User login
No Clear Benefit to Combination Treatment to Prevent Recurrent C. difficile in IBD
TOPLINE:
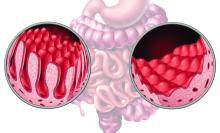
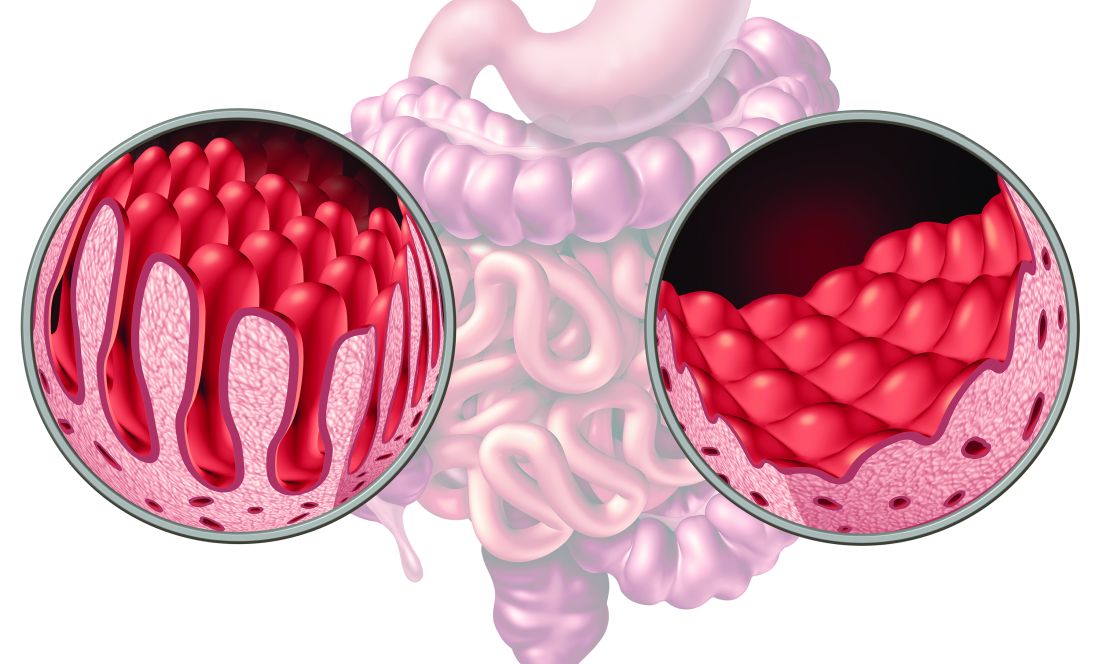
Although the combination of fecal microbiota transplantation (FMT) with bezlotoxumab was well-tolerated in patients with inflammatory bowel disease (IBD) and recurrent Clostridioides difficile infection (CDI), there›s no clear benefit to using both vs FMT alone.
METHODOLOGY:
- Researchers conducted a randomized placebo-controlled trial among 61 patients with IBD (20 with Crohn’s disease and 41 with ulcerative colitis) who had two or more episodes of CDI.
- All participants received a single colonoscopic FMT from a universal stool bank and were randomly assigned to receive a single bezlotoxumab infusion or placebo infusion before or at the time of the FMT.
- Patients were measured for CDI recurrence, defined as presence of diarrhea and positive glutamate dehydrogenase and enzyme immunoassay toxin test results, up to week 8 after treatment.
- Researchers also looked at C difficile decolonization, defined as absence of diarrhea and negative polymerase chain reaction (PCR) test results, and changes in IBD disease activity through week 12.
TAKEAWAY:
- Five participants (8%) had a CDI recurrence, including four who received bezlotoxumab and one who received placebo (13% vs 3%).
- Although participants in the treatment arm had higher odds of CDI recurrence, the difference wasn’t statistically significant.
- More patients who received bezlotoxumab were decolonized compared to placebo, both at week 1 (82% vs 68%) and week 12 (83% vs 72%), though the difference wasn’t statistically significant.
- There weren’t any significant differences in IBD outcomes, although there were higher rates of IBD improvement among those who received bezlotoxumab (56% vs 46%).
IN PRACTICE:
“As bezlotoxumab can be used to prevent recurrence in high-risk patients during their first episode of CDI, it may be more appropriate to use these therapies early and sequentially,” the study authors wrote.
SOURCE:
The study, with first author Jessica R. Allegretti, MD, MPH, from Brigham and Women’s Hospital, Boston, Massachusetts, was published online on March 19 in the American Journal of Gastroenterology.
LIMITATIONS:
The study was limited by the sample size and inclusion of PCR-only testing for the qualifying episode.
DISCLOSURES:
The trial was funded by an investigator-initiated grant from Merck Sharpe and Dohme. Several authors reported consultancy fees, research grants, and advisory board member roles with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Although the combination of fecal microbiota transplantation (FMT) with bezlotoxumab was well-tolerated in patients with inflammatory bowel disease (IBD) and recurrent Clostridioides difficile infection (CDI), there›s no clear benefit to using both vs FMT alone.
METHODOLOGY:
- Researchers conducted a randomized placebo-controlled trial among 61 patients with IBD (20 with Crohn’s disease and 41 with ulcerative colitis) who had two or more episodes of CDI.
- All participants received a single colonoscopic FMT from a universal stool bank and were randomly assigned to receive a single bezlotoxumab infusion or placebo infusion before or at the time of the FMT.
- Patients were measured for CDI recurrence, defined as presence of diarrhea and positive glutamate dehydrogenase and enzyme immunoassay toxin test results, up to week 8 after treatment.
- Researchers also looked at C difficile decolonization, defined as absence of diarrhea and negative polymerase chain reaction (PCR) test results, and changes in IBD disease activity through week 12.
TAKEAWAY:
- Five participants (8%) had a CDI recurrence, including four who received bezlotoxumab and one who received placebo (13% vs 3%).
- Although participants in the treatment arm had higher odds of CDI recurrence, the difference wasn’t statistically significant.
- More patients who received bezlotoxumab were decolonized compared to placebo, both at week 1 (82% vs 68%) and week 12 (83% vs 72%), though the difference wasn’t statistically significant.
- There weren’t any significant differences in IBD outcomes, although there were higher rates of IBD improvement among those who received bezlotoxumab (56% vs 46%).
IN PRACTICE:
“As bezlotoxumab can be used to prevent recurrence in high-risk patients during their first episode of CDI, it may be more appropriate to use these therapies early and sequentially,” the study authors wrote.
SOURCE:
The study, with first author Jessica R. Allegretti, MD, MPH, from Brigham and Women’s Hospital, Boston, Massachusetts, was published online on March 19 in the American Journal of Gastroenterology.
LIMITATIONS:
The study was limited by the sample size and inclusion of PCR-only testing for the qualifying episode.
DISCLOSURES:
The trial was funded by an investigator-initiated grant from Merck Sharpe and Dohme. Several authors reported consultancy fees, research grants, and advisory board member roles with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Although the combination of fecal microbiota transplantation (FMT) with bezlotoxumab was well-tolerated in patients with inflammatory bowel disease (IBD) and recurrent Clostridioides difficile infection (CDI), there›s no clear benefit to using both vs FMT alone.
METHODOLOGY:
- Researchers conducted a randomized placebo-controlled trial among 61 patients with IBD (20 with Crohn’s disease and 41 with ulcerative colitis) who had two or more episodes of CDI.
- All participants received a single colonoscopic FMT from a universal stool bank and were randomly assigned to receive a single bezlotoxumab infusion or placebo infusion before or at the time of the FMT.
- Patients were measured for CDI recurrence, defined as presence of diarrhea and positive glutamate dehydrogenase and enzyme immunoassay toxin test results, up to week 8 after treatment.
- Researchers also looked at C difficile decolonization, defined as absence of diarrhea and negative polymerase chain reaction (PCR) test results, and changes in IBD disease activity through week 12.
TAKEAWAY:
- Five participants (8%) had a CDI recurrence, including four who received bezlotoxumab and one who received placebo (13% vs 3%).
- Although participants in the treatment arm had higher odds of CDI recurrence, the difference wasn’t statistically significant.
- More patients who received bezlotoxumab were decolonized compared to placebo, both at week 1 (82% vs 68%) and week 12 (83% vs 72%), though the difference wasn’t statistically significant.
- There weren’t any significant differences in IBD outcomes, although there were higher rates of IBD improvement among those who received bezlotoxumab (56% vs 46%).
IN PRACTICE:
“As bezlotoxumab can be used to prevent recurrence in high-risk patients during their first episode of CDI, it may be more appropriate to use these therapies early and sequentially,” the study authors wrote.
SOURCE:
The study, with first author Jessica R. Allegretti, MD, MPH, from Brigham and Women’s Hospital, Boston, Massachusetts, was published online on March 19 in the American Journal of Gastroenterology.
LIMITATIONS:
The study was limited by the sample size and inclusion of PCR-only testing for the qualifying episode.
DISCLOSURES:
The trial was funded by an investigator-initiated grant from Merck Sharpe and Dohme. Several authors reported consultancy fees, research grants, and advisory board member roles with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
Teen Pregnancy Linked With Risk for Premature Death
Teen pregnancy is associated with a higher risk for premature mortality, both among those who carry the pregnancies to term and those who miscarry, according to a new study.
Among 2.2 million female teenagers in Ontario, Canada, the risk for premature death by age 31 years was 1.5 times higher among those who had one teen pregnancy and 2.1 times higher among those with two or more teen pregnancies.
“No person should die during childhood or early adulthood. Such deaths, unexpected and tragic, are often from preventable causes, including intentional injury,” lead author Joel Ray, MD, an obstetric medicine specialist and epidemiologist at St. Michael’s Hospital in Toronto, told this news organization.
“Women who experience teen pregnancy appear more vulnerable, often having experienced a history of adverse experiences in childhood, including abuse and economic challenges,” he said.
The study was published online in JAMA Network Open.
Analyzing Pregnancy Associations
The investigators conducted a population-based cohort study of all girls who were alive at age 12 years from April 1991 to March 2021 in Ontario. They evaluated the risk for all-cause mortality from age 12 years onward in association with the number of teen pregnancies between ages 12 and 19 years and the age at first pregnancy. The investigators adjusted the hazard ratios for year of birth, comorbidities at ages 9-11 years, area-level education, income level, and rural status.
Among more than 2.2 million teens, 163,124 (7.3%) had a pregnancy at a median age of 18 years, including 121,276 (74.3%) who had one pregnancy and 41,848 (25.6%) who had two or more. These teens were more likely to live in the lowest neighborhood income quintile and in an area with a lower rate of high school completion. They also had a higher prevalence of self-harm history between ages 12 and 19 years but not a higher prevalence of physical or mental comorbidities.
Among all teens who had a pregnancy, 60,037 (36.8%) ended in a birth, including 59,485 (99.1%) live births. A further 106,135 (65.1%) ended in induced abortion, and 17,945 (11%) ended in miscarriage or ectopic pregnancy.
Overall, there were 6030 premature deaths among those without a teen pregnancy, or 1.9 per 10,000 person-years. There were 701 deaths among those with one teen pregnancy (4.1 per 10,000 person-years) and 345 deaths among those with two or more teen pregnancies (6.1 per 10,000 person-years).
The adjusted hazard ratios (AHRs) for mortality were 1.51 for those with one pregnancy and 2.14 for those with two or more pregnancies. Compared with no teen pregnancy, the AHRs for premature death were 1.41 if the first teen pregnancy ended in an induced abortion and 2.10 if it ended in a miscarriage or birth.
Comparing those with a teen pregnancy and those without, the AHRs for premature death were 1.25 from noninjury, 2.06 from unintentional injury, and 2.02 from intentional injury. Among patients with teen pregnancy, noninjury-related premature mortality was more common, at 2.0 per 10,000 person-years, than unintentional and intentional injuries, at 1.0 per 10,000 person-years and 0.4 per 10,000 person-years, respectively.
A teen pregnancy before age 16 years entailed the highest associated risk for premature death, with an AHR of 2.00.
Next Research Steps
“We were not surprised by our findings, but it was new to us to see that the risk for premature death was higher for women who had an induced abortion in their teen years,” said Dr. Ray. “It was even higher in those whose pregnancy ended in a birth or miscarriage.”
The investigators plan to evaluate whether the future risk for premature death after teen pregnancy differs by the type of induced abortion, such as procedural or pharmaceutical, or by whether the pregnancy ended in a live birth, stillbirth, or miscarriage. Among those with a live birth, the researchers will also analyze the risk for premature death in relation to whether the newborn was taken into custody by child protection services in Canada.
Other factors associated with teen pregnancy and overall mortality, particularly adverse childhood experiences, may point to the reasons for premature mortality and should be studied further, the authors wrote. Structural and systems-related factors should be considered as well.
Stigmatization and Isolation
“Some teens choose to become pregnant, but most teen pregnancies are unintended, which exposes shortcomings in the systems that exist to educate, guide, and support young people,” said Elizabeth Cook, a research scientist at Child Trends in Rockville, Maryland.
Dr. Cook, who wasn’t involved with this study, wrote an accompanying editorial in JAMA Network Open. She conducts studies of sexual and reproductive health for Child Trends.
“Teens who become pregnant often experience stigmatization and isolation that can make it more difficult to thrive in adulthood, especially if they lack the necessary support to navigate such a significant decision,” she said. “Fortunately, the systems that youths encounter, such as healthcare, education, and child welfare, are taking on a larger role in prevention efforts than they have in the past.”
These systems are shifting the burden of unintended teen pregnancy from the teens themselves and their behaviors to the health and education systems, Dr. Cook noted, though more work is needed around local policies and lack of access to healthcare facilities.
“Teen pregnancy may offer an opportunity to intervene in the lives of people at higher risk for premature death, but knowing how best to offer support requires an understanding of the context of their lives,” she said. “As a starting point, we must celebrate and listen to all pregnant young people so they can tell us what they need to live long, fulfilled lives.”
The study was funded by grants from the PSI Foundation and the Canadian Institutes of Health Research, as well as ICES, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Ray and Dr. Cook reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Teen pregnancy is associated with a higher risk for premature mortality, both among those who carry the pregnancies to term and those who miscarry, according to a new study.
Among 2.2 million female teenagers in Ontario, Canada, the risk for premature death by age 31 years was 1.5 times higher among those who had one teen pregnancy and 2.1 times higher among those with two or more teen pregnancies.
“No person should die during childhood or early adulthood. Such deaths, unexpected and tragic, are often from preventable causes, including intentional injury,” lead author Joel Ray, MD, an obstetric medicine specialist and epidemiologist at St. Michael’s Hospital in Toronto, told this news organization.
“Women who experience teen pregnancy appear more vulnerable, often having experienced a history of adverse experiences in childhood, including abuse and economic challenges,” he said.
The study was published online in JAMA Network Open.
Analyzing Pregnancy Associations
The investigators conducted a population-based cohort study of all girls who were alive at age 12 years from April 1991 to March 2021 in Ontario. They evaluated the risk for all-cause mortality from age 12 years onward in association with the number of teen pregnancies between ages 12 and 19 years and the age at first pregnancy. The investigators adjusted the hazard ratios for year of birth, comorbidities at ages 9-11 years, area-level education, income level, and rural status.
Among more than 2.2 million teens, 163,124 (7.3%) had a pregnancy at a median age of 18 years, including 121,276 (74.3%) who had one pregnancy and 41,848 (25.6%) who had two or more. These teens were more likely to live in the lowest neighborhood income quintile and in an area with a lower rate of high school completion. They also had a higher prevalence of self-harm history between ages 12 and 19 years but not a higher prevalence of physical or mental comorbidities.
Among all teens who had a pregnancy, 60,037 (36.8%) ended in a birth, including 59,485 (99.1%) live births. A further 106,135 (65.1%) ended in induced abortion, and 17,945 (11%) ended in miscarriage or ectopic pregnancy.
Overall, there were 6030 premature deaths among those without a teen pregnancy, or 1.9 per 10,000 person-years. There were 701 deaths among those with one teen pregnancy (4.1 per 10,000 person-years) and 345 deaths among those with two or more teen pregnancies (6.1 per 10,000 person-years).
The adjusted hazard ratios (AHRs) for mortality were 1.51 for those with one pregnancy and 2.14 for those with two or more pregnancies. Compared with no teen pregnancy, the AHRs for premature death were 1.41 if the first teen pregnancy ended in an induced abortion and 2.10 if it ended in a miscarriage or birth.
Comparing those with a teen pregnancy and those without, the AHRs for premature death were 1.25 from noninjury, 2.06 from unintentional injury, and 2.02 from intentional injury. Among patients with teen pregnancy, noninjury-related premature mortality was more common, at 2.0 per 10,000 person-years, than unintentional and intentional injuries, at 1.0 per 10,000 person-years and 0.4 per 10,000 person-years, respectively.
A teen pregnancy before age 16 years entailed the highest associated risk for premature death, with an AHR of 2.00.
Next Research Steps
“We were not surprised by our findings, but it was new to us to see that the risk for premature death was higher for women who had an induced abortion in their teen years,” said Dr. Ray. “It was even higher in those whose pregnancy ended in a birth or miscarriage.”
The investigators plan to evaluate whether the future risk for premature death after teen pregnancy differs by the type of induced abortion, such as procedural or pharmaceutical, or by whether the pregnancy ended in a live birth, stillbirth, or miscarriage. Among those with a live birth, the researchers will also analyze the risk for premature death in relation to whether the newborn was taken into custody by child protection services in Canada.
Other factors associated with teen pregnancy and overall mortality, particularly adverse childhood experiences, may point to the reasons for premature mortality and should be studied further, the authors wrote. Structural and systems-related factors should be considered as well.
Stigmatization and Isolation
“Some teens choose to become pregnant, but most teen pregnancies are unintended, which exposes shortcomings in the systems that exist to educate, guide, and support young people,” said Elizabeth Cook, a research scientist at Child Trends in Rockville, Maryland.
Dr. Cook, who wasn’t involved with this study, wrote an accompanying editorial in JAMA Network Open. She conducts studies of sexual and reproductive health for Child Trends.
“Teens who become pregnant often experience stigmatization and isolation that can make it more difficult to thrive in adulthood, especially if they lack the necessary support to navigate such a significant decision,” she said. “Fortunately, the systems that youths encounter, such as healthcare, education, and child welfare, are taking on a larger role in prevention efforts than they have in the past.”
These systems are shifting the burden of unintended teen pregnancy from the teens themselves and their behaviors to the health and education systems, Dr. Cook noted, though more work is needed around local policies and lack of access to healthcare facilities.
“Teen pregnancy may offer an opportunity to intervene in the lives of people at higher risk for premature death, but knowing how best to offer support requires an understanding of the context of their lives,” she said. “As a starting point, we must celebrate and listen to all pregnant young people so they can tell us what they need to live long, fulfilled lives.”
The study was funded by grants from the PSI Foundation and the Canadian Institutes of Health Research, as well as ICES, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Ray and Dr. Cook reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Teen pregnancy is associated with a higher risk for premature mortality, both among those who carry the pregnancies to term and those who miscarry, according to a new study.
Among 2.2 million female teenagers in Ontario, Canada, the risk for premature death by age 31 years was 1.5 times higher among those who had one teen pregnancy and 2.1 times higher among those with two or more teen pregnancies.
“No person should die during childhood or early adulthood. Such deaths, unexpected and tragic, are often from preventable causes, including intentional injury,” lead author Joel Ray, MD, an obstetric medicine specialist and epidemiologist at St. Michael’s Hospital in Toronto, told this news organization.
“Women who experience teen pregnancy appear more vulnerable, often having experienced a history of adverse experiences in childhood, including abuse and economic challenges,” he said.
The study was published online in JAMA Network Open.
Analyzing Pregnancy Associations
The investigators conducted a population-based cohort study of all girls who were alive at age 12 years from April 1991 to March 2021 in Ontario. They evaluated the risk for all-cause mortality from age 12 years onward in association with the number of teen pregnancies between ages 12 and 19 years and the age at first pregnancy. The investigators adjusted the hazard ratios for year of birth, comorbidities at ages 9-11 years, area-level education, income level, and rural status.
Among more than 2.2 million teens, 163,124 (7.3%) had a pregnancy at a median age of 18 years, including 121,276 (74.3%) who had one pregnancy and 41,848 (25.6%) who had two or more. These teens were more likely to live in the lowest neighborhood income quintile and in an area with a lower rate of high school completion. They also had a higher prevalence of self-harm history between ages 12 and 19 years but not a higher prevalence of physical or mental comorbidities.
Among all teens who had a pregnancy, 60,037 (36.8%) ended in a birth, including 59,485 (99.1%) live births. A further 106,135 (65.1%) ended in induced abortion, and 17,945 (11%) ended in miscarriage or ectopic pregnancy.
Overall, there were 6030 premature deaths among those without a teen pregnancy, or 1.9 per 10,000 person-years. There were 701 deaths among those with one teen pregnancy (4.1 per 10,000 person-years) and 345 deaths among those with two or more teen pregnancies (6.1 per 10,000 person-years).
The adjusted hazard ratios (AHRs) for mortality were 1.51 for those with one pregnancy and 2.14 for those with two or more pregnancies. Compared with no teen pregnancy, the AHRs for premature death were 1.41 if the first teen pregnancy ended in an induced abortion and 2.10 if it ended in a miscarriage or birth.
Comparing those with a teen pregnancy and those without, the AHRs for premature death were 1.25 from noninjury, 2.06 from unintentional injury, and 2.02 from intentional injury. Among patients with teen pregnancy, noninjury-related premature mortality was more common, at 2.0 per 10,000 person-years, than unintentional and intentional injuries, at 1.0 per 10,000 person-years and 0.4 per 10,000 person-years, respectively.
A teen pregnancy before age 16 years entailed the highest associated risk for premature death, with an AHR of 2.00.
Next Research Steps
“We were not surprised by our findings, but it was new to us to see that the risk for premature death was higher for women who had an induced abortion in their teen years,” said Dr. Ray. “It was even higher in those whose pregnancy ended in a birth or miscarriage.”
The investigators plan to evaluate whether the future risk for premature death after teen pregnancy differs by the type of induced abortion, such as procedural or pharmaceutical, or by whether the pregnancy ended in a live birth, stillbirth, or miscarriage. Among those with a live birth, the researchers will also analyze the risk for premature death in relation to whether the newborn was taken into custody by child protection services in Canada.
Other factors associated with teen pregnancy and overall mortality, particularly adverse childhood experiences, may point to the reasons for premature mortality and should be studied further, the authors wrote. Structural and systems-related factors should be considered as well.
Stigmatization and Isolation
“Some teens choose to become pregnant, but most teen pregnancies are unintended, which exposes shortcomings in the systems that exist to educate, guide, and support young people,” said Elizabeth Cook, a research scientist at Child Trends in Rockville, Maryland.
Dr. Cook, who wasn’t involved with this study, wrote an accompanying editorial in JAMA Network Open. She conducts studies of sexual and reproductive health for Child Trends.
“Teens who become pregnant often experience stigmatization and isolation that can make it more difficult to thrive in adulthood, especially if they lack the necessary support to navigate such a significant decision,” she said. “Fortunately, the systems that youths encounter, such as healthcare, education, and child welfare, are taking on a larger role in prevention efforts than they have in the past.”
These systems are shifting the burden of unintended teen pregnancy from the teens themselves and their behaviors to the health and education systems, Dr. Cook noted, though more work is needed around local policies and lack of access to healthcare facilities.
“Teen pregnancy may offer an opportunity to intervene in the lives of people at higher risk for premature death, but knowing how best to offer support requires an understanding of the context of their lives,” she said. “As a starting point, we must celebrate and listen to all pregnant young people so they can tell us what they need to live long, fulfilled lives.”
The study was funded by grants from the PSI Foundation and the Canadian Institutes of Health Research, as well as ICES, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Ray and Dr. Cook reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Liquid Biopsy for Colorectal Cancer Appears Promising But Still Lacks Robust Efficacy
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
, according to two new modeling studies and an expert consensus commentary.
Although some patients find blood-based tests more convenient, the higher numbers of false positives and false negatives could lead to more CRC cases and deaths.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor cost-effective and would worsen outcomes,” David Lieberman, MD, AGAF, chair of the American Gastroenterological Association’s CRC Workshop Panel, and lead author of the expert commentary, said in a statement.
The blood tests detect circulating nucleotides, such as cell-free DNA or metabolic products associated with CRC and its precursors. Current tests are in development by Guardant Health and Freenome.
The two modeling studies, published in Gastroenterology on March 26, analyzed the effectiveness and cost-effectiveness of blood-based CRC screening that meets Centers for Medicare & Medicaid Services (CMS) coverage criteria, as well as the comparative effectiveness and cost-effectiveness of CRC screening with blood-based biomarkers versus fecal tests or colonoscopy.
Also published on March 26 in Clinical Gastroenterology and Hepatology, the expert commentary included key conclusions from the AGA CRC Workshop, which analyzed the two modeling studies.
Comparing CRC Screening Methods
In the first modeling study, an international team of researchers ran three microsimulation models for CRC to estimate the effectiveness and cost-effectiveness of triennial blood-based screening for ages 45-75, compared with no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with a FIT assay, and colonoscopy screening every 10 years. The researchers used CMS coverage criteria for blood tests, with a sensitivity of at least 74% for detection of CRC and specificity of at least 90%.
Without screening, the models predicted between 77 and 88 CRC cases and between 32 and 36 deaths per 1,000 individuals, costing between $5.3 million to $5.8 million. Compared with no screening, blood-based screening was considered cost-effective, with an additional cost of $25,600 to $43,700 per quality-adjusted life-year gained (QALYG).
However, compared with the FIT, stool, and colonoscopy options, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT was more effective and less costly, with 5-24 QALYG and nearly $3.5 million cheaper than blood-based screening, even when blood-based uptake was 20 percentage points higher than FIT uptake.
In the second modeling study, US researchers compared triennial blood-based screening with established alternatives at the CMS thresholds of 74% sensitivity and 90% specificity.
Overall, a blood-based test at the CMS minimum reduced CRC incidence by 40% and CRC mortality by 52% versus no screening. However, a blood-based test was significantly less effective than triennial stool DNA testing, annual FIT, and colonoscopy every 10 years, which reduced CRC incidence by 68%-79% and CRC mortality by 73%-81%.
Assuming a blood-based test would cost the same as a multi-target stool test, the blood-based test would cost $28,500 per QALYG versus no screening. At the same time, FIT, colonoscopy, and stool DNA testing were less costly and more effective. In general, the blood-based test would match FIT’s clinical outcomes if it achieved 1.4- to 1.8-fold the participation rate for FIT.
Even still, the sensitivity for advanced precancerous lesion (APL) was a key determinant. A paradigm-changing blood-based test would need to have higher than 90% sensitivity for CRC and 80% for APL, 90% specificity, and cost less than $120 to $140, the study authors wrote.
“High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers,” the authors wrote. “APL detection should not be penalized by a definition of test specificity that focuses on CRC only.”
Additional Considerations
The AGA CRC Workshop Panel met in September 2023 to review the two modeling studies and other data on blood-based tests for CRC. Overall, the group concluded that a triennial blood test that meets minimal CMS criteria would likely result in better outcomes than no screening and provide a simple process to encourage more people to participate in screening.
However, patients who may have declined colonoscopy should understand the need for a colonoscopy if blood-based tests show abnormal results, the commentary authors wrote.
In addition, because blood-based tests for CRC appear to be less effective and more costly than current screening options, they shouldn’t be recommended to replace established screening methods. Although these blood-based tests may improve screening rates and outcomes in unscreened people, substituting blood tests for other effective tests would increase costs and worsen patient outcomes.
Beyond that, they wrote, the industry should consider other potential benchmarks for an effective blood test, such as a sensitivity for stage I-III CRC of greater than 90% and sensitivity for advanced adenomas of 40%-50% or higher.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” John M. Carethers, MD, AGAF, vice chancellor for health sciences at UC San Diego, AGA past president, and a member of the AGA CRC Workshop panel, said in a statement.
Several authors reported consultant roles and funding support from numerous companies, including Guardant Health and Freenome.
Few Childhood Cancer Survivors Get Recommended Screenings
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Among childhood cancer survivors in Ontario, Canada, who faced an elevated risk due to chemotherapy or radiation treatments, 53% followed screening recommendations for cardiomyopathy, 13% met colorectal cancer screening guidelines, and 6% adhered to breast cancer screening guidelines.
“Although over 80% of children newly diagnosed with cancer will become long-term survivors, as many as four out of five of these survivors will develop a serious or life-threatening late effect of their cancer therapy by age 45,” lead author Jennifer Shuldiner, PhD, MPH, a scientist at Women’s College Hospital Institute for Health Systems Solutions and Virtual Care in Toronto, told this news organization.
For instance, the risk for colorectal cancer in childhood cancer survivors is two to three times higher than it is among the general population, and the risk for breast cancer is similar between those who underwent chest radiation and those with a BRCA mutation. As many as 50% of those who received anthracycline chemotherapy or radiation involving the heart later develop cardiotoxicity.
The North American Children’s Oncology Group has published long-term follow-up guidelines for survivors of childhood cancer, yet many survivors don’t follow them because of lack of awareness or other barriers, said Dr. Shuldiner.
“Prior research has shown that many survivors do not complete these recommended tests,” she said. “With better knowledge of this at-risk population, we can design, test, and implement appropriate interventions and supports to tackle the issues.”
The study was published online on March 11 in CMAJ.
Changes in Adherence
The researchers conducted a retrospective population-based cohort study analyzing Ontario healthcare administrative data for adult survivors of childhood cancer diagnosed between 1986 and 2014 who faced an elevated risk for therapy-related colorectal cancer, breast cancer, or cardiomyopathy. The research team then assessed long-term adherence to the North American Children’s Oncology Group guidelines and predictors of adherence.
Among 3241 survivors, 3205 (99%) were at elevated risk for cardiomyopathy, 327 (10%) were at elevated risk for colorectal cancer, and 234 (7%) were at elevated risk for breast cancer. In addition, 2806 (87%) were at risk for one late effect, 345 (11%) were at risk for two late effects, and 90 (3%) were at risk for three late effects.
Overall, 53%, 13%, and 6% were adherent to their recommended surveillance for cardiomyopathy, colorectal cancer, and breast cancer, respectively. Over time, adherence increased for colorectal cancer and cardiomyopathy but decreased for breast cancer.
In addition, patients who were older at diagnosis were more likely to follow screening guidelines for colorectal and breast cancers, whereas those who were younger at diagnosis were more likely to follow screening guidelines for cardiomyopathy.
During a median follow-up of 7.8 years, the proportion of time spent adherent was 43% for cardiomyopathy, 14% for colorectal cancer, and 10% for breast cancer.
Survivors who attended a long-term follow-up clinic in the previous year had low adherence rates as well, though they were higher than in the rest of the cohort. In this group, the proportion of time that was spent adherent was 71% for cardiomyopathy, 27% for colorectal cancer, and 15% for breast cancer.
Shuldiner and colleagues are launching a research trial to determine whether a provincial support system can help childhood cancer survivors receive the recommended surveillance. The support system provides information about screening recommendations to survivors as well as reminders and sends key information to their family doctors.
“We now understand that childhood cancer survivors need help to complete the recommended tests,” said Dr. Shuldiner. “If the trial is successful, we hope it will be implemented in Ontario.”
Survivorship Care Plans
Low screening rates may result from a lack of awareness about screening recommendations and the negative long-term effects of cancer treatments, the study authors wrote. Cancer survivors, caregivers, family physicians, specialists, and survivor support groups can share the responsibility of spreading awareness and adhering to guidelines, they noted. In some cases, a survivorship care plan (SCP) may help.
“SCPs are intended to improve adherence by providing follow-up information and facilitating the transition from cancer treatment to survivorship and from pediatric to adult care,” Adam Yan, MD, a staff oncologist and oncology informatics lead at the Hospital for Sick Children in Toronto, told this news organization.
Dr. Yan, who wasn’t involved with this study, has researched surveillance adherence for secondary cancers and cardiac dysfunction among childhood cancer survivors. He and his colleagues found that screening rates were typically low among survivors who faced high risks for cardiac dysfunction and breast, colorectal, or skin cancers.
However, having a survivorship care plan seemed to help, and survivors treated after 1990 were more likely to have an SCP.
“SCP possession by high-risk survivors was associated with increased breast, skin, and cardiac surveillance,” he said. “It is uncertain whether SCP possession leads to adherence or whether SCP possession is a marker of survivors who are focused on their health and thus likely to adhere to preventive health practices, including surveillance.”
The study was funded by the Canadian Institutes of Health Research and ICES, which receives support from the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Shuldiner received a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in support of the work. Dr. Yan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Systematic Viral Testing in Emergency Departments Has Limited Benefit for General Population
Routine use of rapid respiratory virus testing in the emergency department (ED) appears to show limited benefit among patients with signs and symptoms of acute respiratory infection (ARI), according to a new study.
Rapid viral testing wasn’t associated with reduced antibiotic use, ED length of stay, or rates of ED return visits or hospitalization. However, testing was associated with a small increase in antiviral prescriptions and a small reduction in blood tests and chest x-rays.
“Our interest in studying the benefits of rapid viral testing in emergency departments comes from a commitment to diagnostic stewardship — ensuring that the right tests are administered to the right patients at the right time while also curbing overuse,” said lead author Tilmann Schober, MD, a resident in pediatric infectious disease at McGill University and Montreal Children’s Hospital.
“Following the SARS-CoV-2 pandemic, we have seen a surge in the availability of rapid viral testing, including molecular multiplex panels,” he said. “However, the actual impact of these advancements on patient care in the ED remains uncertain.”
The study was published online on March 4, 2024, in JAMA Internal Medicine).
Rapid Viral Testing
Dr. Schober and colleagues conducted a systematic review and meta-analysis of 11 randomized clinical trials to understand whether rapid testing for respiratory viruses was associated with patient treatment in the ED.
In particular, the research team looked at whether testing in patients with suspected ARI was associated with decreased antibiotic use, ancillary tests, ED length of stay, ED return visits, hospitalization, and increased influenza antiviral treatment.
Among the trials, seven studies included molecular testing, and eight used multiplex panels, including influenza and respiratory syncytial virus (RSV), influenza/RSV/adenovirus/parainfluenza, or a panel of 15 or more respiratory viruses. No study evaluated testing for SARS-CoV-2. The research team reported risk ratios (RRs) and risk difference estimates.
In general, routine rapid viral testing was associated with higher use of influenza antivirals (RR, 1.33) and lower use of chest radiography (RR, 0.88) and blood tests (RR, 0.81). However, the magnitude of these effects was small. For instance, to achieve one additional viral prescription, 70 patients would need to be tested, and to save one x-ray, 30 patients would need to be tested.
“This suggests that, while statistically significant, the practical impact of these secondary outcomes may not justify the extensive effort and resources involved in widespread testing,” Dr. Schober said.
In addition, there was no association between rapid testing and antibiotic use (RR, 0.99), urine testing (RR, 0.95), ED length of stay (0 h), return visits (RR, 0.93), or hospitalization (RR, 1.01).
Notably, there was no association between rapid viral testing and antibiotic use in any prespecified subgroup based on age, test method, publication date, number of viral targets, risk of bias, or industry funding, the authors said. They concluded that rapid virus testing should be reserved for patients for whom the testing will change treatment, such as high-risk patients or those with severe disease.
“It’s crucial to note that our study specifically evaluated the impact of systematic testing of patients with signs and symptoms of acute respiratory infection. Our findings do not advocate against rapid respiratory virus testing in general,” Dr. Schober said. “There is well-established evidence supporting the benefits of viral testing in certain contexts, such as hospitalized patients, to guide infection control practices or in specific high-risk populations.”
Future Research
Additional studies should look at testing among subgroups, particularly those with high-risk conditions, the study authors wrote. In addition, the research team would like to study the implementation of novel diagnostic stewardship programs as compared with well-established antibiotic stewardship programs.
“Acute respiratory tract illnesses represent one of the most common reasons for being evaluated in an acute care setting, especially in pediatrics, and these visits have traditionally resulted in excessive antibiotic prescribing, despite the etiology of the infection mostly being viral,” said Suchitra Rao, MBBS, associate professor of pediatrics at the University of Colorado School of Medicine and associate medical director of infection prevention and control at Children’s Hospital Colorado, Aurora.
Dr. Rao, who wasn’t involved with this study, has surveyed ED providers about respiratory viral testing and changes in clinical decision-making. She and colleagues found that providers most commonly changed clinical decision-making while prescribing an antiviral if influenza was detected or withholding antivirals if influenza wasn’t detected.
“Multiplex testing for respiratory viruses and atypical bacteria is becoming more widespread, with newer-generation platforms having shorter turnaround times, and offers the potential to impact point-of-care decision-making,” she said. “However, these tests are expensive, and more studies are needed to explore whether respiratory pathogen panel testing in the acute care setting has an impact in terms of reduced antibiotic use as well as other outcomes, including ED visits, health-seeking behaviors, and hospitalization.”
For instance, more recent studies around SARS-CoV-2 with newer-generation panels may make a difference, as well as multiplex panels that include numerous viral targets, she said.
“Further RCTs are required to evaluate the impact of influenza/RSV/SARS-CoV-2 panels, as well as respiratory pathogen panel testing in conjunction with antimicrobial and diagnostic stewardship efforts, which have been associated with improved outcomes for other rapid molecular platforms, such as blood culture identification panels,” Rao said.
The study was funded by the Research Institute of the McGill University Health Center. Dr. Schober reported no disclosures, and several study authors reported grants or personal fees from companies outside of this research. Dr. Rao disclosed no relevant relationships.
A version of this article appeared on Medscape.com .
Routine use of rapid respiratory virus testing in the emergency department (ED) appears to show limited benefit among patients with signs and symptoms of acute respiratory infection (ARI), according to a new study.
Rapid viral testing wasn’t associated with reduced antibiotic use, ED length of stay, or rates of ED return visits or hospitalization. However, testing was associated with a small increase in antiviral prescriptions and a small reduction in blood tests and chest x-rays.
“Our interest in studying the benefits of rapid viral testing in emergency departments comes from a commitment to diagnostic stewardship — ensuring that the right tests are administered to the right patients at the right time while also curbing overuse,” said lead author Tilmann Schober, MD, a resident in pediatric infectious disease at McGill University and Montreal Children’s Hospital.
“Following the SARS-CoV-2 pandemic, we have seen a surge in the availability of rapid viral testing, including molecular multiplex panels,” he said. “However, the actual impact of these advancements on patient care in the ED remains uncertain.”
The study was published online on March 4, 2024, in JAMA Internal Medicine).
Rapid Viral Testing
Dr. Schober and colleagues conducted a systematic review and meta-analysis of 11 randomized clinical trials to understand whether rapid testing for respiratory viruses was associated with patient treatment in the ED.
In particular, the research team looked at whether testing in patients with suspected ARI was associated with decreased antibiotic use, ancillary tests, ED length of stay, ED return visits, hospitalization, and increased influenza antiviral treatment.
Among the trials, seven studies included molecular testing, and eight used multiplex panels, including influenza and respiratory syncytial virus (RSV), influenza/RSV/adenovirus/parainfluenza, or a panel of 15 or more respiratory viruses. No study evaluated testing for SARS-CoV-2. The research team reported risk ratios (RRs) and risk difference estimates.
In general, routine rapid viral testing was associated with higher use of influenza antivirals (RR, 1.33) and lower use of chest radiography (RR, 0.88) and blood tests (RR, 0.81). However, the magnitude of these effects was small. For instance, to achieve one additional viral prescription, 70 patients would need to be tested, and to save one x-ray, 30 patients would need to be tested.
“This suggests that, while statistically significant, the practical impact of these secondary outcomes may not justify the extensive effort and resources involved in widespread testing,” Dr. Schober said.
In addition, there was no association between rapid testing and antibiotic use (RR, 0.99), urine testing (RR, 0.95), ED length of stay (0 h), return visits (RR, 0.93), or hospitalization (RR, 1.01).
Notably, there was no association between rapid viral testing and antibiotic use in any prespecified subgroup based on age, test method, publication date, number of viral targets, risk of bias, or industry funding, the authors said. They concluded that rapid virus testing should be reserved for patients for whom the testing will change treatment, such as high-risk patients or those with severe disease.
“It’s crucial to note that our study specifically evaluated the impact of systematic testing of patients with signs and symptoms of acute respiratory infection. Our findings do not advocate against rapid respiratory virus testing in general,” Dr. Schober said. “There is well-established evidence supporting the benefits of viral testing in certain contexts, such as hospitalized patients, to guide infection control practices or in specific high-risk populations.”
Future Research
Additional studies should look at testing among subgroups, particularly those with high-risk conditions, the study authors wrote. In addition, the research team would like to study the implementation of novel diagnostic stewardship programs as compared with well-established antibiotic stewardship programs.
“Acute respiratory tract illnesses represent one of the most common reasons for being evaluated in an acute care setting, especially in pediatrics, and these visits have traditionally resulted in excessive antibiotic prescribing, despite the etiology of the infection mostly being viral,” said Suchitra Rao, MBBS, associate professor of pediatrics at the University of Colorado School of Medicine and associate medical director of infection prevention and control at Children’s Hospital Colorado, Aurora.
Dr. Rao, who wasn’t involved with this study, has surveyed ED providers about respiratory viral testing and changes in clinical decision-making. She and colleagues found that providers most commonly changed clinical decision-making while prescribing an antiviral if influenza was detected or withholding antivirals if influenza wasn’t detected.
“Multiplex testing for respiratory viruses and atypical bacteria is becoming more widespread, with newer-generation platforms having shorter turnaround times, and offers the potential to impact point-of-care decision-making,” she said. “However, these tests are expensive, and more studies are needed to explore whether respiratory pathogen panel testing in the acute care setting has an impact in terms of reduced antibiotic use as well as other outcomes, including ED visits, health-seeking behaviors, and hospitalization.”
For instance, more recent studies around SARS-CoV-2 with newer-generation panels may make a difference, as well as multiplex panels that include numerous viral targets, she said.
“Further RCTs are required to evaluate the impact of influenza/RSV/SARS-CoV-2 panels, as well as respiratory pathogen panel testing in conjunction with antimicrobial and diagnostic stewardship efforts, which have been associated with improved outcomes for other rapid molecular platforms, such as blood culture identification panels,” Rao said.
The study was funded by the Research Institute of the McGill University Health Center. Dr. Schober reported no disclosures, and several study authors reported grants or personal fees from companies outside of this research. Dr. Rao disclosed no relevant relationships.
A version of this article appeared on Medscape.com .
Routine use of rapid respiratory virus testing in the emergency department (ED) appears to show limited benefit among patients with signs and symptoms of acute respiratory infection (ARI), according to a new study.
Rapid viral testing wasn’t associated with reduced antibiotic use, ED length of stay, or rates of ED return visits or hospitalization. However, testing was associated with a small increase in antiviral prescriptions and a small reduction in blood tests and chest x-rays.
“Our interest in studying the benefits of rapid viral testing in emergency departments comes from a commitment to diagnostic stewardship — ensuring that the right tests are administered to the right patients at the right time while also curbing overuse,” said lead author Tilmann Schober, MD, a resident in pediatric infectious disease at McGill University and Montreal Children’s Hospital.
“Following the SARS-CoV-2 pandemic, we have seen a surge in the availability of rapid viral testing, including molecular multiplex panels,” he said. “However, the actual impact of these advancements on patient care in the ED remains uncertain.”
The study was published online on March 4, 2024, in JAMA Internal Medicine).
Rapid Viral Testing
Dr. Schober and colleagues conducted a systematic review and meta-analysis of 11 randomized clinical trials to understand whether rapid testing for respiratory viruses was associated with patient treatment in the ED.
In particular, the research team looked at whether testing in patients with suspected ARI was associated with decreased antibiotic use, ancillary tests, ED length of stay, ED return visits, hospitalization, and increased influenza antiviral treatment.
Among the trials, seven studies included molecular testing, and eight used multiplex panels, including influenza and respiratory syncytial virus (RSV), influenza/RSV/adenovirus/parainfluenza, or a panel of 15 or more respiratory viruses. No study evaluated testing for SARS-CoV-2. The research team reported risk ratios (RRs) and risk difference estimates.
In general, routine rapid viral testing was associated with higher use of influenza antivirals (RR, 1.33) and lower use of chest radiography (RR, 0.88) and blood tests (RR, 0.81). However, the magnitude of these effects was small. For instance, to achieve one additional viral prescription, 70 patients would need to be tested, and to save one x-ray, 30 patients would need to be tested.
“This suggests that, while statistically significant, the practical impact of these secondary outcomes may not justify the extensive effort and resources involved in widespread testing,” Dr. Schober said.
In addition, there was no association between rapid testing and antibiotic use (RR, 0.99), urine testing (RR, 0.95), ED length of stay (0 h), return visits (RR, 0.93), or hospitalization (RR, 1.01).
Notably, there was no association between rapid viral testing and antibiotic use in any prespecified subgroup based on age, test method, publication date, number of viral targets, risk of bias, or industry funding, the authors said. They concluded that rapid virus testing should be reserved for patients for whom the testing will change treatment, such as high-risk patients or those with severe disease.
“It’s crucial to note that our study specifically evaluated the impact of systematic testing of patients with signs and symptoms of acute respiratory infection. Our findings do not advocate against rapid respiratory virus testing in general,” Dr. Schober said. “There is well-established evidence supporting the benefits of viral testing in certain contexts, such as hospitalized patients, to guide infection control practices or in specific high-risk populations.”
Future Research
Additional studies should look at testing among subgroups, particularly those with high-risk conditions, the study authors wrote. In addition, the research team would like to study the implementation of novel diagnostic stewardship programs as compared with well-established antibiotic stewardship programs.
“Acute respiratory tract illnesses represent one of the most common reasons for being evaluated in an acute care setting, especially in pediatrics, and these visits have traditionally resulted in excessive antibiotic prescribing, despite the etiology of the infection mostly being viral,” said Suchitra Rao, MBBS, associate professor of pediatrics at the University of Colorado School of Medicine and associate medical director of infection prevention and control at Children’s Hospital Colorado, Aurora.
Dr. Rao, who wasn’t involved with this study, has surveyed ED providers about respiratory viral testing and changes in clinical decision-making. She and colleagues found that providers most commonly changed clinical decision-making while prescribing an antiviral if influenza was detected or withholding antivirals if influenza wasn’t detected.
“Multiplex testing for respiratory viruses and atypical bacteria is becoming more widespread, with newer-generation platforms having shorter turnaround times, and offers the potential to impact point-of-care decision-making,” she said. “However, these tests are expensive, and more studies are needed to explore whether respiratory pathogen panel testing in the acute care setting has an impact in terms of reduced antibiotic use as well as other outcomes, including ED visits, health-seeking behaviors, and hospitalization.”
For instance, more recent studies around SARS-CoV-2 with newer-generation panels may make a difference, as well as multiplex panels that include numerous viral targets, she said.
“Further RCTs are required to evaluate the impact of influenza/RSV/SARS-CoV-2 panels, as well as respiratory pathogen panel testing in conjunction with antimicrobial and diagnostic stewardship efforts, which have been associated with improved outcomes for other rapid molecular platforms, such as blood culture identification panels,” Rao said.
The study was funded by the Research Institute of the McGill University Health Center. Dr. Schober reported no disclosures, and several study authors reported grants or personal fees from companies outside of this research. Dr. Rao disclosed no relevant relationships.
A version of this article appeared on Medscape.com .
Cell-Free DNA Blood Test Has High Accuracy for Detecting Colorectal Cancer
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM NEJM
No-Biopsy Approach to Celiac Disease Diagnosis Appears Effective for Select Adult Patients
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
FROM GASTROENTEROLOGY
Mood Interventions May Reduce IBD Inflammation
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
Most Americans Believe Bariatric Surgery Is Shortcut, Should Be ‘Last Resort’: Survey
Most Americans’ views about obesity and bariatric surgery are colored by stigmas, according to a new survey from the healthcare system at Orlando Health.
For example, most Americans believe that weight loss surgery should be pursued only as a last resort and that bariatric surgery is a shortcut to shedding pounds, the survey found.
Common stigmas could be deterring people who qualify for bariatric surgery from pursuing it, according to Orlando Health, located in Florida.
“Bariatric surgery is by no means an easy way out. If you have the courage to ask for help and commit to doing the hard work of changing your diet and improving your life, you’re a champion in my book,” said Andre Teixeira, MD, medical director and bariatric surgeon at Orlando Health Weight Loss and Bariatric Surgery Institute, Orlando, Florida.
“Surgery is simply a tool to jumpstart that change,” he said. “After surgery, it is up to the patient to learn how to eat well, implement exercise into their routine, and shift their mindset to maintain their health for the rest of their lives.”
The survey results were published in January by Orlando Health.
Surveying Americans
The national survey, conducted for Orlando Health by the market research firm Ipsos in early November 2023, asked 1017 US adults whether they agreed or disagreed with several statements about weight loss and bariatric surgery. The statements and responses are as follows:
- “Weight loss surgery is a shortcut to shedding pounds” — 60% strongly or somewhat agreed, 38% strongly or somewhat disagreed, and the remainder declined to answer.
- “Weight loss surgery is cosmetic and mainly impacts appearance” — 37% strongly or somewhat agreed, 61% strongly or somewhat disagreed, and the remainder declined to respond.
- “Exercise and diet should be enough for weight loss” — 61% strongly or somewhat agreed, 37% strongly or somewhat disagreed, and the remainder declined to respond.
- “Weight loss surgery should only be pursued as a last resort” — 79% strongly or somewhat agreed, 19% strongly or somewhat disagreed, and the remainder declined to answer.
- “Surgery should be more socially accepted as a way to lose weight” — 46% strongly or somewhat agreed, 52% strongly or somewhat disagreed, and the remainder declined to respond.
Men’s responses indicated that they are more likely to have negative views toward weight loss surgery than women. For example, 66% of men vs 54% of women respondents see weight loss surgery as a shortcut to losing weight. Conversely, 42% of men vs 50% of women said that surgery should be a more socially accepted weight loss method.
Opinions that might interfere with the willingness to have weight loss surgery were apparent among people with obesity. The survey found that 65% of respondents with obesity and 59% with extreme obesity view surgery as a shortcut. Eighty-two percent of respondents with obesity and 68% with extreme obesity see surgery as a last resort.
At the end of 2022, the American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders updated their guidelines for metabolic and bariatric surgery for the first time since 1991, with the aim of expanding access to surgery, Orlando Health noted. However, only 1% of those who are clinically eligible end up undergoing weight loss surgery, even with advancements in laparoscopic and robotic techniques that have made it safer and less invasive, the health system added.
“Because of the stigma around obesity and bariatric surgery, so many of my patients feel defeated if they can’t lose weight on their own,” said Muhammad Ghanem, MD, a bariatric surgeon at Orlando Health.
“But when I tell them obesity is a disease and that many of its causes are outside of their control, you can see their relief,” he said. “They often even shed a tear because they’ve struggled with their weight all their lives and finally have some validation.”
Individualizing Treatment
Obesity treatment plans should be tailored to patients on the basis of individual factors such as body mass index, existing medical conditions, and family history, Dr. Teixeira said.
Besides bariatric surgery, patients also may consider options such as counseling, lifestyle changes, and medications including the latest weight loss drugs, he added.
The clinical approach to obesity treatment has evolved, said Miguel Burch, MD, director of general surgery and chief of minimally invasive and gastrointestinal surgery at Cedars-Sinai, Los Angeles, California, who was not involved in the survey.
“At one point in my career, I could say the only proven durable treatment for obesity is weight loss surgery. This was in the context of patients who were morbidly obese requiring risk reduction, not for a year or two but for decades, and not for 10-20 pounds but for 40-60 pounds of weight loss,” said Dr. Burch, who also directs the bariatric surgery program at Torrance Memorial Medical Center, Torrance, California.
“That was a previous era. We are now in a new one with the weight loss drugs,” Dr. Burch said. “In fact, it’s wonderful to have the opportunity to serve so many patients with an option other than just surgery.”
Still, Dr. Burch added, “we have to change the way we look at obesity management as being either surgery or medicine and start thinking about it more as a multidisciplinary approach to a chronic and potentially relapsing disease.”
A version of this article appeared on Medscape.com.
Most Americans’ views about obesity and bariatric surgery are colored by stigmas, according to a new survey from the healthcare system at Orlando Health.
For example, most Americans believe that weight loss surgery should be pursued only as a last resort and that bariatric surgery is a shortcut to shedding pounds, the survey found.
Common stigmas could be deterring people who qualify for bariatric surgery from pursuing it, according to Orlando Health, located in Florida.
“Bariatric surgery is by no means an easy way out. If you have the courage to ask for help and commit to doing the hard work of changing your diet and improving your life, you’re a champion in my book,” said Andre Teixeira, MD, medical director and bariatric surgeon at Orlando Health Weight Loss and Bariatric Surgery Institute, Orlando, Florida.
“Surgery is simply a tool to jumpstart that change,” he said. “After surgery, it is up to the patient to learn how to eat well, implement exercise into their routine, and shift their mindset to maintain their health for the rest of their lives.”
The survey results were published in January by Orlando Health.
Surveying Americans
The national survey, conducted for Orlando Health by the market research firm Ipsos in early November 2023, asked 1017 US adults whether they agreed or disagreed with several statements about weight loss and bariatric surgery. The statements and responses are as follows:
- “Weight loss surgery is a shortcut to shedding pounds” — 60% strongly or somewhat agreed, 38% strongly or somewhat disagreed, and the remainder declined to answer.
- “Weight loss surgery is cosmetic and mainly impacts appearance” — 37% strongly or somewhat agreed, 61% strongly or somewhat disagreed, and the remainder declined to respond.
- “Exercise and diet should be enough for weight loss” — 61% strongly or somewhat agreed, 37% strongly or somewhat disagreed, and the remainder declined to respond.
- “Weight loss surgery should only be pursued as a last resort” — 79% strongly or somewhat agreed, 19% strongly or somewhat disagreed, and the remainder declined to answer.
- “Surgery should be more socially accepted as a way to lose weight” — 46% strongly or somewhat agreed, 52% strongly or somewhat disagreed, and the remainder declined to respond.
Men’s responses indicated that they are more likely to have negative views toward weight loss surgery than women. For example, 66% of men vs 54% of women respondents see weight loss surgery as a shortcut to losing weight. Conversely, 42% of men vs 50% of women said that surgery should be a more socially accepted weight loss method.
Opinions that might interfere with the willingness to have weight loss surgery were apparent among people with obesity. The survey found that 65% of respondents with obesity and 59% with extreme obesity view surgery as a shortcut. Eighty-two percent of respondents with obesity and 68% with extreme obesity see surgery as a last resort.
At the end of 2022, the American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders updated their guidelines for metabolic and bariatric surgery for the first time since 1991, with the aim of expanding access to surgery, Orlando Health noted. However, only 1% of those who are clinically eligible end up undergoing weight loss surgery, even with advancements in laparoscopic and robotic techniques that have made it safer and less invasive, the health system added.
“Because of the stigma around obesity and bariatric surgery, so many of my patients feel defeated if they can’t lose weight on their own,” said Muhammad Ghanem, MD, a bariatric surgeon at Orlando Health.
“But when I tell them obesity is a disease and that many of its causes are outside of their control, you can see their relief,” he said. “They often even shed a tear because they’ve struggled with their weight all their lives and finally have some validation.”
Individualizing Treatment
Obesity treatment plans should be tailored to patients on the basis of individual factors such as body mass index, existing medical conditions, and family history, Dr. Teixeira said.
Besides bariatric surgery, patients also may consider options such as counseling, lifestyle changes, and medications including the latest weight loss drugs, he added.
The clinical approach to obesity treatment has evolved, said Miguel Burch, MD, director of general surgery and chief of minimally invasive and gastrointestinal surgery at Cedars-Sinai, Los Angeles, California, who was not involved in the survey.
“At one point in my career, I could say the only proven durable treatment for obesity is weight loss surgery. This was in the context of patients who were morbidly obese requiring risk reduction, not for a year or two but for decades, and not for 10-20 pounds but for 40-60 pounds of weight loss,” said Dr. Burch, who also directs the bariatric surgery program at Torrance Memorial Medical Center, Torrance, California.
“That was a previous era. We are now in a new one with the weight loss drugs,” Dr. Burch said. “In fact, it’s wonderful to have the opportunity to serve so many patients with an option other than just surgery.”
Still, Dr. Burch added, “we have to change the way we look at obesity management as being either surgery or medicine and start thinking about it more as a multidisciplinary approach to a chronic and potentially relapsing disease.”
A version of this article appeared on Medscape.com.
Most Americans’ views about obesity and bariatric surgery are colored by stigmas, according to a new survey from the healthcare system at Orlando Health.
For example, most Americans believe that weight loss surgery should be pursued only as a last resort and that bariatric surgery is a shortcut to shedding pounds, the survey found.
Common stigmas could be deterring people who qualify for bariatric surgery from pursuing it, according to Orlando Health, located in Florida.
“Bariatric surgery is by no means an easy way out. If you have the courage to ask for help and commit to doing the hard work of changing your diet and improving your life, you’re a champion in my book,” said Andre Teixeira, MD, medical director and bariatric surgeon at Orlando Health Weight Loss and Bariatric Surgery Institute, Orlando, Florida.
“Surgery is simply a tool to jumpstart that change,” he said. “After surgery, it is up to the patient to learn how to eat well, implement exercise into their routine, and shift their mindset to maintain their health for the rest of their lives.”
The survey results were published in January by Orlando Health.
Surveying Americans
The national survey, conducted for Orlando Health by the market research firm Ipsos in early November 2023, asked 1017 US adults whether they agreed or disagreed with several statements about weight loss and bariatric surgery. The statements and responses are as follows:
- “Weight loss surgery is a shortcut to shedding pounds” — 60% strongly or somewhat agreed, 38% strongly or somewhat disagreed, and the remainder declined to answer.
- “Weight loss surgery is cosmetic and mainly impacts appearance” — 37% strongly or somewhat agreed, 61% strongly or somewhat disagreed, and the remainder declined to respond.
- “Exercise and diet should be enough for weight loss” — 61% strongly or somewhat agreed, 37% strongly or somewhat disagreed, and the remainder declined to respond.
- “Weight loss surgery should only be pursued as a last resort” — 79% strongly or somewhat agreed, 19% strongly or somewhat disagreed, and the remainder declined to answer.
- “Surgery should be more socially accepted as a way to lose weight” — 46% strongly or somewhat agreed, 52% strongly or somewhat disagreed, and the remainder declined to respond.
Men’s responses indicated that they are more likely to have negative views toward weight loss surgery than women. For example, 66% of men vs 54% of women respondents see weight loss surgery as a shortcut to losing weight. Conversely, 42% of men vs 50% of women said that surgery should be a more socially accepted weight loss method.
Opinions that might interfere with the willingness to have weight loss surgery were apparent among people with obesity. The survey found that 65% of respondents with obesity and 59% with extreme obesity view surgery as a shortcut. Eighty-two percent of respondents with obesity and 68% with extreme obesity see surgery as a last resort.
At the end of 2022, the American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders updated their guidelines for metabolic and bariatric surgery for the first time since 1991, with the aim of expanding access to surgery, Orlando Health noted. However, only 1% of those who are clinically eligible end up undergoing weight loss surgery, even with advancements in laparoscopic and robotic techniques that have made it safer and less invasive, the health system added.
“Because of the stigma around obesity and bariatric surgery, so many of my patients feel defeated if they can’t lose weight on their own,” said Muhammad Ghanem, MD, a bariatric surgeon at Orlando Health.
“But when I tell them obesity is a disease and that many of its causes are outside of their control, you can see their relief,” he said. “They often even shed a tear because they’ve struggled with their weight all their lives and finally have some validation.”
Individualizing Treatment
Obesity treatment plans should be tailored to patients on the basis of individual factors such as body mass index, existing medical conditions, and family history, Dr. Teixeira said.
Besides bariatric surgery, patients also may consider options such as counseling, lifestyle changes, and medications including the latest weight loss drugs, he added.
The clinical approach to obesity treatment has evolved, said Miguel Burch, MD, director of general surgery and chief of minimally invasive and gastrointestinal surgery at Cedars-Sinai, Los Angeles, California, who was not involved in the survey.
“At one point in my career, I could say the only proven durable treatment for obesity is weight loss surgery. This was in the context of patients who were morbidly obese requiring risk reduction, not for a year or two but for decades, and not for 10-20 pounds but for 40-60 pounds of weight loss,” said Dr. Burch, who also directs the bariatric surgery program at Torrance Memorial Medical Center, Torrance, California.
“That was a previous era. We are now in a new one with the weight loss drugs,” Dr. Burch said. “In fact, it’s wonderful to have the opportunity to serve so many patients with an option other than just surgery.”
Still, Dr. Burch added, “we have to change the way we look at obesity management as being either surgery or medicine and start thinking about it more as a multidisciplinary approach to a chronic and potentially relapsing disease.”
A version of this article appeared on Medscape.com.
New Guideline Offers Recommendations for Alcohol-Associated Liver Disease
according to a new clinical guideline from the American College of Gastroenterology.
In addition, health systems need to overcome barriers to treating alcohol use disorder (AUD) and commit to creating a multidisciplinary care model with behavioral interventions and pharmacotherapy for patients.
Experts were convened to develop these guidelines because it was “imperative to provide an up-to-date, evidence-based blueprint for how to care for patients, as well as guide prevention and research efforts in the field of ALD for the coming years,” said the first author, Loretta Jophlin, MD, PhD, assistant professor of medicine in gastroenterology, hepatology, and nutrition and medical director of liver transplantation at the University of Louisville in Kentucky.
“In recent years, perhaps fueled by the COVID-19 pandemic, alcohol use has been normalized in an increasing number of situations,” she said. “Drinking was normalized as a coping mechanism to deal with many of the sorrows we experienced during the pandemic, including loss of purposeful work and social isolation, and many more people are struggling with AUD. So many aspects of our culture have been inundated by the presence of alcohol use, and we need to work hard to denormalize this, first focusing on at-risk populations.”
The guideline was published in the January issue of the American Journal of Gastroenterology.
Updating ALD Recommendations
With ALD as the most common cause of advanced hepatic disease and a frequent indicator of eventual liver transplantation, the rising incidence of alcohol use during the past decade has led to rapid growth in ALD-related healthcare burdens, the guideline authors wrote.
In particular, those with ALD tend to present at an advanced stage and progress faster, which can lead to progressive fibrosis, cirrhosis, and hepatocellular carcinoma. This can include alcohol-associated hepatitis (AH), which often presents with a rapid onset or worsening of jaundice and can lead to acute or chronic liver failure.
To update the guideline, Dr. Jophlin and colleagues analyzed data based on a patient-intervention-comparison-outcome format, resulting in 34 key concepts or statements and 21 recommendations.
Among them, the authors recommended screening and treating AUD with the goal of helping patients who have not yet developed significant liver injury and preventing progression to advanced stages of ALD, particularly among at-risk groups who have had an increasing prevalence of severe AUD, including women, younger people, and Hispanic and American Indian patients.
“So many patients are still told to ‘stop drinking’ or ‘cut back’ but are provided no additional resources. Without offering referrals to treatment programs or pharmacologic therapies to assist in abstinence, many patients are not successful,” Dr. Jophlin said. “We hope these guidelines empower providers to consider selected [Food and Drug Administration]-approved medications, well-studied off-label therapies, and nonpharmacologic interventions to aid their patients’ journeys to abstinence and hopefully avert the progression of ALD.”
In addition, the guidelines provide recommendations for AH treatment. In patients with severe AH, the authors offered strong recommendations against the use of pentoxifylline and prophylactic antibiotics, and in support of corticosteroid therapy and intravenous N-acetyl cysteine as an adjuvant to corticosteroids.
Liver transplantation, which may be recommended for carefully selected patients, is being performed at many centers but remains relatively controversial, Dr. Jophlin said.
“Questions remain about ideal patient selection as center practices vary considerably, yet we have started to realize the impacts of relapse after transplantation,” she said. “The guidelines highlight the knowns and unknowns in this area and will hopefully serve as a catalyst for the dissemination of centers’ experiences and the development of a universal set of ethically sound, evidence-based guidelines to be used by all transplant centers.”
Policy Implications
Dr. Jophlin and colleagues noted the importance of policy aimed at alcohol use reduction, multidisciplinary care for AUD and ALD, and additional research around severe AH.
“As a practicing transplant hepatologist and medical director of a liver transplant program in the heart of Bourbon country, I am a part of just one healthcare team experiencing ALD, particularly AH, as a mass casualty event. Healthcare teams are fighting an unrelenting fire that the alcohol industry is pouring gasoline on,” Dr. Jophlin said. “It is imperative that healthcare providers have a voice in the policies that shape this preventable disease. We hope these guidelines inspire practitioners to explore our influence on how alcohol is regulated, marketed, and distributed.”
Additional interventions and public policy considerations could help reduce alcohol-related morbidity and mortality at a moment when the characteristics of those who present with AUD appear to be evolving.
“The typical person I’m seeing now is not someone who has been drinking heavily for decades. Rather, it’s a young person who has been drinking heavily for many months or a couple of years,” said James Burton, MD, a professor of medicine at the University of Colorado School of Medicine and medical director of liver transplantation at the University of Colorado Hospital’s Anschutz Medical Campus in Aurora.
Dr. Burton, who wasn’t involved with the guideline, noted it’s become more common for people to drink multiple alcoholic drinks per day for multiple times per week. Patients often don’t think it’s a problem, even as he discusses their liver-related issues.
“We can’t just keep living and working the way we were 10 years ago,” he said. “We’ve got to change how we approach treatment. We have to treat liver disease and AUD.”
The guideline was supported by several National Institutes of Health grants and an American College of Gastroenterology faculty development grant. The authors declared potential competing interests with various pharmaceutical companies. Dr. Burton reported no financial disclosures.
A version of this article appeared on Medscape.com.
according to a new clinical guideline from the American College of Gastroenterology.
In addition, health systems need to overcome barriers to treating alcohol use disorder (AUD) and commit to creating a multidisciplinary care model with behavioral interventions and pharmacotherapy for patients.
Experts were convened to develop these guidelines because it was “imperative to provide an up-to-date, evidence-based blueprint for how to care for patients, as well as guide prevention and research efforts in the field of ALD for the coming years,” said the first author, Loretta Jophlin, MD, PhD, assistant professor of medicine in gastroenterology, hepatology, and nutrition and medical director of liver transplantation at the University of Louisville in Kentucky.
“In recent years, perhaps fueled by the COVID-19 pandemic, alcohol use has been normalized in an increasing number of situations,” she said. “Drinking was normalized as a coping mechanism to deal with many of the sorrows we experienced during the pandemic, including loss of purposeful work and social isolation, and many more people are struggling with AUD. So many aspects of our culture have been inundated by the presence of alcohol use, and we need to work hard to denormalize this, first focusing on at-risk populations.”
The guideline was published in the January issue of the American Journal of Gastroenterology.
Updating ALD Recommendations
With ALD as the most common cause of advanced hepatic disease and a frequent indicator of eventual liver transplantation, the rising incidence of alcohol use during the past decade has led to rapid growth in ALD-related healthcare burdens, the guideline authors wrote.
In particular, those with ALD tend to present at an advanced stage and progress faster, which can lead to progressive fibrosis, cirrhosis, and hepatocellular carcinoma. This can include alcohol-associated hepatitis (AH), which often presents with a rapid onset or worsening of jaundice and can lead to acute or chronic liver failure.
To update the guideline, Dr. Jophlin and colleagues analyzed data based on a patient-intervention-comparison-outcome format, resulting in 34 key concepts or statements and 21 recommendations.
Among them, the authors recommended screening and treating AUD with the goal of helping patients who have not yet developed significant liver injury and preventing progression to advanced stages of ALD, particularly among at-risk groups who have had an increasing prevalence of severe AUD, including women, younger people, and Hispanic and American Indian patients.
“So many patients are still told to ‘stop drinking’ or ‘cut back’ but are provided no additional resources. Without offering referrals to treatment programs or pharmacologic therapies to assist in abstinence, many patients are not successful,” Dr. Jophlin said. “We hope these guidelines empower providers to consider selected [Food and Drug Administration]-approved medications, well-studied off-label therapies, and nonpharmacologic interventions to aid their patients’ journeys to abstinence and hopefully avert the progression of ALD.”
In addition, the guidelines provide recommendations for AH treatment. In patients with severe AH, the authors offered strong recommendations against the use of pentoxifylline and prophylactic antibiotics, and in support of corticosteroid therapy and intravenous N-acetyl cysteine as an adjuvant to corticosteroids.
Liver transplantation, which may be recommended for carefully selected patients, is being performed at many centers but remains relatively controversial, Dr. Jophlin said.
“Questions remain about ideal patient selection as center practices vary considerably, yet we have started to realize the impacts of relapse after transplantation,” she said. “The guidelines highlight the knowns and unknowns in this area and will hopefully serve as a catalyst for the dissemination of centers’ experiences and the development of a universal set of ethically sound, evidence-based guidelines to be used by all transplant centers.”
Policy Implications
Dr. Jophlin and colleagues noted the importance of policy aimed at alcohol use reduction, multidisciplinary care for AUD and ALD, and additional research around severe AH.
“As a practicing transplant hepatologist and medical director of a liver transplant program in the heart of Bourbon country, I am a part of just one healthcare team experiencing ALD, particularly AH, as a mass casualty event. Healthcare teams are fighting an unrelenting fire that the alcohol industry is pouring gasoline on,” Dr. Jophlin said. “It is imperative that healthcare providers have a voice in the policies that shape this preventable disease. We hope these guidelines inspire practitioners to explore our influence on how alcohol is regulated, marketed, and distributed.”
Additional interventions and public policy considerations could help reduce alcohol-related morbidity and mortality at a moment when the characteristics of those who present with AUD appear to be evolving.
“The typical person I’m seeing now is not someone who has been drinking heavily for decades. Rather, it’s a young person who has been drinking heavily for many months or a couple of years,” said James Burton, MD, a professor of medicine at the University of Colorado School of Medicine and medical director of liver transplantation at the University of Colorado Hospital’s Anschutz Medical Campus in Aurora.
Dr. Burton, who wasn’t involved with the guideline, noted it’s become more common for people to drink multiple alcoholic drinks per day for multiple times per week. Patients often don’t think it’s a problem, even as he discusses their liver-related issues.
“We can’t just keep living and working the way we were 10 years ago,” he said. “We’ve got to change how we approach treatment. We have to treat liver disease and AUD.”
The guideline was supported by several National Institutes of Health grants and an American College of Gastroenterology faculty development grant. The authors declared potential competing interests with various pharmaceutical companies. Dr. Burton reported no financial disclosures.
A version of this article appeared on Medscape.com.
according to a new clinical guideline from the American College of Gastroenterology.
In addition, health systems need to overcome barriers to treating alcohol use disorder (AUD) and commit to creating a multidisciplinary care model with behavioral interventions and pharmacotherapy for patients.
Experts were convened to develop these guidelines because it was “imperative to provide an up-to-date, evidence-based blueprint for how to care for patients, as well as guide prevention and research efforts in the field of ALD for the coming years,” said the first author, Loretta Jophlin, MD, PhD, assistant professor of medicine in gastroenterology, hepatology, and nutrition and medical director of liver transplantation at the University of Louisville in Kentucky.
“In recent years, perhaps fueled by the COVID-19 pandemic, alcohol use has been normalized in an increasing number of situations,” she said. “Drinking was normalized as a coping mechanism to deal with many of the sorrows we experienced during the pandemic, including loss of purposeful work and social isolation, and many more people are struggling with AUD. So many aspects of our culture have been inundated by the presence of alcohol use, and we need to work hard to denormalize this, first focusing on at-risk populations.”
The guideline was published in the January issue of the American Journal of Gastroenterology.
Updating ALD Recommendations
With ALD as the most common cause of advanced hepatic disease and a frequent indicator of eventual liver transplantation, the rising incidence of alcohol use during the past decade has led to rapid growth in ALD-related healthcare burdens, the guideline authors wrote.
In particular, those with ALD tend to present at an advanced stage and progress faster, which can lead to progressive fibrosis, cirrhosis, and hepatocellular carcinoma. This can include alcohol-associated hepatitis (AH), which often presents with a rapid onset or worsening of jaundice and can lead to acute or chronic liver failure.
To update the guideline, Dr. Jophlin and colleagues analyzed data based on a patient-intervention-comparison-outcome format, resulting in 34 key concepts or statements and 21 recommendations.
Among them, the authors recommended screening and treating AUD with the goal of helping patients who have not yet developed significant liver injury and preventing progression to advanced stages of ALD, particularly among at-risk groups who have had an increasing prevalence of severe AUD, including women, younger people, and Hispanic and American Indian patients.
“So many patients are still told to ‘stop drinking’ or ‘cut back’ but are provided no additional resources. Without offering referrals to treatment programs or pharmacologic therapies to assist in abstinence, many patients are not successful,” Dr. Jophlin said. “We hope these guidelines empower providers to consider selected [Food and Drug Administration]-approved medications, well-studied off-label therapies, and nonpharmacologic interventions to aid their patients’ journeys to abstinence and hopefully avert the progression of ALD.”
In addition, the guidelines provide recommendations for AH treatment. In patients with severe AH, the authors offered strong recommendations against the use of pentoxifylline and prophylactic antibiotics, and in support of corticosteroid therapy and intravenous N-acetyl cysteine as an adjuvant to corticosteroids.
Liver transplantation, which may be recommended for carefully selected patients, is being performed at many centers but remains relatively controversial, Dr. Jophlin said.
“Questions remain about ideal patient selection as center practices vary considerably, yet we have started to realize the impacts of relapse after transplantation,” she said. “The guidelines highlight the knowns and unknowns in this area and will hopefully serve as a catalyst for the dissemination of centers’ experiences and the development of a universal set of ethically sound, evidence-based guidelines to be used by all transplant centers.”
Policy Implications
Dr. Jophlin and colleagues noted the importance of policy aimed at alcohol use reduction, multidisciplinary care for AUD and ALD, and additional research around severe AH.
“As a practicing transplant hepatologist and medical director of a liver transplant program in the heart of Bourbon country, I am a part of just one healthcare team experiencing ALD, particularly AH, as a mass casualty event. Healthcare teams are fighting an unrelenting fire that the alcohol industry is pouring gasoline on,” Dr. Jophlin said. “It is imperative that healthcare providers have a voice in the policies that shape this preventable disease. We hope these guidelines inspire practitioners to explore our influence on how alcohol is regulated, marketed, and distributed.”
Additional interventions and public policy considerations could help reduce alcohol-related morbidity and mortality at a moment when the characteristics of those who present with AUD appear to be evolving.
“The typical person I’m seeing now is not someone who has been drinking heavily for decades. Rather, it’s a young person who has been drinking heavily for many months or a couple of years,” said James Burton, MD, a professor of medicine at the University of Colorado School of Medicine and medical director of liver transplantation at the University of Colorado Hospital’s Anschutz Medical Campus in Aurora.
Dr. Burton, who wasn’t involved with the guideline, noted it’s become more common for people to drink multiple alcoholic drinks per day for multiple times per week. Patients often don’t think it’s a problem, even as he discusses their liver-related issues.
“We can’t just keep living and working the way we were 10 years ago,” he said. “We’ve got to change how we approach treatment. We have to treat liver disease and AUD.”
The guideline was supported by several National Institutes of Health grants and an American College of Gastroenterology faculty development grant. The authors declared potential competing interests with various pharmaceutical companies. Dr. Burton reported no financial disclosures.
A version of this article appeared on Medscape.com.