User login
Upfront ASCT overcomes the survival advantage provided by pre-transplant HDAC induction in MCL
Key clinical point: High-dose cytarabine (HDAC)-based pre-autologous stem cell transplantation (ASCT) induction regimens were not associated with improved survival but led to higher overall response rates (ORR) and lower rates of early relapses in ASCT-eligible patients with mantle cell lymphoma (MCL).
Major finding: Patients receiving rituximab + HDAC (R-HDAC)-based regimens vs rituximab + cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) had significantly higher ORR (85.9% vs 65.7%; P = .007), lower 24-month progression rates (61.9% vs 80.4%; P = .043), and lower mortality (43.9% vs 68.6%; P = .004). However, the 2-year overall survival rates were similar between the R-HADC + ASCT and R-CHOP + ASCT groups (88.7% and 78.8%, respectively; P = .289).
Study details: This retrospective single-center study included 165 ASCT-eligible adult patients with MCL, of whom 136 patients received pre-ASCT induction immunochemotherapy with R-CHOP-like or regimens based on R-HDAC and 50 patients received consolidation with high-dose therapy and ASCT.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: de Pádua Covas Lage LA et al. Up-front ASCT overcomes the survival benefit provided by HDAC-based induction regimens in mantle cell lymphoma: Data from a real-life and long-term cohort. Cancers. 2023; 15(19):4759 (Sep 28). doi: 10.3390/cancers15194759
Key clinical point: High-dose cytarabine (HDAC)-based pre-autologous stem cell transplantation (ASCT) induction regimens were not associated with improved survival but led to higher overall response rates (ORR) and lower rates of early relapses in ASCT-eligible patients with mantle cell lymphoma (MCL).
Major finding: Patients receiving rituximab + HDAC (R-HDAC)-based regimens vs rituximab + cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) had significantly higher ORR (85.9% vs 65.7%; P = .007), lower 24-month progression rates (61.9% vs 80.4%; P = .043), and lower mortality (43.9% vs 68.6%; P = .004). However, the 2-year overall survival rates were similar between the R-HADC + ASCT and R-CHOP + ASCT groups (88.7% and 78.8%, respectively; P = .289).
Study details: This retrospective single-center study included 165 ASCT-eligible adult patients with MCL, of whom 136 patients received pre-ASCT induction immunochemotherapy with R-CHOP-like or regimens based on R-HDAC and 50 patients received consolidation with high-dose therapy and ASCT.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: de Pádua Covas Lage LA et al. Up-front ASCT overcomes the survival benefit provided by HDAC-based induction regimens in mantle cell lymphoma: Data from a real-life and long-term cohort. Cancers. 2023; 15(19):4759 (Sep 28). doi: 10.3390/cancers15194759
Key clinical point: High-dose cytarabine (HDAC)-based pre-autologous stem cell transplantation (ASCT) induction regimens were not associated with improved survival but led to higher overall response rates (ORR) and lower rates of early relapses in ASCT-eligible patients with mantle cell lymphoma (MCL).
Major finding: Patients receiving rituximab + HDAC (R-HDAC)-based regimens vs rituximab + cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) had significantly higher ORR (85.9% vs 65.7%; P = .007), lower 24-month progression rates (61.9% vs 80.4%; P = .043), and lower mortality (43.9% vs 68.6%; P = .004). However, the 2-year overall survival rates were similar between the R-HADC + ASCT and R-CHOP + ASCT groups (88.7% and 78.8%, respectively; P = .289).
Study details: This retrospective single-center study included 165 ASCT-eligible adult patients with MCL, of whom 136 patients received pre-ASCT induction immunochemotherapy with R-CHOP-like or regimens based on R-HDAC and 50 patients received consolidation with high-dose therapy and ASCT.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: de Pádua Covas Lage LA et al. Up-front ASCT overcomes the survival benefit provided by HDAC-based induction regimens in mantle cell lymphoma: Data from a real-life and long-term cohort. Cancers. 2023; 15(19):4759 (Sep 28). doi: 10.3390/cancers15194759
Cumulative airborne dioxin exposure increases CLL and SLL risk
Key clinical point: Cumulative airborne dioxin exposure is significantly associated with an increased risk for non-Hodgkin's lymphoma (NHL), particularly for combined chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
Major finding: A significant association was observed between log-transformed cumulative dioxin exposure index scores and the risk for NHL (adjusted odds ratio [aOR] 1.2; 95% CI 1.0-1.4), especially in case of the CLL and SLL subtypes (aOR 1.6; 95% CI 1.1-2.3), for a 4.4 log µg-toxic equivalent quantity/m2 increase corresponding to a standard deviation.
Study details: This case-control study was nested within the prospective French National Institute for Health and Medical Research E3N cohort and included 368 women with NHL and 368 matched control women without NHL.
Disclosures: The E3N cohort was established and maintained with the support of the Mutuelle Générale de l'Education Nationale, France, and other sources. The authors declared no conflicts of interest.
Source: Gaspard E et al. Association between cumulative airborne dioxin exposure and non-Hodgkin's lymphoma risk in a nested case-control study within the French E3N cohort. Sci Total Environ. 2023;906:167330 (Sep 29). doi: 10.1016/j.scitotenv.2023.167330
Key clinical point: Cumulative airborne dioxin exposure is significantly associated with an increased risk for non-Hodgkin's lymphoma (NHL), particularly for combined chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
Major finding: A significant association was observed between log-transformed cumulative dioxin exposure index scores and the risk for NHL (adjusted odds ratio [aOR] 1.2; 95% CI 1.0-1.4), especially in case of the CLL and SLL subtypes (aOR 1.6; 95% CI 1.1-2.3), for a 4.4 log µg-toxic equivalent quantity/m2 increase corresponding to a standard deviation.
Study details: This case-control study was nested within the prospective French National Institute for Health and Medical Research E3N cohort and included 368 women with NHL and 368 matched control women without NHL.
Disclosures: The E3N cohort was established and maintained with the support of the Mutuelle Générale de l'Education Nationale, France, and other sources. The authors declared no conflicts of interest.
Source: Gaspard E et al. Association between cumulative airborne dioxin exposure and non-Hodgkin's lymphoma risk in a nested case-control study within the French E3N cohort. Sci Total Environ. 2023;906:167330 (Sep 29). doi: 10.1016/j.scitotenv.2023.167330
Key clinical point: Cumulative airborne dioxin exposure is significantly associated with an increased risk for non-Hodgkin's lymphoma (NHL), particularly for combined chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
Major finding: A significant association was observed between log-transformed cumulative dioxin exposure index scores and the risk for NHL (adjusted odds ratio [aOR] 1.2; 95% CI 1.0-1.4), especially in case of the CLL and SLL subtypes (aOR 1.6; 95% CI 1.1-2.3), for a 4.4 log µg-toxic equivalent quantity/m2 increase corresponding to a standard deviation.
Study details: This case-control study was nested within the prospective French National Institute for Health and Medical Research E3N cohort and included 368 women with NHL and 368 matched control women without NHL.
Disclosures: The E3N cohort was established and maintained with the support of the Mutuelle Générale de l'Education Nationale, France, and other sources. The authors declared no conflicts of interest.
Source: Gaspard E et al. Association between cumulative airborne dioxin exposure and non-Hodgkin's lymphoma risk in a nested case-control study within the French E3N cohort. Sci Total Environ. 2023;906:167330 (Sep 29). doi: 10.1016/j.scitotenv.2023.167330
Ibrutinib and bortezomib combo durably effective in relapsed or refractory MCL with high-risk features
Key clinical point: The ibrutinib + bortezomib combination shows durable efficacy and manageable safety in patients with relapsed or refractory (R/R) mantle cell lymphoma (MCL), including high-risk patients.
Major finding: The combination led to an objective response rate of 81.8% (90% CI 71.1%-89.8%), which increased to 87.3% (90% CI 77.4%-93.9%) with ibrutinib maintenance. At a 25.4-month median follow-up, the median duration of response and progression-free survival were 22.7 (95% CI 12.3-not achieved) and 18.6 (95% CI 12.5-not achieved) months, respectively. The adverse event profile was consistent with the known safety profiles of individual drugs.
Study details: This phase 2 trial included 55 ibrutinib-naive and bortezomib-naive patients with R/R MCL previously treated with ≤2 lines of chemotherapy (of whom 75.6% had ≥1 high-risk features) who received 6 cycles of ibrutinib + bortezomib followed by ibrutinib maintenance.
Disclosures: This study was supported by Janssen and others. Some authors declared serving on the advisory boards of or receiving research funding, consulting fees, honoraria, or meeting or travel support from various sources, including Janssen.
Source: Novak U et al. Combined therapy with ibrutinib and bortezomib followed by ibrutinib maintenance in relapsed or refractory mantle cell lymphoma and high-risk features: A phase 1/2 trial of the European MCL network (SAKK 36/13). EClinicalMedicine. 2023;64:102221 (Sep 21). doi: 10.1016/j.eclinm.2023.102221
Key clinical point: The ibrutinib + bortezomib combination shows durable efficacy and manageable safety in patients with relapsed or refractory (R/R) mantle cell lymphoma (MCL), including high-risk patients.
Major finding: The combination led to an objective response rate of 81.8% (90% CI 71.1%-89.8%), which increased to 87.3% (90% CI 77.4%-93.9%) with ibrutinib maintenance. At a 25.4-month median follow-up, the median duration of response and progression-free survival were 22.7 (95% CI 12.3-not achieved) and 18.6 (95% CI 12.5-not achieved) months, respectively. The adverse event profile was consistent with the known safety profiles of individual drugs.
Study details: This phase 2 trial included 55 ibrutinib-naive and bortezomib-naive patients with R/R MCL previously treated with ≤2 lines of chemotherapy (of whom 75.6% had ≥1 high-risk features) who received 6 cycles of ibrutinib + bortezomib followed by ibrutinib maintenance.
Disclosures: This study was supported by Janssen and others. Some authors declared serving on the advisory boards of or receiving research funding, consulting fees, honoraria, or meeting or travel support from various sources, including Janssen.
Source: Novak U et al. Combined therapy with ibrutinib and bortezomib followed by ibrutinib maintenance in relapsed or refractory mantle cell lymphoma and high-risk features: A phase 1/2 trial of the European MCL network (SAKK 36/13). EClinicalMedicine. 2023;64:102221 (Sep 21). doi: 10.1016/j.eclinm.2023.102221
Key clinical point: The ibrutinib + bortezomib combination shows durable efficacy and manageable safety in patients with relapsed or refractory (R/R) mantle cell lymphoma (MCL), including high-risk patients.
Major finding: The combination led to an objective response rate of 81.8% (90% CI 71.1%-89.8%), which increased to 87.3% (90% CI 77.4%-93.9%) with ibrutinib maintenance. At a 25.4-month median follow-up, the median duration of response and progression-free survival were 22.7 (95% CI 12.3-not achieved) and 18.6 (95% CI 12.5-not achieved) months, respectively. The adverse event profile was consistent with the known safety profiles of individual drugs.
Study details: This phase 2 trial included 55 ibrutinib-naive and bortezomib-naive patients with R/R MCL previously treated with ≤2 lines of chemotherapy (of whom 75.6% had ≥1 high-risk features) who received 6 cycles of ibrutinib + bortezomib followed by ibrutinib maintenance.
Disclosures: This study was supported by Janssen and others. Some authors declared serving on the advisory boards of or receiving research funding, consulting fees, honoraria, or meeting or travel support from various sources, including Janssen.
Source: Novak U et al. Combined therapy with ibrutinib and bortezomib followed by ibrutinib maintenance in relapsed or refractory mantle cell lymphoma and high-risk features: A phase 1/2 trial of the European MCL network (SAKK 36/13). EClinicalMedicine. 2023;64:102221 (Sep 21). doi: 10.1016/j.eclinm.2023.102221
Ibrutinib maintenance after frontline induction is effective in mantle cell lymphoma
Key clinical point: Ibrutinib maintenance (I-M; dose 560 mg daily) for 4 years is effective in patients with treatment-naive mantle cell lymphoma (MCL) who are responsive to frontline chemo-immunotherapy with significant but manageable toxicities.
Major finding: The 3-year progression-free survival (PFS) and overall survival (OS) rates were 94% and 97%, whereas the 5-year PFS and OS rates were 89% and 91%, respectively. In patients with prior autologous stem cell transplantation (autoSCT), the 5-year PFS and OS rates were 100% each. The most common treatment-related adverse event was infection (86%; grades 1-2), and the most common grade 3-4 toxicities were hematologic.
Study details: This multicenter phase 2 study included patients with treatment-naive MCL who achieved a complete or partial response to frontline intensive induction chemo-immunotherapy with or without autoSCT and received 560 mg I-M daily for 4 years.
Disclosures: This study was supported by Pharmacyclics and Janssen. R Karmali and B Pro declared serving as consultants, speakers, or advisory board members for or receiving research funding or honoraria from various sources.
Source: Karmali R et al. Ibrutinib maintenance following frontline treatment in patients with mantle cell lymphoma. Blood Adv. 2023 (Sep 27). doi: 10.1182/bloodadvances.2023011271
Key clinical point: Ibrutinib maintenance (I-M; dose 560 mg daily) for 4 years is effective in patients with treatment-naive mantle cell lymphoma (MCL) who are responsive to frontline chemo-immunotherapy with significant but manageable toxicities.
Major finding: The 3-year progression-free survival (PFS) and overall survival (OS) rates were 94% and 97%, whereas the 5-year PFS and OS rates were 89% and 91%, respectively. In patients with prior autologous stem cell transplantation (autoSCT), the 5-year PFS and OS rates were 100% each. The most common treatment-related adverse event was infection (86%; grades 1-2), and the most common grade 3-4 toxicities were hematologic.
Study details: This multicenter phase 2 study included patients with treatment-naive MCL who achieved a complete or partial response to frontline intensive induction chemo-immunotherapy with or without autoSCT and received 560 mg I-M daily for 4 years.
Disclosures: This study was supported by Pharmacyclics and Janssen. R Karmali and B Pro declared serving as consultants, speakers, or advisory board members for or receiving research funding or honoraria from various sources.
Source: Karmali R et al. Ibrutinib maintenance following frontline treatment in patients with mantle cell lymphoma. Blood Adv. 2023 (Sep 27). doi: 10.1182/bloodadvances.2023011271
Key clinical point: Ibrutinib maintenance (I-M; dose 560 mg daily) for 4 years is effective in patients with treatment-naive mantle cell lymphoma (MCL) who are responsive to frontline chemo-immunotherapy with significant but manageable toxicities.
Major finding: The 3-year progression-free survival (PFS) and overall survival (OS) rates were 94% and 97%, whereas the 5-year PFS and OS rates were 89% and 91%, respectively. In patients with prior autologous stem cell transplantation (autoSCT), the 5-year PFS and OS rates were 100% each. The most common treatment-related adverse event was infection (86%; grades 1-2), and the most common grade 3-4 toxicities were hematologic.
Study details: This multicenter phase 2 study included patients with treatment-naive MCL who achieved a complete or partial response to frontline intensive induction chemo-immunotherapy with or without autoSCT and received 560 mg I-M daily for 4 years.
Disclosures: This study was supported by Pharmacyclics and Janssen. R Karmali and B Pro declared serving as consultants, speakers, or advisory board members for or receiving research funding or honoraria from various sources.
Source: Karmali R et al. Ibrutinib maintenance following frontline treatment in patients with mantle cell lymphoma. Blood Adv. 2023 (Sep 27). doi: 10.1182/bloodadvances.2023011271
High-dose methotrexate does not reduce CNS progression risk in high-risk aggressive BCL
Key clinical point: Prophylaxis with high-dose methotrexate (HD-MTX) was not associated with a clinically meaningful reduction in the risk for central nervous system (CNS) progression in high-risk patients with aggressive B-cell lymphoma (BCL).
Major finding: Patients who did vs did not receive HD-MTX had a significantly lower risk for CNS progression (adjusted 5-year risk difference 1.6%; adjusted hazard ratio [aHR] 0.59; P = .014), but the significance was lost when considering only those who achieved a complete response at chemoimmunotherapy completion (adjusted 5-year risk difference 1.4%; aHR 0.74; P = .30).
Study details: This multicenter retrospective study included 2418 adults with aggressive BCL and a high risk for CNS progression treated with curative-intent anti-CD20-based chemoimmunotherapy who did or did not receive HD-MTX, of whom 1616 achieved a complete response.
Disclosures: This study was funded by Janssen Pharmaceuticals and others. All authors except TC El-Galaly declared serving as consultants, advisors, or speakers for or receiving honoraria, research funding, or travel support from various sources, including Janssen.
Source: Lewis KL et al on behalf of the International CNS Prophylaxis Study Group. High-dose methotrexate as CNS prophylaxis in high-risk aggressive B-cell lymphoma. J Clin Oncol. 2023 (Oct 5). doi: 10.1200/JCO.23.00365
Key clinical point: Prophylaxis with high-dose methotrexate (HD-MTX) was not associated with a clinically meaningful reduction in the risk for central nervous system (CNS) progression in high-risk patients with aggressive B-cell lymphoma (BCL).
Major finding: Patients who did vs did not receive HD-MTX had a significantly lower risk for CNS progression (adjusted 5-year risk difference 1.6%; adjusted hazard ratio [aHR] 0.59; P = .014), but the significance was lost when considering only those who achieved a complete response at chemoimmunotherapy completion (adjusted 5-year risk difference 1.4%; aHR 0.74; P = .30).
Study details: This multicenter retrospective study included 2418 adults with aggressive BCL and a high risk for CNS progression treated with curative-intent anti-CD20-based chemoimmunotherapy who did or did not receive HD-MTX, of whom 1616 achieved a complete response.
Disclosures: This study was funded by Janssen Pharmaceuticals and others. All authors except TC El-Galaly declared serving as consultants, advisors, or speakers for or receiving honoraria, research funding, or travel support from various sources, including Janssen.
Source: Lewis KL et al on behalf of the International CNS Prophylaxis Study Group. High-dose methotrexate as CNS prophylaxis in high-risk aggressive B-cell lymphoma. J Clin Oncol. 2023 (Oct 5). doi: 10.1200/JCO.23.00365
Key clinical point: Prophylaxis with high-dose methotrexate (HD-MTX) was not associated with a clinically meaningful reduction in the risk for central nervous system (CNS) progression in high-risk patients with aggressive B-cell lymphoma (BCL).
Major finding: Patients who did vs did not receive HD-MTX had a significantly lower risk for CNS progression (adjusted 5-year risk difference 1.6%; adjusted hazard ratio [aHR] 0.59; P = .014), but the significance was lost when considering only those who achieved a complete response at chemoimmunotherapy completion (adjusted 5-year risk difference 1.4%; aHR 0.74; P = .30).
Study details: This multicenter retrospective study included 2418 adults with aggressive BCL and a high risk for CNS progression treated with curative-intent anti-CD20-based chemoimmunotherapy who did or did not receive HD-MTX, of whom 1616 achieved a complete response.
Disclosures: This study was funded by Janssen Pharmaceuticals and others. All authors except TC El-Galaly declared serving as consultants, advisors, or speakers for or receiving honoraria, research funding, or travel support from various sources, including Janssen.
Source: Lewis KL et al on behalf of the International CNS Prophylaxis Study Group. High-dose methotrexate as CNS prophylaxis in high-risk aggressive B-cell lymphoma. J Clin Oncol. 2023 (Oct 5). doi: 10.1200/JCO.23.00365
Second-line vs later-line zanubrutinib improves survival in relapsed or refractory MCL
Key clinical point: Second-line vs later-line zanubrutinib treatment leads to significantly improved long-term survival outcomes in patients with relapsed or refractory mantle cell lymphoma (MCL).
Major finding: At a median follow-up of 35.2 months, patients receiving second-line vs later-line zanubrutinib had significantly improved median overall survival (adjusted hazard ratio 0.459; P = .044) and numerically longer median progression-free survival (27.8 vs 22.1 months). Adverse events observed in both groups were consistent with the known safety profile of zanubrutinib.
Study details: Findings are from an updated pooled analysis of 112 patients from the BGB-3111-AU-003 and BGB-3111-206 clinical trials who had relapsed or refractory MCL and received second-line (n = 41) or later-line (n = 71) zanubrutinib.
Disclosures: The BGB-3111-AU-003 and BGB-3111-206 trials were sponsored by BeiGene. C Fang and S Sun declared being employees of BeiGene Co., Ltd., China. The other authors declared no conflicts of interest.
Source: Song Y et al. Long-term outcomes of second-line versus later-line zanubrutinib treatment in patients with relapsed/refractory mantle cell lymphoma: An updated pooled analysis. Cancer Med. 2023;12(18):18643-18653 (Sep 14). doi: 10.1002/cam4.6473
Key clinical point: Second-line vs later-line zanubrutinib treatment leads to significantly improved long-term survival outcomes in patients with relapsed or refractory mantle cell lymphoma (MCL).
Major finding: At a median follow-up of 35.2 months, patients receiving second-line vs later-line zanubrutinib had significantly improved median overall survival (adjusted hazard ratio 0.459; P = .044) and numerically longer median progression-free survival (27.8 vs 22.1 months). Adverse events observed in both groups were consistent with the known safety profile of zanubrutinib.
Study details: Findings are from an updated pooled analysis of 112 patients from the BGB-3111-AU-003 and BGB-3111-206 clinical trials who had relapsed or refractory MCL and received second-line (n = 41) or later-line (n = 71) zanubrutinib.
Disclosures: The BGB-3111-AU-003 and BGB-3111-206 trials were sponsored by BeiGene. C Fang and S Sun declared being employees of BeiGene Co., Ltd., China. The other authors declared no conflicts of interest.
Source: Song Y et al. Long-term outcomes of second-line versus later-line zanubrutinib treatment in patients with relapsed/refractory mantle cell lymphoma: An updated pooled analysis. Cancer Med. 2023;12(18):18643-18653 (Sep 14). doi: 10.1002/cam4.6473
Key clinical point: Second-line vs later-line zanubrutinib treatment leads to significantly improved long-term survival outcomes in patients with relapsed or refractory mantle cell lymphoma (MCL).
Major finding: At a median follow-up of 35.2 months, patients receiving second-line vs later-line zanubrutinib had significantly improved median overall survival (adjusted hazard ratio 0.459; P = .044) and numerically longer median progression-free survival (27.8 vs 22.1 months). Adverse events observed in both groups were consistent with the known safety profile of zanubrutinib.
Study details: Findings are from an updated pooled analysis of 112 patients from the BGB-3111-AU-003 and BGB-3111-206 clinical trials who had relapsed or refractory MCL and received second-line (n = 41) or later-line (n = 71) zanubrutinib.
Disclosures: The BGB-3111-AU-003 and BGB-3111-206 trials were sponsored by BeiGene. C Fang and S Sun declared being employees of BeiGene Co., Ltd., China. The other authors declared no conflicts of interest.
Source: Song Y et al. Long-term outcomes of second-line versus later-line zanubrutinib treatment in patients with relapsed/refractory mantle cell lymphoma: An updated pooled analysis. Cancer Med. 2023;12(18):18643-18653 (Sep 14). doi: 10.1002/cam4.6473
Second-line axi-cel therapy yields high response rates in high-risk relapsed or refractory LBCL
Key clinical point: Second-line axicabtagene ciloleucel (axi-cel) provides high response rates and manageable safety in patients with high-risk relapsed or refractory (R/R) large B-cell lymphoma (LBCL) who are ineligible for autologous stem-cell transplantation (ASCT).
Major finding: At 3 months from axi-cel infusion, the complete metabolic response rate was 71.0% (95% CI 58.1%-81.8%). At a 12-month median follow-up, the median progression-free survival was 11.8 months (95% CI 8.4-not reached) whereas median overall survival was not reached. Grade ≥3 cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome occurred in 8.1% and 14.5% of patients, respectively.
Study details: Findings are from the phase 2 ALYCANTE trial including 62 ASCT-ineligible patients with high-risk R/R LBCL who underwent leukapheresis and subsequently received second-line axi-cel.
Disclosures: This study was funded by Kite, a Gilead company. Some authors declared serving as members of directors’ boards or advisory committees of or receiving honoraria, research funding, consulting fees, or travel or accommodation expenses from various sources, including Kite and Gilead.
Source: Houot R et al. Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: A phase 2 trial. Nat Med. 2023;29:2593-2601 (Sep 14). doi: 10.1038/s41591-023-02572-5
Key clinical point: Second-line axicabtagene ciloleucel (axi-cel) provides high response rates and manageable safety in patients with high-risk relapsed or refractory (R/R) large B-cell lymphoma (LBCL) who are ineligible for autologous stem-cell transplantation (ASCT).
Major finding: At 3 months from axi-cel infusion, the complete metabolic response rate was 71.0% (95% CI 58.1%-81.8%). At a 12-month median follow-up, the median progression-free survival was 11.8 months (95% CI 8.4-not reached) whereas median overall survival was not reached. Grade ≥3 cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome occurred in 8.1% and 14.5% of patients, respectively.
Study details: Findings are from the phase 2 ALYCANTE trial including 62 ASCT-ineligible patients with high-risk R/R LBCL who underwent leukapheresis and subsequently received second-line axi-cel.
Disclosures: This study was funded by Kite, a Gilead company. Some authors declared serving as members of directors’ boards or advisory committees of or receiving honoraria, research funding, consulting fees, or travel or accommodation expenses from various sources, including Kite and Gilead.
Source: Houot R et al. Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: A phase 2 trial. Nat Med. 2023;29:2593-2601 (Sep 14). doi: 10.1038/s41591-023-02572-5
Key clinical point: Second-line axicabtagene ciloleucel (axi-cel) provides high response rates and manageable safety in patients with high-risk relapsed or refractory (R/R) large B-cell lymphoma (LBCL) who are ineligible for autologous stem-cell transplantation (ASCT).
Major finding: At 3 months from axi-cel infusion, the complete metabolic response rate was 71.0% (95% CI 58.1%-81.8%). At a 12-month median follow-up, the median progression-free survival was 11.8 months (95% CI 8.4-not reached) whereas median overall survival was not reached. Grade ≥3 cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome occurred in 8.1% and 14.5% of patients, respectively.
Study details: Findings are from the phase 2 ALYCANTE trial including 62 ASCT-ineligible patients with high-risk R/R LBCL who underwent leukapheresis and subsequently received second-line axi-cel.
Disclosures: This study was funded by Kite, a Gilead company. Some authors declared serving as members of directors’ boards or advisory committees of or receiving honoraria, research funding, consulting fees, or travel or accommodation expenses from various sources, including Kite and Gilead.
Source: Houot R et al. Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: A phase 2 trial. Nat Med. 2023;29:2593-2601 (Sep 14). doi: 10.1038/s41591-023-02572-5
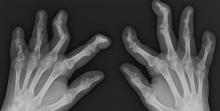
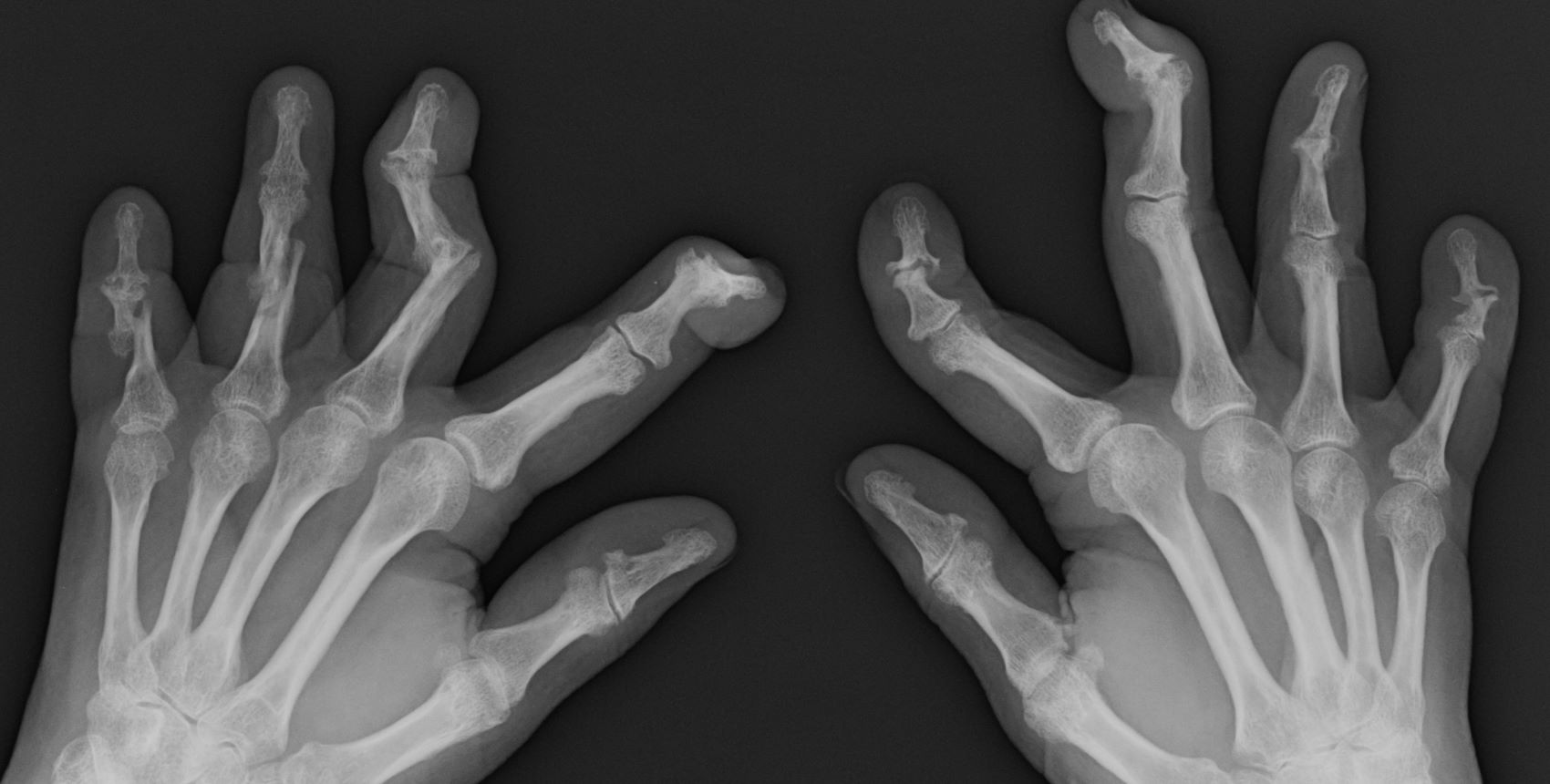
Pain in fingers for several months
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man presents with pain in fingers of several months' duration, which is moderately relieved with over-the-counter naproxen. He is concerned about "crooked" fingers and is worried that his work will be affected. He is overweight (BMI, 28.8), hypertensive, hypercholesterolemic, and a nonsmoker. The patient also reports a 6-month history of itchy "scalp" behind the ears, not relieved with dandruff shampoos. Physical exam reveals advanced deformity in index and middle fingers of both hands and no evident deformity in the wrists or metacarpophalangeal joints. Nails are pitted and discolored. Scalp behind ears shows well-demarcated, scaly patches. Lab work, radiography, and biopsy of the retroauricular area are ordered.
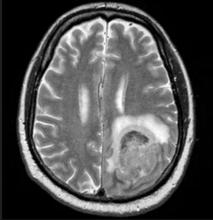
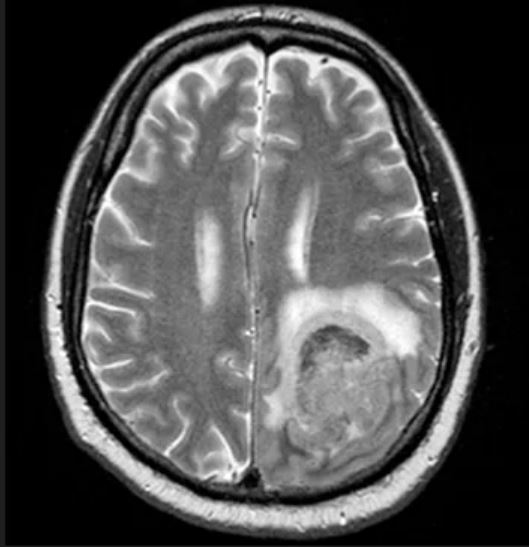
Fatigue and night sweats
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 65-year-old man presents to the oncology clinic with a 6-week history of fatigue, night sweats, and unintentional weight loss of 15 lb. He reports occasional fevers and generalized discomfort in his abdomen and has recently been experiencing painful headaches that are not relieved with nonsteroidal anti-inflammatory drugs. His medical history is otherwise unremarkable except for mild hypertension, for which he takes medication. His family history is unremarkable.
Physical examination reveals palpable lymph nodes in the neck, axilla, and inguinal regions; the spleen is palpable 3 cm below the left costal margin. A complete blood count shows anemia (hemoglobin level, 9.1g/dL) thrombocytopenia (platelet count, 90,000 cells/μL), and lymphocytosis (total leukocyte count, 5000 cells/μL); peripheral blood smear shows small, monomorphic lymphoid cells with oval-shaped nuclei and high nuclear-to-cytoplasmic ratio. Flow cytometry of lymph node biopsy is CD5-positive and pan B-cell antigen positive (eg, CD19, CD20, and CD22) but lacks expression of CD10 and CD23. A T2-weighted MRI is ordered.
No association between atopic dermatitis and non-alcoholic fatty liver disease
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057
Key clinical point: Comparable prevalence rates of non-alcoholic fatty liver disease (NAFLD) in patients with moderate-to-severe atopic dermatitis (AD) and those with in situ melanoma suggest that AD is not a risk factor for NAFLD.
Major finding: The prevalence rate of NAFLD was similar in patients with AD (24.1%) and those with in situ melanoma (23.2%), but it was significantly higher in patients with moderate-to-severe chronic plaque psoriasis (49.8%) compared with the other two groups (both P < .01). AD was not independently associated with NAFLD (adjusted odds ratio 1.02; 95% CI 0.78-1.26).
Study details: Findings are from a retrospective cross-sectional study including adult patients with moderate-to-severe AD (n = 144), moderate-to-severe chronic plaque psoriasis (n = 466), or in situ melanoma (n = 99).
Disclosures: This study was funded by European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and Strengthening of Biomedical Research in the National Health Service. The authors declared no conflicts of interest.
Source: Maurelli M et al. Prevalence of non-alcoholic fatty liver disease in adult individuals with moderate-to-severe atopic dermatitis. J Clin Med. 2023;12(18):6057 (Sep 19). doi: 10.3390/jcm12186057