User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
Seven days of opioids adequate for most hernia and other general surgery procedures
A 7-day limit on the initial opioid prescription may be sufficient for many common general surgery procedures, including hernia surgery and gynecologic procedures, findings of a large retrospective study suggest.
Rebecca E. Scully, MD, of the Center for Surgery and Public Health at Brigham and Women’s Hospital in Boston, and her associates examined opioid pain medication prescriptions and refills from records of the Military Health System Data Repository and the TRICARE insurance program of 215,140 opioid-naive patients. These patients were aged 18-64 years who underwent either cholecystectomy, appendectomy, inguinal hernia repair, anterior cruciate ligament reconstruction, rotator cuff tear repair, discectomy, mastectomy, or hysterectomy (JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.3132). Only 20% of the covered individuals are active members of the U.S. military. The mean age was 40 years; 50% were male, and 60% were white.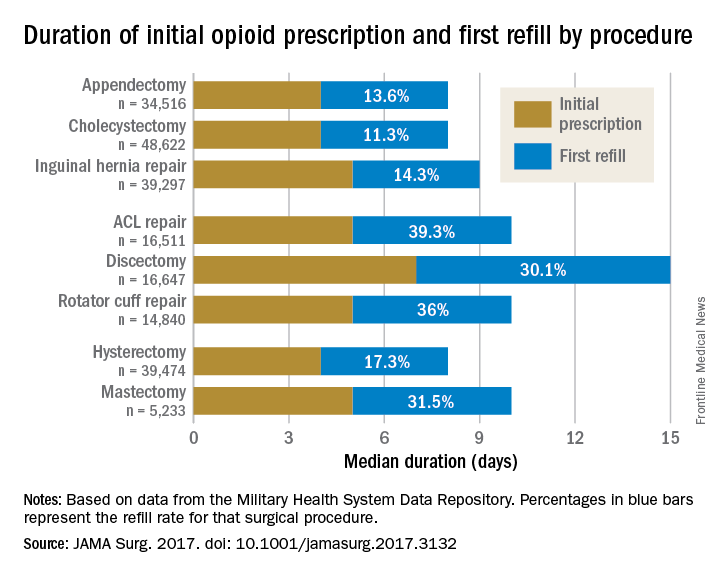
For appendectomy, cholecystectomy, and hysterectomy, the prescription was a median 4 days. For inguinal hernia repair, anterior cruciate ligament repair, rotator cuff repair, and mastectomy, the initial prescription was for 5 days. For discectomy, the median was 7 days.
Refill rates were the least at 11.3% for cholecystectomy and the most at 39.3% after anterior cruciate ligament repair. The time after the initial prescription until a refill was a median 6 days for appendectomy, cholecystectomy, and inguinal hernia repair, compared with a median 10 days for discectomy. The median duration of a refill prescription was 4 days for appendectomy, cholecystectomy, hernia repair, and hysterectomy versus 8 days for discectomy.
“Although 7 days appears to be more than adequate for many patients undergoing common general surgery and gynecologic procedures, prescription lengths likely should be extended to 10 days, particularly after common neurosurgical and musculoskeletal procedures, recognizing that as many as 40% of patients may still require one refill at a 7-day limit,” Dr. Scully and her associates said.
Although this study did not include rates of unused prescriptions or use of nonopioid pain relievers such as acetaminophen or NSAIDs, it did include a large population considered to be nationally representative “in many respects,” and it included a variety of procedures for which patients are commonly discharged to home, the researchers said.
The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
A 7-day limit on the initial opioid prescription may be sufficient for many common general surgery procedures, including hernia surgery and gynecologic procedures, findings of a large retrospective study suggest.
Rebecca E. Scully, MD, of the Center for Surgery and Public Health at Brigham and Women’s Hospital in Boston, and her associates examined opioid pain medication prescriptions and refills from records of the Military Health System Data Repository and the TRICARE insurance program of 215,140 opioid-naive patients. These patients were aged 18-64 years who underwent either cholecystectomy, appendectomy, inguinal hernia repair, anterior cruciate ligament reconstruction, rotator cuff tear repair, discectomy, mastectomy, or hysterectomy (JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.3132). Only 20% of the covered individuals are active members of the U.S. military. The mean age was 40 years; 50% were male, and 60% were white.
For appendectomy, cholecystectomy, and hysterectomy, the prescription was a median 4 days. For inguinal hernia repair, anterior cruciate ligament repair, rotator cuff repair, and mastectomy, the initial prescription was for 5 days. For discectomy, the median was 7 days.
Refill rates were the least at 11.3% for cholecystectomy and the most at 39.3% after anterior cruciate ligament repair. The time after the initial prescription until a refill was a median 6 days for appendectomy, cholecystectomy, and inguinal hernia repair, compared with a median 10 days for discectomy. The median duration of a refill prescription was 4 days for appendectomy, cholecystectomy, hernia repair, and hysterectomy versus 8 days for discectomy.
“Although 7 days appears to be more than adequate for many patients undergoing common general surgery and gynecologic procedures, prescription lengths likely should be extended to 10 days, particularly after common neurosurgical and musculoskeletal procedures, recognizing that as many as 40% of patients may still require one refill at a 7-day limit,” Dr. Scully and her associates said.
Although this study did not include rates of unused prescriptions or use of nonopioid pain relievers such as acetaminophen or NSAIDs, it did include a large population considered to be nationally representative “in many respects,” and it included a variety of procedures for which patients are commonly discharged to home, the researchers said.
The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
A 7-day limit on the initial opioid prescription may be sufficient for many common general surgery procedures, including hernia surgery and gynecologic procedures, findings of a large retrospective study suggest.
Rebecca E. Scully, MD, of the Center for Surgery and Public Health at Brigham and Women’s Hospital in Boston, and her associates examined opioid pain medication prescriptions and refills from records of the Military Health System Data Repository and the TRICARE insurance program of 215,140 opioid-naive patients. These patients were aged 18-64 years who underwent either cholecystectomy, appendectomy, inguinal hernia repair, anterior cruciate ligament reconstruction, rotator cuff tear repair, discectomy, mastectomy, or hysterectomy (JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.3132). Only 20% of the covered individuals are active members of the U.S. military. The mean age was 40 years; 50% were male, and 60% were white.
For appendectomy, cholecystectomy, and hysterectomy, the prescription was a median 4 days. For inguinal hernia repair, anterior cruciate ligament repair, rotator cuff repair, and mastectomy, the initial prescription was for 5 days. For discectomy, the median was 7 days.
Refill rates were the least at 11.3% for cholecystectomy and the most at 39.3% after anterior cruciate ligament repair. The time after the initial prescription until a refill was a median 6 days for appendectomy, cholecystectomy, and inguinal hernia repair, compared with a median 10 days for discectomy. The median duration of a refill prescription was 4 days for appendectomy, cholecystectomy, hernia repair, and hysterectomy versus 8 days for discectomy.
“Although 7 days appears to be more than adequate for many patients undergoing common general surgery and gynecologic procedures, prescription lengths likely should be extended to 10 days, particularly after common neurosurgical and musculoskeletal procedures, recognizing that as many as 40% of patients may still require one refill at a 7-day limit,” Dr. Scully and her associates said.
Although this study did not include rates of unused prescriptions or use of nonopioid pain relievers such as acetaminophen or NSAIDs, it did include a large population considered to be nationally representative “in many respects,” and it included a variety of procedures for which patients are commonly discharged to home, the researchers said.
The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
FROM JAMA SURGERY
Key clinical point:
Major finding: The initial opioid prescription was a median 4 days for appendectomy and cholecystectomy, a median 5 days for inguinal hernia repair and anterior cruciate ligament and rotator cuff repair, and a median 7 days for discectomy.
Data source: A study of opioid prescriptions in 215,140 surgery patients aged 18-64 years.
Disclosures: The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
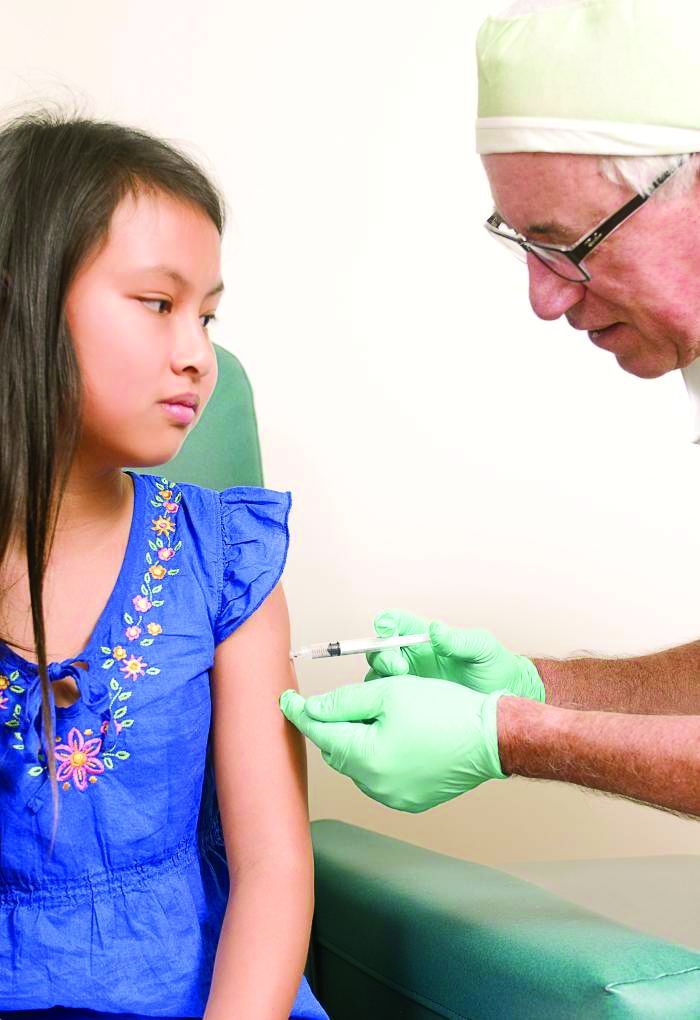
Use of MenACWY-CRM vaccine in 2- to 10-year-olds raised no safety concerns
, reported Sara Yee Tartof, PhD, of Kaiser Permanente Southern California, Pasadena, and her associates.
The study was undertaken to evaluate the safety of the MenACWY-CRM vaccine in the care of children aged 2-10 years in the real world. The other quadrivalent meningococcal conjugate vaccine, MenACWY-D (Menactra), was not used in this study.
In a retrospective, observational study of 327 children aged 2-10 years when they received the MenACWY-CRM vaccine as part of routine clinical care, there was only one event of interest, which was a child who developed asthma during the 1-year observation period, and that occurred 237 days after vaccination with both the MenACWY-CRM and a typhoid vaccine. “A causal link between the two events is unlikely,” Dr. Tartof and her associates wrote.
Most of the serious medically attended events were considered to be unrelated to MenACWY-CRM vaccination, or symptom onset occurred a long time after vaccination. “The remaining events were pneumonia, bronchitis, cough, febrile convulsion, and vomiting identified within 30 days of vaccination among four children,” the investigators reported. “Pneumonia, bronchitis, and cough were diagnosed in the same child; cough, febrile convulsion, and vomiting were diagnosed separately in the other 3 children.
“It appears that many MenACWY-CRM recipients in our study population received the vaccine because of travel to high-risk areas, possible exposure to meningitis, or as routine vaccinations received early as part of the ACIP-recommended routine vaccination for those 11-12 years old,” Dr. Tartof and her associates wrote.
Read more in the Pediatric Infectious Diseases Journal (2017 Nov 1. doi: 10.1097/INF.0000000000001696).
, reported Sara Yee Tartof, PhD, of Kaiser Permanente Southern California, Pasadena, and her associates.
The study was undertaken to evaluate the safety of the MenACWY-CRM vaccine in the care of children aged 2-10 years in the real world. The other quadrivalent meningococcal conjugate vaccine, MenACWY-D (Menactra), was not used in this study.
In a retrospective, observational study of 327 children aged 2-10 years when they received the MenACWY-CRM vaccine as part of routine clinical care, there was only one event of interest, which was a child who developed asthma during the 1-year observation period, and that occurred 237 days after vaccination with both the MenACWY-CRM and a typhoid vaccine. “A causal link between the two events is unlikely,” Dr. Tartof and her associates wrote.
Most of the serious medically attended events were considered to be unrelated to MenACWY-CRM vaccination, or symptom onset occurred a long time after vaccination. “The remaining events were pneumonia, bronchitis, cough, febrile convulsion, and vomiting identified within 30 days of vaccination among four children,” the investigators reported. “Pneumonia, bronchitis, and cough were diagnosed in the same child; cough, febrile convulsion, and vomiting were diagnosed separately in the other 3 children.
“It appears that many MenACWY-CRM recipients in our study population received the vaccine because of travel to high-risk areas, possible exposure to meningitis, or as routine vaccinations received early as part of the ACIP-recommended routine vaccination for those 11-12 years old,” Dr. Tartof and her associates wrote.
Read more in the Pediatric Infectious Diseases Journal (2017 Nov 1. doi: 10.1097/INF.0000000000001696).
, reported Sara Yee Tartof, PhD, of Kaiser Permanente Southern California, Pasadena, and her associates.
The study was undertaken to evaluate the safety of the MenACWY-CRM vaccine in the care of children aged 2-10 years in the real world. The other quadrivalent meningococcal conjugate vaccine, MenACWY-D (Menactra), was not used in this study.
In a retrospective, observational study of 327 children aged 2-10 years when they received the MenACWY-CRM vaccine as part of routine clinical care, there was only one event of interest, which was a child who developed asthma during the 1-year observation period, and that occurred 237 days after vaccination with both the MenACWY-CRM and a typhoid vaccine. “A causal link between the two events is unlikely,” Dr. Tartof and her associates wrote.
Most of the serious medically attended events were considered to be unrelated to MenACWY-CRM vaccination, or symptom onset occurred a long time after vaccination. “The remaining events were pneumonia, bronchitis, cough, febrile convulsion, and vomiting identified within 30 days of vaccination among four children,” the investigators reported. “Pneumonia, bronchitis, and cough were diagnosed in the same child; cough, febrile convulsion, and vomiting were diagnosed separately in the other 3 children.
“It appears that many MenACWY-CRM recipients in our study population received the vaccine because of travel to high-risk areas, possible exposure to meningitis, or as routine vaccinations received early as part of the ACIP-recommended routine vaccination for those 11-12 years old,” Dr. Tartof and her associates wrote.
Read more in the Pediatric Infectious Diseases Journal (2017 Nov 1. doi: 10.1097/INF.0000000000001696).
FROM PEDIATRIC INFECTIOUS DISEASE JOURNAL
Early diagnosis of tuberous sclerosis may be possible in infants
with an echocardiogram for cardiac rhabdomyomas and a skin examination for hypomelanotic macules, noninvasive tests that do not require sedation, said Peter E. Davis, MD, of Boston Children’s Hospital, and his associates on behalf of the Tuberous Sclerosis Complex Autism Center of Excellence Research Network.
“Early TSC diagnosis in infants opens a window of opportunity to treat before the onset of epilepsy or other neurodevelopmental disorders and allows for close surveillance for sequelae of TSC,” the researchers said. “However, this window may be as small as a few months.”
In two concurrent, prospective, longitudinal, observational studies at five medical centers of 130 infants meeting a genetic or clinical diagnosis of TSC, cardiac rhabdomyomas, and hypomelanotic macules were the most common initial presenting features, occurring in 59% and 39% of infants, respectively; 85% of infants had either or both. In terms of prevalence, hypomelanotic macules and tubers or other cortical dysplasias were the most prevalent TSC features both occurring at 94%, followed by subependymal nodules (SENs) at 90%, and cardiac rhabdomyomas at 82%. Every infant had at least one of these diagnostic criteria, and 61% had all four of them, the investigators reported.
Neuroimaging results were available in 115 infants, of whom 94% had tubers or cortical dysplasias, 90% had SENs, and 89% had both; 6% had subependymal giant cell astrocytomas. Seizure onset occurred in 15% of infants before or when other TSC criteria were recorded, “suggesting that seizure was an initial presenting symptom.” Seizure onset occurred within 3 months after initial presentation in 17% of infants, within 6 months in 39%, and within 12 months in 57%. Ultimately, 57% of the infants had infantile spasms, 55% had focal seizures, and 12% had another seizure type, Dr. Davis and his associates said.
“Early, prospective use of EEGs may enable risk stratification in studies of epilepsy prevention in infants with TSC. The antiepileptic medication vigabatrin is particularly effective in treating infantile spasms in TSC and has mTOR [mechanistic target of the rapamycin]–inhibiting effects. Vigabatrin is currently in clinical trials to determine its efficacy at preventing epilepsy in patients with TSC,” they said, although the drug is not currently recommended for infants. “mTOR inhibitors have been successfully used to treat multiple TSC manifestations and have shown some efficacy as adjunctive treatment of refractory epilepsy,” they wrote, adding that studies are needed “to determine the safety and efficacy of mTOR inhibitors in this age group.”
Read more at Pediatrics. 2017;140(6):e20164040 (doi: 10.1542/peds.2016-4040).
with an echocardiogram for cardiac rhabdomyomas and a skin examination for hypomelanotic macules, noninvasive tests that do not require sedation, said Peter E. Davis, MD, of Boston Children’s Hospital, and his associates on behalf of the Tuberous Sclerosis Complex Autism Center of Excellence Research Network.
“Early TSC diagnosis in infants opens a window of opportunity to treat before the onset of epilepsy or other neurodevelopmental disorders and allows for close surveillance for sequelae of TSC,” the researchers said. “However, this window may be as small as a few months.”
In two concurrent, prospective, longitudinal, observational studies at five medical centers of 130 infants meeting a genetic or clinical diagnosis of TSC, cardiac rhabdomyomas, and hypomelanotic macules were the most common initial presenting features, occurring in 59% and 39% of infants, respectively; 85% of infants had either or both. In terms of prevalence, hypomelanotic macules and tubers or other cortical dysplasias were the most prevalent TSC features both occurring at 94%, followed by subependymal nodules (SENs) at 90%, and cardiac rhabdomyomas at 82%. Every infant had at least one of these diagnostic criteria, and 61% had all four of them, the investigators reported.
Neuroimaging results were available in 115 infants, of whom 94% had tubers or cortical dysplasias, 90% had SENs, and 89% had both; 6% had subependymal giant cell astrocytomas. Seizure onset occurred in 15% of infants before or when other TSC criteria were recorded, “suggesting that seizure was an initial presenting symptom.” Seizure onset occurred within 3 months after initial presentation in 17% of infants, within 6 months in 39%, and within 12 months in 57%. Ultimately, 57% of the infants had infantile spasms, 55% had focal seizures, and 12% had another seizure type, Dr. Davis and his associates said.
“Early, prospective use of EEGs may enable risk stratification in studies of epilepsy prevention in infants with TSC. The antiepileptic medication vigabatrin is particularly effective in treating infantile spasms in TSC and has mTOR [mechanistic target of the rapamycin]–inhibiting effects. Vigabatrin is currently in clinical trials to determine its efficacy at preventing epilepsy in patients with TSC,” they said, although the drug is not currently recommended for infants. “mTOR inhibitors have been successfully used to treat multiple TSC manifestations and have shown some efficacy as adjunctive treatment of refractory epilepsy,” they wrote, adding that studies are needed “to determine the safety and efficacy of mTOR inhibitors in this age group.”
Read more at Pediatrics. 2017;140(6):e20164040 (doi: 10.1542/peds.2016-4040).
with an echocardiogram for cardiac rhabdomyomas and a skin examination for hypomelanotic macules, noninvasive tests that do not require sedation, said Peter E. Davis, MD, of Boston Children’s Hospital, and his associates on behalf of the Tuberous Sclerosis Complex Autism Center of Excellence Research Network.
“Early TSC diagnosis in infants opens a window of opportunity to treat before the onset of epilepsy or other neurodevelopmental disorders and allows for close surveillance for sequelae of TSC,” the researchers said. “However, this window may be as small as a few months.”
In two concurrent, prospective, longitudinal, observational studies at five medical centers of 130 infants meeting a genetic or clinical diagnosis of TSC, cardiac rhabdomyomas, and hypomelanotic macules were the most common initial presenting features, occurring in 59% and 39% of infants, respectively; 85% of infants had either or both. In terms of prevalence, hypomelanotic macules and tubers or other cortical dysplasias were the most prevalent TSC features both occurring at 94%, followed by subependymal nodules (SENs) at 90%, and cardiac rhabdomyomas at 82%. Every infant had at least one of these diagnostic criteria, and 61% had all four of them, the investigators reported.
Neuroimaging results were available in 115 infants, of whom 94% had tubers or cortical dysplasias, 90% had SENs, and 89% had both; 6% had subependymal giant cell astrocytomas. Seizure onset occurred in 15% of infants before or when other TSC criteria were recorded, “suggesting that seizure was an initial presenting symptom.” Seizure onset occurred within 3 months after initial presentation in 17% of infants, within 6 months in 39%, and within 12 months in 57%. Ultimately, 57% of the infants had infantile spasms, 55% had focal seizures, and 12% had another seizure type, Dr. Davis and his associates said.
“Early, prospective use of EEGs may enable risk stratification in studies of epilepsy prevention in infants with TSC. The antiepileptic medication vigabatrin is particularly effective in treating infantile spasms in TSC and has mTOR [mechanistic target of the rapamycin]–inhibiting effects. Vigabatrin is currently in clinical trials to determine its efficacy at preventing epilepsy in patients with TSC,” they said, although the drug is not currently recommended for infants. “mTOR inhibitors have been successfully used to treat multiple TSC manifestations and have shown some efficacy as adjunctive treatment of refractory epilepsy,” they wrote, adding that studies are needed “to determine the safety and efficacy of mTOR inhibitors in this age group.”
Read more at Pediatrics. 2017;140(6):e20164040 (doi: 10.1542/peds.2016-4040).
FROM PEDIATRICS
PCV13 vaccination safe, effective in 6- to 17-year-olds
Positive immunogenicity results and lack of adverse events with receipt of the 13-valent pneumococcal conjugate vaccine by 200 children and adolescents in India supports extension of the indication to that population, reported Sharad Agarkhedkar, MD, of the Padmashree Dr. D. Y. Patil Medical College in Pune, India, and associates.
In India, the PCV13 vaccine is indicated for children aged 6 months to 5 years and for adults older than 50 years. The study assessed the safety and immunogenicity of the vaccine in children aged 6-17 years to consider expanding the indication to that age group, the investigators said.
In an open-label study of 200 children and adolescents who received PCV13, 113 were 6 years to less than 10 years of age and 87 were aged 10-17 years; 54% were female. Antibody-mediated opsonophagocytic activity geometric mean titers were significantly higher for all vaccine serotypes 1 month after vaccination with PCV13, and there was no significant difference in psonophagocytic activity geometric mean titers between the two age groups. , the investigators said.
There were no acute reactions within 20 minutes of immunization. No adverse effects or severe adverse effects were recalled by caregivers who were questioned by the investigators 28-42 days following vaccination.
Read more in the Pediatric Infectious Diseases Journal (2017 Nov 1;36[11]:e282-5. doi: 10.1097/INF.0000000000001695).
Positive immunogenicity results and lack of adverse events with receipt of the 13-valent pneumococcal conjugate vaccine by 200 children and adolescents in India supports extension of the indication to that population, reported Sharad Agarkhedkar, MD, of the Padmashree Dr. D. Y. Patil Medical College in Pune, India, and associates.
In India, the PCV13 vaccine is indicated for children aged 6 months to 5 years and for adults older than 50 years. The study assessed the safety and immunogenicity of the vaccine in children aged 6-17 years to consider expanding the indication to that age group, the investigators said.
In an open-label study of 200 children and adolescents who received PCV13, 113 were 6 years to less than 10 years of age and 87 were aged 10-17 years; 54% were female. Antibody-mediated opsonophagocytic activity geometric mean titers were significantly higher for all vaccine serotypes 1 month after vaccination with PCV13, and there was no significant difference in psonophagocytic activity geometric mean titers between the two age groups. , the investigators said.
There were no acute reactions within 20 minutes of immunization. No adverse effects or severe adverse effects were recalled by caregivers who were questioned by the investigators 28-42 days following vaccination.
Read more in the Pediatric Infectious Diseases Journal (2017 Nov 1;36[11]:e282-5. doi: 10.1097/INF.0000000000001695).
Positive immunogenicity results and lack of adverse events with receipt of the 13-valent pneumococcal conjugate vaccine by 200 children and adolescents in India supports extension of the indication to that population, reported Sharad Agarkhedkar, MD, of the Padmashree Dr. D. Y. Patil Medical College in Pune, India, and associates.
In India, the PCV13 vaccine is indicated for children aged 6 months to 5 years and for adults older than 50 years. The study assessed the safety and immunogenicity of the vaccine in children aged 6-17 years to consider expanding the indication to that age group, the investigators said.
In an open-label study of 200 children and adolescents who received PCV13, 113 were 6 years to less than 10 years of age and 87 were aged 10-17 years; 54% were female. Antibody-mediated opsonophagocytic activity geometric mean titers were significantly higher for all vaccine serotypes 1 month after vaccination with PCV13, and there was no significant difference in psonophagocytic activity geometric mean titers between the two age groups. , the investigators said.
There were no acute reactions within 20 minutes of immunization. No adverse effects or severe adverse effects were recalled by caregivers who were questioned by the investigators 28-42 days following vaccination.
Read more in the Pediatric Infectious Diseases Journal (2017 Nov 1;36[11]:e282-5. doi: 10.1097/INF.0000000000001695).
FROM PEDIATRIC INFECTIOUS DISEASE JOURNAL
Up-to-date vaccination status varies by mother’s country of origin
, and, more particularly, on the country of origin of their mother, reported Maureen Leeds and Miriam Halstead Muscoplat of the Minnesota Department of Health, St. Paul.
In a study of 97,885 Minnesota children born during 2011-2012, fewer than half were up to date with their vaccinations by age 18 months and only 70% were up to date by age 36 months.
If children had at least one parent born outside the United States, they were 25% less likely to be up to date with their vaccinations by age 36 months than if both their parents were born in the United States. However, children whose mothers were from Africa (excluding Somalia), the Caribbean, Central and South America, or Mexico were significantly more likely to be up to date at all ages, compared with children whose mothers were born in the United States.
Children whose mothers were born in Asia, Canada, Eastern Europe, Somalia, or Western Europe were significantly less likely to be up to date at all ages than were children whose mothers were born in the United States. At 18 months, fewer than 10% of children whose mothers were born in Somalia were up to date; by 36 months, 44% were.
“Inadequate parental understanding of vaccination and weaker public health education programs in some regions might account for some of these findings, as well as economic and social factors influencing emigration, including fleeing war, religious persecution, or poverty,” Ms. Leeds and Ms. Muscoplat said. “Somali parents in Minnesota have been reported to be more likely than non-Somali parents to have concerns about the safety of measles-mumps-rubella (MMR) vaccine, which has led to a decline in coverage with MMR and possibly other childhood vaccines. From April to August 2017, Minnesota experienced a measles outbreak, ending with 79 confirmed cases, including 65 in children of Somali descent,” they wrote.
“Encouraging medical providers to use interpreters, take time to build trust, and assess vaccination status at every visit might improve vaccination coverage” in immigrant, migrant, and refugee populations, they said.
Read more in Morbidity and Mortality Weekly Report (2017 Oct 27;66[42]:1125-9).
, and, more particularly, on the country of origin of their mother, reported Maureen Leeds and Miriam Halstead Muscoplat of the Minnesota Department of Health, St. Paul.
In a study of 97,885 Minnesota children born during 2011-2012, fewer than half were up to date with their vaccinations by age 18 months and only 70% were up to date by age 36 months.
If children had at least one parent born outside the United States, they were 25% less likely to be up to date with their vaccinations by age 36 months than if both their parents were born in the United States. However, children whose mothers were from Africa (excluding Somalia), the Caribbean, Central and South America, or Mexico were significantly more likely to be up to date at all ages, compared with children whose mothers were born in the United States.
Children whose mothers were born in Asia, Canada, Eastern Europe, Somalia, or Western Europe were significantly less likely to be up to date at all ages than were children whose mothers were born in the United States. At 18 months, fewer than 10% of children whose mothers were born in Somalia were up to date; by 36 months, 44% were.
“Inadequate parental understanding of vaccination and weaker public health education programs in some regions might account for some of these findings, as well as economic and social factors influencing emigration, including fleeing war, religious persecution, or poverty,” Ms. Leeds and Ms. Muscoplat said. “Somali parents in Minnesota have been reported to be more likely than non-Somali parents to have concerns about the safety of measles-mumps-rubella (MMR) vaccine, which has led to a decline in coverage with MMR and possibly other childhood vaccines. From April to August 2017, Minnesota experienced a measles outbreak, ending with 79 confirmed cases, including 65 in children of Somali descent,” they wrote.
“Encouraging medical providers to use interpreters, take time to build trust, and assess vaccination status at every visit might improve vaccination coverage” in immigrant, migrant, and refugee populations, they said.
Read more in Morbidity and Mortality Weekly Report (2017 Oct 27;66[42]:1125-9).
, and, more particularly, on the country of origin of their mother, reported Maureen Leeds and Miriam Halstead Muscoplat of the Minnesota Department of Health, St. Paul.
In a study of 97,885 Minnesota children born during 2011-2012, fewer than half were up to date with their vaccinations by age 18 months and only 70% were up to date by age 36 months.
If children had at least one parent born outside the United States, they were 25% less likely to be up to date with their vaccinations by age 36 months than if both their parents were born in the United States. However, children whose mothers were from Africa (excluding Somalia), the Caribbean, Central and South America, or Mexico were significantly more likely to be up to date at all ages, compared with children whose mothers were born in the United States.
Children whose mothers were born in Asia, Canada, Eastern Europe, Somalia, or Western Europe were significantly less likely to be up to date at all ages than were children whose mothers were born in the United States. At 18 months, fewer than 10% of children whose mothers were born in Somalia were up to date; by 36 months, 44% were.
“Inadequate parental understanding of vaccination and weaker public health education programs in some regions might account for some of these findings, as well as economic and social factors influencing emigration, including fleeing war, religious persecution, or poverty,” Ms. Leeds and Ms. Muscoplat said. “Somali parents in Minnesota have been reported to be more likely than non-Somali parents to have concerns about the safety of measles-mumps-rubella (MMR) vaccine, which has led to a decline in coverage with MMR and possibly other childhood vaccines. From April to August 2017, Minnesota experienced a measles outbreak, ending with 79 confirmed cases, including 65 in children of Somali descent,” they wrote.
“Encouraging medical providers to use interpreters, take time to build trust, and assess vaccination status at every visit might improve vaccination coverage” in immigrant, migrant, and refugee populations, they said.
Read more in Morbidity and Mortality Weekly Report (2017 Oct 27;66[42]:1125-9).
FROM MMWR
Reminder spurs flu vaccination of chronic disease patients
and because their parents are unaware influenza is risky for their children, reported Gilat Livni, MD, and Alina Wainstein, MD, of Tel Aviv University and their associates.
The investigators surveyed 186 parents of children attending a cardiology institute in Israel regarding flu vaccination. Over a third (37%) of the children had received a flu vaccine during the last flu season. Those who had been vaccinated were significantly more likely to have parents and siblings who were vaccinated (P less than .01).The mean age of the children was 8 years.
The main underlying cardiac diseases in the 186 children were left-to-right shunt defect in 31%, obstructive lesions in 30%, and cyanotic defect in 16%. Other cardiac abnormalities included valvular insufficiency in 11%, cardiomyopathy in 8%, and complete atrioventricular block in 4%.
More than half of parents (59%) reported that a pediatrician had recommended that their child get a flu vaccine; of these parents, 53% complied. Only 13% of parents who did not get such a recommendation had their children vaccinated – a statistically significant difference. Findings were similar regarding recommendations from pediatric cardiologists. “The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the investigators concluded.
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination. Recommending the vaccine should be made part of routine patient visits in fall and winter,” concluded Dr. Livni, Dr. Wainstein, and associates.
The full text is available online (Pediatr Infect Dis J. 2017 Nov;36[11]: e268-71. doi: 10.1097/INF.0000000000001579).
and because their parents are unaware influenza is risky for their children, reported Gilat Livni, MD, and Alina Wainstein, MD, of Tel Aviv University and their associates.
The investigators surveyed 186 parents of children attending a cardiology institute in Israel regarding flu vaccination. Over a third (37%) of the children had received a flu vaccine during the last flu season. Those who had been vaccinated were significantly more likely to have parents and siblings who were vaccinated (P less than .01).The mean age of the children was 8 years.
The main underlying cardiac diseases in the 186 children were left-to-right shunt defect in 31%, obstructive lesions in 30%, and cyanotic defect in 16%. Other cardiac abnormalities included valvular insufficiency in 11%, cardiomyopathy in 8%, and complete atrioventricular block in 4%.
More than half of parents (59%) reported that a pediatrician had recommended that their child get a flu vaccine; of these parents, 53% complied. Only 13% of parents who did not get such a recommendation had their children vaccinated – a statistically significant difference. Findings were similar regarding recommendations from pediatric cardiologists. “The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the investigators concluded.
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination. Recommending the vaccine should be made part of routine patient visits in fall and winter,” concluded Dr. Livni, Dr. Wainstein, and associates.
The full text is available online (Pediatr Infect Dis J. 2017 Nov;36[11]: e268-71. doi: 10.1097/INF.0000000000001579).
and because their parents are unaware influenza is risky for their children, reported Gilat Livni, MD, and Alina Wainstein, MD, of Tel Aviv University and their associates.
The investigators surveyed 186 parents of children attending a cardiology institute in Israel regarding flu vaccination. Over a third (37%) of the children had received a flu vaccine during the last flu season. Those who had been vaccinated were significantly more likely to have parents and siblings who were vaccinated (P less than .01).The mean age of the children was 8 years.
The main underlying cardiac diseases in the 186 children were left-to-right shunt defect in 31%, obstructive lesions in 30%, and cyanotic defect in 16%. Other cardiac abnormalities included valvular insufficiency in 11%, cardiomyopathy in 8%, and complete atrioventricular block in 4%.
More than half of parents (59%) reported that a pediatrician had recommended that their child get a flu vaccine; of these parents, 53% complied. Only 13% of parents who did not get such a recommendation had their children vaccinated – a statistically significant difference. Findings were similar regarding recommendations from pediatric cardiologists. “The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the investigators concluded.
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination. Recommending the vaccine should be made part of routine patient visits in fall and winter,” concluded Dr. Livni, Dr. Wainstein, and associates.
The full text is available online (Pediatr Infect Dis J. 2017 Nov;36[11]: e268-71. doi: 10.1097/INF.0000000000001579).
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Flu study shows overall efficacy of LAIV, but weakness for one strain
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
FROM CLINICAL INFECTIOUS DISEASES
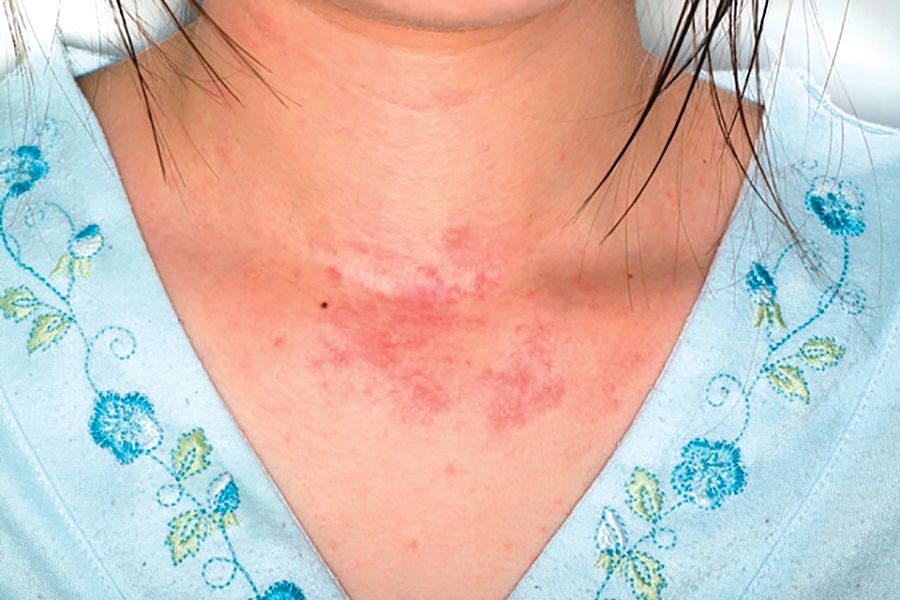
Adverse effects low in long-term crisaborole eczema study
, suggesting that the therapy has the potential to treat atopic dermatitis without the side effects of the current topical treatments, said Lawrence F. Eichenfield, MD, of Rady Children’s Hospital, San Diego, and his associates.
The multicenter, long-term, open-label safety study of 48 weeks assessed 517 patients with mild to moderate atopic dermatitis after they had finished a 28-day phase 3 study of 2% crisaborole ointment. The patients in the extension study were told to apply crisaborole twice daily for 28 days, with an off-treatment period initiated if their disease severity was clear or almost clear after the 28 days. They were told to stop the treatment if they had no improvement in their Investigator’s Static Global Assessment score after three consecutive treatment periods.
Treatment-related adverse events occurred in 10% of patients; 86% of them were mild or moderate. Dermatitis atopic – defined as worsening, exacerbation, flare, or flare-up – occurred in 3% of patients; application-site burning or stinging in 2%; and application-site infection in 1%. The median duration was 18 days for dermatitis atopic, 5 days for application-site burning or stinging, and 12 days for application-site infection. The frequency of these adverse events did not increase over time, the investigators said.
Most patients (78%) did not need rescue therapy, 79% later resumed crisaborole therapy at a later date, and 76% stayed in the study until week 48 or the end of the study.
Read more in the Journal of the American Academy of Dermatology (2017 Oct;77[4]:641-9).
, suggesting that the therapy has the potential to treat atopic dermatitis without the side effects of the current topical treatments, said Lawrence F. Eichenfield, MD, of Rady Children’s Hospital, San Diego, and his associates.
The multicenter, long-term, open-label safety study of 48 weeks assessed 517 patients with mild to moderate atopic dermatitis after they had finished a 28-day phase 3 study of 2% crisaborole ointment. The patients in the extension study were told to apply crisaborole twice daily for 28 days, with an off-treatment period initiated if their disease severity was clear or almost clear after the 28 days. They were told to stop the treatment if they had no improvement in their Investigator’s Static Global Assessment score after three consecutive treatment periods.
Treatment-related adverse events occurred in 10% of patients; 86% of them were mild or moderate. Dermatitis atopic – defined as worsening, exacerbation, flare, or flare-up – occurred in 3% of patients; application-site burning or stinging in 2%; and application-site infection in 1%. The median duration was 18 days for dermatitis atopic, 5 days for application-site burning or stinging, and 12 days for application-site infection. The frequency of these adverse events did not increase over time, the investigators said.
Most patients (78%) did not need rescue therapy, 79% later resumed crisaborole therapy at a later date, and 76% stayed in the study until week 48 or the end of the study.
Read more in the Journal of the American Academy of Dermatology (2017 Oct;77[4]:641-9).
, suggesting that the therapy has the potential to treat atopic dermatitis without the side effects of the current topical treatments, said Lawrence F. Eichenfield, MD, of Rady Children’s Hospital, San Diego, and his associates.
The multicenter, long-term, open-label safety study of 48 weeks assessed 517 patients with mild to moderate atopic dermatitis after they had finished a 28-day phase 3 study of 2% crisaborole ointment. The patients in the extension study were told to apply crisaborole twice daily for 28 days, with an off-treatment period initiated if their disease severity was clear or almost clear after the 28 days. They were told to stop the treatment if they had no improvement in their Investigator’s Static Global Assessment score after three consecutive treatment periods.
Treatment-related adverse events occurred in 10% of patients; 86% of them were mild or moderate. Dermatitis atopic – defined as worsening, exacerbation, flare, or flare-up – occurred in 3% of patients; application-site burning or stinging in 2%; and application-site infection in 1%. The median duration was 18 days for dermatitis atopic, 5 days for application-site burning or stinging, and 12 days for application-site infection. The frequency of these adverse events did not increase over time, the investigators said.
Most patients (78%) did not need rescue therapy, 79% later resumed crisaborole therapy at a later date, and 76% stayed in the study until week 48 or the end of the study.
Read more in the Journal of the American Academy of Dermatology (2017 Oct;77[4]:641-9).
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Vaccination program cut hospital-treated RV gastroenteritis in young children
under 5 years, and it more than adequately pays for itself in secondary health care costs, said Tuija Leino of the National Institute for Health and Welfare, Helsinki, and associates.
Since 2009, all Finnish children younger than 5 years have been offered an RV vaccine.
The investigators conducted a register-based study comparing the RV disease burden before and after introduction of RV vaccination, with the years 1999-2005 as the prevaccine period and the years 2010-2014 as the vaccination period. The study population was all children younger than 5 years living in Finland during the two study periods.
The relative reduction in inpatient RVGE incidence ranged from 86% in the 4-year-old children to 94% in the 0-year-old children, the researchers reported. That amounted to 843 prevented inpatient cases in children under 5 years.
The highest incidence of RVGE, which is rarely treated in hospital outpatients, was 0.4/1,000 person-years in children aged 1 year or younger. The relative reduction in outpatient RVGE incidence was 86% in the 0-year-olds and 100% in the 3- and 4-year-olds. The RV vaccination program prevented only 64 hospital outpatient cases in children younger than 5 years of age in 2014.
RV vaccination also resulted in a reduction of unspecified viral gastroenteritis (UVGE) incidence by 84%. Because the incidence of UVGE in 1-year-olds during the prevaccine era was more than twice the incidence of RVGE, the absolute reduction from 10.7 to 1.7 per 1,000 person-years “reflects a removal of a much larger disease burden than the reduction in the most specific outcome of inpatient RVGE,” the researchers said.
The annually prevented inpatient UVGE cases in children up to 4 years was 1,522, almost twice as many as the prevented inpatient RVGE cases. In the prevaccine era, the UVGE reduction was greatest among 1-year-olds, at 71%. In children younger than 5 years of age, 1,313 UVGE hospital outpatient cases were prevented in 2014.
“Considering secondary health care, the program annually pays for itself almost two times over in Finland,” the investigators concluded.
Read more in Vaccine (2017 Oct 9;35[42]:5611-7).
The researchers had no conflicts of interest.
under 5 years, and it more than adequately pays for itself in secondary health care costs, said Tuija Leino of the National Institute for Health and Welfare, Helsinki, and associates.
Since 2009, all Finnish children younger than 5 years have been offered an RV vaccine.
The investigators conducted a register-based study comparing the RV disease burden before and after introduction of RV vaccination, with the years 1999-2005 as the prevaccine period and the years 2010-2014 as the vaccination period. The study population was all children younger than 5 years living in Finland during the two study periods.
The relative reduction in inpatient RVGE incidence ranged from 86% in the 4-year-old children to 94% in the 0-year-old children, the researchers reported. That amounted to 843 prevented inpatient cases in children under 5 years.
The highest incidence of RVGE, which is rarely treated in hospital outpatients, was 0.4/1,000 person-years in children aged 1 year or younger. The relative reduction in outpatient RVGE incidence was 86% in the 0-year-olds and 100% in the 3- and 4-year-olds. The RV vaccination program prevented only 64 hospital outpatient cases in children younger than 5 years of age in 2014.
RV vaccination also resulted in a reduction of unspecified viral gastroenteritis (UVGE) incidence by 84%. Because the incidence of UVGE in 1-year-olds during the prevaccine era was more than twice the incidence of RVGE, the absolute reduction from 10.7 to 1.7 per 1,000 person-years “reflects a removal of a much larger disease burden than the reduction in the most specific outcome of inpatient RVGE,” the researchers said.
The annually prevented inpatient UVGE cases in children up to 4 years was 1,522, almost twice as many as the prevented inpatient RVGE cases. In the prevaccine era, the UVGE reduction was greatest among 1-year-olds, at 71%. In children younger than 5 years of age, 1,313 UVGE hospital outpatient cases were prevented in 2014.
“Considering secondary health care, the program annually pays for itself almost two times over in Finland,” the investigators concluded.
Read more in Vaccine (2017 Oct 9;35[42]:5611-7).
The researchers had no conflicts of interest.
under 5 years, and it more than adequately pays for itself in secondary health care costs, said Tuija Leino of the National Institute for Health and Welfare, Helsinki, and associates.
Since 2009, all Finnish children younger than 5 years have been offered an RV vaccine.
The investigators conducted a register-based study comparing the RV disease burden before and after introduction of RV vaccination, with the years 1999-2005 as the prevaccine period and the years 2010-2014 as the vaccination period. The study population was all children younger than 5 years living in Finland during the two study periods.
The relative reduction in inpatient RVGE incidence ranged from 86% in the 4-year-old children to 94% in the 0-year-old children, the researchers reported. That amounted to 843 prevented inpatient cases in children under 5 years.
The highest incidence of RVGE, which is rarely treated in hospital outpatients, was 0.4/1,000 person-years in children aged 1 year or younger. The relative reduction in outpatient RVGE incidence was 86% in the 0-year-olds and 100% in the 3- and 4-year-olds. The RV vaccination program prevented only 64 hospital outpatient cases in children younger than 5 years of age in 2014.
RV vaccination also resulted in a reduction of unspecified viral gastroenteritis (UVGE) incidence by 84%. Because the incidence of UVGE in 1-year-olds during the prevaccine era was more than twice the incidence of RVGE, the absolute reduction from 10.7 to 1.7 per 1,000 person-years “reflects a removal of a much larger disease burden than the reduction in the most specific outcome of inpatient RVGE,” the researchers said.
The annually prevented inpatient UVGE cases in children up to 4 years was 1,522, almost twice as many as the prevented inpatient RVGE cases. In the prevaccine era, the UVGE reduction was greatest among 1-year-olds, at 71%. In children younger than 5 years of age, 1,313 UVGE hospital outpatient cases were prevented in 2014.
“Considering secondary health care, the program annually pays for itself almost two times over in Finland,” the investigators concluded.
Read more in Vaccine (2017 Oct 9;35[42]:5611-7).
The researchers had no conflicts of interest.
from vaccine
Maternally derived pneumococcal, meningococcal antibodies may affect vaccine effectiveness
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
FROM VACCINE