User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
High or low birth weight in pediatric NAFLD may increase disease severity
Among children with nonalcoholic fatty liver disease (NAFLD), those with high birth weight had significantly greater odds of having more severe steatosis and nonalcoholic steatohepatitis (NASH) than those with normal birth weight, reported Kimberly P. Newton, MD, of the University of California, San Diego, and her associates.
In contrast, the children with low birth weight had twice the risk of advanced fibrosis, compared with those with normal birth weight.
Of 538 children with a mean age of 13 years, 9% had LBW, 76% had NBW, and 15% had HBW. Definite NASH was present in 27%.
Of the HBW children, 51% had severe steatosis, and those with HBW had significant 1.82-times greater odds of having severe steatosis than those with NBW. There was no significant difference in severe steatosis between children with LBW and NBW.
The odds of having NASH were two-times higher in the HBW children, compared with NBW children. The prevalence of NASH did not differ between the LBW and NBW children. The children with LBW had 2.23-times greater odds of advanced fibrosis, compared with the NBW children. There was no significant difference in advanced fibrosis between the HBW and NBW children.
“The frequency of children born with HBW has greatly increased recently, in parallel with the epidemic of obesity, and, thus, implications of HBW for future health have not been fully characterized,” Dr. Newton and her colleagues. The results of this study suggest there may be long-lasting hepatic consequences.
Read more in the Journal of Pediatrics (2017 Mar 30. doi: 10.1016/j.jpeds.2017.03.007).
Among children with nonalcoholic fatty liver disease (NAFLD), those with high birth weight had significantly greater odds of having more severe steatosis and nonalcoholic steatohepatitis (NASH) than those with normal birth weight, reported Kimberly P. Newton, MD, of the University of California, San Diego, and her associates.
In contrast, the children with low birth weight had twice the risk of advanced fibrosis, compared with those with normal birth weight.
Of 538 children with a mean age of 13 years, 9% had LBW, 76% had NBW, and 15% had HBW. Definite NASH was present in 27%.
Of the HBW children, 51% had severe steatosis, and those with HBW had significant 1.82-times greater odds of having severe steatosis than those with NBW. There was no significant difference in severe steatosis between children with LBW and NBW.
The odds of having NASH were two-times higher in the HBW children, compared with NBW children. The prevalence of NASH did not differ between the LBW and NBW children. The children with LBW had 2.23-times greater odds of advanced fibrosis, compared with the NBW children. There was no significant difference in advanced fibrosis between the HBW and NBW children.
“The frequency of children born with HBW has greatly increased recently, in parallel with the epidemic of obesity, and, thus, implications of HBW for future health have not been fully characterized,” Dr. Newton and her colleagues. The results of this study suggest there may be long-lasting hepatic consequences.
Read more in the Journal of Pediatrics (2017 Mar 30. doi: 10.1016/j.jpeds.2017.03.007).
Among children with nonalcoholic fatty liver disease (NAFLD), those with high birth weight had significantly greater odds of having more severe steatosis and nonalcoholic steatohepatitis (NASH) than those with normal birth weight, reported Kimberly P. Newton, MD, of the University of California, San Diego, and her associates.
In contrast, the children with low birth weight had twice the risk of advanced fibrosis, compared with those with normal birth weight.
Of 538 children with a mean age of 13 years, 9% had LBW, 76% had NBW, and 15% had HBW. Definite NASH was present in 27%.
Of the HBW children, 51% had severe steatosis, and those with HBW had significant 1.82-times greater odds of having severe steatosis than those with NBW. There was no significant difference in severe steatosis between children with LBW and NBW.
The odds of having NASH were two-times higher in the HBW children, compared with NBW children. The prevalence of NASH did not differ between the LBW and NBW children. The children with LBW had 2.23-times greater odds of advanced fibrosis, compared with the NBW children. There was no significant difference in advanced fibrosis between the HBW and NBW children.
“The frequency of children born with HBW has greatly increased recently, in parallel with the epidemic of obesity, and, thus, implications of HBW for future health have not been fully characterized,” Dr. Newton and her colleagues. The results of this study suggest there may be long-lasting hepatic consequences.
Read more in the Journal of Pediatrics (2017 Mar 30. doi: 10.1016/j.jpeds.2017.03.007).
FROM THE JOURNAL OF PEDIATRICS
HMGB1 might be new biomarker of celiac disease in children
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
FROM NUTRITION
Each added day of pediatric MRSA bacteremia upped complication risk 50%
Every additional day of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in hospitalized children was associated with a 50% increased risk of developing a complication, reported Rana F. Hamdy, MD, of Children’s National Health System, Washington, and her associates.
That was one of the findings of a study performed to determine the epidemiology, clinical outcomes, and risk factors for treatment failure in pediatric MRSA bacteremia. It took place in three hospitals, one each in Philadelphia, Baltimore, and Salt Lake City.
“This finding is in contrast to the epidemiology of MRSA bacteremia in adults, in whom bacteremia is more frequently attributed to catheter-related infections (31%-36%), endovascular infections (13%-15%), or an unknown source (15%-20%), and the durations of MRSA bacteremia are typically more prolonged (median duration of bacteremia is 8-9 days),” Dr. Hamdy and her associates wrote.
“Differences in the epidemiology of MRSA bacteremia between children and adults emphasize the need for dedicated pediatric studies to better understand the clinical characteristics and outcomes specific to children,” the researchers noted.
Musculoskeletal infections and endovascular infections were linked with treatment failure, possibly reflecting “the relatively higher burden of bacteria and/or decreased drug penetration into bone and endovascular infection sites,” the investigators said. Catheter-related infections were tied to reduced odds of treatment failure, “these episodes being localized to the catheter and therefore potentially less-invasive S. aureus infections.”
Mortality among these children with MRSA bacteremia was low, at 2%, but “nearly one-quarter of all patients experienced complications,” the study authors said (Pediatrics. 2017 May 5. doi: 10.1542/peds.2017-0183).
There was progression of infection in 7% of cases, and hematogenous complications or sequelae occurred in 23%. Twenty percent of children developed septic emboli or another metastatic focus of infection.
“This association between the duration of bacteremia and the development of complications has been previously reported among adults with S. aureus bacteremia,” Dr. Hamdy noted, “and provides important epidemiologic data that could inform decisions relating to the timing of additional imaging, such as echocardiograms, to identify metastatic foci.”
The children were treated with vancomycin, and some received additional anti-MRSA antibiotics. “Vancomycin trough concentrations or [minimum inhibitory concentrations] were not associated with treatment failure,” the investigators said. “Future studies to determine the appropriate vancomycin dose, duration, and approach to therapeutic drug monitoring are warranted to optimize patient outcomes.”
The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
Every additional day of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in hospitalized children was associated with a 50% increased risk of developing a complication, reported Rana F. Hamdy, MD, of Children’s National Health System, Washington, and her associates.
That was one of the findings of a study performed to determine the epidemiology, clinical outcomes, and risk factors for treatment failure in pediatric MRSA bacteremia. It took place in three hospitals, one each in Philadelphia, Baltimore, and Salt Lake City.
“This finding is in contrast to the epidemiology of MRSA bacteremia in adults, in whom bacteremia is more frequently attributed to catheter-related infections (31%-36%), endovascular infections (13%-15%), or an unknown source (15%-20%), and the durations of MRSA bacteremia are typically more prolonged (median duration of bacteremia is 8-9 days),” Dr. Hamdy and her associates wrote.
“Differences in the epidemiology of MRSA bacteremia between children and adults emphasize the need for dedicated pediatric studies to better understand the clinical characteristics and outcomes specific to children,” the researchers noted.
Musculoskeletal infections and endovascular infections were linked with treatment failure, possibly reflecting “the relatively higher burden of bacteria and/or decreased drug penetration into bone and endovascular infection sites,” the investigators said. Catheter-related infections were tied to reduced odds of treatment failure, “these episodes being localized to the catheter and therefore potentially less-invasive S. aureus infections.”
Mortality among these children with MRSA bacteremia was low, at 2%, but “nearly one-quarter of all patients experienced complications,” the study authors said (Pediatrics. 2017 May 5. doi: 10.1542/peds.2017-0183).
There was progression of infection in 7% of cases, and hematogenous complications or sequelae occurred in 23%. Twenty percent of children developed septic emboli or another metastatic focus of infection.
“This association between the duration of bacteremia and the development of complications has been previously reported among adults with S. aureus bacteremia,” Dr. Hamdy noted, “and provides important epidemiologic data that could inform decisions relating to the timing of additional imaging, such as echocardiograms, to identify metastatic foci.”
The children were treated with vancomycin, and some received additional anti-MRSA antibiotics. “Vancomycin trough concentrations or [minimum inhibitory concentrations] were not associated with treatment failure,” the investigators said. “Future studies to determine the appropriate vancomycin dose, duration, and approach to therapeutic drug monitoring are warranted to optimize patient outcomes.”
The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
Every additional day of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in hospitalized children was associated with a 50% increased risk of developing a complication, reported Rana F. Hamdy, MD, of Children’s National Health System, Washington, and her associates.
That was one of the findings of a study performed to determine the epidemiology, clinical outcomes, and risk factors for treatment failure in pediatric MRSA bacteremia. It took place in three hospitals, one each in Philadelphia, Baltimore, and Salt Lake City.
“This finding is in contrast to the epidemiology of MRSA bacteremia in adults, in whom bacteremia is more frequently attributed to catheter-related infections (31%-36%), endovascular infections (13%-15%), or an unknown source (15%-20%), and the durations of MRSA bacteremia are typically more prolonged (median duration of bacteremia is 8-9 days),” Dr. Hamdy and her associates wrote.
“Differences in the epidemiology of MRSA bacteremia between children and adults emphasize the need for dedicated pediatric studies to better understand the clinical characteristics and outcomes specific to children,” the researchers noted.
Musculoskeletal infections and endovascular infections were linked with treatment failure, possibly reflecting “the relatively higher burden of bacteria and/or decreased drug penetration into bone and endovascular infection sites,” the investigators said. Catheter-related infections were tied to reduced odds of treatment failure, “these episodes being localized to the catheter and therefore potentially less-invasive S. aureus infections.”
Mortality among these children with MRSA bacteremia was low, at 2%, but “nearly one-quarter of all patients experienced complications,” the study authors said (Pediatrics. 2017 May 5. doi: 10.1542/peds.2017-0183).
There was progression of infection in 7% of cases, and hematogenous complications or sequelae occurred in 23%. Twenty percent of children developed septic emboli or another metastatic focus of infection.
“This association between the duration of bacteremia and the development of complications has been previously reported among adults with S. aureus bacteremia,” Dr. Hamdy noted, “and provides important epidemiologic data that could inform decisions relating to the timing of additional imaging, such as echocardiograms, to identify metastatic foci.”
The children were treated with vancomycin, and some received additional anti-MRSA antibiotics. “Vancomycin trough concentrations or [minimum inhibitory concentrations] were not associated with treatment failure,” the investigators said. “Future studies to determine the appropriate vancomycin dose, duration, and approach to therapeutic drug monitoring are warranted to optimize patient outcomes.”
The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
FROM PEDIATRICS
Key clinical point:
Major finding: The primary sources of infection were osteomyelitis (31%), catheter-related bloodstream infections (22%), and skin and soft tissue infections (16%); endocarditis occurred in only 2% – a different epidemiology than in adults.
Data source: A study of 174 hospitalized children (younger than 19 years) with MRSA bacteremia at three hospitals in different states.
Disclosures: The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
Two doses HPV vaccine are as good as three against genital warts
Receiving two doses of human papillomavirus (HPV) vaccine at 5-month intervals or longer appears to provide similar protection against genital warts as three doses among girls initiating the series before age 15 years, reported Rebecca B. Perkins, MD, of Boston University, and her associates.
Of 387,906 adolescent females, 8% received 1 dose of HPV vaccine, 9% received 2 doses, 31% received 3 doses, and 52% remained unvaccinated. The mean age of the girls in the study was 15 years, and average length of follow-up was 6 years. The girls were aged 9-18 years on Jan.1, 2007, and the exposure period began at that time for unvaccinated girls or on the date of the last HPV vaccine injection for those receiving the vaccine. Among girls receiving more than 1 dose, 60% received their second dose within 3 months of their first dose (on time), and 47% received their third dose within 5 months of their second dose.
“Although reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes,” Dr. Perkins and her associates said. “Human papillomavirus vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount,” they noted.
The study used data from the Truven Health Analytics MarketScan Commercial Claims Database, covering enrollees and dependents from about half of provider-sponsored U.S. health insurance plans.
Read more at (Sex Transm Dis. 2017 Jun. doi: 10.1097/OLQ.0000000000000615).
Receiving two doses of human papillomavirus (HPV) vaccine at 5-month intervals or longer appears to provide similar protection against genital warts as three doses among girls initiating the series before age 15 years, reported Rebecca B. Perkins, MD, of Boston University, and her associates.
Of 387,906 adolescent females, 8% received 1 dose of HPV vaccine, 9% received 2 doses, 31% received 3 doses, and 52% remained unvaccinated. The mean age of the girls in the study was 15 years, and average length of follow-up was 6 years. The girls were aged 9-18 years on Jan.1, 2007, and the exposure period began at that time for unvaccinated girls or on the date of the last HPV vaccine injection for those receiving the vaccine. Among girls receiving more than 1 dose, 60% received their second dose within 3 months of their first dose (on time), and 47% received their third dose within 5 months of their second dose.
“Although reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes,” Dr. Perkins and her associates said. “Human papillomavirus vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount,” they noted.
The study used data from the Truven Health Analytics MarketScan Commercial Claims Database, covering enrollees and dependents from about half of provider-sponsored U.S. health insurance plans.
Read more at (Sex Transm Dis. 2017 Jun. doi: 10.1097/OLQ.0000000000000615).
Receiving two doses of human papillomavirus (HPV) vaccine at 5-month intervals or longer appears to provide similar protection against genital warts as three doses among girls initiating the series before age 15 years, reported Rebecca B. Perkins, MD, of Boston University, and her associates.
Of 387,906 adolescent females, 8% received 1 dose of HPV vaccine, 9% received 2 doses, 31% received 3 doses, and 52% remained unvaccinated. The mean age of the girls in the study was 15 years, and average length of follow-up was 6 years. The girls were aged 9-18 years on Jan.1, 2007, and the exposure period began at that time for unvaccinated girls or on the date of the last HPV vaccine injection for those receiving the vaccine. Among girls receiving more than 1 dose, 60% received their second dose within 3 months of their first dose (on time), and 47% received their third dose within 5 months of their second dose.
“Although reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes,” Dr. Perkins and her associates said. “Human papillomavirus vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount,” they noted.
The study used data from the Truven Health Analytics MarketScan Commercial Claims Database, covering enrollees and dependents from about half of provider-sponsored U.S. health insurance plans.
Read more at (Sex Transm Dis. 2017 Jun. doi: 10.1097/OLQ.0000000000000615).
FROM SEXUALLY TRANSMITTED DISEASES
SJS, TEN occur less frequently in children than adults
Although incidences of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are lower in children than in adults, the increased costs, lengths of stay, and mortality still pose a substantial health burden, said Derek Y. Hsu, of Northwestern University, Chicago, and his associates.
Using data from the 2009-2012 Nationwide Inpatient Sample of 1,687,172 pediatric admissions, “estimated frequencies per million children ranged from 4.3 to 5.8 for SJS, 0.6 to 1.4 for SJS/TEN, and 0 to 0.7 for TEN,” the study researchers reported. In adults, those numbers are 9.3, 1.9, and 1.6 per million adults per year, according to a 2016 study by the authors (J Invest Dermatol. 2016 Jul;136[7]:1387-97).
Pediatric SJS, SJS/TEN, and TEN mean hospital costs were $24,947, $63,787, and $102,243, respectively, compared with $10,496 for the control group.
The mean length of stay for patients with SJS, SJS/TEN, and TEN was 9.4 days, 15.7 days, and 20.4 days, compared with 4.6 days in children without these disorders, respectively, and they most often were discharged to their home or to other self-care.
“One in 10 children with SJS, SJS/TEN, and TEN underwent mechanical ventilation,” Mr. Hsu and his associates reported.
Mortality was 0% for SJS, 4% for SJS/TEN, and 16% for TEN.
Read more at the Journal of the American Academy of Dermatology (2017 May;76[5]:811-7).
Although incidences of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are lower in children than in adults, the increased costs, lengths of stay, and mortality still pose a substantial health burden, said Derek Y. Hsu, of Northwestern University, Chicago, and his associates.
Using data from the 2009-2012 Nationwide Inpatient Sample of 1,687,172 pediatric admissions, “estimated frequencies per million children ranged from 4.3 to 5.8 for SJS, 0.6 to 1.4 for SJS/TEN, and 0 to 0.7 for TEN,” the study researchers reported. In adults, those numbers are 9.3, 1.9, and 1.6 per million adults per year, according to a 2016 study by the authors (J Invest Dermatol. 2016 Jul;136[7]:1387-97).
Pediatric SJS, SJS/TEN, and TEN mean hospital costs were $24,947, $63,787, and $102,243, respectively, compared with $10,496 for the control group.
The mean length of stay for patients with SJS, SJS/TEN, and TEN was 9.4 days, 15.7 days, and 20.4 days, compared with 4.6 days in children without these disorders, respectively, and they most often were discharged to their home or to other self-care.
“One in 10 children with SJS, SJS/TEN, and TEN underwent mechanical ventilation,” Mr. Hsu and his associates reported.
Mortality was 0% for SJS, 4% for SJS/TEN, and 16% for TEN.
Read more at the Journal of the American Academy of Dermatology (2017 May;76[5]:811-7).
Although incidences of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are lower in children than in adults, the increased costs, lengths of stay, and mortality still pose a substantial health burden, said Derek Y. Hsu, of Northwestern University, Chicago, and his associates.
Using data from the 2009-2012 Nationwide Inpatient Sample of 1,687,172 pediatric admissions, “estimated frequencies per million children ranged from 4.3 to 5.8 for SJS, 0.6 to 1.4 for SJS/TEN, and 0 to 0.7 for TEN,” the study researchers reported. In adults, those numbers are 9.3, 1.9, and 1.6 per million adults per year, according to a 2016 study by the authors (J Invest Dermatol. 2016 Jul;136[7]:1387-97).
Pediatric SJS, SJS/TEN, and TEN mean hospital costs were $24,947, $63,787, and $102,243, respectively, compared with $10,496 for the control group.
The mean length of stay for patients with SJS, SJS/TEN, and TEN was 9.4 days, 15.7 days, and 20.4 days, compared with 4.6 days in children without these disorders, respectively, and they most often were discharged to their home or to other self-care.
“One in 10 children with SJS, SJS/TEN, and TEN underwent mechanical ventilation,” Mr. Hsu and his associates reported.
Mortality was 0% for SJS, 4% for SJS/TEN, and 16% for TEN.
Read more at the Journal of the American Academy of Dermatology (2017 May;76[5]:811-7).
Rotavirus vaccination in last decade cuts AGE hospitalization
There have been important reductions in hospitalization for acute gastroenteritis (AGE) and mortality since licensure of rotavirus vaccines 10 years ago, even in low-income countries with high child mortality, according to a new analysis of data from 27 countries.
In a search of articles published between Jan. 1, 2006, and Dec. 6, 2016, in the PubMed database, Eleanor Burnett of the division of viral diseases at the National Center for Immunization and Respiratory Diseases, Atlanta, and her associates identified 57 articles reporting on 59 data sources from 27 countries.
Among children younger than 5 years, the median percent reduction in AGE hospitalizations and/or ED visits was 38% overall, and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
The median reduction in AGE mortality among children younger than 1 year was 31% overall. In countries with medium and high child mortality, it was 45% and 30%, respectively.
The median reduction in AGE mortality in children younger than 5 years was 42% overall. In countries with medium and high child mortality, it was 50% and 36%, respectively. No estimates have been published from countries with low child mortality (J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix186).
“In several, but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection,” Ms. Burnett and her associates said. This population was not directly assessed in the studies.
The authors had no funding or conflicts of interest to disclose.
Decisions to introduce a new vaccine into a number of national immunization programs are influenced by many factors, including local disease burden, vaccine efficacy and safety, and the cost-effectiveness of the vaccines.
Introduction of rotavirus vaccination never started in earnest in Asia, a region with large birth cohort countries carrying substantial disease, but that lack has now turned an important corner, with India, Pakistan, and Bangladesh implementing or planning to implement rotavirus vaccination.
Also, two large African countries with high rotavirus mortality, Nigeria and Democratic Republic of Congo, are approved for Gavi (the Vaccine Alliance) funding to introduce the vaccines in 2018. The dramatic impact of rotavirus vaccines on rotavirus-associated hospitalizations and deaths described by Burnett et al. support the decisions by these large countries with high rotavirus mortality to introduce rotavirus vaccines and will lead to greater global health impacts.
Countries considering rotavirus vaccine introduction now will increasingly have additional data on vaccine safety and effectiveness. Burnett and her colleagues’ review will likely provide important information to these national immunization technical advisory groups and other decision-making bodies. This review will also be important for the earlier-adopter countries by way of validating their earlier introduction decisions.
Finally, the information will be of value to Gavi, UNICEF, and other international bodies tasked with providing resources and support for rotavirus vaccine introduction in low-income and lower-middle income countries.
Anthony Nelson, MD , is in the department of paediatrics at the Chinese University of Hong Kong, and Duncan Steele, PhD, is at the Bill & Melinda Gates Foundation, Seattle. These comments were excerpted from an editorial accompanying the article by Burnett et al. ( J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix187 ). The authors had no conflicts of interest or funding to disclose.
Decisions to introduce a new vaccine into a number of national immunization programs are influenced by many factors, including local disease burden, vaccine efficacy and safety, and the cost-effectiveness of the vaccines.
Introduction of rotavirus vaccination never started in earnest in Asia, a region with large birth cohort countries carrying substantial disease, but that lack has now turned an important corner, with India, Pakistan, and Bangladesh implementing or planning to implement rotavirus vaccination.
Also, two large African countries with high rotavirus mortality, Nigeria and Democratic Republic of Congo, are approved for Gavi (the Vaccine Alliance) funding to introduce the vaccines in 2018. The dramatic impact of rotavirus vaccines on rotavirus-associated hospitalizations and deaths described by Burnett et al. support the decisions by these large countries with high rotavirus mortality to introduce rotavirus vaccines and will lead to greater global health impacts.
Countries considering rotavirus vaccine introduction now will increasingly have additional data on vaccine safety and effectiveness. Burnett and her colleagues’ review will likely provide important information to these national immunization technical advisory groups and other decision-making bodies. This review will also be important for the earlier-adopter countries by way of validating their earlier introduction decisions.
Finally, the information will be of value to Gavi, UNICEF, and other international bodies tasked with providing resources and support for rotavirus vaccine introduction in low-income and lower-middle income countries.
Anthony Nelson, MD , is in the department of paediatrics at the Chinese University of Hong Kong, and Duncan Steele, PhD, is at the Bill & Melinda Gates Foundation, Seattle. These comments were excerpted from an editorial accompanying the article by Burnett et al. ( J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix187 ). The authors had no conflicts of interest or funding to disclose.
Decisions to introduce a new vaccine into a number of national immunization programs are influenced by many factors, including local disease burden, vaccine efficacy and safety, and the cost-effectiveness of the vaccines.
Introduction of rotavirus vaccination never started in earnest in Asia, a region with large birth cohort countries carrying substantial disease, but that lack has now turned an important corner, with India, Pakistan, and Bangladesh implementing or planning to implement rotavirus vaccination.
Also, two large African countries with high rotavirus mortality, Nigeria and Democratic Republic of Congo, are approved for Gavi (the Vaccine Alliance) funding to introduce the vaccines in 2018. The dramatic impact of rotavirus vaccines on rotavirus-associated hospitalizations and deaths described by Burnett et al. support the decisions by these large countries with high rotavirus mortality to introduce rotavirus vaccines and will lead to greater global health impacts.
Countries considering rotavirus vaccine introduction now will increasingly have additional data on vaccine safety and effectiveness. Burnett and her colleagues’ review will likely provide important information to these national immunization technical advisory groups and other decision-making bodies. This review will also be important for the earlier-adopter countries by way of validating their earlier introduction decisions.
Finally, the information will be of value to Gavi, UNICEF, and other international bodies tasked with providing resources and support for rotavirus vaccine introduction in low-income and lower-middle income countries.
Anthony Nelson, MD , is in the department of paediatrics at the Chinese University of Hong Kong, and Duncan Steele, PhD, is at the Bill & Melinda Gates Foundation, Seattle. These comments were excerpted from an editorial accompanying the article by Burnett et al. ( J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix187 ). The authors had no conflicts of interest or funding to disclose.
There have been important reductions in hospitalization for acute gastroenteritis (AGE) and mortality since licensure of rotavirus vaccines 10 years ago, even in low-income countries with high child mortality, according to a new analysis of data from 27 countries.
In a search of articles published between Jan. 1, 2006, and Dec. 6, 2016, in the PubMed database, Eleanor Burnett of the division of viral diseases at the National Center for Immunization and Respiratory Diseases, Atlanta, and her associates identified 57 articles reporting on 59 data sources from 27 countries.
Among children younger than 5 years, the median percent reduction in AGE hospitalizations and/or ED visits was 38% overall, and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
The median reduction in AGE mortality among children younger than 1 year was 31% overall. In countries with medium and high child mortality, it was 45% and 30%, respectively.
The median reduction in AGE mortality in children younger than 5 years was 42% overall. In countries with medium and high child mortality, it was 50% and 36%, respectively. No estimates have been published from countries with low child mortality (J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix186).
“In several, but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection,” Ms. Burnett and her associates said. This population was not directly assessed in the studies.
The authors had no funding or conflicts of interest to disclose.
There have been important reductions in hospitalization for acute gastroenteritis (AGE) and mortality since licensure of rotavirus vaccines 10 years ago, even in low-income countries with high child mortality, according to a new analysis of data from 27 countries.
In a search of articles published between Jan. 1, 2006, and Dec. 6, 2016, in the PubMed database, Eleanor Burnett of the division of viral diseases at the National Center for Immunization and Respiratory Diseases, Atlanta, and her associates identified 57 articles reporting on 59 data sources from 27 countries.
Among children younger than 5 years, the median percent reduction in AGE hospitalizations and/or ED visits was 38% overall, and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
The median reduction in AGE mortality among children younger than 1 year was 31% overall. In countries with medium and high child mortality, it was 45% and 30%, respectively.
The median reduction in AGE mortality in children younger than 5 years was 42% overall. In countries with medium and high child mortality, it was 50% and 36%, respectively. No estimates have been published from countries with low child mortality (J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix186).
“In several, but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection,” Ms. Burnett and her associates said. This population was not directly assessed in the studies.
The authors had no funding or conflicts of interest to disclose.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point:
Major finding: In children younger than 5 years, the median percent reduction in AGE hospitalizations and/or emergency department visits was 38% overall and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
Data source: Meta-analysis of 57 articles reporting on 59 data sources from 27 countries between Jan. 1, 2006, and Dec. 6, 2016.
Disclosures: The authors had no funding or conflicts of interest to disclose.
AAP policy addresses nonemergency acute care when it’s not the medical home
The American Academy of Pediatrics’ new policy statement affirms that the medical home is the best location for nonemergency acute care but realizes that parents may take their children to other acute care services because of access, after-hours timing, or other reasons.
The AAP encourages its members to make the medical home available as “the first point of contact for acute care” by offering “enhanced access scheduling, extended hours, and telehealth incorporated inside the medical home.” Also, the AAP “supports innovation, peer-to-peer collaboration, and practice change to promote high-quality and accessible care for all children,” the policy states.
The policy, authored by Gregory P. Conners, MD, chairperson of the AAP Committee on Practice and Ambulatory Medicine, further outlines recommendations for acute care entities serving infants, children, and adolescents outside the medical home, such as maximizing continuity of care by quickly communicating information to the medical home, as well as referring the child for necessary follow-up.
Another recommendation is to have clearly defined scope-of-service limits that are transparent to families.
Other AAP entities involved in developing the policy were the Committee on Pediatric Emergency Medicine, the Section on Telehealth Care Executive Committee, the Task Force on Pediatric Practice Change, and the Section on Emergency Medicine Executive Committee.
Read more in the journal Pediatrics (2017;139[5]:e20170629).
The American Academy of Pediatrics’ new policy statement affirms that the medical home is the best location for nonemergency acute care but realizes that parents may take their children to other acute care services because of access, after-hours timing, or other reasons.
The AAP encourages its members to make the medical home available as “the first point of contact for acute care” by offering “enhanced access scheduling, extended hours, and telehealth incorporated inside the medical home.” Also, the AAP “supports innovation, peer-to-peer collaboration, and practice change to promote high-quality and accessible care for all children,” the policy states.
The policy, authored by Gregory P. Conners, MD, chairperson of the AAP Committee on Practice and Ambulatory Medicine, further outlines recommendations for acute care entities serving infants, children, and adolescents outside the medical home, such as maximizing continuity of care by quickly communicating information to the medical home, as well as referring the child for necessary follow-up.
Another recommendation is to have clearly defined scope-of-service limits that are transparent to families.
Other AAP entities involved in developing the policy were the Committee on Pediatric Emergency Medicine, the Section on Telehealth Care Executive Committee, the Task Force on Pediatric Practice Change, and the Section on Emergency Medicine Executive Committee.
Read more in the journal Pediatrics (2017;139[5]:e20170629).
The American Academy of Pediatrics’ new policy statement affirms that the medical home is the best location for nonemergency acute care but realizes that parents may take their children to other acute care services because of access, after-hours timing, or other reasons.
The AAP encourages its members to make the medical home available as “the first point of contact for acute care” by offering “enhanced access scheduling, extended hours, and telehealth incorporated inside the medical home.” Also, the AAP “supports innovation, peer-to-peer collaboration, and practice change to promote high-quality and accessible care for all children,” the policy states.
The policy, authored by Gregory P. Conners, MD, chairperson of the AAP Committee on Practice and Ambulatory Medicine, further outlines recommendations for acute care entities serving infants, children, and adolescents outside the medical home, such as maximizing continuity of care by quickly communicating information to the medical home, as well as referring the child for necessary follow-up.
Another recommendation is to have clearly defined scope-of-service limits that are transparent to families.
Other AAP entities involved in developing the policy were the Committee on Pediatric Emergency Medicine, the Section on Telehealth Care Executive Committee, the Task Force on Pediatric Practice Change, and the Section on Emergency Medicine Executive Committee.
Read more in the journal Pediatrics (2017;139[5]:e20170629).
Revaccinate HIV-infected teens with hepatitis B vaccine
Consider revaccinating all perinatally HIV-infected adolescents who did not initiate highly active antiretroviral therapy (HAART) at the time of initial infant hepatitis B virus (HBV) vaccination, with a three-dose HBV schedule, recommended researchers at Mahidol University, Bangkok, in a recent study.
In a prospective study from March 2012 to March 2014 ultimately involving 162 perinatally HIV-infected adolescents with immune reconstitution, only 3.6% were receiving HAART at the time of initial infant HBV vaccination and 96.3% had undetectable antihepatitis B surface antibodies (anti-HBs) at baseline. Adolescents with breakthrough HBV infection had been excluded from the cohort.
In a multivariate analysis, there was no independent factor that was associated with the presence of immune memory defined as anti-HBs greater than or equal to 100 mIU/mL, wrote lead researcher Keswadee Lapphra, MD, and her associates.
In a previous study, 71% of three-dose revaccinated persons retained protective antibodies against HBV at 3-year follow-up; this was a similar rate to that reported in healthy HIV-infected children after their infant primary HBV series (Vaccine. 2011 May 23;29[23]:3977-81), they said.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/inf.0000000000001613).
Consider revaccinating all perinatally HIV-infected adolescents who did not initiate highly active antiretroviral therapy (HAART) at the time of initial infant hepatitis B virus (HBV) vaccination, with a three-dose HBV schedule, recommended researchers at Mahidol University, Bangkok, in a recent study.
In a prospective study from March 2012 to March 2014 ultimately involving 162 perinatally HIV-infected adolescents with immune reconstitution, only 3.6% were receiving HAART at the time of initial infant HBV vaccination and 96.3% had undetectable antihepatitis B surface antibodies (anti-HBs) at baseline. Adolescents with breakthrough HBV infection had been excluded from the cohort.
In a multivariate analysis, there was no independent factor that was associated with the presence of immune memory defined as anti-HBs greater than or equal to 100 mIU/mL, wrote lead researcher Keswadee Lapphra, MD, and her associates.
In a previous study, 71% of three-dose revaccinated persons retained protective antibodies against HBV at 3-year follow-up; this was a similar rate to that reported in healthy HIV-infected children after their infant primary HBV series (Vaccine. 2011 May 23;29[23]:3977-81), they said.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/inf.0000000000001613).
Consider revaccinating all perinatally HIV-infected adolescents who did not initiate highly active antiretroviral therapy (HAART) at the time of initial infant hepatitis B virus (HBV) vaccination, with a three-dose HBV schedule, recommended researchers at Mahidol University, Bangkok, in a recent study.
In a prospective study from March 2012 to March 2014 ultimately involving 162 perinatally HIV-infected adolescents with immune reconstitution, only 3.6% were receiving HAART at the time of initial infant HBV vaccination and 96.3% had undetectable antihepatitis B surface antibodies (anti-HBs) at baseline. Adolescents with breakthrough HBV infection had been excluded from the cohort.
In a multivariate analysis, there was no independent factor that was associated with the presence of immune memory defined as anti-HBs greater than or equal to 100 mIU/mL, wrote lead researcher Keswadee Lapphra, MD, and her associates.
In a previous study, 71% of three-dose revaccinated persons retained protective antibodies against HBV at 3-year follow-up; this was a similar rate to that reported in healthy HIV-infected children after their infant primary HBV series (Vaccine. 2011 May 23;29[23]:3977-81), they said.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/inf.0000000000001613).
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
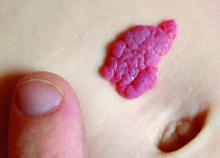
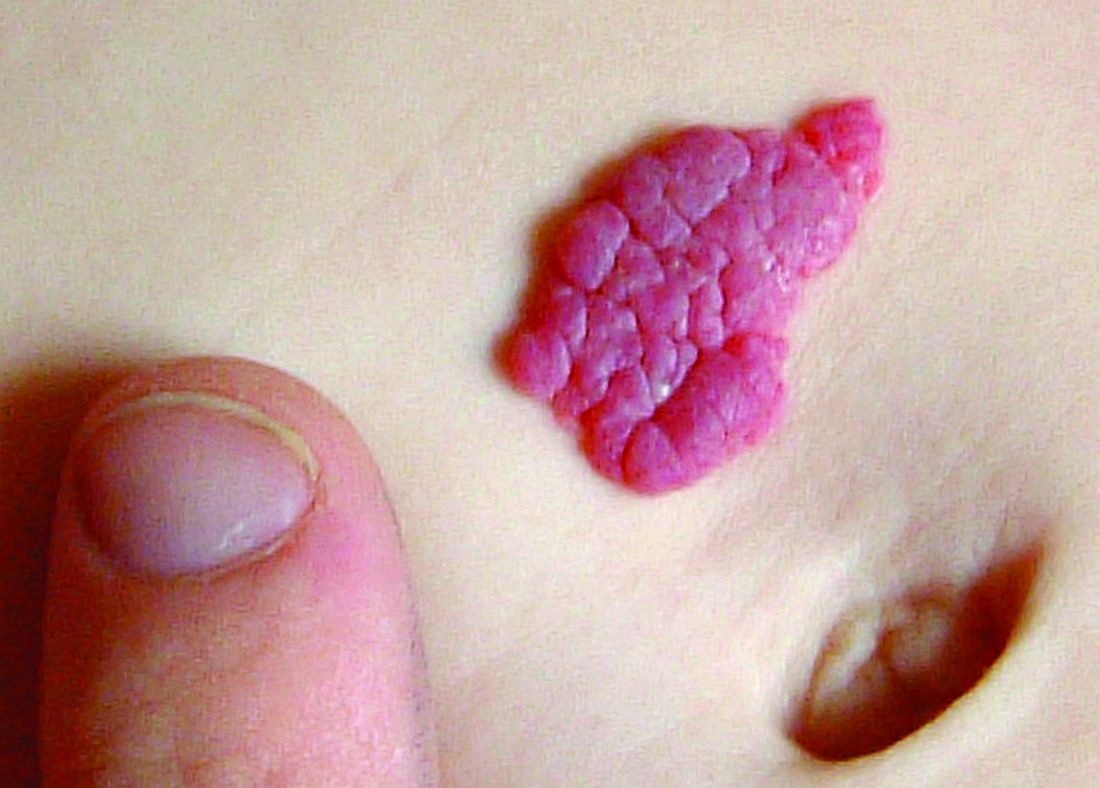
Congenital melanocytic nevi in the very young tend to be benign
Histopathology findings of cytologic atypia, architectural disorder, and pagetoid spread are common in congenital melanocytic nevi (CMN) of all sizes in children aged 0-35 months, and tend to have benign outcomes, according to a retrospective study.
Emily A. Simons, MPH, and her associates at Boston Children’s Hospital studied 197 nevi in 179 patients with an average age of 14 months (range, 4 days to 35 months); 51% were female. Of those, 80% had skin types I-II, and 90% were white. The majority of the lesions involved the head or trunk and were predominantly medium in size, and 58% had a projected adult size of 1.5-10 cm. The study was retrospective; cases had been diagnosed between 1993 and 2013.
Cytologic atypia, architectural disorder, and pagetoid spread were the most frequent features – they were present in 73% of nevi and were closely associated. Combined histologic patterns of a blue nevus, spindle and Spitz nevus, or a deep penetrating nevus were identified in 40% of CMN. Proliferative nodules occurred in 5% of nevi.
Clinical outcomes were available for 130 patients, including 26 with large CMN and 8 with proliferative nodules. The children were alive and had not been diagnosed with melanoma at a mean follow-up of a mean of 8.4 years (range, 7 months to 21.3 years), even though margins of the last excision were positive in 41% of all CMN and in 77% of large CMN.
Malignant transformation of CMN certainly should be recognized, but the morbidity of overdiagnosis also needs to be considered. “Excision of larger CMN might require serial excisions under general anesthesia, the use of tissue expanders, and grafts,” Dr. Simons and her associates said.
Among the limitations of this study were that the majority of patients were white, so the results may not translate to children with darker skin types, they noted.
“The diagnosis of malignant melanoma should be made with great caution in this population,” they concluded, pointing out that the histopathologic features alone (cytologic atypia, architectural disorder, and pagetoid spread) “should not be interpreted as evidence for potential malignant behavior or serve as grounds for further excision.”
Read more in the Journal of the American Academy of Dermatology (2017 May;76[5]941-7).
The authors had no relevant financial disorders.
Histopathology findings of cytologic atypia, architectural disorder, and pagetoid spread are common in congenital melanocytic nevi (CMN) of all sizes in children aged 0-35 months, and tend to have benign outcomes, according to a retrospective study.
Emily A. Simons, MPH, and her associates at Boston Children’s Hospital studied 197 nevi in 179 patients with an average age of 14 months (range, 4 days to 35 months); 51% were female. Of those, 80% had skin types I-II, and 90% were white. The majority of the lesions involved the head or trunk and were predominantly medium in size, and 58% had a projected adult size of 1.5-10 cm. The study was retrospective; cases had been diagnosed between 1993 and 2013.
Cytologic atypia, architectural disorder, and pagetoid spread were the most frequent features – they were present in 73% of nevi and were closely associated. Combined histologic patterns of a blue nevus, spindle and Spitz nevus, or a deep penetrating nevus were identified in 40% of CMN. Proliferative nodules occurred in 5% of nevi.
Clinical outcomes were available for 130 patients, including 26 with large CMN and 8 with proliferative nodules. The children were alive and had not been diagnosed with melanoma at a mean follow-up of a mean of 8.4 years (range, 7 months to 21.3 years), even though margins of the last excision were positive in 41% of all CMN and in 77% of large CMN.
Malignant transformation of CMN certainly should be recognized, but the morbidity of overdiagnosis also needs to be considered. “Excision of larger CMN might require serial excisions under general anesthesia, the use of tissue expanders, and grafts,” Dr. Simons and her associates said.
Among the limitations of this study were that the majority of patients were white, so the results may not translate to children with darker skin types, they noted.
“The diagnosis of malignant melanoma should be made with great caution in this population,” they concluded, pointing out that the histopathologic features alone (cytologic atypia, architectural disorder, and pagetoid spread) “should not be interpreted as evidence for potential malignant behavior or serve as grounds for further excision.”
Read more in the Journal of the American Academy of Dermatology (2017 May;76[5]941-7).
The authors had no relevant financial disorders.
Histopathology findings of cytologic atypia, architectural disorder, and pagetoid spread are common in congenital melanocytic nevi (CMN) of all sizes in children aged 0-35 months, and tend to have benign outcomes, according to a retrospective study.
Emily A. Simons, MPH, and her associates at Boston Children’s Hospital studied 197 nevi in 179 patients with an average age of 14 months (range, 4 days to 35 months); 51% were female. Of those, 80% had skin types I-II, and 90% were white. The majority of the lesions involved the head or trunk and were predominantly medium in size, and 58% had a projected adult size of 1.5-10 cm. The study was retrospective; cases had been diagnosed between 1993 and 2013.
Cytologic atypia, architectural disorder, and pagetoid spread were the most frequent features – they were present in 73% of nevi and were closely associated. Combined histologic patterns of a blue nevus, spindle and Spitz nevus, or a deep penetrating nevus were identified in 40% of CMN. Proliferative nodules occurred in 5% of nevi.
Clinical outcomes were available for 130 patients, including 26 with large CMN and 8 with proliferative nodules. The children were alive and had not been diagnosed with melanoma at a mean follow-up of a mean of 8.4 years (range, 7 months to 21.3 years), even though margins of the last excision were positive in 41% of all CMN and in 77% of large CMN.
Malignant transformation of CMN certainly should be recognized, but the morbidity of overdiagnosis also needs to be considered. “Excision of larger CMN might require serial excisions under general anesthesia, the use of tissue expanders, and grafts,” Dr. Simons and her associates said.
Among the limitations of this study were that the majority of patients were white, so the results may not translate to children with darker skin types, they noted.
“The diagnosis of malignant melanoma should be made with great caution in this population,” they concluded, pointing out that the histopathologic features alone (cytologic atypia, architectural disorder, and pagetoid spread) “should not be interpreted as evidence for potential malignant behavior or serve as grounds for further excision.”
Read more in the Journal of the American Academy of Dermatology (2017 May;76[5]941-7).
The authors had no relevant financial disorders.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Infantile hemangiomas: Calculating dose for propranolol is tricky
Use of Hemangeol was associated with fewer dosing errors than use of generic propranolol hydrochloride oral solutions in treating infantile hemangiomas, according to an online questionnaire survey.
Anastasia O. Kurta, DO, of Saint Louis University and her associates emailed a questionnaire to 531 physicians who were members of the Society for Pediatric Dermatology and physicians known to treat infantile hemangiomas. Most of the 220 physicians who responded were pediatric dermatologists. Of those, 90% had prescribed generic propranolol available in concentrations of 4 mg/mL and 8 mg/mL, and 58.6% had prescribed Hemangeol, the Food and Drug Administration-approved formulation of propranolol hydrochloride available in a concentration of 4.28 mg/mL.
“A dosing chart accompanies propranolol 4.28 mg/mL [Hemangeol] using mL/kg doses, which eliminates conversion from milligrams to milliliters and could potentially explain the lower reported dose calculation error reported with propranolol 4.28 mg/mL,” Dr. Kurta and her associates said. “The risk of dispensing errors is increased for medications that are commercially available in different concentrations. Liquid medications prescribed for pediatric patients require additional computation for weight-based dosing and conversion, increasing the possibility of miscalculation.”
Daisy Dai, PhD, is employed by Pierre Fabre Pharmaceuticals and Elaine C. Siegfried, PhD, is a consultant for the company. Dr. Kurta and Eric S. Ambrecht, PhD, have no relevant financial disclosures.
Read more at J Am Acad Dermatol. 2017 May;76(5):999-1000.
Dr. Kurta reported no relevant financial disclosures.
Use of Hemangeol was associated with fewer dosing errors than use of generic propranolol hydrochloride oral solutions in treating infantile hemangiomas, according to an online questionnaire survey.
Anastasia O. Kurta, DO, of Saint Louis University and her associates emailed a questionnaire to 531 physicians who were members of the Society for Pediatric Dermatology and physicians known to treat infantile hemangiomas. Most of the 220 physicians who responded were pediatric dermatologists. Of those, 90% had prescribed generic propranolol available in concentrations of 4 mg/mL and 8 mg/mL, and 58.6% had prescribed Hemangeol, the Food and Drug Administration-approved formulation of propranolol hydrochloride available in a concentration of 4.28 mg/mL.
“A dosing chart accompanies propranolol 4.28 mg/mL [Hemangeol] using mL/kg doses, which eliminates conversion from milligrams to milliliters and could potentially explain the lower reported dose calculation error reported with propranolol 4.28 mg/mL,” Dr. Kurta and her associates said. “The risk of dispensing errors is increased for medications that are commercially available in different concentrations. Liquid medications prescribed for pediatric patients require additional computation for weight-based dosing and conversion, increasing the possibility of miscalculation.”
Daisy Dai, PhD, is employed by Pierre Fabre Pharmaceuticals and Elaine C. Siegfried, PhD, is a consultant for the company. Dr. Kurta and Eric S. Ambrecht, PhD, have no relevant financial disclosures.
Read more at J Am Acad Dermatol. 2017 May;76(5):999-1000.
Dr. Kurta reported no relevant financial disclosures.
Use of Hemangeol was associated with fewer dosing errors than use of generic propranolol hydrochloride oral solutions in treating infantile hemangiomas, according to an online questionnaire survey.
Anastasia O. Kurta, DO, of Saint Louis University and her associates emailed a questionnaire to 531 physicians who were members of the Society for Pediatric Dermatology and physicians known to treat infantile hemangiomas. Most of the 220 physicians who responded were pediatric dermatologists. Of those, 90% had prescribed generic propranolol available in concentrations of 4 mg/mL and 8 mg/mL, and 58.6% had prescribed Hemangeol, the Food and Drug Administration-approved formulation of propranolol hydrochloride available in a concentration of 4.28 mg/mL.
“A dosing chart accompanies propranolol 4.28 mg/mL [Hemangeol] using mL/kg doses, which eliminates conversion from milligrams to milliliters and could potentially explain the lower reported dose calculation error reported with propranolol 4.28 mg/mL,” Dr. Kurta and her associates said. “The risk of dispensing errors is increased for medications that are commercially available in different concentrations. Liquid medications prescribed for pediatric patients require additional computation for weight-based dosing and conversion, increasing the possibility of miscalculation.”
Daisy Dai, PhD, is employed by Pierre Fabre Pharmaceuticals and Elaine C. Siegfried, PhD, is a consultant for the company. Dr. Kurta and Eric S. Ambrecht, PhD, have no relevant financial disclosures.
Read more at J Am Acad Dermatol. 2017 May;76(5):999-1000.
Dr. Kurta reported no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY