User login
Official Newspaper of the American College of Surgeons
Comprehensive mechanical circulatory support guidelines issued
As a further sign of how much mechanical circulatory support for advanced heart failure has matured, the International Society of Heart and Lung Transplantation issued on Jan. 10 the first comprehensive guidelines for all phases of evaluating, implanting, and managing patients who receive left ventricular assist devices or related equipment.
"Traditionally management of patients with mechanical circulatory support [MCS] was very center specific, but because the number of treated patients has increased, and because patients now live with these devices for years, we reached a point where we needed best practices guidelines, an expert consensus on what is the best way to approach this treatment" said Dr. Salpy V. Pamboukian, a cardiologist and one of three cochairs of the guidelines-writing project.
"When MSC started, the role of the devices was as a bridge to heart transplantation, but the field has evolved over the past decade and now MCS for destination therapy has opened a new array of patients who could benefit from these devices," said Dr. Pamboukian, medical director of the MCS device program at the University of Alabama, Birmingham. "We hope these guidelines will serve as a springboard for further research into the long-term management of these patients," she said in an interview.
"As pumps improve and the number of patients with advanced heart failure increases more and more patients will receive a ventricular assist device [VAD], and heart transplant will grow less relevant. These guidelines are much more comprehensive [than anything previously published] and they represent the opinions of the physicians, surgeons, nurses, and other providers who care for these patients," said Dr. David S. Feldman, a cardiologist who is director of the heart failure, VAD, and cardiac transplantation program at the Minneapolis Heart Institute at Abbott Northwestern Hospital, and another cochair of the guidelines committee.
The guidelines, which took about 3 years to produce, came from a committee of 35 health care providers, with initial review by three independent experts followed by additional peer review and then a period of open comment from the society’s membership. The 146-page document consists of more than 250 individual recommendations presented in five sections: patient selection; risk management prior to surgery; intraoperative procedures and immediate postoperative management; in-patient management during the immediate postoperative period; and long-term outpatient management (J. Heart Lung Transplant. 2013;32:157-87).
The writing committee admitted up front in the paper that most of the recommendations are consensus opinions with no clear evidence base. "It’s a limitation," admitted Dr. Pamboukian, "but you need a common approach to patients. Even a busy center may put in 50 or 60 VADs a year. Hopefully, a result of the guidelines is that they will help centers get together and produce the critical mass of patients needed to conduct meaningful trials. It was time to get something on paper; the new guidelines are what we will now work off of." But despite an absence of evidence on which to base many recommendations, "I was pleasantly surprised that there was more consensus than controversy. There was more commonality in our approaches than differences," she added.
The most limited number of recommendations came from the third task force of the panel, which handled intraoperative procedures and immediate postoperative care. Though this section runs 17 pages and deals with topics such as anesthesia, implantation techniques, establishing hemostasis, performing concomitant procedures, methods for explantation, and management of postoperative hemodynamics and bleeding, it contains just three specific recommendations, all dealing with anesthesia. "There are essentially no studies that have looked at how to make things better in the surgical suite," explained Dr. Feldman.
"It’s very challenging to standardize a surgical procedure," added Dr. Pamboukian. "We tried to summarize useful practices, but consensus-based recommendations are difficult to do."
Another topic the guidelines finesse is patient selection. The field is currently trying to sort out the best stage of advanced heart failure for patients to receive mechanical circulatory support. "You’d be amazed at the disparity of who gets these devices now," Dr. Feldman said. In addition, the guideline writing committee decided to defer definitive choices until results are available from a large study starting later this year. The study, Evaluation of VAD Intervention Before Inotropic Therapy (REVIVE-IT), will examine the outcomes of patients with advanced New York Heart Association stage III heart failure who receive a VAD. "It didn’t seem appropriate to address this because of the trial," he added.
Both Dr. Pamboukian and Dr. Feldman agreed that the newly released guidelines will likely be in place for only a couple of years before a revision comes out, testament to the rapid changes in this field. Dr. Feldman cited new VADs from at least two manufacturers expected to enter first-in-man studies this year, and the continued snowballing of VAD implantation rates. The most recent 2012 numbers (through Sept. 30, 2012) from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) showed nearly 2,000 VADS getting implanted into U.S. patients last year, the highest annual rate ever.
"Because the field is growing, a lot of new centers want to establish programs. We want this treatment to reach as many appropriate patients as possible, but we want it to grow responsibly. These guidelines help establish the best practices, and help ensure that patients get the best care wherever they go," Dr. Pamboukian said.
Dr. Pamboukian said that she had no disclosures. Dr. Feldman said that he has received research support from Terumo.
As a further sign of how much mechanical circulatory support for advanced heart failure has matured, the International Society of Heart and Lung Transplantation issued on Jan. 10 the first comprehensive guidelines for all phases of evaluating, implanting, and managing patients who receive left ventricular assist devices or related equipment.
"Traditionally management of patients with mechanical circulatory support [MCS] was very center specific, but because the number of treated patients has increased, and because patients now live with these devices for years, we reached a point where we needed best practices guidelines, an expert consensus on what is the best way to approach this treatment" said Dr. Salpy V. Pamboukian, a cardiologist and one of three cochairs of the guidelines-writing project.
"When MSC started, the role of the devices was as a bridge to heart transplantation, but the field has evolved over the past decade and now MCS for destination therapy has opened a new array of patients who could benefit from these devices," said Dr. Pamboukian, medical director of the MCS device program at the University of Alabama, Birmingham. "We hope these guidelines will serve as a springboard for further research into the long-term management of these patients," she said in an interview.
"As pumps improve and the number of patients with advanced heart failure increases more and more patients will receive a ventricular assist device [VAD], and heart transplant will grow less relevant. These guidelines are much more comprehensive [than anything previously published] and they represent the opinions of the physicians, surgeons, nurses, and other providers who care for these patients," said Dr. David S. Feldman, a cardiologist who is director of the heart failure, VAD, and cardiac transplantation program at the Minneapolis Heart Institute at Abbott Northwestern Hospital, and another cochair of the guidelines committee.
The guidelines, which took about 3 years to produce, came from a committee of 35 health care providers, with initial review by three independent experts followed by additional peer review and then a period of open comment from the society’s membership. The 146-page document consists of more than 250 individual recommendations presented in five sections: patient selection; risk management prior to surgery; intraoperative procedures and immediate postoperative management; in-patient management during the immediate postoperative period; and long-term outpatient management (J. Heart Lung Transplant. 2013;32:157-87).
The writing committee admitted up front in the paper that most of the recommendations are consensus opinions with no clear evidence base. "It’s a limitation," admitted Dr. Pamboukian, "but you need a common approach to patients. Even a busy center may put in 50 or 60 VADs a year. Hopefully, a result of the guidelines is that they will help centers get together and produce the critical mass of patients needed to conduct meaningful trials. It was time to get something on paper; the new guidelines are what we will now work off of." But despite an absence of evidence on which to base many recommendations, "I was pleasantly surprised that there was more consensus than controversy. There was more commonality in our approaches than differences," she added.
The most limited number of recommendations came from the third task force of the panel, which handled intraoperative procedures and immediate postoperative care. Though this section runs 17 pages and deals with topics such as anesthesia, implantation techniques, establishing hemostasis, performing concomitant procedures, methods for explantation, and management of postoperative hemodynamics and bleeding, it contains just three specific recommendations, all dealing with anesthesia. "There are essentially no studies that have looked at how to make things better in the surgical suite," explained Dr. Feldman.
"It’s very challenging to standardize a surgical procedure," added Dr. Pamboukian. "We tried to summarize useful practices, but consensus-based recommendations are difficult to do."
Another topic the guidelines finesse is patient selection. The field is currently trying to sort out the best stage of advanced heart failure for patients to receive mechanical circulatory support. "You’d be amazed at the disparity of who gets these devices now," Dr. Feldman said. In addition, the guideline writing committee decided to defer definitive choices until results are available from a large study starting later this year. The study, Evaluation of VAD Intervention Before Inotropic Therapy (REVIVE-IT), will examine the outcomes of patients with advanced New York Heart Association stage III heart failure who receive a VAD. "It didn’t seem appropriate to address this because of the trial," he added.
Both Dr. Pamboukian and Dr. Feldman agreed that the newly released guidelines will likely be in place for only a couple of years before a revision comes out, testament to the rapid changes in this field. Dr. Feldman cited new VADs from at least two manufacturers expected to enter first-in-man studies this year, and the continued snowballing of VAD implantation rates. The most recent 2012 numbers (through Sept. 30, 2012) from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) showed nearly 2,000 VADS getting implanted into U.S. patients last year, the highest annual rate ever.
"Because the field is growing, a lot of new centers want to establish programs. We want this treatment to reach as many appropriate patients as possible, but we want it to grow responsibly. These guidelines help establish the best practices, and help ensure that patients get the best care wherever they go," Dr. Pamboukian said.
Dr. Pamboukian said that she had no disclosures. Dr. Feldman said that he has received research support from Terumo.
As a further sign of how much mechanical circulatory support for advanced heart failure has matured, the International Society of Heart and Lung Transplantation issued on Jan. 10 the first comprehensive guidelines for all phases of evaluating, implanting, and managing patients who receive left ventricular assist devices or related equipment.
"Traditionally management of patients with mechanical circulatory support [MCS] was very center specific, but because the number of treated patients has increased, and because patients now live with these devices for years, we reached a point where we needed best practices guidelines, an expert consensus on what is the best way to approach this treatment" said Dr. Salpy V. Pamboukian, a cardiologist and one of three cochairs of the guidelines-writing project.
"When MSC started, the role of the devices was as a bridge to heart transplantation, but the field has evolved over the past decade and now MCS for destination therapy has opened a new array of patients who could benefit from these devices," said Dr. Pamboukian, medical director of the MCS device program at the University of Alabama, Birmingham. "We hope these guidelines will serve as a springboard for further research into the long-term management of these patients," she said in an interview.
"As pumps improve and the number of patients with advanced heart failure increases more and more patients will receive a ventricular assist device [VAD], and heart transplant will grow less relevant. These guidelines are much more comprehensive [than anything previously published] and they represent the opinions of the physicians, surgeons, nurses, and other providers who care for these patients," said Dr. David S. Feldman, a cardiologist who is director of the heart failure, VAD, and cardiac transplantation program at the Minneapolis Heart Institute at Abbott Northwestern Hospital, and another cochair of the guidelines committee.
The guidelines, which took about 3 years to produce, came from a committee of 35 health care providers, with initial review by three independent experts followed by additional peer review and then a period of open comment from the society’s membership. The 146-page document consists of more than 250 individual recommendations presented in five sections: patient selection; risk management prior to surgery; intraoperative procedures and immediate postoperative management; in-patient management during the immediate postoperative period; and long-term outpatient management (J. Heart Lung Transplant. 2013;32:157-87).
The writing committee admitted up front in the paper that most of the recommendations are consensus opinions with no clear evidence base. "It’s a limitation," admitted Dr. Pamboukian, "but you need a common approach to patients. Even a busy center may put in 50 or 60 VADs a year. Hopefully, a result of the guidelines is that they will help centers get together and produce the critical mass of patients needed to conduct meaningful trials. It was time to get something on paper; the new guidelines are what we will now work off of." But despite an absence of evidence on which to base many recommendations, "I was pleasantly surprised that there was more consensus than controversy. There was more commonality in our approaches than differences," she added.
The most limited number of recommendations came from the third task force of the panel, which handled intraoperative procedures and immediate postoperative care. Though this section runs 17 pages and deals with topics such as anesthesia, implantation techniques, establishing hemostasis, performing concomitant procedures, methods for explantation, and management of postoperative hemodynamics and bleeding, it contains just three specific recommendations, all dealing with anesthesia. "There are essentially no studies that have looked at how to make things better in the surgical suite," explained Dr. Feldman.
"It’s very challenging to standardize a surgical procedure," added Dr. Pamboukian. "We tried to summarize useful practices, but consensus-based recommendations are difficult to do."
Another topic the guidelines finesse is patient selection. The field is currently trying to sort out the best stage of advanced heart failure for patients to receive mechanical circulatory support. "You’d be amazed at the disparity of who gets these devices now," Dr. Feldman said. In addition, the guideline writing committee decided to defer definitive choices until results are available from a large study starting later this year. The study, Evaluation of VAD Intervention Before Inotropic Therapy (REVIVE-IT), will examine the outcomes of patients with advanced New York Heart Association stage III heart failure who receive a VAD. "It didn’t seem appropriate to address this because of the trial," he added.
Both Dr. Pamboukian and Dr. Feldman agreed that the newly released guidelines will likely be in place for only a couple of years before a revision comes out, testament to the rapid changes in this field. Dr. Feldman cited new VADs from at least two manufacturers expected to enter first-in-man studies this year, and the continued snowballing of VAD implantation rates. The most recent 2012 numbers (through Sept. 30, 2012) from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) showed nearly 2,000 VADS getting implanted into U.S. patients last year, the highest annual rate ever.
"Because the field is growing, a lot of new centers want to establish programs. We want this treatment to reach as many appropriate patients as possible, but we want it to grow responsibly. These guidelines help establish the best practices, and help ensure that patients get the best care wherever they go," Dr. Pamboukian said.
Dr. Pamboukian said that she had no disclosures. Dr. Feldman said that he has received research support from Terumo.
FROM THE JOURNAL OF HEART AND LUNG TRANSPLANTATION
Night on call has no effect on next-day operations
PALM BEACH, FLA. – Surgeons who performed routine, elective operations the day after they spent all night working an in-hospital shift in the trauma unit had no increased rate of complications, no need for hospital readmissions, and no mortality among their elective cases in a single-center review of 869 cases.
The findings suggest that "there remains no compelling evidence to mandate work-hour restrictions for attending general surgeons," Dr. Martin A. Croce said at the annual meeting of the Southern Surgical Association.
"No study to date has evaluated the effect of sleep deprivation on outcomes specific to the practice of general surgery. This study looked at the impact of an overnight shift in a busy trauma center on the outcomes of typical general surgery procedures performed the next day" and found no significant difference in outcomes between post-call and non–post-call surgeons with respect to postoperative complications, readmissions, or deaths, said Dr. Croce, professor of surgery and chief of the division of trauma and critical care at the University of Tennessee Health Science Center in Memphis.
"These results confirm my bias, but the study was limited by its retrospective, single-institution nature, and by only including tertiary teaching patients. Each [surgeon] must continue to use judgment when deciding whether to perform elective operations after a busy night on call," commented Dr. William G. Cioffi Jr., professor and chairman of surgery at Brown University, Providence, R.I.
"It is unclear to me that the surgical community will need to present these results because there has been no call for work-hour restrictions for attendings, nor do I feel there will be," said Dr. L.D. Britt, professor and chairman of surgery at Eastern Virginia Medical School, Norfolk.
In his study, Dr. Croce reviewed patients who underwent any of three types of general surgeries at his institution during January 2006 to April 2009 by one of the nine attending surgeons in the department. The review comprised 869 procedures: 46% were hernia repairs, 35% were cholecystectomies, and 19% were bowel operations. The primary outcomes were 30-day postsurgical mortality, 30-day postsurgical readmissions, and procedure-related complications: wound infections, seroma, injured adjacent structures, intra-abdominal abscess, hemorrhage, dehiscence, leak, or perforation of the gallbladder during laparoscopic cholecystectomy. Overall, patients had a 13% complication rate, a 5% readmission rate, and mortality of less than 1%.
Among the 869 procedures, 132 (15%) were done by surgeons following a night on call and the remaining 737 (85%) were done by surgeons not on call the prior night. In a multivariable regression analysis that controlled for patient’s age, urgency of the elective surgery, and comorbidities, the rates of complications, readmissions, and deaths were similar between those done by surgeons following a night on call and those done without working the night before, Dr. Croce reported.
Further analyses showed no significant difference between the two surgeon subgroups when the types of procedures were broken down into hernia repair, cholecystectomy, or bowel operation.
A limitation of the study was that the findings apply only to these three types of surgeries; they should not be extrapolated to other, more complex surgeries, said Dr. John P. Sharpe, a surgeon at the University of Tennessee who collaborated with Dr. Croce on the study.
Dr. Croce, Dr. Cioffi, Dr. Britt, and Dr. Sharpe had no disclosures.
PALM BEACH, FLA. – Surgeons who performed routine, elective operations the day after they spent all night working an in-hospital shift in the trauma unit had no increased rate of complications, no need for hospital readmissions, and no mortality among their elective cases in a single-center review of 869 cases.
The findings suggest that "there remains no compelling evidence to mandate work-hour restrictions for attending general surgeons," Dr. Martin A. Croce said at the annual meeting of the Southern Surgical Association.
"No study to date has evaluated the effect of sleep deprivation on outcomes specific to the practice of general surgery. This study looked at the impact of an overnight shift in a busy trauma center on the outcomes of typical general surgery procedures performed the next day" and found no significant difference in outcomes between post-call and non–post-call surgeons with respect to postoperative complications, readmissions, or deaths, said Dr. Croce, professor of surgery and chief of the division of trauma and critical care at the University of Tennessee Health Science Center in Memphis.
"These results confirm my bias, but the study was limited by its retrospective, single-institution nature, and by only including tertiary teaching patients. Each [surgeon] must continue to use judgment when deciding whether to perform elective operations after a busy night on call," commented Dr. William G. Cioffi Jr., professor and chairman of surgery at Brown University, Providence, R.I.
"It is unclear to me that the surgical community will need to present these results because there has been no call for work-hour restrictions for attendings, nor do I feel there will be," said Dr. L.D. Britt, professor and chairman of surgery at Eastern Virginia Medical School, Norfolk.
In his study, Dr. Croce reviewed patients who underwent any of three types of general surgeries at his institution during January 2006 to April 2009 by one of the nine attending surgeons in the department. The review comprised 869 procedures: 46% were hernia repairs, 35% were cholecystectomies, and 19% were bowel operations. The primary outcomes were 30-day postsurgical mortality, 30-day postsurgical readmissions, and procedure-related complications: wound infections, seroma, injured adjacent structures, intra-abdominal abscess, hemorrhage, dehiscence, leak, or perforation of the gallbladder during laparoscopic cholecystectomy. Overall, patients had a 13% complication rate, a 5% readmission rate, and mortality of less than 1%.
Among the 869 procedures, 132 (15%) were done by surgeons following a night on call and the remaining 737 (85%) were done by surgeons not on call the prior night. In a multivariable regression analysis that controlled for patient’s age, urgency of the elective surgery, and comorbidities, the rates of complications, readmissions, and deaths were similar between those done by surgeons following a night on call and those done without working the night before, Dr. Croce reported.
Further analyses showed no significant difference between the two surgeon subgroups when the types of procedures were broken down into hernia repair, cholecystectomy, or bowel operation.
A limitation of the study was that the findings apply only to these three types of surgeries; they should not be extrapolated to other, more complex surgeries, said Dr. John P. Sharpe, a surgeon at the University of Tennessee who collaborated with Dr. Croce on the study.
Dr. Croce, Dr. Cioffi, Dr. Britt, and Dr. Sharpe had no disclosures.
PALM BEACH, FLA. – Surgeons who performed routine, elective operations the day after they spent all night working an in-hospital shift in the trauma unit had no increased rate of complications, no need for hospital readmissions, and no mortality among their elective cases in a single-center review of 869 cases.
The findings suggest that "there remains no compelling evidence to mandate work-hour restrictions for attending general surgeons," Dr. Martin A. Croce said at the annual meeting of the Southern Surgical Association.
"No study to date has evaluated the effect of sleep deprivation on outcomes specific to the practice of general surgery. This study looked at the impact of an overnight shift in a busy trauma center on the outcomes of typical general surgery procedures performed the next day" and found no significant difference in outcomes between post-call and non–post-call surgeons with respect to postoperative complications, readmissions, or deaths, said Dr. Croce, professor of surgery and chief of the division of trauma and critical care at the University of Tennessee Health Science Center in Memphis.
"These results confirm my bias, but the study was limited by its retrospective, single-institution nature, and by only including tertiary teaching patients. Each [surgeon] must continue to use judgment when deciding whether to perform elective operations after a busy night on call," commented Dr. William G. Cioffi Jr., professor and chairman of surgery at Brown University, Providence, R.I.
"It is unclear to me that the surgical community will need to present these results because there has been no call for work-hour restrictions for attendings, nor do I feel there will be," said Dr. L.D. Britt, professor and chairman of surgery at Eastern Virginia Medical School, Norfolk.
In his study, Dr. Croce reviewed patients who underwent any of three types of general surgeries at his institution during January 2006 to April 2009 by one of the nine attending surgeons in the department. The review comprised 869 procedures: 46% were hernia repairs, 35% were cholecystectomies, and 19% were bowel operations. The primary outcomes were 30-day postsurgical mortality, 30-day postsurgical readmissions, and procedure-related complications: wound infections, seroma, injured adjacent structures, intra-abdominal abscess, hemorrhage, dehiscence, leak, or perforation of the gallbladder during laparoscopic cholecystectomy. Overall, patients had a 13% complication rate, a 5% readmission rate, and mortality of less than 1%.
Among the 869 procedures, 132 (15%) were done by surgeons following a night on call and the remaining 737 (85%) were done by surgeons not on call the prior night. In a multivariable regression analysis that controlled for patient’s age, urgency of the elective surgery, and comorbidities, the rates of complications, readmissions, and deaths were similar between those done by surgeons following a night on call and those done without working the night before, Dr. Croce reported.
Further analyses showed no significant difference between the two surgeon subgroups when the types of procedures were broken down into hernia repair, cholecystectomy, or bowel operation.
A limitation of the study was that the findings apply only to these three types of surgeries; they should not be extrapolated to other, more complex surgeries, said Dr. John P. Sharpe, a surgeon at the University of Tennessee who collaborated with Dr. Croce on the study.
Dr. Croce, Dr. Cioffi, Dr. Britt, and Dr. Sharpe had no disclosures.
AT THE ANNUAL MEETING OF THE SOUTHERN SURGICAL ASSOCIATION
Major Finding: General surgeons who performed 132 elective operations after a night on call had no increased complications, readmissions, or deaths, compared with surgeons not on call the previous night (737 surgeries).
Data Source: A review of 869 surgeries at one U.S. center during 2006-2009.
Disclosures: Dr. Croce, Dr. Cioffi, Dr. Britt, and Dr. Sharpe had no disclosures.
Clinical decision support in search of a smarter EHR
We have written routinely about the positive impact of implementing an electronic health record, citing potential improvements in areas such as charge capture, data sharing, and population management. In an attempt to be balanced, we’ve also discussed the financial implications and the risks of decreased productivity and provider frustration, among others. One area that we have not focused on – but which has been attracting increasingly more attention – is that of the advantages and limitations of Clinical Decision Support Systems (CDSSs).
CDSSs are tools that add evidence-based clinical intelligence to patient care, providing assistance to the provider as he or she treats patients and makes decisions about their management. A simple example of this would be an alert, reminding a physician to provide an immunization to age-appropriate patients while seeing them in the office. Some EHRs ship with this capability built-in and ready for deployment "right out of the box," while others completely lack real-decision support. Most commonly, however, an EHR will have the capability to provide support but rely heavily on end-user customization prior to implementation. The question that many are beginning to ask is how using a clinical decision support system will ultimately affect patient outcomes.
The promise and liability of clinical intelligence
There is no question that the medical community has accepted the concept of guideline-based workflows and the importance of evidence-based medicine at the point of care. More recently, though, several studies have begun to look at how CDSS tools that are packaged into EHRs have affected care delivery. Surprisingly, the results are inconsistent; while many studies have demonstrated the benefits of decision support, others have not shown impressive changes in patient outcomes.
Findings from a review of 100 studies comparing the outcomes in care provided with and without a CDSS showed that 64% of the studies demonstrated improvements in practitioner performance when using a Clinical Decision Support System. While the specific systems varied in type and purpose, improvements in performance were "associated with CDSSs that automatically prompted users," compared with those "requiring users to activate the system," (JAMA 2005;293:1223-38).
Similar results were found in a multidisciplinary randomized trial pin which investigators analyzed data from 21 centers and demonstrated that "computerized decision support increased concordance with guideline-recommended therapeutic decisions" for numerous treatment options and "reduced cases of both overtreatment and undertreatment" (BMJ 2009;338:b1440 [doi:10.1136/bmj.b1440]).
But not all of the studies have been so optimistic. Findings from a more recent study showed that there is little benefit to having a CDSS in place. Using survey data collected from over 250,000 ambulatory patient visits (sourced from the National Ambulatory Medical Care Survey), they discovered that only 1 of 20 quality indicators proved better in the group of patients treated using EHRs with a CDSS in place, compared with those treated without decision support. The investigators offered little explanation for these unexpected results, but they did cite some limitations in their methods and theorized that the value of current support systems may be minimal in the absence of standardization and better quality control (Arch. Intern. Med. 2011;171:897-903).
Searching for help
To meet certification for meaningful use, electronic records are required to have some minimal CDSS functionality available from Day 1. But in our experience with most products, the depth and breadth of this built-in support is sorely lacking. For some practitioners who simply view the EMR as a more complicated way of documenting progress notes and telephone calls, this might not seem like a big deal. After all, the world of paper offered no clinical intelligence to speak of. But for others hoping to realize the true promises of health information technology, high-quality decision support may be essential.
It is again important to point out that the usefulness of clinical decision support systems is typically limited by the EHR itself, so it’s critical to start investigating CDSS capability when first selecting an EHR. We would encourage everyone to request to see a demonstration of what – if any – decision support is present in the EHRs they are considering, and ask a lot of questions about how the information is accessed and kept current. Does the product have a standard toolset based on outdated practice suggestions or is it updated as new guidelines are published and research is released? Is the information customizable to meet the needs of the implementation, or is it a "one-size-fits-all" solution? Finally, is the information passive or active? In other words, does the provider need to go searching for the support, or is the software smart enough to offer support when appropriate in the form of an "alert" or "pop-up"?
A tale of art and science
When chess champion Gary Kasparov defeated IBM’s Deep Blue Supercomputer back in 1996, people around the globe shared in a warm feeling of vindication. More than a simple win, Kasparov’s victory proved that humans still had the advantage over machines. In the same way, it is possible to find the data questioning the value of CDSSs oddly reassuring. But the irony of history reminds us not to get comfortable in our assertions; just 1 year later, after extensive enhancements, Deep Blue returned to defeat Kasparov in a devastating rematch. We suggest viewing this irony as instructive; if one accepts – as we do unequivocally – the value of evidence-based medicine, one must also accept that the right decision support delivered in a timely fashion will ultimately lead to better care and improved clinical outcomes.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is also editor in chief of Redi-Reference, a software company that creates medical handheld references. Dr. Notte practices family medicine and health care informatics for Abington Memorial Hospital. They are partners in EHR Practice Consultants, helping practices move to EHR systems. Contact them at [email protected].
We have written routinely about the positive impact of implementing an electronic health record, citing potential improvements in areas such as charge capture, data sharing, and population management. In an attempt to be balanced, we’ve also discussed the financial implications and the risks of decreased productivity and provider frustration, among others. One area that we have not focused on – but which has been attracting increasingly more attention – is that of the advantages and limitations of Clinical Decision Support Systems (CDSSs).
CDSSs are tools that add evidence-based clinical intelligence to patient care, providing assistance to the provider as he or she treats patients and makes decisions about their management. A simple example of this would be an alert, reminding a physician to provide an immunization to age-appropriate patients while seeing them in the office. Some EHRs ship with this capability built-in and ready for deployment "right out of the box," while others completely lack real-decision support. Most commonly, however, an EHR will have the capability to provide support but rely heavily on end-user customization prior to implementation. The question that many are beginning to ask is how using a clinical decision support system will ultimately affect patient outcomes.
The promise and liability of clinical intelligence
There is no question that the medical community has accepted the concept of guideline-based workflows and the importance of evidence-based medicine at the point of care. More recently, though, several studies have begun to look at how CDSS tools that are packaged into EHRs have affected care delivery. Surprisingly, the results are inconsistent; while many studies have demonstrated the benefits of decision support, others have not shown impressive changes in patient outcomes.
Findings from a review of 100 studies comparing the outcomes in care provided with and without a CDSS showed that 64% of the studies demonstrated improvements in practitioner performance when using a Clinical Decision Support System. While the specific systems varied in type and purpose, improvements in performance were "associated with CDSSs that automatically prompted users," compared with those "requiring users to activate the system," (JAMA 2005;293:1223-38).
Similar results were found in a multidisciplinary randomized trial pin which investigators analyzed data from 21 centers and demonstrated that "computerized decision support increased concordance with guideline-recommended therapeutic decisions" for numerous treatment options and "reduced cases of both overtreatment and undertreatment" (BMJ 2009;338:b1440 [doi:10.1136/bmj.b1440]).
But not all of the studies have been so optimistic. Findings from a more recent study showed that there is little benefit to having a CDSS in place. Using survey data collected from over 250,000 ambulatory patient visits (sourced from the National Ambulatory Medical Care Survey), they discovered that only 1 of 20 quality indicators proved better in the group of patients treated using EHRs with a CDSS in place, compared with those treated without decision support. The investigators offered little explanation for these unexpected results, but they did cite some limitations in their methods and theorized that the value of current support systems may be minimal in the absence of standardization and better quality control (Arch. Intern. Med. 2011;171:897-903).
Searching for help
To meet certification for meaningful use, electronic records are required to have some minimal CDSS functionality available from Day 1. But in our experience with most products, the depth and breadth of this built-in support is sorely lacking. For some practitioners who simply view the EMR as a more complicated way of documenting progress notes and telephone calls, this might not seem like a big deal. After all, the world of paper offered no clinical intelligence to speak of. But for others hoping to realize the true promises of health information technology, high-quality decision support may be essential.
It is again important to point out that the usefulness of clinical decision support systems is typically limited by the EHR itself, so it’s critical to start investigating CDSS capability when first selecting an EHR. We would encourage everyone to request to see a demonstration of what – if any – decision support is present in the EHRs they are considering, and ask a lot of questions about how the information is accessed and kept current. Does the product have a standard toolset based on outdated practice suggestions or is it updated as new guidelines are published and research is released? Is the information customizable to meet the needs of the implementation, or is it a "one-size-fits-all" solution? Finally, is the information passive or active? In other words, does the provider need to go searching for the support, or is the software smart enough to offer support when appropriate in the form of an "alert" or "pop-up"?
A tale of art and science
When chess champion Gary Kasparov defeated IBM’s Deep Blue Supercomputer back in 1996, people around the globe shared in a warm feeling of vindication. More than a simple win, Kasparov’s victory proved that humans still had the advantage over machines. In the same way, it is possible to find the data questioning the value of CDSSs oddly reassuring. But the irony of history reminds us not to get comfortable in our assertions; just 1 year later, after extensive enhancements, Deep Blue returned to defeat Kasparov in a devastating rematch. We suggest viewing this irony as instructive; if one accepts – as we do unequivocally – the value of evidence-based medicine, one must also accept that the right decision support delivered in a timely fashion will ultimately lead to better care and improved clinical outcomes.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is also editor in chief of Redi-Reference, a software company that creates medical handheld references. Dr. Notte practices family medicine and health care informatics for Abington Memorial Hospital. They are partners in EHR Practice Consultants, helping practices move to EHR systems. Contact them at [email protected].
We have written routinely about the positive impact of implementing an electronic health record, citing potential improvements in areas such as charge capture, data sharing, and population management. In an attempt to be balanced, we’ve also discussed the financial implications and the risks of decreased productivity and provider frustration, among others. One area that we have not focused on – but which has been attracting increasingly more attention – is that of the advantages and limitations of Clinical Decision Support Systems (CDSSs).
CDSSs are tools that add evidence-based clinical intelligence to patient care, providing assistance to the provider as he or she treats patients and makes decisions about their management. A simple example of this would be an alert, reminding a physician to provide an immunization to age-appropriate patients while seeing them in the office. Some EHRs ship with this capability built-in and ready for deployment "right out of the box," while others completely lack real-decision support. Most commonly, however, an EHR will have the capability to provide support but rely heavily on end-user customization prior to implementation. The question that many are beginning to ask is how using a clinical decision support system will ultimately affect patient outcomes.
The promise and liability of clinical intelligence
There is no question that the medical community has accepted the concept of guideline-based workflows and the importance of evidence-based medicine at the point of care. More recently, though, several studies have begun to look at how CDSS tools that are packaged into EHRs have affected care delivery. Surprisingly, the results are inconsistent; while many studies have demonstrated the benefits of decision support, others have not shown impressive changes in patient outcomes.
Findings from a review of 100 studies comparing the outcomes in care provided with and without a CDSS showed that 64% of the studies demonstrated improvements in practitioner performance when using a Clinical Decision Support System. While the specific systems varied in type and purpose, improvements in performance were "associated with CDSSs that automatically prompted users," compared with those "requiring users to activate the system," (JAMA 2005;293:1223-38).
Similar results were found in a multidisciplinary randomized trial pin which investigators analyzed data from 21 centers and demonstrated that "computerized decision support increased concordance with guideline-recommended therapeutic decisions" for numerous treatment options and "reduced cases of both overtreatment and undertreatment" (BMJ 2009;338:b1440 [doi:10.1136/bmj.b1440]).
But not all of the studies have been so optimistic. Findings from a more recent study showed that there is little benefit to having a CDSS in place. Using survey data collected from over 250,000 ambulatory patient visits (sourced from the National Ambulatory Medical Care Survey), they discovered that only 1 of 20 quality indicators proved better in the group of patients treated using EHRs with a CDSS in place, compared with those treated without decision support. The investigators offered little explanation for these unexpected results, but they did cite some limitations in their methods and theorized that the value of current support systems may be minimal in the absence of standardization and better quality control (Arch. Intern. Med. 2011;171:897-903).
Searching for help
To meet certification for meaningful use, electronic records are required to have some minimal CDSS functionality available from Day 1. But in our experience with most products, the depth and breadth of this built-in support is sorely lacking. For some practitioners who simply view the EMR as a more complicated way of documenting progress notes and telephone calls, this might not seem like a big deal. After all, the world of paper offered no clinical intelligence to speak of. But for others hoping to realize the true promises of health information technology, high-quality decision support may be essential.
It is again important to point out that the usefulness of clinical decision support systems is typically limited by the EHR itself, so it’s critical to start investigating CDSS capability when first selecting an EHR. We would encourage everyone to request to see a demonstration of what – if any – decision support is present in the EHRs they are considering, and ask a lot of questions about how the information is accessed and kept current. Does the product have a standard toolset based on outdated practice suggestions or is it updated as new guidelines are published and research is released? Is the information customizable to meet the needs of the implementation, or is it a "one-size-fits-all" solution? Finally, is the information passive or active? In other words, does the provider need to go searching for the support, or is the software smart enough to offer support when appropriate in the form of an "alert" or "pop-up"?
A tale of art and science
When chess champion Gary Kasparov defeated IBM’s Deep Blue Supercomputer back in 1996, people around the globe shared in a warm feeling of vindication. More than a simple win, Kasparov’s victory proved that humans still had the advantage over machines. In the same way, it is possible to find the data questioning the value of CDSSs oddly reassuring. But the irony of history reminds us not to get comfortable in our assertions; just 1 year later, after extensive enhancements, Deep Blue returned to defeat Kasparov in a devastating rematch. We suggest viewing this irony as instructive; if one accepts – as we do unequivocally – the value of evidence-based medicine, one must also accept that the right decision support delivered in a timely fashion will ultimately lead to better care and improved clinical outcomes.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is also editor in chief of Redi-Reference, a software company that creates medical handheld references. Dr. Notte practices family medicine and health care informatics for Abington Memorial Hospital. They are partners in EHR Practice Consultants, helping practices move to EHR systems. Contact them at [email protected].
My angry patient
"Dr. Pistoria, they need you over in the preop holding area."
There was no urgency in the request, no indication that I was needed immediately. Nonetheless, I went right over. Once there, I was briefed on the situation – a patient, scheduled to go to the OR for joint replacement had coughed up some blood. The surgery was cancelled and now she was angry. I was being asked to see the patient for the possible hemoptysis and determine whether she could be safely sent home or if we should admit her.
The anesthesiologist and the nursing staff concurred: This patient was mad and rude and, everyone’s favorite word, "difficult." I took a deep breath and wandered back into the bay where she lay on the litter. I introduced myself and explained that I was a hospital medicine physician there to help evaluate her. She stared at me with a look that bordered on disdain and launched into her story.
I let her talk, maintaining eye contact the whole time. I nodded and made appropriately empathetic facial expressions as she spoke. She told me how the surgery was initially set up for 2 weeks prior but was cancelled for reasons beyond her control. She explained how much her shoulder was bothering her and limiting her ability to function in the way she once did. She talked about the fact that she had an upper respiratory infection that had left her with a nagging cough. She expressed her anger that her surgery was cancelled again.
When she was done, I told her that I was sorry she had two surgeries cancelled within the span of several weeks. I said I could not imagine how frustrating it must be to have constant pain and limitations in ability, and that I would be angry as well if my surgery – the surgery I was hoping would ease my pain – were cancelled yet again. I also explained that her surgeon felt she was at too much risk to operate safely today given the blood she had coughed up. I said that my role was to determine whether this incident was something we needed to worry about, and I walked her through how I would do that.
I could see the anger slowly dissipate as I spoke. This was an individual who wanted to feel better and wanted someone to acknowledge that the situation stank.
I took a full history and examined her. I reviewed her chest x-ray and went over my findings and thoughts with her. I was not concerned about the blood – it was likely due to irritation to her throat from her persistent and occasionally violent coughing. When she said she could not get a ride home, I put her on my service for observation and to allow her nerve block to wear off. In the end, the angry and suspicious patient who had greeted me was kind and appreciative.
A key part of health care reform is the concept of shared decision making. We need to work with our patients and find mutually agreeable treatment plans, based upon the best available evidence. In order to get there, we need to develop better relationships with our patients. Sometimes patients just need to vent, and they need to have an affirmation that, yes, their situation stinks. Those needs often run counter to our collective need and desire to have things run smoothly and without complication – particularly a complication that will require our time to listen. However, that time and listening can make all the difference in the world for that patient.
A little bit of empathy and a caring ear can show exactly how important that patient is to us. As we move toward a model of shared decision making, listening is a skill we must acquire and hone. A little bit can go a long way.
Michael Pistoria, D.O., is chief of hospital medicine at Coordinated Health in Bethlehem, Pa.
"Dr. Pistoria, they need you over in the preop holding area."
There was no urgency in the request, no indication that I was needed immediately. Nonetheless, I went right over. Once there, I was briefed on the situation – a patient, scheduled to go to the OR for joint replacement had coughed up some blood. The surgery was cancelled and now she was angry. I was being asked to see the patient for the possible hemoptysis and determine whether she could be safely sent home or if we should admit her.
The anesthesiologist and the nursing staff concurred: This patient was mad and rude and, everyone’s favorite word, "difficult." I took a deep breath and wandered back into the bay where she lay on the litter. I introduced myself and explained that I was a hospital medicine physician there to help evaluate her. She stared at me with a look that bordered on disdain and launched into her story.
I let her talk, maintaining eye contact the whole time. I nodded and made appropriately empathetic facial expressions as she spoke. She told me how the surgery was initially set up for 2 weeks prior but was cancelled for reasons beyond her control. She explained how much her shoulder was bothering her and limiting her ability to function in the way she once did. She talked about the fact that she had an upper respiratory infection that had left her with a nagging cough. She expressed her anger that her surgery was cancelled again.
When she was done, I told her that I was sorry she had two surgeries cancelled within the span of several weeks. I said I could not imagine how frustrating it must be to have constant pain and limitations in ability, and that I would be angry as well if my surgery – the surgery I was hoping would ease my pain – were cancelled yet again. I also explained that her surgeon felt she was at too much risk to operate safely today given the blood she had coughed up. I said that my role was to determine whether this incident was something we needed to worry about, and I walked her through how I would do that.
I could see the anger slowly dissipate as I spoke. This was an individual who wanted to feel better and wanted someone to acknowledge that the situation stank.
I took a full history and examined her. I reviewed her chest x-ray and went over my findings and thoughts with her. I was not concerned about the blood – it was likely due to irritation to her throat from her persistent and occasionally violent coughing. When she said she could not get a ride home, I put her on my service for observation and to allow her nerve block to wear off. In the end, the angry and suspicious patient who had greeted me was kind and appreciative.
A key part of health care reform is the concept of shared decision making. We need to work with our patients and find mutually agreeable treatment plans, based upon the best available evidence. In order to get there, we need to develop better relationships with our patients. Sometimes patients just need to vent, and they need to have an affirmation that, yes, their situation stinks. Those needs often run counter to our collective need and desire to have things run smoothly and without complication – particularly a complication that will require our time to listen. However, that time and listening can make all the difference in the world for that patient.
A little bit of empathy and a caring ear can show exactly how important that patient is to us. As we move toward a model of shared decision making, listening is a skill we must acquire and hone. A little bit can go a long way.
Michael Pistoria, D.O., is chief of hospital medicine at Coordinated Health in Bethlehem, Pa.
"Dr. Pistoria, they need you over in the preop holding area."
There was no urgency in the request, no indication that I was needed immediately. Nonetheless, I went right over. Once there, I was briefed on the situation – a patient, scheduled to go to the OR for joint replacement had coughed up some blood. The surgery was cancelled and now she was angry. I was being asked to see the patient for the possible hemoptysis and determine whether she could be safely sent home or if we should admit her.
The anesthesiologist and the nursing staff concurred: This patient was mad and rude and, everyone’s favorite word, "difficult." I took a deep breath and wandered back into the bay where she lay on the litter. I introduced myself and explained that I was a hospital medicine physician there to help evaluate her. She stared at me with a look that bordered on disdain and launched into her story.
I let her talk, maintaining eye contact the whole time. I nodded and made appropriately empathetic facial expressions as she spoke. She told me how the surgery was initially set up for 2 weeks prior but was cancelled for reasons beyond her control. She explained how much her shoulder was bothering her and limiting her ability to function in the way she once did. She talked about the fact that she had an upper respiratory infection that had left her with a nagging cough. She expressed her anger that her surgery was cancelled again.
When she was done, I told her that I was sorry she had two surgeries cancelled within the span of several weeks. I said I could not imagine how frustrating it must be to have constant pain and limitations in ability, and that I would be angry as well if my surgery – the surgery I was hoping would ease my pain – were cancelled yet again. I also explained that her surgeon felt she was at too much risk to operate safely today given the blood she had coughed up. I said that my role was to determine whether this incident was something we needed to worry about, and I walked her through how I would do that.
I could see the anger slowly dissipate as I spoke. This was an individual who wanted to feel better and wanted someone to acknowledge that the situation stank.
I took a full history and examined her. I reviewed her chest x-ray and went over my findings and thoughts with her. I was not concerned about the blood – it was likely due to irritation to her throat from her persistent and occasionally violent coughing. When she said she could not get a ride home, I put her on my service for observation and to allow her nerve block to wear off. In the end, the angry and suspicious patient who had greeted me was kind and appreciative.
A key part of health care reform is the concept of shared decision making. We need to work with our patients and find mutually agreeable treatment plans, based upon the best available evidence. In order to get there, we need to develop better relationships with our patients. Sometimes patients just need to vent, and they need to have an affirmation that, yes, their situation stinks. Those needs often run counter to our collective need and desire to have things run smoothly and without complication – particularly a complication that will require our time to listen. However, that time and listening can make all the difference in the world for that patient.
A little bit of empathy and a caring ear can show exactly how important that patient is to us. As we move toward a model of shared decision making, listening is a skill we must acquire and hone. A little bit can go a long way.
Michael Pistoria, D.O., is chief of hospital medicine at Coordinated Health in Bethlehem, Pa.
Health spending, doc pay: The Policy & Practice Podcast
Health care spending continues to grow – no surprise there – according to an annual federal government report. While hospital payments are still responsible for the largest portion of the nation’s health spending, provisions of the Affordable Care Act have already started to slow spending growth on hospital services. That’s not yet the case for physician and clinical services, for which Medicare spending grew by 8 percent in 2011 (the most recent data).
At the same time, the administration is touting their progress in approving a total of 250 Accountable Care Organizations, or ACOs, half of which are led by physicians.
The Department of Health and Human Services estimates it can save up to $940 million over four years by using this model.
Still, the need for a permanent solution to physician Medicare payments was the source of exasperation at the most recent meeting of the Medicare Payment Advisory Commission. Commissioners said they’re tired of the year-to-year patches and that replacing the flawed SGR system just makes sense. For more details on that, check out this week’s Policy & Practice podcast.
-- Frances Correa (FMCReporting)
Health care spending continues to grow – no surprise there – according to an annual federal government report. While hospital payments are still responsible for the largest portion of the nation’s health spending, provisions of the Affordable Care Act have already started to slow spending growth on hospital services. That’s not yet the case for physician and clinical services, for which Medicare spending grew by 8 percent in 2011 (the most recent data).
At the same time, the administration is touting their progress in approving a total of 250 Accountable Care Organizations, or ACOs, half of which are led by physicians.
The Department of Health and Human Services estimates it can save up to $940 million over four years by using this model.
Still, the need for a permanent solution to physician Medicare payments was the source of exasperation at the most recent meeting of the Medicare Payment Advisory Commission. Commissioners said they’re tired of the year-to-year patches and that replacing the flawed SGR system just makes sense. For more details on that, check out this week’s Policy & Practice podcast.
-- Frances Correa (FMCReporting)
Health care spending continues to grow – no surprise there – according to an annual federal government report. While hospital payments are still responsible for the largest portion of the nation’s health spending, provisions of the Affordable Care Act have already started to slow spending growth on hospital services. That’s not yet the case for physician and clinical services, for which Medicare spending grew by 8 percent in 2011 (the most recent data).
At the same time, the administration is touting their progress in approving a total of 250 Accountable Care Organizations, or ACOs, half of which are led by physicians.
The Department of Health and Human Services estimates it can save up to $940 million over four years by using this model.
Still, the need for a permanent solution to physician Medicare payments was the source of exasperation at the most recent meeting of the Medicare Payment Advisory Commission. Commissioners said they’re tired of the year-to-year patches and that replacing the flawed SGR system just makes sense. For more details on that, check out this week’s Policy & Practice podcast.
-- Frances Correa (FMCReporting)
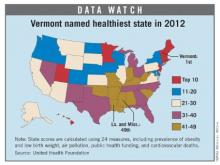
Vermont tops 'America's Health Rankings' for 2012
For the fourth consecutive year, Vermont earned the top health rating in the United States, according to the 2012 edition of "America’s Health Rankings."
Hawaii, which has never finished out of the top six since the rankings began in 1990, was second, followed by New Hampshire, Massachusetts, and Minnesota. Mississippi and Louisiana were tied for 49th as the least healthy states, with Arkansas, West Virginia, and South Carolina making up the rest of the bottom five.
The states that showed the greatest improvement since last year were Alaska (up seven places) and Oklahoma (up five slots). The states that fell the furthest in the rankings from 2011 were Michigan (down seven) and West Virginia (down six), according to the report.
"America’s Health Rankings" is published jointly by United Health Foundation, the American Public Health Association, and the Partnership for Prevention. The private, not-for-profit United Health Foundation was founded in 1999 by UnitedHealth Group, which operates UnitedHealthcare.
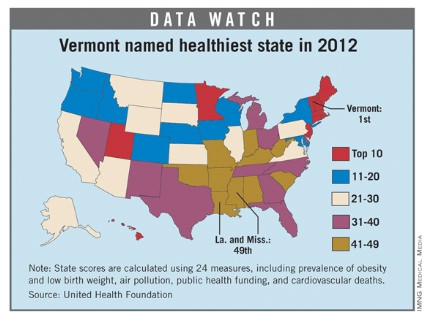
For the fourth consecutive year, Vermont earned the top health rating in the United States, according to the 2012 edition of "America’s Health Rankings."
Hawaii, which has never finished out of the top six since the rankings began in 1990, was second, followed by New Hampshire, Massachusetts, and Minnesota. Mississippi and Louisiana were tied for 49th as the least healthy states, with Arkansas, West Virginia, and South Carolina making up the rest of the bottom five.
The states that showed the greatest improvement since last year were Alaska (up seven places) and Oklahoma (up five slots). The states that fell the furthest in the rankings from 2011 were Michigan (down seven) and West Virginia (down six), according to the report.
"America’s Health Rankings" is published jointly by United Health Foundation, the American Public Health Association, and the Partnership for Prevention. The private, not-for-profit United Health Foundation was founded in 1999 by UnitedHealth Group, which operates UnitedHealthcare.
For the fourth consecutive year, Vermont earned the top health rating in the United States, according to the 2012 edition of "America’s Health Rankings."
Hawaii, which has never finished out of the top six since the rankings began in 1990, was second, followed by New Hampshire, Massachusetts, and Minnesota. Mississippi and Louisiana were tied for 49th as the least healthy states, with Arkansas, West Virginia, and South Carolina making up the rest of the bottom five.
The states that showed the greatest improvement since last year were Alaska (up seven places) and Oklahoma (up five slots). The states that fell the furthest in the rankings from 2011 were Michigan (down seven) and West Virginia (down six), according to the report.
"America’s Health Rankings" is published jointly by United Health Foundation, the American Public Health Association, and the Partnership for Prevention. The private, not-for-profit United Health Foundation was founded in 1999 by UnitedHealth Group, which operates UnitedHealthcare.
Point-Counterpoint: Are there significant advantages for robotic vs. laparoscopic surgery for abdominal procedures?
YES – Robotics Holds Promise for Surgery’s Future
Robotic surgery offers some real advantages over laparoscopic techniques in the abdominal area, but so far it has been difficult to prove the clinical benefits and cost-effectiveness of robotic systems.
It will likely take some time before there is ample evidence to show that robotic procedures produce better outcomes. And it may take even longer before the price tag on these systems comes down to earth.
But most surgeons will agree that there are real benefits to using robotic systems. As laparoscopic surgeons, we lost our tactile feedback, our three-dimensional visibility, wrist degrees of freedom, and even a comfortable operating posture. Robotic surgery can give a lot of that back.
The vivid, three-dimensional visibility of the robotic system is like nothing else that I’ve seen to date. Robotic systems also return the ability to get behind tissues. And these systems offer a more comfortable posture. Unfortunately, there are no gains in tactile feedback, and it may even be a little worse.
More than 40% of prostatectomies are now being done using the robotic da Vinci system – yet, for the most part, urologic surgeons weren’t even doing laparoscopic surgery before this device came along. But this device is very well suited for that particular operation. Similarly, robotic techniques are making inroads in colorectal pelvic surgery and esophageal surgery.
Robotic technology improves our ability to precisely manipulate tissue, minimize tissue trauma, improve visibility, and reduce surgeon fatigue. But how do you put a value on these improvements? We have a serious cost problem in American health care, and because surgical technology is expensive, we must continue to try to find ways to measure and assess advanced technologies.
Imagine that I took a superb open surgeon and put Crisco on his glasses. Then, instead of handing him a pair of his favorite 6-inch Metzenbaum scissors, I gave him a pair that was a foot-and-a-half long. Would that great surgeon still be able to get his Whipple operation done? Of course. And he would do it well because he’s a great surgeon. But I’m equally sure that same excellent surgeon would rather be able to see the way he likes and use instruments that can get the job done more effectively. To me, that’s a little bit like what robotics can give to surgeons.
I’m not ready to say that robotic surgery is the future for our field. It’s a good fit for specific procedures, but not for all. However, using computer technology and advanced imaging to improve what we do for patients is absolutely the future of surgery – and the current generation of robotic devices is the very beginning of that revolution.
Dr. Talamini is Professor and Chairman of the Department of Surgery at the University of California, San Diego. He is a pioneer in minimally invasive abdominal surgery and specializes in robotic surgery.
NO – A Question of Value
It’s true that robotic-assisted surgery has given us back some of the advantages we lost with the move to laparoscopic surgery. We have gained back the degrees of freedom. The three-dimensional vision that we lost has been restored. We can put surgeons in a more comfortable position while operating.
But there aren’t any data that show beyond a doubt that robotic-assisted surgery results in decisively better patient outcomes – especially in abdominal operations, for which there is currently no indication.
The heart of the matter is value – which we can see as an equation of quality divided by outcomes and costs. With robotic surgery, the quality is generally equivalent or goes down slightly, but the cost goes up – a lot.
The question of value even arises in prostate surgery, the robotic procedure with the largest body of clinical data.
A 2009 study of 2,600 men who underwent minimally invasive or robotic radical prostatectomy is a case in point. The laparoscopic group had a shorter length of stay as well as fewer blood transfusions, respiratory complications, and surgical complications. Men who had the robot-assisted surgery were more likely have to genitourinary complications, to become incontinent, and to experience erectile dysfunction (JAMA 2009;302:1557-64).
The New England Journal of Medicine also looked at the cost issue. At a price tag of more than $2 million, the robot added about $1,600-$3,200 to the cost of every procedure – without any increase in reimbursement (N. Engl. J. Med. 2010;363:701-4).
Let’s look at outcomes specifically in abdominal procedures. One recent study examined robotic and laparoscopic liver resection in 29 patients. Patients who underwent the robotic procedure had more ICU admissions, more minor complications, and longer hospital stays. In addition, the robotic surgery cost almost $5,000 more (J. Gastrointest. Surg. 2012;16:2233-8).
Another recent study looked at outcomes in a group of 68 patients who had either hand-assisted or robot-assisted nephrectomy. Length of stay was significantly shorter for the laparoscopy group. In the robot-assisted group, extended operating room time and the robot’s cost tacked $1,165 onto the bottom line (J. Endourol. 2012 Nov. 7 [e-pub ahead of print]).
A recent randomized study provides even stronger evidence. It compared surgical outcomes between laparoscopic and robotic surgery for right-sided colonic cancer in 70 patients who were randomized to the procedures. The group that underwent the robotic surgery had longer surgery times, and the cost was about $2,000 more than that of the laparoscopic procedure (Br. J. Surg. 2012;99:1219-26).
Given the current state of affairs, both in terms of pricing and clinical data, surgical robots don’t make a lot of sense for abdominal procedures. There is a clear ergonomic advantage with the robot, and the three-dimensional imaging allows you to do some very fine maneuvers in small places. But almost any operation that can be done by a skilled laparoscopic surgeon can be done with identical fidelity in less time, with equivalent outcomes, and for a lot less money.
Dr. Soper is Surgeon-in-Chief at Northwestern Memorial Hospital, and the Loyal and Edith Davis Professor and Chairman of Surgery at Northwestern University Feinberg School of Medicine, Chicago.
YES – Robotics Holds Promise for Surgery’s Future
Robotic surgery offers some real advantages over laparoscopic techniques in the abdominal area, but so far it has been difficult to prove the clinical benefits and cost-effectiveness of robotic systems.
It will likely take some time before there is ample evidence to show that robotic procedures produce better outcomes. And it may take even longer before the price tag on these systems comes down to earth.
But most surgeons will agree that there are real benefits to using robotic systems. As laparoscopic surgeons, we lost our tactile feedback, our three-dimensional visibility, wrist degrees of freedom, and even a comfortable operating posture. Robotic surgery can give a lot of that back.
The vivid, three-dimensional visibility of the robotic system is like nothing else that I’ve seen to date. Robotic systems also return the ability to get behind tissues. And these systems offer a more comfortable posture. Unfortunately, there are no gains in tactile feedback, and it may even be a little worse.
More than 40% of prostatectomies are now being done using the robotic da Vinci system – yet, for the most part, urologic surgeons weren’t even doing laparoscopic surgery before this device came along. But this device is very well suited for that particular operation. Similarly, robotic techniques are making inroads in colorectal pelvic surgery and esophageal surgery.
Robotic technology improves our ability to precisely manipulate tissue, minimize tissue trauma, improve visibility, and reduce surgeon fatigue. But how do you put a value on these improvements? We have a serious cost problem in American health care, and because surgical technology is expensive, we must continue to try to find ways to measure and assess advanced technologies.
Imagine that I took a superb open surgeon and put Crisco on his glasses. Then, instead of handing him a pair of his favorite 6-inch Metzenbaum scissors, I gave him a pair that was a foot-and-a-half long. Would that great surgeon still be able to get his Whipple operation done? Of course. And he would do it well because he’s a great surgeon. But I’m equally sure that same excellent surgeon would rather be able to see the way he likes and use instruments that can get the job done more effectively. To me, that’s a little bit like what robotics can give to surgeons.
I’m not ready to say that robotic surgery is the future for our field. It’s a good fit for specific procedures, but not for all. However, using computer technology and advanced imaging to improve what we do for patients is absolutely the future of surgery – and the current generation of robotic devices is the very beginning of that revolution.
Dr. Talamini is Professor and Chairman of the Department of Surgery at the University of California, San Diego. He is a pioneer in minimally invasive abdominal surgery and specializes in robotic surgery.
NO – A Question of Value
It’s true that robotic-assisted surgery has given us back some of the advantages we lost with the move to laparoscopic surgery. We have gained back the degrees of freedom. The three-dimensional vision that we lost has been restored. We can put surgeons in a more comfortable position while operating.
But there aren’t any data that show beyond a doubt that robotic-assisted surgery results in decisively better patient outcomes – especially in abdominal operations, for which there is currently no indication.
The heart of the matter is value – which we can see as an equation of quality divided by outcomes and costs. With robotic surgery, the quality is generally equivalent or goes down slightly, but the cost goes up – a lot.
The question of value even arises in prostate surgery, the robotic procedure with the largest body of clinical data.
A 2009 study of 2,600 men who underwent minimally invasive or robotic radical prostatectomy is a case in point. The laparoscopic group had a shorter length of stay as well as fewer blood transfusions, respiratory complications, and surgical complications. Men who had the robot-assisted surgery were more likely have to genitourinary complications, to become incontinent, and to experience erectile dysfunction (JAMA 2009;302:1557-64).
The New England Journal of Medicine also looked at the cost issue. At a price tag of more than $2 million, the robot added about $1,600-$3,200 to the cost of every procedure – without any increase in reimbursement (N. Engl. J. Med. 2010;363:701-4).
Let’s look at outcomes specifically in abdominal procedures. One recent study examined robotic and laparoscopic liver resection in 29 patients. Patients who underwent the robotic procedure had more ICU admissions, more minor complications, and longer hospital stays. In addition, the robotic surgery cost almost $5,000 more (J. Gastrointest. Surg. 2012;16:2233-8).
Another recent study looked at outcomes in a group of 68 patients who had either hand-assisted or robot-assisted nephrectomy. Length of stay was significantly shorter for the laparoscopy group. In the robot-assisted group, extended operating room time and the robot’s cost tacked $1,165 onto the bottom line (J. Endourol. 2012 Nov. 7 [e-pub ahead of print]).
A recent randomized study provides even stronger evidence. It compared surgical outcomes between laparoscopic and robotic surgery for right-sided colonic cancer in 70 patients who were randomized to the procedures. The group that underwent the robotic surgery had longer surgery times, and the cost was about $2,000 more than that of the laparoscopic procedure (Br. J. Surg. 2012;99:1219-26).
Given the current state of affairs, both in terms of pricing and clinical data, surgical robots don’t make a lot of sense for abdominal procedures. There is a clear ergonomic advantage with the robot, and the three-dimensional imaging allows you to do some very fine maneuvers in small places. But almost any operation that can be done by a skilled laparoscopic surgeon can be done with identical fidelity in less time, with equivalent outcomes, and for a lot less money.
Dr. Soper is Surgeon-in-Chief at Northwestern Memorial Hospital, and the Loyal and Edith Davis Professor and Chairman of Surgery at Northwestern University Feinberg School of Medicine, Chicago.
YES – Robotics Holds Promise for Surgery’s Future
Robotic surgery offers some real advantages over laparoscopic techniques in the abdominal area, but so far it has been difficult to prove the clinical benefits and cost-effectiveness of robotic systems.
It will likely take some time before there is ample evidence to show that robotic procedures produce better outcomes. And it may take even longer before the price tag on these systems comes down to earth.
But most surgeons will agree that there are real benefits to using robotic systems. As laparoscopic surgeons, we lost our tactile feedback, our three-dimensional visibility, wrist degrees of freedom, and even a comfortable operating posture. Robotic surgery can give a lot of that back.
The vivid, three-dimensional visibility of the robotic system is like nothing else that I’ve seen to date. Robotic systems also return the ability to get behind tissues. And these systems offer a more comfortable posture. Unfortunately, there are no gains in tactile feedback, and it may even be a little worse.
More than 40% of prostatectomies are now being done using the robotic da Vinci system – yet, for the most part, urologic surgeons weren’t even doing laparoscopic surgery before this device came along. But this device is very well suited for that particular operation. Similarly, robotic techniques are making inroads in colorectal pelvic surgery and esophageal surgery.
Robotic technology improves our ability to precisely manipulate tissue, minimize tissue trauma, improve visibility, and reduce surgeon fatigue. But how do you put a value on these improvements? We have a serious cost problem in American health care, and because surgical technology is expensive, we must continue to try to find ways to measure and assess advanced technologies.
Imagine that I took a superb open surgeon and put Crisco on his glasses. Then, instead of handing him a pair of his favorite 6-inch Metzenbaum scissors, I gave him a pair that was a foot-and-a-half long. Would that great surgeon still be able to get his Whipple operation done? Of course. And he would do it well because he’s a great surgeon. But I’m equally sure that same excellent surgeon would rather be able to see the way he likes and use instruments that can get the job done more effectively. To me, that’s a little bit like what robotics can give to surgeons.
I’m not ready to say that robotic surgery is the future for our field. It’s a good fit for specific procedures, but not for all. However, using computer technology and advanced imaging to improve what we do for patients is absolutely the future of surgery – and the current generation of robotic devices is the very beginning of that revolution.
Dr. Talamini is Professor and Chairman of the Department of Surgery at the University of California, San Diego. He is a pioneer in minimally invasive abdominal surgery and specializes in robotic surgery.
NO – A Question of Value
It’s true that robotic-assisted surgery has given us back some of the advantages we lost with the move to laparoscopic surgery. We have gained back the degrees of freedom. The three-dimensional vision that we lost has been restored. We can put surgeons in a more comfortable position while operating.
But there aren’t any data that show beyond a doubt that robotic-assisted surgery results in decisively better patient outcomes – especially in abdominal operations, for which there is currently no indication.
The heart of the matter is value – which we can see as an equation of quality divided by outcomes and costs. With robotic surgery, the quality is generally equivalent or goes down slightly, but the cost goes up – a lot.
The question of value even arises in prostate surgery, the robotic procedure with the largest body of clinical data.
A 2009 study of 2,600 men who underwent minimally invasive or robotic radical prostatectomy is a case in point. The laparoscopic group had a shorter length of stay as well as fewer blood transfusions, respiratory complications, and surgical complications. Men who had the robot-assisted surgery were more likely have to genitourinary complications, to become incontinent, and to experience erectile dysfunction (JAMA 2009;302:1557-64).
The New England Journal of Medicine also looked at the cost issue. At a price tag of more than $2 million, the robot added about $1,600-$3,200 to the cost of every procedure – without any increase in reimbursement (N. Engl. J. Med. 2010;363:701-4).
Let’s look at outcomes specifically in abdominal procedures. One recent study examined robotic and laparoscopic liver resection in 29 patients. Patients who underwent the robotic procedure had more ICU admissions, more minor complications, and longer hospital stays. In addition, the robotic surgery cost almost $5,000 more (J. Gastrointest. Surg. 2012;16:2233-8).
Another recent study looked at outcomes in a group of 68 patients who had either hand-assisted or robot-assisted nephrectomy. Length of stay was significantly shorter for the laparoscopy group. In the robot-assisted group, extended operating room time and the robot’s cost tacked $1,165 onto the bottom line (J. Endourol. 2012 Nov. 7 [e-pub ahead of print]).
A recent randomized study provides even stronger evidence. It compared surgical outcomes between laparoscopic and robotic surgery for right-sided colonic cancer in 70 patients who were randomized to the procedures. The group that underwent the robotic surgery had longer surgery times, and the cost was about $2,000 more than that of the laparoscopic procedure (Br. J. Surg. 2012;99:1219-26).
Given the current state of affairs, both in terms of pricing and clinical data, surgical robots don’t make a lot of sense for abdominal procedures. There is a clear ergonomic advantage with the robot, and the three-dimensional imaging allows you to do some very fine maneuvers in small places. But almost any operation that can be done by a skilled laparoscopic surgeon can be done with identical fidelity in less time, with equivalent outcomes, and for a lot less money.
Dr. Soper is Surgeon-in-Chief at Northwestern Memorial Hospital, and the Loyal and Edith Davis Professor and Chairman of Surgery at Northwestern University Feinberg School of Medicine, Chicago.
FDA proposal encourages development of abuse deterrent opioids
Encouraging manufacturers to develop opioid products that are formulated to deter abuse is one of the main goals of a new draft guidance released Jan. 9 by the Food and Drug Administration.
"Guidance for Industry: Abuse-Deterrent Opioids – Evaluation and Labeling" provides information for companies interested in developing formulations of opioids with potential abuse deterrent properties, Dr. Douglas Throckmorton, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said at a briefing.
The document explains the agency’s views on the types of studies that should be conducted to show that a product can deter abuse, how those studies will be evaluated by the agency, and what labeling claims would be allowed based on the results of the FDA’s evaluations of those data. The ability to add abuse deterrent claims to a product’s label is expected to encourage companies to develop these products, he said.
The technology of formulations that deter abuse and the clinical epidemiology and statistical methods used to evaluate the potential of such products for abuse deterrence is a relatively new area of research, and the FDA plans "to take a flexible approach" in evaluating these products as the science evolves, Dr. Throckmorton said.
During the briefing, he pointed out that the guidance is one of the components of the FDA’s effort to prevent prescription drug abuse and misuse, "while ensuring patients in pain continue to have access to these medicines." In 2009, almost 425,000 visits to emergency departments involving nonmedical or inappropriate use of opioids and an estimated 15,600 deaths in the United States were attributed to opioid products, he said.
This draft guidance fulfills mandates under the Food and Drug Administration Safety and Innovation Act and the Office of National Drug Control Policy’s Prescription Drug Abuse Prevention Plan.
The FDA statement announcing the guidance explains that abuse deterrent formulations "target the known or expected routes of abuse, such as crushing in order to snort or dissolving in order to inject, for the specific opioid drug substance in that formulation" and that the agency believes these formulations have "promise to help reduce prescription drug abuse."
Two opioid products with abuse deterrent formulations are currently available: Since 2010, OxyContin (controlled-release oxycodone) has been available in a formulation that prevents it from being chewed, crushed, or dissolved. A crush-resistant formulation of hydromorphone (Opana ER) was approved in September 2012. But the prescribing information for these products do not include claims regarding abuse deterrence properties.
The lack of abuse deterrent features was the main reason an FDA advisory panel recommended against approval of an extended-release, single-ingredient capsule formulation of hydrocodone at a meeting in December. Without such a feature, the product would quickly become widely abused once it was approved and marketed, some of the panelists predicted.
The FDA plans to hold a public meeting to discuss public feedback on the draft guidance.
During the briefing, Dr. Throckmorton said that the FDA had not yet decided what to do about generic formulations of opioids that have no abuse deterrent properties.
The guidance "is an important part of a larger effort by FDA aimed at preventing prescription drug abuse and misuse," and the agency is "extremely concerned about the inappropriate use of prescription opioids, which is a major public health challenge for our nation," FDA commissioner Dr. Margaret Hamburg said in the statement.
Comments and suggestions can be submitted to the FDA within 60 days of publication in the Federal Register, to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Room 1061, Rockville, Md., 20852 , with the docket number listed.
Encouraging manufacturers to develop opioid products that are formulated to deter abuse is one of the main goals of a new draft guidance released Jan. 9 by the Food and Drug Administration.
"Guidance for Industry: Abuse-Deterrent Opioids – Evaluation and Labeling" provides information for companies interested in developing formulations of opioids with potential abuse deterrent properties, Dr. Douglas Throckmorton, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said at a briefing.
The document explains the agency’s views on the types of studies that should be conducted to show that a product can deter abuse, how those studies will be evaluated by the agency, and what labeling claims would be allowed based on the results of the FDA’s evaluations of those data. The ability to add abuse deterrent claims to a product’s label is expected to encourage companies to develop these products, he said.
The technology of formulations that deter abuse and the clinical epidemiology and statistical methods used to evaluate the potential of such products for abuse deterrence is a relatively new area of research, and the FDA plans "to take a flexible approach" in evaluating these products as the science evolves, Dr. Throckmorton said.
During the briefing, he pointed out that the guidance is one of the components of the FDA’s effort to prevent prescription drug abuse and misuse, "while ensuring patients in pain continue to have access to these medicines." In 2009, almost 425,000 visits to emergency departments involving nonmedical or inappropriate use of opioids and an estimated 15,600 deaths in the United States were attributed to opioid products, he said.
This draft guidance fulfills mandates under the Food and Drug Administration Safety and Innovation Act and the Office of National Drug Control Policy’s Prescription Drug Abuse Prevention Plan.
The FDA statement announcing the guidance explains that abuse deterrent formulations "target the known or expected routes of abuse, such as crushing in order to snort or dissolving in order to inject, for the specific opioid drug substance in that formulation" and that the agency believes these formulations have "promise to help reduce prescription drug abuse."
Two opioid products with abuse deterrent formulations are currently available: Since 2010, OxyContin (controlled-release oxycodone) has been available in a formulation that prevents it from being chewed, crushed, or dissolved. A crush-resistant formulation of hydromorphone (Opana ER) was approved in September 2012. But the prescribing information for these products do not include claims regarding abuse deterrence properties.
The lack of abuse deterrent features was the main reason an FDA advisory panel recommended against approval of an extended-release, single-ingredient capsule formulation of hydrocodone at a meeting in December. Without such a feature, the product would quickly become widely abused once it was approved and marketed, some of the panelists predicted.
The FDA plans to hold a public meeting to discuss public feedback on the draft guidance.
During the briefing, Dr. Throckmorton said that the FDA had not yet decided what to do about generic formulations of opioids that have no abuse deterrent properties.
The guidance "is an important part of a larger effort by FDA aimed at preventing prescription drug abuse and misuse," and the agency is "extremely concerned about the inappropriate use of prescription opioids, which is a major public health challenge for our nation," FDA commissioner Dr. Margaret Hamburg said in the statement.
Comments and suggestions can be submitted to the FDA within 60 days of publication in the Federal Register, to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Room 1061, Rockville, Md., 20852 , with the docket number listed.
Encouraging manufacturers to develop opioid products that are formulated to deter abuse is one of the main goals of a new draft guidance released Jan. 9 by the Food and Drug Administration.
"Guidance for Industry: Abuse-Deterrent Opioids – Evaluation and Labeling" provides information for companies interested in developing formulations of opioids with potential abuse deterrent properties, Dr. Douglas Throckmorton, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said at a briefing.
The document explains the agency’s views on the types of studies that should be conducted to show that a product can deter abuse, how those studies will be evaluated by the agency, and what labeling claims would be allowed based on the results of the FDA’s evaluations of those data. The ability to add abuse deterrent claims to a product’s label is expected to encourage companies to develop these products, he said.
The technology of formulations that deter abuse and the clinical epidemiology and statistical methods used to evaluate the potential of such products for abuse deterrence is a relatively new area of research, and the FDA plans "to take a flexible approach" in evaluating these products as the science evolves, Dr. Throckmorton said.
During the briefing, he pointed out that the guidance is one of the components of the FDA’s effort to prevent prescription drug abuse and misuse, "while ensuring patients in pain continue to have access to these medicines." In 2009, almost 425,000 visits to emergency departments involving nonmedical or inappropriate use of opioids and an estimated 15,600 deaths in the United States were attributed to opioid products, he said.
This draft guidance fulfills mandates under the Food and Drug Administration Safety and Innovation Act and the Office of National Drug Control Policy’s Prescription Drug Abuse Prevention Plan.
The FDA statement announcing the guidance explains that abuse deterrent formulations "target the known or expected routes of abuse, such as crushing in order to snort or dissolving in order to inject, for the specific opioid drug substance in that formulation" and that the agency believes these formulations have "promise to help reduce prescription drug abuse."
Two opioid products with abuse deterrent formulations are currently available: Since 2010, OxyContin (controlled-release oxycodone) has been available in a formulation that prevents it from being chewed, crushed, or dissolved. A crush-resistant formulation of hydromorphone (Opana ER) was approved in September 2012. But the prescribing information for these products do not include claims regarding abuse deterrence properties.
The lack of abuse deterrent features was the main reason an FDA advisory panel recommended against approval of an extended-release, single-ingredient capsule formulation of hydrocodone at a meeting in December. Without such a feature, the product would quickly become widely abused once it was approved and marketed, some of the panelists predicted.
The FDA plans to hold a public meeting to discuss public feedback on the draft guidance.
During the briefing, Dr. Throckmorton said that the FDA had not yet decided what to do about generic formulations of opioids that have no abuse deterrent properties.
The guidance "is an important part of a larger effort by FDA aimed at preventing prescription drug abuse and misuse," and the agency is "extremely concerned about the inappropriate use of prescription opioids, which is a major public health challenge for our nation," FDA commissioner Dr. Margaret Hamburg said in the statement.
Comments and suggestions can be submitted to the FDA within 60 days of publication in the Federal Register, to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Room 1061, Rockville, Md., 20852 , with the docket number listed.
Lymph node count may not be best predictor of colon cancer survival
Lymph node counts of 12 or higher, widely considered a key marker of surgical quality in colon cancer resection, had no significant effect on 5-year survival rates in settings where surgeons are audited and credentialed and where surgical techniques are standardized.
Lymph node (LN) count is one of several measures used to determine the extent of surgical resection and an indicator of clear surgical margins, which have been assumed to result in better survival outcomes for colon cancer patients. A new data analysis, however, of the COST (Clinical Outcomes of Surgical Therapy) trial suggests reevaluating the use of surgical surrogates such as 12 LNs and margins.
The COST trial, a large, multicenter randomized trial of colon cancer procedures, compared outcomes for laparoscopic and open techniques in treating colon adenocarcinoma. The trial collected data on a number of surgical variables, including tumor location and LN count. A total of 787 patients were included: 267 with stage I disease, 284 with stage II, and 236 with stage III. Their median age was 70 years, and 50% were male.
In the current study, Dr. Kellie L. Mathis of the Mayo Clinic in Rochester, Minn., and her colleagues found that 5-year overall and disease-free survival were not influenced by LN count of above or below 12 (Ann. Surg. 2012;257:102-7 [doi:10.1097/SLA.0b013e318260a8e6]). When they adjusted for age and cancer stage, LN count was seen as not predictive of overall or disease-free survival (P = .60).
Other surgical surrogates, including total bowel length, margins, or mesenteric length, likewise did not have a significant effect on survival (P greater than .05 for all), nor did tumor location (right, left, or sigmoid), surgical technique (laparoscopic or open), and sex. Only patient age and cancer stage were found to be predictive of survival.
"On the basis of abundant literature and the acceptance of the 12 LN count as a surgical quality surrogate by National Quality Forum, most would expect the 12 LN count or other surgical variables to be predictive of survival," Dr. Mathis and her associates wrote. However, they hypothesized that procedural standardization, monitoring, and credentialing may provide a better strategy for quality control.
Overall 5-year survival results from the COST trial, they noted, were 77.2% – better than national rates for comparable patient groups in the same time period. All enrolling surgeons underwent pretrial credentialing and had performed a minimum of 20 laparoscopic colon resections, for which they had submitted operative and pathology reports. All laparoscopic resections were video recorded, and videos were randomly audited by an external review committee.
If the observations in the current study can be validated by others, Dr. Mathis and her colleagues said, "we submit that now is the time to invest in the development of technical quality control programs that directly measure and monitor surgical procedures."
Dr. Mathis and her colleagues stated that they had no conflicts of interest related to their findings.
Lymph node counts of 12 or higher, widely considered a key marker of surgical quality in colon cancer resection, had no significant effect on 5-year survival rates in settings where surgeons are audited and credentialed and where surgical techniques are standardized.
Lymph node (LN) count is one of several measures used to determine the extent of surgical resection and an indicator of clear surgical margins, which have been assumed to result in better survival outcomes for colon cancer patients. A new data analysis, however, of the COST (Clinical Outcomes of Surgical Therapy) trial suggests reevaluating the use of surgical surrogates such as 12 LNs and margins.
The COST trial, a large, multicenter randomized trial of colon cancer procedures, compared outcomes for laparoscopic and open techniques in treating colon adenocarcinoma. The trial collected data on a number of surgical variables, including tumor location and LN count. A total of 787 patients were included: 267 with stage I disease, 284 with stage II, and 236 with stage III. Their median age was 70 years, and 50% were male.
In the current study, Dr. Kellie L. Mathis of the Mayo Clinic in Rochester, Minn., and her colleagues found that 5-year overall and disease-free survival were not influenced by LN count of above or below 12 (Ann. Surg. 2012;257:102-7 [doi:10.1097/SLA.0b013e318260a8e6]). When they adjusted for age and cancer stage, LN count was seen as not predictive of overall or disease-free survival (P = .60).
Other surgical surrogates, including total bowel length, margins, or mesenteric length, likewise did not have a significant effect on survival (P greater than .05 for all), nor did tumor location (right, left, or sigmoid), surgical technique (laparoscopic or open), and sex. Only patient age and cancer stage were found to be predictive of survival.
"On the basis of abundant literature and the acceptance of the 12 LN count as a surgical quality surrogate by National Quality Forum, most would expect the 12 LN count or other surgical variables to be predictive of survival," Dr. Mathis and her associates wrote. However, they hypothesized that procedural standardization, monitoring, and credentialing may provide a better strategy for quality control.
Overall 5-year survival results from the COST trial, they noted, were 77.2% – better than national rates for comparable patient groups in the same time period. All enrolling surgeons underwent pretrial credentialing and had performed a minimum of 20 laparoscopic colon resections, for which they had submitted operative and pathology reports. All laparoscopic resections were video recorded, and videos were randomly audited by an external review committee.
If the observations in the current study can be validated by others, Dr. Mathis and her colleagues said, "we submit that now is the time to invest in the development of technical quality control programs that directly measure and monitor surgical procedures."
Dr. Mathis and her colleagues stated that they had no conflicts of interest related to their findings.
Lymph node counts of 12 or higher, widely considered a key marker of surgical quality in colon cancer resection, had no significant effect on 5-year survival rates in settings where surgeons are audited and credentialed and where surgical techniques are standardized.
Lymph node (LN) count is one of several measures used to determine the extent of surgical resection and an indicator of clear surgical margins, which have been assumed to result in better survival outcomes for colon cancer patients. A new data analysis, however, of the COST (Clinical Outcomes of Surgical Therapy) trial suggests reevaluating the use of surgical surrogates such as 12 LNs and margins.
The COST trial, a large, multicenter randomized trial of colon cancer procedures, compared outcomes for laparoscopic and open techniques in treating colon adenocarcinoma. The trial collected data on a number of surgical variables, including tumor location and LN count. A total of 787 patients were included: 267 with stage I disease, 284 with stage II, and 236 with stage III. Their median age was 70 years, and 50% were male.
In the current study, Dr. Kellie L. Mathis of the Mayo Clinic in Rochester, Minn., and her colleagues found that 5-year overall and disease-free survival were not influenced by LN count of above or below 12 (Ann. Surg. 2012;257:102-7 [doi:10.1097/SLA.0b013e318260a8e6]). When they adjusted for age and cancer stage, LN count was seen as not predictive of overall or disease-free survival (P = .60).
Other surgical surrogates, including total bowel length, margins, or mesenteric length, likewise did not have a significant effect on survival (P greater than .05 for all), nor did tumor location (right, left, or sigmoid), surgical technique (laparoscopic or open), and sex. Only patient age and cancer stage were found to be predictive of survival.
"On the basis of abundant literature and the acceptance of the 12 LN count as a surgical quality surrogate by National Quality Forum, most would expect the 12 LN count or other surgical variables to be predictive of survival," Dr. Mathis and her associates wrote. However, they hypothesized that procedural standardization, monitoring, and credentialing may provide a better strategy for quality control.
Overall 5-year survival results from the COST trial, they noted, were 77.2% – better than national rates for comparable patient groups in the same time period. All enrolling surgeons underwent pretrial credentialing and had performed a minimum of 20 laparoscopic colon resections, for which they had submitted operative and pathology reports. All laparoscopic resections were video recorded, and videos were randomly audited by an external review committee.
If the observations in the current study can be validated by others, Dr. Mathis and her colleagues said, "we submit that now is the time to invest in the development of technical quality control programs that directly measure and monitor surgical procedures."
Dr. Mathis and her colleagues stated that they had no conflicts of interest related to their findings.
FROM ANNALS OF SURGERY
Major Finding: Lymph node count of 12 or higher was not associated with better 5-year survival rates in patients undergoing surgery to treat colon cancer.
Data Source: A secondary analysis of data from the COST trial comparing laparoscopic vs. open colectomy in 787patients with stages I-III colon cancer.
Disclosures: Dr. Mathis and colleagues stated that they had no conflicts of interest related to their findings.
Evidence-based medicine depends on quality evidence
Efforts to improve the quality of health care often emphasize evidence-based medicine, but flaws in how research is designed, conducted, and reported make this "a great time for skeptics, in looking at clinical trials," according to Dr. J. Russell Hoverman.
Multiple studies in recent years suggest that increasing influence from industry and researchers’ desire to emphasize positive results, as well as other factors, may be distorting choices about which studies get done and how they get reported, said Dr. Hoverman, a medical oncologist and hematologist at Texas Oncology in Austin, Tex.
If researchers don’t improve the way they conduct and assess clinical trials, a lot of money could be wasted on misguided research, he said at a quality care symposium sponsored by the American Society of Clinical Oncology.
He’s not the only one making the case. Physicians at Yale recently argued for greater transparency in pharmaceutical industry–sponsored research to improve the integrity of medical research (Am. J. Public Health 2012;102:72-80).
Over the last three decades, sponsorship of breast, colon, and lung cancer studies by for-profit companies increased from 4% to 57%, Dr. Hoverman noted. Industry sponsorship was associated with trial results that endorsed the experimental agent, according to one study (J. Clin. Oncol. 2008;26:5458-64).
A separate study showed that abstracts of study results presented at major oncology meetings before final publication were discordant from the published article 63% of the time. In 10% of cases, the abstract and article presented substantially different conclusions (J. Clin. Oncol. 2009;3938-44).
One example of this was a trial of a cancer treatment regimen using gemcitabine, cisplatin, and bevacizumab. The investigators initially released an early abstract reporting an improvement in progression-free survival using the regimen. "That actually changed some [oncologists’] practices," he noted. But that was before the study reached its main outcome measure – overall survival – which, in the end, did not improve significantly with the new regimen.
Only half of phase II clinical trials with positive findings lead to positive phase III trials, another study found. For some reason, industry-sponsored trials are much more likely to report positive findings, compared with all other trials – 90% and 45%, respectively (J. Clin. Oncol. 2008;26:1511-8).
When reading or interpreting abstract summaries from a medical conference, "one needs to be a little careful," Dr. Hoverman advised.
Yet another study found that only 45% of randomized clinical trials were registered, even though trial registration has been required since 2005 by the International Committee of Medical Journal Editors in order for the results to be published in participating journals.
Among the registered studies, 31% showed discrepancies between what the investigators said they would be studying and the published outcomes. Half of the studies with discrepancies could be assessed to try to figure out why this was so; of those, 83% of the time it appeared that the investigators decided to favor statistically significant findings in the published article (JAMA 2009;302:977-84).
One set of experts from within industry and from Johns Hopkins University called for "transformational change" in how randomized clinical trials are conducted (Ann. Intern. Med. 2009;151:206-209).
"Without major changes in how we conceive, design, conduct, and analyze randomized controlled trials, the nation risks spending large sums of money inefficiently to answer the wrong questions, or the right questions too late," Dr. Hoverman said.
"In fact, we probably can’t do randomized clinical trials on everything we want to know about. It’s simply impossible. There’s not enough money, and many things involve competing industries or competing members within an industry," making it unlikely that some head-to-head comparisons will ever be done, he added. "So, we are challenged to make decisions based on evidence."
The broader challenge for clinicians and researchers will be to improve the quality and integrity of medical studies while maintaining a healthy skepticism about the available evidence. Medicine has always been an art and a science. Where the science behind medicine is lacking, the art takes over.
Dr. Hoverman reported having no financial disclosures.
-- Sherry Boschert
On Twitter @sherryboschert
Efforts to improve the quality of health care often emphasize evidence-based medicine, but flaws in how research is designed, conducted, and reported make this "a great time for skeptics, in looking at clinical trials," according to Dr. J. Russell Hoverman.
Multiple studies in recent years suggest that increasing influence from industry and researchers’ desire to emphasize positive results, as well as other factors, may be distorting choices about which studies get done and how they get reported, said Dr. Hoverman, a medical oncologist and hematologist at Texas Oncology in Austin, Tex.
If researchers don’t improve the way they conduct and assess clinical trials, a lot of money could be wasted on misguided research, he said at a quality care symposium sponsored by the American Society of Clinical Oncology.
He’s not the only one making the case. Physicians at Yale recently argued for greater transparency in pharmaceutical industry–sponsored research to improve the integrity of medical research (Am. J. Public Health 2012;102:72-80).
Over the last three decades, sponsorship of breast, colon, and lung cancer studies by for-profit companies increased from 4% to 57%, Dr. Hoverman noted. Industry sponsorship was associated with trial results that endorsed the experimental agent, according to one study (J. Clin. Oncol. 2008;26:5458-64).
A separate study showed that abstracts of study results presented at major oncology meetings before final publication were discordant from the published article 63% of the time. In 10% of cases, the abstract and article presented substantially different conclusions (J. Clin. Oncol. 2009;3938-44).
One example of this was a trial of a cancer treatment regimen using gemcitabine, cisplatin, and bevacizumab. The investigators initially released an early abstract reporting an improvement in progression-free survival using the regimen. "That actually changed some [oncologists’] practices," he noted. But that was before the study reached its main outcome measure – overall survival – which, in the end, did not improve significantly with the new regimen.
Only half of phase II clinical trials with positive findings lead to positive phase III trials, another study found. For some reason, industry-sponsored trials are much more likely to report positive findings, compared with all other trials – 90% and 45%, respectively (J. Clin. Oncol. 2008;26:1511-8).
When reading or interpreting abstract summaries from a medical conference, "one needs to be a little careful," Dr. Hoverman advised.
Yet another study found that only 45% of randomized clinical trials were registered, even though trial registration has been required since 2005 by the International Committee of Medical Journal Editors in order for the results to be published in participating journals.
Among the registered studies, 31% showed discrepancies between what the investigators said they would be studying and the published outcomes. Half of the studies with discrepancies could be assessed to try to figure out why this was so; of those, 83% of the time it appeared that the investigators decided to favor statistically significant findings in the published article (JAMA 2009;302:977-84).
One set of experts from within industry and from Johns Hopkins University called for "transformational change" in how randomized clinical trials are conducted (Ann. Intern. Med. 2009;151:206-209).
"Without major changes in how we conceive, design, conduct, and analyze randomized controlled trials, the nation risks spending large sums of money inefficiently to answer the wrong questions, or the right questions too late," Dr. Hoverman said.
"In fact, we probably can’t do randomized clinical trials on everything we want to know about. It’s simply impossible. There’s not enough money, and many things involve competing industries or competing members within an industry," making it unlikely that some head-to-head comparisons will ever be done, he added. "So, we are challenged to make decisions based on evidence."
The broader challenge for clinicians and researchers will be to improve the quality and integrity of medical studies while maintaining a healthy skepticism about the available evidence. Medicine has always been an art and a science. Where the science behind medicine is lacking, the art takes over.
Dr. Hoverman reported having no financial disclosures.
-- Sherry Boschert
On Twitter @sherryboschert
Efforts to improve the quality of health care often emphasize evidence-based medicine, but flaws in how research is designed, conducted, and reported make this "a great time for skeptics, in looking at clinical trials," according to Dr. J. Russell Hoverman.
Multiple studies in recent years suggest that increasing influence from industry and researchers’ desire to emphasize positive results, as well as other factors, may be distorting choices about which studies get done and how they get reported, said Dr. Hoverman, a medical oncologist and hematologist at Texas Oncology in Austin, Tex.
If researchers don’t improve the way they conduct and assess clinical trials, a lot of money could be wasted on misguided research, he said at a quality care symposium sponsored by the American Society of Clinical Oncology.
He’s not the only one making the case. Physicians at Yale recently argued for greater transparency in pharmaceutical industry–sponsored research to improve the integrity of medical research (Am. J. Public Health 2012;102:72-80).
Over the last three decades, sponsorship of breast, colon, and lung cancer studies by for-profit companies increased from 4% to 57%, Dr. Hoverman noted. Industry sponsorship was associated with trial results that endorsed the experimental agent, according to one study (J. Clin. Oncol. 2008;26:5458-64).
A separate study showed that abstracts of study results presented at major oncology meetings before final publication were discordant from the published article 63% of the time. In 10% of cases, the abstract and article presented substantially different conclusions (J. Clin. Oncol. 2009;3938-44).
One example of this was a trial of a cancer treatment regimen using gemcitabine, cisplatin, and bevacizumab. The investigators initially released an early abstract reporting an improvement in progression-free survival using the regimen. "That actually changed some [oncologists’] practices," he noted. But that was before the study reached its main outcome measure – overall survival – which, in the end, did not improve significantly with the new regimen.
Only half of phase II clinical trials with positive findings lead to positive phase III trials, another study found. For some reason, industry-sponsored trials are much more likely to report positive findings, compared with all other trials – 90% and 45%, respectively (J. Clin. Oncol. 2008;26:1511-8).
When reading or interpreting abstract summaries from a medical conference, "one needs to be a little careful," Dr. Hoverman advised.
Yet another study found that only 45% of randomized clinical trials were registered, even though trial registration has been required since 2005 by the International Committee of Medical Journal Editors in order for the results to be published in participating journals.
Among the registered studies, 31% showed discrepancies between what the investigators said they would be studying and the published outcomes. Half of the studies with discrepancies could be assessed to try to figure out why this was so; of those, 83% of the time it appeared that the investigators decided to favor statistically significant findings in the published article (JAMA 2009;302:977-84).
One set of experts from within industry and from Johns Hopkins University called for "transformational change" in how randomized clinical trials are conducted (Ann. Intern. Med. 2009;151:206-209).
"Without major changes in how we conceive, design, conduct, and analyze randomized controlled trials, the nation risks spending large sums of money inefficiently to answer the wrong questions, or the right questions too late," Dr. Hoverman said.
"In fact, we probably can’t do randomized clinical trials on everything we want to know about. It’s simply impossible. There’s not enough money, and many things involve competing industries or competing members within an industry," making it unlikely that some head-to-head comparisons will ever be done, he added. "So, we are challenged to make decisions based on evidence."
The broader challenge for clinicians and researchers will be to improve the quality and integrity of medical studies while maintaining a healthy skepticism about the available evidence. Medicine has always been an art and a science. Where the science behind medicine is lacking, the art takes over.
Dr. Hoverman reported having no financial disclosures.
-- Sherry Boschert
On Twitter @sherryboschert