User login
Colchicine May Benefit Patients With Diabetes and Recent MI
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
Eli Lilly Offers Obesity Drug Directly to Consumers
Eli Lilly, maker of the anti-obesity drug Zepbound, announced this week the launch of LillyDirect, a direct-to-patient portal, allowing some patients to obtain its drug for as little as $25 a month.
The move is seen as a major shift in the way these popular medications can reach patients.
For many of the 42 million Americans with obesity, weight loss medications such as Wegovy, Saxenda, and the brand-new Zepbound can be a godsend, helping them lose the excess pounds they’ve struggled with for decades or a lifetime.
But getting these medications has been a struggle for many who are eligible. Shortages of the drugs have been one barrier, and costs of up to $1,300 monthly — the price tag without insurance coverage — are another hurdle.
But 2024 may be a much brighter year, thanks to Lilly’s new portal as well as other developments:
Insurance coverage on private health plans, while still spotty, may be improving. Federal legislators are fighting a 2003 law that forbids Medicare from paying for the medications when prescribed for obesity.
New research found that semaglutide (Wegovy) can reduce the risk of recurrent strokes and heart attacks as well as deaths from cardiovascular events in those with obesity and preexisting cardiovascular disease (or diseases of the heart and blood vessels), a finding experts said should get the attention of health insurers.
The medications, also referred to as GLP-1 agonists, work by activating the receptors of hormones (called glucagon-like peptide 1 and others) that are naturally released after eating. That, in turn, makes you feel more full, leading to weight loss of up to 22% for some. The medications are approved for those with a body mass index (BMI) of 30 or a BMI of 27 with at least one other weight-related health condition such as high blood pressure or high cholesterol. The medicines, injected weekly or more often, are prescribed along with advice about a reduced-calorie diet and increased physical activity.
LillyDirect
Patients can access the obesity medicines through the telehealth platform FORM. Patients reach independent telehealth providers, according to Lilly, who can complement a patient’s current doctor or be an alternative to in-patient care in some cases.
Eli Lilly officials did not respond to requests for comment.
Some obesity experts welcomed the new service. “Any program that improves availability and affordability of these ground-breaking medications is welcome news for our long-suffering patients,” said Louis Aronne, MD, director of the Comprehensive Weight Control Center at Weill Cornell Medicine in New York City, a long-time obesity researcher.
“It’s a great move for Lilly to do,” agreed Caroline Apovian, MD, a professor of medicine at Harvard Medical School and co-director of the Center for Weight Management and Wellness at Brigham & Women’s Hospital in Boston, who is also a veteran obesity specialist. “It is trying to help the accessibility issue and do it responsibly.”
“The bottom line is, there is an overwhelming amount of consumer need and desire for these medications and not enough channels [to provide them],” said Zeev Neuwirth, MD, a former executive at Atrium Health who writes about health care trends. “Eli Lilly is responding to a market need that is out there and quite honestly continuing to grow.”
There are still concerns and questions, Dr. Neuwirth said, “especially since this is to my knowledge the first of its kind in terms of a pharmaceutical manufacturer directly dispensing medication in this nontraditional way.”
He called for transparency between telehealth providers and the pharmaceutical company to rule out any conflicts of interest.
The American College of Physicians, an organization of internal medicine doctors and others, issued a statement expressing concern. Omar T. Atiq, MD, group’s president, said his organization is “concerned by the development of websites that enable patients to order prescription medications directly from the drugmakers. While information on in-person care is available, this direct-to-consumer approach is primarily oriented around the use of telehealth services to prescribe a drug maker’s products.”
The group urged that an established patient-doctor relationship be present, or that care should happen in consultation with a doctor who does have an established relationship (the latter an option offered by Lilly). “These direct-to-consumer services have the potential to leave patients confused and misinformed about medications.”
Heart Attack, Stroke Reduction Benefits
Previous research has found that the GLP-1 medicines such as Ozempic (semaglutide), which the FDA approved to treat diabetes, also reduce the risk of cardiovascular issues such as strokes and heart attacks. Now, new research finds that semaglutide at the Wegovy dose (usually slightly higher than the Ozempic dose for diabetes) also has those benefits in those who don›t have a diabetes diagnosis but do have obesity and cardiovascular disease.
In a clinical trial sponsored by Novo Nordisk, the maker of Wegovy, half of more than 17,000 people with obesity were given semaglutide (Wegovy); the other half got a placebo. Compared to those on the placebo, those who took the Wegovy had a 20% reduction in strokes, heart attacks, and deaths from cardiovascular causes over a 33-month period.
The study results are a “big deal,” Dr. Aronne said. The results make it clear that those with obesity but not diabetes will get the cardiovascular benefits from the treatment as well. While more analysis is necessary, he said the important point is that the study showed that reducing body weight is linked to improvement in critical health outcomes.
As the research evolves, he said, it’s going to be difficult for insurers to deny medications in the face of those findings, which promise reductions in long-term health care costs.
Insurance Coverage
In November, the American Medical Association voted to adopt a policy to urge insurance coverage for evidence-based treatment for obesity, including the new obesity medications.
“No single organization is going to be able to convince insurers and employers to cover this,” Dr. Aronne said. “But I think a prominent organization like the AMA adding their voice to the rising chorus is going to help.”
Coverage of GLP-1 medications could nearly double in 2024, according to a survey of 500 human resources decision-makers released in October by Accolade, a personalized health care advocacy and delivery company. While 25% of respondents said they currently offered coverage when the survey was done in August and September, 43% said they intend to offer coverage in 2024.
In an email, David Allen, a spokesperson for America’s Health Insurance Plans, a health care industry association, said: “Every American deserves affordable coverage and high-quality care, and that includes coverage and care for evidence-based obesity treatments and therapies.”
He said “clinical leaders and other experts at health insurance providers routinely review the evidence for all types of treatments, including treatments for obesity, and offer multiple options to patients — ranging from lifestyle changes and nutrition counseling, to surgical interventions, to prescription drugs.”
Mr. Allen said the evidence that obesity drugs help with weight loss “is still evolving.”
“And some patients are experiencing bad effects related to these drugs such as vomiting and nausea, for example, and the likelihood of gaining the weight back when discontinuing the drugs,” he said.
Others are fighting for Medicare coverage, while some experts contend the costs of that coverage would be overwhelming. A bipartisan bill, the Treat and Reduce Obesity Act of 2023, would allow coverage under Medicare›s prescription drug benefit for drugs used for the treatment of obesity or for weigh loss management for people who are overweight. Some say it›s an uphill climb, citing a Vanderbilt University analysis that found giving just 10% of Medicare-eligible patients the drugs would cost $13.6 billion to more than $26 billion.
However, a white paper from the University of Southern California concluded that the value to society of covering the drugs for Medicare recipients would equal nearly $1 trillion over 10 years, citing savings in hospitalizations and other health care costs.
Comprehensive insurance coverage is needed, Dr. Apovian said. Private insurance plans, Medicare, and Medicaid must all realize the importance of covering what has been now shown to be life-saving drugs, she said.
Broader coverage might also reduce the number of patients getting obesity drugs from unreliable sources, in an effort to save money, and having adverse effects. The FDA warned against counterfeit semaglutide in December.
Long-Term Picture
Research suggests the obesity medications must be taken continuously, at least for most people, to maintain the weight loss. In a study of patients on Zepbound, Dr. Aronne and colleagues found that withdrawing the medication led people to regain weight, while continuing it led to maintaining and even increasing the initial weight loss. While some may be able to use the medications only from time to time, “the majority will have to take these on a chronic basis,” Dr. Aronne said.
Obesity, like high blood pressure and other chronic conditions, needs continuous treatment, Dr. Apovian said. No one would suggest withdrawing blood pressure medications that stabilize blood pressure; the same should be true for the obesity drugs, she said.
Dr. Apovian consults for FORM, the telehealth platform Lilly uses for LillyDirect, and consults for Novo Nordisk, which makes Saxenda and Wegovy. Dr. Aronne is a consultant and investigator for Novo Nordisk, Eli Lilly, and other companies.
A version of this article appeared on WebMD.com.
Eli Lilly, maker of the anti-obesity drug Zepbound, announced this week the launch of LillyDirect, a direct-to-patient portal, allowing some patients to obtain its drug for as little as $25 a month.
The move is seen as a major shift in the way these popular medications can reach patients.
For many of the 42 million Americans with obesity, weight loss medications such as Wegovy, Saxenda, and the brand-new Zepbound can be a godsend, helping them lose the excess pounds they’ve struggled with for decades or a lifetime.
But getting these medications has been a struggle for many who are eligible. Shortages of the drugs have been one barrier, and costs of up to $1,300 monthly — the price tag without insurance coverage — are another hurdle.
But 2024 may be a much brighter year, thanks to Lilly’s new portal as well as other developments:
Insurance coverage on private health plans, while still spotty, may be improving. Federal legislators are fighting a 2003 law that forbids Medicare from paying for the medications when prescribed for obesity.
New research found that semaglutide (Wegovy) can reduce the risk of recurrent strokes and heart attacks as well as deaths from cardiovascular events in those with obesity and preexisting cardiovascular disease (or diseases of the heart and blood vessels), a finding experts said should get the attention of health insurers.
The medications, also referred to as GLP-1 agonists, work by activating the receptors of hormones (called glucagon-like peptide 1 and others) that are naturally released after eating. That, in turn, makes you feel more full, leading to weight loss of up to 22% for some. The medications are approved for those with a body mass index (BMI) of 30 or a BMI of 27 with at least one other weight-related health condition such as high blood pressure or high cholesterol. The medicines, injected weekly or more often, are prescribed along with advice about a reduced-calorie diet and increased physical activity.
LillyDirect
Patients can access the obesity medicines through the telehealth platform FORM. Patients reach independent telehealth providers, according to Lilly, who can complement a patient’s current doctor or be an alternative to in-patient care in some cases.
Eli Lilly officials did not respond to requests for comment.
Some obesity experts welcomed the new service. “Any program that improves availability and affordability of these ground-breaking medications is welcome news for our long-suffering patients,” said Louis Aronne, MD, director of the Comprehensive Weight Control Center at Weill Cornell Medicine in New York City, a long-time obesity researcher.
“It’s a great move for Lilly to do,” agreed Caroline Apovian, MD, a professor of medicine at Harvard Medical School and co-director of the Center for Weight Management and Wellness at Brigham & Women’s Hospital in Boston, who is also a veteran obesity specialist. “It is trying to help the accessibility issue and do it responsibly.”
“The bottom line is, there is an overwhelming amount of consumer need and desire for these medications and not enough channels [to provide them],” said Zeev Neuwirth, MD, a former executive at Atrium Health who writes about health care trends. “Eli Lilly is responding to a market need that is out there and quite honestly continuing to grow.”
There are still concerns and questions, Dr. Neuwirth said, “especially since this is to my knowledge the first of its kind in terms of a pharmaceutical manufacturer directly dispensing medication in this nontraditional way.”
He called for transparency between telehealth providers and the pharmaceutical company to rule out any conflicts of interest.
The American College of Physicians, an organization of internal medicine doctors and others, issued a statement expressing concern. Omar T. Atiq, MD, group’s president, said his organization is “concerned by the development of websites that enable patients to order prescription medications directly from the drugmakers. While information on in-person care is available, this direct-to-consumer approach is primarily oriented around the use of telehealth services to prescribe a drug maker’s products.”
The group urged that an established patient-doctor relationship be present, or that care should happen in consultation with a doctor who does have an established relationship (the latter an option offered by Lilly). “These direct-to-consumer services have the potential to leave patients confused and misinformed about medications.”
Heart Attack, Stroke Reduction Benefits
Previous research has found that the GLP-1 medicines such as Ozempic (semaglutide), which the FDA approved to treat diabetes, also reduce the risk of cardiovascular issues such as strokes and heart attacks. Now, new research finds that semaglutide at the Wegovy dose (usually slightly higher than the Ozempic dose for diabetes) also has those benefits in those who don›t have a diabetes diagnosis but do have obesity and cardiovascular disease.
In a clinical trial sponsored by Novo Nordisk, the maker of Wegovy, half of more than 17,000 people with obesity were given semaglutide (Wegovy); the other half got a placebo. Compared to those on the placebo, those who took the Wegovy had a 20% reduction in strokes, heart attacks, and deaths from cardiovascular causes over a 33-month period.
The study results are a “big deal,” Dr. Aronne said. The results make it clear that those with obesity but not diabetes will get the cardiovascular benefits from the treatment as well. While more analysis is necessary, he said the important point is that the study showed that reducing body weight is linked to improvement in critical health outcomes.
As the research evolves, he said, it’s going to be difficult for insurers to deny medications in the face of those findings, which promise reductions in long-term health care costs.
Insurance Coverage
In November, the American Medical Association voted to adopt a policy to urge insurance coverage for evidence-based treatment for obesity, including the new obesity medications.
“No single organization is going to be able to convince insurers and employers to cover this,” Dr. Aronne said. “But I think a prominent organization like the AMA adding their voice to the rising chorus is going to help.”
Coverage of GLP-1 medications could nearly double in 2024, according to a survey of 500 human resources decision-makers released in October by Accolade, a personalized health care advocacy and delivery company. While 25% of respondents said they currently offered coverage when the survey was done in August and September, 43% said they intend to offer coverage in 2024.
In an email, David Allen, a spokesperson for America’s Health Insurance Plans, a health care industry association, said: “Every American deserves affordable coverage and high-quality care, and that includes coverage and care for evidence-based obesity treatments and therapies.”
He said “clinical leaders and other experts at health insurance providers routinely review the evidence for all types of treatments, including treatments for obesity, and offer multiple options to patients — ranging from lifestyle changes and nutrition counseling, to surgical interventions, to prescription drugs.”
Mr. Allen said the evidence that obesity drugs help with weight loss “is still evolving.”
“And some patients are experiencing bad effects related to these drugs such as vomiting and nausea, for example, and the likelihood of gaining the weight back when discontinuing the drugs,” he said.
Others are fighting for Medicare coverage, while some experts contend the costs of that coverage would be overwhelming. A bipartisan bill, the Treat and Reduce Obesity Act of 2023, would allow coverage under Medicare›s prescription drug benefit for drugs used for the treatment of obesity or for weigh loss management for people who are overweight. Some say it›s an uphill climb, citing a Vanderbilt University analysis that found giving just 10% of Medicare-eligible patients the drugs would cost $13.6 billion to more than $26 billion.
However, a white paper from the University of Southern California concluded that the value to society of covering the drugs for Medicare recipients would equal nearly $1 trillion over 10 years, citing savings in hospitalizations and other health care costs.
Comprehensive insurance coverage is needed, Dr. Apovian said. Private insurance plans, Medicare, and Medicaid must all realize the importance of covering what has been now shown to be life-saving drugs, she said.
Broader coverage might also reduce the number of patients getting obesity drugs from unreliable sources, in an effort to save money, and having adverse effects. The FDA warned against counterfeit semaglutide in December.
Long-Term Picture
Research suggests the obesity medications must be taken continuously, at least for most people, to maintain the weight loss. In a study of patients on Zepbound, Dr. Aronne and colleagues found that withdrawing the medication led people to regain weight, while continuing it led to maintaining and even increasing the initial weight loss. While some may be able to use the medications only from time to time, “the majority will have to take these on a chronic basis,” Dr. Aronne said.
Obesity, like high blood pressure and other chronic conditions, needs continuous treatment, Dr. Apovian said. No one would suggest withdrawing blood pressure medications that stabilize blood pressure; the same should be true for the obesity drugs, she said.
Dr. Apovian consults for FORM, the telehealth platform Lilly uses for LillyDirect, and consults for Novo Nordisk, which makes Saxenda and Wegovy. Dr. Aronne is a consultant and investigator for Novo Nordisk, Eli Lilly, and other companies.
A version of this article appeared on WebMD.com.
Eli Lilly, maker of the anti-obesity drug Zepbound, announced this week the launch of LillyDirect, a direct-to-patient portal, allowing some patients to obtain its drug for as little as $25 a month.
The move is seen as a major shift in the way these popular medications can reach patients.
For many of the 42 million Americans with obesity, weight loss medications such as Wegovy, Saxenda, and the brand-new Zepbound can be a godsend, helping them lose the excess pounds they’ve struggled with for decades or a lifetime.
But getting these medications has been a struggle for many who are eligible. Shortages of the drugs have been one barrier, and costs of up to $1,300 monthly — the price tag without insurance coverage — are another hurdle.
But 2024 may be a much brighter year, thanks to Lilly’s new portal as well as other developments:
Insurance coverage on private health plans, while still spotty, may be improving. Federal legislators are fighting a 2003 law that forbids Medicare from paying for the medications when prescribed for obesity.
New research found that semaglutide (Wegovy) can reduce the risk of recurrent strokes and heart attacks as well as deaths from cardiovascular events in those with obesity and preexisting cardiovascular disease (or diseases of the heart and blood vessels), a finding experts said should get the attention of health insurers.
The medications, also referred to as GLP-1 agonists, work by activating the receptors of hormones (called glucagon-like peptide 1 and others) that are naturally released after eating. That, in turn, makes you feel more full, leading to weight loss of up to 22% for some. The medications are approved for those with a body mass index (BMI) of 30 or a BMI of 27 with at least one other weight-related health condition such as high blood pressure or high cholesterol. The medicines, injected weekly or more often, are prescribed along with advice about a reduced-calorie diet and increased physical activity.
LillyDirect
Patients can access the obesity medicines through the telehealth platform FORM. Patients reach independent telehealth providers, according to Lilly, who can complement a patient’s current doctor or be an alternative to in-patient care in some cases.
Eli Lilly officials did not respond to requests for comment.
Some obesity experts welcomed the new service. “Any program that improves availability and affordability of these ground-breaking medications is welcome news for our long-suffering patients,” said Louis Aronne, MD, director of the Comprehensive Weight Control Center at Weill Cornell Medicine in New York City, a long-time obesity researcher.
“It’s a great move for Lilly to do,” agreed Caroline Apovian, MD, a professor of medicine at Harvard Medical School and co-director of the Center for Weight Management and Wellness at Brigham & Women’s Hospital in Boston, who is also a veteran obesity specialist. “It is trying to help the accessibility issue and do it responsibly.”
“The bottom line is, there is an overwhelming amount of consumer need and desire for these medications and not enough channels [to provide them],” said Zeev Neuwirth, MD, a former executive at Atrium Health who writes about health care trends. “Eli Lilly is responding to a market need that is out there and quite honestly continuing to grow.”
There are still concerns and questions, Dr. Neuwirth said, “especially since this is to my knowledge the first of its kind in terms of a pharmaceutical manufacturer directly dispensing medication in this nontraditional way.”
He called for transparency between telehealth providers and the pharmaceutical company to rule out any conflicts of interest.
The American College of Physicians, an organization of internal medicine doctors and others, issued a statement expressing concern. Omar T. Atiq, MD, group’s president, said his organization is “concerned by the development of websites that enable patients to order prescription medications directly from the drugmakers. While information on in-person care is available, this direct-to-consumer approach is primarily oriented around the use of telehealth services to prescribe a drug maker’s products.”
The group urged that an established patient-doctor relationship be present, or that care should happen in consultation with a doctor who does have an established relationship (the latter an option offered by Lilly). “These direct-to-consumer services have the potential to leave patients confused and misinformed about medications.”
Heart Attack, Stroke Reduction Benefits
Previous research has found that the GLP-1 medicines such as Ozempic (semaglutide), which the FDA approved to treat diabetes, also reduce the risk of cardiovascular issues such as strokes and heart attacks. Now, new research finds that semaglutide at the Wegovy dose (usually slightly higher than the Ozempic dose for diabetes) also has those benefits in those who don›t have a diabetes diagnosis but do have obesity and cardiovascular disease.
In a clinical trial sponsored by Novo Nordisk, the maker of Wegovy, half of more than 17,000 people with obesity were given semaglutide (Wegovy); the other half got a placebo. Compared to those on the placebo, those who took the Wegovy had a 20% reduction in strokes, heart attacks, and deaths from cardiovascular causes over a 33-month period.
The study results are a “big deal,” Dr. Aronne said. The results make it clear that those with obesity but not diabetes will get the cardiovascular benefits from the treatment as well. While more analysis is necessary, he said the important point is that the study showed that reducing body weight is linked to improvement in critical health outcomes.
As the research evolves, he said, it’s going to be difficult for insurers to deny medications in the face of those findings, which promise reductions in long-term health care costs.
Insurance Coverage
In November, the American Medical Association voted to adopt a policy to urge insurance coverage for evidence-based treatment for obesity, including the new obesity medications.
“No single organization is going to be able to convince insurers and employers to cover this,” Dr. Aronne said. “But I think a prominent organization like the AMA adding their voice to the rising chorus is going to help.”
Coverage of GLP-1 medications could nearly double in 2024, according to a survey of 500 human resources decision-makers released in October by Accolade, a personalized health care advocacy and delivery company. While 25% of respondents said they currently offered coverage when the survey was done in August and September, 43% said they intend to offer coverage in 2024.
In an email, David Allen, a spokesperson for America’s Health Insurance Plans, a health care industry association, said: “Every American deserves affordable coverage and high-quality care, and that includes coverage and care for evidence-based obesity treatments and therapies.”
He said “clinical leaders and other experts at health insurance providers routinely review the evidence for all types of treatments, including treatments for obesity, and offer multiple options to patients — ranging from lifestyle changes and nutrition counseling, to surgical interventions, to prescription drugs.”
Mr. Allen said the evidence that obesity drugs help with weight loss “is still evolving.”
“And some patients are experiencing bad effects related to these drugs such as vomiting and nausea, for example, and the likelihood of gaining the weight back when discontinuing the drugs,” he said.
Others are fighting for Medicare coverage, while some experts contend the costs of that coverage would be overwhelming. A bipartisan bill, the Treat and Reduce Obesity Act of 2023, would allow coverage under Medicare›s prescription drug benefit for drugs used for the treatment of obesity or for weigh loss management for people who are overweight. Some say it›s an uphill climb, citing a Vanderbilt University analysis that found giving just 10% of Medicare-eligible patients the drugs would cost $13.6 billion to more than $26 billion.
However, a white paper from the University of Southern California concluded that the value to society of covering the drugs for Medicare recipients would equal nearly $1 trillion over 10 years, citing savings in hospitalizations and other health care costs.
Comprehensive insurance coverage is needed, Dr. Apovian said. Private insurance plans, Medicare, and Medicaid must all realize the importance of covering what has been now shown to be life-saving drugs, she said.
Broader coverage might also reduce the number of patients getting obesity drugs from unreliable sources, in an effort to save money, and having adverse effects. The FDA warned against counterfeit semaglutide in December.
Long-Term Picture
Research suggests the obesity medications must be taken continuously, at least for most people, to maintain the weight loss. In a study of patients on Zepbound, Dr. Aronne and colleagues found that withdrawing the medication led people to regain weight, while continuing it led to maintaining and even increasing the initial weight loss. While some may be able to use the medications only from time to time, “the majority will have to take these on a chronic basis,” Dr. Aronne said.
Obesity, like high blood pressure and other chronic conditions, needs continuous treatment, Dr. Apovian said. No one would suggest withdrawing blood pressure medications that stabilize blood pressure; the same should be true for the obesity drugs, she said.
Dr. Apovian consults for FORM, the telehealth platform Lilly uses for LillyDirect, and consults for Novo Nordisk, which makes Saxenda and Wegovy. Dr. Aronne is a consultant and investigator for Novo Nordisk, Eli Lilly, and other companies.
A version of this article appeared on WebMD.com.
Walking Fast May Help Prevent Type 2 Diabetes
Walking is a simple, cost-free form of exercise that benefits physical, social, and mental health in many ways. Several clinical trials have shown that walking regularly is associated with a lower risk for cardiovascular events and all-cause mortality, and having a higher daily step count is linked to a decreased risk for premature death.
Walking and Diabetes
In recent years, the link between walking speed and the risk for multiple health problems has sparked keen interest. Data suggest that a faster walking pace may have a greater physiological response and may be associated with more favorable health advantages than a slow walking pace. A previous meta-analysis of eight cohort studies suggested that individuals in the fastest walking-pace category (median = 5.6 km/h) had a 44% lower risk for stroke than those in the slowest walking-pace category (median = 1.6 km/h). The risk for the former decreased by 13% for every 1 km/h increment in baseline walking pace.
Type 2 diabetes (T2D) is one of the most common metabolic diseases in the world. People with this type of diabetes have an increased risk for microvascular and macrovascular complications and a shorter life expectancy. Approximately 537 million adults are estimated to be living with diabetes worldwide, and this number is expected to reach 783 million by 2045.
Physical activity is an essential component of T2D prevention programs and can favorably affect blood sugar control. A meta-analysis of cohort studies showed that being physically active was associated with a 35% reduction in the risk of acquiring T2D in the general population, and regular walking was associated with a 15% reduction in the risk of developing T2D.
However, no studies have investigated the link between different walking speeds and the risk for T2D. A team from the Research Center at the Semnan University of Medical Sciences in Iran carried out a systematic review of the association between walking speed and the risk of developing T2D in adults; this review was published in the British Journal of Sports Medicine.
10 Cohort Studies
This systematic review used publications (1999-2022) available in the usual data sources (PubMed, Scopus, CENTRAL, and Web of Science). Random-effects meta-analyses were used to calculate relative risk (RR) and risk difference (RD) based on different walking speeds. The researchers rated the credibility of subgroup differences and the certainty of evidence using the Instrument to assess the Credibility of Effect Modification ANalyses (ICEMAN) and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tools, respectively.
Of the 508,121 potential participants, 18,410 adults from 10 prospective cohort studies conducted in the United States, Japan, and the United Kingdom were deemed eligible. The proportion of women was between 52% and 73%, depending on the cohort. Follow-up duration varied from 3 to 11.1 years (median, 8 years).
Five cohort studies measured walking speed using stopwatch testing, while the other five used self-assessed questionnaires. To define cases of T2D, seven studies used objective methods such as blood glucose measurement or linkage with medical records, and in three cohorts, self-assessment questionnaires were used (these were checked against patient records). All studies controlled age, sex, and tobacco consumption in the multivariate analyses, and some controlled just alcohol consumption, blood pressure, total physical activity volume, body mass index, time spent walking or daily step count, and a family history of diabetes.
The Right Speed
The authors first categorized walking speed into four prespecified levels: Easy or casual (< 2 mph or 3.2 km/h), average or normal (2-3 mph or 3.2-4.8 km/h), fairly brisk (3-4 mph or 4.8-6.4 km/h), and very brisk or brisk/striding (> 4 mph or > 6.4 km/h).
Four cohort studies with 6,520 cases of T2D among 160,321 participants reported information on average or normal walking. Participants with average or normal walking were at a 15% lower risk for T2D than those with easy or casual walking (RR = 0.85 [95% CI, 0.70-1.00]; RD = 0.86 [1.72-0]). Ten cohort studies with 18,410 cases among 508,121 participants reported information on fairly brisk walking. Those with fairly brisk walking were at a 24% lower risk for T2D than those with easy or casual walking (RR = 0.76 [0.65-0.87]; I2 = 90%; RD = 1.38 [2.01-0.75]).
There was no significant or credible subgroup difference by adjustment for the total physical activity or time spent walking per day. The dose-response analysis suggested that the risk for T2D decreased significantly at a walking speed of 4 km/h and above.
Study Limitations
This meta-analysis has strengths that may increase the generalizability of its results. The researchers included cohort studies, which allowed them to consider the temporal sequence of exposure and outcome. Cohort studies are less affected by recall and selection biases compared with retrospective case–control studies, which increase the likelihood of causality. The researchers also assessed the credibility of subgroup differences using the recently developed ICEMAN tool, calculated both relative and absolute risks, and rated the certainty of evidence using the GRADE approach.
Some shortcomings must be considered. Most of the studies included in the present review were rated as having a serious risk for bias, with the most important biases resulting from inadequate adjustment for potential confounders and the methods used for walking speed assessment and diagnosis of T2D. In addition, the findings could have been subject to reverse causality bias because participants with faster walking speed are more likely to perform more physical activity and have better cardiorespiratory fitness, greater muscle mass, and better health status. However, the subgroup analyses of fairly brisk and brisk/striding walking indicated that there were no significant subgroup differences by follow-up duration and that the significant inverse associations remained stable in the subgroup of cohort studies with a follow-up duration of > 10 years.
The authors concluded that While current strategies to increase total walking time are beneficial, it may also be reasonable to encourage people to walk at faster speeds to further increase the health benefits of walking.”
This article was translated from JIM, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Walking is a simple, cost-free form of exercise that benefits physical, social, and mental health in many ways. Several clinical trials have shown that walking regularly is associated with a lower risk for cardiovascular events and all-cause mortality, and having a higher daily step count is linked to a decreased risk for premature death.
Walking and Diabetes
In recent years, the link between walking speed and the risk for multiple health problems has sparked keen interest. Data suggest that a faster walking pace may have a greater physiological response and may be associated with more favorable health advantages than a slow walking pace. A previous meta-analysis of eight cohort studies suggested that individuals in the fastest walking-pace category (median = 5.6 km/h) had a 44% lower risk for stroke than those in the slowest walking-pace category (median = 1.6 km/h). The risk for the former decreased by 13% for every 1 km/h increment in baseline walking pace.
Type 2 diabetes (T2D) is one of the most common metabolic diseases in the world. People with this type of diabetes have an increased risk for microvascular and macrovascular complications and a shorter life expectancy. Approximately 537 million adults are estimated to be living with diabetes worldwide, and this number is expected to reach 783 million by 2045.
Physical activity is an essential component of T2D prevention programs and can favorably affect blood sugar control. A meta-analysis of cohort studies showed that being physically active was associated with a 35% reduction in the risk of acquiring T2D in the general population, and regular walking was associated with a 15% reduction in the risk of developing T2D.
However, no studies have investigated the link between different walking speeds and the risk for T2D. A team from the Research Center at the Semnan University of Medical Sciences in Iran carried out a systematic review of the association between walking speed and the risk of developing T2D in adults; this review was published in the British Journal of Sports Medicine.
10 Cohort Studies
This systematic review used publications (1999-2022) available in the usual data sources (PubMed, Scopus, CENTRAL, and Web of Science). Random-effects meta-analyses were used to calculate relative risk (RR) and risk difference (RD) based on different walking speeds. The researchers rated the credibility of subgroup differences and the certainty of evidence using the Instrument to assess the Credibility of Effect Modification ANalyses (ICEMAN) and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tools, respectively.
Of the 508,121 potential participants, 18,410 adults from 10 prospective cohort studies conducted in the United States, Japan, and the United Kingdom were deemed eligible. The proportion of women was between 52% and 73%, depending on the cohort. Follow-up duration varied from 3 to 11.1 years (median, 8 years).
Five cohort studies measured walking speed using stopwatch testing, while the other five used self-assessed questionnaires. To define cases of T2D, seven studies used objective methods such as blood glucose measurement or linkage with medical records, and in three cohorts, self-assessment questionnaires were used (these were checked against patient records). All studies controlled age, sex, and tobacco consumption in the multivariate analyses, and some controlled just alcohol consumption, blood pressure, total physical activity volume, body mass index, time spent walking or daily step count, and a family history of diabetes.
The Right Speed
The authors first categorized walking speed into four prespecified levels: Easy or casual (< 2 mph or 3.2 km/h), average or normal (2-3 mph or 3.2-4.8 km/h), fairly brisk (3-4 mph or 4.8-6.4 km/h), and very brisk or brisk/striding (> 4 mph or > 6.4 km/h).
Four cohort studies with 6,520 cases of T2D among 160,321 participants reported information on average or normal walking. Participants with average or normal walking were at a 15% lower risk for T2D than those with easy or casual walking (RR = 0.85 [95% CI, 0.70-1.00]; RD = 0.86 [1.72-0]). Ten cohort studies with 18,410 cases among 508,121 participants reported information on fairly brisk walking. Those with fairly brisk walking were at a 24% lower risk for T2D than those with easy or casual walking (RR = 0.76 [0.65-0.87]; I2 = 90%; RD = 1.38 [2.01-0.75]).
There was no significant or credible subgroup difference by adjustment for the total physical activity or time spent walking per day. The dose-response analysis suggested that the risk for T2D decreased significantly at a walking speed of 4 km/h and above.
Study Limitations
This meta-analysis has strengths that may increase the generalizability of its results. The researchers included cohort studies, which allowed them to consider the temporal sequence of exposure and outcome. Cohort studies are less affected by recall and selection biases compared with retrospective case–control studies, which increase the likelihood of causality. The researchers also assessed the credibility of subgroup differences using the recently developed ICEMAN tool, calculated both relative and absolute risks, and rated the certainty of evidence using the GRADE approach.
Some shortcomings must be considered. Most of the studies included in the present review were rated as having a serious risk for bias, with the most important biases resulting from inadequate adjustment for potential confounders and the methods used for walking speed assessment and diagnosis of T2D. In addition, the findings could have been subject to reverse causality bias because participants with faster walking speed are more likely to perform more physical activity and have better cardiorespiratory fitness, greater muscle mass, and better health status. However, the subgroup analyses of fairly brisk and brisk/striding walking indicated that there were no significant subgroup differences by follow-up duration and that the significant inverse associations remained stable in the subgroup of cohort studies with a follow-up duration of > 10 years.
The authors concluded that While current strategies to increase total walking time are beneficial, it may also be reasonable to encourage people to walk at faster speeds to further increase the health benefits of walking.”
This article was translated from JIM, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Walking is a simple, cost-free form of exercise that benefits physical, social, and mental health in many ways. Several clinical trials have shown that walking regularly is associated with a lower risk for cardiovascular events and all-cause mortality, and having a higher daily step count is linked to a decreased risk for premature death.
Walking and Diabetes
In recent years, the link between walking speed and the risk for multiple health problems has sparked keen interest. Data suggest that a faster walking pace may have a greater physiological response and may be associated with more favorable health advantages than a slow walking pace. A previous meta-analysis of eight cohort studies suggested that individuals in the fastest walking-pace category (median = 5.6 km/h) had a 44% lower risk for stroke than those in the slowest walking-pace category (median = 1.6 km/h). The risk for the former decreased by 13% for every 1 km/h increment in baseline walking pace.
Type 2 diabetes (T2D) is one of the most common metabolic diseases in the world. People with this type of diabetes have an increased risk for microvascular and macrovascular complications and a shorter life expectancy. Approximately 537 million adults are estimated to be living with diabetes worldwide, and this number is expected to reach 783 million by 2045.
Physical activity is an essential component of T2D prevention programs and can favorably affect blood sugar control. A meta-analysis of cohort studies showed that being physically active was associated with a 35% reduction in the risk of acquiring T2D in the general population, and regular walking was associated with a 15% reduction in the risk of developing T2D.
However, no studies have investigated the link between different walking speeds and the risk for T2D. A team from the Research Center at the Semnan University of Medical Sciences in Iran carried out a systematic review of the association between walking speed and the risk of developing T2D in adults; this review was published in the British Journal of Sports Medicine.
10 Cohort Studies
This systematic review used publications (1999-2022) available in the usual data sources (PubMed, Scopus, CENTRAL, and Web of Science). Random-effects meta-analyses were used to calculate relative risk (RR) and risk difference (RD) based on different walking speeds. The researchers rated the credibility of subgroup differences and the certainty of evidence using the Instrument to assess the Credibility of Effect Modification ANalyses (ICEMAN) and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tools, respectively.
Of the 508,121 potential participants, 18,410 adults from 10 prospective cohort studies conducted in the United States, Japan, and the United Kingdom were deemed eligible. The proportion of women was between 52% and 73%, depending on the cohort. Follow-up duration varied from 3 to 11.1 years (median, 8 years).
Five cohort studies measured walking speed using stopwatch testing, while the other five used self-assessed questionnaires. To define cases of T2D, seven studies used objective methods such as blood glucose measurement or linkage with medical records, and in three cohorts, self-assessment questionnaires were used (these were checked against patient records). All studies controlled age, sex, and tobacco consumption in the multivariate analyses, and some controlled just alcohol consumption, blood pressure, total physical activity volume, body mass index, time spent walking or daily step count, and a family history of diabetes.
The Right Speed
The authors first categorized walking speed into four prespecified levels: Easy or casual (< 2 mph or 3.2 km/h), average or normal (2-3 mph or 3.2-4.8 km/h), fairly brisk (3-4 mph or 4.8-6.4 km/h), and very brisk or brisk/striding (> 4 mph or > 6.4 km/h).
Four cohort studies with 6,520 cases of T2D among 160,321 participants reported information on average or normal walking. Participants with average or normal walking were at a 15% lower risk for T2D than those with easy or casual walking (RR = 0.85 [95% CI, 0.70-1.00]; RD = 0.86 [1.72-0]). Ten cohort studies with 18,410 cases among 508,121 participants reported information on fairly brisk walking. Those with fairly brisk walking were at a 24% lower risk for T2D than those with easy or casual walking (RR = 0.76 [0.65-0.87]; I2 = 90%; RD = 1.38 [2.01-0.75]).
There was no significant or credible subgroup difference by adjustment for the total physical activity or time spent walking per day. The dose-response analysis suggested that the risk for T2D decreased significantly at a walking speed of 4 km/h and above.
Study Limitations
This meta-analysis has strengths that may increase the generalizability of its results. The researchers included cohort studies, which allowed them to consider the temporal sequence of exposure and outcome. Cohort studies are less affected by recall and selection biases compared with retrospective case–control studies, which increase the likelihood of causality. The researchers also assessed the credibility of subgroup differences using the recently developed ICEMAN tool, calculated both relative and absolute risks, and rated the certainty of evidence using the GRADE approach.
Some shortcomings must be considered. Most of the studies included in the present review were rated as having a serious risk for bias, with the most important biases resulting from inadequate adjustment for potential confounders and the methods used for walking speed assessment and diagnosis of T2D. In addition, the findings could have been subject to reverse causality bias because participants with faster walking speed are more likely to perform more physical activity and have better cardiorespiratory fitness, greater muscle mass, and better health status. However, the subgroup analyses of fairly brisk and brisk/striding walking indicated that there were no significant subgroup differences by follow-up duration and that the significant inverse associations remained stable in the subgroup of cohort studies with a follow-up duration of > 10 years.
The authors concluded that While current strategies to increase total walking time are beneficial, it may also be reasonable to encourage people to walk at faster speeds to further increase the health benefits of walking.”
This article was translated from JIM, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF SPORTS MEDICINE
Spending the Holidays With GLP-1 Receptor Agonists: 5 Things to Know
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
As an endocrinologist, I treat many patients who have diabetes, obesity, or both. Antiobesity medications, particularly the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), are our first support tools when nutrition and physical activity aren’t enough.
1. Be mindful of fullness cues.
GLP-1 RAs increase satiety; they help patients feel fuller sooner within a meal and longer in between meals. This means consuming the “usual” at a holiday gathering makes them feel as if they ate too much, and often this will result in more side effects, such as nausea and reflux.
Patient tip: A good rule of thumb is to anticipate feeling full with half of your usual portion. Start with half a plate and reassess your hunger level after finishing.
2. Distinguish between hunger and “food noise.”
Ask your patients, “Do you ever find yourself eating even when you’re not hungry?” Many people eat because of emotions (eg, stress, anxiety, happiness), social situations, or cultural expectations. This type of food consumption is what scientists call “hedonic food intake” and may be driven by the “food noise” that patients describe as persistent thoughts about food in the absence of physiologic hunger. Semaglutide (Ozempic, Wegovy) has been found to reduce cravings, though other research has shown that emotional eating may blunt the effect of GLP-1 RAs.
Patient tip: Recognize when you might be seeking food for reasons other than hunger, and try a different way to address the cue (eg, chat with a friend or family member, go for a walk).
3. Be careful with alcohol.
GLP-1 RAs are being researched as potential treatments for alcohol use disorder. Many patients report less interest in alcohol and a lower tolerance to alcohol when they are taking a GLP-1 RA. Additionally, GLP-1 RAs may be a risk factor for pancreatitis, which can be caused by consuming too much alcohol.
Patient tip: The standard recommendation remains true: If drinking alcohol, limit to one to two servings per day, but also know that reduced intake or interest is normal when taking a GLP-1 RA.
4. Be aware of sickness vs side effects.
With holiday travel and the winter season, it is common for people to catch a cold or a stomach bug. Symptoms of common illnesses might include fatigue, loss of appetite, or diarrhea. These symptoms overlap with side effects of antiobesity medications like semaglutide and tirzepatide.
Patient tip: If you are experiencing constitutional or gastrointestinal symptoms due to illness, speak with your board-certified obesity medicine doctor, who may recommend a temporary medication adjustment to avoid excess side effects.
5. Stay strong against weight stigma.
The holiday season can be a stressful time, especially as patients are reconnecting with people who have not been a part of their health or weight loss journey. Unfortunately, weight bias and weight stigma remain rampant. Many people don’t understand the biology of obesity and refuse to accept the necessity of medical treatment. They may be surrounded by opinions, often louder and less informed.
Patient tip: Remember that obesity is a medical disease. Tell your nosy cousin that it’s a private health matter and that your decisions are your own.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed financial relationships with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
ED Visits for Diabetes on the Rise in the US
Emergency department (ED) visits by adults with diabetes increased by more than 25% since 2012, with the highest rates among Blacks and those aged over 65 years, a new data brief from the Centers for Disease Control and Prevention’s National Center for Health Statistics shows.
In 2021, diabetes was the eighth leading cause of death in the United States, according to the brief, published online on December 19, 2023. Its frequency is increasing in young people, and increasing age is a risk factor for hospitalization.
The latest data show that in 2020-2021, the overall annual ED visit rate was 72.2 visits per 1000 adults with diabetes, with no significant difference in terms of sex (75.1 visits per 1000 women vs 69.1 visits per 1000 men). By race/ethnicity, Blacks had the highest rates, at 135.5 visits per 1000 adults, followed by Whites (69.9) and Hispanics (52.3). The rates increased with age for both women and men, and among the three race/ethnic groups.
Comorbidities Count
The most ED visits were made by patients with diabetes and two to four other chronic conditions (541.4 visits per 1000 visits). Rates for patients without other chronic conditions were the lowest (90.2).
Among individuals with diabetes aged 18-44 years, ED visit rates were the highest for those with two to four other chronic conditions (402.0) and lowest among those with five or more other conditions (93.8).
Among patients aged 45-64 years, ED visit rates were the highest for those with two to four other chronic conditions (526.4) and lowest for those without other conditions (87.7). In the 65 years and older group, rates were the highest for individuals with two to four other chronic conditions (605.2), followed by five or more conditions (217.7), one other condition (140.6), and no other conditions (36.5).
Notably, the ED visit rates for those with two to four or five or more other chronic conditions increased with age, whereas visits for those with no other chronic conditions or one other condition decreased with age.
Decade-Long Trend
ED visit rates among adults with diabetes increased throughout the past decade, from 48.6 visits per 1000 adults in 2012 to 74.9 per 1000 adults in 2021. Rates for those aged 65 and older were higher than all other age groups, increasing from 113.4 to 156.8. Increases were also seen among those aged 45-64 years (53.1 in 2012 to 89.2 in 2021) and 18-44 (20.9 in 2012 to 26.4 in 2016, then plateauing from 2016-2021).
Data are based on a sample of 4051 ED visits, representing about 18,238,000 average annual visits made by adults with diabetes to nonfederal, general, and short-stay hospitals during 2020-2021.
Taken together, these most recent estimates “show an increasing trend in rates by adults with diabetes in the ED setting,” the authors concluded.
A version of this article appeared on Medscape.com.
Emergency department (ED) visits by adults with diabetes increased by more than 25% since 2012, with the highest rates among Blacks and those aged over 65 years, a new data brief from the Centers for Disease Control and Prevention’s National Center for Health Statistics shows.
In 2021, diabetes was the eighth leading cause of death in the United States, according to the brief, published online on December 19, 2023. Its frequency is increasing in young people, and increasing age is a risk factor for hospitalization.
The latest data show that in 2020-2021, the overall annual ED visit rate was 72.2 visits per 1000 adults with diabetes, with no significant difference in terms of sex (75.1 visits per 1000 women vs 69.1 visits per 1000 men). By race/ethnicity, Blacks had the highest rates, at 135.5 visits per 1000 adults, followed by Whites (69.9) and Hispanics (52.3). The rates increased with age for both women and men, and among the three race/ethnic groups.
Comorbidities Count
The most ED visits were made by patients with diabetes and two to four other chronic conditions (541.4 visits per 1000 visits). Rates for patients without other chronic conditions were the lowest (90.2).
Among individuals with diabetes aged 18-44 years, ED visit rates were the highest for those with two to four other chronic conditions (402.0) and lowest among those with five or more other conditions (93.8).
Among patients aged 45-64 years, ED visit rates were the highest for those with two to four other chronic conditions (526.4) and lowest for those without other conditions (87.7). In the 65 years and older group, rates were the highest for individuals with two to four other chronic conditions (605.2), followed by five or more conditions (217.7), one other condition (140.6), and no other conditions (36.5).
Notably, the ED visit rates for those with two to four or five or more other chronic conditions increased with age, whereas visits for those with no other chronic conditions or one other condition decreased with age.
Decade-Long Trend
ED visit rates among adults with diabetes increased throughout the past decade, from 48.6 visits per 1000 adults in 2012 to 74.9 per 1000 adults in 2021. Rates for those aged 65 and older were higher than all other age groups, increasing from 113.4 to 156.8. Increases were also seen among those aged 45-64 years (53.1 in 2012 to 89.2 in 2021) and 18-44 (20.9 in 2012 to 26.4 in 2016, then plateauing from 2016-2021).
Data are based on a sample of 4051 ED visits, representing about 18,238,000 average annual visits made by adults with diabetes to nonfederal, general, and short-stay hospitals during 2020-2021.
Taken together, these most recent estimates “show an increasing trend in rates by adults with diabetes in the ED setting,” the authors concluded.
A version of this article appeared on Medscape.com.
Emergency department (ED) visits by adults with diabetes increased by more than 25% since 2012, with the highest rates among Blacks and those aged over 65 years, a new data brief from the Centers for Disease Control and Prevention’s National Center for Health Statistics shows.
In 2021, diabetes was the eighth leading cause of death in the United States, according to the brief, published online on December 19, 2023. Its frequency is increasing in young people, and increasing age is a risk factor for hospitalization.
The latest data show that in 2020-2021, the overall annual ED visit rate was 72.2 visits per 1000 adults with diabetes, with no significant difference in terms of sex (75.1 visits per 1000 women vs 69.1 visits per 1000 men). By race/ethnicity, Blacks had the highest rates, at 135.5 visits per 1000 adults, followed by Whites (69.9) and Hispanics (52.3). The rates increased with age for both women and men, and among the three race/ethnic groups.
Comorbidities Count
The most ED visits were made by patients with diabetes and two to four other chronic conditions (541.4 visits per 1000 visits). Rates for patients without other chronic conditions were the lowest (90.2).
Among individuals with diabetes aged 18-44 years, ED visit rates were the highest for those with two to four other chronic conditions (402.0) and lowest among those with five or more other conditions (93.8).
Among patients aged 45-64 years, ED visit rates were the highest for those with two to four other chronic conditions (526.4) and lowest for those without other conditions (87.7). In the 65 years and older group, rates were the highest for individuals with two to four other chronic conditions (605.2), followed by five or more conditions (217.7), one other condition (140.6), and no other conditions (36.5).
Notably, the ED visit rates for those with two to four or five or more other chronic conditions increased with age, whereas visits for those with no other chronic conditions or one other condition decreased with age.
Decade-Long Trend
ED visit rates among adults with diabetes increased throughout the past decade, from 48.6 visits per 1000 adults in 2012 to 74.9 per 1000 adults in 2021. Rates for those aged 65 and older were higher than all other age groups, increasing from 113.4 to 156.8. Increases were also seen among those aged 45-64 years (53.1 in 2012 to 89.2 in 2021) and 18-44 (20.9 in 2012 to 26.4 in 2016, then plateauing from 2016-2021).
Data are based on a sample of 4051 ED visits, representing about 18,238,000 average annual visits made by adults with diabetes to nonfederal, general, and short-stay hospitals during 2020-2021.
Taken together, these most recent estimates “show an increasing trend in rates by adults with diabetes in the ED setting,” the authors concluded.
A version of this article appeared on Medscape.com.
GLP-1 RAs Associated With Reduced Colorectal Cancer Risk in Patients With Type 2 Diabetes
according to a new analysis.
In particular, GLP-1 RAs were associated with decreased risk compared with other antidiabetic treatments, including insulin, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, sulfonylureas, and thiazolidinediones.
More profound effects were seen in patients with overweight or obesity, “suggesting a potential protective effect against CRC partially mediated by weight loss and other mechanisms related to weight loss,” Lindsey Wang, an undergraduate student at Case Western Reserve University, Cleveland, Ohio, and colleagues wrote in JAMA Oncology.
Testing Treatments
GLP-1 RAs, usually given by injection, are approved by the US Food and Drug Administration to treat type 2 diabetes. They can lower blood sugar levels, improve insulin sensitivity, and help patients manage their weight.
Diabetes, overweight, and obesity are known risk factors for CRC and make prognosis worse. Ms. Wang and colleagues hypothesized that GLP-1 RAs might reduce CRC risk compared with other antidiabetics, including metformin and insulin, which have also been shown to reduce CRC risk.
Using a national database of more than 101 million electronic health records, Ms. Wang and colleagues conducted a population-based study of more than 1.2 million patients who had medical encounters for type 2 diabetes and were subsequently prescribed antidiabetic medications between 2005 and 2019. The patients had no prior antidiabetic medication use nor CRC diagnosis.
The researchers analyzed the effects of GLP-1 RAs on CRC incidence compared with the other prescribed antidiabetic drugs, matching for demographics, adverse socioeconomic determinants of health, preexisting medical conditions, family and personal history of cancers and colonic polyps, lifestyle factors, and procedures such as colonoscopy.
During a 15-year follow-up, GLP-1 RAs were associated with decreased risk for CRC compared with insulin (hazard ratio [HR], 0.56), metformin (HR, 0.75), SGLT2 inhibitors (HR, 0.77), sulfonylureas (HR, 0.82), and thiazolidinediones (HR, 0.82) in the overall study population.
For instance, among 22,572 patients who took insulin, 167 cases of CRC occurred, compared with 94 cases among the matched GLP-1 RA cohort. Among 18,518 patients who took metformin, 153 cases of CRC occurred compared with 96 cases among the matched GLP-1 RA cohort.
GLP-1 RAs also were associated with lower but not statistically significant risk than alpha-glucosidase inhibitors (HR, 0.59) and dipeptidyl-peptidase-4 (DPP-4) inhibitors (HR, 0.93).
In patients with overweight or obesity, GLP-1 RAs were associated with a lower risk for CRC than most of the other antidiabetics, including insulin (HR, 0.5), metformin (HR, 0.58), SGLT2 inhibitors (HR, 0.68), sulfonylureas (HR, 0.63), thiazolidinediones (HR, 0.73), and DPP-4 inhibitors (HR, 0.77).
Consistent findings were observed in women and men.
“Our results clearly demonstrate that GLP-1 RAs are significantly more effective than popular antidiabetic drugs, such as metformin or insulin, at preventing the development of CRC,” said Nathan Berger, MD, co-lead researcher, professor of experimental medicine, and member of the Case Comprehensive Cancer Center.
Targets for Future Research
Study limitations include potential unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases involved in observational studies, the research team noted.
Further research is warranted to investigate the effects in patients with prior antidiabetic treatments, underlying mechanisms, potential variation in effects among different GLP-1 RAs, and the potential of GLP-1 RAs to reduce the risks for other obesity-associated cancers, the researchers wrote.
“To our knowledge, this is the first indication this popular weight loss and antidiabetic class of drugs reduces incidence of CRC, relative to other antidiabetic agents,” said Rong Xu, PhD, co-lead researcher, professor of medicine, and member of the Case Comprehensive Cancer Center.
The study was supported by the National Cancer Institute Case Comprehensive Cancer Center, American Cancer Society, Landon Foundation-American Association for Cancer Research, National Institutes of Health Director’s New Innovator Award Program, National Institute on Aging, and National Institute on Alcohol Abuse and Alcoholism. Several authors reported grants from the National Institutes of Health during the conduct of the study.
A version of this article appeared on Medscape.com.
according to a new analysis.
In particular, GLP-1 RAs were associated with decreased risk compared with other antidiabetic treatments, including insulin, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, sulfonylureas, and thiazolidinediones.
More profound effects were seen in patients with overweight or obesity, “suggesting a potential protective effect against CRC partially mediated by weight loss and other mechanisms related to weight loss,” Lindsey Wang, an undergraduate student at Case Western Reserve University, Cleveland, Ohio, and colleagues wrote in JAMA Oncology.
Testing Treatments
GLP-1 RAs, usually given by injection, are approved by the US Food and Drug Administration to treat type 2 diabetes. They can lower blood sugar levels, improve insulin sensitivity, and help patients manage their weight.
Diabetes, overweight, and obesity are known risk factors for CRC and make prognosis worse. Ms. Wang and colleagues hypothesized that GLP-1 RAs might reduce CRC risk compared with other antidiabetics, including metformin and insulin, which have also been shown to reduce CRC risk.
Using a national database of more than 101 million electronic health records, Ms. Wang and colleagues conducted a population-based study of more than 1.2 million patients who had medical encounters for type 2 diabetes and were subsequently prescribed antidiabetic medications between 2005 and 2019. The patients had no prior antidiabetic medication use nor CRC diagnosis.
The researchers analyzed the effects of GLP-1 RAs on CRC incidence compared with the other prescribed antidiabetic drugs, matching for demographics, adverse socioeconomic determinants of health, preexisting medical conditions, family and personal history of cancers and colonic polyps, lifestyle factors, and procedures such as colonoscopy.
During a 15-year follow-up, GLP-1 RAs were associated with decreased risk for CRC compared with insulin (hazard ratio [HR], 0.56), metformin (HR, 0.75), SGLT2 inhibitors (HR, 0.77), sulfonylureas (HR, 0.82), and thiazolidinediones (HR, 0.82) in the overall study population.
For instance, among 22,572 patients who took insulin, 167 cases of CRC occurred, compared with 94 cases among the matched GLP-1 RA cohort. Among 18,518 patients who took metformin, 153 cases of CRC occurred compared with 96 cases among the matched GLP-1 RA cohort.
GLP-1 RAs also were associated with lower but not statistically significant risk than alpha-glucosidase inhibitors (HR, 0.59) and dipeptidyl-peptidase-4 (DPP-4) inhibitors (HR, 0.93).
In patients with overweight or obesity, GLP-1 RAs were associated with a lower risk for CRC than most of the other antidiabetics, including insulin (HR, 0.5), metformin (HR, 0.58), SGLT2 inhibitors (HR, 0.68), sulfonylureas (HR, 0.63), thiazolidinediones (HR, 0.73), and DPP-4 inhibitors (HR, 0.77).
Consistent findings were observed in women and men.
“Our results clearly demonstrate that GLP-1 RAs are significantly more effective than popular antidiabetic drugs, such as metformin or insulin, at preventing the development of CRC,” said Nathan Berger, MD, co-lead researcher, professor of experimental medicine, and member of the Case Comprehensive Cancer Center.
Targets for Future Research
Study limitations include potential unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases involved in observational studies, the research team noted.
Further research is warranted to investigate the effects in patients with prior antidiabetic treatments, underlying mechanisms, potential variation in effects among different GLP-1 RAs, and the potential of GLP-1 RAs to reduce the risks for other obesity-associated cancers, the researchers wrote.
“To our knowledge, this is the first indication this popular weight loss and antidiabetic class of drugs reduces incidence of CRC, relative to other antidiabetic agents,” said Rong Xu, PhD, co-lead researcher, professor of medicine, and member of the Case Comprehensive Cancer Center.
The study was supported by the National Cancer Institute Case Comprehensive Cancer Center, American Cancer Society, Landon Foundation-American Association for Cancer Research, National Institutes of Health Director’s New Innovator Award Program, National Institute on Aging, and National Institute on Alcohol Abuse and Alcoholism. Several authors reported grants from the National Institutes of Health during the conduct of the study.
A version of this article appeared on Medscape.com.
according to a new analysis.
In particular, GLP-1 RAs were associated with decreased risk compared with other antidiabetic treatments, including insulin, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, sulfonylureas, and thiazolidinediones.
More profound effects were seen in patients with overweight or obesity, “suggesting a potential protective effect against CRC partially mediated by weight loss and other mechanisms related to weight loss,” Lindsey Wang, an undergraduate student at Case Western Reserve University, Cleveland, Ohio, and colleagues wrote in JAMA Oncology.
Testing Treatments
GLP-1 RAs, usually given by injection, are approved by the US Food and Drug Administration to treat type 2 diabetes. They can lower blood sugar levels, improve insulin sensitivity, and help patients manage their weight.
Diabetes, overweight, and obesity are known risk factors for CRC and make prognosis worse. Ms. Wang and colleagues hypothesized that GLP-1 RAs might reduce CRC risk compared with other antidiabetics, including metformin and insulin, which have also been shown to reduce CRC risk.
Using a national database of more than 101 million electronic health records, Ms. Wang and colleagues conducted a population-based study of more than 1.2 million patients who had medical encounters for type 2 diabetes and were subsequently prescribed antidiabetic medications between 2005 and 2019. The patients had no prior antidiabetic medication use nor CRC diagnosis.
The researchers analyzed the effects of GLP-1 RAs on CRC incidence compared with the other prescribed antidiabetic drugs, matching for demographics, adverse socioeconomic determinants of health, preexisting medical conditions, family and personal history of cancers and colonic polyps, lifestyle factors, and procedures such as colonoscopy.
During a 15-year follow-up, GLP-1 RAs were associated with decreased risk for CRC compared with insulin (hazard ratio [HR], 0.56), metformin (HR, 0.75), SGLT2 inhibitors (HR, 0.77), sulfonylureas (HR, 0.82), and thiazolidinediones (HR, 0.82) in the overall study population.
For instance, among 22,572 patients who took insulin, 167 cases of CRC occurred, compared with 94 cases among the matched GLP-1 RA cohort. Among 18,518 patients who took metformin, 153 cases of CRC occurred compared with 96 cases among the matched GLP-1 RA cohort.
GLP-1 RAs also were associated with lower but not statistically significant risk than alpha-glucosidase inhibitors (HR, 0.59) and dipeptidyl-peptidase-4 (DPP-4) inhibitors (HR, 0.93).
In patients with overweight or obesity, GLP-1 RAs were associated with a lower risk for CRC than most of the other antidiabetics, including insulin (HR, 0.5), metformin (HR, 0.58), SGLT2 inhibitors (HR, 0.68), sulfonylureas (HR, 0.63), thiazolidinediones (HR, 0.73), and DPP-4 inhibitors (HR, 0.77).
Consistent findings were observed in women and men.
“Our results clearly demonstrate that GLP-1 RAs are significantly more effective than popular antidiabetic drugs, such as metformin or insulin, at preventing the development of CRC,” said Nathan Berger, MD, co-lead researcher, professor of experimental medicine, and member of the Case Comprehensive Cancer Center.
Targets for Future Research
Study limitations include potential unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases involved in observational studies, the research team noted.
Further research is warranted to investigate the effects in patients with prior antidiabetic treatments, underlying mechanisms, potential variation in effects among different GLP-1 RAs, and the potential of GLP-1 RAs to reduce the risks for other obesity-associated cancers, the researchers wrote.
“To our knowledge, this is the first indication this popular weight loss and antidiabetic class of drugs reduces incidence of CRC, relative to other antidiabetic agents,” said Rong Xu, PhD, co-lead researcher, professor of medicine, and member of the Case Comprehensive Cancer Center.
The study was supported by the National Cancer Institute Case Comprehensive Cancer Center, American Cancer Society, Landon Foundation-American Association for Cancer Research, National Institutes of Health Director’s New Innovator Award Program, National Institute on Aging, and National Institute on Alcohol Abuse and Alcoholism. Several authors reported grants from the National Institutes of Health during the conduct of the study.
A version of this article appeared on Medscape.com.
‘We Will Rock You’ Into Real-time Diabetes Control
, reveals a series of experiments.
The research was published in The Lancet Diabetes & Endocrinology.
After developing a cell line in which music-sensitive calcium channels triggered the release of insulin-containing vesicles, the researchers conducted a series of studies identifying the optimal frequency, pitch, and volume of sounds for triggering release.
After settling on low-bass heavy popular music, they tested their system on mice with type 1 diabetes that had the insulin-releasing cells implanted in their abdomen. Applying the music directly at 60 dB led to near wild-type levels of insulin in the blood within 15 minutes.
“With only 4 hours required for a full refill, [the system] can provide several therapeutic doses a day,” says Martin Fussenegger, PhD, professor of biotechnology and bioengineering, Department of Biosystems Science and Engineering, ETH Zurich, Basel, Switzerland, and colleagues.
“This would match the typical needs of people with type 2 diabetes consuming three meals a day, and for whom administration of prandial insulin is an established treatment option, as they do not have capability for early postprandial insulin secretion from preformed insulin.”
As the system requires nothing more than portable battery-powered commercially available loudspeakers, the multiple daily dosing of biopharmaceuticals becomes “straightforward in the absence of medical infrastructure or staff, simply by having the patient listen to the prescribed music.”
It therefore “could be an interesting option for cell-based therapies, especially where the need for frequent dosing raises compliance issues.”
It is a “very exciting piece of work, no doubt,” said Anandwardhan A. Hardikar, PhD, group leader, Diabetes and Islet Biology Group, Translational Health Research Institute, Western Sydney University, Penrith NSW, Australia.
He pointed out that the concept of using music to drive gene expression “is something we’ve known for the last 20 years,” but bringing the different strands of research together to generate cells that can be implanted into mice is “an amazing idea.”
Dr. Hardikar, who was not involved in the study, said, however, the publication of the study as a correspondence “does not allow for a lot of the detail that I would have expected as an academic,” and consequently some questions remain.
The most important is whether the music itself is required to trigger the insulin release, as opposed simply to sounds in general.
Is Music or Sound the “Trigger?”
Music is “frequency, it’s the amplitude of the waveform, and it’s the duration for which those waveforms are present,” he noted, but the same profile can be achieved by cutting up and editing the melody so it becomes a jumble of sounds.
For Dr. Hardikar, the “best control” for the study would be to have no music as well as the edited song, with “bits of pieces” played randomly so “it sounds like it’s the same frequency and amplitude.”
Then it would be clear whether the effect is owing to the “noise, or we have to appreciate the melody.”
The other outstanding question is whether the results “can directly translate to larger animals,” such as humans, Dr. Hardikar said.
The authors point out that when translated into mechanical vibrations in the middle ear, the acoustic waves of music activate mechanosensitive ion channels, a form of trigger that is seen across the animal kingdom.
They go on to highlight that while gene switches have been developed for use in next-generation cell-based therapies for a range of conditions, small-molecular trigger compounds face a number of challenges and may cause adverse effects.
With “traceless triggers” such as light, ultrasound, magnetic fields, radio waves, electricity, and heat also facing issues, there is a “need for new switching modalities.”
The researchers therefore developed a music-inducible cellular control (MUSIC) system, which leverages the known intracellular calcium surge in response to music, via calcium-permeable mechanosensitive channels, to drive the release of biopharmaceuticals from vesicles.
They then generated MUSIC-controlled insulin-releasing cell lines, finding that, using a customized box containing off-the-shelf loudspeakers, they could induce channel activation and insulin release with 60 dB at 50 Hz, which is “within the safe range for the human ear.”
Further experiments revealed that insulin release was greatest at 50-100 Hz, and higher than that seen with potassium chloride, the “gold-standard” depolarization control for calcium channels.
The researchers then showed that with optimal stimulation at 50 Hz and 60 dB, channel activation and subsequent insulin release required at least 3 seconds of continuous music, “which might protect the cellular device from inadvertent activation during everyday activities.”
Next, they examined the impact of different musical genres on insulin release, finding that low-bass heavy popular music and movie soundtracks induced maximum release, while the responses were more diverse to classical and guitar-based music.
Specifically, “We Will Rock You,” by the British rock band Queen, induced the release of 70% of available insulin within 5 minutes and 100% within 15 minutes. This, the team notes, is “similar to the dynamics of glucose-triggered insulin release by human pancreatic islets.”
Exposing the cells to a second music session at different intervals revealed that full insulin refill was achieved within 4 hours, which “would be appropriate to attenuate glycemic excursions associated with typical dietary habits.”
Finally, the researchers tested the system in vivo, constructing a box with two off-the-shelf loudspeakers that focuses acoustic waves, via deflectors, onto the abdomens of mice with type 1 diabetes.
Exposing the mice, which had been implanted with microencapsulated MUSIC cells in the peritoneum, to low-bass acoustic waves at 60 dB (50 m/s2) for 15 minutes allowed them to achieve near wild-type levels of insulin in the blood and restored normoglycemia.
Moreover, “Queen’s song ‘We Will Rock You’ generated sufficient insulin to rapidly attenuate postprandial glycemic excursions during glucose tolerance tests,” the team says.
In contrast, animals without implants, or those that had implants but did not have music immersion, remained severely hyperglycemic, they add.
They also note that the effect was seen only when the sound waves “directly impinge on the skin just above the implantation site” for at least 15 minutes, with no increase in insulin release observed with commercially available headphones or ear plugs, such as Apple AirPods, or with loud environmental noises.
Consequently, “therapeutic MUSIC sessions would still be compatible with listening to other types of music or listening to all types of music via headphones,” the researchers write, and are “compatible with standard drug administration schemes.”
The study was supported by a European Research Council advanced grant and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering. One author acknowledges the support of the Chinese Scholarship Council.
No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
, reveals a series of experiments.
The research was published in The Lancet Diabetes & Endocrinology.
After developing a cell line in which music-sensitive calcium channels triggered the release of insulin-containing vesicles, the researchers conducted a series of studies identifying the optimal frequency, pitch, and volume of sounds for triggering release.
After settling on low-bass heavy popular music, they tested their system on mice with type 1 diabetes that had the insulin-releasing cells implanted in their abdomen. Applying the music directly at 60 dB led to near wild-type levels of insulin in the blood within 15 minutes.
“With only 4 hours required for a full refill, [the system] can provide several therapeutic doses a day,” says Martin Fussenegger, PhD, professor of biotechnology and bioengineering, Department of Biosystems Science and Engineering, ETH Zurich, Basel, Switzerland, and colleagues.
“This would match the typical needs of people with type 2 diabetes consuming three meals a day, and for whom administration of prandial insulin is an established treatment option, as they do not have capability for early postprandial insulin secretion from preformed insulin.”
As the system requires nothing more than portable battery-powered commercially available loudspeakers, the multiple daily dosing of biopharmaceuticals becomes “straightforward in the absence of medical infrastructure or staff, simply by having the patient listen to the prescribed music.”
It therefore “could be an interesting option for cell-based therapies, especially where the need for frequent dosing raises compliance issues.”
It is a “very exciting piece of work, no doubt,” said Anandwardhan A. Hardikar, PhD, group leader, Diabetes and Islet Biology Group, Translational Health Research Institute, Western Sydney University, Penrith NSW, Australia.
He pointed out that the concept of using music to drive gene expression “is something we’ve known for the last 20 years,” but bringing the different strands of research together to generate cells that can be implanted into mice is “an amazing idea.”
Dr. Hardikar, who was not involved in the study, said, however, the publication of the study as a correspondence “does not allow for a lot of the detail that I would have expected as an academic,” and consequently some questions remain.
The most important is whether the music itself is required to trigger the insulin release, as opposed simply to sounds in general.
Is Music or Sound the “Trigger?”
Music is “frequency, it’s the amplitude of the waveform, and it’s the duration for which those waveforms are present,” he noted, but the same profile can be achieved by cutting up and editing the melody so it becomes a jumble of sounds.
For Dr. Hardikar, the “best control” for the study would be to have no music as well as the edited song, with “bits of pieces” played randomly so “it sounds like it’s the same frequency and amplitude.”
Then it would be clear whether the effect is owing to the “noise, or we have to appreciate the melody.”
The other outstanding question is whether the results “can directly translate to larger animals,” such as humans, Dr. Hardikar said.
The authors point out that when translated into mechanical vibrations in the middle ear, the acoustic waves of music activate mechanosensitive ion channels, a form of trigger that is seen across the animal kingdom.
They go on to highlight that while gene switches have been developed for use in next-generation cell-based therapies for a range of conditions, small-molecular trigger compounds face a number of challenges and may cause adverse effects.
With “traceless triggers” such as light, ultrasound, magnetic fields, radio waves, electricity, and heat also facing issues, there is a “need for new switching modalities.”
The researchers therefore developed a music-inducible cellular control (MUSIC) system, which leverages the known intracellular calcium surge in response to music, via calcium-permeable mechanosensitive channels, to drive the release of biopharmaceuticals from vesicles.
They then generated MUSIC-controlled insulin-releasing cell lines, finding that, using a customized box containing off-the-shelf loudspeakers, they could induce channel activation and insulin release with 60 dB at 50 Hz, which is “within the safe range for the human ear.”
Further experiments revealed that insulin release was greatest at 50-100 Hz, and higher than that seen with potassium chloride, the “gold-standard” depolarization control for calcium channels.
The researchers then showed that with optimal stimulation at 50 Hz and 60 dB, channel activation and subsequent insulin release required at least 3 seconds of continuous music, “which might protect the cellular device from inadvertent activation during everyday activities.”
Next, they examined the impact of different musical genres on insulin release, finding that low-bass heavy popular music and movie soundtracks induced maximum release, while the responses were more diverse to classical and guitar-based music.
Specifically, “We Will Rock You,” by the British rock band Queen, induced the release of 70% of available insulin within 5 minutes and 100% within 15 minutes. This, the team notes, is “similar to the dynamics of glucose-triggered insulin release by human pancreatic islets.”
Exposing the cells to a second music session at different intervals revealed that full insulin refill was achieved within 4 hours, which “would be appropriate to attenuate glycemic excursions associated with typical dietary habits.”
Finally, the researchers tested the system in vivo, constructing a box with two off-the-shelf loudspeakers that focuses acoustic waves, via deflectors, onto the abdomens of mice with type 1 diabetes.
Exposing the mice, which had been implanted with microencapsulated MUSIC cells in the peritoneum, to low-bass acoustic waves at 60 dB (50 m/s2) for 15 minutes allowed them to achieve near wild-type levels of insulin in the blood and restored normoglycemia.
Moreover, “Queen’s song ‘We Will Rock You’ generated sufficient insulin to rapidly attenuate postprandial glycemic excursions during glucose tolerance tests,” the team says.
In contrast, animals without implants, or those that had implants but did not have music immersion, remained severely hyperglycemic, they add.
They also note that the effect was seen only when the sound waves “directly impinge on the skin just above the implantation site” for at least 15 minutes, with no increase in insulin release observed with commercially available headphones or ear plugs, such as Apple AirPods, or with loud environmental noises.
Consequently, “therapeutic MUSIC sessions would still be compatible with listening to other types of music or listening to all types of music via headphones,” the researchers write, and are “compatible with standard drug administration schemes.”
The study was supported by a European Research Council advanced grant and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering. One author acknowledges the support of the Chinese Scholarship Council.
No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
, reveals a series of experiments.
The research was published in The Lancet Diabetes & Endocrinology.
After developing a cell line in which music-sensitive calcium channels triggered the release of insulin-containing vesicles, the researchers conducted a series of studies identifying the optimal frequency, pitch, and volume of sounds for triggering release.
After settling on low-bass heavy popular music, they tested their system on mice with type 1 diabetes that had the insulin-releasing cells implanted in their abdomen. Applying the music directly at 60 dB led to near wild-type levels of insulin in the blood within 15 minutes.
“With only 4 hours required for a full refill, [the system] can provide several therapeutic doses a day,” says Martin Fussenegger, PhD, professor of biotechnology and bioengineering, Department of Biosystems Science and Engineering, ETH Zurich, Basel, Switzerland, and colleagues.
“This would match the typical needs of people with type 2 diabetes consuming three meals a day, and for whom administration of prandial insulin is an established treatment option, as they do not have capability for early postprandial insulin secretion from preformed insulin.”
As the system requires nothing more than portable battery-powered commercially available loudspeakers, the multiple daily dosing of biopharmaceuticals becomes “straightforward in the absence of medical infrastructure or staff, simply by having the patient listen to the prescribed music.”
It therefore “could be an interesting option for cell-based therapies, especially where the need for frequent dosing raises compliance issues.”
It is a “very exciting piece of work, no doubt,” said Anandwardhan A. Hardikar, PhD, group leader, Diabetes and Islet Biology Group, Translational Health Research Institute, Western Sydney University, Penrith NSW, Australia.
He pointed out that the concept of using music to drive gene expression “is something we’ve known for the last 20 years,” but bringing the different strands of research together to generate cells that can be implanted into mice is “an amazing idea.”
Dr. Hardikar, who was not involved in the study, said, however, the publication of the study as a correspondence “does not allow for a lot of the detail that I would have expected as an academic,” and consequently some questions remain.
The most important is whether the music itself is required to trigger the insulin release, as opposed simply to sounds in general.
Is Music or Sound the “Trigger?”
Music is “frequency, it’s the amplitude of the waveform, and it’s the duration for which those waveforms are present,” he noted, but the same profile can be achieved by cutting up and editing the melody so it becomes a jumble of sounds.
For Dr. Hardikar, the “best control” for the study would be to have no music as well as the edited song, with “bits of pieces” played randomly so “it sounds like it’s the same frequency and amplitude.”
Then it would be clear whether the effect is owing to the “noise, or we have to appreciate the melody.”
The other outstanding question is whether the results “can directly translate to larger animals,” such as humans, Dr. Hardikar said.
The authors point out that when translated into mechanical vibrations in the middle ear, the acoustic waves of music activate mechanosensitive ion channels, a form of trigger that is seen across the animal kingdom.
They go on to highlight that while gene switches have been developed for use in next-generation cell-based therapies for a range of conditions, small-molecular trigger compounds face a number of challenges and may cause adverse effects.
With “traceless triggers” such as light, ultrasound, magnetic fields, radio waves, electricity, and heat also facing issues, there is a “need for new switching modalities.”
The researchers therefore developed a music-inducible cellular control (MUSIC) system, which leverages the known intracellular calcium surge in response to music, via calcium-permeable mechanosensitive channels, to drive the release of biopharmaceuticals from vesicles.
They then generated MUSIC-controlled insulin-releasing cell lines, finding that, using a customized box containing off-the-shelf loudspeakers, they could induce channel activation and insulin release with 60 dB at 50 Hz, which is “within the safe range for the human ear.”
Further experiments revealed that insulin release was greatest at 50-100 Hz, and higher than that seen with potassium chloride, the “gold-standard” depolarization control for calcium channels.
The researchers then showed that with optimal stimulation at 50 Hz and 60 dB, channel activation and subsequent insulin release required at least 3 seconds of continuous music, “which might protect the cellular device from inadvertent activation during everyday activities.”
Next, they examined the impact of different musical genres on insulin release, finding that low-bass heavy popular music and movie soundtracks induced maximum release, while the responses were more diverse to classical and guitar-based music.
Specifically, “We Will Rock You,” by the British rock band Queen, induced the release of 70% of available insulin within 5 minutes and 100% within 15 minutes. This, the team notes, is “similar to the dynamics of glucose-triggered insulin release by human pancreatic islets.”
Exposing the cells to a second music session at different intervals revealed that full insulin refill was achieved within 4 hours, which “would be appropriate to attenuate glycemic excursions associated with typical dietary habits.”
Finally, the researchers tested the system in vivo, constructing a box with two off-the-shelf loudspeakers that focuses acoustic waves, via deflectors, onto the abdomens of mice with type 1 diabetes.
Exposing the mice, which had been implanted with microencapsulated MUSIC cells in the peritoneum, to low-bass acoustic waves at 60 dB (50 m/s2) for 15 minutes allowed them to achieve near wild-type levels of insulin in the blood and restored normoglycemia.
Moreover, “Queen’s song ‘We Will Rock You’ generated sufficient insulin to rapidly attenuate postprandial glycemic excursions during glucose tolerance tests,” the team says.
In contrast, animals without implants, or those that had implants but did not have music immersion, remained severely hyperglycemic, they add.
They also note that the effect was seen only when the sound waves “directly impinge on the skin just above the implantation site” for at least 15 minutes, with no increase in insulin release observed with commercially available headphones or ear plugs, such as Apple AirPods, or with loud environmental noises.
Consequently, “therapeutic MUSIC sessions would still be compatible with listening to other types of music or listening to all types of music via headphones,” the researchers write, and are “compatible with standard drug administration schemes.”
The study was supported by a European Research Council advanced grant and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering. One author acknowledges the support of the Chinese Scholarship Council.
No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
What if a single GLP-1 shot could last for months?
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
How to prescribe Zepbound
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 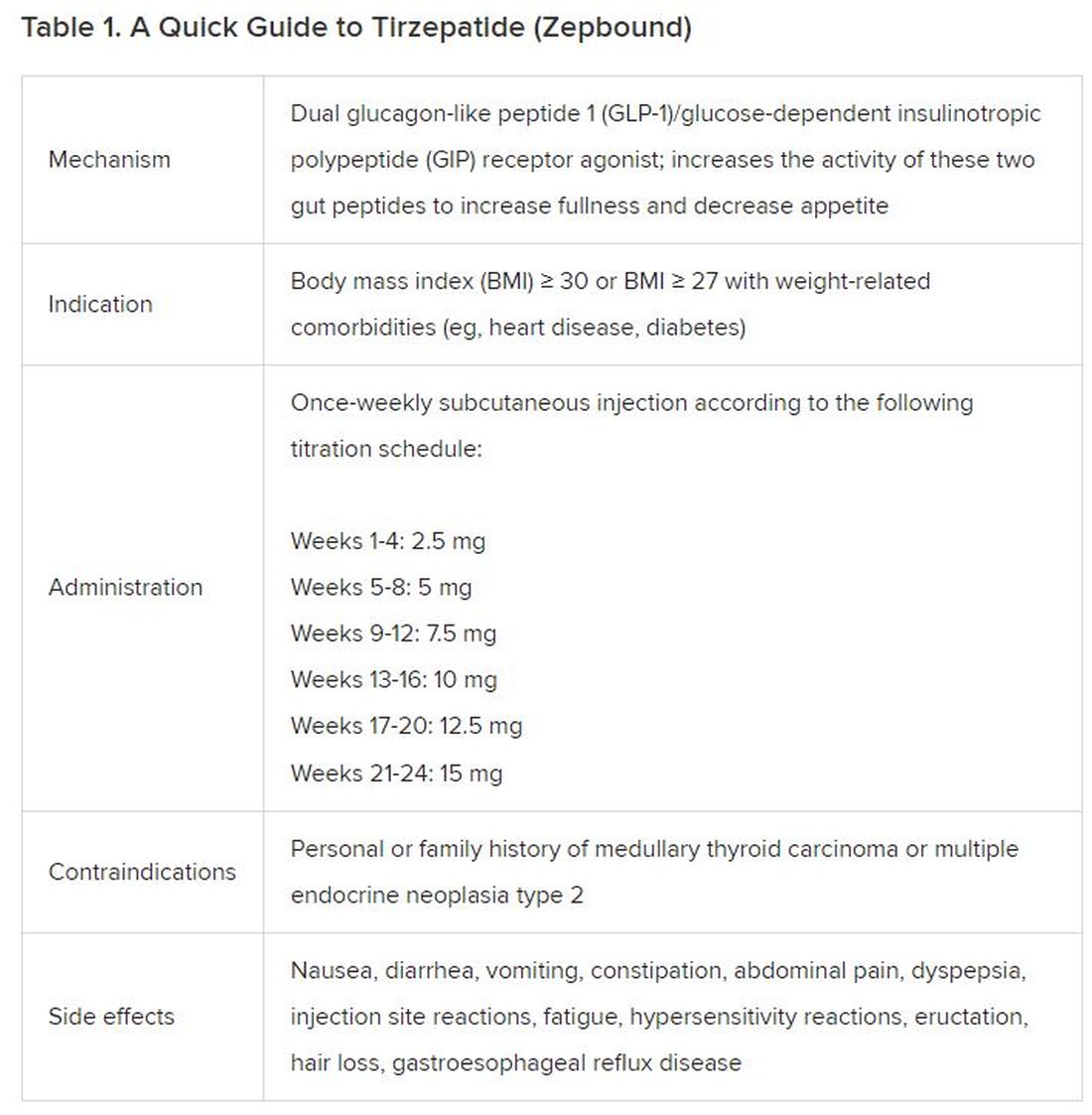
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).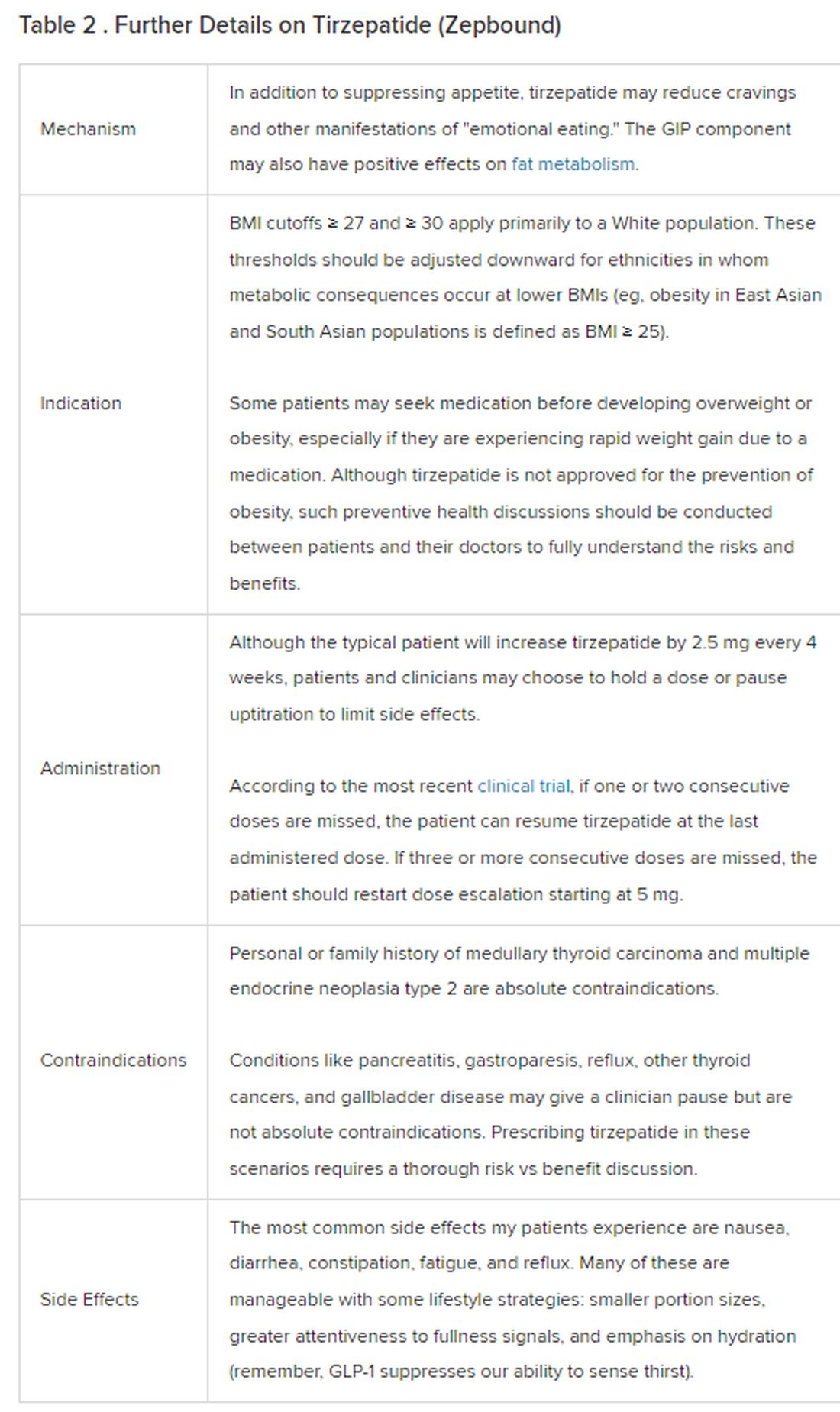
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
ADA issues new screening, obesity management recommendations
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.