User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
FDA approves epinephrine autoinjector for infants, small children
The Food and Drug Administration approved an epinephrine autoinjector constructed specifically to treat life-threatening allergic reactions in infants and small children weighing 16.5-33 pounds.
The Auvi-Q 0.1 mg autoinjector by kaléo was approved after a priority review by the FDA, with features such as “a voice prompt system that guides a user with step-by-step instructions through the delivery process,” according to a written statement from the company. This auto-injector has a shorter needle length and lower dose of epinephrine than other FDA-approved 0.15-mg and 0.3-mg epinephrine autoinjectors.
In a previous study of 51 infants with a mean weight of 24 pounds who were treated with a 0.15-mg epinephrine auto-injector with a standard 12.7-mm needle length, 43% were at risk of having the needle strike the bone. Unintentional injection of epinephrine into the intraosseous space can cause systemic absorption of the epinephrine and possible cardiac complications (Ann Allergy Asthma Immunol. 2017 Jun;118[6]:719-25.e1).
This new autoinjector with a shorter needle length was designed to obviate this problem, according to kaléo’s statement.
The Auvi-Q 0.1 mg autoinjector should be available to patients in the first half of 2018, the company said.
The Food and Drug Administration approved an epinephrine autoinjector constructed specifically to treat life-threatening allergic reactions in infants and small children weighing 16.5-33 pounds.
The Auvi-Q 0.1 mg autoinjector by kaléo was approved after a priority review by the FDA, with features such as “a voice prompt system that guides a user with step-by-step instructions through the delivery process,” according to a written statement from the company. This auto-injector has a shorter needle length and lower dose of epinephrine than other FDA-approved 0.15-mg and 0.3-mg epinephrine autoinjectors.
In a previous study of 51 infants with a mean weight of 24 pounds who were treated with a 0.15-mg epinephrine auto-injector with a standard 12.7-mm needle length, 43% were at risk of having the needle strike the bone. Unintentional injection of epinephrine into the intraosseous space can cause systemic absorption of the epinephrine and possible cardiac complications (Ann Allergy Asthma Immunol. 2017 Jun;118[6]:719-25.e1).
This new autoinjector with a shorter needle length was designed to obviate this problem, according to kaléo’s statement.
The Auvi-Q 0.1 mg autoinjector should be available to patients in the first half of 2018, the company said.
The Food and Drug Administration approved an epinephrine autoinjector constructed specifically to treat life-threatening allergic reactions in infants and small children weighing 16.5-33 pounds.
The Auvi-Q 0.1 mg autoinjector by kaléo was approved after a priority review by the FDA, with features such as “a voice prompt system that guides a user with step-by-step instructions through the delivery process,” according to a written statement from the company. This auto-injector has a shorter needle length and lower dose of epinephrine than other FDA-approved 0.15-mg and 0.3-mg epinephrine autoinjectors.
In a previous study of 51 infants with a mean weight of 24 pounds who were treated with a 0.15-mg epinephrine auto-injector with a standard 12.7-mm needle length, 43% were at risk of having the needle strike the bone. Unintentional injection of epinephrine into the intraosseous space can cause systemic absorption of the epinephrine and possible cardiac complications (Ann Allergy Asthma Immunol. 2017 Jun;118[6]:719-25.e1).
This new autoinjector with a shorter needle length was designed to obviate this problem, according to kaléo’s statement.
The Auvi-Q 0.1 mg autoinjector should be available to patients in the first half of 2018, the company said.
Parents have diverse reasons for refusing HPV vaccine for their child
, so health care providers have much work to do to educate parents about this anticancer vaccine, said Frances DiAnna Kinder, PhD, RN, CPNP-PC, of the La Salle University, Philadelphia.
Pediatricians, nurse practitioners, and physician assistants from numerous private pediatric settings in Philadelphia discussed HPV vaccination during a well-child visit with parents and children who were eligible for the vaccine, which generally starts at age 9 years. After a health visit in which the HPV vaccine was refused, the parent was asked to complete a survey in a private room. Of 72 surveys by 63 mothers, 8 fathers, and 1 grandparent of children who were mostly female and mostly between ages 11-16 years, 58% said the vaccine was too new, and 50% said there was not enough research. However, 63% said they believed in the HPV vaccine’s efficacy, and all reported their child was up to date with other recommended vaccines.
There was an open-ended question for parents to indicate other reasons for refusal; some themes of refusal included fear, anxiety, and misunderstanding the facts of the HPV vaccine; 21 parents whose children were aged 11-13 years said their child was too young to be vaccinated with the HPV vaccine. Three parents said that they had “witnessed severe side effects such as becoming paralyzed” or “acquiring an autoimmune disease after the vaccine was administered” to either a family member or friend’s child, Dr. Kinder noted. One parent said, “this vaccine was a money maker for the pharmaceutical companies” and there was “too much controversy for her to be comfortable giving this to her child.”
Read more at (J Pediatr Health Care. 2017. 10.1016/j.pedhc.2017.09.003).
, so health care providers have much work to do to educate parents about this anticancer vaccine, said Frances DiAnna Kinder, PhD, RN, CPNP-PC, of the La Salle University, Philadelphia.
Pediatricians, nurse practitioners, and physician assistants from numerous private pediatric settings in Philadelphia discussed HPV vaccination during a well-child visit with parents and children who were eligible for the vaccine, which generally starts at age 9 years. After a health visit in which the HPV vaccine was refused, the parent was asked to complete a survey in a private room. Of 72 surveys by 63 mothers, 8 fathers, and 1 grandparent of children who were mostly female and mostly between ages 11-16 years, 58% said the vaccine was too new, and 50% said there was not enough research. However, 63% said they believed in the HPV vaccine’s efficacy, and all reported their child was up to date with other recommended vaccines.
There was an open-ended question for parents to indicate other reasons for refusal; some themes of refusal included fear, anxiety, and misunderstanding the facts of the HPV vaccine; 21 parents whose children were aged 11-13 years said their child was too young to be vaccinated with the HPV vaccine. Three parents said that they had “witnessed severe side effects such as becoming paralyzed” or “acquiring an autoimmune disease after the vaccine was administered” to either a family member or friend’s child, Dr. Kinder noted. One parent said, “this vaccine was a money maker for the pharmaceutical companies” and there was “too much controversy for her to be comfortable giving this to her child.”
Read more at (J Pediatr Health Care. 2017. 10.1016/j.pedhc.2017.09.003).
, so health care providers have much work to do to educate parents about this anticancer vaccine, said Frances DiAnna Kinder, PhD, RN, CPNP-PC, of the La Salle University, Philadelphia.
Pediatricians, nurse practitioners, and physician assistants from numerous private pediatric settings in Philadelphia discussed HPV vaccination during a well-child visit with parents and children who were eligible for the vaccine, which generally starts at age 9 years. After a health visit in which the HPV vaccine was refused, the parent was asked to complete a survey in a private room. Of 72 surveys by 63 mothers, 8 fathers, and 1 grandparent of children who were mostly female and mostly between ages 11-16 years, 58% said the vaccine was too new, and 50% said there was not enough research. However, 63% said they believed in the HPV vaccine’s efficacy, and all reported their child was up to date with other recommended vaccines.
There was an open-ended question for parents to indicate other reasons for refusal; some themes of refusal included fear, anxiety, and misunderstanding the facts of the HPV vaccine; 21 parents whose children were aged 11-13 years said their child was too young to be vaccinated with the HPV vaccine. Three parents said that they had “witnessed severe side effects such as becoming paralyzed” or “acquiring an autoimmune disease after the vaccine was administered” to either a family member or friend’s child, Dr. Kinder noted. One parent said, “this vaccine was a money maker for the pharmaceutical companies” and there was “too much controversy for her to be comfortable giving this to her child.”
Read more at (J Pediatr Health Care. 2017. 10.1016/j.pedhc.2017.09.003).
FROM THE JOURNAL OF PEDIATRIC HEALTH CARE
Chinese school-based flu vaccination program reduced outbreaks
, said Yang Pan, PhD, of the Institute for Infectious Disease and Endemic Disease Control, Beijing Center for Disease Prevention and Control, and associates.
School-based trivalent inactivated influenza vaccination programs generally occurred Oct. 15-Nov. 30 each year since 2007, with greater than 50% vaccination coverage. In an 11-year retrospective study of school outbreaks of influenza in elementary, middle, and high schools in the Beijing area during Sept. 1, 2006-March 31, 2017, there were 286 febrile outbreaks in schools, involving 6,863 children.
During the 11 years, a mismatch between circulating strains and vaccine strains was identified in two influenza seasons, such as “the A(H3N2) 3C.1 (vaccine strain)-A(H3N2) 3C.3a (circulating strains) mismatch in 2014-2015, the B(Yamagata) Clade 2 (vaccine strain)-B(Yamagata) Clade 3 (circulating strain) mismatch in the 2014-2015 influenza season, and B(Yamagata) (vaccine strain)-B(Victoria) (circulating strains) mismatch in 2015-2016,” they reported.
A combination of high flu vaccine coverage because of school-based vaccinations and a good vaccine match reduced influenza outbreaks in schools by 89% (odds ratio, 0.111), Dr. Pan and associates concluded.
“The school-based influenza vaccination program has been in operation for nearly 10 years in the Beijing area, is unique in China, and is one of the few school-based influenza programs in the world,” the researchers explained. “These data can inform and improve vaccination policy locally and nationally.”
Read more in Vaccine (2017 Nov 8. doi: 10.1016/j.vaccine.2017.10.096).
, said Yang Pan, PhD, of the Institute for Infectious Disease and Endemic Disease Control, Beijing Center for Disease Prevention and Control, and associates.
School-based trivalent inactivated influenza vaccination programs generally occurred Oct. 15-Nov. 30 each year since 2007, with greater than 50% vaccination coverage. In an 11-year retrospective study of school outbreaks of influenza in elementary, middle, and high schools in the Beijing area during Sept. 1, 2006-March 31, 2017, there were 286 febrile outbreaks in schools, involving 6,863 children.
During the 11 years, a mismatch between circulating strains and vaccine strains was identified in two influenza seasons, such as “the A(H3N2) 3C.1 (vaccine strain)-A(H3N2) 3C.3a (circulating strains) mismatch in 2014-2015, the B(Yamagata) Clade 2 (vaccine strain)-B(Yamagata) Clade 3 (circulating strain) mismatch in the 2014-2015 influenza season, and B(Yamagata) (vaccine strain)-B(Victoria) (circulating strains) mismatch in 2015-2016,” they reported.
A combination of high flu vaccine coverage because of school-based vaccinations and a good vaccine match reduced influenza outbreaks in schools by 89% (odds ratio, 0.111), Dr. Pan and associates concluded.
“The school-based influenza vaccination program has been in operation for nearly 10 years in the Beijing area, is unique in China, and is one of the few school-based influenza programs in the world,” the researchers explained. “These data can inform and improve vaccination policy locally and nationally.”
Read more in Vaccine (2017 Nov 8. doi: 10.1016/j.vaccine.2017.10.096).
, said Yang Pan, PhD, of the Institute for Infectious Disease and Endemic Disease Control, Beijing Center for Disease Prevention and Control, and associates.
School-based trivalent inactivated influenza vaccination programs generally occurred Oct. 15-Nov. 30 each year since 2007, with greater than 50% vaccination coverage. In an 11-year retrospective study of school outbreaks of influenza in elementary, middle, and high schools in the Beijing area during Sept. 1, 2006-March 31, 2017, there were 286 febrile outbreaks in schools, involving 6,863 children.
During the 11 years, a mismatch between circulating strains and vaccine strains was identified in two influenza seasons, such as “the A(H3N2) 3C.1 (vaccine strain)-A(H3N2) 3C.3a (circulating strains) mismatch in 2014-2015, the B(Yamagata) Clade 2 (vaccine strain)-B(Yamagata) Clade 3 (circulating strain) mismatch in the 2014-2015 influenza season, and B(Yamagata) (vaccine strain)-B(Victoria) (circulating strains) mismatch in 2015-2016,” they reported.
A combination of high flu vaccine coverage because of school-based vaccinations and a good vaccine match reduced influenza outbreaks in schools by 89% (odds ratio, 0.111), Dr. Pan and associates concluded.
“The school-based influenza vaccination program has been in operation for nearly 10 years in the Beijing area, is unique in China, and is one of the few school-based influenza programs in the world,” the researchers explained. “These data can inform and improve vaccination policy locally and nationally.”
Read more in Vaccine (2017 Nov 8. doi: 10.1016/j.vaccine.2017.10.096).
FROM VACCINE
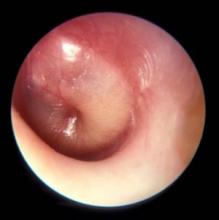
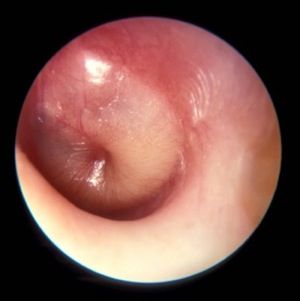
PCVs may reduce AOM severity, although they do not decrease incidence
Use of the 7-valent pneumococcal conjugate vaccine (PCV7) may have had a part in reducing the incidence of severe middle ear inflammation of acute otitis media (AOM) in Japanese children, decreasing the rate of myringotomies for AOM, said Atsushi Sasaki, of the Hiroshima (Japan) University, and associates.
To assess whether use of PCV7 and then PCV13 affected AOM incidence, the investigators looked at the incidence of visits to medical institutions (VtMI) due to all-cause AOM in children younger than 15 years in the Japan Medical Data Center Claims Database between January 2005 and December 2015. Data for children aged 10 years to younger than 15 years served as the control. The rate of myringotomies for AOM (MyfA) from January 2007 to December 2015 also was assessed.
Numerous other studies have “concluded that the preventive effect of PCV7 against AOM was very modest,” the researchers said. “In contrast, our study proposes the hypothesis that PCV7 use in 1-year-olds may contribute to the decreased incidence of severe middle ear inflammation of AOM. When evaluating the effectiveness of PCV, measures to evaluate severity may be as important as evaluating disease prevention.”
Read more in Auris Nasus Larynx (2017 Nov 1. doi: 10.1016/j.anl.2017.10.006).
Use of the 7-valent pneumococcal conjugate vaccine (PCV7) may have had a part in reducing the incidence of severe middle ear inflammation of acute otitis media (AOM) in Japanese children, decreasing the rate of myringotomies for AOM, said Atsushi Sasaki, of the Hiroshima (Japan) University, and associates.
To assess whether use of PCV7 and then PCV13 affected AOM incidence, the investigators looked at the incidence of visits to medical institutions (VtMI) due to all-cause AOM in children younger than 15 years in the Japan Medical Data Center Claims Database between January 2005 and December 2015. Data for children aged 10 years to younger than 15 years served as the control. The rate of myringotomies for AOM (MyfA) from January 2007 to December 2015 also was assessed.
Numerous other studies have “concluded that the preventive effect of PCV7 against AOM was very modest,” the researchers said. “In contrast, our study proposes the hypothesis that PCV7 use in 1-year-olds may contribute to the decreased incidence of severe middle ear inflammation of AOM. When evaluating the effectiveness of PCV, measures to evaluate severity may be as important as evaluating disease prevention.”
Read more in Auris Nasus Larynx (2017 Nov 1. doi: 10.1016/j.anl.2017.10.006).
Use of the 7-valent pneumococcal conjugate vaccine (PCV7) may have had a part in reducing the incidence of severe middle ear inflammation of acute otitis media (AOM) in Japanese children, decreasing the rate of myringotomies for AOM, said Atsushi Sasaki, of the Hiroshima (Japan) University, and associates.
To assess whether use of PCV7 and then PCV13 affected AOM incidence, the investigators looked at the incidence of visits to medical institutions (VtMI) due to all-cause AOM in children younger than 15 years in the Japan Medical Data Center Claims Database between January 2005 and December 2015. Data for children aged 10 years to younger than 15 years served as the control. The rate of myringotomies for AOM (MyfA) from January 2007 to December 2015 also was assessed.
Numerous other studies have “concluded that the preventive effect of PCV7 against AOM was very modest,” the researchers said. “In contrast, our study proposes the hypothesis that PCV7 use in 1-year-olds may contribute to the decreased incidence of severe middle ear inflammation of AOM. When evaluating the effectiveness of PCV, measures to evaluate severity may be as important as evaluating disease prevention.”
Read more in Auris Nasus Larynx (2017 Nov 1. doi: 10.1016/j.anl.2017.10.006).
FROM AURIS NASUS LARYNX
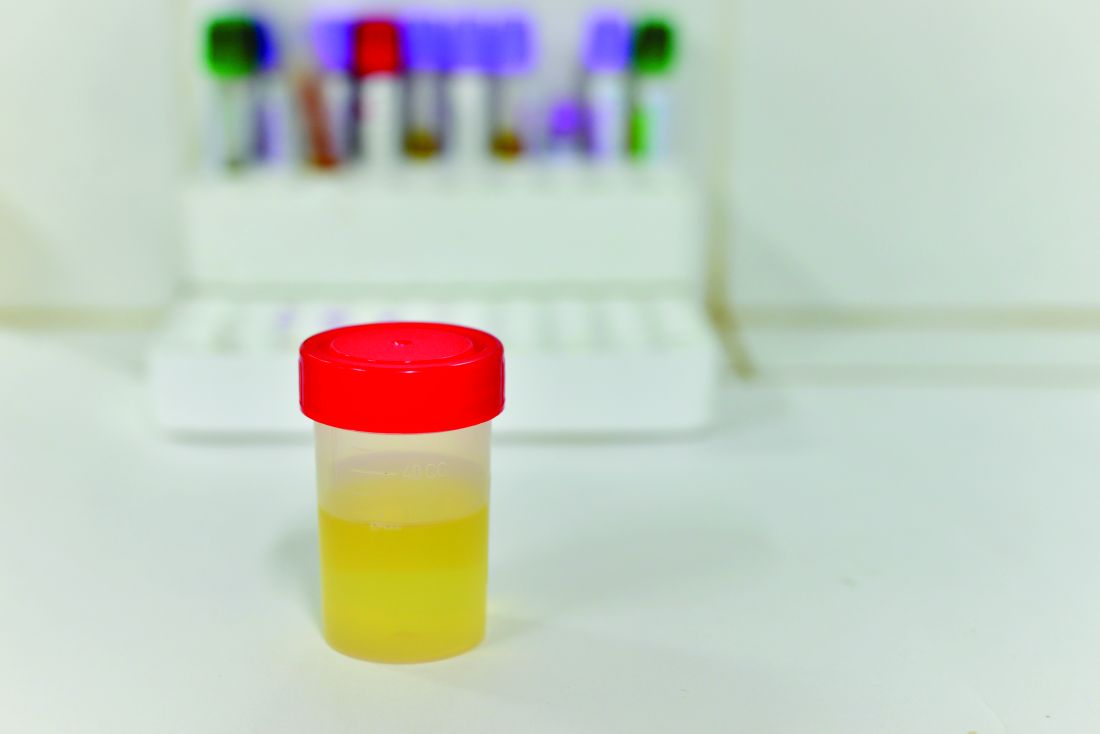
Novel biomarker test appears accurate for UTI diagnosis in febrile children under 2 years
Novel test for the urinary neutrophil gelatinase–associated lipocalin (uNGAL) biomarker have good sensitivity and specificity to distinguish whether infants and children younger than 2 years old have a urinary tract infection (UTI), said Tamar R. Lubell, MD, and her associates at Columbia University, New York.
“NGAL is a protein expressed in neutrophils and several other human tissues, including alpha-intercalated cells in the collecting duct of the kidney,” the researchers explained. “The urine and serum contain very low levels of NGAL protein at steady state, with expression of NGAL rising rapidly in response to cell damage caused by ischemia-reperfusion injury, presence of cytotoxins, and sepsis.”
Of the 260 children whose catheterized urine was analyzed, 14% had UTIs. The median uNGAL concentration was 215.1 ng/mL in the UTI group, compared with 4.4 ng/mL in the culture-negative group. Urinalysis and Gram stain also were performed.
“uNGAL had higher sensitivity than [urinalysis], with similar specificity,” the investigators said. “Gram stain had a somewhat lower sensitivity than uNGAL, but with high specificity.”
The researchers identified a cutoff point for uNGAL levels to be 39.1 ng/mL. A previous case-control study of 108 infants with UTI had a cutoff for uNGAL levels of 38 ng/mL, with sensitivity of 93% and specificity of 95%. Most urine samples in that study were obtained by clean catch rather than by catheterization, as in the current study.
“Further studies will need to both confirm our findings and determine the benefit and cost effectiveness of uNGAL testing, compared with [urinalysis],” Dr. Lubell and her associates said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/peds.2017-1090).
Novel test for the urinary neutrophil gelatinase–associated lipocalin (uNGAL) biomarker have good sensitivity and specificity to distinguish whether infants and children younger than 2 years old have a urinary tract infection (UTI), said Tamar R. Lubell, MD, and her associates at Columbia University, New York.
“NGAL is a protein expressed in neutrophils and several other human tissues, including alpha-intercalated cells in the collecting duct of the kidney,” the researchers explained. “The urine and serum contain very low levels of NGAL protein at steady state, with expression of NGAL rising rapidly in response to cell damage caused by ischemia-reperfusion injury, presence of cytotoxins, and sepsis.”
Of the 260 children whose catheterized urine was analyzed, 14% had UTIs. The median uNGAL concentration was 215.1 ng/mL in the UTI group, compared with 4.4 ng/mL in the culture-negative group. Urinalysis and Gram stain also were performed.
“uNGAL had higher sensitivity than [urinalysis], with similar specificity,” the investigators said. “Gram stain had a somewhat lower sensitivity than uNGAL, but with high specificity.”
The researchers identified a cutoff point for uNGAL levels to be 39.1 ng/mL. A previous case-control study of 108 infants with UTI had a cutoff for uNGAL levels of 38 ng/mL, with sensitivity of 93% and specificity of 95%. Most urine samples in that study were obtained by clean catch rather than by catheterization, as in the current study.
“Further studies will need to both confirm our findings and determine the benefit and cost effectiveness of uNGAL testing, compared with [urinalysis],” Dr. Lubell and her associates said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/peds.2017-1090).
Novel test for the urinary neutrophil gelatinase–associated lipocalin (uNGAL) biomarker have good sensitivity and specificity to distinguish whether infants and children younger than 2 years old have a urinary tract infection (UTI), said Tamar R. Lubell, MD, and her associates at Columbia University, New York.
“NGAL is a protein expressed in neutrophils and several other human tissues, including alpha-intercalated cells in the collecting duct of the kidney,” the researchers explained. “The urine and serum contain very low levels of NGAL protein at steady state, with expression of NGAL rising rapidly in response to cell damage caused by ischemia-reperfusion injury, presence of cytotoxins, and sepsis.”
Of the 260 children whose catheterized urine was analyzed, 14% had UTIs. The median uNGAL concentration was 215.1 ng/mL in the UTI group, compared with 4.4 ng/mL in the culture-negative group. Urinalysis and Gram stain also were performed.
“uNGAL had higher sensitivity than [urinalysis], with similar specificity,” the investigators said. “Gram stain had a somewhat lower sensitivity than uNGAL, but with high specificity.”
The researchers identified a cutoff point for uNGAL levels to be 39.1 ng/mL. A previous case-control study of 108 infants with UTI had a cutoff for uNGAL levels of 38 ng/mL, with sensitivity of 93% and specificity of 95%. Most urine samples in that study were obtained by clean catch rather than by catheterization, as in the current study.
“Further studies will need to both confirm our findings and determine the benefit and cost effectiveness of uNGAL testing, compared with [urinalysis],” Dr. Lubell and her associates said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/peds.2017-1090).
FROM PEDIATRICS
After warning, codeine use after tonsillectomy drops, doesn’t stop
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
FROM PEDIATRICS
Vaccine coverage high among U.S. toddlers in 2016, but gaps remain
, said Holly A. Hill, MD, PhD, and her associates at the National Center for Immunization and Respiratory Diseases, Atlanta.
Coverage still was below 90% for vaccines that needed booster doses during the second year of life (four or more doses of DTaP and pneumococcal conjugate vaccine [PCV] and Haemophilus influenzae type b [Hib] full series) and for other recommended vaccines (hepatitis B [HepB] birth dose, rotavirus, and hepatitis A [HepA]), they reported in Morbidity and Mortality Weekly Report.
Coverage was estimated to be 61% for two or more doses of HepA vaccine, 71% of the HepB birth dose, 74% of a completed series of rotavirus vaccine, and 71% of the combined seven-vaccine series (four or more doses of DTaP; three or more doses of poliovirus vaccine; one or more doses of measles-containing vaccine; three or four doses of Hib [depending upon product type of vaccine]; three or more doses of HepB; one or more doses of varicella vaccine; and four or more doses of PCV).
Fewer than 1% of children received no vaccinations.
Coverage of most vaccines in 2016 was lower in non-Hispanic black children, compared with non-Hispanic white children. It also was lower for children living below the federal poverty level, compared with children living at or above the poverty level. For Medicaid children, vaccination coverage was lower by 3%-13% than among children who had private insurance; for children with no insurance, vaccination coverage was lower by 12%-25% than among children with private insurance, the investigators reported.
Uninsured children “are eligible for the Vaccines for Children (VFC) program, which was designed to increase access to vaccination among children through age 18 years who might not otherwise be vaccinated because of inability to pay,” the researchers said. “Some families might not be aware of the VFC program, be unable to afford fees associated with visits to a vaccine provider, or might need assistance locating a physician who participates in the VFC program. Children living below poverty and up to a certain percentage above the poverty level are eligible for Medicaid … and are entitled to VFC vaccines.”
The investigators cited language barriers, lack of trust in providers, transportation problems, inconvenient office hours, and other provider- and system-level factors as health care–access barriers among publicly insured children.
“These data indicate that the immunization safety net is not reaching all children early in life,” Dr. Hill and her associates said. “Health care providers can increase vaccination coverage using evidence-based strategies such as provider reminders, standing orders to provide vaccination whenever appropriate, and immunization information systems” such as www.thecommunityguide.org/topic/vaccination.
Read more in MMWR (2017 Nov 3;66[43]:1171-7).
, said Holly A. Hill, MD, PhD, and her associates at the National Center for Immunization and Respiratory Diseases, Atlanta.
Coverage still was below 90% for vaccines that needed booster doses during the second year of life (four or more doses of DTaP and pneumococcal conjugate vaccine [PCV] and Haemophilus influenzae type b [Hib] full series) and for other recommended vaccines (hepatitis B [HepB] birth dose, rotavirus, and hepatitis A [HepA]), they reported in Morbidity and Mortality Weekly Report.
Coverage was estimated to be 61% for two or more doses of HepA vaccine, 71% of the HepB birth dose, 74% of a completed series of rotavirus vaccine, and 71% of the combined seven-vaccine series (four or more doses of DTaP; three or more doses of poliovirus vaccine; one or more doses of measles-containing vaccine; three or four doses of Hib [depending upon product type of vaccine]; three or more doses of HepB; one or more doses of varicella vaccine; and four or more doses of PCV).
Fewer than 1% of children received no vaccinations.
Coverage of most vaccines in 2016 was lower in non-Hispanic black children, compared with non-Hispanic white children. It also was lower for children living below the federal poverty level, compared with children living at or above the poverty level. For Medicaid children, vaccination coverage was lower by 3%-13% than among children who had private insurance; for children with no insurance, vaccination coverage was lower by 12%-25% than among children with private insurance, the investigators reported.
Uninsured children “are eligible for the Vaccines for Children (VFC) program, which was designed to increase access to vaccination among children through age 18 years who might not otherwise be vaccinated because of inability to pay,” the researchers said. “Some families might not be aware of the VFC program, be unable to afford fees associated with visits to a vaccine provider, or might need assistance locating a physician who participates in the VFC program. Children living below poverty and up to a certain percentage above the poverty level are eligible for Medicaid … and are entitled to VFC vaccines.”
The investigators cited language barriers, lack of trust in providers, transportation problems, inconvenient office hours, and other provider- and system-level factors as health care–access barriers among publicly insured children.
“These data indicate that the immunization safety net is not reaching all children early in life,” Dr. Hill and her associates said. “Health care providers can increase vaccination coverage using evidence-based strategies such as provider reminders, standing orders to provide vaccination whenever appropriate, and immunization information systems” such as www.thecommunityguide.org/topic/vaccination.
Read more in MMWR (2017 Nov 3;66[43]:1171-7).
, said Holly A. Hill, MD, PhD, and her associates at the National Center for Immunization and Respiratory Diseases, Atlanta.
Coverage still was below 90% for vaccines that needed booster doses during the second year of life (four or more doses of DTaP and pneumococcal conjugate vaccine [PCV] and Haemophilus influenzae type b [Hib] full series) and for other recommended vaccines (hepatitis B [HepB] birth dose, rotavirus, and hepatitis A [HepA]), they reported in Morbidity and Mortality Weekly Report.
Coverage was estimated to be 61% for two or more doses of HepA vaccine, 71% of the HepB birth dose, 74% of a completed series of rotavirus vaccine, and 71% of the combined seven-vaccine series (four or more doses of DTaP; three or more doses of poliovirus vaccine; one or more doses of measles-containing vaccine; three or four doses of Hib [depending upon product type of vaccine]; three or more doses of HepB; one or more doses of varicella vaccine; and four or more doses of PCV).
Fewer than 1% of children received no vaccinations.
Coverage of most vaccines in 2016 was lower in non-Hispanic black children, compared with non-Hispanic white children. It also was lower for children living below the federal poverty level, compared with children living at or above the poverty level. For Medicaid children, vaccination coverage was lower by 3%-13% than among children who had private insurance; for children with no insurance, vaccination coverage was lower by 12%-25% than among children with private insurance, the investigators reported.
Uninsured children “are eligible for the Vaccines for Children (VFC) program, which was designed to increase access to vaccination among children through age 18 years who might not otherwise be vaccinated because of inability to pay,” the researchers said. “Some families might not be aware of the VFC program, be unable to afford fees associated with visits to a vaccine provider, or might need assistance locating a physician who participates in the VFC program. Children living below poverty and up to a certain percentage above the poverty level are eligible for Medicaid … and are entitled to VFC vaccines.”
The investigators cited language barriers, lack of trust in providers, transportation problems, inconvenient office hours, and other provider- and system-level factors as health care–access barriers among publicly insured children.
“These data indicate that the immunization safety net is not reaching all children early in life,” Dr. Hill and her associates said. “Health care providers can increase vaccination coverage using evidence-based strategies such as provider reminders, standing orders to provide vaccination whenever appropriate, and immunization information systems” such as www.thecommunityguide.org/topic/vaccination.
Read more in MMWR (2017 Nov 3;66[43]:1171-7).
FROM MMWR
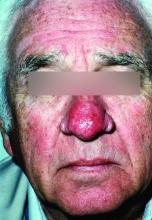
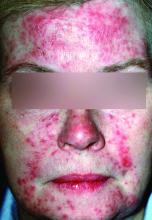
New standard classification, pathophysiology of rosacea announced
Rosacea’s phenotypes may occur in different combinations and at different times, but all are considered to be “manifestations of the same underlying inflammatory continuum,” the expert panel wrote (J Am Acad Dermatol. 2017. doi: 10.1016/j.jaad.2017.08.037).
Rosacea’s phenotypes may occur in different combinations and at different times, but all are considered to be “manifestations of the same underlying inflammatory continuum,” the expert panel wrote (J Am Acad Dermatol. 2017. doi: 10.1016/j.jaad.2017.08.037).
Rosacea’s phenotypes may occur in different combinations and at different times, but all are considered to be “manifestations of the same underlying inflammatory continuum,” the expert panel wrote (J Am Acad Dermatol. 2017. doi: 10.1016/j.jaad.2017.08.037).
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Pregnant women’s access to vaccination website, social media improved infants’ up-to-date immunizations
than were those receiving usual care, said Jason M. Glanz, PhD, of the Institute for Health Research, Kaiser Permanente Colorado, Denver, and his associates.
“These results suggest that interactive, informational interventions administered outside of the physician’s office can improve vaccine acceptance,” the investigators said. “The information appears to be effective when presented to parents before their children are born.”
The women in the VSM group had access to vaccine content, social media technologies that included a blog, discussion forum, chat rooms with experts, and “Ask a Question” portal through which the women could directly ask experts questions about vaccination. The experts were a pediatrician, a vaccine safety researcher, and a risk communication specialist.
Monthly, one to two blog posts were created on topics such as new vaccine safety research, vaccine-preventable disease outbreaks, changes in immunization policy, and the importance of adhering to the recommended immunization schedule, the researchers said. The women also could submit questions privately through e-mail, and receive personalized responses within 2 business days.
At the end of 200 days of follow-up, the proportion of infants up to date with their vaccinations were 93% for the VSM group, 91% for the VI group, and 87% for the UC arms. Infants in the VSM group were more likely to be up to date at age 200 days, compared with infants in the UC group (odds ratio, 1.92; 95% confidence interval, 1.07-3.47).
“Up-to-date status did not differ significantly between the VI and UC arms or the VSM and VI arms,” Dr. Glanz and his associates wrote.
Of 739 women in the VSM and VI groups, 35% visited the website at least once, with a mean of 1.8 visits (range, 1-15 visits). Of 75 vaccine-hesitant women, 44% visited the website, compared with 34% of 664 nonhesitant women. Median vaccine hesitancy scores were 13 for the VSM group, 17 for the VI group, and 15 for the UC group.
The study authors had no relevant disclosures.
Read more in Pediatrics (2017 Nov. doi: 10.1542/peds.2017-1117).
than were those receiving usual care, said Jason M. Glanz, PhD, of the Institute for Health Research, Kaiser Permanente Colorado, Denver, and his associates.
“These results suggest that interactive, informational interventions administered outside of the physician’s office can improve vaccine acceptance,” the investigators said. “The information appears to be effective when presented to parents before their children are born.”
The women in the VSM group had access to vaccine content, social media technologies that included a blog, discussion forum, chat rooms with experts, and “Ask a Question” portal through which the women could directly ask experts questions about vaccination. The experts were a pediatrician, a vaccine safety researcher, and a risk communication specialist.
Monthly, one to two blog posts were created on topics such as new vaccine safety research, vaccine-preventable disease outbreaks, changes in immunization policy, and the importance of adhering to the recommended immunization schedule, the researchers said. The women also could submit questions privately through e-mail, and receive personalized responses within 2 business days.
At the end of 200 days of follow-up, the proportion of infants up to date with their vaccinations were 93% for the VSM group, 91% for the VI group, and 87% for the UC arms. Infants in the VSM group were more likely to be up to date at age 200 days, compared with infants in the UC group (odds ratio, 1.92; 95% confidence interval, 1.07-3.47).
“Up-to-date status did not differ significantly between the VI and UC arms or the VSM and VI arms,” Dr. Glanz and his associates wrote.
Of 739 women in the VSM and VI groups, 35% visited the website at least once, with a mean of 1.8 visits (range, 1-15 visits). Of 75 vaccine-hesitant women, 44% visited the website, compared with 34% of 664 nonhesitant women. Median vaccine hesitancy scores were 13 for the VSM group, 17 for the VI group, and 15 for the UC group.
The study authors had no relevant disclosures.
Read more in Pediatrics (2017 Nov. doi: 10.1542/peds.2017-1117).
than were those receiving usual care, said Jason M. Glanz, PhD, of the Institute for Health Research, Kaiser Permanente Colorado, Denver, and his associates.
“These results suggest that interactive, informational interventions administered outside of the physician’s office can improve vaccine acceptance,” the investigators said. “The information appears to be effective when presented to parents before their children are born.”
The women in the VSM group had access to vaccine content, social media technologies that included a blog, discussion forum, chat rooms with experts, and “Ask a Question” portal through which the women could directly ask experts questions about vaccination. The experts were a pediatrician, a vaccine safety researcher, and a risk communication specialist.
Monthly, one to two blog posts were created on topics such as new vaccine safety research, vaccine-preventable disease outbreaks, changes in immunization policy, and the importance of adhering to the recommended immunization schedule, the researchers said. The women also could submit questions privately through e-mail, and receive personalized responses within 2 business days.
At the end of 200 days of follow-up, the proportion of infants up to date with their vaccinations were 93% for the VSM group, 91% for the VI group, and 87% for the UC arms. Infants in the VSM group were more likely to be up to date at age 200 days, compared with infants in the UC group (odds ratio, 1.92; 95% confidence interval, 1.07-3.47).
“Up-to-date status did not differ significantly between the VI and UC arms or the VSM and VI arms,” Dr. Glanz and his associates wrote.
Of 739 women in the VSM and VI groups, 35% visited the website at least once, with a mean of 1.8 visits (range, 1-15 visits). Of 75 vaccine-hesitant women, 44% visited the website, compared with 34% of 664 nonhesitant women. Median vaccine hesitancy scores were 13 for the VSM group, 17 for the VI group, and 15 for the UC group.
The study authors had no relevant disclosures.
Read more in Pediatrics (2017 Nov. doi: 10.1542/peds.2017-1117).
FROM PEDIATRICS
Immunization information systems show progress over recent years
From 2013 to 2016, all U.S. immunization information systems showed progress in bidirectional information exchange with EHRs, said Neil Murthy, MD, and his associates at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta.
Across all 55 jurisdictions in 49 states and six cities in the United States using immunization information systems (IIS), 106% of U.S. births were registered in IIS in 2016, which is an increase from 102% in 2013; percentages may exceed 100%, because a child who is born in one state but who lives in a different state might be recorded in both IISs.
Bidirectional exchange of data with EHRs is an important part of an IIS. In 2016, 91% of jurisdictions had an IIS that used a platform-independent messaging system that received vaccination histories from providers and returned acknowledgment messages, compared with 87% in 2013, the investigators said.
“Clinical Decision Support (CDS) functionalities enable providers to evaluate the validity of vaccine doses administered to patients and forecast future vaccines that will be needed, based on recommendations developed by the Advisory Committee on Immunization Practices,” Dr. Murthy and his associates said. In 2016, 58% of the 55 jurisdictions sent a vaccine forecast to another system, compared with 31% in 2013.
In 2016, 95% of the 55 jurisdictions gave a “predefined, automatic report on immunization coverage by provider site,” compared with 89% of jurisdictions in 2013.
“IISs are integral components of routine clinical practice and public health surveillance for immunization,” Dr. Murthy and his associates said. “Availability of more complete IIS data also offers many benefits to health care providers and public health practitioners, including consolidating patients’ vaccination histories, identifying undervaccinated subgroups, and forecasting the needs of individual patients for recommended vaccines.”
Read more in Morbidity and Mortality Weekly Report (2017 Nov 3;66[43]:1178-81).
From 2013 to 2016, all U.S. immunization information systems showed progress in bidirectional information exchange with EHRs, said Neil Murthy, MD, and his associates at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta.
Across all 55 jurisdictions in 49 states and six cities in the United States using immunization information systems (IIS), 106% of U.S. births were registered in IIS in 2016, which is an increase from 102% in 2013; percentages may exceed 100%, because a child who is born in one state but who lives in a different state might be recorded in both IISs.
Bidirectional exchange of data with EHRs is an important part of an IIS. In 2016, 91% of jurisdictions had an IIS that used a platform-independent messaging system that received vaccination histories from providers and returned acknowledgment messages, compared with 87% in 2013, the investigators said.
“Clinical Decision Support (CDS) functionalities enable providers to evaluate the validity of vaccine doses administered to patients and forecast future vaccines that will be needed, based on recommendations developed by the Advisory Committee on Immunization Practices,” Dr. Murthy and his associates said. In 2016, 58% of the 55 jurisdictions sent a vaccine forecast to another system, compared with 31% in 2013.
In 2016, 95% of the 55 jurisdictions gave a “predefined, automatic report on immunization coverage by provider site,” compared with 89% of jurisdictions in 2013.
“IISs are integral components of routine clinical practice and public health surveillance for immunization,” Dr. Murthy and his associates said. “Availability of more complete IIS data also offers many benefits to health care providers and public health practitioners, including consolidating patients’ vaccination histories, identifying undervaccinated subgroups, and forecasting the needs of individual patients for recommended vaccines.”
Read more in Morbidity and Mortality Weekly Report (2017 Nov 3;66[43]:1178-81).
From 2013 to 2016, all U.S. immunization information systems showed progress in bidirectional information exchange with EHRs, said Neil Murthy, MD, and his associates at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta.
Across all 55 jurisdictions in 49 states and six cities in the United States using immunization information systems (IIS), 106% of U.S. births were registered in IIS in 2016, which is an increase from 102% in 2013; percentages may exceed 100%, because a child who is born in one state but who lives in a different state might be recorded in both IISs.
Bidirectional exchange of data with EHRs is an important part of an IIS. In 2016, 91% of jurisdictions had an IIS that used a platform-independent messaging system that received vaccination histories from providers and returned acknowledgment messages, compared with 87% in 2013, the investigators said.
“Clinical Decision Support (CDS) functionalities enable providers to evaluate the validity of vaccine doses administered to patients and forecast future vaccines that will be needed, based on recommendations developed by the Advisory Committee on Immunization Practices,” Dr. Murthy and his associates said. In 2016, 58% of the 55 jurisdictions sent a vaccine forecast to another system, compared with 31% in 2013.
In 2016, 95% of the 55 jurisdictions gave a “predefined, automatic report on immunization coverage by provider site,” compared with 89% of jurisdictions in 2013.
“IISs are integral components of routine clinical practice and public health surveillance for immunization,” Dr. Murthy and his associates said. “Availability of more complete IIS data also offers many benefits to health care providers and public health practitioners, including consolidating patients’ vaccination histories, identifying undervaccinated subgroups, and forecasting the needs of individual patients for recommended vaccines.”
Read more in Morbidity and Mortality Weekly Report (2017 Nov 3;66[43]:1178-81).
FROM MMWR