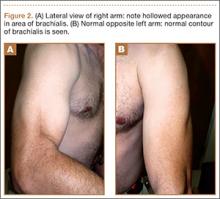
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Analysis of Direct Costs of Outpatient Arthroscopic Rotator Cuff Repair
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
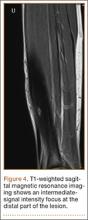
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
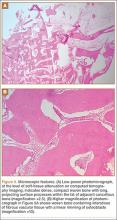
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
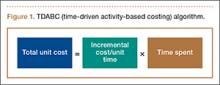
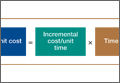
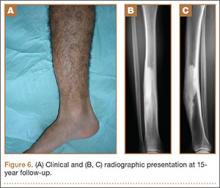
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
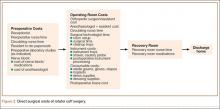
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
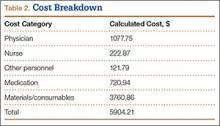
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
A Bariatric Surgery Primer for Orthopedic Surgeons
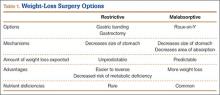
An estimated 220,000 bariatric surgeries are performed annually in the United States and Canada, and 344,221 procedures worldwide.1 Not only are orthopedic surgeons seeing more patients who have had bariatric surgery, they are also referring morbidly obese patients to bariatric surgeons before elective procedures.2 Patients with body mass index (BMI) over 40 kg/m2 are candidates for surgical treatment of obesity. Comorbid conditions directly related to obesity, including diabetes, respiratory insufficiency, and pseudotumor cerebri, decrease the BMI of eligibility to 35 kg/m2. Other considerations are failure of nonsurgical weight-loss methods, such as dietary programs for weight reduction, behavioral modification programs, and pharmacotherapy. Patients’ psychological stability is extremely important given the rigorous dietary changes required after surgery.3 Although weight-loss surgery can eliminate many of the complications of obesity, bariatric patients even with weight loss have increased operative and postoperative risks, likely because of alterations in nutrient absorption. Knowledge of the pathophysiology associated with bariatric surgery can assist orthopedic surgeons in optimizing medical and surgical management of patients’ musculoskeletal issues.
Bariatric Surgery
Surgically induced weight loss works by reducing quantity of food consumed and absorption of calories. Jejunoileal bypass, one of the first procedures used, significantly decreased the absorptive area for nutrients, which led to complications such as diarrhea, cirrhosis, and nephrolithiasis.4 This surgery is no longer performed, and current procedures try to minimize the risks of malabsorption.5
The 2 types of bariatric surgeries now available in the United States are gastroplasty and gastric bypass, both of which are performed laparoscopically.6 Gastroplasties are purely restrictive procedures, which reduce stomach volume. In gastric banding, the most common gastroplasty, a silicone band is placed around the proximal stomach to create a 15-mL pouch in the cardia. Sleeve gastrectomy also reduces stomach volume, to about 25%, by stapling along the greater curvature. In both procedures, consumed calories are restricted, but the gastrointestinal tract is left in continuity, and essential nutrients are properly absorbed.7 However, failure rates are higher, and weight loss more variable, than with gastric bypass procedures.8
Gastric bypass uses both restriction and malabsorption to increase weight loss.7 A gastric pouch (15-30 mL) is created by stapling across the cardia of the stomach. The jejunum is then divided, and the distal portion of the divided jejunum anastomosed to the small proximal stomach pouch. This creates the roux limb where food passes. The duodenum is excluded, and the proximal portion of the jejunum is attached to the roux limb to provide a conduit for biliary and pancreatic digestive secretions. Weight loss is caused by both reduction in stomach size and malabsorption of calories owing to the diversion of digestive enzymes and the decrease in absorptive surface area. Only 28% of ingested fat and 57% of ingested protein are absorbed9 (Table 1).
Metabolic Consequences
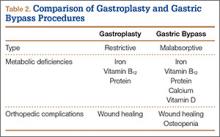
Nutrient deficiencies are seen more often in the malabsorptive procedures; however, patients with restrictive procedures may have poor eating habits and are therefore also at risk.10 In fact, many patients have nutritional deficiencies predating their bariatric surgery, as obesity creates a chronic inflammatory state that leads to anemia of chronic disease. Schweiger and colleagues11 assessed the nutritional status of bariatric surgery candidates and noted a high incidence of iron and folic acid deficiencies with corresponding anemia. They concluded these deficiencies stemmed from calorie-dense diets high in carbohydrates and fats. Although patients may improve their diet after surgery with concomitant nutritional counseling, deficiencies in iron, calcium, vitamin B12, folate, and vitamin D are common12 (Table 2).
Iron deficiency continues after bariatric surgery because dietary iron must be converted to its absorbable form by hydrochloric acid secreted from the stomach. As stomach volume is reduced, there is a corresponding decrease in acid secretion. The result is that iron deficiency occurs in both restrictive and malabsorptive procedures.13 Moreover, with the diversion from the duodenum and the proximal jejunum in bypass surgery, the main areas of absorption are excluded.10 Patients may require intravenous therapy for iron-deficiency anemia—or oral supplementation combined with ascorbic acid to increase stomach acidity.
As calcium is absorbed mainly in the duodenum and the jejunum, patients who undergo malabsorptive procedures can absorb only 20% of the amount ingested.14 Restrictive procedures do not have the same effect on calcium absorption; however, patients may have reduced dietary lactose intake and be at risk for deficiency.
A study by Ducloux and colleagues15 found that 96% of bariatric surgery patients had vitamin D deficiency before the procedure. After malabsorptive procedures, the decrease in bile salts leads to an inability to break down fat-soluble vitamins and to uncoordinated mixing of food and bile secretions.16 Restrictive procedures do not carry this risk, though many patients still require supplementation because of their underlying deficiency.
The decrease in stomach size causes a decrease in intrinsic factor from parietal cells, with subsequent inability to appropriately transport vitamin B12. Exclusion of the duodenum also eliminates the site of absorption; therefore, B12 should be replaced orally.11 Megaloblastic anemia is a rarely reported sequela.17,18 Folate deficiency is less common because it can take place in the entire intestine after surgery, even though absorption occurs primarily in the proximal portion of the small intestine.10
Protein deficiency can result in loss of muscle mass and subsequent muscle weakness, edema, and anomalies of the skin, mucosa, and nails.12 It is seen after both types of procedures because of decreased dietary intake from intolerance. Malabsorptive procedures also decrease pepsinogen secretion and reduce the intestinal absorption surface.
Considerations for Orthopedic Surgeons
Wound Healing
Much of our knowledge of the effects of bariatric surgery on skin and wound healing has been gleaned from samples obtained from patients during abdominoplasty or other body-contouring procedures. These samples have all shown a decrease in hydroxyproline, the major constituent of collagen and the main factor in determining the tensile strength of a wound.19 D’Ettorre and colleagues20 performed biopsies of abdominal skin before and after biliopancreatic diversion and noted that tissue proteins, including hydroxyproline, were significantly reduced. Histologic examination revealed disorganized dermal elastic and collagen fibers. In addition, all patients involved in the study had wound-healing problems, with delayed healing of 25 days, compared with 12 days in nonbariatric patients. Deficiencies in vitamins B12, D, and E, as well as folate and total tissue protein, were implicated as causative factors.
Effects on Bone
Malabsorptive procedures decrease bone mineral density (BMD) through their effects on calcium and vitamin D. BMD is also decreased because these procedures lower levels of plasma leptin and ghrelin, increase adiponectin, and reduce estrogen in women.21 The BMD decline correlates with amount of weight lost.22 This complication is not seen in restrictive procedures, even though patients may have decreased calcium and vitamin D levels.23 The exact effect on BMD and on subsequent risk for osteopenia and osteoporosis is difficult to quantify, as obese patients have higher BMD than age-matched controls do, because of increased mechanical loading. In a prospective study, Vilarrasa and colleagues24 found a 10.9% decrease in femoral neck BMD in women 1 year after Roux-en-Y with 34% weight loss, despite supplementation with 800 IU of vitamin D and 1200 mg of calcium daily.
Fracture Healing
Although BMD is decreased in patients after gastric bypass surgery, there has been only 1 recorded case of fracture nonunion after bariatric surgery—involving a distal radius fracture in a patient who had undergone jejunoileal bypass surgery.25 Hypovitaminosis has a detrimental effect on bone repair and BMD, increasing the risk for fracture from minor trauma; however, delayed union and nonunion have not been reported as consequences.26
Pharmacology
Both restrictive and malabsorptive procedures decrease drug bioavailabilty from tablet preparations by shortening the surface area available for absorption and diminishing stomach acidity.27 These consequences pose a problem particularly for extended-release formulations, as these formulations are not given enough time to dissolve and reach therapeutic concentrations.28 Also affected is warfarin, which requires a larger dose to maintain therapeutic international normalized ratio. Antibiotics may have reduced bioavailability because of decreased transit time. Therefore, liquid preparations are preferred, as they need not be dissolved.
As there is no reported change in intravenous bioavailability with preoperative and postoperative antimicrobial prophylaxis, this is the preferred administration method.29 However, obese patients in general may have altered pharmacokinetics, including increased glomerular filtration rate, and in most cases they should be treated with higher levels of antibiotics.30
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in all patients. The acidic composition of NSAIDs causes direct injury to the gastric pouch. NSAIDs also injure the gastrointestinal lining by inhibiting prostaglandin synthesis, which thins the mucosa. In turn, erosions and ulcers may form in the epithelial layer.31 Acetaminophen or a centrally acting agent (eg, tramadol) is recommended instead. Aspirin has a chemical structure similar to that of NSAIDs and should not be used either. Alendronate causes esophageal ulceration; however, no such complication has been reported with teriparatide32 (Table 3).
Preoperative Evaluation
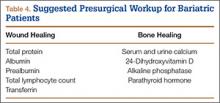
As already discussed, patients who undergo weight-loss surgery are at higher risk for wound-healing complications because of nutritional deficiencies. Total protein, albumin, and prealbumin levels and total lymphocyte count should be used to identify protein deficiency, which can decrease the likelihood of organized collagen formation. Huang and colleagues33 noted a statistically significant increase in complications after total knee arthroplasty (TKA) in patients with a prealbumin level under 3.5 mg/dL or a transferrin level under 200 mg/dL. Rates of prosthetic joint infection and development of hematoma or seroma requiring operative management were much higher, as were rates of postoperative neurovascular, renal, and cardiovascular complications.
Serum levels of vitamin A, folate, vitamin B12, and vitamin C should also be ordered, as many patients are deficient. Transferrin levels should be checked before surgery, as iron-deficiency anemia is common. Naghshineh and colleagues34 noted an anecdotal decrease in wound-healing complications in body-contouring surgery after correction of subclinical and clinical deficiencies in protein, arginine, glutamine, vitamin A, vitamin B12, vitamin C, folate, thiamine, iron, zinc, and selenium. Zinc deficiency was similarly implicated in wound-healing complications by Zorrilla and colleagues,35,36 who found a statistically significant delay in wound healing in patients with serum zinc levels under 95 mg/dL after total hip arthroplasty (THA)35 and hip hemiarthroplasty.36 To facilitate bone healing, physicians should give patients a thorough workup of levels of serum and urine calcium, 24-hydroxyvitamin D, and alkaline phosphatase. Osteomalacia typically presents with high alkaline phosphatase levels37 and secondary hyperparathyroidism. Therefore, physicians should monitor for these conditions. Although nonunion and aseptic loosening have not been reported as consequences of bariatric surgery, bone health should nevertheless be optimized when possible (Table 4).
Elective Orthopedic Surgery
Little is known about the true effect of weight-loss surgery on subsequent orthopedic procedures. Few investigators have explored the effect of surgery on arthrodesis, and the only recommendation for orthopedic surgeons is to be prepared for poor bone healing and the possibility of nonunion.38 Hidalgo and colleagues39 studied laparoscopic sleeve gastrectomy performed a minimum of 6 months before another elective surgery. Two patients had lumbar laminectomies, 2 had lumbar discectomies, 1 had a cervical discectomy, and 1 had a rotator cuff repair. By most recent follow-up, there were no complications of any of the orthopedic procedures, and all patients had healed.
There is no recommended amount of time between bariatric surgery and elective orthopedic surgery. Maximum weight loss and stabilization are typically achieved 2 years after surgery.40 However, elective orthopedic surgery has been performed as early as 6 months after bariatric surgery. Inacio and colleagues41 studied 3 groups of patients who underwent total joint arthroplasty (TJA): those who had it less than 2 years after bariatric surgery, those who had it more than 2 years after bariatric surgery, and those who were obese but did not have bariatric surgery. Complications of TJA occurred within the first year in 2.9% of the patients who had it more than 2 years after bariatric surgery, in 5.9% of the patients who had it less than 2 years after bariatric surgery, and in 4.1% of the patients who did not undergo bariatric surgery. Similarly, Severson and colleagues2 evaluated intraoperative and postoperative complications of TKA in 3 groups of obese patients: those who had TKA before bariatric surgery, those who had TKA less than 2 years after bariatric surgery, and those who had TKA more than 2 years after bariatric surgery. Gastroplasty and bypass patients were included. BMI was statistically significantly higher in the preoperative group than in the other 2 groups, though mean BMI for all groups was above 35 kg/m2. Operative time and tourniquet time were reduced in patients who had TKA more than 2 years after bariatric surgery, but there was no significant difference in anesthesia time. There was also no difference in 90-day complication rates between patients who had TKA before bariatric surgery and those who had it afterward. Severson and colleagues2 recommended communicating the risks to all obese patients, whether they undergo weight-loss surgery or not.
Arthroplasty
Obese patients have a higher rate of complications after arthroplasty—hence the referrals to bariatric surgeons. Bariatric surgery and its associated weight loss might improve joint pain and delay the need for arthroplasty in some cases.42 Obese people are prone to joint degeneration from the excess weight, and from altered gait patterns (eg, exaggerated step width, slower walking speed, increased time in double-limb stance).43 Gait changes are reversible after weight loss.44 Hooper and colleagues45 found a 37% decrease in lower extremity complaints after surgical weight loss, even though mean BMI at final follow-up was still in the obese range.
Obesity itself is a risk factor for ligamentous instability, but it is unclear whether the risk is mitigated by bariatric surgery. Disruption of the anterior fibers of the medial collateral ligament is more common in obese patients, as are complications involving the extensor mechanism (eg, patellofemoral dislocation). As a result, slower postoperative rehabilitation is recommended.46 Although there is no recorded link between bariatric surgery and the development of ligamentous laxity, surgeons should be aware of the potential for medial collateral ligament avulsion in obese and formerly obese patients and have appropriate implants available.
Kulkarni and colleagues47 compared the rates of hip and knee arthroplasty complications in patients who were obese before bariatric surgery and patients who were still obese after bariatric surgery. Gastroplasty and bypass patients were included. Data on superficial wound infections were excluded; however, the bariatric surgery group’s deep wound infection rate was 3.5 times lower, and its 30-day readmission rate was 7 times lower. There was no difference in dislocation and hip revision rates at 1 year. Although 1 patient in the bariatric surgery group died of an unknown cause 9 days after surgery, Kulkarni and colleagues47 concluded it is safer to operate on obese patients after versus before bariatric surgery. However, their study did not include mean BMI, so no conclusion can be drawn about the risk of operating on patients who were still obese after bariatric surgery.
Studies of weight loss in primary TJA patients have had conflicting findings.48 Trofa and colleagues49 reported that 15 patients who underwent arthroplasty a mean of 42.4 months after bariatric surgery lost 27.9% more of their original BMI compared with patients who underwent bariatric surgery but not arthroplasty. This relationship between arthroplasty and weight loss was strongest in patients who underwent knee arthroplasty, with an average of 43.9% more BMI lost compared to patients who did not undergo TKA. There was no significant change in BMI in patients who underwent THA and bariatric surgery compared with patients who underwent bariatric surgery but not THA.
Parvizi and colleagues50 assessed the results of 20 arthroplasties (8 THAs, 12 TKAs) performed in 14 patients a mean of 23 months after bariatric surgery (2 gastroplasties, 12 bypass surgeries). Mean BMI was 29 kg/m2. At final follow-up, 1 patient required revision THA for aseptic loosening, but all the others showed no evidence of radiographic loosening or wear. One patient had a superficial wound infection, and 1 had a deep wound infection. Parvizi and colleagues50 reported that arthroplasty after bariatric surgery is a viable option and is preferable to operating on morbidly obese patients.
Summary
Orthopedic surgeons are increasingly performing elective hip and knee arthroplasties on patients who have undergone bariatric surgery. Although bariatric surgery may alleviate some of the complications associated with surgery on morbidly obese patients, it should be approached with caution. Studies have shown that bariatric surgery patients are at increased risk for wound-healing and other complications, often caused by unrecognized preoperative nutrient deficiencies. In addition, patients are often unable to tolerate commonly used medications. The exact timing of bariatric surgery relative to elective orthopedic procedures is unclear. Surgeons should perform a preoperative evaluation based on type of bariatric surgery in order to reduce the likelihood of adverse events. Such preemptive therapy may improve the short- and long-term results of major reconstructive surgery. Further research is needed to determine the true effect of bariatric surgery on orthopedic procedures.
1. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611.
2. Severson EP, Singh JA, Browne JA, Trousdale RT, Sarr MG, Lewallen DG. Total knee arthroplasty in morbidly obese patients treated with bariatric surgery. J Arthroplasty. 2012;27(9):1696-1700.
3. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient [published correction appears in Endocr Pract. 2009;15(7):768]. Endocr Pract. 2008;14(suppl 1):1-83.
4. Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoilial bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308(17):995-999.
5. DeMaria EJ. Morbid obesity. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:736-743.
6. O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358-1365.
7. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
8. DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809-818.
9. Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48-55.
10. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569-575.
11. Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193-197.
12. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13-24.
13. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447-453.
14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40-47.
15. Ducloux R, Nobécourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21(5):556-560.
16. Wang A, Powell A. The effects of obesity surgery on bone metabolism: what orthopedic surgeons need to know. Am J Orthop. 2009;38(2):77-79.
17. Baghdasarian KL. Gastric bypass and megaloblastic anemia. J Am Diet Assoc. 1982;80(4):368-371.
18. Crowley LV, Olson RW. Megaloblastic anemia after gastric bypass for obesity. Am J Gastroenterol. 1983;78(7):406-410.
19. Sorg H, Schulz T, Krueger C, Vollmar B. Consequences of surgical stress on the kinetics of skin wound healing: partial hepatectomy delays and functionally alters dermal repair. Wound Repair Regen. 2009;17(3):367-377.
20. D’Ettorre M, Gniuli D, Iaconelli A, Massi G, Mingrone G, Bracaglia R. Wound healing process in post-bariatric patients: an experimental evaluation. Obes Surg. 2010;20(11):1552-1558.
21. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1);41-46.
22. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
23. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918-921.
24. Vilarrasa N, Gómez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860-866.
25. Hey H, Lund B, Sørensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34(1):13-15.
26. Borrelli J Jr, Pape C, Hak D, et al. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708-711.
27. Sardo P, Walker JH. Bariatric surgery: impact on medication management. Hosp Pharm. 2008;43(2):113-120.
28. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
29. Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):317-328.
30. Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829-854.
31. Sasse KC, Ganser J, Kozar M, et al. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18(5):530-534.
32. Miller AD, Smith KM. Medication use in bariatric surgery patients: what orthopedists need to know. Orthopedics. 2006;29(2):121-123.
33. Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28(8 suppl):21-24.
34. Naghshineh N, O’Brien Coon D, McTigue K, Courcoulas AP, Fernstrom M, Rubin JP. Nutritional assessment of bariatric surgery patients presenting for plastic surgery: a prospective analysis. Plast Reconstr Surg. 2010;126(2):602-610.
35. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14(2):119-122.
36. Zorrilla P, Salido JA, López-Alonso A, Silva A. Serum zinc as a prognostic tool for wound healing in hip hemiarthroplasty. Clin Orthop Relat Res. 2004;(420):304-308.
37. Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med. 2008;75(5):333-349.
38. Kini S, Kannan U. Effect of bariatric surgery on future general surgical procedures. J Minim Access Surg. 2011;7(2):126-131.
39. Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obes Surg. 2012;22(4):555-559.
40. O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032-1040.
41. Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364.
42. Gill RS, Al‐Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083-1089.
43. Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery–induced weight loss. J Biomech. 2012;45(10):1769-1774.
44. Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346-354.
45. Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114-120.
46. Booth RE Jr. Total knee arthroplasty in the obese patient: tips and quips. J Arthroplasty. 2002;17(4 suppl 1):69-70.
47. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
48. Inacio MC, Silverstein DK, Raman R, et al. Weight patterns before and after total joint arthroplasty and characteristics associated with weight change. Perm J. 2014;18(1):25-31.
49. Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014;10(2):335-339.
50. Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15(8):1003-1008.
An estimated 220,000 bariatric surgeries are performed annually in the United States and Canada, and 344,221 procedures worldwide.1 Not only are orthopedic surgeons seeing more patients who have had bariatric surgery, they are also referring morbidly obese patients to bariatric surgeons before elective procedures.2 Patients with body mass index (BMI) over 40 kg/m2 are candidates for surgical treatment of obesity. Comorbid conditions directly related to obesity, including diabetes, respiratory insufficiency, and pseudotumor cerebri, decrease the BMI of eligibility to 35 kg/m2. Other considerations are failure of nonsurgical weight-loss methods, such as dietary programs for weight reduction, behavioral modification programs, and pharmacotherapy. Patients’ psychological stability is extremely important given the rigorous dietary changes required after surgery.3 Although weight-loss surgery can eliminate many of the complications of obesity, bariatric patients even with weight loss have increased operative and postoperative risks, likely because of alterations in nutrient absorption. Knowledge of the pathophysiology associated with bariatric surgery can assist orthopedic surgeons in optimizing medical and surgical management of patients’ musculoskeletal issues.
Bariatric Surgery
Surgically induced weight loss works by reducing quantity of food consumed and absorption of calories. Jejunoileal bypass, one of the first procedures used, significantly decreased the absorptive area for nutrients, which led to complications such as diarrhea, cirrhosis, and nephrolithiasis.4 This surgery is no longer performed, and current procedures try to minimize the risks of malabsorption.5
The 2 types of bariatric surgeries now available in the United States are gastroplasty and gastric bypass, both of which are performed laparoscopically.6 Gastroplasties are purely restrictive procedures, which reduce stomach volume. In gastric banding, the most common gastroplasty, a silicone band is placed around the proximal stomach to create a 15-mL pouch in the cardia. Sleeve gastrectomy also reduces stomach volume, to about 25%, by stapling along the greater curvature. In both procedures, consumed calories are restricted, but the gastrointestinal tract is left in continuity, and essential nutrients are properly absorbed.7 However, failure rates are higher, and weight loss more variable, than with gastric bypass procedures.8
Gastric bypass uses both restriction and malabsorption to increase weight loss.7 A gastric pouch (15-30 mL) is created by stapling across the cardia of the stomach. The jejunum is then divided, and the distal portion of the divided jejunum anastomosed to the small proximal stomach pouch. This creates the roux limb where food passes. The duodenum is excluded, and the proximal portion of the jejunum is attached to the roux limb to provide a conduit for biliary and pancreatic digestive secretions. Weight loss is caused by both reduction in stomach size and malabsorption of calories owing to the diversion of digestive enzymes and the decrease in absorptive surface area. Only 28% of ingested fat and 57% of ingested protein are absorbed9 (Table 1).
Metabolic Consequences
Nutrient deficiencies are seen more often in the malabsorptive procedures; however, patients with restrictive procedures may have poor eating habits and are therefore also at risk.10 In fact, many patients have nutritional deficiencies predating their bariatric surgery, as obesity creates a chronic inflammatory state that leads to anemia of chronic disease. Schweiger and colleagues11 assessed the nutritional status of bariatric surgery candidates and noted a high incidence of iron and folic acid deficiencies with corresponding anemia. They concluded these deficiencies stemmed from calorie-dense diets high in carbohydrates and fats. Although patients may improve their diet after surgery with concomitant nutritional counseling, deficiencies in iron, calcium, vitamin B12, folate, and vitamin D are common12 (Table 2).
Iron deficiency continues after bariatric surgery because dietary iron must be converted to its absorbable form by hydrochloric acid secreted from the stomach. As stomach volume is reduced, there is a corresponding decrease in acid secretion. The result is that iron deficiency occurs in both restrictive and malabsorptive procedures.13 Moreover, with the diversion from the duodenum and the proximal jejunum in bypass surgery, the main areas of absorption are excluded.10 Patients may require intravenous therapy for iron-deficiency anemia—or oral supplementation combined with ascorbic acid to increase stomach acidity.
As calcium is absorbed mainly in the duodenum and the jejunum, patients who undergo malabsorptive procedures can absorb only 20% of the amount ingested.14 Restrictive procedures do not have the same effect on calcium absorption; however, patients may have reduced dietary lactose intake and be at risk for deficiency.
A study by Ducloux and colleagues15 found that 96% of bariatric surgery patients had vitamin D deficiency before the procedure. After malabsorptive procedures, the decrease in bile salts leads to an inability to break down fat-soluble vitamins and to uncoordinated mixing of food and bile secretions.16 Restrictive procedures do not carry this risk, though many patients still require supplementation because of their underlying deficiency.
The decrease in stomach size causes a decrease in intrinsic factor from parietal cells, with subsequent inability to appropriately transport vitamin B12. Exclusion of the duodenum also eliminates the site of absorption; therefore, B12 should be replaced orally.11 Megaloblastic anemia is a rarely reported sequela.17,18 Folate deficiency is less common because it can take place in the entire intestine after surgery, even though absorption occurs primarily in the proximal portion of the small intestine.10
Protein deficiency can result in loss of muscle mass and subsequent muscle weakness, edema, and anomalies of the skin, mucosa, and nails.12 It is seen after both types of procedures because of decreased dietary intake from intolerance. Malabsorptive procedures also decrease pepsinogen secretion and reduce the intestinal absorption surface.
Considerations for Orthopedic Surgeons
Wound Healing
Much of our knowledge of the effects of bariatric surgery on skin and wound healing has been gleaned from samples obtained from patients during abdominoplasty or other body-contouring procedures. These samples have all shown a decrease in hydroxyproline, the major constituent of collagen and the main factor in determining the tensile strength of a wound.19 D’Ettorre and colleagues20 performed biopsies of abdominal skin before and after biliopancreatic diversion and noted that tissue proteins, including hydroxyproline, were significantly reduced. Histologic examination revealed disorganized dermal elastic and collagen fibers. In addition, all patients involved in the study had wound-healing problems, with delayed healing of 25 days, compared with 12 days in nonbariatric patients. Deficiencies in vitamins B12, D, and E, as well as folate and total tissue protein, were implicated as causative factors.
Effects on Bone
Malabsorptive procedures decrease bone mineral density (BMD) through their effects on calcium and vitamin D. BMD is also decreased because these procedures lower levels of plasma leptin and ghrelin, increase adiponectin, and reduce estrogen in women.21 The BMD decline correlates with amount of weight lost.22 This complication is not seen in restrictive procedures, even though patients may have decreased calcium and vitamin D levels.23 The exact effect on BMD and on subsequent risk for osteopenia and osteoporosis is difficult to quantify, as obese patients have higher BMD than age-matched controls do, because of increased mechanical loading. In a prospective study, Vilarrasa and colleagues24 found a 10.9% decrease in femoral neck BMD in women 1 year after Roux-en-Y with 34% weight loss, despite supplementation with 800 IU of vitamin D and 1200 mg of calcium daily.
Fracture Healing
Although BMD is decreased in patients after gastric bypass surgery, there has been only 1 recorded case of fracture nonunion after bariatric surgery—involving a distal radius fracture in a patient who had undergone jejunoileal bypass surgery.25 Hypovitaminosis has a detrimental effect on bone repair and BMD, increasing the risk for fracture from minor trauma; however, delayed union and nonunion have not been reported as consequences.26
Pharmacology
Both restrictive and malabsorptive procedures decrease drug bioavailabilty from tablet preparations by shortening the surface area available for absorption and diminishing stomach acidity.27 These consequences pose a problem particularly for extended-release formulations, as these formulations are not given enough time to dissolve and reach therapeutic concentrations.28 Also affected is warfarin, which requires a larger dose to maintain therapeutic international normalized ratio. Antibiotics may have reduced bioavailability because of decreased transit time. Therefore, liquid preparations are preferred, as they need not be dissolved.
As there is no reported change in intravenous bioavailability with preoperative and postoperative antimicrobial prophylaxis, this is the preferred administration method.29 However, obese patients in general may have altered pharmacokinetics, including increased glomerular filtration rate, and in most cases they should be treated with higher levels of antibiotics.30
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in all patients. The acidic composition of NSAIDs causes direct injury to the gastric pouch. NSAIDs also injure the gastrointestinal lining by inhibiting prostaglandin synthesis, which thins the mucosa. In turn, erosions and ulcers may form in the epithelial layer.31 Acetaminophen or a centrally acting agent (eg, tramadol) is recommended instead. Aspirin has a chemical structure similar to that of NSAIDs and should not be used either. Alendronate causes esophageal ulceration; however, no such complication has been reported with teriparatide32 (Table 3).
Preoperative Evaluation
As already discussed, patients who undergo weight-loss surgery are at higher risk for wound-healing complications because of nutritional deficiencies. Total protein, albumin, and prealbumin levels and total lymphocyte count should be used to identify protein deficiency, which can decrease the likelihood of organized collagen formation. Huang and colleagues33 noted a statistically significant increase in complications after total knee arthroplasty (TKA) in patients with a prealbumin level under 3.5 mg/dL or a transferrin level under 200 mg/dL. Rates of prosthetic joint infection and development of hematoma or seroma requiring operative management were much higher, as were rates of postoperative neurovascular, renal, and cardiovascular complications.
Serum levels of vitamin A, folate, vitamin B12, and vitamin C should also be ordered, as many patients are deficient. Transferrin levels should be checked before surgery, as iron-deficiency anemia is common. Naghshineh and colleagues34 noted an anecdotal decrease in wound-healing complications in body-contouring surgery after correction of subclinical and clinical deficiencies in protein, arginine, glutamine, vitamin A, vitamin B12, vitamin C, folate, thiamine, iron, zinc, and selenium. Zinc deficiency was similarly implicated in wound-healing complications by Zorrilla and colleagues,35,36 who found a statistically significant delay in wound healing in patients with serum zinc levels under 95 mg/dL after total hip arthroplasty (THA)35 and hip hemiarthroplasty.36 To facilitate bone healing, physicians should give patients a thorough workup of levels of serum and urine calcium, 24-hydroxyvitamin D, and alkaline phosphatase. Osteomalacia typically presents with high alkaline phosphatase levels37 and secondary hyperparathyroidism. Therefore, physicians should monitor for these conditions. Although nonunion and aseptic loosening have not been reported as consequences of bariatric surgery, bone health should nevertheless be optimized when possible (Table 4).
Elective Orthopedic Surgery
Little is known about the true effect of weight-loss surgery on subsequent orthopedic procedures. Few investigators have explored the effect of surgery on arthrodesis, and the only recommendation for orthopedic surgeons is to be prepared for poor bone healing and the possibility of nonunion.38 Hidalgo and colleagues39 studied laparoscopic sleeve gastrectomy performed a minimum of 6 months before another elective surgery. Two patients had lumbar laminectomies, 2 had lumbar discectomies, 1 had a cervical discectomy, and 1 had a rotator cuff repair. By most recent follow-up, there were no complications of any of the orthopedic procedures, and all patients had healed.
There is no recommended amount of time between bariatric surgery and elective orthopedic surgery. Maximum weight loss and stabilization are typically achieved 2 years after surgery.40 However, elective orthopedic surgery has been performed as early as 6 months after bariatric surgery. Inacio and colleagues41 studied 3 groups of patients who underwent total joint arthroplasty (TJA): those who had it less than 2 years after bariatric surgery, those who had it more than 2 years after bariatric surgery, and those who were obese but did not have bariatric surgery. Complications of TJA occurred within the first year in 2.9% of the patients who had it more than 2 years after bariatric surgery, in 5.9% of the patients who had it less than 2 years after bariatric surgery, and in 4.1% of the patients who did not undergo bariatric surgery. Similarly, Severson and colleagues2 evaluated intraoperative and postoperative complications of TKA in 3 groups of obese patients: those who had TKA before bariatric surgery, those who had TKA less than 2 years after bariatric surgery, and those who had TKA more than 2 years after bariatric surgery. Gastroplasty and bypass patients were included. BMI was statistically significantly higher in the preoperative group than in the other 2 groups, though mean BMI for all groups was above 35 kg/m2. Operative time and tourniquet time were reduced in patients who had TKA more than 2 years after bariatric surgery, but there was no significant difference in anesthesia time. There was also no difference in 90-day complication rates between patients who had TKA before bariatric surgery and those who had it afterward. Severson and colleagues2 recommended communicating the risks to all obese patients, whether they undergo weight-loss surgery or not.
Arthroplasty
Obese patients have a higher rate of complications after arthroplasty—hence the referrals to bariatric surgeons. Bariatric surgery and its associated weight loss might improve joint pain and delay the need for arthroplasty in some cases.42 Obese people are prone to joint degeneration from the excess weight, and from altered gait patterns (eg, exaggerated step width, slower walking speed, increased time in double-limb stance).43 Gait changes are reversible after weight loss.44 Hooper and colleagues45 found a 37% decrease in lower extremity complaints after surgical weight loss, even though mean BMI at final follow-up was still in the obese range.
Obesity itself is a risk factor for ligamentous instability, but it is unclear whether the risk is mitigated by bariatric surgery. Disruption of the anterior fibers of the medial collateral ligament is more common in obese patients, as are complications involving the extensor mechanism (eg, patellofemoral dislocation). As a result, slower postoperative rehabilitation is recommended.46 Although there is no recorded link between bariatric surgery and the development of ligamentous laxity, surgeons should be aware of the potential for medial collateral ligament avulsion in obese and formerly obese patients and have appropriate implants available.
Kulkarni and colleagues47 compared the rates of hip and knee arthroplasty complications in patients who were obese before bariatric surgery and patients who were still obese after bariatric surgery. Gastroplasty and bypass patients were included. Data on superficial wound infections were excluded; however, the bariatric surgery group’s deep wound infection rate was 3.5 times lower, and its 30-day readmission rate was 7 times lower. There was no difference in dislocation and hip revision rates at 1 year. Although 1 patient in the bariatric surgery group died of an unknown cause 9 days after surgery, Kulkarni and colleagues47 concluded it is safer to operate on obese patients after versus before bariatric surgery. However, their study did not include mean BMI, so no conclusion can be drawn about the risk of operating on patients who were still obese after bariatric surgery.
Studies of weight loss in primary TJA patients have had conflicting findings.48 Trofa and colleagues49 reported that 15 patients who underwent arthroplasty a mean of 42.4 months after bariatric surgery lost 27.9% more of their original BMI compared with patients who underwent bariatric surgery but not arthroplasty. This relationship between arthroplasty and weight loss was strongest in patients who underwent knee arthroplasty, with an average of 43.9% more BMI lost compared to patients who did not undergo TKA. There was no significant change in BMI in patients who underwent THA and bariatric surgery compared with patients who underwent bariatric surgery but not THA.
Parvizi and colleagues50 assessed the results of 20 arthroplasties (8 THAs, 12 TKAs) performed in 14 patients a mean of 23 months after bariatric surgery (2 gastroplasties, 12 bypass surgeries). Mean BMI was 29 kg/m2. At final follow-up, 1 patient required revision THA for aseptic loosening, but all the others showed no evidence of radiographic loosening or wear. One patient had a superficial wound infection, and 1 had a deep wound infection. Parvizi and colleagues50 reported that arthroplasty after bariatric surgery is a viable option and is preferable to operating on morbidly obese patients.
Summary
Orthopedic surgeons are increasingly performing elective hip and knee arthroplasties on patients who have undergone bariatric surgery. Although bariatric surgery may alleviate some of the complications associated with surgery on morbidly obese patients, it should be approached with caution. Studies have shown that bariatric surgery patients are at increased risk for wound-healing and other complications, often caused by unrecognized preoperative nutrient deficiencies. In addition, patients are often unable to tolerate commonly used medications. The exact timing of bariatric surgery relative to elective orthopedic procedures is unclear. Surgeons should perform a preoperative evaluation based on type of bariatric surgery in order to reduce the likelihood of adverse events. Such preemptive therapy may improve the short- and long-term results of major reconstructive surgery. Further research is needed to determine the true effect of bariatric surgery on orthopedic procedures.
An estimated 220,000 bariatric surgeries are performed annually in the United States and Canada, and 344,221 procedures worldwide.1 Not only are orthopedic surgeons seeing more patients who have had bariatric surgery, they are also referring morbidly obese patients to bariatric surgeons before elective procedures.2 Patients with body mass index (BMI) over 40 kg/m2 are candidates for surgical treatment of obesity. Comorbid conditions directly related to obesity, including diabetes, respiratory insufficiency, and pseudotumor cerebri, decrease the BMI of eligibility to 35 kg/m2. Other considerations are failure of nonsurgical weight-loss methods, such as dietary programs for weight reduction, behavioral modification programs, and pharmacotherapy. Patients’ psychological stability is extremely important given the rigorous dietary changes required after surgery.3 Although weight-loss surgery can eliminate many of the complications of obesity, bariatric patients even with weight loss have increased operative and postoperative risks, likely because of alterations in nutrient absorption. Knowledge of the pathophysiology associated with bariatric surgery can assist orthopedic surgeons in optimizing medical and surgical management of patients’ musculoskeletal issues.
Bariatric Surgery
Surgically induced weight loss works by reducing quantity of food consumed and absorption of calories. Jejunoileal bypass, one of the first procedures used, significantly decreased the absorptive area for nutrients, which led to complications such as diarrhea, cirrhosis, and nephrolithiasis.4 This surgery is no longer performed, and current procedures try to minimize the risks of malabsorption.5
The 2 types of bariatric surgeries now available in the United States are gastroplasty and gastric bypass, both of which are performed laparoscopically.6 Gastroplasties are purely restrictive procedures, which reduce stomach volume. In gastric banding, the most common gastroplasty, a silicone band is placed around the proximal stomach to create a 15-mL pouch in the cardia. Sleeve gastrectomy also reduces stomach volume, to about 25%, by stapling along the greater curvature. In both procedures, consumed calories are restricted, but the gastrointestinal tract is left in continuity, and essential nutrients are properly absorbed.7 However, failure rates are higher, and weight loss more variable, than with gastric bypass procedures.8
Gastric bypass uses both restriction and malabsorption to increase weight loss.7 A gastric pouch (15-30 mL) is created by stapling across the cardia of the stomach. The jejunum is then divided, and the distal portion of the divided jejunum anastomosed to the small proximal stomach pouch. This creates the roux limb where food passes. The duodenum is excluded, and the proximal portion of the jejunum is attached to the roux limb to provide a conduit for biliary and pancreatic digestive secretions. Weight loss is caused by both reduction in stomach size and malabsorption of calories owing to the diversion of digestive enzymes and the decrease in absorptive surface area. Only 28% of ingested fat and 57% of ingested protein are absorbed9 (Table 1).
Metabolic Consequences
Nutrient deficiencies are seen more often in the malabsorptive procedures; however, patients with restrictive procedures may have poor eating habits and are therefore also at risk.10 In fact, many patients have nutritional deficiencies predating their bariatric surgery, as obesity creates a chronic inflammatory state that leads to anemia of chronic disease. Schweiger and colleagues11 assessed the nutritional status of bariatric surgery candidates and noted a high incidence of iron and folic acid deficiencies with corresponding anemia. They concluded these deficiencies stemmed from calorie-dense diets high in carbohydrates and fats. Although patients may improve their diet after surgery with concomitant nutritional counseling, deficiencies in iron, calcium, vitamin B12, folate, and vitamin D are common12 (Table 2).
Iron deficiency continues after bariatric surgery because dietary iron must be converted to its absorbable form by hydrochloric acid secreted from the stomach. As stomach volume is reduced, there is a corresponding decrease in acid secretion. The result is that iron deficiency occurs in both restrictive and malabsorptive procedures.13 Moreover, with the diversion from the duodenum and the proximal jejunum in bypass surgery, the main areas of absorption are excluded.10 Patients may require intravenous therapy for iron-deficiency anemia—or oral supplementation combined with ascorbic acid to increase stomach acidity.
As calcium is absorbed mainly in the duodenum and the jejunum, patients who undergo malabsorptive procedures can absorb only 20% of the amount ingested.14 Restrictive procedures do not have the same effect on calcium absorption; however, patients may have reduced dietary lactose intake and be at risk for deficiency.
A study by Ducloux and colleagues15 found that 96% of bariatric surgery patients had vitamin D deficiency before the procedure. After malabsorptive procedures, the decrease in bile salts leads to an inability to break down fat-soluble vitamins and to uncoordinated mixing of food and bile secretions.16 Restrictive procedures do not carry this risk, though many patients still require supplementation because of their underlying deficiency.
The decrease in stomach size causes a decrease in intrinsic factor from parietal cells, with subsequent inability to appropriately transport vitamin B12. Exclusion of the duodenum also eliminates the site of absorption; therefore, B12 should be replaced orally.11 Megaloblastic anemia is a rarely reported sequela.17,18 Folate deficiency is less common because it can take place in the entire intestine after surgery, even though absorption occurs primarily in the proximal portion of the small intestine.10
Protein deficiency can result in loss of muscle mass and subsequent muscle weakness, edema, and anomalies of the skin, mucosa, and nails.12 It is seen after both types of procedures because of decreased dietary intake from intolerance. Malabsorptive procedures also decrease pepsinogen secretion and reduce the intestinal absorption surface.
Considerations for Orthopedic Surgeons
Wound Healing
Much of our knowledge of the effects of bariatric surgery on skin and wound healing has been gleaned from samples obtained from patients during abdominoplasty or other body-contouring procedures. These samples have all shown a decrease in hydroxyproline, the major constituent of collagen and the main factor in determining the tensile strength of a wound.19 D’Ettorre and colleagues20 performed biopsies of abdominal skin before and after biliopancreatic diversion and noted that tissue proteins, including hydroxyproline, were significantly reduced. Histologic examination revealed disorganized dermal elastic and collagen fibers. In addition, all patients involved in the study had wound-healing problems, with delayed healing of 25 days, compared with 12 days in nonbariatric patients. Deficiencies in vitamins B12, D, and E, as well as folate and total tissue protein, were implicated as causative factors.
Effects on Bone
Malabsorptive procedures decrease bone mineral density (BMD) through their effects on calcium and vitamin D. BMD is also decreased because these procedures lower levels of plasma leptin and ghrelin, increase adiponectin, and reduce estrogen in women.21 The BMD decline correlates with amount of weight lost.22 This complication is not seen in restrictive procedures, even though patients may have decreased calcium and vitamin D levels.23 The exact effect on BMD and on subsequent risk for osteopenia and osteoporosis is difficult to quantify, as obese patients have higher BMD than age-matched controls do, because of increased mechanical loading. In a prospective study, Vilarrasa and colleagues24 found a 10.9% decrease in femoral neck BMD in women 1 year after Roux-en-Y with 34% weight loss, despite supplementation with 800 IU of vitamin D and 1200 mg of calcium daily.
Fracture Healing
Although BMD is decreased in patients after gastric bypass surgery, there has been only 1 recorded case of fracture nonunion after bariatric surgery—involving a distal radius fracture in a patient who had undergone jejunoileal bypass surgery.25 Hypovitaminosis has a detrimental effect on bone repair and BMD, increasing the risk for fracture from minor trauma; however, delayed union and nonunion have not been reported as consequences.26
Pharmacology
Both restrictive and malabsorptive procedures decrease drug bioavailabilty from tablet preparations by shortening the surface area available for absorption and diminishing stomach acidity.27 These consequences pose a problem particularly for extended-release formulations, as these formulations are not given enough time to dissolve and reach therapeutic concentrations.28 Also affected is warfarin, which requires a larger dose to maintain therapeutic international normalized ratio. Antibiotics may have reduced bioavailability because of decreased transit time. Therefore, liquid preparations are preferred, as they need not be dissolved.
As there is no reported change in intravenous bioavailability with preoperative and postoperative antimicrobial prophylaxis, this is the preferred administration method.29 However, obese patients in general may have altered pharmacokinetics, including increased glomerular filtration rate, and in most cases they should be treated with higher levels of antibiotics.30
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in all patients. The acidic composition of NSAIDs causes direct injury to the gastric pouch. NSAIDs also injure the gastrointestinal lining by inhibiting prostaglandin synthesis, which thins the mucosa. In turn, erosions and ulcers may form in the epithelial layer.31 Acetaminophen or a centrally acting agent (eg, tramadol) is recommended instead. Aspirin has a chemical structure similar to that of NSAIDs and should not be used either. Alendronate causes esophageal ulceration; however, no such complication has been reported with teriparatide32 (Table 3).
Preoperative Evaluation
As already discussed, patients who undergo weight-loss surgery are at higher risk for wound-healing complications because of nutritional deficiencies. Total protein, albumin, and prealbumin levels and total lymphocyte count should be used to identify protein deficiency, which can decrease the likelihood of organized collagen formation. Huang and colleagues33 noted a statistically significant increase in complications after total knee arthroplasty (TKA) in patients with a prealbumin level under 3.5 mg/dL or a transferrin level under 200 mg/dL. Rates of prosthetic joint infection and development of hematoma or seroma requiring operative management were much higher, as were rates of postoperative neurovascular, renal, and cardiovascular complications.
Serum levels of vitamin A, folate, vitamin B12, and vitamin C should also be ordered, as many patients are deficient. Transferrin levels should be checked before surgery, as iron-deficiency anemia is common. Naghshineh and colleagues34 noted an anecdotal decrease in wound-healing complications in body-contouring surgery after correction of subclinical and clinical deficiencies in protein, arginine, glutamine, vitamin A, vitamin B12, vitamin C, folate, thiamine, iron, zinc, and selenium. Zinc deficiency was similarly implicated in wound-healing complications by Zorrilla and colleagues,35,36 who found a statistically significant delay in wound healing in patients with serum zinc levels under 95 mg/dL after total hip arthroplasty (THA)35 and hip hemiarthroplasty.36 To facilitate bone healing, physicians should give patients a thorough workup of levels of serum and urine calcium, 24-hydroxyvitamin D, and alkaline phosphatase. Osteomalacia typically presents with high alkaline phosphatase levels37 and secondary hyperparathyroidism. Therefore, physicians should monitor for these conditions. Although nonunion and aseptic loosening have not been reported as consequences of bariatric surgery, bone health should nevertheless be optimized when possible (Table 4).
Elective Orthopedic Surgery
Little is known about the true effect of weight-loss surgery on subsequent orthopedic procedures. Few investigators have explored the effect of surgery on arthrodesis, and the only recommendation for orthopedic surgeons is to be prepared for poor bone healing and the possibility of nonunion.38 Hidalgo and colleagues39 studied laparoscopic sleeve gastrectomy performed a minimum of 6 months before another elective surgery. Two patients had lumbar laminectomies, 2 had lumbar discectomies, 1 had a cervical discectomy, and 1 had a rotator cuff repair. By most recent follow-up, there were no complications of any of the orthopedic procedures, and all patients had healed.
There is no recommended amount of time between bariatric surgery and elective orthopedic surgery. Maximum weight loss and stabilization are typically achieved 2 years after surgery.40 However, elective orthopedic surgery has been performed as early as 6 months after bariatric surgery. Inacio and colleagues41 studied 3 groups of patients who underwent total joint arthroplasty (TJA): those who had it less than 2 years after bariatric surgery, those who had it more than 2 years after bariatric surgery, and those who were obese but did not have bariatric surgery. Complications of TJA occurred within the first year in 2.9% of the patients who had it more than 2 years after bariatric surgery, in 5.9% of the patients who had it less than 2 years after bariatric surgery, and in 4.1% of the patients who did not undergo bariatric surgery. Similarly, Severson and colleagues2 evaluated intraoperative and postoperative complications of TKA in 3 groups of obese patients: those who had TKA before bariatric surgery, those who had TKA less than 2 years after bariatric surgery, and those who had TKA more than 2 years after bariatric surgery. Gastroplasty and bypass patients were included. BMI was statistically significantly higher in the preoperative group than in the other 2 groups, though mean BMI for all groups was above 35 kg/m2. Operative time and tourniquet time were reduced in patients who had TKA more than 2 years after bariatric surgery, but there was no significant difference in anesthesia time. There was also no difference in 90-day complication rates between patients who had TKA before bariatric surgery and those who had it afterward. Severson and colleagues2 recommended communicating the risks to all obese patients, whether they undergo weight-loss surgery or not.
Arthroplasty
Obese patients have a higher rate of complications after arthroplasty—hence the referrals to bariatric surgeons. Bariatric surgery and its associated weight loss might improve joint pain and delay the need for arthroplasty in some cases.42 Obese people are prone to joint degeneration from the excess weight, and from altered gait patterns (eg, exaggerated step width, slower walking speed, increased time in double-limb stance).43 Gait changes are reversible after weight loss.44 Hooper and colleagues45 found a 37% decrease in lower extremity complaints after surgical weight loss, even though mean BMI at final follow-up was still in the obese range.
Obesity itself is a risk factor for ligamentous instability, but it is unclear whether the risk is mitigated by bariatric surgery. Disruption of the anterior fibers of the medial collateral ligament is more common in obese patients, as are complications involving the extensor mechanism (eg, patellofemoral dislocation). As a result, slower postoperative rehabilitation is recommended.46 Although there is no recorded link between bariatric surgery and the development of ligamentous laxity, surgeons should be aware of the potential for medial collateral ligament avulsion in obese and formerly obese patients and have appropriate implants available.
Kulkarni and colleagues47 compared the rates of hip and knee arthroplasty complications in patients who were obese before bariatric surgery and patients who were still obese after bariatric surgery. Gastroplasty and bypass patients were included. Data on superficial wound infections were excluded; however, the bariatric surgery group’s deep wound infection rate was 3.5 times lower, and its 30-day readmission rate was 7 times lower. There was no difference in dislocation and hip revision rates at 1 year. Although 1 patient in the bariatric surgery group died of an unknown cause 9 days after surgery, Kulkarni and colleagues47 concluded it is safer to operate on obese patients after versus before bariatric surgery. However, their study did not include mean BMI, so no conclusion can be drawn about the risk of operating on patients who were still obese after bariatric surgery.
Studies of weight loss in primary TJA patients have had conflicting findings.48 Trofa and colleagues49 reported that 15 patients who underwent arthroplasty a mean of 42.4 months after bariatric surgery lost 27.9% more of their original BMI compared with patients who underwent bariatric surgery but not arthroplasty. This relationship between arthroplasty and weight loss was strongest in patients who underwent knee arthroplasty, with an average of 43.9% more BMI lost compared to patients who did not undergo TKA. There was no significant change in BMI in patients who underwent THA and bariatric surgery compared with patients who underwent bariatric surgery but not THA.
Parvizi and colleagues50 assessed the results of 20 arthroplasties (8 THAs, 12 TKAs) performed in 14 patients a mean of 23 months after bariatric surgery (2 gastroplasties, 12 bypass surgeries). Mean BMI was 29 kg/m2. At final follow-up, 1 patient required revision THA for aseptic loosening, but all the others showed no evidence of radiographic loosening or wear. One patient had a superficial wound infection, and 1 had a deep wound infection. Parvizi and colleagues50 reported that arthroplasty after bariatric surgery is a viable option and is preferable to operating on morbidly obese patients.
Summary
Orthopedic surgeons are increasingly performing elective hip and knee arthroplasties on patients who have undergone bariatric surgery. Although bariatric surgery may alleviate some of the complications associated with surgery on morbidly obese patients, it should be approached with caution. Studies have shown that bariatric surgery patients are at increased risk for wound-healing and other complications, often caused by unrecognized preoperative nutrient deficiencies. In addition, patients are often unable to tolerate commonly used medications. The exact timing of bariatric surgery relative to elective orthopedic procedures is unclear. Surgeons should perform a preoperative evaluation based on type of bariatric surgery in order to reduce the likelihood of adverse events. Such preemptive therapy may improve the short- and long-term results of major reconstructive surgery. Further research is needed to determine the true effect of bariatric surgery on orthopedic procedures.
1. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611.
2. Severson EP, Singh JA, Browne JA, Trousdale RT, Sarr MG, Lewallen DG. Total knee arthroplasty in morbidly obese patients treated with bariatric surgery. J Arthroplasty. 2012;27(9):1696-1700.
3. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient [published correction appears in Endocr Pract. 2009;15(7):768]. Endocr Pract. 2008;14(suppl 1):1-83.
4. Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoilial bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308(17):995-999.
5. DeMaria EJ. Morbid obesity. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:736-743.
6. O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358-1365.
7. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
8. DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809-818.
9. Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48-55.
10. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569-575.
11. Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193-197.
12. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13-24.
13. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447-453.
14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40-47.
15. Ducloux R, Nobécourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21(5):556-560.
16. Wang A, Powell A. The effects of obesity surgery on bone metabolism: what orthopedic surgeons need to know. Am J Orthop. 2009;38(2):77-79.
17. Baghdasarian KL. Gastric bypass and megaloblastic anemia. J Am Diet Assoc. 1982;80(4):368-371.
18. Crowley LV, Olson RW. Megaloblastic anemia after gastric bypass for obesity. Am J Gastroenterol. 1983;78(7):406-410.
19. Sorg H, Schulz T, Krueger C, Vollmar B. Consequences of surgical stress on the kinetics of skin wound healing: partial hepatectomy delays and functionally alters dermal repair. Wound Repair Regen. 2009;17(3):367-377.
20. D’Ettorre M, Gniuli D, Iaconelli A, Massi G, Mingrone G, Bracaglia R. Wound healing process in post-bariatric patients: an experimental evaluation. Obes Surg. 2010;20(11):1552-1558.
21. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1);41-46.
22. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
23. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918-921.
24. Vilarrasa N, Gómez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860-866.
25. Hey H, Lund B, Sørensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34(1):13-15.
26. Borrelli J Jr, Pape C, Hak D, et al. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708-711.
27. Sardo P, Walker JH. Bariatric surgery: impact on medication management. Hosp Pharm. 2008;43(2):113-120.
28. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
29. Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):317-328.
30. Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829-854.
31. Sasse KC, Ganser J, Kozar M, et al. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18(5):530-534.
32. Miller AD, Smith KM. Medication use in bariatric surgery patients: what orthopedists need to know. Orthopedics. 2006;29(2):121-123.
33. Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28(8 suppl):21-24.
34. Naghshineh N, O’Brien Coon D, McTigue K, Courcoulas AP, Fernstrom M, Rubin JP. Nutritional assessment of bariatric surgery patients presenting for plastic surgery: a prospective analysis. Plast Reconstr Surg. 2010;126(2):602-610.
35. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14(2):119-122.
36. Zorrilla P, Salido JA, López-Alonso A, Silva A. Serum zinc as a prognostic tool for wound healing in hip hemiarthroplasty. Clin Orthop Relat Res. 2004;(420):304-308.
37. Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med. 2008;75(5):333-349.
38. Kini S, Kannan U. Effect of bariatric surgery on future general surgical procedures. J Minim Access Surg. 2011;7(2):126-131.
39. Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obes Surg. 2012;22(4):555-559.
40. O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032-1040.
41. Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364.
42. Gill RS, Al‐Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083-1089.
43. Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery–induced weight loss. J Biomech. 2012;45(10):1769-1774.
44. Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346-354.
45. Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114-120.
46. Booth RE Jr. Total knee arthroplasty in the obese patient: tips and quips. J Arthroplasty. 2002;17(4 suppl 1):69-70.
47. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
48. Inacio MC, Silverstein DK, Raman R, et al. Weight patterns before and after total joint arthroplasty and characteristics associated with weight change. Perm J. 2014;18(1):25-31.
49. Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014;10(2):335-339.
50. Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15(8):1003-1008.
1. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611.
2. Severson EP, Singh JA, Browne JA, Trousdale RT, Sarr MG, Lewallen DG. Total knee arthroplasty in morbidly obese patients treated with bariatric surgery. J Arthroplasty. 2012;27(9):1696-1700.
3. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient [published correction appears in Endocr Pract. 2009;15(7):768]. Endocr Pract. 2008;14(suppl 1):1-83.
4. Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoilial bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308(17):995-999.
5. DeMaria EJ. Morbid obesity. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:736-743.
6. O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358-1365.
7. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
8. DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809-818.
9. Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48-55.
10. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569-575.
11. Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193-197.
12. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13-24.
13. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447-453.
14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40-47.
15. Ducloux R, Nobécourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21(5):556-560.
16. Wang A, Powell A. The effects of obesity surgery on bone metabolism: what orthopedic surgeons need to know. Am J Orthop. 2009;38(2):77-79.
17. Baghdasarian KL. Gastric bypass and megaloblastic anemia. J Am Diet Assoc. 1982;80(4):368-371.
18. Crowley LV, Olson RW. Megaloblastic anemia after gastric bypass for obesity. Am J Gastroenterol. 1983;78(7):406-410.
19. Sorg H, Schulz T, Krueger C, Vollmar B. Consequences of surgical stress on the kinetics of skin wound healing: partial hepatectomy delays and functionally alters dermal repair. Wound Repair Regen. 2009;17(3):367-377.
20. D’Ettorre M, Gniuli D, Iaconelli A, Massi G, Mingrone G, Bracaglia R. Wound healing process in post-bariatric patients: an experimental evaluation. Obes Surg. 2010;20(11):1552-1558.
21. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1);41-46.
22. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
23. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918-921.
24. Vilarrasa N, Gómez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860-866.
25. Hey H, Lund B, Sørensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34(1):13-15.
26. Borrelli J Jr, Pape C, Hak D, et al. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708-711.
27. Sardo P, Walker JH. Bariatric surgery: impact on medication management. Hosp Pharm. 2008;43(2):113-120.
28. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
29. Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):317-328.
30. Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829-854.
31. Sasse KC, Ganser J, Kozar M, et al. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18(5):530-534.
32. Miller AD, Smith KM. Medication use in bariatric surgery patients: what orthopedists need to know. Orthopedics. 2006;29(2):121-123.
33. Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28(8 suppl):21-24.
34. Naghshineh N, O’Brien Coon D, McTigue K, Courcoulas AP, Fernstrom M, Rubin JP. Nutritional assessment of bariatric surgery patients presenting for plastic surgery: a prospective analysis. Plast Reconstr Surg. 2010;126(2):602-610.
35. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14(2):119-122.
36. Zorrilla P, Salido JA, López-Alonso A, Silva A. Serum zinc as a prognostic tool for wound healing in hip hemiarthroplasty. Clin Orthop Relat Res. 2004;(420):304-308.
37. Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med. 2008;75(5):333-349.
38. Kini S, Kannan U. Effect of bariatric surgery on future general surgical procedures. J Minim Access Surg. 2011;7(2):126-131.
39. Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obes Surg. 2012;22(4):555-559.
40. O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032-1040.
41. Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364.
42. Gill RS, Al‐Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083-1089.
43. Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery–induced weight loss. J Biomech. 2012;45(10):1769-1774.
44. Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346-354.
45. Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114-120.
46. Booth RE Jr. Total knee arthroplasty in the obese patient: tips and quips. J Arthroplasty. 2002;17(4 suppl 1):69-70.
47. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
48. Inacio MC, Silverstein DK, Raman R, et al. Weight patterns before and after total joint arthroplasty and characteristics associated with weight change. Perm J. 2014;18(1):25-31.
49. Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014;10(2):335-339.
50. Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15(8):1003-1008.
Female Athletes: Unique Challenges Facing Women Warriors
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Isolated Brachialis Muscle Atrophy
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Concomitant Ulnar Styloid Fracture and Distal Radius Fracture Portend Poorer Outcome
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Radiofrequency Microtenotomy for Elbow Epicondylitis: Midterm Results
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
Elbow epicondylitis is a painful condition caused by overuse and development of tendon degeneration. It is one of the most common elbow problems in adults, occurring both laterally and medially. “Tennis elbow” or lateral epicondylitis is diagnosed 7 to 10 times more often than the medial form, “golfer’s elbow.”1 Although these injuries are often associated with racquet sports, activities such as bowling and weightlifting and the professions of carpentry, plumbing, and meat-cutting have been described as causes.2,3
Elbow epicondylitis is thought to be the result of multiple microtraumatic events that cause disruption of the internal structure of the tendon and degeneration of the cells and matrix.4 Lesions caused by chronic overuse are now commonly called tendinosis and are not considered inflammatory in nature. Although the term tendinitis is used frequently and indiscriminately, histopathologic studies have shown that specimens of tendon obtained from areas of chronic overuse do not contain large numbers of macrophages, lymphocytes, or neutrophils.5 Rather, tendinosis appears to be a degenerative process that is characterized by the presence of dense populations of fibroblasts, vascular hyperplasia, and disorganized collagen. This constellation of findings has been termed by some authors as angiofibroblastic hyperplasia.6
Conservative care for the treatment of chronic tendinosis has been well described and is often successful. Treatment consists of rest, ice, compression, and elevation in the acute phase. This can be followed with bracing, activity modification, physical therapy, oral nonsteroidal anti-inflammatory drugs, topical applications, and injections of cortisone or platelet-rich plasma. When conservative treatment fails, surgical intervention may be considered. Procedures for the treatment of lateral epicondylitis include open débridement and release, arthroscopic débridement, percutaneous release, and radiofrequency (RF) coblation. The goals of operative treatment are to resect pathological material, to stimulate neovascularization by producing focused local bleeding, and to create a healthy scar while doing the least possible structural damage to surrounding tissues.4
The efficacy of a bipolar RF-based approach for using microtenotomy was first recognized when researchers studied the effects of transmyocardial revascularization for treating congestive heart failure.7 The use of RF- and laser-based transmyocardial revascularization initiated an angiogenic response in degenerated (ischemic) heart tissue. This success led to investigating the use of a RF-based approach for performing microtenotomy. Preclinical studies demonstrated that RF-based microtenotomy was effective for stimulating an angiogenic-healing response in tendon tissue.8 Histologic evaluation of treated tendons showed an early inflammatory response, with new blood-vessel formation by 28 days. In 2005, short-term results of this technique were published.9 This preliminary prospective case series showed that the treatment was safe and effectively improved or eliminated clinical symptoms.9 In the present midterm study, we hypothesized that pain scores would improve after RF microtenotomy and that these favorable results would continue to be observed over a longer term postoperatively.
Materials and Methods
Patients
This was a prospective, nonrandomized, single-center clinical study. After receiving institutional review board approval, patients who were 18 to 65 years of age with a diagnosis of tendinosis were approached for enrollment. For inclusion, patients had to be symptomatic for at least 6 months and had to have failed extensive conservative treatments. Nonoperative treatment included activity modification, enrollment in a facility- or home-based exercise program, bracing, oral nonsteroidal anti-inflammatory medication, and cortisone injection. Candidates with diabetes, confirmed or suspected pregnancy, surgery in the same tendon, implanted hardware adjacent to the target treatment region, or who were receiving care under workers’ compensation or had litigation-related injury were excluded. A single clinician performed a thorough medical history and clinical evaluation. The clinical follow-up and data collection were performed by an independent medical technician.
Clinical Outcomes
Pain status was assessed by using a visual analog scale (VAS). Postoperative clinical assessment was conducted within the first 2 days; at 7 to 10 days; at 4 to 6 weeks; and at 3, 6, 12, and 24 months, up to 9 years postoperatively. The VAS scales were completed annually up to 9 years after the procedure.
The percent improvement of VAS score was calculated. This value represented the difference between the patient’s preoperative and most recent VAS assessments. Failure of the procedure was defined as less than 50% improvement of the VAS score.
The RF-Based Microtenotomy Device
The Topaz Microdebrider (ArthroCare), connected to a System 2000 generator at setting 4 (175 V-RMS), was used to perform the RF-based microtenotomy. The device uses a controlled plasma-mediated RF-based process (coblation). Radiofrequency energy is used to excite the electrolytes in a conductive medium, such as a saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds,10,11 excising or dissolving (ie, ablating) soft tissue at relatively low temperatures (typically, 40°-70° C).12,13 The diameter of the active tip of the Topaz device is 0.8 mm.
Surgical Procedure
The senior author performed the majority of procedures in this study. Near the end of the series, the senior author’s associate also performed procedures. The symptomatic area of the tendon was identified and marked while the patient was alert. After the patient was positioned appropriately, light sedation was administered. A tourniquet was placed over the treatment limb and inflated to 250 mm Hg. A small incision, approximately 3 cm in length, was made over the marked treatment site to expose the involved tendon. After initiating sterile isotonic saline flow of 1 drop every 1 to 2 seconds from a line connected to the RF system, the tip of the device was placed on the tendon perpendicular to its surface (Figure 1). Using a light touch, it was activated for 500 milliseconds using a timer accessory for the control box. Five to 8 grams of pressure were applied with the device to penetrate the tendon and achieve successful ablation. The RF applications were performed at 5-mm intervals, to create a grid-like pattern on and throughout the symptomatic tendon area. The tendon was perforated to a depth of several millimeters on every second or third application throughout the treatment grid. After treatment of the symptomatic area, the wound was irrigated with copious amounts of normal saline solution and closed with interrupted nylon suture. Local anesthetic was injected only in the skin and in subcutaneous tissue. Standard wound dressings were applied. In the immediate postoperative period, the patient was advised to begin gentle active and passive range-of-motion exercises. Each patient was evaluated at 1 week postoperatively. At 6 weeks, patients were permitted to increase the intensity of their activities. Return to sports and heavy lifting was allowed once the patient was asymptomatic and had achieved full strength and range of motion; this typically occurred at 6 to 9 weeks after surgery.
Statistical Analysis
Normally distributed data were described using standard parametric statistics (ie, mean and standard deviation); non-normally distributed data were characterized using nonparametric descriptors (ie, median and quartiles). Statistical evaluation of improvement in pain status was performed by calculating 99% confidence intervals and using the Student t test for change between subsequent time points. Use of confidence intervals provides a descriptive analysis of the observed treatment effect, while permitting determination of statistical relevance. In all statistical testing, confidence bounds not including 0 were considered statistically significant. Probability of P ≤ .01 for committing type I experiment-wise error (rejecting a true null hypothesis) was selected for all statistical testing because of our lack of a control group, small sample size, and evaluation of multiple postoperative time points.
Results
Eighty consecutive patients with tendinosis of the elbow were included in this study. Sixty-nine patients were treated for lateral epicondylitis and 11 for medial epicondylitis. The average age of the patients (33 women, 47 men) was 50 years. The duration of follow-up evaluation ranged from 6 months to 9 years (mean, 2.5 years; median, 2 years). The Table presents the VAS improvement for these patients after the RF microtenotomy.
Within the lateral epicondylitis group, 91% (63/69) of the patients reported a successful outcome. The postoperative VAS improved to 1.3 from 6.9, which demonstrated an 81% improvement. Of the 6 patients that did not improve, 2 underwent repeat surgery.
Among the patients treated for medial epicondylitis, 91% (10/11) reported improvement in symptoms. The postoperative VAS improved to 1.3 from 6.1, a 79% improvement. One patient did not improve and did not undergo repeat surgery.
Discussion
For the treatment of medial and lateral elbow epicondylitis, RF microtenotomy is successful in 91% of patients. Symptomatic improvement was observed up to 9 years postoperatively. During this study, no complications were recorded; 7 treatment failures occurred. When compared with other techniques, the results with RF microtenotomy are equivalent or better.
In a retrospective study, Szabo and colleagues14 compared open, arthroscopic, and percutaneous release for lateral elbow tendinosis. They found the 3 methods to be highly effective for the treatment of tendinosis with no significant difference between them. Resection of the epicondyle and transfer of the anconeus muscle was found to be effective (94%) in a retrospective study by Almquist and colleagues.15 Dunn and coauthors16 reported a 97% success rate at 10 to 14 years postoperatively with a mini-open technique. Rubenthaler and colleagues17 showed 88% effectiveness for the open technique and 93% for the arthroscopic technique. With arthroscopic release of the extensor carpi radialis brevis tendon, Lattermann and coauthors18 reported clinical improvement in 94% of patients. In a study by Rose and colleagues,19 denervation of the lateral epicondyle was effective in relieving pain in 80% of patients who had had a positive response to a local anesthetic block. In a recently published study by Koh and coauthors,20 19 of 20 patients experienced a favorable outcome after treatment with ultrasonic microresection.
Regardless of surgical methods and their reported success rate, complications are associated with elbow surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma of the posterior cutaneous nerve.10,21,22 The recent introduction of arthroscopic release offers the potential for less morbidity and enables visualization of the elbow joint. However, disadvantages of the arthroscopic approach include violation of the joint for extra-articular pathology, increased operative time and cost, and neurovascular complications. Additionally, it is possible that the entire spectrum of extra-articular tendinosis cannot be effectively identified arthroscopically.23 In a prospective, randomized study, Meknas and colleagues24 compared RF microtenotomy with extensor tendon release and repair. They showed that patients treated with RF-microtenotomy experienced earlier pain relief and improved grip strength over the release group.
Different proposed mechanisms of action have been described to explain the favorable effects of the RF-based microtenotomy procedure, such as induced healing by an angiogenic response in the tendon tissue. In an animal study, Harwood and colleagues8 showed that low-dose RF-based plasma microtenotomy has the ability to stimulate angiogenic growth factors in tendons, such as αv integrin and vascular endothelial growth factor. These factors have been shown to be associated with healing.8 Early inflammatory response with new-vessel formation after 28 days was found in another animal study using the same method.25 Evaluation of RF-based methods in a prospective controlled laboratory study using a rabbit-tendon model showed histologic evidence of early inflammation with development of neovasculature after treatment.8 A later histologic study using an aged Achilles rabbit tendon model was performed to evaluate the effect of RF-based plasma microtenotomy on collagen remodeling.25 The degenerated tendon showed gaps, few normal crimpings, and a lack of reflectivity under polarized light. At 9 days after treatment, the treated tendon showed localized irregular crimpings, and, at 30 days, it showed regular crimping, tightly dense collagen fibers, and hypercellularity with good reflectivity. This was similar in appearance to a normal nondegenerated tendon (Figures 2A-2D). The RF-treated tendon also demonstrated an increase in production of insulin-like growth factor-1, β-fibroblast growth factor-1, αv integrin, and vascular endothelial growth factor.
Pathologic nerve ingrowth or nerve irritation in the tendon substance has been considered a possible cause of the pain experienced with tendinosis. Radiofrequency treatment has been shown to induce acute degeneration and ablation of sensory nerve fibers.26 These degenerated nerve fibers were observed to regenerate at 90 days after treatment.27 These findings provide potential evidence for early pain relief that is maintained long term as the nerves regenerate.
This midterm follow-up of patients with elbow epicondylitis has shown that RF-based microtenotomy can produce successful, durable results. Microtenotomy is a technically simple procedure to perform and is associated with a rapid and uncomplicated recovery. It is safe and can effectively eliminate or markedly reduce clinical symptoms.
Limitations
Lateral epicondylitis has been described as a self-limited disease, with resolution of symptoms at 12 to 18 months with conservative treatment. This perspective challenges the indication of any proposed surgical treatment for the condition. Although the results of this research demonstrated the benefits of RF microtenotomy, there are inherent limitations of the study design. The study lacks a control group, and randomization would improve the strength of the study. Additional outcome measures, such as Disabilities of the Arm, Shoulder, and Hand score, and grip strength could complement pain scores to provide more data. These data were collected in a preliminary study.9 Postoperative histologic analysis of treated human tissue would be ideal, but ethical considerations limit study to animal models. An additional limitation is potential examiner bias. Data collection was performed by an independent medical technician; a third-party blinded evaluation could have been performed, but this was not feasible in a clinical setting.
Conclusion
Radiofrequency-based microtenotomy is a safe and effective procedure for elbow epicondylitis. The results are durable with successful outcomes observed 9 years after surgery.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
1. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6(2):259-272.
2. Vangsness CT Jr, Jobe FW. Surgical technique of medial epicondylitis: Results in 35 elbows. J Bone Joint Surg Br. 1991;73(3):409-411.
3. Galloway M, DeMaio M, Mangine R. Rehabilitative techniques in the treatment of medial and lateral epicondylitis. Orthopedics. 1992;15(9):1089-1096.
4. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259-278.
5. Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11(3):533-578.
6. Nirschl RP. Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Fine LJ, ed. Repetitive Motion Disorders of the Upper Extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:467-479.
7. Chu V, Kuang J, Aiaid A, Korkola S, Chiu RC. Angiogenic response induced by mechanical transmyocardial revascularization. J Thorac Cardiovasc Surg 1999;118:849-856.
8. Harwood R, Bowden K, Amiel M, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit achilles tendon: a potential application to the treatment of tendinosis. Trans Orthop Res Soc. 2003;28:819.
9. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
10. Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE Trans Plasma Sci. 2002;30:1376-1383.
11. Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503-4505.
12. Woloszko J, Gilbride C. Coblation technology (plasma mediated ablation for otolaryngology applications). Proc SPIE. 2000;3907:306–316.
13. Woloszko J, Kwende MM, Stalder KR. Coblation in otolaryngology. Proc SPIE. 2003;4949:341–352.
14. Szabo SJ, Savoie FH 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg Am. 2006;15(6):721-727.
15. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
16. Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266.
17. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690.
18. Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for the treatment of recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656.
19. Rose NE, Forman SK, Dellon AL. Denervation of the lateral epicondyle for treatment of chronic lateral epicondylitis. J Hand Surg Am. 2013;38(2):344-349.
20. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendonopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
21. Nirschl RP, Ashman ES. Elbow tendonopathy: tennis elbow. Clin Sports Med. 2003;22(4):813-836.
22. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29(3):387-390.
23. Cummins CA. Lateral epicondylitis: in-vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491.
24. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965.
25. Takahashi N, Tasto JP, Locke J, et al. The use of radiofrequency (RF) for the treatment of chronic tendinosis. Paper presented at: 6th Biennial Congress of the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress; May 2007; Florence, Italy. Abstract 1433.
26. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35(5):805-810.
27. Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency ablation. Am J Sports Med. 2007;35(11):1940-1944.
Pigmented Villonodular Synovitis of the Hip: A Systematic Review
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Giant Bone Island of the Tibia in a Child
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.