User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Cryo-Compression Therapy
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
All-Inside Meniscal Repair Devices
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Meniscal Root Tears: Identification and Repair
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
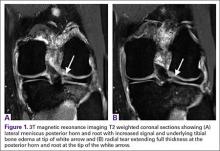
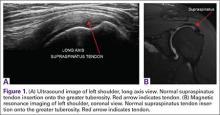
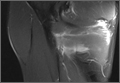
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
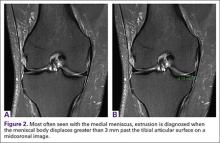
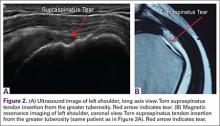
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
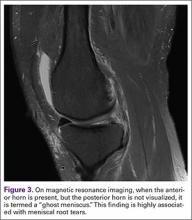
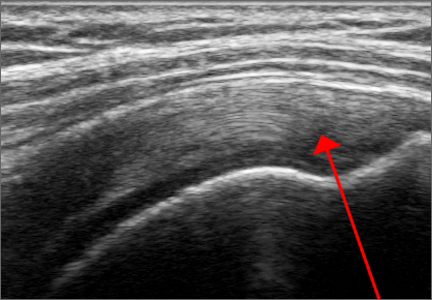
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
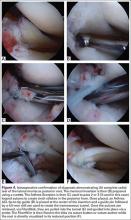
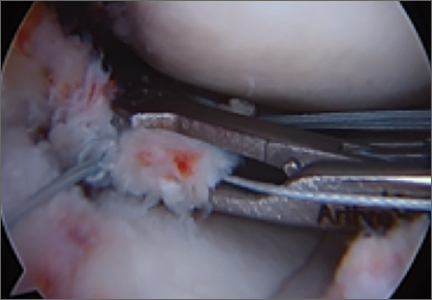
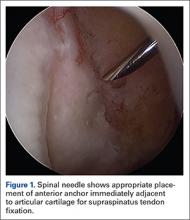
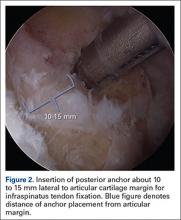
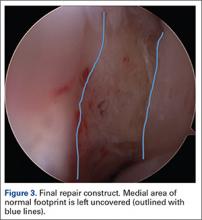
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
A Guide to Ultrasound of the Shoulder, Part 1: Coding and Reimbursement
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
The Epidemiology of Hip and Groin Injuries in Professional Baseball Players
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
Injury Trends in Major League Baseball Over 18 Seasons: 1998-2015
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Latissimus Dorsi and Teres Major Injuries in Major League Baseball Pitchers: A Systematic Review
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
Interval Throwing and Hitting Programs in Baseball: Biomechanics and Rehabilitation
Throwing and batting each require repetitive motions that can result in injuries unique to baseball. Fortuantely, advances in operative and nonoperative treatments have allowed players to return to competition after sustaining what previously would have been considered a career-ending injury. Once a player has been deemed ready to return to throwing or hitting, a comprehensive, multiphased approach to rehabilitation is necessary to reintroduce the athlete back to baseball activities and avoid re-injury. This article reviews the biomechanics of both throwing and hitting, and outlines the phases of rehabilitation necessary to allow the athlete to return to competition.
Throwing
Biomechanical Overview
The overhead throwing motion is complex and involves full body coordination from the initial force generation through the follow-through phase of throwing. The “kinetic chain”—the concept that movements in the body are connected through segments culminating with the highest energy in the final segment—is paramount to achieving the force and energy needed for throwing.1-8 The kinetic chain begins in the lower body and trunk and transmits the energy distally to the shoulder, elbow, and hand, ending with kinetic energy transfer to the ball.3-5,7 The progression of motion through the kinetic chain during throwing includes stride, pelvis rotation, upper torso rotation, elbow extension, shoulder internal rotation, and wrist flexion. Disruptions in this chain due to muscle imbalance or weakness can lead to injury downstream, particularly in the upper extremity.3,7,9
The importance of the kinetic chain can be highlighted in the 6 phases of throwing motion. These include wind-up, early arm cocking, late arm cocking, arm acceleration, arm deceleration, and follow-through (Figure 1).1,2,9,10
The wind-up phase starts with initiation of motion and ends with maximal knee lift of the lead leg; its objective is to place the body in an optimal stance to throw.3-5,7 There are minimal forces, torques, and muscle activity in the upper extremity during this phase, but up to 50% of throw speed is created through stride and trunk rotation.6 During the early cocking phase, the thrower keeps his stance foot planted and drives his lead leg towards the target, while bringing both arms into abduction. This is coupled with internal rotation of the stance hip, external rotation of the lead hip, and external rotation of the throwing shoulder. This creates linear velocity by maximizing the length of the elastic components of the body. Elbow, wrist, and finger extensors are also contracting during this phase to control elbow flexion and wrist hyperextension.3
The late cocking phase begins when the lead foot contacts the ground and ends with maximum shoulder external rotation.3-5 Lead foot contact is followed by quadriceps contraction to decelerate and stabilize the lead leg. This is followed by rotation of the pelvis and upper torso. The result is energy transfer to the throwing arm with a shear force across the anterior shoulder of 400 N.4 The shoulder stays in 90° of abduction, 15° of horizontal adduction, and externally rotates to between 150° and 180°. This produces a maximum horizontal adduction moment of 100 N.m and internal rotation torque of 70 N.m.4 Simultaneously, the elbow generates maximum flexion and a 65 N.m varus torque.7 Forces about the elbow are generated to resist the large angular velocity experienced (up to 3000°/second). This places an extreme amount of valgus stress along the medial elbow, particularly on the ulnar collateral ligament. The shoulder girdle and rotator cuff muscles simultaneously act to stabilize the scapula and glenohumeral joint.
The arm acceleration phase is from maximal shoulder external rotation until ball release.3-5 In this phase, the thrower flexes his trunk from an extended position, returning to neutral by the time of ball release while the lead leg straightens. The shoulder stays abducted at 90° throughout while the rotator cuff internal rotators and scapular stabilizers contract to explosively internally rotate the shoulder, creating a maximal internal rotation velocity greater than 7000°/second by ball release.1,4,7 The elbow also begins to extend, reaching maximum velocity during mid-acceleration phase from a combination of triceps contraction and torque generated from rotation at the shoulder and upper trunk.3 Finally, the wrist flexors contract to move the wrist to a neutral position from hyperextension as the ball is released.
During arm deceleration, the shoulder achieves maximum internal rotation until reaching a neutral position and horizontally adducts across the body. This is controlled by contraction of the shoulder girdle musculature; the teres minor has the highest activity.3,4 The greatest forces produced during the throwing motion act at the shoulder and elbow during deceleration and can contribute to injury.2 These include compressive forces of greater than 1000 N, posterior shear forces of 400 N, and inferior shear forces of 300 N.4,7
The final phase, the follow-through phase, starts at shoulder maximum internal rotation and ends when the arm assumes a balanced position across the trunk. Lower extremity extension and trunk flexion help distribute forces throughout the body, taking stress away from the throwing arm. The posterior shoulder musculature and scapular protractors contribute to continued deceleration and muscle firing returns to resting levels. This complex motion of throwing fueled by the kinetic chain lasts less than 2 seconds and can result in ball release speeds as high as 100 miles per hour.3,4
Return to Throwing: Principles
Nonoperative and postoperative rehabilitation programs allow restoration of motion, strength, static and dynamic stability, and neuromuscular control. The initiation of an interval throwing program (ITP) is based on the assumption that tissue healing is complete and a complete physical examination has been conducted to the treating physician’s approval.11 An ITP progressively applies forces along the kinetic chain in a controlled manner through graduated throwing distances, while minimizing the risk of re-injury.
Reinold and colleagues12 described guidelines that were used in the development of the ITP.12 These factors include: (1) The act of throwing a baseball involves the transfer of energy from the feet up to the hand and therefore careful attention must be paid along the entire kinetic chain; (2) gradual progression of interval throwing decreases the chance for re-injury; (3) proper warm-up; and (4) proper throwing mechanics minimizes the chance of re-injury.
Variability. Unlike traditional rehabilitation programs that advance an athlete based on a specific timetable, the ITP requires that each level or phase to be completed pain-free or without complications prior to starting the next level. Therefore, an ITP can be used for overhead athletes of varying skill levels because progression will be different from one athlete to another. It is also important to have the athlete adhere strictly to the program, as over-eagerness to complete the ITP as quickly as possible can increase the chance of re-injury and thus slow the rehabilitation process.12
Warm-up. An adequate warm-up is recommended prior to initiating ITP. An athlete should jog or cycle to develop a light sweat and then progress to stretching and flexibility exercises. As emphasized before, throwing involves nearly all the muscles in the body. Therefore, all muscle groups should be stretched beginning with the legs and working distally along the kinetic chain.
Mechanics. Analysis, correction, and maintenance of proper throwing mechanics is essential throughout the early phases of rehabilitation and ITP. Improper pitching mechanics places increased stress on the throwing arm, potentially leading to re-injury. Therefore, it would be valuable to have a pitching coach available to emphasize proper mechanics throughout the rehabilitation process.
The Interval Throwing Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 1.
Phase 1. We have adopted the ITP as described by Reinold and colleagues.12 Phase begins with the overhead athlete throwing on flat ground. He or she begins tossing from 45 feet and gradually progresses to 60, 90, 120, 150, and 180 feet.
As discussed earlier, it is critical to use proper mechanics throughout the ITP. The “crow hop” method simulates a throwing act and helps maintain proper pitching mechanics. Crow hop has 3 components: hop, skip, and throw. Using this technique, the pitcher begins warm-up throws at a comfortable distance (generally 30 feet) and then progresses to the distance as indicated on the ITP. The athlete will then need to perform each step 2 times, with 1 day of rest between steps, before advancing to the next step. The ball should be thrown with an arc and have only enough momentum to reach the desired distance.
For example, Step 1 calls for the athlete to perform 2 sets of 25 throws at 45 feet, with adequate rest (5 minutes) between sets. This step will be repeated following 1 day of rest. If the athlete demonstrates the ability to throw at the prescribed distance without pain, he or she can progress to Step 2, which calls for 3 sets of 25 throws at 45 feet. If pain is present at any step, the thrower returns to the previous asymptomatic step and can progress once he is pain-free.
Positional players are instructed to complete Phase 1 prior to starting position-specific drills. Pitchers, on the other hand, are instructed to stop once they reach and complete 120 feet. They will then progress to tossing at progressive distances of 60, 90, and 120 feet, followed by throwing at 60 feet 6 inches with normal pitching mechanics, initiating straight line throws with little to no arc.
Phase II (Throwing off the Mound). Once a pitcher completes Phase 1 without pain or complications, he is ready to begin throwing off the mound. The same principle remains in Phase 2: pitchers must complete each step pain-free before advancing to the next stage. Pitchers should first throw fastballs at 50% effort and progress to 75% and 100% effort. Because athletes often find it difficult to gauge their own effort, it is important to emphasize the importance of strictly adhering to the program. Fleisig and colleagues13 studied healthy pitchers’ ability to estimate their throwing effort. When targeting 50% effort, athletes generated ball speeds of 85% with forces and torque approaching 75% of maximum. A radar gun may be valuable in guiding effort control.
As the player advances through Phase 2, he will increase the volume of pitches as well as the effort in a gradual manner. The player may introduce breaking ball pitches once he demonstrates the ability to throw light batting practice. Phase 2 concludes with the pitcher throwing simulated games, progressing by 15 throws per workout.
Hitting
Biomechanics Overview
The mechanics of hitting a baseball can be broken down into 6 phases: the preparatory phase, stance phase, stride phase, drive phase, bat acceleration phase, and follow-through phase.14 While progressing through a return-to-play protocol, it is important to understand and teach the player proper swing mechanics during each phase in order to minimize the risk of re-injury (Figure 2).
The preparatory phase occurs as the player positions himself into the batter’s box. This phase is highly individualized, depending on each player’s personal preference. Though significant variability in approach exists, there are 3 basic stances a player can take in preparation to bat. In the closed stance, the batter’s front foot is positioned closer to the plate than the back foot. A more popular stance is the open stance, where the player’s back foot is placed closer to the plate than the front foot. The square batting stance is the most common stance. This stance is where both feet are in line with the pitcher and parallel with the edge of the batter’s box. Most authors agree that the square stance is the optimal position because it provides batters the best opportunity to hit pitches anywhere in the strike zone and limits compensatory or extra motion to their swing.15
Once the player begins the swing, he has entered the loading period, which is divided into the stance, stride, and drive phases. The loading period, also known as coiling or triggering, begins as the athlete eccentrically stretches agonist muscles and rotates the body away from the incoming ball. The elastic energy stored during this stretching is released during the concentric contraction of the same muscles and transferred through the entire kinetic chain as different segments of the body are rotated; it culminates in effort directed at hitting the baseball.16
In each phase of the loading period, certain critical motions should be monitored and corrected in order to return the player to his previous level of competition. Stride length has been shown to be critical in the timing of a batter’s swing. A short stride length can cause early initiation of the swing, while a longer stride can produce delayed activation of hip rotation. As the player enters the drive phase, he should have increased elbow flexion in the back elbow compared to the front elbow. The bat should be placed at a position approximately 45° in the frontal plane, and the bat should bisect the batter’s helmet. The back elbow should be down, both upper extremities should be positioned close to the hitter’s body, and the proximal interphalangeal joints of the hands should align on the handle of the bat. Athletic trainers and coaches should be aware that subtle compensations due to deficits during these movements could cause injury during the swing by disrupting the body’s natural motion.
The bat acceleration phase occurs from maximal bat loading through striking the ball. In this time, the linear force that has been exerted by the player must be transferred into rotational force through the trunk and upper extremities. When the lead leg contacts the ground, the player has created a closed kinetic chain, where the elastic energy gathered during the loading period is used to produce segmental rotation beginning in the hips and rising through the trunk and out to the arms and hands, finally producing contact with the baseball.16 To produce effective bat velocity, each segment must rotate in a sequential manner. If the upper extremities reach peak velocity before any lower segment, then the player has lost the ability to efficiently transfer kinetic energy up the kinetic chain.
Finally, the follow-through phase occurs after contact with the baseball and ends with complete deceleration, completing the swing. In order to achieve optimal effort, full hip rotation is needed, which is aided by rotation of the trail foot. Both hips and back laces should face the pitcher upon completion of the swing producing maximum power output.15
Return to Hitting: Principles
As with the initiation of the ITP, an interval hitting protocol (IHP) is designed to begin only after the player has been assessed on impairment measures, physical performance measures, and self-assessment.17 The player should have minimum to no pain, have no tenderness to palpation, and show adequate range of motion and strength to meet the demands of performing a full hitting cycle.12 It is recommended that before beginning a return-to-play protocol, the involved extremity should be at least 80% as strong as the uninvolved extremity.18 Physical measures challenging an athlete’s ability to perform tasks specific to hitting a baseball must also be considered through standardized examinations of the involved area.19 Finally, the athlete’s self-perception of functional abilities must be taken into account. This gives a subjective account of what the hitter perceives they are able to perform, providing useful insight into whether they are mentally prepared to participate in the protocol.
Like the ITP, progression through the IHP is also based on the player’s level of pain and soreness rather than following a specific timetable (Table). The program features a 1 day on, 1 day off schedule during which the player completes 1 step per day. The athlete must remain pain-free to progress to the next step and monitor his level of soreness during their workout. If pain or soreness persists, the player should rest for 2 days and be reevaluated upon return.17
The same principles of proper warm-up and mechanics apply in the IHP. An athlete should jog or cycle for a minimum of 10 minutes and perform stretching exercises focused on both upper and lower extremity muscles, as batting involves whole body movement. As the athlete progresses through the IHP, having a hitting coach to analyze, correct and maintain proper swing mechanics is valuable in enhancing performance as well as decreasing risk of re-injury.
The Interval Hitting Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 2.
Phase 1 (Dry Swings). Only the most basic fundamentals are stressed during this phase. The player should focus on properly moving from one phase of the swing to the next, without the goal of hitting the baseball. Trainers should measure critical points in the swing and correct deficits early.
Phase 2 (Batting Off a Tee). In this phase, the player is reintroduced to batting at low intensity with a fixed position target. The initial steps have the batter swing in a position of greatest comfort and natural movement, while the final steps in this phase test the athlete’s range of motion and confidence in the previous, healed injury.
Phase 3 (Soft Toss). As the player progresses to this phase, a baseball with trajectory is used to simulate differences in placement of pitches used during a game. As the hitter is able to pick up differences in target position, his performance and confidence should both increase.20 The coach should sit about 30 feet away, facing the hitter at an angle of 45°, and toss the ball in an underhand motion.
Phase 4 (Simulated Hitting). In this phase, the player and coach should focus on the timing of sequential body movements in order to elicit proper loading and force production. With the randomized pitch delivery and increased velocity, the hitter will practice against pitches similar to those delivered in competition.
Conclusion
Interval throwing and hitting programs are designed to allow the athlete to return to competition through a gradual, stepwise program. This permits the player to prepare his body for the unique stresses associated with throwing and hitting. The medical personnel should familiarize themselves with the philosophy of the interval throwing and hitting programs and individualize them to each athlete. Emphasis on proper warm-up, mechanics, and effort control is paramount in expediting return to play while preventing re-injury.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med Auckl NZ. 1996;21(6):421-437.
4. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
5. Kaczmarek PK, Lubiatowski P, Cisowski P, et al. Shoulder problems in overhead sports. Part I - biomechanics of throwing. Pol Orthop Traumatol. 2014;79:50-58.
6. Toyoshima S, Hoshikawa T, Miyashita M, Oguri T. Contribution of the body parts to throwing performance. Biomech IV. 1974;5:169-174.
7. Weber AE, Kontaxis A, O’Brien SJ, Bedi A. The biomechanics of throwing: simplified and cogent. Sports Med Arthrosc Rev. 2014;22(2):72-79.
8. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
9. Chang ES, Greco NJ, McClincy MP, Bradley JP. Posterior shoulder instability in overhead athletes. Orthop Clin North Am. 2016;47(1):179-187.
10. Digiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
11. Axe M, Hurd W, Snyder-Mackler L. Data-based interval throwing programs for baseball players. Sports Health. 2009;1(2):145-153.
12. Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32(6):293-298.
13. Fleisig GS, Zheng N, Barrentine SW, Escamilla RF, Andrews JR, Lemak LF. Kinematic and kinetic comparison of full and partial effort baseball pitching. Conference proceedings of the 20th Annual Meeting. Atlanta, GA: American Society of Biomechanics; 1996:151-152.
14. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
15. Monti R. Return to hitting: an interval hitting progression and overview of hitting mechanics following injury. Int J Sports Phys Ther. 2015;10(7):1059-1073.
16. Welch CM, Banks SA, Cook FF, Draovitch P. Hitting a baseball: a biomechanical description. J Orthop Sports Phys Ther. 1995;22(5):193-201.
17. Axe MJ, Snyder-Mackler L, Konin JG, Strube MJ. Development of a distance-based interval throwing program for Little League-aged athletes. Am J Sports Med. 1996;24(5):594-602.
18. Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194-203.
19. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642-648.
20. Higuchi T, Nagami T, Morohoshi J, Nakata H, Kanosue K. Disturbance in hitting accuracy by professional and collegiate baseball players due to intentional change of target position. Percept Mot Skills. 2013;116(2):627-639.
Throwing and batting each require repetitive motions that can result in injuries unique to baseball. Fortuantely, advances in operative and nonoperative treatments have allowed players to return to competition after sustaining what previously would have been considered a career-ending injury. Once a player has been deemed ready to return to throwing or hitting, a comprehensive, multiphased approach to rehabilitation is necessary to reintroduce the athlete back to baseball activities and avoid re-injury. This article reviews the biomechanics of both throwing and hitting, and outlines the phases of rehabilitation necessary to allow the athlete to return to competition.
Throwing
Biomechanical Overview
The overhead throwing motion is complex and involves full body coordination from the initial force generation through the follow-through phase of throwing. The “kinetic chain”—the concept that movements in the body are connected through segments culminating with the highest energy in the final segment—is paramount to achieving the force and energy needed for throwing.1-8 The kinetic chain begins in the lower body and trunk and transmits the energy distally to the shoulder, elbow, and hand, ending with kinetic energy transfer to the ball.3-5,7 The progression of motion through the kinetic chain during throwing includes stride, pelvis rotation, upper torso rotation, elbow extension, shoulder internal rotation, and wrist flexion. Disruptions in this chain due to muscle imbalance or weakness can lead to injury downstream, particularly in the upper extremity.3,7,9
The importance of the kinetic chain can be highlighted in the 6 phases of throwing motion. These include wind-up, early arm cocking, late arm cocking, arm acceleration, arm deceleration, and follow-through (Figure 1).1,2,9,10
The wind-up phase starts with initiation of motion and ends with maximal knee lift of the lead leg; its objective is to place the body in an optimal stance to throw.3-5,7 There are minimal forces, torques, and muscle activity in the upper extremity during this phase, but up to 50% of throw speed is created through stride and trunk rotation.6 During the early cocking phase, the thrower keeps his stance foot planted and drives his lead leg towards the target, while bringing both arms into abduction. This is coupled with internal rotation of the stance hip, external rotation of the lead hip, and external rotation of the throwing shoulder. This creates linear velocity by maximizing the length of the elastic components of the body. Elbow, wrist, and finger extensors are also contracting during this phase to control elbow flexion and wrist hyperextension.3
The late cocking phase begins when the lead foot contacts the ground and ends with maximum shoulder external rotation.3-5 Lead foot contact is followed by quadriceps contraction to decelerate and stabilize the lead leg. This is followed by rotation of the pelvis and upper torso. The result is energy transfer to the throwing arm with a shear force across the anterior shoulder of 400 N.4 The shoulder stays in 90° of abduction, 15° of horizontal adduction, and externally rotates to between 150° and 180°. This produces a maximum horizontal adduction moment of 100 N.m and internal rotation torque of 70 N.m.4 Simultaneously, the elbow generates maximum flexion and a 65 N.m varus torque.7 Forces about the elbow are generated to resist the large angular velocity experienced (up to 3000°/second). This places an extreme amount of valgus stress along the medial elbow, particularly on the ulnar collateral ligament. The shoulder girdle and rotator cuff muscles simultaneously act to stabilize the scapula and glenohumeral joint.
The arm acceleration phase is from maximal shoulder external rotation until ball release.3-5 In this phase, the thrower flexes his trunk from an extended position, returning to neutral by the time of ball release while the lead leg straightens. The shoulder stays abducted at 90° throughout while the rotator cuff internal rotators and scapular stabilizers contract to explosively internally rotate the shoulder, creating a maximal internal rotation velocity greater than 7000°/second by ball release.1,4,7 The elbow also begins to extend, reaching maximum velocity during mid-acceleration phase from a combination of triceps contraction and torque generated from rotation at the shoulder and upper trunk.3 Finally, the wrist flexors contract to move the wrist to a neutral position from hyperextension as the ball is released.
During arm deceleration, the shoulder achieves maximum internal rotation until reaching a neutral position and horizontally adducts across the body. This is controlled by contraction of the shoulder girdle musculature; the teres minor has the highest activity.3,4 The greatest forces produced during the throwing motion act at the shoulder and elbow during deceleration and can contribute to injury.2 These include compressive forces of greater than 1000 N, posterior shear forces of 400 N, and inferior shear forces of 300 N.4,7
The final phase, the follow-through phase, starts at shoulder maximum internal rotation and ends when the arm assumes a balanced position across the trunk. Lower extremity extension and trunk flexion help distribute forces throughout the body, taking stress away from the throwing arm. The posterior shoulder musculature and scapular protractors contribute to continued deceleration and muscle firing returns to resting levels. This complex motion of throwing fueled by the kinetic chain lasts less than 2 seconds and can result in ball release speeds as high as 100 miles per hour.3,4
Return to Throwing: Principles
Nonoperative and postoperative rehabilitation programs allow restoration of motion, strength, static and dynamic stability, and neuromuscular control. The initiation of an interval throwing program (ITP) is based on the assumption that tissue healing is complete and a complete physical examination has been conducted to the treating physician’s approval.11 An ITP progressively applies forces along the kinetic chain in a controlled manner through graduated throwing distances, while minimizing the risk of re-injury.
Reinold and colleagues12 described guidelines that were used in the development of the ITP.12 These factors include: (1) The act of throwing a baseball involves the transfer of energy from the feet up to the hand and therefore careful attention must be paid along the entire kinetic chain; (2) gradual progression of interval throwing decreases the chance for re-injury; (3) proper warm-up; and (4) proper throwing mechanics minimizes the chance of re-injury.
Variability. Unlike traditional rehabilitation programs that advance an athlete based on a specific timetable, the ITP requires that each level or phase to be completed pain-free or without complications prior to starting the next level. Therefore, an ITP can be used for overhead athletes of varying skill levels because progression will be different from one athlete to another. It is also important to have the athlete adhere strictly to the program, as over-eagerness to complete the ITP as quickly as possible can increase the chance of re-injury and thus slow the rehabilitation process.12
Warm-up. An adequate warm-up is recommended prior to initiating ITP. An athlete should jog or cycle to develop a light sweat and then progress to stretching and flexibility exercises. As emphasized before, throwing involves nearly all the muscles in the body. Therefore, all muscle groups should be stretched beginning with the legs and working distally along the kinetic chain.
Mechanics. Analysis, correction, and maintenance of proper throwing mechanics is essential throughout the early phases of rehabilitation and ITP. Improper pitching mechanics places increased stress on the throwing arm, potentially leading to re-injury. Therefore, it would be valuable to have a pitching coach available to emphasize proper mechanics throughout the rehabilitation process.
The Interval Throwing Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 1.
Phase 1. We have adopted the ITP as described by Reinold and colleagues.12 Phase begins with the overhead athlete throwing on flat ground. He or she begins tossing from 45 feet and gradually progresses to 60, 90, 120, 150, and 180 feet.
As discussed earlier, it is critical to use proper mechanics throughout the ITP. The “crow hop” method simulates a throwing act and helps maintain proper pitching mechanics. Crow hop has 3 components: hop, skip, and throw. Using this technique, the pitcher begins warm-up throws at a comfortable distance (generally 30 feet) and then progresses to the distance as indicated on the ITP. The athlete will then need to perform each step 2 times, with 1 day of rest between steps, before advancing to the next step. The ball should be thrown with an arc and have only enough momentum to reach the desired distance.
For example, Step 1 calls for the athlete to perform 2 sets of 25 throws at 45 feet, with adequate rest (5 minutes) between sets. This step will be repeated following 1 day of rest. If the athlete demonstrates the ability to throw at the prescribed distance without pain, he or she can progress to Step 2, which calls for 3 sets of 25 throws at 45 feet. If pain is present at any step, the thrower returns to the previous asymptomatic step and can progress once he is pain-free.
Positional players are instructed to complete Phase 1 prior to starting position-specific drills. Pitchers, on the other hand, are instructed to stop once they reach and complete 120 feet. They will then progress to tossing at progressive distances of 60, 90, and 120 feet, followed by throwing at 60 feet 6 inches with normal pitching mechanics, initiating straight line throws with little to no arc.
Phase II (Throwing off the Mound). Once a pitcher completes Phase 1 without pain or complications, he is ready to begin throwing off the mound. The same principle remains in Phase 2: pitchers must complete each step pain-free before advancing to the next stage. Pitchers should first throw fastballs at 50% effort and progress to 75% and 100% effort. Because athletes often find it difficult to gauge their own effort, it is important to emphasize the importance of strictly adhering to the program. Fleisig and colleagues13 studied healthy pitchers’ ability to estimate their throwing effort. When targeting 50% effort, athletes generated ball speeds of 85% with forces and torque approaching 75% of maximum. A radar gun may be valuable in guiding effort control.
As the player advances through Phase 2, he will increase the volume of pitches as well as the effort in a gradual manner. The player may introduce breaking ball pitches once he demonstrates the ability to throw light batting practice. Phase 2 concludes with the pitcher throwing simulated games, progressing by 15 throws per workout.
Hitting
Biomechanics Overview
The mechanics of hitting a baseball can be broken down into 6 phases: the preparatory phase, stance phase, stride phase, drive phase, bat acceleration phase, and follow-through phase.14 While progressing through a return-to-play protocol, it is important to understand and teach the player proper swing mechanics during each phase in order to minimize the risk of re-injury (Figure 2).
The preparatory phase occurs as the player positions himself into the batter’s box. This phase is highly individualized, depending on each player’s personal preference. Though significant variability in approach exists, there are 3 basic stances a player can take in preparation to bat. In the closed stance, the batter’s front foot is positioned closer to the plate than the back foot. A more popular stance is the open stance, where the player’s back foot is placed closer to the plate than the front foot. The square batting stance is the most common stance. This stance is where both feet are in line with the pitcher and parallel with the edge of the batter’s box. Most authors agree that the square stance is the optimal position because it provides batters the best opportunity to hit pitches anywhere in the strike zone and limits compensatory or extra motion to their swing.15
Once the player begins the swing, he has entered the loading period, which is divided into the stance, stride, and drive phases. The loading period, also known as coiling or triggering, begins as the athlete eccentrically stretches agonist muscles and rotates the body away from the incoming ball. The elastic energy stored during this stretching is released during the concentric contraction of the same muscles and transferred through the entire kinetic chain as different segments of the body are rotated; it culminates in effort directed at hitting the baseball.16
In each phase of the loading period, certain critical motions should be monitored and corrected in order to return the player to his previous level of competition. Stride length has been shown to be critical in the timing of a batter’s swing. A short stride length can cause early initiation of the swing, while a longer stride can produce delayed activation of hip rotation. As the player enters the drive phase, he should have increased elbow flexion in the back elbow compared to the front elbow. The bat should be placed at a position approximately 45° in the frontal plane, and the bat should bisect the batter’s helmet. The back elbow should be down, both upper extremities should be positioned close to the hitter’s body, and the proximal interphalangeal joints of the hands should align on the handle of the bat. Athletic trainers and coaches should be aware that subtle compensations due to deficits during these movements could cause injury during the swing by disrupting the body’s natural motion.
The bat acceleration phase occurs from maximal bat loading through striking the ball. In this time, the linear force that has been exerted by the player must be transferred into rotational force through the trunk and upper extremities. When the lead leg contacts the ground, the player has created a closed kinetic chain, where the elastic energy gathered during the loading period is used to produce segmental rotation beginning in the hips and rising through the trunk and out to the arms and hands, finally producing contact with the baseball.16 To produce effective bat velocity, each segment must rotate in a sequential manner. If the upper extremities reach peak velocity before any lower segment, then the player has lost the ability to efficiently transfer kinetic energy up the kinetic chain.
Finally, the follow-through phase occurs after contact with the baseball and ends with complete deceleration, completing the swing. In order to achieve optimal effort, full hip rotation is needed, which is aided by rotation of the trail foot. Both hips and back laces should face the pitcher upon completion of the swing producing maximum power output.15
Return to Hitting: Principles
As with the initiation of the ITP, an interval hitting protocol (IHP) is designed to begin only after the player has been assessed on impairment measures, physical performance measures, and self-assessment.17 The player should have minimum to no pain, have no tenderness to palpation, and show adequate range of motion and strength to meet the demands of performing a full hitting cycle.12 It is recommended that before beginning a return-to-play protocol, the involved extremity should be at least 80% as strong as the uninvolved extremity.18 Physical measures challenging an athlete’s ability to perform tasks specific to hitting a baseball must also be considered through standardized examinations of the involved area.19 Finally, the athlete’s self-perception of functional abilities must be taken into account. This gives a subjective account of what the hitter perceives they are able to perform, providing useful insight into whether they are mentally prepared to participate in the protocol.
Like the ITP, progression through the IHP is also based on the player’s level of pain and soreness rather than following a specific timetable (Table). The program features a 1 day on, 1 day off schedule during which the player completes 1 step per day. The athlete must remain pain-free to progress to the next step and monitor his level of soreness during their workout. If pain or soreness persists, the player should rest for 2 days and be reevaluated upon return.17
The same principles of proper warm-up and mechanics apply in the IHP. An athlete should jog or cycle for a minimum of 10 minutes and perform stretching exercises focused on both upper and lower extremity muscles, as batting involves whole body movement. As the athlete progresses through the IHP, having a hitting coach to analyze, correct and maintain proper swing mechanics is valuable in enhancing performance as well as decreasing risk of re-injury.
The Interval Hitting Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 2.
Phase 1 (Dry Swings). Only the most basic fundamentals are stressed during this phase. The player should focus on properly moving from one phase of the swing to the next, without the goal of hitting the baseball. Trainers should measure critical points in the swing and correct deficits early.
Phase 2 (Batting Off a Tee). In this phase, the player is reintroduced to batting at low intensity with a fixed position target. The initial steps have the batter swing in a position of greatest comfort and natural movement, while the final steps in this phase test the athlete’s range of motion and confidence in the previous, healed injury.
Phase 3 (Soft Toss). As the player progresses to this phase, a baseball with trajectory is used to simulate differences in placement of pitches used during a game. As the hitter is able to pick up differences in target position, his performance and confidence should both increase.20 The coach should sit about 30 feet away, facing the hitter at an angle of 45°, and toss the ball in an underhand motion.
Phase 4 (Simulated Hitting). In this phase, the player and coach should focus on the timing of sequential body movements in order to elicit proper loading and force production. With the randomized pitch delivery and increased velocity, the hitter will practice against pitches similar to those delivered in competition.
Conclusion
Interval throwing and hitting programs are designed to allow the athlete to return to competition through a gradual, stepwise program. This permits the player to prepare his body for the unique stresses associated with throwing and hitting. The medical personnel should familiarize themselves with the philosophy of the interval throwing and hitting programs and individualize them to each athlete. Emphasis on proper warm-up, mechanics, and effort control is paramount in expediting return to play while preventing re-injury.
Throwing and batting each require repetitive motions that can result in injuries unique to baseball. Fortuantely, advances in operative and nonoperative treatments have allowed players to return to competition after sustaining what previously would have been considered a career-ending injury. Once a player has been deemed ready to return to throwing or hitting, a comprehensive, multiphased approach to rehabilitation is necessary to reintroduce the athlete back to baseball activities and avoid re-injury. This article reviews the biomechanics of both throwing and hitting, and outlines the phases of rehabilitation necessary to allow the athlete to return to competition.
Throwing
Biomechanical Overview
The overhead throwing motion is complex and involves full body coordination from the initial force generation through the follow-through phase of throwing. The “kinetic chain”—the concept that movements in the body are connected through segments culminating with the highest energy in the final segment—is paramount to achieving the force and energy needed for throwing.1-8 The kinetic chain begins in the lower body and trunk and transmits the energy distally to the shoulder, elbow, and hand, ending with kinetic energy transfer to the ball.3-5,7 The progression of motion through the kinetic chain during throwing includes stride, pelvis rotation, upper torso rotation, elbow extension, shoulder internal rotation, and wrist flexion. Disruptions in this chain due to muscle imbalance or weakness can lead to injury downstream, particularly in the upper extremity.3,7,9
The importance of the kinetic chain can be highlighted in the 6 phases of throwing motion. These include wind-up, early arm cocking, late arm cocking, arm acceleration, arm deceleration, and follow-through (Figure 1).1,2,9,10
The wind-up phase starts with initiation of motion and ends with maximal knee lift of the lead leg; its objective is to place the body in an optimal stance to throw.3-5,7 There are minimal forces, torques, and muscle activity in the upper extremity during this phase, but up to 50% of throw speed is created through stride and trunk rotation.6 During the early cocking phase, the thrower keeps his stance foot planted and drives his lead leg towards the target, while bringing both arms into abduction. This is coupled with internal rotation of the stance hip, external rotation of the lead hip, and external rotation of the throwing shoulder. This creates linear velocity by maximizing the length of the elastic components of the body. Elbow, wrist, and finger extensors are also contracting during this phase to control elbow flexion and wrist hyperextension.3
The late cocking phase begins when the lead foot contacts the ground and ends with maximum shoulder external rotation.3-5 Lead foot contact is followed by quadriceps contraction to decelerate and stabilize the lead leg. This is followed by rotation of the pelvis and upper torso. The result is energy transfer to the throwing arm with a shear force across the anterior shoulder of 400 N.4 The shoulder stays in 90° of abduction, 15° of horizontal adduction, and externally rotates to between 150° and 180°. This produces a maximum horizontal adduction moment of 100 N.m and internal rotation torque of 70 N.m.4 Simultaneously, the elbow generates maximum flexion and a 65 N.m varus torque.7 Forces about the elbow are generated to resist the large angular velocity experienced (up to 3000°/second). This places an extreme amount of valgus stress along the medial elbow, particularly on the ulnar collateral ligament. The shoulder girdle and rotator cuff muscles simultaneously act to stabilize the scapula and glenohumeral joint.
The arm acceleration phase is from maximal shoulder external rotation until ball release.3-5 In this phase, the thrower flexes his trunk from an extended position, returning to neutral by the time of ball release while the lead leg straightens. The shoulder stays abducted at 90° throughout while the rotator cuff internal rotators and scapular stabilizers contract to explosively internally rotate the shoulder, creating a maximal internal rotation velocity greater than 7000°/second by ball release.1,4,7 The elbow also begins to extend, reaching maximum velocity during mid-acceleration phase from a combination of triceps contraction and torque generated from rotation at the shoulder and upper trunk.3 Finally, the wrist flexors contract to move the wrist to a neutral position from hyperextension as the ball is released.
During arm deceleration, the shoulder achieves maximum internal rotation until reaching a neutral position and horizontally adducts across the body. This is controlled by contraction of the shoulder girdle musculature; the teres minor has the highest activity.3,4 The greatest forces produced during the throwing motion act at the shoulder and elbow during deceleration and can contribute to injury.2 These include compressive forces of greater than 1000 N, posterior shear forces of 400 N, and inferior shear forces of 300 N.4,7
The final phase, the follow-through phase, starts at shoulder maximum internal rotation and ends when the arm assumes a balanced position across the trunk. Lower extremity extension and trunk flexion help distribute forces throughout the body, taking stress away from the throwing arm. The posterior shoulder musculature and scapular protractors contribute to continued deceleration and muscle firing returns to resting levels. This complex motion of throwing fueled by the kinetic chain lasts less than 2 seconds and can result in ball release speeds as high as 100 miles per hour.3,4
Return to Throwing: Principles
Nonoperative and postoperative rehabilitation programs allow restoration of motion, strength, static and dynamic stability, and neuromuscular control. The initiation of an interval throwing program (ITP) is based on the assumption that tissue healing is complete and a complete physical examination has been conducted to the treating physician’s approval.11 An ITP progressively applies forces along the kinetic chain in a controlled manner through graduated throwing distances, while minimizing the risk of re-injury.
Reinold and colleagues12 described guidelines that were used in the development of the ITP.12 These factors include: (1) The act of throwing a baseball involves the transfer of energy from the feet up to the hand and therefore careful attention must be paid along the entire kinetic chain; (2) gradual progression of interval throwing decreases the chance for re-injury; (3) proper warm-up; and (4) proper throwing mechanics minimizes the chance of re-injury.
Variability. Unlike traditional rehabilitation programs that advance an athlete based on a specific timetable, the ITP requires that each level or phase to be completed pain-free or without complications prior to starting the next level. Therefore, an ITP can be used for overhead athletes of varying skill levels because progression will be different from one athlete to another. It is also important to have the athlete adhere strictly to the program, as over-eagerness to complete the ITP as quickly as possible can increase the chance of re-injury and thus slow the rehabilitation process.12
Warm-up. An adequate warm-up is recommended prior to initiating ITP. An athlete should jog or cycle to develop a light sweat and then progress to stretching and flexibility exercises. As emphasized before, throwing involves nearly all the muscles in the body. Therefore, all muscle groups should be stretched beginning with the legs and working distally along the kinetic chain.
Mechanics. Analysis, correction, and maintenance of proper throwing mechanics is essential throughout the early phases of rehabilitation and ITP. Improper pitching mechanics places increased stress on the throwing arm, potentially leading to re-injury. Therefore, it would be valuable to have a pitching coach available to emphasize proper mechanics throughout the rehabilitation process.
The Interval Throwing Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 1.
Phase 1. We have adopted the ITP as described by Reinold and colleagues.12 Phase begins with the overhead athlete throwing on flat ground. He or she begins tossing from 45 feet and gradually progresses to 60, 90, 120, 150, and 180 feet.
As discussed earlier, it is critical to use proper mechanics throughout the ITP. The “crow hop” method simulates a throwing act and helps maintain proper pitching mechanics. Crow hop has 3 components: hop, skip, and throw. Using this technique, the pitcher begins warm-up throws at a comfortable distance (generally 30 feet) and then progresses to the distance as indicated on the ITP. The athlete will then need to perform each step 2 times, with 1 day of rest between steps, before advancing to the next step. The ball should be thrown with an arc and have only enough momentum to reach the desired distance.
For example, Step 1 calls for the athlete to perform 2 sets of 25 throws at 45 feet, with adequate rest (5 minutes) between sets. This step will be repeated following 1 day of rest. If the athlete demonstrates the ability to throw at the prescribed distance without pain, he or she can progress to Step 2, which calls for 3 sets of 25 throws at 45 feet. If pain is present at any step, the thrower returns to the previous asymptomatic step and can progress once he is pain-free.
Positional players are instructed to complete Phase 1 prior to starting position-specific drills. Pitchers, on the other hand, are instructed to stop once they reach and complete 120 feet. They will then progress to tossing at progressive distances of 60, 90, and 120 feet, followed by throwing at 60 feet 6 inches with normal pitching mechanics, initiating straight line throws with little to no arc.
Phase II (Throwing off the Mound). Once a pitcher completes Phase 1 without pain or complications, he is ready to begin throwing off the mound. The same principle remains in Phase 2: pitchers must complete each step pain-free before advancing to the next stage. Pitchers should first throw fastballs at 50% effort and progress to 75% and 100% effort. Because athletes often find it difficult to gauge their own effort, it is important to emphasize the importance of strictly adhering to the program. Fleisig and colleagues13 studied healthy pitchers’ ability to estimate their throwing effort. When targeting 50% effort, athletes generated ball speeds of 85% with forces and torque approaching 75% of maximum. A radar gun may be valuable in guiding effort control.
As the player advances through Phase 2, he will increase the volume of pitches as well as the effort in a gradual manner. The player may introduce breaking ball pitches once he demonstrates the ability to throw light batting practice. Phase 2 concludes with the pitcher throwing simulated games, progressing by 15 throws per workout.
Hitting
Biomechanics Overview
The mechanics of hitting a baseball can be broken down into 6 phases: the preparatory phase, stance phase, stride phase, drive phase, bat acceleration phase, and follow-through phase.14 While progressing through a return-to-play protocol, it is important to understand and teach the player proper swing mechanics during each phase in order to minimize the risk of re-injury (Figure 2).
The preparatory phase occurs as the player positions himself into the batter’s box. This phase is highly individualized, depending on each player’s personal preference. Though significant variability in approach exists, there are 3 basic stances a player can take in preparation to bat. In the closed stance, the batter’s front foot is positioned closer to the plate than the back foot. A more popular stance is the open stance, where the player’s back foot is placed closer to the plate than the front foot. The square batting stance is the most common stance. This stance is where both feet are in line with the pitcher and parallel with the edge of the batter’s box. Most authors agree that the square stance is the optimal position because it provides batters the best opportunity to hit pitches anywhere in the strike zone and limits compensatory or extra motion to their swing.15
Once the player begins the swing, he has entered the loading period, which is divided into the stance, stride, and drive phases. The loading period, also known as coiling or triggering, begins as the athlete eccentrically stretches agonist muscles and rotates the body away from the incoming ball. The elastic energy stored during this stretching is released during the concentric contraction of the same muscles and transferred through the entire kinetic chain as different segments of the body are rotated; it culminates in effort directed at hitting the baseball.16
In each phase of the loading period, certain critical motions should be monitored and corrected in order to return the player to his previous level of competition. Stride length has been shown to be critical in the timing of a batter’s swing. A short stride length can cause early initiation of the swing, while a longer stride can produce delayed activation of hip rotation. As the player enters the drive phase, he should have increased elbow flexion in the back elbow compared to the front elbow. The bat should be placed at a position approximately 45° in the frontal plane, and the bat should bisect the batter’s helmet. The back elbow should be down, both upper extremities should be positioned close to the hitter’s body, and the proximal interphalangeal joints of the hands should align on the handle of the bat. Athletic trainers and coaches should be aware that subtle compensations due to deficits during these movements could cause injury during the swing by disrupting the body’s natural motion.
The bat acceleration phase occurs from maximal bat loading through striking the ball. In this time, the linear force that has been exerted by the player must be transferred into rotational force through the trunk and upper extremities. When the lead leg contacts the ground, the player has created a closed kinetic chain, where the elastic energy gathered during the loading period is used to produce segmental rotation beginning in the hips and rising through the trunk and out to the arms and hands, finally producing contact with the baseball.16 To produce effective bat velocity, each segment must rotate in a sequential manner. If the upper extremities reach peak velocity before any lower segment, then the player has lost the ability to efficiently transfer kinetic energy up the kinetic chain.
Finally, the follow-through phase occurs after contact with the baseball and ends with complete deceleration, completing the swing. In order to achieve optimal effort, full hip rotation is needed, which is aided by rotation of the trail foot. Both hips and back laces should face the pitcher upon completion of the swing producing maximum power output.15
Return to Hitting: Principles
As with the initiation of the ITP, an interval hitting protocol (IHP) is designed to begin only after the player has been assessed on impairment measures, physical performance measures, and self-assessment.17 The player should have minimum to no pain, have no tenderness to palpation, and show adequate range of motion and strength to meet the demands of performing a full hitting cycle.12 It is recommended that before beginning a return-to-play protocol, the involved extremity should be at least 80% as strong as the uninvolved extremity.18 Physical measures challenging an athlete’s ability to perform tasks specific to hitting a baseball must also be considered through standardized examinations of the involved area.19 Finally, the athlete’s self-perception of functional abilities must be taken into account. This gives a subjective account of what the hitter perceives they are able to perform, providing useful insight into whether they are mentally prepared to participate in the protocol.
Like the ITP, progression through the IHP is also based on the player’s level of pain and soreness rather than following a specific timetable (Table). The program features a 1 day on, 1 day off schedule during which the player completes 1 step per day. The athlete must remain pain-free to progress to the next step and monitor his level of soreness during their workout. If pain or soreness persists, the player should rest for 2 days and be reevaluated upon return.17
The same principles of proper warm-up and mechanics apply in the IHP. An athlete should jog or cycle for a minimum of 10 minutes and perform stretching exercises focused on both upper and lower extremity muscles, as batting involves whole body movement. As the athlete progresses through the IHP, having a hitting coach to analyze, correct and maintain proper swing mechanics is valuable in enhancing performance as well as decreasing risk of re-injury.
The Interval Hitting Program
For a PDF patient handout that summarizes the phases of this program, see Appendix 2.
Phase 1 (Dry Swings). Only the most basic fundamentals are stressed during this phase. The player should focus on properly moving from one phase of the swing to the next, without the goal of hitting the baseball. Trainers should measure critical points in the swing and correct deficits early.
Phase 2 (Batting Off a Tee). In this phase, the player is reintroduced to batting at low intensity with a fixed position target. The initial steps have the batter swing in a position of greatest comfort and natural movement, while the final steps in this phase test the athlete’s range of motion and confidence in the previous, healed injury.
Phase 3 (Soft Toss). As the player progresses to this phase, a baseball with trajectory is used to simulate differences in placement of pitches used during a game. As the hitter is able to pick up differences in target position, his performance and confidence should both increase.20 The coach should sit about 30 feet away, facing the hitter at an angle of 45°, and toss the ball in an underhand motion.
Phase 4 (Simulated Hitting). In this phase, the player and coach should focus on the timing of sequential body movements in order to elicit proper loading and force production. With the randomized pitch delivery and increased velocity, the hitter will practice against pitches similar to those delivered in competition.
Conclusion
Interval throwing and hitting programs are designed to allow the athlete to return to competition through a gradual, stepwise program. This permits the player to prepare his body for the unique stresses associated with throwing and hitting. The medical personnel should familiarize themselves with the philosophy of the interval throwing and hitting programs and individualize them to each athlete. Emphasis on proper warm-up, mechanics, and effort control is paramount in expediting return to play while preventing re-injury.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med Auckl NZ. 1996;21(6):421-437.
4. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
5. Kaczmarek PK, Lubiatowski P, Cisowski P, et al. Shoulder problems in overhead sports. Part I - biomechanics of throwing. Pol Orthop Traumatol. 2014;79:50-58.
6. Toyoshima S, Hoshikawa T, Miyashita M, Oguri T. Contribution of the body parts to throwing performance. Biomech IV. 1974;5:169-174.
7. Weber AE, Kontaxis A, O’Brien SJ, Bedi A. The biomechanics of throwing: simplified and cogent. Sports Med Arthrosc Rev. 2014;22(2):72-79.
8. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
9. Chang ES, Greco NJ, McClincy MP, Bradley JP. Posterior shoulder instability in overhead athletes. Orthop Clin North Am. 2016;47(1):179-187.
10. Digiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
11. Axe M, Hurd W, Snyder-Mackler L. Data-based interval throwing programs for baseball players. Sports Health. 2009;1(2):145-153.
12. Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32(6):293-298.
13. Fleisig GS, Zheng N, Barrentine SW, Escamilla RF, Andrews JR, Lemak LF. Kinematic and kinetic comparison of full and partial effort baseball pitching. Conference proceedings of the 20th Annual Meeting. Atlanta, GA: American Society of Biomechanics; 1996:151-152.
14. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
15. Monti R. Return to hitting: an interval hitting progression and overview of hitting mechanics following injury. Int J Sports Phys Ther. 2015;10(7):1059-1073.
16. Welch CM, Banks SA, Cook FF, Draovitch P. Hitting a baseball: a biomechanical description. J Orthop Sports Phys Ther. 1995;22(5):193-201.
17. Axe MJ, Snyder-Mackler L, Konin JG, Strube MJ. Development of a distance-based interval throwing program for Little League-aged athletes. Am J Sports Med. 1996;24(5):594-602.
18. Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194-203.
19. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642-648.
20. Higuchi T, Nagami T, Morohoshi J, Nakata H, Kanosue K. Disturbance in hitting accuracy by professional and collegiate baseball players due to intentional change of target position. Percept Mot Skills. 2013;116(2):627-639.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med Auckl NZ. 1996;21(6):421-437.
4. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
5. Kaczmarek PK, Lubiatowski P, Cisowski P, et al. Shoulder problems in overhead sports. Part I - biomechanics of throwing. Pol Orthop Traumatol. 2014;79:50-58.
6. Toyoshima S, Hoshikawa T, Miyashita M, Oguri T. Contribution of the body parts to throwing performance. Biomech IV. 1974;5:169-174.
7. Weber AE, Kontaxis A, O’Brien SJ, Bedi A. The biomechanics of throwing: simplified and cogent. Sports Med Arthrosc Rev. 2014;22(2):72-79.
8. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
9. Chang ES, Greco NJ, McClincy MP, Bradley JP. Posterior shoulder instability in overhead athletes. Orthop Clin North Am. 2016;47(1):179-187.
10. Digiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
11. Axe M, Hurd W, Snyder-Mackler L. Data-based interval throwing programs for baseball players. Sports Health. 2009;1(2):145-153.
12. Reinold MM, Wilk KE, Reed J, Crenshaw K, Andrews JR. Interval sport programs: guidelines for baseball, tennis, and golf. J Orthop Sports Phys Ther. 2002;32(6):293-298.
13. Fleisig GS, Zheng N, Barrentine SW, Escamilla RF, Andrews JR, Lemak LF. Kinematic and kinetic comparison of full and partial effort baseball pitching. Conference proceedings of the 20th Annual Meeting. Atlanta, GA: American Society of Biomechanics; 1996:151-152.
14. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
15. Monti R. Return to hitting: an interval hitting progression and overview of hitting mechanics following injury. Int J Sports Phys Ther. 2015;10(7):1059-1073.
16. Welch CM, Banks SA, Cook FF, Draovitch P. Hitting a baseball: a biomechanical description. J Orthop Sports Phys Ther. 1995;22(5):193-201.
17. Axe MJ, Snyder-Mackler L, Konin JG, Strube MJ. Development of a distance-based interval throwing program for Little League-aged athletes. Am J Sports Med. 1996;24(5):594-602.
18. Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194-203.
19. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury, part 1. The tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642-648.
20. Higuchi T, Nagami T, Morohoshi J, Nakata H, Kanosue K. Disturbance in hitting accuracy by professional and collegiate baseball players due to intentional change of target position. Percept Mot Skills. 2013;116(2):627-639.