canned light tuna, tilapia, catfish, and cod,” according to an FDA press statement.
The potential health benefits of eating fish were highlighted in the U.S. Department of Health and Human Services/Department of Agriculture 2015-2020 Dietary Guidelines for Americans, and in 2017 the FDA and the Environmental Protection Agency released advice on fish consumption, including a user-friendly reference chart regarding mercury levels in various types of fish.
Although the information in the chart has not changed, the FDA revised its advice to expand on the “information about the benefits of fish as part of healthy eating patterns by promoting the science-based recommendations of the 2015-2020 Dietary Guidelines for Americans.”
The advice calls for consumption of at least 8 ounces of seafood per week for adults (less for children) based on a 2,000 calorie diet, and for women who are pregnant or breastfeeding, 8-12 ounces of a variety of seafood per week selected from choices lower in mercury.
“The FDA’s revised advice highlights the many nutrients found in fish, several of which have important roles in growth and development during pregnancy and early childhood. It also highlights the potential health benefits of eating fish as part of a healthy eating pattern, particularly for improving heart health and lowering the risk of obesity,” the press release states.
Despite these benefits – and the recommendations for intake – concerns about mercury in fish have led many pregnant women in the United States to consume far less than the recommended amount of seafood, according to Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition.
“Our goal is to make sure Americans are equipped with this knowledge so that they can reap the benefits of eating fish, while choosing types of fish that are safe for them and their families to eat,” Dr. Mayne said in the FDA statement.
In response to the revised guidance, John S. Cullen, MD, president of the American Academy of Family Physicians, said that all women should be counseled to eat a well-balanced and varied diet including meats, dairy products, fruits, vegetables, and grains, and pregnant women should limit their intake of fish and seafood products to 8-12 ounces, or about 2-3 fish meals, per week.
Pregnant women may eat salmon in moderation, but should avoid raw seafood of any type because of possible contamination with parasites and Norwalk-like viruses, he said, adding that seafood like shark, swordfish, king mackerel, tilefish, Bigeye (Ahi) tuna steaks, and other long-lived fish high on the food chain should be avoided completely because of high mercury levels.
“While the AAFP did not review the revised advice to the dietary guidelines, family physicians are on the front lines encouraging healthy nutrition for pregnant and breastfeeding women and young children. It’s an ongoing, important part of the patient-physician conversation that begins with the initial prenatal visit,” Dr. Cullen said in a statement.
Similarly, Christopher M. Zahn, MD, vice president of practice activities for the American College of Obstetricians and Gynecologists, said the FDA/EPA updated guidance is in line with ACOG recommendations.
“The guidance continues to underscore the value of eating seafood 2-3 times per week during pregnancy and the importance of avoiding fish products that are high in mercury. The additional emphasis on healthy eating patterns mirrors ACOG’s long-standing guidance on the importance of a well-balanced, varied, nutritional diet that is consistent with a woman’s access to food and food preferences,” he said in a statement, noting that “seafood is a nutrient-rich food that has proven beneficial to women and in aiding the development of a fetus throughout pregnancy.”
Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Claims data suggest endometriosis ups risk of chronic opioid use
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
REPORTING FROM ACOG 2019
2019 Update on abnormal uterine bleeding
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Breast density alone should not prompt supplemental imaging discussions
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
FROM JAMA INTERNAL MEDICINE
Infertility: A practical framework
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
KEY POINTS
- A primary care physician can provide advice and testing regarding most fertility concerns.
- Female reproductive aging is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 and older.
- Male factor infertility can now often be overcome with assisted reproductive technologies.
- Polycystic ovary syndrome can cause anovulation and has metabolic effects that can evolve into metabolic syndrome, with serious health consequences.
Long-term trend: Women receiving fewer pelvic exams
according to the National Center for Health Statistics.
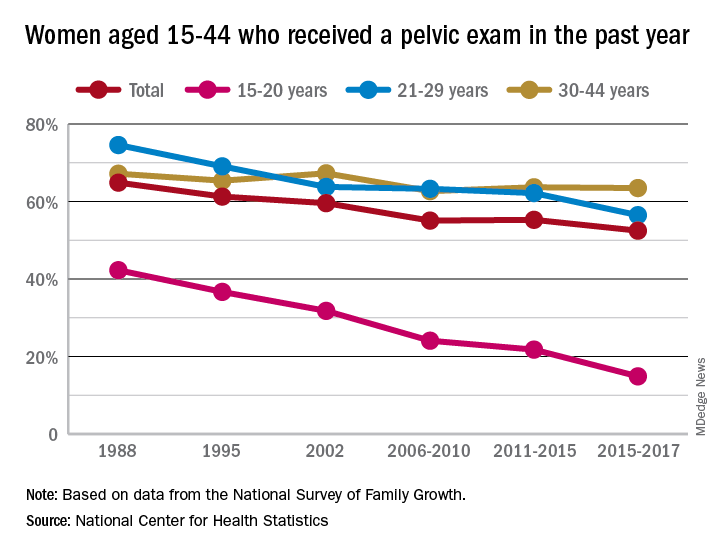
Sixty-five percent of women aged 15-44 years had received a pelvic examination in the past year when asked in 1988 as part of the National Survey of Family Growth, but the 3-year average for the 2015-2017 surveys was 53%, a significant decline, the NCHS said in a recent report.
The decrease was seen in all three of the age subgroups – 15-20 years, 21-29 years, and 30-44 years – over the length of the study period, with the trend in only the oldest women not reaching significance. The 30-44 group also was the only one of the three in which the rate ever increased at any point, the survey data show.
Data for other subgroups focused on the last 3-year period. From 2015 to 2017, non-Hispanic black women were more likely to have received a pelvic examination in the past year (60%) than were non-Hispanic white (54%) or Hispanic women (45%). An association with education level also was seen: Women with a bachelor’s degree or higher were most likely to get an exam (69%), and those with less than a high-school degree were least likely (52%), the researchers reported.
In 2018, the American College of Obstetricians and Gynecologists altered its recommendation that annual pelvic examinations be part of the well-woman visit for those aged 21 years and over, advising instead “that pelvic examinations be performed when indicated by medical history or symptoms,” the NCHS authors explained. They also suggested that their data “could provide a benchmark for estimates of the prevalence of pelvic examinations before the 2018 ACOG-updated guidelines.”
according to the National Center for Health Statistics.

Sixty-five percent of women aged 15-44 years had received a pelvic examination in the past year when asked in 1988 as part of the National Survey of Family Growth, but the 3-year average for the 2015-2017 surveys was 53%, a significant decline, the NCHS said in a recent report.
The decrease was seen in all three of the age subgroups – 15-20 years, 21-29 years, and 30-44 years – over the length of the study period, with the trend in only the oldest women not reaching significance. The 30-44 group also was the only one of the three in which the rate ever increased at any point, the survey data show.
Data for other subgroups focused on the last 3-year period. From 2015 to 2017, non-Hispanic black women were more likely to have received a pelvic examination in the past year (60%) than were non-Hispanic white (54%) or Hispanic women (45%). An association with education level also was seen: Women with a bachelor’s degree or higher were most likely to get an exam (69%), and those with less than a high-school degree were least likely (52%), the researchers reported.
In 2018, the American College of Obstetricians and Gynecologists altered its recommendation that annual pelvic examinations be part of the well-woman visit for those aged 21 years and over, advising instead “that pelvic examinations be performed when indicated by medical history or symptoms,” the NCHS authors explained. They also suggested that their data “could provide a benchmark for estimates of the prevalence of pelvic examinations before the 2018 ACOG-updated guidelines.”
according to the National Center for Health Statistics.

Sixty-five percent of women aged 15-44 years had received a pelvic examination in the past year when asked in 1988 as part of the National Survey of Family Growth, but the 3-year average for the 2015-2017 surveys was 53%, a significant decline, the NCHS said in a recent report.
The decrease was seen in all three of the age subgroups – 15-20 years, 21-29 years, and 30-44 years – over the length of the study period, with the trend in only the oldest women not reaching significance. The 30-44 group also was the only one of the three in which the rate ever increased at any point, the survey data show.
Data for other subgroups focused on the last 3-year period. From 2015 to 2017, non-Hispanic black women were more likely to have received a pelvic examination in the past year (60%) than were non-Hispanic white (54%) or Hispanic women (45%). An association with education level also was seen: Women with a bachelor’s degree or higher were most likely to get an exam (69%), and those with less than a high-school degree were least likely (52%), the researchers reported.
In 2018, the American College of Obstetricians and Gynecologists altered its recommendation that annual pelvic examinations be part of the well-woman visit for those aged 21 years and over, advising instead “that pelvic examinations be performed when indicated by medical history or symptoms,” the NCHS authors explained. They also suggested that their data “could provide a benchmark for estimates of the prevalence of pelvic examinations before the 2018 ACOG-updated guidelines.”
Dr. Eve Espey: Some good news in her 2019 contraceptive update
NASHVILLE, TENN. – There’s some good news on the contraception and reproductive health front, according to a recent update from Eve Espey, MD.
The unintended pregnancy rate in the United States, including among adolescents and young women, is declining, and the U.S. abortion rate is at its lowest level since Roe v. Wade, she said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
A 2016 article based on 2008-2011 data showed that after hovering around 50% for nearly 3 decades, the unintended pregnancy rate dropped “for the first time in a very long period of time,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology, division of family planning at the University of New Mexico, Albuquerque (N Engl J Med. 2016; 374[9]:843-52).
“It doesn’t look that impressive – it basically went down to 45%, but considering the scope and the number of women who are affected by unplanned pregnancy, this is actually a huge public health achievement,” she said. “And I think ... in the next cycles of the [Center for Disease Control and Prevention’s] National Survey of Family Growth ... we’ll hopefully continue to see this and potentially more [decline].”
As for abortion rates, an increase occurred following Roe v. Wade, but rates are now down to pre-Roe levels.
“One of the things that we know about the abortion rate is that the most important determinant ... is access to contraceptives,” Dr. Espey said, noting that both the abortion and unintended pregnancy rate declines are attributable to better and more consistent use of contraceptives, increased abstinence as teens are waiting longer to have sex, and the “meteoric rise in long-acting reversible contraceptive (LARC) use.”
Importantly, while improvements in public health have traditionally only impacted upper-class white women, a reduction is finally occurring in disparities with women of color, but those disparities still remain,” she added. “Just like we’re focusing so much on this relative to maternal mortality, the same kinds of disparities occur in access to reproductive health.”
Dr. Espey also provided updates on other aspects of contraception.
IUDs and other LARC methods
The use of LARCs increased from 2% of contraceptive types used by reproductive-aged women in 2002 to 12% in 2012. The majority of that change was in IUD use, with a small increase in implant use, she said, noting that the latest data from the 2015-2017 cycle of the National Survey of Family Growth shows that the rate is now up to 16%.
“The rise has been nothing that I ever imagined that I would see, certainly in my professional career,” she said.
The huge impact of LARCs on the unintended pregnancy rate is attributable to consistent effectiveness over time, compared with an increasing failure rate over time with short-acting contraceptive methods, she said, explaining that while the failure rate with oral contraceptives is about 8%-9% over the first 3 years, it increases to 53% at 8 years.
It’s a matter of looking at both “typical use” effectiveness and continuation rates: LARCs have continuation rates of about 75%-85%; Depot-Provera, for example, has a 25%-30% continuation rate at 1 year, she noted.
Dr. Espey also attributed the gains to improved access via the Affordable Care Act’s contraceptive mandate, which has been shown in numerous studies to have improved access and consistency of contraceptive use, but which is “currently being chipped away,” and to the federal Title X program that covers family planning care for low income women, including undocumented women.
“These two programs have made a huge impact for us, and I hope that we as ob.gyns. will continue to support them,” she said.
Reproductive justice
Despite their effectiveness, it is important to remember that LARC methods are not right for everyone, Dr. Espey said.
“It’s not all about effectiveness. Women have many reasons for accessing contraception, and our job is not to reduce unintended pregnancy. ... The idea really is that we empower women. ... We should really give choices and trust women to make the best choices for them,” she explained.
Barriers to IUD removal also should be eliminated, she noted, explaining that a woman who wants her IUD removed a month after insertion should have that option.
She said she has “changed her language,” from asking why a woman wants an $800 IUD removed after a month to asking whether she would like to hear about ways to make it better or if she is “just ready to have it removed.”
For those not interested in a discussion about birth control, she suggested providing information about the bedsider.org site.
“This is a great resource for patients,” she said, noting that it is available in both English and Spanish.
U.S. Medical Eligibility Criteria and Selected Practice Recommendations on contraceptive use
The MEC contraceptive guidance, a regularly updated, evidence-based project of the CDC, provides “best practices” information on candidate selection, or the “who” of contraceptive selection (who is a candidate for a particular method), Dr. Espy said, noting that it’s a “handy resource” for in-office use.
The SPR is more of a “how-to” guide that provides specifics on contraceptive use, such as when a woman can rely on the pill for contraception after she starts taking it, or how a woman should be followed after IUD placement, she said.
A free CDC app provides access to both.
Emergency contraception
The best overall emergency contraceptive method is the copper IUD, but often it is less accessible than oral methods, of which ulipristal acetate (ella), is the best choice, Dr. Espy said.
“Ulipristal is kind of a best-kept secret. It’s a selective estrogen-receptor modulator – it actually works better and longer than Plan B (levonorgestrel). What’s great about Plan B is that you can get it over the counter, but ulipristal delays ovulation longer,” she explained.
Contraceptives and obesity
Oral contraceptive efficacy is “so much more about adherence,” than about weight, she said.
With respect to the contraceptive patch, limited evidence suggests that obesity may reduce effectiveness, but “it’s still way better than barrier methods,” and for the contraceptive ring, no evidence suggests that obesity affects efficacy, she said.
For emergency contraception, evidence suggests that ulipristal is more effective than Plan B in women with high body mass index.
OTC contraceptive access
Pharmacy and OTC access are a good idea, Dr. Espy said.
“ACOG now supports both, which is great, and there are now a number of states where women can access contraception through the pharmacy. There are a lot of barriers there as well, and really the answer is OTC access,” she said. “There is a pill right now that is seeking [Food and Drug Administration] approval; it will be a progestin-only pill – the first one to be available over the counter, so I think this is something that we’ll see in the next 5-10 years.”
Additional future directions
One technology in development is a longer-acting injectable, such as a 6- or 9-month Depot-type shot.
Biodegradable implants also are in development. “What a cool idea – it just disappears in your arm, no need to remove it,” Dr. Espey said, adding that nonsurgical permanent sterilization is another possible advance, which would be “a holy grail.”
As for male contraception?
“I’ve been saying for about 25 years that in 5 years we’ll have a male contraceptive, so I’m not going to say it anymore with any kind of time frame, but it’s possible,” she said.
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – There’s some good news on the contraception and reproductive health front, according to a recent update from Eve Espey, MD.
The unintended pregnancy rate in the United States, including among adolescents and young women, is declining, and the U.S. abortion rate is at its lowest level since Roe v. Wade, she said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
A 2016 article based on 2008-2011 data showed that after hovering around 50% for nearly 3 decades, the unintended pregnancy rate dropped “for the first time in a very long period of time,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology, division of family planning at the University of New Mexico, Albuquerque (N Engl J Med. 2016; 374[9]:843-52).
“It doesn’t look that impressive – it basically went down to 45%, but considering the scope and the number of women who are affected by unplanned pregnancy, this is actually a huge public health achievement,” she said. “And I think ... in the next cycles of the [Center for Disease Control and Prevention’s] National Survey of Family Growth ... we’ll hopefully continue to see this and potentially more [decline].”
As for abortion rates, an increase occurred following Roe v. Wade, but rates are now down to pre-Roe levels.
“One of the things that we know about the abortion rate is that the most important determinant ... is access to contraceptives,” Dr. Espey said, noting that both the abortion and unintended pregnancy rate declines are attributable to better and more consistent use of contraceptives, increased abstinence as teens are waiting longer to have sex, and the “meteoric rise in long-acting reversible contraceptive (LARC) use.”
Importantly, while improvements in public health have traditionally only impacted upper-class white women, a reduction is finally occurring in disparities with women of color, but those disparities still remain,” she added. “Just like we’re focusing so much on this relative to maternal mortality, the same kinds of disparities occur in access to reproductive health.”
Dr. Espey also provided updates on other aspects of contraception.
IUDs and other LARC methods
The use of LARCs increased from 2% of contraceptive types used by reproductive-aged women in 2002 to 12% in 2012. The majority of that change was in IUD use, with a small increase in implant use, she said, noting that the latest data from the 2015-2017 cycle of the National Survey of Family Growth shows that the rate is now up to 16%.
“The rise has been nothing that I ever imagined that I would see, certainly in my professional career,” she said.
The huge impact of LARCs on the unintended pregnancy rate is attributable to consistent effectiveness over time, compared with an increasing failure rate over time with short-acting contraceptive methods, she said, explaining that while the failure rate with oral contraceptives is about 8%-9% over the first 3 years, it increases to 53% at 8 years.
It’s a matter of looking at both “typical use” effectiveness and continuation rates: LARCs have continuation rates of about 75%-85%; Depot-Provera, for example, has a 25%-30% continuation rate at 1 year, she noted.
Dr. Espey also attributed the gains to improved access via the Affordable Care Act’s contraceptive mandate, which has been shown in numerous studies to have improved access and consistency of contraceptive use, but which is “currently being chipped away,” and to the federal Title X program that covers family planning care for low income women, including undocumented women.
“These two programs have made a huge impact for us, and I hope that we as ob.gyns. will continue to support them,” she said.
Reproductive justice
Despite their effectiveness, it is important to remember that LARC methods are not right for everyone, Dr. Espey said.
“It’s not all about effectiveness. Women have many reasons for accessing contraception, and our job is not to reduce unintended pregnancy. ... The idea really is that we empower women. ... We should really give choices and trust women to make the best choices for them,” she explained.
Barriers to IUD removal also should be eliminated, she noted, explaining that a woman who wants her IUD removed a month after insertion should have that option.
She said she has “changed her language,” from asking why a woman wants an $800 IUD removed after a month to asking whether she would like to hear about ways to make it better or if she is “just ready to have it removed.”
For those not interested in a discussion about birth control, she suggested providing information about the bedsider.org site.
“This is a great resource for patients,” she said, noting that it is available in both English and Spanish.
U.S. Medical Eligibility Criteria and Selected Practice Recommendations on contraceptive use
The MEC contraceptive guidance, a regularly updated, evidence-based project of the CDC, provides “best practices” information on candidate selection, or the “who” of contraceptive selection (who is a candidate for a particular method), Dr. Espy said, noting that it’s a “handy resource” for in-office use.
The SPR is more of a “how-to” guide that provides specifics on contraceptive use, such as when a woman can rely on the pill for contraception after she starts taking it, or how a woman should be followed after IUD placement, she said.
A free CDC app provides access to both.
Emergency contraception
The best overall emergency contraceptive method is the copper IUD, but often it is less accessible than oral methods, of which ulipristal acetate (ella), is the best choice, Dr. Espy said.
“Ulipristal is kind of a best-kept secret. It’s a selective estrogen-receptor modulator – it actually works better and longer than Plan B (levonorgestrel). What’s great about Plan B is that you can get it over the counter, but ulipristal delays ovulation longer,” she explained.
Contraceptives and obesity
Oral contraceptive efficacy is “so much more about adherence,” than about weight, she said.
With respect to the contraceptive patch, limited evidence suggests that obesity may reduce effectiveness, but “it’s still way better than barrier methods,” and for the contraceptive ring, no evidence suggests that obesity affects efficacy, she said.
For emergency contraception, evidence suggests that ulipristal is more effective than Plan B in women with high body mass index.
OTC contraceptive access
Pharmacy and OTC access are a good idea, Dr. Espy said.
“ACOG now supports both, which is great, and there are now a number of states where women can access contraception through the pharmacy. There are a lot of barriers there as well, and really the answer is OTC access,” she said. “There is a pill right now that is seeking [Food and Drug Administration] approval; it will be a progestin-only pill – the first one to be available over the counter, so I think this is something that we’ll see in the next 5-10 years.”
Additional future directions
One technology in development is a longer-acting injectable, such as a 6- or 9-month Depot-type shot.
Biodegradable implants also are in development. “What a cool idea – it just disappears in your arm, no need to remove it,” Dr. Espey said, adding that nonsurgical permanent sterilization is another possible advance, which would be “a holy grail.”
As for male contraception?
“I’ve been saying for about 25 years that in 5 years we’ll have a male contraceptive, so I’m not going to say it anymore with any kind of time frame, but it’s possible,” she said.
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – There’s some good news on the contraception and reproductive health front, according to a recent update from Eve Espey, MD.
The unintended pregnancy rate in the United States, including among adolescents and young women, is declining, and the U.S. abortion rate is at its lowest level since Roe v. Wade, she said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
A 2016 article based on 2008-2011 data showed that after hovering around 50% for nearly 3 decades, the unintended pregnancy rate dropped “for the first time in a very long period of time,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology, division of family planning at the University of New Mexico, Albuquerque (N Engl J Med. 2016; 374[9]:843-52).
“It doesn’t look that impressive – it basically went down to 45%, but considering the scope and the number of women who are affected by unplanned pregnancy, this is actually a huge public health achievement,” she said. “And I think ... in the next cycles of the [Center for Disease Control and Prevention’s] National Survey of Family Growth ... we’ll hopefully continue to see this and potentially more [decline].”
As for abortion rates, an increase occurred following Roe v. Wade, but rates are now down to pre-Roe levels.
“One of the things that we know about the abortion rate is that the most important determinant ... is access to contraceptives,” Dr. Espey said, noting that both the abortion and unintended pregnancy rate declines are attributable to better and more consistent use of contraceptives, increased abstinence as teens are waiting longer to have sex, and the “meteoric rise in long-acting reversible contraceptive (LARC) use.”
Importantly, while improvements in public health have traditionally only impacted upper-class white women, a reduction is finally occurring in disparities with women of color, but those disparities still remain,” she added. “Just like we’re focusing so much on this relative to maternal mortality, the same kinds of disparities occur in access to reproductive health.”
Dr. Espey also provided updates on other aspects of contraception.
IUDs and other LARC methods
The use of LARCs increased from 2% of contraceptive types used by reproductive-aged women in 2002 to 12% in 2012. The majority of that change was in IUD use, with a small increase in implant use, she said, noting that the latest data from the 2015-2017 cycle of the National Survey of Family Growth shows that the rate is now up to 16%.
“The rise has been nothing that I ever imagined that I would see, certainly in my professional career,” she said.
The huge impact of LARCs on the unintended pregnancy rate is attributable to consistent effectiveness over time, compared with an increasing failure rate over time with short-acting contraceptive methods, she said, explaining that while the failure rate with oral contraceptives is about 8%-9% over the first 3 years, it increases to 53% at 8 years.
It’s a matter of looking at both “typical use” effectiveness and continuation rates: LARCs have continuation rates of about 75%-85%; Depot-Provera, for example, has a 25%-30% continuation rate at 1 year, she noted.
Dr. Espey also attributed the gains to improved access via the Affordable Care Act’s contraceptive mandate, which has been shown in numerous studies to have improved access and consistency of contraceptive use, but which is “currently being chipped away,” and to the federal Title X program that covers family planning care for low income women, including undocumented women.
“These two programs have made a huge impact for us, and I hope that we as ob.gyns. will continue to support them,” she said.
Reproductive justice
Despite their effectiveness, it is important to remember that LARC methods are not right for everyone, Dr. Espey said.
“It’s not all about effectiveness. Women have many reasons for accessing contraception, and our job is not to reduce unintended pregnancy. ... The idea really is that we empower women. ... We should really give choices and trust women to make the best choices for them,” she explained.
Barriers to IUD removal also should be eliminated, she noted, explaining that a woman who wants her IUD removed a month after insertion should have that option.
She said she has “changed her language,” from asking why a woman wants an $800 IUD removed after a month to asking whether she would like to hear about ways to make it better or if she is “just ready to have it removed.”
For those not interested in a discussion about birth control, she suggested providing information about the bedsider.org site.
“This is a great resource for patients,” she said, noting that it is available in both English and Spanish.
U.S. Medical Eligibility Criteria and Selected Practice Recommendations on contraceptive use
The MEC contraceptive guidance, a regularly updated, evidence-based project of the CDC, provides “best practices” information on candidate selection, or the “who” of contraceptive selection (who is a candidate for a particular method), Dr. Espy said, noting that it’s a “handy resource” for in-office use.
The SPR is more of a “how-to” guide that provides specifics on contraceptive use, such as when a woman can rely on the pill for contraception after she starts taking it, or how a woman should be followed after IUD placement, she said.
A free CDC app provides access to both.
Emergency contraception
The best overall emergency contraceptive method is the copper IUD, but often it is less accessible than oral methods, of which ulipristal acetate (ella), is the best choice, Dr. Espy said.
“Ulipristal is kind of a best-kept secret. It’s a selective estrogen-receptor modulator – it actually works better and longer than Plan B (levonorgestrel). What’s great about Plan B is that you can get it over the counter, but ulipristal delays ovulation longer,” she explained.
Contraceptives and obesity
Oral contraceptive efficacy is “so much more about adherence,” than about weight, she said.
With respect to the contraceptive patch, limited evidence suggests that obesity may reduce effectiveness, but “it’s still way better than barrier methods,” and for the contraceptive ring, no evidence suggests that obesity affects efficacy, she said.
For emergency contraception, evidence suggests that ulipristal is more effective than Plan B in women with high body mass index.
OTC contraceptive access
Pharmacy and OTC access are a good idea, Dr. Espy said.
“ACOG now supports both, which is great, and there are now a number of states where women can access contraception through the pharmacy. There are a lot of barriers there as well, and really the answer is OTC access,” she said. “There is a pill right now that is seeking [Food and Drug Administration] approval; it will be a progestin-only pill – the first one to be available over the counter, so I think this is something that we’ll see in the next 5-10 years.”
Additional future directions
One technology in development is a longer-acting injectable, such as a 6- or 9-month Depot-type shot.
Biodegradable implants also are in development. “What a cool idea – it just disappears in your arm, no need to remove it,” Dr. Espey said, adding that nonsurgical permanent sterilization is another possible advance, which would be “a holy grail.”
As for male contraception?
“I’ve been saying for about 25 years that in 5 years we’ll have a male contraceptive, so I’m not going to say it anymore with any kind of time frame, but it’s possible,” she said.
Dr. Espey reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019