User login
Mailing out fecal tests may improve CRC screening rates
Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening in community health centers, results of a recent randomized trial show.
Outreach by mail led to a 3.4–percentage point increase in completion of FIT, compared with clinics who did not participate in the intervention, according to results of the randomized Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) trial.
Although that difference was statistically significant, investigators said the improvement was less than expected based on previous experience, including a pilot study showing that the strategy of mailing fecal tests boosted completion rates by 38%.
Based on that discrepancy, additional strategies may be needed to support implementation of FIT mailing programs in low-resource health centers, reported Gloria D. Coronado, PhD, Kaiser Permanente Center for Health Research, Portland, Ore., and coinvestigators.
“This work demonstrates that mailed FIT outreach programs can have clinical impact when integrated into clinical work flows, but emphasizes the need to identify additional strategies to support program implementation in low-resource health centers,” Dr. Coronado and coauthors said in JAMA Internal Medicine.
The STOP CRC study included 26 federally qualified health center clinics serving low-income populations in Oregon and California. Investigators identified a total of 41,193 adults overdue for colorectal cancer screening between Feb. 4, 2014 and Feb. 3, 2015.
The core of the intervention was a set of electronic health record–embedded tools that identified adults due for screening and allowed staff to generate letters and mailing labels for a series of three mailings. The first mailing was an introductory letter, the second was a FIT kit packet that included wordless instructions, and the third was a reminder letter.
For clinics that participated in the intervention, the rate of FIT completion was 13.9%, versus 10.4%, a difference that was statistically significant (95% confidence interval, 0.1%-6.8%; P = .047), according to investigators. Likewise, the proportion of participants completing any CRC screening was significantly higher in the intervention clinics (18.3% versus 14.5%; 3.8 percentage points difference; 95% CI, 0.6%-7.0%; P = .024).
Somewhat larger effects were seen in an analysis that accounted for delays in implementation of the program. In that analysis, FIT completion rates were 17.6% for the intervention clinics and 12.8% for the usual care clinics (95% CI, 0.9-8.6%; P = .020), with similar increases seen in the proportion of patients receiving any CRC screening.
These increases in screening occurred despite “relatively low” implementation of the program, Dr. Coronado and colleagues said.
In the pilot study, a concerted effort was made to ensure all eligible adults got the intervention; in this study, 6,925 out of 21,134 intervention participants (33%) got an introductory letter, and of those, 91% received the FIT and 59% got the reminder letter.
Implementation varied widely by health center, ranging from 6.5% to 68.2%, investigators said in their report.
One reason for low implementation may be that the program competed with other priorities in the clinics. In interviews, health center leaders said challenges in the clinic included time burden, limited organizational capacity, and challenges with the EHR and associated reporting tools.
“For most participating health centers, STOP CRC represented the first time EHR tools were used to deliver cancer screening services outside the clinic,” Dr. Coronado said. “Implementation might have increased with experience.”
The research reported by Dr. Coronado and coinvestigators was supported by the National Institutes of Health. Dr. Coronado reported serving as a coinvestigator on a study of an experimental blood test for colorectal cancer funded by EpiGenomics and as principal investigator on a study of an experimental FIT funded by Quidel Corporation. No other disclosures were reported.
AGA patient education materials on colorectal cancer will help your patients better understand all of their screening options. Learn more at patient.gastro.org.
SOURCE: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening in community health centers, results of a recent randomized trial show.
Outreach by mail led to a 3.4–percentage point increase in completion of FIT, compared with clinics who did not participate in the intervention, according to results of the randomized Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) trial.
Although that difference was statistically significant, investigators said the improvement was less than expected based on previous experience, including a pilot study showing that the strategy of mailing fecal tests boosted completion rates by 38%.
Based on that discrepancy, additional strategies may be needed to support implementation of FIT mailing programs in low-resource health centers, reported Gloria D. Coronado, PhD, Kaiser Permanente Center for Health Research, Portland, Ore., and coinvestigators.
“This work demonstrates that mailed FIT outreach programs can have clinical impact when integrated into clinical work flows, but emphasizes the need to identify additional strategies to support program implementation in low-resource health centers,” Dr. Coronado and coauthors said in JAMA Internal Medicine.
The STOP CRC study included 26 federally qualified health center clinics serving low-income populations in Oregon and California. Investigators identified a total of 41,193 adults overdue for colorectal cancer screening between Feb. 4, 2014 and Feb. 3, 2015.
The core of the intervention was a set of electronic health record–embedded tools that identified adults due for screening and allowed staff to generate letters and mailing labels for a series of three mailings. The first mailing was an introductory letter, the second was a FIT kit packet that included wordless instructions, and the third was a reminder letter.
For clinics that participated in the intervention, the rate of FIT completion was 13.9%, versus 10.4%, a difference that was statistically significant (95% confidence interval, 0.1%-6.8%; P = .047), according to investigators. Likewise, the proportion of participants completing any CRC screening was significantly higher in the intervention clinics (18.3% versus 14.5%; 3.8 percentage points difference; 95% CI, 0.6%-7.0%; P = .024).
Somewhat larger effects were seen in an analysis that accounted for delays in implementation of the program. In that analysis, FIT completion rates were 17.6% for the intervention clinics and 12.8% for the usual care clinics (95% CI, 0.9-8.6%; P = .020), with similar increases seen in the proportion of patients receiving any CRC screening.
These increases in screening occurred despite “relatively low” implementation of the program, Dr. Coronado and colleagues said.
In the pilot study, a concerted effort was made to ensure all eligible adults got the intervention; in this study, 6,925 out of 21,134 intervention participants (33%) got an introductory letter, and of those, 91% received the FIT and 59% got the reminder letter.
Implementation varied widely by health center, ranging from 6.5% to 68.2%, investigators said in their report.
One reason for low implementation may be that the program competed with other priorities in the clinics. In interviews, health center leaders said challenges in the clinic included time burden, limited organizational capacity, and challenges with the EHR and associated reporting tools.
“For most participating health centers, STOP CRC represented the first time EHR tools were used to deliver cancer screening services outside the clinic,” Dr. Coronado said. “Implementation might have increased with experience.”
The research reported by Dr. Coronado and coinvestigators was supported by the National Institutes of Health. Dr. Coronado reported serving as a coinvestigator on a study of an experimental blood test for colorectal cancer funded by EpiGenomics and as principal investigator on a study of an experimental FIT funded by Quidel Corporation. No other disclosures were reported.
AGA patient education materials on colorectal cancer will help your patients better understand all of their screening options. Learn more at patient.gastro.org.
SOURCE: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening in community health centers, results of a recent randomized trial show.
Outreach by mail led to a 3.4–percentage point increase in completion of FIT, compared with clinics who did not participate in the intervention, according to results of the randomized Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) trial.
Although that difference was statistically significant, investigators said the improvement was less than expected based on previous experience, including a pilot study showing that the strategy of mailing fecal tests boosted completion rates by 38%.
Based on that discrepancy, additional strategies may be needed to support implementation of FIT mailing programs in low-resource health centers, reported Gloria D. Coronado, PhD, Kaiser Permanente Center for Health Research, Portland, Ore., and coinvestigators.
“This work demonstrates that mailed FIT outreach programs can have clinical impact when integrated into clinical work flows, but emphasizes the need to identify additional strategies to support program implementation in low-resource health centers,” Dr. Coronado and coauthors said in JAMA Internal Medicine.
The STOP CRC study included 26 federally qualified health center clinics serving low-income populations in Oregon and California. Investigators identified a total of 41,193 adults overdue for colorectal cancer screening between Feb. 4, 2014 and Feb. 3, 2015.
The core of the intervention was a set of electronic health record–embedded tools that identified adults due for screening and allowed staff to generate letters and mailing labels for a series of three mailings. The first mailing was an introductory letter, the second was a FIT kit packet that included wordless instructions, and the third was a reminder letter.
For clinics that participated in the intervention, the rate of FIT completion was 13.9%, versus 10.4%, a difference that was statistically significant (95% confidence interval, 0.1%-6.8%; P = .047), according to investigators. Likewise, the proportion of participants completing any CRC screening was significantly higher in the intervention clinics (18.3% versus 14.5%; 3.8 percentage points difference; 95% CI, 0.6%-7.0%; P = .024).
Somewhat larger effects were seen in an analysis that accounted for delays in implementation of the program. In that analysis, FIT completion rates were 17.6% for the intervention clinics and 12.8% for the usual care clinics (95% CI, 0.9-8.6%; P = .020), with similar increases seen in the proportion of patients receiving any CRC screening.
These increases in screening occurred despite “relatively low” implementation of the program, Dr. Coronado and colleagues said.
In the pilot study, a concerted effort was made to ensure all eligible adults got the intervention; in this study, 6,925 out of 21,134 intervention participants (33%) got an introductory letter, and of those, 91% received the FIT and 59% got the reminder letter.
Implementation varied widely by health center, ranging from 6.5% to 68.2%, investigators said in their report.
One reason for low implementation may be that the program competed with other priorities in the clinics. In interviews, health center leaders said challenges in the clinic included time burden, limited organizational capacity, and challenges with the EHR and associated reporting tools.
“For most participating health centers, STOP CRC represented the first time EHR tools were used to deliver cancer screening services outside the clinic,” Dr. Coronado said. “Implementation might have increased with experience.”
The research reported by Dr. Coronado and coinvestigators was supported by the National Institutes of Health. Dr. Coronado reported serving as a coinvestigator on a study of an experimental blood test for colorectal cancer funded by EpiGenomics and as principal investigator on a study of an experimental FIT funded by Quidel Corporation. No other disclosures were reported.
AGA patient education materials on colorectal cancer will help your patients better understand all of their screening options. Learn more at patient.gastro.org.
SOURCE: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening, though not to the extent that had been seen in a pilot study.
Major finding: Outreach by mail led to a 3.4–percentage point increase in FIT completion for participating clinics versus clinics that implemented usual care.
Study details: STOP CRC, a cluster-randomized pragmatic clinical trial including 26 federally qualified health center clinics and a total of 41,193 adults overdue for colorectal cancer screening.
Disclosures: The research was supported by the National Institutes of Health. One investigator reported disclosures related to EpiGenomics and Quidel Corporation.
Source: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
Baseline steroids may reduce efficacy of checkpoint inhibitors
Immune checkpoint inhibitor therapy may be less effective if the patient is receiving corticosteroids at the time that treatment is initiated, results of a retrospective, two-center analysis suggest.
Corticosteroid use at the time of starting a PD-1 or PD-L1 inhibitor was associated with significantly reduced overall survival and progression-free survival in patients with non–small-cell lung cancer, the authors of the analysis reported.
“Prudent use of corticosteroids at the time of initiating PD-1/PD-L1 blockade is warranted,” said Matthew D. Hellmann, MD, of Memorial Sloan Kettering Cancer Center, New York, and his coauthors. The report is in the Journal of Clinical Oncology.
The retrospective analysis from Dr. Hellmann and his colleagues comprised 640 patients treated with single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab at Memorial Sloan Kettering Cancer Center or Gustave Roussy Cancer Center, Villejuif, France, between April 2011 and September 2017.
Ninety of those patients (14%) were receiving at least 10 mg of prednisone equivalent at the time the immune checkpoint inhibitor was started, a review of patient records revealed. About one-third were receiving corticosteroids for dyspnea or other respiratory symptoms. Another 21% had fatigue and 19% had brain metastases prompting corticosteroid treatment.
Corticosteroid use at the time of PD-1/PD-L1 blockade was associated with decreased progression-free survival (hazard ratio, 1.31; P = .03) and overall survival (HR, 1.66; P less than .001), as well as decreased overall response rate in a multivariable analysis adjusted for performance status, history of smoking, and history of brain metastases, the investigators reported.
Reduced efficacy also was seen in analyses that looked at each center separately. For the Memorial Sloan Kettering cohort (455 patients), baseline corticosteroid use was associated with significantly decreased overall response rate, shorter progression-free survival, and shorter overall survival.
For the smaller French cohort, (185 patients), there was a statistically nonsignificant decrease in overall response rate, along with significant reductions in progression-free survival and overall survival.
Based on these data, it may be prudent to try other pharmacologic or nonpharmacologic strategies to manage cancer symptoms if treatment with a PD-1/PD-L1 blocker is planned, Dr. Hellmann and his coauthors said.
“These strategies could enable patients to be tapered off corticosteroids before the start of PD-1/PD-L1 blockade to potentially achieve maximum benefit from these agents,” they wrote.
However, medically necessary corticosteroids should not be avoided, such as those given for management of brain metastases, they added.
Interestingly, other recent studies have suggested that patients already on PD-1/PD-L1 inhibitors do not seem to have decreased efficacy when corticosteroids are prescribed to manage emergent immune-related adverse events.
That’s “fortunate,” given that corticosteroids are a mainstay for the management of these characteristic adverse events in patients receiving immune checkpoint inhibitors, they said.
Dr. Hellmann reported research funding from Bristol-Myers Squibb, and a consulting or advisory role with AstraZeneca, Bristol-Myers Squibb, Genentech, Janssen Pharmaceuticals, MedImmune, Merck, Mirati Therapeutics, Shattuck Labs, and Syndax. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Pfizer, and Roche, among others.
SOURCE: Arbour KC et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.79.0006.
Immune checkpoint inhibitor therapy may be less effective if the patient is receiving corticosteroids at the time that treatment is initiated, results of a retrospective, two-center analysis suggest.
Corticosteroid use at the time of starting a PD-1 or PD-L1 inhibitor was associated with significantly reduced overall survival and progression-free survival in patients with non–small-cell lung cancer, the authors of the analysis reported.
“Prudent use of corticosteroids at the time of initiating PD-1/PD-L1 blockade is warranted,” said Matthew D. Hellmann, MD, of Memorial Sloan Kettering Cancer Center, New York, and his coauthors. The report is in the Journal of Clinical Oncology.
The retrospective analysis from Dr. Hellmann and his colleagues comprised 640 patients treated with single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab at Memorial Sloan Kettering Cancer Center or Gustave Roussy Cancer Center, Villejuif, France, between April 2011 and September 2017.
Ninety of those patients (14%) were receiving at least 10 mg of prednisone equivalent at the time the immune checkpoint inhibitor was started, a review of patient records revealed. About one-third were receiving corticosteroids for dyspnea or other respiratory symptoms. Another 21% had fatigue and 19% had brain metastases prompting corticosteroid treatment.
Corticosteroid use at the time of PD-1/PD-L1 blockade was associated with decreased progression-free survival (hazard ratio, 1.31; P = .03) and overall survival (HR, 1.66; P less than .001), as well as decreased overall response rate in a multivariable analysis adjusted for performance status, history of smoking, and history of brain metastases, the investigators reported.
Reduced efficacy also was seen in analyses that looked at each center separately. For the Memorial Sloan Kettering cohort (455 patients), baseline corticosteroid use was associated with significantly decreased overall response rate, shorter progression-free survival, and shorter overall survival.
For the smaller French cohort, (185 patients), there was a statistically nonsignificant decrease in overall response rate, along with significant reductions in progression-free survival and overall survival.
Based on these data, it may be prudent to try other pharmacologic or nonpharmacologic strategies to manage cancer symptoms if treatment with a PD-1/PD-L1 blocker is planned, Dr. Hellmann and his coauthors said.
“These strategies could enable patients to be tapered off corticosteroids before the start of PD-1/PD-L1 blockade to potentially achieve maximum benefit from these agents,” they wrote.
However, medically necessary corticosteroids should not be avoided, such as those given for management of brain metastases, they added.
Interestingly, other recent studies have suggested that patients already on PD-1/PD-L1 inhibitors do not seem to have decreased efficacy when corticosteroids are prescribed to manage emergent immune-related adverse events.
That’s “fortunate,” given that corticosteroids are a mainstay for the management of these characteristic adverse events in patients receiving immune checkpoint inhibitors, they said.
Dr. Hellmann reported research funding from Bristol-Myers Squibb, and a consulting or advisory role with AstraZeneca, Bristol-Myers Squibb, Genentech, Janssen Pharmaceuticals, MedImmune, Merck, Mirati Therapeutics, Shattuck Labs, and Syndax. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Pfizer, and Roche, among others.
SOURCE: Arbour KC et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.79.0006.
Immune checkpoint inhibitor therapy may be less effective if the patient is receiving corticosteroids at the time that treatment is initiated, results of a retrospective, two-center analysis suggest.
Corticosteroid use at the time of starting a PD-1 or PD-L1 inhibitor was associated with significantly reduced overall survival and progression-free survival in patients with non–small-cell lung cancer, the authors of the analysis reported.
“Prudent use of corticosteroids at the time of initiating PD-1/PD-L1 blockade is warranted,” said Matthew D. Hellmann, MD, of Memorial Sloan Kettering Cancer Center, New York, and his coauthors. The report is in the Journal of Clinical Oncology.
The retrospective analysis from Dr. Hellmann and his colleagues comprised 640 patients treated with single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab at Memorial Sloan Kettering Cancer Center or Gustave Roussy Cancer Center, Villejuif, France, between April 2011 and September 2017.
Ninety of those patients (14%) were receiving at least 10 mg of prednisone equivalent at the time the immune checkpoint inhibitor was started, a review of patient records revealed. About one-third were receiving corticosteroids for dyspnea or other respiratory symptoms. Another 21% had fatigue and 19% had brain metastases prompting corticosteroid treatment.
Corticosteroid use at the time of PD-1/PD-L1 blockade was associated with decreased progression-free survival (hazard ratio, 1.31; P = .03) and overall survival (HR, 1.66; P less than .001), as well as decreased overall response rate in a multivariable analysis adjusted for performance status, history of smoking, and history of brain metastases, the investigators reported.
Reduced efficacy also was seen in analyses that looked at each center separately. For the Memorial Sloan Kettering cohort (455 patients), baseline corticosteroid use was associated with significantly decreased overall response rate, shorter progression-free survival, and shorter overall survival.
For the smaller French cohort, (185 patients), there was a statistically nonsignificant decrease in overall response rate, along with significant reductions in progression-free survival and overall survival.
Based on these data, it may be prudent to try other pharmacologic or nonpharmacologic strategies to manage cancer symptoms if treatment with a PD-1/PD-L1 blocker is planned, Dr. Hellmann and his coauthors said.
“These strategies could enable patients to be tapered off corticosteroids before the start of PD-1/PD-L1 blockade to potentially achieve maximum benefit from these agents,” they wrote.
However, medically necessary corticosteroids should not be avoided, such as those given for management of brain metastases, they added.
Interestingly, other recent studies have suggested that patients already on PD-1/PD-L1 inhibitors do not seem to have decreased efficacy when corticosteroids are prescribed to manage emergent immune-related adverse events.
That’s “fortunate,” given that corticosteroids are a mainstay for the management of these characteristic adverse events in patients receiving immune checkpoint inhibitors, they said.
Dr. Hellmann reported research funding from Bristol-Myers Squibb, and a consulting or advisory role with AstraZeneca, Bristol-Myers Squibb, Genentech, Janssen Pharmaceuticals, MedImmune, Merck, Mirati Therapeutics, Shattuck Labs, and Syndax. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Pfizer, and Roche, among others.
SOURCE: Arbour KC et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.79.0006.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Immune checkpoint inhibitors may be less effective if the patient is receiving corticosteroids at the time treatment is started.
Major finding: Corticosteroid use at the time of PD-1/PD-L1 blockade was associated with decreased progression-free survival (hazard ratio, 1.31; P = .03) and overall survival (HR, 1.66; P less than .001) in multivariate analysis.
Study details: Retrospective analysis of 640 patients treated with atezolizumab, durvalumab, nivolumab, or pembrolizumab at Memorial Sloan Kettering Cancer Center or Gustave Roussy Cancer Center.
Disclosures: The authors reported disclosures related to Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen Pharmaceuticals, MedImmune, Merck, Mirati Therapeutics, Pfizer, Roche, Shattuck Labs, and Syndax, among others.
Source: Arbour KC et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.79.0006.
Prealbumin level predicts outcomes for HCC resection
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Preoperative prealbumin levels independently predicted survival after curative liver resection for hepatocellular carcinoma (HCC) in a recent multicenter, retrospective study.
By contrast, preoperative albumin levels did not predict long-term overall or relapse-free survival in the analysis, which was reported by Tian Yang, MD, and Feng Shen, MD, along with their coinvestigators, in the journal HPB.
Those findings suggest that serum prealbumin is superior to the widely used serum albumin level as a marker of nutritional status and liver function in this setting, according to Dr. Yang and Dr. Shen, who are with the department of hepatobiliary surgery at Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
“The importance of preoperative prealbumin level in predicting long-term prognosis after liver resection for HCC should be given adequate attention by hepatic surgeons,” they wrote in their report.
The retrospective analysis included a total of 1,483 patients with HCC newly diagnosed at one of six medical institutions in China during 2001-2014. Of those patients, 1,046 (71%) had normal prealbumin levels (above 170 mg/L) measured within a week before surgery, while the remaining 437 (29%) had low prealbumin levels.
Overall survival was a mean of 72 months for the low prealbumin group versus 99 months for the normal prealbumin group (P less than .001), with a corresponding 5-year overall survival of 31% versus 43%, respectively, investigators reported
Likewise, relapse-free survival was a mean of 56 months for the low prealbumin group versus 77 months for the normal prealbumin groups (P less than .001), with 5-year relapse-free survival rates of 20% and 28%, respectively.
In multivariable Cox-regression analyses, the hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
By contrast, preoperative albumin level was not an independent predictor of either overall or relapse-free survival in multivariate analyses, according to investigators.
Despite these findings, it remains controversial as to which marker is more accurate as a measure of nutritional status, investigators wrote in their report.
While albumin is more commonly used in clinical practice, they explained, multiple studies have shown prealbumin is more specific and sensitive in evaluating protein malnutrition and liver function.
The present study, although retrospective, is multicenter, has a large sample size, and includes adequately long follow-up. Nevertheless, further studies will be required to determine whether prealbumin could replace albumin for assessments of nutritional status and liver function after curative liver resection for HCC, investigators concluded.
The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. Dr. Yang, Dr. Shen, and their coauthors had no conflicts of interest to disclose.
SOURCE: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
FROM HPB
Key clinical point: Preoperative prealbumin levels independently predicted survival after curative liver resection for HCC, while preoperative albumin levels did not.
Major finding: The hazard ratios of low preoperative prealbumin level for risk of decreased overall survival and for risk of decreased relapse-free survival were 1.45 (95% confidence interval, 1.24-1.70) and 1.28 (95% CI, 1.10-1.48), respectively.
Study details: Retrospective analysis that included 1,483 patients with HCC newly diagnosed at one of six institutions in China during 2001-2014.
Disclosures: The research was supported in part by the National Natural Science Foundation of China and the Shanghai Pujiang Program. The study authors had no conflicts of interest to disclose.
Source: Li J-D et al. HPB (Oxford). 2018 Aug 3. doi: 10.1016/j.hpb.2018.06.1803.
Mailing out fecal tests may improve CRC screening rates
Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening in community health centers, results of a recent randomized trial show.
Outreach by mail led to a 3.4–percentage point increase in completion of FIT, compared with clinics who did not participate in the intervention, according to results of the randomized Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) trial.
Although that difference was statistically significant, investigators said the improvement was less than expected based on previous experience, including a pilot study showing that the strategy of mailing fecal tests boosted completion rates by 38%.
Based on that discrepancy, additional strategies may be needed to support implementation of FIT mailing programs in low-resource health centers, reported Gloria D. Coronado, PhD, Kaiser Permanente Center for Health Research, Portland, Ore., and coinvestigators.
“This work demonstrates that mailed FIT outreach programs can have clinical impact when integrated into clinical work flows, but emphasizes the need to identify additional strategies to support program implementation in low-resource health centers,” Dr. Coronado and coauthors said in JAMA Internal Medicine.
The STOP CRC study included 26 federally qualified health center clinics serving low-income populations in Oregon and California. Investigators identified a total of 41,193 adults overdue for colorectal cancer screening between Feb. 4, 2014 and Feb. 3, 2015.
The core of the intervention was a set of electronic health record–embedded tools that identified adults due for screening and allowed staff to generate letters and mailing labels for a series of three mailings. The first mailing was an introductory letter, the second was a FIT kit packet that included wordless instructions, and the third was a reminder letter.
For clinics that participated in the intervention, the rate of FIT completion was 13.9%, versus 10.4%, a difference that was statistically significant (95% confidence interval, 0.1%-6.8%; P = .047), according to investigators. Likewise, the proportion of participants completing any CRC screening was significantly higher in the intervention clinics (18.3% versus 14.5%; 3.8 percentage points difference; 95% CI, 0.6%-7.0%; P = .024).
Somewhat larger effects were seen in an analysis that accounted for delays in implementation of the program. In that analysis, FIT completion rates were 17.6% for the intervention clinics and 12.8% for the usual care clinics (95% CI, 0.9-8.6%; P = .020), with similar increases seen in the proportion of patients receiving any CRC screening.
These increases in screening occurred despite “relatively low” implementation of the program, Dr. Coronado and colleagues said.
In the pilot study, a concerted effort was made to ensure all eligible adults got the intervention; in this study, 6,925 out of 21,134 intervention participants (33%) got an introductory letter, and of those, 91% received the FIT and 59% got the reminder letter.
Implementation varied widely by health center, ranging from 6.5% to 68.2%, investigators said in their report.
One reason for low implementation may be that the program competed with other priorities in the clinics. In interviews, health center leaders said challenges in the clinic included time burden, limited organizational capacity, and challenges with the EHR and associated reporting tools.
“For most participating health centers, STOP CRC represented the first time EHR tools were used to deliver cancer screening services outside the clinic,” Dr. Coronado said. “Implementation might have increased with experience.”
The research reported by Dr. Coronado and coinvestigators was supported by the National Institutes of Health. Dr. Coronado reported serving as a coinvestigator on a study of an experimental blood test for colorectal cancer funded by EpiGenomics and as principal investigator on a study of an experimental FIT funded by Quidel Corporation. No other disclosures were reported.
SOURCE: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening in community health centers, results of a recent randomized trial show.
Outreach by mail led to a 3.4–percentage point increase in completion of FIT, compared with clinics who did not participate in the intervention, according to results of the randomized Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) trial.
Although that difference was statistically significant, investigators said the improvement was less than expected based on previous experience, including a pilot study showing that the strategy of mailing fecal tests boosted completion rates by 38%.
Based on that discrepancy, additional strategies may be needed to support implementation of FIT mailing programs in low-resource health centers, reported Gloria D. Coronado, PhD, Kaiser Permanente Center for Health Research, Portland, Ore., and coinvestigators.
“This work demonstrates that mailed FIT outreach programs can have clinical impact when integrated into clinical work flows, but emphasizes the need to identify additional strategies to support program implementation in low-resource health centers,” Dr. Coronado and coauthors said in JAMA Internal Medicine.
The STOP CRC study included 26 federally qualified health center clinics serving low-income populations in Oregon and California. Investigators identified a total of 41,193 adults overdue for colorectal cancer screening between Feb. 4, 2014 and Feb. 3, 2015.
The core of the intervention was a set of electronic health record–embedded tools that identified adults due for screening and allowed staff to generate letters and mailing labels for a series of three mailings. The first mailing was an introductory letter, the second was a FIT kit packet that included wordless instructions, and the third was a reminder letter.
For clinics that participated in the intervention, the rate of FIT completion was 13.9%, versus 10.4%, a difference that was statistically significant (95% confidence interval, 0.1%-6.8%; P = .047), according to investigators. Likewise, the proportion of participants completing any CRC screening was significantly higher in the intervention clinics (18.3% versus 14.5%; 3.8 percentage points difference; 95% CI, 0.6%-7.0%; P = .024).
Somewhat larger effects were seen in an analysis that accounted for delays in implementation of the program. In that analysis, FIT completion rates were 17.6% for the intervention clinics and 12.8% for the usual care clinics (95% CI, 0.9-8.6%; P = .020), with similar increases seen in the proportion of patients receiving any CRC screening.
These increases in screening occurred despite “relatively low” implementation of the program, Dr. Coronado and colleagues said.
In the pilot study, a concerted effort was made to ensure all eligible adults got the intervention; in this study, 6,925 out of 21,134 intervention participants (33%) got an introductory letter, and of those, 91% received the FIT and 59% got the reminder letter.
Implementation varied widely by health center, ranging from 6.5% to 68.2%, investigators said in their report.
One reason for low implementation may be that the program competed with other priorities in the clinics. In interviews, health center leaders said challenges in the clinic included time burden, limited organizational capacity, and challenges with the EHR and associated reporting tools.
“For most participating health centers, STOP CRC represented the first time EHR tools were used to deliver cancer screening services outside the clinic,” Dr. Coronado said. “Implementation might have increased with experience.”
The research reported by Dr. Coronado and coinvestigators was supported by the National Institutes of Health. Dr. Coronado reported serving as a coinvestigator on a study of an experimental blood test for colorectal cancer funded by EpiGenomics and as principal investigator on a study of an experimental FIT funded by Quidel Corporation. No other disclosures were reported.
SOURCE: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening in community health centers, results of a recent randomized trial show.
Outreach by mail led to a 3.4–percentage point increase in completion of FIT, compared with clinics who did not participate in the intervention, according to results of the randomized Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) trial.
Although that difference was statistically significant, investigators said the improvement was less than expected based on previous experience, including a pilot study showing that the strategy of mailing fecal tests boosted completion rates by 38%.
Based on that discrepancy, additional strategies may be needed to support implementation of FIT mailing programs in low-resource health centers, reported Gloria D. Coronado, PhD, Kaiser Permanente Center for Health Research, Portland, Ore., and coinvestigators.
“This work demonstrates that mailed FIT outreach programs can have clinical impact when integrated into clinical work flows, but emphasizes the need to identify additional strategies to support program implementation in low-resource health centers,” Dr. Coronado and coauthors said in JAMA Internal Medicine.
The STOP CRC study included 26 federally qualified health center clinics serving low-income populations in Oregon and California. Investigators identified a total of 41,193 adults overdue for colorectal cancer screening between Feb. 4, 2014 and Feb. 3, 2015.
The core of the intervention was a set of electronic health record–embedded tools that identified adults due for screening and allowed staff to generate letters and mailing labels for a series of three mailings. The first mailing was an introductory letter, the second was a FIT kit packet that included wordless instructions, and the third was a reminder letter.
For clinics that participated in the intervention, the rate of FIT completion was 13.9%, versus 10.4%, a difference that was statistically significant (95% confidence interval, 0.1%-6.8%; P = .047), according to investigators. Likewise, the proportion of participants completing any CRC screening was significantly higher in the intervention clinics (18.3% versus 14.5%; 3.8 percentage points difference; 95% CI, 0.6%-7.0%; P = .024).
Somewhat larger effects were seen in an analysis that accounted for delays in implementation of the program. In that analysis, FIT completion rates were 17.6% for the intervention clinics and 12.8% for the usual care clinics (95% CI, 0.9-8.6%; P = .020), with similar increases seen in the proportion of patients receiving any CRC screening.
These increases in screening occurred despite “relatively low” implementation of the program, Dr. Coronado and colleagues said.
In the pilot study, a concerted effort was made to ensure all eligible adults got the intervention; in this study, 6,925 out of 21,134 intervention participants (33%) got an introductory letter, and of those, 91% received the FIT and 59% got the reminder letter.
Implementation varied widely by health center, ranging from 6.5% to 68.2%, investigators said in their report.
One reason for low implementation may be that the program competed with other priorities in the clinics. In interviews, health center leaders said challenges in the clinic included time burden, limited organizational capacity, and challenges with the EHR and associated reporting tools.
“For most participating health centers, STOP CRC represented the first time EHR tools were used to deliver cancer screening services outside the clinic,” Dr. Coronado said. “Implementation might have increased with experience.”
The research reported by Dr. Coronado and coinvestigators was supported by the National Institutes of Health. Dr. Coronado reported serving as a coinvestigator on a study of an experimental blood test for colorectal cancer funded by EpiGenomics and as principal investigator on a study of an experimental FIT funded by Quidel Corporation. No other disclosures were reported.
SOURCE: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Mailing fecal immunochemical tests (FITs) to overdue patients improved the rate of colorectal cancer screening, though not to the extent that had been seen in a pilot study.
Major finding: Outreach by mail led to a 3.4–percentage point increase in FIT completion for participating clinics versus clinics that implemented usual care.
Study details: STOP CRC, a cluster-randomized pragmatic clinical trial including 26 federally qualified health center clinics and a total of 41,193 adults overdue for colorectal cancer screening.
Disclosures: The research was supported by the National Institutes of Health. One investigator reported disclosures related to EpiGenomics and Quidel Corporation.
Source: Coronado GD et al. JAMA Intern Med. 2018 Aug 6. doi: 10.1001/jamainternmed.2018.3629.
Signal strength tied to potency of CAR T-cell therapy
Investigators found that chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model.
Intracellular signaling strength was a key determinant of T-cell fate in the study, which was published in Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
These findings suggest tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center in Seattle, Wash.
For this study, Salter and his colleagues used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T-cell signaling pathways, Salter said.
That overlap was somewhat surprising, according to Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor but the speed and strength of signaling, he added.
In particular, the CARs that evoked stronger signals also had increased T-cell dysfunction, decreasing their potency in the mouse model of lymphoma.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model.
By contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, Salter said.
These findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense.
“This is a modification that we think should be considered in future CAR design,” Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at efficacy, noted Stanley Riddell, MD, of Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said.
“But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics. He and Salter have filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
Investigators found that chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model.
Intracellular signaling strength was a key determinant of T-cell fate in the study, which was published in Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
These findings suggest tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center in Seattle, Wash.
For this study, Salter and his colleagues used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T-cell signaling pathways, Salter said.
That overlap was somewhat surprising, according to Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor but the speed and strength of signaling, he added.
In particular, the CARs that evoked stronger signals also had increased T-cell dysfunction, decreasing their potency in the mouse model of lymphoma.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model.
By contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, Salter said.
These findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense.
“This is a modification that we think should be considered in future CAR design,” Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at efficacy, noted Stanley Riddell, MD, of Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said.
“But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics. He and Salter have filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
Investigators found that chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model.
Intracellular signaling strength was a key determinant of T-cell fate in the study, which was published in Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
These findings suggest tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center in Seattle, Wash.
For this study, Salter and his colleagues used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T-cell signaling pathways, Salter said.
That overlap was somewhat surprising, according to Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor but the speed and strength of signaling, he added.
In particular, the CARs that evoked stronger signals also had increased T-cell dysfunction, decreasing their potency in the mouse model of lymphoma.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model.
By contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, Salter said.
These findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense.
“This is a modification that we think should be considered in future CAR design,” Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at efficacy, noted Stanley Riddell, MD, of Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said.
“But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics. He and Salter have filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
Signal strength may limit potency of CAR T-cell therapy
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
Contrary to what might be expected, chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model, investigators reported.
Intracellular signaling strength was a key determinant of T cell fate in the study, which was published in the journal Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
Based on those findings, tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center, Seattle, Wash.
In a press conference, Mr. Salter described results of the study, which used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T cell signaling pathways, Mr. Salter said.
That overlap was somewhat surprising, according to Mr. Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Mr. Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor, but the speed and strength of signaling, he added. In particular, the CARs that evoked stronger signals also had increased T cell dysfunction, decreasing their potency in the mouse lymphoma model.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model of lymphoma; by contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, he said.
Those findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Mr. Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense. “This is a modification that we think should be considered in future CAR design,” Mr. Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at the efficacy, noted Stanley Riddell, MD, scientific director of the Immunotherapy Integrated Research Center at Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said during the press conference. “But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics, and together with Mr. Salter, he has filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
SOURCE: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
FROM SCIENCE SIGNALING
Key clinical point:
Major finding: T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in a mouse model of lymphoma, while the 4-1BB CAR signal led to T cells that better retained their function in vivo and had a longer median survival in the model.
Study details: Analysis of CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells using mass spectrometry, plus analysis of efficacy in a mouse model of lymphoma.
Disclosures: Study authors reported disclosures related to Juno therapeutics and a patent application related to use of mutant CD28 CARs for cellular therapy.
Source: Salter AI et al., Sci Signal. 2018 Aug 21;11. pii:eaat6753.
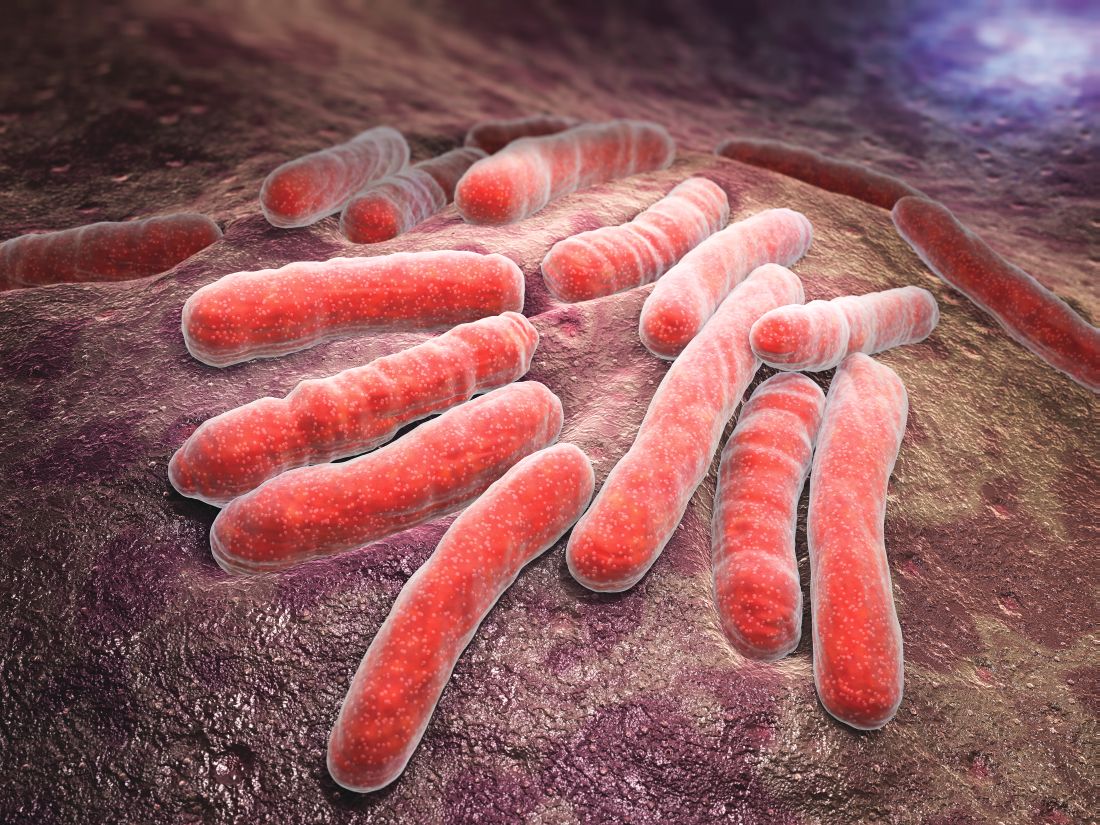
Better adherence, shorter course with rifampin for tuberculosis
Four months of rifampin is effective in prevention of active tuberculosis, with significantly higher adherence rates versus 9 months of isoniazid in adults and children, a pair of recent studies suggest.
In one randomized, open-label trial that included adults with latent Mycobacterium tuberculosis infection, the 4-month rifampin regimen was not inferior to the 9-month isoniazid regimen in preventing active tuberculosis, had better safety, and had a rate of treatment completion 15.1 percentage points higher than the comparator.
“This trial adds to the mounting evidence of benefits of rifamycin-containing regimens of 3 or 4 months’ duration,” investigators reported in the New England Journal of Medicine.
Similarly, in an open-label study in children with latent M. tuberculosis infection, the shorter rifampin regimen had comparable efficacy and safety, according to investigators, along with a rate of treatment completion 13.4 percentage points higher than the longer isoniazid regimen.
“Rifampin has the advantage of being a single-drug regimen with existing palatable formulations for children,” reported authors of this companion study, also published in the journal.
Treatment challenges
Treating latent tuberculosis infection is central to the World Health Organization End TB Strategy and other tuberculosis elimination plans. An estimated 1.7 billion individuals, or about one-quarter of the global population, harbor latent tuberculosis infection, according to one recent estimate.
The WHO recommends treatment of latent tuberculosis infection, as well as for children under 5 years of age who are household contacts of individuals with tuberculosis. The recommended treatment is 6 or 9 months of isoniazid, with the longer duration being associated with better efficacy, previous studies have shown.
However, isoniazid treatment has been associated with low rates of regimen completion because of the hepatotoxic effects, according to authors of the current studies comparing isoniazid to rifampin.
The 4-month daily rifampin regimen has been associated with superior treatment adherence rates and fewer hepatotoxic effects, compared with the 9-month isoniazid regimen in previous observational studies. Moreover, an earlier randomized trial including 679 men in Hong Kong demonstrated that 3 months of rifampin was superior to placebo and comparable to 6 months of isoniazid as tuberculosis prophylaxis.
Rifampin: Latest data
The adult trial just published in the New England Journal of Medicine demonstrates the efficacy and real-world effectiveness of the 4-month rifampin regimen versus the 9-month isoniazid regimen for prevention of active tuberculosis, according to lead author Dick Menzies, MD, of the Montreal Chest Institute at McGill University Health Centre.
The 4-month rifampin regimen is a “fundamental game-changer in TB prevention” based on its comparable efficacy is adults, along with improved safety and acceptability, Dr. Menzies said in a recent press release.
Dr. Menzies and his colleagues reported on 6,063 adults (aged 18 years or older) randomized to the 4-month rifampin or 9-month isoniazid regimen at trial sites in Australia, Benin, Brazil, Canada, Ghana, Guinea, Indonesia, Saudi Arabia, and South Korea.
Treatment was completed by 78.8% of individuals in the rifampin arm, compared with 63.2% of patients in the isoniazid arm, for a difference of 15.1 percentage points (95% confidence interval, 12.7-17.4; P less than .001), the researchers reported.
Rifampin was not inferior to isoniazid in preventing tuberculosis, according to the report. In the per-protocol analysis, there were a total of five confirmed or clinically diagnosed cases of active tuberculosis in each of the trial arms. All active cases were treated successfully, including two cases that had demonstrated drug resistance, investigators added.
The rifampin group had consistently lower rates of grade 3-grade 5 adverse events, particularly hepatotoxic events, versus the isoniazid group, according to analyses outlined in the report.
“We believe this 4-month rifampin treatment should replace the 9 months on isoniazid for most people who need therapy for latent tuberculosis,” said Dr. Menzies, a respirologist with the Montreal Chest Institute and a professor of medicine, epidemiology and biostatistics at McGill University, also in Montreal.
Experience in children
In the related study, reported by lead author Thierno Diallo, MD, of Hôpital National Ignace Deen, in Conakry, Guinea, along with Dr. Menzies, and their coauthors, 829 children were randomized to 4 months of rifampin or 9 months of isoniazid.
The study population included 79 children under 2 years, the age group that has the highest risk of life-threatening TB, Dr. Diallo and his colleagues wrote in their report.
Treatment was completed in 86.5% of all children randomized to rifampin, compared with 77.1% in the isoniazid arm (difference of 13.6 percentage points; 95% confidence interval, 7.9-19.3; P less than .001), according to the investigators.
Two active tuberculosis cases were diagnosed in the isoniazid group over 542 person-years of follow-up, versus no cases in the rifampin group over a similar follow-up period.
“Although the only cases of active tuberculosis were diagnosed in the isoniazid group, we cannot conclude that 4 months of rifampin was either superior or noninferior to 9 months of isoniazid for the prevention of active tuberculosis,” the authors wrote.
“However, since there were no cases of active tuberculosis in the rifampin group in our trial or among 434 children who received 3 months of once-weekly isoniazid plus rifapentine in another trial, we suggest that these shorter rifamycin containing regimens are effective,” they added.
In contrast to the adult trial, safety profiles in this study were similar for rifampin and isoniazid, investigators said.
The lack of difference is side effects was possibly because of the differences in the pharmacokinetic activity of rifampin in younger patients, a topic that deserves further study, they concluded.
No potential conflicts of interest relevant to the studies were reported by Dr. Menzies, Dr. Diallo, or their coauthors.
Both studies were supported by grants from the Canadian Institutes of Health Research. The adult study was supported in part by a grant from the Australian National Health and Medical Research Council, while the companion study in children was supported in part by a grant from the Conselho Nacional de Pesquisa in Brazil.
SOURCES: Menzies D et al. N Engl J Med. 2018 Aug 2;379(5):440-53; Diallo T et al. N Engl J Med. 2018 Aug 2;379(5):454-63.
Four months of rifampin is effective in prevention of active tuberculosis, with significantly higher adherence rates versus 9 months of isoniazid in adults and children, a pair of recent studies suggest.
In one randomized, open-label trial that included adults with latent Mycobacterium tuberculosis infection, the 4-month rifampin regimen was not inferior to the 9-month isoniazid regimen in preventing active tuberculosis, had better safety, and had a rate of treatment completion 15.1 percentage points higher than the comparator.
“This trial adds to the mounting evidence of benefits of rifamycin-containing regimens of 3 or 4 months’ duration,” investigators reported in the New England Journal of Medicine.
Similarly, in an open-label study in children with latent M. tuberculosis infection, the shorter rifampin regimen had comparable efficacy and safety, according to investigators, along with a rate of treatment completion 13.4 percentage points higher than the longer isoniazid regimen.
“Rifampin has the advantage of being a single-drug regimen with existing palatable formulations for children,” reported authors of this companion study, also published in the journal.
Treatment challenges
Treating latent tuberculosis infection is central to the World Health Organization End TB Strategy and other tuberculosis elimination plans. An estimated 1.7 billion individuals, or about one-quarter of the global population, harbor latent tuberculosis infection, according to one recent estimate.
The WHO recommends treatment of latent tuberculosis infection, as well as for children under 5 years of age who are household contacts of individuals with tuberculosis. The recommended treatment is 6 or 9 months of isoniazid, with the longer duration being associated with better efficacy, previous studies have shown.
However, isoniazid treatment has been associated with low rates of regimen completion because of the hepatotoxic effects, according to authors of the current studies comparing isoniazid to rifampin.
The 4-month daily rifampin regimen has been associated with superior treatment adherence rates and fewer hepatotoxic effects, compared with the 9-month isoniazid regimen in previous observational studies. Moreover, an earlier randomized trial including 679 men in Hong Kong demonstrated that 3 months of rifampin was superior to placebo and comparable to 6 months of isoniazid as tuberculosis prophylaxis.
Rifampin: Latest data
The adult trial just published in the New England Journal of Medicine demonstrates the efficacy and real-world effectiveness of the 4-month rifampin regimen versus the 9-month isoniazid regimen for prevention of active tuberculosis, according to lead author Dick Menzies, MD, of the Montreal Chest Institute at McGill University Health Centre.
The 4-month rifampin regimen is a “fundamental game-changer in TB prevention” based on its comparable efficacy is adults, along with improved safety and acceptability, Dr. Menzies said in a recent press release.
Dr. Menzies and his colleagues reported on 6,063 adults (aged 18 years or older) randomized to the 4-month rifampin or 9-month isoniazid regimen at trial sites in Australia, Benin, Brazil, Canada, Ghana, Guinea, Indonesia, Saudi Arabia, and South Korea.
Treatment was completed by 78.8% of individuals in the rifampin arm, compared with 63.2% of patients in the isoniazid arm, for a difference of 15.1 percentage points (95% confidence interval, 12.7-17.4; P less than .001), the researchers reported.
Rifampin was not inferior to isoniazid in preventing tuberculosis, according to the report. In the per-protocol analysis, there were a total of five confirmed or clinically diagnosed cases of active tuberculosis in each of the trial arms. All active cases were treated successfully, including two cases that had demonstrated drug resistance, investigators added.
The rifampin group had consistently lower rates of grade 3-grade 5 adverse events, particularly hepatotoxic events, versus the isoniazid group, according to analyses outlined in the report.
“We believe this 4-month rifampin treatment should replace the 9 months on isoniazid for most people who need therapy for latent tuberculosis,” said Dr. Menzies, a respirologist with the Montreal Chest Institute and a professor of medicine, epidemiology and biostatistics at McGill University, also in Montreal.
Experience in children
In the related study, reported by lead author Thierno Diallo, MD, of Hôpital National Ignace Deen, in Conakry, Guinea, along with Dr. Menzies, and their coauthors, 829 children were randomized to 4 months of rifampin or 9 months of isoniazid.
The study population included 79 children under 2 years, the age group that has the highest risk of life-threatening TB, Dr. Diallo and his colleagues wrote in their report.
Treatment was completed in 86.5% of all children randomized to rifampin, compared with 77.1% in the isoniazid arm (difference of 13.6 percentage points; 95% confidence interval, 7.9-19.3; P less than .001), according to the investigators.
Two active tuberculosis cases were diagnosed in the isoniazid group over 542 person-years of follow-up, versus no cases in the rifampin group over a similar follow-up period.
“Although the only cases of active tuberculosis were diagnosed in the isoniazid group, we cannot conclude that 4 months of rifampin was either superior or noninferior to 9 months of isoniazid for the prevention of active tuberculosis,” the authors wrote.
“However, since there were no cases of active tuberculosis in the rifampin group in our trial or among 434 children who received 3 months of once-weekly isoniazid plus rifapentine in another trial, we suggest that these shorter rifamycin containing regimens are effective,” they added.
In contrast to the adult trial, safety profiles in this study were similar for rifampin and isoniazid, investigators said.
The lack of difference is side effects was possibly because of the differences in the pharmacokinetic activity of rifampin in younger patients, a topic that deserves further study, they concluded.
No potential conflicts of interest relevant to the studies were reported by Dr. Menzies, Dr. Diallo, or their coauthors.
Both studies were supported by grants from the Canadian Institutes of Health Research. The adult study was supported in part by a grant from the Australian National Health and Medical Research Council, while the companion study in children was supported in part by a grant from the Conselho Nacional de Pesquisa in Brazil.
SOURCES: Menzies D et al. N Engl J Med. 2018 Aug 2;379(5):440-53; Diallo T et al. N Engl J Med. 2018 Aug 2;379(5):454-63.
Four months of rifampin is effective in prevention of active tuberculosis, with significantly higher adherence rates versus 9 months of isoniazid in adults and children, a pair of recent studies suggest.
In one randomized, open-label trial that included adults with latent Mycobacterium tuberculosis infection, the 4-month rifampin regimen was not inferior to the 9-month isoniazid regimen in preventing active tuberculosis, had better safety, and had a rate of treatment completion 15.1 percentage points higher than the comparator.
“This trial adds to the mounting evidence of benefits of rifamycin-containing regimens of 3 or 4 months’ duration,” investigators reported in the New England Journal of Medicine.
Similarly, in an open-label study in children with latent M. tuberculosis infection, the shorter rifampin regimen had comparable efficacy and safety, according to investigators, along with a rate of treatment completion 13.4 percentage points higher than the longer isoniazid regimen.
“Rifampin has the advantage of being a single-drug regimen with existing palatable formulations for children,” reported authors of this companion study, also published in the journal.
Treatment challenges
Treating latent tuberculosis infection is central to the World Health Organization End TB Strategy and other tuberculosis elimination plans. An estimated 1.7 billion individuals, or about one-quarter of the global population, harbor latent tuberculosis infection, according to one recent estimate.
The WHO recommends treatment of latent tuberculosis infection, as well as for children under 5 years of age who are household contacts of individuals with tuberculosis. The recommended treatment is 6 or 9 months of isoniazid, with the longer duration being associated with better efficacy, previous studies have shown.
However, isoniazid treatment has been associated with low rates of regimen completion because of the hepatotoxic effects, according to authors of the current studies comparing isoniazid to rifampin.
The 4-month daily rifampin regimen has been associated with superior treatment adherence rates and fewer hepatotoxic effects, compared with the 9-month isoniazid regimen in previous observational studies. Moreover, an earlier randomized trial including 679 men in Hong Kong demonstrated that 3 months of rifampin was superior to placebo and comparable to 6 months of isoniazid as tuberculosis prophylaxis.
Rifampin: Latest data
The adult trial just published in the New England Journal of Medicine demonstrates the efficacy and real-world effectiveness of the 4-month rifampin regimen versus the 9-month isoniazid regimen for prevention of active tuberculosis, according to lead author Dick Menzies, MD, of the Montreal Chest Institute at McGill University Health Centre.
The 4-month rifampin regimen is a “fundamental game-changer in TB prevention” based on its comparable efficacy is adults, along with improved safety and acceptability, Dr. Menzies said in a recent press release.
Dr. Menzies and his colleagues reported on 6,063 adults (aged 18 years or older) randomized to the 4-month rifampin or 9-month isoniazid regimen at trial sites in Australia, Benin, Brazil, Canada, Ghana, Guinea, Indonesia, Saudi Arabia, and South Korea.
Treatment was completed by 78.8% of individuals in the rifampin arm, compared with 63.2% of patients in the isoniazid arm, for a difference of 15.1 percentage points (95% confidence interval, 12.7-17.4; P less than .001), the researchers reported.
Rifampin was not inferior to isoniazid in preventing tuberculosis, according to the report. In the per-protocol analysis, there were a total of five confirmed or clinically diagnosed cases of active tuberculosis in each of the trial arms. All active cases were treated successfully, including two cases that had demonstrated drug resistance, investigators added.
The rifampin group had consistently lower rates of grade 3-grade 5 adverse events, particularly hepatotoxic events, versus the isoniazid group, according to analyses outlined in the report.
“We believe this 4-month rifampin treatment should replace the 9 months on isoniazid for most people who need therapy for latent tuberculosis,” said Dr. Menzies, a respirologist with the Montreal Chest Institute and a professor of medicine, epidemiology and biostatistics at McGill University, also in Montreal.
Experience in children
In the related study, reported by lead author Thierno Diallo, MD, of Hôpital National Ignace Deen, in Conakry, Guinea, along with Dr. Menzies, and their coauthors, 829 children were randomized to 4 months of rifampin or 9 months of isoniazid.
The study population included 79 children under 2 years, the age group that has the highest risk of life-threatening TB, Dr. Diallo and his colleagues wrote in their report.
Treatment was completed in 86.5% of all children randomized to rifampin, compared with 77.1% in the isoniazid arm (difference of 13.6 percentage points; 95% confidence interval, 7.9-19.3; P less than .001), according to the investigators.
Two active tuberculosis cases were diagnosed in the isoniazid group over 542 person-years of follow-up, versus no cases in the rifampin group over a similar follow-up period.
“Although the only cases of active tuberculosis were diagnosed in the isoniazid group, we cannot conclude that 4 months of rifampin was either superior or noninferior to 9 months of isoniazid for the prevention of active tuberculosis,” the authors wrote.
“However, since there were no cases of active tuberculosis in the rifampin group in our trial or among 434 children who received 3 months of once-weekly isoniazid plus rifapentine in another trial, we suggest that these shorter rifamycin containing regimens are effective,” they added.
In contrast to the adult trial, safety profiles in this study were similar for rifampin and isoniazid, investigators said.
The lack of difference is side effects was possibly because of the differences in the pharmacokinetic activity of rifampin in younger patients, a topic that deserves further study, they concluded.
No potential conflicts of interest relevant to the studies were reported by Dr. Menzies, Dr. Diallo, or their coauthors.
Both studies were supported by grants from the Canadian Institutes of Health Research. The adult study was supported in part by a grant from the Australian National Health and Medical Research Council, while the companion study in children was supported in part by a grant from the Conselho Nacional de Pesquisa in Brazil.
SOURCES: Menzies D et al. N Engl J Med. 2018 Aug 2;379(5):440-53; Diallo T et al. N Engl J Med. 2018 Aug 2;379(5):454-63.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Four months of rifampin is effective in prevention of active tuberculosis, with significantly higher adherence rates versus 9 months of isoniazid in adults and children.
Major finding: Treatment was completed by 78.8% and 63.2% of adults randomized to rifampin or isoniazid, respectively.
Study details: A randomized, open-label trial including 6,063 randomized adults, and a companion study including 829 children, with latent Mycobacterium tuberculosis infection.
Disclosures: Authors reported no potential conflicts of interest relevant to the research. Support for the research came from the Canadian Institutes of Health Research, the Australian National Health and Medical Research Council, and the Conselho Nacional de Pesquisa in Brazil.
Sources: Menzies D et al. N Engl J Med. 2018 Aug 2;379(5):440-53; Diallo T, et al. N Engl J Med. 2018 Aug 2;379(5):454-63.
Many physicians don’t discuss MenB vaccine in teen well visits
survey results showed.
About half of pediatricians and one-third of family physicians said they always or often initiate discussion of MenB vaccines for adolescents aged 16- 18 year, according to a report on the survey published in Pediatrics.
However, it is challenging to say whether or not that level of discussion is on track with ideal clinical practice, according to Allison Kempe, MD, MPH, of the University of Colorado at Denver and Children’s Hospital Colorado, Aurora, and her coauthors. While MenB vaccines are recommended in this setting, the new Category B designation used for the recommendation indicates that the vaccines “may be administered” in the context of individual clinical decision making.
While some interpret the new Category B recommendation to mean that a discussion should always occur, others may interpret the recommendation as applicable to their own assessment of risks and benefits, without the need to involve patients and parents.
“Providers not initiating a discussion may not think the time required to discuss the MenB vaccine is justified by the risks posed by the disease or the benefits offered by these vaccines,” wrote Dr. Kempe and her associates. “Alternatively, they may have a low level of awareness regarding the disease or the MenB vaccine and feel insufficiently knowledgeable to have an informed discussion about the pros and cons of vaccination. They also may have been entirely unaware of the ACIP [Advisory Committee on Immunization Practices] recommendation for MenB vaccination.”
Dr. Kempe and her colleagues invited a nationally representative sample of primary care physicians to complete the survey, which was administered via Internet or mail between October and December 2016. They heard back from 374 of 475 (79%) pediatricians and 286 of 441 (65%) family physicians.
A total of 50% of pediatricians and 31% of family physicians said they always or often discussed MenB vaccines during routine visits with adolescents aged 16-18 years, with slightly higher percentages saying they initiated discussions during precollege physical exams, according to the report. Of the pediatricians, 58% recommended the MenB vaccine to those in this age group, compared with 50% of family physicians. Not all physicians who recommended the vaccine reported consistently initiating a discussion about it.
Nearly three-fourths of pediatricians and 41% of family physicians reported currently administering the MenB vaccine in their practices, the authors said, adding that greater awareness of disease outbreaks was linked to higher likelihood of discussing the vaccine, while working in an HMO setting was linked to lower likelihood of initiating that discussion.
Recommending MenB vaccination was closely tied to discussing the vaccine. Physicians who said they initiated discussion almost always reported making a recommendation to vaccinate, and conversely, those who rarely initiated discussions were unlikely to recommend it, according to Dr. Kempe and her colleagues.
Factors that made physicians more likely to recommend vaccine included awareness of outbreaks, effectiveness and safety data, and duration of vaccine protection.
The Category B recommendation, on the other hand, was one of the key factors that made physicians less likely to recommend MenB vaccine, according to this survey. ACIP made the Category B recommendation in October 2015, stating that those aged 16- 23 years may be vaccinated, with a preferred age of 16-18 years for administration. The accompanying rationale for the Category B designation referenced the low disease prevalence and insufficient data on effectiveness and safety for the two vaccines, which were both licensed under an accelerated approval mechanism following the outbreaks that have occurred on college campuses.
The Centers for Disease Control and Prevention did not provide additional guidance on how that Category B recommendation should be implemented, Dr. Kempe and her coauthors noted in their report.
“With our data, we highlight the challenges providers face with implementing recommendations for vaccination based on individual clinical decision making when they have limited experience with a disease and limited knowledge of a new vaccine,” they wrote.
The research was funded by the CDC. Dr. Kempe and her coauthors reported no relevant financial relationships or potential conflicts of interest.
SOURCE: Kempe A et al. Pediatrics. 2018 Aug 20. doi: 10.1542/peds.2018-0344.
These survey results suggest primary care physicians’ zeal for discussing meningococcal serogroup B (MenB) vaccines during adolescent well visits is affected by the recommendation that they “may be administered” in this setting, according to Michael T. Brady, MD.
“When pediatricians are fortunate to have 16- to 18-year-old patients come to a routine visit, there are many important issues to discuss, such as sexual activity, tobacco, alcohol and illicit drug use, contraception, and mental health,” Dr. Brady wrote in an editorial discussing the survey results.
The new Category B designation, used by the Advisory Committee on Immunization Practices to recommend MenB vaccines for adolescents and young adults not at increased risk for meningococcal B disease, puts the recommendation in the realm of individual clinical decision making, Dr. Brady noted. “Without specific and clear guidance as to how to quantify benefits, risks, and costs for their individual patients, it is easy to understand why providers would have disparate responses reflecting the challenge associated with a new vaccine and a new vaccine recommendation classification.”
Pediatricians can achieve “exceptional rates of immunization” when recommendations are “evidence based, clear, and unequivocal,” but by contrast, they will remain challenged by Category B or permissive recommendations when clear guidance on how to implement the recommendation is not provided, he concluded.
Dr. Brad is a pediatric infectious diseases specialist at Nationwide Children’s Hospital and the Ohio State University, Columbus. These comments are from his editorial in Pediatrics (2018 Aug 20. doi: 10.1542/peds.2018-1633) . Dr. Brady reported receiving royalties from Up-To-Date for a chapter on human herpesvirus 6, but received no external funding for this editorial. He reported no potential conflicts of interest.
These survey results suggest primary care physicians’ zeal for discussing meningococcal serogroup B (MenB) vaccines during adolescent well visits is affected by the recommendation that they “may be administered” in this setting, according to Michael T. Brady, MD.
“When pediatricians are fortunate to have 16- to 18-year-old patients come to a routine visit, there are many important issues to discuss, such as sexual activity, tobacco, alcohol and illicit drug use, contraception, and mental health,” Dr. Brady wrote in an editorial discussing the survey results.
The new Category B designation, used by the Advisory Committee on Immunization Practices to recommend MenB vaccines for adolescents and young adults not at increased risk for meningococcal B disease, puts the recommendation in the realm of individual clinical decision making, Dr. Brady noted. “Without specific and clear guidance as to how to quantify benefits, risks, and costs for their individual patients, it is easy to understand why providers would have disparate responses reflecting the challenge associated with a new vaccine and a new vaccine recommendation classification.”
Pediatricians can achieve “exceptional rates of immunization” when recommendations are “evidence based, clear, and unequivocal,” but by contrast, they will remain challenged by Category B or permissive recommendations when clear guidance on how to implement the recommendation is not provided, he concluded.
Dr. Brad is a pediatric infectious diseases specialist at Nationwide Children’s Hospital and the Ohio State University, Columbus. These comments are from his editorial in Pediatrics (2018 Aug 20. doi: 10.1542/peds.2018-1633) . Dr. Brady reported receiving royalties from Up-To-Date for a chapter on human herpesvirus 6, but received no external funding for this editorial. He reported no potential conflicts of interest.
These survey results suggest primary care physicians’ zeal for discussing meningococcal serogroup B (MenB) vaccines during adolescent well visits is affected by the recommendation that they “may be administered” in this setting, according to Michael T. Brady, MD.
“When pediatricians are fortunate to have 16- to 18-year-old patients come to a routine visit, there are many important issues to discuss, such as sexual activity, tobacco, alcohol and illicit drug use, contraception, and mental health,” Dr. Brady wrote in an editorial discussing the survey results.
The new Category B designation, used by the Advisory Committee on Immunization Practices to recommend MenB vaccines for adolescents and young adults not at increased risk for meningococcal B disease, puts the recommendation in the realm of individual clinical decision making, Dr. Brady noted. “Without specific and clear guidance as to how to quantify benefits, risks, and costs for their individual patients, it is easy to understand why providers would have disparate responses reflecting the challenge associated with a new vaccine and a new vaccine recommendation classification.”
Pediatricians can achieve “exceptional rates of immunization” when recommendations are “evidence based, clear, and unequivocal,” but by contrast, they will remain challenged by Category B or permissive recommendations when clear guidance on how to implement the recommendation is not provided, he concluded.
Dr. Brad is a pediatric infectious diseases specialist at Nationwide Children’s Hospital and the Ohio State University, Columbus. These comments are from his editorial in Pediatrics (2018 Aug 20. doi: 10.1542/peds.2018-1633) . Dr. Brady reported receiving royalties from Up-To-Date for a chapter on human herpesvirus 6, but received no external funding for this editorial. He reported no potential conflicts of interest.
survey results showed.
About half of pediatricians and one-third of family physicians said they always or often initiate discussion of MenB vaccines for adolescents aged 16- 18 year, according to a report on the survey published in Pediatrics.
However, it is challenging to say whether or not that level of discussion is on track with ideal clinical practice, according to Allison Kempe, MD, MPH, of the University of Colorado at Denver and Children’s Hospital Colorado, Aurora, and her coauthors. While MenB vaccines are recommended in this setting, the new Category B designation used for the recommendation indicates that the vaccines “may be administered” in the context of individual clinical decision making.
While some interpret the new Category B recommendation to mean that a discussion should always occur, others may interpret the recommendation as applicable to their own assessment of risks and benefits, without the need to involve patients and parents.
“Providers not initiating a discussion may not think the time required to discuss the MenB vaccine is justified by the risks posed by the disease or the benefits offered by these vaccines,” wrote Dr. Kempe and her associates. “Alternatively, they may have a low level of awareness regarding the disease or the MenB vaccine and feel insufficiently knowledgeable to have an informed discussion about the pros and cons of vaccination. They also may have been entirely unaware of the ACIP [Advisory Committee on Immunization Practices] recommendation for MenB vaccination.”
Dr. Kempe and her colleagues invited a nationally representative sample of primary care physicians to complete the survey, which was administered via Internet or mail between October and December 2016. They heard back from 374 of 475 (79%) pediatricians and 286 of 441 (65%) family physicians.
A total of 50% of pediatricians and 31% of family physicians said they always or often discussed MenB vaccines during routine visits with adolescents aged 16-18 years, with slightly higher percentages saying they initiated discussions during precollege physical exams, according to the report. Of the pediatricians, 58% recommended the MenB vaccine to those in this age group, compared with 50% of family physicians. Not all physicians who recommended the vaccine reported consistently initiating a discussion about it.
Nearly three-fourths of pediatricians and 41% of family physicians reported currently administering the MenB vaccine in their practices, the authors said, adding that greater awareness of disease outbreaks was linked to higher likelihood of discussing the vaccine, while working in an HMO setting was linked to lower likelihood of initiating that discussion.
Recommending MenB vaccination was closely tied to discussing the vaccine. Physicians who said they initiated discussion almost always reported making a recommendation to vaccinate, and conversely, those who rarely initiated discussions were unlikely to recommend it, according to Dr. Kempe and her colleagues.
Factors that made physicians more likely to recommend vaccine included awareness of outbreaks, effectiveness and safety data, and duration of vaccine protection.
The Category B recommendation, on the other hand, was one of the key factors that made physicians less likely to recommend MenB vaccine, according to this survey. ACIP made the Category B recommendation in October 2015, stating that those aged 16- 23 years may be vaccinated, with a preferred age of 16-18 years for administration. The accompanying rationale for the Category B designation referenced the low disease prevalence and insufficient data on effectiveness and safety for the two vaccines, which were both licensed under an accelerated approval mechanism following the outbreaks that have occurred on college campuses.
The Centers for Disease Control and Prevention did not provide additional guidance on how that Category B recommendation should be implemented, Dr. Kempe and her coauthors noted in their report.
“With our data, we highlight the challenges providers face with implementing recommendations for vaccination based on individual clinical decision making when they have limited experience with a disease and limited knowledge of a new vaccine,” they wrote.
The research was funded by the CDC. Dr. Kempe and her coauthors reported no relevant financial relationships or potential conflicts of interest.
SOURCE: Kempe A et al. Pediatrics. 2018 Aug 20. doi: 10.1542/peds.2018-0344.
survey results showed.
About half of pediatricians and one-third of family physicians said they always or often initiate discussion of MenB vaccines for adolescents aged 16- 18 year, according to a report on the survey published in Pediatrics.
However, it is challenging to say whether or not that level of discussion is on track with ideal clinical practice, according to Allison Kempe, MD, MPH, of the University of Colorado at Denver and Children’s Hospital Colorado, Aurora, and her coauthors. While MenB vaccines are recommended in this setting, the new Category B designation used for the recommendation indicates that the vaccines “may be administered” in the context of individual clinical decision making.
While some interpret the new Category B recommendation to mean that a discussion should always occur, others may interpret the recommendation as applicable to their own assessment of risks and benefits, without the need to involve patients and parents.
“Providers not initiating a discussion may not think the time required to discuss the MenB vaccine is justified by the risks posed by the disease or the benefits offered by these vaccines,” wrote Dr. Kempe and her associates. “Alternatively, they may have a low level of awareness regarding the disease or the MenB vaccine and feel insufficiently knowledgeable to have an informed discussion about the pros and cons of vaccination. They also may have been entirely unaware of the ACIP [Advisory Committee on Immunization Practices] recommendation for MenB vaccination.”
Dr. Kempe and her colleagues invited a nationally representative sample of primary care physicians to complete the survey, which was administered via Internet or mail between October and December 2016. They heard back from 374 of 475 (79%) pediatricians and 286 of 441 (65%) family physicians.
A total of 50% of pediatricians and 31% of family physicians said they always or often discussed MenB vaccines during routine visits with adolescents aged 16-18 years, with slightly higher percentages saying they initiated discussions during precollege physical exams, according to the report. Of the pediatricians, 58% recommended the MenB vaccine to those in this age group, compared with 50% of family physicians. Not all physicians who recommended the vaccine reported consistently initiating a discussion about it.
Nearly three-fourths of pediatricians and 41% of family physicians reported currently administering the MenB vaccine in their practices, the authors said, adding that greater awareness of disease outbreaks was linked to higher likelihood of discussing the vaccine, while working in an HMO setting was linked to lower likelihood of initiating that discussion.
Recommending MenB vaccination was closely tied to discussing the vaccine. Physicians who said they initiated discussion almost always reported making a recommendation to vaccinate, and conversely, those who rarely initiated discussions were unlikely to recommend it, according to Dr. Kempe and her colleagues.
Factors that made physicians more likely to recommend vaccine included awareness of outbreaks, effectiveness and safety data, and duration of vaccine protection.
The Category B recommendation, on the other hand, was one of the key factors that made physicians less likely to recommend MenB vaccine, according to this survey. ACIP made the Category B recommendation in October 2015, stating that those aged 16- 23 years may be vaccinated, with a preferred age of 16-18 years for administration. The accompanying rationale for the Category B designation referenced the low disease prevalence and insufficient data on effectiveness and safety for the two vaccines, which were both licensed under an accelerated approval mechanism following the outbreaks that have occurred on college campuses.
The Centers for Disease Control and Prevention did not provide additional guidance on how that Category B recommendation should be implemented, Dr. Kempe and her coauthors noted in their report.
“With our data, we highlight the challenges providers face with implementing recommendations for vaccination based on individual clinical decision making when they have limited experience with a disease and limited knowledge of a new vaccine,” they wrote.
The research was funded by the CDC. Dr. Kempe and her coauthors reported no relevant financial relationships or potential conflicts of interest.
SOURCE: Kempe A et al. Pediatrics. 2018 Aug 20. doi: 10.1542/peds.2018-0344.
FROM PEDIATRICS
Key clinical point: Many primary care physicians are not discussing serogroup B meningococcal (MenB) vaccines during routine adolescent visits, possibly because of uncertainty about how current recommendations should be implemented.
Major finding: Half of pediatricians and 31% of family physicians said they always or often discussed MenB during routine visits with those aged 16- 18 years. Of the pediatricians, 58% recommended the MenB vaccine to adolescents in this age group, compared with 50% of family physicians.
Study details: A late 2016 survey of a nationally representative sample of 374 pediatricians and 286 family physicians.
Disclosures: The research was funded by the Centers for Disease Control and Prevention. Dr. Kempe and her coauthors reported no relevant financial relationships or potential conflicts of interest.
Source: Kempe A et al. Pediatrics. 2018 Aug 20. doi: 10.1542/peds.2018-0344.
CDC: 2017 worst year yet for drug overdoses
An estimated 72,000 drug overdose deaths occurred in 2017 in the United States, making it the worst year on record, according to preliminary data released by the Centers for Disease Control and Prevention.
The record high was driven by a sharp increase in deaths attributed to synthetic opioids, such as fentanyl and tramadol, data published on the agency’s website show.
The provisional counts are based on death records sent to the CDC’s National Center for Health Statistics from state vital registration offices in all 50 states and the District of Columbia, reported lead author Farida B. Ahmad, MPH, of the division of vital statistics at the NCHS. Overall, the predicted number of drug overdose deaths has climbed steadily, from 54,207 in November 2015 to 66,012 in November 2016, and to 72,287 in November 2017, according to an interactive chart accessible on the website.
Deaths attributable to synthetic opioids have climbed faster than any other drug class, soaring from just 9,983 in 2015 to 20,310 in 2016, and to 29,418 in 2017.
The next-largest category, heroin-related deaths, increased from 13,407 in 2015 to 16,012 in 2016, but appeared to plateau at 15,959 in 2017. However, the CDC cautioned that flat or declining numbers could be attributable to incomplete data, true decreases in deaths, or some combination of the two. “True declines or plateaus in the numbers of drug overdose deaths across the U.S. cannot be ascertained until final data become available.”
Cocaine-related deaths were fewer in number but appear to have risen substantially to the point where the number of deaths now nearly rival that of heroin. The number of deaths was 7,106 in 2015, 10,868 in 2016, and 14,614 in 2017.
The count of drug overdose deaths varied by state. coming in at 33.3%, though the absolute numbers of cases were low (114 through January 2017 and 152 through January 2018). North Carolina also showed substantial increases (from 2,053 to 2,515 cases, 22.5%), as did New Jersey (2,219 to 2,687 cases, 21.1%), the CDC data showed.
Provisional data will be updated on a monthly basis as additional records are received, the CDC said.
An estimated 72,000 drug overdose deaths occurred in 2017 in the United States, making it the worst year on record, according to preliminary data released by the Centers for Disease Control and Prevention.
The record high was driven by a sharp increase in deaths attributed to synthetic opioids, such as fentanyl and tramadol, data published on the agency’s website show.
The provisional counts are based on death records sent to the CDC’s National Center for Health Statistics from state vital registration offices in all 50 states and the District of Columbia, reported lead author Farida B. Ahmad, MPH, of the division of vital statistics at the NCHS. Overall, the predicted number of drug overdose deaths has climbed steadily, from 54,207 in November 2015 to 66,012 in November 2016, and to 72,287 in November 2017, according to an interactive chart accessible on the website.
Deaths attributable to synthetic opioids have climbed faster than any other drug class, soaring from just 9,983 in 2015 to 20,310 in 2016, and to 29,418 in 2017.
The next-largest category, heroin-related deaths, increased from 13,407 in 2015 to 16,012 in 2016, but appeared to plateau at 15,959 in 2017. However, the CDC cautioned that flat or declining numbers could be attributable to incomplete data, true decreases in deaths, or some combination of the two. “True declines or plateaus in the numbers of drug overdose deaths across the U.S. cannot be ascertained until final data become available.”
Cocaine-related deaths were fewer in number but appear to have risen substantially to the point where the number of deaths now nearly rival that of heroin. The number of deaths was 7,106 in 2015, 10,868 in 2016, and 14,614 in 2017.
The count of drug overdose deaths varied by state. coming in at 33.3%, though the absolute numbers of cases were low (114 through January 2017 and 152 through January 2018). North Carolina also showed substantial increases (from 2,053 to 2,515 cases, 22.5%), as did New Jersey (2,219 to 2,687 cases, 21.1%), the CDC data showed.
Provisional data will be updated on a monthly basis as additional records are received, the CDC said.
An estimated 72,000 drug overdose deaths occurred in 2017 in the United States, making it the worst year on record, according to preliminary data released by the Centers for Disease Control and Prevention.
The record high was driven by a sharp increase in deaths attributed to synthetic opioids, such as fentanyl and tramadol, data published on the agency’s website show.
The provisional counts are based on death records sent to the CDC’s National Center for Health Statistics from state vital registration offices in all 50 states and the District of Columbia, reported lead author Farida B. Ahmad, MPH, of the division of vital statistics at the NCHS. Overall, the predicted number of drug overdose deaths has climbed steadily, from 54,207 in November 2015 to 66,012 in November 2016, and to 72,287 in November 2017, according to an interactive chart accessible on the website.
Deaths attributable to synthetic opioids have climbed faster than any other drug class, soaring from just 9,983 in 2015 to 20,310 in 2016, and to 29,418 in 2017.
The next-largest category, heroin-related deaths, increased from 13,407 in 2015 to 16,012 in 2016, but appeared to plateau at 15,959 in 2017. However, the CDC cautioned that flat or declining numbers could be attributable to incomplete data, true decreases in deaths, or some combination of the two. “True declines or plateaus in the numbers of drug overdose deaths across the U.S. cannot be ascertained until final data become available.”
Cocaine-related deaths were fewer in number but appear to have risen substantially to the point where the number of deaths now nearly rival that of heroin. The number of deaths was 7,106 in 2015, 10,868 in 2016, and 14,614 in 2017.
The count of drug overdose deaths varied by state. coming in at 33.3%, though the absolute numbers of cases were low (114 through January 2017 and 152 through January 2018). North Carolina also showed substantial increases (from 2,053 to 2,515 cases, 22.5%), as did New Jersey (2,219 to 2,687 cases, 21.1%), the CDC data showed.
Provisional data will be updated on a monthly basis as additional records are received, the CDC said.
Leptin expression may help differentiate renal lesions
Expression of leptin could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC), results of a recent pathology study suggest.
Renal oncocytomas had significantly increased nuclear expression of leptin, compared with the eosinophilic subtype of chromophobe RCC, according to the study.
If translated into clinical urology and pathology practice, quantification of leptin expression could help in the difficult task of differentiating between these renal lesions, according to the investigators, led by Glenda Gobe, PhD, of the Kidney Disease Research Group at the University of Queensland in Brisbane, Australia.
“This may have a major clinical impact in reducing unnecessary intervention and treatment-related harm,” Dr. Gobe and her colleagues reported in Pathology.
They obtained archived paraffin blocks from human renal tumor tissue obtained between 2009 and 2014. Paraffin sections were immunostained for leptin and leptin receptors.
The investigators evaluated 30 chromophobe RCC specimens – 15 eosinophilic and 15 noneosinophilic variants – and 15 renal oncocytomas. They also included 30 clear cell RCCs, the most common histological subtype of RCC, to verify immunohistochemistry (IHC) staining patterns.
Results showed that both leptin and leptin receptor IHC was significantly increased in tumor versus in matched, noncancerous kidney tissue.
Expression of both leptin and leptin receptor was highest for renal oncocytomas versus both the chromophobe and clear cell RCCs. On closer scrutiny, the investigators said, nuclear expression of leptin in renal oncocytomas had a significantly higher intensity versus chromophobe RCC.
“This important finding could prove to be helpful in the distinction between chromophobe RCC and renal oncocytoma,” Dr. Gobe and her coauthors wrote in their report.
In a subgroup analysis, they found a significantly increased nuclear leptin intensity for the renal oncocytomas as compared to the eosinophilic variants of chromophobe RCC (P = .016 by Dunn’s multiple comparisons test).
By contrast, testing did not reveal a significant difference for renal oncocytomas versus noneosinophilic chromophobe RCC (P = .0939) or versus clear cell RCC (P greater than .999).
Leptin, secreted by adipose cells that help regulate energy balance through inhibition of hunger, may play a role in carcinogenesis, according to investigators. Studies to date have shown that the hormone may impact carcinogenesis through cell proliferation, inhibition of apoptosis, and other potential mechanisms.
Applying this biomarker in clinical practice could more reliably characterize renal lesions with no or limited malignant potential, according to the investigators.
“The distinction of renal oncocytoma from chromophobe RCC will dictate different management pathways, as renal oncocytoma is benign while chromophobe RCC is a malignant subtype which will require further surveillance,” Dr. Gobe and her coinvestigators wrote.
The University of Malaya, Kuala Lumpur, Malaysia, funded the study. Dr. Gobe and her coauthors stated that they had no conflicts of interest.
SOURCE: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
Expression of leptin could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC), results of a recent pathology study suggest.
Renal oncocytomas had significantly increased nuclear expression of leptin, compared with the eosinophilic subtype of chromophobe RCC, according to the study.
If translated into clinical urology and pathology practice, quantification of leptin expression could help in the difficult task of differentiating between these renal lesions, according to the investigators, led by Glenda Gobe, PhD, of the Kidney Disease Research Group at the University of Queensland in Brisbane, Australia.
“This may have a major clinical impact in reducing unnecessary intervention and treatment-related harm,” Dr. Gobe and her colleagues reported in Pathology.
They obtained archived paraffin blocks from human renal tumor tissue obtained between 2009 and 2014. Paraffin sections were immunostained for leptin and leptin receptors.
The investigators evaluated 30 chromophobe RCC specimens – 15 eosinophilic and 15 noneosinophilic variants – and 15 renal oncocytomas. They also included 30 clear cell RCCs, the most common histological subtype of RCC, to verify immunohistochemistry (IHC) staining patterns.
Results showed that both leptin and leptin receptor IHC was significantly increased in tumor versus in matched, noncancerous kidney tissue.
Expression of both leptin and leptin receptor was highest for renal oncocytomas versus both the chromophobe and clear cell RCCs. On closer scrutiny, the investigators said, nuclear expression of leptin in renal oncocytomas had a significantly higher intensity versus chromophobe RCC.
“This important finding could prove to be helpful in the distinction between chromophobe RCC and renal oncocytoma,” Dr. Gobe and her coauthors wrote in their report.
In a subgroup analysis, they found a significantly increased nuclear leptin intensity for the renal oncocytomas as compared to the eosinophilic variants of chromophobe RCC (P = .016 by Dunn’s multiple comparisons test).
By contrast, testing did not reveal a significant difference for renal oncocytomas versus noneosinophilic chromophobe RCC (P = .0939) or versus clear cell RCC (P greater than .999).
Leptin, secreted by adipose cells that help regulate energy balance through inhibition of hunger, may play a role in carcinogenesis, according to investigators. Studies to date have shown that the hormone may impact carcinogenesis through cell proliferation, inhibition of apoptosis, and other potential mechanisms.
Applying this biomarker in clinical practice could more reliably characterize renal lesions with no or limited malignant potential, according to the investigators.
“The distinction of renal oncocytoma from chromophobe RCC will dictate different management pathways, as renal oncocytoma is benign while chromophobe RCC is a malignant subtype which will require further surveillance,” Dr. Gobe and her coinvestigators wrote.
The University of Malaya, Kuala Lumpur, Malaysia, funded the study. Dr. Gobe and her coauthors stated that they had no conflicts of interest.
SOURCE: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
Expression of leptin could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC), results of a recent pathology study suggest.
Renal oncocytomas had significantly increased nuclear expression of leptin, compared with the eosinophilic subtype of chromophobe RCC, according to the study.
If translated into clinical urology and pathology practice, quantification of leptin expression could help in the difficult task of differentiating between these renal lesions, according to the investigators, led by Glenda Gobe, PhD, of the Kidney Disease Research Group at the University of Queensland in Brisbane, Australia.
“This may have a major clinical impact in reducing unnecessary intervention and treatment-related harm,” Dr. Gobe and her colleagues reported in Pathology.
They obtained archived paraffin blocks from human renal tumor tissue obtained between 2009 and 2014. Paraffin sections were immunostained for leptin and leptin receptors.
The investigators evaluated 30 chromophobe RCC specimens – 15 eosinophilic and 15 noneosinophilic variants – and 15 renal oncocytomas. They also included 30 clear cell RCCs, the most common histological subtype of RCC, to verify immunohistochemistry (IHC) staining patterns.
Results showed that both leptin and leptin receptor IHC was significantly increased in tumor versus in matched, noncancerous kidney tissue.
Expression of both leptin and leptin receptor was highest for renal oncocytomas versus both the chromophobe and clear cell RCCs. On closer scrutiny, the investigators said, nuclear expression of leptin in renal oncocytomas had a significantly higher intensity versus chromophobe RCC.
“This important finding could prove to be helpful in the distinction between chromophobe RCC and renal oncocytoma,” Dr. Gobe and her coauthors wrote in their report.
In a subgroup analysis, they found a significantly increased nuclear leptin intensity for the renal oncocytomas as compared to the eosinophilic variants of chromophobe RCC (P = .016 by Dunn’s multiple comparisons test).
By contrast, testing did not reveal a significant difference for renal oncocytomas versus noneosinophilic chromophobe RCC (P = .0939) or versus clear cell RCC (P greater than .999).
Leptin, secreted by adipose cells that help regulate energy balance through inhibition of hunger, may play a role in carcinogenesis, according to investigators. Studies to date have shown that the hormone may impact carcinogenesis through cell proliferation, inhibition of apoptosis, and other potential mechanisms.
Applying this biomarker in clinical practice could more reliably characterize renal lesions with no or limited malignant potential, according to the investigators.
“The distinction of renal oncocytoma from chromophobe RCC will dictate different management pathways, as renal oncocytoma is benign while chromophobe RCC is a malignant subtype which will require further surveillance,” Dr. Gobe and her coinvestigators wrote.
The University of Malaya, Kuala Lumpur, Malaysia, funded the study. Dr. Gobe and her coauthors stated that they had no conflicts of interest.
SOURCE: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
FROM PATHOLOGY
Key clinical point: Measuring leptin expression could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC).
Major finding: Nuclear leptin intensity was significantly increased for renal oncocytomas versus eosinophilic variants of chromophobe RCC (P = 0.016).
Study details: Evaluation of 75 archived tissue samples, including 30 chromophobe RCC specimens, 15 renal oncocytomas, and 30 clear cell RCCs, along with matched, noncancerous kidney tissue specimens.
Disclosures: The University of Malaya, Kuala Lumpur, Malaysia, funded the study. The authors stated that they had no conflicts of interest.
Source: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.