User login
Official Newspaper of the American College of Surgeons
Normothermic machine perfusion found to salvage fatty livers for transplantation
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
AT THE LIVER MEETING 2018
Key clinical point: The addition of apheresis filtration and defatting agents to normothermic machine perfusion led to significant improvements in liver function.
Major finding: Livers which received apheresis filtration and defatting agents fared better than those that did not.
Study details: An analysis of 18 discarded human steatotic livers that were perfused on a normothermic, blood-based circuit for 48 hours.
Disclosures: Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
Despite interest, few liver transplant candidates discuss advance care planning with clinicians
SAN FRANCISCO – .
“Recent studies have shown that there have been low rates of these types of discussions in all areas of medicine, not just in liver transplantation per se,” Connie W. Wang, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “We were curious to see what it looked like in our practice setting.”
In an effort to evaluate current advanced care planning documentation practices in the liver transplantation setting, she and her colleagues reviewed the medical charts of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco, from January 2017 to June 2017. Next, to assess readiness to complete advanced care planning among liver transplant candidates, the researchers administered the Advanced Care Planning Engagement Survey to 41 adults who underwent an initial liver transplant evaluation from March 2018 to May 2018. The survey was scored on a Likert scale of 1-4, in which a score of 4 equaled “ready” or “confident,” and a score of 5 equaled “very ready” or “very confident.”
The mean age of the 168 transplant candidates was 53 years, 35% were female, and 52% were non-Hispanic white. Only 15 patients (9%) reported completing advanced care planning prior to their liver transplant evaluation and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record. Durable power of attorney for health care was discussed with 17 patients (10%). On logistic regression analysis, only white race was associated with completion of advanced care planning (OR 4.16; P = .03), but age, Child-Pugh score, and MELD-Na score were not.
The mean age of the 41 transplant candidates who completed the Advanced Care Planning Engagement Survey was 58 years, 39% were female, and 58% were non-Hispanic white. Nearly all respondents (93%) indicated that they were ready to appoint a durable power of attorney, 85% were ready to discuss end-of-life care, and 93% were ready to ask physicians questions about medical decisions. Similarly, 93% of patients felt confident to appoint a durable power of attorney, 88% felt confident to discuss end-of-life care, and 93% felt confident to ask physicians questions about medical decisions.
“It seems like from the patients’ perspective, they are very much open to having these conversations, but there hasn’t been [the right] environment or setting to have them,” said Dr. Wang, a third-year internal medicine resident at UCSF. “Or, there may be a barrier from the provider’s perspective. Clearly, there is a huge need that can be filled.” She noted that future research should focus on development of tools to facilitate discussions and documentation between transplant clinicians, patients, and their caregivers.
One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]: Abstract 771.
SAN FRANCISCO – .
“Recent studies have shown that there have been low rates of these types of discussions in all areas of medicine, not just in liver transplantation per se,” Connie W. Wang, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “We were curious to see what it looked like in our practice setting.”
In an effort to evaluate current advanced care planning documentation practices in the liver transplantation setting, she and her colleagues reviewed the medical charts of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco, from January 2017 to June 2017. Next, to assess readiness to complete advanced care planning among liver transplant candidates, the researchers administered the Advanced Care Planning Engagement Survey to 41 adults who underwent an initial liver transplant evaluation from March 2018 to May 2018. The survey was scored on a Likert scale of 1-4, in which a score of 4 equaled “ready” or “confident,” and a score of 5 equaled “very ready” or “very confident.”
The mean age of the 168 transplant candidates was 53 years, 35% were female, and 52% were non-Hispanic white. Only 15 patients (9%) reported completing advanced care planning prior to their liver transplant evaluation and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record. Durable power of attorney for health care was discussed with 17 patients (10%). On logistic regression analysis, only white race was associated with completion of advanced care planning (OR 4.16; P = .03), but age, Child-Pugh score, and MELD-Na score were not.
The mean age of the 41 transplant candidates who completed the Advanced Care Planning Engagement Survey was 58 years, 39% were female, and 58% were non-Hispanic white. Nearly all respondents (93%) indicated that they were ready to appoint a durable power of attorney, 85% were ready to discuss end-of-life care, and 93% were ready to ask physicians questions about medical decisions. Similarly, 93% of patients felt confident to appoint a durable power of attorney, 88% felt confident to discuss end-of-life care, and 93% felt confident to ask physicians questions about medical decisions.
“It seems like from the patients’ perspective, they are very much open to having these conversations, but there hasn’t been [the right] environment or setting to have them,” said Dr. Wang, a third-year internal medicine resident at UCSF. “Or, there may be a barrier from the provider’s perspective. Clearly, there is a huge need that can be filled.” She noted that future research should focus on development of tools to facilitate discussions and documentation between transplant clinicians, patients, and their caregivers.
One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]: Abstract 771.
SAN FRANCISCO – .
“Recent studies have shown that there have been low rates of these types of discussions in all areas of medicine, not just in liver transplantation per se,” Connie W. Wang, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “We were curious to see what it looked like in our practice setting.”
In an effort to evaluate current advanced care planning documentation practices in the liver transplantation setting, she and her colleagues reviewed the medical charts of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco, from January 2017 to June 2017. Next, to assess readiness to complete advanced care planning among liver transplant candidates, the researchers administered the Advanced Care Planning Engagement Survey to 41 adults who underwent an initial liver transplant evaluation from March 2018 to May 2018. The survey was scored on a Likert scale of 1-4, in which a score of 4 equaled “ready” or “confident,” and a score of 5 equaled “very ready” or “very confident.”
The mean age of the 168 transplant candidates was 53 years, 35% were female, and 52% were non-Hispanic white. Only 15 patients (9%) reported completing advanced care planning prior to their liver transplant evaluation and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record. Durable power of attorney for health care was discussed with 17 patients (10%). On logistic regression analysis, only white race was associated with completion of advanced care planning (OR 4.16; P = .03), but age, Child-Pugh score, and MELD-Na score were not.
The mean age of the 41 transplant candidates who completed the Advanced Care Planning Engagement Survey was 58 years, 39% were female, and 58% were non-Hispanic white. Nearly all respondents (93%) indicated that they were ready to appoint a durable power of attorney, 85% were ready to discuss end-of-life care, and 93% were ready to ask physicians questions about medical decisions. Similarly, 93% of patients felt confident to appoint a durable power of attorney, 88% felt confident to discuss end-of-life care, and 93% felt confident to ask physicians questions about medical decisions.
“It seems like from the patients’ perspective, they are very much open to having these conversations, but there hasn’t been [the right] environment or setting to have them,” said Dr. Wang, a third-year internal medicine resident at UCSF. “Or, there may be a barrier from the provider’s perspective. Clearly, there is a huge need that can be filled.” She noted that future research should focus on development of tools to facilitate discussions and documentation between transplant clinicians, patients, and their caregivers.
One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]: Abstract 771.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: There is a paucity of documentation of advance care planning or identification of a durable power of attorney in the medical record of liver transplant candidates.
Major finding: Only 9% of liver transplant candidates reported completing advanced care planning prior to their liver transplant evaluations and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record.
Study details: A retrospective review of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco.
Disclosures: One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]:Abstract 771.
Laparoscopic hysterectomy with obliterated cul-de-sac needs specialist care
LAS VEGAS – When stage IV endometriosis with obliterated posterior cul-de-sac is discovered during laparoscopic hysterectomy, or suspected beforehand, women should be referred to a minimally invasive gynecologic surgery specialist because the procedure will be much more difficult, investigators said at the meeting sponsored by AAGL.
They reviewed 333 laparoscopic hysterectomies where endometriosis was discovered in the operating room. The disease is known to increase the complexity of hysterectomy; the investigators wanted to quantify the risk by endometriosis severity. Among their subjects, 237 women (71%) had stage I, II, or III endometriosis; 96 (29%) had stage IV disease, including 55 women (57%) with obliterated posterior cul-de-sacs.
Surgery was longer among stage IV cases (137 vs. 116 minutes), and there was greater blood loss; 66% of stage IV women required laparoscopic-modified radical hysterectomy versus about a quarter of women with stage I-III endometriosis.
A total of 93% required modified radical hysterectomies versus 29% of stage IV women with intact cul-de-sacs. Additional procedures were far more likely in this population, including salpingectomy, ureterolysis, enterolysis, cystoscopy, ureteral stenting, proctoscopy, bowel oversew, and anterior resection anastomosis. The differences all were statistically significant.
Among stage IV cases, mean operating time was longer in obliterated cul-de-sac cases (159 vs. 108 minutes), with higher blood loss, 100 mL versus 50 mL.
“Patients with obliterated cul-de-sacs identified intraoperatively should be referred to minimally invasive gynecologic surgeons because of the ... extra training required to safely perform [laparoscopic hysterectomy] with limited morbidity,” said lead investigator Alexandra Melnyk, MD, a University of Pittsburgh ob.gyn resident.
There was no industry funding and the investigators reported no disclosures.
SOURCE: Melnyk A et al. 2018 AAGL Global Congress, Abstract 81.
LAS VEGAS – When stage IV endometriosis with obliterated posterior cul-de-sac is discovered during laparoscopic hysterectomy, or suspected beforehand, women should be referred to a minimally invasive gynecologic surgery specialist because the procedure will be much more difficult, investigators said at the meeting sponsored by AAGL.
They reviewed 333 laparoscopic hysterectomies where endometriosis was discovered in the operating room. The disease is known to increase the complexity of hysterectomy; the investigators wanted to quantify the risk by endometriosis severity. Among their subjects, 237 women (71%) had stage I, II, or III endometriosis; 96 (29%) had stage IV disease, including 55 women (57%) with obliterated posterior cul-de-sacs.
Surgery was longer among stage IV cases (137 vs. 116 minutes), and there was greater blood loss; 66% of stage IV women required laparoscopic-modified radical hysterectomy versus about a quarter of women with stage I-III endometriosis.
A total of 93% required modified radical hysterectomies versus 29% of stage IV women with intact cul-de-sacs. Additional procedures were far more likely in this population, including salpingectomy, ureterolysis, enterolysis, cystoscopy, ureteral stenting, proctoscopy, bowel oversew, and anterior resection anastomosis. The differences all were statistically significant.
Among stage IV cases, mean operating time was longer in obliterated cul-de-sac cases (159 vs. 108 minutes), with higher blood loss, 100 mL versus 50 mL.
“Patients with obliterated cul-de-sacs identified intraoperatively should be referred to minimally invasive gynecologic surgeons because of the ... extra training required to safely perform [laparoscopic hysterectomy] with limited morbidity,” said lead investigator Alexandra Melnyk, MD, a University of Pittsburgh ob.gyn resident.
There was no industry funding and the investigators reported no disclosures.
SOURCE: Melnyk A et al. 2018 AAGL Global Congress, Abstract 81.
LAS VEGAS – When stage IV endometriosis with obliterated posterior cul-de-sac is discovered during laparoscopic hysterectomy, or suspected beforehand, women should be referred to a minimally invasive gynecologic surgery specialist because the procedure will be much more difficult, investigators said at the meeting sponsored by AAGL.
They reviewed 333 laparoscopic hysterectomies where endometriosis was discovered in the operating room. The disease is known to increase the complexity of hysterectomy; the investigators wanted to quantify the risk by endometriosis severity. Among their subjects, 237 women (71%) had stage I, II, or III endometriosis; 96 (29%) had stage IV disease, including 55 women (57%) with obliterated posterior cul-de-sacs.
Surgery was longer among stage IV cases (137 vs. 116 minutes), and there was greater blood loss; 66% of stage IV women required laparoscopic-modified radical hysterectomy versus about a quarter of women with stage I-III endometriosis.
A total of 93% required modified radical hysterectomies versus 29% of stage IV women with intact cul-de-sacs. Additional procedures were far more likely in this population, including salpingectomy, ureterolysis, enterolysis, cystoscopy, ureteral stenting, proctoscopy, bowel oversew, and anterior resection anastomosis. The differences all were statistically significant.
Among stage IV cases, mean operating time was longer in obliterated cul-de-sac cases (159 vs. 108 minutes), with higher blood loss, 100 mL versus 50 mL.
“Patients with obliterated cul-de-sacs identified intraoperatively should be referred to minimally invasive gynecologic surgeons because of the ... extra training required to safely perform [laparoscopic hysterectomy] with limited morbidity,” said lead investigator Alexandra Melnyk, MD, a University of Pittsburgh ob.gyn resident.
There was no industry funding and the investigators reported no disclosures.
SOURCE: Melnyk A et al. 2018 AAGL Global Congress, Abstract 81.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Study eyed endoscopic submucosal dissection for early-stage esophageal cancer
according to the findings of a single-center retrospective cohort study.
After a median of 21 months of follow-up (range, 6-73 months), rates of all-cause mortality were 7% with ESD and 11% with esophagectomy, said Yiqun Zhang of Zhongshan Hospital, Shanghai, China, and his associates. Rates of cancer recurrence or metastasis were 9.1% and 8.9%, respectively, while disease-specific mortality was lower with ESD (3.4% vs. 7.4% with esophagectomy; P = .049). Severe nonfatal adverse perioperative events occurred in 15% of ESD cases versus 28% of esophagectomy cases (P less than .001). The findings justify more studies of ESD in carefully selected patients with early-stage (T1a-m2/m3 or T1b) esophageal squamous cell carcinoma, the researchers wrote in Clinical Gastroenterology and Hepatology.
Esophagectomy is standard for managing early-stage esophageal squamous cell carcinoma but is associated with high rates of morbidity and mortality. While ESD is minimally invasive, it is considered risky because esophageal squamous cell carcinoma so frequently metastasizes to the lymph nodes, the investigators noted. For the study, they retrospectively compared 322 ESDs and 274 esophagectomies performed during 2011-2016 in patients with T1a-m2/m3 or T1b esophageal squamous cell carcinoma. All cases were pathologically confirmed, and none were premalignant (that is, high-grade intraepithelial neoplasias).
Endoscopic submucosal dissection was associated with significantly lower rates of esophageal fistula (0.3% with ESD vs. 16% with esophagectomy; P less than .001) and pulmonary complications (0.3% vs. 3.6%, respectively; P less than .001), which explained its overall superiority in terms of severe adverse perioperative events, the researchers wrote. Perioperative deaths were rare but occurred more often with esophagectomy (four patients) than with ESD (one patient). Depth of tumor invasion was the only significant correlate of all-cause mortality (hazard ratio for T1a–m3 or deeper tumors versus T1a–m2 tumors, 3.54; 95% confidence interval, 1.08-11.62; P = .04) in a Cox regression analysis that accounted for many potential confounders, such as demographic and tumor characteristics, hypertension, chronic obstructive pulmonary disease (COPD), nodal metastasis, chemotherapy, and radiotherapy.
Perhaps esophagectomy did not improve survival in this retrospective study because follow-up time was too short, because adjuvant therapy compensated for the increased risk of tumor relapse with ESD, or because of the confounding effects of unmeasured variables, such as submucosal stages of T1b cancer, lymphovascular invasion, or tumor morphology, the researchers wrote. “Since a randomized study comparing esophagectomy and ESD alone would not be practical, a potential strategy for future research may include serial treatments – that is, ESD first, followed by esophagectomy, radiotherapy, or chemotherapy, depending on the ESD pathology findings,” they added. “A quality-of-life analysis of ESD would also be helpful because this might be one of the biggest advantages of ESD over esophagectomy and was beyond the scope of this study.”
The study was supported by the National Natural Science Foundation of China, the Shanghai Committee of Science and Technology, and Zhongshan Hospital. The investigators reported having no relevant conflicts of interest.
SOURCE: Zhang Y et al. Clin Gastroenterol Hepatol. 2018 Apr 25. doi: 10.1016/j.cgh.2018.04.038.
This study adds more evidence supporting the use of endoscopic submucosal dissection (ESD) in early esophageal cancer. Unlike esophageal adenocarcinoma, esophageal squamous cell carcinoma (ESCC) has a higher risk of lymph node metastasis and tends to be multifocal. ESCC lesions invading the submucosa (T1b) have the highest risk of lymph node metastasis (up to 60% in lesions with deep submucosal invasion).
Historically, endoscopic resection was reserved for mucosal tumors while submucosal tumors were managed surgically. Several trials have investigated the role of ESD in ESCC limited to the mucosa with excellent results. However, data for ESCC invading the submucosa (T1b lesions) are lacking. This study included 596 patients, almost half of included patients (282 patients) had T1b lesions. Although most of the T1b lesions were treated surgically (200 patients), there was a large cohort of 82 T1b ESCC lesions treated by ESD.
Interestingly, there was no difference in tumor recurrence or overall mortality in patients treated with ESD, compared with surgery for both mucosal and submucosal lesions.
Another interesting finding in this study was the use of adjuvant treatment such as radiotherapy and chemotherapy for patients treated with ESD who were found to have evidence of lymphovascular invasion. The outcome of this subset of patients was not different from patients who underwent esophagectomy. Recent evidence from this study and other published data suggest that there is a subset of submucosal ESCC lesions that can be managed endoscopically, especially submucosal lesions limited to the upper third of the submucosa. Further studies investigating the role of adjuvant treatment after ESD for deep submucosal lesions or lesions with lymphovascular invasion are needed.
Mohamed O. Othman, MD, is an associate professor of medicine, director of advanced endoscopy, and chief of the section of gastroenterology, Baylor College of Medicine, Houston. He is a consultant for Olympus and Boston Scientific.
This study adds more evidence supporting the use of endoscopic submucosal dissection (ESD) in early esophageal cancer. Unlike esophageal adenocarcinoma, esophageal squamous cell carcinoma (ESCC) has a higher risk of lymph node metastasis and tends to be multifocal. ESCC lesions invading the submucosa (T1b) have the highest risk of lymph node metastasis (up to 60% in lesions with deep submucosal invasion).
Historically, endoscopic resection was reserved for mucosal tumors while submucosal tumors were managed surgically. Several trials have investigated the role of ESD in ESCC limited to the mucosa with excellent results. However, data for ESCC invading the submucosa (T1b lesions) are lacking. This study included 596 patients, almost half of included patients (282 patients) had T1b lesions. Although most of the T1b lesions were treated surgically (200 patients), there was a large cohort of 82 T1b ESCC lesions treated by ESD.
Interestingly, there was no difference in tumor recurrence or overall mortality in patients treated with ESD, compared with surgery for both mucosal and submucosal lesions.
Another interesting finding in this study was the use of adjuvant treatment such as radiotherapy and chemotherapy for patients treated with ESD who were found to have evidence of lymphovascular invasion. The outcome of this subset of patients was not different from patients who underwent esophagectomy. Recent evidence from this study and other published data suggest that there is a subset of submucosal ESCC lesions that can be managed endoscopically, especially submucosal lesions limited to the upper third of the submucosa. Further studies investigating the role of adjuvant treatment after ESD for deep submucosal lesions or lesions with lymphovascular invasion are needed.
Mohamed O. Othman, MD, is an associate professor of medicine, director of advanced endoscopy, and chief of the section of gastroenterology, Baylor College of Medicine, Houston. He is a consultant for Olympus and Boston Scientific.
This study adds more evidence supporting the use of endoscopic submucosal dissection (ESD) in early esophageal cancer. Unlike esophageal adenocarcinoma, esophageal squamous cell carcinoma (ESCC) has a higher risk of lymph node metastasis and tends to be multifocal. ESCC lesions invading the submucosa (T1b) have the highest risk of lymph node metastasis (up to 60% in lesions with deep submucosal invasion).
Historically, endoscopic resection was reserved for mucosal tumors while submucosal tumors were managed surgically. Several trials have investigated the role of ESD in ESCC limited to the mucosa with excellent results. However, data for ESCC invading the submucosa (T1b lesions) are lacking. This study included 596 patients, almost half of included patients (282 patients) had T1b lesions. Although most of the T1b lesions were treated surgically (200 patients), there was a large cohort of 82 T1b ESCC lesions treated by ESD.
Interestingly, there was no difference in tumor recurrence or overall mortality in patients treated with ESD, compared with surgery for both mucosal and submucosal lesions.
Another interesting finding in this study was the use of adjuvant treatment such as radiotherapy and chemotherapy for patients treated with ESD who were found to have evidence of lymphovascular invasion. The outcome of this subset of patients was not different from patients who underwent esophagectomy. Recent evidence from this study and other published data suggest that there is a subset of submucosal ESCC lesions that can be managed endoscopically, especially submucosal lesions limited to the upper third of the submucosa. Further studies investigating the role of adjuvant treatment after ESD for deep submucosal lesions or lesions with lymphovascular invasion are needed.
Mohamed O. Othman, MD, is an associate professor of medicine, director of advanced endoscopy, and chief of the section of gastroenterology, Baylor College of Medicine, Houston. He is a consultant for Olympus and Boston Scientific.
according to the findings of a single-center retrospective cohort study.
After a median of 21 months of follow-up (range, 6-73 months), rates of all-cause mortality were 7% with ESD and 11% with esophagectomy, said Yiqun Zhang of Zhongshan Hospital, Shanghai, China, and his associates. Rates of cancer recurrence or metastasis were 9.1% and 8.9%, respectively, while disease-specific mortality was lower with ESD (3.4% vs. 7.4% with esophagectomy; P = .049). Severe nonfatal adverse perioperative events occurred in 15% of ESD cases versus 28% of esophagectomy cases (P less than .001). The findings justify more studies of ESD in carefully selected patients with early-stage (T1a-m2/m3 or T1b) esophageal squamous cell carcinoma, the researchers wrote in Clinical Gastroenterology and Hepatology.
Esophagectomy is standard for managing early-stage esophageal squamous cell carcinoma but is associated with high rates of morbidity and mortality. While ESD is minimally invasive, it is considered risky because esophageal squamous cell carcinoma so frequently metastasizes to the lymph nodes, the investigators noted. For the study, they retrospectively compared 322 ESDs and 274 esophagectomies performed during 2011-2016 in patients with T1a-m2/m3 or T1b esophageal squamous cell carcinoma. All cases were pathologically confirmed, and none were premalignant (that is, high-grade intraepithelial neoplasias).
Endoscopic submucosal dissection was associated with significantly lower rates of esophageal fistula (0.3% with ESD vs. 16% with esophagectomy; P less than .001) and pulmonary complications (0.3% vs. 3.6%, respectively; P less than .001), which explained its overall superiority in terms of severe adverse perioperative events, the researchers wrote. Perioperative deaths were rare but occurred more often with esophagectomy (four patients) than with ESD (one patient). Depth of tumor invasion was the only significant correlate of all-cause mortality (hazard ratio for T1a–m3 or deeper tumors versus T1a–m2 tumors, 3.54; 95% confidence interval, 1.08-11.62; P = .04) in a Cox regression analysis that accounted for many potential confounders, such as demographic and tumor characteristics, hypertension, chronic obstructive pulmonary disease (COPD), nodal metastasis, chemotherapy, and radiotherapy.
Perhaps esophagectomy did not improve survival in this retrospective study because follow-up time was too short, because adjuvant therapy compensated for the increased risk of tumor relapse with ESD, or because of the confounding effects of unmeasured variables, such as submucosal stages of T1b cancer, lymphovascular invasion, or tumor morphology, the researchers wrote. “Since a randomized study comparing esophagectomy and ESD alone would not be practical, a potential strategy for future research may include serial treatments – that is, ESD first, followed by esophagectomy, radiotherapy, or chemotherapy, depending on the ESD pathology findings,” they added. “A quality-of-life analysis of ESD would also be helpful because this might be one of the biggest advantages of ESD over esophagectomy and was beyond the scope of this study.”
The study was supported by the National Natural Science Foundation of China, the Shanghai Committee of Science and Technology, and Zhongshan Hospital. The investigators reported having no relevant conflicts of interest.
SOURCE: Zhang Y et al. Clin Gastroenterol Hepatol. 2018 Apr 25. doi: 10.1016/j.cgh.2018.04.038.
according to the findings of a single-center retrospective cohort study.
After a median of 21 months of follow-up (range, 6-73 months), rates of all-cause mortality were 7% with ESD and 11% with esophagectomy, said Yiqun Zhang of Zhongshan Hospital, Shanghai, China, and his associates. Rates of cancer recurrence or metastasis were 9.1% and 8.9%, respectively, while disease-specific mortality was lower with ESD (3.4% vs. 7.4% with esophagectomy; P = .049). Severe nonfatal adverse perioperative events occurred in 15% of ESD cases versus 28% of esophagectomy cases (P less than .001). The findings justify more studies of ESD in carefully selected patients with early-stage (T1a-m2/m3 or T1b) esophageal squamous cell carcinoma, the researchers wrote in Clinical Gastroenterology and Hepatology.
Esophagectomy is standard for managing early-stage esophageal squamous cell carcinoma but is associated with high rates of morbidity and mortality. While ESD is minimally invasive, it is considered risky because esophageal squamous cell carcinoma so frequently metastasizes to the lymph nodes, the investigators noted. For the study, they retrospectively compared 322 ESDs and 274 esophagectomies performed during 2011-2016 in patients with T1a-m2/m3 or T1b esophageal squamous cell carcinoma. All cases were pathologically confirmed, and none were premalignant (that is, high-grade intraepithelial neoplasias).
Endoscopic submucosal dissection was associated with significantly lower rates of esophageal fistula (0.3% with ESD vs. 16% with esophagectomy; P less than .001) and pulmonary complications (0.3% vs. 3.6%, respectively; P less than .001), which explained its overall superiority in terms of severe adverse perioperative events, the researchers wrote. Perioperative deaths were rare but occurred more often with esophagectomy (four patients) than with ESD (one patient). Depth of tumor invasion was the only significant correlate of all-cause mortality (hazard ratio for T1a–m3 or deeper tumors versus T1a–m2 tumors, 3.54; 95% confidence interval, 1.08-11.62; P = .04) in a Cox regression analysis that accounted for many potential confounders, such as demographic and tumor characteristics, hypertension, chronic obstructive pulmonary disease (COPD), nodal metastasis, chemotherapy, and radiotherapy.
Perhaps esophagectomy did not improve survival in this retrospective study because follow-up time was too short, because adjuvant therapy compensated for the increased risk of tumor relapse with ESD, or because of the confounding effects of unmeasured variables, such as submucosal stages of T1b cancer, lymphovascular invasion, or tumor morphology, the researchers wrote. “Since a randomized study comparing esophagectomy and ESD alone would not be practical, a potential strategy for future research may include serial treatments – that is, ESD first, followed by esophagectomy, radiotherapy, or chemotherapy, depending on the ESD pathology findings,” they added. “A quality-of-life analysis of ESD would also be helpful because this might be one of the biggest advantages of ESD over esophagectomy and was beyond the scope of this study.”
The study was supported by the National Natural Science Foundation of China, the Shanghai Committee of Science and Technology, and Zhongshan Hospital. The investigators reported having no relevant conflicts of interest.
SOURCE: Zhang Y et al. Clin Gastroenterol Hepatol. 2018 Apr 25. doi: 10.1016/j.cgh.2018.04.038.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Compared with esophagectomy, endoscopic submucosal dissection (ESD) was associated with significantly fewer severe adverse perioperative events and a similar rate of all-cause mortality in patients with early-stage esophageal squamous cell carcinoma.
Major finding: After a median of 21 months of follow-up, rates of all-cause mortality were 7% with ESD and 11% with esophagectomy (P = .21). Severe adverse perioperative events occurred in 15% of ESDs and 28% of esophagectomies.
Study details: Retrospective study of 596 patients with T1a-m2/m3 or T1b esophageal squamous cell carcinoma.
Disclosures: The study was supported by the National Natural Science Foundation of China, the Shanghai Committee of Science and Technology, and Zhongshan Hospital. The investigators reported having no relevant conflicts of interest.
Source: Zhang Y et al. Clin Gastroenterol Hepatol. 2018 Apr 25. doi: 10.1016/j.cgh.2018.04.038.
Medicaid patients have higher MELD scores at time of liver transplantation
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
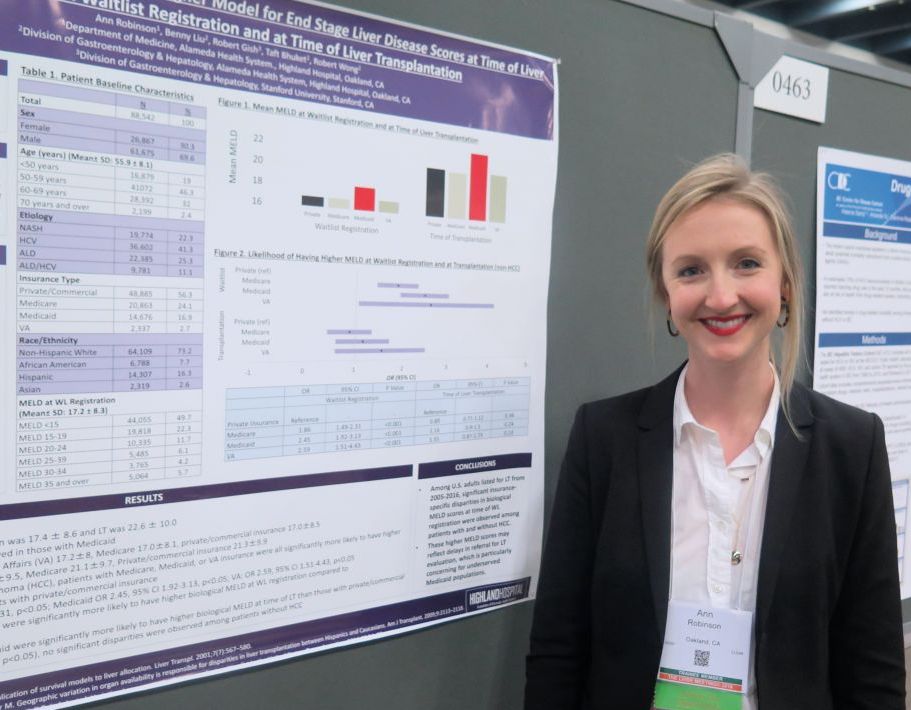
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AT THE LIVER MEETING 2018
Key clinical point: Significant insurance-specific disparities in MELD scores at time of wait-list registration were observed among patients with and without hepatocellular carcinoma.
Major finding: Among patients without hepatocellular carcinoma, those with Medicaid coverage were 2.45 times more likely to have higher MELD scores at wait-list registration, compared with those covered by commercial or private insurance (P less than .01).
Study details: A retrospective analysis of 88,542 liver transplantation wait-list registrants.
Disclosures: Dr. Robinson reported having no disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
Overprescribing opioids leads to higher levels of consumption
according to a population-based study of surgery patients.
Ryan Howard, MD, FACS, of the department of surgery at the University of Michigan, Ann Arbor, and his coauthors analyzed data from the Michigan Surgical Quality Collaborative and sampled 2,392 patients who underwent 1 of 12 common surgical procedures in Michigan between Jan. 1 and Sept. 30, 2017, and were prescribed opioids for pain. For all patients, the quantity of opioid prescribed – converted to oral morphine equivalents (OMEs) to adjust for varying potency – was considerably greater than the quantity actually consumed by the patient, wrote Dr. Howard and his colleagues in JAMA Surgery.
The study findings have troubling implications, the authors suggested. “Overprescribing was universally observed in this cohort, affecting each of the 12 procedures analyzed. This phenomenon was not limited to single, outlier institutions, but was widespread across many hospitals. This resulted in increased opioid consumption among patients who received larger prescriptions, as well as tens of thousands of leftover pills in 9 months that entered communities across the state of Michigan.”
The median amount prescribed was 150 OMEs, the equivalent of 30 pills of hydrocodone/acetaminophen, 5/325 mg. The median consumed, as reported by patients, was 45 OMEs, or 9 pills, meaning only 27% of the prescribed amount was used. Prescription size was also strongly associated with higher consumption; patients used an additional 0.53 OMEs (95% confidence interval, 0.40-0.65; P less than .001), or 5.3 more pills, for every 10 extra pills prescribed. The larger the initial prescription, the more patients used, an association that persisted when the data were adjusted for procedure and patient-specific factors such as postoperative pain.
The study’s acknowledged limitations included an inability to estimate how many patients were contacted for patient-reported outcome collection, which obscures how representative this sample may be of the patient population in general. There was also no data gathered regarding preoperative opioid use, a near certainty in this cohort given a 3%-4% prevalence of chronic opioid use.
That said, the investigators noted that “intentionally keeping future recommendations liberal in quantity may ultimately aid with widespread adoption, especially for clinicians concerned that prescribing reductions may lead to increased pain and calls for refills after surgery.” They commended local efforts already underway to combat this issue– including their own work at the University of Michigan, where evidence-based prescribing recommendations resulted in a 63% reduction in opioid prescription size without an increase in refills or pain – but reiterated that more needs to be done at a state level.
The authors offered a possible reason for the link between prescription size and patient consumption. “A plausible explanation for the association between prescription size and medication use is the anchoring and adjustment heuristic. This is a psychologic heuristic wherein a piece of information serves as an anchor on which adjustments are made to reach an estimation or decision. For example, obesity literature has shown that food intake increases with portion size. In this case, a larger amount of opioids may serve as a mental anchor by which patients estimate their analgesic needs.”
Michael Englesbe, MD, Jennifer Waljee,MD, and Chad Brummett, MD, reported receiving funding from the Michigan Department of Health and Human Services and the National Institute on Drug Abuse. Joceline Vu, MD, reported receiving funding from the National Institutes of Health Ruth L. Kirschstein National Research Service Award; Jay Lee, MD, reported receiving funding from the National Cancer Institute.
SOURCE: Howard R et al. JAMA Surg. 2018 Nov 7. doi: 10.1001/jamasurg.2018.4234.
according to a population-based study of surgery patients.
Ryan Howard, MD, FACS, of the department of surgery at the University of Michigan, Ann Arbor, and his coauthors analyzed data from the Michigan Surgical Quality Collaborative and sampled 2,392 patients who underwent 1 of 12 common surgical procedures in Michigan between Jan. 1 and Sept. 30, 2017, and were prescribed opioids for pain. For all patients, the quantity of opioid prescribed – converted to oral morphine equivalents (OMEs) to adjust for varying potency – was considerably greater than the quantity actually consumed by the patient, wrote Dr. Howard and his colleagues in JAMA Surgery.
The study findings have troubling implications, the authors suggested. “Overprescribing was universally observed in this cohort, affecting each of the 12 procedures analyzed. This phenomenon was not limited to single, outlier institutions, but was widespread across many hospitals. This resulted in increased opioid consumption among patients who received larger prescriptions, as well as tens of thousands of leftover pills in 9 months that entered communities across the state of Michigan.”
The median amount prescribed was 150 OMEs, the equivalent of 30 pills of hydrocodone/acetaminophen, 5/325 mg. The median consumed, as reported by patients, was 45 OMEs, or 9 pills, meaning only 27% of the prescribed amount was used. Prescription size was also strongly associated with higher consumption; patients used an additional 0.53 OMEs (95% confidence interval, 0.40-0.65; P less than .001), or 5.3 more pills, for every 10 extra pills prescribed. The larger the initial prescription, the more patients used, an association that persisted when the data were adjusted for procedure and patient-specific factors such as postoperative pain.
The study’s acknowledged limitations included an inability to estimate how many patients were contacted for patient-reported outcome collection, which obscures how representative this sample may be of the patient population in general. There was also no data gathered regarding preoperative opioid use, a near certainty in this cohort given a 3%-4% prevalence of chronic opioid use.
That said, the investigators noted that “intentionally keeping future recommendations liberal in quantity may ultimately aid with widespread adoption, especially for clinicians concerned that prescribing reductions may lead to increased pain and calls for refills after surgery.” They commended local efforts already underway to combat this issue– including their own work at the University of Michigan, where evidence-based prescribing recommendations resulted in a 63% reduction in opioid prescription size without an increase in refills or pain – but reiterated that more needs to be done at a state level.
The authors offered a possible reason for the link between prescription size and patient consumption. “A plausible explanation for the association between prescription size and medication use is the anchoring and adjustment heuristic. This is a psychologic heuristic wherein a piece of information serves as an anchor on which adjustments are made to reach an estimation or decision. For example, obesity literature has shown that food intake increases with portion size. In this case, a larger amount of opioids may serve as a mental anchor by which patients estimate their analgesic needs.”
Michael Englesbe, MD, Jennifer Waljee,MD, and Chad Brummett, MD, reported receiving funding from the Michigan Department of Health and Human Services and the National Institute on Drug Abuse. Joceline Vu, MD, reported receiving funding from the National Institutes of Health Ruth L. Kirschstein National Research Service Award; Jay Lee, MD, reported receiving funding from the National Cancer Institute.
SOURCE: Howard R et al. JAMA Surg. 2018 Nov 7. doi: 10.1001/jamasurg.2018.4234.
according to a population-based study of surgery patients.
Ryan Howard, MD, FACS, of the department of surgery at the University of Michigan, Ann Arbor, and his coauthors analyzed data from the Michigan Surgical Quality Collaborative and sampled 2,392 patients who underwent 1 of 12 common surgical procedures in Michigan between Jan. 1 and Sept. 30, 2017, and were prescribed opioids for pain. For all patients, the quantity of opioid prescribed – converted to oral morphine equivalents (OMEs) to adjust for varying potency – was considerably greater than the quantity actually consumed by the patient, wrote Dr. Howard and his colleagues in JAMA Surgery.
The study findings have troubling implications, the authors suggested. “Overprescribing was universally observed in this cohort, affecting each of the 12 procedures analyzed. This phenomenon was not limited to single, outlier institutions, but was widespread across many hospitals. This resulted in increased opioid consumption among patients who received larger prescriptions, as well as tens of thousands of leftover pills in 9 months that entered communities across the state of Michigan.”
The median amount prescribed was 150 OMEs, the equivalent of 30 pills of hydrocodone/acetaminophen, 5/325 mg. The median consumed, as reported by patients, was 45 OMEs, or 9 pills, meaning only 27% of the prescribed amount was used. Prescription size was also strongly associated with higher consumption; patients used an additional 0.53 OMEs (95% confidence interval, 0.40-0.65; P less than .001), or 5.3 more pills, for every 10 extra pills prescribed. The larger the initial prescription, the more patients used, an association that persisted when the data were adjusted for procedure and patient-specific factors such as postoperative pain.
The study’s acknowledged limitations included an inability to estimate how many patients were contacted for patient-reported outcome collection, which obscures how representative this sample may be of the patient population in general. There was also no data gathered regarding preoperative opioid use, a near certainty in this cohort given a 3%-4% prevalence of chronic opioid use.
That said, the investigators noted that “intentionally keeping future recommendations liberal in quantity may ultimately aid with widespread adoption, especially for clinicians concerned that prescribing reductions may lead to increased pain and calls for refills after surgery.” They commended local efforts already underway to combat this issue– including their own work at the University of Michigan, where evidence-based prescribing recommendations resulted in a 63% reduction in opioid prescription size without an increase in refills or pain – but reiterated that more needs to be done at a state level.
The authors offered a possible reason for the link between prescription size and patient consumption. “A plausible explanation for the association between prescription size and medication use is the anchoring and adjustment heuristic. This is a psychologic heuristic wherein a piece of information serves as an anchor on which adjustments are made to reach an estimation or decision. For example, obesity literature has shown that food intake increases with portion size. In this case, a larger amount of opioids may serve as a mental anchor by which patients estimate their analgesic needs.”
Michael Englesbe, MD, Jennifer Waljee,MD, and Chad Brummett, MD, reported receiving funding from the Michigan Department of Health and Human Services and the National Institute on Drug Abuse. Joceline Vu, MD, reported receiving funding from the National Institutes of Health Ruth L. Kirschstein National Research Service Award; Jay Lee, MD, reported receiving funding from the National Cancer Institute.
SOURCE: Howard R et al. JAMA Surg. 2018 Nov 7. doi: 10.1001/jamasurg.2018.4234.
FROM JAMA SURGERY
Key clinical point: Patients recovering from 12 common surgical procedures were universally overprescribed opioids.
Major finding: Surgery patients used 5.3 more pills for every 10 additional pills prescribed.
Study details: A retrospective, population-based study of 2,392 patients who underwent 1 of 12 surgeries in Michigan between Jan. 1 and Sept. 30, 2017, and were prescribed opioids for pain.
Disclosures: Michael Englesbe, MD, Jennifer Waljee, MD, and Chad Brummett, MD, reported receiving funding from the Michigan Department of Health and Human Services and the National Institute on Drug Abuse. Joceline Vu, MD, reported receiving funding from the National Institutes of Health Ruth L. Kirschstein National Research Service Award; Jay Lee, MD, reported receiving funding from the National Cancer Institute.
Source: Howard R et al. JAMA Surg. 2018 Nov 7. doi: 10.1001/jamasurg.2018.4234.
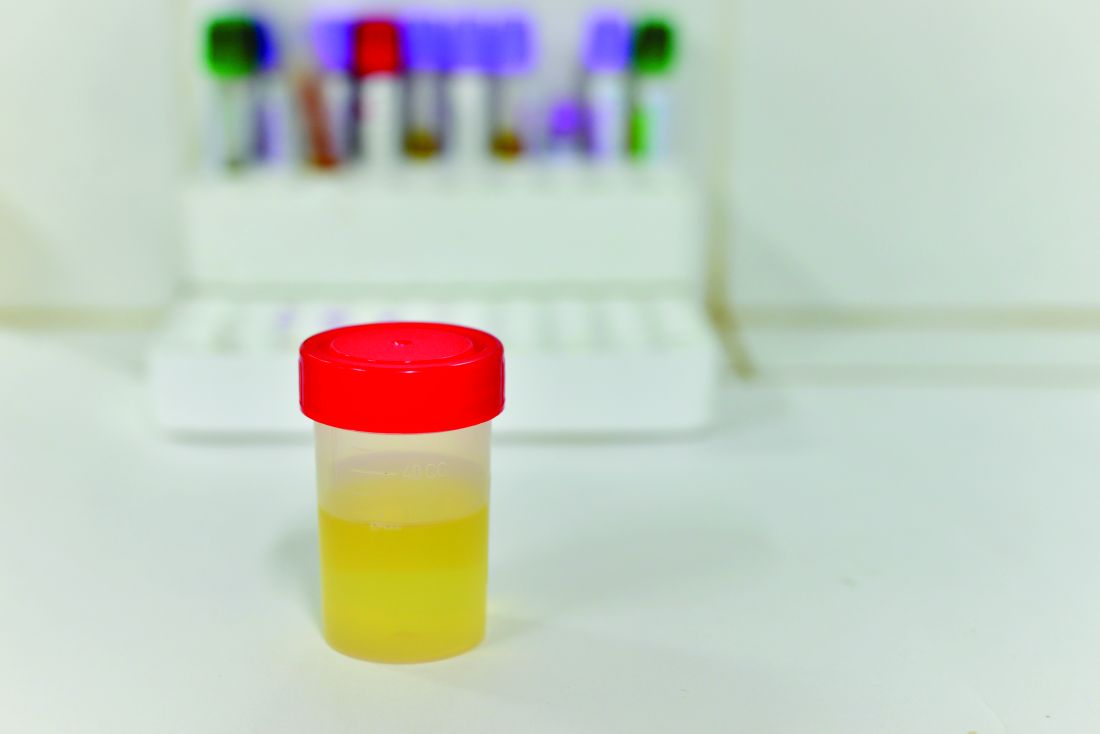
Harnessing the power of urine tests in pain care
SAN DIEGO – Clinicians have few tools to in their patients. However, addiction specialist and internist Edwin Salsitz, MD, says an inexpensive and simple tool, the urine test, can provide an impressive amount of useful information.
“The urine drug test, or another matrix for testing, gives one of the only objective factors we have to see how a patient is doing, if they’re following the treatment plan,” said Dr. Salsitz, of Mount Sinai Beth Israel, New York, in a presentation at Pain Care for Primary Care, a symposium offered by the American Pain Society and the Global Academy for Medical Education.
Dr. Salsitz offered these tips about urine tests in pain care:
Consider urine tests before beginning opioid therapy
Dr. Salsitz pointed to this 2016 recommendation from the Centers for Disease Control and Prevention: “When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.” As Dr. Salsitz puts it, these tests “can help identify misuse, which hopefully hasn’t gotten to addiction yet.”
Ask the patient what the urine test will reveal
Dr. Salsitz likes to tell patients: “If you tell me the truth, no matter what’s in the urine, it’s going to be OK. I’m not going to stop prescribing or do anything harmful to you.” But, he tells patients, if they lie, “you’re going to start breaking the trust between us. Once you do that, it becomes a problem. I don’t know what’s true or not.” In some cases, he said, patients will fess up to drug use that wouldn’t have shown up in the urine tests because it didn’t happen recently enough. “We’ll talk about whether it’s a problem,” he said.
Begin with an immunoassay panel test (IA)
The CDC recommends using an immunoassay panel first in most situations. “You can do this in the office,” Dr. Salsitz said, using a dipstick-style test. Or you can “send it out to a lab, and they’ll do the same thing.”
Understand what IA tests do and don’t do
Standard 5-drug IA screening tests detect marijuana, cocaine, amphetamine/methamphetamine, PCP, and opiates (morphine/codeine). Keep in mind, Dr. Salsitz said, that opiates and opioids aren’t the same. That means IA tests don’t pick up oxycodone use, for example, he said. More sophisticated (and more expensive) tests can distinguish between types of drugs (for example, morphine vs. codeine) and can detect drugs that aren’t included in the IA tests.
Don’t make assumptions about positive or negative tests
A positive drug test for cocaine doesn’t necessarily mean the person is addicted, Dr. Salsitz said. “It just means they used that molecule in the last 3 days. It’s up to you to figure out what it actually means.” And if a patient’s urine fails to show that he or she is taking a prescribed medication, that doesn’t necessarily indicate that the drug is being illegally diverted. The patient could have run out of the drug or lost insurance coverage, Dr. Salsitz said.
Be aware that patients may fake urine tests
“Cheating is a huge problem,” Dr. Salsitz said. “Is it their urine or not their urine?” Many kits promise to help people provide fake urine, and some have even provided fake penises to foil observed urine collection. What to do? Alternative tests that rely on hair, saliva, and even sweat are available, Dr. Salsitz said, and these make cheating more difficult. However, they have various limitations. Saliva, for example, only tells you what patients are using now, not what they used days ago, he said, and it’s not sensitive for marijuana.
Dr. Salsitz reported no disclosures.
The Global Academy for Medical Education, which offered the Pain Care for Primary Care symposium, and this news organization are owned by the same parent company.
SAN DIEGO – Clinicians have few tools to in their patients. However, addiction specialist and internist Edwin Salsitz, MD, says an inexpensive and simple tool, the urine test, can provide an impressive amount of useful information.
“The urine drug test, or another matrix for testing, gives one of the only objective factors we have to see how a patient is doing, if they’re following the treatment plan,” said Dr. Salsitz, of Mount Sinai Beth Israel, New York, in a presentation at Pain Care for Primary Care, a symposium offered by the American Pain Society and the Global Academy for Medical Education.
Dr. Salsitz offered these tips about urine tests in pain care:
Consider urine tests before beginning opioid therapy
Dr. Salsitz pointed to this 2016 recommendation from the Centers for Disease Control and Prevention: “When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.” As Dr. Salsitz puts it, these tests “can help identify misuse, which hopefully hasn’t gotten to addiction yet.”
Ask the patient what the urine test will reveal
Dr. Salsitz likes to tell patients: “If you tell me the truth, no matter what’s in the urine, it’s going to be OK. I’m not going to stop prescribing or do anything harmful to you.” But, he tells patients, if they lie, “you’re going to start breaking the trust between us. Once you do that, it becomes a problem. I don’t know what’s true or not.” In some cases, he said, patients will fess up to drug use that wouldn’t have shown up in the urine tests because it didn’t happen recently enough. “We’ll talk about whether it’s a problem,” he said.
Begin with an immunoassay panel test (IA)
The CDC recommends using an immunoassay panel first in most situations. “You can do this in the office,” Dr. Salsitz said, using a dipstick-style test. Or you can “send it out to a lab, and they’ll do the same thing.”
Understand what IA tests do and don’t do
Standard 5-drug IA screening tests detect marijuana, cocaine, amphetamine/methamphetamine, PCP, and opiates (morphine/codeine). Keep in mind, Dr. Salsitz said, that opiates and opioids aren’t the same. That means IA tests don’t pick up oxycodone use, for example, he said. More sophisticated (and more expensive) tests can distinguish between types of drugs (for example, morphine vs. codeine) and can detect drugs that aren’t included in the IA tests.
Don’t make assumptions about positive or negative tests
A positive drug test for cocaine doesn’t necessarily mean the person is addicted, Dr. Salsitz said. “It just means they used that molecule in the last 3 days. It’s up to you to figure out what it actually means.” And if a patient’s urine fails to show that he or she is taking a prescribed medication, that doesn’t necessarily indicate that the drug is being illegally diverted. The patient could have run out of the drug or lost insurance coverage, Dr. Salsitz said.
Be aware that patients may fake urine tests
“Cheating is a huge problem,” Dr. Salsitz said. “Is it their urine or not their urine?” Many kits promise to help people provide fake urine, and some have even provided fake penises to foil observed urine collection. What to do? Alternative tests that rely on hair, saliva, and even sweat are available, Dr. Salsitz said, and these make cheating more difficult. However, they have various limitations. Saliva, for example, only tells you what patients are using now, not what they used days ago, he said, and it’s not sensitive for marijuana.
Dr. Salsitz reported no disclosures.
The Global Academy for Medical Education, which offered the Pain Care for Primary Care symposium, and this news organization are owned by the same parent company.
SAN DIEGO – Clinicians have few tools to in their patients. However, addiction specialist and internist Edwin Salsitz, MD, says an inexpensive and simple tool, the urine test, can provide an impressive amount of useful information.
“The urine drug test, or another matrix for testing, gives one of the only objective factors we have to see how a patient is doing, if they’re following the treatment plan,” said Dr. Salsitz, of Mount Sinai Beth Israel, New York, in a presentation at Pain Care for Primary Care, a symposium offered by the American Pain Society and the Global Academy for Medical Education.
Dr. Salsitz offered these tips about urine tests in pain care:
Consider urine tests before beginning opioid therapy
Dr. Salsitz pointed to this 2016 recommendation from the Centers for Disease Control and Prevention: “When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.” As Dr. Salsitz puts it, these tests “can help identify misuse, which hopefully hasn’t gotten to addiction yet.”
Ask the patient what the urine test will reveal
Dr. Salsitz likes to tell patients: “If you tell me the truth, no matter what’s in the urine, it’s going to be OK. I’m not going to stop prescribing or do anything harmful to you.” But, he tells patients, if they lie, “you’re going to start breaking the trust between us. Once you do that, it becomes a problem. I don’t know what’s true or not.” In some cases, he said, patients will fess up to drug use that wouldn’t have shown up in the urine tests because it didn’t happen recently enough. “We’ll talk about whether it’s a problem,” he said.
Begin with an immunoassay panel test (IA)
The CDC recommends using an immunoassay panel first in most situations. “You can do this in the office,” Dr. Salsitz said, using a dipstick-style test. Or you can “send it out to a lab, and they’ll do the same thing.”
Understand what IA tests do and don’t do
Standard 5-drug IA screening tests detect marijuana, cocaine, amphetamine/methamphetamine, PCP, and opiates (morphine/codeine). Keep in mind, Dr. Salsitz said, that opiates and opioids aren’t the same. That means IA tests don’t pick up oxycodone use, for example, he said. More sophisticated (and more expensive) tests can distinguish between types of drugs (for example, morphine vs. codeine) and can detect drugs that aren’t included in the IA tests.
Don’t make assumptions about positive or negative tests
A positive drug test for cocaine doesn’t necessarily mean the person is addicted, Dr. Salsitz said. “It just means they used that molecule in the last 3 days. It’s up to you to figure out what it actually means.” And if a patient’s urine fails to show that he or she is taking a prescribed medication, that doesn’t necessarily indicate that the drug is being illegally diverted. The patient could have run out of the drug or lost insurance coverage, Dr. Salsitz said.
Be aware that patients may fake urine tests
“Cheating is a huge problem,” Dr. Salsitz said. “Is it their urine or not their urine?” Many kits promise to help people provide fake urine, and some have even provided fake penises to foil observed urine collection. What to do? Alternative tests that rely on hair, saliva, and even sweat are available, Dr. Salsitz said, and these make cheating more difficult. However, they have various limitations. Saliva, for example, only tells you what patients are using now, not what they used days ago, he said, and it’s not sensitive for marijuana.
Dr. Salsitz reported no disclosures.
The Global Academy for Medical Education, which offered the Pain Care for Primary Care symposium, and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
Open AAA repair mortality rates doubled for very-low-volume surgeons
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: In-hospital mortality is approximately double (OR 2.09; P less than .001) for very low-volume relative to high-volume surgeon.
Study details: Retrospective database review.
Disclosures: Dr. Cronenwett reports no conflicts of interest.
Source: Cronenwett JL et al. 2018; 45th VEITHsymposium.
Smaller inguinal hernias linked to more postoperative pain
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
FROM ANNALS OF SURGERY
Key clinical point: Smaller inguinal hernias associated with more postoperative pain than larger hernias.
Major finding: Smaller hernias are associated with a 100% higher odds of pain on exertion after surgery than those with large hernias.
Study details: An analysis of registry data from 57,999 patients who underwent hernia repair.
Disclosures: The Herniamed registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
Source: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
Staying up to date on screening may cut risk of death from CRC
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
FROM GASTROENTEROLOGY
Key clinical point: Being up to date on screening was associated with a significant reduction in the risk of dying from colon cancer.
Major finding: Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44).
Study details: Retrospective cohort study of 1,750 patients who died from colorectal cancer during 2002-2012 and 3,486 matched controls.
Disclosures: The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of Gastroenterology.
Source: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.