User login
AAP: Protect from vaccine refusal with documentation
Protecting oneself when parents refuse vaccinations for their children entails documenting all discussions – including a conversation about how parents should respond to illness or fever – as well as creating a system in the office to identify incompletely immunized patients for proper triage and care should the need arise, Dr. James P. Scibilia said at the annual meeting of the American Academy of Pediatrics.
Pediatricians should also document that parents have received a vaccine information sheet at each relevant visit and ensure that parents sign appropriate and unaltered “informed refusal” forms, preferably the AAP’s Refusal to Vaccinate form, each time vaccination is refused, said Dr. Scibilia, who is not an attorney but serves on the AAP Committee on Medical Liability and Risk Management.
“If patients bring their own form, make sure you read it properly. I received one that had an AAP logo and looked like our form but had a different set of information,” he said.
Physicians may be at legal risk if parents claim in the wake of a bad outcome that they weren’t informed of the risk of nonvaccination; courts have favored the plaintiffs in at least a couple of reported cases thus far, he explained.
Documentation of repeated vaccine discussions – with chart notes indicating that “you’ve reemphasized the risks of not getting a vaccine,” for instance – is important. “Have some little phrase to integrate into your records so you can show that each time the patient came in, you readdressed the issue with them,” said Dr. Scibilia, of Heritage Valley Health System, Beaver, Pa.
“Then make sure you tell your parents that their [unvaccinated] child may require more aggressive evaluation when ill,” he said. “And make sure you have some kind of triage in your phone protocol system.”
Not properly triaging or caring for an unimmunized or underimmunized child may be legally risky, he explained. “If you have a 10-month-old child who hasn’t had their pneumococcal vaccine and develops a 104° fever, you’re going to treat that child differently than one who had the vaccine,” he said. “You need to have some way to identify kids who aren’t immunized and triage them properly.”
Less clear is a situation in which a child infected with a vaccine-preventable illness is seen in the office and infects another child while there. “There is no case law in the U.S. on this. It may happen at some point, but there is nothing right now that suggests you’re at special legal risk [in such a case],” said Dr. Scibilia.
Just as vaccine refusal has been a growing problem, so have requests for altered vaccine schedules. In a recent survey of pediatricians and family physicians, 93% of physicians reported that some parents had requested altered vaccine schedules within the prior month. A significant number of physicians agreed to spread out vaccines, either always or often (37%) or sometimes (37%).
In a minority of cases, physicians decided to dismiss families from their practice: 2% said they “always or often” dismissed patients, and 4% said they “sometimes” did (Pediatrics. 2015 Apr;135:666-77).
“As a group, it seems like pediatricians are following the AAP’s guidance in that we’re trying to convince parents [that vaccination is in their child’s best interest], but it seems that we’re willing to alter schedules in order to get kids vaccinated,” he said.
Whether to agree to alternative scheduling requests is a “philosophical question” for the individual physician to answer, with the understanding that “when you alter the established vaccine schedule, you’re putting yourself at some risk if a patient develops a vaccine-preventable illness during the time frame when you’ve altered the schedule,” he said.
If a physician-patient relationship must be severed, the termination should be done properly and formally in order to avoid possible claims of abandonment. This means providing written notification and documenting that the patient received the notification in a timely fashion, usually with a return receipt. The notification must include an offer to provide emergency care for a specified period of time, Dr. Scibilia said.
Protecting oneself when parents refuse vaccinations for their children entails documenting all discussions – including a conversation about how parents should respond to illness or fever – as well as creating a system in the office to identify incompletely immunized patients for proper triage and care should the need arise, Dr. James P. Scibilia said at the annual meeting of the American Academy of Pediatrics.
Pediatricians should also document that parents have received a vaccine information sheet at each relevant visit and ensure that parents sign appropriate and unaltered “informed refusal” forms, preferably the AAP’s Refusal to Vaccinate form, each time vaccination is refused, said Dr. Scibilia, who is not an attorney but serves on the AAP Committee on Medical Liability and Risk Management.
“If patients bring their own form, make sure you read it properly. I received one that had an AAP logo and looked like our form but had a different set of information,” he said.
Physicians may be at legal risk if parents claim in the wake of a bad outcome that they weren’t informed of the risk of nonvaccination; courts have favored the plaintiffs in at least a couple of reported cases thus far, he explained.
Documentation of repeated vaccine discussions – with chart notes indicating that “you’ve reemphasized the risks of not getting a vaccine,” for instance – is important. “Have some little phrase to integrate into your records so you can show that each time the patient came in, you readdressed the issue with them,” said Dr. Scibilia, of Heritage Valley Health System, Beaver, Pa.
“Then make sure you tell your parents that their [unvaccinated] child may require more aggressive evaluation when ill,” he said. “And make sure you have some kind of triage in your phone protocol system.”
Not properly triaging or caring for an unimmunized or underimmunized child may be legally risky, he explained. “If you have a 10-month-old child who hasn’t had their pneumococcal vaccine and develops a 104° fever, you’re going to treat that child differently than one who had the vaccine,” he said. “You need to have some way to identify kids who aren’t immunized and triage them properly.”
Less clear is a situation in which a child infected with a vaccine-preventable illness is seen in the office and infects another child while there. “There is no case law in the U.S. on this. It may happen at some point, but there is nothing right now that suggests you’re at special legal risk [in such a case],” said Dr. Scibilia.
Just as vaccine refusal has been a growing problem, so have requests for altered vaccine schedules. In a recent survey of pediatricians and family physicians, 93% of physicians reported that some parents had requested altered vaccine schedules within the prior month. A significant number of physicians agreed to spread out vaccines, either always or often (37%) or sometimes (37%).
In a minority of cases, physicians decided to dismiss families from their practice: 2% said they “always or often” dismissed patients, and 4% said they “sometimes” did (Pediatrics. 2015 Apr;135:666-77).
“As a group, it seems like pediatricians are following the AAP’s guidance in that we’re trying to convince parents [that vaccination is in their child’s best interest], but it seems that we’re willing to alter schedules in order to get kids vaccinated,” he said.
Whether to agree to alternative scheduling requests is a “philosophical question” for the individual physician to answer, with the understanding that “when you alter the established vaccine schedule, you’re putting yourself at some risk if a patient develops a vaccine-preventable illness during the time frame when you’ve altered the schedule,” he said.
If a physician-patient relationship must be severed, the termination should be done properly and formally in order to avoid possible claims of abandonment. This means providing written notification and documenting that the patient received the notification in a timely fashion, usually with a return receipt. The notification must include an offer to provide emergency care for a specified period of time, Dr. Scibilia said.
Protecting oneself when parents refuse vaccinations for their children entails documenting all discussions – including a conversation about how parents should respond to illness or fever – as well as creating a system in the office to identify incompletely immunized patients for proper triage and care should the need arise, Dr. James P. Scibilia said at the annual meeting of the American Academy of Pediatrics.
Pediatricians should also document that parents have received a vaccine information sheet at each relevant visit and ensure that parents sign appropriate and unaltered “informed refusal” forms, preferably the AAP’s Refusal to Vaccinate form, each time vaccination is refused, said Dr. Scibilia, who is not an attorney but serves on the AAP Committee on Medical Liability and Risk Management.
“If patients bring their own form, make sure you read it properly. I received one that had an AAP logo and looked like our form but had a different set of information,” he said.
Physicians may be at legal risk if parents claim in the wake of a bad outcome that they weren’t informed of the risk of nonvaccination; courts have favored the plaintiffs in at least a couple of reported cases thus far, he explained.
Documentation of repeated vaccine discussions – with chart notes indicating that “you’ve reemphasized the risks of not getting a vaccine,” for instance – is important. “Have some little phrase to integrate into your records so you can show that each time the patient came in, you readdressed the issue with them,” said Dr. Scibilia, of Heritage Valley Health System, Beaver, Pa.
“Then make sure you tell your parents that their [unvaccinated] child may require more aggressive evaluation when ill,” he said. “And make sure you have some kind of triage in your phone protocol system.”
Not properly triaging or caring for an unimmunized or underimmunized child may be legally risky, he explained. “If you have a 10-month-old child who hasn’t had their pneumococcal vaccine and develops a 104° fever, you’re going to treat that child differently than one who had the vaccine,” he said. “You need to have some way to identify kids who aren’t immunized and triage them properly.”
Less clear is a situation in which a child infected with a vaccine-preventable illness is seen in the office and infects another child while there. “There is no case law in the U.S. on this. It may happen at some point, but there is nothing right now that suggests you’re at special legal risk [in such a case],” said Dr. Scibilia.
Just as vaccine refusal has been a growing problem, so have requests for altered vaccine schedules. In a recent survey of pediatricians and family physicians, 93% of physicians reported that some parents had requested altered vaccine schedules within the prior month. A significant number of physicians agreed to spread out vaccines, either always or often (37%) or sometimes (37%).
In a minority of cases, physicians decided to dismiss families from their practice: 2% said they “always or often” dismissed patients, and 4% said they “sometimes” did (Pediatrics. 2015 Apr;135:666-77).
“As a group, it seems like pediatricians are following the AAP’s guidance in that we’re trying to convince parents [that vaccination is in their child’s best interest], but it seems that we’re willing to alter schedules in order to get kids vaccinated,” he said.
Whether to agree to alternative scheduling requests is a “philosophical question” for the individual physician to answer, with the understanding that “when you alter the established vaccine schedule, you’re putting yourself at some risk if a patient develops a vaccine-preventable illness during the time frame when you’ve altered the schedule,” he said.
If a physician-patient relationship must be severed, the termination should be done properly and formally in order to avoid possible claims of abandonment. This means providing written notification and documenting that the patient received the notification in a timely fashion, usually with a return receipt. The notification must include an offer to provide emergency care for a specified period of time, Dr. Scibilia said.
AT THE AAP NATIONAL CONFERENCE
Complicated concussions drive new clinics, management approaches
The Fairfax Family Practice Comprehensive Concussion Center is an example of what dedicated physicians can accomplish when they undertake to improve upon an accepted medical approach to a health problem because it falls far short of effective. The concussion center’s hall wall is adorned with a broad and colorful sign autographed by scores of patients – many of them student-athletes – who have been cleared to return to their sports and activities and to lives free of restrictions and struggle following concussion.
Dr. Garry W.K. Ho, the double-credentialed family practice and sports medicine physician who directs the 2-year-old concussion center in Fairfax, Va., takes pride in the skills his team has acquired to facilitate these recoveries.
He and his colleagues within the practice’s sports medicine division established the center, teaming up with several certified athletic trainers, to meet what they saw as a growing, urgent need: to help concussed patients who were taking longer than the oft-mentioned 7-10 days or 2 weeks to heal.
“While many of the people we’d been treating in the clinic with typical 15- to 30-minute appointments and relatively simple follow-up were able to get better, there was clearly a subset of the population that was more complicated and needed a more dedicated, multidimensional approach,” Dr. Ho said.
Findings from a growing number of studies have shown that concussions can involve a vast array of deficits – problems with the vestibular and vision systems, for example – and that a more comprehensive initial evaluation and a multipronged approach to management could shorten recovery time for any individual with the brain injury, he said.
“We’d started to realize how much patients can benefit from new and creative approaches,” said Dr. Ho. “We were also appreciating that no one person can own this disorder. You can’t say that only the neurologist can treat a concussion, or only the family physician or sports medicine physician.
‘Silent epidemic’
A small but growing number of concussion-care programs and clinics are being established across the country. Most often, the programs are incorporated into hospitals and sports medicine clinics. But some, like the program Dr. Ho leads, are embedded within larger primary care practices with a sports medicine component.
And, in other traditional family practices, some physicians are deepening their knowledge of concussions and building the networks necessary for managing prolonged impairment.
“If we have a good understanding of brain injury, we can often facilitate recovery and help [our patients] get back on track seamlessly,” said Dr. Rebecca Jaffe, a family physician in Wilmington, Del. “And when recovery is protracted, when issues don’t resolve in 2-3 weeks, there’s often more to the situation than is initially apparent, and it’s often better that we have a team of individuals who can help us.”
According to the Institute of Medicine (IOM), there are not enough data for an accurate estimate of the incidence of sports-related concussions in youth (Institute of Medicine and National Research Council. 2014. Sports-related concussions in youth: Improving the science, changing the culture. Washington, D.C.: The National Academies Press.)
But among high school athletes, an increase in concussions has been documented in several emergency department studies and other longitudinal studies published over the past 7-8 years. Most recently, the first national study to look at trends among high school athletes showed the numbers of concussions reported by certified athletic trainers increasing from 0.23/1,000 athlete-exposures in the 2005-2006 academic year to 0.51/1,000 in the 2011-2012 academic year (Am. J. Sports Med. 2014;42:1710-5).
Experts attribute such increases largely to improved awareness and new state laws. The Centers for Disease Control and Prevention’s launched its “Heads Up” educational program in 2004, for instance. And as of last year, every state and the District of Columbia had enacted at least one law to protect young athletes with concussions.
State concussion laws vary, but most require that parents, athletes, and coaches be educated about concussions, and that athletes with suspected concussions be evaluated and cleared by a health care professional with knowledge in concussions before returning to play.
It is likely, some experts say, that the upward curve is leveling off with improved awareness and diagnosis. But with such improvements there lurks a void of data on concussions in youth outside the realm of high school sports. And, according to the 2014 IOM report, the acute and long-term health threat from concussions is “not fully appreciated or acted upon” in many settings. “Kids are getting removed from play more frequently, but then it’s not really clear what anyone should do after that,” said Dr. Shireen Atabaki, an emergency medicine specialist at Children’s National Medical Center in Washington, who is leading several national initiatives to improve concussion care.
Too often, concussed children and teens receive little besides unnecessary CT scans in emergency departments and concussions go undiagnosed. And when concussions are diagnosed, discharge instructions are poor and “kids are sent out into a no-man’s land” where recovery is often poorly managed, she said.
When Dr. Ho and his colleagues opened their concussion center in March 2013, they began seeing patients who remained symptomatic after concussions that had occurred 6-12 months ago, and longer. “It was almost like a silent epidemic,” he said. “We had one young woman who’d been injured in her senior year of high school and was still symptomatic after 2 years.”
To build the program at Fairfax Family Practice, Dr. Ho and his colleagues, Dr. Thomas Howard and Dr. Marc Childress (who, like Dr. Ho, are board certified in sports medicine as well as family medicine), capitalized on what they saw as a valuable synergy with high school athletic trainers.
Dr. Ho had worked closely with athletic trainers as a volunteer medical adviser to the Fairfax County Public School System’s athletic training program, which provides care to student-athletes in 25 high schools. He drew on his relationships to recruit three athletic trainers who were at transition points in their careers and who had both experience and “passion” in the treatment of concussions.
One of them, Jon Almquist, VATL, ATC, had led the development of return-to-learn protocols and other management practices for the high schools. He had also partnered with investigators at MedStar Health Research Institute in Baltimore on research that documented a 4.2-fold increase in concussions among the school system’s high school athletes between 1997 and 2008 (Am. J. Sports Med. 2011;39:958-63).
More surprising than this increase, however, were some data collected after the study period ended. When Mr. Almquist looked at recovery times for the concussions reported between 2011 and 2013, he found that about 25% of concussions took 30 days or longer to recover – a portion greater than he’d seen anywhere in the literature.
According to the 2014 IOM report, youth athletes typically recover from a concussion within 2 weeks of the injury, but in 10-20% of cases the symptoms persist for a number of weeks, months, or even years.
“Families would come to us for advice,” Mr. Almquist recalled. Other than referring them to the University of Pittsburgh Medical Center’s (UPMC) Sports Medicine Concussion Program, there were few local providers whom they believed offered comprehensive care and had experience with prolonged impairment.
Mr. Almquist and his fellow athletic trainers, and Dr. Ho and his physician colleagues, all had been following developments at UPMC’s Sports Medicine Concussion Program since it was established in 2000. The program had evolved to assess and manage diverse facets of concussions, including vision, vestibular, exertion, and medication components—not all of which are fully addressed in clinical practice guidelines and position statements. By 2010, the program was drawing 10,000 patients a year.
Care at UPMC is guided by a clinical neuropsychologist who assesses head injuries and then refers patients on as needed to other members of the team – like a neurovestibular expert for vestibular assessment and therapy, a neuro-optometrist for vision therapy, a physical therapist for exertion training, or a sports medicine physician for medication.
In planning their own concussion center, Dr. Ho and his team visited various programs in the country but homed in on UPMC. They decided to mimic several aspects of UPMC’s approach by providing comprehensive and individualized care, and by having the certified athletic trainers serve as the “quarterbacks.”
“The athletic trainer in our center does all the symptom inventories and history taking, a concussion-focused physical exam, vestibular-ocular-motor screening, and neurocognitive tests if appropriate,” said Dr. Ho.
Dr. Ho or one of his sports medicine physician colleagues then discusses results with the athletic trainer and completes the patient visit – with the trainer – by asking any necessary follow-up questions, probing further with additional evaluation, and writing medication prescriptions when appropriate.
Throughout the course of care – visits occur weekly to monthly and often last 30-90 minutes – the athletic trainer and physician work together on clinical issues such as sleep and mental health and on subtyping headaches (cognitive fatigue, cervicogenic, or migrainelike) and “determining where the headaches lie relative to other areas of dysfunction,” said Dr. Ho.
Much of the individualized counseling, monitoring, and coordination that’s required to address sleep hygiene, rest, nutrition, and graded return to physical and cognitive activity can be led by the athletic trainer, he said.
For the first year or so, patients were referred out for such special services as vision or vestibular therapy. But there were problems with this approach: One frequent issue was poor insurance coverage for these therapies. Another was the stress involved in getting to additional appointments on Fairfax’s congested roads. “Traffic and busy environments aren’t always tolerable for a concussed patient,” Dr. Ho said.
Dr. Ho searched the Internet, worked the phone, read the neuro-optometry and vestibular therapy literature, and attended conferences to learn what kinds of therapeutic exercises they could integrate into office visits and take-home care plans. Dr. Ho said he was “surprised at how willing some of [their] colleagues were to give us some basic skills” to help patients who “don’t all need the Cadillac version” of therapy.
About the same time they integrated basic vision and vestibular therapy, they also revamped their billing strategy, moving away from the use of prolonged service billing codes, which Dr. Ho said were frequently denied on the first submission, to using separate codes for the encounter, therapeutic exercise (which encompasses vision therapy and vestibular therapy), and various physical tests.
Concussion care networks
Fairfax Family Practice is not the only practice to center its concussion program on physician-athletic trainer partnerships. Carolina Family Practice and Sports Medicine, a 12-provider practice with three offices in and around Raleigh, N.C., took a similar approach when it established its concussion clinic in 2008.
“Concussion is the ultimate example of an injury that benefits from a team approach,” said Dr. Josh Bloom, medical director of the Carolina Sports Concussion Clinic that is embedded into the practice. “And it’s the one injury – the only injury in sports medicine – where the standard of care calls for the injury to be 100% resolved prior to being cleared to return to play.”
Dr. Jaffe, the family physician in Delaware, said she is fortunate to have a neuro-optometrist practicing in the medical building where her three-physician family practice resides, and a concussion care team in the nearby Nemours/Alfred I. Dupont Hospital for Children, also in Wilmington.
Someday, she and others hope, physicians who see patients soon after injury will have tools to better predict clinical trajectories and identify who could benefit from earlier referral to a “more sophisticated team.”
For now, Dr. Jaffe said, she seeks assistance when headache and initial symptoms persist after 2-3 weeks or when their symptoms “seem out of proportion to the incident.”
Dr. Scott Ross of Novant Health Bull Run Family Medicine based in Manassas, Va., said that family physicians are well suited to guide management and should learn to evaluate each patient’s concussion-related impairments as thoroughly as possible.
About 3 years ago, he and a partner established an official “Sports Medicine and Concussion Management” service line in their practice. “We don’t have a concussion center all under one roof, but we have the knowledge, skills, and resources that we need,” he said.
This article was updated April 13, 2015.
The Fairfax Family Practice Comprehensive Concussion Center is an example of what dedicated physicians can accomplish when they undertake to improve upon an accepted medical approach to a health problem because it falls far short of effective. The concussion center’s hall wall is adorned with a broad and colorful sign autographed by scores of patients – many of them student-athletes – who have been cleared to return to their sports and activities and to lives free of restrictions and struggle following concussion.
Dr. Garry W.K. Ho, the double-credentialed family practice and sports medicine physician who directs the 2-year-old concussion center in Fairfax, Va., takes pride in the skills his team has acquired to facilitate these recoveries.
He and his colleagues within the practice’s sports medicine division established the center, teaming up with several certified athletic trainers, to meet what they saw as a growing, urgent need: to help concussed patients who were taking longer than the oft-mentioned 7-10 days or 2 weeks to heal.
“While many of the people we’d been treating in the clinic with typical 15- to 30-minute appointments and relatively simple follow-up were able to get better, there was clearly a subset of the population that was more complicated and needed a more dedicated, multidimensional approach,” Dr. Ho said.
Findings from a growing number of studies have shown that concussions can involve a vast array of deficits – problems with the vestibular and vision systems, for example – and that a more comprehensive initial evaluation and a multipronged approach to management could shorten recovery time for any individual with the brain injury, he said.
“We’d started to realize how much patients can benefit from new and creative approaches,” said Dr. Ho. “We were also appreciating that no one person can own this disorder. You can’t say that only the neurologist can treat a concussion, or only the family physician or sports medicine physician.
‘Silent epidemic’
A small but growing number of concussion-care programs and clinics are being established across the country. Most often, the programs are incorporated into hospitals and sports medicine clinics. But some, like the program Dr. Ho leads, are embedded within larger primary care practices with a sports medicine component.
And, in other traditional family practices, some physicians are deepening their knowledge of concussions and building the networks necessary for managing prolonged impairment.
“If we have a good understanding of brain injury, we can often facilitate recovery and help [our patients] get back on track seamlessly,” said Dr. Rebecca Jaffe, a family physician in Wilmington, Del. “And when recovery is protracted, when issues don’t resolve in 2-3 weeks, there’s often more to the situation than is initially apparent, and it’s often better that we have a team of individuals who can help us.”
According to the Institute of Medicine (IOM), there are not enough data for an accurate estimate of the incidence of sports-related concussions in youth (Institute of Medicine and National Research Council. 2014. Sports-related concussions in youth: Improving the science, changing the culture. Washington, D.C.: The National Academies Press.)
But among high school athletes, an increase in concussions has been documented in several emergency department studies and other longitudinal studies published over the past 7-8 years. Most recently, the first national study to look at trends among high school athletes showed the numbers of concussions reported by certified athletic trainers increasing from 0.23/1,000 athlete-exposures in the 2005-2006 academic year to 0.51/1,000 in the 2011-2012 academic year (Am. J. Sports Med. 2014;42:1710-5).
Experts attribute such increases largely to improved awareness and new state laws. The Centers for Disease Control and Prevention’s launched its “Heads Up” educational program in 2004, for instance. And as of last year, every state and the District of Columbia had enacted at least one law to protect young athletes with concussions.
State concussion laws vary, but most require that parents, athletes, and coaches be educated about concussions, and that athletes with suspected concussions be evaluated and cleared by a health care professional with knowledge in concussions before returning to play.
It is likely, some experts say, that the upward curve is leveling off with improved awareness and diagnosis. But with such improvements there lurks a void of data on concussions in youth outside the realm of high school sports. And, according to the 2014 IOM report, the acute and long-term health threat from concussions is “not fully appreciated or acted upon” in many settings. “Kids are getting removed from play more frequently, but then it’s not really clear what anyone should do after that,” said Dr. Shireen Atabaki, an emergency medicine specialist at Children’s National Medical Center in Washington, who is leading several national initiatives to improve concussion care.
Too often, concussed children and teens receive little besides unnecessary CT scans in emergency departments and concussions go undiagnosed. And when concussions are diagnosed, discharge instructions are poor and “kids are sent out into a no-man’s land” where recovery is often poorly managed, she said.
When Dr. Ho and his colleagues opened their concussion center in March 2013, they began seeing patients who remained symptomatic after concussions that had occurred 6-12 months ago, and longer. “It was almost like a silent epidemic,” he said. “We had one young woman who’d been injured in her senior year of high school and was still symptomatic after 2 years.”
To build the program at Fairfax Family Practice, Dr. Ho and his colleagues, Dr. Thomas Howard and Dr. Marc Childress (who, like Dr. Ho, are board certified in sports medicine as well as family medicine), capitalized on what they saw as a valuable synergy with high school athletic trainers.
Dr. Ho had worked closely with athletic trainers as a volunteer medical adviser to the Fairfax County Public School System’s athletic training program, which provides care to student-athletes in 25 high schools. He drew on his relationships to recruit three athletic trainers who were at transition points in their careers and who had both experience and “passion” in the treatment of concussions.
One of them, Jon Almquist, VATL, ATC, had led the development of return-to-learn protocols and other management practices for the high schools. He had also partnered with investigators at MedStar Health Research Institute in Baltimore on research that documented a 4.2-fold increase in concussions among the school system’s high school athletes between 1997 and 2008 (Am. J. Sports Med. 2011;39:958-63).
More surprising than this increase, however, were some data collected after the study period ended. When Mr. Almquist looked at recovery times for the concussions reported between 2011 and 2013, he found that about 25% of concussions took 30 days or longer to recover – a portion greater than he’d seen anywhere in the literature.
According to the 2014 IOM report, youth athletes typically recover from a concussion within 2 weeks of the injury, but in 10-20% of cases the symptoms persist for a number of weeks, months, or even years.
“Families would come to us for advice,” Mr. Almquist recalled. Other than referring them to the University of Pittsburgh Medical Center’s (UPMC) Sports Medicine Concussion Program, there were few local providers whom they believed offered comprehensive care and had experience with prolonged impairment.
Mr. Almquist and his fellow athletic trainers, and Dr. Ho and his physician colleagues, all had been following developments at UPMC’s Sports Medicine Concussion Program since it was established in 2000. The program had evolved to assess and manage diverse facets of concussions, including vision, vestibular, exertion, and medication components—not all of which are fully addressed in clinical practice guidelines and position statements. By 2010, the program was drawing 10,000 patients a year.
Care at UPMC is guided by a clinical neuropsychologist who assesses head injuries and then refers patients on as needed to other members of the team – like a neurovestibular expert for vestibular assessment and therapy, a neuro-optometrist for vision therapy, a physical therapist for exertion training, or a sports medicine physician for medication.
In planning their own concussion center, Dr. Ho and his team visited various programs in the country but homed in on UPMC. They decided to mimic several aspects of UPMC’s approach by providing comprehensive and individualized care, and by having the certified athletic trainers serve as the “quarterbacks.”
“The athletic trainer in our center does all the symptom inventories and history taking, a concussion-focused physical exam, vestibular-ocular-motor screening, and neurocognitive tests if appropriate,” said Dr. Ho.
Dr. Ho or one of his sports medicine physician colleagues then discusses results with the athletic trainer and completes the patient visit – with the trainer – by asking any necessary follow-up questions, probing further with additional evaluation, and writing medication prescriptions when appropriate.
Throughout the course of care – visits occur weekly to monthly and often last 30-90 minutes – the athletic trainer and physician work together on clinical issues such as sleep and mental health and on subtyping headaches (cognitive fatigue, cervicogenic, or migrainelike) and “determining where the headaches lie relative to other areas of dysfunction,” said Dr. Ho.
Much of the individualized counseling, monitoring, and coordination that’s required to address sleep hygiene, rest, nutrition, and graded return to physical and cognitive activity can be led by the athletic trainer, he said.
For the first year or so, patients were referred out for such special services as vision or vestibular therapy. But there were problems with this approach: One frequent issue was poor insurance coverage for these therapies. Another was the stress involved in getting to additional appointments on Fairfax’s congested roads. “Traffic and busy environments aren’t always tolerable for a concussed patient,” Dr. Ho said.
Dr. Ho searched the Internet, worked the phone, read the neuro-optometry and vestibular therapy literature, and attended conferences to learn what kinds of therapeutic exercises they could integrate into office visits and take-home care plans. Dr. Ho said he was “surprised at how willing some of [their] colleagues were to give us some basic skills” to help patients who “don’t all need the Cadillac version” of therapy.
About the same time they integrated basic vision and vestibular therapy, they also revamped their billing strategy, moving away from the use of prolonged service billing codes, which Dr. Ho said were frequently denied on the first submission, to using separate codes for the encounter, therapeutic exercise (which encompasses vision therapy and vestibular therapy), and various physical tests.
Concussion care networks
Fairfax Family Practice is not the only practice to center its concussion program on physician-athletic trainer partnerships. Carolina Family Practice and Sports Medicine, a 12-provider practice with three offices in and around Raleigh, N.C., took a similar approach when it established its concussion clinic in 2008.
“Concussion is the ultimate example of an injury that benefits from a team approach,” said Dr. Josh Bloom, medical director of the Carolina Sports Concussion Clinic that is embedded into the practice. “And it’s the one injury – the only injury in sports medicine – where the standard of care calls for the injury to be 100% resolved prior to being cleared to return to play.”
Dr. Jaffe, the family physician in Delaware, said she is fortunate to have a neuro-optometrist practicing in the medical building where her three-physician family practice resides, and a concussion care team in the nearby Nemours/Alfred I. Dupont Hospital for Children, also in Wilmington.
Someday, she and others hope, physicians who see patients soon after injury will have tools to better predict clinical trajectories and identify who could benefit from earlier referral to a “more sophisticated team.”
For now, Dr. Jaffe said, she seeks assistance when headache and initial symptoms persist after 2-3 weeks or when their symptoms “seem out of proportion to the incident.”
Dr. Scott Ross of Novant Health Bull Run Family Medicine based in Manassas, Va., said that family physicians are well suited to guide management and should learn to evaluate each patient’s concussion-related impairments as thoroughly as possible.
About 3 years ago, he and a partner established an official “Sports Medicine and Concussion Management” service line in their practice. “We don’t have a concussion center all under one roof, but we have the knowledge, skills, and resources that we need,” he said.
This article was updated April 13, 2015.
The Fairfax Family Practice Comprehensive Concussion Center is an example of what dedicated physicians can accomplish when they undertake to improve upon an accepted medical approach to a health problem because it falls far short of effective. The concussion center’s hall wall is adorned with a broad and colorful sign autographed by scores of patients – many of them student-athletes – who have been cleared to return to their sports and activities and to lives free of restrictions and struggle following concussion.
Dr. Garry W.K. Ho, the double-credentialed family practice and sports medicine physician who directs the 2-year-old concussion center in Fairfax, Va., takes pride in the skills his team has acquired to facilitate these recoveries.
He and his colleagues within the practice’s sports medicine division established the center, teaming up with several certified athletic trainers, to meet what they saw as a growing, urgent need: to help concussed patients who were taking longer than the oft-mentioned 7-10 days or 2 weeks to heal.
“While many of the people we’d been treating in the clinic with typical 15- to 30-minute appointments and relatively simple follow-up were able to get better, there was clearly a subset of the population that was more complicated and needed a more dedicated, multidimensional approach,” Dr. Ho said.
Findings from a growing number of studies have shown that concussions can involve a vast array of deficits – problems with the vestibular and vision systems, for example – and that a more comprehensive initial evaluation and a multipronged approach to management could shorten recovery time for any individual with the brain injury, he said.
“We’d started to realize how much patients can benefit from new and creative approaches,” said Dr. Ho. “We were also appreciating that no one person can own this disorder. You can’t say that only the neurologist can treat a concussion, or only the family physician or sports medicine physician.
‘Silent epidemic’
A small but growing number of concussion-care programs and clinics are being established across the country. Most often, the programs are incorporated into hospitals and sports medicine clinics. But some, like the program Dr. Ho leads, are embedded within larger primary care practices with a sports medicine component.
And, in other traditional family practices, some physicians are deepening their knowledge of concussions and building the networks necessary for managing prolonged impairment.
“If we have a good understanding of brain injury, we can often facilitate recovery and help [our patients] get back on track seamlessly,” said Dr. Rebecca Jaffe, a family physician in Wilmington, Del. “And when recovery is protracted, when issues don’t resolve in 2-3 weeks, there’s often more to the situation than is initially apparent, and it’s often better that we have a team of individuals who can help us.”
According to the Institute of Medicine (IOM), there are not enough data for an accurate estimate of the incidence of sports-related concussions in youth (Institute of Medicine and National Research Council. 2014. Sports-related concussions in youth: Improving the science, changing the culture. Washington, D.C.: The National Academies Press.)
But among high school athletes, an increase in concussions has been documented in several emergency department studies and other longitudinal studies published over the past 7-8 years. Most recently, the first national study to look at trends among high school athletes showed the numbers of concussions reported by certified athletic trainers increasing from 0.23/1,000 athlete-exposures in the 2005-2006 academic year to 0.51/1,000 in the 2011-2012 academic year (Am. J. Sports Med. 2014;42:1710-5).
Experts attribute such increases largely to improved awareness and new state laws. The Centers for Disease Control and Prevention’s launched its “Heads Up” educational program in 2004, for instance. And as of last year, every state and the District of Columbia had enacted at least one law to protect young athletes with concussions.
State concussion laws vary, but most require that parents, athletes, and coaches be educated about concussions, and that athletes with suspected concussions be evaluated and cleared by a health care professional with knowledge in concussions before returning to play.
It is likely, some experts say, that the upward curve is leveling off with improved awareness and diagnosis. But with such improvements there lurks a void of data on concussions in youth outside the realm of high school sports. And, according to the 2014 IOM report, the acute and long-term health threat from concussions is “not fully appreciated or acted upon” in many settings. “Kids are getting removed from play more frequently, but then it’s not really clear what anyone should do after that,” said Dr. Shireen Atabaki, an emergency medicine specialist at Children’s National Medical Center in Washington, who is leading several national initiatives to improve concussion care.
Too often, concussed children and teens receive little besides unnecessary CT scans in emergency departments and concussions go undiagnosed. And when concussions are diagnosed, discharge instructions are poor and “kids are sent out into a no-man’s land” where recovery is often poorly managed, she said.
When Dr. Ho and his colleagues opened their concussion center in March 2013, they began seeing patients who remained symptomatic after concussions that had occurred 6-12 months ago, and longer. “It was almost like a silent epidemic,” he said. “We had one young woman who’d been injured in her senior year of high school and was still symptomatic after 2 years.”
To build the program at Fairfax Family Practice, Dr. Ho and his colleagues, Dr. Thomas Howard and Dr. Marc Childress (who, like Dr. Ho, are board certified in sports medicine as well as family medicine), capitalized on what they saw as a valuable synergy with high school athletic trainers.
Dr. Ho had worked closely with athletic trainers as a volunteer medical adviser to the Fairfax County Public School System’s athletic training program, which provides care to student-athletes in 25 high schools. He drew on his relationships to recruit three athletic trainers who were at transition points in their careers and who had both experience and “passion” in the treatment of concussions.
One of them, Jon Almquist, VATL, ATC, had led the development of return-to-learn protocols and other management practices for the high schools. He had also partnered with investigators at MedStar Health Research Institute in Baltimore on research that documented a 4.2-fold increase in concussions among the school system’s high school athletes between 1997 and 2008 (Am. J. Sports Med. 2011;39:958-63).
More surprising than this increase, however, were some data collected after the study period ended. When Mr. Almquist looked at recovery times for the concussions reported between 2011 and 2013, he found that about 25% of concussions took 30 days or longer to recover – a portion greater than he’d seen anywhere in the literature.
According to the 2014 IOM report, youth athletes typically recover from a concussion within 2 weeks of the injury, but in 10-20% of cases the symptoms persist for a number of weeks, months, or even years.
“Families would come to us for advice,” Mr. Almquist recalled. Other than referring them to the University of Pittsburgh Medical Center’s (UPMC) Sports Medicine Concussion Program, there were few local providers whom they believed offered comprehensive care and had experience with prolonged impairment.
Mr. Almquist and his fellow athletic trainers, and Dr. Ho and his physician colleagues, all had been following developments at UPMC’s Sports Medicine Concussion Program since it was established in 2000. The program had evolved to assess and manage diverse facets of concussions, including vision, vestibular, exertion, and medication components—not all of which are fully addressed in clinical practice guidelines and position statements. By 2010, the program was drawing 10,000 patients a year.
Care at UPMC is guided by a clinical neuropsychologist who assesses head injuries and then refers patients on as needed to other members of the team – like a neurovestibular expert for vestibular assessment and therapy, a neuro-optometrist for vision therapy, a physical therapist for exertion training, or a sports medicine physician for medication.
In planning their own concussion center, Dr. Ho and his team visited various programs in the country but homed in on UPMC. They decided to mimic several aspects of UPMC’s approach by providing comprehensive and individualized care, and by having the certified athletic trainers serve as the “quarterbacks.”
“The athletic trainer in our center does all the symptom inventories and history taking, a concussion-focused physical exam, vestibular-ocular-motor screening, and neurocognitive tests if appropriate,” said Dr. Ho.
Dr. Ho or one of his sports medicine physician colleagues then discusses results with the athletic trainer and completes the patient visit – with the trainer – by asking any necessary follow-up questions, probing further with additional evaluation, and writing medication prescriptions when appropriate.
Throughout the course of care – visits occur weekly to monthly and often last 30-90 minutes – the athletic trainer and physician work together on clinical issues such as sleep and mental health and on subtyping headaches (cognitive fatigue, cervicogenic, or migrainelike) and “determining where the headaches lie relative to other areas of dysfunction,” said Dr. Ho.
Much of the individualized counseling, monitoring, and coordination that’s required to address sleep hygiene, rest, nutrition, and graded return to physical and cognitive activity can be led by the athletic trainer, he said.
For the first year or so, patients were referred out for such special services as vision or vestibular therapy. But there were problems with this approach: One frequent issue was poor insurance coverage for these therapies. Another was the stress involved in getting to additional appointments on Fairfax’s congested roads. “Traffic and busy environments aren’t always tolerable for a concussed patient,” Dr. Ho said.
Dr. Ho searched the Internet, worked the phone, read the neuro-optometry and vestibular therapy literature, and attended conferences to learn what kinds of therapeutic exercises they could integrate into office visits and take-home care plans. Dr. Ho said he was “surprised at how willing some of [their] colleagues were to give us some basic skills” to help patients who “don’t all need the Cadillac version” of therapy.
About the same time they integrated basic vision and vestibular therapy, they also revamped their billing strategy, moving away from the use of prolonged service billing codes, which Dr. Ho said were frequently denied on the first submission, to using separate codes for the encounter, therapeutic exercise (which encompasses vision therapy and vestibular therapy), and various physical tests.
Concussion care networks
Fairfax Family Practice is not the only practice to center its concussion program on physician-athletic trainer partnerships. Carolina Family Practice and Sports Medicine, a 12-provider practice with three offices in and around Raleigh, N.C., took a similar approach when it established its concussion clinic in 2008.
“Concussion is the ultimate example of an injury that benefits from a team approach,” said Dr. Josh Bloom, medical director of the Carolina Sports Concussion Clinic that is embedded into the practice. “And it’s the one injury – the only injury in sports medicine – where the standard of care calls for the injury to be 100% resolved prior to being cleared to return to play.”
Dr. Jaffe, the family physician in Delaware, said she is fortunate to have a neuro-optometrist practicing in the medical building where her three-physician family practice resides, and a concussion care team in the nearby Nemours/Alfred I. Dupont Hospital for Children, also in Wilmington.
Someday, she and others hope, physicians who see patients soon after injury will have tools to better predict clinical trajectories and identify who could benefit from earlier referral to a “more sophisticated team.”
For now, Dr. Jaffe said, she seeks assistance when headache and initial symptoms persist after 2-3 weeks or when their symptoms “seem out of proportion to the incident.”
Dr. Scott Ross of Novant Health Bull Run Family Medicine based in Manassas, Va., said that family physicians are well suited to guide management and should learn to evaluate each patient’s concussion-related impairments as thoroughly as possible.
About 3 years ago, he and a partner established an official “Sports Medicine and Concussion Management” service line in their practice. “We don’t have a concussion center all under one roof, but we have the knowledge, skills, and resources that we need,” he said.
This article was updated April 13, 2015.
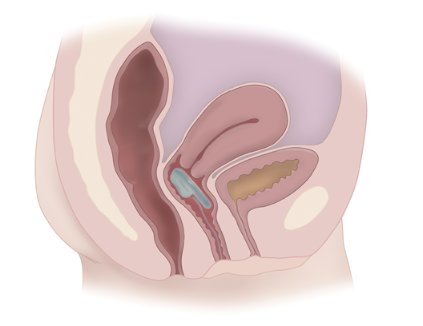
Women Are Not Seeking Care for Urinary Incontinence
WASHINGTON – Women are not seeking care for even moderate to severe urinary incontinence because they have a fear of treatment and lack knowledge of the etiology of the condition and available treatment options, according to a qualitative focus group study at Kaiser Permanente Orange County (Calif.).
Embarrassment is another barrier. So are physicians themselves, said Dr. Jennifer K. Lee, a urogynecologist at Kaiser in Anaheim, Calif., who reported the findings at the annual meeting of the American Urogynecologic Society.
"Providers were mentioned (in the focus groups) to be barriers," Dr. Lee said. "Patients were either dismissed, or they were given misinformation."
By 2050, an estimated 50 million Americans will have urinary incontinence. Previous research has shown, however, that less than 50% of women with moderate incontinence seek care.
The focus group study involved 19 women – 11 who had not sought care but were found after recruitment to have moderate to severe incontinence by the Sandvik severity index, and 8 who had previous treatment or had been evaluated for urinary incontinence.
A moderator led discussions among small groups of these women, all of whom were fluent in English and most of whom (90%) had a college education or higher. The participants were of varying ethnicities, including Caucasian, Hispanic, and Asian.
A fear of medical or surgical treatment, including medication side effects and recovery time for surgery, was one of the themes to emerge. Women also said they felt embarrassed and were therefore not likely to raise the issue, particularly with time constraints in their medical visits.
There was a prevailing idea that urinary incontinence is a normal part of aging or childbearing, or that it is hereditary, and that other health issues take priority. Physicians, moreover, did not ask about the issue, and in cases in which it was mentioned, physicians were dismissive or gave misinformation, the study showed.
Dr. Lee said the barriers are significant, and that "there’s a lot of work to be done" to facilitate communication and change practices.
Leaders at Kaiser Permanente Orange County, Anaheim, Calif., plan to give physicians question prompts to incorporate into routine and primary care visits, she noted during a press conference at the meeting. "We also want to work on educational tools for the public," she said.
"There are urinary incontinence questions [for] the Medicare wellness visit, but these are for women over 65," she said. "There certainly are plenty of women under 65 who have incontinence."
Food and Drug Administration warnings about complications associated with transvaginal placement of surgical mesh did not come up in the focus group discussions, but Dr. Lee said that in her practice she has seen this controversy feed patients’ fear of incontinence and its treatment.
Study participants were recruited through e-mail research announcements and flyers, as well as more actively through the offices of urogynecology providers.
Dr. Lee said she had no disclosures to report. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
WASHINGTON – Women are not seeking care for even moderate to severe urinary incontinence because they have a fear of treatment and lack knowledge of the etiology of the condition and available treatment options, according to a qualitative focus group study at Kaiser Permanente Orange County (Calif.).
Embarrassment is another barrier. So are physicians themselves, said Dr. Jennifer K. Lee, a urogynecologist at Kaiser in Anaheim, Calif., who reported the findings at the annual meeting of the American Urogynecologic Society.
"Providers were mentioned (in the focus groups) to be barriers," Dr. Lee said. "Patients were either dismissed, or they were given misinformation."
By 2050, an estimated 50 million Americans will have urinary incontinence. Previous research has shown, however, that less than 50% of women with moderate incontinence seek care.
The focus group study involved 19 women – 11 who had not sought care but were found after recruitment to have moderate to severe incontinence by the Sandvik severity index, and 8 who had previous treatment or had been evaluated for urinary incontinence.
A moderator led discussions among small groups of these women, all of whom were fluent in English and most of whom (90%) had a college education or higher. The participants were of varying ethnicities, including Caucasian, Hispanic, and Asian.
A fear of medical or surgical treatment, including medication side effects and recovery time for surgery, was one of the themes to emerge. Women also said they felt embarrassed and were therefore not likely to raise the issue, particularly with time constraints in their medical visits.
There was a prevailing idea that urinary incontinence is a normal part of aging or childbearing, or that it is hereditary, and that other health issues take priority. Physicians, moreover, did not ask about the issue, and in cases in which it was mentioned, physicians were dismissive or gave misinformation, the study showed.
Dr. Lee said the barriers are significant, and that "there’s a lot of work to be done" to facilitate communication and change practices.
Leaders at Kaiser Permanente Orange County, Anaheim, Calif., plan to give physicians question prompts to incorporate into routine and primary care visits, she noted during a press conference at the meeting. "We also want to work on educational tools for the public," she said.
"There are urinary incontinence questions [for] the Medicare wellness visit, but these are for women over 65," she said. "There certainly are plenty of women under 65 who have incontinence."
Food and Drug Administration warnings about complications associated with transvaginal placement of surgical mesh did not come up in the focus group discussions, but Dr. Lee said that in her practice she has seen this controversy feed patients’ fear of incontinence and its treatment.
Study participants were recruited through e-mail research announcements and flyers, as well as more actively through the offices of urogynecology providers.
Dr. Lee said she had no disclosures to report. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
WASHINGTON – Women are not seeking care for even moderate to severe urinary incontinence because they have a fear of treatment and lack knowledge of the etiology of the condition and available treatment options, according to a qualitative focus group study at Kaiser Permanente Orange County (Calif.).
Embarrassment is another barrier. So are physicians themselves, said Dr. Jennifer K. Lee, a urogynecologist at Kaiser in Anaheim, Calif., who reported the findings at the annual meeting of the American Urogynecologic Society.
"Providers were mentioned (in the focus groups) to be barriers," Dr. Lee said. "Patients were either dismissed, or they were given misinformation."
By 2050, an estimated 50 million Americans will have urinary incontinence. Previous research has shown, however, that less than 50% of women with moderate incontinence seek care.
The focus group study involved 19 women – 11 who had not sought care but were found after recruitment to have moderate to severe incontinence by the Sandvik severity index, and 8 who had previous treatment or had been evaluated for urinary incontinence.
A moderator led discussions among small groups of these women, all of whom were fluent in English and most of whom (90%) had a college education or higher. The participants were of varying ethnicities, including Caucasian, Hispanic, and Asian.
A fear of medical or surgical treatment, including medication side effects and recovery time for surgery, was one of the themes to emerge. Women also said they felt embarrassed and were therefore not likely to raise the issue, particularly with time constraints in their medical visits.
There was a prevailing idea that urinary incontinence is a normal part of aging or childbearing, or that it is hereditary, and that other health issues take priority. Physicians, moreover, did not ask about the issue, and in cases in which it was mentioned, physicians were dismissive or gave misinformation, the study showed.
Dr. Lee said the barriers are significant, and that "there’s a lot of work to be done" to facilitate communication and change practices.
Leaders at Kaiser Permanente Orange County, Anaheim, Calif., plan to give physicians question prompts to incorporate into routine and primary care visits, she noted during a press conference at the meeting. "We also want to work on educational tools for the public," she said.
"There are urinary incontinence questions [for] the Medicare wellness visit, but these are for women over 65," she said. "There certainly are plenty of women under 65 who have incontinence."
Food and Drug Administration warnings about complications associated with transvaginal placement of surgical mesh did not come up in the focus group discussions, but Dr. Lee said that in her practice she has seen this controversy feed patients’ fear of incontinence and its treatment.
Study participants were recruited through e-mail research announcements and flyers, as well as more actively through the offices of urogynecology providers.
Dr. Lee said she had no disclosures to report. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
AT THE AUGS ANNUAL MEETING
Mediolateral episiotomy shines in 10-year Netherlands analysis
WASHINGTON – The use of mediolateral episiotomy in women undergoing operative vaginal delivery – a common practice in the Netherlands – was associated with a large reduction in the risk of obstetrical anal sphincter injuries, according to an analysis of 10 years of data from the Netherlands Perinatal Registry.
For physicians and other obstetrical providers in the small European country, the findings reinforce current practice of favoring mediolateral episiotomies in operative vaginal deliveries as protection against obstetrical anal sphincter injuries (OASIS) – a risk factor for fecal incontinence, the investigators said.
"In the Netherlands, a mediolateral episiotomy is common practice. ... Our opinion is that the mediolateral episiotomies are causing less morbidity than an anal sphincter rupture would cause," Dr. Jeroen van Bavel reported during a press conference at the scientific meetings of the American Urogynecologic Society (AUGS) and the International Urogynecological Association.
But for physicians here in the United States, the risks are weighed differently. "It’s not that we don’t know that a mediolateral episiotomy decreases the risk of sphincter injury," Dr. Haywood Brown, professor and chair of obstetrics and gynecology at Duke University, Durham, N.C., said in a telephone interview after the conference.
"The problem is, mediolateral incisions are so uncomfortable," said Dr. Brown, who also is chair of the American College of Obstetricians and Gynecologists, District IV.
"They heal poorly, they heal with defects, ... and as a result the patient has more pain related to the mediolateral [episiotomy] than they would have with a third- or fourth-degree tear," he said. "Doing the mediolateral is really not the answer."
The Netherlands Perinatal Registry contains information on 96% of the 1.5 million deliveries that occurred during 2000-2009. Dr. van Bavel, of Amphia Hospital in Breda, the Netherlands, and his coinvestigators focused their analysis on the 170,974 women who had operative vaginal deliveries, comparing the rates of OASIS in women who had mediolateral episiotomies with those who did not.
Among primiparous women who had vacuum deliveries, OASIS occurred in 2.5% of those who had mediolateral episiotomies, compared with 14% who did not. Among multiparous women who had vacuum deliveries, OASIS rates were 2.1% with mediolateral episiotomy versus 7.5% without.
The differences were more striking with forceps deliveries. Anal sphincter injuries occurred in 3.4% versus 26.7% in primiparous women with and without mediolateral episiotomies, respectively. Among multiparous women, the risk of OASIS was 2.6% versus 14.2%.
For primiparous women, the number of mediolateral episiotomies needed to prevent one OASIS was 8 for vacuum delivery and 4 for forceps delivery, according to the analysis. For multiparous women, 18 mediolateral episiotomies were needed to prevent one OASIS for vacuum delivery, and 8 for forceps delivery.
ACOG’s Practice Bulletin No. 71 on episiotomy, which was written in 2006 (Obstet. Gynecol. 2006;107:957-62) and reaffirmed in 2013, states that median episiotomy is associated with higher rates of injury to the anal sphincter and rectum than mediolateral episiotomy (level A evidence), and that mediolateral episiotomy may be preferable in selected cases (level B evidence).
Overall, "restricted use of episiotomy is preferable to routine use of episiotomy," the guidelines say (level A evidence). Postpartum recovery, the guidelines note, is an area of obstetrics that lacks systematic study and analysis.
Dr. Brown said he stands firmly with his belief that mediolateral episiotomy as a routine prophylactic procedure in operative vaginal deliveries cannot be justified. "Having lived through an era of mediolaterals and seeing how long they take to heal, and the discomfort that patients have, I can’t justify it," he said.
"We’ve moved away from episiotomies [in the United States], period," he said. "We’ve moved away from them primarily because of the data showing that midline episiotomies increase the risk of sphincter injury. And the mediolateral episiotomies are just too painful."
The risk of OASIS can be minimized through good delivery technique, he noted.
"The trend here is toward more vacuum deliveries, which have [been shown to be less risky] than forceps deliveries, although its depends on the type of forceps used and the skill of the [obstetrician]," Dr. Brown said. "The challenge we face is that we don’t have enough forceps and vacuum deliveries to easily keep skill levels up."
Dr. Charles W. Nager, president of AUGS and director the urogynecological and reconstructive pelvic surgery division at the University of California, San Diego, said that rates of both episiotomy and operative vaginal delivery have been declining in the United States, and that simultaneously, "there’s been a parallel drop in OASIS."
There also is more training ongoing in U.S. hospitals on repairing third- and fourth-degree obstetric lacerations, he said in an interview at the meeting.
The Netherlands analysis excluded women with preterm delivery, stillbirth, multiple gestation, transverse position, and breech delivery, as well as women whose deliveries involved both forceps and vacuum and women who had a midline episiotomy.
Factors controlled for in the study included parity, fetal position, birth weight, augmentation with oxytocin, and duration of the second stage of labor.
Dr. van Bavel and all but one of his coinvestigators reported no disclosures.
WASHINGTON – The use of mediolateral episiotomy in women undergoing operative vaginal delivery – a common practice in the Netherlands – was associated with a large reduction in the risk of obstetrical anal sphincter injuries, according to an analysis of 10 years of data from the Netherlands Perinatal Registry.
For physicians and other obstetrical providers in the small European country, the findings reinforce current practice of favoring mediolateral episiotomies in operative vaginal deliveries as protection against obstetrical anal sphincter injuries (OASIS) – a risk factor for fecal incontinence, the investigators said.
"In the Netherlands, a mediolateral episiotomy is common practice. ... Our opinion is that the mediolateral episiotomies are causing less morbidity than an anal sphincter rupture would cause," Dr. Jeroen van Bavel reported during a press conference at the scientific meetings of the American Urogynecologic Society (AUGS) and the International Urogynecological Association.
But for physicians here in the United States, the risks are weighed differently. "It’s not that we don’t know that a mediolateral episiotomy decreases the risk of sphincter injury," Dr. Haywood Brown, professor and chair of obstetrics and gynecology at Duke University, Durham, N.C., said in a telephone interview after the conference.
"The problem is, mediolateral incisions are so uncomfortable," said Dr. Brown, who also is chair of the American College of Obstetricians and Gynecologists, District IV.
"They heal poorly, they heal with defects, ... and as a result the patient has more pain related to the mediolateral [episiotomy] than they would have with a third- or fourth-degree tear," he said. "Doing the mediolateral is really not the answer."
The Netherlands Perinatal Registry contains information on 96% of the 1.5 million deliveries that occurred during 2000-2009. Dr. van Bavel, of Amphia Hospital in Breda, the Netherlands, and his coinvestigators focused their analysis on the 170,974 women who had operative vaginal deliveries, comparing the rates of OASIS in women who had mediolateral episiotomies with those who did not.
Among primiparous women who had vacuum deliveries, OASIS occurred in 2.5% of those who had mediolateral episiotomies, compared with 14% who did not. Among multiparous women who had vacuum deliveries, OASIS rates were 2.1% with mediolateral episiotomy versus 7.5% without.
The differences were more striking with forceps deliveries. Anal sphincter injuries occurred in 3.4% versus 26.7% in primiparous women with and without mediolateral episiotomies, respectively. Among multiparous women, the risk of OASIS was 2.6% versus 14.2%.
For primiparous women, the number of mediolateral episiotomies needed to prevent one OASIS was 8 for vacuum delivery and 4 for forceps delivery, according to the analysis. For multiparous women, 18 mediolateral episiotomies were needed to prevent one OASIS for vacuum delivery, and 8 for forceps delivery.
ACOG’s Practice Bulletin No. 71 on episiotomy, which was written in 2006 (Obstet. Gynecol. 2006;107:957-62) and reaffirmed in 2013, states that median episiotomy is associated with higher rates of injury to the anal sphincter and rectum than mediolateral episiotomy (level A evidence), and that mediolateral episiotomy may be preferable in selected cases (level B evidence).
Overall, "restricted use of episiotomy is preferable to routine use of episiotomy," the guidelines say (level A evidence). Postpartum recovery, the guidelines note, is an area of obstetrics that lacks systematic study and analysis.
Dr. Brown said he stands firmly with his belief that mediolateral episiotomy as a routine prophylactic procedure in operative vaginal deliveries cannot be justified. "Having lived through an era of mediolaterals and seeing how long they take to heal, and the discomfort that patients have, I can’t justify it," he said.
"We’ve moved away from episiotomies [in the United States], period," he said. "We’ve moved away from them primarily because of the data showing that midline episiotomies increase the risk of sphincter injury. And the mediolateral episiotomies are just too painful."
The risk of OASIS can be minimized through good delivery technique, he noted.
"The trend here is toward more vacuum deliveries, which have [been shown to be less risky] than forceps deliveries, although its depends on the type of forceps used and the skill of the [obstetrician]," Dr. Brown said. "The challenge we face is that we don’t have enough forceps and vacuum deliveries to easily keep skill levels up."
Dr. Charles W. Nager, president of AUGS and director the urogynecological and reconstructive pelvic surgery division at the University of California, San Diego, said that rates of both episiotomy and operative vaginal delivery have been declining in the United States, and that simultaneously, "there’s been a parallel drop in OASIS."
There also is more training ongoing in U.S. hospitals on repairing third- and fourth-degree obstetric lacerations, he said in an interview at the meeting.
The Netherlands analysis excluded women with preterm delivery, stillbirth, multiple gestation, transverse position, and breech delivery, as well as women whose deliveries involved both forceps and vacuum and women who had a midline episiotomy.
Factors controlled for in the study included parity, fetal position, birth weight, augmentation with oxytocin, and duration of the second stage of labor.
Dr. van Bavel and all but one of his coinvestigators reported no disclosures.
WASHINGTON – The use of mediolateral episiotomy in women undergoing operative vaginal delivery – a common practice in the Netherlands – was associated with a large reduction in the risk of obstetrical anal sphincter injuries, according to an analysis of 10 years of data from the Netherlands Perinatal Registry.
For physicians and other obstetrical providers in the small European country, the findings reinforce current practice of favoring mediolateral episiotomies in operative vaginal deliveries as protection against obstetrical anal sphincter injuries (OASIS) – a risk factor for fecal incontinence, the investigators said.
"In the Netherlands, a mediolateral episiotomy is common practice. ... Our opinion is that the mediolateral episiotomies are causing less morbidity than an anal sphincter rupture would cause," Dr. Jeroen van Bavel reported during a press conference at the scientific meetings of the American Urogynecologic Society (AUGS) and the International Urogynecological Association.
But for physicians here in the United States, the risks are weighed differently. "It’s not that we don’t know that a mediolateral episiotomy decreases the risk of sphincter injury," Dr. Haywood Brown, professor and chair of obstetrics and gynecology at Duke University, Durham, N.C., said in a telephone interview after the conference.
"The problem is, mediolateral incisions are so uncomfortable," said Dr. Brown, who also is chair of the American College of Obstetricians and Gynecologists, District IV.
"They heal poorly, they heal with defects, ... and as a result the patient has more pain related to the mediolateral [episiotomy] than they would have with a third- or fourth-degree tear," he said. "Doing the mediolateral is really not the answer."
The Netherlands Perinatal Registry contains information on 96% of the 1.5 million deliveries that occurred during 2000-2009. Dr. van Bavel, of Amphia Hospital in Breda, the Netherlands, and his coinvestigators focused their analysis on the 170,974 women who had operative vaginal deliveries, comparing the rates of OASIS in women who had mediolateral episiotomies with those who did not.
Among primiparous women who had vacuum deliveries, OASIS occurred in 2.5% of those who had mediolateral episiotomies, compared with 14% who did not. Among multiparous women who had vacuum deliveries, OASIS rates were 2.1% with mediolateral episiotomy versus 7.5% without.
The differences were more striking with forceps deliveries. Anal sphincter injuries occurred in 3.4% versus 26.7% in primiparous women with and without mediolateral episiotomies, respectively. Among multiparous women, the risk of OASIS was 2.6% versus 14.2%.
For primiparous women, the number of mediolateral episiotomies needed to prevent one OASIS was 8 for vacuum delivery and 4 for forceps delivery, according to the analysis. For multiparous women, 18 mediolateral episiotomies were needed to prevent one OASIS for vacuum delivery, and 8 for forceps delivery.
ACOG’s Practice Bulletin No. 71 on episiotomy, which was written in 2006 (Obstet. Gynecol. 2006;107:957-62) and reaffirmed in 2013, states that median episiotomy is associated with higher rates of injury to the anal sphincter and rectum than mediolateral episiotomy (level A evidence), and that mediolateral episiotomy may be preferable in selected cases (level B evidence).
Overall, "restricted use of episiotomy is preferable to routine use of episiotomy," the guidelines say (level A evidence). Postpartum recovery, the guidelines note, is an area of obstetrics that lacks systematic study and analysis.
Dr. Brown said he stands firmly with his belief that mediolateral episiotomy as a routine prophylactic procedure in operative vaginal deliveries cannot be justified. "Having lived through an era of mediolaterals and seeing how long they take to heal, and the discomfort that patients have, I can’t justify it," he said.
"We’ve moved away from episiotomies [in the United States], period," he said. "We’ve moved away from them primarily because of the data showing that midline episiotomies increase the risk of sphincter injury. And the mediolateral episiotomies are just too painful."
The risk of OASIS can be minimized through good delivery technique, he noted.
"The trend here is toward more vacuum deliveries, which have [been shown to be less risky] than forceps deliveries, although its depends on the type of forceps used and the skill of the [obstetrician]," Dr. Brown said. "The challenge we face is that we don’t have enough forceps and vacuum deliveries to easily keep skill levels up."
Dr. Charles W. Nager, president of AUGS and director the urogynecological and reconstructive pelvic surgery division at the University of California, San Diego, said that rates of both episiotomy and operative vaginal delivery have been declining in the United States, and that simultaneously, "there’s been a parallel drop in OASIS."
There also is more training ongoing in U.S. hospitals on repairing third- and fourth-degree obstetric lacerations, he said in an interview at the meeting.
The Netherlands analysis excluded women with preterm delivery, stillbirth, multiple gestation, transverse position, and breech delivery, as well as women whose deliveries involved both forceps and vacuum and women who had a midline episiotomy.
Factors controlled for in the study included parity, fetal position, birth weight, augmentation with oxytocin, and duration of the second stage of labor.
Dr. van Bavel and all but one of his coinvestigators reported no disclosures.
AT AUGS/IUGA 2014
Key clinical point: The findings reinforce the Dutch practice of favoring mediolateral episiotomies in operative vaginal deliveries as protection against obstetrical anal sphincter injuries, but American obstetricians disagree on the basis that mediolateral episiotomies are quite painful and vacuum and forceps deliveries are becoming less common in the United States.
Major finding: The use of mediolateral episiotomy in vaginal operative deliveries is associated with significant reductions in the risk of obstetric anal sphincter injuries, across vacuum and forceps deliveries and primiparous and multiparous births.
Data source: A retrospective cohort study of 170,974 vaginal operative deliveries in the Netherlands.
Disclosures: Dr. Jeroen van Bavel and all but one of his coinvestigators reported no disclosures.
Vaginal bowel control device shown safe, effective in pivotal trial
WASHINGTON – Use of a vaginal bowel control device currently under review by the Food and Drug Administration resulted in a significant reduction of fecal incontinence episodes and significant improvement in quality of life, with only mild and transient adverse events, a pivotal study has shown.
The device – the Vaginal Bowel Control (VBC) System made by Pelvalon (Sunnyvale, Calif.) – consists of a silicone base and a posteriorly directed balloon. The patient controls the inflation pump, inflating the balloon to deflect the rectovaginal septum and interrupt stool passage, and deflating it to allow bowel movements.
"It provides [the patient with] dynamic control of the rectum," said Holly E. Richter, Ph.D., M.D., who served as the national principal investigator of the study. "It’s a new paradigm for treating patients with fecal incontinence."
At the 1-month primary endpoint, 79% of the 61 patients in the study’s intent-to-treat cohort – and 86% of patients in the per-protocol cohort – had experienced treatment success, which was defined as a 50% or greater reduction in fecal incontinence episodes, Dr. Richter reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Mean weekly fecal incontinence episodes were reduced from 5.9 per week at baseline to 1.1 at 1 month.
After the 1-month treatment period, women were given the option of continuing with an additional 2-month study period. The rate of treatment success held steady among the 44 women who continued, with 86% seeing a 50% or greater reduction in fecal incontinence at 3 months, said Dr. Richter, director of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham.
Total continence was achieved in approximately 40% of women in the 1-month and 3-month per-protocol cohorts, she noted.
Scores on the Fecal Incontinence Quality of Life survey and the Modified Manchester Health Questionnaire improved significantly across all subscales. At 1 month, 86% reported their fecal incontinence to be "very much better" (57%) or "much better" (29%). Almost all – 96% – reported their insert to be comfortable, and half said they could not feel it.
To qualify for the six-center study, women had to have a history of fecal incontinence of at least 6 months, with 4 or more incontinence episodes recorded during a 2-week baseline bowel diary.
Study participants had a mean age of 61 years and a mean of 5.9 weekly fecal incontinence episodes (or 12 in their diaries).Thirty percent were obese. Nearly 50% had prior hysterectomy, 8% had prior pelvic prolapse surgery, and 15% had prior urinary incontinence surgery.
There were no device-related serious adverse events. The most common complaint was cramping or discomfort, which most often occurred during the fitting period. There were approximately a half-dozen reported changes in urinary incontinence or overactive bladder symptoms.
"We believe that the Vaginal Bowel Control System can be tried early in the algorithm of treatment for fecal incontinence," Dr. Richter said.
It won’t be a good option for everyone, however. After being fitted with the device (it is made with three base sizes and two balloon sizes), women in the study were instructed to try it for a week before entering the 1-month treatment period. Approximately 40 women who were screened and fitted did not continue. This rate of discomfort or nonacceptance is similar to that associated with pessaries, Dr. Richter noted.
The initial fitting procedure for the new bowel control device "requires a little more provider and patient training, beyond [what is needed] for use of a traditional vaginal pessary," she said.
"Patients in the study could put the device in and take it out as they wished. Most patients used it all day long, and some took it out at night," she said. Patients were instructed to "take it out once or twice a week to wash it."
Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
Fecal incontinence is one of the most emotionally devastating of all nonfatal medical conditions, and there is a need for new and effective strategies when medical therapy fails. The vaginal bowel control device appears to hold promise for some women with fecal incontinence by mechanically impeding stool passage to the anorectum by means of an inflatable balloon inserted into the vagina. The premise is that it can be used during times when the patient is not near to toileting facilities, can be inserted and removed at will, and is not a safety concern.
It is important to emphasize that this was not a randomized, placebo-controlled trial but rather a proof-of-concept study. The 40% complete continence response is 1) similar to that observed with the sacral nerve stimulation trial that resulted in Food and Drug Administration approval in the absence of a double-blind, controlled trial (Ann. Surg. 2010;1251:441), and 2) superior to the injectable bulking agent (6% complete continence), which was approved on the basis of a randomized, double-blind, sham-controlled trial (Lancet 2011;377:997).
However, complete continence is not necessary to improve quality of life in patients with incontinence. All three of these techniques require future studies to determine which incontinent patients are likely to benefit from each of these modalities. It must be recognized that there are many causes of incontinence, which often require different therapeutic approaches.
I look forward to reading a peer-reviewed paper on this new technique and to future randomized, controlled studies on this innovative approach to a condition with a large unmet need.
Dr. Arnold Wald is professor of medicine at the University of Wisconsin School of Medicine and Public Health, Madison.
Fecal incontinence is one of the most emotionally devastating of all nonfatal medical conditions, and there is a need for new and effective strategies when medical therapy fails. The vaginal bowel control device appears to hold promise for some women with fecal incontinence by mechanically impeding stool passage to the anorectum by means of an inflatable balloon inserted into the vagina. The premise is that it can be used during times when the patient is not near to toileting facilities, can be inserted and removed at will, and is not a safety concern.
It is important to emphasize that this was not a randomized, placebo-controlled trial but rather a proof-of-concept study. The 40% complete continence response is 1) similar to that observed with the sacral nerve stimulation trial that resulted in Food and Drug Administration approval in the absence of a double-blind, controlled trial (Ann. Surg. 2010;1251:441), and 2) superior to the injectable bulking agent (6% complete continence), which was approved on the basis of a randomized, double-blind, sham-controlled trial (Lancet 2011;377:997).
However, complete continence is not necessary to improve quality of life in patients with incontinence. All three of these techniques require future studies to determine which incontinent patients are likely to benefit from each of these modalities. It must be recognized that there are many causes of incontinence, which often require different therapeutic approaches.
I look forward to reading a peer-reviewed paper on this new technique and to future randomized, controlled studies on this innovative approach to a condition with a large unmet need.
Dr. Arnold Wald is professor of medicine at the University of Wisconsin School of Medicine and Public Health, Madison.
Fecal incontinence is one of the most emotionally devastating of all nonfatal medical conditions, and there is a need for new and effective strategies when medical therapy fails. The vaginal bowel control device appears to hold promise for some women with fecal incontinence by mechanically impeding stool passage to the anorectum by means of an inflatable balloon inserted into the vagina. The premise is that it can be used during times when the patient is not near to toileting facilities, can be inserted and removed at will, and is not a safety concern.
It is important to emphasize that this was not a randomized, placebo-controlled trial but rather a proof-of-concept study. The 40% complete continence response is 1) similar to that observed with the sacral nerve stimulation trial that resulted in Food and Drug Administration approval in the absence of a double-blind, controlled trial (Ann. Surg. 2010;1251:441), and 2) superior to the injectable bulking agent (6% complete continence), which was approved on the basis of a randomized, double-blind, sham-controlled trial (Lancet 2011;377:997).
However, complete continence is not necessary to improve quality of life in patients with incontinence. All three of these techniques require future studies to determine which incontinent patients are likely to benefit from each of these modalities. It must be recognized that there are many causes of incontinence, which often require different therapeutic approaches.
I look forward to reading a peer-reviewed paper on this new technique and to future randomized, controlled studies on this innovative approach to a condition with a large unmet need.
Dr. Arnold Wald is professor of medicine at the University of Wisconsin School of Medicine and Public Health, Madison.
WASHINGTON – Use of a vaginal bowel control device currently under review by the Food and Drug Administration resulted in a significant reduction of fecal incontinence episodes and significant improvement in quality of life, with only mild and transient adverse events, a pivotal study has shown.
The device – the Vaginal Bowel Control (VBC) System made by Pelvalon (Sunnyvale, Calif.) – consists of a silicone base and a posteriorly directed balloon. The patient controls the inflation pump, inflating the balloon to deflect the rectovaginal septum and interrupt stool passage, and deflating it to allow bowel movements.
"It provides [the patient with] dynamic control of the rectum," said Holly E. Richter, Ph.D., M.D., who served as the national principal investigator of the study. "It’s a new paradigm for treating patients with fecal incontinence."
At the 1-month primary endpoint, 79% of the 61 patients in the study’s intent-to-treat cohort – and 86% of patients in the per-protocol cohort – had experienced treatment success, which was defined as a 50% or greater reduction in fecal incontinence episodes, Dr. Richter reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Mean weekly fecal incontinence episodes were reduced from 5.9 per week at baseline to 1.1 at 1 month.
After the 1-month treatment period, women were given the option of continuing with an additional 2-month study period. The rate of treatment success held steady among the 44 women who continued, with 86% seeing a 50% or greater reduction in fecal incontinence at 3 months, said Dr. Richter, director of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham.
Total continence was achieved in approximately 40% of women in the 1-month and 3-month per-protocol cohorts, she noted.
Scores on the Fecal Incontinence Quality of Life survey and the Modified Manchester Health Questionnaire improved significantly across all subscales. At 1 month, 86% reported their fecal incontinence to be "very much better" (57%) or "much better" (29%). Almost all – 96% – reported their insert to be comfortable, and half said they could not feel it.
To qualify for the six-center study, women had to have a history of fecal incontinence of at least 6 months, with 4 or more incontinence episodes recorded during a 2-week baseline bowel diary.
Study participants had a mean age of 61 years and a mean of 5.9 weekly fecal incontinence episodes (or 12 in their diaries).Thirty percent were obese. Nearly 50% had prior hysterectomy, 8% had prior pelvic prolapse surgery, and 15% had prior urinary incontinence surgery.
There were no device-related serious adverse events. The most common complaint was cramping or discomfort, which most often occurred during the fitting period. There were approximately a half-dozen reported changes in urinary incontinence or overactive bladder symptoms.
"We believe that the Vaginal Bowel Control System can be tried early in the algorithm of treatment for fecal incontinence," Dr. Richter said.
It won’t be a good option for everyone, however. After being fitted with the device (it is made with three base sizes and two balloon sizes), women in the study were instructed to try it for a week before entering the 1-month treatment period. Approximately 40 women who were screened and fitted did not continue. This rate of discomfort or nonacceptance is similar to that associated with pessaries, Dr. Richter noted.
The initial fitting procedure for the new bowel control device "requires a little more provider and patient training, beyond [what is needed] for use of a traditional vaginal pessary," she said.
"Patients in the study could put the device in and take it out as they wished. Most patients used it all day long, and some took it out at night," she said. Patients were instructed to "take it out once or twice a week to wash it."
Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
WASHINGTON – Use of a vaginal bowel control device currently under review by the Food and Drug Administration resulted in a significant reduction of fecal incontinence episodes and significant improvement in quality of life, with only mild and transient adverse events, a pivotal study has shown.
The device – the Vaginal Bowel Control (VBC) System made by Pelvalon (Sunnyvale, Calif.) – consists of a silicone base and a posteriorly directed balloon. The patient controls the inflation pump, inflating the balloon to deflect the rectovaginal septum and interrupt stool passage, and deflating it to allow bowel movements.
"It provides [the patient with] dynamic control of the rectum," said Holly E. Richter, Ph.D., M.D., who served as the national principal investigator of the study. "It’s a new paradigm for treating patients with fecal incontinence."
At the 1-month primary endpoint, 79% of the 61 patients in the study’s intent-to-treat cohort – and 86% of patients in the per-protocol cohort – had experienced treatment success, which was defined as a 50% or greater reduction in fecal incontinence episodes, Dr. Richter reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Mean weekly fecal incontinence episodes were reduced from 5.9 per week at baseline to 1.1 at 1 month.
After the 1-month treatment period, women were given the option of continuing with an additional 2-month study period. The rate of treatment success held steady among the 44 women who continued, with 86% seeing a 50% or greater reduction in fecal incontinence at 3 months, said Dr. Richter, director of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham.
Total continence was achieved in approximately 40% of women in the 1-month and 3-month per-protocol cohorts, she noted.
Scores on the Fecal Incontinence Quality of Life survey and the Modified Manchester Health Questionnaire improved significantly across all subscales. At 1 month, 86% reported their fecal incontinence to be "very much better" (57%) or "much better" (29%). Almost all – 96% – reported their insert to be comfortable, and half said they could not feel it.
To qualify for the six-center study, women had to have a history of fecal incontinence of at least 6 months, with 4 or more incontinence episodes recorded during a 2-week baseline bowel diary.
Study participants had a mean age of 61 years and a mean of 5.9 weekly fecal incontinence episodes (or 12 in their diaries).Thirty percent were obese. Nearly 50% had prior hysterectomy, 8% had prior pelvic prolapse surgery, and 15% had prior urinary incontinence surgery.
There were no device-related serious adverse events. The most common complaint was cramping or discomfort, which most often occurred during the fitting period. There were approximately a half-dozen reported changes in urinary incontinence or overactive bladder symptoms.
"We believe that the Vaginal Bowel Control System can be tried early in the algorithm of treatment for fecal incontinence," Dr. Richter said.
It won’t be a good option for everyone, however. After being fitted with the device (it is made with three base sizes and two balloon sizes), women in the study were instructed to try it for a week before entering the 1-month treatment period. Approximately 40 women who were screened and fitted did not continue. This rate of discomfort or nonacceptance is similar to that associated with pessaries, Dr. Richter noted.
The initial fitting procedure for the new bowel control device "requires a little more provider and patient training, beyond [what is needed] for use of a traditional vaginal pessary," she said.
"Patients in the study could put the device in and take it out as they wished. Most patients used it all day long, and some took it out at night," she said. Patients were instructed to "take it out once or twice a week to wash it."
Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
AT AUGS/IUGA 2014
Key clinical finding: A controllable vaginal approach to bowel continence may be useful in the future.
Major finding: Almost 80% of 61 patients in an intent-to-treat cohort saw a 50% or greater reduction in fecal incontinence episodes with use of the Vaginal Bowel Control System, a device placed in the vagina to restore bowel control.
Data source: A multicenter, prospective, open-label study of women with fecal incontinence.
Disclosures: Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
Repeat Botox treatments sustain improvements for overactive bladder
WASHINGTON – Repeat treatment with onabotulinumtoxin A provided sustained improvements in the symptoms of overactive bladder and was well tolerated in women who were followed for a median 2.4 years beyond their participation in one of the two large clinical trials that established the agent’s effectiveness and safety for this indication.
"This treatment remains a safe and effective therapeutic option" for patients with overactive bladder, said Dr. Tomasz Rechberger of the Medical University of Lublin (Poland). It’s "an attractive alternative [in the longer term] for patients who were inadequately treated with anticholinergics."
Women who completed either of the placebo-controlled, multicenter phase III studies were given the option of entering a 3-year open-label extension study in which they could receive additional treatments upon request and as needed to maintain symptom control. The intradetrusor injections of 100 U of onabotulinumtoxin A (Botox) needed to be at least 12 weeks apart.
At the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, Dr. Rechberger reported on an interim analysis of this extension study, which was scheduled to end in August 2014.
As of June 2014, the overall discontinuation rate among the 749 patients who entered the extension study was 38%, but only 4.5% discontinued because of adverse effects or a lack of efficacy. "It was extremely low – much lower than [we expected]," said Dr. Rechberger.
The women had a mean age of 60 and had experienced symptoms of overactive bladder (OAB) for more than 6 years. At the start of the extension study, they had a mean of 5.6 episodes per day of urinary incontinence, 11.6 micturitions per day, and 8.4 urgency episodes per day.
In the original studies, the results after 12 weeks showed that patients treated with Botox experienced urinary incontinence an average of 1.6-1.9 times fewer per day than did patients treated with placebo, and they needed to urinate on average 1.0-1.7 times fewer per day, according to the Food and Drug Administration. In 2013, the FDA approved the use of Botox to treat adults with OAB who cannot use or do not adequately respond to anticholinergics.
Consistent reductions in symptoms were seen after the one to five treatments evaluated as part of the interim analysis of the extension study. Investigators checked all endpoints against the baseline 12 weeks after each treatment. The number of urinary incontinence episodes per day, for instance, was reduced by 3.3 after the first treatment cycle, 3.7 after two treatments, 3.9 after the third treatment, and 3.2 after the fourth and fifth treatment cycles.
Approximately 63%-72% of patients achieved a reduction of incontinence episodes of at least 50% across the treatment cycles.
Additionally, women saw mean reductions in micturition ranging from 2.4 to 3 over the five treatment cycles, and mean reductions in urgency episodes ranging from 3.6 to 4.2. A consistent increase in the volume voided per micturition also was seen.
Urinary tract infection was the most common side effect in each repeat treatment, as was the case in the original studies, but declined slightly with each treatment cycle, from approximately 30% after the first treatment to approximately 20% after the fifth. There were no new safety signals, Dr. Rechberger reported.
Each treatment consisted of 20 cystoscopic injections of 0.5 mL each. The median time between treatments ranged from 24 to 33 weeks – significantly longer than the required 12-week minimum.
Dr. Rechberger is an advisory committee member for Allergan (maker of Botox) and consults for Astellas and Johnson & Johnson. The study was funded by Allergan.
WASHINGTON – Repeat treatment with onabotulinumtoxin A provided sustained improvements in the symptoms of overactive bladder and was well tolerated in women who were followed for a median 2.4 years beyond their participation in one of the two large clinical trials that established the agent’s effectiveness and safety for this indication.
"This treatment remains a safe and effective therapeutic option" for patients with overactive bladder, said Dr. Tomasz Rechberger of the Medical University of Lublin (Poland). It’s "an attractive alternative [in the longer term] for patients who were inadequately treated with anticholinergics."
Women who completed either of the placebo-controlled, multicenter phase III studies were given the option of entering a 3-year open-label extension study in which they could receive additional treatments upon request and as needed to maintain symptom control. The intradetrusor injections of 100 U of onabotulinumtoxin A (Botox) needed to be at least 12 weeks apart.
At the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, Dr. Rechberger reported on an interim analysis of this extension study, which was scheduled to end in August 2014.
As of June 2014, the overall discontinuation rate among the 749 patients who entered the extension study was 38%, but only 4.5% discontinued because of adverse effects or a lack of efficacy. "It was extremely low – much lower than [we expected]," said Dr. Rechberger.
The women had a mean age of 60 and had experienced symptoms of overactive bladder (OAB) for more than 6 years. At the start of the extension study, they had a mean of 5.6 episodes per day of urinary incontinence, 11.6 micturitions per day, and 8.4 urgency episodes per day.
In the original studies, the results after 12 weeks showed that patients treated with Botox experienced urinary incontinence an average of 1.6-1.9 times fewer per day than did patients treated with placebo, and they needed to urinate on average 1.0-1.7 times fewer per day, according to the Food and Drug Administration. In 2013, the FDA approved the use of Botox to treat adults with OAB who cannot use or do not adequately respond to anticholinergics.
Consistent reductions in symptoms were seen after the one to five treatments evaluated as part of the interim analysis of the extension study. Investigators checked all endpoints against the baseline 12 weeks after each treatment. The number of urinary incontinence episodes per day, for instance, was reduced by 3.3 after the first treatment cycle, 3.7 after two treatments, 3.9 after the third treatment, and 3.2 after the fourth and fifth treatment cycles.
Approximately 63%-72% of patients achieved a reduction of incontinence episodes of at least 50% across the treatment cycles.
Additionally, women saw mean reductions in micturition ranging from 2.4 to 3 over the five treatment cycles, and mean reductions in urgency episodes ranging from 3.6 to 4.2. A consistent increase in the volume voided per micturition also was seen.
Urinary tract infection was the most common side effect in each repeat treatment, as was the case in the original studies, but declined slightly with each treatment cycle, from approximately 30% after the first treatment to approximately 20% after the fifth. There were no new safety signals, Dr. Rechberger reported.
Each treatment consisted of 20 cystoscopic injections of 0.5 mL each. The median time between treatments ranged from 24 to 33 weeks – significantly longer than the required 12-week minimum.
Dr. Rechberger is an advisory committee member for Allergan (maker of Botox) and consults for Astellas and Johnson & Johnson. The study was funded by Allergan.
WASHINGTON – Repeat treatment with onabotulinumtoxin A provided sustained improvements in the symptoms of overactive bladder and was well tolerated in women who were followed for a median 2.4 years beyond their participation in one of the two large clinical trials that established the agent’s effectiveness and safety for this indication.
"This treatment remains a safe and effective therapeutic option" for patients with overactive bladder, said Dr. Tomasz Rechberger of the Medical University of Lublin (Poland). It’s "an attractive alternative [in the longer term] for patients who were inadequately treated with anticholinergics."
Women who completed either of the placebo-controlled, multicenter phase III studies were given the option of entering a 3-year open-label extension study in which they could receive additional treatments upon request and as needed to maintain symptom control. The intradetrusor injections of 100 U of onabotulinumtoxin A (Botox) needed to be at least 12 weeks apart.
At the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, Dr. Rechberger reported on an interim analysis of this extension study, which was scheduled to end in August 2014.
As of June 2014, the overall discontinuation rate among the 749 patients who entered the extension study was 38%, but only 4.5% discontinued because of adverse effects or a lack of efficacy. "It was extremely low – much lower than [we expected]," said Dr. Rechberger.
The women had a mean age of 60 and had experienced symptoms of overactive bladder (OAB) for more than 6 years. At the start of the extension study, they had a mean of 5.6 episodes per day of urinary incontinence, 11.6 micturitions per day, and 8.4 urgency episodes per day.
In the original studies, the results after 12 weeks showed that patients treated with Botox experienced urinary incontinence an average of 1.6-1.9 times fewer per day than did patients treated with placebo, and they needed to urinate on average 1.0-1.7 times fewer per day, according to the Food and Drug Administration. In 2013, the FDA approved the use of Botox to treat adults with OAB who cannot use or do not adequately respond to anticholinergics.
Consistent reductions in symptoms were seen after the one to five treatments evaluated as part of the interim analysis of the extension study. Investigators checked all endpoints against the baseline 12 weeks after each treatment. The number of urinary incontinence episodes per day, for instance, was reduced by 3.3 after the first treatment cycle, 3.7 after two treatments, 3.9 after the third treatment, and 3.2 after the fourth and fifth treatment cycles.
Approximately 63%-72% of patients achieved a reduction of incontinence episodes of at least 50% across the treatment cycles.
Additionally, women saw mean reductions in micturition ranging from 2.4 to 3 over the five treatment cycles, and mean reductions in urgency episodes ranging from 3.6 to 4.2. A consistent increase in the volume voided per micturition also was seen.
Urinary tract infection was the most common side effect in each repeat treatment, as was the case in the original studies, but declined slightly with each treatment cycle, from approximately 30% after the first treatment to approximately 20% after the fifth. There were no new safety signals, Dr. Rechberger reported.
Each treatment consisted of 20 cystoscopic injections of 0.5 mL each. The median time between treatments ranged from 24 to 33 weeks – significantly longer than the required 12-week minimum.
Dr. Rechberger is an advisory committee member for Allergan (maker of Botox) and consults for Astellas and Johnson & Johnson. The study was funded by Allergan.
AT AUGS/IUGA 2014
Key clinical point: Consider Botox treatments in women with overactive bladder who could not use or did not adequately respond to anticholinergics.
Major finding: Repeat treatments of 100 U of onabotulinumtoxin A sustained improvements and led to consistent reductions in symptoms overall in a group of almost 750 women with overactive bladder who could not use or did not adequately respond to anticholinergics.
Data source: An interim analysis of an open-label, multicenter extension study of two placebo-controlled phase III trials.
Disclosures: Dr. Rechberger is an advisory committee member of Allergan and consults for Astellas and Johnson & Johnson. The study was funded by Allergan.
Pelvic floor disorders prevalent in female triathletes
WASHINGTON – A large proportion of female triathletes who responded to a national Web-based survey have symptoms of pelvic floor disorders, and one in four screened positive for the female athlete triad.
These findings from women in the general population, with a median age range of 35-44 years, echo findings from studies of Olympic and professional athletes, and "show that even young, thin, and otherwise healthy endurance athletes [who are not professional athletes] have pelvic floor disorders," said Dr. Johnny Yi after presenting his findings in a press conference at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Currently, "these women may not get screened because they are not believed to be in a high-risk group," said Dr. Yi, a urogynecologist in group practice in Denver.
Of 311 women who responded to the survey, 37% reported symptoms of stress urinary incontinence, and 16% reported symptoms of urgency urinary incontinence. Anal incontinence was also a problem, with 37% reporting symptoms of this condition. Pelvic organ prolapse symptoms were reported by 5%.
Most of the participants were white (90%), nonsmokers (99%), and premenopausal (80%), and had a mean body mass index of 22 kg/m2. About half had borne children, almost all of them had vaginal deliveries.
The women were invited to participate through a database of nationwide triathlon interest groups and a triathlete Internet forum. The survey included the Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ) and the Pelvic Girdle Pain Questionnaire (PGPQ), both of which are validated tools; the Female Athlete Triad Screening Questionnaire, which is endorsed by the National Collegiate Athletic Association; and various medical and demographic questions.
Approximately 18% of the women had pelvic girdle pain, although the mean score on the PGPQ (35.6) was relatively low. Respondents with various types of incontinence reported higher levels of pelvic girdle pain, Dr. Yi said.
Of the 75% of respondents who completed the triad questionnaire, 22% screened positive for low energy availability, 24% for menstrual irregularities, and 29% for abnormal bone strength. Eight percent screened positive for all three components of the female athlete triad, which is characterized by a hypoestrogenic state similar to menopause.
Dr. Yi said he is particularly interested in possible associations between the female athlete triad and pelvic floor disorders, and in the pelvic floor consequences of coupling high-impact, high-endurance activity with the potential for a hypoestrogenic state.
This study showed no significant association between the triad and pelvic floor disorders, but the findings emphasize that "both disorders are prevalent" and should be screened for and treated to avoid long-term sequelae and improve quality of life, he said.
Weekly training of the study participants consisted of running for a mean of 3.7 days, biking for a mean of 2.9 days, and swimming for a mean of 2.4 days. Training mileage and intensity were not associated with pelvic floor disorders. Not unexpectedly, parity was associated with a higher prevalence of stress urinary incontinence and pelvic organ prolapse.
Dr. Yi and his coinvestigators reported no disclosures.
WASHINGTON – A large proportion of female triathletes who responded to a national Web-based survey have symptoms of pelvic floor disorders, and one in four screened positive for the female athlete triad.
These findings from women in the general population, with a median age range of 35-44 years, echo findings from studies of Olympic and professional athletes, and "show that even young, thin, and otherwise healthy endurance athletes [who are not professional athletes] have pelvic floor disorders," said Dr. Johnny Yi after presenting his findings in a press conference at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Currently, "these women may not get screened because they are not believed to be in a high-risk group," said Dr. Yi, a urogynecologist in group practice in Denver.
Of 311 women who responded to the survey, 37% reported symptoms of stress urinary incontinence, and 16% reported symptoms of urgency urinary incontinence. Anal incontinence was also a problem, with 37% reporting symptoms of this condition. Pelvic organ prolapse symptoms were reported by 5%.
Most of the participants were white (90%), nonsmokers (99%), and premenopausal (80%), and had a mean body mass index of 22 kg/m2. About half had borne children, almost all of them had vaginal deliveries.
The women were invited to participate through a database of nationwide triathlon interest groups and a triathlete Internet forum. The survey included the Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ) and the Pelvic Girdle Pain Questionnaire (PGPQ), both of which are validated tools; the Female Athlete Triad Screening Questionnaire, which is endorsed by the National Collegiate Athletic Association; and various medical and demographic questions.
Approximately 18% of the women had pelvic girdle pain, although the mean score on the PGPQ (35.6) was relatively low. Respondents with various types of incontinence reported higher levels of pelvic girdle pain, Dr. Yi said.
Of the 75% of respondents who completed the triad questionnaire, 22% screened positive for low energy availability, 24% for menstrual irregularities, and 29% for abnormal bone strength. Eight percent screened positive for all three components of the female athlete triad, which is characterized by a hypoestrogenic state similar to menopause.
Dr. Yi said he is particularly interested in possible associations between the female athlete triad and pelvic floor disorders, and in the pelvic floor consequences of coupling high-impact, high-endurance activity with the potential for a hypoestrogenic state.
This study showed no significant association between the triad and pelvic floor disorders, but the findings emphasize that "both disorders are prevalent" and should be screened for and treated to avoid long-term sequelae and improve quality of life, he said.
Weekly training of the study participants consisted of running for a mean of 3.7 days, biking for a mean of 2.9 days, and swimming for a mean of 2.4 days. Training mileage and intensity were not associated with pelvic floor disorders. Not unexpectedly, parity was associated with a higher prevalence of stress urinary incontinence and pelvic organ prolapse.
Dr. Yi and his coinvestigators reported no disclosures.
WASHINGTON – A large proportion of female triathletes who responded to a national Web-based survey have symptoms of pelvic floor disorders, and one in four screened positive for the female athlete triad.
These findings from women in the general population, with a median age range of 35-44 years, echo findings from studies of Olympic and professional athletes, and "show that even young, thin, and otherwise healthy endurance athletes [who are not professional athletes] have pelvic floor disorders," said Dr. Johnny Yi after presenting his findings in a press conference at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Currently, "these women may not get screened because they are not believed to be in a high-risk group," said Dr. Yi, a urogynecologist in group practice in Denver.
Of 311 women who responded to the survey, 37% reported symptoms of stress urinary incontinence, and 16% reported symptoms of urgency urinary incontinence. Anal incontinence was also a problem, with 37% reporting symptoms of this condition. Pelvic organ prolapse symptoms were reported by 5%.
Most of the participants were white (90%), nonsmokers (99%), and premenopausal (80%), and had a mean body mass index of 22 kg/m2. About half had borne children, almost all of them had vaginal deliveries.
The women were invited to participate through a database of nationwide triathlon interest groups and a triathlete Internet forum. The survey included the Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ) and the Pelvic Girdle Pain Questionnaire (PGPQ), both of which are validated tools; the Female Athlete Triad Screening Questionnaire, which is endorsed by the National Collegiate Athletic Association; and various medical and demographic questions.
Approximately 18% of the women had pelvic girdle pain, although the mean score on the PGPQ (35.6) was relatively low. Respondents with various types of incontinence reported higher levels of pelvic girdle pain, Dr. Yi said.
Of the 75% of respondents who completed the triad questionnaire, 22% screened positive for low energy availability, 24% for menstrual irregularities, and 29% for abnormal bone strength. Eight percent screened positive for all three components of the female athlete triad, which is characterized by a hypoestrogenic state similar to menopause.
Dr. Yi said he is particularly interested in possible associations between the female athlete triad and pelvic floor disorders, and in the pelvic floor consequences of coupling high-impact, high-endurance activity with the potential for a hypoestrogenic state.
This study showed no significant association between the triad and pelvic floor disorders, but the findings emphasize that "both disorders are prevalent" and should be screened for and treated to avoid long-term sequelae and improve quality of life, he said.
Weekly training of the study participants consisted of running for a mean of 3.7 days, biking for a mean of 2.9 days, and swimming for a mean of 2.4 days. Training mileage and intensity were not associated with pelvic floor disorders. Not unexpectedly, parity was associated with a higher prevalence of stress urinary incontinence and pelvic organ prolapse.
Dr. Yi and his coinvestigators reported no disclosures.
AT AUGS/IUGA 2014
Key clinical point: Consider stress urinary incontinence and pelvic floor disorders in female triathletes.
Major finding: Women training for triathlons have a high prevalence of stress urinary incontinence (37%), urgency urinary incontinence (16%), anal incontinence (37%), and components of the female athlete triad.
Data source: A survey of 311 female triathletes with a median age range of 35-44 years.
Disclosures: Dr. Yi and his colleagues reported no disclosures.
Bowel prep before vaginal prolapse surgery offers no postop benefit
WASHINGTON – Bowel preparation for vaginal reconstructive surgery does not affect postoperative bowel function and bowel symptoms one way or the other, according to a secondary analysis of a single-blind randomized trial examining various effects of mechanical bowel preparation.
In a primary analysis published earlier this year, investigators found no benefit to mechanical bowel preparation with regard to the operative field quality. They also found that patients who were randomized not to receive bowel preparation were more satisfied and had fewer abdominal symptoms than those who received the intervention – a clear-liquid diet and enemas (Obstet. Gynecol. 2014;123:232-8).
The new analysis, reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, looked more closely at postoperative bowel habits after vaginal reconstructive surgery.
"As surgical intervention for pelvic organ prolapse increases, so does our need for knowledge of [best] perioperative management practices," said Dr. Alicia C. Ballard of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham, where the study was conducted.
"Concerns about painful defecation and GI symptoms such as nausea and vomiting are significant concerns for women undergoing surgery," she said.
Women may be predisposed to postsurgical constipation as a result of preoperative bowel preparation (including diet), the lasting effects of anesthesia, the use of narcotics, and the surgery itself. Prior research has shown, moreover, that constipation and incomplete bowel evacuation are not uncommon preoperatively in women with pelvic organ prolapse, she noted.
The study randomized 150 women scheduled to undergo vaginal prolapse surgery with, at a minimum, a planned apical suspension and posterior compartment repair. Surgeries included other prolapse and incontinence procedures.
Women randomized to the bowel preparation group were instructed to have a clear-liquid diet and to self-administer two saline enemas in the late afternoon of the day before surgery.
Those who were randomized to receive no intervention were allowed to have a regular diet. Women in both groups were instructed to eat nothing after midnight on the day of surgery.
All study participants were instructed to complete a bowel diary for 7 days preoperatively and 14 days postoperatively. Those who completed the preoperative diary and 10 of 14 days of the postoperative diary were included in the analysis.
Of the 150 women randomized at the preoperative visit in a 1:1 fashion, 5 withdrew from the study or had surgery canceled, and 121 completed the bowel diary.
The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel prep group and 3.2 days in the control group. The groups were also similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement. The use of antiemetics postoperatively was similar as well (48% and 55% in the bowel prep and no-prep groups, respectively.)
Most of the 121 women – across both groups – used at least one laxative postoperatively, mainly osmotic laxatives. Women who had not received bowel preparation were more likely, however, to report daily fiber use.
"Bowel preparation ... doesn’t appear to affect the return of bowel function and other bowel symptoms postoperatively," Dr. Ballard said. Moreover, "the lack of bowel preparation doesn’t seem to impact painful defecation symptoms, and most women use some type of laxative."
The study’s exclusion criteria included colorectal cancer, inflammatory bowel disease, a history of bowel resection, neurological disorders, and symptoms of colonic inertia (fewer than three bowel movements per week). The decision to exclude patients with significant preoperative constipation was made in order to "decrease the risk of bowel dysfunction and was important for our primary outcomes," Dr. Ballard explained. "But there actually were not that many women excluded."
Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
WASHINGTON – Bowel preparation for vaginal reconstructive surgery does not affect postoperative bowel function and bowel symptoms one way or the other, according to a secondary analysis of a single-blind randomized trial examining various effects of mechanical bowel preparation.
In a primary analysis published earlier this year, investigators found no benefit to mechanical bowel preparation with regard to the operative field quality. They also found that patients who were randomized not to receive bowel preparation were more satisfied and had fewer abdominal symptoms than those who received the intervention – a clear-liquid diet and enemas (Obstet. Gynecol. 2014;123:232-8).
The new analysis, reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, looked more closely at postoperative bowel habits after vaginal reconstructive surgery.
"As surgical intervention for pelvic organ prolapse increases, so does our need for knowledge of [best] perioperative management practices," said Dr. Alicia C. Ballard of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham, where the study was conducted.
"Concerns about painful defecation and GI symptoms such as nausea and vomiting are significant concerns for women undergoing surgery," she said.
Women may be predisposed to postsurgical constipation as a result of preoperative bowel preparation (including diet), the lasting effects of anesthesia, the use of narcotics, and the surgery itself. Prior research has shown, moreover, that constipation and incomplete bowel evacuation are not uncommon preoperatively in women with pelvic organ prolapse, she noted.
The study randomized 150 women scheduled to undergo vaginal prolapse surgery with, at a minimum, a planned apical suspension and posterior compartment repair. Surgeries included other prolapse and incontinence procedures.
Women randomized to the bowel preparation group were instructed to have a clear-liquid diet and to self-administer two saline enemas in the late afternoon of the day before surgery.
Those who were randomized to receive no intervention were allowed to have a regular diet. Women in both groups were instructed to eat nothing after midnight on the day of surgery.
All study participants were instructed to complete a bowel diary for 7 days preoperatively and 14 days postoperatively. Those who completed the preoperative diary and 10 of 14 days of the postoperative diary were included in the analysis.
Of the 150 women randomized at the preoperative visit in a 1:1 fashion, 5 withdrew from the study or had surgery canceled, and 121 completed the bowel diary.
The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel prep group and 3.2 days in the control group. The groups were also similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement. The use of antiemetics postoperatively was similar as well (48% and 55% in the bowel prep and no-prep groups, respectively.)
Most of the 121 women – across both groups – used at least one laxative postoperatively, mainly osmotic laxatives. Women who had not received bowel preparation were more likely, however, to report daily fiber use.
"Bowel preparation ... doesn’t appear to affect the return of bowel function and other bowel symptoms postoperatively," Dr. Ballard said. Moreover, "the lack of bowel preparation doesn’t seem to impact painful defecation symptoms, and most women use some type of laxative."
The study’s exclusion criteria included colorectal cancer, inflammatory bowel disease, a history of bowel resection, neurological disorders, and symptoms of colonic inertia (fewer than three bowel movements per week). The decision to exclude patients with significant preoperative constipation was made in order to "decrease the risk of bowel dysfunction and was important for our primary outcomes," Dr. Ballard explained. "But there actually were not that many women excluded."
Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
WASHINGTON – Bowel preparation for vaginal reconstructive surgery does not affect postoperative bowel function and bowel symptoms one way or the other, according to a secondary analysis of a single-blind randomized trial examining various effects of mechanical bowel preparation.
In a primary analysis published earlier this year, investigators found no benefit to mechanical bowel preparation with regard to the operative field quality. They also found that patients who were randomized not to receive bowel preparation were more satisfied and had fewer abdominal symptoms than those who received the intervention – a clear-liquid diet and enemas (Obstet. Gynecol. 2014;123:232-8).
The new analysis, reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, looked more closely at postoperative bowel habits after vaginal reconstructive surgery.
"As surgical intervention for pelvic organ prolapse increases, so does our need for knowledge of [best] perioperative management practices," said Dr. Alicia C. Ballard of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham, where the study was conducted.
"Concerns about painful defecation and GI symptoms such as nausea and vomiting are significant concerns for women undergoing surgery," she said.
Women may be predisposed to postsurgical constipation as a result of preoperative bowel preparation (including diet), the lasting effects of anesthesia, the use of narcotics, and the surgery itself. Prior research has shown, moreover, that constipation and incomplete bowel evacuation are not uncommon preoperatively in women with pelvic organ prolapse, she noted.
The study randomized 150 women scheduled to undergo vaginal prolapse surgery with, at a minimum, a planned apical suspension and posterior compartment repair. Surgeries included other prolapse and incontinence procedures.
Women randomized to the bowel preparation group were instructed to have a clear-liquid diet and to self-administer two saline enemas in the late afternoon of the day before surgery.
Those who were randomized to receive no intervention were allowed to have a regular diet. Women in both groups were instructed to eat nothing after midnight on the day of surgery.
All study participants were instructed to complete a bowel diary for 7 days preoperatively and 14 days postoperatively. Those who completed the preoperative diary and 10 of 14 days of the postoperative diary were included in the analysis.
Of the 150 women randomized at the preoperative visit in a 1:1 fashion, 5 withdrew from the study or had surgery canceled, and 121 completed the bowel diary.
The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel prep group and 3.2 days in the control group. The groups were also similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement. The use of antiemetics postoperatively was similar as well (48% and 55% in the bowel prep and no-prep groups, respectively.)
Most of the 121 women – across both groups – used at least one laxative postoperatively, mainly osmotic laxatives. Women who had not received bowel preparation were more likely, however, to report daily fiber use.
"Bowel preparation ... doesn’t appear to affect the return of bowel function and other bowel symptoms postoperatively," Dr. Ballard said. Moreover, "the lack of bowel preparation doesn’t seem to impact painful defecation symptoms, and most women use some type of laxative."
The study’s exclusion criteria included colorectal cancer, inflammatory bowel disease, a history of bowel resection, neurological disorders, and symptoms of colonic inertia (fewer than three bowel movements per week). The decision to exclude patients with significant preoperative constipation was made in order to "decrease the risk of bowel dysfunction and was important for our primary outcomes," Dr. Ballard explained. "But there actually were not that many women excluded."
Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
AT AUGS/IUGA 2014
Key clinical point: Bowel preparation prior to surgery for vaginal prolapse does not improve postoperative bowel function.
Major finding: The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel preparation group and 3.2 days in the control group. The groups also were similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement.
Data source: A secondary analysis of a single-blind randomized trial of 150 women undergoing vaginal prolapse surgery.
Disclosures: Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
Women are not seeking care for urinary incontinence, and physicians can be a barrier
WASHINGTON – Women are not seeking care for even moderate to severe urinary incontinence because they have a fear of treatment and lack knowledge of the etiology of the condition and available treatment options, according to a qualitative focus group study at Kaiser Permanente Orange County (Calif.).
Embarrassment is another barrier. So are physicians themselves, said Dr. Jennifer K. Lee, a urogynecologist at Kaiser in Anaheim, Calif., who reported the findings at the annual meeting of the American Urogynecologic Society.
"Providers were mentioned (in the focus groups) to be barriers," Dr. Lee said. "Patients were either dismissed, or they were given misinformation."
By 2050, an estimated 50 million Americans will have urinary incontinence. Previous research has shown, however, that less than 50% of women with moderate incontinence seek care.
The focus group study involved 19 women – 11 who had not sought care but were found after recruitment to have moderate to severe incontinence by the Sandvik severity index, and 8 who had previous treatment or had been evaluated for urinary incontinence.
A moderator led discussions among small groups of these women, all of whom were fluent in English and most of whom (90%) had a college education or higher. The participants were of varying ethnicities, including Caucasian, Hispanic, and Asian.
A fear of medical or surgical treatment, including medication side effects and recovery time for surgery, was one of the themes to emerge. Women also said they felt embarrassed and were therefore not likely to raise the issue, particularly with time constraints in their medical visits.
There was a prevailing idea that urinary incontinence is a normal part of aging or childbearing, or that it is hereditary, and that other health issues take priority. Physicians, moreover, did not ask about the issue, and in cases in which it was mentioned, physicians were dismissive or gave misinformation, the study showed.
Dr. Lee said the barriers are significant, and that "there’s a lot of work to be done" to facilitate communication and change practices.
Leaders at Kaiser Permanente Orange County, Anaheim, Calif., plan to give physicians question prompts to incorporate into routine and primary care visits, she noted during a press conference at the meeting. "We also want to work on educational tools for the public," she said.
"There are urinary incontinence questions [for] the Medicare wellness visit, but these are for women over 65," she said. "There certainly are plenty of women under 65 who have incontinence."
Food and Drug Administration warnings about complications associated with transvaginal placement of surgical mesh did not come up in the focus group discussions, but Dr. Lee said that in her practice she has seen this controversy feed patients’ fear of incontinence and its treatment.
Study participants were recruited through e-mail research announcements and flyers, as well as more actively through the offices of urogynecology providers.
Dr. Lee said she had no disclosures to report. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
WASHINGTON – Women are not seeking care for even moderate to severe urinary incontinence because they have a fear of treatment and lack knowledge of the etiology of the condition and available treatment options, according to a qualitative focus group study at Kaiser Permanente Orange County (Calif.).
Embarrassment is another barrier. So are physicians themselves, said Dr. Jennifer K. Lee, a urogynecologist at Kaiser in Anaheim, Calif., who reported the findings at the annual meeting of the American Urogynecologic Society.
"Providers were mentioned (in the focus groups) to be barriers," Dr. Lee said. "Patients were either dismissed, or they were given misinformation."
By 2050, an estimated 50 million Americans will have urinary incontinence. Previous research has shown, however, that less than 50% of women with moderate incontinence seek care.
The focus group study involved 19 women – 11 who had not sought care but were found after recruitment to have moderate to severe incontinence by the Sandvik severity index, and 8 who had previous treatment or had been evaluated for urinary incontinence.
A moderator led discussions among small groups of these women, all of whom were fluent in English and most of whom (90%) had a college education or higher. The participants were of varying ethnicities, including Caucasian, Hispanic, and Asian.
A fear of medical or surgical treatment, including medication side effects and recovery time for surgery, was one of the themes to emerge. Women also said they felt embarrassed and were therefore not likely to raise the issue, particularly with time constraints in their medical visits.
There was a prevailing idea that urinary incontinence is a normal part of aging or childbearing, or that it is hereditary, and that other health issues take priority. Physicians, moreover, did not ask about the issue, and in cases in which it was mentioned, physicians were dismissive or gave misinformation, the study showed.
Dr. Lee said the barriers are significant, and that "there’s a lot of work to be done" to facilitate communication and change practices.
Leaders at Kaiser Permanente Orange County, Anaheim, Calif., plan to give physicians question prompts to incorporate into routine and primary care visits, she noted during a press conference at the meeting. "We also want to work on educational tools for the public," she said.
"There are urinary incontinence questions [for] the Medicare wellness visit, but these are for women over 65," she said. "There certainly are plenty of women under 65 who have incontinence."
Food and Drug Administration warnings about complications associated with transvaginal placement of surgical mesh did not come up in the focus group discussions, but Dr. Lee said that in her practice she has seen this controversy feed patients’ fear of incontinence and its treatment.
Study participants were recruited through e-mail research announcements and flyers, as well as more actively through the offices of urogynecology providers.
Dr. Lee said she had no disclosures to report. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
WASHINGTON – Women are not seeking care for even moderate to severe urinary incontinence because they have a fear of treatment and lack knowledge of the etiology of the condition and available treatment options, according to a qualitative focus group study at Kaiser Permanente Orange County (Calif.).
Embarrassment is another barrier. So are physicians themselves, said Dr. Jennifer K. Lee, a urogynecologist at Kaiser in Anaheim, Calif., who reported the findings at the annual meeting of the American Urogynecologic Society.
"Providers were mentioned (in the focus groups) to be barriers," Dr. Lee said. "Patients were either dismissed, or they were given misinformation."
By 2050, an estimated 50 million Americans will have urinary incontinence. Previous research has shown, however, that less than 50% of women with moderate incontinence seek care.
The focus group study involved 19 women – 11 who had not sought care but were found after recruitment to have moderate to severe incontinence by the Sandvik severity index, and 8 who had previous treatment or had been evaluated for urinary incontinence.
A moderator led discussions among small groups of these women, all of whom were fluent in English and most of whom (90%) had a college education or higher. The participants were of varying ethnicities, including Caucasian, Hispanic, and Asian.
A fear of medical or surgical treatment, including medication side effects and recovery time for surgery, was one of the themes to emerge. Women also said they felt embarrassed and were therefore not likely to raise the issue, particularly with time constraints in their medical visits.
There was a prevailing idea that urinary incontinence is a normal part of aging or childbearing, or that it is hereditary, and that other health issues take priority. Physicians, moreover, did not ask about the issue, and in cases in which it was mentioned, physicians were dismissive or gave misinformation, the study showed.
Dr. Lee said the barriers are significant, and that "there’s a lot of work to be done" to facilitate communication and change practices.
Leaders at Kaiser Permanente Orange County, Anaheim, Calif., plan to give physicians question prompts to incorporate into routine and primary care visits, she noted during a press conference at the meeting. "We also want to work on educational tools for the public," she said.
"There are urinary incontinence questions [for] the Medicare wellness visit, but these are for women over 65," she said. "There certainly are plenty of women under 65 who have incontinence."
Food and Drug Administration warnings about complications associated with transvaginal placement of surgical mesh did not come up in the focus group discussions, but Dr. Lee said that in her practice she has seen this controversy feed patients’ fear of incontinence and its treatment.
Study participants were recruited through e-mail research announcements and flyers, as well as more actively through the offices of urogynecology providers.
Dr. Lee said she had no disclosures to report. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
AT THE AUGS ANNUAL MEETING
Key clinical finding: Be sure to ask women about urinary incontinence.
Major finding: There are significant but modifiable barriers to women seeking care for incontinence.
Data source: A small focus group study involving 19 insured women.
Disclosures: Lead author Jennifer K. Lee, MD, reported that she had no disclosures. Her coauthors and research colleagues at the University of California, Irvine, reported numerous disclosures ranging from speakers’ bureau participation and consulting fees at Hospira and Cadence Pharmaceuticals to consultant positions with Medtronic and Boston Scientific.
Quality measurement of children’s health care: A new stage of growth
Quality measurement for pediatric health care is accelerating – and maturing, some say – with an increasing focus among policy makers, providers, and other experts on developing common quality measures that can be used more consistently and widely and that better capture outcomes and the patient experience.
The ideas aren’t new, but current efforts may be more successful than past ones in addressing what have been unrelenting challenges. Finding quality measures that work in pediatrics is even tougher than in adult health care: Children are developing, dependent, and less often fit into neat categorical condition groups. The scientific evidence base is much weaker, and the overall goals for children in health care involve maximizing development and growth – an upward trajectory that is challenging to measure.
"The measures we use today for child and adolescent health care are mostly process measures, and it’s not always clear that these correlate with the outcomes we want to see," said Dr. David Keller, professor of pediatrics at the University of Colorado and vice chair of clinical affairs and health care transformation at Children’s Hospital Colorado, Aurora.
Increasingly, however, experts involved in measuring child and adolescent health are pursuing common and consistent quality measures that, when used more widely, will facilitate more comparison and the collection of more data to be used in looking at long-term outcomes and population impact.
"It’s mind-boggling to see all the available [pediatric] measures ... all the indices and projects out there. Yet there’s little pooled data, and no common language – especially for routine, general pediatric primary care," said Judith S. Shaw, Ed.D., M.P.H., R.N., executive director of the Vermont Child Health Improvement Program (VCHIP).
Dr. Shaw’s organization is housed at the University of Vermont, in a small state with an advanced health information infrastructure and a history of multi-insurer payment reforms that support medical homes and community health teams. VCHIP has supported and led a string of quality improvement projects over the years, but "we realized we have no longitudinal database to look at what’s transpiring across the state," she explained.
Dr. Shaw recently led a team of pediatricians and other experts from across the state in culling through almost 200 pediatric quality measures developed by the National Committee for Quality Assurance (NCQA), Bright Futures, and an array of other state and national organizations and efforts. They narrowed the list to a core set of about 40 measures that VCHIP will monitor over the long term in an attempt to bring measures in line with each other, identify gaps and areas for refinement, and more easily inform improvements in child health and health care. Chart auditors will collect data from a random sample of charts from participating practices.
Over time, Dr. Shaw hopes to pool her state’s data with data from other states. VCHIP leads and advises a network of more than 15 states with similar Improvement Partnerships (IPs), or collaborative efforts to measure and improve children’s health care. The network provides an opportunity to create the large data pools that are important for pediatrics and measurement of long-term outcomes. First, though, measures will need to be streamlined.
Dr. Shaw and her colleagues recently surveyed the IPs in 12 states and found that, of 100 primary care measures described in the survey, only 8 measures were utilized by more than one state IP. Similarly, among three states measuring asthma care, only 2 of the 14 available measures were used by all three states. "There’s not a lot of overlap in the measures that state QI projects are using," and in the big picture, this is limiting, said Dr. Shaw, also associate professor of pediatrics at the University of Vermont, Burlington.
On a national level, development of the 2013 first "core set" of children’s health care quality measures for voluntary use by Medicaid and State Children’s Health Insurance Programs was driven by the desire to "get everyone [across states] aligned and to be able to make fair comparisons of children’s health across the country," said Sarah H. Scholle, Dr.P.H., vice president of research and analysis at NCQA.
A core set of children’s health care quality measures was mandated by the Children’s Health Insurance Program Reauthorization Act of 2009 (CHIPRA) and developed under the auspices of the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS). It is meant to be annually updated and enhanced – a 2014 version is now in use – and to be usable across different settings and by multiple stakeholders including payers, providers, and consumers. This isn’t an easy task: Many experts maintain that data can rarely be used for both accountability and quality improvement.
A CHIPRA-mandated $55-million Pediatric Quality Measures Program (PQMP) has been supporting seven Centers of Excellence in an effort to refine existing measures in both primary and specialty care and develop new ones that are more evidence based. "[Finally], there has been substantial federal investment for the design of pediatric measures to be used nationally," said Dr. Mark Schuster, who is William Berenberg professor of pediatrics at Harvard Medical School and the principal investigator for CHIPRA’s Center of Excellence for Pediatric Quality Measurement at Boston Children’s Hospital.
Thus far, it "has been hard to learn from each other ... To get a sense of what and how well your peer groups are doing," he said. "We want to be able to go to a hospital that’s consistently doing better on a certain measure and ask, ‘How are you doing this? Can you help us?’ "
Experts in quality measurement say that interest and efforts are increasingly focused on measures that address more than the structure and process of care, as well as on measures that address behavioral and mental health. The national Children’s Core Set includes three measures relating to mental and behavioral health.
NCQA’s Healthcare Effectiveness Data and Information Set (HEDIS) measures – which comprise a large portion of the Children’s Core Set – have "provided a basic floor of measures" for various stakeholders in the health care system by looking at factors such as immunization status and whether or not children receive well-child visits, for instance, "but they don’t say anything about what happens when the child is there or" how the child fares afterwards, said Dr. Charles Homer, chief executive officer of the National Initiative for Children’s Healthcare Quality in Boston, which assists practices, hospitals, and others in using data and measurements to improve.
"You can do very well on the HEDIS measures and still not deliver the kind of care you or I would want our children to get," he said. "As part of our efforts to improve quality, we need data-driven, higher-bar measures. ... I don\'t think we’re very far from adding some of these kinds of metrics."
NCQA’s Dr. Scholle doesn’t disagree. Like Dr. Schuster, she also is serving as a principal investigator for the PQMP. One of her main areas of focus has been the development of measures on adolescent depression care. "We need to see whether adolescents [who receive depression care] are actually improving, with a reduction in symptoms over time," she said.
Also of growing importance in pediatric quality measurement is what experts call the patient or family "experiences of care." Dr. Scholle, for one, can attest to the value of such input when it comes to measuring the quality of depression care. NCQA has been incorporating measurement of the patient/ family experience in some of its HEDIS measures, and Dr. Schuster and his colleagues in Boston have been testing a family experience of care measure in pediatric hospitals across the country.
"We’re not talking about patient satisfaction – we’re talking about the experience of care and the outcomes," said Christina Bethell, Ph.D., M.B.A., M.P.H., professor of pediatrics at the Oregon Health & Science University, Portland, and founding director of the Child and Adolescent Health Measurement Initiative, which focuses on developing patient- and family-centered measures of quality.
"We can’t ask patients about what kinds of lab tests they had, but we can ask them about their behaviors and their functioning and their outcome," she said. "Is the child going to school, for instance? Sleeping well?"
Growing use of electronic medical records and other technologies – as well as financial incentives – may help support the collection of such information. And, Dr. Bethell maintains, there is a wealth of information to tap into from national surveys like the National Survey of Children’s Health that can be used to support state and regional-level measurement and quality improvement.
Overall, "we have a lot of what we need right now to" better measure the quality of children’s health and health care. "We just have to use it better," she said, adding that the main supportive pillars of good quality measurement – policy, culture, technology, and financial incentives – "are coming together." That, she said, "is what’s really changing."
Quality measurement for pediatric health care is accelerating – and maturing, some say – with an increasing focus among policy makers, providers, and other experts on developing common quality measures that can be used more consistently and widely and that better capture outcomes and the patient experience.
The ideas aren’t new, but current efforts may be more successful than past ones in addressing what have been unrelenting challenges. Finding quality measures that work in pediatrics is even tougher than in adult health care: Children are developing, dependent, and less often fit into neat categorical condition groups. The scientific evidence base is much weaker, and the overall goals for children in health care involve maximizing development and growth – an upward trajectory that is challenging to measure.
"The measures we use today for child and adolescent health care are mostly process measures, and it’s not always clear that these correlate with the outcomes we want to see," said Dr. David Keller, professor of pediatrics at the University of Colorado and vice chair of clinical affairs and health care transformation at Children’s Hospital Colorado, Aurora.
Increasingly, however, experts involved in measuring child and adolescent health are pursuing common and consistent quality measures that, when used more widely, will facilitate more comparison and the collection of more data to be used in looking at long-term outcomes and population impact.
"It’s mind-boggling to see all the available [pediatric] measures ... all the indices and projects out there. Yet there’s little pooled data, and no common language – especially for routine, general pediatric primary care," said Judith S. Shaw, Ed.D., M.P.H., R.N., executive director of the Vermont Child Health Improvement Program (VCHIP).
Dr. Shaw’s organization is housed at the University of Vermont, in a small state with an advanced health information infrastructure and a history of multi-insurer payment reforms that support medical homes and community health teams. VCHIP has supported and led a string of quality improvement projects over the years, but "we realized we have no longitudinal database to look at what’s transpiring across the state," she explained.
Dr. Shaw recently led a team of pediatricians and other experts from across the state in culling through almost 200 pediatric quality measures developed by the National Committee for Quality Assurance (NCQA), Bright Futures, and an array of other state and national organizations and efforts. They narrowed the list to a core set of about 40 measures that VCHIP will monitor over the long term in an attempt to bring measures in line with each other, identify gaps and areas for refinement, and more easily inform improvements in child health and health care. Chart auditors will collect data from a random sample of charts from participating practices.
Over time, Dr. Shaw hopes to pool her state’s data with data from other states. VCHIP leads and advises a network of more than 15 states with similar Improvement Partnerships (IPs), or collaborative efforts to measure and improve children’s health care. The network provides an opportunity to create the large data pools that are important for pediatrics and measurement of long-term outcomes. First, though, measures will need to be streamlined.
Dr. Shaw and her colleagues recently surveyed the IPs in 12 states and found that, of 100 primary care measures described in the survey, only 8 measures were utilized by more than one state IP. Similarly, among three states measuring asthma care, only 2 of the 14 available measures were used by all three states. "There’s not a lot of overlap in the measures that state QI projects are using," and in the big picture, this is limiting, said Dr. Shaw, also associate professor of pediatrics at the University of Vermont, Burlington.
On a national level, development of the 2013 first "core set" of children’s health care quality measures for voluntary use by Medicaid and State Children’s Health Insurance Programs was driven by the desire to "get everyone [across states] aligned and to be able to make fair comparisons of children’s health across the country," said Sarah H. Scholle, Dr.P.H., vice president of research and analysis at NCQA.
A core set of children’s health care quality measures was mandated by the Children’s Health Insurance Program Reauthorization Act of 2009 (CHIPRA) and developed under the auspices of the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS). It is meant to be annually updated and enhanced – a 2014 version is now in use – and to be usable across different settings and by multiple stakeholders including payers, providers, and consumers. This isn’t an easy task: Many experts maintain that data can rarely be used for both accountability and quality improvement.
A CHIPRA-mandated $55-million Pediatric Quality Measures Program (PQMP) has been supporting seven Centers of Excellence in an effort to refine existing measures in both primary and specialty care and develop new ones that are more evidence based. "[Finally], there has been substantial federal investment for the design of pediatric measures to be used nationally," said Dr. Mark Schuster, who is William Berenberg professor of pediatrics at Harvard Medical School and the principal investigator for CHIPRA’s Center of Excellence for Pediatric Quality Measurement at Boston Children’s Hospital.
Thus far, it "has been hard to learn from each other ... To get a sense of what and how well your peer groups are doing," he said. "We want to be able to go to a hospital that’s consistently doing better on a certain measure and ask, ‘How are you doing this? Can you help us?’ "
Experts in quality measurement say that interest and efforts are increasingly focused on measures that address more than the structure and process of care, as well as on measures that address behavioral and mental health. The national Children’s Core Set includes three measures relating to mental and behavioral health.
NCQA’s Healthcare Effectiveness Data and Information Set (HEDIS) measures – which comprise a large portion of the Children’s Core Set – have "provided a basic floor of measures" for various stakeholders in the health care system by looking at factors such as immunization status and whether or not children receive well-child visits, for instance, "but they don’t say anything about what happens when the child is there or" how the child fares afterwards, said Dr. Charles Homer, chief executive officer of the National Initiative for Children’s Healthcare Quality in Boston, which assists practices, hospitals, and others in using data and measurements to improve.
"You can do very well on the HEDIS measures and still not deliver the kind of care you or I would want our children to get," he said. "As part of our efforts to improve quality, we need data-driven, higher-bar measures. ... I don\'t think we’re very far from adding some of these kinds of metrics."
NCQA’s Dr. Scholle doesn’t disagree. Like Dr. Schuster, she also is serving as a principal investigator for the PQMP. One of her main areas of focus has been the development of measures on adolescent depression care. "We need to see whether adolescents [who receive depression care] are actually improving, with a reduction in symptoms over time," she said.
Also of growing importance in pediatric quality measurement is what experts call the patient or family "experiences of care." Dr. Scholle, for one, can attest to the value of such input when it comes to measuring the quality of depression care. NCQA has been incorporating measurement of the patient/ family experience in some of its HEDIS measures, and Dr. Schuster and his colleagues in Boston have been testing a family experience of care measure in pediatric hospitals across the country.
"We’re not talking about patient satisfaction – we’re talking about the experience of care and the outcomes," said Christina Bethell, Ph.D., M.B.A., M.P.H., professor of pediatrics at the Oregon Health & Science University, Portland, and founding director of the Child and Adolescent Health Measurement Initiative, which focuses on developing patient- and family-centered measures of quality.
"We can’t ask patients about what kinds of lab tests they had, but we can ask them about their behaviors and their functioning and their outcome," she said. "Is the child going to school, for instance? Sleeping well?"
Growing use of electronic medical records and other technologies – as well as financial incentives – may help support the collection of such information. And, Dr. Bethell maintains, there is a wealth of information to tap into from national surveys like the National Survey of Children’s Health that can be used to support state and regional-level measurement and quality improvement.
Overall, "we have a lot of what we need right now to" better measure the quality of children’s health and health care. "We just have to use it better," she said, adding that the main supportive pillars of good quality measurement – policy, culture, technology, and financial incentives – "are coming together." That, she said, "is what’s really changing."
Quality measurement for pediatric health care is accelerating – and maturing, some say – with an increasing focus among policy makers, providers, and other experts on developing common quality measures that can be used more consistently and widely and that better capture outcomes and the patient experience.
The ideas aren’t new, but current efforts may be more successful than past ones in addressing what have been unrelenting challenges. Finding quality measures that work in pediatrics is even tougher than in adult health care: Children are developing, dependent, and less often fit into neat categorical condition groups. The scientific evidence base is much weaker, and the overall goals for children in health care involve maximizing development and growth – an upward trajectory that is challenging to measure.
"The measures we use today for child and adolescent health care are mostly process measures, and it’s not always clear that these correlate with the outcomes we want to see," said Dr. David Keller, professor of pediatrics at the University of Colorado and vice chair of clinical affairs and health care transformation at Children’s Hospital Colorado, Aurora.
Increasingly, however, experts involved in measuring child and adolescent health are pursuing common and consistent quality measures that, when used more widely, will facilitate more comparison and the collection of more data to be used in looking at long-term outcomes and population impact.
"It’s mind-boggling to see all the available [pediatric] measures ... all the indices and projects out there. Yet there’s little pooled data, and no common language – especially for routine, general pediatric primary care," said Judith S. Shaw, Ed.D., M.P.H., R.N., executive director of the Vermont Child Health Improvement Program (VCHIP).
Dr. Shaw’s organization is housed at the University of Vermont, in a small state with an advanced health information infrastructure and a history of multi-insurer payment reforms that support medical homes and community health teams. VCHIP has supported and led a string of quality improvement projects over the years, but "we realized we have no longitudinal database to look at what’s transpiring across the state," she explained.
Dr. Shaw recently led a team of pediatricians and other experts from across the state in culling through almost 200 pediatric quality measures developed by the National Committee for Quality Assurance (NCQA), Bright Futures, and an array of other state and national organizations and efforts. They narrowed the list to a core set of about 40 measures that VCHIP will monitor over the long term in an attempt to bring measures in line with each other, identify gaps and areas for refinement, and more easily inform improvements in child health and health care. Chart auditors will collect data from a random sample of charts from participating practices.
Over time, Dr. Shaw hopes to pool her state’s data with data from other states. VCHIP leads and advises a network of more than 15 states with similar Improvement Partnerships (IPs), or collaborative efforts to measure and improve children’s health care. The network provides an opportunity to create the large data pools that are important for pediatrics and measurement of long-term outcomes. First, though, measures will need to be streamlined.
Dr. Shaw and her colleagues recently surveyed the IPs in 12 states and found that, of 100 primary care measures described in the survey, only 8 measures were utilized by more than one state IP. Similarly, among three states measuring asthma care, only 2 of the 14 available measures were used by all three states. "There’s not a lot of overlap in the measures that state QI projects are using," and in the big picture, this is limiting, said Dr. Shaw, also associate professor of pediatrics at the University of Vermont, Burlington.
On a national level, development of the 2013 first "core set" of children’s health care quality measures for voluntary use by Medicaid and State Children’s Health Insurance Programs was driven by the desire to "get everyone [across states] aligned and to be able to make fair comparisons of children’s health across the country," said Sarah H. Scholle, Dr.P.H., vice president of research and analysis at NCQA.
A core set of children’s health care quality measures was mandated by the Children’s Health Insurance Program Reauthorization Act of 2009 (CHIPRA) and developed under the auspices of the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS). It is meant to be annually updated and enhanced – a 2014 version is now in use – and to be usable across different settings and by multiple stakeholders including payers, providers, and consumers. This isn’t an easy task: Many experts maintain that data can rarely be used for both accountability and quality improvement.
A CHIPRA-mandated $55-million Pediatric Quality Measures Program (PQMP) has been supporting seven Centers of Excellence in an effort to refine existing measures in both primary and specialty care and develop new ones that are more evidence based. "[Finally], there has been substantial federal investment for the design of pediatric measures to be used nationally," said Dr. Mark Schuster, who is William Berenberg professor of pediatrics at Harvard Medical School and the principal investigator for CHIPRA’s Center of Excellence for Pediatric Quality Measurement at Boston Children’s Hospital.
Thus far, it "has been hard to learn from each other ... To get a sense of what and how well your peer groups are doing," he said. "We want to be able to go to a hospital that’s consistently doing better on a certain measure and ask, ‘How are you doing this? Can you help us?’ "
Experts in quality measurement say that interest and efforts are increasingly focused on measures that address more than the structure and process of care, as well as on measures that address behavioral and mental health. The national Children’s Core Set includes three measures relating to mental and behavioral health.
NCQA’s Healthcare Effectiveness Data and Information Set (HEDIS) measures – which comprise a large portion of the Children’s Core Set – have "provided a basic floor of measures" for various stakeholders in the health care system by looking at factors such as immunization status and whether or not children receive well-child visits, for instance, "but they don’t say anything about what happens when the child is there or" how the child fares afterwards, said Dr. Charles Homer, chief executive officer of the National Initiative for Children’s Healthcare Quality in Boston, which assists practices, hospitals, and others in using data and measurements to improve.
"You can do very well on the HEDIS measures and still not deliver the kind of care you or I would want our children to get," he said. "As part of our efforts to improve quality, we need data-driven, higher-bar measures. ... I don\'t think we’re very far from adding some of these kinds of metrics."
NCQA’s Dr. Scholle doesn’t disagree. Like Dr. Schuster, she also is serving as a principal investigator for the PQMP. One of her main areas of focus has been the development of measures on adolescent depression care. "We need to see whether adolescents [who receive depression care] are actually improving, with a reduction in symptoms over time," she said.
Also of growing importance in pediatric quality measurement is what experts call the patient or family "experiences of care." Dr. Scholle, for one, can attest to the value of such input when it comes to measuring the quality of depression care. NCQA has been incorporating measurement of the patient/ family experience in some of its HEDIS measures, and Dr. Schuster and his colleagues in Boston have been testing a family experience of care measure in pediatric hospitals across the country.
"We’re not talking about patient satisfaction – we’re talking about the experience of care and the outcomes," said Christina Bethell, Ph.D., M.B.A., M.P.H., professor of pediatrics at the Oregon Health & Science University, Portland, and founding director of the Child and Adolescent Health Measurement Initiative, which focuses on developing patient- and family-centered measures of quality.
"We can’t ask patients about what kinds of lab tests they had, but we can ask them about their behaviors and their functioning and their outcome," she said. "Is the child going to school, for instance? Sleeping well?"
Growing use of electronic medical records and other technologies – as well as financial incentives – may help support the collection of such information. And, Dr. Bethell maintains, there is a wealth of information to tap into from national surveys like the National Survey of Children’s Health that can be used to support state and regional-level measurement and quality improvement.
Overall, "we have a lot of what we need right now to" better measure the quality of children’s health and health care. "We just have to use it better," she said, adding that the main supportive pillars of good quality measurement – policy, culture, technology, and financial incentives – "are coming together." That, she said, "is what’s really changing."