User login
DDSEP quick quiz December question 2
A 54-year-old woman presents for management of moderately severe ileocolonic Crohn’s disease. She has a strong family history of multiple sclerosis and recently noted some tingling in her toes for which she is undergoing neurologic evaluation. She has had two small basal cell carcinomas removed from her cheek in the last year. She received the BCG vaccine as a child and had a positive PPD skin test within the last year. Laboratory evaluation reveals HBsAg negative, anti-HBs positive, and anti-HBc positive; JC virus antibody is positive.
January 2019 Question 1
A 31-year-old man is seen for a 1-week history of epigastric pain and scleral icterus. One month earlier, he developed diarrhea and fatigue, which has continued to persist. He denies any prior medical problems, though he admits he has not seen a doctor in years. He is currently visiting family in the United States, but he resides in South Africa. His laboratory tests are as follows: total bilirubin, 3.5 mg/dL; direct bilirubin, 2.9 mg/dL; alkaline phosphatase, 720 U/L; ALT, 105 U/L; AST, 117 U/L; albumin, 2.1 g/dL; INR, 1.2; HIV viral load 450,000 copies/mL, CD4 count, 25 cells/mm3. An abdominal ultrasound shows intra- and extrahepatic ductal dilation and an ERCP shows strictured intrahepatic ducts with papillary stenosis.
December 2018
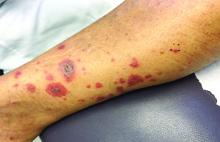
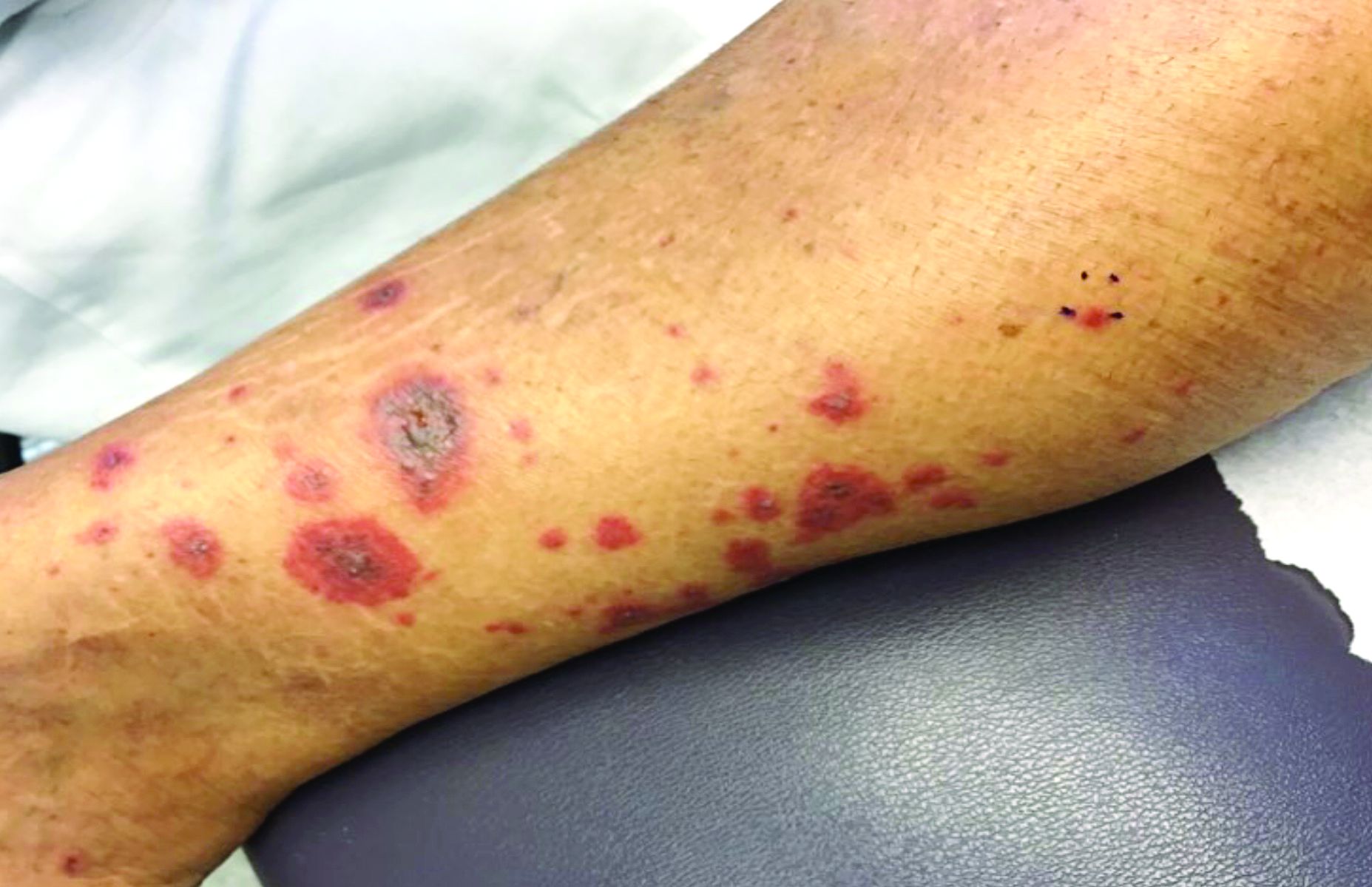
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 63-year-old white female presented with a 2-week history of hemorrhagic purpuric lesions and necrotic vesicles on the bilateral lower extremities.
Nearly 1 year prior to presentation, the patient underwent surgical resection for lung cancer. The patient also complained of joint swelling and pain in her ankles. She denied abdominal pain. She denied recent illness, including sore throat and upper respiratory infection. Skin biopsies were performed, including for direct immunofluorescence.
December 2018 Question 2
A 26-year-old woman presents for an evaluation of an 8-month history of intermittent abdominal pain, which is associated with diarrhea. Her pain improves with bowel movements. She denies weight loss, GI bleeding, or nocturnal symptoms. There is no family history of IBD or celiac disease. Physical examination is normal. Thyroid function testing, C-reactive protein, celiac serology, stool studies for infectious pathogens, stool calprotectin, and colonoscopy with biopsies are all negative.
December 2018 Question 1
A 45-year-old woman presents with a 3-year history of a sense of incomplete evacuation, excessive straining to defecate, and rectal bleeding. Colonoscopy demonstrates an irregular, polypoid lesion on the anterior wall of the rectum. Biopsies reveal fibromuscular obliteration of the lamina propria and hypertrophied muscularis mucosa with extension of muscle fibers upward between the crypts.
What is your diagnosis?
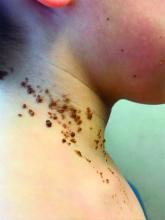
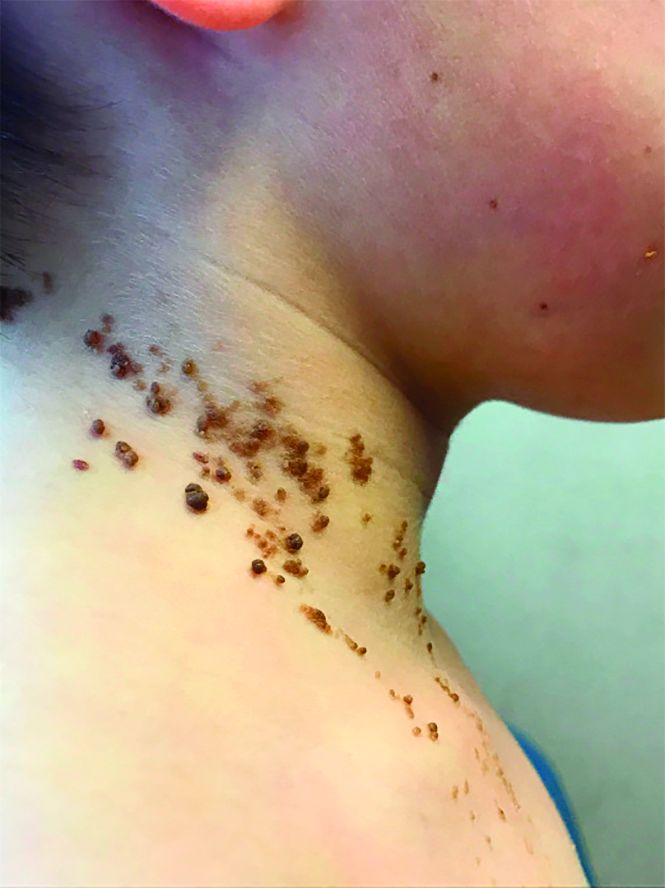
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
A 6-year-old, otherwise-healthy male is brought into clinic for evaluation of papules on his neck. The rash has been present since 1 year of age and has been growing in size proportionately. He claims there is occasional itching but no pain or redness. He does not seem to be disturbed by his rash. He has two siblings, aged 2 and 4 years, without lesions.
On physical exam, he is noted to have a linear plaque of hyperpigmented verrucous papules on his neck.
December 2018
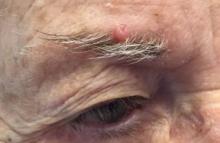
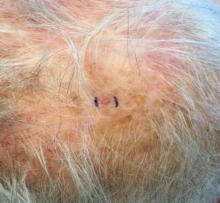
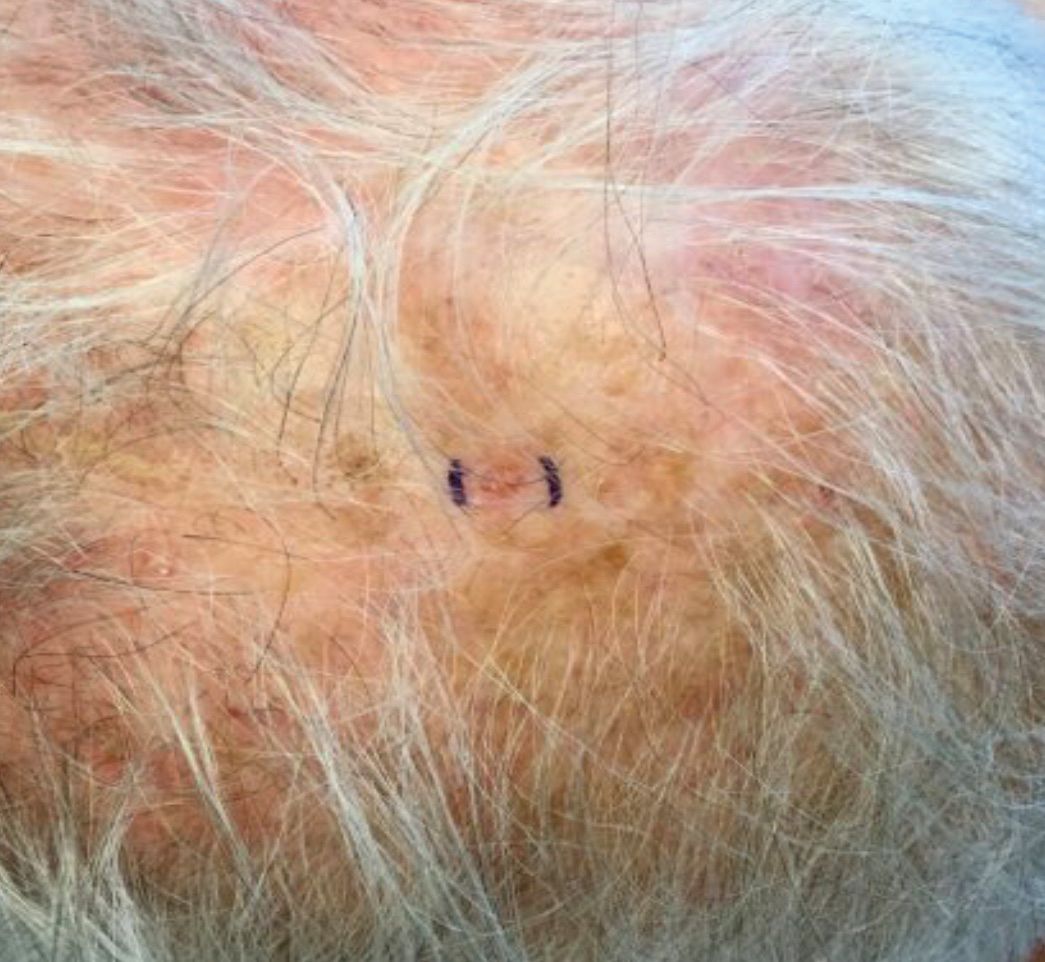
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
. White individuals over aged 50 years are more frequently affected. Both genders are equally affected, and 25% of cases occur on the covered areas (trunk or extremities) of younger patients. Clinically, lesions present as pink to red plaques or nodules that exhibit rapid growth. Ulceration or crusting may be present. Causes of AFX include ultraviolet radiation and ionizing radiation. AFX is considered a superficial variant of malignant fibrous histiocytoma (MFH), the most common soft tissue sarcoma of adults. Clinically, MFH involves deeper tissues than does AFX, often on the thighs or buttocks. MFH is a more aggressive malignancy that regularly metastasizes.
Histologically, the tumor occurs as a dermal proliferation of “bizarre” spindle cells, epithelioid cells, and atypical histiocytes. Vesicular changes may be present in the nucleus or cytoplasm of the spindle cells. Mitotic figures are present. Multinucleated giant cells may be present. Solar elastosis is often seen, as well. Vimentin and histiocyte stains are positive. Unlike melanoma, S-100 staining is minimal. Unlike squamous cell carcinoma, prekeratin staining is negative. CD34 is negative. AFX resembles MFH histologically.
Surgical excision by the Mohs procedure is preferred over wide excision as there is a risk of recurrence. AFX rarely metastasizes. This is more likely if inadequately excised or the patient is immunosuppressed. Sun protective practices, such as applying and reapplying sunscreen regularly, wearing sun protective clothing, and avoiding excessive UV exposure during peak hours is recommended.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
August 2018 Question 2
Rationale
For patients who have small varices with either red wale signs or the presence of severe liver disease (Child Pugh class C), the risk of first hemorrhage is as high as for patients with large varices. Because these small varices are difficult to ligate, therapy with a nonselective beta-blocker such as nadolol is recommended. Nonselective beta-adrenergic blockers (propranolol, nadolol) reduce portal pressure by reducing portal venous inflow through both a beta-1 (reduction in cardiac output) and a beta-2 (splanchnic vasoconstriction). A decrease in HVPG greater than 20% in patients treated with nonselective beta-blockers has been associated with a lower rate of first variceal hemorrhage, ascites, and death. Clinical targets include a heart rate below 60 bpm or a 25% reduction from baseline heart rate. Metoprolol is a selective beta-blocker and is not effective in reducing portal pressure. Nitrates alone are not effective in preventing first variceal hemorrhage and are associated with increased long-term mortality in patients over the age of 50. Diltiazem is a calcium channel blocker, which has not been shown to be effective in the treatment of esophageal varices. Observation is not an appropriate option given the high risk of bleeding for these varices, which should be addressed.
References
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-76.
[email protected]
Rationale
For patients who have small varices with either red wale signs or the presence of severe liver disease (Child Pugh class C), the risk of first hemorrhage is as high as for patients with large varices. Because these small varices are difficult to ligate, therapy with a nonselective beta-blocker such as nadolol is recommended. Nonselective beta-adrenergic blockers (propranolol, nadolol) reduce portal pressure by reducing portal venous inflow through both a beta-1 (reduction in cardiac output) and a beta-2 (splanchnic vasoconstriction). A decrease in HVPG greater than 20% in patients treated with nonselective beta-blockers has been associated with a lower rate of first variceal hemorrhage, ascites, and death. Clinical targets include a heart rate below 60 bpm or a 25% reduction from baseline heart rate. Metoprolol is a selective beta-blocker and is not effective in reducing portal pressure. Nitrates alone are not effective in preventing first variceal hemorrhage and are associated with increased long-term mortality in patients over the age of 50. Diltiazem is a calcium channel blocker, which has not been shown to be effective in the treatment of esophageal varices. Observation is not an appropriate option given the high risk of bleeding for these varices, which should be addressed.
References
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-76.
[email protected]
Rationale
For patients who have small varices with either red wale signs or the presence of severe liver disease (Child Pugh class C), the risk of first hemorrhage is as high as for patients with large varices. Because these small varices are difficult to ligate, therapy with a nonselective beta-blocker such as nadolol is recommended. Nonselective beta-adrenergic blockers (propranolol, nadolol) reduce portal pressure by reducing portal venous inflow through both a beta-1 (reduction in cardiac output) and a beta-2 (splanchnic vasoconstriction). A decrease in HVPG greater than 20% in patients treated with nonselective beta-blockers has been associated with a lower rate of first variceal hemorrhage, ascites, and death. Clinical targets include a heart rate below 60 bpm or a 25% reduction from baseline heart rate. Metoprolol is a selective beta-blocker and is not effective in reducing portal pressure. Nitrates alone are not effective in preventing first variceal hemorrhage and are associated with increased long-term mortality in patients over the age of 50. Diltiazem is a calcium channel blocker, which has not been shown to be effective in the treatment of esophageal varices. Observation is not an appropriate option given the high risk of bleeding for these varices, which should be addressed.
References
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-76.
[email protected]
A 56-year-old man with hepatitis C cirrhosis presents for variceal screening. He has mild ascites and grade 1-2 encephalopathy, both controlled with pharmacologic treatment. Recent blood work reveals a total bilirubin of 2.1 mg/dL, albumin of 3.1 g/dL, and an INR of 1.8. Endoscopy reveals small varices.
Which is the next best step in management?
August 2018 Question 1
References
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-76.
[email protected]
References
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-76.
[email protected]
References
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-76.
[email protected]
A 44-year-old man with history of renal transplant, on immunosupression with prednisone and tacrolimus, presents to the emergency department with high-grade fever of 105 °C and confusion. He initially developed nausea, vomiting, and diarrhea for a few days after attending a dinner party. Later, he developed fever and headache. His symptoms progressed, with worsening neurological features, manifested as ataxia and tremors. Soon after admission to the emergency department, he developed seizures and lost consciousness, and was intubated to protect his airway. Routine labs were sent for investigation, and both CT head scan and lumbar puncture were performed. CBC revealed leukocytosis. CT scan was negative for any acute findings, and CSF fluid analysis revealed increased white blood cells, mainly lymphocytes, and low glucose.
Which of the following organisms is the most likely cause of this illness?