User login
Past spontaneous abortion raises risk for gestational diabetes
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA NETWORK OPEN
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
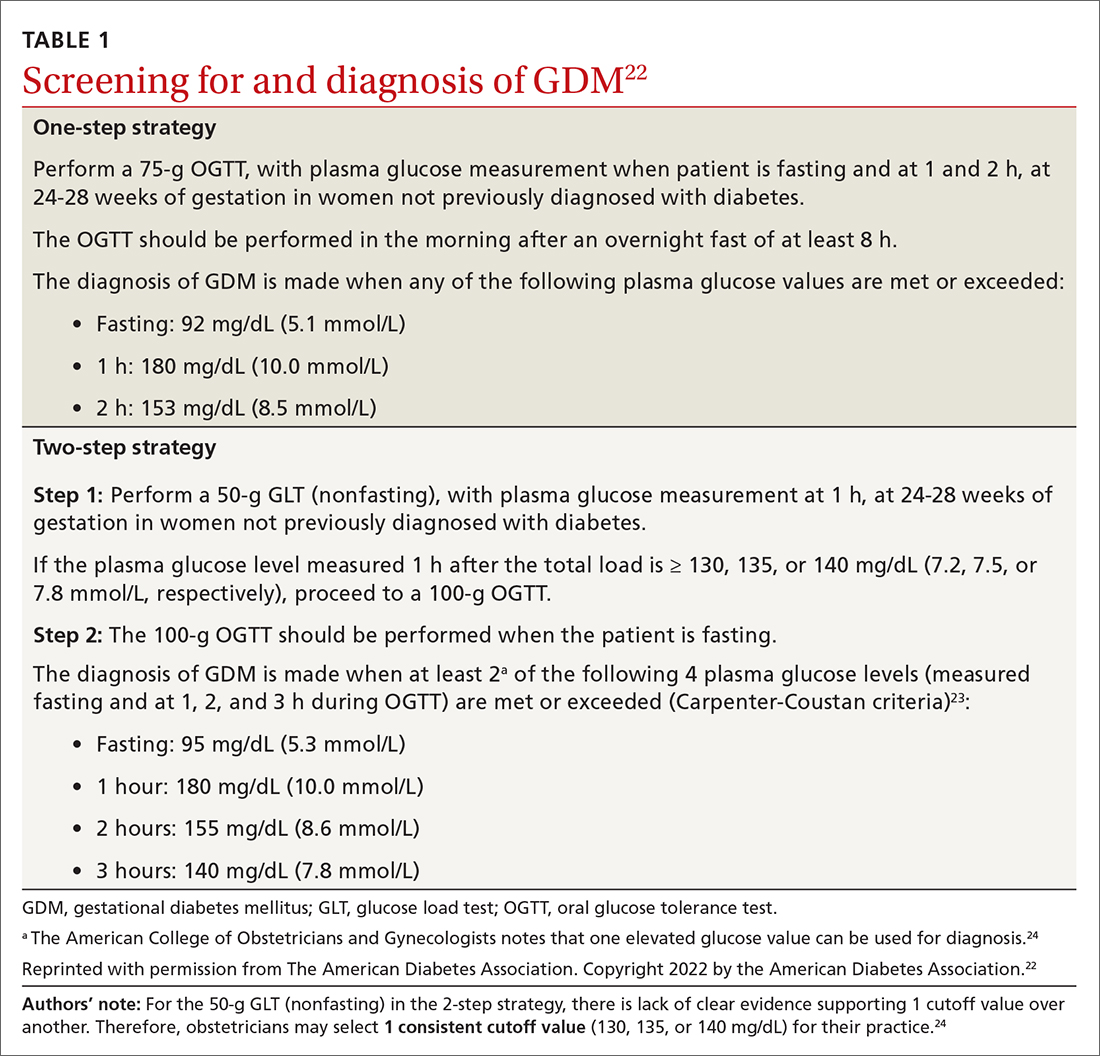
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24

Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24

Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Lawsuit: 18-inch sponge left in stomach for 5 years; migrates internally
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
What is the psychological impact of type 1 diabetes?
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
Finerenone + SGLT2 inhibitor of benefit in diabetes with CKD?
New signals of a potential additive benefit from the nonsteroidal mineralocorticoid antagonist finerenone (Kerendia) and a sodium-glucose transporter 2 inhibitor in patients with type 2 diabetes and chronic kidney disease (CKD) emerged in a follow-up report from the FIDELITY analysis, which combined data from more than 13,000 patients who received finerenone in either of the two pivotal trials with the agent.
The analysis showed that the 877 patients enrolled in either the FIDELIO DKD or FIGARO DKD trials taking an SGLT2 inhibitor at baseline had a 37% relative reduction in their urinary albumin-to-creatinine ratio (UACR), compared with placebo-treated patients after a median of 3 years on treatment.
Among the remaining 12,149 patients who did not receive an SGLT2 inhibitor, finerenone cut the average UACR by 32%, compared with placebo, said Peter Rossing, DMSc, MD, who presented the findings on Feb. 27 at the World Congress of Nephrology 2022 in Kuala Lumpur, Malaysia.
Primary endpoint results for FIDELIO-DKD and FIGARO-DKD also suggest similar additive effects of finerenone plus an SGLT2 inhibitor.
Results of the composite renal endpoint in each study – progression to kidney failure, renal death, or at least a 57% decline in estimated glomerular filtration rate (eGFR) from baseline – showed a 58% relative risk reduction in patients who received agents from both drug classes and a 20% relative risk reduction in those who only received finerenone, a between-group difference that was not significant.
For the composite cardiovascular event endpoint – cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure – the rate fell by 37%, compared with placebo, in patients who also received an SGLT2 inhibitor, and by 13%, compared with placebo, in those who received finerenone but no SGLT2 inhibitor, also a difference that was not significant.
‘A lot of interest in finerenone’ in U.S.
“The benefits of finerenone on cardiovascular and kidney outcomes were consistent, irrespective of SGLT2 inhibitor use at baseline,” concluded Dr. Rossing, professor and head of research at the Steno Diabetes Center in Copenhagen.
The new findings are a “suggestion that the two classes might be additive [in their effects], but more data are needed,” Dr. Rossing said during his presentation.
But he cautioned that in both pivotal trials randomization did not consider SGLT2 inhibitor use. All patients in the two trials were already receiving a renin-angiotensin system (RAS) inhibitor as background treatment, either an ACE inhibitor or angiotensin-receptor blocker.
The consequence of treatment with finerenone combined with an SGLT2 inhibitor is of growing importance because “an SGLT2 inhibitor is now recommended in most guidelines” for the type of patients enrolled in the two finerenone trials, explained Dr. Rossing.
He also noted that the first guideline to recommend routine use of finerenone in indicated patients appeared recently in the annual update to Standards of Medical Care in Diabetes – 2022 published by the American Diabetes Association.
The 2022 Standards states: “In patients with CKD who are at increased risk for cardiovascular events or CKD progression or are unable to use an SGLT2 inhibitor, a nonsteroidal mineralocorticoid receptor antagonist (finerenone) is recommended to reduce CKD progression and cardiovascular events.”
Results from FIDELIO-DKD, reported in the New England Journal of Medicine in 2020, and the main study, FIGARO-DKD, published in the same journal in 2021, led the Food and Drug Administration to approve finerenone in July 2021 to slow the progression of renal disease in patients with type 2 diabetes and CKD.
“My impression is that in the United States there is a lot of interest in finerenone,” Dr. Rossing said during the discussion following his presentation.
Finerenone has also been recently approved in the European Union.
‘Consistent’ benefits irrespective of SGLT2 inhibitors
“The cardiovascular and kidney benefits of finerenone were consistent irrespective of SGLT2 inhibitor use. This is definitely interesting and warrants a randomized controlled trial” to examine the relationship in a more rigorous way, commented Tejas Desai, MD, a nephrologist with the Department of Veterans Affairs, Charlotte, N.C.
That study, CONFIDENCE, is on its way, Dr. Rossing said during his talk. The randomized phase 2 trial has a planned enrollment of 800 patients with type 2 diabetes and CKD and three treatment groups: finerenone plus placebo, the SGLT2 inhibitor empagliflozin (Jardiance) plus placebo, or finerenone plus empagliflozin. The study will launch later in 2022 and has a planned completion date of late 2023.
“SGLT2 inhibitors, compared with finerenone, is where all of this is headed. We need a large trial that adjudicates the best medication to use with a RAS inhibitor,” Dr. Desai said in an interview.
The new analyses from the combined FIDELITY study expand on a previous report presented at the 2021 annual congress of the European Society of Cardiology and published in the European Heart Journal.
‘Impressive’ effect on cardiovascular events
The main findings from FIDELITY presented in those earlier reports, in 13,026 patients, showed there was a significant 14% relative reduction in the composite cardiovascular endpoint with finerenone, compared with placebo, during a median 3 years of follow-up.
The same report documented, in the total combined cohort, a significant 23% relative reduction in the composite renal endpoint in those taking finerenone compared with placebo.
“Reducing the risk of cardiovascular endpoints by a relative 14% is impressive,” and the time course showed a “relatively quick onset of action,” Dr. Desai noted.
He also characterized the enrolled patients, which included many with stage 3 or 4 CKD, as “not the sickest population of patients with CKD,” but rather “relatively healthier patients with CKD.”
Dr. Desai also downplayed the importance of the observed reduction in UACR associated with finerenone in FIDELITY.
“UACR is a surrogate marker. Results from many studies have shown improvements in UACR only to not show protection against falls in eGFR rate,” Dr. Desai said.
He was also reassured by the low incidence of hyperkalemia that led to discontinuation, which occurred in 1.7% of patients taking finerenone and in 0.6% of those taking placebo.
The types of patients enrolled in FIDELIO-DKD and FIGARO-DKD, who did not have eGFR rates below 25 mL/min per 1.73 m2, are not particularly susceptible to this adverse effect, he said, noting, “I’m not overly concerned with hyperkalemia in this CKD population.
“I’m more concerned about [hyperkalemia in] patients with CKD and an eGFR of less than 25 mL/min per 1.73 m2, but this was less than 1% of the enrolled population,” Dr. Desai observed.
FIDELIO-DKD, FIGARO-DKD, and FIDELITY were sponsored by Bayer, which markets finerenone (Kerendia). Dr. Rossing reported being a consultant for Bayer and other drug companies, and receiving research funding from AstraZeneca and Novo Nordisk. Dr. Desai reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New signals of a potential additive benefit from the nonsteroidal mineralocorticoid antagonist finerenone (Kerendia) and a sodium-glucose transporter 2 inhibitor in patients with type 2 diabetes and chronic kidney disease (CKD) emerged in a follow-up report from the FIDELITY analysis, which combined data from more than 13,000 patients who received finerenone in either of the two pivotal trials with the agent.
The analysis showed that the 877 patients enrolled in either the FIDELIO DKD or FIGARO DKD trials taking an SGLT2 inhibitor at baseline had a 37% relative reduction in their urinary albumin-to-creatinine ratio (UACR), compared with placebo-treated patients after a median of 3 years on treatment.
Among the remaining 12,149 patients who did not receive an SGLT2 inhibitor, finerenone cut the average UACR by 32%, compared with placebo, said Peter Rossing, DMSc, MD, who presented the findings on Feb. 27 at the World Congress of Nephrology 2022 in Kuala Lumpur, Malaysia.
Primary endpoint results for FIDELIO-DKD and FIGARO-DKD also suggest similar additive effects of finerenone plus an SGLT2 inhibitor.
Results of the composite renal endpoint in each study – progression to kidney failure, renal death, or at least a 57% decline in estimated glomerular filtration rate (eGFR) from baseline – showed a 58% relative risk reduction in patients who received agents from both drug classes and a 20% relative risk reduction in those who only received finerenone, a between-group difference that was not significant.
For the composite cardiovascular event endpoint – cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure – the rate fell by 37%, compared with placebo, in patients who also received an SGLT2 inhibitor, and by 13%, compared with placebo, in those who received finerenone but no SGLT2 inhibitor, also a difference that was not significant.
‘A lot of interest in finerenone’ in U.S.
“The benefits of finerenone on cardiovascular and kidney outcomes were consistent, irrespective of SGLT2 inhibitor use at baseline,” concluded Dr. Rossing, professor and head of research at the Steno Diabetes Center in Copenhagen.
The new findings are a “suggestion that the two classes might be additive [in their effects], but more data are needed,” Dr. Rossing said during his presentation.
But he cautioned that in both pivotal trials randomization did not consider SGLT2 inhibitor use. All patients in the two trials were already receiving a renin-angiotensin system (RAS) inhibitor as background treatment, either an ACE inhibitor or angiotensin-receptor blocker.
The consequence of treatment with finerenone combined with an SGLT2 inhibitor is of growing importance because “an SGLT2 inhibitor is now recommended in most guidelines” for the type of patients enrolled in the two finerenone trials, explained Dr. Rossing.
He also noted that the first guideline to recommend routine use of finerenone in indicated patients appeared recently in the annual update to Standards of Medical Care in Diabetes – 2022 published by the American Diabetes Association.
The 2022 Standards states: “In patients with CKD who are at increased risk for cardiovascular events or CKD progression or are unable to use an SGLT2 inhibitor, a nonsteroidal mineralocorticoid receptor antagonist (finerenone) is recommended to reduce CKD progression and cardiovascular events.”
Results from FIDELIO-DKD, reported in the New England Journal of Medicine in 2020, and the main study, FIGARO-DKD, published in the same journal in 2021, led the Food and Drug Administration to approve finerenone in July 2021 to slow the progression of renal disease in patients with type 2 diabetes and CKD.
“My impression is that in the United States there is a lot of interest in finerenone,” Dr. Rossing said during the discussion following his presentation.
Finerenone has also been recently approved in the European Union.
‘Consistent’ benefits irrespective of SGLT2 inhibitors
“The cardiovascular and kidney benefits of finerenone were consistent irrespective of SGLT2 inhibitor use. This is definitely interesting and warrants a randomized controlled trial” to examine the relationship in a more rigorous way, commented Tejas Desai, MD, a nephrologist with the Department of Veterans Affairs, Charlotte, N.C.
That study, CONFIDENCE, is on its way, Dr. Rossing said during his talk. The randomized phase 2 trial has a planned enrollment of 800 patients with type 2 diabetes and CKD and three treatment groups: finerenone plus placebo, the SGLT2 inhibitor empagliflozin (Jardiance) plus placebo, or finerenone plus empagliflozin. The study will launch later in 2022 and has a planned completion date of late 2023.
“SGLT2 inhibitors, compared with finerenone, is where all of this is headed. We need a large trial that adjudicates the best medication to use with a RAS inhibitor,” Dr. Desai said in an interview.
The new analyses from the combined FIDELITY study expand on a previous report presented at the 2021 annual congress of the European Society of Cardiology and published in the European Heart Journal.
‘Impressive’ effect on cardiovascular events
The main findings from FIDELITY presented in those earlier reports, in 13,026 patients, showed there was a significant 14% relative reduction in the composite cardiovascular endpoint with finerenone, compared with placebo, during a median 3 years of follow-up.
The same report documented, in the total combined cohort, a significant 23% relative reduction in the composite renal endpoint in those taking finerenone compared with placebo.
“Reducing the risk of cardiovascular endpoints by a relative 14% is impressive,” and the time course showed a “relatively quick onset of action,” Dr. Desai noted.
He also characterized the enrolled patients, which included many with stage 3 or 4 CKD, as “not the sickest population of patients with CKD,” but rather “relatively healthier patients with CKD.”
Dr. Desai also downplayed the importance of the observed reduction in UACR associated with finerenone in FIDELITY.
“UACR is a surrogate marker. Results from many studies have shown improvements in UACR only to not show protection against falls in eGFR rate,” Dr. Desai said.
He was also reassured by the low incidence of hyperkalemia that led to discontinuation, which occurred in 1.7% of patients taking finerenone and in 0.6% of those taking placebo.
The types of patients enrolled in FIDELIO-DKD and FIGARO-DKD, who did not have eGFR rates below 25 mL/min per 1.73 m2, are not particularly susceptible to this adverse effect, he said, noting, “I’m not overly concerned with hyperkalemia in this CKD population.
“I’m more concerned about [hyperkalemia in] patients with CKD and an eGFR of less than 25 mL/min per 1.73 m2, but this was less than 1% of the enrolled population,” Dr. Desai observed.
FIDELIO-DKD, FIGARO-DKD, and FIDELITY were sponsored by Bayer, which markets finerenone (Kerendia). Dr. Rossing reported being a consultant for Bayer and other drug companies, and receiving research funding from AstraZeneca and Novo Nordisk. Dr. Desai reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New signals of a potential additive benefit from the nonsteroidal mineralocorticoid antagonist finerenone (Kerendia) and a sodium-glucose transporter 2 inhibitor in patients with type 2 diabetes and chronic kidney disease (CKD) emerged in a follow-up report from the FIDELITY analysis, which combined data from more than 13,000 patients who received finerenone in either of the two pivotal trials with the agent.
The analysis showed that the 877 patients enrolled in either the FIDELIO DKD or FIGARO DKD trials taking an SGLT2 inhibitor at baseline had a 37% relative reduction in their urinary albumin-to-creatinine ratio (UACR), compared with placebo-treated patients after a median of 3 years on treatment.
Among the remaining 12,149 patients who did not receive an SGLT2 inhibitor, finerenone cut the average UACR by 32%, compared with placebo, said Peter Rossing, DMSc, MD, who presented the findings on Feb. 27 at the World Congress of Nephrology 2022 in Kuala Lumpur, Malaysia.
Primary endpoint results for FIDELIO-DKD and FIGARO-DKD also suggest similar additive effects of finerenone plus an SGLT2 inhibitor.
Results of the composite renal endpoint in each study – progression to kidney failure, renal death, or at least a 57% decline in estimated glomerular filtration rate (eGFR) from baseline – showed a 58% relative risk reduction in patients who received agents from both drug classes and a 20% relative risk reduction in those who only received finerenone, a between-group difference that was not significant.
For the composite cardiovascular event endpoint – cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure – the rate fell by 37%, compared with placebo, in patients who also received an SGLT2 inhibitor, and by 13%, compared with placebo, in those who received finerenone but no SGLT2 inhibitor, also a difference that was not significant.
‘A lot of interest in finerenone’ in U.S.
“The benefits of finerenone on cardiovascular and kidney outcomes were consistent, irrespective of SGLT2 inhibitor use at baseline,” concluded Dr. Rossing, professor and head of research at the Steno Diabetes Center in Copenhagen.
The new findings are a “suggestion that the two classes might be additive [in their effects], but more data are needed,” Dr. Rossing said during his presentation.
But he cautioned that in both pivotal trials randomization did not consider SGLT2 inhibitor use. All patients in the two trials were already receiving a renin-angiotensin system (RAS) inhibitor as background treatment, either an ACE inhibitor or angiotensin-receptor blocker.
The consequence of treatment with finerenone combined with an SGLT2 inhibitor is of growing importance because “an SGLT2 inhibitor is now recommended in most guidelines” for the type of patients enrolled in the two finerenone trials, explained Dr. Rossing.
He also noted that the first guideline to recommend routine use of finerenone in indicated patients appeared recently in the annual update to Standards of Medical Care in Diabetes – 2022 published by the American Diabetes Association.
The 2022 Standards states: “In patients with CKD who are at increased risk for cardiovascular events or CKD progression or are unable to use an SGLT2 inhibitor, a nonsteroidal mineralocorticoid receptor antagonist (finerenone) is recommended to reduce CKD progression and cardiovascular events.”
Results from FIDELIO-DKD, reported in the New England Journal of Medicine in 2020, and the main study, FIGARO-DKD, published in the same journal in 2021, led the Food and Drug Administration to approve finerenone in July 2021 to slow the progression of renal disease in patients with type 2 diabetes and CKD.
“My impression is that in the United States there is a lot of interest in finerenone,” Dr. Rossing said during the discussion following his presentation.
Finerenone has also been recently approved in the European Union.
‘Consistent’ benefits irrespective of SGLT2 inhibitors
“The cardiovascular and kidney benefits of finerenone were consistent irrespective of SGLT2 inhibitor use. This is definitely interesting and warrants a randomized controlled trial” to examine the relationship in a more rigorous way, commented Tejas Desai, MD, a nephrologist with the Department of Veterans Affairs, Charlotte, N.C.
That study, CONFIDENCE, is on its way, Dr. Rossing said during his talk. The randomized phase 2 trial has a planned enrollment of 800 patients with type 2 diabetes and CKD and three treatment groups: finerenone plus placebo, the SGLT2 inhibitor empagliflozin (Jardiance) plus placebo, or finerenone plus empagliflozin. The study will launch later in 2022 and has a planned completion date of late 2023.
“SGLT2 inhibitors, compared with finerenone, is where all of this is headed. We need a large trial that adjudicates the best medication to use with a RAS inhibitor,” Dr. Desai said in an interview.
The new analyses from the combined FIDELITY study expand on a previous report presented at the 2021 annual congress of the European Society of Cardiology and published in the European Heart Journal.
‘Impressive’ effect on cardiovascular events
The main findings from FIDELITY presented in those earlier reports, in 13,026 patients, showed there was a significant 14% relative reduction in the composite cardiovascular endpoint with finerenone, compared with placebo, during a median 3 years of follow-up.
The same report documented, in the total combined cohort, a significant 23% relative reduction in the composite renal endpoint in those taking finerenone compared with placebo.
“Reducing the risk of cardiovascular endpoints by a relative 14% is impressive,” and the time course showed a “relatively quick onset of action,” Dr. Desai noted.
He also characterized the enrolled patients, which included many with stage 3 or 4 CKD, as “not the sickest population of patients with CKD,” but rather “relatively healthier patients with CKD.”
Dr. Desai also downplayed the importance of the observed reduction in UACR associated with finerenone in FIDELITY.
“UACR is a surrogate marker. Results from many studies have shown improvements in UACR only to not show protection against falls in eGFR rate,” Dr. Desai said.
He was also reassured by the low incidence of hyperkalemia that led to discontinuation, which occurred in 1.7% of patients taking finerenone and in 0.6% of those taking placebo.
The types of patients enrolled in FIDELIO-DKD and FIGARO-DKD, who did not have eGFR rates below 25 mL/min per 1.73 m2, are not particularly susceptible to this adverse effect, he said, noting, “I’m not overly concerned with hyperkalemia in this CKD population.
“I’m more concerned about [hyperkalemia in] patients with CKD and an eGFR of less than 25 mL/min per 1.73 m2, but this was less than 1% of the enrolled population,” Dr. Desai observed.
FIDELIO-DKD, FIGARO-DKD, and FIDELITY were sponsored by Bayer, which markets finerenone (Kerendia). Dr. Rossing reported being a consultant for Bayer and other drug companies, and receiving research funding from AstraZeneca and Novo Nordisk. Dr. Desai reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE WORLD CONGRESS OF NEPHROLOGY
Is a blood test for type 1 diabetes in kids worth the cost?
Universal screening for presymptomatic type 1 diabetes among schoolchildren would cost approximately 22 euros (about $25) per child screened, and about 7,000 euros (about $7,900) per child diagnosed, a new analysis of data from a German program finds.
The data come from the Fr1da study, in which a blood test for type 1 diabetes–associated islet autoantibodies is offered to all children aged 21 months to 6 years old in Bavaria.
Families of those who test positive are offered participation in a program of diabetes education, metabolic staging, psychological evaluation for stress, and prospective follow-up.
The researchers explain that, worldwide, 4 in 1,000 people under the age of 20 years have type 1 diabetes. It is the most common metabolic disease in children and adolescents. Only about 1 in 10 of those affected has a close relative with the disease. This means that type 1 diabetes can affect any child.
However, in many cases, the disease does not become known until a severe to life-threatening metabolic derailment known as diabetic ketoacidosis (DKA) occurs. This often leads to intensive medical treatment, a longer hospitalization, and poorer blood glucose control, which can result in an increased risk of secondary diseases and very high costs for the health care system.
“We want to protect as many children as possible from serious metabolic derailments. This is only possible with type 1 diabetes screenings. Therefore, we strongly support to include early detection tests in standard medical care,” Peter Achenbach, DrMed, senior author of the study, said in a statement from his institution, Helmholtz Zentrum München in Neuherberg, Germany.
The new findings were published in Diabetes Care by Florian M. Karl, also of Helmholtz Zentrum München, and colleagues.
In 2020, the Fr1da investigators reported that, of 90,632 children who participated from February 2015 to May 2019, 0.31% (280) were diagnosed with presymptomatic type 1 diabetes through the presence of two or more islet autoantibodies.
This news organization asked Brett McQueen, PhD, who led a similar study examining cost and cost-effectiveness in the Autoimmunity Screening for Kids (ASK) program, in which Denver-area children aged 2-17 years are offered autoantibody screening for both type 1 diabetes and celiac disease, for comment.
“If we have a chance to change a child’s life from when they’re 2 or 3 years old and there’s even a small chance that this thing potentially improves health outcomes for a decent price, what are we waiting for?” said Dr. McQueen, who is assistant professor in the department of clinical pharmacy at the University of Colorado, Aurora.
Is DKA prevention enough to justify universal screening?
Although identifying type 1 diabetes before symptoms arise could help avoid DKA, currently no therapeutic interventions are available to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes.
A possible future intervention – the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio) – had a setback in July 2021 when the Food and Drug Administration declined to approve it for the delay of type 1 diabetes in at-risk individuals.
However, on Feb. 22 Provention Bio announced that it has resubmitted the Biologics License Application for teplizumab for the delay of clinical type 1 diabetes in at-risk individuals. The FDA now has 30 days to review the resubmission, determine whether it is complete and acceptable for review, and provide a review goal date, according to a company statement.
But even without the ability to forestall the development of type 1 diabetes, screening proponents point to the potential benefit from educating families about early signs of diabetes onset and thereby preventing progression to DKA and both its short-term and possible long-term sequelae.
Prevention of DKA at diagnosis has been linked to improved long-term glycemic control and other potential health benefits.
And the frequency of DKA at the onset of type 1 diabetes has increased in recent years, to more than 20% in Germany and over 45% in the United States.
But, prior data have suggested that universal screening for presymptomatic type 1 diabetes is unlikely to be cost effective if only the health and economic benefits of prevention of DKA at type 1 diabetes onset is considered, unless the screening costs are exceedingly low.
What will it take to implement universal screening?
“What this paper does is contribute really to our understanding of more around resource utilization,” noted Dr. McQueen. “As they correctly identify, it’s really hard to compare country prices. It’s easier to compare utilization.”
In Dr. McQueen’s ASK program, the cost per child screened and per case detected in that program were similar to those found in the German study, even though the cost of the antibody testing itself was considerably lower in Germany than in the United States.
Fr1da included more components of screening and monitoring than did ASK, Dr. McQueen told this news organization.
The conclusions of the ASK study were that “presymptomatic type 1 diabetes screening may be cost effective in areas with a high prevalence of DKA and an infrastructure facilitating screening and monitoring if the benefits of avoiding DKA events and improved [hemoglobin] A1c persist over long-run time horizons.”
Nonetheless, Dr. McQueen thinks it’s unlikely that universal screening will be recommended by professional societies or covered by payers in the United States until a pharmacologic intervention to forestall disease progression is available.“Teplizumab approval could move this along. ... We’re just trying to take one factor, the economics of it, to create the most efficient scenario so that if it were to be adopted we would catch the most cases, prevent the most complications, benefit children the most in terms of their lifetime health outcomes – all at the minimum cost possible.”
‘A benchmark for the expected implementation cost of screening’
Mr. Karl and colleagues simulated the cost of implementation of this screening as standard care in Germany – assuming the same 0.31% prevalence found in Fr1da – the average cost per child was estimated at 21.73 euros, including 9.34 euros for laboratory costs, 12.25 euros for pediatrician costs, and 0.14 euros for local diabetes clinics to perform metabolic staging and education for children diagnosed with presymptomatic type 1 diabetes.
The model included 50% of the costs incurred in Fr1da for obtaining informed consent. Negative autoantibody results from the initial screening were not communicated to families, and all children with presymptomatic type 1 diabetes received staging and education. The estimated average cost per diagnosed child was 7,035 euros.
“Although our analyses are subject to some level of uncertainty, they provide a benchmark for the expected implementation cost of screening,” said coauthor Michael Laxy, MSc, PhD, also at Helmholtz Zentrum München.
“Next, we aim to evaluate the long-term ratio of screening costs, potential cost savings through the prevention of metabolic derailment and its consequences, and potentially increased quality of life with a type 1 diabetes screening compared to the costs and quality of life without a screening.”
Dr. McQueen is working along similar lines in Colorado, attempting to create a model that incorporates all the different possibilities including DKA monitoring, teplizumab availability, screening children at different ages, and the effect of including blood glucose monitoring in children identified with presymptomatic type 1 diabetes.
“There are so many different potential answers and avenues and no one has really put it all together,” he observed.
But he believes that economics shouldn’t be the only factor used in deciding whether to institute widespread screening.
This study was supported by grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD). The Fr1da study was supported by grants from the LifeScience-Stiftung, JDRF International, the Bavarian State Ministry of Health and Care, the Leona M. and Harry B. Helmsley Charitable Trust, Deutsche Diabetes-Stiftung, Landesverband Bayern der Betriebskrankenkassen, B. Braun-Stiftung, Deutsche Diabetes Hilfe, and the German Federal Ministry of Education and Research to the DZD. The authors disclosed no relevant financial relationships. The ASK study was funded by JDRF International, the Leona M. and Harry B. Helmsley Charitable Trust, and Janssen Research and Development. Dr. McQueen has received institutional funding for value assessment applications from the Institute for Clinical and Economic Review, the PhRMA Foundation, and PhRMA.
A version of this article first appeared on Medscape.com.
Universal screening for presymptomatic type 1 diabetes among schoolchildren would cost approximately 22 euros (about $25) per child screened, and about 7,000 euros (about $7,900) per child diagnosed, a new analysis of data from a German program finds.
The data come from the Fr1da study, in which a blood test for type 1 diabetes–associated islet autoantibodies is offered to all children aged 21 months to 6 years old in Bavaria.
Families of those who test positive are offered participation in a program of diabetes education, metabolic staging, psychological evaluation for stress, and prospective follow-up.
The researchers explain that, worldwide, 4 in 1,000 people under the age of 20 years have type 1 diabetes. It is the most common metabolic disease in children and adolescents. Only about 1 in 10 of those affected has a close relative with the disease. This means that type 1 diabetes can affect any child.
However, in many cases, the disease does not become known until a severe to life-threatening metabolic derailment known as diabetic ketoacidosis (DKA) occurs. This often leads to intensive medical treatment, a longer hospitalization, and poorer blood glucose control, which can result in an increased risk of secondary diseases and very high costs for the health care system.
“We want to protect as many children as possible from serious metabolic derailments. This is only possible with type 1 diabetes screenings. Therefore, we strongly support to include early detection tests in standard medical care,” Peter Achenbach, DrMed, senior author of the study, said in a statement from his institution, Helmholtz Zentrum München in Neuherberg, Germany.
The new findings were published in Diabetes Care by Florian M. Karl, also of Helmholtz Zentrum München, and colleagues.
In 2020, the Fr1da investigators reported that, of 90,632 children who participated from February 2015 to May 2019, 0.31% (280) were diagnosed with presymptomatic type 1 diabetes through the presence of two or more islet autoantibodies.
This news organization asked Brett McQueen, PhD, who led a similar study examining cost and cost-effectiveness in the Autoimmunity Screening for Kids (ASK) program, in which Denver-area children aged 2-17 years are offered autoantibody screening for both type 1 diabetes and celiac disease, for comment.
“If we have a chance to change a child’s life from when they’re 2 or 3 years old and there’s even a small chance that this thing potentially improves health outcomes for a decent price, what are we waiting for?” said Dr. McQueen, who is assistant professor in the department of clinical pharmacy at the University of Colorado, Aurora.
Is DKA prevention enough to justify universal screening?
Although identifying type 1 diabetes before symptoms arise could help avoid DKA, currently no therapeutic interventions are available to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes.
A possible future intervention – the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio) – had a setback in July 2021 when the Food and Drug Administration declined to approve it for the delay of type 1 diabetes in at-risk individuals.
However, on Feb. 22 Provention Bio announced that it has resubmitted the Biologics License Application for teplizumab for the delay of clinical type 1 diabetes in at-risk individuals. The FDA now has 30 days to review the resubmission, determine whether it is complete and acceptable for review, and provide a review goal date, according to a company statement.
But even without the ability to forestall the development of type 1 diabetes, screening proponents point to the potential benefit from educating families about early signs of diabetes onset and thereby preventing progression to DKA and both its short-term and possible long-term sequelae.
Prevention of DKA at diagnosis has been linked to improved long-term glycemic control and other potential health benefits.
And the frequency of DKA at the onset of type 1 diabetes has increased in recent years, to more than 20% in Germany and over 45% in the United States.
But, prior data have suggested that universal screening for presymptomatic type 1 diabetes is unlikely to be cost effective if only the health and economic benefits of prevention of DKA at type 1 diabetes onset is considered, unless the screening costs are exceedingly low.
What will it take to implement universal screening?
“What this paper does is contribute really to our understanding of more around resource utilization,” noted Dr. McQueen. “As they correctly identify, it’s really hard to compare country prices. It’s easier to compare utilization.”
In Dr. McQueen’s ASK program, the cost per child screened and per case detected in that program were similar to those found in the German study, even though the cost of the antibody testing itself was considerably lower in Germany than in the United States.
Fr1da included more components of screening and monitoring than did ASK, Dr. McQueen told this news organization.
The conclusions of the ASK study were that “presymptomatic type 1 diabetes screening may be cost effective in areas with a high prevalence of DKA and an infrastructure facilitating screening and monitoring if the benefits of avoiding DKA events and improved [hemoglobin] A1c persist over long-run time horizons.”
Nonetheless, Dr. McQueen thinks it’s unlikely that universal screening will be recommended by professional societies or covered by payers in the United States until a pharmacologic intervention to forestall disease progression is available.“Teplizumab approval could move this along. ... We’re just trying to take one factor, the economics of it, to create the most efficient scenario so that if it were to be adopted we would catch the most cases, prevent the most complications, benefit children the most in terms of their lifetime health outcomes – all at the minimum cost possible.”
‘A benchmark for the expected implementation cost of screening’
Mr. Karl and colleagues simulated the cost of implementation of this screening as standard care in Germany – assuming the same 0.31% prevalence found in Fr1da – the average cost per child was estimated at 21.73 euros, including 9.34 euros for laboratory costs, 12.25 euros for pediatrician costs, and 0.14 euros for local diabetes clinics to perform metabolic staging and education for children diagnosed with presymptomatic type 1 diabetes.
The model included 50% of the costs incurred in Fr1da for obtaining informed consent. Negative autoantibody results from the initial screening were not communicated to families, and all children with presymptomatic type 1 diabetes received staging and education. The estimated average cost per diagnosed child was 7,035 euros.
“Although our analyses are subject to some level of uncertainty, they provide a benchmark for the expected implementation cost of screening,” said coauthor Michael Laxy, MSc, PhD, also at Helmholtz Zentrum München.
“Next, we aim to evaluate the long-term ratio of screening costs, potential cost savings through the prevention of metabolic derailment and its consequences, and potentially increased quality of life with a type 1 diabetes screening compared to the costs and quality of life without a screening.”
Dr. McQueen is working along similar lines in Colorado, attempting to create a model that incorporates all the different possibilities including DKA monitoring, teplizumab availability, screening children at different ages, and the effect of including blood glucose monitoring in children identified with presymptomatic type 1 diabetes.
“There are so many different potential answers and avenues and no one has really put it all together,” he observed.
But he believes that economics shouldn’t be the only factor used in deciding whether to institute widespread screening.
This study was supported by grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD). The Fr1da study was supported by grants from the LifeScience-Stiftung, JDRF International, the Bavarian State Ministry of Health and Care, the Leona M. and Harry B. Helmsley Charitable Trust, Deutsche Diabetes-Stiftung, Landesverband Bayern der Betriebskrankenkassen, B. Braun-Stiftung, Deutsche Diabetes Hilfe, and the German Federal Ministry of Education and Research to the DZD. The authors disclosed no relevant financial relationships. The ASK study was funded by JDRF International, the Leona M. and Harry B. Helmsley Charitable Trust, and Janssen Research and Development. Dr. McQueen has received institutional funding for value assessment applications from the Institute for Clinical and Economic Review, the PhRMA Foundation, and PhRMA.
A version of this article first appeared on Medscape.com.
Universal screening for presymptomatic type 1 diabetes among schoolchildren would cost approximately 22 euros (about $25) per child screened, and about 7,000 euros (about $7,900) per child diagnosed, a new analysis of data from a German program finds.
The data come from the Fr1da study, in which a blood test for type 1 diabetes–associated islet autoantibodies is offered to all children aged 21 months to 6 years old in Bavaria.
Families of those who test positive are offered participation in a program of diabetes education, metabolic staging, psychological evaluation for stress, and prospective follow-up.
The researchers explain that, worldwide, 4 in 1,000 people under the age of 20 years have type 1 diabetes. It is the most common metabolic disease in children and adolescents. Only about 1 in 10 of those affected has a close relative with the disease. This means that type 1 diabetes can affect any child.
However, in many cases, the disease does not become known until a severe to life-threatening metabolic derailment known as diabetic ketoacidosis (DKA) occurs. This often leads to intensive medical treatment, a longer hospitalization, and poorer blood glucose control, which can result in an increased risk of secondary diseases and very high costs for the health care system.
“We want to protect as many children as possible from serious metabolic derailments. This is only possible with type 1 diabetes screenings. Therefore, we strongly support to include early detection tests in standard medical care,” Peter Achenbach, DrMed, senior author of the study, said in a statement from his institution, Helmholtz Zentrum München in Neuherberg, Germany.
The new findings were published in Diabetes Care by Florian M. Karl, also of Helmholtz Zentrum München, and colleagues.
In 2020, the Fr1da investigators reported that, of 90,632 children who participated from February 2015 to May 2019, 0.31% (280) were diagnosed with presymptomatic type 1 diabetes through the presence of two or more islet autoantibodies.
This news organization asked Brett McQueen, PhD, who led a similar study examining cost and cost-effectiveness in the Autoimmunity Screening for Kids (ASK) program, in which Denver-area children aged 2-17 years are offered autoantibody screening for both type 1 diabetes and celiac disease, for comment.
“If we have a chance to change a child’s life from when they’re 2 or 3 years old and there’s even a small chance that this thing potentially improves health outcomes for a decent price, what are we waiting for?” said Dr. McQueen, who is assistant professor in the department of clinical pharmacy at the University of Colorado, Aurora.
Is DKA prevention enough to justify universal screening?
Although identifying type 1 diabetes before symptoms arise could help avoid DKA, currently no therapeutic interventions are available to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes.
A possible future intervention – the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio) – had a setback in July 2021 when the Food and Drug Administration declined to approve it for the delay of type 1 diabetes in at-risk individuals.
However, on Feb. 22 Provention Bio announced that it has resubmitted the Biologics License Application for teplizumab for the delay of clinical type 1 diabetes in at-risk individuals. The FDA now has 30 days to review the resubmission, determine whether it is complete and acceptable for review, and provide a review goal date, according to a company statement.
But even without the ability to forestall the development of type 1 diabetes, screening proponents point to the potential benefit from educating families about early signs of diabetes onset and thereby preventing progression to DKA and both its short-term and possible long-term sequelae.
Prevention of DKA at diagnosis has been linked to improved long-term glycemic control and other potential health benefits.
And the frequency of DKA at the onset of type 1 diabetes has increased in recent years, to more than 20% in Germany and over 45% in the United States.
But, prior data have suggested that universal screening for presymptomatic type 1 diabetes is unlikely to be cost effective if only the health and economic benefits of prevention of DKA at type 1 diabetes onset is considered, unless the screening costs are exceedingly low.
What will it take to implement universal screening?
“What this paper does is contribute really to our understanding of more around resource utilization,” noted Dr. McQueen. “As they correctly identify, it’s really hard to compare country prices. It’s easier to compare utilization.”
In Dr. McQueen’s ASK program, the cost per child screened and per case detected in that program were similar to those found in the German study, even though the cost of the antibody testing itself was considerably lower in Germany than in the United States.
Fr1da included more components of screening and monitoring than did ASK, Dr. McQueen told this news organization.
The conclusions of the ASK study were that “presymptomatic type 1 diabetes screening may be cost effective in areas with a high prevalence of DKA and an infrastructure facilitating screening and monitoring if the benefits of avoiding DKA events and improved [hemoglobin] A1c persist over long-run time horizons.”
Nonetheless, Dr. McQueen thinks it’s unlikely that universal screening will be recommended by professional societies or covered by payers in the United States until a pharmacologic intervention to forestall disease progression is available.“Teplizumab approval could move this along. ... We’re just trying to take one factor, the economics of it, to create the most efficient scenario so that if it were to be adopted we would catch the most cases, prevent the most complications, benefit children the most in terms of their lifetime health outcomes – all at the minimum cost possible.”
‘A benchmark for the expected implementation cost of screening’
Mr. Karl and colleagues simulated the cost of implementation of this screening as standard care in Germany – assuming the same 0.31% prevalence found in Fr1da – the average cost per child was estimated at 21.73 euros, including 9.34 euros for laboratory costs, 12.25 euros for pediatrician costs, and 0.14 euros for local diabetes clinics to perform metabolic staging and education for children diagnosed with presymptomatic type 1 diabetes.
The model included 50% of the costs incurred in Fr1da for obtaining informed consent. Negative autoantibody results from the initial screening were not communicated to families, and all children with presymptomatic type 1 diabetes received staging and education. The estimated average cost per diagnosed child was 7,035 euros.
“Although our analyses are subject to some level of uncertainty, they provide a benchmark for the expected implementation cost of screening,” said coauthor Michael Laxy, MSc, PhD, also at Helmholtz Zentrum München.
“Next, we aim to evaluate the long-term ratio of screening costs, potential cost savings through the prevention of metabolic derailment and its consequences, and potentially increased quality of life with a type 1 diabetes screening compared to the costs and quality of life without a screening.”
Dr. McQueen is working along similar lines in Colorado, attempting to create a model that incorporates all the different possibilities including DKA monitoring, teplizumab availability, screening children at different ages, and the effect of including blood glucose monitoring in children identified with presymptomatic type 1 diabetes.
“There are so many different potential answers and avenues and no one has really put it all together,” he observed.
But he believes that economics shouldn’t be the only factor used in deciding whether to institute widespread screening.
This study was supported by grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD). The Fr1da study was supported by grants from the LifeScience-Stiftung, JDRF International, the Bavarian State Ministry of Health and Care, the Leona M. and Harry B. Helmsley Charitable Trust, Deutsche Diabetes-Stiftung, Landesverband Bayern der Betriebskrankenkassen, B. Braun-Stiftung, Deutsche Diabetes Hilfe, and the German Federal Ministry of Education and Research to the DZD. The authors disclosed no relevant financial relationships. The ASK study was funded by JDRF International, the Leona M. and Harry B. Helmsley Charitable Trust, and Janssen Research and Development. Dr. McQueen has received institutional funding for value assessment applications from the Institute for Clinical and Economic Review, the PhRMA Foundation, and PhRMA.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
More than half of U.S. women enter pregnancy at higher CVD risk
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
FDA okays empagliflozin for HF regardless of ejection fraction
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
Next-generation Dexcom CGM, G7, accurate and easier to use
The Dexcom G7 continuous glucose monitor (CGM) is as accurate or better than other currently marketed CGM devices for measuring glucose in those with diabetes, new data from a pivotal study suggest.
Currently under review by the U.S. Food and Drug Administration, the G7 is expected to be an improvement over the Dexcom G6 version in several ways.
The on-body size will be 60% smaller, “roughly the size of three stacked quarters,” according to the authors, and will incorporate the sensor with a single-use transmitter, as opposed to the current separate 3-month transmitter used with the G6 sensor. This will eliminate the need for using a transmitter across multiple sensor sessions (as is the case for G6).
The warm-up period after insertion is reduced from 2 hours to 27 minutes, and users are given an extra 12-hour “grace period” after the 10-day wear period to change the device before it stops displaying glucose data. Up to 24 hours of missed data can also be recaptured.
“The enhanced features of G7 may increase clinical adoption, encourage sustained use, and reduce the burden of diabetes management,” write Satish K. Garg, MD, of the University of Colorado, Aurora, and colleagues, in their article, published online Feb. 14 in Diabetes Technology & Therapeutics.
Several features of the G6 remain unchanged, including factory calibration, but also the capacity for optional user calibrations, use of Bluetooth to transmit data up to 20 feet (approximately 6 meters), and data displays on either a dedicated receiver or a variety of iOS and Android smart devices.
It will also allow for user-customized settings and alerts, as well as the option for real-time remote “share” monitoring with caregivers or providers. The G7 will also not be susceptible to interference by acetaminophen (paracetamol) or ascorbic acid.
And, like the G6, the G7 was submitted for approval to the FDA as an “integrated CGM,” meaning that it will be interoperable with other compatible devices, including insulin pumps, glucose meters, or other electronic devices used for diabetes management.
Accuracy shown on abdomen, arm
The prospective, multicenter, single-arm study reported by Dr. Garg and colleagues was conducted at 12 U.S. sites between February and June 2021.
In-clinic visits were conducted on days 1 or 2, 4 or 7, and one additional day for comparisons with a reference glucose measure (YSI 2300 Stat Plus glucose analyzer). Participants wore blinded G7 sensors concurrently on the upper arm and abdomen while continuing to use their own personal glucose monitoring method (CGM or fingerstick) at home.
A total of 316 insulin-using adults with type 1 or type 2 diabetes contributed data from 308 arm- and 311 abdomen-placed blinded devices, which generated 77,774 matched pairs of data within the blood glucose reportable range of 40-400 mg/dL.
The overall mean absolute relative difference (MARD) of each CGM-YSI pair (a standard metric for CGM analysis) was 8.2%, with 9.1% for the abdomen and 8.2% for the arm.
Accuracy remained high in both arm- and abdomen-placed sensors across the 10-day wear period through the 12-hour grace period and across glucose ranges. There were no significant differences between G7 accuracy by diabetes type, insulin regimen, or body mass index.
The highest agreement rates and lowest MARDs occurred when CGM readings were increasing or decreasing by no more than 1 mg/dL per minute. However, even at the highest rates of glucose concentration change, MARD values below 10% were found for arm-placed sensors and below 10.5% for abdomen-placed sensors, Dr. Garg and colleagues report.
When the hypoglycemia threshold alert was set to 55 mg/dL, true alert rates for detection of hypoglycemia below 70 mg/dL by sensors worn on the arm and abdomen were 91.3% and 85.2%, respectively. With hyperglycemia threshold alerts set to 300 mg/dL, the true alert rates for detection of hyperglycemia greater than 250 mg/dL by sensors worn on the arm and abdomen were 99.9% and 99.8% respectively.
The overall mean time lag for the sensors was 3.5 minutes, 3.6 minutes for the arm, and 3.4 minutes for the abdomen. There were no serious adverse events during the study.
The study excluded children and adolescents; data from these populations will be reported separately, the authors note.
Accuracy at least as good as prior Dexcom versions, competitors
The MARD values of 8.2% on the arm and 9.1% on the abdomen were similar to or better than accuracy measurements of other commercially available CGM systems, note Dr. Garg and colleagues, although they acknowledge that few head-to-head studies at different anatomic locations have been conducted.
A study of an older Dexcom version (G4 Platinum) found MARD estimates of 12.0% on the arm and 12.3% on the abdomen, the authors note.
The newly FDA-approved implantable Eversense E3 (Senseonics) CGM, worn on the upper arm, has a MARD of 9.1%, while the arm-placed Abbott FreeStyle Libre 2, approved in the U.S. in June 2020, has an overall MARD of 9.3%.
Lag-time differences between the reference and G7 were also similar to or better than time delays in prior-generation Dexcom CGMs, Dr. Garg and colleagues say.
Participants also completed a survey. “The redesigned applicator allows for sensor deployment with one hand, and most participants found G7 easier to insert than their prior CGM system,” the researchers say.
Finally, “unlike G6, G7 allows for temporary silencing of all audible alerts, including Urgent Low. Taken together, these attributes are anticipated to provide for a better end-user experience with G7 and help reduce diabetes burden,” they conclude.
The study was supported by a grant from Dexcom. Dr. Garg has reported receiving consultant fees from Medtronic, Novo Nordisk, Zealand, LifeScan, Roche, and Lilly, as well as research grants through the University of Colorado from Lilly, Novo Nordisk, Medtronic, Dexcom, T1D Exchange, Helmsley Trust, NIDDK, and JDRF.
A version of this article first appeared on Medscape.com.
The Dexcom G7 continuous glucose monitor (CGM) is as accurate or better than other currently marketed CGM devices for measuring glucose in those with diabetes, new data from a pivotal study suggest.
Currently under review by the U.S. Food and Drug Administration, the G7 is expected to be an improvement over the Dexcom G6 version in several ways.
The on-body size will be 60% smaller, “roughly the size of three stacked quarters,” according to the authors, and will incorporate the sensor with a single-use transmitter, as opposed to the current separate 3-month transmitter used with the G6 sensor. This will eliminate the need for using a transmitter across multiple sensor sessions (as is the case for G6).
The warm-up period after insertion is reduced from 2 hours to 27 minutes, and users are given an extra 12-hour “grace period” after the 10-day wear period to change the device before it stops displaying glucose data. Up to 24 hours of missed data can also be recaptured.
“The enhanced features of G7 may increase clinical adoption, encourage sustained use, and reduce the burden of diabetes management,” write Satish K. Garg, MD, of the University of Colorado, Aurora, and colleagues, in their article, published online Feb. 14 in Diabetes Technology & Therapeutics.
Several features of the G6 remain unchanged, including factory calibration, but also the capacity for optional user calibrations, use of Bluetooth to transmit data up to 20 feet (approximately 6 meters), and data displays on either a dedicated receiver or a variety of iOS and Android smart devices.
It will also allow for user-customized settings and alerts, as well as the option for real-time remote “share” monitoring with caregivers or providers. The G7 will also not be susceptible to interference by acetaminophen (paracetamol) or ascorbic acid.
And, like the G6, the G7 was submitted for approval to the FDA as an “integrated CGM,” meaning that it will be interoperable with other compatible devices, including insulin pumps, glucose meters, or other electronic devices used for diabetes management.
Accuracy shown on abdomen, arm
The prospective, multicenter, single-arm study reported by Dr. Garg and colleagues was conducted at 12 U.S. sites between February and June 2021.
In-clinic visits were conducted on days 1 or 2, 4 or 7, and one additional day for comparisons with a reference glucose measure (YSI 2300 Stat Plus glucose analyzer). Participants wore blinded G7 sensors concurrently on the upper arm and abdomen while continuing to use their own personal glucose monitoring method (CGM or fingerstick) at home.
A total of 316 insulin-using adults with type 1 or type 2 diabetes contributed data from 308 arm- and 311 abdomen-placed blinded devices, which generated 77,774 matched pairs of data within the blood glucose reportable range of 40-400 mg/dL.
The overall mean absolute relative difference (MARD) of each CGM-YSI pair (a standard metric for CGM analysis) was 8.2%, with 9.1% for the abdomen and 8.2% for the arm.
Accuracy remained high in both arm- and abdomen-placed sensors across the 10-day wear period through the 12-hour grace period and across glucose ranges. There were no significant differences between G7 accuracy by diabetes type, insulin regimen, or body mass index.
The highest agreement rates and lowest MARDs occurred when CGM readings were increasing or decreasing by no more than 1 mg/dL per minute. However, even at the highest rates of glucose concentration change, MARD values below 10% were found for arm-placed sensors and below 10.5% for abdomen-placed sensors, Dr. Garg and colleagues report.
When the hypoglycemia threshold alert was set to 55 mg/dL, true alert rates for detection of hypoglycemia below 70 mg/dL by sensors worn on the arm and abdomen were 91.3% and 85.2%, respectively. With hyperglycemia threshold alerts set to 300 mg/dL, the true alert rates for detection of hyperglycemia greater than 250 mg/dL by sensors worn on the arm and abdomen were 99.9% and 99.8% respectively.
The overall mean time lag for the sensors was 3.5 minutes, 3.6 minutes for the arm, and 3.4 minutes for the abdomen. There were no serious adverse events during the study.
The study excluded children and adolescents; data from these populations will be reported separately, the authors note.
Accuracy at least as good as prior Dexcom versions, competitors
The MARD values of 8.2% on the arm and 9.1% on the abdomen were similar to or better than accuracy measurements of other commercially available CGM systems, note Dr. Garg and colleagues, although they acknowledge that few head-to-head studies at different anatomic locations have been conducted.
A study of an older Dexcom version (G4 Platinum) found MARD estimates of 12.0% on the arm and 12.3% on the abdomen, the authors note.
The newly FDA-approved implantable Eversense E3 (Senseonics) CGM, worn on the upper arm, has a MARD of 9.1%, while the arm-placed Abbott FreeStyle Libre 2, approved in the U.S. in June 2020, has an overall MARD of 9.3%.
Lag-time differences between the reference and G7 were also similar to or better than time delays in prior-generation Dexcom CGMs, Dr. Garg and colleagues say.
Participants also completed a survey. “The redesigned applicator allows for sensor deployment with one hand, and most participants found G7 easier to insert than their prior CGM system,” the researchers say.
Finally, “unlike G6, G7 allows for temporary silencing of all audible alerts, including Urgent Low. Taken together, these attributes are anticipated to provide for a better end-user experience with G7 and help reduce diabetes burden,” they conclude.
The study was supported by a grant from Dexcom. Dr. Garg has reported receiving consultant fees from Medtronic, Novo Nordisk, Zealand, LifeScan, Roche, and Lilly, as well as research grants through the University of Colorado from Lilly, Novo Nordisk, Medtronic, Dexcom, T1D Exchange, Helmsley Trust, NIDDK, and JDRF.
A version of this article first appeared on Medscape.com.
The Dexcom G7 continuous glucose monitor (CGM) is as accurate or better than other currently marketed CGM devices for measuring glucose in those with diabetes, new data from a pivotal study suggest.
Currently under review by the U.S. Food and Drug Administration, the G7 is expected to be an improvement over the Dexcom G6 version in several ways.
The on-body size will be 60% smaller, “roughly the size of three stacked quarters,” according to the authors, and will incorporate the sensor with a single-use transmitter, as opposed to the current separate 3-month transmitter used with the G6 sensor. This will eliminate the need for using a transmitter across multiple sensor sessions (as is the case for G6).
The warm-up period after insertion is reduced from 2 hours to 27 minutes, and users are given an extra 12-hour “grace period” after the 10-day wear period to change the device before it stops displaying glucose data. Up to 24 hours of missed data can also be recaptured.
“The enhanced features of G7 may increase clinical adoption, encourage sustained use, and reduce the burden of diabetes management,” write Satish K. Garg, MD, of the University of Colorado, Aurora, and colleagues, in their article, published online Feb. 14 in Diabetes Technology & Therapeutics.
Several features of the G6 remain unchanged, including factory calibration, but also the capacity for optional user calibrations, use of Bluetooth to transmit data up to 20 feet (approximately 6 meters), and data displays on either a dedicated receiver or a variety of iOS and Android smart devices.
It will also allow for user-customized settings and alerts, as well as the option for real-time remote “share” monitoring with caregivers or providers. The G7 will also not be susceptible to interference by acetaminophen (paracetamol) or ascorbic acid.
And, like the G6, the G7 was submitted for approval to the FDA as an “integrated CGM,” meaning that it will be interoperable with other compatible devices, including insulin pumps, glucose meters, or other electronic devices used for diabetes management.
Accuracy shown on abdomen, arm
The prospective, multicenter, single-arm study reported by Dr. Garg and colleagues was conducted at 12 U.S. sites between February and June 2021.
In-clinic visits were conducted on days 1 or 2, 4 or 7, and one additional day for comparisons with a reference glucose measure (YSI 2300 Stat Plus glucose analyzer). Participants wore blinded G7 sensors concurrently on the upper arm and abdomen while continuing to use their own personal glucose monitoring method (CGM or fingerstick) at home.
A total of 316 insulin-using adults with type 1 or type 2 diabetes contributed data from 308 arm- and 311 abdomen-placed blinded devices, which generated 77,774 matched pairs of data within the blood glucose reportable range of 40-400 mg/dL.
The overall mean absolute relative difference (MARD) of each CGM-YSI pair (a standard metric for CGM analysis) was 8.2%, with 9.1% for the abdomen and 8.2% for the arm.
Accuracy remained high in both arm- and abdomen-placed sensors across the 10-day wear period through the 12-hour grace period and across glucose ranges. There were no significant differences between G7 accuracy by diabetes type, insulin regimen, or body mass index.
The highest agreement rates and lowest MARDs occurred when CGM readings were increasing or decreasing by no more than 1 mg/dL per minute. However, even at the highest rates of glucose concentration change, MARD values below 10% were found for arm-placed sensors and below 10.5% for abdomen-placed sensors, Dr. Garg and colleagues report.
When the hypoglycemia threshold alert was set to 55 mg/dL, true alert rates for detection of hypoglycemia below 70 mg/dL by sensors worn on the arm and abdomen were 91.3% and 85.2%, respectively. With hyperglycemia threshold alerts set to 300 mg/dL, the true alert rates for detection of hyperglycemia greater than 250 mg/dL by sensors worn on the arm and abdomen were 99.9% and 99.8% respectively.
The overall mean time lag for the sensors was 3.5 minutes, 3.6 minutes for the arm, and 3.4 minutes for the abdomen. There were no serious adverse events during the study.
The study excluded children and adolescents; data from these populations will be reported separately, the authors note.
Accuracy at least as good as prior Dexcom versions, competitors
The MARD values of 8.2% on the arm and 9.1% on the abdomen were similar to or better than accuracy measurements of other commercially available CGM systems, note Dr. Garg and colleagues, although they acknowledge that few head-to-head studies at different anatomic locations have been conducted.
A study of an older Dexcom version (G4 Platinum) found MARD estimates of 12.0% on the arm and 12.3% on the abdomen, the authors note.
The newly FDA-approved implantable Eversense E3 (Senseonics) CGM, worn on the upper arm, has a MARD of 9.1%, while the arm-placed Abbott FreeStyle Libre 2, approved in the U.S. in June 2020, has an overall MARD of 9.3%.
Lag-time differences between the reference and G7 were also similar to or better than time delays in prior-generation Dexcom CGMs, Dr. Garg and colleagues say.
Participants also completed a survey. “The redesigned applicator allows for sensor deployment with one hand, and most participants found G7 easier to insert than their prior CGM system,” the researchers say.
Finally, “unlike G6, G7 allows for temporary silencing of all audible alerts, including Urgent Low. Taken together, these attributes are anticipated to provide for a better end-user experience with G7 and help reduce diabetes burden,” they conclude.
The study was supported by a grant from Dexcom. Dr. Garg has reported receiving consultant fees from Medtronic, Novo Nordisk, Zealand, LifeScan, Roche, and Lilly, as well as research grants through the University of Colorado from Lilly, Novo Nordisk, Medtronic, Dexcom, T1D Exchange, Helmsley Trust, NIDDK, and JDRF.
A version of this article first appeared on Medscape.com.
2021 in Review: Key Trials in Type 2 Diabetes (T2D)
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.