User login
Official Newspaper of the American College of Surgeons
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Adding mitral valve repair to CABG found risky, with limited benefits
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
FROM ACC 16
Key clinical point: In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect left ventricular remodeling at two years.
Major finding: Mean decreases in left ventricular end systolic volume index were 14.1 mL and 14.6 mL, respectively.
Data source: A multicenter randomized trial of 301 patients with moderate to severe baseline mitral regurgitation.
Disclosures: The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
Self-expanding TAVR bests surgery based on 3-year stroke and death risks
Patients with severe aortic stenosis that puts them at increased risk for surgery continue to do better at 3 years after receiving a self-expanding transcatheter aortic valve replacement than do similar patients who have an open surgical valve replacement, according to new results from a randomized trial presented at the annual meeting of the American College of Cardiology.
Two-year follow-up results from the same trial cohort, the CoreValve U.S. Pivotal High Risk Trial, showed superior survival and stroke outcomes for TAVR compared with open surgery (J Am Coll Cardiol. 2015;66[2]:113-21). The difference in outcomes was thought to stem mainly from fewer postprocedural complications and faster recovery in the TAVR group.
The new study, presented at the meeting and simultaneously published online April 3 in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.03.506) aimed to determine whether the previously seen benefits extended into the third year and whether these were accompanied by differences in valve hemodynamics.
Dr. G. Michael Deeb, Herbert Sloan Collegiate Professor of Cardiac Surgery at the University of Michigan, Ann Arbor, and his colleagues evaluated three-year clinical and echocardiographic outcomes from the 391 patients who underwent TAVR and 359 who had SAVR. At baseline all patients had severe aortic stenosis and were considered to be at increased risk for SAVR, with an estimated 30-day mortality risk 15% or greater and a combined 30-day surgical mortality and major morbidity risk less than 50%.
At 3 years follow-up in the treated groups, combined all-cause mortality or stroke was significantly lower at 37% in TAVR patients as compared to nearly 47% in SAVR patients. All-cause mortality was 33% with TAVR and 39% with SAVR, a difference that did not reach statistical significance. Stroke rates were nearly 13% with TAVR and 19% with SAVR; major adverse cardiovascular or cerebrovascular events were 40% with TAVR and 48% for SAVR. Both were significant differences.
While mean aortic valve gradient measures were more favorable – 7.62 ± 3.57 mm Hg with TAVR and 11.40 ± 6.81 mm Hg with SAVR – regurgitation was significantly higher at nearly 7% with TAVR and no regurgitation with SAVR. Valve thrombosis and valve structural deterioration were not observed in either group.
While the findings show sustained 3-year clinical benefit of self-expanding TAVR over SAVR in patients with aortic stenosis at increased risk for surgery, longer studies are needed to determine whether the crimping and re-crimping of the transcatheter valve would have an impact on long-term bioprosthesis durability.
The study was funded by the device manufacturer Medtronic, and 21 of its 28 authors disclosed financial relationships with Medtronic and/or other manufacturers; one is a Medtronic employee. Dr. Deeb disclosed serving as an unpaid advisor to Medtronic.
Patients with severe aortic stenosis that puts them at increased risk for surgery continue to do better at 3 years after receiving a self-expanding transcatheter aortic valve replacement than do similar patients who have an open surgical valve replacement, according to new results from a randomized trial presented at the annual meeting of the American College of Cardiology.
Two-year follow-up results from the same trial cohort, the CoreValve U.S. Pivotal High Risk Trial, showed superior survival and stroke outcomes for TAVR compared with open surgery (J Am Coll Cardiol. 2015;66[2]:113-21). The difference in outcomes was thought to stem mainly from fewer postprocedural complications and faster recovery in the TAVR group.
The new study, presented at the meeting and simultaneously published online April 3 in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.03.506) aimed to determine whether the previously seen benefits extended into the third year and whether these were accompanied by differences in valve hemodynamics.
Dr. G. Michael Deeb, Herbert Sloan Collegiate Professor of Cardiac Surgery at the University of Michigan, Ann Arbor, and his colleagues evaluated three-year clinical and echocardiographic outcomes from the 391 patients who underwent TAVR and 359 who had SAVR. At baseline all patients had severe aortic stenosis and were considered to be at increased risk for SAVR, with an estimated 30-day mortality risk 15% or greater and a combined 30-day surgical mortality and major morbidity risk less than 50%.
At 3 years follow-up in the treated groups, combined all-cause mortality or stroke was significantly lower at 37% in TAVR patients as compared to nearly 47% in SAVR patients. All-cause mortality was 33% with TAVR and 39% with SAVR, a difference that did not reach statistical significance. Stroke rates were nearly 13% with TAVR and 19% with SAVR; major adverse cardiovascular or cerebrovascular events were 40% with TAVR and 48% for SAVR. Both were significant differences.
While mean aortic valve gradient measures were more favorable – 7.62 ± 3.57 mm Hg with TAVR and 11.40 ± 6.81 mm Hg with SAVR – regurgitation was significantly higher at nearly 7% with TAVR and no regurgitation with SAVR. Valve thrombosis and valve structural deterioration were not observed in either group.
While the findings show sustained 3-year clinical benefit of self-expanding TAVR over SAVR in patients with aortic stenosis at increased risk for surgery, longer studies are needed to determine whether the crimping and re-crimping of the transcatheter valve would have an impact on long-term bioprosthesis durability.
The study was funded by the device manufacturer Medtronic, and 21 of its 28 authors disclosed financial relationships with Medtronic and/or other manufacturers; one is a Medtronic employee. Dr. Deeb disclosed serving as an unpaid advisor to Medtronic.
Patients with severe aortic stenosis that puts them at increased risk for surgery continue to do better at 3 years after receiving a self-expanding transcatheter aortic valve replacement than do similar patients who have an open surgical valve replacement, according to new results from a randomized trial presented at the annual meeting of the American College of Cardiology.
Two-year follow-up results from the same trial cohort, the CoreValve U.S. Pivotal High Risk Trial, showed superior survival and stroke outcomes for TAVR compared with open surgery (J Am Coll Cardiol. 2015;66[2]:113-21). The difference in outcomes was thought to stem mainly from fewer postprocedural complications and faster recovery in the TAVR group.
The new study, presented at the meeting and simultaneously published online April 3 in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.03.506) aimed to determine whether the previously seen benefits extended into the third year and whether these were accompanied by differences in valve hemodynamics.
Dr. G. Michael Deeb, Herbert Sloan Collegiate Professor of Cardiac Surgery at the University of Michigan, Ann Arbor, and his colleagues evaluated three-year clinical and echocardiographic outcomes from the 391 patients who underwent TAVR and 359 who had SAVR. At baseline all patients had severe aortic stenosis and were considered to be at increased risk for SAVR, with an estimated 30-day mortality risk 15% or greater and a combined 30-day surgical mortality and major morbidity risk less than 50%.
At 3 years follow-up in the treated groups, combined all-cause mortality or stroke was significantly lower at 37% in TAVR patients as compared to nearly 47% in SAVR patients. All-cause mortality was 33% with TAVR and 39% with SAVR, a difference that did not reach statistical significance. Stroke rates were nearly 13% with TAVR and 19% with SAVR; major adverse cardiovascular or cerebrovascular events were 40% with TAVR and 48% for SAVR. Both were significant differences.
While mean aortic valve gradient measures were more favorable – 7.62 ± 3.57 mm Hg with TAVR and 11.40 ± 6.81 mm Hg with SAVR – regurgitation was significantly higher at nearly 7% with TAVR and no regurgitation with SAVR. Valve thrombosis and valve structural deterioration were not observed in either group.
While the findings show sustained 3-year clinical benefit of self-expanding TAVR over SAVR in patients with aortic stenosis at increased risk for surgery, longer studies are needed to determine whether the crimping and re-crimping of the transcatheter valve would have an impact on long-term bioprosthesis durability.
The study was funded by the device manufacturer Medtronic, and 21 of its 28 authors disclosed financial relationships with Medtronic and/or other manufacturers; one is a Medtronic employee. Dr. Deeb disclosed serving as an unpaid advisor to Medtronic.
FROM ACC 2016
Key clinical point: Patients randomized to self-expanding TAVR or open surgical aortic valve replacement were less likely to have died or had a stroke at 3 years post-procedure
Major finding:. Three-year all-cause mortality and stroke rate was significantly lower in TAVR patients – 37% versus nearly 47% in SAVR patients.
Data source: A cohort of 750 patients deemed high risk who underwent open aortic valve replacement or TAVR after randomization; procedures performed at 45 sites
Disclosures: The study was sponsored by Medtronic, maker of the self-expanding transcatheter technology. Most study authors, though not the lead author, had direct financial involvement.
TAVR matches surgery in intermediate-risk patients
CHICAGO – Transcatheter aortic-valve replacement performed as well as surgical-valve replacement in patients with an intermediate mortality risk in a prospective, randomized trial with more than 2,000 patients followed for 2 years, the first randomized trial to compare the efficacy and safety of transcatheter aortic-valve replacement against surgical replacement in patients who did not have a high mortality risk.
The results “support TAVR [transcatheter aortic-valve replacement] as an alternative to surgery in intermediate-risk patients similar to those included in this trial,” said Dr. Craig R. Smith at the annual meeting of the American College of Cardiology. The findings from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A trial “will increase use of TAVR,” predicted Dr. Smith, professor and chairman of surgery at New York–Presbyterian Hospital/Columbia University in New York.
Until now, TAVR had been compared with surgical aortic-valve replacement in two prospective, randomized trials that both enrolled either high-risk or inoperable patients with severe aortic stenosis, the PARTNER 1 trial that tested the original Sapien TAVR system, and the U.S. CoreValve High-Risk Study that tested the original CoreValve system (often now called CoreValve classic). The average Society of Thoracic Surgeons (STS) operative risk score of high-risk patients enrolled in PARTNER 1 was 11.8%, and the average risk score in patients enrolled in the CoreValve study was 7.3%. In contrast, the design of PARTNER 2A specified that enrolled patients have a STS risk score of 4%-8%, a criterion actually met by 81% of the enrolled patients, and the average STS risk score of all patients enrolled in PARTNER 2A was 5.8%.
Although U.S. labeling for both the Sapien valve and its later iterations, Sapien XT and S3, and for CoreValve and its later iteration, Evolut R, specify that treated patients should have a STS risk score of at least 8%, the labeling also gives the heart teams that perform TAVR the latitude to treat patients with risk scores below 8% when the heart teams identify other patient factors that confer high risk such as frailty or comorbidities. U.S. and European TAVR registries have documented that many patients with STS risk scores below 8% have undergone TAVR since these systems received regulatory approval. The new results from PARTNER 2A may change that by leading to revised labeling that cuts the STS risk-score threshold.
“These findings might lead to a labeling change that would avoid a lot of the patient-evaluation gymnastics that have been used to justify” TAVR treatment, noted Dr. Smith. New labeling like this “would sanction what is already going on” in terms of which patients undergo TAVR.
Others who heard these results at the meeting agreed they were an important milestone in TAVR development and its expanding use.
The new results “make a huge difference,” commented Dr. David R. Holmes Jr., an interventional cardiologist and professor at the Mayo Clinic in Rochester, Minn. “We base many of our guidelines on the results from randomized, controlled trials. It’s true that there are reports of lower-risk patients undergoing TAVR, but we now have results from a well-designed trial with well-controlled and adjudicated endpoints that documents the safety and efficacy of TAVR in intermediate-risk patients,” Dr. Holmes said in an interview.
“The results will have a very important influence on the choice between TAVR and surgery,” commented Dr. Duane S. Pinto, an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “It validates the strategy” of using TAVR in patients with a risk score of 4%-8%. “TAVR has already been used in these patients, but these results validate this, especially when used in a transfemoral approach,” Dr. Pinto said in an interview.
One aspect of PARTNER 2A that received a lot of discussion at the meeting was whether enrolled patients could appropriately be characterized as “intermediate” in their risk level. Although their average STS risk score of 5.8% fell squarely within the target range specified for the study, they averaged 82 years old, and other clinical features at baseline suggested a higher risk population. The published report of the PARTNER 2A results that appeared online concurrent with Dr. Smith’s report at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616) acknowledged that STS risk scores of 4%-8% place the enrolled patients into the upper 20% for risk of all U.S. patients who undergo surgical aortic-valve replacement
“I would characterize the enrolled patients as ‘less high risk’ rather than intermediate risk,” said Dr. Pinto.
But as Dr. Smith explained “even if the enrolled patients are not ‘intermediate’ risk they are at a different risk level” than were the patients enrolled in the prior TAVR randomized trials.
In the PARTNER 1 high-risk trial, the overall 1-year rate of all-cause mortality was 24% and 27% in the TAVR and surgical arms of the study, respectively. In the CoreValve trial these rates were 14% with TAVR and 19% with surgery. In PARTNER 2A 1-year all-cause mortality was 12% with TAVR and 13% with surgery.
Two other notable findings of PARTNER 2A were the superior outcomes of patients who underwent TAVR using a transfemoral approach, and the improved outcomes that all TAVR patients had compared with surgical valve replacement for several secondary outcomes.
The rate of the study’s primary outcome, all-cause death or disabling stroke after 2 years, was cut by a relative 21% in the 77% of TAVR patients who underwent a transfemoral procedure, compared with the surgery patients, a difference that was of borderline statistical significance. In contrast, the entire group of TAVR patients, including those treated via nontransfemoral routes, had an 11% relative reduction of the primary endpoint, compared with surgery, a difference that was not statistically significant but did easily meet the study’s prespecified definition of noninferority. Dr. Smith and others were especially encouraged by these findings as PARTNER 2A used the older Sapien XT TAVR system that is not often used today in U.S. practice. When U.S. patients undergo TAVR with a balloon-expandable valve they most often receive treatment with the S3 system, much smaller than XT and hence much more likely to be used with a transfemoral approach.
Other secondary outcomes included life-threatening or disabling bleeding events, which after 2 years had occurred in 17% of the TAVR patients and 47% of those who underwent surgery; atrial fibrillation, which occurred in 11% of the TAVR patients and 27% of those undergoing surgery; and acute kidney injury which occurred in 4% of TAVR patients and 6% of the surgery patients. With 2-year follow-up, the rate of disabling strokes was 6% in both arms of the study.
PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
On Twitter @mitchelzoler
Registries of patients who have undergone transcatheter aortic-valve replacement in Europe and the United States show that this procedure has already been frequently used in selected patients with Society of Thoracic Surgeons operative-risk scores of 4%-8%. Even though regulatory approval specifies using the procedure in high-risk patients with risk scores of at least 8%, the labeling leaves the decision of which patients are at high risk up to local heart teams, and factors other than the risk score play into a patient’s overall risk assessment including frailty and comorbidities.
Despite the prior experience using TAVR in patients with STS risk scores of 4%-8% the results of PARTNER 2A are a game changer because they come from a prospective, randomized, controlled trial.
The PARTNER 2A results are also notable because this is the second randomized trial (in addition to the CoreValve high-risk trial) with results that show or suggest that transcatheter aortic-valve replacement (TAVR) produces better outcomes than surgery, especially in patients who undergo TAVR via a transfemoral approach. Other notable advantages of TAVR over surgery seen in PARTNER 2A include substantial reductions in disabling or life-threatening bleeding events and in new-onset atrial fibrillation, a statistically significant reduction in acute kidney injury, and no significant difference in the incidence of disabling strokes. In the past, we expected stroke rates to be higher with TAVR, but in PARTNER 2A, with neurologists adjudicating the strokes, we saw no difference in the TAVR and surgical stroke rates, a finding that was probably unexpected for many people.

|
Dr. Ajay J. Kirtane |
The patients enrolled in PARTNER 2A were clearly at lower risk for all-cause mortality than the patients enrolled in the earlier TAVR trials. The operative risk score is just one of several ways to estimate patient risk. The data collected in PARTNER 2A provide a robust resource for finding new, additional ways to assess patients who are at intermediate risk and to match patients seen during routine practice to those who entered this trial.
Dr. Ajay J. Kirtane is an interventional cardiologist and director of the coronary catheterization laboratory at New York–Presbyterian/Columbia University in New York. He was a coinvestigator on prior Sapien TAVR studies but did not participate in PARTNER 2. His institution has received research support from Edwards and from Boston Scientific. He made these comments in an interview.
Registries of patients who have undergone transcatheter aortic-valve replacement in Europe and the United States show that this procedure has already been frequently used in selected patients with Society of Thoracic Surgeons operative-risk scores of 4%-8%. Even though regulatory approval specifies using the procedure in high-risk patients with risk scores of at least 8%, the labeling leaves the decision of which patients are at high risk up to local heart teams, and factors other than the risk score play into a patient’s overall risk assessment including frailty and comorbidities.
Despite the prior experience using TAVR in patients with STS risk scores of 4%-8% the results of PARTNER 2A are a game changer because they come from a prospective, randomized, controlled trial.
The PARTNER 2A results are also notable because this is the second randomized trial (in addition to the CoreValve high-risk trial) with results that show or suggest that transcatheter aortic-valve replacement (TAVR) produces better outcomes than surgery, especially in patients who undergo TAVR via a transfemoral approach. Other notable advantages of TAVR over surgery seen in PARTNER 2A include substantial reductions in disabling or life-threatening bleeding events and in new-onset atrial fibrillation, a statistically significant reduction in acute kidney injury, and no significant difference in the incidence of disabling strokes. In the past, we expected stroke rates to be higher with TAVR, but in PARTNER 2A, with neurologists adjudicating the strokes, we saw no difference in the TAVR and surgical stroke rates, a finding that was probably unexpected for many people.

|
Dr. Ajay J. Kirtane |
The patients enrolled in PARTNER 2A were clearly at lower risk for all-cause mortality than the patients enrolled in the earlier TAVR trials. The operative risk score is just one of several ways to estimate patient risk. The data collected in PARTNER 2A provide a robust resource for finding new, additional ways to assess patients who are at intermediate risk and to match patients seen during routine practice to those who entered this trial.
Dr. Ajay J. Kirtane is an interventional cardiologist and director of the coronary catheterization laboratory at New York–Presbyterian/Columbia University in New York. He was a coinvestigator on prior Sapien TAVR studies but did not participate in PARTNER 2. His institution has received research support from Edwards and from Boston Scientific. He made these comments in an interview.
Registries of patients who have undergone transcatheter aortic-valve replacement in Europe and the United States show that this procedure has already been frequently used in selected patients with Society of Thoracic Surgeons operative-risk scores of 4%-8%. Even though regulatory approval specifies using the procedure in high-risk patients with risk scores of at least 8%, the labeling leaves the decision of which patients are at high risk up to local heart teams, and factors other than the risk score play into a patient’s overall risk assessment including frailty and comorbidities.
Despite the prior experience using TAVR in patients with STS risk scores of 4%-8% the results of PARTNER 2A are a game changer because they come from a prospective, randomized, controlled trial.
The PARTNER 2A results are also notable because this is the second randomized trial (in addition to the CoreValve high-risk trial) with results that show or suggest that transcatheter aortic-valve replacement (TAVR) produces better outcomes than surgery, especially in patients who undergo TAVR via a transfemoral approach. Other notable advantages of TAVR over surgery seen in PARTNER 2A include substantial reductions in disabling or life-threatening bleeding events and in new-onset atrial fibrillation, a statistically significant reduction in acute kidney injury, and no significant difference in the incidence of disabling strokes. In the past, we expected stroke rates to be higher with TAVR, but in PARTNER 2A, with neurologists adjudicating the strokes, we saw no difference in the TAVR and surgical stroke rates, a finding that was probably unexpected for many people.

|
Dr. Ajay J. Kirtane |
The patients enrolled in PARTNER 2A were clearly at lower risk for all-cause mortality than the patients enrolled in the earlier TAVR trials. The operative risk score is just one of several ways to estimate patient risk. The data collected in PARTNER 2A provide a robust resource for finding new, additional ways to assess patients who are at intermediate risk and to match patients seen during routine practice to those who entered this trial.
Dr. Ajay J. Kirtane is an interventional cardiologist and director of the coronary catheterization laboratory at New York–Presbyterian/Columbia University in New York. He was a coinvestigator on prior Sapien TAVR studies but did not participate in PARTNER 2. His institution has received research support from Edwards and from Boston Scientific. He made these comments in an interview.
CHICAGO – Transcatheter aortic-valve replacement performed as well as surgical-valve replacement in patients with an intermediate mortality risk in a prospective, randomized trial with more than 2,000 patients followed for 2 years, the first randomized trial to compare the efficacy and safety of transcatheter aortic-valve replacement against surgical replacement in patients who did not have a high mortality risk.
The results “support TAVR [transcatheter aortic-valve replacement] as an alternative to surgery in intermediate-risk patients similar to those included in this trial,” said Dr. Craig R. Smith at the annual meeting of the American College of Cardiology. The findings from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A trial “will increase use of TAVR,” predicted Dr. Smith, professor and chairman of surgery at New York–Presbyterian Hospital/Columbia University in New York.
Until now, TAVR had been compared with surgical aortic-valve replacement in two prospective, randomized trials that both enrolled either high-risk or inoperable patients with severe aortic stenosis, the PARTNER 1 trial that tested the original Sapien TAVR system, and the U.S. CoreValve High-Risk Study that tested the original CoreValve system (often now called CoreValve classic). The average Society of Thoracic Surgeons (STS) operative risk score of high-risk patients enrolled in PARTNER 1 was 11.8%, and the average risk score in patients enrolled in the CoreValve study was 7.3%. In contrast, the design of PARTNER 2A specified that enrolled patients have a STS risk score of 4%-8%, a criterion actually met by 81% of the enrolled patients, and the average STS risk score of all patients enrolled in PARTNER 2A was 5.8%.
Although U.S. labeling for both the Sapien valve and its later iterations, Sapien XT and S3, and for CoreValve and its later iteration, Evolut R, specify that treated patients should have a STS risk score of at least 8%, the labeling also gives the heart teams that perform TAVR the latitude to treat patients with risk scores below 8% when the heart teams identify other patient factors that confer high risk such as frailty or comorbidities. U.S. and European TAVR registries have documented that many patients with STS risk scores below 8% have undergone TAVR since these systems received regulatory approval. The new results from PARTNER 2A may change that by leading to revised labeling that cuts the STS risk-score threshold.
“These findings might lead to a labeling change that would avoid a lot of the patient-evaluation gymnastics that have been used to justify” TAVR treatment, noted Dr. Smith. New labeling like this “would sanction what is already going on” in terms of which patients undergo TAVR.
Others who heard these results at the meeting agreed they were an important milestone in TAVR development and its expanding use.
The new results “make a huge difference,” commented Dr. David R. Holmes Jr., an interventional cardiologist and professor at the Mayo Clinic in Rochester, Minn. “We base many of our guidelines on the results from randomized, controlled trials. It’s true that there are reports of lower-risk patients undergoing TAVR, but we now have results from a well-designed trial with well-controlled and adjudicated endpoints that documents the safety and efficacy of TAVR in intermediate-risk patients,” Dr. Holmes said in an interview.
“The results will have a very important influence on the choice between TAVR and surgery,” commented Dr. Duane S. Pinto, an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “It validates the strategy” of using TAVR in patients with a risk score of 4%-8%. “TAVR has already been used in these patients, but these results validate this, especially when used in a transfemoral approach,” Dr. Pinto said in an interview.
One aspect of PARTNER 2A that received a lot of discussion at the meeting was whether enrolled patients could appropriately be characterized as “intermediate” in their risk level. Although their average STS risk score of 5.8% fell squarely within the target range specified for the study, they averaged 82 years old, and other clinical features at baseline suggested a higher risk population. The published report of the PARTNER 2A results that appeared online concurrent with Dr. Smith’s report at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616) acknowledged that STS risk scores of 4%-8% place the enrolled patients into the upper 20% for risk of all U.S. patients who undergo surgical aortic-valve replacement
“I would characterize the enrolled patients as ‘less high risk’ rather than intermediate risk,” said Dr. Pinto.
But as Dr. Smith explained “even if the enrolled patients are not ‘intermediate’ risk they are at a different risk level” than were the patients enrolled in the prior TAVR randomized trials.
In the PARTNER 1 high-risk trial, the overall 1-year rate of all-cause mortality was 24% and 27% in the TAVR and surgical arms of the study, respectively. In the CoreValve trial these rates were 14% with TAVR and 19% with surgery. In PARTNER 2A 1-year all-cause mortality was 12% with TAVR and 13% with surgery.
Two other notable findings of PARTNER 2A were the superior outcomes of patients who underwent TAVR using a transfemoral approach, and the improved outcomes that all TAVR patients had compared with surgical valve replacement for several secondary outcomes.
The rate of the study’s primary outcome, all-cause death or disabling stroke after 2 years, was cut by a relative 21% in the 77% of TAVR patients who underwent a transfemoral procedure, compared with the surgery patients, a difference that was of borderline statistical significance. In contrast, the entire group of TAVR patients, including those treated via nontransfemoral routes, had an 11% relative reduction of the primary endpoint, compared with surgery, a difference that was not statistically significant but did easily meet the study’s prespecified definition of noninferority. Dr. Smith and others were especially encouraged by these findings as PARTNER 2A used the older Sapien XT TAVR system that is not often used today in U.S. practice. When U.S. patients undergo TAVR with a balloon-expandable valve they most often receive treatment with the S3 system, much smaller than XT and hence much more likely to be used with a transfemoral approach.
Other secondary outcomes included life-threatening or disabling bleeding events, which after 2 years had occurred in 17% of the TAVR patients and 47% of those who underwent surgery; atrial fibrillation, which occurred in 11% of the TAVR patients and 27% of those undergoing surgery; and acute kidney injury which occurred in 4% of TAVR patients and 6% of the surgery patients. With 2-year follow-up, the rate of disabling strokes was 6% in both arms of the study.
PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
On Twitter @mitchelzoler
CHICAGO – Transcatheter aortic-valve replacement performed as well as surgical-valve replacement in patients with an intermediate mortality risk in a prospective, randomized trial with more than 2,000 patients followed for 2 years, the first randomized trial to compare the efficacy and safety of transcatheter aortic-valve replacement against surgical replacement in patients who did not have a high mortality risk.
The results “support TAVR [transcatheter aortic-valve replacement] as an alternative to surgery in intermediate-risk patients similar to those included in this trial,” said Dr. Craig R. Smith at the annual meeting of the American College of Cardiology. The findings from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A trial “will increase use of TAVR,” predicted Dr. Smith, professor and chairman of surgery at New York–Presbyterian Hospital/Columbia University in New York.
Until now, TAVR had been compared with surgical aortic-valve replacement in two prospective, randomized trials that both enrolled either high-risk or inoperable patients with severe aortic stenosis, the PARTNER 1 trial that tested the original Sapien TAVR system, and the U.S. CoreValve High-Risk Study that tested the original CoreValve system (often now called CoreValve classic). The average Society of Thoracic Surgeons (STS) operative risk score of high-risk patients enrolled in PARTNER 1 was 11.8%, and the average risk score in patients enrolled in the CoreValve study was 7.3%. In contrast, the design of PARTNER 2A specified that enrolled patients have a STS risk score of 4%-8%, a criterion actually met by 81% of the enrolled patients, and the average STS risk score of all patients enrolled in PARTNER 2A was 5.8%.
Although U.S. labeling for both the Sapien valve and its later iterations, Sapien XT and S3, and for CoreValve and its later iteration, Evolut R, specify that treated patients should have a STS risk score of at least 8%, the labeling also gives the heart teams that perform TAVR the latitude to treat patients with risk scores below 8% when the heart teams identify other patient factors that confer high risk such as frailty or comorbidities. U.S. and European TAVR registries have documented that many patients with STS risk scores below 8% have undergone TAVR since these systems received regulatory approval. The new results from PARTNER 2A may change that by leading to revised labeling that cuts the STS risk-score threshold.
“These findings might lead to a labeling change that would avoid a lot of the patient-evaluation gymnastics that have been used to justify” TAVR treatment, noted Dr. Smith. New labeling like this “would sanction what is already going on” in terms of which patients undergo TAVR.
Others who heard these results at the meeting agreed they were an important milestone in TAVR development and its expanding use.
The new results “make a huge difference,” commented Dr. David R. Holmes Jr., an interventional cardiologist and professor at the Mayo Clinic in Rochester, Minn. “We base many of our guidelines on the results from randomized, controlled trials. It’s true that there are reports of lower-risk patients undergoing TAVR, but we now have results from a well-designed trial with well-controlled and adjudicated endpoints that documents the safety and efficacy of TAVR in intermediate-risk patients,” Dr. Holmes said in an interview.
“The results will have a very important influence on the choice between TAVR and surgery,” commented Dr. Duane S. Pinto, an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “It validates the strategy” of using TAVR in patients with a risk score of 4%-8%. “TAVR has already been used in these patients, but these results validate this, especially when used in a transfemoral approach,” Dr. Pinto said in an interview.
One aspect of PARTNER 2A that received a lot of discussion at the meeting was whether enrolled patients could appropriately be characterized as “intermediate” in their risk level. Although their average STS risk score of 5.8% fell squarely within the target range specified for the study, they averaged 82 years old, and other clinical features at baseline suggested a higher risk population. The published report of the PARTNER 2A results that appeared online concurrent with Dr. Smith’s report at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616) acknowledged that STS risk scores of 4%-8% place the enrolled patients into the upper 20% for risk of all U.S. patients who undergo surgical aortic-valve replacement
“I would characterize the enrolled patients as ‘less high risk’ rather than intermediate risk,” said Dr. Pinto.
But as Dr. Smith explained “even if the enrolled patients are not ‘intermediate’ risk they are at a different risk level” than were the patients enrolled in the prior TAVR randomized trials.
In the PARTNER 1 high-risk trial, the overall 1-year rate of all-cause mortality was 24% and 27% in the TAVR and surgical arms of the study, respectively. In the CoreValve trial these rates were 14% with TAVR and 19% with surgery. In PARTNER 2A 1-year all-cause mortality was 12% with TAVR and 13% with surgery.
Two other notable findings of PARTNER 2A were the superior outcomes of patients who underwent TAVR using a transfemoral approach, and the improved outcomes that all TAVR patients had compared with surgical valve replacement for several secondary outcomes.
The rate of the study’s primary outcome, all-cause death or disabling stroke after 2 years, was cut by a relative 21% in the 77% of TAVR patients who underwent a transfemoral procedure, compared with the surgery patients, a difference that was of borderline statistical significance. In contrast, the entire group of TAVR patients, including those treated via nontransfemoral routes, had an 11% relative reduction of the primary endpoint, compared with surgery, a difference that was not statistically significant but did easily meet the study’s prespecified definition of noninferority. Dr. Smith and others were especially encouraged by these findings as PARTNER 2A used the older Sapien XT TAVR system that is not often used today in U.S. practice. When U.S. patients undergo TAVR with a balloon-expandable valve they most often receive treatment with the S3 system, much smaller than XT and hence much more likely to be used with a transfemoral approach.
Other secondary outcomes included life-threatening or disabling bleeding events, which after 2 years had occurred in 17% of the TAVR patients and 47% of those who underwent surgery; atrial fibrillation, which occurred in 11% of the TAVR patients and 27% of those undergoing surgery; and acute kidney injury which occurred in 4% of TAVR patients and 6% of the surgery patients. With 2-year follow-up, the rate of disabling strokes was 6% in both arms of the study.
PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
On Twitter @mitchelzoler
AT ACC 16
Key clinical point: Intermediate-risk patients who had transcatheter aortic valve replacement had a 2-year death or disabling stroke rate that was no different from patients who had surgical valve replacement.
Major finding: The 2-year rate of death or disabling stroke was 19% with transcatheter valve replacement and 21% with surgical replacement.
Data source: PARTNER 2A, a prospective, multicenter, North American trial with 2,032 enrolled patients with severe aortic-valve stenosis.
Disclosures: PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
VIDEO: PARTNER 2 results extend TAVR to intermediate-risk patients
CHICAGO – The “groundbreaking” results from the PARTNER 2 trial of transcatheter aortic valve replacement in patients at intermediate risk for death or complications from surgical replacement of a stenotic aortic valve provide substantially increased certainty that the transcatheter approach is at least as good as surgical valve replacement, commented Dr. Roxana Mehran in a video interview at the annual meeting of the American College of Cardiology.
In addition to providing important new data on the equivalence of surgical and transcatheter aortic valve replacement (TAVR) in a new target population--intermediate-risk patients, the findings from the randomized, multicenter PARTNER trial, which enrolled 2,032 patients, may also lead to expansion of the Food and Drug Administration labeling of the balloon-expandable TAVR device used in the study, the Sapien XT, to intermediate-risk patients. This expanded indication may also apply to the newer, smaller and more advanced iteration of this device, the Sapien 3, which has largely replaced the XT in U.S. practice, Dr. Mehran suggested.
Although the results came from the XT TAVR system, “these results have big relevance” to current U.S. practice said Dr. Mehran, professor and director of interventional cardiovascular research at Mount Sinai Hospital in New York.
The primary outcome of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial showed that all-cause mortality or disabling stroke after 2 years follow-up occurred in 21.2% (695) of the surgery patients and 19.3% (775) of the TAVR patients, meeting the prespecified noninferiority endpoint, with a P value of .001. Full results from the study were published online concurrent with their presentation at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616).
Dr. Mehran has received consultant fees and honoraria from Abbott Vascular, AstraZeneca, and Bayer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
CHICAGO – The “groundbreaking” results from the PARTNER 2 trial of transcatheter aortic valve replacement in patients at intermediate risk for death or complications from surgical replacement of a stenotic aortic valve provide substantially increased certainty that the transcatheter approach is at least as good as surgical valve replacement, commented Dr. Roxana Mehran in a video interview at the annual meeting of the American College of Cardiology.
In addition to providing important new data on the equivalence of surgical and transcatheter aortic valve replacement (TAVR) in a new target population--intermediate-risk patients, the findings from the randomized, multicenter PARTNER trial, which enrolled 2,032 patients, may also lead to expansion of the Food and Drug Administration labeling of the balloon-expandable TAVR device used in the study, the Sapien XT, to intermediate-risk patients. This expanded indication may also apply to the newer, smaller and more advanced iteration of this device, the Sapien 3, which has largely replaced the XT in U.S. practice, Dr. Mehran suggested.
Although the results came from the XT TAVR system, “these results have big relevance” to current U.S. practice said Dr. Mehran, professor and director of interventional cardiovascular research at Mount Sinai Hospital in New York.
The primary outcome of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial showed that all-cause mortality or disabling stroke after 2 years follow-up occurred in 21.2% (695) of the surgery patients and 19.3% (775) of the TAVR patients, meeting the prespecified noninferiority endpoint, with a P value of .001. Full results from the study were published online concurrent with their presentation at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616).
Dr. Mehran has received consultant fees and honoraria from Abbott Vascular, AstraZeneca, and Bayer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
CHICAGO – The “groundbreaking” results from the PARTNER 2 trial of transcatheter aortic valve replacement in patients at intermediate risk for death or complications from surgical replacement of a stenotic aortic valve provide substantially increased certainty that the transcatheter approach is at least as good as surgical valve replacement, commented Dr. Roxana Mehran in a video interview at the annual meeting of the American College of Cardiology.
In addition to providing important new data on the equivalence of surgical and transcatheter aortic valve replacement (TAVR) in a new target population--intermediate-risk patients, the findings from the randomized, multicenter PARTNER trial, which enrolled 2,032 patients, may also lead to expansion of the Food and Drug Administration labeling of the balloon-expandable TAVR device used in the study, the Sapien XT, to intermediate-risk patients. This expanded indication may also apply to the newer, smaller and more advanced iteration of this device, the Sapien 3, which has largely replaced the XT in U.S. practice, Dr. Mehran suggested.
Although the results came from the XT TAVR system, “these results have big relevance” to current U.S. practice said Dr. Mehran, professor and director of interventional cardiovascular research at Mount Sinai Hospital in New York.
The primary outcome of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial showed that all-cause mortality or disabling stroke after 2 years follow-up occurred in 21.2% (695) of the surgery patients and 19.3% (775) of the TAVR patients, meeting the prespecified noninferiority endpoint, with a P value of .001. Full results from the study were published online concurrent with their presentation at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616).
Dr. Mehran has received consultant fees and honoraria from Abbott Vascular, AstraZeneca, and Bayer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT ACC 16
Gynecologic oncologists often missing from pediatric pelvic evaluations
SAN DIEGO – Young women and girls with gynecologic malignancies more often present with pain and masses greater than 5 cm in size, compared with their counterparts who have benign disease. Additionally, gynecologic oncologists are inconsistently involved in the management of this patient population.
Those are key findings from a study that set out to compare the clinical presentation and surgical outcomes of women and girls younger than 21 years old who had a pelvic mass.
“If something is suspicious, it’s not a bad idea to get your colleagues who specialize in gynecologic cancer involved sooner rather than later,” Dr. Teuta Shemshedini, the lead study author, said in an interview at the annual meeting of the Society of Gynecologic Oncology. Clinicians who specialize in gynecologic oncology “were often talked to either intraoperatively or postoperatively, so we were kind of working backwards when we could have sat with patients and the families before the surgery and worked forward.”
Dr. Shemshedini, who is a fourth-year resident in the department of obstetrics and gynecology at Westchester Medical Center, Valhalla, N.Y., and her associates reviewed medical records of all women and girls younger than 21 years old who underwent primary surgery for a pelvic mass at the medical center from 2010 to 2015.
Of the 138 patients evaluated, 77 were included in the final analysis: 57 who had benign disease and 20 who had malignant disease. The mean age of the patients was 13.5 years and the mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P = .005). The most common presentation was pain, which occurred in 75% of all cases.
Gynecologic oncologists were consulted on 10 cases (13%), with six of the 10 consults (60%) requested by pediatric gynecologists. However, only two of eight (25%) were preoperative consults in malignant cases.
The researchers also observed that tumors greater than 10 cm in size were found in 75% of malignancies, and all tumors 5 cm or smaller were benign (14%). Clinicians did not use tumor markers in 29% of the entire study group, even though tumor markers were elevated in 70% of the malignant cases.
Laparoscopic surgery was performed in 35 patients (45%), with a majority of cases being benign. The most common benign tumors were mature teratomas (70%). The most common malignant tumors were borderline ovarian tumors (35%), followed by immature teratomas (20%), and mixed germ cell tumors (20%). More than half of malignant tumors (55%) were stage I.
“The most surprising part was that we weren’t getting gynecologic oncology involved soon enough,” Dr. Shemshedini said. “I think most people are very surprised when a mass comes back as cancer in kids, especially ovarian cancer. In adults we see epithelial cancer most commonly, while in kids it’s more of the germ cell tumors. Those are rare.”
Dr. Shemshedini reported having no financial disclosures.
SAN DIEGO – Young women and girls with gynecologic malignancies more often present with pain and masses greater than 5 cm in size, compared with their counterparts who have benign disease. Additionally, gynecologic oncologists are inconsistently involved in the management of this patient population.
Those are key findings from a study that set out to compare the clinical presentation and surgical outcomes of women and girls younger than 21 years old who had a pelvic mass.
“If something is suspicious, it’s not a bad idea to get your colleagues who specialize in gynecologic cancer involved sooner rather than later,” Dr. Teuta Shemshedini, the lead study author, said in an interview at the annual meeting of the Society of Gynecologic Oncology. Clinicians who specialize in gynecologic oncology “were often talked to either intraoperatively or postoperatively, so we were kind of working backwards when we could have sat with patients and the families before the surgery and worked forward.”
Dr. Shemshedini, who is a fourth-year resident in the department of obstetrics and gynecology at Westchester Medical Center, Valhalla, N.Y., and her associates reviewed medical records of all women and girls younger than 21 years old who underwent primary surgery for a pelvic mass at the medical center from 2010 to 2015.
Of the 138 patients evaluated, 77 were included in the final analysis: 57 who had benign disease and 20 who had malignant disease. The mean age of the patients was 13.5 years and the mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P = .005). The most common presentation was pain, which occurred in 75% of all cases.
Gynecologic oncologists were consulted on 10 cases (13%), with six of the 10 consults (60%) requested by pediatric gynecologists. However, only two of eight (25%) were preoperative consults in malignant cases.
The researchers also observed that tumors greater than 10 cm in size were found in 75% of malignancies, and all tumors 5 cm or smaller were benign (14%). Clinicians did not use tumor markers in 29% of the entire study group, even though tumor markers were elevated in 70% of the malignant cases.
Laparoscopic surgery was performed in 35 patients (45%), with a majority of cases being benign. The most common benign tumors were mature teratomas (70%). The most common malignant tumors were borderline ovarian tumors (35%), followed by immature teratomas (20%), and mixed germ cell tumors (20%). More than half of malignant tumors (55%) were stage I.
“The most surprising part was that we weren’t getting gynecologic oncology involved soon enough,” Dr. Shemshedini said. “I think most people are very surprised when a mass comes back as cancer in kids, especially ovarian cancer. In adults we see epithelial cancer most commonly, while in kids it’s more of the germ cell tumors. Those are rare.”
Dr. Shemshedini reported having no financial disclosures.
SAN DIEGO – Young women and girls with gynecologic malignancies more often present with pain and masses greater than 5 cm in size, compared with their counterparts who have benign disease. Additionally, gynecologic oncologists are inconsistently involved in the management of this patient population.
Those are key findings from a study that set out to compare the clinical presentation and surgical outcomes of women and girls younger than 21 years old who had a pelvic mass.
“If something is suspicious, it’s not a bad idea to get your colleagues who specialize in gynecologic cancer involved sooner rather than later,” Dr. Teuta Shemshedini, the lead study author, said in an interview at the annual meeting of the Society of Gynecologic Oncology. Clinicians who specialize in gynecologic oncology “were often talked to either intraoperatively or postoperatively, so we were kind of working backwards when we could have sat with patients and the families before the surgery and worked forward.”
Dr. Shemshedini, who is a fourth-year resident in the department of obstetrics and gynecology at Westchester Medical Center, Valhalla, N.Y., and her associates reviewed medical records of all women and girls younger than 21 years old who underwent primary surgery for a pelvic mass at the medical center from 2010 to 2015.
Of the 138 patients evaluated, 77 were included in the final analysis: 57 who had benign disease and 20 who had malignant disease. The mean age of the patients was 13.5 years and the mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P = .005). The most common presentation was pain, which occurred in 75% of all cases.
Gynecologic oncologists were consulted on 10 cases (13%), with six of the 10 consults (60%) requested by pediatric gynecologists. However, only two of eight (25%) were preoperative consults in malignant cases.
The researchers also observed that tumors greater than 10 cm in size were found in 75% of malignancies, and all tumors 5 cm or smaller were benign (14%). Clinicians did not use tumor markers in 29% of the entire study group, even though tumor markers were elevated in 70% of the malignant cases.
Laparoscopic surgery was performed in 35 patients (45%), with a majority of cases being benign. The most common benign tumors were mature teratomas (70%). The most common malignant tumors were borderline ovarian tumors (35%), followed by immature teratomas (20%), and mixed germ cell tumors (20%). More than half of malignant tumors (55%) were stage I.
“The most surprising part was that we weren’t getting gynecologic oncology involved soon enough,” Dr. Shemshedini said. “I think most people are very surprised when a mass comes back as cancer in kids, especially ovarian cancer. In adults we see epithelial cancer most commonly, while in kids it’s more of the germ cell tumors. Those are rare.”
Dr. Shemshedini reported having no financial disclosures.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Among young women and girls with pelvic malignancies, the mass is often greater than 5 cm in size.
Major finding: The mean adnexal mass size was 9.8 cm in the benign group, compared with 15.5 cm in the malignant group (P =.005).
Data source: A review of medical records from 77 women and girls younger than 21 years old who underwent primary surgery for a pelvic mass from 2010 to 2015.
Disclosures: Dr. Shemshedini reported having no financial disclosures.
VIDEO: Anesthesia services during colonoscopy increase risk of near-term complications
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Using anesthesia services on individuals receiving colonoscopy increases the overall risk of complications associated with the procedure.
Major finding: Colonoscopy patients who received anesthesia had a 13% higher risk of complication within 30 days, including perforation, hemorrhage, abdominal pain, and stroke.
Data source: A prospective cohort study of claims data from 3,168,228 colonoscopy procedures in the Truven Health MarketScan Research Databases from 2008 to 2011.
Disclosures: Funding provided by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Carpal tunnel syndrome: Guidelines rate evidence for diagnosis, treatment
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.
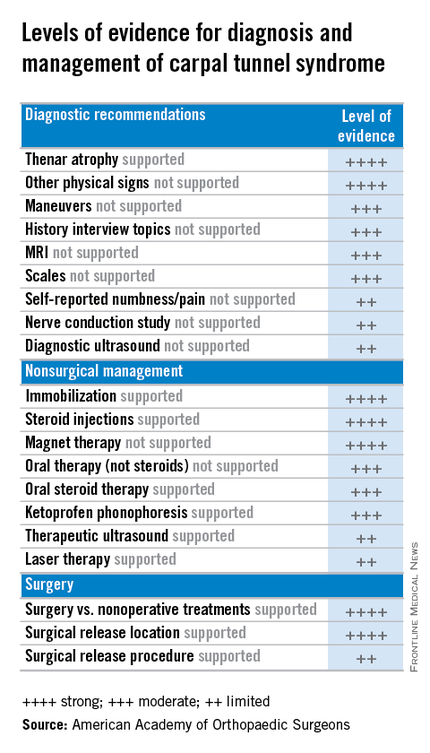
Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
Study ranks risk factors for cervical cancer recurrence
SAN DIEGO – Among patients with stage IB cervical cancer, deep stromal invasion and large tumor size are the two biggest factors associated with increased risk of recurrence. Higher risk-weighted surgical-pathological scores were also associated with decreased benefit of concurrent chemoradiotherapy after surgery.
Those are key findings from a Gynecologic Oncology Group (GOG) ancillary data analysis reported by Dr. Koji Matsuo at the annual meeting of the Society of Gynecologic Oncology.
“Surgery remains the mainstay of treatment for early-stage cervical cancer,” Dr. Matsuo, assistant director of the gynecologic oncology clinic at the University of Southern California, Los Angeles, said in an interview prior to the meeting. “Surgical specimen is useful to identify certain types of factors that can benefit from postoperative adjuvant therapy with concurrent chemoradiotherapy. Traditionally, tumor factors are grouped into high, intermediate, and low risk. Tumors often exhibit multiple risk factors and magnitude of significance for survival may differ across the tumor factors. In this study, we examined the effects of combination of multiple risk factors by weighing magnitude of significance for recurrence.”
The researchers analyzed data from 1,538 stage IB cervical cancer patients who underwent primary radical hysterectomy and pelvic lymphadenectomy. They used a multivariate model to examine hazard ratios associated with disease-free survival (DFS) for seven surgical-pathological risk factors: nodal metastasis, parametrial involvement, surgical margin, lymphovascular space invasion (LVSI), deep stromal invasion, large tumor, and histology. Next, they used a risk-weighted surgical-pathological score (a sum of HR scores) to determine DFS and compared it to a traditional risk factor model.
The median age of patients in the study was 41 years, the median follow-up time was 84 months, the recurrence rate was 26%, and the mortality rate was 27%.
Dr. Matsuo reported that based on the risk-weighted surgical-pathological score model, factors associated with the highest risk of recurrence were deep stromal invasion (HR 1.85), large tumor size (HR 1.81), parametrial involvement (HR 1.73), LVSI (HR 1.37), histology (HR 1.30), and nodal metastasis (HR 1.29; P less than .05 for all).
The 5-year DFS rates based on risk-weighted scores were 85.6% for score 0, 89.1% for the first quartile, 79.6% for the second quartile, 69.3% for the third quartile, and 50.2% for fourth quartile (P less than .001). A fourth-quartile score in the risk-weighted model had a significantly lower 5-year DFS rate, compared with the traditional risk factor model high-risk group (50.2% vs. 60.9%; P less than .001).
Dr. Matsuo and his associates also found that higher risk-weighted surgical-pathological scores were associated with decreased benefit of concurrent chemoradiotherapy after surgery.
“That has been the mainstay of postoperative treatment in adjuvant therapy for a group of cervical cancer with high risk of recurrence,” he said. “It is beneficial to be aware that each tumor factor has a different risk for recurrence and tumors may exhibit multiple risk factors that can be associated with decreased benefit of concurrent chemoradiotherapy after surgical treatment.”
He acknowledged certain limitations of the study, including the lack of information regarding the site of recurrence.
Dr. Matsuo reported having no financial disclosures.
SAN DIEGO – Among patients with stage IB cervical cancer, deep stromal invasion and large tumor size are the two biggest factors associated with increased risk of recurrence. Higher risk-weighted surgical-pathological scores were also associated with decreased benefit of concurrent chemoradiotherapy after surgery.
Those are key findings from a Gynecologic Oncology Group (GOG) ancillary data analysis reported by Dr. Koji Matsuo at the annual meeting of the Society of Gynecologic Oncology.
“Surgery remains the mainstay of treatment for early-stage cervical cancer,” Dr. Matsuo, assistant director of the gynecologic oncology clinic at the University of Southern California, Los Angeles, said in an interview prior to the meeting. “Surgical specimen is useful to identify certain types of factors that can benefit from postoperative adjuvant therapy with concurrent chemoradiotherapy. Traditionally, tumor factors are grouped into high, intermediate, and low risk. Tumors often exhibit multiple risk factors and magnitude of significance for survival may differ across the tumor factors. In this study, we examined the effects of combination of multiple risk factors by weighing magnitude of significance for recurrence.”
The researchers analyzed data from 1,538 stage IB cervical cancer patients who underwent primary radical hysterectomy and pelvic lymphadenectomy. They used a multivariate model to examine hazard ratios associated with disease-free survival (DFS) for seven surgical-pathological risk factors: nodal metastasis, parametrial involvement, surgical margin, lymphovascular space invasion (LVSI), deep stromal invasion, large tumor, and histology. Next, they used a risk-weighted surgical-pathological score (a sum of HR scores) to determine DFS and compared it to a traditional risk factor model.
The median age of patients in the study was 41 years, the median follow-up time was 84 months, the recurrence rate was 26%, and the mortality rate was 27%.
Dr. Matsuo reported that based on the risk-weighted surgical-pathological score model, factors associated with the highest risk of recurrence were deep stromal invasion (HR 1.85), large tumor size (HR 1.81), parametrial involvement (HR 1.73), LVSI (HR 1.37), histology (HR 1.30), and nodal metastasis (HR 1.29; P less than .05 for all).
The 5-year DFS rates based on risk-weighted scores were 85.6% for score 0, 89.1% for the first quartile, 79.6% for the second quartile, 69.3% for the third quartile, and 50.2% for fourth quartile (P less than .001). A fourth-quartile score in the risk-weighted model had a significantly lower 5-year DFS rate, compared with the traditional risk factor model high-risk group (50.2% vs. 60.9%; P less than .001).
Dr. Matsuo and his associates also found that higher risk-weighted surgical-pathological scores were associated with decreased benefit of concurrent chemoradiotherapy after surgery.
“That has been the mainstay of postoperative treatment in adjuvant therapy for a group of cervical cancer with high risk of recurrence,” he said. “It is beneficial to be aware that each tumor factor has a different risk for recurrence and tumors may exhibit multiple risk factors that can be associated with decreased benefit of concurrent chemoradiotherapy after surgical treatment.”
He acknowledged certain limitations of the study, including the lack of information regarding the site of recurrence.
Dr. Matsuo reported having no financial disclosures.
SAN DIEGO – Among patients with stage IB cervical cancer, deep stromal invasion and large tumor size are the two biggest factors associated with increased risk of recurrence. Higher risk-weighted surgical-pathological scores were also associated with decreased benefit of concurrent chemoradiotherapy after surgery.
Those are key findings from a Gynecologic Oncology Group (GOG) ancillary data analysis reported by Dr. Koji Matsuo at the annual meeting of the Society of Gynecologic Oncology.
“Surgery remains the mainstay of treatment for early-stage cervical cancer,” Dr. Matsuo, assistant director of the gynecologic oncology clinic at the University of Southern California, Los Angeles, said in an interview prior to the meeting. “Surgical specimen is useful to identify certain types of factors that can benefit from postoperative adjuvant therapy with concurrent chemoradiotherapy. Traditionally, tumor factors are grouped into high, intermediate, and low risk. Tumors often exhibit multiple risk factors and magnitude of significance for survival may differ across the tumor factors. In this study, we examined the effects of combination of multiple risk factors by weighing magnitude of significance for recurrence.”
The researchers analyzed data from 1,538 stage IB cervical cancer patients who underwent primary radical hysterectomy and pelvic lymphadenectomy. They used a multivariate model to examine hazard ratios associated with disease-free survival (DFS) for seven surgical-pathological risk factors: nodal metastasis, parametrial involvement, surgical margin, lymphovascular space invasion (LVSI), deep stromal invasion, large tumor, and histology. Next, they used a risk-weighted surgical-pathological score (a sum of HR scores) to determine DFS and compared it to a traditional risk factor model.
The median age of patients in the study was 41 years, the median follow-up time was 84 months, the recurrence rate was 26%, and the mortality rate was 27%.
Dr. Matsuo reported that based on the risk-weighted surgical-pathological score model, factors associated with the highest risk of recurrence were deep stromal invasion (HR 1.85), large tumor size (HR 1.81), parametrial involvement (HR 1.73), LVSI (HR 1.37), histology (HR 1.30), and nodal metastasis (HR 1.29; P less than .05 for all).
The 5-year DFS rates based on risk-weighted scores were 85.6% for score 0, 89.1% for the first quartile, 79.6% for the second quartile, 69.3% for the third quartile, and 50.2% for fourth quartile (P less than .001). A fourth-quartile score in the risk-weighted model had a significantly lower 5-year DFS rate, compared with the traditional risk factor model high-risk group (50.2% vs. 60.9%; P less than .001).
Dr. Matsuo and his associates also found that higher risk-weighted surgical-pathological scores were associated with decreased benefit of concurrent chemoradiotherapy after surgery.
“That has been the mainstay of postoperative treatment in adjuvant therapy for a group of cervical cancer with high risk of recurrence,” he said. “It is beneficial to be aware that each tumor factor has a different risk for recurrence and tumors may exhibit multiple risk factors that can be associated with decreased benefit of concurrent chemoradiotherapy after surgical treatment.”
He acknowledged certain limitations of the study, including the lack of information regarding the site of recurrence.
Dr. Matsuo reported having no financial disclosures.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Deep stromal invasion and large tumor size are two risk factors associated with increased risk of stage IB cervical cancer recurrence.
Major finding: A risk-weighted model for determining recurrence of cervical cancer had a significantly lower 5-year disease-free survival rate, compared with the traditional high-risk group (50.2% vs. 60.9%; P less than .001).
Data source: An ancillary analysis of Gynecologic Oncology Group data from 1,538 stage IB cervical cancer patients who underwent primary radical hysterectomy and pelvic lymphadenectomy.
Disclosures: Dr. Matsuo reported having no financial disclosures.