User login
Social determinants of health gaining prominence
Fragmented, essentializing, simplistic. That’s how students at Perelman School of Medicine at the University of Pennsylvania, Philadelphia, described their required course on cultural competence. Lectures and discussions about cultural groups and communication issues weren’t providing them with the skills they needed to navigate doctor-patient relationships.
Their criticism was a wake-up call that Horace Delisser, MD, associate dean for diversity and inclusion at the school, took to heart. He enlisted medical students to help reinvent the curriculum. The result, Introduction to Medicine and Society, launched in 2013 and described in an article published in 2017 (Acad Med. 2017;92[3]:335-43), emphasizes self-awareness and reflection about one’s own biases and the adoption of a less hierarchical and more respectful “other-oriented” approach to the patient relationship.
The course examines social determinants of health (SDHs) – the influences of society, government, culture, and health systems. Students analyze how health and health outcomes are affected by a patient’s income, education, and living and working conditions, as well as access to healthy food, safe water, and transportation.
A host of policy makers, advisory groups, and organized medicine groups have called in recent years for educational efforts to boost all physicians’ working knowledge of health inequities and SDHs.
Dr. Delisser, associate professor of medicine who also practices as a pulmonologist at the Harron Lung Center in the Perelman Center for Advanced Medicine, said SDHs play into daily care.
Consider the patient who is chronically late for appointments. “It may not be an issue of the patient being disinterested in their health care, but maybe the public transportation system is unreliable, or maybe the patient has to take two buses and a subway to get there. I need [this knowledge] to inform my care and to engage my patient. I need to know, ‘what does it take for you to get here?’ That factors into how I [make the care plan],” said Dr. Delisser.
Malika Fair, MD, MPH, who teaches a longitudinal professional development class at George Washington University, Washington, and is senior director of health equity partnerships and programs at the American Association of Medical Colleges, provided the example of how her medical students intervened during their rotation in the emergency department on behalf of a newly-diagnosed patient with diabetes who had been unable to fill a prescribed medication. After determining where the patient lived, the students ensured that she had transportation and was able to get the needed medication at a local grocery store. They asked about her barriers to healthy eating, researched local grocery stores, and made practical recommendations that the patient was amenable to implementing. They identified a clinic closer to the patient’s home, and worked with her on making an appointment at a time when she could take off from work.
“Because of their training, these students were able to identify and address social risks in their first month on the ward,” said Dr. Fair, who also practices emergency medicine. They had learned about how to ask about food access and how safe it was for the patient to walk and exercise in her neighborhood.
At Perelman, most students work in student-led community clinics, and some fourth-year students participate in an elective rotation as apprentices to community health workers, learning to address SDHs and develop the cultural humility that they learned about in the classroom. The rotation was similarly created in 2013 and is described in a 2018 article (J Health Care Poor Underserved. 2018;29[2]:581-90).“Being a good physician involves being technically competent as well as what I call relationally competent,” Dr. Delisser said. “And [this involves] being aware that my relationship with a patient doesn’t exist in a vacuum ... that there’s a bigger, broader social and structural context that I need to know and understand. I [then need] to use that to inform how I mediate and empower that relationship.”
Aletha Maybank, MD, who became the American Medical Association’s first chief health equity officer earlier this year, explained that “the medical profession had a very strong social context at one point in time,” but this was dampened by the Flexner Report of 1910.*
The report revolutionized medical education by increasing its rigor, but “it was really focused on clinical and basic science and took out the social context, the context of what medicine is about,” said Dr. Maybank, a pediatrician with a board certification in preventive medicine/public health. “[Now] we’re asking, how do we revolutionize medical education again at this point in time, recognizing the confluence of information and data that we now have available to us about inequities and disparities ... and the sense of urgency from students.”
Students driving practice change
Students nationally are “the most important” drivers of the increasing focus on SDHs in medical education, according to Dr. Fair. “They are demanding experiences to learn about the entire patient. We know that only 20% of a patient’s health is dependent on their health care. Our students are demanding education about the other 80%.”
More and more, communities are identifying needs and “students will then come up with initiatives to meet those needs,” Dr. Fair said.
Others interviewed for this story predicted this trend will only intensify, since not-for-profit hospitals are required under the Affordable Care Act regulations to assess community health needs every few years and to intervene accordingly.
Education on health care systems is also advancing. Penn State University, for instance, utilized a million-dollar grant from the AMA’s Accelerating Change in Medical Education initiative to design and implement a 4-year curriculum on the health system sciences that started in 2014. The curriculum includes an immersive experience in patient navigation.
“Students were taught to be patient navigators, and they were assigned within the clinical context to work on issues like, why are [patients] having trouble getting their medications?” said Susan E. Skochelak, MD, MPH, who leads the 6-year-old Accelerating Change initiative as vice president for medical education at the AMA.
From the start, she noted, students at Penn State are encouraged to question inequities, social and structural barriers to health, and faults in the health care system. “The message given at their white coat ceremony is ‘Welcome to medicine. Now that you’re here, you’re a member of the health care team, and we want you to speak up if you think there are things that need to be addressed. We want you to tell us when the system is working and not working,’ ” said Dr Skochelak, who previously served as the senior associate dean for academic affairs at the University of Wisconsin School of Medicine and Public Health, where she had been a tenured professor of family medicine.
Tomorrow’s physician partners
Approximately 80% of medical school graduates who participated in the AAMC’s 2018 survey of graduates said they had received significant training on health disparities—up from 71% in 2014.
“There’s a huge amount [of innovation] happening, but on the flip side, there’s not really a set of accepted tools and practices, and certainly no robust evaluation [of the training],” said Philip M. Alberti, PhD, senior director for health equity research and policy at the American Association of Medical Colleges. A recently published review (J Gen Intern Med. 2019;34[5]:720-30) shows growing interest in the teaching of SDHs in undergraduate medical education but variable content, strategies, and instructional practices.
Health care systems and practicing physicians are still very much feeling their way with SDHs. Screening tools are being developed and tested, and academic medical centers are trying to determine their roles in addressing issues such as transportation and housing – and what funding and structural levers can be pulled to fulfill these roles. “As we learn more about [these issues], it will become clearer what the right baseline set of competencies might be for all physicians,” Dr. Alberti noted.
In the meantime, some basic expectations for medical education are taking root officially. The National Board of Medical Examiners, with whom the AMA has partnered in its Accelerating Change initiative, has included questions in the United States Medical Licensing Examination on population health and SDHs, and plans to add more exam content on these topics and on health systems science, said Dr. Skochelak.
And through its site visit program (the Clinical Learning Environment Review program), the Accreditation Council for Graduate Medical Education has “made it pretty clear that there’s an expectation that residents and fellows are learning about the health system’s approach to identifying and addressing health care disparities – and that they’re given opportunities to develop quality improvement initiatives that target those disparities,” Dr. Alberti said.
In hopes of achieving consistency across medical specialties and in national accreditation and board certifications exams, the American Association of Medical Colleges is developing its first set of competencies in quality improvement and patient safety, with health equity being one of these competencies’ domains .
The competencies are tiered for medical school graduates, residency graduates, and faculty physicians who are 3-5 years post residency. At this point in time, said Dr. Alberti, the consensus among medical educators has been that physicians “need to be able to understand and consider [social, economic, and structural] contexts when they’re seeing patients, when they’re developing care plans, when they’re talking with caregivers, and when they’re looking at their own quality data.”
Elisabeth Poorman, MD, MPH, an internist at UW Medicine in Kent, Washington, said she worries that the passion of medical students for SDHs will too often be crushed, especially during residency and with immersion in the productivity-focused health care system. Studies show a drop in mental wellness and empathy and a rise in cynicism as training advances, said Dr. Poorman, who also writes about health care and issues of equity and serves on the editorial advisory board of Internal Medicine News.
With similar concerns, the AMA has recently launched a “Reimagining Residency” initiative that aims to improve transitions from medical school to residency and the wellness of residents and faculty, and expand educational content relating to SDHs.
Dr. Fair is optimistic that new physicians’ knowledge of SDHs will permeate medical practices.
“Physicians who are out practicing are going to be working with our graduates, and they’re going to be asking in [job] interviews, do you have flexible hours for patients? What community partnerships do you have? Are there other professionals on staff to help us address social determinants of health? What data [relating to SDHs] are you collecting?” she said.
Correction, 8/26/2019: An earlier version of this story misstated the title of Aletha Maybank, MD. Dr. Maybank's correct title is the first chief health equity officer of the American Medical Association.
Fragmented, essentializing, simplistic. That’s how students at Perelman School of Medicine at the University of Pennsylvania, Philadelphia, described their required course on cultural competence. Lectures and discussions about cultural groups and communication issues weren’t providing them with the skills they needed to navigate doctor-patient relationships.
Their criticism was a wake-up call that Horace Delisser, MD, associate dean for diversity and inclusion at the school, took to heart. He enlisted medical students to help reinvent the curriculum. The result, Introduction to Medicine and Society, launched in 2013 and described in an article published in 2017 (Acad Med. 2017;92[3]:335-43), emphasizes self-awareness and reflection about one’s own biases and the adoption of a less hierarchical and more respectful “other-oriented” approach to the patient relationship.
The course examines social determinants of health (SDHs) – the influences of society, government, culture, and health systems. Students analyze how health and health outcomes are affected by a patient’s income, education, and living and working conditions, as well as access to healthy food, safe water, and transportation.
A host of policy makers, advisory groups, and organized medicine groups have called in recent years for educational efforts to boost all physicians’ working knowledge of health inequities and SDHs.
Dr. Delisser, associate professor of medicine who also practices as a pulmonologist at the Harron Lung Center in the Perelman Center for Advanced Medicine, said SDHs play into daily care.
Consider the patient who is chronically late for appointments. “It may not be an issue of the patient being disinterested in their health care, but maybe the public transportation system is unreliable, or maybe the patient has to take two buses and a subway to get there. I need [this knowledge] to inform my care and to engage my patient. I need to know, ‘what does it take for you to get here?’ That factors into how I [make the care plan],” said Dr. Delisser.
Malika Fair, MD, MPH, who teaches a longitudinal professional development class at George Washington University, Washington, and is senior director of health equity partnerships and programs at the American Association of Medical Colleges, provided the example of how her medical students intervened during their rotation in the emergency department on behalf of a newly-diagnosed patient with diabetes who had been unable to fill a prescribed medication. After determining where the patient lived, the students ensured that she had transportation and was able to get the needed medication at a local grocery store. They asked about her barriers to healthy eating, researched local grocery stores, and made practical recommendations that the patient was amenable to implementing. They identified a clinic closer to the patient’s home, and worked with her on making an appointment at a time when she could take off from work.
“Because of their training, these students were able to identify and address social risks in their first month on the ward,” said Dr. Fair, who also practices emergency medicine. They had learned about how to ask about food access and how safe it was for the patient to walk and exercise in her neighborhood.
At Perelman, most students work in student-led community clinics, and some fourth-year students participate in an elective rotation as apprentices to community health workers, learning to address SDHs and develop the cultural humility that they learned about in the classroom. The rotation was similarly created in 2013 and is described in a 2018 article (J Health Care Poor Underserved. 2018;29[2]:581-90).“Being a good physician involves being technically competent as well as what I call relationally competent,” Dr. Delisser said. “And [this involves] being aware that my relationship with a patient doesn’t exist in a vacuum ... that there’s a bigger, broader social and structural context that I need to know and understand. I [then need] to use that to inform how I mediate and empower that relationship.”
Aletha Maybank, MD, who became the American Medical Association’s first chief health equity officer earlier this year, explained that “the medical profession had a very strong social context at one point in time,” but this was dampened by the Flexner Report of 1910.*
The report revolutionized medical education by increasing its rigor, but “it was really focused on clinical and basic science and took out the social context, the context of what medicine is about,” said Dr. Maybank, a pediatrician with a board certification in preventive medicine/public health. “[Now] we’re asking, how do we revolutionize medical education again at this point in time, recognizing the confluence of information and data that we now have available to us about inequities and disparities ... and the sense of urgency from students.”
Students driving practice change
Students nationally are “the most important” drivers of the increasing focus on SDHs in medical education, according to Dr. Fair. “They are demanding experiences to learn about the entire patient. We know that only 20% of a patient’s health is dependent on their health care. Our students are demanding education about the other 80%.”
More and more, communities are identifying needs and “students will then come up with initiatives to meet those needs,” Dr. Fair said.
Others interviewed for this story predicted this trend will only intensify, since not-for-profit hospitals are required under the Affordable Care Act regulations to assess community health needs every few years and to intervene accordingly.
Education on health care systems is also advancing. Penn State University, for instance, utilized a million-dollar grant from the AMA’s Accelerating Change in Medical Education initiative to design and implement a 4-year curriculum on the health system sciences that started in 2014. The curriculum includes an immersive experience in patient navigation.
“Students were taught to be patient navigators, and they were assigned within the clinical context to work on issues like, why are [patients] having trouble getting their medications?” said Susan E. Skochelak, MD, MPH, who leads the 6-year-old Accelerating Change initiative as vice president for medical education at the AMA.
From the start, she noted, students at Penn State are encouraged to question inequities, social and structural barriers to health, and faults in the health care system. “The message given at their white coat ceremony is ‘Welcome to medicine. Now that you’re here, you’re a member of the health care team, and we want you to speak up if you think there are things that need to be addressed. We want you to tell us when the system is working and not working,’ ” said Dr Skochelak, who previously served as the senior associate dean for academic affairs at the University of Wisconsin School of Medicine and Public Health, where she had been a tenured professor of family medicine.
Tomorrow’s physician partners
Approximately 80% of medical school graduates who participated in the AAMC’s 2018 survey of graduates said they had received significant training on health disparities—up from 71% in 2014.
“There’s a huge amount [of innovation] happening, but on the flip side, there’s not really a set of accepted tools and practices, and certainly no robust evaluation [of the training],” said Philip M. Alberti, PhD, senior director for health equity research and policy at the American Association of Medical Colleges. A recently published review (J Gen Intern Med. 2019;34[5]:720-30) shows growing interest in the teaching of SDHs in undergraduate medical education but variable content, strategies, and instructional practices.
Health care systems and practicing physicians are still very much feeling their way with SDHs. Screening tools are being developed and tested, and academic medical centers are trying to determine their roles in addressing issues such as transportation and housing – and what funding and structural levers can be pulled to fulfill these roles. “As we learn more about [these issues], it will become clearer what the right baseline set of competencies might be for all physicians,” Dr. Alberti noted.
In the meantime, some basic expectations for medical education are taking root officially. The National Board of Medical Examiners, with whom the AMA has partnered in its Accelerating Change initiative, has included questions in the United States Medical Licensing Examination on population health and SDHs, and plans to add more exam content on these topics and on health systems science, said Dr. Skochelak.
And through its site visit program (the Clinical Learning Environment Review program), the Accreditation Council for Graduate Medical Education has “made it pretty clear that there’s an expectation that residents and fellows are learning about the health system’s approach to identifying and addressing health care disparities – and that they’re given opportunities to develop quality improvement initiatives that target those disparities,” Dr. Alberti said.
In hopes of achieving consistency across medical specialties and in national accreditation and board certifications exams, the American Association of Medical Colleges is developing its first set of competencies in quality improvement and patient safety, with health equity being one of these competencies’ domains .
The competencies are tiered for medical school graduates, residency graduates, and faculty physicians who are 3-5 years post residency. At this point in time, said Dr. Alberti, the consensus among medical educators has been that physicians “need to be able to understand and consider [social, economic, and structural] contexts when they’re seeing patients, when they’re developing care plans, when they’re talking with caregivers, and when they’re looking at their own quality data.”
Elisabeth Poorman, MD, MPH, an internist at UW Medicine in Kent, Washington, said she worries that the passion of medical students for SDHs will too often be crushed, especially during residency and with immersion in the productivity-focused health care system. Studies show a drop in mental wellness and empathy and a rise in cynicism as training advances, said Dr. Poorman, who also writes about health care and issues of equity and serves on the editorial advisory board of Internal Medicine News.
With similar concerns, the AMA has recently launched a “Reimagining Residency” initiative that aims to improve transitions from medical school to residency and the wellness of residents and faculty, and expand educational content relating to SDHs.
Dr. Fair is optimistic that new physicians’ knowledge of SDHs will permeate medical practices.
“Physicians who are out practicing are going to be working with our graduates, and they’re going to be asking in [job] interviews, do you have flexible hours for patients? What community partnerships do you have? Are there other professionals on staff to help us address social determinants of health? What data [relating to SDHs] are you collecting?” she said.
Correction, 8/26/2019: An earlier version of this story misstated the title of Aletha Maybank, MD. Dr. Maybank's correct title is the first chief health equity officer of the American Medical Association.
Fragmented, essentializing, simplistic. That’s how students at Perelman School of Medicine at the University of Pennsylvania, Philadelphia, described their required course on cultural competence. Lectures and discussions about cultural groups and communication issues weren’t providing them with the skills they needed to navigate doctor-patient relationships.
Their criticism was a wake-up call that Horace Delisser, MD, associate dean for diversity and inclusion at the school, took to heart. He enlisted medical students to help reinvent the curriculum. The result, Introduction to Medicine and Society, launched in 2013 and described in an article published in 2017 (Acad Med. 2017;92[3]:335-43), emphasizes self-awareness and reflection about one’s own biases and the adoption of a less hierarchical and more respectful “other-oriented” approach to the patient relationship.
The course examines social determinants of health (SDHs) – the influences of society, government, culture, and health systems. Students analyze how health and health outcomes are affected by a patient’s income, education, and living and working conditions, as well as access to healthy food, safe water, and transportation.
A host of policy makers, advisory groups, and organized medicine groups have called in recent years for educational efforts to boost all physicians’ working knowledge of health inequities and SDHs.
Dr. Delisser, associate professor of medicine who also practices as a pulmonologist at the Harron Lung Center in the Perelman Center for Advanced Medicine, said SDHs play into daily care.
Consider the patient who is chronically late for appointments. “It may not be an issue of the patient being disinterested in their health care, but maybe the public transportation system is unreliable, or maybe the patient has to take two buses and a subway to get there. I need [this knowledge] to inform my care and to engage my patient. I need to know, ‘what does it take for you to get here?’ That factors into how I [make the care plan],” said Dr. Delisser.
Malika Fair, MD, MPH, who teaches a longitudinal professional development class at George Washington University, Washington, and is senior director of health equity partnerships and programs at the American Association of Medical Colleges, provided the example of how her medical students intervened during their rotation in the emergency department on behalf of a newly-diagnosed patient with diabetes who had been unable to fill a prescribed medication. After determining where the patient lived, the students ensured that she had transportation and was able to get the needed medication at a local grocery store. They asked about her barriers to healthy eating, researched local grocery stores, and made practical recommendations that the patient was amenable to implementing. They identified a clinic closer to the patient’s home, and worked with her on making an appointment at a time when she could take off from work.
“Because of their training, these students were able to identify and address social risks in their first month on the ward,” said Dr. Fair, who also practices emergency medicine. They had learned about how to ask about food access and how safe it was for the patient to walk and exercise in her neighborhood.
At Perelman, most students work in student-led community clinics, and some fourth-year students participate in an elective rotation as apprentices to community health workers, learning to address SDHs and develop the cultural humility that they learned about in the classroom. The rotation was similarly created in 2013 and is described in a 2018 article (J Health Care Poor Underserved. 2018;29[2]:581-90).“Being a good physician involves being technically competent as well as what I call relationally competent,” Dr. Delisser said. “And [this involves] being aware that my relationship with a patient doesn’t exist in a vacuum ... that there’s a bigger, broader social and structural context that I need to know and understand. I [then need] to use that to inform how I mediate and empower that relationship.”
Aletha Maybank, MD, who became the American Medical Association’s first chief health equity officer earlier this year, explained that “the medical profession had a very strong social context at one point in time,” but this was dampened by the Flexner Report of 1910.*
The report revolutionized medical education by increasing its rigor, but “it was really focused on clinical and basic science and took out the social context, the context of what medicine is about,” said Dr. Maybank, a pediatrician with a board certification in preventive medicine/public health. “[Now] we’re asking, how do we revolutionize medical education again at this point in time, recognizing the confluence of information and data that we now have available to us about inequities and disparities ... and the sense of urgency from students.”
Students driving practice change
Students nationally are “the most important” drivers of the increasing focus on SDHs in medical education, according to Dr. Fair. “They are demanding experiences to learn about the entire patient. We know that only 20% of a patient’s health is dependent on their health care. Our students are demanding education about the other 80%.”
More and more, communities are identifying needs and “students will then come up with initiatives to meet those needs,” Dr. Fair said.
Others interviewed for this story predicted this trend will only intensify, since not-for-profit hospitals are required under the Affordable Care Act regulations to assess community health needs every few years and to intervene accordingly.
Education on health care systems is also advancing. Penn State University, for instance, utilized a million-dollar grant from the AMA’s Accelerating Change in Medical Education initiative to design and implement a 4-year curriculum on the health system sciences that started in 2014. The curriculum includes an immersive experience in patient navigation.
“Students were taught to be patient navigators, and they were assigned within the clinical context to work on issues like, why are [patients] having trouble getting their medications?” said Susan E. Skochelak, MD, MPH, who leads the 6-year-old Accelerating Change initiative as vice president for medical education at the AMA.
From the start, she noted, students at Penn State are encouraged to question inequities, social and structural barriers to health, and faults in the health care system. “The message given at their white coat ceremony is ‘Welcome to medicine. Now that you’re here, you’re a member of the health care team, and we want you to speak up if you think there are things that need to be addressed. We want you to tell us when the system is working and not working,’ ” said Dr Skochelak, who previously served as the senior associate dean for academic affairs at the University of Wisconsin School of Medicine and Public Health, where she had been a tenured professor of family medicine.
Tomorrow’s physician partners
Approximately 80% of medical school graduates who participated in the AAMC’s 2018 survey of graduates said they had received significant training on health disparities—up from 71% in 2014.
“There’s a huge amount [of innovation] happening, but on the flip side, there’s not really a set of accepted tools and practices, and certainly no robust evaluation [of the training],” said Philip M. Alberti, PhD, senior director for health equity research and policy at the American Association of Medical Colleges. A recently published review (J Gen Intern Med. 2019;34[5]:720-30) shows growing interest in the teaching of SDHs in undergraduate medical education but variable content, strategies, and instructional practices.
Health care systems and practicing physicians are still very much feeling their way with SDHs. Screening tools are being developed and tested, and academic medical centers are trying to determine their roles in addressing issues such as transportation and housing – and what funding and structural levers can be pulled to fulfill these roles. “As we learn more about [these issues], it will become clearer what the right baseline set of competencies might be for all physicians,” Dr. Alberti noted.
In the meantime, some basic expectations for medical education are taking root officially. The National Board of Medical Examiners, with whom the AMA has partnered in its Accelerating Change initiative, has included questions in the United States Medical Licensing Examination on population health and SDHs, and plans to add more exam content on these topics and on health systems science, said Dr. Skochelak.
And through its site visit program (the Clinical Learning Environment Review program), the Accreditation Council for Graduate Medical Education has “made it pretty clear that there’s an expectation that residents and fellows are learning about the health system’s approach to identifying and addressing health care disparities – and that they’re given opportunities to develop quality improvement initiatives that target those disparities,” Dr. Alberti said.
In hopes of achieving consistency across medical specialties and in national accreditation and board certifications exams, the American Association of Medical Colleges is developing its first set of competencies in quality improvement and patient safety, with health equity being one of these competencies’ domains .
The competencies are tiered for medical school graduates, residency graduates, and faculty physicians who are 3-5 years post residency. At this point in time, said Dr. Alberti, the consensus among medical educators has been that physicians “need to be able to understand and consider [social, economic, and structural] contexts when they’re seeing patients, when they’re developing care plans, when they’re talking with caregivers, and when they’re looking at their own quality data.”
Elisabeth Poorman, MD, MPH, an internist at UW Medicine in Kent, Washington, said she worries that the passion of medical students for SDHs will too often be crushed, especially during residency and with immersion in the productivity-focused health care system. Studies show a drop in mental wellness and empathy and a rise in cynicism as training advances, said Dr. Poorman, who also writes about health care and issues of equity and serves on the editorial advisory board of Internal Medicine News.
With similar concerns, the AMA has recently launched a “Reimagining Residency” initiative that aims to improve transitions from medical school to residency and the wellness of residents and faculty, and expand educational content relating to SDHs.
Dr. Fair is optimistic that new physicians’ knowledge of SDHs will permeate medical practices.
“Physicians who are out practicing are going to be working with our graduates, and they’re going to be asking in [job] interviews, do you have flexible hours for patients? What community partnerships do you have? Are there other professionals on staff to help us address social determinants of health? What data [relating to SDHs] are you collecting?” she said.
Correction, 8/26/2019: An earlier version of this story misstated the title of Aletha Maybank, MD. Dr. Maybank's correct title is the first chief health equity officer of the American Medical Association.
Sexual assault in military linked to sexual pain
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
FROM OBSTETRICS & GYNECOLOGY
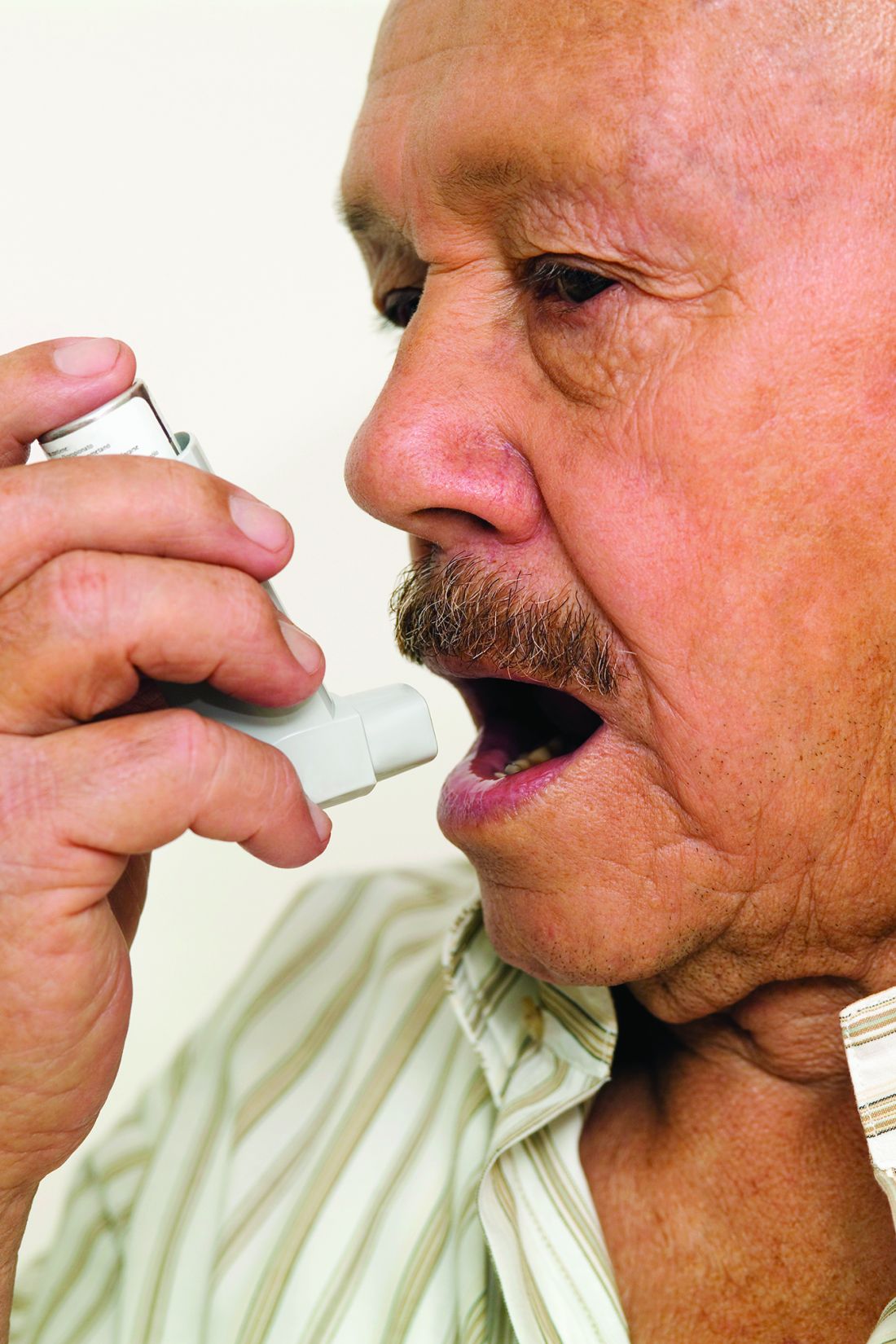
Inhaler technique not to blame for uncontrolled asthma in inner-city study
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Key clinical point: Factors other than inhaler technique should be considered to explain uncontrolled asthma in a low-income, inner-city population.
Major finding: In the study, 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers were using their devices correctly.
Study details: In all, 586 patients were observed using their inhalers, and their technique was scored by way of a checklist developed for the study.
Disclosures: The National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute supported the study. Coinvestigator Andrea J. Apter, MD, consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
Source: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
Preprints come to medicine: medRxiv launches with safeguards
A new preprint server for the medical and health sciences – medRxiv – has launched, along with safeguards designed to mitigate the risk of non–peer-reviewed findings prematurely guiding clinical practice or misinforming the public.
The new repository of preprints is intended for researcher-to-researcher communication – and mainly to facilitate faster sharing of research findings before publication in peer-reviewed journals. Papers will not be scrutinized for study design or the strength of the science, but they will be screened by an external clinical scientist and – at least for now – by an editor funded by BMJ, the London-based publisher and one of the three cofounding organizations of medRxiv (pronounced “med archive”).
The server’s six-person leadership team – comprising leaders from BMJ and cofounders, Cold Spring Harbor Laboratory in New York, and Yale University in New Haven, Conn. – will make final decisions about whether to post papers that generate concerns.
“We’ve put in place more stringent screening procedures than existed for bioRxiv, [a biological preprint server launched in 2013 by Cold Spring Harbor Laboratory],”said Theodora Bloom, PhD, executive editor of BMJ. “We’ll specifically ask the question, is there a risk to public health or health-related behaviors if this preprint is posted and [turns out to be] wrong?”
Concerns that poor information will be disseminated to the public or that the public will misinterpret information published, were heard by the medRxiv founders as they “work-shopped the idea and talked with the community,” said Joseph Ross, MD, an associate professor of medicine and public health at Yale and codirector of the Yale Open Data Access (YODA) Project
“We’re taking a cautious approach, particularly in the early days as we learn from the process,” he said. “How a paper [could potentially influence clinical practice] will be a guiding question.”
The cofounders had several conversations, Dr. Ross said, with Howard Bauchner, MD, editor in chief of JAMA, who took a strong stance against preprints and shortcutting the peer review process in a 2017 editorial titled “The rush to publication: An editorial and scientific mistake.” (Dr. Ross is an associate editor at JAMA Internal Medicine. Dr. Bauchner was unavailable for comment on the safeguards built into medRvix.)
Aaron D. Viny, MD, a hematologist-oncologist at Memorial Sloan Kettering in New York, said he has mixed feelings about preprints and believes the stakes are higher with medRvix, given that it will house clinical content – including, he anticipates, single-institution, nonprospective outcome studies of off-label drug uses. “These aren’t bona fide clinical trials and may not have the best data,” he said.
Still, there are advantages for investigators – and for the progress of research – with earlier dissemination of findings, Dr. Viny said. He recently had a paper posted on bioRvix for the first time. The paper was undergoing revision for a peer-reviewed journal and was being presented at a national meeting at the time it was posted.
“We timed it [as such], so that not only were we presenting it at a national meeting, but it also got more Twitter buzz,” he said. “I thought it was a good body of work, and I was excited to discuss it online with the scientific community.”
Dr. Viny’s decision is common among preprint authors and reflects the values of the preprint server, Dr. Ross said. “When people are reading or hearing about [new findings] at a meeting, they can go to the papers to get more complete information.” And, he said, the investigators themselves can get more feedback than they otherwise would.
In addition to papers that are well on their way to publication in peer-reviewed journals, Dr. Ross anticipates that medRvix will house papers on qualitative studies and observational research that face more arduous publication paths. He said he expects to see research on medical education and hopes to see papers on “quality improvement work, which typically involve small interventions at a single institution, and have important insights but are hard to publish because of generalizability and controls.”
And while there has been a “positive shift” in the past 10 years in the publication of negative results in peer-reviewed literature, medRvix may well capture studies that have negative results “because they have challenges with recruitment or other [elements of study design],” Dr. Ross said. “There is still a lot that can be learned by the scientific community from these negative studies, but they’re very difficult to publish in a peer-reviewed journal.”
Road to preprints
BMJ has a history with preprints. The publisher established a preprint server for biomedical research in the late 1990s, but it never took off and was shut down in the early 2000s. “It just didn’t get the uptake,” said Dr. Bloom. “It’s hard to know exactly why.”
What is clear, she said, is what has changed in the past 20 years: Copious use of the Internet overall, a growing desire to stake out one’s research turf online, requests from funders to have preprints listed on grant applications, and disease outbreaks involving the Zika virus and Ebola that have highlighted the advantages of faster dissemination of research findings.
BMJ had begun discussions with John Inglis, PhD, of Cold Spring Harbor Laboratory about launching a preprint server for the medical sciences (building on the experience of bioRxiv) when they heard Harlan Krumholz, MD, professor of medicine at Yale and head of the YODA project, speak at the 2017 meeting of the International Congress on Peer Review & Scientific Publication. In his keynote address, Dr. Krumholz described Yale’s plans to launch a preprint server.
“We all felt it would be better working together than apart,” Dr. Bloom said.
Getting published
Each preprint on medRxiv will get a permanent DOI link and a disclaimer stating that preprints are not peer reviewed, should not be relied on to guide clinical practice, and should not be reported in the news media as established information.
Authors will be required to meet various standards and requirements common in the clinical and medical sciences, such as including details on ethics approvals, patient consent, funding sources and conflicts of interest, and trial registration numbers. They will have the option of adding a revision(s) of their preprint (each preprint will have a “history”), as well as the option of having their preprint marked as “withdrawn” if they can no longer stand by the findings or conclusions. Preprints will automatically be linked to final published papers.
Journals have wrestled with how to handle preprints. A look at several major peer-reviewed journals shows that they’ll consider articles that have appeared in early form as preprints (including the New England Journal of Medicine, according to media relations manager Jennifer Zeis), but there are caveats. JAMA, for instance, will look at whether submitted manuscripts add “meaningfully new” information above what the preprint disseminated.
Similarly, the American Society of Clinical Oncology (ASCO) will consider how preprints affect the “novelty” of the manuscript’s findings for its ASCO journal readers. Editors of the journal Blood will consider “public comments or coverage about [the] preprint” in its evaluation of the manuscript’s impact. Several of the major journals specify that preprints cannot be updated while manuscripts are under review.
A recent review of bioRxiv preprints shows that approximately two-thirds went on to peer-reviewed publication.
And according to the BMJ’s Dr. Bloom, “there is definitely evidence that preprints [overall] are getting cited [in the scientific literature] before peer-reviewed articles appear.”
The server medRvix began accepting manuscripts on June 6 and will go live on June 25. It will accept only research papers – not commentaries or case reports, Dr. Ross emphasized.
For now, Dr. Bloom said, the most immediate and “real question for us is, will clinical researchers embrace preprints? And if they do, can we continue to provide a light touch but rapid way to screen papers while ensuring the safety of what we’re posting?”
For his part, Dr. Viny is bracing for “public consumption” of medRxiv content, especially in the oncology community in which patients are often extraordinarily well educated about their disease and determined to learn about all possible treatment options. “My job as a clinician,” he said, “will be to contextualize the patient’s reference information.”
A new preprint server for the medical and health sciences – medRxiv – has launched, along with safeguards designed to mitigate the risk of non–peer-reviewed findings prematurely guiding clinical practice or misinforming the public.
The new repository of preprints is intended for researcher-to-researcher communication – and mainly to facilitate faster sharing of research findings before publication in peer-reviewed journals. Papers will not be scrutinized for study design or the strength of the science, but they will be screened by an external clinical scientist and – at least for now – by an editor funded by BMJ, the London-based publisher and one of the three cofounding organizations of medRxiv (pronounced “med archive”).
The server’s six-person leadership team – comprising leaders from BMJ and cofounders, Cold Spring Harbor Laboratory in New York, and Yale University in New Haven, Conn. – will make final decisions about whether to post papers that generate concerns.
“We’ve put in place more stringent screening procedures than existed for bioRxiv, [a biological preprint server launched in 2013 by Cold Spring Harbor Laboratory],”said Theodora Bloom, PhD, executive editor of BMJ. “We’ll specifically ask the question, is there a risk to public health or health-related behaviors if this preprint is posted and [turns out to be] wrong?”
Concerns that poor information will be disseminated to the public or that the public will misinterpret information published, were heard by the medRxiv founders as they “work-shopped the idea and talked with the community,” said Joseph Ross, MD, an associate professor of medicine and public health at Yale and codirector of the Yale Open Data Access (YODA) Project
“We’re taking a cautious approach, particularly in the early days as we learn from the process,” he said. “How a paper [could potentially influence clinical practice] will be a guiding question.”
The cofounders had several conversations, Dr. Ross said, with Howard Bauchner, MD, editor in chief of JAMA, who took a strong stance against preprints and shortcutting the peer review process in a 2017 editorial titled “The rush to publication: An editorial and scientific mistake.” (Dr. Ross is an associate editor at JAMA Internal Medicine. Dr. Bauchner was unavailable for comment on the safeguards built into medRvix.)
Aaron D. Viny, MD, a hematologist-oncologist at Memorial Sloan Kettering in New York, said he has mixed feelings about preprints and believes the stakes are higher with medRvix, given that it will house clinical content – including, he anticipates, single-institution, nonprospective outcome studies of off-label drug uses. “These aren’t bona fide clinical trials and may not have the best data,” he said.
Still, there are advantages for investigators – and for the progress of research – with earlier dissemination of findings, Dr. Viny said. He recently had a paper posted on bioRvix for the first time. The paper was undergoing revision for a peer-reviewed journal and was being presented at a national meeting at the time it was posted.
“We timed it [as such], so that not only were we presenting it at a national meeting, but it also got more Twitter buzz,” he said. “I thought it was a good body of work, and I was excited to discuss it online with the scientific community.”
Dr. Viny’s decision is common among preprint authors and reflects the values of the preprint server, Dr. Ross said. “When people are reading or hearing about [new findings] at a meeting, they can go to the papers to get more complete information.” And, he said, the investigators themselves can get more feedback than they otherwise would.
In addition to papers that are well on their way to publication in peer-reviewed journals, Dr. Ross anticipates that medRvix will house papers on qualitative studies and observational research that face more arduous publication paths. He said he expects to see research on medical education and hopes to see papers on “quality improvement work, which typically involve small interventions at a single institution, and have important insights but are hard to publish because of generalizability and controls.”
And while there has been a “positive shift” in the past 10 years in the publication of negative results in peer-reviewed literature, medRvix may well capture studies that have negative results “because they have challenges with recruitment or other [elements of study design],” Dr. Ross said. “There is still a lot that can be learned by the scientific community from these negative studies, but they’re very difficult to publish in a peer-reviewed journal.”
Road to preprints
BMJ has a history with preprints. The publisher established a preprint server for biomedical research in the late 1990s, but it never took off and was shut down in the early 2000s. “It just didn’t get the uptake,” said Dr. Bloom. “It’s hard to know exactly why.”
What is clear, she said, is what has changed in the past 20 years: Copious use of the Internet overall, a growing desire to stake out one’s research turf online, requests from funders to have preprints listed on grant applications, and disease outbreaks involving the Zika virus and Ebola that have highlighted the advantages of faster dissemination of research findings.
BMJ had begun discussions with John Inglis, PhD, of Cold Spring Harbor Laboratory about launching a preprint server for the medical sciences (building on the experience of bioRxiv) when they heard Harlan Krumholz, MD, professor of medicine at Yale and head of the YODA project, speak at the 2017 meeting of the International Congress on Peer Review & Scientific Publication. In his keynote address, Dr. Krumholz described Yale’s plans to launch a preprint server.
“We all felt it would be better working together than apart,” Dr. Bloom said.
Getting published
Each preprint on medRxiv will get a permanent DOI link and a disclaimer stating that preprints are not peer reviewed, should not be relied on to guide clinical practice, and should not be reported in the news media as established information.
Authors will be required to meet various standards and requirements common in the clinical and medical sciences, such as including details on ethics approvals, patient consent, funding sources and conflicts of interest, and trial registration numbers. They will have the option of adding a revision(s) of their preprint (each preprint will have a “history”), as well as the option of having their preprint marked as “withdrawn” if they can no longer stand by the findings or conclusions. Preprints will automatically be linked to final published papers.
Journals have wrestled with how to handle preprints. A look at several major peer-reviewed journals shows that they’ll consider articles that have appeared in early form as preprints (including the New England Journal of Medicine, according to media relations manager Jennifer Zeis), but there are caveats. JAMA, for instance, will look at whether submitted manuscripts add “meaningfully new” information above what the preprint disseminated.
Similarly, the American Society of Clinical Oncology (ASCO) will consider how preprints affect the “novelty” of the manuscript’s findings for its ASCO journal readers. Editors of the journal Blood will consider “public comments or coverage about [the] preprint” in its evaluation of the manuscript’s impact. Several of the major journals specify that preprints cannot be updated while manuscripts are under review.
A recent review of bioRxiv preprints shows that approximately two-thirds went on to peer-reviewed publication.
And according to the BMJ’s Dr. Bloom, “there is definitely evidence that preprints [overall] are getting cited [in the scientific literature] before peer-reviewed articles appear.”
The server medRvix began accepting manuscripts on June 6 and will go live on June 25. It will accept only research papers – not commentaries or case reports, Dr. Ross emphasized.
For now, Dr. Bloom said, the most immediate and “real question for us is, will clinical researchers embrace preprints? And if they do, can we continue to provide a light touch but rapid way to screen papers while ensuring the safety of what we’re posting?”
For his part, Dr. Viny is bracing for “public consumption” of medRxiv content, especially in the oncology community in which patients are often extraordinarily well educated about their disease and determined to learn about all possible treatment options. “My job as a clinician,” he said, “will be to contextualize the patient’s reference information.”
A new preprint server for the medical and health sciences – medRxiv – has launched, along with safeguards designed to mitigate the risk of non–peer-reviewed findings prematurely guiding clinical practice or misinforming the public.
The new repository of preprints is intended for researcher-to-researcher communication – and mainly to facilitate faster sharing of research findings before publication in peer-reviewed journals. Papers will not be scrutinized for study design or the strength of the science, but they will be screened by an external clinical scientist and – at least for now – by an editor funded by BMJ, the London-based publisher and one of the three cofounding organizations of medRxiv (pronounced “med archive”).
The server’s six-person leadership team – comprising leaders from BMJ and cofounders, Cold Spring Harbor Laboratory in New York, and Yale University in New Haven, Conn. – will make final decisions about whether to post papers that generate concerns.
“We’ve put in place more stringent screening procedures than existed for bioRxiv, [a biological preprint server launched in 2013 by Cold Spring Harbor Laboratory],”said Theodora Bloom, PhD, executive editor of BMJ. “We’ll specifically ask the question, is there a risk to public health or health-related behaviors if this preprint is posted and [turns out to be] wrong?”
Concerns that poor information will be disseminated to the public or that the public will misinterpret information published, were heard by the medRxiv founders as they “work-shopped the idea and talked with the community,” said Joseph Ross, MD, an associate professor of medicine and public health at Yale and codirector of the Yale Open Data Access (YODA) Project
“We’re taking a cautious approach, particularly in the early days as we learn from the process,” he said. “How a paper [could potentially influence clinical practice] will be a guiding question.”
The cofounders had several conversations, Dr. Ross said, with Howard Bauchner, MD, editor in chief of JAMA, who took a strong stance against preprints and shortcutting the peer review process in a 2017 editorial titled “The rush to publication: An editorial and scientific mistake.” (Dr. Ross is an associate editor at JAMA Internal Medicine. Dr. Bauchner was unavailable for comment on the safeguards built into medRvix.)
Aaron D. Viny, MD, a hematologist-oncologist at Memorial Sloan Kettering in New York, said he has mixed feelings about preprints and believes the stakes are higher with medRvix, given that it will house clinical content – including, he anticipates, single-institution, nonprospective outcome studies of off-label drug uses. “These aren’t bona fide clinical trials and may not have the best data,” he said.
Still, there are advantages for investigators – and for the progress of research – with earlier dissemination of findings, Dr. Viny said. He recently had a paper posted on bioRvix for the first time. The paper was undergoing revision for a peer-reviewed journal and was being presented at a national meeting at the time it was posted.
“We timed it [as such], so that not only were we presenting it at a national meeting, but it also got more Twitter buzz,” he said. “I thought it was a good body of work, and I was excited to discuss it online with the scientific community.”
Dr. Viny’s decision is common among preprint authors and reflects the values of the preprint server, Dr. Ross said. “When people are reading or hearing about [new findings] at a meeting, they can go to the papers to get more complete information.” And, he said, the investigators themselves can get more feedback than they otherwise would.
In addition to papers that are well on their way to publication in peer-reviewed journals, Dr. Ross anticipates that medRvix will house papers on qualitative studies and observational research that face more arduous publication paths. He said he expects to see research on medical education and hopes to see papers on “quality improvement work, which typically involve small interventions at a single institution, and have important insights but are hard to publish because of generalizability and controls.”
And while there has been a “positive shift” in the past 10 years in the publication of negative results in peer-reviewed literature, medRvix may well capture studies that have negative results “because they have challenges with recruitment or other [elements of study design],” Dr. Ross said. “There is still a lot that can be learned by the scientific community from these negative studies, but they’re very difficult to publish in a peer-reviewed journal.”
Road to preprints
BMJ has a history with preprints. The publisher established a preprint server for biomedical research in the late 1990s, but it never took off and was shut down in the early 2000s. “It just didn’t get the uptake,” said Dr. Bloom. “It’s hard to know exactly why.”
What is clear, she said, is what has changed in the past 20 years: Copious use of the Internet overall, a growing desire to stake out one’s research turf online, requests from funders to have preprints listed on grant applications, and disease outbreaks involving the Zika virus and Ebola that have highlighted the advantages of faster dissemination of research findings.
BMJ had begun discussions with John Inglis, PhD, of Cold Spring Harbor Laboratory about launching a preprint server for the medical sciences (building on the experience of bioRxiv) when they heard Harlan Krumholz, MD, professor of medicine at Yale and head of the YODA project, speak at the 2017 meeting of the International Congress on Peer Review & Scientific Publication. In his keynote address, Dr. Krumholz described Yale’s plans to launch a preprint server.
“We all felt it would be better working together than apart,” Dr. Bloom said.
Getting published
Each preprint on medRxiv will get a permanent DOI link and a disclaimer stating that preprints are not peer reviewed, should not be relied on to guide clinical practice, and should not be reported in the news media as established information.
Authors will be required to meet various standards and requirements common in the clinical and medical sciences, such as including details on ethics approvals, patient consent, funding sources and conflicts of interest, and trial registration numbers. They will have the option of adding a revision(s) of their preprint (each preprint will have a “history”), as well as the option of having their preprint marked as “withdrawn” if they can no longer stand by the findings or conclusions. Preprints will automatically be linked to final published papers.
Journals have wrestled with how to handle preprints. A look at several major peer-reviewed journals shows that they’ll consider articles that have appeared in early form as preprints (including the New England Journal of Medicine, according to media relations manager Jennifer Zeis), but there are caveats. JAMA, for instance, will look at whether submitted manuscripts add “meaningfully new” information above what the preprint disseminated.
Similarly, the American Society of Clinical Oncology (ASCO) will consider how preprints affect the “novelty” of the manuscript’s findings for its ASCO journal readers. Editors of the journal Blood will consider “public comments or coverage about [the] preprint” in its evaluation of the manuscript’s impact. Several of the major journals specify that preprints cannot be updated while manuscripts are under review.
A recent review of bioRxiv preprints shows that approximately two-thirds went on to peer-reviewed publication.
And according to the BMJ’s Dr. Bloom, “there is definitely evidence that preprints [overall] are getting cited [in the scientific literature] before peer-reviewed articles appear.”
The server medRvix began accepting manuscripts on June 6 and will go live on June 25. It will accept only research papers – not commentaries or case reports, Dr. Ross emphasized.
For now, Dr. Bloom said, the most immediate and “real question for us is, will clinical researchers embrace preprints? And if they do, can we continue to provide a light touch but rapid way to screen papers while ensuring the safety of what we’re posting?”
For his part, Dr. Viny is bracing for “public consumption” of medRxiv content, especially in the oncology community in which patients are often extraordinarily well educated about their disease and determined to learn about all possible treatment options. “My job as a clinician,” he said, “will be to contextualize the patient’s reference information.”
Beyond disclosure: Industry relationships face renewed scrutiny in oncology
Just as conflict of interest (COI) disclosure has moved center stage in recent months, so too have certain physician-industry relationships. Some cancer centers and academic medical institutions across the country have reinvigorated or renewed discussions about the participation of leaders on outside corporate boards and about how to best navigate an increasingly complex web of individual and institutional relationships with industry.
Memorial Sloan Kettering Cancer Center (MSK), which was thrust into the spotlight last fall with news coverage of a top leader’s disclosure failures and coverage of other leaders’ financial relationships with start-up companies, has decided that its senior executives may no longer serve on boards of directors of for-profit health- or science-related companies.
MSK officials also decided that its board members may not serve on the boards of MSK-affiliated start-up companies or make any direct investments in them.
Other institutions, such as the Fred Hutchinson Cancer Research Center in Seattle, were also reviewing their conflict of interest policies.
Participation of academic medical center leaders on the boards of public companies is one of the “most important topics” of discussion – along with disclosure – among those who oversee and manage COI through institutional research offices and COI committees, said Raed Dweik, MD, MBA, chair of Cleveland Clinic’s Innovation Management and COI Committee.
Discussions cover “whether [participation on outside boards] should be allowed in the first place, how relationships should be managed if they’re allowed, and whether there should be limits on compensation,” said Dr. Dweik, who also chairs the American Association of Medical Colleges’ Forum on Conflict of Interest in Academe, a group of over 600 representatives from academic health centers, medical schools, teaching hospitals, and other hospitals and centers with substantive research programs.
Institutions have been grappling with these issues for years. But “what was reported [about] MSK has brought these topics into hyperfocus in a way,” he said, along with questions concerning the magnitude of compensation and financial interest more broadly.
Institutional approaches
One of the reports on MSK’s financial ties involved a vice president and expert in technology transfer who was appointed to the board of a biotech company, Y-mAbs, in which MSK had an equity stake; the vice president had stock options that soared when the start-up went public. (He later turned over a nearly $1.4 million windfall profit to the hospital.)
In addition to barring appointments of its leaders to boards of MSK start-ups and any direct investments in them, MSK announced that going forward, “any potential equity that could be attained by employees appointed as MSK designees to outside boards will be returned to the institution and dedicated to research.”
A letter to MSK staff also said that “when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
Broadly speaking, conflict of interest deliberations within institutions center on how financial relationships with industry can potentially compromise the integrity of research (from patient selection to data analysis and the reporting of findings), the safety of research subjects and other patients, and the protection of public trust in physicians and their institutions.
Public trust is important, said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the Association of American Medical Colleges.
“In some cases, institutions may decide,” she said, “that they can control for any potential bias in research, but they can’t control for a perception that a person should not be engaged, or should not be compensated by a company because of appearance.”
Institutions are guided by federal regulations and institutional considerations that deal mainly with “how to evaluate and assess [potential conflicts] and the types of information needed [to do so] – not with specific delineations on what types of relationships are prohibited or not,” said Ms. Pierce. “There are some bright lines, but most things are a lot more complicated.”
Since 2001, the AAMC has recommended the use of a rebuttable presumption framework, in which individuals with a financial interest are prohibited from participating in related research unless “compelling circumstances” justify an exemption. Institutions typically use a “de minimis threshold” to define what financial interests are significant enough for application of the rebuttable presumptions framework.
Cleveland Clinic’s COI Committee considers anything above $20,000 from one company to be significant for the purposes of rebuttable presumption. Compensation between $5,000 and $20,000 (per company per year) triggers a COI management plan that typically focuses on disclosure, but can also include elements such as limits on compensation or the involvement of nonconflicted individuals in data analysis, Dr. Dweik said.
“Anything above $20,000, or equity, we more deeply scrutinize,” he said. “You have to have a really good reason to participate in research related to that company or product. There have to be compelling circumstances.”
Any amount of equity, he emphasized, “is automatically treated as high compensation, because the potential value is high. We are very keenly aware of this in our decisions about what research goes through and what doesn’t.”
Compensation of $5,000 or less is generally considered low at Cleveland Clinic and other institutions (and not in need of COI management), in keeping with U.S. Department of Health and Human Services regulations designed to promote objectivity in research. Thresholds for rebuttable presumption vary from institution to institution, with some being much higher – around $50,000 – than at the Cleveland Clinic, said Dr. Dweik, a pulmonary and critical care medicine specialist who chairs the clinic’s Respiratory Institute.
The big challenge with industry ties involving key executives and leaders – chief medical officers, deans, department chairs, and division chiefs – is that these ties involve institutional COI (not only individual COI) since an individual’s conflicts can be imputed to the institution.
Institutional COI issues generally are much “trickier” for the AAMC’s COI forum to discuss and guide because institutions have different structures and cultures, Dr. Dweik said. Institutional start-ups, moreover, have no standard structure.
“Some institutions, once a company is spun off, will build a firewall between the institution and the company,” he said. “Some institutions will keep the company embedded within the inventor’s lab, or provide infrastructure. And there’s a lot in between.”
Regarding the participation of academic medical center leaders on outside boards, it appears “that institutions are all over the map” in how they regard and evaluate such relationships, Dr. Dweik said. Some institutions address these relationships in their COI policies, while others don’t. And “some are more liberal,” he said. “Others are stricter.”
The magnitude of personal financial interest among MSK leaders (both in institutional start-ups and other companies) is “way outside the norm,” Dr. Dweik said.
Still, board participation can be quite lucrative. One study described in a research letter in JAMA 2014;311[13]:1353-5 5 years ago reported that the mean financial compensation of pharmaceutical company board members who held academic medical center leadership positions was more than $300,000 corporate board participation is “one of the most egregious examples of conflict of interest,” said Vinay Prasad, MD, MPH , a hematologist-oncologist and associate professor of medicine at Oregon Health & Science University, who researches COI and bias. “You have a fiduciary duty to the company’s best interest. … so there will inevitably be a tension between that oath and your other duties,” from responsibilities for institutional oversight to one’s individual research and clinical practices, he said. Physicians at academic medical centers and cancer centers “need to interact with industry,” he said. “But it’s about creating independence.”
A muddied field
S. Vincent Rajkumar, MD, a hematologist-oncologist and active researcher at the Mayo Clinic in Rochester, agrees. “Being on the board of directors of a pharmaceutical company, particularly by institutional leaders, is one of the genuine COIs” amid a field of COI that’s become increasingly muddied with the shift toward more comprehensive, general disclosure practices.
“It’s important that we don’t lose sight of clarity in what types of financial ties and relationships we’re really concerned about,” he said. “The ties that are really concerning – the significant conflicts of interest – are serving on boards of companies or getting large personal payments for participating in speakers’ bureaus or single, company-sponsored CME lectures, for instance, or having stocks, investments, or significant royalties or large payments to your lab or research program [outside of clinical trial funding].”
Other financial transactions such as “reasonable” payments for participation in data-monitoring committees and steering committees are financial ties that should be disclosed, but generally aren’t concerning, Dr. Rajkumar said.
Dr. Rajkumar serves as an editor-in-chief of the Blood Cancer Journal and is an author of several UptoDate chapters. Because of these roles and in keeping with his own views on COI, he has worked deliberately over the past decade to be free of industry payments.
This has become increasingly difficult given the breadth of payments attributed to individual physicians on the federal Open Payments website – some of which are “not truly personal financial ties.” He cited industry payments to support multicompany-sponsored meetings or to institutions for clinical trials.
“It’s nearly impossible to have zero dollars against your name unless you do no clinical trials,” Dr. Rajkumar said.
Still, he said, transparency and disclosure programs like Open Payments are important. “I do think bias arising from COI is very real. There are people with significant conflicts of interest who are writing influential reviews, speaking at influential meetings, and writing influential guidelines – and you can sense the bias pretty quickly,” he said, with endpoints that aren’t appropriate for the trial at hand, for instance, or with overly rosy assessments and the exclusion of negative results.
Collaboration benefits
But Thomas Stossel, MD, American Cancer Society Professor of Medicine Emeritus at Harvard Medical School and founder and chief science advisor at BioAegis Therapeutics, worries that the benefit of physician-industry collaboration is being drowned out with all the attention paid to COI.
His experience on a scientific advisory board in the late 1980s was a “transforming experience that opened my eyes to [the complexities] of product development. … and [later] enabled me to turn my basic research into a potentially life-saving product,” he said.
The “conflict-of-interest narrative” falsely maintains that collaboration causes corruption, he said, and the resultant “hand wringing over [assumed risks]” has slowed innovation by preventing or delaying research and development projects. “It’s like death by a thousand cuts,” said Dr. Stossel, who wrote a book, Pharmacophobia – How the Conflict-of-Interest Myth Undermines American Medical Innovation, to document his concerns.
Dr. Dweik said he has seen the “field move in a good direction” with most academic institutions now deeming physician participation in drug company speakers’ bureaus as “no longer acceptable,” unless physicians discuss drugs in balanced, “disease-specific, not drug-specific” presentations. At this point, it’s unclear exactly how the needle will move on other types of relationships.
“I wouldn’t be surprised if over the next couple of years there is more consistency, more standardization” on issues of participation on outside boards. “The [academic medical center] community is certainly trying to get a better handle on this,” Dr. Dweik said.
Future institutional changes will come, said Ms. Pierce of the AAMC, “but in more subtle ways than we’ve seen in the past, given [progress] already made” through major reports, guidance, and laws and regulations relating to COI. Today, she said, “technology transfer is incrementally more complicated, relationships are more complicated, and corporate structures are more complicated. This makes teasing apart what the issues are a little bit harder.”
Just as conflict of interest (COI) disclosure has moved center stage in recent months, so too have certain physician-industry relationships. Some cancer centers and academic medical institutions across the country have reinvigorated or renewed discussions about the participation of leaders on outside corporate boards and about how to best navigate an increasingly complex web of individual and institutional relationships with industry.
Memorial Sloan Kettering Cancer Center (MSK), which was thrust into the spotlight last fall with news coverage of a top leader’s disclosure failures and coverage of other leaders’ financial relationships with start-up companies, has decided that its senior executives may no longer serve on boards of directors of for-profit health- or science-related companies.
MSK officials also decided that its board members may not serve on the boards of MSK-affiliated start-up companies or make any direct investments in them.
Other institutions, such as the Fred Hutchinson Cancer Research Center in Seattle, were also reviewing their conflict of interest policies.
Participation of academic medical center leaders on the boards of public companies is one of the “most important topics” of discussion – along with disclosure – among those who oversee and manage COI through institutional research offices and COI committees, said Raed Dweik, MD, MBA, chair of Cleveland Clinic’s Innovation Management and COI Committee.
Discussions cover “whether [participation on outside boards] should be allowed in the first place, how relationships should be managed if they’re allowed, and whether there should be limits on compensation,” said Dr. Dweik, who also chairs the American Association of Medical Colleges’ Forum on Conflict of Interest in Academe, a group of over 600 representatives from academic health centers, medical schools, teaching hospitals, and other hospitals and centers with substantive research programs.
Institutions have been grappling with these issues for years. But “what was reported [about] MSK has brought these topics into hyperfocus in a way,” he said, along with questions concerning the magnitude of compensation and financial interest more broadly.
Institutional approaches
One of the reports on MSK’s financial ties involved a vice president and expert in technology transfer who was appointed to the board of a biotech company, Y-mAbs, in which MSK had an equity stake; the vice president had stock options that soared when the start-up went public. (He later turned over a nearly $1.4 million windfall profit to the hospital.)
In addition to barring appointments of its leaders to boards of MSK start-ups and any direct investments in them, MSK announced that going forward, “any potential equity that could be attained by employees appointed as MSK designees to outside boards will be returned to the institution and dedicated to research.”
A letter to MSK staff also said that “when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
Broadly speaking, conflict of interest deliberations within institutions center on how financial relationships with industry can potentially compromise the integrity of research (from patient selection to data analysis and the reporting of findings), the safety of research subjects and other patients, and the protection of public trust in physicians and their institutions.
Public trust is important, said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the Association of American Medical Colleges.
“In some cases, institutions may decide,” she said, “that they can control for any potential bias in research, but they can’t control for a perception that a person should not be engaged, or should not be compensated by a company because of appearance.”
Institutions are guided by federal regulations and institutional considerations that deal mainly with “how to evaluate and assess [potential conflicts] and the types of information needed [to do so] – not with specific delineations on what types of relationships are prohibited or not,” said Ms. Pierce. “There are some bright lines, but most things are a lot more complicated.”
Since 2001, the AAMC has recommended the use of a rebuttable presumption framework, in which individuals with a financial interest are prohibited from participating in related research unless “compelling circumstances” justify an exemption. Institutions typically use a “de minimis threshold” to define what financial interests are significant enough for application of the rebuttable presumptions framework.
Cleveland Clinic’s COI Committee considers anything above $20,000 from one company to be significant for the purposes of rebuttable presumption. Compensation between $5,000 and $20,000 (per company per year) triggers a COI management plan that typically focuses on disclosure, but can also include elements such as limits on compensation or the involvement of nonconflicted individuals in data analysis, Dr. Dweik said.
“Anything above $20,000, or equity, we more deeply scrutinize,” he said. “You have to have a really good reason to participate in research related to that company or product. There have to be compelling circumstances.”
Any amount of equity, he emphasized, “is automatically treated as high compensation, because the potential value is high. We are very keenly aware of this in our decisions about what research goes through and what doesn’t.”
Compensation of $5,000 or less is generally considered low at Cleveland Clinic and other institutions (and not in need of COI management), in keeping with U.S. Department of Health and Human Services regulations designed to promote objectivity in research. Thresholds for rebuttable presumption vary from institution to institution, with some being much higher – around $50,000 – than at the Cleveland Clinic, said Dr. Dweik, a pulmonary and critical care medicine specialist who chairs the clinic’s Respiratory Institute.
The big challenge with industry ties involving key executives and leaders – chief medical officers, deans, department chairs, and division chiefs – is that these ties involve institutional COI (not only individual COI) since an individual’s conflicts can be imputed to the institution.
Institutional COI issues generally are much “trickier” for the AAMC’s COI forum to discuss and guide because institutions have different structures and cultures, Dr. Dweik said. Institutional start-ups, moreover, have no standard structure.
“Some institutions, once a company is spun off, will build a firewall between the institution and the company,” he said. “Some institutions will keep the company embedded within the inventor’s lab, or provide infrastructure. And there’s a lot in between.”
Regarding the participation of academic medical center leaders on outside boards, it appears “that institutions are all over the map” in how they regard and evaluate such relationships, Dr. Dweik said. Some institutions address these relationships in their COI policies, while others don’t. And “some are more liberal,” he said. “Others are stricter.”
The magnitude of personal financial interest among MSK leaders (both in institutional start-ups and other companies) is “way outside the norm,” Dr. Dweik said.
Still, board participation can be quite lucrative. One study described in a research letter in JAMA 2014;311[13]:1353-5 5 years ago reported that the mean financial compensation of pharmaceutical company board members who held academic medical center leadership positions was more than $300,000 corporate board participation is “one of the most egregious examples of conflict of interest,” said Vinay Prasad, MD, MPH , a hematologist-oncologist and associate professor of medicine at Oregon Health & Science University, who researches COI and bias. “You have a fiduciary duty to the company’s best interest. … so there will inevitably be a tension between that oath and your other duties,” from responsibilities for institutional oversight to one’s individual research and clinical practices, he said. Physicians at academic medical centers and cancer centers “need to interact with industry,” he said. “But it’s about creating independence.”
A muddied field
S. Vincent Rajkumar, MD, a hematologist-oncologist and active researcher at the Mayo Clinic in Rochester, agrees. “Being on the board of directors of a pharmaceutical company, particularly by institutional leaders, is one of the genuine COIs” amid a field of COI that’s become increasingly muddied with the shift toward more comprehensive, general disclosure practices.
“It’s important that we don’t lose sight of clarity in what types of financial ties and relationships we’re really concerned about,” he said. “The ties that are really concerning – the significant conflicts of interest – are serving on boards of companies or getting large personal payments for participating in speakers’ bureaus or single, company-sponsored CME lectures, for instance, or having stocks, investments, or significant royalties or large payments to your lab or research program [outside of clinical trial funding].”
Other financial transactions such as “reasonable” payments for participation in data-monitoring committees and steering committees are financial ties that should be disclosed, but generally aren’t concerning, Dr. Rajkumar said.
Dr. Rajkumar serves as an editor-in-chief of the Blood Cancer Journal and is an author of several UptoDate chapters. Because of these roles and in keeping with his own views on COI, he has worked deliberately over the past decade to be free of industry payments.
This has become increasingly difficult given the breadth of payments attributed to individual physicians on the federal Open Payments website – some of which are “not truly personal financial ties.” He cited industry payments to support multicompany-sponsored meetings or to institutions for clinical trials.
“It’s nearly impossible to have zero dollars against your name unless you do no clinical trials,” Dr. Rajkumar said.
Still, he said, transparency and disclosure programs like Open Payments are important. “I do think bias arising from COI is very real. There are people with significant conflicts of interest who are writing influential reviews, speaking at influential meetings, and writing influential guidelines – and you can sense the bias pretty quickly,” he said, with endpoints that aren’t appropriate for the trial at hand, for instance, or with overly rosy assessments and the exclusion of negative results.
Collaboration benefits
But Thomas Stossel, MD, American Cancer Society Professor of Medicine Emeritus at Harvard Medical School and founder and chief science advisor at BioAegis Therapeutics, worries that the benefit of physician-industry collaboration is being drowned out with all the attention paid to COI.
His experience on a scientific advisory board in the late 1980s was a “transforming experience that opened my eyes to [the complexities] of product development. … and [later] enabled me to turn my basic research into a potentially life-saving product,” he said.
The “conflict-of-interest narrative” falsely maintains that collaboration causes corruption, he said, and the resultant “hand wringing over [assumed risks]” has slowed innovation by preventing or delaying research and development projects. “It’s like death by a thousand cuts,” said Dr. Stossel, who wrote a book, Pharmacophobia – How the Conflict-of-Interest Myth Undermines American Medical Innovation, to document his concerns.
Dr. Dweik said he has seen the “field move in a good direction” with most academic institutions now deeming physician participation in drug company speakers’ bureaus as “no longer acceptable,” unless physicians discuss drugs in balanced, “disease-specific, not drug-specific” presentations. At this point, it’s unclear exactly how the needle will move on other types of relationships.
“I wouldn’t be surprised if over the next couple of years there is more consistency, more standardization” on issues of participation on outside boards. “The [academic medical center] community is certainly trying to get a better handle on this,” Dr. Dweik said.
Future institutional changes will come, said Ms. Pierce of the AAMC, “but in more subtle ways than we’ve seen in the past, given [progress] already made” through major reports, guidance, and laws and regulations relating to COI. Today, she said, “technology transfer is incrementally more complicated, relationships are more complicated, and corporate structures are more complicated. This makes teasing apart what the issues are a little bit harder.”
Just as conflict of interest (COI) disclosure has moved center stage in recent months, so too have certain physician-industry relationships. Some cancer centers and academic medical institutions across the country have reinvigorated or renewed discussions about the participation of leaders on outside corporate boards and about how to best navigate an increasingly complex web of individual and institutional relationships with industry.
Memorial Sloan Kettering Cancer Center (MSK), which was thrust into the spotlight last fall with news coverage of a top leader’s disclosure failures and coverage of other leaders’ financial relationships with start-up companies, has decided that its senior executives may no longer serve on boards of directors of for-profit health- or science-related companies.
MSK officials also decided that its board members may not serve on the boards of MSK-affiliated start-up companies or make any direct investments in them.
Other institutions, such as the Fred Hutchinson Cancer Research Center in Seattle, were also reviewing their conflict of interest policies.
Participation of academic medical center leaders on the boards of public companies is one of the “most important topics” of discussion – along with disclosure – among those who oversee and manage COI through institutional research offices and COI committees, said Raed Dweik, MD, MBA, chair of Cleveland Clinic’s Innovation Management and COI Committee.
Discussions cover “whether [participation on outside boards] should be allowed in the first place, how relationships should be managed if they’re allowed, and whether there should be limits on compensation,” said Dr. Dweik, who also chairs the American Association of Medical Colleges’ Forum on Conflict of Interest in Academe, a group of over 600 representatives from academic health centers, medical schools, teaching hospitals, and other hospitals and centers with substantive research programs.
Institutions have been grappling with these issues for years. But “what was reported [about] MSK has brought these topics into hyperfocus in a way,” he said, along with questions concerning the magnitude of compensation and financial interest more broadly.
Institutional approaches
One of the reports on MSK’s financial ties involved a vice president and expert in technology transfer who was appointed to the board of a biotech company, Y-mAbs, in which MSK had an equity stake; the vice president had stock options that soared when the start-up went public. (He later turned over a nearly $1.4 million windfall profit to the hospital.)
In addition to barring appointments of its leaders to boards of MSK start-ups and any direct investments in them, MSK announced that going forward, “any potential equity that could be attained by employees appointed as MSK designees to outside boards will be returned to the institution and dedicated to research.”
A letter to MSK staff also said that “when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
Broadly speaking, conflict of interest deliberations within institutions center on how financial relationships with industry can potentially compromise the integrity of research (from patient selection to data analysis and the reporting of findings), the safety of research subjects and other patients, and the protection of public trust in physicians and their institutions.
Public trust is important, said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the Association of American Medical Colleges.
“In some cases, institutions may decide,” she said, “that they can control for any potential bias in research, but they can’t control for a perception that a person should not be engaged, or should not be compensated by a company because of appearance.”
Institutions are guided by federal regulations and institutional considerations that deal mainly with “how to evaluate and assess [potential conflicts] and the types of information needed [to do so] – not with specific delineations on what types of relationships are prohibited or not,” said Ms. Pierce. “There are some bright lines, but most things are a lot more complicated.”
Since 2001, the AAMC has recommended the use of a rebuttable presumption framework, in which individuals with a financial interest are prohibited from participating in related research unless “compelling circumstances” justify an exemption. Institutions typically use a “de minimis threshold” to define what financial interests are significant enough for application of the rebuttable presumptions framework.
Cleveland Clinic’s COI Committee considers anything above $20,000 from one company to be significant for the purposes of rebuttable presumption. Compensation between $5,000 and $20,000 (per company per year) triggers a COI management plan that typically focuses on disclosure, but can also include elements such as limits on compensation or the involvement of nonconflicted individuals in data analysis, Dr. Dweik said.
“Anything above $20,000, or equity, we more deeply scrutinize,” he said. “You have to have a really good reason to participate in research related to that company or product. There have to be compelling circumstances.”
Any amount of equity, he emphasized, “is automatically treated as high compensation, because the potential value is high. We are very keenly aware of this in our decisions about what research goes through and what doesn’t.”
Compensation of $5,000 or less is generally considered low at Cleveland Clinic and other institutions (and not in need of COI management), in keeping with U.S. Department of Health and Human Services regulations designed to promote objectivity in research. Thresholds for rebuttable presumption vary from institution to institution, with some being much higher – around $50,000 – than at the Cleveland Clinic, said Dr. Dweik, a pulmonary and critical care medicine specialist who chairs the clinic’s Respiratory Institute.
The big challenge with industry ties involving key executives and leaders – chief medical officers, deans, department chairs, and division chiefs – is that these ties involve institutional COI (not only individual COI) since an individual’s conflicts can be imputed to the institution.
Institutional COI issues generally are much “trickier” for the AAMC’s COI forum to discuss and guide because institutions have different structures and cultures, Dr. Dweik said. Institutional start-ups, moreover, have no standard structure.
“Some institutions, once a company is spun off, will build a firewall between the institution and the company,” he said. “Some institutions will keep the company embedded within the inventor’s lab, or provide infrastructure. And there’s a lot in between.”
Regarding the participation of academic medical center leaders on outside boards, it appears “that institutions are all over the map” in how they regard and evaluate such relationships, Dr. Dweik said. Some institutions address these relationships in their COI policies, while others don’t. And “some are more liberal,” he said. “Others are stricter.”
The magnitude of personal financial interest among MSK leaders (both in institutional start-ups and other companies) is “way outside the norm,” Dr. Dweik said.
Still, board participation can be quite lucrative. One study described in a research letter in JAMA 2014;311[13]:1353-5 5 years ago reported that the mean financial compensation of pharmaceutical company board members who held academic medical center leadership positions was more than $300,000 corporate board participation is “one of the most egregious examples of conflict of interest,” said Vinay Prasad, MD, MPH , a hematologist-oncologist and associate professor of medicine at Oregon Health & Science University, who researches COI and bias. “You have a fiduciary duty to the company’s best interest. … so there will inevitably be a tension between that oath and your other duties,” from responsibilities for institutional oversight to one’s individual research and clinical practices, he said. Physicians at academic medical centers and cancer centers “need to interact with industry,” he said. “But it’s about creating independence.”
A muddied field
S. Vincent Rajkumar, MD, a hematologist-oncologist and active researcher at the Mayo Clinic in Rochester, agrees. “Being on the board of directors of a pharmaceutical company, particularly by institutional leaders, is one of the genuine COIs” amid a field of COI that’s become increasingly muddied with the shift toward more comprehensive, general disclosure practices.
“It’s important that we don’t lose sight of clarity in what types of financial ties and relationships we’re really concerned about,” he said. “The ties that are really concerning – the significant conflicts of interest – are serving on boards of companies or getting large personal payments for participating in speakers’ bureaus or single, company-sponsored CME lectures, for instance, or having stocks, investments, or significant royalties or large payments to your lab or research program [outside of clinical trial funding].”
Other financial transactions such as “reasonable” payments for participation in data-monitoring committees and steering committees are financial ties that should be disclosed, but generally aren’t concerning, Dr. Rajkumar said.
Dr. Rajkumar serves as an editor-in-chief of the Blood Cancer Journal and is an author of several UptoDate chapters. Because of these roles and in keeping with his own views on COI, he has worked deliberately over the past decade to be free of industry payments.
This has become increasingly difficult given the breadth of payments attributed to individual physicians on the federal Open Payments website – some of which are “not truly personal financial ties.” He cited industry payments to support multicompany-sponsored meetings or to institutions for clinical trials.
“It’s nearly impossible to have zero dollars against your name unless you do no clinical trials,” Dr. Rajkumar said.
Still, he said, transparency and disclosure programs like Open Payments are important. “I do think bias arising from COI is very real. There are people with significant conflicts of interest who are writing influential reviews, speaking at influential meetings, and writing influential guidelines – and you can sense the bias pretty quickly,” he said, with endpoints that aren’t appropriate for the trial at hand, for instance, or with overly rosy assessments and the exclusion of negative results.
Collaboration benefits
But Thomas Stossel, MD, American Cancer Society Professor of Medicine Emeritus at Harvard Medical School and founder and chief science advisor at BioAegis Therapeutics, worries that the benefit of physician-industry collaboration is being drowned out with all the attention paid to COI.
His experience on a scientific advisory board in the late 1980s was a “transforming experience that opened my eyes to [the complexities] of product development. … and [later] enabled me to turn my basic research into a potentially life-saving product,” he said.
The “conflict-of-interest narrative” falsely maintains that collaboration causes corruption, he said, and the resultant “hand wringing over [assumed risks]” has slowed innovation by preventing or delaying research and development projects. “It’s like death by a thousand cuts,” said Dr. Stossel, who wrote a book, Pharmacophobia – How the Conflict-of-Interest Myth Undermines American Medical Innovation, to document his concerns.
Dr. Dweik said he has seen the “field move in a good direction” with most academic institutions now deeming physician participation in drug company speakers’ bureaus as “no longer acceptable,” unless physicians discuss drugs in balanced, “disease-specific, not drug-specific” presentations. At this point, it’s unclear exactly how the needle will move on other types of relationships.
“I wouldn’t be surprised if over the next couple of years there is more consistency, more standardization” on issues of participation on outside boards. “The [academic medical center] community is certainly trying to get a better handle on this,” Dr. Dweik said.
Future institutional changes will come, said Ms. Pierce of the AAMC, “but in more subtle ways than we’ve seen in the past, given [progress] already made” through major reports, guidance, and laws and regulations relating to COI. Today, she said, “technology transfer is incrementally more complicated, relationships are more complicated, and corporate structures are more complicated. This makes teasing apart what the issues are a little bit harder.”
FDA urges caution with robotic devices in cancer surgery
A new safety communication from the Food and Drug Administration on the use of robotically assisted surgical devices for mastectomy and other cancer-related surgeries in women encourages physician-patient dialogue and suggests that, moving forward, data on specific oncologic outcomes – not only perioperative and short-term outcomes – are key.
The FDA is “warning patients and providers that the use of robotically assisted surgical devices for any cancer-related surgery has not been granted marketing authorization by the agency, and therefore the survival benefits to patients when compared to traditional surgery have not been established,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement.
The safety communication focuses on women and calls attention specifically to robotically-assisted mastectomy and hysterectomy for early cervical cancers. It says there is “limited, preliminary evidence that the use of robotically-assisted surgical devices for treatment or prevention of cancers that primarily (breast) or exclusively (cervical) affect women may be associated with diminished long-term survival.”
The FDA cited a multicenter randomized trial that found that minimally invasive radical hysterectomy in women with cervical cancer (laparoscopic and robotically assisted) was associated with a lower rate of long-term survival compared with open surgery (N Engl J Med. 2018;379:1895-1904).
The communication does not refer to any other specific studies. Regarding current evidence on robotically-assisted mastectomies, the FDA safety communication says simply that safety and effectiveness have not been established and that the agency is “aware of scientific literature and media publications describing surgeons and hospital systems that use robotically-assisted surgical devices for mastectomy.”
Robotically-assisted mastectomy
Walton Taylor, MD, president of the American Society of Breast Surgeons and a surgeon with Texas Health Physicians Group in Dallas, said that the FDA’s concern is valid. “I really hope that robotic surgery turns out to be good [for mastectomy]. It’s awesome technology that can be great for patients,” he said. “But we have to gather real data that shows that long-term and short-term outcomes – from a cancer standpoint – are as good as with the open procedure ... that there aren’t negative unintended consequences.”
Right now, Dr. Taylor said, robotic mastectomy “is not commonplace by any means.”
The technique for robotic nipple-sparing mastectomy (NSM) was first described by Antonio Toesca, MD, of the European Institute of Oncology in Milan (Ann Surg. 2017;266[2]:e28-e30).
In an editorial published recently in Annals of Surgical Oncology, Jesse C. Selber, MD, MPH, of the department of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston, described the technique as a “natural next step in the evolution of minimally invasive breast surgery that has the potential to mitigate the challenges associated with traditional NSM” (Ann Surg Oncol. 2019;26[1]:10-11). Robotic nipple-sparing mastectomy is catching on in Europe” with very promising early results, he wrote.
At least a couple of practices promoted their performance of robotic mastectomy last year. Northwell Health, a large network of hospitals, outpatient facilities, and physicians in New York, announced in March 2018 that Neil Tanna, MD, and Alan Kadison, MD, of the divisions of plastic and reconstructive surgery and surgical oncology, respectively, had performed the first robotic nipple-sparing mastectomy and breast reconstructive surgery in the United States. Their patient carried the BRCA gene and had a preventive mastectomy at Northwell Health’s Long Island Jewish Medical Center.
In October 2018, a surgeon in Tinton Falls, N.J., Stephen Chagares, MD, announced that he had performed the first robotic nipple-sparing mastectomy with reconstruction in a patient with breast cancer at Monmouth Medical Center. His press release described a 3-cm incision “to the side of the breast, tucked neatly behind the armpit.” Both Dr. Chagares and Dr. Tanna had traveled to Milan to train with Dr. Toesca, according to the press releases.
Both of these cases – as well as a decision by Monmouth Medical Center in December 2018 to suspend the surgery pending further review – were mentioned in a letter submitted to the FDA in mid-December by Hooman Noorchashm, MD, PhD, a Philadelphia cardiothoracic surgeon-turned-patient-advocate whose wife Amy Josephine Reed, MD, PhD, died of uterine cancer in May 2017 following a laparoscopic hysterectomy performed with a power morcellator.
In his complaint, Dr. Noorchashm urged the agency to issue a warning about the “potentially dangerous/premature application” of robotic mastectomy for the treatment of breast cancer or BRCA carrier status outside the setting of randomized controlled trials with primary cancer–related outcomes metrics or an investigational device exemption from the FDA. (Receipt of the letter was acknowledged by the Allegation of Regulatory Misconduct Branch of the FDA several days later.)
In an interview, Dr. Noorchashm said he wants to see a regulatory framework that doesn’t allow 510(k) devices (devices requiring a premarket notification to the FDA) to modify an existing standard of care without having been shown to have noninferior primary outcomes. When devices are used in the diagnosis or treatment of cancerous or potentially cancerous tissue, he said, this means primary oncologic outcomes must be shown to be noninferior.
“When you have 510(k) devices able to inject themselves and affect existing standards of care without any sort of clinical trial requirement, you get the standard of care changing without any outcomes data to back it up,” he said. “That’s what happened with the power morcellator. Physicians started using it without any sort of prospective data, level 1 outcomes data, and it dramatically changed the conduct of hysterectomies.”
In its safety communication, the FDA encourages the establishment of patient registries to gather data on robotically-assisted surgical devices for all uses, including the prevention and treatment of cancer. It also says that while the agency’s evaluation of the devices has generally focused on complication rates at 30 days, the FDA “anticipates” that their use in the prevention or treatment of cancer “would be supported by specific clinical outcomes, such as local cancer recurrence, disease-free survival, or overall survival at time periods much longer than 30 days.”
The American Society of Breast Surgeons has a Nipple Sparing Mastectomy Registry that is collecting oncologic outcomes as well as aesthetic outcomes and other metrics on 2,000 patients. “In the last year or two, we’ve seen nipple-sparing mastectomy become much more commonplace,” said Dr. Taylor. Thus far, the registry does not include robotic procedures, but “if there were interest in a registry specifically for robotic nipple-sparing mastectomy, we would do it in a heartbeat.”
Gynecologic oncology surgery
The randomized controlled study on radical hysterectomy for cervical cancer that caught the FDA’s attention reported lower rates of disease-free survival at 4.5 years with minimally invasive surgery than with open abdominal surgery (86% versus 96.5%) and lower rates of overall survival at 3 years.
The phase 3 multicenter Laparoscopic Approach to Cervical Cancer trial recruited more than 600 women with stage IA1, IA2, or IB1 cervical cancer. Most (91.9%) had IB1 disease and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Differences in the outcomes remained after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. The findings led to early termination of the study.
The study did not single out robotically-assisted surgery. It was a two-arm study and was “not powered to analyze laparoscopy versus robotics,” lead author Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, said in an interview. “But based on our numbers, we saw no difference [in outcomes] between the two groups.” Of the patients who underwent minimally invasive surgery, 84.4% underwent laparoscopy and 15.6% underwent robot-assisted surgery.
The study, funded by MD Anderson and Medtronic, has been criticized for potential design and conduct issues. Outside experts pointed out that the study involved extremely small numbers of patients at each of the 33 participating centers, and that cancer recurrences were clustered at 14 of these centers. It’s important to appreciate, Dr. Ramirez said in the interview, that the majority of patients were accrued in these 14 centers.
In its safety communication, the FDA noted that other researchers have reported no statistically significant difference in long-term survival when open and minimally invasive approaches to radical hysterectomy for cervical cancer have been compared.
Asked to comment on the FDA’s safety communication, Dwight D. Im, MD, who leads the National Institute of Robotic Surgery at Mercy in Baltimore, said in an e-mail that “while robotic surgery may advance into new areas, such as mastectomy and cancer prevention, more research must be done and this should be part of any conversation between gyn-surgeons who are experienced in the realm of robotic surgery, and their patients.”
Regarding the treatment of cervical cancer, “I think it is safe to say that most gynecologic oncologists now offer only open laparotomies until we have more data comparing open to minimally invasive (laparoscopic and robotic) approaches,” he said.
The FDA said in a briefing document accompanying the safety communication that it has received a “small number of medical device reports of patient injury when [robotically-assisted surgical devices] are used in cancer-related procedures.”
According to the FDA spokesperson, 5 of 32 medical device reports received between January 2016 and December 2018 describe patients who underwent hysterectomy and experienced metastases afterward. It does not appear that any of the 5 cases were a direct result of a system error or device malfunction, and the complications described in the reports are not unique to robotically-assisted surgical devices, the spokesperson said.
The safety communication “reflects the agency’s commitment to enhancing the oversight of device safety as part of our Medical Device Action Plan, as well as the agency’s ongoing commitment to advancing women’s health.”
Dr. Taylor reported that he has no current financial disclosures. Dr. Ramirez reported to the New England Journal of Medicine that he had no relevant disclosures. Dr. Im reported that he is a speaker for Intuitive Surgical, which manufacturers the da Vinci Surgical System, as well as for Conmed and Ethicon.
A new safety communication from the Food and Drug Administration on the use of robotically assisted surgical devices for mastectomy and other cancer-related surgeries in women encourages physician-patient dialogue and suggests that, moving forward, data on specific oncologic outcomes – not only perioperative and short-term outcomes – are key.
The FDA is “warning patients and providers that the use of robotically assisted surgical devices for any cancer-related surgery has not been granted marketing authorization by the agency, and therefore the survival benefits to patients when compared to traditional surgery have not been established,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement.
The safety communication focuses on women and calls attention specifically to robotically-assisted mastectomy and hysterectomy for early cervical cancers. It says there is “limited, preliminary evidence that the use of robotically-assisted surgical devices for treatment or prevention of cancers that primarily (breast) or exclusively (cervical) affect women may be associated with diminished long-term survival.”
The FDA cited a multicenter randomized trial that found that minimally invasive radical hysterectomy in women with cervical cancer (laparoscopic and robotically assisted) was associated with a lower rate of long-term survival compared with open surgery (N Engl J Med. 2018;379:1895-1904).
The communication does not refer to any other specific studies. Regarding current evidence on robotically-assisted mastectomies, the FDA safety communication says simply that safety and effectiveness have not been established and that the agency is “aware of scientific literature and media publications describing surgeons and hospital systems that use robotically-assisted surgical devices for mastectomy.”
Robotically-assisted mastectomy
Walton Taylor, MD, president of the American Society of Breast Surgeons and a surgeon with Texas Health Physicians Group in Dallas, said that the FDA’s concern is valid. “I really hope that robotic surgery turns out to be good [for mastectomy]. It’s awesome technology that can be great for patients,” he said. “But we have to gather real data that shows that long-term and short-term outcomes – from a cancer standpoint – are as good as with the open procedure ... that there aren’t negative unintended consequences.”
Right now, Dr. Taylor said, robotic mastectomy “is not commonplace by any means.”
The technique for robotic nipple-sparing mastectomy (NSM) was first described by Antonio Toesca, MD, of the European Institute of Oncology in Milan (Ann Surg. 2017;266[2]:e28-e30).
In an editorial published recently in Annals of Surgical Oncology, Jesse C. Selber, MD, MPH, of the department of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston, described the technique as a “natural next step in the evolution of minimally invasive breast surgery that has the potential to mitigate the challenges associated with traditional NSM” (Ann Surg Oncol. 2019;26[1]:10-11). Robotic nipple-sparing mastectomy is catching on in Europe” with very promising early results, he wrote.
At least a couple of practices promoted their performance of robotic mastectomy last year. Northwell Health, a large network of hospitals, outpatient facilities, and physicians in New York, announced in March 2018 that Neil Tanna, MD, and Alan Kadison, MD, of the divisions of plastic and reconstructive surgery and surgical oncology, respectively, had performed the first robotic nipple-sparing mastectomy and breast reconstructive surgery in the United States. Their patient carried the BRCA gene and had a preventive mastectomy at Northwell Health’s Long Island Jewish Medical Center.
In October 2018, a surgeon in Tinton Falls, N.J., Stephen Chagares, MD, announced that he had performed the first robotic nipple-sparing mastectomy with reconstruction in a patient with breast cancer at Monmouth Medical Center. His press release described a 3-cm incision “to the side of the breast, tucked neatly behind the armpit.” Both Dr. Chagares and Dr. Tanna had traveled to Milan to train with Dr. Toesca, according to the press releases.
Both of these cases – as well as a decision by Monmouth Medical Center in December 2018 to suspend the surgery pending further review – were mentioned in a letter submitted to the FDA in mid-December by Hooman Noorchashm, MD, PhD, a Philadelphia cardiothoracic surgeon-turned-patient-advocate whose wife Amy Josephine Reed, MD, PhD, died of uterine cancer in May 2017 following a laparoscopic hysterectomy performed with a power morcellator.
In his complaint, Dr. Noorchashm urged the agency to issue a warning about the “potentially dangerous/premature application” of robotic mastectomy for the treatment of breast cancer or BRCA carrier status outside the setting of randomized controlled trials with primary cancer–related outcomes metrics or an investigational device exemption from the FDA. (Receipt of the letter was acknowledged by the Allegation of Regulatory Misconduct Branch of the FDA several days later.)
In an interview, Dr. Noorchashm said he wants to see a regulatory framework that doesn’t allow 510(k) devices (devices requiring a premarket notification to the FDA) to modify an existing standard of care without having been shown to have noninferior primary outcomes. When devices are used in the diagnosis or treatment of cancerous or potentially cancerous tissue, he said, this means primary oncologic outcomes must be shown to be noninferior.
“When you have 510(k) devices able to inject themselves and affect existing standards of care without any sort of clinical trial requirement, you get the standard of care changing without any outcomes data to back it up,” he said. “That’s what happened with the power morcellator. Physicians started using it without any sort of prospective data, level 1 outcomes data, and it dramatically changed the conduct of hysterectomies.”
In its safety communication, the FDA encourages the establishment of patient registries to gather data on robotically-assisted surgical devices for all uses, including the prevention and treatment of cancer. It also says that while the agency’s evaluation of the devices has generally focused on complication rates at 30 days, the FDA “anticipates” that their use in the prevention or treatment of cancer “would be supported by specific clinical outcomes, such as local cancer recurrence, disease-free survival, or overall survival at time periods much longer than 30 days.”
The American Society of Breast Surgeons has a Nipple Sparing Mastectomy Registry that is collecting oncologic outcomes as well as aesthetic outcomes and other metrics on 2,000 patients. “In the last year or two, we’ve seen nipple-sparing mastectomy become much more commonplace,” said Dr. Taylor. Thus far, the registry does not include robotic procedures, but “if there were interest in a registry specifically for robotic nipple-sparing mastectomy, we would do it in a heartbeat.”
Gynecologic oncology surgery
The randomized controlled study on radical hysterectomy for cervical cancer that caught the FDA’s attention reported lower rates of disease-free survival at 4.5 years with minimally invasive surgery than with open abdominal surgery (86% versus 96.5%) and lower rates of overall survival at 3 years.
The phase 3 multicenter Laparoscopic Approach to Cervical Cancer trial recruited more than 600 women with stage IA1, IA2, or IB1 cervical cancer. Most (91.9%) had IB1 disease and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Differences in the outcomes remained after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. The findings led to early termination of the study.
The study did not single out robotically-assisted surgery. It was a two-arm study and was “not powered to analyze laparoscopy versus robotics,” lead author Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, said in an interview. “But based on our numbers, we saw no difference [in outcomes] between the two groups.” Of the patients who underwent minimally invasive surgery, 84.4% underwent laparoscopy and 15.6% underwent robot-assisted surgery.
The study, funded by MD Anderson and Medtronic, has been criticized for potential design and conduct issues. Outside experts pointed out that the study involved extremely small numbers of patients at each of the 33 participating centers, and that cancer recurrences were clustered at 14 of these centers. It’s important to appreciate, Dr. Ramirez said in the interview, that the majority of patients were accrued in these 14 centers.
In its safety communication, the FDA noted that other researchers have reported no statistically significant difference in long-term survival when open and minimally invasive approaches to radical hysterectomy for cervical cancer have been compared.
Asked to comment on the FDA’s safety communication, Dwight D. Im, MD, who leads the National Institute of Robotic Surgery at Mercy in Baltimore, said in an e-mail that “while robotic surgery may advance into new areas, such as mastectomy and cancer prevention, more research must be done and this should be part of any conversation between gyn-surgeons who are experienced in the realm of robotic surgery, and their patients.”
Regarding the treatment of cervical cancer, “I think it is safe to say that most gynecologic oncologists now offer only open laparotomies until we have more data comparing open to minimally invasive (laparoscopic and robotic) approaches,” he said.
The FDA said in a briefing document accompanying the safety communication that it has received a “small number of medical device reports of patient injury when [robotically-assisted surgical devices] are used in cancer-related procedures.”
According to the FDA spokesperson, 5 of 32 medical device reports received between January 2016 and December 2018 describe patients who underwent hysterectomy and experienced metastases afterward. It does not appear that any of the 5 cases were a direct result of a system error or device malfunction, and the complications described in the reports are not unique to robotically-assisted surgical devices, the spokesperson said.
The safety communication “reflects the agency’s commitment to enhancing the oversight of device safety as part of our Medical Device Action Plan, as well as the agency’s ongoing commitment to advancing women’s health.”
Dr. Taylor reported that he has no current financial disclosures. Dr. Ramirez reported to the New England Journal of Medicine that he had no relevant disclosures. Dr. Im reported that he is a speaker for Intuitive Surgical, which manufacturers the da Vinci Surgical System, as well as for Conmed and Ethicon.
A new safety communication from the Food and Drug Administration on the use of robotically assisted surgical devices for mastectomy and other cancer-related surgeries in women encourages physician-patient dialogue and suggests that, moving forward, data on specific oncologic outcomes – not only perioperative and short-term outcomes – are key.
The FDA is “warning patients and providers that the use of robotically assisted surgical devices for any cancer-related surgery has not been granted marketing authorization by the agency, and therefore the survival benefits to patients when compared to traditional surgery have not been established,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement.
The safety communication focuses on women and calls attention specifically to robotically-assisted mastectomy and hysterectomy for early cervical cancers. It says there is “limited, preliminary evidence that the use of robotically-assisted surgical devices for treatment or prevention of cancers that primarily (breast) or exclusively (cervical) affect women may be associated with diminished long-term survival.”
The FDA cited a multicenter randomized trial that found that minimally invasive radical hysterectomy in women with cervical cancer (laparoscopic and robotically assisted) was associated with a lower rate of long-term survival compared with open surgery (N Engl J Med. 2018;379:1895-1904).
The communication does not refer to any other specific studies. Regarding current evidence on robotically-assisted mastectomies, the FDA safety communication says simply that safety and effectiveness have not been established and that the agency is “aware of scientific literature and media publications describing surgeons and hospital systems that use robotically-assisted surgical devices for mastectomy.”
Robotically-assisted mastectomy
Walton Taylor, MD, president of the American Society of Breast Surgeons and a surgeon with Texas Health Physicians Group in Dallas, said that the FDA’s concern is valid. “I really hope that robotic surgery turns out to be good [for mastectomy]. It’s awesome technology that can be great for patients,” he said. “But we have to gather real data that shows that long-term and short-term outcomes – from a cancer standpoint – are as good as with the open procedure ... that there aren’t negative unintended consequences.”
Right now, Dr. Taylor said, robotic mastectomy “is not commonplace by any means.”
The technique for robotic nipple-sparing mastectomy (NSM) was first described by Antonio Toesca, MD, of the European Institute of Oncology in Milan (Ann Surg. 2017;266[2]:e28-e30).
In an editorial published recently in Annals of Surgical Oncology, Jesse C. Selber, MD, MPH, of the department of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston, described the technique as a “natural next step in the evolution of minimally invasive breast surgery that has the potential to mitigate the challenges associated with traditional NSM” (Ann Surg Oncol. 2019;26[1]:10-11). Robotic nipple-sparing mastectomy is catching on in Europe” with very promising early results, he wrote.
At least a couple of practices promoted their performance of robotic mastectomy last year. Northwell Health, a large network of hospitals, outpatient facilities, and physicians in New York, announced in March 2018 that Neil Tanna, MD, and Alan Kadison, MD, of the divisions of plastic and reconstructive surgery and surgical oncology, respectively, had performed the first robotic nipple-sparing mastectomy and breast reconstructive surgery in the United States. Their patient carried the BRCA gene and had a preventive mastectomy at Northwell Health’s Long Island Jewish Medical Center.
In October 2018, a surgeon in Tinton Falls, N.J., Stephen Chagares, MD, announced that he had performed the first robotic nipple-sparing mastectomy with reconstruction in a patient with breast cancer at Monmouth Medical Center. His press release described a 3-cm incision “to the side of the breast, tucked neatly behind the armpit.” Both Dr. Chagares and Dr. Tanna had traveled to Milan to train with Dr. Toesca, according to the press releases.
Both of these cases – as well as a decision by Monmouth Medical Center in December 2018 to suspend the surgery pending further review – were mentioned in a letter submitted to the FDA in mid-December by Hooman Noorchashm, MD, PhD, a Philadelphia cardiothoracic surgeon-turned-patient-advocate whose wife Amy Josephine Reed, MD, PhD, died of uterine cancer in May 2017 following a laparoscopic hysterectomy performed with a power morcellator.
In his complaint, Dr. Noorchashm urged the agency to issue a warning about the “potentially dangerous/premature application” of robotic mastectomy for the treatment of breast cancer or BRCA carrier status outside the setting of randomized controlled trials with primary cancer–related outcomes metrics or an investigational device exemption from the FDA. (Receipt of the letter was acknowledged by the Allegation of Regulatory Misconduct Branch of the FDA several days later.)
In an interview, Dr. Noorchashm said he wants to see a regulatory framework that doesn’t allow 510(k) devices (devices requiring a premarket notification to the FDA) to modify an existing standard of care without having been shown to have noninferior primary outcomes. When devices are used in the diagnosis or treatment of cancerous or potentially cancerous tissue, he said, this means primary oncologic outcomes must be shown to be noninferior.
“When you have 510(k) devices able to inject themselves and affect existing standards of care without any sort of clinical trial requirement, you get the standard of care changing without any outcomes data to back it up,” he said. “That’s what happened with the power morcellator. Physicians started using it without any sort of prospective data, level 1 outcomes data, and it dramatically changed the conduct of hysterectomies.”
In its safety communication, the FDA encourages the establishment of patient registries to gather data on robotically-assisted surgical devices for all uses, including the prevention and treatment of cancer. It also says that while the agency’s evaluation of the devices has generally focused on complication rates at 30 days, the FDA “anticipates” that their use in the prevention or treatment of cancer “would be supported by specific clinical outcomes, such as local cancer recurrence, disease-free survival, or overall survival at time periods much longer than 30 days.”
The American Society of Breast Surgeons has a Nipple Sparing Mastectomy Registry that is collecting oncologic outcomes as well as aesthetic outcomes and other metrics on 2,000 patients. “In the last year or two, we’ve seen nipple-sparing mastectomy become much more commonplace,” said Dr. Taylor. Thus far, the registry does not include robotic procedures, but “if there were interest in a registry specifically for robotic nipple-sparing mastectomy, we would do it in a heartbeat.”
Gynecologic oncology surgery
The randomized controlled study on radical hysterectomy for cervical cancer that caught the FDA’s attention reported lower rates of disease-free survival at 4.5 years with minimally invasive surgery than with open abdominal surgery (86% versus 96.5%) and lower rates of overall survival at 3 years.
The phase 3 multicenter Laparoscopic Approach to Cervical Cancer trial recruited more than 600 women with stage IA1, IA2, or IB1 cervical cancer. Most (91.9%) had IB1 disease and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Differences in the outcomes remained after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. The findings led to early termination of the study.
The study did not single out robotically-assisted surgery. It was a two-arm study and was “not powered to analyze laparoscopy versus robotics,” lead author Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, said in an interview. “But based on our numbers, we saw no difference [in outcomes] between the two groups.” Of the patients who underwent minimally invasive surgery, 84.4% underwent laparoscopy and 15.6% underwent robot-assisted surgery.
The study, funded by MD Anderson and Medtronic, has been criticized for potential design and conduct issues. Outside experts pointed out that the study involved extremely small numbers of patients at each of the 33 participating centers, and that cancer recurrences were clustered at 14 of these centers. It’s important to appreciate, Dr. Ramirez said in the interview, that the majority of patients were accrued in these 14 centers.
In its safety communication, the FDA noted that other researchers have reported no statistically significant difference in long-term survival when open and minimally invasive approaches to radical hysterectomy for cervical cancer have been compared.
Asked to comment on the FDA’s safety communication, Dwight D. Im, MD, who leads the National Institute of Robotic Surgery at Mercy in Baltimore, said in an e-mail that “while robotic surgery may advance into new areas, such as mastectomy and cancer prevention, more research must be done and this should be part of any conversation between gyn-surgeons who are experienced in the realm of robotic surgery, and their patients.”
Regarding the treatment of cervical cancer, “I think it is safe to say that most gynecologic oncologists now offer only open laparotomies until we have more data comparing open to minimally invasive (laparoscopic and robotic) approaches,” he said.
The FDA said in a briefing document accompanying the safety communication that it has received a “small number of medical device reports of patient injury when [robotically-assisted surgical devices] are used in cancer-related procedures.”
According to the FDA spokesperson, 5 of 32 medical device reports received between January 2016 and December 2018 describe patients who underwent hysterectomy and experienced metastases afterward. It does not appear that any of the 5 cases were a direct result of a system error or device malfunction, and the complications described in the reports are not unique to robotically-assisted surgical devices, the spokesperson said.
The safety communication “reflects the agency’s commitment to enhancing the oversight of device safety as part of our Medical Device Action Plan, as well as the agency’s ongoing commitment to advancing women’s health.”
Dr. Taylor reported that he has no current financial disclosures. Dr. Ramirez reported to the New England Journal of Medicine that he had no relevant disclosures. Dr. Im reported that he is a speaker for Intuitive Surgical, which manufacturers the da Vinci Surgical System, as well as for Conmed and Ethicon.
Medicine grapples with COI reporting
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, spokeswoman for journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
AGA COI and interactions with industry policies
AGA has a set of robust policies to manage potential conflicts of interest and interactions with industry for its leadership, boards of editors, task forces, educational activities and staff. These policies can be found on AGA’s website at www.gastro.org/aga-leadership/committees and http://partner.gastro.org/.
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, spokeswoman for journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
AGA COI and interactions with industry policies
AGA has a set of robust policies to manage potential conflicts of interest and interactions with industry for its leadership, boards of editors, task forces, educational activities and staff. These policies can be found on AGA’s website at www.gastro.org/aga-leadership/committees and http://partner.gastro.org/.
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, spokeswoman for journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
AGA COI and interactions with industry policies
AGA has a set of robust policies to manage potential conflicts of interest and interactions with industry for its leadership, boards of editors, task forces, educational activities and staff. These policies can be found on AGA’s website at www.gastro.org/aga-leadership/committees and http://partner.gastro.org/.
Medicine grapples with COI reporting
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, a spokeswoman forthe journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, a spokeswoman forthe journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, a spokeswoman forthe journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
FDA panel tackles mesh for anterior repair of POP
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Newer cholangioscopy system safe and effective for difficult biliary stones
“D-SOC with either [electrohydraulic lithotripsy or laser lithotripsy] represents an efficient, highly valuable, safe and minimally invasive alternative to percutaneous or surgical approaches in the management of difficult biliary stones,” reported Olaya I. Brewer Gutierrez, MD, of Johns Hopkins Hospital, Baltimore, and her colleagues.
The study, published in Clinical Gastroenterology and Hepatology, involved 22 tertiary centers in the United States, the United Kingdom, and Korea. Patients had difficult biliary stones, defined as stones greater than 15 mm in size, multiple (more than three) stones, intrahepatic duct/cystic duct stones, impacted stones, and stones associated with Mirizzi syndrome or a difficult biliary anatomy such as stricture below the stone or duodenal diverticula.
Most patients (85.7%) had a prior failed treatment with endoscopic retrograde cholangiopancreatography (ERCP) for stone clearance at outside hospitals, with biliary sphincterotomy and the use of an extraction balloon attempted in 62.2% and 73.2% of these cases, respectively.
Of the 407 study patients, 306 (75.2%) were treated with electrohydraulic lithotripsy (EHL), and 101 (24.8%) were treated with laser lithotripsy (LL). Complete ductal clearance was achieved in 396 (97.3%) of patients with a median of one lithotripsy session (range of one to four sessions), and in 77.4% of patients in a single session. Stone clearance rates and procedure outcomes were similar between the two approaches, but the mean procedure time was significantly longer with EHL than with LL (74 vs. 50 minutes).
Eleven (2.7%) of the 407 patients failed EHL/LL and were treated either with surgery, extracorporeal shock-wave lithotripsy, or both. Adverse events were observed in 15 patients (3.7%), with cholangitis and abdominal pain most commonly reported.
In addition to evaluating the technical success of D-SOC for difficult bile duct stones and comparing the effectiveness of EHL and LL, the investigators looked for predictors of outcomes. On multivariate analysis, they found difficult anatomy/cannulation was associated with incomplete ductal clearance, and the duration of the procedure was a predictor of the need for more than one D-SOC.
The introduction of D-SOC several years ago has “improved the ease of SOC and markedly improved the image quality,” Dr. Gutierrez and her colleagues wrote. “These [improvements] have led to greater use of EHL or LL as part of the ERCP armamentarium to attempt stone clearance in difficult biliary or pancreatic stones.”
Still, given the retrospective nature of the study and subsequent selection bias, randomized controlled trials comparing D-SOC and EHL/LL with conventional techniques for stone extraction are needed, they say.
In an accompanying editorial, Dennis Yang, MD, of the University of Florida, and Jonathan M. Buscaglia, MD, AGAF, of State University of New York at Stony Brook commended the study for critically evaluating a new technology, but said that “it still remains unclear in whom D-SOC with EHL or LL should be considered over conventional ERCP with stone extraction techniques.”
Although most patients in the study had a prior failed ERCP, conventional stone extraction techniques such as endoscopic sphincterotomy with endoscopic papillary large balloon dilation (EPLBD) or mechanical lithotripsy were attempted in less than a quarter of the cases before D-SOC, they wrote. “In the era of high-cost health care, it is important that proven and effective measures for the treatment of stones ... be used adequately before moving up the ladder to cholangioscopy-based techniques.”
Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
SOURCE: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
“D-SOC with either [electrohydraulic lithotripsy or laser lithotripsy] represents an efficient, highly valuable, safe and minimally invasive alternative to percutaneous or surgical approaches in the management of difficult biliary stones,” reported Olaya I. Brewer Gutierrez, MD, of Johns Hopkins Hospital, Baltimore, and her colleagues.
The study, published in Clinical Gastroenterology and Hepatology, involved 22 tertiary centers in the United States, the United Kingdom, and Korea. Patients had difficult biliary stones, defined as stones greater than 15 mm in size, multiple (more than three) stones, intrahepatic duct/cystic duct stones, impacted stones, and stones associated with Mirizzi syndrome or a difficult biliary anatomy such as stricture below the stone or duodenal diverticula.
Most patients (85.7%) had a prior failed treatment with endoscopic retrograde cholangiopancreatography (ERCP) for stone clearance at outside hospitals, with biliary sphincterotomy and the use of an extraction balloon attempted in 62.2% and 73.2% of these cases, respectively.
Of the 407 study patients, 306 (75.2%) were treated with electrohydraulic lithotripsy (EHL), and 101 (24.8%) were treated with laser lithotripsy (LL). Complete ductal clearance was achieved in 396 (97.3%) of patients with a median of one lithotripsy session (range of one to four sessions), and in 77.4% of patients in a single session. Stone clearance rates and procedure outcomes were similar between the two approaches, but the mean procedure time was significantly longer with EHL than with LL (74 vs. 50 minutes).
Eleven (2.7%) of the 407 patients failed EHL/LL and were treated either with surgery, extracorporeal shock-wave lithotripsy, or both. Adverse events were observed in 15 patients (3.7%), with cholangitis and abdominal pain most commonly reported.
In addition to evaluating the technical success of D-SOC for difficult bile duct stones and comparing the effectiveness of EHL and LL, the investigators looked for predictors of outcomes. On multivariate analysis, they found difficult anatomy/cannulation was associated with incomplete ductal clearance, and the duration of the procedure was a predictor of the need for more than one D-SOC.
The introduction of D-SOC several years ago has “improved the ease of SOC and markedly improved the image quality,” Dr. Gutierrez and her colleagues wrote. “These [improvements] have led to greater use of EHL or LL as part of the ERCP armamentarium to attempt stone clearance in difficult biliary or pancreatic stones.”
Still, given the retrospective nature of the study and subsequent selection bias, randomized controlled trials comparing D-SOC and EHL/LL with conventional techniques for stone extraction are needed, they say.
In an accompanying editorial, Dennis Yang, MD, of the University of Florida, and Jonathan M. Buscaglia, MD, AGAF, of State University of New York at Stony Brook commended the study for critically evaluating a new technology, but said that “it still remains unclear in whom D-SOC with EHL or LL should be considered over conventional ERCP with stone extraction techniques.”
Although most patients in the study had a prior failed ERCP, conventional stone extraction techniques such as endoscopic sphincterotomy with endoscopic papillary large balloon dilation (EPLBD) or mechanical lithotripsy were attempted in less than a quarter of the cases before D-SOC, they wrote. “In the era of high-cost health care, it is important that proven and effective measures for the treatment of stones ... be used adequately before moving up the ladder to cholangioscopy-based techniques.”
Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
SOURCE: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
“D-SOC with either [electrohydraulic lithotripsy or laser lithotripsy] represents an efficient, highly valuable, safe and minimally invasive alternative to percutaneous or surgical approaches in the management of difficult biliary stones,” reported Olaya I. Brewer Gutierrez, MD, of Johns Hopkins Hospital, Baltimore, and her colleagues.
The study, published in Clinical Gastroenterology and Hepatology, involved 22 tertiary centers in the United States, the United Kingdom, and Korea. Patients had difficult biliary stones, defined as stones greater than 15 mm in size, multiple (more than three) stones, intrahepatic duct/cystic duct stones, impacted stones, and stones associated with Mirizzi syndrome or a difficult biliary anatomy such as stricture below the stone or duodenal diverticula.
Most patients (85.7%) had a prior failed treatment with endoscopic retrograde cholangiopancreatography (ERCP) for stone clearance at outside hospitals, with biliary sphincterotomy and the use of an extraction balloon attempted in 62.2% and 73.2% of these cases, respectively.
Of the 407 study patients, 306 (75.2%) were treated with electrohydraulic lithotripsy (EHL), and 101 (24.8%) were treated with laser lithotripsy (LL). Complete ductal clearance was achieved in 396 (97.3%) of patients with a median of one lithotripsy session (range of one to four sessions), and in 77.4% of patients in a single session. Stone clearance rates and procedure outcomes were similar between the two approaches, but the mean procedure time was significantly longer with EHL than with LL (74 vs. 50 minutes).
Eleven (2.7%) of the 407 patients failed EHL/LL and were treated either with surgery, extracorporeal shock-wave lithotripsy, or both. Adverse events were observed in 15 patients (3.7%), with cholangitis and abdominal pain most commonly reported.
In addition to evaluating the technical success of D-SOC for difficult bile duct stones and comparing the effectiveness of EHL and LL, the investigators looked for predictors of outcomes. On multivariate analysis, they found difficult anatomy/cannulation was associated with incomplete ductal clearance, and the duration of the procedure was a predictor of the need for more than one D-SOC.
The introduction of D-SOC several years ago has “improved the ease of SOC and markedly improved the image quality,” Dr. Gutierrez and her colleagues wrote. “These [improvements] have led to greater use of EHL or LL as part of the ERCP armamentarium to attempt stone clearance in difficult biliary or pancreatic stones.”
Still, given the retrospective nature of the study and subsequent selection bias, randomized controlled trials comparing D-SOC and EHL/LL with conventional techniques for stone extraction are needed, they say.
In an accompanying editorial, Dennis Yang, MD, of the University of Florida, and Jonathan M. Buscaglia, MD, AGAF, of State University of New York at Stony Brook commended the study for critically evaluating a new technology, but said that “it still remains unclear in whom D-SOC with EHL or LL should be considered over conventional ERCP with stone extraction techniques.”
Although most patients in the study had a prior failed ERCP, conventional stone extraction techniques such as endoscopic sphincterotomy with endoscopic papillary large balloon dilation (EPLBD) or mechanical lithotripsy were attempted in less than a quarter of the cases before D-SOC, they wrote. “In the era of high-cost health care, it is important that proven and effective measures for the treatment of stones ... be used adequately before moving up the ladder to cholangioscopy-based techniques.”
Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
SOURCE: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Digital single-operator cholangioscopy (D-SOC) is effective and safe for difficult biliary stones
Major finding: More than 95% of 407 consecutive patients with difficult bile duct stones had complete bile duct clearance with the use of D-SOC with electrohydraulic or laser lithotripsy.
Study details: International, multicenter, retrospective analysis.
Disclosures: Eleven of the study authors reported serving as consultants for Boston Scientific, which manufactures the D-SOC system used at the study sites, and two of the investigators reported serving as speakers for the company. Dr. Gutierrez reported no conflicts of interest.
Source: Brewer Gutierrez OI et al. Clin Gastroenterol Hepatol. 2018;16:918-26.