User login
Official Newspaper of the American College of Surgeons
Could European data privacy rules cost you big?
U.S. health providers who treat foreign patients may want to take a closer look at their privacy policies to make sure they comply with new European Union data protection rules.
May 25 heralds the enforcement of the European Union’s General Data Protection Regulation (GDPR), a set of rules designed to strengthen and harmonize record protection for EU citizens and tighten how their data privacy is managed. The regulations protect various forms of electronic data including basic identity information, health and genetic data, and biometric information.
Treating a vacationing EU patient who needs unplanned treatment in the states is not likely to subject physicians to the GDPR, said Cynthia J. Larose, a privacy and data security attorney based in Boston.
“In general, the GDPR should not impact U.S. doctors who may incidentally treat an EU patient while that patient is here in the U.S.,” Ms. Larose said in an interview … If the EU patient presents at a U.S. health care provider for treatment, then the GDPR does not apply to her personal data in the possession of the U.S. health care provider – HIPAA applies. While the [GDPR] does have extraterritorial reach, you have to be doing something in the EU for the GDPR to apply.”
But other scenarios that could prove problematic, such as U.S. researchers studying patients in the EU, U.S. physicians providing telemedicine care to EU patients, and doctors who continue to monitor EU patients following treatment in the United States once patients return to their home country.
About 200,000 international visitors fly to the United States yearly for health treatment, of whom about 25% are from Europe, according to a 2015 report by the United States International Trade Commission.
Advertising medical services in the European Union is another way that U.S. physicians could be subject to the GDPR. For example, if a practice or hospital markets their specialty care on websites or other materials in the EU, this could fall under the GDPR umbrella, according to security experts.
“If you are advertising services to patients in the EU, and then they decide to obtain such services, that could trigger GDPR because the data subjects are in the EU and you are offering services to them,” said Elaine C. Zacharakis Loumbas, a health and security law attorney based in Chicago. “It becomes very fact specific.”
Like HIPAA, the GDPR requires that health providers disclose information to patients about where and how their data may be used. Mr. Barchie notes that in the United States, patient consent forms may generally include two or three potential uses for patient data such as marketing and medical research. The GDPR specifies that each potential usage of patient data requires its own separate consent form, he said.
“Let’s say you’re a clinic that specializes in diabetes [and] you’re used to taking data and sending it to a general database to [collect information] about diabetes,” Mr. Barchie said. “You can’t do that under GDPR. You would have to have a separate consent form for that. So one consent to provide your diabetes service, one consent form to maybe market to the [patient], and a separate consent form [regarding] the database.”
GDPR also requires the minimizing of personal data copies stored within multiple systems. In the United States, it’s not uncommon for there to be multiple copies of a person’s data in several places, which makes sense from an IT perspective, Mr. Barchie said. The GDPR however requires that data keepers limit the number of copies they maintain to only the most necessary information.
“[Under GDPR], you should send only the data that you need for that particular process,” he said. “For example, [in the case of] address, user name, and patient ID. If you only need the patient ID number, you should not send the patient name and address. You minimize the amount of data that you’re sending to be processed.”
Breach notification also is more stringent under the GDPR, compared with U.S. regulations. Under HIPAA, covered entities must notify the U.S. Department of Health & Human Services and affected patients of a data breach without unreasonable delay no later than 60 days following discovery of a breach. The GDPR requires that effected entities notify the supervisory authority “without undue delay and, where feasible, not later than 72 hours after having become aware of [the breach].” (The GDPR supervisory authority depends on the EU country affected.)
If determined that the personal data breach is “likely to result in a high risk to the rights and freedoms of individuals,” efforts must be made to communicate about the personal data breach with the affected data subjects “without undue delay,” according to the rules.
If unsure of whether your practices may fall under GDPR, experts advise discussing the question with a legal counselor, GDPR expert, or risk management team.
U.S. health providers who treat foreign patients may want to take a closer look at their privacy policies to make sure they comply with new European Union data protection rules.
May 25 heralds the enforcement of the European Union’s General Data Protection Regulation (GDPR), a set of rules designed to strengthen and harmonize record protection for EU citizens and tighten how their data privacy is managed. The regulations protect various forms of electronic data including basic identity information, health and genetic data, and biometric information.
Treating a vacationing EU patient who needs unplanned treatment in the states is not likely to subject physicians to the GDPR, said Cynthia J. Larose, a privacy and data security attorney based in Boston.
“In general, the GDPR should not impact U.S. doctors who may incidentally treat an EU patient while that patient is here in the U.S.,” Ms. Larose said in an interview … If the EU patient presents at a U.S. health care provider for treatment, then the GDPR does not apply to her personal data in the possession of the U.S. health care provider – HIPAA applies. While the [GDPR] does have extraterritorial reach, you have to be doing something in the EU for the GDPR to apply.”
But other scenarios that could prove problematic, such as U.S. researchers studying patients in the EU, U.S. physicians providing telemedicine care to EU patients, and doctors who continue to monitor EU patients following treatment in the United States once patients return to their home country.
About 200,000 international visitors fly to the United States yearly for health treatment, of whom about 25% are from Europe, according to a 2015 report by the United States International Trade Commission.
Advertising medical services in the European Union is another way that U.S. physicians could be subject to the GDPR. For example, if a practice or hospital markets their specialty care on websites or other materials in the EU, this could fall under the GDPR umbrella, according to security experts.
“If you are advertising services to patients in the EU, and then they decide to obtain such services, that could trigger GDPR because the data subjects are in the EU and you are offering services to them,” said Elaine C. Zacharakis Loumbas, a health and security law attorney based in Chicago. “It becomes very fact specific.”
Like HIPAA, the GDPR requires that health providers disclose information to patients about where and how their data may be used. Mr. Barchie notes that in the United States, patient consent forms may generally include two or three potential uses for patient data such as marketing and medical research. The GDPR specifies that each potential usage of patient data requires its own separate consent form, he said.
“Let’s say you’re a clinic that specializes in diabetes [and] you’re used to taking data and sending it to a general database to [collect information] about diabetes,” Mr. Barchie said. “You can’t do that under GDPR. You would have to have a separate consent form for that. So one consent to provide your diabetes service, one consent form to maybe market to the [patient], and a separate consent form [regarding] the database.”
GDPR also requires the minimizing of personal data copies stored within multiple systems. In the United States, it’s not uncommon for there to be multiple copies of a person’s data in several places, which makes sense from an IT perspective, Mr. Barchie said. The GDPR however requires that data keepers limit the number of copies they maintain to only the most necessary information.
“[Under GDPR], you should send only the data that you need for that particular process,” he said. “For example, [in the case of] address, user name, and patient ID. If you only need the patient ID number, you should not send the patient name and address. You minimize the amount of data that you’re sending to be processed.”
Breach notification also is more stringent under the GDPR, compared with U.S. regulations. Under HIPAA, covered entities must notify the U.S. Department of Health & Human Services and affected patients of a data breach without unreasonable delay no later than 60 days following discovery of a breach. The GDPR requires that effected entities notify the supervisory authority “without undue delay and, where feasible, not later than 72 hours after having become aware of [the breach].” (The GDPR supervisory authority depends on the EU country affected.)
If determined that the personal data breach is “likely to result in a high risk to the rights and freedoms of individuals,” efforts must be made to communicate about the personal data breach with the affected data subjects “without undue delay,” according to the rules.
If unsure of whether your practices may fall under GDPR, experts advise discussing the question with a legal counselor, GDPR expert, or risk management team.
U.S. health providers who treat foreign patients may want to take a closer look at their privacy policies to make sure they comply with new European Union data protection rules.
May 25 heralds the enforcement of the European Union’s General Data Protection Regulation (GDPR), a set of rules designed to strengthen and harmonize record protection for EU citizens and tighten how their data privacy is managed. The regulations protect various forms of electronic data including basic identity information, health and genetic data, and biometric information.
Treating a vacationing EU patient who needs unplanned treatment in the states is not likely to subject physicians to the GDPR, said Cynthia J. Larose, a privacy and data security attorney based in Boston.
“In general, the GDPR should not impact U.S. doctors who may incidentally treat an EU patient while that patient is here in the U.S.,” Ms. Larose said in an interview … If the EU patient presents at a U.S. health care provider for treatment, then the GDPR does not apply to her personal data in the possession of the U.S. health care provider – HIPAA applies. While the [GDPR] does have extraterritorial reach, you have to be doing something in the EU for the GDPR to apply.”
But other scenarios that could prove problematic, such as U.S. researchers studying patients in the EU, U.S. physicians providing telemedicine care to EU patients, and doctors who continue to monitor EU patients following treatment in the United States once patients return to their home country.
About 200,000 international visitors fly to the United States yearly for health treatment, of whom about 25% are from Europe, according to a 2015 report by the United States International Trade Commission.
Advertising medical services in the European Union is another way that U.S. physicians could be subject to the GDPR. For example, if a practice or hospital markets their specialty care on websites or other materials in the EU, this could fall under the GDPR umbrella, according to security experts.
“If you are advertising services to patients in the EU, and then they decide to obtain such services, that could trigger GDPR because the data subjects are in the EU and you are offering services to them,” said Elaine C. Zacharakis Loumbas, a health and security law attorney based in Chicago. “It becomes very fact specific.”
Like HIPAA, the GDPR requires that health providers disclose information to patients about where and how their data may be used. Mr. Barchie notes that in the United States, patient consent forms may generally include two or three potential uses for patient data such as marketing and medical research. The GDPR specifies that each potential usage of patient data requires its own separate consent form, he said.
“Let’s say you’re a clinic that specializes in diabetes [and] you’re used to taking data and sending it to a general database to [collect information] about diabetes,” Mr. Barchie said. “You can’t do that under GDPR. You would have to have a separate consent form for that. So one consent to provide your diabetes service, one consent form to maybe market to the [patient], and a separate consent form [regarding] the database.”
GDPR also requires the minimizing of personal data copies stored within multiple systems. In the United States, it’s not uncommon for there to be multiple copies of a person’s data in several places, which makes sense from an IT perspective, Mr. Barchie said. The GDPR however requires that data keepers limit the number of copies they maintain to only the most necessary information.
“[Under GDPR], you should send only the data that you need for that particular process,” he said. “For example, [in the case of] address, user name, and patient ID. If you only need the patient ID number, you should not send the patient name and address. You minimize the amount of data that you’re sending to be processed.”
Breach notification also is more stringent under the GDPR, compared with U.S. regulations. Under HIPAA, covered entities must notify the U.S. Department of Health & Human Services and affected patients of a data breach without unreasonable delay no later than 60 days following discovery of a breach. The GDPR requires that effected entities notify the supervisory authority “without undue delay and, where feasible, not later than 72 hours after having become aware of [the breach].” (The GDPR supervisory authority depends on the EU country affected.)
If determined that the personal data breach is “likely to result in a high risk to the rights and freedoms of individuals,” efforts must be made to communicate about the personal data breach with the affected data subjects “without undue delay,” according to the rules.
If unsure of whether your practices may fall under GDPR, experts advise discussing the question with a legal counselor, GDPR expert, or risk management team.
Ranking points physicians toward South Dakota
South Dakota’s nickname may be the Mount Rushmore State, but for physicians it’s the land of opportunity, according to personal finance website WalletHub.
South Dakota took the top spot in the 2018 list of the “best states to practice medicine” with 75.97 out of a possible 100 points while New Jersey took up residence at the other end of the list by finishing 51st (the District of Columbia was included) with a score of 40.24, WalletHub reported.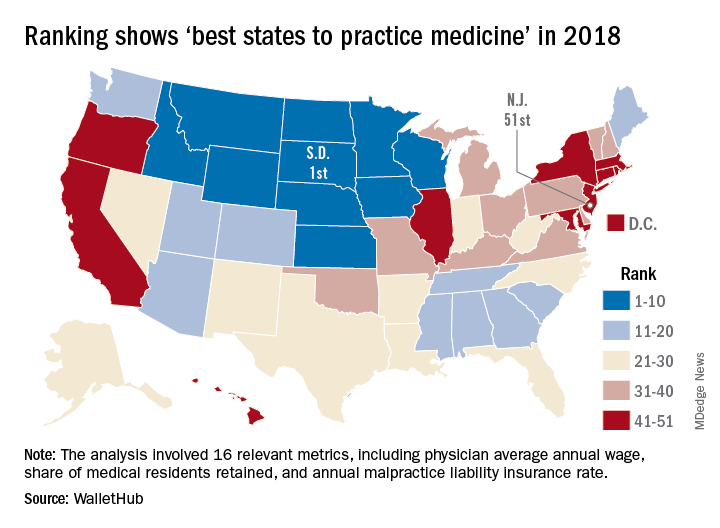
South Dakota headed a solid block of states in the upper Midwest and Rocky Mountain regions that made up the entire top 10, with Nebraska second overall, Idaho third, Iowa (last year’s winner) fourth, and Minnesota fifth. Finishing just above New Jersey were Rhode Island in 50th, New York in 49th, Hawaii in 48th, and D.C. in 47th.
WalletHub compared the 50 states and D.C. using 16 different metrics across two broad categories: “opportunity and competition” (11 metrics worth 70 points) and “medical environment” (5 metrics worth 30 points). Metrics included physicians’ average annual wage (adjusted for cost of living), employer-based insurance rates, projected share of elderly population, physician assistants per capita, and malpractice award payout amount per capita.
South Dakota, which ranked first in the medical environment category and third in opportunity and competition, posted top-5 scores in such areas as adjusted physician annual wage, lowest projected competition, and annual malpractice liability insurance rate, according to the survey.
South Dakota’s nickname may be the Mount Rushmore State, but for physicians it’s the land of opportunity, according to personal finance website WalletHub.
South Dakota took the top spot in the 2018 list of the “best states to practice medicine” with 75.97 out of a possible 100 points while New Jersey took up residence at the other end of the list by finishing 51st (the District of Columbia was included) with a score of 40.24, WalletHub reported.
South Dakota headed a solid block of states in the upper Midwest and Rocky Mountain regions that made up the entire top 10, with Nebraska second overall, Idaho third, Iowa (last year’s winner) fourth, and Minnesota fifth. Finishing just above New Jersey were Rhode Island in 50th, New York in 49th, Hawaii in 48th, and D.C. in 47th.
WalletHub compared the 50 states and D.C. using 16 different metrics across two broad categories: “opportunity and competition” (11 metrics worth 70 points) and “medical environment” (5 metrics worth 30 points). Metrics included physicians’ average annual wage (adjusted for cost of living), employer-based insurance rates, projected share of elderly population, physician assistants per capita, and malpractice award payout amount per capita.
South Dakota, which ranked first in the medical environment category and third in opportunity and competition, posted top-5 scores in such areas as adjusted physician annual wage, lowest projected competition, and annual malpractice liability insurance rate, according to the survey.
South Dakota’s nickname may be the Mount Rushmore State, but for physicians it’s the land of opportunity, according to personal finance website WalletHub.
South Dakota took the top spot in the 2018 list of the “best states to practice medicine” with 75.97 out of a possible 100 points while New Jersey took up residence at the other end of the list by finishing 51st (the District of Columbia was included) with a score of 40.24, WalletHub reported.
South Dakota headed a solid block of states in the upper Midwest and Rocky Mountain regions that made up the entire top 10, with Nebraska second overall, Idaho third, Iowa (last year’s winner) fourth, and Minnesota fifth. Finishing just above New Jersey were Rhode Island in 50th, New York in 49th, Hawaii in 48th, and D.C. in 47th.
WalletHub compared the 50 states and D.C. using 16 different metrics across two broad categories: “opportunity and competition” (11 metrics worth 70 points) and “medical environment” (5 metrics worth 30 points). Metrics included physicians’ average annual wage (adjusted for cost of living), employer-based insurance rates, projected share of elderly population, physician assistants per capita, and malpractice award payout amount per capita.
South Dakota, which ranked first in the medical environment category and third in opportunity and competition, posted top-5 scores in such areas as adjusted physician annual wage, lowest projected competition, and annual malpractice liability insurance rate, according to the survey.
Thousands mistakenly enrolled during state’s Medicaid expansion, feds find
California signed up an estimated 450,000 people under Medicaid expansion who may not have been eligible for coverage, according to a report by the Health & Human Services’ chief watchdog.
In a Feb. 21 report, the HHS’s inspector general estimated that California spent $738.2 million on 366,078 expansion beneficiaries who were ineligible. It spent an additional $416.5 million for 79,055 expansion enrollees who were “potentially” ineligible, auditors found.
Auditors said nearly 90% of the $1.15 billion in questionable payments involved federal money, while the rest came from the state’s Medicaid program, known as Medi-Cal. They examined a 6-month period from Oct. 1, 2014, to March 31, 2015, when Medicaid payments of $6.2 billion were made related to 1.9 million newly eligible enrollees.
There were limitations to the California review, however. The audit extrapolated from a sample of 150 beneficiaries. The authors reported a 90% confidence level in their results – whereas 95% would be more common. That meant that the number of those ineligible could have been as low as 260,000 or as high as 630,000.
“If HHS has a strong reason to believe that California is systematically making enrollment errors, it would be helpful to show that in a more robust analysis,” said Ben Ippolito, a health care economist at the American Enterprise Institute, a conservative think tank. “The federal government should ensure that states are being good stewards of federal money.”
Nonetheless, the audit highlighted weaknesses in California’s Medicaid program, the largest in the nation with 13.4 million enrollees and an annual budget topping $100 billion, counting federal and state money. Medicaid covers one in three Californians.
The inspector general found deficiencies in the state’s computer system for verifying eligibility and discovered errors by caseworkers. The Medicaid payments cited in the report covered people in the state’s fee-for-service system, managed-care plans, drug treatment programs, and those receiving mental health services.
California’s Department of Health Care Services, which runs Medi-Cal, said in a statement that it agreed with nearly all of the auditors’ recommendations and that the agency “has taken steps to address all of the findings.”
In a written response to the inspector general, California officials said several computer upgrades were made after the audit period and before publication of the report that should improve the accuracy of eligibility decisions.
Among the 150 expansion enrollees analyzed in detail, 75%, or 112, were deemed eligible for the Medicaid program in California. Auditors discovered a variety of problems with the other 38 enrollees.
During the audit period, 12 enrollees in the sample group had incomes above 138% of the federal poverty level, making them ineligible financially for public assistance, according to the report.
In other instances, beneficiaries were already enrolled in Medicare, the federal health insurance for people 65 and older or who have severe disabilities, and did not qualify for Medi-Cal. One woman indicated she didn’t want Medi-Cal but was enrolled anyway.
In 2014, the state struggled to clear a massive backlog of Medi-Cal applications, which reached about 900,000 at one point. Many people complained about being mistakenly rejected for coverage or that their applications were lost in the state or county computer systems.
California was one of 31 states to expand Medicaid under the 2010 Affordable Care Act. The health law established a higher federal reimbursement for these newly eligible patients, primarily low-income adults without children. After expansion started in 2014, the HHS inspector general’s office began reviewing whether states were determining eligibility correctly and spending taxpayer dollars appropriately.
In a similar audit released in January, the inspector general estimated that New York spent $26.2 million in federal Medicaid money on 47,271 expansion enrollees who were ineligible for coverage. (The sample size there was 130 enrollees.) Overall, New York had far fewer expansion enrollees and related spending, compared with California.
Audits of other states’ records are planned
“It is inevitable that in a big rollout of new eligibility for any public program there are going to be glitches in implementation,” said Kathy Hempstead, a health-policy expert and senior adviser at the Robert Wood Johnson Foundation. “The inspector general wants to make sure that states are being sufficiently careful.”
Nationwide, Medicaid, the state-federal health insurance program designed for the poor, is the country’s largest health insurance program, covering 74 million Americans. In the past year, Republican efforts to reduce Medicaid funding and enrollment have sparked intense political debates and loud protests over the size and scope of the public program.
The federal government footed the entire cost of Medicaid expansion during the first three years, instead of taking the usual approach of splitting the costs with states. Now, states are picking up more of the bill. Their share of the costs will grow to 10 percent by 2020.
The California audit didn’t request a specific repayment from the state, but the findings were sent to the Centers for Medicare & Medicaid Services for review. CMS officials didn’t return a request for comment.
Donald White, a spokesman for the inspector general’s office, said the agency stood by the report’s findings and declined to comment further.
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
California signed up an estimated 450,000 people under Medicaid expansion who may not have been eligible for coverage, according to a report by the Health & Human Services’ chief watchdog.
In a Feb. 21 report, the HHS’s inspector general estimated that California spent $738.2 million on 366,078 expansion beneficiaries who were ineligible. It spent an additional $416.5 million for 79,055 expansion enrollees who were “potentially” ineligible, auditors found.
Auditors said nearly 90% of the $1.15 billion in questionable payments involved federal money, while the rest came from the state’s Medicaid program, known as Medi-Cal. They examined a 6-month period from Oct. 1, 2014, to March 31, 2015, when Medicaid payments of $6.2 billion were made related to 1.9 million newly eligible enrollees.
There were limitations to the California review, however. The audit extrapolated from a sample of 150 beneficiaries. The authors reported a 90% confidence level in their results – whereas 95% would be more common. That meant that the number of those ineligible could have been as low as 260,000 or as high as 630,000.
“If HHS has a strong reason to believe that California is systematically making enrollment errors, it would be helpful to show that in a more robust analysis,” said Ben Ippolito, a health care economist at the American Enterprise Institute, a conservative think tank. “The federal government should ensure that states are being good stewards of federal money.”
Nonetheless, the audit highlighted weaknesses in California’s Medicaid program, the largest in the nation with 13.4 million enrollees and an annual budget topping $100 billion, counting federal and state money. Medicaid covers one in three Californians.
The inspector general found deficiencies in the state’s computer system for verifying eligibility and discovered errors by caseworkers. The Medicaid payments cited in the report covered people in the state’s fee-for-service system, managed-care plans, drug treatment programs, and those receiving mental health services.
California’s Department of Health Care Services, which runs Medi-Cal, said in a statement that it agreed with nearly all of the auditors’ recommendations and that the agency “has taken steps to address all of the findings.”
In a written response to the inspector general, California officials said several computer upgrades were made after the audit period and before publication of the report that should improve the accuracy of eligibility decisions.
Among the 150 expansion enrollees analyzed in detail, 75%, or 112, were deemed eligible for the Medicaid program in California. Auditors discovered a variety of problems with the other 38 enrollees.
During the audit period, 12 enrollees in the sample group had incomes above 138% of the federal poverty level, making them ineligible financially for public assistance, according to the report.
In other instances, beneficiaries were already enrolled in Medicare, the federal health insurance for people 65 and older or who have severe disabilities, and did not qualify for Medi-Cal. One woman indicated she didn’t want Medi-Cal but was enrolled anyway.
In 2014, the state struggled to clear a massive backlog of Medi-Cal applications, which reached about 900,000 at one point. Many people complained about being mistakenly rejected for coverage or that their applications were lost in the state or county computer systems.
California was one of 31 states to expand Medicaid under the 2010 Affordable Care Act. The health law established a higher federal reimbursement for these newly eligible patients, primarily low-income adults without children. After expansion started in 2014, the HHS inspector general’s office began reviewing whether states were determining eligibility correctly and spending taxpayer dollars appropriately.
In a similar audit released in January, the inspector general estimated that New York spent $26.2 million in federal Medicaid money on 47,271 expansion enrollees who were ineligible for coverage. (The sample size there was 130 enrollees.) Overall, New York had far fewer expansion enrollees and related spending, compared with California.
Audits of other states’ records are planned
“It is inevitable that in a big rollout of new eligibility for any public program there are going to be glitches in implementation,” said Kathy Hempstead, a health-policy expert and senior adviser at the Robert Wood Johnson Foundation. “The inspector general wants to make sure that states are being sufficiently careful.”
Nationwide, Medicaid, the state-federal health insurance program designed for the poor, is the country’s largest health insurance program, covering 74 million Americans. In the past year, Republican efforts to reduce Medicaid funding and enrollment have sparked intense political debates and loud protests over the size and scope of the public program.
The federal government footed the entire cost of Medicaid expansion during the first three years, instead of taking the usual approach of splitting the costs with states. Now, states are picking up more of the bill. Their share of the costs will grow to 10 percent by 2020.
The California audit didn’t request a specific repayment from the state, but the findings were sent to the Centers for Medicare & Medicaid Services for review. CMS officials didn’t return a request for comment.
Donald White, a spokesman for the inspector general’s office, said the agency stood by the report’s findings and declined to comment further.
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
California signed up an estimated 450,000 people under Medicaid expansion who may not have been eligible for coverage, according to a report by the Health & Human Services’ chief watchdog.
In a Feb. 21 report, the HHS’s inspector general estimated that California spent $738.2 million on 366,078 expansion beneficiaries who were ineligible. It spent an additional $416.5 million for 79,055 expansion enrollees who were “potentially” ineligible, auditors found.
Auditors said nearly 90% of the $1.15 billion in questionable payments involved federal money, while the rest came from the state’s Medicaid program, known as Medi-Cal. They examined a 6-month period from Oct. 1, 2014, to March 31, 2015, when Medicaid payments of $6.2 billion were made related to 1.9 million newly eligible enrollees.
There were limitations to the California review, however. The audit extrapolated from a sample of 150 beneficiaries. The authors reported a 90% confidence level in their results – whereas 95% would be more common. That meant that the number of those ineligible could have been as low as 260,000 or as high as 630,000.
“If HHS has a strong reason to believe that California is systematically making enrollment errors, it would be helpful to show that in a more robust analysis,” said Ben Ippolito, a health care economist at the American Enterprise Institute, a conservative think tank. “The federal government should ensure that states are being good stewards of federal money.”
Nonetheless, the audit highlighted weaknesses in California’s Medicaid program, the largest in the nation with 13.4 million enrollees and an annual budget topping $100 billion, counting federal and state money. Medicaid covers one in three Californians.
The inspector general found deficiencies in the state’s computer system for verifying eligibility and discovered errors by caseworkers. The Medicaid payments cited in the report covered people in the state’s fee-for-service system, managed-care plans, drug treatment programs, and those receiving mental health services.
California’s Department of Health Care Services, which runs Medi-Cal, said in a statement that it agreed with nearly all of the auditors’ recommendations and that the agency “has taken steps to address all of the findings.”
In a written response to the inspector general, California officials said several computer upgrades were made after the audit period and before publication of the report that should improve the accuracy of eligibility decisions.
Among the 150 expansion enrollees analyzed in detail, 75%, or 112, were deemed eligible for the Medicaid program in California. Auditors discovered a variety of problems with the other 38 enrollees.
During the audit period, 12 enrollees in the sample group had incomes above 138% of the federal poverty level, making them ineligible financially for public assistance, according to the report.
In other instances, beneficiaries were already enrolled in Medicare, the federal health insurance for people 65 and older or who have severe disabilities, and did not qualify for Medi-Cal. One woman indicated she didn’t want Medi-Cal but was enrolled anyway.
In 2014, the state struggled to clear a massive backlog of Medi-Cal applications, which reached about 900,000 at one point. Many people complained about being mistakenly rejected for coverage or that their applications were lost in the state or county computer systems.
California was one of 31 states to expand Medicaid under the 2010 Affordable Care Act. The health law established a higher federal reimbursement for these newly eligible patients, primarily low-income adults without children. After expansion started in 2014, the HHS inspector general’s office began reviewing whether states were determining eligibility correctly and spending taxpayer dollars appropriately.
In a similar audit released in January, the inspector general estimated that New York spent $26.2 million in federal Medicaid money on 47,271 expansion enrollees who were ineligible for coverage. (The sample size there was 130 enrollees.) Overall, New York had far fewer expansion enrollees and related spending, compared with California.
Audits of other states’ records are planned
“It is inevitable that in a big rollout of new eligibility for any public program there are going to be glitches in implementation,” said Kathy Hempstead, a health-policy expert and senior adviser at the Robert Wood Johnson Foundation. “The inspector general wants to make sure that states are being sufficiently careful.”
Nationwide, Medicaid, the state-federal health insurance program designed for the poor, is the country’s largest health insurance program, covering 74 million Americans. In the past year, Republican efforts to reduce Medicaid funding and enrollment have sparked intense political debates and loud protests over the size and scope of the public program.
The federal government footed the entire cost of Medicaid expansion during the first three years, instead of taking the usual approach of splitting the costs with states. Now, states are picking up more of the bill. Their share of the costs will grow to 10 percent by 2020.
The California audit didn’t request a specific repayment from the state, but the findings were sent to the Centers for Medicare & Medicaid Services for review. CMS officials didn’t return a request for comment.
Donald White, a spokesman for the inspector general’s office, said the agency stood by the report’s findings and declined to comment further.
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Hot Threads in ACS Communities
Your colleagues have a lot to say to each other. Here are the top discussion threads in ACS Communities this week:
1. ABS Continuous Certification Program (General Surgery)
2. ACS Leadership Response to ABS Continuous Certification Program (General Surgery)
3. Update on ACS Violence Prevention Strategy (General Surgery)
4. FNA parathyroid? (Endocrine Surgery)
5. Risk reduction mastectomy with mastectomy for unilateral breast cancer (Breast Surgery)
6. First mammogram after treatment (Breast Surgery)
7. Transferring for “pt preference” (Rural Surgery)
8. Where will the Gen X surgeon work? (Young Fellows)
9. Hiatal hernias and the sleeve (Bariatric Surgery)
10. Level 3 trauma (Trauma Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org, click “Communities” on the blue bar, select “All Communities,” and then click the “Join” button next to the communities you’d like to join. If you have any questions, please send them to [email protected].
Your colleagues have a lot to say to each other. Here are the top discussion threads in ACS Communities this week:
1. ABS Continuous Certification Program (General Surgery)
2. ACS Leadership Response to ABS Continuous Certification Program (General Surgery)
3. Update on ACS Violence Prevention Strategy (General Surgery)
4. FNA parathyroid? (Endocrine Surgery)
5. Risk reduction mastectomy with mastectomy for unilateral breast cancer (Breast Surgery)
6. First mammogram after treatment (Breast Surgery)
7. Transferring for “pt preference” (Rural Surgery)
8. Where will the Gen X surgeon work? (Young Fellows)
9. Hiatal hernias and the sleeve (Bariatric Surgery)
10. Level 3 trauma (Trauma Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org, click “Communities” on the blue bar, select “All Communities,” and then click the “Join” button next to the communities you’d like to join. If you have any questions, please send them to [email protected].
Your colleagues have a lot to say to each other. Here are the top discussion threads in ACS Communities this week:
1. ABS Continuous Certification Program (General Surgery)
2. ACS Leadership Response to ABS Continuous Certification Program (General Surgery)
3. Update on ACS Violence Prevention Strategy (General Surgery)
4. FNA parathyroid? (Endocrine Surgery)
5. Risk reduction mastectomy with mastectomy for unilateral breast cancer (Breast Surgery)
6. First mammogram after treatment (Breast Surgery)
7. Transferring for “pt preference” (Rural Surgery)
8. Where will the Gen X surgeon work? (Young Fellows)
9. Hiatal hernias and the sleeve (Bariatric Surgery)
10. Level 3 trauma (Trauma Surgery)
To join communities, log in to ACS Communities at http://acscommunities.facs.org, click “Communities” on the blue bar, select “All Communities,” and then click the “Join” button next to the communities you’d like to join. If you have any questions, please send them to [email protected].
Transgender trauma patients: What surgeons need to know
The likelihood that a is increasing every year.
The number of patients who self-identify as transgender and who have undergone both medical and/or surgical gender-affirming treatment is on the rise. The trend has accelerated since private insurers, Medicare, and Medicaid are now covering some of the costs (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231).
Lead author Samuel Mandell, MD, FACS, a trauma surgeon at the University of Washington Harborview Medical Center, Seattle, and his colleagues quote an estimate of 1 million transgender people in the United States. These individuals, many of whom have experienced gender dysphoria, in addition to stigma and negative psychosocial sequelae, may or may not have sought medical treatment. Medical interventions range from hormonal treatments to craniofacial plastic surgery and/or genital surgery.
“As transgender patients are more likely to be victims of assault and intimate partner violence or suicide, they are at increased risk for traumatic injury,” Dr. Mandell and coauthors said. More than 60% of the transgender population has been subjected to assault and more than 40% have attempted suicide. A recent study found that 42% of transgender individuals had a history on nonsuicidal self-injury (Psychiatr Clin North Am. 2017;40:41-50). The research team based their recommendations on managing transgender trauma patients on their own experience, and suggest some topics for future research
The authors searched the MEDLINE database for articles with the key words “trauma” or “injury” and “transgender/transsexual,” in addition to “surgery” and “transgender.” While the search yielded 388 articles, only 6 were relevant to acute care surgery or physical trauma/injury in the transgender population. “No articles were identified that addressed trauma/injury from the perspective of caring for the injured transgender patient,” Dr. Mandell and coauthors said.
The researchers recommend that the trauma surgeon begin if possible by working to establish patient-provider trust. “During surgical consultation, it is important to be aware that any transgender patient may have limited or negative interactions with general health care providers due to the significant discrimination this population faces,” the investigators wrote. Among the steps they suggest for the initial encounter with transgender patients are respectful questions about gender identity, asking what name they prefer, as well as what pronoun should be used.
Privacy concerns can be of particular sensitivity. “Care must be taken to maintain privacy for the patient, as others outside of the hospital may not know they are transgender. Consultation with the patient’s primary care provider may be beneficial to determine the extent of gender-affirmation and the patient’s disclosure to family and friends,” the investigator advised. In addition, the clinician needs to establish which if any nonmedical interventions the transgender patients has had. These may include nonprescription hormone therapy and silicone injections.
The encounter should include an evaluation for injury to genitalia. “Transgender patients may have significant dysphoria associated with their preoperative genitals,” Dr. Mandell and his coauthors said. In these cases, “involvement of providers experienced with examination of transgender patients should be sought, if possible.” These patients should be screened for potential abuse by a companion or self-injury, the investigators suggested.
Dr. Mandell and his coauthors also discussed some of the nuances of trauma care for this population. For example, transgender women may need a smaller endotracheal tube for establishing an airway as intubation to avoid damaging surgically altered vocal chords. Other craniofacial alterations can get in the way of establishing an airway. Clinicians also should keep in mind the increased likelihood of a venous thromboembolism from estrogen hormone therapy in immobilized transgender patients in the trauma setting. Implants and surgical alterations can add a layer of complexity to reading images. Anatomical rearrangement can make catheterization challenging.
Dr. Mandell and his coauthors concluded, “Further research is needed on the appropriate management of cross-gender hormones, dosing of medications and nutrition, and the special considerations for injury patterns and risks in transgender patients. Development of a system for quickly determining the state of gender-affirmation of the patient in regards to hormone therapy, surgeries, and social aspects may prove beneficial to providers in the setting of trauma, but involvement of the transgender population in the development of any such system is crucial.”
Eileen M. Bulger, MD, FACS, Chair ACS Committee on Trauma and one of the coauthors of the study, views the findings as potentially useful to meet the training deficit on transgender trauma issues. “As trauma surgeons, we strive to provide optimal care by attending to the physical, psychological, and social needs of our patients. This review raises awareness of critical issues to consider when caring for transgender patients and should be included in our educational programs for trauma fellowship training and used as a resource to raise awareness in our trauma centers.”
Dr. Mandell and his coauthors reported having no financial disclosures.
Education on the care of transgender and gender nonbinary population is lacking in both medical schools as well as surgical residencies, and it is often left to individual surgeons to seek out their own training. Unfortunately, this leaves many uncertain how to ask a patient about his/her/their history without making the patient uncomfortable. If we don’t ask the right questions, some patients may not disclose information that could be very detrimental to their care. Documentation in EHRs can be made difficult if the software doesn’t include transgender female, transgender male, and gender nonbinary options in addition to the binary choice of female or male. This can contribute to the misgendering and distress of the patient.
Asking which pronouns a transgender individual uses can be a big first step because it allows that person know that you are being respectful. Be prepared for pronouns you may not be used to: Some may use she/her or he/his, and some may use they/their, ze/hir, ze/zir or xe/xyr. It is important to have appropriate registration forms, gender neutral bathrooms, and respect and discretion from every individual provider for all of our patients. Providers should seek out education and training so that the patients aren’t forced to do the educating themselves. As trauma and acute care surgeons, we are used to caring for a diverse patient population with many unique needs. However, we don’t know enough about the trauma and surgery risks in the transgender and gender nonbinary population as only a limited research has been done. Studies such as this by Dr. Mandell et al. are encouraging and hopefully more will follow.
Andrea Long, MD, is an acute care surgeon and an assistant clinical professor at University of San Francisco, Fresno.
Education on the care of transgender and gender nonbinary population is lacking in both medical schools as well as surgical residencies, and it is often left to individual surgeons to seek out their own training. Unfortunately, this leaves many uncertain how to ask a patient about his/her/their history without making the patient uncomfortable. If we don’t ask the right questions, some patients may not disclose information that could be very detrimental to their care. Documentation in EHRs can be made difficult if the software doesn’t include transgender female, transgender male, and gender nonbinary options in addition to the binary choice of female or male. This can contribute to the misgendering and distress of the patient.
Asking which pronouns a transgender individual uses can be a big first step because it allows that person know that you are being respectful. Be prepared for pronouns you may not be used to: Some may use she/her or he/his, and some may use they/their, ze/hir, ze/zir or xe/xyr. It is important to have appropriate registration forms, gender neutral bathrooms, and respect and discretion from every individual provider for all of our patients. Providers should seek out education and training so that the patients aren’t forced to do the educating themselves. As trauma and acute care surgeons, we are used to caring for a diverse patient population with many unique needs. However, we don’t know enough about the trauma and surgery risks in the transgender and gender nonbinary population as only a limited research has been done. Studies such as this by Dr. Mandell et al. are encouraging and hopefully more will follow.
Andrea Long, MD, is an acute care surgeon and an assistant clinical professor at University of San Francisco, Fresno.
Education on the care of transgender and gender nonbinary population is lacking in both medical schools as well as surgical residencies, and it is often left to individual surgeons to seek out their own training. Unfortunately, this leaves many uncertain how to ask a patient about his/her/their history without making the patient uncomfortable. If we don’t ask the right questions, some patients may not disclose information that could be very detrimental to their care. Documentation in EHRs can be made difficult if the software doesn’t include transgender female, transgender male, and gender nonbinary options in addition to the binary choice of female or male. This can contribute to the misgendering and distress of the patient.
Asking which pronouns a transgender individual uses can be a big first step because it allows that person know that you are being respectful. Be prepared for pronouns you may not be used to: Some may use she/her or he/his, and some may use they/their, ze/hir, ze/zir or xe/xyr. It is important to have appropriate registration forms, gender neutral bathrooms, and respect and discretion from every individual provider for all of our patients. Providers should seek out education and training so that the patients aren’t forced to do the educating themselves. As trauma and acute care surgeons, we are used to caring for a diverse patient population with many unique needs. However, we don’t know enough about the trauma and surgery risks in the transgender and gender nonbinary population as only a limited research has been done. Studies such as this by Dr. Mandell et al. are encouraging and hopefully more will follow.
Andrea Long, MD, is an acute care surgeon and an assistant clinical professor at University of San Francisco, Fresno.
The likelihood that a is increasing every year.
The number of patients who self-identify as transgender and who have undergone both medical and/or surgical gender-affirming treatment is on the rise. The trend has accelerated since private insurers, Medicare, and Medicaid are now covering some of the costs (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231).
Lead author Samuel Mandell, MD, FACS, a trauma surgeon at the University of Washington Harborview Medical Center, Seattle, and his colleagues quote an estimate of 1 million transgender people in the United States. These individuals, many of whom have experienced gender dysphoria, in addition to stigma and negative psychosocial sequelae, may or may not have sought medical treatment. Medical interventions range from hormonal treatments to craniofacial plastic surgery and/or genital surgery.
“As transgender patients are more likely to be victims of assault and intimate partner violence or suicide, they are at increased risk for traumatic injury,” Dr. Mandell and coauthors said. More than 60% of the transgender population has been subjected to assault and more than 40% have attempted suicide. A recent study found that 42% of transgender individuals had a history on nonsuicidal self-injury (Psychiatr Clin North Am. 2017;40:41-50). The research team based their recommendations on managing transgender trauma patients on their own experience, and suggest some topics for future research
The authors searched the MEDLINE database for articles with the key words “trauma” or “injury” and “transgender/transsexual,” in addition to “surgery” and “transgender.” While the search yielded 388 articles, only 6 were relevant to acute care surgery or physical trauma/injury in the transgender population. “No articles were identified that addressed trauma/injury from the perspective of caring for the injured transgender patient,” Dr. Mandell and coauthors said.
The researchers recommend that the trauma surgeon begin if possible by working to establish patient-provider trust. “During surgical consultation, it is important to be aware that any transgender patient may have limited or negative interactions with general health care providers due to the significant discrimination this population faces,” the investigators wrote. Among the steps they suggest for the initial encounter with transgender patients are respectful questions about gender identity, asking what name they prefer, as well as what pronoun should be used.
Privacy concerns can be of particular sensitivity. “Care must be taken to maintain privacy for the patient, as others outside of the hospital may not know they are transgender. Consultation with the patient’s primary care provider may be beneficial to determine the extent of gender-affirmation and the patient’s disclosure to family and friends,” the investigator advised. In addition, the clinician needs to establish which if any nonmedical interventions the transgender patients has had. These may include nonprescription hormone therapy and silicone injections.
The encounter should include an evaluation for injury to genitalia. “Transgender patients may have significant dysphoria associated with their preoperative genitals,” Dr. Mandell and his coauthors said. In these cases, “involvement of providers experienced with examination of transgender patients should be sought, if possible.” These patients should be screened for potential abuse by a companion or self-injury, the investigators suggested.
Dr. Mandell and his coauthors also discussed some of the nuances of trauma care for this population. For example, transgender women may need a smaller endotracheal tube for establishing an airway as intubation to avoid damaging surgically altered vocal chords. Other craniofacial alterations can get in the way of establishing an airway. Clinicians also should keep in mind the increased likelihood of a venous thromboembolism from estrogen hormone therapy in immobilized transgender patients in the trauma setting. Implants and surgical alterations can add a layer of complexity to reading images. Anatomical rearrangement can make catheterization challenging.
Dr. Mandell and his coauthors concluded, “Further research is needed on the appropriate management of cross-gender hormones, dosing of medications and nutrition, and the special considerations for injury patterns and risks in transgender patients. Development of a system for quickly determining the state of gender-affirmation of the patient in regards to hormone therapy, surgeries, and social aspects may prove beneficial to providers in the setting of trauma, but involvement of the transgender population in the development of any such system is crucial.”
Eileen M. Bulger, MD, FACS, Chair ACS Committee on Trauma and one of the coauthors of the study, views the findings as potentially useful to meet the training deficit on transgender trauma issues. “As trauma surgeons, we strive to provide optimal care by attending to the physical, psychological, and social needs of our patients. This review raises awareness of critical issues to consider when caring for transgender patients and should be included in our educational programs for trauma fellowship training and used as a resource to raise awareness in our trauma centers.”
Dr. Mandell and his coauthors reported having no financial disclosures.
The likelihood that a is increasing every year.
The number of patients who self-identify as transgender and who have undergone both medical and/or surgical gender-affirming treatment is on the rise. The trend has accelerated since private insurers, Medicare, and Medicaid are now covering some of the costs (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231).
Lead author Samuel Mandell, MD, FACS, a trauma surgeon at the University of Washington Harborview Medical Center, Seattle, and his colleagues quote an estimate of 1 million transgender people in the United States. These individuals, many of whom have experienced gender dysphoria, in addition to stigma and negative psychosocial sequelae, may or may not have sought medical treatment. Medical interventions range from hormonal treatments to craniofacial plastic surgery and/or genital surgery.
“As transgender patients are more likely to be victims of assault and intimate partner violence or suicide, they are at increased risk for traumatic injury,” Dr. Mandell and coauthors said. More than 60% of the transgender population has been subjected to assault and more than 40% have attempted suicide. A recent study found that 42% of transgender individuals had a history on nonsuicidal self-injury (Psychiatr Clin North Am. 2017;40:41-50). The research team based their recommendations on managing transgender trauma patients on their own experience, and suggest some topics for future research
The authors searched the MEDLINE database for articles with the key words “trauma” or “injury” and “transgender/transsexual,” in addition to “surgery” and “transgender.” While the search yielded 388 articles, only 6 were relevant to acute care surgery or physical trauma/injury in the transgender population. “No articles were identified that addressed trauma/injury from the perspective of caring for the injured transgender patient,” Dr. Mandell and coauthors said.
The researchers recommend that the trauma surgeon begin if possible by working to establish patient-provider trust. “During surgical consultation, it is important to be aware that any transgender patient may have limited or negative interactions with general health care providers due to the significant discrimination this population faces,” the investigators wrote. Among the steps they suggest for the initial encounter with transgender patients are respectful questions about gender identity, asking what name they prefer, as well as what pronoun should be used.
Privacy concerns can be of particular sensitivity. “Care must be taken to maintain privacy for the patient, as others outside of the hospital may not know they are transgender. Consultation with the patient’s primary care provider may be beneficial to determine the extent of gender-affirmation and the patient’s disclosure to family and friends,” the investigator advised. In addition, the clinician needs to establish which if any nonmedical interventions the transgender patients has had. These may include nonprescription hormone therapy and silicone injections.
The encounter should include an evaluation for injury to genitalia. “Transgender patients may have significant dysphoria associated with their preoperative genitals,” Dr. Mandell and his coauthors said. In these cases, “involvement of providers experienced with examination of transgender patients should be sought, if possible.” These patients should be screened for potential abuse by a companion or self-injury, the investigators suggested.
Dr. Mandell and his coauthors also discussed some of the nuances of trauma care for this population. For example, transgender women may need a smaller endotracheal tube for establishing an airway as intubation to avoid damaging surgically altered vocal chords. Other craniofacial alterations can get in the way of establishing an airway. Clinicians also should keep in mind the increased likelihood of a venous thromboembolism from estrogen hormone therapy in immobilized transgender patients in the trauma setting. Implants and surgical alterations can add a layer of complexity to reading images. Anatomical rearrangement can make catheterization challenging.
Dr. Mandell and his coauthors concluded, “Further research is needed on the appropriate management of cross-gender hormones, dosing of medications and nutrition, and the special considerations for injury patterns and risks in transgender patients. Development of a system for quickly determining the state of gender-affirmation of the patient in regards to hormone therapy, surgeries, and social aspects may prove beneficial to providers in the setting of trauma, but involvement of the transgender population in the development of any such system is crucial.”
Eileen M. Bulger, MD, FACS, Chair ACS Committee on Trauma and one of the coauthors of the study, views the findings as potentially useful to meet the training deficit on transgender trauma issues. “As trauma surgeons, we strive to provide optimal care by attending to the physical, psychological, and social needs of our patients. This review raises awareness of critical issues to consider when caring for transgender patients and should be included in our educational programs for trauma fellowship training and used as a resource to raise awareness in our trauma centers.”
Dr. Mandell and his coauthors reported having no financial disclosures.
FROM THE JOURNAL OF TRAUMA AND ACUTE CARE SURGERY
FDA advisors recommend lofexidine for opioid withdrawal
SILVER SPRING, MD. – Members of the Food and Drug Administration Psychopharmacologic Drugs Advisory Committee voted 11 to 1 to recommend approval of lofexidine as the first nonopioid treatment option for the symptomatic treatment of opioid withdrawal.
Opioid withdrawal symptoms are the largest barrier to discontinuing opioid use, according to Louis Baxter, MD, executive medical director of the Professional Assistance Program in Princeton, N.J., who presented on behalf of U.S. WorldMeds, which plans to market lofexidine as Lucemyra.
Lofexidine, a selective alpha2-adrenergic receptor agonist that regulates norepinephrine release has been approved for management of opioid withdrawal in the United Kingdom since 1992.
The advisory committee voted to recommend lofexidine on the strength of the results of two randomized, double-blind, and placebo controlled phase 3 studies on the safety and efficacy of lofexidine for symptomatic treatment of opioid withdrawal between days 1 through 7. One study randomized 264 patients to lofexidine (134) or placebo (130), with patients in the treatment arm received 3.2 mg of lofexidine on days 1-5, then placebo until day 7. The second study randomized 603 patients to three groups, comparing high dose (3.2 mg/day) and low dose (2.4 mg/day) regimens of lofexidine to placebo; patients in the treatment arms took four smaller doses of lofexidine throughout the day to achieve the cumulative dose.
Researchers enrolled heavy users of short-acting opioids; heroin was the predominant agent. Both studies were conducted in the scenario of abrupt withdrawal, or the most intense withdrawal situation.
Symptomatic benefit was measured using the Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop), a patient reported outcome. Patients were asked to rank their symptoms as none, mild, moderate or severe across measures like feeling sick, stomach cramps, and heart pounding among other symptoms.
Lofexidine increased completion of withdrawal treatment compared to placebo. Patients in the first study had a 5-day completion rate of 53%, compared to 35% for the placebo group. Researchers observed similar results in the 7-day completion rates the second study, with low and high dose completion rates of 42% and 40%, respectively, both of which were much higher than placebo (28%).
Lofexidine also reduced patient withdrawal symptoms, according to SOWS-Gossop scores during peak withdrawal. In the first study, SOWS-Gossop scores were 2-4 points lower in the lofexidine group compared to placebo. Similarly, the scores were significantly better in both lofexidine groups in the second study, compared to placebo, particularly on days 1 to 4. Decreasing withdrawal symptoms during this period is particularly important because this is the most vulnerable window for patient dropout, briefing documents from US WorldMeds.
Several notable adverse events occurred during the study, particularly at higher doses of lofexidine. The risk of bradycardia and hypotension are prominent in patients taking lofexidine, but these are risks associated with this class of drug, according to Mark Pirner, MD, senior medical director at US WorldMeds, who noted that “the lower dose, if that’s what ultimately gets approved [by the FDA], is safe and effective too.”
Development of lofexidine was conducted in collaboration with the National Institute on Drug Abuse and the FDA, according to briefing documents from US WorldMeds.
The Prescription Drug User Fee Act (PDUFA) for lofexidine is May 26; FDA actions on new drug applications often occur at near the PDUFA date.
The FDA is not obligated to follow the recommendations of its advisory committees.
SILVER SPRING, MD. – Members of the Food and Drug Administration Psychopharmacologic Drugs Advisory Committee voted 11 to 1 to recommend approval of lofexidine as the first nonopioid treatment option for the symptomatic treatment of opioid withdrawal.
Opioid withdrawal symptoms are the largest barrier to discontinuing opioid use, according to Louis Baxter, MD, executive medical director of the Professional Assistance Program in Princeton, N.J., who presented on behalf of U.S. WorldMeds, which plans to market lofexidine as Lucemyra.
Lofexidine, a selective alpha2-adrenergic receptor agonist that regulates norepinephrine release has been approved for management of opioid withdrawal in the United Kingdom since 1992.
The advisory committee voted to recommend lofexidine on the strength of the results of two randomized, double-blind, and placebo controlled phase 3 studies on the safety and efficacy of lofexidine for symptomatic treatment of opioid withdrawal between days 1 through 7. One study randomized 264 patients to lofexidine (134) or placebo (130), with patients in the treatment arm received 3.2 mg of lofexidine on days 1-5, then placebo until day 7. The second study randomized 603 patients to three groups, comparing high dose (3.2 mg/day) and low dose (2.4 mg/day) regimens of lofexidine to placebo; patients in the treatment arms took four smaller doses of lofexidine throughout the day to achieve the cumulative dose.
Researchers enrolled heavy users of short-acting opioids; heroin was the predominant agent. Both studies were conducted in the scenario of abrupt withdrawal, or the most intense withdrawal situation.
Symptomatic benefit was measured using the Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop), a patient reported outcome. Patients were asked to rank their symptoms as none, mild, moderate or severe across measures like feeling sick, stomach cramps, and heart pounding among other symptoms.
Lofexidine increased completion of withdrawal treatment compared to placebo. Patients in the first study had a 5-day completion rate of 53%, compared to 35% for the placebo group. Researchers observed similar results in the 7-day completion rates the second study, with low and high dose completion rates of 42% and 40%, respectively, both of which were much higher than placebo (28%).
Lofexidine also reduced patient withdrawal symptoms, according to SOWS-Gossop scores during peak withdrawal. In the first study, SOWS-Gossop scores were 2-4 points lower in the lofexidine group compared to placebo. Similarly, the scores were significantly better in both lofexidine groups in the second study, compared to placebo, particularly on days 1 to 4. Decreasing withdrawal symptoms during this period is particularly important because this is the most vulnerable window for patient dropout, briefing documents from US WorldMeds.
Several notable adverse events occurred during the study, particularly at higher doses of lofexidine. The risk of bradycardia and hypotension are prominent in patients taking lofexidine, but these are risks associated with this class of drug, according to Mark Pirner, MD, senior medical director at US WorldMeds, who noted that “the lower dose, if that’s what ultimately gets approved [by the FDA], is safe and effective too.”
Development of lofexidine was conducted in collaboration with the National Institute on Drug Abuse and the FDA, according to briefing documents from US WorldMeds.
The Prescription Drug User Fee Act (PDUFA) for lofexidine is May 26; FDA actions on new drug applications often occur at near the PDUFA date.
The FDA is not obligated to follow the recommendations of its advisory committees.
SILVER SPRING, MD. – Members of the Food and Drug Administration Psychopharmacologic Drugs Advisory Committee voted 11 to 1 to recommend approval of lofexidine as the first nonopioid treatment option for the symptomatic treatment of opioid withdrawal.
Opioid withdrawal symptoms are the largest barrier to discontinuing opioid use, according to Louis Baxter, MD, executive medical director of the Professional Assistance Program in Princeton, N.J., who presented on behalf of U.S. WorldMeds, which plans to market lofexidine as Lucemyra.
Lofexidine, a selective alpha2-adrenergic receptor agonist that regulates norepinephrine release has been approved for management of opioid withdrawal in the United Kingdom since 1992.
The advisory committee voted to recommend lofexidine on the strength of the results of two randomized, double-blind, and placebo controlled phase 3 studies on the safety and efficacy of lofexidine for symptomatic treatment of opioid withdrawal between days 1 through 7. One study randomized 264 patients to lofexidine (134) or placebo (130), with patients in the treatment arm received 3.2 mg of lofexidine on days 1-5, then placebo until day 7. The second study randomized 603 patients to three groups, comparing high dose (3.2 mg/day) and low dose (2.4 mg/day) regimens of lofexidine to placebo; patients in the treatment arms took four smaller doses of lofexidine throughout the day to achieve the cumulative dose.
Researchers enrolled heavy users of short-acting opioids; heroin was the predominant agent. Both studies were conducted in the scenario of abrupt withdrawal, or the most intense withdrawal situation.
Symptomatic benefit was measured using the Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop), a patient reported outcome. Patients were asked to rank their symptoms as none, mild, moderate or severe across measures like feeling sick, stomach cramps, and heart pounding among other symptoms.
Lofexidine increased completion of withdrawal treatment compared to placebo. Patients in the first study had a 5-day completion rate of 53%, compared to 35% for the placebo group. Researchers observed similar results in the 7-day completion rates the second study, with low and high dose completion rates of 42% and 40%, respectively, both of which were much higher than placebo (28%).
Lofexidine also reduced patient withdrawal symptoms, according to SOWS-Gossop scores during peak withdrawal. In the first study, SOWS-Gossop scores were 2-4 points lower in the lofexidine group compared to placebo. Similarly, the scores were significantly better in both lofexidine groups in the second study, compared to placebo, particularly on days 1 to 4. Decreasing withdrawal symptoms during this period is particularly important because this is the most vulnerable window for patient dropout, briefing documents from US WorldMeds.
Several notable adverse events occurred during the study, particularly at higher doses of lofexidine. The risk of bradycardia and hypotension are prominent in patients taking lofexidine, but these are risks associated with this class of drug, according to Mark Pirner, MD, senior medical director at US WorldMeds, who noted that “the lower dose, if that’s what ultimately gets approved [by the FDA], is safe and effective too.”
Development of lofexidine was conducted in collaboration with the National Institute on Drug Abuse and the FDA, according to briefing documents from US WorldMeds.
The Prescription Drug User Fee Act (PDUFA) for lofexidine is May 26; FDA actions on new drug applications often occur at near the PDUFA date.
The FDA is not obligated to follow the recommendations of its advisory committees.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
From the Washington Office: An opportunity to address policymakers on the concerns of Fellows
On March 15, 2018, I had the opportunity to present on behalf of the ACS at a roundtable discussion on Capitol Hill to members of the House Ways and Means Committee on the topic of Medicare red tape relief
The roundtable provided members of this key committee of jurisdiction over Medicare policy the opportunity to hear from representatives from a variety of health care professional organizations on how Congress can improve Medicare to work more effectively and efficiently for both patients and providers. Each group was allotted just three minutes for their presentation. A summary of my presentation is included below:
E/M Documentation Guidelines
The ACS has significant concerns regarding Evaluation and Management (E/M) Documentation Guidelines. Though CMS created the E/M documentation guidelines 23 years ago with the laudable goal of adding structure to the various levels of E/M services, and in an effort to create a sense of equivalency of E/M services across the multitude of specialties, ACS believes the time has come to re-examine and revise these guidelines to be more appropriate in the modern digital information era.
Again, the primary goal of all medical record documentation is to provide an accurate, chronologic record of patient care. That said, the medical record also serves other important goals including communication between providers, data exchange to facilitate clinical decisions, and a legal document. The payment-focused E/M documentation guidelines do not serve any of these objectives.
There must be some level of trust of the provider by the payers. Physicians should have the ability to meet the primary goal of the medical record without being required to repeatedly enter the same information. If a family history is recorded on Monday, there should be no requirement to re-record it on Thursday unless something cogent changes in the interim. ACS believes that documentation should focus on the minimum data elements needed to establish an accurate chronologic record of patient care.
The ACS is prepared to assist in an effort to explore ways to revise the current paper-based E/M documentation guidelines such that they more efficiently and accurately document patient care information in the modern digital era.
Meaningful Measurement of Surgical Quality
I also addressed concerns relative to the meaningful measurement of surgical quality. Despite having expended significant human and financial resources toward helping Fellows succeed in MIPS, the College is becoming increasingly concerned that MIPS is not actually measuring surgical quality, and therefore, is not a quality program for surgery and serves primarily as a payment program.
As evidence, the most recent quality metric data available (from the 2015 Physician Quality Reporting System) show that many of the CMS quality measures reported by surgeons have little to do with improving the quality of the actual surgical care provided to patients. For general surgeons, the two most commonly reported measures were the documentation of a patient’s medications in the medical record and tobacco use screening. While no one would deny the importance of either of these activities, neither is of much real value in the effort to measure the quality of surgical care provided. In another, perhaps even more illustrative example, one of the most common quality measures reported by urologists was inquiring of their patients whether they had received a pneumovax. This obviously has little to do with why one would see an urologist, much less the quality of care provided.
As an organization, the ACS and its members are absolutely dedicated to improving the quality of care they provide to their patients. However, the quality measures forming the basis of the assessment of their care must first be relevant to the surgical care they provide, and second be achievable. Fellows are increasingly expressing concerns about the burdens imposed by the Quality component of MIPS and believe their efforts to participate do little to meaningfully measure the quality of surgical care they provide. I asked that the Ways and Means Committee hold a hearing specifically addressing issues relative to the Quality component of MIPS.
Standardizing Electronic Prior Authorization for Safe Prescribing Act
I expressed ACS’ support for the Standardizing Electronic Prior Authorization for Safe Prescribing Act, H.R. 4841, which would allow for electronic prior authorization under Medicare Part D and allow for the creation of technical standards for the electronic transmission of prior authorization. While the College believes this legislation is a good first step for electronic prior authorization, I asked that the scope of the legislation be expanded to include all medical services, supplies, and prescription drugs covered by the Medicare program, and also requested prior authorization policies be standardized across all insurers and that prior authorization requests, decisions, and appeals processes be automated through uniform electronic transaction portals for all services and supplies.
As evidence, I provided data from a 2017 ACS survey of nearly 300 surgeons and their staff, which indicated that, on average, a medical practice receives approximately 37 prior authorization requests per provider, per week. Action on these requests requires 25 hours to complete. This exorbitant expenditure of time and resources required for prior authorization is largely due to a lack of automated prior authorization processes that integrate with current electronic health record systems. The ACS is committed to working with the bill’s sponsors and the Ways and Means Committee toward a goal of swift passage.
Questions from and discussion with members of the Ways and Means Committee were truncated because of votes on the House floor. We look forward to continuing the dialogue in the coming weeks when the roundtable is reconvened.
Until next month ….
On March 15, 2018, I had the opportunity to present on behalf of the ACS at a roundtable discussion on Capitol Hill to members of the House Ways and Means Committee on the topic of Medicare red tape relief
The roundtable provided members of this key committee of jurisdiction over Medicare policy the opportunity to hear from representatives from a variety of health care professional organizations on how Congress can improve Medicare to work more effectively and efficiently for both patients and providers. Each group was allotted just three minutes for their presentation. A summary of my presentation is included below:
E/M Documentation Guidelines
The ACS has significant concerns regarding Evaluation and Management (E/M) Documentation Guidelines. Though CMS created the E/M documentation guidelines 23 years ago with the laudable goal of adding structure to the various levels of E/M services, and in an effort to create a sense of equivalency of E/M services across the multitude of specialties, ACS believes the time has come to re-examine and revise these guidelines to be more appropriate in the modern digital information era.
Again, the primary goal of all medical record documentation is to provide an accurate, chronologic record of patient care. That said, the medical record also serves other important goals including communication between providers, data exchange to facilitate clinical decisions, and a legal document. The payment-focused E/M documentation guidelines do not serve any of these objectives.
There must be some level of trust of the provider by the payers. Physicians should have the ability to meet the primary goal of the medical record without being required to repeatedly enter the same information. If a family history is recorded on Monday, there should be no requirement to re-record it on Thursday unless something cogent changes in the interim. ACS believes that documentation should focus on the minimum data elements needed to establish an accurate chronologic record of patient care.
The ACS is prepared to assist in an effort to explore ways to revise the current paper-based E/M documentation guidelines such that they more efficiently and accurately document patient care information in the modern digital era.
Meaningful Measurement of Surgical Quality
I also addressed concerns relative to the meaningful measurement of surgical quality. Despite having expended significant human and financial resources toward helping Fellows succeed in MIPS, the College is becoming increasingly concerned that MIPS is not actually measuring surgical quality, and therefore, is not a quality program for surgery and serves primarily as a payment program.
As evidence, the most recent quality metric data available (from the 2015 Physician Quality Reporting System) show that many of the CMS quality measures reported by surgeons have little to do with improving the quality of the actual surgical care provided to patients. For general surgeons, the two most commonly reported measures were the documentation of a patient’s medications in the medical record and tobacco use screening. While no one would deny the importance of either of these activities, neither is of much real value in the effort to measure the quality of surgical care provided. In another, perhaps even more illustrative example, one of the most common quality measures reported by urologists was inquiring of their patients whether they had received a pneumovax. This obviously has little to do with why one would see an urologist, much less the quality of care provided.
As an organization, the ACS and its members are absolutely dedicated to improving the quality of care they provide to their patients. However, the quality measures forming the basis of the assessment of their care must first be relevant to the surgical care they provide, and second be achievable. Fellows are increasingly expressing concerns about the burdens imposed by the Quality component of MIPS and believe their efforts to participate do little to meaningfully measure the quality of surgical care they provide. I asked that the Ways and Means Committee hold a hearing specifically addressing issues relative to the Quality component of MIPS.
Standardizing Electronic Prior Authorization for Safe Prescribing Act
I expressed ACS’ support for the Standardizing Electronic Prior Authorization for Safe Prescribing Act, H.R. 4841, which would allow for electronic prior authorization under Medicare Part D and allow for the creation of technical standards for the electronic transmission of prior authorization. While the College believes this legislation is a good first step for electronic prior authorization, I asked that the scope of the legislation be expanded to include all medical services, supplies, and prescription drugs covered by the Medicare program, and also requested prior authorization policies be standardized across all insurers and that prior authorization requests, decisions, and appeals processes be automated through uniform electronic transaction portals for all services and supplies.
As evidence, I provided data from a 2017 ACS survey of nearly 300 surgeons and their staff, which indicated that, on average, a medical practice receives approximately 37 prior authorization requests per provider, per week. Action on these requests requires 25 hours to complete. This exorbitant expenditure of time and resources required for prior authorization is largely due to a lack of automated prior authorization processes that integrate with current electronic health record systems. The ACS is committed to working with the bill’s sponsors and the Ways and Means Committee toward a goal of swift passage.
Questions from and discussion with members of the Ways and Means Committee were truncated because of votes on the House floor. We look forward to continuing the dialogue in the coming weeks when the roundtable is reconvened.
Until next month ….
On March 15, 2018, I had the opportunity to present on behalf of the ACS at a roundtable discussion on Capitol Hill to members of the House Ways and Means Committee on the topic of Medicare red tape relief
The roundtable provided members of this key committee of jurisdiction over Medicare policy the opportunity to hear from representatives from a variety of health care professional organizations on how Congress can improve Medicare to work more effectively and efficiently for both patients and providers. Each group was allotted just three minutes for their presentation. A summary of my presentation is included below:
E/M Documentation Guidelines
The ACS has significant concerns regarding Evaluation and Management (E/M) Documentation Guidelines. Though CMS created the E/M documentation guidelines 23 years ago with the laudable goal of adding structure to the various levels of E/M services, and in an effort to create a sense of equivalency of E/M services across the multitude of specialties, ACS believes the time has come to re-examine and revise these guidelines to be more appropriate in the modern digital information era.
Again, the primary goal of all medical record documentation is to provide an accurate, chronologic record of patient care. That said, the medical record also serves other important goals including communication between providers, data exchange to facilitate clinical decisions, and a legal document. The payment-focused E/M documentation guidelines do not serve any of these objectives.
There must be some level of trust of the provider by the payers. Physicians should have the ability to meet the primary goal of the medical record without being required to repeatedly enter the same information. If a family history is recorded on Monday, there should be no requirement to re-record it on Thursday unless something cogent changes in the interim. ACS believes that documentation should focus on the minimum data elements needed to establish an accurate chronologic record of patient care.
The ACS is prepared to assist in an effort to explore ways to revise the current paper-based E/M documentation guidelines such that they more efficiently and accurately document patient care information in the modern digital era.
Meaningful Measurement of Surgical Quality
I also addressed concerns relative to the meaningful measurement of surgical quality. Despite having expended significant human and financial resources toward helping Fellows succeed in MIPS, the College is becoming increasingly concerned that MIPS is not actually measuring surgical quality, and therefore, is not a quality program for surgery and serves primarily as a payment program.
As evidence, the most recent quality metric data available (from the 2015 Physician Quality Reporting System) show that many of the CMS quality measures reported by surgeons have little to do with improving the quality of the actual surgical care provided to patients. For general surgeons, the two most commonly reported measures were the documentation of a patient’s medications in the medical record and tobacco use screening. While no one would deny the importance of either of these activities, neither is of much real value in the effort to measure the quality of surgical care provided. In another, perhaps even more illustrative example, one of the most common quality measures reported by urologists was inquiring of their patients whether they had received a pneumovax. This obviously has little to do with why one would see an urologist, much less the quality of care provided.
As an organization, the ACS and its members are absolutely dedicated to improving the quality of care they provide to their patients. However, the quality measures forming the basis of the assessment of their care must first be relevant to the surgical care they provide, and second be achievable. Fellows are increasingly expressing concerns about the burdens imposed by the Quality component of MIPS and believe their efforts to participate do little to meaningfully measure the quality of surgical care they provide. I asked that the Ways and Means Committee hold a hearing specifically addressing issues relative to the Quality component of MIPS.
Standardizing Electronic Prior Authorization for Safe Prescribing Act
I expressed ACS’ support for the Standardizing Electronic Prior Authorization for Safe Prescribing Act, H.R. 4841, which would allow for electronic prior authorization under Medicare Part D and allow for the creation of technical standards for the electronic transmission of prior authorization. While the College believes this legislation is a good first step for electronic prior authorization, I asked that the scope of the legislation be expanded to include all medical services, supplies, and prescription drugs covered by the Medicare program, and also requested prior authorization policies be standardized across all insurers and that prior authorization requests, decisions, and appeals processes be automated through uniform electronic transaction portals for all services and supplies.
As evidence, I provided data from a 2017 ACS survey of nearly 300 surgeons and their staff, which indicated that, on average, a medical practice receives approximately 37 prior authorization requests per provider, per week. Action on these requests requires 25 hours to complete. This exorbitant expenditure of time and resources required for prior authorization is largely due to a lack of automated prior authorization processes that integrate with current electronic health record systems. The ACS is committed to working with the bill’s sponsors and the Ways and Means Committee toward a goal of swift passage.
Questions from and discussion with members of the Ways and Means Committee were truncated because of votes on the House floor. We look forward to continuing the dialogue in the coming weeks when the roundtable is reconvened.
Until next month ….
Femoral artery endarterectomy still ‘gold standard’
CHICAGO – Additional higher quality supporting evidence is needed before , Jeffrey J. Siracuse, MD, FACS, asserted at a symposium on vascular surgery sponsored by Northwestern University.
“Open surgery in the CFA [common femoral artery] is probably still the gold standard in most cases,” said Dr. Siracuse, a vascular surgeon at Boston University.
He was quick to note that others would disagree. Stenting and other endovascular interventions in the CFA are booming in popularity, particularly among cardiologists, interventional radiologists, and the patients to whom the clinicians present the option in a favorable light. But this enthusiasm is based almost entirely on small, single-center, retrospective studies conducted in patients with heterogeneous profiles. The one prospective randomized multicenter trial of stenting versus surgery for CFA stenosis published to date – the French TECCO study – has a number of key limitations, flaws, and unanswered questions, which endovascular proponents have overlooked in their enthusiasm to promote an “endo-first” approach in the CFA, according to Dr. Siracuse.
“Everyone’s pretty much jumping on the bandwagon now. I think endovascular therapy of the CFA is here to stay. You’re going to see more people doing it, and potentially doing it incorrectly,” he predicted.
“The biggest thing I worry about with stenting is covering or jailing out the deep femoral artery. On multiple occasions – including a case just 2 weeks ago – I’ve taken out stents placed in the CFA by others that developed in-stent hyperplasia to the extent that the entire stent goes down, the DFA is covered, and now all of a sudden you’ve lost all flow to the leg. That’s my biggest concern with stenting,” he said.
Dr. Siracuse has other reservations as well. The CFA has traditionally been considered a “no-stent zone” because of the unique biomechanical stresses the artery is subjected to as a result of torsion, flexion, and extension at the hip joint. These forces render the area particularly vulnerable to neointimal hyperplasia, acute thrombosis, and stent fracture.
In addition, he noted, CFA endarterectomy for atherosclerotic lesions is a mature, well-established operation with an excellent track record for safety and durability. Dr. Siracuse’s review of procedural safety in 1,513 patients in the American College of Surgeons National Surgical Quality Improvement Project database during 2007-2010 showed a 30-day mortality of 1.5% and a 7.9% rate of major or minor complications (Vasc Endovascular Surg. 2014 Jan;48[1]:27-33).
In contrast, his review of 1,014 patients who underwent nonemergent endovascular CFA interventions for CFA stenosis without acute limb ischemia in the Vascular Quality Initiative registry demonstrated a 1-year patency rate of 85.3%, significantly lower than historically observed patency rates for endarterectomy. The 30-day mortality rate of 1.6% associated with endovascular interventions was essentially the same as in his earlier analysis of endarterectomy in the ACS NSQIP database, and the average 1.5-day hospital length of stay was shorter than with open surgery. Of considerable concern, however, stent implantation, which was performed in 35% of the endovascular interventions, was an independent predictor of amputation or death, with an associated 195% increased risk (J Vasc Surg. 2017 Apr;[4]:1039-46).
The travails of TECCO
The 17-center French TECCO study randomized 117 patients with de novo CFA atherosclerotic lesions to treatment via self-expanding stents or open surgery. A total of 98 participants were Rutherford stage 3, making TECCO primarily a study of claudicants. The primary outcome – the 30-day combined rate of morbidity and mortality – occurred in 26% of the surgical patients, a significantly higher rate than the 12.5% in the stent population. After a median follow-up of 24 months, the rates of primary patency, target lesion and extremity revascularization, and sustained clinical improvement were similar in the two groups (JACC Cardiovasc Interv. 2017 Jul 10;10[13]:1344-54).
The TECCO findings were hailed by endovascular therapy partisans as a big win. However, closer examination tells a different story, according to Dr. Siracuse.
There was no 30-day mortality in this rather small study. All 16 morbidity events occurring in the open surgery group within 30 days were relatively minor: 10 cases of delayed wound healing, 4 cases of postoperative paresthesia requiring medication, and 2 cases of lymphorrhea lasting longer than 3 days. In contrast, the seven morbidity events in the stent group included a complication requiring urgent open surgical repair at the time of stenting, one stent fracture, and a major amputation.
“The investigators didn’t elaborate on that major amputation, but I thought it was a little alarming because you should not have a major amputation with CFA interventions for claudicants,” the vascular surgeon commented. “Really, do people care about a lymphatic leak or do they care about amputation? I think more needs to be fleshed out about what really happened in that case.”
He was also puzzled by the hospital lengths of stay: a mean of 3.2 days in the stent group and 6.3 days in the open surgery group. “I think those lengths of stay are astounding. Very high and unusual,” he observed.
Dr. Siracuse predicted that much-needed high-quality data comparing treatments of the CFA will be provided by the BEST-CLI trial (Best Endovascular versus Surgical Treatment for Critical Limb Ischemia), which has been updated to include both open and endovascular interventions.
He reported having no financial conflicts of interest regarding his presentation.
CHICAGO – Additional higher quality supporting evidence is needed before , Jeffrey J. Siracuse, MD, FACS, asserted at a symposium on vascular surgery sponsored by Northwestern University.
“Open surgery in the CFA [common femoral artery] is probably still the gold standard in most cases,” said Dr. Siracuse, a vascular surgeon at Boston University.
He was quick to note that others would disagree. Stenting and other endovascular interventions in the CFA are booming in popularity, particularly among cardiologists, interventional radiologists, and the patients to whom the clinicians present the option in a favorable light. But this enthusiasm is based almost entirely on small, single-center, retrospective studies conducted in patients with heterogeneous profiles. The one prospective randomized multicenter trial of stenting versus surgery for CFA stenosis published to date – the French TECCO study – has a number of key limitations, flaws, and unanswered questions, which endovascular proponents have overlooked in their enthusiasm to promote an “endo-first” approach in the CFA, according to Dr. Siracuse.
“Everyone’s pretty much jumping on the bandwagon now. I think endovascular therapy of the CFA is here to stay. You’re going to see more people doing it, and potentially doing it incorrectly,” he predicted.
“The biggest thing I worry about with stenting is covering or jailing out the deep femoral artery. On multiple occasions – including a case just 2 weeks ago – I’ve taken out stents placed in the CFA by others that developed in-stent hyperplasia to the extent that the entire stent goes down, the DFA is covered, and now all of a sudden you’ve lost all flow to the leg. That’s my biggest concern with stenting,” he said.
Dr. Siracuse has other reservations as well. The CFA has traditionally been considered a “no-stent zone” because of the unique biomechanical stresses the artery is subjected to as a result of torsion, flexion, and extension at the hip joint. These forces render the area particularly vulnerable to neointimal hyperplasia, acute thrombosis, and stent fracture.
In addition, he noted, CFA endarterectomy for atherosclerotic lesions is a mature, well-established operation with an excellent track record for safety and durability. Dr. Siracuse’s review of procedural safety in 1,513 patients in the American College of Surgeons National Surgical Quality Improvement Project database during 2007-2010 showed a 30-day mortality of 1.5% and a 7.9% rate of major or minor complications (Vasc Endovascular Surg. 2014 Jan;48[1]:27-33).
In contrast, his review of 1,014 patients who underwent nonemergent endovascular CFA interventions for CFA stenosis without acute limb ischemia in the Vascular Quality Initiative registry demonstrated a 1-year patency rate of 85.3%, significantly lower than historically observed patency rates for endarterectomy. The 30-day mortality rate of 1.6% associated with endovascular interventions was essentially the same as in his earlier analysis of endarterectomy in the ACS NSQIP database, and the average 1.5-day hospital length of stay was shorter than with open surgery. Of considerable concern, however, stent implantation, which was performed in 35% of the endovascular interventions, was an independent predictor of amputation or death, with an associated 195% increased risk (J Vasc Surg. 2017 Apr;[4]:1039-46).
The travails of TECCO
The 17-center French TECCO study randomized 117 patients with de novo CFA atherosclerotic lesions to treatment via self-expanding stents or open surgery. A total of 98 participants were Rutherford stage 3, making TECCO primarily a study of claudicants. The primary outcome – the 30-day combined rate of morbidity and mortality – occurred in 26% of the surgical patients, a significantly higher rate than the 12.5% in the stent population. After a median follow-up of 24 months, the rates of primary patency, target lesion and extremity revascularization, and sustained clinical improvement were similar in the two groups (JACC Cardiovasc Interv. 2017 Jul 10;10[13]:1344-54).
The TECCO findings were hailed by endovascular therapy partisans as a big win. However, closer examination tells a different story, according to Dr. Siracuse.
There was no 30-day mortality in this rather small study. All 16 morbidity events occurring in the open surgery group within 30 days were relatively minor: 10 cases of delayed wound healing, 4 cases of postoperative paresthesia requiring medication, and 2 cases of lymphorrhea lasting longer than 3 days. In contrast, the seven morbidity events in the stent group included a complication requiring urgent open surgical repair at the time of stenting, one stent fracture, and a major amputation.
“The investigators didn’t elaborate on that major amputation, but I thought it was a little alarming because you should not have a major amputation with CFA interventions for claudicants,” the vascular surgeon commented. “Really, do people care about a lymphatic leak or do they care about amputation? I think more needs to be fleshed out about what really happened in that case.”
He was also puzzled by the hospital lengths of stay: a mean of 3.2 days in the stent group and 6.3 days in the open surgery group. “I think those lengths of stay are astounding. Very high and unusual,” he observed.
Dr. Siracuse predicted that much-needed high-quality data comparing treatments of the CFA will be provided by the BEST-CLI trial (Best Endovascular versus Surgical Treatment for Critical Limb Ischemia), which has been updated to include both open and endovascular interventions.
He reported having no financial conflicts of interest regarding his presentation.
CHICAGO – Additional higher quality supporting evidence is needed before , Jeffrey J. Siracuse, MD, FACS, asserted at a symposium on vascular surgery sponsored by Northwestern University.
“Open surgery in the CFA [common femoral artery] is probably still the gold standard in most cases,” said Dr. Siracuse, a vascular surgeon at Boston University.
He was quick to note that others would disagree. Stenting and other endovascular interventions in the CFA are booming in popularity, particularly among cardiologists, interventional radiologists, and the patients to whom the clinicians present the option in a favorable light. But this enthusiasm is based almost entirely on small, single-center, retrospective studies conducted in patients with heterogeneous profiles. The one prospective randomized multicenter trial of stenting versus surgery for CFA stenosis published to date – the French TECCO study – has a number of key limitations, flaws, and unanswered questions, which endovascular proponents have overlooked in their enthusiasm to promote an “endo-first” approach in the CFA, according to Dr. Siracuse.
“Everyone’s pretty much jumping on the bandwagon now. I think endovascular therapy of the CFA is here to stay. You’re going to see more people doing it, and potentially doing it incorrectly,” he predicted.
“The biggest thing I worry about with stenting is covering or jailing out the deep femoral artery. On multiple occasions – including a case just 2 weeks ago – I’ve taken out stents placed in the CFA by others that developed in-stent hyperplasia to the extent that the entire stent goes down, the DFA is covered, and now all of a sudden you’ve lost all flow to the leg. That’s my biggest concern with stenting,” he said.
Dr. Siracuse has other reservations as well. The CFA has traditionally been considered a “no-stent zone” because of the unique biomechanical stresses the artery is subjected to as a result of torsion, flexion, and extension at the hip joint. These forces render the area particularly vulnerable to neointimal hyperplasia, acute thrombosis, and stent fracture.
In addition, he noted, CFA endarterectomy for atherosclerotic lesions is a mature, well-established operation with an excellent track record for safety and durability. Dr. Siracuse’s review of procedural safety in 1,513 patients in the American College of Surgeons National Surgical Quality Improvement Project database during 2007-2010 showed a 30-day mortality of 1.5% and a 7.9% rate of major or minor complications (Vasc Endovascular Surg. 2014 Jan;48[1]:27-33).
In contrast, his review of 1,014 patients who underwent nonemergent endovascular CFA interventions for CFA stenosis without acute limb ischemia in the Vascular Quality Initiative registry demonstrated a 1-year patency rate of 85.3%, significantly lower than historically observed patency rates for endarterectomy. The 30-day mortality rate of 1.6% associated with endovascular interventions was essentially the same as in his earlier analysis of endarterectomy in the ACS NSQIP database, and the average 1.5-day hospital length of stay was shorter than with open surgery. Of considerable concern, however, stent implantation, which was performed in 35% of the endovascular interventions, was an independent predictor of amputation or death, with an associated 195% increased risk (J Vasc Surg. 2017 Apr;[4]:1039-46).
The travails of TECCO
The 17-center French TECCO study randomized 117 patients with de novo CFA atherosclerotic lesions to treatment via self-expanding stents or open surgery. A total of 98 participants were Rutherford stage 3, making TECCO primarily a study of claudicants. The primary outcome – the 30-day combined rate of morbidity and mortality – occurred in 26% of the surgical patients, a significantly higher rate than the 12.5% in the stent population. After a median follow-up of 24 months, the rates of primary patency, target lesion and extremity revascularization, and sustained clinical improvement were similar in the two groups (JACC Cardiovasc Interv. 2017 Jul 10;10[13]:1344-54).
The TECCO findings were hailed by endovascular therapy partisans as a big win. However, closer examination tells a different story, according to Dr. Siracuse.
There was no 30-day mortality in this rather small study. All 16 morbidity events occurring in the open surgery group within 30 days were relatively minor: 10 cases of delayed wound healing, 4 cases of postoperative paresthesia requiring medication, and 2 cases of lymphorrhea lasting longer than 3 days. In contrast, the seven morbidity events in the stent group included a complication requiring urgent open surgical repair at the time of stenting, one stent fracture, and a major amputation.
“The investigators didn’t elaborate on that major amputation, but I thought it was a little alarming because you should not have a major amputation with CFA interventions for claudicants,” the vascular surgeon commented. “Really, do people care about a lymphatic leak or do they care about amputation? I think more needs to be fleshed out about what really happened in that case.”
He was also puzzled by the hospital lengths of stay: a mean of 3.2 days in the stent group and 6.3 days in the open surgery group. “I think those lengths of stay are astounding. Very high and unusual,” he observed.
Dr. Siracuse predicted that much-needed high-quality data comparing treatments of the CFA will be provided by the BEST-CLI trial (Best Endovascular versus Surgical Treatment for Critical Limb Ischemia), which has been updated to include both open and endovascular interventions.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
VIDEO: It is an exciting time in obesity treatment
BOSTON – For those in obesity treatment, things are looking up, said Reem Z. Sharaiha, MD, MSc, in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. There are several new therapies to choose from, said Dr. Sharaiha, assistant professor of medicine at Cornell University, New York – and a variety of therapies coming down the pipeline. The key is to choose the right treatment, or right combination of treatments – surgical, endoscopic, or medical – for the right patient at the right time and to follow up. Obesity is a chronic disease that needs long-term, team treatment. With obesity treatments there is sometimes a trade-off between risk and results, but the innovations coming along may balance that risk-results equation for some patients, she said.
BOSTON – For those in obesity treatment, things are looking up, said Reem Z. Sharaiha, MD, MSc, in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. There are several new therapies to choose from, said Dr. Sharaiha, assistant professor of medicine at Cornell University, New York – and a variety of therapies coming down the pipeline. The key is to choose the right treatment, or right combination of treatments – surgical, endoscopic, or medical – for the right patient at the right time and to follow up. Obesity is a chronic disease that needs long-term, team treatment. With obesity treatments there is sometimes a trade-off between risk and results, but the innovations coming along may balance that risk-results equation for some patients, she said.
BOSTON – For those in obesity treatment, things are looking up, said Reem Z. Sharaiha, MD, MSc, in a video interview at the AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. There are several new therapies to choose from, said Dr. Sharaiha, assistant professor of medicine at Cornell University, New York – and a variety of therapies coming down the pipeline. The key is to choose the right treatment, or right combination of treatments – surgical, endoscopic, or medical – for the right patient at the right time and to follow up. Obesity is a chronic disease that needs long-term, team treatment. With obesity treatments there is sometimes a trade-off between risk and results, but the innovations coming along may balance that risk-results equation for some patients, she said.
REPORTING FROM 2018 AGA TECH SUMMIT
VIDEO: Organ-sparing resection techniques should be way of the future
BOSTON – Organ-sparing resection techniques that remove lesions from the esophagus, stomach, and colon are being developed, Amrita Sethi, MD, said in a video interview at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Dr. Sethi, an assistant professor of medicine at Columbia University Medical Center, New York, said these techniques improve patient outcomes by maintaining organ integrity, whereas older techniques often led to removal of large amounts of tissue around lesions. And while organ-sparing techniques reduce recovery time and hospital stays, training and reimbursement for these procedures remain problematic. As much as new endoscopic package devices are needed from industry to make these procedures easier, automated or artificial intelligence is needed to help make the decisions on when these techniques are applicable. Reimbursement structures are needed so that these procedures make financial sense, she noted.
We live in a health care system now, Dr. Sethi said, in which benign polyps of the colon are being sent for surgical resection when what is really needed is referral for more advanced endoscopic treatment. This is a matter of training, and perhaps showing through comparative trials that organ-sparing techniques cost less and improve patient outcomes.
BOSTON – Organ-sparing resection techniques that remove lesions from the esophagus, stomach, and colon are being developed, Amrita Sethi, MD, said in a video interview at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Dr. Sethi, an assistant professor of medicine at Columbia University Medical Center, New York, said these techniques improve patient outcomes by maintaining organ integrity, whereas older techniques often led to removal of large amounts of tissue around lesions. And while organ-sparing techniques reduce recovery time and hospital stays, training and reimbursement for these procedures remain problematic. As much as new endoscopic package devices are needed from industry to make these procedures easier, automated or artificial intelligence is needed to help make the decisions on when these techniques are applicable. Reimbursement structures are needed so that these procedures make financial sense, she noted.
We live in a health care system now, Dr. Sethi said, in which benign polyps of the colon are being sent for surgical resection when what is really needed is referral for more advanced endoscopic treatment. This is a matter of training, and perhaps showing through comparative trials that organ-sparing techniques cost less and improve patient outcomes.
BOSTON – Organ-sparing resection techniques that remove lesions from the esophagus, stomach, and colon are being developed, Amrita Sethi, MD, said in a video interview at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Dr. Sethi, an assistant professor of medicine at Columbia University Medical Center, New York, said these techniques improve patient outcomes by maintaining organ integrity, whereas older techniques often led to removal of large amounts of tissue around lesions. And while organ-sparing techniques reduce recovery time and hospital stays, training and reimbursement for these procedures remain problematic. As much as new endoscopic package devices are needed from industry to make these procedures easier, automated or artificial intelligence is needed to help make the decisions on when these techniques are applicable. Reimbursement structures are needed so that these procedures make financial sense, she noted.
We live in a health care system now, Dr. Sethi said, in which benign polyps of the colon are being sent for surgical resection when what is really needed is referral for more advanced endoscopic treatment. This is a matter of training, and perhaps showing through comparative trials that organ-sparing techniques cost less and improve patient outcomes.
REPORTING FROM 2018 AGA TECH SUMMIT











