User login
Diet packed with fast food found hard on the liver
The study finds that getting one-fifth or more of total daily calories from fast food can increase the risk of nonalcoholic fatty liver disease, which can lead to cirrhosis and its complications, including liver failure and liver cancer.
Although the magnitude of association was modest among the general population, “striking” elevations in steatosis were evident among persons with obesity and diabetes who consumed fast food, in comparison with their counterparts who did not have obesity and diabetes, the researchers reported.
“My hope is that this study encourages people to seek out more nutritious, healthy food options and provides information that clinicians can use to counsel their patients, particularly those with underlying metabolic risk factors, of the importance of avoiding foods that are high in fat, carbohydrates, and processed sugars,” lead investigator Ani Kardashian, MD, hepatologist with the University of Southern California, Los Angeles, said in an interview.
“At a policy level, public health efforts are needed to improve access to affordable, healthy, and nutritious food options across the U.S. This is especially important as more people have turned to fast foods during the pandemic and as the price of food as risen dramatically over the past year due to food inflation,” Dr. Kardashian added.
The study was published online in Clinical Gastroenterology and Hepatology.
More fast food, greater steatosis
The findings are based on data from 3,954 adults who participated in the National Health and Nutrition Examination Survey (NHANES) of 2017-2018 and who underwent vibration-controlled transient elastography. Of these participants, data regarding 1- or 2-day dietary recall were available.
Steatosis, the primary outcome, was measured via controlled attenuation parameter (CAP). Two validated cutoffs were utilized (CAP ≥ 263 dB/m and CAP ≥ 285 dB/m).
Of those surveyed, 52% consumed any fast food, and 29% derived 20% or more of their daily calories from fast food.
Fast-food intake of 20% or more of daily calories was significantly associated with greater steatosis after multivariable adjustment, both as a continuous measure (4.6 dB/m higher CAP score) and with respect to the CAP ≥ 263 dB/m cutoff (odds ratio [OR], 1.45).
“The negative effects are particularly severe in people who already have diabetes and obesity,” Dr. Kardashian told this news organization.
For example, with diabetes and fast-food intake of 20% or more of daily calories, the ORs of meeting the CAP ≥ 263 dB/m cutoff and the CAP ≥ 285 dB/m cutoff were 2.3 and 2.48, respectively.
The researchers said their findings are particularly “alarming,” given the overall increase in fast-food consumption over the past 50 years in the United States, regardless of socioeconomic status.
Diet coaching
The finding that fast food has more deleterious impact on those with obesity and diabetes “emphasizes that it is not just one insult but multiple factors that contribute to overall health,” said Nancy Reau, MD, section chief of hepatology at Rush University Medical Center in Chicago.
“This is actually great news, because diet is modifiable, vs. your genetics, which you currently can’t change. This doesn’t mean if you’re lean you can eat whatever you want, but if you are overweight, being careful with your diet does have impact, even if it doesn’t lead to substantial weight changes,” said Dr. Reau, who is not affiliated with the study.
For people who have limited options and need to eat fast food, “there are healthy choices at most restaurants; you just need to be smart about reading labels, watching calories, and ordering the healthier options,” Dr. Reau said in an interview.
Fast food and fatty liver go “hand in hand,” Lisa Ganjhu, DO, gastroenterologist and hepatologist at NYU Langone Health in New York, told this news organization.
“I counsel and coach my patients on healthy diet and exercise, and I’ve been pretty successful,” said Dr. Ganjhu, who was not involved with the study.
“If my patient is eating at McDonald’s a lot, I basically walk through the menu with them and help them find something healthy. When patients see the benefits of cutting out fat and reducing carbohydrates, they are more apt to continue,” Dr. Ganjhu said.
The study was funded by the University of Southern California. Dr. Kardashian, Dr. Reau, and Dr. Ganjhu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study finds that getting one-fifth or more of total daily calories from fast food can increase the risk of nonalcoholic fatty liver disease, which can lead to cirrhosis and its complications, including liver failure and liver cancer.
Although the magnitude of association was modest among the general population, “striking” elevations in steatosis were evident among persons with obesity and diabetes who consumed fast food, in comparison with their counterparts who did not have obesity and diabetes, the researchers reported.
“My hope is that this study encourages people to seek out more nutritious, healthy food options and provides information that clinicians can use to counsel their patients, particularly those with underlying metabolic risk factors, of the importance of avoiding foods that are high in fat, carbohydrates, and processed sugars,” lead investigator Ani Kardashian, MD, hepatologist with the University of Southern California, Los Angeles, said in an interview.
“At a policy level, public health efforts are needed to improve access to affordable, healthy, and nutritious food options across the U.S. This is especially important as more people have turned to fast foods during the pandemic and as the price of food as risen dramatically over the past year due to food inflation,” Dr. Kardashian added.
The study was published online in Clinical Gastroenterology and Hepatology.
More fast food, greater steatosis
The findings are based on data from 3,954 adults who participated in the National Health and Nutrition Examination Survey (NHANES) of 2017-2018 and who underwent vibration-controlled transient elastography. Of these participants, data regarding 1- or 2-day dietary recall were available.
Steatosis, the primary outcome, was measured via controlled attenuation parameter (CAP). Two validated cutoffs were utilized (CAP ≥ 263 dB/m and CAP ≥ 285 dB/m).
Of those surveyed, 52% consumed any fast food, and 29% derived 20% or more of their daily calories from fast food.
Fast-food intake of 20% or more of daily calories was significantly associated with greater steatosis after multivariable adjustment, both as a continuous measure (4.6 dB/m higher CAP score) and with respect to the CAP ≥ 263 dB/m cutoff (odds ratio [OR], 1.45).
“The negative effects are particularly severe in people who already have diabetes and obesity,” Dr. Kardashian told this news organization.
For example, with diabetes and fast-food intake of 20% or more of daily calories, the ORs of meeting the CAP ≥ 263 dB/m cutoff and the CAP ≥ 285 dB/m cutoff were 2.3 and 2.48, respectively.
The researchers said their findings are particularly “alarming,” given the overall increase in fast-food consumption over the past 50 years in the United States, regardless of socioeconomic status.
Diet coaching
The finding that fast food has more deleterious impact on those with obesity and diabetes “emphasizes that it is not just one insult but multiple factors that contribute to overall health,” said Nancy Reau, MD, section chief of hepatology at Rush University Medical Center in Chicago.
“This is actually great news, because diet is modifiable, vs. your genetics, which you currently can’t change. This doesn’t mean if you’re lean you can eat whatever you want, but if you are overweight, being careful with your diet does have impact, even if it doesn’t lead to substantial weight changes,” said Dr. Reau, who is not affiliated with the study.
For people who have limited options and need to eat fast food, “there are healthy choices at most restaurants; you just need to be smart about reading labels, watching calories, and ordering the healthier options,” Dr. Reau said in an interview.
Fast food and fatty liver go “hand in hand,” Lisa Ganjhu, DO, gastroenterologist and hepatologist at NYU Langone Health in New York, told this news organization.
“I counsel and coach my patients on healthy diet and exercise, and I’ve been pretty successful,” said Dr. Ganjhu, who was not involved with the study.
“If my patient is eating at McDonald’s a lot, I basically walk through the menu with them and help them find something healthy. When patients see the benefits of cutting out fat and reducing carbohydrates, they are more apt to continue,” Dr. Ganjhu said.
The study was funded by the University of Southern California. Dr. Kardashian, Dr. Reau, and Dr. Ganjhu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study finds that getting one-fifth or more of total daily calories from fast food can increase the risk of nonalcoholic fatty liver disease, which can lead to cirrhosis and its complications, including liver failure and liver cancer.
Although the magnitude of association was modest among the general population, “striking” elevations in steatosis were evident among persons with obesity and diabetes who consumed fast food, in comparison with their counterparts who did not have obesity and diabetes, the researchers reported.
“My hope is that this study encourages people to seek out more nutritious, healthy food options and provides information that clinicians can use to counsel their patients, particularly those with underlying metabolic risk factors, of the importance of avoiding foods that are high in fat, carbohydrates, and processed sugars,” lead investigator Ani Kardashian, MD, hepatologist with the University of Southern California, Los Angeles, said in an interview.
“At a policy level, public health efforts are needed to improve access to affordable, healthy, and nutritious food options across the U.S. This is especially important as more people have turned to fast foods during the pandemic and as the price of food as risen dramatically over the past year due to food inflation,” Dr. Kardashian added.
The study was published online in Clinical Gastroenterology and Hepatology.
More fast food, greater steatosis
The findings are based on data from 3,954 adults who participated in the National Health and Nutrition Examination Survey (NHANES) of 2017-2018 and who underwent vibration-controlled transient elastography. Of these participants, data regarding 1- or 2-day dietary recall were available.
Steatosis, the primary outcome, was measured via controlled attenuation parameter (CAP). Two validated cutoffs were utilized (CAP ≥ 263 dB/m and CAP ≥ 285 dB/m).
Of those surveyed, 52% consumed any fast food, and 29% derived 20% or more of their daily calories from fast food.
Fast-food intake of 20% or more of daily calories was significantly associated with greater steatosis after multivariable adjustment, both as a continuous measure (4.6 dB/m higher CAP score) and with respect to the CAP ≥ 263 dB/m cutoff (odds ratio [OR], 1.45).
“The negative effects are particularly severe in people who already have diabetes and obesity,” Dr. Kardashian told this news organization.
For example, with diabetes and fast-food intake of 20% or more of daily calories, the ORs of meeting the CAP ≥ 263 dB/m cutoff and the CAP ≥ 285 dB/m cutoff were 2.3 and 2.48, respectively.
The researchers said their findings are particularly “alarming,” given the overall increase in fast-food consumption over the past 50 years in the United States, regardless of socioeconomic status.
Diet coaching
The finding that fast food has more deleterious impact on those with obesity and diabetes “emphasizes that it is not just one insult but multiple factors that contribute to overall health,” said Nancy Reau, MD, section chief of hepatology at Rush University Medical Center in Chicago.
“This is actually great news, because diet is modifiable, vs. your genetics, which you currently can’t change. This doesn’t mean if you’re lean you can eat whatever you want, but if you are overweight, being careful with your diet does have impact, even if it doesn’t lead to substantial weight changes,” said Dr. Reau, who is not affiliated with the study.
For people who have limited options and need to eat fast food, “there are healthy choices at most restaurants; you just need to be smart about reading labels, watching calories, and ordering the healthier options,” Dr. Reau said in an interview.
Fast food and fatty liver go “hand in hand,” Lisa Ganjhu, DO, gastroenterologist and hepatologist at NYU Langone Health in New York, told this news organization.
“I counsel and coach my patients on healthy diet and exercise, and I’ve been pretty successful,” said Dr. Ganjhu, who was not involved with the study.
“If my patient is eating at McDonald’s a lot, I basically walk through the menu with them and help them find something healthy. When patients see the benefits of cutting out fat and reducing carbohydrates, they are more apt to continue,” Dr. Ganjhu said.
The study was funded by the University of Southern California. Dr. Kardashian, Dr. Reau, and Dr. Ganjhu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Metformin monotherapy not always best start in type 2 diabetes
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin failure in people with type 2 diabetes is very common, particularly among those with high hemoglobin A1c levels at the time of diagnosis, new findings suggest.
An analysis of electronic health record data for more than 22,000 patients starting metformin at three U.S. clinical sites found that over 40% experienced metformin failure.
This was defined as either failure to achieve or maintain A1c less than 7% within 18 months or the use of additional glucose-lowering medications.
Other predictors that metformin use wouldn’t be successful included increasing age, male sex, and race/ethnicity. However, the latter ceased to be linked after adjustment for other clinical risk factors.
“Our study results suggest increased monitoring with potentially earlier treatment intensification to achieve glycemic control may be appropriate in patients with clinical parameters described in this paper,” Suzette J. Bielinski, PhD, and colleagues wrote.
“Further, these results call into question the ubiquitous use of metformin as the first-line therapy and suggest a more individualized approach may be needed to optimize therapy,” they added in their article, published online in the Journal of Clinical Endocrinology and Metabolism.
The study is also noteworthy in that it demonstrated the feasibility of using EHR data with a machine-learning approach to discover risk biomarkers, Dr. Bielinski, professor of epidemiology at the Mayo Clinic, Rochester, Minn., said in an interview.
“We wanted to repurpose clinical data to answer questions ... I think more studies using these types of techniques repurposing data meant for one thing could potentially impact care in other domains. ... If we can get the bang for the buck from all these data that we generate on people I just think it will improve health care and maybe save health care dollars.”
Baseline A1c strongest predictor of metformin failure
The investigators identified a total of 22,047 metformin initiators from three clinical primary care sites: the University of Mississippi’s Jackson centers, which serves a mostly African American population, the Mountain Park Health Center in Arizona, a seven-clinic federally qualified community health center in Phoenix that serves a mostly Latino population, and the Rochester Epidemiology Project, which includes the Mayo Clinic and serves a primarily White population.
Overall, a total of 43% (9,407) of patients met one of two criteria for metformin failure by 18 months. Among those, median time to failure on metformin was 3.9 months.
Unadjusted failure rates were higher among African Americans, Hispanics, and other racial groups, compared with non-Hispanic White patients.
However, the racial groups also differed by baseline characteristics. Mean A1c was 7.7% overall, 8.1% for the African American group, 7.9% for Asians, and 8.2% for Hispanics, compared with 7.6% for non-Hispanic Whites.
Of 150 clinical factors examined, higher A1c was the strongest predictor of metformin failure, with a rapid increase in risk appearing between 7.5% and 8.0%.
“The slope is steep. It gives us some clinical guidance,” Dr. Bielinski said.
Other variables positively correlated with metformin failure included “diabetes with complications,” increased age, and higher levels of potassium, triglycerides, heart rate, and mean cell hemoglobin.
Factors inversely correlated with metformin failure were having received screening for other suspected conditions and medical examination/evaluation, and lower levels of sodium, albumin, and HDL cholesterol.
Three variables – body mass index, LDL cholesterol, and creatinine – had a U-shaped relationship with metformin failure, so that both high and low values were associated with increased risk.
“The racial/ethnic differences disappeared once other clinical factors were considered suggesting that the biological response to metformin is similar regardless of race/ethnicity,” Dr. Bielinski and colleagues wrote.
They also noted that the abnormal lab results which correlated with metformin failure “likely represent biomarkers for chronic illnesses. However, the effect size for lab abnormalities was small compared with that of baseline A1c.”
Dr. Bielinski urged caution in interpreting the findings. “Electronic health records data have limitations. We have evidence that these people were prescribed metformin. We have no idea if they took it. ... I would really be hesitant to be too strong in making clinical recommendations.”
However, she said that the data are “suggestive to say maybe we need to have some kind of threshold where if someone comes in with an A1c of X that they go on dual therapy right away. I think this is opening the door to that.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oramed oral insulin fails to meet goal in type 2 diabetes
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Oramed Pharmaceuticals’ investigational oral insulin failed to achieve its primary endpoint in a phase 3 trial, according to top-line results announced by the company.
“Therefore, Oramed expects to discontinue its oral insulin clinical activities for [type 2 diabetes],” according to a company statement.
, ORA-D-013-1, comparing the efficacy of the insulin product ORMD-0801 to placebo in 710 people with type 2 diabetes with inadequate glycemic control on two or three oral glucose-lowering agents.
The participants were randomized 2:2:1:1 into ORMD-0801 dosed at 8 mg once or twice daily, or placebo dosed once or twice daily. They completed a 21-day screening period, followed by a 26-week double-blind treatment period.
The product didn’t achieve the primary endpoint comparing reduction in hemoglobin A1c from baseline to 26 weeks, or the secondary endpoint of mean change in fasting plasma glucose at 26 weeks. There were no serious adverse events.
Oramed Pharmaceuticals specializes in developing oral delivery formulations of drugs currently delivered via injection. The company has offices in the United States and Israel.
Oramed CEO Nadav Kidron commented in the statement, “Today’s outcome is very disappointing, given the positive results from prior trials. Once full data from the studies are available, we expect to share relevant learnings and future plans. We thank all the patients, families, and health care professionals who participated in the trial.”
Insulin manufacturer Novo Nordisk had also been developing an oral insulin product. Successful phase 2a results were presented at the American Diabetes Association’s 2017 Scientific Sessions and full phase 2 feasibility results were published in Lancet Diabetes & Endocrinology in 2019.
However, Novo Nordisk, which manufactures the oral glucagon-like peptide-1 receptor agonist semaglutide (Rybelsus), subsequently discontinued development of their oral insulin product. According to a statement, “Initial results raised questions about truly addressing patients’ unmet needs with insulin therapy. Therefore, we discontinued this work to focus on projects that could in fact improve cardiometabolic outcomes for people living with diabetes.”
A version of this article first appeared on Medscape.com.
Fair access crucial for new diabetes/kidney disease drugs, say guidelines
The 2022 guideline update released by the KDIGO organization for managing people with diabetes and chronic kidney disease (CKD) highlighted the safety and expanded, evidence-based role for agents from three drug classes: the SGLT2 inhibitors, the GLP-1 receptor agonists, and the nonsteroidal mineralocorticoid receptor antagonists.
But this key take-away from the guideline also underscored the challenges for ensuring fair and affordable access among US patients to these practice-changing medications.
The impact of widespread adoption of these three drug classes into routine US management of people with diabetes and CKD “will be determined by how effective the health care system and its patients and clinicians are at overcoming individual and structural barriers,” write Milda Saunders, MD, and Neda Laiteerapong, MD, in an editorial that accompanied the publication of a synopsis of the 2022 guideline update in Annals of Internal Medicine.
The synopsis is an 11-page distillation of the full 128-page guideline released by the Kidney Disease: Improving Global Outcomes (KDIGO) organization in 2022.
The recommendations in the 2022 guideline update “are exciting for their potential to change the natural history of CKD and diabetes, but their effect could be highly limited by barriers at multiple levels,” write Dr. Saunders and Dr. Laiteerapong, two internal medicine physicians at the University of Chicago.
“Without equitable implementation of the KDIGO 2022 guidelines there is a potential that clinical practice variation will increase and widen health inequities for minoritized people with CKD and diabetes,” they warn.
Generics to the rescue
One potentially effective, and likely imminent, way to level the prescribing field for patients with CKD and diabetes is for agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor, glucagonlike peptide-1 (GLP-1) receptor agonist, and nonsteroidal mineralocorticoid receptor antagonist classes to become available in generic formulations.
That should lower prices and thereby boost wider access and will likely occur fairly soon for at least two of the three drug classes, Dr. Laiteerapong predicts.
Some GLP-1 receptor agonists have already escaped patent exclusivity or will do so in 2023, she notes, including the anticipated ability of one drugmaker to start U.S. marketing of generic liraglutide by the end of 2023.
However, whether that manufacturer, Teva, proceeds with generic liraglutide “is a big question,” Dr. Laiteerapong said in an interview. She cited Teva’s history of failing to introduce a generic formulation of exenatide onto the U.S. market even though it has had a green light to do so since 2017.
The only nonsteroidal mineralocorticoid receptor antagonist now on the market is finerenone (Kerendia), which will not go off patent for several more years, but for some branded SGLT2 inhibitors, U.S. patents will expire in 2025. In addition, remogliflozin is an SGLT2 inhibitor that “may have already lost patent exclusivity,” noted Dr. Laiteerapong, although it has also never received U.S. marketing approval.
Dr. Laiteerapong expressed optimism that the overall trajectory of access is on the rise. “Many people have type 2 diabetes, and these drugs are in demand,” she noted. She also pointed to progress recently made on insulin affordability. “Things will get better as long as people advocate and argue for equity,” she maintained.
Incentivize formulary listings
Dr. Laiteerapong cited other approaches that could boost access to these medications, such as “creating incentives for pharmaceutical companies to ensure that [these drugs] are on formularies” of large, government-affiliated U.S. health insurance programs, such as Medicare Advantage plans, Medicare Part D, state Medicaid plans, and coverage through U.S. Veterans Affairs and the Tricare health insurance plans available to active members of the US military.
The editorial she coauthored with Dr. Saunders also calls for future collaborations among various medical societies to create “a more unified and streamlined set of recommendations” that benefits patients with diabetes, CKD, and multiple other chronic conditions.
“Over the last decade, we have seen more societies willing to present cooperative guidelines, as well as a surge in research on patients who live with multiple chronic conditions. There is momentum that will allow these different societies to work together,” Dr. Laiteerapong said.
Dr. Laiteerapong and Dr. Saunders have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 2022 guideline update released by the KDIGO organization for managing people with diabetes and chronic kidney disease (CKD) highlighted the safety and expanded, evidence-based role for agents from three drug classes: the SGLT2 inhibitors, the GLP-1 receptor agonists, and the nonsteroidal mineralocorticoid receptor antagonists.
But this key take-away from the guideline also underscored the challenges for ensuring fair and affordable access among US patients to these practice-changing medications.
The impact of widespread adoption of these three drug classes into routine US management of people with diabetes and CKD “will be determined by how effective the health care system and its patients and clinicians are at overcoming individual and structural barriers,” write Milda Saunders, MD, and Neda Laiteerapong, MD, in an editorial that accompanied the publication of a synopsis of the 2022 guideline update in Annals of Internal Medicine.
The synopsis is an 11-page distillation of the full 128-page guideline released by the Kidney Disease: Improving Global Outcomes (KDIGO) organization in 2022.
The recommendations in the 2022 guideline update “are exciting for their potential to change the natural history of CKD and diabetes, but their effect could be highly limited by barriers at multiple levels,” write Dr. Saunders and Dr. Laiteerapong, two internal medicine physicians at the University of Chicago.
“Without equitable implementation of the KDIGO 2022 guidelines there is a potential that clinical practice variation will increase and widen health inequities for minoritized people with CKD and diabetes,” they warn.
Generics to the rescue
One potentially effective, and likely imminent, way to level the prescribing field for patients with CKD and diabetes is for agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor, glucagonlike peptide-1 (GLP-1) receptor agonist, and nonsteroidal mineralocorticoid receptor antagonist classes to become available in generic formulations.
That should lower prices and thereby boost wider access and will likely occur fairly soon for at least two of the three drug classes, Dr. Laiteerapong predicts.
Some GLP-1 receptor agonists have already escaped patent exclusivity or will do so in 2023, she notes, including the anticipated ability of one drugmaker to start U.S. marketing of generic liraglutide by the end of 2023.
However, whether that manufacturer, Teva, proceeds with generic liraglutide “is a big question,” Dr. Laiteerapong said in an interview. She cited Teva’s history of failing to introduce a generic formulation of exenatide onto the U.S. market even though it has had a green light to do so since 2017.
The only nonsteroidal mineralocorticoid receptor antagonist now on the market is finerenone (Kerendia), which will not go off patent for several more years, but for some branded SGLT2 inhibitors, U.S. patents will expire in 2025. In addition, remogliflozin is an SGLT2 inhibitor that “may have already lost patent exclusivity,” noted Dr. Laiteerapong, although it has also never received U.S. marketing approval.
Dr. Laiteerapong expressed optimism that the overall trajectory of access is on the rise. “Many people have type 2 diabetes, and these drugs are in demand,” she noted. She also pointed to progress recently made on insulin affordability. “Things will get better as long as people advocate and argue for equity,” she maintained.
Incentivize formulary listings
Dr. Laiteerapong cited other approaches that could boost access to these medications, such as “creating incentives for pharmaceutical companies to ensure that [these drugs] are on formularies” of large, government-affiliated U.S. health insurance programs, such as Medicare Advantage plans, Medicare Part D, state Medicaid plans, and coverage through U.S. Veterans Affairs and the Tricare health insurance plans available to active members of the US military.
The editorial she coauthored with Dr. Saunders also calls for future collaborations among various medical societies to create “a more unified and streamlined set of recommendations” that benefits patients with diabetes, CKD, and multiple other chronic conditions.
“Over the last decade, we have seen more societies willing to present cooperative guidelines, as well as a surge in research on patients who live with multiple chronic conditions. There is momentum that will allow these different societies to work together,” Dr. Laiteerapong said.
Dr. Laiteerapong and Dr. Saunders have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 2022 guideline update released by the KDIGO organization for managing people with diabetes and chronic kidney disease (CKD) highlighted the safety and expanded, evidence-based role for agents from three drug classes: the SGLT2 inhibitors, the GLP-1 receptor agonists, and the nonsteroidal mineralocorticoid receptor antagonists.
But this key take-away from the guideline also underscored the challenges for ensuring fair and affordable access among US patients to these practice-changing medications.
The impact of widespread adoption of these three drug classes into routine US management of people with diabetes and CKD “will be determined by how effective the health care system and its patients and clinicians are at overcoming individual and structural barriers,” write Milda Saunders, MD, and Neda Laiteerapong, MD, in an editorial that accompanied the publication of a synopsis of the 2022 guideline update in Annals of Internal Medicine.
The synopsis is an 11-page distillation of the full 128-page guideline released by the Kidney Disease: Improving Global Outcomes (KDIGO) organization in 2022.
The recommendations in the 2022 guideline update “are exciting for their potential to change the natural history of CKD and diabetes, but their effect could be highly limited by barriers at multiple levels,” write Dr. Saunders and Dr. Laiteerapong, two internal medicine physicians at the University of Chicago.
“Without equitable implementation of the KDIGO 2022 guidelines there is a potential that clinical practice variation will increase and widen health inequities for minoritized people with CKD and diabetes,” they warn.
Generics to the rescue
One potentially effective, and likely imminent, way to level the prescribing field for patients with CKD and diabetes is for agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor, glucagonlike peptide-1 (GLP-1) receptor agonist, and nonsteroidal mineralocorticoid receptor antagonist classes to become available in generic formulations.
That should lower prices and thereby boost wider access and will likely occur fairly soon for at least two of the three drug classes, Dr. Laiteerapong predicts.
Some GLP-1 receptor agonists have already escaped patent exclusivity or will do so in 2023, she notes, including the anticipated ability of one drugmaker to start U.S. marketing of generic liraglutide by the end of 2023.
However, whether that manufacturer, Teva, proceeds with generic liraglutide “is a big question,” Dr. Laiteerapong said in an interview. She cited Teva’s history of failing to introduce a generic formulation of exenatide onto the U.S. market even though it has had a green light to do so since 2017.
The only nonsteroidal mineralocorticoid receptor antagonist now on the market is finerenone (Kerendia), which will not go off patent for several more years, but for some branded SGLT2 inhibitors, U.S. patents will expire in 2025. In addition, remogliflozin is an SGLT2 inhibitor that “may have already lost patent exclusivity,” noted Dr. Laiteerapong, although it has also never received U.S. marketing approval.
Dr. Laiteerapong expressed optimism that the overall trajectory of access is on the rise. “Many people have type 2 diabetes, and these drugs are in demand,” she noted. She also pointed to progress recently made on insulin affordability. “Things will get better as long as people advocate and argue for equity,” she maintained.
Incentivize formulary listings
Dr. Laiteerapong cited other approaches that could boost access to these medications, such as “creating incentives for pharmaceutical companies to ensure that [these drugs] are on formularies” of large, government-affiliated U.S. health insurance programs, such as Medicare Advantage plans, Medicare Part D, state Medicaid plans, and coverage through U.S. Veterans Affairs and the Tricare health insurance plans available to active members of the US military.
The editorial she coauthored with Dr. Saunders also calls for future collaborations among various medical societies to create “a more unified and streamlined set of recommendations” that benefits patients with diabetes, CKD, and multiple other chronic conditions.
“Over the last decade, we have seen more societies willing to present cooperative guidelines, as well as a surge in research on patients who live with multiple chronic conditions. There is momentum that will allow these different societies to work together,” Dr. Laiteerapong said.
Dr. Laiteerapong and Dr. Saunders have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
PPI use in type 2 diabetes links with cardiovascular events
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Principles and Process for Reducing the Need for Insulin in Patients With Type 2 Diabetes
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
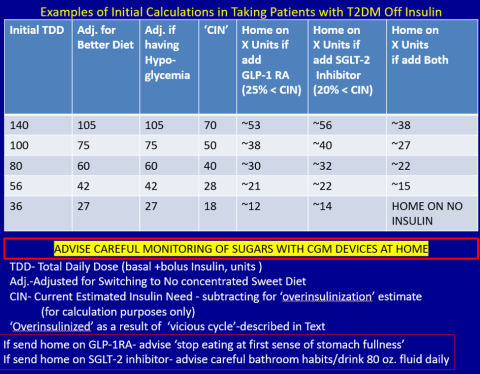
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at [email protected] with any questions about his protocol or diet.
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at [email protected] with any questions about his protocol or diet.
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at [email protected] with any questions about his protocol or diet.
Intermittent fasting can lead to type 2 diabetes remission
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
ADA issues 2023 ‘Standards of Care’ for diabetes: Focus on tight BP, lipids
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
Cognitive behavioral therapy app lowers A1c in type 2 diabetes
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
CHICAGO – A smartphone app that delivers nutritional cognitive behavioral therapy (CBT) to people with type 2 diabetes produced an average 0.29 percentage point drop in hemoglobin A1c during 180 days of use compared with controls, and an average 0.37 percentage point reduction in A1c compared with baseline values in a randomized, pivotal trial with 669 adults.
Use of the app for 180 days also significantly linked with a reduced need for additional medications, reduced weight and blood pressure, and improved patient-reported outcomes, and it led to fewer adverse effects than seen in control subjects, Marc P. Bonaca, MD, reported at the American Heart Association scientific sessions.
The findings also showed a clear dose-response relationship: The more CBT lessons a person completed with the app, the greater the A1c reduction.
The results suggest that the app, called BT-001, “potentially provides a scalable treatment option for patients with type 2 diabetes,” concluded Dr. Bonaca.
On the basis of the results from this trial, also called BT-001, the company developing the app, Better Therapeutics, announced in September 2022 that it had filed a classification request with the Food and Drug Administration that would allow marketing authorization for the BT-001 app. Better Therapeutics envisions that once authorized by the FDA, the app would be available to people with type 2 diabetes by prescriptions written by health care providers and that the cost for the app would be covered by health insurance, explained a company spokesperson.
A ‘modest positive impact’
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” said Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression, “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira said in an interview.
Based on published results, the BT-001 app “seems to have a modest positive impact on glycemic control, especially among people who completed more than 10 [lesson] modules.” The evidence appears to suggest that the app “might be a good supplement to working with a behavioral health counselor.”
The BT-001 trial enrolled 669 adults with type 2 diabetes for an average of 11 years and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antidiabetes medications, including 90% on metformin and 42% on a sulfonylurea. The researchers also highlighted that the enrolled cohort of people with type 2 diabetes had a demographic profile that was “generally representative” of U.S. adults with type 2 diabetes.
The researchers told the 326 people who were randomized to the active intervention group to use the app but subjects were free to determine their frequency of use. The app introduced a new lesson module weekly that took 10-20 minutes to complete, and each weekly lesson came with associated exercises aimed at practicing skills related to behavioral beliefs.
The study’s primary efficacy endpoint was the average change from baseline in A1c compared with the 343 control participants after 90 days of app use, and 610 of the 669 enrolled participants (91%) had paired baseline and 90-day measurements. At 90 days, people in the app group had an average 0.28 percentage point decrease in their A1c compared with an average 0.11 percentage point increase among the controls, a between-group difference of 0.39 percentage points. Both the reduction from baseline with app use and the reduction relative to the controls were significant. These results appeared in an article published online in in Diabetes Care.
At the scientific sessions, Dr. Bonaca presented additional outcome data after 180 days of app use. He reported an average 0.37 percentage point reduction from baseline in A1c among app users and a 0.08 percentage point decrease from baseline among the controls, for a net 0.29 percentage point incremental decline with the app, a significant difference. At 180 days, 50% of the people in the app group had an A1c decline from baseline of at least 0.4 percentage points compared with 34% of the controls, a significant difference.
A dose-response relationship
Notably, app use showed a clear dose-response pattern. During 180 days of app availability, people who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use) average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times, also one-third of the intervention group, the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
Dr. Bonaca, a cardiologist and vascular medicine specialist and executive director of CPC Clinical Research and CPC Community Health, an academic research organization created by and affiliated with the University of Colorado Anschutz Medical Campus in Aurora, Colo., reported several other 180-day outcomes in the BT-001 trial:
- A 33% relative decrease in the percentage of subjects who needed during the study an additional antidiabetes medication or increased dosages of their baseline medications, which occurred at a rate of 21% among the controls and 14% among those who used the app.
- An average weight loss from baseline of 5.5 pounds using the app compared with an average 1.9 pound decrease among controls, a significant difference.
- A decline in average systolic blood pressure of 4.7 mm Hg with app use compared with a 1.8 mm Hg average decline among the controls, a significant difference.
- Significant incremental average improvements in a self-reported Short Form-12 physical component score with the app compared with controls, and increased average improvement in the PHQ9 self-reported measure of depression in app users compared with controls.
- Significantly fewer treatment-emergent adverse effects, and significantly fewer serious treatment-emergent adverse effects among the app users compared with the controls.
‘Ready for clinical use’
Based on these findings, “in my view the app is ready for [routine] clinical use,” declared Judith Hsia, MD, a cardiologist and professor of medicine at the University of Colorado in Aurora, and with Dr. Bonaca a co-lead investigator for the study.
The BT-001 app can serve as “an addition to the toolkit of diabetes treatments,” Dr. Hsia said in an interview. One key advantage of the app is that, once approved, it could be available to many more people with type 2 diabetes than would be able to receive CBT directly from a therapist. Another potential plus for the CBT app is that “the effects should be durable in contrast to medications,” which must be taken on an ongoing basis to maintain effectiveness. In addition, the safety profile “is favorable compared with drug therapies, which should appeal to health care providers,” said Dr. Hsia, chief science officer for CPC Clinical Research.
However, Dr. Shapira cited the issue that therapeutic apps “raise privacy and licensing liability concerns.”
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira had no disclosures. Dr. Hsia is a stockholder of AstraZeneca.
AT AHA 2022
Guideline stresses new strategies for hypoglycemia management
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM