User login
Official Newspaper of the American College of Surgeons
Elective CRC resections increase with universal insurance
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
BOSTON – Expanding access to health insurance for low- and moderate-income families has apparently improved colorectal cancer care in Massachusetts, and may do the same for other states that participate in Medicaid expansion under the Affordable Care Act.
That assertion comes from investigators at Massachusetts General Hospital in Boston. They found that following the introduction in 2006 of a universal health insurance law in the Bay State – the law that would serve as a model for the Affordable Care Act – the rate of elective colorectal resections increased while the rate of emergent resections decreased.
In contrast, in three states used as controls, the opposite occurred.
“This could be due to a variety of different factors, including earlier diagnosis, presenting with disease more amenable to surgical resection. It could also be due to increased referrals from primary care providers or GI doctors,” said Dr. Andrew P. Loehrer from the Massachusetts General Hospital Department of Surgery, at the annual Society of Surgical Oncology Cancer Symposium.
He acknowledged, however, that the administrative dataset he and his colleagues used in the study lacks information about clinical staging or use of neoadjuvant therapy, making it difficult to determine whether insured patients actually present at an earlier, more readily treatable disease stage.
Nonetheless, “from a cancer standpoint, my study provides early, hopeful evidence. In order to definitively say that this improves care, we need to have some more of the cancer-specific variables, but with this study, combined with some other work that we and other groups have done, we see that patients in Massachusetts are presenting with earlier stage disease, whether it’s acute disease or cancer, and they’re getting more appropriate care in a more timely fashion,” he said in an interview.
Role model
Dr. Loehrer noted that disparities in access to health care have been shown in previous studies to be associated with the likelihood of unfavorable outcomes for patients with colorectal cancer. For example, a 2008 study (Lancet Oncol. 2008 Mar;9:222-31) showed that uninsured patients with colorectal cancer had a twofold greater risk for presenting with advanced disease than privately insured patients. Additionally, a 2004 study (Br J Surg. 91:605-9) showed that patients who presented with colorectal cancer requiring emergent resection had significantly lower 5-year overall survival than patients who underwent elective resection.
Massachusetts implemented its pioneering health insurance reform law in 2006. The law increased eligibility for persons with incomes up to 150% of the Federal Poverty Level, created government-subsidized insurance for those with incomes from 150% to 300% of the poverty line, mandated that all Bay State residents have some form of health insurance, and allowed young adults up to the age of 26 to remain on their parents’ plans.
To see whether insurance reform could have a salutary effect on cancer care, the investigators drew on Agency for Health Research and Quality (AHRQ) State Inpatient Databases for Massachusetts and for Florida, New Jersey, and New York as control states. They used ICD-9 diagnosis codes to identify patients with colorectal cancer, including those who underwent resection.
To establish procedure rates, they used U.S. Census Bureau data to establish the population of denominators, which included all adults 18-54 years of age who were insured either through Medicaid, Commonwealth Care (in Massachusetts), or were listed as uninsured or self-pay. Medicare-insured patients were not included, as they were not directly affected by the reform law.
They identified 18,598 patients admitted to Massachusetts hospitals for colorectal cancer from 2001 through 2011, and 147,482 admitted during the same period to hospitals in the control states.
The authors created Poisson difference-in-differences models which compare changes in the selected outcomes in Massachusetts with changes in the control states. The models were adjusted for age, sex, race, hospital type, and secular trends.
They found that admission rates for colorectal cancer increased over time in Massachusetts by 13.3 per 100,000 residents per quarter, compared with 8.3/100,000 in the control states, translating into an adjusted rate ratio (ARR) of 1.13. Resection rates for cancer, the primary study outcome, also grew by a significantly larger margin in Massachusetts, by 5.5/100,000, compared with 0.5/100,000 in control states, with an ARR of 1.37 (P less than .001 for both comparisons).
For the secondary outcome of changes in emergent and elective resections after admission, they found that emergent surgeries in Massachusetts declined by 2.7/100,000, but increased by 4.4/100,000 in the states without insurance reform. Similarly, elective resections after admission increased in the Bay State by 7.4/100,000, but decreased by 1.8/100,000 in control states.
Relative to controls, the adjusted probability that a patient with colorectal cancer in Massachusetts would have emergent surgery after admission declined by 6.1% (P = .014) and the probability that he or she would have elective resection increased by 7.8% (P = .005).
An analysis of the odds ratio of resection during admission, adjusted for age, race, presentation with metastatic disease, hospital type, and secular trends, showed that prior to reform uninsured patients in both Massachusetts and control states were significantly less likely than privately insured patients to have resections (odds ratio, 0.42 in Mass.; 0.45 in control states).
However, after the implementation of reform the gap between previously uninsured and privately insured in Massachusetts narrowed (OR, 0.63) but remained the same in control states (OR, 0.44).
Dr. Loehrer acknowledged in an interview that Massachusetts differs from other states in some regards, including in concentrations of health providers and in requirements for insurance coverage that were in place even before the 2006 reforms, but is optimistic that improvements in colorectal cancer care can occur in states that have embraced the Affordable Care Act.
“There are a lot of services that were available and we had high colonoscopy rates prior to all of this, but that said, the mechanism is exactly the same, there are still vulnerable populations, and at this point I think it’s hopeful and promising that we will see similar results in other states,” he said.
FROM SSO 2016
Key clinical point: Outcomes for patients with colorectal cancer (CRC) who undergo elective resection are better than for those who require emergent resections.
Major finding: Elective CRC resection rates increased and emergent resections decreased after universal insurance was instituted in Massachusetts in 2006.
Data source: Retrospective study comparing differences over time between CRC resection rates in Massachusetts vs. those in Florida, New Jersey, and New York.
Disclosures: The study was supported in part by a grant from the National Institute on Aging. Dr. Loehrer and his coauthors reported no conflicts of interest.
Risk-prediction tool for early TAVR mortality
Experts in the Society for Thoracic Surgeons and the American College of Cardiology used data from more than 13,000 consecutive transcatheter aortic valve replacement procedures to develop a new tool for predicting the risk of in-hospital mortality in patients undergoing TAVR, according to a report published online March 9 in JAMA Cardiology.
Their risk-prediction model was only “modestly” accurate but performed better than any existing methods for assessing risk in this patient population. It should be considered the first iteration of this tool and will be modified as the procedure itself evolves and as more data concerning TAVR are collected and analyzed. Ongoing analysis “may well define clinical subsets of patients who accrue particular benefit from the procedure or, conversely, reveal subsets not well served by TAVR,” said Dr. Fred H. Edwards and his associates on the steering committee of the STS/ACC Transcatheter Valve Therapy Registry.
They noted that more models soon will be developed to predict 30-day and 1-year mortality after TAVR. Models to predict the risk of neurologic deficit following TAVR are currently being developed, and models for other nonfatal outcomes will be developed soon.
This tool predicting in-hospital mortality is expected to become “a valuable adjunct for patient counseling, performance assessment, local quality improvement, and national monitoring of the appropriateness of patient selection for TAVR,” said Dr. Edwards, who is also in the department of surgery, University of Florida, Jacksonville, and his associates.
They began by analyzing the registry data for virtually every commercial TAVR performed at 265 participating sites in the United States during a 27-month period. In general, patients were selected for TAVR because they were considered unsuitable candidates for surgical aortic valve replacement. The mean patient age was 82.1 years. A total of 730 patients died before leaving the hospital, for an in-hospital mortality of 5.3%.
Working from an initial list of 39 possible patient variables to include in their statistical prediction model, the researchers narrowed it down to the 7 most predictive factors available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state (i.e., preprocedural acuity status), and need for a nonfemoral approach during the procedure.
The model was then tested in a separate validation cohort of 6,868 patients (52% men) treated at 314 sites during a 7-month period. It performed better at predicting in-hospital mortality than did either the EuroSCORE (European System for Cardiac Operative Risk Evaluation) or the FRANCE 2 (French Aortic National Corevalve and Edwards 2) models.
This STS/ACC model should assist clinicians in patient selection for TAVR, not by dictating which patients are candidates for TAVR, but by being used as “one element in the selection process, to be considered in concert with history, physical examination, laboratory information, and clinical judgment. The model may also provide useful information for patient counseling,” the investigators said (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2015.0326). One factor that is generally recognized as an important risk predictor but isn’t yet incorporated into this tool is a measure of patient frailty. Data on frailty are not yet collected consistently in the STS/ACC registry. As more complete data become available, frailty likely will be included as a predictive factor in this tool.
Another important issue that eventually should be considered alongside survival prediction is the effect TAVR has on quality of life. The STS/ACC registry “is one of the few clinical registries to collect quality-of-life data,” and it could prove to be a critical adjunct to patient selection. A given patient might have a favorable outlook regarding mortality after the procedure, but would still be a poor candidate if he or she wouldn’t derive significant benefit from it, Dr. Edwards and his associates said.
It is encouraging that Edwards et al. plan to refine this predictive model further, with the goal of developing a tool that provides a fuller picture of anticipated survival and functional outcomes for the TAVR population, because the demographics of this patient population are likely to change considerably in the coming years.

|
Dr. Laura Mauri |
The experience in Europe shows that TAVR is no longer reserved for high-risk patients there but is disseminating into the population at intermediate surgical risk. A similar trend is widely expected to occur in the United States after publication of favorable results from randomized clinical trials.
A reliable tool for predicting risk might eventually give providers and treatment centers a way to benchmark their current outcomes against those in the past and against those of other sites. Thus, it could serve as an instrument for continuous quality improvement for local heart care teams.
Dr. Laura Mauri and Dr. Patrick T. O’Gara are in the cardiovascular division at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported that their institution receives grants from Abbott, Boston Scientific, and Medtronic. Dr. Mauri and Dr. O’Gara made these remarks in an invited commentary accompanying Dr. Edwards’ report (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2016.0006).
It is encouraging that Edwards et al. plan to refine this predictive model further, with the goal of developing a tool that provides a fuller picture of anticipated survival and functional outcomes for the TAVR population, because the demographics of this patient population are likely to change considerably in the coming years.

|
Dr. Laura Mauri |
The experience in Europe shows that TAVR is no longer reserved for high-risk patients there but is disseminating into the population at intermediate surgical risk. A similar trend is widely expected to occur in the United States after publication of favorable results from randomized clinical trials.
A reliable tool for predicting risk might eventually give providers and treatment centers a way to benchmark their current outcomes against those in the past and against those of other sites. Thus, it could serve as an instrument for continuous quality improvement for local heart care teams.
Dr. Laura Mauri and Dr. Patrick T. O’Gara are in the cardiovascular division at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported that their institution receives grants from Abbott, Boston Scientific, and Medtronic. Dr. Mauri and Dr. O’Gara made these remarks in an invited commentary accompanying Dr. Edwards’ report (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2016.0006).
It is encouraging that Edwards et al. plan to refine this predictive model further, with the goal of developing a tool that provides a fuller picture of anticipated survival and functional outcomes for the TAVR population, because the demographics of this patient population are likely to change considerably in the coming years.

|
Dr. Laura Mauri |
The experience in Europe shows that TAVR is no longer reserved for high-risk patients there but is disseminating into the population at intermediate surgical risk. A similar trend is widely expected to occur in the United States after publication of favorable results from randomized clinical trials.
A reliable tool for predicting risk might eventually give providers and treatment centers a way to benchmark their current outcomes against those in the past and against those of other sites. Thus, it could serve as an instrument for continuous quality improvement for local heart care teams.
Dr. Laura Mauri and Dr. Patrick T. O’Gara are in the cardiovascular division at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported that their institution receives grants from Abbott, Boston Scientific, and Medtronic. Dr. Mauri and Dr. O’Gara made these remarks in an invited commentary accompanying Dr. Edwards’ report (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2016.0006).
Experts in the Society for Thoracic Surgeons and the American College of Cardiology used data from more than 13,000 consecutive transcatheter aortic valve replacement procedures to develop a new tool for predicting the risk of in-hospital mortality in patients undergoing TAVR, according to a report published online March 9 in JAMA Cardiology.
Their risk-prediction model was only “modestly” accurate but performed better than any existing methods for assessing risk in this patient population. It should be considered the first iteration of this tool and will be modified as the procedure itself evolves and as more data concerning TAVR are collected and analyzed. Ongoing analysis “may well define clinical subsets of patients who accrue particular benefit from the procedure or, conversely, reveal subsets not well served by TAVR,” said Dr. Fred H. Edwards and his associates on the steering committee of the STS/ACC Transcatheter Valve Therapy Registry.
They noted that more models soon will be developed to predict 30-day and 1-year mortality after TAVR. Models to predict the risk of neurologic deficit following TAVR are currently being developed, and models for other nonfatal outcomes will be developed soon.
This tool predicting in-hospital mortality is expected to become “a valuable adjunct for patient counseling, performance assessment, local quality improvement, and national monitoring of the appropriateness of patient selection for TAVR,” said Dr. Edwards, who is also in the department of surgery, University of Florida, Jacksonville, and his associates.
They began by analyzing the registry data for virtually every commercial TAVR performed at 265 participating sites in the United States during a 27-month period. In general, patients were selected for TAVR because they were considered unsuitable candidates for surgical aortic valve replacement. The mean patient age was 82.1 years. A total of 730 patients died before leaving the hospital, for an in-hospital mortality of 5.3%.
Working from an initial list of 39 possible patient variables to include in their statistical prediction model, the researchers narrowed it down to the 7 most predictive factors available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state (i.e., preprocedural acuity status), and need for a nonfemoral approach during the procedure.
The model was then tested in a separate validation cohort of 6,868 patients (52% men) treated at 314 sites during a 7-month period. It performed better at predicting in-hospital mortality than did either the EuroSCORE (European System for Cardiac Operative Risk Evaluation) or the FRANCE 2 (French Aortic National Corevalve and Edwards 2) models.
This STS/ACC model should assist clinicians in patient selection for TAVR, not by dictating which patients are candidates for TAVR, but by being used as “one element in the selection process, to be considered in concert with history, physical examination, laboratory information, and clinical judgment. The model may also provide useful information for patient counseling,” the investigators said (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2015.0326). One factor that is generally recognized as an important risk predictor but isn’t yet incorporated into this tool is a measure of patient frailty. Data on frailty are not yet collected consistently in the STS/ACC registry. As more complete data become available, frailty likely will be included as a predictive factor in this tool.
Another important issue that eventually should be considered alongside survival prediction is the effect TAVR has on quality of life. The STS/ACC registry “is one of the few clinical registries to collect quality-of-life data,” and it could prove to be a critical adjunct to patient selection. A given patient might have a favorable outlook regarding mortality after the procedure, but would still be a poor candidate if he or she wouldn’t derive significant benefit from it, Dr. Edwards and his associates said.
Experts in the Society for Thoracic Surgeons and the American College of Cardiology used data from more than 13,000 consecutive transcatheter aortic valve replacement procedures to develop a new tool for predicting the risk of in-hospital mortality in patients undergoing TAVR, according to a report published online March 9 in JAMA Cardiology.
Their risk-prediction model was only “modestly” accurate but performed better than any existing methods for assessing risk in this patient population. It should be considered the first iteration of this tool and will be modified as the procedure itself evolves and as more data concerning TAVR are collected and analyzed. Ongoing analysis “may well define clinical subsets of patients who accrue particular benefit from the procedure or, conversely, reveal subsets not well served by TAVR,” said Dr. Fred H. Edwards and his associates on the steering committee of the STS/ACC Transcatheter Valve Therapy Registry.
They noted that more models soon will be developed to predict 30-day and 1-year mortality after TAVR. Models to predict the risk of neurologic deficit following TAVR are currently being developed, and models for other nonfatal outcomes will be developed soon.
This tool predicting in-hospital mortality is expected to become “a valuable adjunct for patient counseling, performance assessment, local quality improvement, and national monitoring of the appropriateness of patient selection for TAVR,” said Dr. Edwards, who is also in the department of surgery, University of Florida, Jacksonville, and his associates.
They began by analyzing the registry data for virtually every commercial TAVR performed at 265 participating sites in the United States during a 27-month period. In general, patients were selected for TAVR because they were considered unsuitable candidates for surgical aortic valve replacement. The mean patient age was 82.1 years. A total of 730 patients died before leaving the hospital, for an in-hospital mortality of 5.3%.
Working from an initial list of 39 possible patient variables to include in their statistical prediction model, the researchers narrowed it down to the 7 most predictive factors available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state (i.e., preprocedural acuity status), and need for a nonfemoral approach during the procedure.
The model was then tested in a separate validation cohort of 6,868 patients (52% men) treated at 314 sites during a 7-month period. It performed better at predicting in-hospital mortality than did either the EuroSCORE (European System for Cardiac Operative Risk Evaluation) or the FRANCE 2 (French Aortic National Corevalve and Edwards 2) models.
This STS/ACC model should assist clinicians in patient selection for TAVR, not by dictating which patients are candidates for TAVR, but by being used as “one element in the selection process, to be considered in concert with history, physical examination, laboratory information, and clinical judgment. The model may also provide useful information for patient counseling,” the investigators said (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2015.0326). One factor that is generally recognized as an important risk predictor but isn’t yet incorporated into this tool is a measure of patient frailty. Data on frailty are not yet collected consistently in the STS/ACC registry. As more complete data become available, frailty likely will be included as a predictive factor in this tool.
Another important issue that eventually should be considered alongside survival prediction is the effect TAVR has on quality of life. The STS/ACC registry “is one of the few clinical registries to collect quality-of-life data,” and it could prove to be a critical adjunct to patient selection. A given patient might have a favorable outlook regarding mortality after the procedure, but would still be a poor candidate if he or she wouldn’t derive significant benefit from it, Dr. Edwards and his associates said.
FROM JAMA CARDIOLOGY
Key clinical point: The STS and ACC developed a new tool for predicting the risk of in-hospital mortality after transcatheter aortic valve replacement.
Major finding: The new model includes the seven most predictive patient variables available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state, and need for a nonfemoral approach.
Data source: An analysis of data for 13,718 consecutive TAVR patients to develop a predictive risk model, and a validation study involving 6,868 patients to test the performance of that model.
Disclosures: This study was supported by the American College of Cardiology’s National Cardiovascular Data Registry and the Society of Thoracic Surgeons. Dr. Edwards reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Ultrasound bested tomosynthesis for screening dense breast tissue
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Ultrasound outperformed tomosynthesis for the incremental detection of breast cancer in women with negative mammograms and radiologically dense breast tissue.
Major finding: Ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 tomosynthesis screens (95% CI, 1.8-6.2; P = .006).
Data source: A multicenter, prospective trial comparing the two modalities in 3,231 women.
Disclosures: The University of Genoa sponsors the ASTOUND study. Dr. Tagliafico disclosed honoraria from Esaote-Philips, patents, royalties, or other intellectual property from Springer, and travel, accommodations, and expense support from Hologic and Technologic. Four coinvestigators reported financial relationships with several device and pharmaceutical companies. The senior author and the other seven coinvestigators had no disclosures.
New prediction tool forecasts long-term ICU outcomes for very elderly
ORLANDO – A new clinical prediction rule correlates well with performance status at 1 year after ICU hospitalization in patients over age 80.
Illness severity, comorbidities, baseline frailty, a primary diagnosis of stroke, and being male were all predictors of poor performance status at 1 year. A primary diagnosis of emergency coronary artery bypass grafting or valve replacement, a high baseline performance status, and being married were associated with good performance status at 1 year. The c-statistic for the model, a standard indicator of predictive power, was 0.811, a figure that indicates good predictive ability.
The findings from the REALISTIC 80 (Realities, Expectations ,and Attitudes to Life Support Technologies in Intensive Care for Octogenarians) study of 17 patient and illness characteristics allowed Dr. Daren Heyland, professor of medicine and epidemiology at Queen’s University, Kingston, Ont., and his coinvestigators in the Canadian Critical Care Trials Group (CCCTG), to conclude that eight factors were most predictive of performance status at 12 months for ICU patients aged 80 and over. REALISTIC 80 is a CCCTG project.
The values for the predictors are derived from responses to an online guided questionnaire called the ICU Workbook. The questionnaire is completed by patients’ family members or surrogates, and the responses are used to calculate the values that constitute the clinical prediction rule’s components.
Gathering this information may help health care providers and family members in end-of-life decision making, said Dr. Heyland. “For the very elderly, it is plausible that poor communication and decision making lead to overutilization of ICU resources and poor-quality end-of-life care,” he said. “Hopefully, with these strategies, we’ll improve clinical decision making and improve the quality of end-of-life care we provide for our older patients,” said Dr. Heyland, who is also director of CARENET, which hosts the online guided questionnaire and is an affiliation of Canadian researchers focused on end-of-life care. He spoke at the Society of Critical Care Medicine’s Critical Care Congress.
REALISTIC 80 enrolled 434 patients, aged 80-100 years (mean age, 84.6) who were admitted to ICUs at participating institutions.
Previous European and U.S. studies have shown an ICU mortality of 30%-35%, and an overall mortality rate of 60%-70% in the 12 months following ICU admission. In those studies, illness severity most strongly predicted short-term survival, and comorbidities best predicted long-term survival.
The primary outcome measure of REALISTIC 80 was the 12-month survival and health-related quality of life; “recovery from critical illness” was defined as a Palliative Performance Scale (PPS) score of greater than or equal to 60% at 12 months. Patients scoring at 60% on the 0%-100% scale of this functional status measure may have reduced ambulation, be unable to engage in housework or hobbies, have significant disease, need assistance, and be confused at times. An advantage of this scale, said Dr. Heyland, is that it eliminates survivorship bias in analyzing data, since a score of 0 is assigned to individuals who die.
About 50% of patients had died by 12 months; about 21% were alive, with a reduced health status below the threshold of 60 on the PPS; and about 29% were alive, with a PPS score above the predetermined quality of life threshold.
Dr. Heyland acknowledged that the study lost a significant number of participants – about 17% – to follow-up. The predictive model was derived from completed cases, and a sensitivity analysis using imputed data for missing patients showed that it retained its predictive value.
Dr. Heyland said the presence of advance directives didn’t appear to affect outcomes. “We subsequently in a different analysis showed that whether they had [a directive] or not, did not affect subsequent process or outcome of care in the ICU,” he said.
Dying in the ICU after days of mechanical ventilation or surviving with very low performance status “doesn’t sound like good quality of life to me, and it illustrates the challenge we have as clinicians in getting to what’s best for patients,” said Dr. Heyland. “Hopefully, prediction models will help, as well as better elicitation of authentic values and preferences from patients.”
The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no other relevant financial disclosures.
On Twitter @karioakes
ORLANDO – A new clinical prediction rule correlates well with performance status at 1 year after ICU hospitalization in patients over age 80.
Illness severity, comorbidities, baseline frailty, a primary diagnosis of stroke, and being male were all predictors of poor performance status at 1 year. A primary diagnosis of emergency coronary artery bypass grafting or valve replacement, a high baseline performance status, and being married were associated with good performance status at 1 year. The c-statistic for the model, a standard indicator of predictive power, was 0.811, a figure that indicates good predictive ability.
The findings from the REALISTIC 80 (Realities, Expectations ,and Attitudes to Life Support Technologies in Intensive Care for Octogenarians) study of 17 patient and illness characteristics allowed Dr. Daren Heyland, professor of medicine and epidemiology at Queen’s University, Kingston, Ont., and his coinvestigators in the Canadian Critical Care Trials Group (CCCTG), to conclude that eight factors were most predictive of performance status at 12 months for ICU patients aged 80 and over. REALISTIC 80 is a CCCTG project.
The values for the predictors are derived from responses to an online guided questionnaire called the ICU Workbook. The questionnaire is completed by patients’ family members or surrogates, and the responses are used to calculate the values that constitute the clinical prediction rule’s components.
Gathering this information may help health care providers and family members in end-of-life decision making, said Dr. Heyland. “For the very elderly, it is plausible that poor communication and decision making lead to overutilization of ICU resources and poor-quality end-of-life care,” he said. “Hopefully, with these strategies, we’ll improve clinical decision making and improve the quality of end-of-life care we provide for our older patients,” said Dr. Heyland, who is also director of CARENET, which hosts the online guided questionnaire and is an affiliation of Canadian researchers focused on end-of-life care. He spoke at the Society of Critical Care Medicine’s Critical Care Congress.
REALISTIC 80 enrolled 434 patients, aged 80-100 years (mean age, 84.6) who were admitted to ICUs at participating institutions.
Previous European and U.S. studies have shown an ICU mortality of 30%-35%, and an overall mortality rate of 60%-70% in the 12 months following ICU admission. In those studies, illness severity most strongly predicted short-term survival, and comorbidities best predicted long-term survival.
The primary outcome measure of REALISTIC 80 was the 12-month survival and health-related quality of life; “recovery from critical illness” was defined as a Palliative Performance Scale (PPS) score of greater than or equal to 60% at 12 months. Patients scoring at 60% on the 0%-100% scale of this functional status measure may have reduced ambulation, be unable to engage in housework or hobbies, have significant disease, need assistance, and be confused at times. An advantage of this scale, said Dr. Heyland, is that it eliminates survivorship bias in analyzing data, since a score of 0 is assigned to individuals who die.
About 50% of patients had died by 12 months; about 21% were alive, with a reduced health status below the threshold of 60 on the PPS; and about 29% were alive, with a PPS score above the predetermined quality of life threshold.
Dr. Heyland acknowledged that the study lost a significant number of participants – about 17% – to follow-up. The predictive model was derived from completed cases, and a sensitivity analysis using imputed data for missing patients showed that it retained its predictive value.
Dr. Heyland said the presence of advance directives didn’t appear to affect outcomes. “We subsequently in a different analysis showed that whether they had [a directive] or not, did not affect subsequent process or outcome of care in the ICU,” he said.
Dying in the ICU after days of mechanical ventilation or surviving with very low performance status “doesn’t sound like good quality of life to me, and it illustrates the challenge we have as clinicians in getting to what’s best for patients,” said Dr. Heyland. “Hopefully, prediction models will help, as well as better elicitation of authentic values and preferences from patients.”
The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no other relevant financial disclosures.
On Twitter @karioakes
ORLANDO – A new clinical prediction rule correlates well with performance status at 1 year after ICU hospitalization in patients over age 80.
Illness severity, comorbidities, baseline frailty, a primary diagnosis of stroke, and being male were all predictors of poor performance status at 1 year. A primary diagnosis of emergency coronary artery bypass grafting or valve replacement, a high baseline performance status, and being married were associated with good performance status at 1 year. The c-statistic for the model, a standard indicator of predictive power, was 0.811, a figure that indicates good predictive ability.
The findings from the REALISTIC 80 (Realities, Expectations ,and Attitudes to Life Support Technologies in Intensive Care for Octogenarians) study of 17 patient and illness characteristics allowed Dr. Daren Heyland, professor of medicine and epidemiology at Queen’s University, Kingston, Ont., and his coinvestigators in the Canadian Critical Care Trials Group (CCCTG), to conclude that eight factors were most predictive of performance status at 12 months for ICU patients aged 80 and over. REALISTIC 80 is a CCCTG project.
The values for the predictors are derived from responses to an online guided questionnaire called the ICU Workbook. The questionnaire is completed by patients’ family members or surrogates, and the responses are used to calculate the values that constitute the clinical prediction rule’s components.
Gathering this information may help health care providers and family members in end-of-life decision making, said Dr. Heyland. “For the very elderly, it is plausible that poor communication and decision making lead to overutilization of ICU resources and poor-quality end-of-life care,” he said. “Hopefully, with these strategies, we’ll improve clinical decision making and improve the quality of end-of-life care we provide for our older patients,” said Dr. Heyland, who is also director of CARENET, which hosts the online guided questionnaire and is an affiliation of Canadian researchers focused on end-of-life care. He spoke at the Society of Critical Care Medicine’s Critical Care Congress.
REALISTIC 80 enrolled 434 patients, aged 80-100 years (mean age, 84.6) who were admitted to ICUs at participating institutions.
Previous European and U.S. studies have shown an ICU mortality of 30%-35%, and an overall mortality rate of 60%-70% in the 12 months following ICU admission. In those studies, illness severity most strongly predicted short-term survival, and comorbidities best predicted long-term survival.
The primary outcome measure of REALISTIC 80 was the 12-month survival and health-related quality of life; “recovery from critical illness” was defined as a Palliative Performance Scale (PPS) score of greater than or equal to 60% at 12 months. Patients scoring at 60% on the 0%-100% scale of this functional status measure may have reduced ambulation, be unable to engage in housework or hobbies, have significant disease, need assistance, and be confused at times. An advantage of this scale, said Dr. Heyland, is that it eliminates survivorship bias in analyzing data, since a score of 0 is assigned to individuals who die.
About 50% of patients had died by 12 months; about 21% were alive, with a reduced health status below the threshold of 60 on the PPS; and about 29% were alive, with a PPS score above the predetermined quality of life threshold.
Dr. Heyland acknowledged that the study lost a significant number of participants – about 17% – to follow-up. The predictive model was derived from completed cases, and a sensitivity analysis using imputed data for missing patients showed that it retained its predictive value.
Dr. Heyland said the presence of advance directives didn’t appear to affect outcomes. “We subsequently in a different analysis showed that whether they had [a directive] or not, did not affect subsequent process or outcome of care in the ICU,” he said.
Dying in the ICU after days of mechanical ventilation or surviving with very low performance status “doesn’t sound like good quality of life to me, and it illustrates the challenge we have as clinicians in getting to what’s best for patients,” said Dr. Heyland. “Hopefully, prediction models will help, as well as better elicitation of authentic values and preferences from patients.”
The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no other relevant financial disclosures.
On Twitter @karioakes
AT THE CRITICAL CARE CONGRESS
Key clinical point: Eight patient and illness factors were associated with long-term outcomes for ICU patients over age 80.
Major finding: Illness severity, baseline frailty, and comorbidities were associated with lower performance status at 12 months.
Data source: A multicenter study examining 17 patient and illness factors for 434 patients aged 80 and older, admitted to ICUs and followed for 12 months.
Disclosures: The study was funded by the Canadian Institutes of Health Research and conducted under the auspices of the CCCTG and CARENET. The study investigators reported no relevant financial disclosures.
Transition of care plans crucial to cutting readmissions
SAN DIEGO – The way Dr. Michael Kedansky sees it, a hospitalist’s responsibility to a patient doesn’t end when that person is discharged.
“What happens beyond the walls of the hospital matters to us as clinicians,” he said at the annual meeting of the Society of Hospital Medicine. “Readmission is both an undesirable clinical outcome for our patients and a significant cost to the hospital.”
He defined a successful hospital discharge as one in which the patient is not readmitted and transitions to his or her home with an eventual recovery of function. This means that they’re taking the correct medications, follow-up visits are scheduled and honored, and that they feel safe in their home environment, said Dr. Kedansky, chief medical officer of transitional care services for Sound Physicians, which has more than 2,000 physicians in more than 180 hospitals and postacute facilities in the United States. Common barriers that prevent successful care transitions, he said, include the patient not understanding discharge instructions, ineffective medication reconciliation, lack of follow-up appointment availability, need for caregiver training/education, poor continuity of care and transfer of information, and psychosocial factors.
Dr. Kedansky defined effective transitional care as “a set of actions designed to ensure the coordination and continuity of health care as patients move one from one health care setting to another or home. It’s a way to address the current gaps in care, so patients can move safely from hospital to home and back to their PCP.” The problem is, transitional care sometimes takes a back seat to competing demands in the health care landscape. For example, one study found that only 50% of Medicare patients readmitted to the hospital within 30 days of discharge were seen by a follow-up provider (N Engl J Med. 2009;360:1418-28).
One novel care transitions intervention is the so-called Coleman model, in which a “transitions coach” works with patients for 30 days after discharge to help them understand and manage their complex postdischarge needs and ensure continuity of care across settings. Developed by Dr. Eric Coleman, four key aspects of the model include medication self-management, use of a patient-centered record, primary care and specialist follow-up, and knowledge of “red flags.” The process includes an initial visit in the hospital, telephone contact, and a home visit. A randomized trial of 750 community-dwelling adults aged 65 and older showed that those who received the intervention had 20%-40% lower overall hospital readmission rates, compared with controls (Arch Intern Med. 2006;166[17]:1822-8). In addition, they were about 50% less likely to be readmitted at 30, 90, and 180 days for the same condition that caused the initial hospitalization. Barriers to implementing the intervention, Dr. Kedansky said, include costs of startup, training staff, and the question of exactly who sees the savings.
Three more common models of delivering care transitions involve the following:
Traditional internist or family physician. Advantages of this model, he said, include better continuity of care, “both in relationships and transfer of information,” no additional resources required, and a high potential for patient satisfaction. Limitations of this model include a reduction in the physician presence, a high workload, and risk for burnout.
Extensivist. Advantages of this model, which was outlined in a recent JAMA article based on the experience of CareMore Health System (JAMA. 2016;315[1]:23-4), include improved continuity at the time of high-risk transitions, and evidence which demonstrates a reduction in readmission rates and lower costs of care. Drawbacks include the fact that it’s a high-cost model that has not been scaled beyond health plan settings. “There is no model to use extensivists in the fee-for-service world,” Dr. Kedansky said.
Hospitalist + post–acute care provider + PCP. Advantages of this model, he said, are that it’s easier to scale, physicians can develop an area of expertise, and they have a presence in the hospital or skilled nursing facility. One limitation is that it can lead to reduced continuity of care. “Until recently, this has been a volume-based model of care,” he added.
According to Dr. Kedansky, postacute expenses account for 65% of spending during a 90-day acute episode of care. The Bundled Payments for Care Improvement Initiative (BPCI), developed by the Centers for Medicare & Medicaid Services, is a shared savings model aimed at reducing post–acute care spending. Creative components of initiative, he said, include waivers for telemedicine and home health, waiver of the 3-night stay rule, and sharing and using data to define preferred, high performance networks. “I see BPCI as a game changer, as it turbocharges our physician-based models and incentivizes the right behavior,” he said. “The key to working in a bundled payment world is to teach hospitalists and other providers how to think differently, while managing patients across a full 90-day episode of care. Care redesign to improve clinical outcomes for patients leads to success and shared savings in this type of payment model.”
He concluded his remarks by advising physicians to “work through the list of items that ensure a successful care transition: patient education, caregiver training, medication reconciliation, and warm hand-offs of information.”
Dr. Kedansky reported having no financial disclosures.
SAN DIEGO – The way Dr. Michael Kedansky sees it, a hospitalist’s responsibility to a patient doesn’t end when that person is discharged.
“What happens beyond the walls of the hospital matters to us as clinicians,” he said at the annual meeting of the Society of Hospital Medicine. “Readmission is both an undesirable clinical outcome for our patients and a significant cost to the hospital.”
He defined a successful hospital discharge as one in which the patient is not readmitted and transitions to his or her home with an eventual recovery of function. This means that they’re taking the correct medications, follow-up visits are scheduled and honored, and that they feel safe in their home environment, said Dr. Kedansky, chief medical officer of transitional care services for Sound Physicians, which has more than 2,000 physicians in more than 180 hospitals and postacute facilities in the United States. Common barriers that prevent successful care transitions, he said, include the patient not understanding discharge instructions, ineffective medication reconciliation, lack of follow-up appointment availability, need for caregiver training/education, poor continuity of care and transfer of information, and psychosocial factors.
Dr. Kedansky defined effective transitional care as “a set of actions designed to ensure the coordination and continuity of health care as patients move one from one health care setting to another or home. It’s a way to address the current gaps in care, so patients can move safely from hospital to home and back to their PCP.” The problem is, transitional care sometimes takes a back seat to competing demands in the health care landscape. For example, one study found that only 50% of Medicare patients readmitted to the hospital within 30 days of discharge were seen by a follow-up provider (N Engl J Med. 2009;360:1418-28).
One novel care transitions intervention is the so-called Coleman model, in which a “transitions coach” works with patients for 30 days after discharge to help them understand and manage their complex postdischarge needs and ensure continuity of care across settings. Developed by Dr. Eric Coleman, four key aspects of the model include medication self-management, use of a patient-centered record, primary care and specialist follow-up, and knowledge of “red flags.” The process includes an initial visit in the hospital, telephone contact, and a home visit. A randomized trial of 750 community-dwelling adults aged 65 and older showed that those who received the intervention had 20%-40% lower overall hospital readmission rates, compared with controls (Arch Intern Med. 2006;166[17]:1822-8). In addition, they were about 50% less likely to be readmitted at 30, 90, and 180 days for the same condition that caused the initial hospitalization. Barriers to implementing the intervention, Dr. Kedansky said, include costs of startup, training staff, and the question of exactly who sees the savings.
Three more common models of delivering care transitions involve the following:
Traditional internist or family physician. Advantages of this model, he said, include better continuity of care, “both in relationships and transfer of information,” no additional resources required, and a high potential for patient satisfaction. Limitations of this model include a reduction in the physician presence, a high workload, and risk for burnout.
Extensivist. Advantages of this model, which was outlined in a recent JAMA article based on the experience of CareMore Health System (JAMA. 2016;315[1]:23-4), include improved continuity at the time of high-risk transitions, and evidence which demonstrates a reduction in readmission rates and lower costs of care. Drawbacks include the fact that it’s a high-cost model that has not been scaled beyond health plan settings. “There is no model to use extensivists in the fee-for-service world,” Dr. Kedansky said.
Hospitalist + post–acute care provider + PCP. Advantages of this model, he said, are that it’s easier to scale, physicians can develop an area of expertise, and they have a presence in the hospital or skilled nursing facility. One limitation is that it can lead to reduced continuity of care. “Until recently, this has been a volume-based model of care,” he added.
According to Dr. Kedansky, postacute expenses account for 65% of spending during a 90-day acute episode of care. The Bundled Payments for Care Improvement Initiative (BPCI), developed by the Centers for Medicare & Medicaid Services, is a shared savings model aimed at reducing post–acute care spending. Creative components of initiative, he said, include waivers for telemedicine and home health, waiver of the 3-night stay rule, and sharing and using data to define preferred, high performance networks. “I see BPCI as a game changer, as it turbocharges our physician-based models and incentivizes the right behavior,” he said. “The key to working in a bundled payment world is to teach hospitalists and other providers how to think differently, while managing patients across a full 90-day episode of care. Care redesign to improve clinical outcomes for patients leads to success and shared savings in this type of payment model.”
He concluded his remarks by advising physicians to “work through the list of items that ensure a successful care transition: patient education, caregiver training, medication reconciliation, and warm hand-offs of information.”
Dr. Kedansky reported having no financial disclosures.
SAN DIEGO – The way Dr. Michael Kedansky sees it, a hospitalist’s responsibility to a patient doesn’t end when that person is discharged.
“What happens beyond the walls of the hospital matters to us as clinicians,” he said at the annual meeting of the Society of Hospital Medicine. “Readmission is both an undesirable clinical outcome for our patients and a significant cost to the hospital.”
He defined a successful hospital discharge as one in which the patient is not readmitted and transitions to his or her home with an eventual recovery of function. This means that they’re taking the correct medications, follow-up visits are scheduled and honored, and that they feel safe in their home environment, said Dr. Kedansky, chief medical officer of transitional care services for Sound Physicians, which has more than 2,000 physicians in more than 180 hospitals and postacute facilities in the United States. Common barriers that prevent successful care transitions, he said, include the patient not understanding discharge instructions, ineffective medication reconciliation, lack of follow-up appointment availability, need for caregiver training/education, poor continuity of care and transfer of information, and psychosocial factors.
Dr. Kedansky defined effective transitional care as “a set of actions designed to ensure the coordination and continuity of health care as patients move one from one health care setting to another or home. It’s a way to address the current gaps in care, so patients can move safely from hospital to home and back to their PCP.” The problem is, transitional care sometimes takes a back seat to competing demands in the health care landscape. For example, one study found that only 50% of Medicare patients readmitted to the hospital within 30 days of discharge were seen by a follow-up provider (N Engl J Med. 2009;360:1418-28).
One novel care transitions intervention is the so-called Coleman model, in which a “transitions coach” works with patients for 30 days after discharge to help them understand and manage their complex postdischarge needs and ensure continuity of care across settings. Developed by Dr. Eric Coleman, four key aspects of the model include medication self-management, use of a patient-centered record, primary care and specialist follow-up, and knowledge of “red flags.” The process includes an initial visit in the hospital, telephone contact, and a home visit. A randomized trial of 750 community-dwelling adults aged 65 and older showed that those who received the intervention had 20%-40% lower overall hospital readmission rates, compared with controls (Arch Intern Med. 2006;166[17]:1822-8). In addition, they were about 50% less likely to be readmitted at 30, 90, and 180 days for the same condition that caused the initial hospitalization. Barriers to implementing the intervention, Dr. Kedansky said, include costs of startup, training staff, and the question of exactly who sees the savings.
Three more common models of delivering care transitions involve the following:
Traditional internist or family physician. Advantages of this model, he said, include better continuity of care, “both in relationships and transfer of information,” no additional resources required, and a high potential for patient satisfaction. Limitations of this model include a reduction in the physician presence, a high workload, and risk for burnout.
Extensivist. Advantages of this model, which was outlined in a recent JAMA article based on the experience of CareMore Health System (JAMA. 2016;315[1]:23-4), include improved continuity at the time of high-risk transitions, and evidence which demonstrates a reduction in readmission rates and lower costs of care. Drawbacks include the fact that it’s a high-cost model that has not been scaled beyond health plan settings. “There is no model to use extensivists in the fee-for-service world,” Dr. Kedansky said.
Hospitalist + post–acute care provider + PCP. Advantages of this model, he said, are that it’s easier to scale, physicians can develop an area of expertise, and they have a presence in the hospital or skilled nursing facility. One limitation is that it can lead to reduced continuity of care. “Until recently, this has been a volume-based model of care,” he added.
According to Dr. Kedansky, postacute expenses account for 65% of spending during a 90-day acute episode of care. The Bundled Payments for Care Improvement Initiative (BPCI), developed by the Centers for Medicare & Medicaid Services, is a shared savings model aimed at reducing post–acute care spending. Creative components of initiative, he said, include waivers for telemedicine and home health, waiver of the 3-night stay rule, and sharing and using data to define preferred, high performance networks. “I see BPCI as a game changer, as it turbocharges our physician-based models and incentivizes the right behavior,” he said. “The key to working in a bundled payment world is to teach hospitalists and other providers how to think differently, while managing patients across a full 90-day episode of care. Care redesign to improve clinical outcomes for patients leads to success and shared savings in this type of payment model.”
He concluded his remarks by advising physicians to “work through the list of items that ensure a successful care transition: patient education, caregiver training, medication reconciliation, and warm hand-offs of information.”
Dr. Kedansky reported having no financial disclosures.
AT HOSPITAL MEDICINE 16
VIDEO: Surgeon General calls for culture of ‘emotional well-being’
SAN DIEGO – Surgeon General Dr. Vivek H. Murthy called for a culture of “emotional well-being” to curb physician burnout and reduce the number of distressed physicians who take their lives each year.
“I think we have to have a focus on emotional well-being from the time people get into medical school,” he said during a press briefing at the annual meeting of the Society of Hospital Medicine. “We’re not just talking about trying to build a couple of intervention programs where people meet in small groups once a week. This is about shifting perspective in culture, recognizing that emotional well-being is an essential tool for clinicians to be able to do their jobs well.”
Dr. Murthy expressed concern for medical students who enter the profession “with the highest of ideals. But once they get into medicine, they run into challenges and obstacles. They find that the system isn’t always set up to allow them to live up to those ideals.”
Those challenges, he continued, “can wear on people’s emotional well-being. It can lead them to a sense of futility. It can increase burnout, and it can tax people to the point where, sadly, in some cases, people are driven to harm themselves.”
Dr. Murthy also is the cofounder of VISIONS, an HIV/AIDS education program in India and the United States, which he led for 8 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Surgeon General Dr. Vivek H. Murthy called for a culture of “emotional well-being” to curb physician burnout and reduce the number of distressed physicians who take their lives each year.
“I think we have to have a focus on emotional well-being from the time people get into medical school,” he said during a press briefing at the annual meeting of the Society of Hospital Medicine. “We’re not just talking about trying to build a couple of intervention programs where people meet in small groups once a week. This is about shifting perspective in culture, recognizing that emotional well-being is an essential tool for clinicians to be able to do their jobs well.”
Dr. Murthy expressed concern for medical students who enter the profession “with the highest of ideals. But once they get into medicine, they run into challenges and obstacles. They find that the system isn’t always set up to allow them to live up to those ideals.”
Those challenges, he continued, “can wear on people’s emotional well-being. It can lead them to a sense of futility. It can increase burnout, and it can tax people to the point where, sadly, in some cases, people are driven to harm themselves.”
Dr. Murthy also is the cofounder of VISIONS, an HIV/AIDS education program in India and the United States, which he led for 8 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Surgeon General Dr. Vivek H. Murthy called for a culture of “emotional well-being” to curb physician burnout and reduce the number of distressed physicians who take their lives each year.
“I think we have to have a focus on emotional well-being from the time people get into medical school,” he said during a press briefing at the annual meeting of the Society of Hospital Medicine. “We’re not just talking about trying to build a couple of intervention programs where people meet in small groups once a week. This is about shifting perspective in culture, recognizing that emotional well-being is an essential tool for clinicians to be able to do their jobs well.”
Dr. Murthy expressed concern for medical students who enter the profession “with the highest of ideals. But once they get into medicine, they run into challenges and obstacles. They find that the system isn’t always set up to allow them to live up to those ideals.”
Those challenges, he continued, “can wear on people’s emotional well-being. It can lead them to a sense of futility. It can increase burnout, and it can tax people to the point where, sadly, in some cases, people are driven to harm themselves.”
Dr. Murthy also is the cofounder of VISIONS, an HIV/AIDS education program in India and the United States, which he led for 8 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First U.S. uterus transplant raises questions about ethics, cost
The recipient of the first uterus transplant performed in the United States is recovering well and looking forward to the next phase of the research project: pregnancy, according to the team of Cleveland Clinic surgeons who performed the groundbreaking Feb. 24 transplant.
“I’m pleased to report to you that our patient is doing very well,” lead surgeon Dr. Andreas G. Tzakis said during a press conference on March 7.
The transplant recipient, known only as “Lindsey” to protect her privacy, is a 26-year-old woman with uterine factor infertility (UFI). She was selected from among more than 250 applicants to undergo the 9-hour procedure involving transplantation of a uterus from a deceased organ donor of reproductive age. She is the first of 10 women set to undergo the procedure as part of the Cleveland Clinic study.
“Lindsey” made an appearance at the press conference and expressed, first and foremost, her “immense gratitude” to the donor’s family.
“They have provided me with a gift that I will never be able to repay,” she said in an emotional statement in which she shared about learning at age 16 that she would never be able to have children.
“From that moment on I prayed that God would allow me the opportunity to experience pregnancy, and here we are today at the beginning of that journey,” she said.
Lindsey will undergo a year of anti-rejection treatment prior to undergoing in vitro fertilization; her eggs were previously fertilized using her husband’s sperm, and the embryos are in cryogenic storage, according to Dr. Rebecca Flyckt, another member of the surgical team.
The embryos will be transferred one by one until the goal of a healthy pregnancy and healthy baby delivered by cesarean section is achieved, Dr. Flyckt said.
After one or two successful pregnancies and births, the uterus will be removed. Although substantial evidence suggests that anti-rejection medications are relatively safe in pregnancy, minimizing and ultimately eliminating the need for them is advisable, thus uterus transplants, at least under the Cleveland Clinic research protocol, are meant to be temporary.
Uterus transplants are performed to enable patients – who have either lost their uterus to disease or who were born without a uterus or a functioning uterus – to experience pregnancy and childbirth.
Prior to the Cleveland Clinic transplant, nine had been performed successfully with healthy outcomes (the first post-transplant baby was born healthy in 2014). All were performed at the University of Gothenburg in Sweden. Dr. Tzakis traveled there to work with surgeons prior to performing the procedure at the Cleveland Clinic, where he is the Transplant Center program director.
Ethical issues
Addressing the bioethical issues surrounding uterine transplant was an important part of this project, according to team member Dr. Ruth Farrell, a bioethicist and ob.gyn. surgeon at the Cleveland Clinic.
“Despite the name uterine transplant, the focus of this procedure is not on the uterus. It’s on women and children and families,” she said, adding that to understand the ethical issues of uterine transplant, it’s important to consider the perspectives of women with UFI.
“For instance, women born without a uterus have a medical condition that affects every aspect of their lives, from the time they are diagnosed in adolescence, to when they are adults looking for relationships and trying to decide if and how to have a family,” she said, adding that “these women face the real possibility of never having children.”
But this advance in reproductive medicine and science also requires a close look at how the potential risks and benefits of uterine transplant weigh against existing options of adoption and surrogacy. While there are many successful stories involving surrogacy and adoption, these options are not available to all women because of “legal, cultural, religious, and other very personal reasons,” Dr. Farrell said.
“Our research on uterine transplant, we hope, may give women another option which may work better for them and their families,” she said, also noting that while living donor transplants have been performed, these come with some risk, thus deceased donors are being used for the current study.
Insurance coverage
As for the feasibility of uterine transplant, many questions remain to be answered, according to Dr. Tommaso Falcone, chair of the department of obstetrics and gynecology at the Cleveland Clinic, who said that the procedures done as part of the current study will be paid for by an institutional grant.
While the American Society for Reproductive Medicine contends that infertility is a disease worthy of insurance coverage, that is “outside of our hands,” he said.
Indeed, while this first uterine transplant in the United States is a “huge and exciting breakthrough,” and while “the folks at Cleveland Clinic should be congratulated,” the possibility of this procedure ever being covered by insurance and being available to women outside of the research protocol is questionable, especially given the available alternatives, such as gestational carriers and surrogacy, Dr. David A. Forstein, a reproductive endocrinologist at the University of South Carolina, Greenville, said in an interview.
In a patient-centered model, this “tremendous, wonderful gift from science” would give patients – like the 1 in 5,000 women in the United States who are born without a uterus – a very viable alternative, but the question is whether having one’s own children is a right, and whether extensive financial resources should be committed to helping women achieve that, he said.
Dr. Charles E. Miller, a reproductive endocrinologist in Chicago, and head of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., agreed that this transplant is to be celebrated.
“It’s a big moment, to say the least,” he said in an interview.
But Dr. Miller also questioned the feasibility of the procedure and whether society will “look favorably upon donation,” given the availability of alternatives for which there is now great success.
“I salute the pioneering effort,” he said, “But at the end of the day, can society take on this burden of a procedure performed not to sustain life, but to help create life? That’s a tough one.”
The recipient of the first uterus transplant performed in the United States is recovering well and looking forward to the next phase of the research project: pregnancy, according to the team of Cleveland Clinic surgeons who performed the groundbreaking Feb. 24 transplant.
“I’m pleased to report to you that our patient is doing very well,” lead surgeon Dr. Andreas G. Tzakis said during a press conference on March 7.
The transplant recipient, known only as “Lindsey” to protect her privacy, is a 26-year-old woman with uterine factor infertility (UFI). She was selected from among more than 250 applicants to undergo the 9-hour procedure involving transplantation of a uterus from a deceased organ donor of reproductive age. She is the first of 10 women set to undergo the procedure as part of the Cleveland Clinic study.
“Lindsey” made an appearance at the press conference and expressed, first and foremost, her “immense gratitude” to the donor’s family.
“They have provided me with a gift that I will never be able to repay,” she said in an emotional statement in which she shared about learning at age 16 that she would never be able to have children.
“From that moment on I prayed that God would allow me the opportunity to experience pregnancy, and here we are today at the beginning of that journey,” she said.
Lindsey will undergo a year of anti-rejection treatment prior to undergoing in vitro fertilization; her eggs were previously fertilized using her husband’s sperm, and the embryos are in cryogenic storage, according to Dr. Rebecca Flyckt, another member of the surgical team.
The embryos will be transferred one by one until the goal of a healthy pregnancy and healthy baby delivered by cesarean section is achieved, Dr. Flyckt said.
After one or two successful pregnancies and births, the uterus will be removed. Although substantial evidence suggests that anti-rejection medications are relatively safe in pregnancy, minimizing and ultimately eliminating the need for them is advisable, thus uterus transplants, at least under the Cleveland Clinic research protocol, are meant to be temporary.
Uterus transplants are performed to enable patients – who have either lost their uterus to disease or who were born without a uterus or a functioning uterus – to experience pregnancy and childbirth.
Prior to the Cleveland Clinic transplant, nine had been performed successfully with healthy outcomes (the first post-transplant baby was born healthy in 2014). All were performed at the University of Gothenburg in Sweden. Dr. Tzakis traveled there to work with surgeons prior to performing the procedure at the Cleveland Clinic, where he is the Transplant Center program director.
Ethical issues
Addressing the bioethical issues surrounding uterine transplant was an important part of this project, according to team member Dr. Ruth Farrell, a bioethicist and ob.gyn. surgeon at the Cleveland Clinic.
“Despite the name uterine transplant, the focus of this procedure is not on the uterus. It’s on women and children and families,” she said, adding that to understand the ethical issues of uterine transplant, it’s important to consider the perspectives of women with UFI.
“For instance, women born without a uterus have a medical condition that affects every aspect of their lives, from the time they are diagnosed in adolescence, to when they are adults looking for relationships and trying to decide if and how to have a family,” she said, adding that “these women face the real possibility of never having children.”
But this advance in reproductive medicine and science also requires a close look at how the potential risks and benefits of uterine transplant weigh against existing options of adoption and surrogacy. While there are many successful stories involving surrogacy and adoption, these options are not available to all women because of “legal, cultural, religious, and other very personal reasons,” Dr. Farrell said.
“Our research on uterine transplant, we hope, may give women another option which may work better for them and their families,” she said, also noting that while living donor transplants have been performed, these come with some risk, thus deceased donors are being used for the current study.
Insurance coverage
As for the feasibility of uterine transplant, many questions remain to be answered, according to Dr. Tommaso Falcone, chair of the department of obstetrics and gynecology at the Cleveland Clinic, who said that the procedures done as part of the current study will be paid for by an institutional grant.
While the American Society for Reproductive Medicine contends that infertility is a disease worthy of insurance coverage, that is “outside of our hands,” he said.
Indeed, while this first uterine transplant in the United States is a “huge and exciting breakthrough,” and while “the folks at Cleveland Clinic should be congratulated,” the possibility of this procedure ever being covered by insurance and being available to women outside of the research protocol is questionable, especially given the available alternatives, such as gestational carriers and surrogacy, Dr. David A. Forstein, a reproductive endocrinologist at the University of South Carolina, Greenville, said in an interview.
In a patient-centered model, this “tremendous, wonderful gift from science” would give patients – like the 1 in 5,000 women in the United States who are born without a uterus – a very viable alternative, but the question is whether having one’s own children is a right, and whether extensive financial resources should be committed to helping women achieve that, he said.
Dr. Charles E. Miller, a reproductive endocrinologist in Chicago, and head of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., agreed that this transplant is to be celebrated.
“It’s a big moment, to say the least,” he said in an interview.
But Dr. Miller also questioned the feasibility of the procedure and whether society will “look favorably upon donation,” given the availability of alternatives for which there is now great success.
“I salute the pioneering effort,” he said, “But at the end of the day, can society take on this burden of a procedure performed not to sustain life, but to help create life? That’s a tough one.”
The recipient of the first uterus transplant performed in the United States is recovering well and looking forward to the next phase of the research project: pregnancy, according to the team of Cleveland Clinic surgeons who performed the groundbreaking Feb. 24 transplant.
“I’m pleased to report to you that our patient is doing very well,” lead surgeon Dr. Andreas G. Tzakis said during a press conference on March 7.
The transplant recipient, known only as “Lindsey” to protect her privacy, is a 26-year-old woman with uterine factor infertility (UFI). She was selected from among more than 250 applicants to undergo the 9-hour procedure involving transplantation of a uterus from a deceased organ donor of reproductive age. She is the first of 10 women set to undergo the procedure as part of the Cleveland Clinic study.
“Lindsey” made an appearance at the press conference and expressed, first and foremost, her “immense gratitude” to the donor’s family.
“They have provided me with a gift that I will never be able to repay,” she said in an emotional statement in which she shared about learning at age 16 that she would never be able to have children.
“From that moment on I prayed that God would allow me the opportunity to experience pregnancy, and here we are today at the beginning of that journey,” she said.
Lindsey will undergo a year of anti-rejection treatment prior to undergoing in vitro fertilization; her eggs were previously fertilized using her husband’s sperm, and the embryos are in cryogenic storage, according to Dr. Rebecca Flyckt, another member of the surgical team.
The embryos will be transferred one by one until the goal of a healthy pregnancy and healthy baby delivered by cesarean section is achieved, Dr. Flyckt said.
After one or two successful pregnancies and births, the uterus will be removed. Although substantial evidence suggests that anti-rejection medications are relatively safe in pregnancy, minimizing and ultimately eliminating the need for them is advisable, thus uterus transplants, at least under the Cleveland Clinic research protocol, are meant to be temporary.
Uterus transplants are performed to enable patients – who have either lost their uterus to disease or who were born without a uterus or a functioning uterus – to experience pregnancy and childbirth.
Prior to the Cleveland Clinic transplant, nine had been performed successfully with healthy outcomes (the first post-transplant baby was born healthy in 2014). All were performed at the University of Gothenburg in Sweden. Dr. Tzakis traveled there to work with surgeons prior to performing the procedure at the Cleveland Clinic, where he is the Transplant Center program director.
Ethical issues
Addressing the bioethical issues surrounding uterine transplant was an important part of this project, according to team member Dr. Ruth Farrell, a bioethicist and ob.gyn. surgeon at the Cleveland Clinic.
“Despite the name uterine transplant, the focus of this procedure is not on the uterus. It’s on women and children and families,” she said, adding that to understand the ethical issues of uterine transplant, it’s important to consider the perspectives of women with UFI.
“For instance, women born without a uterus have a medical condition that affects every aspect of their lives, from the time they are diagnosed in adolescence, to when they are adults looking for relationships and trying to decide if and how to have a family,” she said, adding that “these women face the real possibility of never having children.”
But this advance in reproductive medicine and science also requires a close look at how the potential risks and benefits of uterine transplant weigh against existing options of adoption and surrogacy. While there are many successful stories involving surrogacy and adoption, these options are not available to all women because of “legal, cultural, religious, and other very personal reasons,” Dr. Farrell said.
“Our research on uterine transplant, we hope, may give women another option which may work better for them and their families,” she said, also noting that while living donor transplants have been performed, these come with some risk, thus deceased donors are being used for the current study.
Insurance coverage
As for the feasibility of uterine transplant, many questions remain to be answered, according to Dr. Tommaso Falcone, chair of the department of obstetrics and gynecology at the Cleveland Clinic, who said that the procedures done as part of the current study will be paid for by an institutional grant.
While the American Society for Reproductive Medicine contends that infertility is a disease worthy of insurance coverage, that is “outside of our hands,” he said.
Indeed, while this first uterine transplant in the United States is a “huge and exciting breakthrough,” and while “the folks at Cleveland Clinic should be congratulated,” the possibility of this procedure ever being covered by insurance and being available to women outside of the research protocol is questionable, especially given the available alternatives, such as gestational carriers and surrogacy, Dr. David A. Forstein, a reproductive endocrinologist at the University of South Carolina, Greenville, said in an interview.
In a patient-centered model, this “tremendous, wonderful gift from science” would give patients – like the 1 in 5,000 women in the United States who are born without a uterus – a very viable alternative, but the question is whether having one’s own children is a right, and whether extensive financial resources should be committed to helping women achieve that, he said.
Dr. Charles E. Miller, a reproductive endocrinologist in Chicago, and head of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., agreed that this transplant is to be celebrated.
“It’s a big moment, to say the least,” he said in an interview.
But Dr. Miller also questioned the feasibility of the procedure and whether society will “look favorably upon donation,” given the availability of alternatives for which there is now great success.
“I salute the pioneering effort,” he said, “But at the end of the day, can society take on this burden of a procedure performed not to sustain life, but to help create life? That’s a tough one.”
Health spending less concentrated among highest-cost population
Good news for the Occupy movement: The 99% have gained some ground.
The 1% of the U.S. population with the highest health care expenses accounted for 21.5% of all health care expenditures in 2013. That may be a lot, but it’s down from 28% in 1996, according to the Agency for Healthcare Research and Quality.
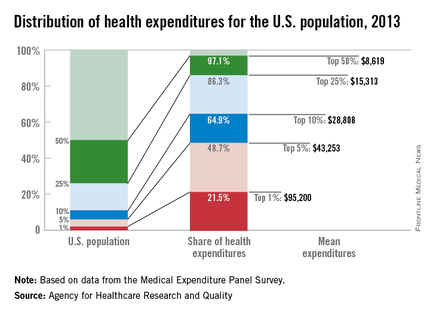
What’s more, the top 5% of the population ranked by their health care expenses dropped to 48.7% of total spending in 2013, after accounting for more than half in 1996, the AHRQ reported.
Health care expenditures for the U.S. civilian noninstitutionalized population totaled $1.4 trillion in 2013, with mean spending of $4,436 per person. The top 1%, by comparison, had mean spending of $95,200 each, and for the top 5% the figure was $43,253 per person. The top 50% of the population had mean per-person spending of $8,619 and accounted for 97.1% of all health care expenditures, leaving just 2.9% of the total to the lower-cost 50% of the population, the AHRQ said.
Looking at the distribution by race and ethnicity, the top 5% of whites accounted for 45.3% of all spending incurred by whites, compared with 50.8% of all Asian spending for the top 5% of Asians, with corresponding numbers of 56.3% for blacks and 57.3% for Hispanics. Mean spending per person was somewhat reversed, however, at $26,491 for Asian five-percenters, $28,868 for Hispanics, $45,574 for blacks, and $46,809 for whites, according to data from the Medical Expenditure Panel Survey.
Good news for the Occupy movement: The 99% have gained some ground.
The 1% of the U.S. population with the highest health care expenses accounted for 21.5% of all health care expenditures in 2013. That may be a lot, but it’s down from 28% in 1996, according to the Agency for Healthcare Research and Quality.

What’s more, the top 5% of the population ranked by their health care expenses dropped to 48.7% of total spending in 2013, after accounting for more than half in 1996, the AHRQ reported.
Health care expenditures for the U.S. civilian noninstitutionalized population totaled $1.4 trillion in 2013, with mean spending of $4,436 per person. The top 1%, by comparison, had mean spending of $95,200 each, and for the top 5% the figure was $43,253 per person. The top 50% of the population had mean per-person spending of $8,619 and accounted for 97.1% of all health care expenditures, leaving just 2.9% of the total to the lower-cost 50% of the population, the AHRQ said.
Looking at the distribution by race and ethnicity, the top 5% of whites accounted for 45.3% of all spending incurred by whites, compared with 50.8% of all Asian spending for the top 5% of Asians, with corresponding numbers of 56.3% for blacks and 57.3% for Hispanics. Mean spending per person was somewhat reversed, however, at $26,491 for Asian five-percenters, $28,868 for Hispanics, $45,574 for blacks, and $46,809 for whites, according to data from the Medical Expenditure Panel Survey.
Good news for the Occupy movement: The 99% have gained some ground.
The 1% of the U.S. population with the highest health care expenses accounted for 21.5% of all health care expenditures in 2013. That may be a lot, but it’s down from 28% in 1996, according to the Agency for Healthcare Research and Quality.

What’s more, the top 5% of the population ranked by their health care expenses dropped to 48.7% of total spending in 2013, after accounting for more than half in 1996, the AHRQ reported.
Health care expenditures for the U.S. civilian noninstitutionalized population totaled $1.4 trillion in 2013, with mean spending of $4,436 per person. The top 1%, by comparison, had mean spending of $95,200 each, and for the top 5% the figure was $43,253 per person. The top 50% of the population had mean per-person spending of $8,619 and accounted for 97.1% of all health care expenditures, leaving just 2.9% of the total to the lower-cost 50% of the population, the AHRQ said.
Looking at the distribution by race and ethnicity, the top 5% of whites accounted for 45.3% of all spending incurred by whites, compared with 50.8% of all Asian spending for the top 5% of Asians, with corresponding numbers of 56.3% for blacks and 57.3% for Hispanics. Mean spending per person was somewhat reversed, however, at $26,491 for Asian five-percenters, $28,868 for Hispanics, $45,574 for blacks, and $46,809 for whites, according to data from the Medical Expenditure Panel Survey.
VIDEO: What are physicians’ top legal risks in 2016?
AUSTIN, TEX. – With ongoing changes in health delivery and ever-increasing government scrutiny over care, physicians face a number of pressing legal risks this year.
At an American Health Lawyers Association meeting, Birmingham, Ala., health law attorney William W. Horton shared the top legal dangers for doctors in 2016.
From False Claims Act investigations and whistle-blower claims to liability connected to value-based care, clinicians have a lot to consider, said Mr. Horton, who is chair of the American Bar Association Health Law Section.
In a video interview, Mr. Horton also discussed how to reduce liability and limit government inquiries.
On Twitter @legal_med
AUSTIN, TEX. – With ongoing changes in health delivery and ever-increasing government scrutiny over care, physicians face a number of pressing legal risks this year.
At an American Health Lawyers Association meeting, Birmingham, Ala., health law attorney William W. Horton shared the top legal dangers for doctors in 2016.
From False Claims Act investigations and whistle-blower claims to liability connected to value-based care, clinicians have a lot to consider, said Mr. Horton, who is chair of the American Bar Association Health Law Section.
In a video interview, Mr. Horton also discussed how to reduce liability and limit government inquiries.
On Twitter @legal_med
AUSTIN, TEX. – With ongoing changes in health delivery and ever-increasing government scrutiny over care, physicians face a number of pressing legal risks this year.
At an American Health Lawyers Association meeting, Birmingham, Ala., health law attorney William W. Horton shared the top legal dangers for doctors in 2016.
From False Claims Act investigations and whistle-blower claims to liability connected to value-based care, clinicians have a lot to consider, said Mr. Horton, who is chair of the American Bar Association Health Law Section.
In a video interview, Mr. Horton also discussed how to reduce liability and limit government inquiries.
On Twitter @legal_med
EXPERT ANALYSIS FROM THE PHYSICIANS AND HOSPITALS LAW INSTITUTE
Antibiotic-resistant infections remain a persistent threat
One in every seven infections in acute care hospitals related to catheters and surgeries was caused by antibiotic-resistant bacteria. In long-term acute care hospitals, that number increased to one in four.
Those are key findings from a study published March 3 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report that is the first to combine national data on antibiotic-resistant (AR) bacteria threats with progress on health care–associated infections (HAIs).
“Antibiotic resistance threatens to return us to a time when a simple infection could kill,” CDC Director Thomas Frieden said during a March 3 telebriefing. “The more people who get infected with resistant bacteria, the more people who suffer complications, the more who, tragically, may die from preventable infections. On any given day about one in 25 hospitalized patients has at least one health care–associated infection that they didn’t come in with. No one should get sick when they’re trying to get well.”
For the study, researchers led by Dr. Clifford McDonald of the CDC’s Division of Healthcare Quality Promotion, collected data on specific infections that were reported to the National Healthcare Safety Network in 2014 by approximately 4,000 short-term acute care hospitals, 501 long-term acute care hospitals, and 1,135 inpatient rehabilitation facilities in all 50 states (MMWR. 2016 Mar 3. doi: 10.15585/mmwr.mm6509e1er). Next, they determined the proportions of AR pathogens and HAIs caused by any of six resistant bacteria highlighted by the CDC in 2013 as urgent or serious threats: CRE (carbapenem-resistant Enterobacteriaceae), MRSA (methicillin-resistant Staphylococcus aureus), ESBL-producing Enterobacteriaceae (extended-spectrum beta-lactamases), VRE (vancomycin-resistant enterococci), multidrug-resistant pseudomonas, and multidrug-resistant Acinetobacter.
The researchers found that, compared with historical data from 5-8 years earlier, central line–associated bloodstream infections decreased by 50% and surgical site infections (SSIs) by 17% in 2014.
“There is encouraging news here,” Dr. Frieden said. “Doctors, nurses, hospitals, health care systems and other partners have made progress preventing some health care–associated infections.” However, the study found that one in six remaining central line-associated bloodstream infections were caused by urgent or serious antibiotic-resistant bacteria, while one in seven remaining surgical site infections were caused by urgent or serious antibiotic-resistant bacteria.
While catheter-associated urinary tract infections appear unchanged from baseline, there have been recent decreases, according to the study. In addition, C. difficile infections in hospitals decreased 8% between 2011 and 2014.
Dr. McDonald and his associates determined that in 2014, one in seven infections in acute care hospitals related to catheters and surgeries was caused by one of the six antibiotic-resistance threat bacteria, “which is deeply concerning,” Dr. Frieden said. That number increased to one in four infections in long-term acute care hospitals, a proportion that he characterized as “chilling.”
The CDC recommends three strategies that doctors, nurses, and other health care providers should take with every patient, to prevent HAIs and stop the spread of antibiotic resistance:
• Prevent the spread of bacteria between patients. Dr. Peter Pronovost, who participated in the telebriefing, said that he and his associates at Johns Hopkins University in Baltimore “do this by practicing good hand hygiene techniques by wearing sterile equipment when inserting lines.”
• Prevent surgery-related infections and/or placement of a catheter. “Check catheters frequently and remove them when you no longer need them,” advised Dr. Pronovost, director of the Armstrong Institute for Patient Safety and Quality at Johns Hopkins. “Ask if you actually need them before you even place them.”
• Improve antibiotic use through stewardship. This means using “the right antibiotics for the right duration,” Dr. Pronovost said. “Antibiotics could be lifesaving and are necessary for critically ill patients, especially those with septic shock. But these antibiotics need to be adjusted based on lab results and new information about the organisms that are causing these infections. Forty-eight hours after antibiotics are initiated, take a ‘time out.’ Perform a brief but focused assessment to determine if antibiotic therapy is still needed, or if it should be refined. A common mistake we make is to continue vancomycin when there is no presence of MRSA. We often tell our staff at Johns Hopkins, ‘if it doesn’t grow, let it go.’ ”
Dr. Frieden concluded his remarks by noting that physicians and other clinicians on the front lines “need support of their facility leadership,” to prevent HAIs. “Health care facilities, CEOs, and administrators are a major part of the solution. It’s important that they make a priority of infection prevention, sepsis prevention, and antibiotic stewardship. Know your facility’s data and target prevention efforts to ensure improvements in patient safety.”
One in every seven infections in acute care hospitals related to catheters and surgeries was caused by antibiotic-resistant bacteria. In long-term acute care hospitals, that number increased to one in four.
Those are key findings from a study published March 3 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report that is the first to combine national data on antibiotic-resistant (AR) bacteria threats with progress on health care–associated infections (HAIs).
“Antibiotic resistance threatens to return us to a time when a simple infection could kill,” CDC Director Thomas Frieden said during a March 3 telebriefing. “The more people who get infected with resistant bacteria, the more people who suffer complications, the more who, tragically, may die from preventable infections. On any given day about one in 25 hospitalized patients has at least one health care–associated infection that they didn’t come in with. No one should get sick when they’re trying to get well.”
For the study, researchers led by Dr. Clifford McDonald of the CDC’s Division of Healthcare Quality Promotion, collected data on specific infections that were reported to the National Healthcare Safety Network in 2014 by approximately 4,000 short-term acute care hospitals, 501 long-term acute care hospitals, and 1,135 inpatient rehabilitation facilities in all 50 states (MMWR. 2016 Mar 3. doi: 10.15585/mmwr.mm6509e1er). Next, they determined the proportions of AR pathogens and HAIs caused by any of six resistant bacteria highlighted by the CDC in 2013 as urgent or serious threats: CRE (carbapenem-resistant Enterobacteriaceae), MRSA (methicillin-resistant Staphylococcus aureus), ESBL-producing Enterobacteriaceae (extended-spectrum beta-lactamases), VRE (vancomycin-resistant enterococci), multidrug-resistant pseudomonas, and multidrug-resistant Acinetobacter.
The researchers found that, compared with historical data from 5-8 years earlier, central line–associated bloodstream infections decreased by 50% and surgical site infections (SSIs) by 17% in 2014.
“There is encouraging news here,” Dr. Frieden said. “Doctors, nurses, hospitals, health care systems and other partners have made progress preventing some health care–associated infections.” However, the study found that one in six remaining central line-associated bloodstream infections were caused by urgent or serious antibiotic-resistant bacteria, while one in seven remaining surgical site infections were caused by urgent or serious antibiotic-resistant bacteria.
While catheter-associated urinary tract infections appear unchanged from baseline, there have been recent decreases, according to the study. In addition, C. difficile infections in hospitals decreased 8% between 2011 and 2014.
Dr. McDonald and his associates determined that in 2014, one in seven infections in acute care hospitals related to catheters and surgeries was caused by one of the six antibiotic-resistance threat bacteria, “which is deeply concerning,” Dr. Frieden said. That number increased to one in four infections in long-term acute care hospitals, a proportion that he characterized as “chilling.”
The CDC recommends three strategies that doctors, nurses, and other health care providers should take with every patient, to prevent HAIs and stop the spread of antibiotic resistance:
• Prevent the spread of bacteria between patients. Dr. Peter Pronovost, who participated in the telebriefing, said that he and his associates at Johns Hopkins University in Baltimore “do this by practicing good hand hygiene techniques by wearing sterile equipment when inserting lines.”
• Prevent surgery-related infections and/or placement of a catheter. “Check catheters frequently and remove them when you no longer need them,” advised Dr. Pronovost, director of the Armstrong Institute for Patient Safety and Quality at Johns Hopkins. “Ask if you actually need them before you even place them.”
• Improve antibiotic use through stewardship. This means using “the right antibiotics for the right duration,” Dr. Pronovost said. “Antibiotics could be lifesaving and are necessary for critically ill patients, especially those with septic shock. But these antibiotics need to be adjusted based on lab results and new information about the organisms that are causing these infections. Forty-eight hours after antibiotics are initiated, take a ‘time out.’ Perform a brief but focused assessment to determine if antibiotic therapy is still needed, or if it should be refined. A common mistake we make is to continue vancomycin when there is no presence of MRSA. We often tell our staff at Johns Hopkins, ‘if it doesn’t grow, let it go.’ ”
Dr. Frieden concluded his remarks by noting that physicians and other clinicians on the front lines “need support of their facility leadership,” to prevent HAIs. “Health care facilities, CEOs, and administrators are a major part of the solution. It’s important that they make a priority of infection prevention, sepsis prevention, and antibiotic stewardship. Know your facility’s data and target prevention efforts to ensure improvements in patient safety.”
One in every seven infections in acute care hospitals related to catheters and surgeries was caused by antibiotic-resistant bacteria. In long-term acute care hospitals, that number increased to one in four.
Those are key findings from a study published March 3 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report that is the first to combine national data on antibiotic-resistant (AR) bacteria threats with progress on health care–associated infections (HAIs).
“Antibiotic resistance threatens to return us to a time when a simple infection could kill,” CDC Director Thomas Frieden said during a March 3 telebriefing. “The more people who get infected with resistant bacteria, the more people who suffer complications, the more who, tragically, may die from preventable infections. On any given day about one in 25 hospitalized patients has at least one health care–associated infection that they didn’t come in with. No one should get sick when they’re trying to get well.”
For the study, researchers led by Dr. Clifford McDonald of the CDC’s Division of Healthcare Quality Promotion, collected data on specific infections that were reported to the National Healthcare Safety Network in 2014 by approximately 4,000 short-term acute care hospitals, 501 long-term acute care hospitals, and 1,135 inpatient rehabilitation facilities in all 50 states (MMWR. 2016 Mar 3. doi: 10.15585/mmwr.mm6509e1er). Next, they determined the proportions of AR pathogens and HAIs caused by any of six resistant bacteria highlighted by the CDC in 2013 as urgent or serious threats: CRE (carbapenem-resistant Enterobacteriaceae), MRSA (methicillin-resistant Staphylococcus aureus), ESBL-producing Enterobacteriaceae (extended-spectrum beta-lactamases), VRE (vancomycin-resistant enterococci), multidrug-resistant pseudomonas, and multidrug-resistant Acinetobacter.
The researchers found that, compared with historical data from 5-8 years earlier, central line–associated bloodstream infections decreased by 50% and surgical site infections (SSIs) by 17% in 2014.
“There is encouraging news here,” Dr. Frieden said. “Doctors, nurses, hospitals, health care systems and other partners have made progress preventing some health care–associated infections.” However, the study found that one in six remaining central line-associated bloodstream infections were caused by urgent or serious antibiotic-resistant bacteria, while one in seven remaining surgical site infections were caused by urgent or serious antibiotic-resistant bacteria.
While catheter-associated urinary tract infections appear unchanged from baseline, there have been recent decreases, according to the study. In addition, C. difficile infections in hospitals decreased 8% between 2011 and 2014.
Dr. McDonald and his associates determined that in 2014, one in seven infections in acute care hospitals related to catheters and surgeries was caused by one of the six antibiotic-resistance threat bacteria, “which is deeply concerning,” Dr. Frieden said. That number increased to one in four infections in long-term acute care hospitals, a proportion that he characterized as “chilling.”
The CDC recommends three strategies that doctors, nurses, and other health care providers should take with every patient, to prevent HAIs and stop the spread of antibiotic resistance:
• Prevent the spread of bacteria between patients. Dr. Peter Pronovost, who participated in the telebriefing, said that he and his associates at Johns Hopkins University in Baltimore “do this by practicing good hand hygiene techniques by wearing sterile equipment when inserting lines.”
• Prevent surgery-related infections and/or placement of a catheter. “Check catheters frequently and remove them when you no longer need them,” advised Dr. Pronovost, director of the Armstrong Institute for Patient Safety and Quality at Johns Hopkins. “Ask if you actually need them before you even place them.”
• Improve antibiotic use through stewardship. This means using “the right antibiotics for the right duration,” Dr. Pronovost said. “Antibiotics could be lifesaving and are necessary for critically ill patients, especially those with septic shock. But these antibiotics need to be adjusted based on lab results and new information about the organisms that are causing these infections. Forty-eight hours after antibiotics are initiated, take a ‘time out.’ Perform a brief but focused assessment to determine if antibiotic therapy is still needed, or if it should be refined. A common mistake we make is to continue vancomycin when there is no presence of MRSA. We often tell our staff at Johns Hopkins, ‘if it doesn’t grow, let it go.’ ”
Dr. Frieden concluded his remarks by noting that physicians and other clinicians on the front lines “need support of their facility leadership,” to prevent HAIs. “Health care facilities, CEOs, and administrators are a major part of the solution. It’s important that they make a priority of infection prevention, sepsis prevention, and antibiotic stewardship. Know your facility’s data and target prevention efforts to ensure improvements in patient safety.”
FROM MMWR