User login
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
NY radiation oncologist loses license, poses ‘potential danger’
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
Less Lumens-Less Risk: A Pilot Intervention to Increase the Use of Single-Lumen Peripherally Inserted Central Catheters
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
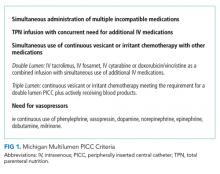
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
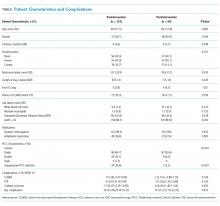
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
© 2019 Society of Hospital Medicine