User login
Clinical Outcomes of Minimally Invasive Versus Open TLIF: A Propensity-Matched Cohort Study
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
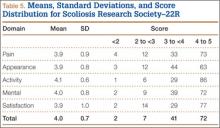
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
Characteristics Associated With Active Defects in Juvenile Spondylolysis
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
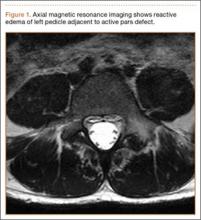
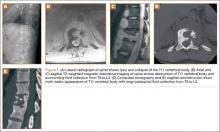
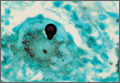
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
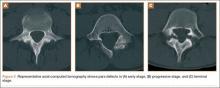
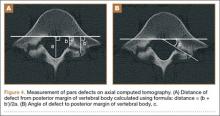
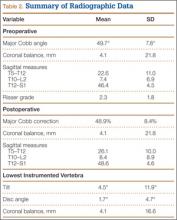
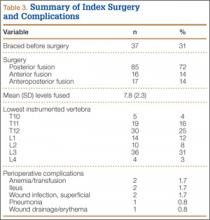
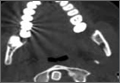
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
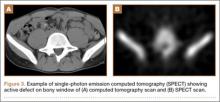
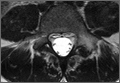
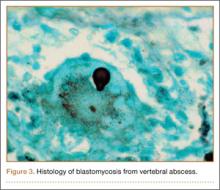
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
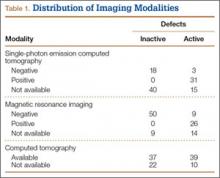
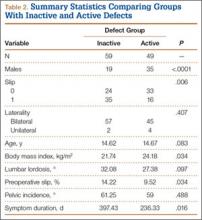
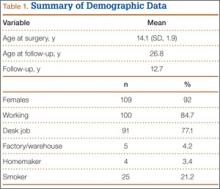
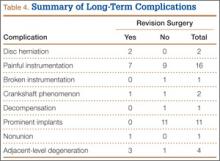
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
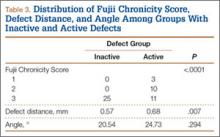
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
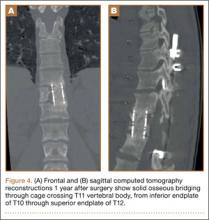
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Health-Related Quality-of-Life Scores, Spine-Related Symptoms, and Reoperations in Young Adults 7 to 17 Years After Surgical Treatment of Adolescent Idiopathic Scoliosis
The goal of surgical treatment of adolescent idiopathic scoliosis (AIS) is to prevent disability associated with curve progression.1 Early studies tended to focus on radiographic measures, such as curve correction and sagittal balance, rather than on improvements in quality of life (QOL).2-5 Although studies have reported on QOL in patients treated surgically for scoliosis,6-11 these studies were largely limited by small sample size and inclusion of patients with congenital and neuromuscular scoliosis,9 lack of a generic measure of QOL,6,7 or lack of surgical treatment of patients in the cohort.10
We conducted a study to determine disease-specific and general health-related QOL (HR-QOL) in young adults who underwent surgical correction of their spinal deformity during adolescence and to evaluate associated complications and reoperations.
Materials and Methods
After obtaining institutional review board approval, we queried the surgical database of a large metropolitan tertiary referral center for consecutive patients who had undergone spine deformity correction between the ages of 10 and 17 years (January 1993–December 2003). Hospital and medical records were retrospectively reviewed to confirm the diagnosis of AIS. Patients with congenital, neuromuscular, juvenile, or infantile scoliosis were excluded. Patients with intraspinal pathology (eg, tethered cord, syringomyelia), developmental delay, chromosomal abnormality, or congenital heart disease were also excluded. Patients were contacted by mail or telephone, and the Scoliosis Research Society–22R (SRS-22R)12-15 and the Short Form–12 (SF-12)16 were administered. Standard demographic and surgical data were also collected.
The SRS-22R is a scoliosis-specific HR-QOL questionnaire with 22 items, 5 domains (pain, activity, appearance, mental, satisfaction), and a total score.12-15 Each domain score ranges from 1 to 5 (higher scores indicating better outcomes). The SRS-22R is the outcome instrument most widely used to measure HR-QOL changes in patients with scoliosis, and it is available in several languages.17-26
The SF-12, a 12-item self-administered short-form health status survey developed in the Medical Outcomes Study, measures patient-based health status. Two composite scores can be calculated: physical composite summary (PCS) and mental composite summary (MCS).16 Using norm-based scoring, all domain scales have a mean (SD) of 50 (10) based on the general 1998 US population. Thus, scores under 50 fall below the general population mean.
In addition, patients were surveyed to determine the incidence of spine-related symptoms and complaints, including activity limitations, rib prominence, waistline asymmetry, back pain, limited range of motion (ROM), shortness of breath, wound/scar problems, lung disease/asthma, heart disease, high blood pressure, and arthritis. Data regarding postoperative treatment regimens of physical therapy, narcotic pain medication, spinal/epidural injections, and nonsteroidal anti-inflammatory drug (NSAID) use were collected. Patients were also queried regarding their current working status and smoking status.
Standard demographic and surgical data were collected from hospital and office charts and radiographs. Data collected included history of bracing, age at index surgery, number of levels fused, surgical approach (anterior, posterior, combined), postoperative complications (eg, ileus, wound infection, anemia, pneumonia), and immediate preoperative and final postoperative radiographic measures. Data on need for subsequent revision surgery and indications for revision surgery were also collected.
Preoperative and latest follow-up radiographs were measured to determine curve magnitude, sagittal and coronal balance, and percentage curve correction. Coronal balance was defined as the distance between a plumb line drawn vertically from the spinous process of C7 and the central sacral line on full-length posteroanterior radiographs. Sagittal balance was defined as the distance of a plumb line drawn vertically from the center of the body of C7 and the posterosuperior endplate of S1.27
Regression analysis was performed to identify factors predictive of SRS-22R total scores. Factors included in the analysis were sex, age at surgery, Lenke type, surgery type (anterior, posterior, anteroposterior), number of levels fused, lowest instrumented vertebra, perioperative complications, percentage curve correction, postoperative coronal and sagittal balance, smoking status, and need for revision surgery. Although age and sex were considered variables outside the surgeon’s control, they were included in the model, as previous studies have shown that SRS scores varied by age and sex both in adolescents28 and adults.29 Significance was set at P < .01. All data analysis was performed with IBM SPSS Version 19.0 (Somers, New York).
Results
Of the 384 postoperative patients identified for study inclusion, 134 (35%) completed surveys. Sixteen patients with nonidiopathic scoliosis were excluded, leaving 118 available for analysis. Of the remaining patients, 248 (64%) could not be contacted because of a change in address or phone number. Two patients (1%) were unwilling to complete survey requests. There was no statistically significant difference in demographics between patients with and without follow-up data available. Demographics are summarized in Table 1. There were 109 females (92%). Mean (SD) age at surgery was 14.1 (1.9) years. Only 37 (31%) were braced before surgery. Table 2 summarizes the radiographic data. Mean (SD) major Cobb angle was 49.7° (7.8°). Eighty-five patients (72%) underwent posterior fusion with instrumentation using hooks only; another 16 (14%) had anterior-only surgery, and another 17 (14%) had combined anterior-posterior surgery. A mean of 7.8 levels were fused. Index surgery data and lowest instrumented vertebra distribution are summarized in Table 3. Mean (SD) percentage curve correction was 48.9% (8.4%).
Seven patients had a total of 8 perioperative complications: anemia requiring transfusion (2), ileus necessitating nasogastric tube insertion (2), superficial wound infection treated with oral antibiotics and local wound care (2), wound drainage and erythema (1), and pneumonia (1). Mean (SD) length of clinical and radiographic follow-up was 57.9 (36.3) months.
Table 4 summarizes the long-term complications. Of the 38 patients with long-term complications, 14 required reoperation. The indications were disc herniation (2 patients), painful instrumentation (7), crankshaft phenomenon (1), nonunion (1), and adjacent-level degeneration (3). Both disc herniations were at L5–S1, several segments below the distal extent of the fusion. Of the 7 patients who had painful instrumentation removed, 6 had the entire construct removed, and 1 had the proximal half of a rod taken out. The 3 patients with adjacent-level degeneration had stenosis at the distal end of the construct—at L5–S1 (2 patients) or L2–L3 (1 patient).
Mean (SD) time between surgery and completion of the surveys/questionnaires was 12.7 (3.2) years (range, 10-18 years). Mean age of respondents was 26.8 years. Twenty-five respondents (21%) were smokers. Mean (SD) outcome scores were 50.9 (9.4) for SF-12 PCS and 49.4 (10.2) for SF-12 MCS. Eighteen patients (15%) had SF-12 PCS scores 1 SD below normal, and 15 (13%) had SF-12 MCS scores 1 SD below normal. Mean (SD) SRS-22R Total score was 4.0 (0.7). Means, standard deviations, and distribution of SRS domain scores are summarized in Table 5. Of the variables, only current smoking (P < .001) was predictive of SRS-22R Total scores, accounting for 20% of their variability (Table 6).
One hundred patients (85%) had jobs, mostly desk jobs. The postoperative limitations most commonly reported are summarized in Table 7. These included at least intermittent back pain in 90 patients (76%), limited ROM in 52 (44%), and activity limitations in 54 (46%). Less common limitations were waistline imbalance in 41 (35%), rib prominence in 28 (24%), wound/scar problems in 18 (15%), and shortness of breath in 18 (15%). Other related medical problems were lung disease/asthma in 11 (9%), osteoarthritis/degenerative arthritis in 11 (9%), heart disease in 3 (3%), and high blood pressure in 2 (2%).
A minority of patients also participated in postoperative treatment regimens. The most common treatment was regular use of NSAIDs (25 patients, 21%). Other treatments were physical therapy (14, 12%), narcotic pain medication use (5, 4%), and epidural steroid injections (5, 4%). Table 8 summarizes the postoperative treatments used by patients with scoliosis.
Discussion
A major concern about prophylactic interventions for diseases is that the treatment will harm the patient. This is especially true for major spine surgery performed on adolescents with minimal symptoms. Although the incidence of perioperative complications in children undergoing corrective spinal surgery for AIS has been reported,30-32 the effect of the surgery on the disease-specific HR-QOL outcomes of these individuals as young adults has not been previously studied. Over the past few decades, a paradigm shift in understanding health and disability has occurred, with increased emphasis being placed on HR-QOL outcomes measures and understanding disability as relating to a measureable impact of the functioning of an individual after a change in health or environment. This change was substantiated when the World Health Organization endorsed the International Classification of Functioning, Disability and Health.33 In light of this shift, we present the disease-specific and general HR-QOL outcomes of young adults who had undergone surgical correction for spinal deformity during adolescence, as well as their associated complications and reoperations, in an attempt to identify targets for improvement.
Our patient-reported outcomes demonstrated a high incidence of occasional back pain, activity-related complaints, and limited ROM. Comparison of our cohort’s SRS-22R outcomes with previously published normative values for the unaffected adolescent population28,34 suggests worse scores for the disease-specific SRS-22R domains of pain and appearance. In 2012, Daubs and colleagues34 reported that normative scores on various SRS-22 domains were statistically lower with age (scores decreased from age 10 to age 19 years). Both Verma and colleagues28 and Daubs and colleagues34 reported lower scores for females than for males. Therefore, it is unclear whether the differences observed in our cohort may be accounted for by the larger proportion of females compared with the normative data.
General health scores on the SF-12 were similar to the population norm (mean [SD]) of 50 (10) referenced by Ware and colleagues.16 These findings suggest that, though pain and appearance may be statistically lower in our cohort—as measured with the SRS-22R—the cohort’s spine-related symptoms do not seem to lower its general health. Eighty-five percent of the patients were working at the time of the survey, further supporting a relatively normal level of overall function. In a retrospective review by Takayama and colleagues,9 similar results were found with regard to working after AIS fusion surgery. Of 32 patients treated surgically for scoliosis, at a mean of 21.1 years after the index fusion 27 (84.4%) were or had been engaged in various occupations without marked difficulty.
Our results in a cohort of patients with segmental instrumentation using hooks are similar to results in other studies of long-term HR-QOL measures in patients with AIS and Harrington rod instrumentation. Danielsson and Nachemson35 evaluated patients with surgically treated AIS with at least 20-year follow-up and reported that, in their surgical cohort with a mean age of 39.7 years, mean SF-36 PCS score was 50.9, and mean SF-36 MCS score was 50.2. In a recent study of patients with AIS and Harrington rod instrumentation, those of a mean age of 32.3 years had a mean score of 50.9 for both SF-36 PCS and SF-36 MCS.36
Regression analysis identified only smoking as a predictor of SRS-22R Total scores. This finding, that smokers have a lower health state, is expected even in the general population.37 Interestingly, bracing before surgery, Lenke type, surgery type, number of levels fused, lowest instrumented vertebra, incidence of perioperative complications, percentage curve correction, postoperative sagittal and coronal balance, and need for revision surgery did not influence HR-QOL measures in this cohort.
Our cohort’s incidence of occasional back pain was 76% (90/118 patients). Other reports have had similar findings. In 2012, Bas and colleagues38 studied self-reported pain in 126 consecutive patients with scoliosis and instrumented fusion. In their cohort, “most participants reported ‘no pain’ (38.5%) or ‘mild pain’ (30.8%) and 72.1% of participants reported a current work/school activity level of 100% normal.” Also in 2012, Rushton and Grevitt39 reported on a review and statistical analysis of the literature on HR-QOL in adolescents with untreated AIS and in unaffected adolescents. Their goal was to identify whether there were any differences in HR-QOL and, if so, whether they were clinically relevant. The authors concluded that pain and self-image tended to be statistically lower among cohorts with AIS but that only self-image was consistently different clinically between untreated patients with AIS and their unaffected peers.
Cosmetic complaints, though less common than functional concerns, affected a substantial percentage of our cohort. Waistline imbalance complaints were more common than rib prominence or scar-related complaints. The validity of patient-reported waistline imbalance is not known but may contribute to the SRS-22R outcomes in this cohort, particularly with regard to appearance scores. Respiratory symptoms, particularly those related to shortness of breath, were reported by 15% of patients. Respiratory symptoms may be in part secondary to underlying lung disease; smoking was reported by 21% of patients and asthma by 9%.
Few additional postoperative treatments were reported by patients. The most common treatment was regular use of NSAIDs (21%), followed by postoperative physical therapy (12%). Opiate medication use and spinal injections were rare—consistent with results reported by Danielsson and Nachemson35 in 2003.
Implant-related complaints, including painful instrumentation (13%) and implant prominence (9%), were some of the most common complaints in our study group. Although not all symptomatic instrumentation required surgical revision, 7 (50%) of the 14 additional spine surgeries were related to painful and/or prominent posterior instrumentation. Additional spine surgery was reported in 11.9% of our patients. Other indications for reoperation were disc herniation, crankshaft phenomenon, nonunion, and adjacent-level degeneration. Our rate of revision surgery is supported by the literature. In 2009, Luhmann and colleagues40 reported that 41 (3.9%) of 1057 primary spine fusions for idiopathic scoliosis required reoperation; the indications included infection (16/1057, 1.5%), pseudarthrosis (12, 1.1%), and painful/prominent implant (7, 0.7%). Richards and colleagues41 similarly reported on 1046 patients who underwent fusion for AIS. Of these patients, 135 underwent 172 repeat surgical interventions (12.9% reoperation rate), with 29 (21.5%) of the 135 undergoing 2 or more separate procedures. The most common reasons for reoperation were infection, symptomatic implant, and pseudarthrosis. The authors concluded that repeat surgeries were relatively common after the initial surgical procedures. Having a clearer understanding of instrumentation-related complaints and reoperations may lead to improvement in this surgeon-controlled variable.
There are limitations to this study. The data regarding clinical courses were collected by retrospective chart review, which has known limitations. To offset this, we collected prospective outcome data with use of the SF-12, the SRS-22R, and a spine-related complaints questionnaire. No control group was available for comparison of outcomes in our cohort. We used the SF-12 and previously published normative values for the SRS-22R for comparison with population norms. Such comparisons have inherent limitations, as the groups vary by sex and mean age; our cohort was primarily female and more than a decade older than the controls.
Only 35% of the patients who met the inclusion criteria had complete data that could be included in our analysis. Although there was no statistically significant difference in demographics between patients with and without follow-up data available, this low response rate could have introduced selection bias. Ideally, patients should have been seen in clinic, standing radiographs should have been taken, and pulmonary function tests should have been performed. However, these patients were asymptomatic, and ethical and insurance issues prevented those actions. Thus, any radiographic changes occurring over the intervening years, from the last clinic visit to completion of the surveys, were not documented. This situation may or may not have limited our findings, as other authors have found low correlation between radiographic outcomes and clinical outcome measures.13,14,19,36 During the period when these surgeries were performed, segmental spine instrumentation with hooks was the standard of care for deformity correction in AIS; therefore, all posterior instrumentations were done with hook-only segmental fixation. Current pedicle screw–based techniques that allow for additional correction of the deformity may provide different outcomes in the future.
We think that, despite the inherent limitations of this study, our data will be useful in the treatment of AIS. Our results suggest that postoperative spinal complaints are common and that, compared with an unaffected adolescent population, patients with AIS score significantly lower on pain and appearance domains of outcomes testing at a mean of 12.7 years after index fusion. Nevertheless, the outcomes do not seem to be of sufficient severity to affect general health and QOL as measured by outcomes testing.
Spinal deformity correction is performed to prevent impaired pulmonary function and spine-related disability later in life.42,43 Thus, longer-term studies, involving patients in their fifth and sixth decades of life, are needed to determine whether patients with AIS will have QOL outcomes, pulmonary function, and spine-related problems similar to those in the general population. In this cohort of young adults, smoking status was the only predictor of HR-QOL measures, and spinal deformity correction did not lead to decreased HR-QOL.
1. Tsutsui S, Pawelek J, Bastrom T, et al. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine. 2009;34(18):E653-E658.
2. Bonnett C, Brown JC, Cross B, Barron R. Posterior spinal fusion with Harrington rod instrumentation in 100 consecutive patients. Contemp Orthop. 1980;2:396-399.
3. Harrington PR, Dixon JR. An eleven year clinical investigation of Harrington instrument. Clin Orthop. 1973;(93):113-130.
4. Mielke CH, Lonstein JE, Denis F, Vandenbrink K, Winter RB. Surgical treatment of adolescent idiopathic scoliosis. A comparative analysis. J Bone Joint Surg Am. 1989;71(8):1170-1177.
5. Moskowitz A, Moe JH, Winter RB, Binner H. Long-term follow-up of scoliosis fusion. J Bone Joint Surg Am. 1980;62(3):529-554.
6. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Health-related quality of life and low back pain of patients surgically treated for scoliosis after 21 years or more of follow-up: comparison among non-idiopathic scoliosis, idiopathic scoliosis, and healthy subjects. Spine. 2012;37(22):1899-1903.
7. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Long-term clinical outcomes of surgery for adolescent idiopathic scoliosis 21 to 41 years later. Spine. 2012;37(5):402-405.
8. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46(7):474-478.
9. Takayama K, Nakamura H, Matsuda H. Quality of life in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine. 2009;34(20):2179-2184.
10. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527-1537.
11. Westrick ER, Ward WT. Adolescent idiopathic scoliosis: 5-year to 20-year evidence-based surgical results. J Pediatr Orthop. 2011;31(1 suppl):S61-S68.
12. Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 health-related quality of life questionnaire Function domain. Spine. 2006;31(5):593-597.
13. Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society–22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28(1):70-73.
14. Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society–22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28(1):63-69.
15. Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society–22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine. 2003;28(1):74-78.
16. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
17. Alanay A, Cil A, Berk H, et al. Reliability and validity of adapted Turkish version of Scoliosis Research Society–22 (SRS-22) questionnaire. Spine. 2005;30(21):2464-2468.
18. Beauséjour M, Joncas J, Goulet L, et al. Reliability and validity of adapted French Canadian version of Scoliosis Research Society outcomes questionnaire (SRS-22) in Quebec. Spine. 2009;34(6):623-628.
19. Climent JM, Bago J, Ey A, Perez-Grueso FJ, Izquierdo E. Validity of the Spanish version of the Scoliosis Research Society–22 (SRS-22) patient questionnaire. Spine. 2005;30(6):705-709.
20. Glowacki M, Misterska E, Laurentowska M, Mankowski P. Polish adaptation of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(10):1060-1065.
21. Hashimoto H, Sase T, Arai Y, Maruyama T, Isobe K, Shouno Y. Validation of a Japanese version of the Scoliosis Research Society–22 patient questionnaire among idiopathic scoliosis patients in Japan. Spine. 2007;32(4):E141-E146.
22. Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(12):1321-1324.
23. Monticone M, Carabalona R, Negrini S. Reliability of the Scoliosis Research Society–22 patient questionnaire (Italian version) in mild adolescent vertebral deformities. Eura Medicophys. 2004;40(3):191-197.
24. Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted German version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(8):818-821.
25. Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31(4):473-478.
26. Glattes RC, Burton DC, Lai SM, Frasier E, Asher MA. The reliability and concurrent validity of the Scoliosis Research Society–22R patient questionnaire compared with the Child Health Questionnaire–CF87 patient questionnaire for adolescent spinal deformity. Spine. 2007;32(16):1778-1784.
27. Blanke KM, Kuklo TR, Lenke LG, et al. Adolescent idiopathic scoliosis. In O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. Spinal Deformity Study Group Radiographic Measurement Manual. Memphis, TN: Medtronic; 2004.
28. Verma K, Lonner B, Hoashi JS, et al. Demographic factors affect Scoliosis Research Society–22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine. 2010;35(24):2134-2139.
29. Baldus C, Bridwell KH, Harrast J, et al. Age-gender matched comparison of SRS instrument scores between adult deformity and normal adults: are all SRS domains disease specific? Spine. 2008;33(20):2214-2218.
30. Brown CA, Lenke LG, Bridwell KH, Geideman WM, Hasan SA, Blanke K. Complication of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23(14):1566-1571.
31. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345-349.
32. Fu KM, Smith JS, Polly DW, et al. Scoliosis Research Society Morbidity and Mortality Committee. Morbidity and mortality associated with spinal surgery in children: a review of the Scoliosis Research Society morbidity and mortality database. J Neurosurg Pediatr. 2011;7(1):37-41.
33. World Health Organization. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva, Switzerland: World Health Organization; 2001.
34. Daubs M, Lawrence B, Hung M, et al. Scoliosis Research Society–22 results in 3,052 healthy adolescents age ten to 19 years. Abstract presented at: 47th Annual Meeting and Course of the Scoliosis Research Society; September 5-8, 2012; Chicago, IL. Abstract 72.
35. Danielsson AL, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case–control study—part II. Spine. 2003;28(18):E373-E383.
36. Götze C, Liljenqvist UR, Slomka A, Götze HG, Steinbeck J. Quality of life and back pain: outcome 16.7 years after Harrington instrumentation. Spine. 2002;27(13):1456-1463.
37. Quercioli C, Messina G, Barbini E, Carriero G, Fanì M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health-related quality of life: performances of three tools: comparison of three questionnaire scores. Eur J Health Econ. 2009;10(4):389-397.
38. Bas T, Franco N, Bas P, Bas JL. Pain and disability following fusion for idiopathic adolescent scoliosis: prevalence and associated factors. Evid Based Spine Care J. 2012;3(2):17-24.
39. Rushton PR, Grevitt MP. Comparison of untreated adolescent idiopathic scoliosis with normal controls: a review and statistical analysis of the literature. Spine. 2013;38(9):778-785.
40. Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine. 2009;34(20):2191-2197.
41. Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine. 2006;31(26):3018-3026.
42. Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25(4):451-456.
43. Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006;31(3):355-366.
The goal of surgical treatment of adolescent idiopathic scoliosis (AIS) is to prevent disability associated with curve progression.1 Early studies tended to focus on radiographic measures, such as curve correction and sagittal balance, rather than on improvements in quality of life (QOL).2-5 Although studies have reported on QOL in patients treated surgically for scoliosis,6-11 these studies were largely limited by small sample size and inclusion of patients with congenital and neuromuscular scoliosis,9 lack of a generic measure of QOL,6,7 or lack of surgical treatment of patients in the cohort.10
We conducted a study to determine disease-specific and general health-related QOL (HR-QOL) in young adults who underwent surgical correction of their spinal deformity during adolescence and to evaluate associated complications and reoperations.
Materials and Methods
After obtaining institutional review board approval, we queried the surgical database of a large metropolitan tertiary referral center for consecutive patients who had undergone spine deformity correction between the ages of 10 and 17 years (January 1993–December 2003). Hospital and medical records were retrospectively reviewed to confirm the diagnosis of AIS. Patients with congenital, neuromuscular, juvenile, or infantile scoliosis were excluded. Patients with intraspinal pathology (eg, tethered cord, syringomyelia), developmental delay, chromosomal abnormality, or congenital heart disease were also excluded. Patients were contacted by mail or telephone, and the Scoliosis Research Society–22R (SRS-22R)12-15 and the Short Form–12 (SF-12)16 were administered. Standard demographic and surgical data were also collected.
The SRS-22R is a scoliosis-specific HR-QOL questionnaire with 22 items, 5 domains (pain, activity, appearance, mental, satisfaction), and a total score.12-15 Each domain score ranges from 1 to 5 (higher scores indicating better outcomes). The SRS-22R is the outcome instrument most widely used to measure HR-QOL changes in patients with scoliosis, and it is available in several languages.17-26
The SF-12, a 12-item self-administered short-form health status survey developed in the Medical Outcomes Study, measures patient-based health status. Two composite scores can be calculated: physical composite summary (PCS) and mental composite summary (MCS).16 Using norm-based scoring, all domain scales have a mean (SD) of 50 (10) based on the general 1998 US population. Thus, scores under 50 fall below the general population mean.
In addition, patients were surveyed to determine the incidence of spine-related symptoms and complaints, including activity limitations, rib prominence, waistline asymmetry, back pain, limited range of motion (ROM), shortness of breath, wound/scar problems, lung disease/asthma, heart disease, high blood pressure, and arthritis. Data regarding postoperative treatment regimens of physical therapy, narcotic pain medication, spinal/epidural injections, and nonsteroidal anti-inflammatory drug (NSAID) use were collected. Patients were also queried regarding their current working status and smoking status.
Standard demographic and surgical data were collected from hospital and office charts and radiographs. Data collected included history of bracing, age at index surgery, number of levels fused, surgical approach (anterior, posterior, combined), postoperative complications (eg, ileus, wound infection, anemia, pneumonia), and immediate preoperative and final postoperative radiographic measures. Data on need for subsequent revision surgery and indications for revision surgery were also collected.
Preoperative and latest follow-up radiographs were measured to determine curve magnitude, sagittal and coronal balance, and percentage curve correction. Coronal balance was defined as the distance between a plumb line drawn vertically from the spinous process of C7 and the central sacral line on full-length posteroanterior radiographs. Sagittal balance was defined as the distance of a plumb line drawn vertically from the center of the body of C7 and the posterosuperior endplate of S1.27
Regression analysis was performed to identify factors predictive of SRS-22R total scores. Factors included in the analysis were sex, age at surgery, Lenke type, surgery type (anterior, posterior, anteroposterior), number of levels fused, lowest instrumented vertebra, perioperative complications, percentage curve correction, postoperative coronal and sagittal balance, smoking status, and need for revision surgery. Although age and sex were considered variables outside the surgeon’s control, they were included in the model, as previous studies have shown that SRS scores varied by age and sex both in adolescents28 and adults.29 Significance was set at P < .01. All data analysis was performed with IBM SPSS Version 19.0 (Somers, New York).
Results
Of the 384 postoperative patients identified for study inclusion, 134 (35%) completed surveys. Sixteen patients with nonidiopathic scoliosis were excluded, leaving 118 available for analysis. Of the remaining patients, 248 (64%) could not be contacted because of a change in address or phone number. Two patients (1%) were unwilling to complete survey requests. There was no statistically significant difference in demographics between patients with and without follow-up data available. Demographics are summarized in Table 1. There were 109 females (92%). Mean (SD) age at surgery was 14.1 (1.9) years. Only 37 (31%) were braced before surgery. Table 2 summarizes the radiographic data. Mean (SD) major Cobb angle was 49.7° (7.8°). Eighty-five patients (72%) underwent posterior fusion with instrumentation using hooks only; another 16 (14%) had anterior-only surgery, and another 17 (14%) had combined anterior-posterior surgery. A mean of 7.8 levels were fused. Index surgery data and lowest instrumented vertebra distribution are summarized in Table 3. Mean (SD) percentage curve correction was 48.9% (8.4%).
Seven patients had a total of 8 perioperative complications: anemia requiring transfusion (2), ileus necessitating nasogastric tube insertion (2), superficial wound infection treated with oral antibiotics and local wound care (2), wound drainage and erythema (1), and pneumonia (1). Mean (SD) length of clinical and radiographic follow-up was 57.9 (36.3) months.
Table 4 summarizes the long-term complications. Of the 38 patients with long-term complications, 14 required reoperation. The indications were disc herniation (2 patients), painful instrumentation (7), crankshaft phenomenon (1), nonunion (1), and adjacent-level degeneration (3). Both disc herniations were at L5–S1, several segments below the distal extent of the fusion. Of the 7 patients who had painful instrumentation removed, 6 had the entire construct removed, and 1 had the proximal half of a rod taken out. The 3 patients with adjacent-level degeneration had stenosis at the distal end of the construct—at L5–S1 (2 patients) or L2–L3 (1 patient).
Mean (SD) time between surgery and completion of the surveys/questionnaires was 12.7 (3.2) years (range, 10-18 years). Mean age of respondents was 26.8 years. Twenty-five respondents (21%) were smokers. Mean (SD) outcome scores were 50.9 (9.4) for SF-12 PCS and 49.4 (10.2) for SF-12 MCS. Eighteen patients (15%) had SF-12 PCS scores 1 SD below normal, and 15 (13%) had SF-12 MCS scores 1 SD below normal. Mean (SD) SRS-22R Total score was 4.0 (0.7). Means, standard deviations, and distribution of SRS domain scores are summarized in Table 5. Of the variables, only current smoking (P < .001) was predictive of SRS-22R Total scores, accounting for 20% of their variability (Table 6).
One hundred patients (85%) had jobs, mostly desk jobs. The postoperative limitations most commonly reported are summarized in Table 7. These included at least intermittent back pain in 90 patients (76%), limited ROM in 52 (44%), and activity limitations in 54 (46%). Less common limitations were waistline imbalance in 41 (35%), rib prominence in 28 (24%), wound/scar problems in 18 (15%), and shortness of breath in 18 (15%). Other related medical problems were lung disease/asthma in 11 (9%), osteoarthritis/degenerative arthritis in 11 (9%), heart disease in 3 (3%), and high blood pressure in 2 (2%).
A minority of patients also participated in postoperative treatment regimens. The most common treatment was regular use of NSAIDs (25 patients, 21%). Other treatments were physical therapy (14, 12%), narcotic pain medication use (5, 4%), and epidural steroid injections (5, 4%). Table 8 summarizes the postoperative treatments used by patients with scoliosis.
Discussion
A major concern about prophylactic interventions for diseases is that the treatment will harm the patient. This is especially true for major spine surgery performed on adolescents with minimal symptoms. Although the incidence of perioperative complications in children undergoing corrective spinal surgery for AIS has been reported,30-32 the effect of the surgery on the disease-specific HR-QOL outcomes of these individuals as young adults has not been previously studied. Over the past few decades, a paradigm shift in understanding health and disability has occurred, with increased emphasis being placed on HR-QOL outcomes measures and understanding disability as relating to a measureable impact of the functioning of an individual after a change in health or environment. This change was substantiated when the World Health Organization endorsed the International Classification of Functioning, Disability and Health.33 In light of this shift, we present the disease-specific and general HR-QOL outcomes of young adults who had undergone surgical correction for spinal deformity during adolescence, as well as their associated complications and reoperations, in an attempt to identify targets for improvement.
Our patient-reported outcomes demonstrated a high incidence of occasional back pain, activity-related complaints, and limited ROM. Comparison of our cohort’s SRS-22R outcomes with previously published normative values for the unaffected adolescent population28,34 suggests worse scores for the disease-specific SRS-22R domains of pain and appearance. In 2012, Daubs and colleagues34 reported that normative scores on various SRS-22 domains were statistically lower with age (scores decreased from age 10 to age 19 years). Both Verma and colleagues28 and Daubs and colleagues34 reported lower scores for females than for males. Therefore, it is unclear whether the differences observed in our cohort may be accounted for by the larger proportion of females compared with the normative data.
General health scores on the SF-12 were similar to the population norm (mean [SD]) of 50 (10) referenced by Ware and colleagues.16 These findings suggest that, though pain and appearance may be statistically lower in our cohort—as measured with the SRS-22R—the cohort’s spine-related symptoms do not seem to lower its general health. Eighty-five percent of the patients were working at the time of the survey, further supporting a relatively normal level of overall function. In a retrospective review by Takayama and colleagues,9 similar results were found with regard to working after AIS fusion surgery. Of 32 patients treated surgically for scoliosis, at a mean of 21.1 years after the index fusion 27 (84.4%) were or had been engaged in various occupations without marked difficulty.
Our results in a cohort of patients with segmental instrumentation using hooks are similar to results in other studies of long-term HR-QOL measures in patients with AIS and Harrington rod instrumentation. Danielsson and Nachemson35 evaluated patients with surgically treated AIS with at least 20-year follow-up and reported that, in their surgical cohort with a mean age of 39.7 years, mean SF-36 PCS score was 50.9, and mean SF-36 MCS score was 50.2. In a recent study of patients with AIS and Harrington rod instrumentation, those of a mean age of 32.3 years had a mean score of 50.9 for both SF-36 PCS and SF-36 MCS.36
Regression analysis identified only smoking as a predictor of SRS-22R Total scores. This finding, that smokers have a lower health state, is expected even in the general population.37 Interestingly, bracing before surgery, Lenke type, surgery type, number of levels fused, lowest instrumented vertebra, incidence of perioperative complications, percentage curve correction, postoperative sagittal and coronal balance, and need for revision surgery did not influence HR-QOL measures in this cohort.
Our cohort’s incidence of occasional back pain was 76% (90/118 patients). Other reports have had similar findings. In 2012, Bas and colleagues38 studied self-reported pain in 126 consecutive patients with scoliosis and instrumented fusion. In their cohort, “most participants reported ‘no pain’ (38.5%) or ‘mild pain’ (30.8%) and 72.1% of participants reported a current work/school activity level of 100% normal.” Also in 2012, Rushton and Grevitt39 reported on a review and statistical analysis of the literature on HR-QOL in adolescents with untreated AIS and in unaffected adolescents. Their goal was to identify whether there were any differences in HR-QOL and, if so, whether they were clinically relevant. The authors concluded that pain and self-image tended to be statistically lower among cohorts with AIS but that only self-image was consistently different clinically between untreated patients with AIS and their unaffected peers.
Cosmetic complaints, though less common than functional concerns, affected a substantial percentage of our cohort. Waistline imbalance complaints were more common than rib prominence or scar-related complaints. The validity of patient-reported waistline imbalance is not known but may contribute to the SRS-22R outcomes in this cohort, particularly with regard to appearance scores. Respiratory symptoms, particularly those related to shortness of breath, were reported by 15% of patients. Respiratory symptoms may be in part secondary to underlying lung disease; smoking was reported by 21% of patients and asthma by 9%.
Few additional postoperative treatments were reported by patients. The most common treatment was regular use of NSAIDs (21%), followed by postoperative physical therapy (12%). Opiate medication use and spinal injections were rare—consistent with results reported by Danielsson and Nachemson35 in 2003.
Implant-related complaints, including painful instrumentation (13%) and implant prominence (9%), were some of the most common complaints in our study group. Although not all symptomatic instrumentation required surgical revision, 7 (50%) of the 14 additional spine surgeries were related to painful and/or prominent posterior instrumentation. Additional spine surgery was reported in 11.9% of our patients. Other indications for reoperation were disc herniation, crankshaft phenomenon, nonunion, and adjacent-level degeneration. Our rate of revision surgery is supported by the literature. In 2009, Luhmann and colleagues40 reported that 41 (3.9%) of 1057 primary spine fusions for idiopathic scoliosis required reoperation; the indications included infection (16/1057, 1.5%), pseudarthrosis (12, 1.1%), and painful/prominent implant (7, 0.7%). Richards and colleagues41 similarly reported on 1046 patients who underwent fusion for AIS. Of these patients, 135 underwent 172 repeat surgical interventions (12.9% reoperation rate), with 29 (21.5%) of the 135 undergoing 2 or more separate procedures. The most common reasons for reoperation were infection, symptomatic implant, and pseudarthrosis. The authors concluded that repeat surgeries were relatively common after the initial surgical procedures. Having a clearer understanding of instrumentation-related complaints and reoperations may lead to improvement in this surgeon-controlled variable.
There are limitations to this study. The data regarding clinical courses were collected by retrospective chart review, which has known limitations. To offset this, we collected prospective outcome data with use of the SF-12, the SRS-22R, and a spine-related complaints questionnaire. No control group was available for comparison of outcomes in our cohort. We used the SF-12 and previously published normative values for the SRS-22R for comparison with population norms. Such comparisons have inherent limitations, as the groups vary by sex and mean age; our cohort was primarily female and more than a decade older than the controls.
Only 35% of the patients who met the inclusion criteria had complete data that could be included in our analysis. Although there was no statistically significant difference in demographics between patients with and without follow-up data available, this low response rate could have introduced selection bias. Ideally, patients should have been seen in clinic, standing radiographs should have been taken, and pulmonary function tests should have been performed. However, these patients were asymptomatic, and ethical and insurance issues prevented those actions. Thus, any radiographic changes occurring over the intervening years, from the last clinic visit to completion of the surveys, were not documented. This situation may or may not have limited our findings, as other authors have found low correlation between radiographic outcomes and clinical outcome measures.13,14,19,36 During the period when these surgeries were performed, segmental spine instrumentation with hooks was the standard of care for deformity correction in AIS; therefore, all posterior instrumentations were done with hook-only segmental fixation. Current pedicle screw–based techniques that allow for additional correction of the deformity may provide different outcomes in the future.
We think that, despite the inherent limitations of this study, our data will be useful in the treatment of AIS. Our results suggest that postoperative spinal complaints are common and that, compared with an unaffected adolescent population, patients with AIS score significantly lower on pain and appearance domains of outcomes testing at a mean of 12.7 years after index fusion. Nevertheless, the outcomes do not seem to be of sufficient severity to affect general health and QOL as measured by outcomes testing.
Spinal deformity correction is performed to prevent impaired pulmonary function and spine-related disability later in life.42,43 Thus, longer-term studies, involving patients in their fifth and sixth decades of life, are needed to determine whether patients with AIS will have QOL outcomes, pulmonary function, and spine-related problems similar to those in the general population. In this cohort of young adults, smoking status was the only predictor of HR-QOL measures, and spinal deformity correction did not lead to decreased HR-QOL.
The goal of surgical treatment of adolescent idiopathic scoliosis (AIS) is to prevent disability associated with curve progression.1 Early studies tended to focus on radiographic measures, such as curve correction and sagittal balance, rather than on improvements in quality of life (QOL).2-5 Although studies have reported on QOL in patients treated surgically for scoliosis,6-11 these studies were largely limited by small sample size and inclusion of patients with congenital and neuromuscular scoliosis,9 lack of a generic measure of QOL,6,7 or lack of surgical treatment of patients in the cohort.10
We conducted a study to determine disease-specific and general health-related QOL (HR-QOL) in young adults who underwent surgical correction of their spinal deformity during adolescence and to evaluate associated complications and reoperations.
Materials and Methods
After obtaining institutional review board approval, we queried the surgical database of a large metropolitan tertiary referral center for consecutive patients who had undergone spine deformity correction between the ages of 10 and 17 years (January 1993–December 2003). Hospital and medical records were retrospectively reviewed to confirm the diagnosis of AIS. Patients with congenital, neuromuscular, juvenile, or infantile scoliosis were excluded. Patients with intraspinal pathology (eg, tethered cord, syringomyelia), developmental delay, chromosomal abnormality, or congenital heart disease were also excluded. Patients were contacted by mail or telephone, and the Scoliosis Research Society–22R (SRS-22R)12-15 and the Short Form–12 (SF-12)16 were administered. Standard demographic and surgical data were also collected.
The SRS-22R is a scoliosis-specific HR-QOL questionnaire with 22 items, 5 domains (pain, activity, appearance, mental, satisfaction), and a total score.12-15 Each domain score ranges from 1 to 5 (higher scores indicating better outcomes). The SRS-22R is the outcome instrument most widely used to measure HR-QOL changes in patients with scoliosis, and it is available in several languages.17-26
The SF-12, a 12-item self-administered short-form health status survey developed in the Medical Outcomes Study, measures patient-based health status. Two composite scores can be calculated: physical composite summary (PCS) and mental composite summary (MCS).16 Using norm-based scoring, all domain scales have a mean (SD) of 50 (10) based on the general 1998 US population. Thus, scores under 50 fall below the general population mean.
In addition, patients were surveyed to determine the incidence of spine-related symptoms and complaints, including activity limitations, rib prominence, waistline asymmetry, back pain, limited range of motion (ROM), shortness of breath, wound/scar problems, lung disease/asthma, heart disease, high blood pressure, and arthritis. Data regarding postoperative treatment regimens of physical therapy, narcotic pain medication, spinal/epidural injections, and nonsteroidal anti-inflammatory drug (NSAID) use were collected. Patients were also queried regarding their current working status and smoking status.
Standard demographic and surgical data were collected from hospital and office charts and radiographs. Data collected included history of bracing, age at index surgery, number of levels fused, surgical approach (anterior, posterior, combined), postoperative complications (eg, ileus, wound infection, anemia, pneumonia), and immediate preoperative and final postoperative radiographic measures. Data on need for subsequent revision surgery and indications for revision surgery were also collected.
Preoperative and latest follow-up radiographs were measured to determine curve magnitude, sagittal and coronal balance, and percentage curve correction. Coronal balance was defined as the distance between a plumb line drawn vertically from the spinous process of C7 and the central sacral line on full-length posteroanterior radiographs. Sagittal balance was defined as the distance of a plumb line drawn vertically from the center of the body of C7 and the posterosuperior endplate of S1.27
Regression analysis was performed to identify factors predictive of SRS-22R total scores. Factors included in the analysis were sex, age at surgery, Lenke type, surgery type (anterior, posterior, anteroposterior), number of levels fused, lowest instrumented vertebra, perioperative complications, percentage curve correction, postoperative coronal and sagittal balance, smoking status, and need for revision surgery. Although age and sex were considered variables outside the surgeon’s control, they were included in the model, as previous studies have shown that SRS scores varied by age and sex both in adolescents28 and adults.29 Significance was set at P < .01. All data analysis was performed with IBM SPSS Version 19.0 (Somers, New York).
Results
Of the 384 postoperative patients identified for study inclusion, 134 (35%) completed surveys. Sixteen patients with nonidiopathic scoliosis were excluded, leaving 118 available for analysis. Of the remaining patients, 248 (64%) could not be contacted because of a change in address or phone number. Two patients (1%) were unwilling to complete survey requests. There was no statistically significant difference in demographics between patients with and without follow-up data available. Demographics are summarized in Table 1. There were 109 females (92%). Mean (SD) age at surgery was 14.1 (1.9) years. Only 37 (31%) were braced before surgery. Table 2 summarizes the radiographic data. Mean (SD) major Cobb angle was 49.7° (7.8°). Eighty-five patients (72%) underwent posterior fusion with instrumentation using hooks only; another 16 (14%) had anterior-only surgery, and another 17 (14%) had combined anterior-posterior surgery. A mean of 7.8 levels were fused. Index surgery data and lowest instrumented vertebra distribution are summarized in Table 3. Mean (SD) percentage curve correction was 48.9% (8.4%).
Seven patients had a total of 8 perioperative complications: anemia requiring transfusion (2), ileus necessitating nasogastric tube insertion (2), superficial wound infection treated with oral antibiotics and local wound care (2), wound drainage and erythema (1), and pneumonia (1). Mean (SD) length of clinical and radiographic follow-up was 57.9 (36.3) months.
Table 4 summarizes the long-term complications. Of the 38 patients with long-term complications, 14 required reoperation. The indications were disc herniation (2 patients), painful instrumentation (7), crankshaft phenomenon (1), nonunion (1), and adjacent-level degeneration (3). Both disc herniations were at L5–S1, several segments below the distal extent of the fusion. Of the 7 patients who had painful instrumentation removed, 6 had the entire construct removed, and 1 had the proximal half of a rod taken out. The 3 patients with adjacent-level degeneration had stenosis at the distal end of the construct—at L5–S1 (2 patients) or L2–L3 (1 patient).
Mean (SD) time between surgery and completion of the surveys/questionnaires was 12.7 (3.2) years (range, 10-18 years). Mean age of respondents was 26.8 years. Twenty-five respondents (21%) were smokers. Mean (SD) outcome scores were 50.9 (9.4) for SF-12 PCS and 49.4 (10.2) for SF-12 MCS. Eighteen patients (15%) had SF-12 PCS scores 1 SD below normal, and 15 (13%) had SF-12 MCS scores 1 SD below normal. Mean (SD) SRS-22R Total score was 4.0 (0.7). Means, standard deviations, and distribution of SRS domain scores are summarized in Table 5. Of the variables, only current smoking (P < .001) was predictive of SRS-22R Total scores, accounting for 20% of their variability (Table 6).
One hundred patients (85%) had jobs, mostly desk jobs. The postoperative limitations most commonly reported are summarized in Table 7. These included at least intermittent back pain in 90 patients (76%), limited ROM in 52 (44%), and activity limitations in 54 (46%). Less common limitations were waistline imbalance in 41 (35%), rib prominence in 28 (24%), wound/scar problems in 18 (15%), and shortness of breath in 18 (15%). Other related medical problems were lung disease/asthma in 11 (9%), osteoarthritis/degenerative arthritis in 11 (9%), heart disease in 3 (3%), and high blood pressure in 2 (2%).
A minority of patients also participated in postoperative treatment regimens. The most common treatment was regular use of NSAIDs (25 patients, 21%). Other treatments were physical therapy (14, 12%), narcotic pain medication use (5, 4%), and epidural steroid injections (5, 4%). Table 8 summarizes the postoperative treatments used by patients with scoliosis.
Discussion
A major concern about prophylactic interventions for diseases is that the treatment will harm the patient. This is especially true for major spine surgery performed on adolescents with minimal symptoms. Although the incidence of perioperative complications in children undergoing corrective spinal surgery for AIS has been reported,30-32 the effect of the surgery on the disease-specific HR-QOL outcomes of these individuals as young adults has not been previously studied. Over the past few decades, a paradigm shift in understanding health and disability has occurred, with increased emphasis being placed on HR-QOL outcomes measures and understanding disability as relating to a measureable impact of the functioning of an individual after a change in health or environment. This change was substantiated when the World Health Organization endorsed the International Classification of Functioning, Disability and Health.33 In light of this shift, we present the disease-specific and general HR-QOL outcomes of young adults who had undergone surgical correction for spinal deformity during adolescence, as well as their associated complications and reoperations, in an attempt to identify targets for improvement.
Our patient-reported outcomes demonstrated a high incidence of occasional back pain, activity-related complaints, and limited ROM. Comparison of our cohort’s SRS-22R outcomes with previously published normative values for the unaffected adolescent population28,34 suggests worse scores for the disease-specific SRS-22R domains of pain and appearance. In 2012, Daubs and colleagues34 reported that normative scores on various SRS-22 domains were statistically lower with age (scores decreased from age 10 to age 19 years). Both Verma and colleagues28 and Daubs and colleagues34 reported lower scores for females than for males. Therefore, it is unclear whether the differences observed in our cohort may be accounted for by the larger proportion of females compared with the normative data.
General health scores on the SF-12 were similar to the population norm (mean [SD]) of 50 (10) referenced by Ware and colleagues.16 These findings suggest that, though pain and appearance may be statistically lower in our cohort—as measured with the SRS-22R—the cohort’s spine-related symptoms do not seem to lower its general health. Eighty-five percent of the patients were working at the time of the survey, further supporting a relatively normal level of overall function. In a retrospective review by Takayama and colleagues,9 similar results were found with regard to working after AIS fusion surgery. Of 32 patients treated surgically for scoliosis, at a mean of 21.1 years after the index fusion 27 (84.4%) were or had been engaged in various occupations without marked difficulty.
Our results in a cohort of patients with segmental instrumentation using hooks are similar to results in other studies of long-term HR-QOL measures in patients with AIS and Harrington rod instrumentation. Danielsson and Nachemson35 evaluated patients with surgically treated AIS with at least 20-year follow-up and reported that, in their surgical cohort with a mean age of 39.7 years, mean SF-36 PCS score was 50.9, and mean SF-36 MCS score was 50.2. In a recent study of patients with AIS and Harrington rod instrumentation, those of a mean age of 32.3 years had a mean score of 50.9 for both SF-36 PCS and SF-36 MCS.36
Regression analysis identified only smoking as a predictor of SRS-22R Total scores. This finding, that smokers have a lower health state, is expected even in the general population.37 Interestingly, bracing before surgery, Lenke type, surgery type, number of levels fused, lowest instrumented vertebra, incidence of perioperative complications, percentage curve correction, postoperative sagittal and coronal balance, and need for revision surgery did not influence HR-QOL measures in this cohort.
Our cohort’s incidence of occasional back pain was 76% (90/118 patients). Other reports have had similar findings. In 2012, Bas and colleagues38 studied self-reported pain in 126 consecutive patients with scoliosis and instrumented fusion. In their cohort, “most participants reported ‘no pain’ (38.5%) or ‘mild pain’ (30.8%) and 72.1% of participants reported a current work/school activity level of 100% normal.” Also in 2012, Rushton and Grevitt39 reported on a review and statistical analysis of the literature on HR-QOL in adolescents with untreated AIS and in unaffected adolescents. Their goal was to identify whether there were any differences in HR-QOL and, if so, whether they were clinically relevant. The authors concluded that pain and self-image tended to be statistically lower among cohorts with AIS but that only self-image was consistently different clinically between untreated patients with AIS and their unaffected peers.
Cosmetic complaints, though less common than functional concerns, affected a substantial percentage of our cohort. Waistline imbalance complaints were more common than rib prominence or scar-related complaints. The validity of patient-reported waistline imbalance is not known but may contribute to the SRS-22R outcomes in this cohort, particularly with regard to appearance scores. Respiratory symptoms, particularly those related to shortness of breath, were reported by 15% of patients. Respiratory symptoms may be in part secondary to underlying lung disease; smoking was reported by 21% of patients and asthma by 9%.
Few additional postoperative treatments were reported by patients. The most common treatment was regular use of NSAIDs (21%), followed by postoperative physical therapy (12%). Opiate medication use and spinal injections were rare—consistent with results reported by Danielsson and Nachemson35 in 2003.
Implant-related complaints, including painful instrumentation (13%) and implant prominence (9%), were some of the most common complaints in our study group. Although not all symptomatic instrumentation required surgical revision, 7 (50%) of the 14 additional spine surgeries were related to painful and/or prominent posterior instrumentation. Additional spine surgery was reported in 11.9% of our patients. Other indications for reoperation were disc herniation, crankshaft phenomenon, nonunion, and adjacent-level degeneration. Our rate of revision surgery is supported by the literature. In 2009, Luhmann and colleagues40 reported that 41 (3.9%) of 1057 primary spine fusions for idiopathic scoliosis required reoperation; the indications included infection (16/1057, 1.5%), pseudarthrosis (12, 1.1%), and painful/prominent implant (7, 0.7%). Richards and colleagues41 similarly reported on 1046 patients who underwent fusion for AIS. Of these patients, 135 underwent 172 repeat surgical interventions (12.9% reoperation rate), with 29 (21.5%) of the 135 undergoing 2 or more separate procedures. The most common reasons for reoperation were infection, symptomatic implant, and pseudarthrosis. The authors concluded that repeat surgeries were relatively common after the initial surgical procedures. Having a clearer understanding of instrumentation-related complaints and reoperations may lead to improvement in this surgeon-controlled variable.
There are limitations to this study. The data regarding clinical courses were collected by retrospective chart review, which has known limitations. To offset this, we collected prospective outcome data with use of the SF-12, the SRS-22R, and a spine-related complaints questionnaire. No control group was available for comparison of outcomes in our cohort. We used the SF-12 and previously published normative values for the SRS-22R for comparison with population norms. Such comparisons have inherent limitations, as the groups vary by sex and mean age; our cohort was primarily female and more than a decade older than the controls.
Only 35% of the patients who met the inclusion criteria had complete data that could be included in our analysis. Although there was no statistically significant difference in demographics between patients with and without follow-up data available, this low response rate could have introduced selection bias. Ideally, patients should have been seen in clinic, standing radiographs should have been taken, and pulmonary function tests should have been performed. However, these patients were asymptomatic, and ethical and insurance issues prevented those actions. Thus, any radiographic changes occurring over the intervening years, from the last clinic visit to completion of the surveys, were not documented. This situation may or may not have limited our findings, as other authors have found low correlation between radiographic outcomes and clinical outcome measures.13,14,19,36 During the period when these surgeries were performed, segmental spine instrumentation with hooks was the standard of care for deformity correction in AIS; therefore, all posterior instrumentations were done with hook-only segmental fixation. Current pedicle screw–based techniques that allow for additional correction of the deformity may provide different outcomes in the future.
We think that, despite the inherent limitations of this study, our data will be useful in the treatment of AIS. Our results suggest that postoperative spinal complaints are common and that, compared with an unaffected adolescent population, patients with AIS score significantly lower on pain and appearance domains of outcomes testing at a mean of 12.7 years after index fusion. Nevertheless, the outcomes do not seem to be of sufficient severity to affect general health and QOL as measured by outcomes testing.
Spinal deformity correction is performed to prevent impaired pulmonary function and spine-related disability later in life.42,43 Thus, longer-term studies, involving patients in their fifth and sixth decades of life, are needed to determine whether patients with AIS will have QOL outcomes, pulmonary function, and spine-related problems similar to those in the general population. In this cohort of young adults, smoking status was the only predictor of HR-QOL measures, and spinal deformity correction did not lead to decreased HR-QOL.
1. Tsutsui S, Pawelek J, Bastrom T, et al. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine. 2009;34(18):E653-E658.
2. Bonnett C, Brown JC, Cross B, Barron R. Posterior spinal fusion with Harrington rod instrumentation in 100 consecutive patients. Contemp Orthop. 1980;2:396-399.
3. Harrington PR, Dixon JR. An eleven year clinical investigation of Harrington instrument. Clin Orthop. 1973;(93):113-130.
4. Mielke CH, Lonstein JE, Denis F, Vandenbrink K, Winter RB. Surgical treatment of adolescent idiopathic scoliosis. A comparative analysis. J Bone Joint Surg Am. 1989;71(8):1170-1177.
5. Moskowitz A, Moe JH, Winter RB, Binner H. Long-term follow-up of scoliosis fusion. J Bone Joint Surg Am. 1980;62(3):529-554.
6. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Health-related quality of life and low back pain of patients surgically treated for scoliosis after 21 years or more of follow-up: comparison among non-idiopathic scoliosis, idiopathic scoliosis, and healthy subjects. Spine. 2012;37(22):1899-1903.
7. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Long-term clinical outcomes of surgery for adolescent idiopathic scoliosis 21 to 41 years later. Spine. 2012;37(5):402-405.
8. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46(7):474-478.
9. Takayama K, Nakamura H, Matsuda H. Quality of life in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine. 2009;34(20):2179-2184.
10. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527-1537.
11. Westrick ER, Ward WT. Adolescent idiopathic scoliosis: 5-year to 20-year evidence-based surgical results. J Pediatr Orthop. 2011;31(1 suppl):S61-S68.
12. Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 health-related quality of life questionnaire Function domain. Spine. 2006;31(5):593-597.
13. Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society–22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28(1):70-73.
14. Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society–22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28(1):63-69.
15. Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society–22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine. 2003;28(1):74-78.
16. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
17. Alanay A, Cil A, Berk H, et al. Reliability and validity of adapted Turkish version of Scoliosis Research Society–22 (SRS-22) questionnaire. Spine. 2005;30(21):2464-2468.
18. Beauséjour M, Joncas J, Goulet L, et al. Reliability and validity of adapted French Canadian version of Scoliosis Research Society outcomes questionnaire (SRS-22) in Quebec. Spine. 2009;34(6):623-628.
19. Climent JM, Bago J, Ey A, Perez-Grueso FJ, Izquierdo E. Validity of the Spanish version of the Scoliosis Research Society–22 (SRS-22) patient questionnaire. Spine. 2005;30(6):705-709.
20. Glowacki M, Misterska E, Laurentowska M, Mankowski P. Polish adaptation of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(10):1060-1065.
21. Hashimoto H, Sase T, Arai Y, Maruyama T, Isobe K, Shouno Y. Validation of a Japanese version of the Scoliosis Research Society–22 patient questionnaire among idiopathic scoliosis patients in Japan. Spine. 2007;32(4):E141-E146.
22. Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(12):1321-1324.
23. Monticone M, Carabalona R, Negrini S. Reliability of the Scoliosis Research Society–22 patient questionnaire (Italian version) in mild adolescent vertebral deformities. Eura Medicophys. 2004;40(3):191-197.
24. Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted German version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(8):818-821.
25. Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31(4):473-478.
26. Glattes RC, Burton DC, Lai SM, Frasier E, Asher MA. The reliability and concurrent validity of the Scoliosis Research Society–22R patient questionnaire compared with the Child Health Questionnaire–CF87 patient questionnaire for adolescent spinal deformity. Spine. 2007;32(16):1778-1784.
27. Blanke KM, Kuklo TR, Lenke LG, et al. Adolescent idiopathic scoliosis. In O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. Spinal Deformity Study Group Radiographic Measurement Manual. Memphis, TN: Medtronic; 2004.
28. Verma K, Lonner B, Hoashi JS, et al. Demographic factors affect Scoliosis Research Society–22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine. 2010;35(24):2134-2139.
29. Baldus C, Bridwell KH, Harrast J, et al. Age-gender matched comparison of SRS instrument scores between adult deformity and normal adults: are all SRS domains disease specific? Spine. 2008;33(20):2214-2218.
30. Brown CA, Lenke LG, Bridwell KH, Geideman WM, Hasan SA, Blanke K. Complication of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23(14):1566-1571.
31. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345-349.
32. Fu KM, Smith JS, Polly DW, et al. Scoliosis Research Society Morbidity and Mortality Committee. Morbidity and mortality associated with spinal surgery in children: a review of the Scoliosis Research Society morbidity and mortality database. J Neurosurg Pediatr. 2011;7(1):37-41.
33. World Health Organization. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva, Switzerland: World Health Organization; 2001.
34. Daubs M, Lawrence B, Hung M, et al. Scoliosis Research Society–22 results in 3,052 healthy adolescents age ten to 19 years. Abstract presented at: 47th Annual Meeting and Course of the Scoliosis Research Society; September 5-8, 2012; Chicago, IL. Abstract 72.
35. Danielsson AL, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case–control study—part II. Spine. 2003;28(18):E373-E383.
36. Götze C, Liljenqvist UR, Slomka A, Götze HG, Steinbeck J. Quality of life and back pain: outcome 16.7 years after Harrington instrumentation. Spine. 2002;27(13):1456-1463.
37. Quercioli C, Messina G, Barbini E, Carriero G, Fanì M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health-related quality of life: performances of three tools: comparison of three questionnaire scores. Eur J Health Econ. 2009;10(4):389-397.
38. Bas T, Franco N, Bas P, Bas JL. Pain and disability following fusion for idiopathic adolescent scoliosis: prevalence and associated factors. Evid Based Spine Care J. 2012;3(2):17-24.
39. Rushton PR, Grevitt MP. Comparison of untreated adolescent idiopathic scoliosis with normal controls: a review and statistical analysis of the literature. Spine. 2013;38(9):778-785.
40. Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine. 2009;34(20):2191-2197.
41. Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine. 2006;31(26):3018-3026.
42. Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25(4):451-456.
43. Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006;31(3):355-366.
1. Tsutsui S, Pawelek J, Bastrom T, et al. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine. 2009;34(18):E653-E658.
2. Bonnett C, Brown JC, Cross B, Barron R. Posterior spinal fusion with Harrington rod instrumentation in 100 consecutive patients. Contemp Orthop. 1980;2:396-399.
3. Harrington PR, Dixon JR. An eleven year clinical investigation of Harrington instrument. Clin Orthop. 1973;(93):113-130.
4. Mielke CH, Lonstein JE, Denis F, Vandenbrink K, Winter RB. Surgical treatment of adolescent idiopathic scoliosis. A comparative analysis. J Bone Joint Surg Am. 1989;71(8):1170-1177.
5. Moskowitz A, Moe JH, Winter RB, Binner H. Long-term follow-up of scoliosis fusion. J Bone Joint Surg Am. 1980;62(3):529-554.
6. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Health-related quality of life and low back pain of patients surgically treated for scoliosis after 21 years or more of follow-up: comparison among non-idiopathic scoliosis, idiopathic scoliosis, and healthy subjects. Spine. 2012;37(22):1899-1903.
7. Akazawa T, Minami S, Kotani T, Nemoto T, Koshi T, Takahashi K. Long-term clinical outcomes of surgery for adolescent idiopathic scoliosis 21 to 41 years later. Spine. 2012;37(5):402-405.
8. Pehrsson K, Bake B, Larsson S, Nachemson A. Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax. 1991;46(7):474-478.
9. Takayama K, Nakamura H, Matsuda H. Quality of life in patients treated surgically for scoliosis: longer than sixteen-year follow-up. Spine. 2009;34(20):2179-2184.
10. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527-1537.
11. Westrick ER, Ward WT. Adolescent idiopathic scoliosis: 5-year to 20-year evidence-based surgical results. J Pediatr Orthop. 2011;31(1 suppl):S61-S68.
12. Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 health-related quality of life questionnaire Function domain. Spine. 2006;31(5):593-597.
13. Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society–22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28(1):70-73.
14. Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society–22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28(1):63-69.
15. Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society–22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine. 2003;28(1):74-78.
16. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
17. Alanay A, Cil A, Berk H, et al. Reliability and validity of adapted Turkish version of Scoliosis Research Society–22 (SRS-22) questionnaire. Spine. 2005;30(21):2464-2468.
18. Beauséjour M, Joncas J, Goulet L, et al. Reliability and validity of adapted French Canadian version of Scoliosis Research Society outcomes questionnaire (SRS-22) in Quebec. Spine. 2009;34(6):623-628.
19. Climent JM, Bago J, Ey A, Perez-Grueso FJ, Izquierdo E. Validity of the Spanish version of the Scoliosis Research Society–22 (SRS-22) patient questionnaire. Spine. 2005;30(6):705-709.
20. Glowacki M, Misterska E, Laurentowska M, Mankowski P. Polish adaptation of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(10):1060-1065.
21. Hashimoto H, Sase T, Arai Y, Maruyama T, Isobe K, Shouno Y. Validation of a Japanese version of the Scoliosis Research Society–22 patient questionnaire among idiopathic scoliosis patients in Japan. Spine. 2007;32(4):E141-E146.
22. Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(12):1321-1324.
23. Monticone M, Carabalona R, Negrini S. Reliability of the Scoliosis Research Society–22 patient questionnaire (Italian version) in mild adolescent vertebral deformities. Eura Medicophys. 2004;40(3):191-197.
24. Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M. Validity and reliability of an adapted German version of Scoliosis Research Society–22 questionnaire. Spine. 2009;34(8):818-821.
25. Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31(4):473-478.
26. Glattes RC, Burton DC, Lai SM, Frasier E, Asher MA. The reliability and concurrent validity of the Scoliosis Research Society–22R patient questionnaire compared with the Child Health Questionnaire–CF87 patient questionnaire for adolescent spinal deformity. Spine. 2007;32(16):1778-1784.
27. Blanke KM, Kuklo TR, Lenke LG, et al. Adolescent idiopathic scoliosis. In O’Brien MF, Kuklo TR, Blanke KM, Lenke LG, eds. Spinal Deformity Study Group Radiographic Measurement Manual. Memphis, TN: Medtronic; 2004.
28. Verma K, Lonner B, Hoashi JS, et al. Demographic factors affect Scoliosis Research Society–22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine. 2010;35(24):2134-2139.
29. Baldus C, Bridwell KH, Harrast J, et al. Age-gender matched comparison of SRS instrument scores between adult deformity and normal adults: are all SRS domains disease specific? Spine. 2008;33(20):2214-2218.
30. Brown CA, Lenke LG, Bridwell KH, Geideman WM, Hasan SA, Blanke K. Complication of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23(14):1566-1571.
31. Coe JD, Arlet V, Donaldson W, et al. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2006;31(3):345-349.
32. Fu KM, Smith JS, Polly DW, et al. Scoliosis Research Society Morbidity and Mortality Committee. Morbidity and mortality associated with spinal surgery in children: a review of the Scoliosis Research Society morbidity and mortality database. J Neurosurg Pediatr. 2011;7(1):37-41.
33. World Health Organization. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva, Switzerland: World Health Organization; 2001.
34. Daubs M, Lawrence B, Hung M, et al. Scoliosis Research Society–22 results in 3,052 healthy adolescents age ten to 19 years. Abstract presented at: 47th Annual Meeting and Course of the Scoliosis Research Society; September 5-8, 2012; Chicago, IL. Abstract 72.
35. Danielsson AL, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case–control study—part II. Spine. 2003;28(18):E373-E383.
36. Götze C, Liljenqvist UR, Slomka A, Götze HG, Steinbeck J. Quality of life and back pain: outcome 16.7 years after Harrington instrumentation. Spine. 2002;27(13):1456-1463.
37. Quercioli C, Messina G, Barbini E, Carriero G, Fanì M, Nante N. Importance of sociodemographic and morbidity aspects in measuring health-related quality of life: performances of three tools: comparison of three questionnaire scores. Eur J Health Econ. 2009;10(4):389-397.
38. Bas T, Franco N, Bas P, Bas JL. Pain and disability following fusion for idiopathic adolescent scoliosis: prevalence and associated factors. Evid Based Spine Care J. 2012;3(2):17-24.
39. Rushton PR, Grevitt MP. Comparison of untreated adolescent idiopathic scoliosis with normal controls: a review and statistical analysis of the literature. Spine. 2013;38(9):778-785.
40. Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine. 2009;34(20):2191-2197.
41. Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine. 2006;31(26):3018-3026.
42. Bjure J, Grimby G, Kasalický J, Lindh M, Nachemson A. Respiratory impairment and airway closure in patients with untreated idiopathic scoliosis. Thorax. 1970;25(4):451-456.
43. Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10- to 60-year follow-up with special reference to health-related quality of life. Spine. 2006;31(3):355-366.
Surgery for Blastomycosis of the Spine
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.