User login
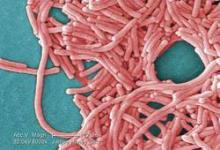
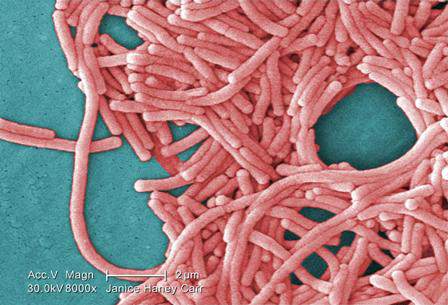
Legionellosis cases continue to increase nationwide
Data from the first 3 years of the Center for Disease Control and Prevention’s Active Bacterial Core surveillance (ABCs) program on legionellosis confirm that incidences of disease caused by the bacteria are increasing across the United States, according to the latest Morbidity and Mortality Weekly Report (MMWR. 2015 Oct 30;64[42]:1190-1193).
The ABCs program, which launched in 2011, identified 1,426 cases of legionellosis over the 3-year time span, and incidence rates of 1.3 (2011), 1.1 (2012), and 1.4 (2013) cases per 100,000 individuals in the general population. This corroborates similar findings made by the National Notifiable Diseases Surveillance System (NNDSS) between 2000 and 2011, which reported an increase in the crude incidence rate of legionellosis nationwide from 0.39 to 1.36 cases per 100,000 individuals.
The two main clinical syndromes associated with legionellosis are Legionnaires’ disease, which is a severe form of pneumonia; and Pontiac fever, a milder illness without pneumonia.
“During 2000-2011, passive surveillance for legionellosis in the United States demonstrated a 249% increase in crude incidence, although little was known about the clinical course and method of diagnosis,” says the report, led by Dr. Kathleen I. Dooling of the CDC’s National Center for Immunization and Respiratory Diseases.
The report also states that “ABCs data during 2011-2013 showed that approximately 44% of patients with legionellosis required intensive care, and 9% died.” Furthermore, incidence increased with age among the ABCs program cohort. Those younger than 50 years of age had a 0.4 incidence rate per 100,000 individuals; patients between 50 and 64 years old had a 2.5 per 100,000 incidence rate; those who were 65-79 years old had a 3.6 per 100,000 incidence rate; and individuals 80 years and older had an incidence rate of 4.7 per 100,000 individuals.
“Among cases identified during 2011-2013, 79% occurred in persons aged >50 years, 65% were in males, and 72% of patients were white,” says the report, adding that “1,300 (91%) received a diagnosis of legionellosis on the basis of urine antigen testing, which only detects Lp1 species.”
The ABCs program defined a confirmed case of legionellosis as “the isolation of Legionella from respiratory culture, detection of Legionella antigen in urine, or seroconversion (a more than fourfold rise in antibody titer between acute and convalescent sera) to Lp1.” Unlike NNDSS, ABCs recorded clinical and race data for each patient found to have legionellosis, finding that incidence rates among blacks were higher than among whites per 100,000 individuals: 1.0 vs. 1.5, respectively.
The report concludes by calling for further research into the disparities in legionellosis cases based on race, age, and geography, as well as the need for “more sensitive laboratory tests for legionellosis because proper diagnosis is needed for treatment and public health action.”
This study was supported by the CDC.
Data from the first 3 years of the Center for Disease Control and Prevention’s Active Bacterial Core surveillance (ABCs) program on legionellosis confirm that incidences of disease caused by the bacteria are increasing across the United States, according to the latest Morbidity and Mortality Weekly Report (MMWR. 2015 Oct 30;64[42]:1190-1193).
The ABCs program, which launched in 2011, identified 1,426 cases of legionellosis over the 3-year time span, and incidence rates of 1.3 (2011), 1.1 (2012), and 1.4 (2013) cases per 100,000 individuals in the general population. This corroborates similar findings made by the National Notifiable Diseases Surveillance System (NNDSS) between 2000 and 2011, which reported an increase in the crude incidence rate of legionellosis nationwide from 0.39 to 1.36 cases per 100,000 individuals.
The two main clinical syndromes associated with legionellosis are Legionnaires’ disease, which is a severe form of pneumonia; and Pontiac fever, a milder illness without pneumonia.
“During 2000-2011, passive surveillance for legionellosis in the United States demonstrated a 249% increase in crude incidence, although little was known about the clinical course and method of diagnosis,” says the report, led by Dr. Kathleen I. Dooling of the CDC’s National Center for Immunization and Respiratory Diseases.
The report also states that “ABCs data during 2011-2013 showed that approximately 44% of patients with legionellosis required intensive care, and 9% died.” Furthermore, incidence increased with age among the ABCs program cohort. Those younger than 50 years of age had a 0.4 incidence rate per 100,000 individuals; patients between 50 and 64 years old had a 2.5 per 100,000 incidence rate; those who were 65-79 years old had a 3.6 per 100,000 incidence rate; and individuals 80 years and older had an incidence rate of 4.7 per 100,000 individuals.
“Among cases identified during 2011-2013, 79% occurred in persons aged >50 years, 65% were in males, and 72% of patients were white,” says the report, adding that “1,300 (91%) received a diagnosis of legionellosis on the basis of urine antigen testing, which only detects Lp1 species.”
The ABCs program defined a confirmed case of legionellosis as “the isolation of Legionella from respiratory culture, detection of Legionella antigen in urine, or seroconversion (a more than fourfold rise in antibody titer between acute and convalescent sera) to Lp1.” Unlike NNDSS, ABCs recorded clinical and race data for each patient found to have legionellosis, finding that incidence rates among blacks were higher than among whites per 100,000 individuals: 1.0 vs. 1.5, respectively.
The report concludes by calling for further research into the disparities in legionellosis cases based on race, age, and geography, as well as the need for “more sensitive laboratory tests for legionellosis because proper diagnosis is needed for treatment and public health action.”
This study was supported by the CDC.
Data from the first 3 years of the Center for Disease Control and Prevention’s Active Bacterial Core surveillance (ABCs) program on legionellosis confirm that incidences of disease caused by the bacteria are increasing across the United States, according to the latest Morbidity and Mortality Weekly Report (MMWR. 2015 Oct 30;64[42]:1190-1193).
The ABCs program, which launched in 2011, identified 1,426 cases of legionellosis over the 3-year time span, and incidence rates of 1.3 (2011), 1.1 (2012), and 1.4 (2013) cases per 100,000 individuals in the general population. This corroborates similar findings made by the National Notifiable Diseases Surveillance System (NNDSS) between 2000 and 2011, which reported an increase in the crude incidence rate of legionellosis nationwide from 0.39 to 1.36 cases per 100,000 individuals.
The two main clinical syndromes associated with legionellosis are Legionnaires’ disease, which is a severe form of pneumonia; and Pontiac fever, a milder illness without pneumonia.
“During 2000-2011, passive surveillance for legionellosis in the United States demonstrated a 249% increase in crude incidence, although little was known about the clinical course and method of diagnosis,” says the report, led by Dr. Kathleen I. Dooling of the CDC’s National Center for Immunization and Respiratory Diseases.
The report also states that “ABCs data during 2011-2013 showed that approximately 44% of patients with legionellosis required intensive care, and 9% died.” Furthermore, incidence increased with age among the ABCs program cohort. Those younger than 50 years of age had a 0.4 incidence rate per 100,000 individuals; patients between 50 and 64 years old had a 2.5 per 100,000 incidence rate; those who were 65-79 years old had a 3.6 per 100,000 incidence rate; and individuals 80 years and older had an incidence rate of 4.7 per 100,000 individuals.
“Among cases identified during 2011-2013, 79% occurred in persons aged >50 years, 65% were in males, and 72% of patients were white,” says the report, adding that “1,300 (91%) received a diagnosis of legionellosis on the basis of urine antigen testing, which only detects Lp1 species.”
The ABCs program defined a confirmed case of legionellosis as “the isolation of Legionella from respiratory culture, detection of Legionella antigen in urine, or seroconversion (a more than fourfold rise in antibody titer between acute and convalescent sera) to Lp1.” Unlike NNDSS, ABCs recorded clinical and race data for each patient found to have legionellosis, finding that incidence rates among blacks were higher than among whites per 100,000 individuals: 1.0 vs. 1.5, respectively.
The report concludes by calling for further research into the disparities in legionellosis cases based on race, age, and geography, as well as the need for “more sensitive laboratory tests for legionellosis because proper diagnosis is needed for treatment and public health action.”
This study was supported by the CDC.
FROM MMWR
Key clinical point: Data from the first 3 years of the CDC’s Active Bacterial Core surveillance program provides evidence confirming that legionellosis rates across the United States are steadily rising.
Major finding: ABCs identified 1,426 legionellosis cases during 2011-2013, for an incidence of 1.3 cases per 100,000 population over the 3 years.
Data source: Analysis of 1,426 legionellosis cases from 2011-2013 collected by the CDC’s ABCs program.
Disclosures: Study supported by the CDC.
FDA approves T-VEC for metastatic melanoma
The Food and Drug Administration has approved talimogene laherparepvec (T-VEC), an oncolytic immunotherapy, for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma that has recurred after initial surgery, the manufacturer Amgen announced today.
The approval officially makes T-VEC (Imlygic) the first virus-based cancer treatment ever approved, as it is derived from the herpes simplex virus. The FDA’s approval comes just months after an FDA advisory panel voiced their support for T-VEC’s approval in a 22-1 vote, based on a phase III study published in the Journal of Clinical Oncology (2015;33 [25]2780-8).
The study looked at 436 individuals with either stage IIIB/C or stage IV (with limited visceral disease burden considered unresectable) and found that patients treated with T-VEC had a significantly higher overall response rate (26.4%) and complete response rate (10.8%) than did those who were treated with subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF) (5.7% and 0.7%, respectively).
The durable response rate was also significantly higher among T-VEC–treated patients (16.3%) than among those on GM-CSF (2.1%). T-VEC patients also experienced a median overall survival time of 23.3 months, compared with 18.9 months for those on GM-CSF. In the study, cellulitis was noted in about 2% of patients on T-VEC. Fifty-four percent of T-VEC patients who responded to the treatment initially had disease progression before response began.
“The evidence of local and systemic immune responses with T-VEC supports combinations with other immunotherapies as a rational approach. A phase 1b/2 study of T-VEC and ipilimumab is evaluating the safety and efficacy of this combination,” wrote the investigators of the phase III study, which was led by Dr. Robert Andtbacka of the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
T-VEC was also scrutinized in five other studies, all of which reported that the most common adverse events related to the drug were fatigue, chills, and pyrexia and flulike symptoms; severity of these symptoms ranged from mild to moderate.
The manufacturer intends to make the therapy available to patients almost immediately and anticipates its cost to average $65,000, according to a statement.
The Food and Drug Administration has approved talimogene laherparepvec (T-VEC), an oncolytic immunotherapy, for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma that has recurred after initial surgery, the manufacturer Amgen announced today.
The approval officially makes T-VEC (Imlygic) the first virus-based cancer treatment ever approved, as it is derived from the herpes simplex virus. The FDA’s approval comes just months after an FDA advisory panel voiced their support for T-VEC’s approval in a 22-1 vote, based on a phase III study published in the Journal of Clinical Oncology (2015;33 [25]2780-8).
The study looked at 436 individuals with either stage IIIB/C or stage IV (with limited visceral disease burden considered unresectable) and found that patients treated with T-VEC had a significantly higher overall response rate (26.4%) and complete response rate (10.8%) than did those who were treated with subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF) (5.7% and 0.7%, respectively).
The durable response rate was also significantly higher among T-VEC–treated patients (16.3%) than among those on GM-CSF (2.1%). T-VEC patients also experienced a median overall survival time of 23.3 months, compared with 18.9 months for those on GM-CSF. In the study, cellulitis was noted in about 2% of patients on T-VEC. Fifty-four percent of T-VEC patients who responded to the treatment initially had disease progression before response began.
“The evidence of local and systemic immune responses with T-VEC supports combinations with other immunotherapies as a rational approach. A phase 1b/2 study of T-VEC and ipilimumab is evaluating the safety and efficacy of this combination,” wrote the investigators of the phase III study, which was led by Dr. Robert Andtbacka of the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
T-VEC was also scrutinized in five other studies, all of which reported that the most common adverse events related to the drug were fatigue, chills, and pyrexia and flulike symptoms; severity of these symptoms ranged from mild to moderate.
The manufacturer intends to make the therapy available to patients almost immediately and anticipates its cost to average $65,000, according to a statement.
The Food and Drug Administration has approved talimogene laherparepvec (T-VEC), an oncolytic immunotherapy, for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma that has recurred after initial surgery, the manufacturer Amgen announced today.
The approval officially makes T-VEC (Imlygic) the first virus-based cancer treatment ever approved, as it is derived from the herpes simplex virus. The FDA’s approval comes just months after an FDA advisory panel voiced their support for T-VEC’s approval in a 22-1 vote, based on a phase III study published in the Journal of Clinical Oncology (2015;33 [25]2780-8).
The study looked at 436 individuals with either stage IIIB/C or stage IV (with limited visceral disease burden considered unresectable) and found that patients treated with T-VEC had a significantly higher overall response rate (26.4%) and complete response rate (10.8%) than did those who were treated with subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF) (5.7% and 0.7%, respectively).
The durable response rate was also significantly higher among T-VEC–treated patients (16.3%) than among those on GM-CSF (2.1%). T-VEC patients also experienced a median overall survival time of 23.3 months, compared with 18.9 months for those on GM-CSF. In the study, cellulitis was noted in about 2% of patients on T-VEC. Fifty-four percent of T-VEC patients who responded to the treatment initially had disease progression before response began.
“The evidence of local and systemic immune responses with T-VEC supports combinations with other immunotherapies as a rational approach. A phase 1b/2 study of T-VEC and ipilimumab is evaluating the safety and efficacy of this combination,” wrote the investigators of the phase III study, which was led by Dr. Robert Andtbacka of the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
T-VEC was also scrutinized in five other studies, all of which reported that the most common adverse events related to the drug were fatigue, chills, and pyrexia and flulike symptoms; severity of these symptoms ranged from mild to moderate.
The manufacturer intends to make the therapy available to patients almost immediately and anticipates its cost to average $65,000, according to a statement.
FROM THE FDA
VIDEO: Identifying preexisting conditions crucial before pneumonectomy, even for benign disease
BOSTON – When performing pneumonectomy on patients with benign disease, it is important to be aware of specific preexisting conditions that could complicate surgery before bringing patients into the operating room.
“Sometimes the usual, standard operative procedure is not appropriate, given the circumstances of a particular patient, [and] typically, these pneumonectomies for benign disease are very challenging operations because the inflamed lung is usually quite densely adherent to the inside of the chest cavity,” explained Dr. G. Alex Patterson of Washington University in St. Louis.
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting sponsored by the American Association for Thoracic Surgeons, Dr. Patterson talked about the challenges associated with pneumonectomies for benign disease and how surgeons can safely navigate them.
Dr. Patterson had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When performing pneumonectomy on patients with benign disease, it is important to be aware of specific preexisting conditions that could complicate surgery before bringing patients into the operating room.
“Sometimes the usual, standard operative procedure is not appropriate, given the circumstances of a particular patient, [and] typically, these pneumonectomies for benign disease are very challenging operations because the inflamed lung is usually quite densely adherent to the inside of the chest cavity,” explained Dr. G. Alex Patterson of Washington University in St. Louis.
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting sponsored by the American Association for Thoracic Surgeons, Dr. Patterson talked about the challenges associated with pneumonectomies for benign disease and how surgeons can safely navigate them.
Dr. Patterson had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When performing pneumonectomy on patients with benign disease, it is important to be aware of specific preexisting conditions that could complicate surgery before bringing patients into the operating room.
“Sometimes the usual, standard operative procedure is not appropriate, given the circumstances of a particular patient, [and] typically, these pneumonectomies for benign disease are very challenging operations because the inflamed lung is usually quite densely adherent to the inside of the chest cavity,” explained Dr. G. Alex Patterson of Washington University in St. Louis.
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting sponsored by the American Association for Thoracic Surgeons, Dr. Patterson talked about the challenges associated with pneumonectomies for benign disease and how surgeons can safely navigate them.
Dr. Patterson had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AATS FOCUS ON THORACIC SURGERY
Pharmacogenetics could be the key to more effective opioid prescription
NATIONAL HARBOR, MD – Use of pharmacogenetic testing could be the key to increasing the effectivweness of opioids in certain patients and the overall effectiveness of therapies and treatments for chronic pain, according to Dr. Anita Gupta.
“Pharmacogenetic testing may help determine if genetic variations [can] impact the metabolism of certain medications [and] lead to unexpected outcomes, such as lack of efficacy or increased or unexpected toxicity,” explained Dr. Gupta of Drexel University in Philadelphia, citing earlier research by another investigator (Clin J Pain. 2010 Jan;26 Suppl 10:S16-20), which found that pharmacogenetic testing can help identify patients who are more likely to respond to opioid treatment, exactly which opioids would produce the most favorable responses, which patients would be at higher risks for opioid-related adverse events and drug interactions, and which patients would require higher-than-average opioid doses.
“In the near future, pharmacogenetic approaches may be implemented to best predict which medicine from the outset may be most appropriate for an individual – the therapy with the most sustained efficacy and the best side effect profile,” the investigators wrote, adding that in current clinical practice, each patient is effectively his own analgesic trial as providers try to hit a moving target as to which and how much opioid treatment is effective for each patient’s needs.
Dr. Gupta also briefly discussed findings from a similar study (Pharmacogenomics. 2009 Jul;10[7]:1157-67), in which the investigators concluded that genotyping drugs could lead to “increased efficiency in proper drug selection, dose optimization, and minimization of adverse drug reactions to improve patient outcome and safety.”
Furthermore, the study “clearly demonstrated a relationship between oxycodone steady-state drug concentrations and pain relief,” and called for more large-scale studies in order to confirm these findings and focus pain management therapy on pharmacogenetics for prescribing opioids.
The need for opioid optimization is very real, Dr. Gupta said at the annual meeting of the American Academy on Pain Medicine. According to findings from another study, about 33% of patients who take antidepressants experience little to no efficacy (Am J Psychiatry. 2006 Nov;163[11]:1905-17). The study examined long-term treatment outcomes as part of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, and found that “those who required more treatment steps had higher relapse rates during the naturalistic follow-up phase,” concluding that “development of more broadly effective treatments [is] needed.”
However, questions linger as to the practical applications of pharmacogenetics, even if the science is widely accepted, Dr. Gupta said. Among the questions that still need to be answered are whether pharmacogenetic testing is reliable, whether providers will be ready and willing to use the information given to them via such testing, whether insurance companies will cover the costs of these tests, and whether tailor-made medicine can lead to discrimination or ethnic biases in treatment.
“We’re at a place where medicine is changing, and it’s an exciting time,” Dr. Gupta noted, pointing to the Human Genome Project as a tool that can be used to help with pharmacogenetics moving forward. “We have so much data overwhelming us, but the future is bright [for] patients and clinicians to improve outcomes.”
Dr. Gupta reported that she receives consulting fees from Millenium Labs.
NATIONAL HARBOR, MD – Use of pharmacogenetic testing could be the key to increasing the effectivweness of opioids in certain patients and the overall effectiveness of therapies and treatments for chronic pain, according to Dr. Anita Gupta.
“Pharmacogenetic testing may help determine if genetic variations [can] impact the metabolism of certain medications [and] lead to unexpected outcomes, such as lack of efficacy or increased or unexpected toxicity,” explained Dr. Gupta of Drexel University in Philadelphia, citing earlier research by another investigator (Clin J Pain. 2010 Jan;26 Suppl 10:S16-20), which found that pharmacogenetic testing can help identify patients who are more likely to respond to opioid treatment, exactly which opioids would produce the most favorable responses, which patients would be at higher risks for opioid-related adverse events and drug interactions, and which patients would require higher-than-average opioid doses.
“In the near future, pharmacogenetic approaches may be implemented to best predict which medicine from the outset may be most appropriate for an individual – the therapy with the most sustained efficacy and the best side effect profile,” the investigators wrote, adding that in current clinical practice, each patient is effectively his own analgesic trial as providers try to hit a moving target as to which and how much opioid treatment is effective for each patient’s needs.
Dr. Gupta also briefly discussed findings from a similar study (Pharmacogenomics. 2009 Jul;10[7]:1157-67), in which the investigators concluded that genotyping drugs could lead to “increased efficiency in proper drug selection, dose optimization, and minimization of adverse drug reactions to improve patient outcome and safety.”
Furthermore, the study “clearly demonstrated a relationship between oxycodone steady-state drug concentrations and pain relief,” and called for more large-scale studies in order to confirm these findings and focus pain management therapy on pharmacogenetics for prescribing opioids.
The need for opioid optimization is very real, Dr. Gupta said at the annual meeting of the American Academy on Pain Medicine. According to findings from another study, about 33% of patients who take antidepressants experience little to no efficacy (Am J Psychiatry. 2006 Nov;163[11]:1905-17). The study examined long-term treatment outcomes as part of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, and found that “those who required more treatment steps had higher relapse rates during the naturalistic follow-up phase,” concluding that “development of more broadly effective treatments [is] needed.”
However, questions linger as to the practical applications of pharmacogenetics, even if the science is widely accepted, Dr. Gupta said. Among the questions that still need to be answered are whether pharmacogenetic testing is reliable, whether providers will be ready and willing to use the information given to them via such testing, whether insurance companies will cover the costs of these tests, and whether tailor-made medicine can lead to discrimination or ethnic biases in treatment.
“We’re at a place where medicine is changing, and it’s an exciting time,” Dr. Gupta noted, pointing to the Human Genome Project as a tool that can be used to help with pharmacogenetics moving forward. “We have so much data overwhelming us, but the future is bright [for] patients and clinicians to improve outcomes.”
Dr. Gupta reported that she receives consulting fees from Millenium Labs.
NATIONAL HARBOR, MD – Use of pharmacogenetic testing could be the key to increasing the effectivweness of opioids in certain patients and the overall effectiveness of therapies and treatments for chronic pain, according to Dr. Anita Gupta.
“Pharmacogenetic testing may help determine if genetic variations [can] impact the metabolism of certain medications [and] lead to unexpected outcomes, such as lack of efficacy or increased or unexpected toxicity,” explained Dr. Gupta of Drexel University in Philadelphia, citing earlier research by another investigator (Clin J Pain. 2010 Jan;26 Suppl 10:S16-20), which found that pharmacogenetic testing can help identify patients who are more likely to respond to opioid treatment, exactly which opioids would produce the most favorable responses, which patients would be at higher risks for opioid-related adverse events and drug interactions, and which patients would require higher-than-average opioid doses.
“In the near future, pharmacogenetic approaches may be implemented to best predict which medicine from the outset may be most appropriate for an individual – the therapy with the most sustained efficacy and the best side effect profile,” the investigators wrote, adding that in current clinical practice, each patient is effectively his own analgesic trial as providers try to hit a moving target as to which and how much opioid treatment is effective for each patient’s needs.
Dr. Gupta also briefly discussed findings from a similar study (Pharmacogenomics. 2009 Jul;10[7]:1157-67), in which the investigators concluded that genotyping drugs could lead to “increased efficiency in proper drug selection, dose optimization, and minimization of adverse drug reactions to improve patient outcome and safety.”
Furthermore, the study “clearly demonstrated a relationship between oxycodone steady-state drug concentrations and pain relief,” and called for more large-scale studies in order to confirm these findings and focus pain management therapy on pharmacogenetics for prescribing opioids.
The need for opioid optimization is very real, Dr. Gupta said at the annual meeting of the American Academy on Pain Medicine. According to findings from another study, about 33% of patients who take antidepressants experience little to no efficacy (Am J Psychiatry. 2006 Nov;163[11]:1905-17). The study examined long-term treatment outcomes as part of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, and found that “those who required more treatment steps had higher relapse rates during the naturalistic follow-up phase,” concluding that “development of more broadly effective treatments [is] needed.”
However, questions linger as to the practical applications of pharmacogenetics, even if the science is widely accepted, Dr. Gupta said. Among the questions that still need to be answered are whether pharmacogenetic testing is reliable, whether providers will be ready and willing to use the information given to them via such testing, whether insurance companies will cover the costs of these tests, and whether tailor-made medicine can lead to discrimination or ethnic biases in treatment.
“We’re at a place where medicine is changing, and it’s an exciting time,” Dr. Gupta noted, pointing to the Human Genome Project as a tool that can be used to help with pharmacogenetics moving forward. “We have so much data overwhelming us, but the future is bright [for] patients and clinicians to improve outcomes.”
Dr. Gupta reported that she receives consulting fees from Millenium Labs.
EXPERT ANALYSIS FROM PAIN 2015
VIDEO: Complications during thoracoscopic lobectomy are surmountable
BOSTON – When it comes to intraoperative complications during thoracoscopic lobectomy, the mantra for success is to always have a preoperative plan, but be flexible enough to improvise should anything out of the norm arise.
“Many surgeons, when they ask [me] about this specific topic, ask what specific tricks [I] have, but I don’t like to use the word ‘trick’ [because] it’s not something we can do that other people can’t,” explained Dr. Thomas A. D’Amico, chief of general thoracic surgery at Duke University in Durham, North Carolina.
“It’s really just about strategy – how you start an operation, what the conduct of it should be, and when you see things that are less common or more difficult cases, how you think about those and manage those.”
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting held by the American Association for Thoracic Surgeons, Dr. D’Amico talked about why surgeons around the world are apprehensive about thoracoscopic lobectomy and why it’s important to begin training residents on how to properly perform the procedure as soon as possible, as it helps mitigate uncertainty while giving them valuable experience to solve any issues that may come up during an operation.
Dr. D’Amico disclosed that he is a consultant for Scanlan, but that it is not relevant to this discussion.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When it comes to intraoperative complications during thoracoscopic lobectomy, the mantra for success is to always have a preoperative plan, but be flexible enough to improvise should anything out of the norm arise.
“Many surgeons, when they ask [me] about this specific topic, ask what specific tricks [I] have, but I don’t like to use the word ‘trick’ [because] it’s not something we can do that other people can’t,” explained Dr. Thomas A. D’Amico, chief of general thoracic surgery at Duke University in Durham, North Carolina.
“It’s really just about strategy – how you start an operation, what the conduct of it should be, and when you see things that are less common or more difficult cases, how you think about those and manage those.”
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting held by the American Association for Thoracic Surgeons, Dr. D’Amico talked about why surgeons around the world are apprehensive about thoracoscopic lobectomy and why it’s important to begin training residents on how to properly perform the procedure as soon as possible, as it helps mitigate uncertainty while giving them valuable experience to solve any issues that may come up during an operation.
Dr. D’Amico disclosed that he is a consultant for Scanlan, but that it is not relevant to this discussion.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When it comes to intraoperative complications during thoracoscopic lobectomy, the mantra for success is to always have a preoperative plan, but be flexible enough to improvise should anything out of the norm arise.
“Many surgeons, when they ask [me] about this specific topic, ask what specific tricks [I] have, but I don’t like to use the word ‘trick’ [because] it’s not something we can do that other people can’t,” explained Dr. Thomas A. D’Amico, chief of general thoracic surgery at Duke University in Durham, North Carolina.
“It’s really just about strategy – how you start an operation, what the conduct of it should be, and when you see things that are less common or more difficult cases, how you think about those and manage those.”
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting held by the American Association for Thoracic Surgeons, Dr. D’Amico talked about why surgeons around the world are apprehensive about thoracoscopic lobectomy and why it’s important to begin training residents on how to properly perform the procedure as soon as possible, as it helps mitigate uncertainty while giving them valuable experience to solve any issues that may come up during an operation.
Dr. D’Amico disclosed that he is a consultant for Scanlan, but that it is not relevant to this discussion.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AATS FOCUS ON THORACIC SURGERY
Getting a good night’s sleep key to reducing chronic pain
NATIONAL HARBOR, MD – While Americans search high and low for solutions to their chronic pain problems, one of the simplest fixes may be right under their noses: getting a good night’s sleep.
Dr. Ashwin Mehta of the University of Miami spoke at length about the need for deep, restful sleep, and the relationship between circadian rhythms and chronic lower back pain, during a presentation at the annual meeting of the American Academy of Pain Medicine.
“REM [rapid eye movement] sleep [can] optimize regulation of physiological functions,” explained Dr. Mehta, adding that sleep loss can lead to altered immune responses, changes in circulating levels of leukocytes and cytokines, and decreased levels of antibodies.
Citing earlier research by another investigator, Dr. Mehta explained that there is evidence pain is directly correlated to fatigue, with sleep holding the key to solving problems with both (J Pain Symptom Manage. 2010 Jan;39[1]:126-38). Particularly in cancer patients, restfulness was shown to improve mood and well-being in a similar study (J Clin Oncol. 2012 Mar 19;30[12]:1335-42).
Dr. Mehta also reviewed known information that neuropeptides, specifically, serotonin, glutamate, gama-aminobutyric acid, and substance P, are linked to poor sleep, poor mood, and chronic pain. Multidisciplinary approaches should be considered by physicians and patients to find the most effective strategy for getting good sleep and mitigating chronic pain. These approaches can include, among other things, cognitive-behavioral interventions, increased exercise, breathing exercises, and yoga (Sleep. 2011 Dec 1;34[12]:1631-40).
Magnesium, vitamin D, and products with lavender (Am J Crit Care. 2014 Jan;23[1]:24-9) and passionflower (Phytother Res. 2011 Aug;25[8]:1153-9) – such as oil and tea – can help with achieving restful sleep on a consistent basis, and have been studied as recently as 2014 and 2011, respectively, said Dr. Mehta.
Naps can also be effective, although impractical for most patients. Finding time either in the middle of the day or after work for a nap can help improve sleep at night, while walking a mile after dinner is also highly recommended. Good “sleep hygiene” is also encouraged; this includes sleeping someplace that is both quiet and dark, without any bright lights – this includes the omnipresent cell phones, laptops, tablet computers, and televisions. Patients should also never eat while in bed, and refrain from alcohol and caffeine before going to bed.
Dr. Mehta did not report any relevant financial disclosures.
NATIONAL HARBOR, MD – While Americans search high and low for solutions to their chronic pain problems, one of the simplest fixes may be right under their noses: getting a good night’s sleep.
Dr. Ashwin Mehta of the University of Miami spoke at length about the need for deep, restful sleep, and the relationship between circadian rhythms and chronic lower back pain, during a presentation at the annual meeting of the American Academy of Pain Medicine.
“REM [rapid eye movement] sleep [can] optimize regulation of physiological functions,” explained Dr. Mehta, adding that sleep loss can lead to altered immune responses, changes in circulating levels of leukocytes and cytokines, and decreased levels of antibodies.
Citing earlier research by another investigator, Dr. Mehta explained that there is evidence pain is directly correlated to fatigue, with sleep holding the key to solving problems with both (J Pain Symptom Manage. 2010 Jan;39[1]:126-38). Particularly in cancer patients, restfulness was shown to improve mood and well-being in a similar study (J Clin Oncol. 2012 Mar 19;30[12]:1335-42).
Dr. Mehta also reviewed known information that neuropeptides, specifically, serotonin, glutamate, gama-aminobutyric acid, and substance P, are linked to poor sleep, poor mood, and chronic pain. Multidisciplinary approaches should be considered by physicians and patients to find the most effective strategy for getting good sleep and mitigating chronic pain. These approaches can include, among other things, cognitive-behavioral interventions, increased exercise, breathing exercises, and yoga (Sleep. 2011 Dec 1;34[12]:1631-40).
Magnesium, vitamin D, and products with lavender (Am J Crit Care. 2014 Jan;23[1]:24-9) and passionflower (Phytother Res. 2011 Aug;25[8]:1153-9) – such as oil and tea – can help with achieving restful sleep on a consistent basis, and have been studied as recently as 2014 and 2011, respectively, said Dr. Mehta.
Naps can also be effective, although impractical for most patients. Finding time either in the middle of the day or after work for a nap can help improve sleep at night, while walking a mile after dinner is also highly recommended. Good “sleep hygiene” is also encouraged; this includes sleeping someplace that is both quiet and dark, without any bright lights – this includes the omnipresent cell phones, laptops, tablet computers, and televisions. Patients should also never eat while in bed, and refrain from alcohol and caffeine before going to bed.
Dr. Mehta did not report any relevant financial disclosures.
NATIONAL HARBOR, MD – While Americans search high and low for solutions to their chronic pain problems, one of the simplest fixes may be right under their noses: getting a good night’s sleep.
Dr. Ashwin Mehta of the University of Miami spoke at length about the need for deep, restful sleep, and the relationship between circadian rhythms and chronic lower back pain, during a presentation at the annual meeting of the American Academy of Pain Medicine.
“REM [rapid eye movement] sleep [can] optimize regulation of physiological functions,” explained Dr. Mehta, adding that sleep loss can lead to altered immune responses, changes in circulating levels of leukocytes and cytokines, and decreased levels of antibodies.
Citing earlier research by another investigator, Dr. Mehta explained that there is evidence pain is directly correlated to fatigue, with sleep holding the key to solving problems with both (J Pain Symptom Manage. 2010 Jan;39[1]:126-38). Particularly in cancer patients, restfulness was shown to improve mood and well-being in a similar study (J Clin Oncol. 2012 Mar 19;30[12]:1335-42).
Dr. Mehta also reviewed known information that neuropeptides, specifically, serotonin, glutamate, gama-aminobutyric acid, and substance P, are linked to poor sleep, poor mood, and chronic pain. Multidisciplinary approaches should be considered by physicians and patients to find the most effective strategy for getting good sleep and mitigating chronic pain. These approaches can include, among other things, cognitive-behavioral interventions, increased exercise, breathing exercises, and yoga (Sleep. 2011 Dec 1;34[12]:1631-40).
Magnesium, vitamin D, and products with lavender (Am J Crit Care. 2014 Jan;23[1]:24-9) and passionflower (Phytother Res. 2011 Aug;25[8]:1153-9) – such as oil and tea – can help with achieving restful sleep on a consistent basis, and have been studied as recently as 2014 and 2011, respectively, said Dr. Mehta.
Naps can also be effective, although impractical for most patients. Finding time either in the middle of the day or after work for a nap can help improve sleep at night, while walking a mile after dinner is also highly recommended. Good “sleep hygiene” is also encouraged; this includes sleeping someplace that is both quiet and dark, without any bright lights – this includes the omnipresent cell phones, laptops, tablet computers, and televisions. Patients should also never eat while in bed, and refrain from alcohol and caffeine before going to bed.
Dr. Mehta did not report any relevant financial disclosures.
AT PAIN 2015
ACIP recommends changes to 2016 adult immunization schedule
The Advisory Committee on Immunization Practices (ACIP) has unanimously approved a series of changes to the adult immunization schedule for several vaccines.
The changes, which must next be approved by the director of the Centers for Disease Control and Prevention, will take effect in 2016. The biggest shifts are related to meningococcal B vaccinations, which are now listed separately in the schedule from vaccination recommendations for all other meningococcal strains.
Adults with asplenia “or complement deficiencies,” microbiologists, and those who routinely work in outbreak settings are recommended to get either a two-dose series of MenB-4C (Bexsero) or a three-dose series of MenB-FHbp (Trumenba) in the 2016 guidelines, according to Dr. David Kim of the CDC’s National Center for Immunization and Respiratory Diseases, who presented the changes at a meeting of the CDC’s ACIP on Oct. 21. The same vaccine type should be taken through an immunization series, as these are not interchangeable.
However, vaccination is not recommended for individuals who travel or live in countries where meningococcal disease is hyperendemic or epidemic, as the disease in these countries is generally not known to be caused by serogroup B. Also, young adults aged 16-23 years maybe receive the vaccine to provide short-term protection against most strains of MenB disease; however, it is mostly recommended for individuals aged 16-18 years. The recommendation for young adults is a Category B recommendation, which means it has not yet been fully integrated into the 2016 schedule and will be listed in the footnotes of the schedule.
For the human papillomavirus vaccine, all women aged 19-26 years are recommended to receive a three-dose series of either 2-valent, 4-valent, or 9-valent HPV vaccine unless they have been vaccinated previously or did not complete the previous series. Men aged 19-21 years should also get a three-dose series of either 4-valent or 9-valent HPV vaccine, according to the recommendations. Homosexual and immunocompromised men, including those with HIV infections, should receive a three-dose series of either 4-valent or 9-valent HPV vaccines through age 26.
Updates were also approved for pneumococcal virus vaccinations, as there are now changes in intervals for PCV13 and PPSV23. Immunocompetent adults 65 years and older should receive PPSV23 at least 1 year after PCV13, not 6-12 months afterward as recommended under the 2015 schedule.
The Advisory Committee on Immunization Practices (ACIP) has unanimously approved a series of changes to the adult immunization schedule for several vaccines.
The changes, which must next be approved by the director of the Centers for Disease Control and Prevention, will take effect in 2016. The biggest shifts are related to meningococcal B vaccinations, which are now listed separately in the schedule from vaccination recommendations for all other meningococcal strains.
Adults with asplenia “or complement deficiencies,” microbiologists, and those who routinely work in outbreak settings are recommended to get either a two-dose series of MenB-4C (Bexsero) or a three-dose series of MenB-FHbp (Trumenba) in the 2016 guidelines, according to Dr. David Kim of the CDC’s National Center for Immunization and Respiratory Diseases, who presented the changes at a meeting of the CDC’s ACIP on Oct. 21. The same vaccine type should be taken through an immunization series, as these are not interchangeable.
However, vaccination is not recommended for individuals who travel or live in countries where meningococcal disease is hyperendemic or epidemic, as the disease in these countries is generally not known to be caused by serogroup B. Also, young adults aged 16-23 years maybe receive the vaccine to provide short-term protection against most strains of MenB disease; however, it is mostly recommended for individuals aged 16-18 years. The recommendation for young adults is a Category B recommendation, which means it has not yet been fully integrated into the 2016 schedule and will be listed in the footnotes of the schedule.
For the human papillomavirus vaccine, all women aged 19-26 years are recommended to receive a three-dose series of either 2-valent, 4-valent, or 9-valent HPV vaccine unless they have been vaccinated previously or did not complete the previous series. Men aged 19-21 years should also get a three-dose series of either 4-valent or 9-valent HPV vaccine, according to the recommendations. Homosexual and immunocompromised men, including those with HIV infections, should receive a three-dose series of either 4-valent or 9-valent HPV vaccines through age 26.
Updates were also approved for pneumococcal virus vaccinations, as there are now changes in intervals for PCV13 and PPSV23. Immunocompetent adults 65 years and older should receive PPSV23 at least 1 year after PCV13, not 6-12 months afterward as recommended under the 2015 schedule.
The Advisory Committee on Immunization Practices (ACIP) has unanimously approved a series of changes to the adult immunization schedule for several vaccines.
The changes, which must next be approved by the director of the Centers for Disease Control and Prevention, will take effect in 2016. The biggest shifts are related to meningococcal B vaccinations, which are now listed separately in the schedule from vaccination recommendations for all other meningococcal strains.
Adults with asplenia “or complement deficiencies,” microbiologists, and those who routinely work in outbreak settings are recommended to get either a two-dose series of MenB-4C (Bexsero) or a three-dose series of MenB-FHbp (Trumenba) in the 2016 guidelines, according to Dr. David Kim of the CDC’s National Center for Immunization and Respiratory Diseases, who presented the changes at a meeting of the CDC’s ACIP on Oct. 21. The same vaccine type should be taken through an immunization series, as these are not interchangeable.
However, vaccination is not recommended for individuals who travel or live in countries where meningococcal disease is hyperendemic or epidemic, as the disease in these countries is generally not known to be caused by serogroup B. Also, young adults aged 16-23 years maybe receive the vaccine to provide short-term protection against most strains of MenB disease; however, it is mostly recommended for individuals aged 16-18 years. The recommendation for young adults is a Category B recommendation, which means it has not yet been fully integrated into the 2016 schedule and will be listed in the footnotes of the schedule.
For the human papillomavirus vaccine, all women aged 19-26 years are recommended to receive a three-dose series of either 2-valent, 4-valent, or 9-valent HPV vaccine unless they have been vaccinated previously or did not complete the previous series. Men aged 19-21 years should also get a three-dose series of either 4-valent or 9-valent HPV vaccine, according to the recommendations. Homosexual and immunocompromised men, including those with HIV infections, should receive a three-dose series of either 4-valent or 9-valent HPV vaccines through age 26.
Updates were also approved for pneumococcal virus vaccinations, as there are now changes in intervals for PCV13 and PPSV23. Immunocompetent adults 65 years and older should receive PPSV23 at least 1 year after PCV13, not 6-12 months afterward as recommended under the 2015 schedule.
FROM AN ACIP MEETING
ACIP Updates Immunization Schedules for Children, Adolescents
Changes to meningococcal B, DTaP, polio, and human papillomavirus (HPV) vaccination recommendations were among those unanimously approved at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) for child and adolescent immunization schedules.
Meningococcal B vaccine has now been added to the 2016 immunization schedule, and is recommended for high-risk children beginning at age 10 years. The schedule also includes recommendations for adolescents age 16 years and older in “not high-risk groups that may receive this vaccine subject to individual clinical decision-making,” according to Dr. Candice Robinson of the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. Relevant information for administration of meningococcal B vaccination has been added to the footnotes and routine vaccination sections as well.
In the footnotes section, DTaP recommendations have been revised to add Quadracel to the minimum age exception line, and administration instructions of inadvertent doses have been clarified. The new instructions will now read: “If the fourth dose of DTaP was administered at least 4 months, but less than 6 months, after the third dose of DTaP, it need not be repeated [and] the 4-day grace period may not be used for the fourth dose given less than 6 months after the third dose of DTaP.”
“We use the description of ‘inadvertent dose’ to indicate that this shortened interval between doses should not be used prospectively; however, the dose could be counted as valid after the fact,” Dr. Robinson explained.
Footnotes for inactivated polio vaccine (IPV) will now include guidance for IPV use in patients who have previously received only oral polio vaccine (OPV) and did not receive any doses after their fourth birthday. This addition, which was added after repeated inquiries from providers, will read: “If only OPV were administered, and all doses were given prior to 4 years of age, one dose of IPV should be given at 4 years or older at least 4 weeks after the last dose of [OPV].”
For HPV, children aged 9-10 years will now be recommended for vaccination if they are “high risk” – indicated by a purple bar on Figure 1 of the 2016 immunization schedule – due to a history of sexual abuse in their youth. Furthermore, all HPV nomenclature in the 2016 recommendations will be updated to reflect currently accepted naming formats; for example, HPV4 will now be called 4-valent or 4V HPV. In the footnotes, the 9-valent HPV vaccine is now included along with the 5-valent and 4-valent variants, along with restructured language to clarify administration instructions.
Other changes to Figure 1 of next year’s immunization schedules include moving the Tdap recommendations to a lower section of the table, and moving the pneumococcal polysaccharide vaccination schedule toward the bottom of the chart because it is “not routinely recommended for any population.” Haemophilus influenzae type b (Hib) vaccinations are now recommended for certain high-risk groups in patients aged 5-18 years, in accordance with text in the recommendations’ footnotes that suggested vaccination in this age group for those who had previously been vaccinated or have other high-risk conditions.
The catch-up schedule will have one minor change – “in the Tdap line’s Dose 2-3 column, we added Tdap and Td to the list of possible previous vaccines,” Dr. Robinson said.
Revisions will be made to be submitted for approval by Dr. Tom Frieden, CDC director. The edited copy of these updated recommendations will be submitted to the American Academy of Pediatrics, American Academy of Family Physicians, and American Congress of Obstetricians and Gynecologists by Jan. 1, 2016, with publication by the CDC and partner publications following in January and February.
Changes to meningococcal B, DTaP, polio, and human papillomavirus (HPV) vaccination recommendations were among those unanimously approved at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) for child and adolescent immunization schedules.
Meningococcal B vaccine has now been added to the 2016 immunization schedule, and is recommended for high-risk children beginning at age 10 years. The schedule also includes recommendations for adolescents age 16 years and older in “not high-risk groups that may receive this vaccine subject to individual clinical decision-making,” according to Dr. Candice Robinson of the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. Relevant information for administration of meningococcal B vaccination has been added to the footnotes and routine vaccination sections as well.
In the footnotes section, DTaP recommendations have been revised to add Quadracel to the minimum age exception line, and administration instructions of inadvertent doses have been clarified. The new instructions will now read: “If the fourth dose of DTaP was administered at least 4 months, but less than 6 months, after the third dose of DTaP, it need not be repeated [and] the 4-day grace period may not be used for the fourth dose given less than 6 months after the third dose of DTaP.”
“We use the description of ‘inadvertent dose’ to indicate that this shortened interval between doses should not be used prospectively; however, the dose could be counted as valid after the fact,” Dr. Robinson explained.
Footnotes for inactivated polio vaccine (IPV) will now include guidance for IPV use in patients who have previously received only oral polio vaccine (OPV) and did not receive any doses after their fourth birthday. This addition, which was added after repeated inquiries from providers, will read: “If only OPV were administered, and all doses were given prior to 4 years of age, one dose of IPV should be given at 4 years or older at least 4 weeks after the last dose of [OPV].”
For HPV, children aged 9-10 years will now be recommended for vaccination if they are “high risk” – indicated by a purple bar on Figure 1 of the 2016 immunization schedule – due to a history of sexual abuse in their youth. Furthermore, all HPV nomenclature in the 2016 recommendations will be updated to reflect currently accepted naming formats; for example, HPV4 will now be called 4-valent or 4V HPV. In the footnotes, the 9-valent HPV vaccine is now included along with the 5-valent and 4-valent variants, along with restructured language to clarify administration instructions.
Other changes to Figure 1 of next year’s immunization schedules include moving the Tdap recommendations to a lower section of the table, and moving the pneumococcal polysaccharide vaccination schedule toward the bottom of the chart because it is “not routinely recommended for any population.” Haemophilus influenzae type b (Hib) vaccinations are now recommended for certain high-risk groups in patients aged 5-18 years, in accordance with text in the recommendations’ footnotes that suggested vaccination in this age group for those who had previously been vaccinated or have other high-risk conditions.
The catch-up schedule will have one minor change – “in the Tdap line’s Dose 2-3 column, we added Tdap and Td to the list of possible previous vaccines,” Dr. Robinson said.
Revisions will be made to be submitted for approval by Dr. Tom Frieden, CDC director. The edited copy of these updated recommendations will be submitted to the American Academy of Pediatrics, American Academy of Family Physicians, and American Congress of Obstetricians and Gynecologists by Jan. 1, 2016, with publication by the CDC and partner publications following in January and February.
Changes to meningococcal B, DTaP, polio, and human papillomavirus (HPV) vaccination recommendations were among those unanimously approved at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) for child and adolescent immunization schedules.
Meningococcal B vaccine has now been added to the 2016 immunization schedule, and is recommended for high-risk children beginning at age 10 years. The schedule also includes recommendations for adolescents age 16 years and older in “not high-risk groups that may receive this vaccine subject to individual clinical decision-making,” according to Dr. Candice Robinson of the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. Relevant information for administration of meningococcal B vaccination has been added to the footnotes and routine vaccination sections as well.
In the footnotes section, DTaP recommendations have been revised to add Quadracel to the minimum age exception line, and administration instructions of inadvertent doses have been clarified. The new instructions will now read: “If the fourth dose of DTaP was administered at least 4 months, but less than 6 months, after the third dose of DTaP, it need not be repeated [and] the 4-day grace period may not be used for the fourth dose given less than 6 months after the third dose of DTaP.”
“We use the description of ‘inadvertent dose’ to indicate that this shortened interval between doses should not be used prospectively; however, the dose could be counted as valid after the fact,” Dr. Robinson explained.
Footnotes for inactivated polio vaccine (IPV) will now include guidance for IPV use in patients who have previously received only oral polio vaccine (OPV) and did not receive any doses after their fourth birthday. This addition, which was added after repeated inquiries from providers, will read: “If only OPV were administered, and all doses were given prior to 4 years of age, one dose of IPV should be given at 4 years or older at least 4 weeks after the last dose of [OPV].”
For HPV, children aged 9-10 years will now be recommended for vaccination if they are “high risk” – indicated by a purple bar on Figure 1 of the 2016 immunization schedule – due to a history of sexual abuse in their youth. Furthermore, all HPV nomenclature in the 2016 recommendations will be updated to reflect currently accepted naming formats; for example, HPV4 will now be called 4-valent or 4V HPV. In the footnotes, the 9-valent HPV vaccine is now included along with the 5-valent and 4-valent variants, along with restructured language to clarify administration instructions.
Other changes to Figure 1 of next year’s immunization schedules include moving the Tdap recommendations to a lower section of the table, and moving the pneumococcal polysaccharide vaccination schedule toward the bottom of the chart because it is “not routinely recommended for any population.” Haemophilus influenzae type b (Hib) vaccinations are now recommended for certain high-risk groups in patients aged 5-18 years, in accordance with text in the recommendations’ footnotes that suggested vaccination in this age group for those who had previously been vaccinated or have other high-risk conditions.
The catch-up schedule will have one minor change – “in the Tdap line’s Dose 2-3 column, we added Tdap and Td to the list of possible previous vaccines,” Dr. Robinson said.
Revisions will be made to be submitted for approval by Dr. Tom Frieden, CDC director. The edited copy of these updated recommendations will be submitted to the American Academy of Pediatrics, American Academy of Family Physicians, and American Congress of Obstetricians and Gynecologists by Jan. 1, 2016, with publication by the CDC and partner publications following in January and February.
FROM AN ACIP MEETING
ACIP updates immunization schedules for children, adolescents
Changes to meningococcal B, DTaP, polio, and human papillomavirus (HPV) vaccination recommendations were among those unanimously approved at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) for child and adolescent immunization schedules.
Meningococcal B vaccine has now been added to the 2016 immunization schedule, and is recommended for high-risk children beginning at age 10 years. The schedule also includes recommendations for adolescents age 16 years and older in “not high-risk groups that may receive this vaccine subject to individual clinical decision-making,” according to Dr. Candice Robinson of the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. Relevant information for administration of meningococcal B vaccination has been added to the footnotes and routine vaccination sections as well.
In the footnotes section, DTaP recommendations have been revised to add Quadracel to the minimum age exception line, and administration instructions of inadvertent doses have been clarified. The new instructions will now read: “If the fourth dose of DTaP was administered at least 4 months, but less than 6 months, after the third dose of DTaP, it need not be repeated [and] the 4-day grace period may not be used for the fourth dose given less than 6 months after the third dose of DTaP.”
“We use the description of ‘inadvertent dose’ to indicate that this shortened interval between doses should not be used prospectively; however, the dose could be counted as valid after the fact,” Dr. Robinson explained.
Footnotes for inactivated polio vaccine (IPV) will now include guidance for IPV use in patients who have previously received only oral polio vaccine (OPV) and did not receive any doses after their fourth birthday. This addition, which was added after repeated inquiries from providers, will read: “If only OPV were administered, and all doses were given prior to 4 years of age, one dose of IPV should be given at 4 years or older at least 4 weeks after the last dose of [OPV].”
For HPV, children aged 9-10 years will now be recommended for vaccination if they are “high risk” – indicated by a purple bar on Figure 1 of the 2016 immunization schedule – due to a history of sexual abuse in their youth. Furthermore, all HPV nomenclature in the 2016 recommendations will be updated to reflect currently accepted naming formats; for example, HPV4 will now be called 4-valent or 4V HPV. In the footnotes, the 9-valent HPV vaccine is now included along with the 5-valent and 4-valent variants, along with restructured language to clarify administration instructions.
Other changes to Figure 1 of next year’s immunization schedules include moving the Tdap recommendations to a lower section of the table, and moving the pneumococcal polysaccharide vaccination schedule toward the bottom of the chart because it is “not routinely recommended for any population.” Haemophilus influenzae type b (Hib) vaccinations are now recommended for certain high-risk groups in patients aged 5-18 years, in accordance with text in the recommendations’ footnotes that suggested vaccination in this age group for those who had previously been vaccinated or have other high-risk conditions.
The catch-up schedule will have one minor change – “in the Tdap line’s Dose 2-3 column, we added Tdap and Td to the list of possible previous vaccines,” Dr. Robinson said.
Revisions will be made to be submitted for approval by Dr. Tom Frieden, CDC director. The edited copy of these updated recommendations will be submitted to the American Academy of Pediatrics, American Academy of Family Physicians, and American Congress of Obstetricians and Gynecologists by Jan. 1, 2016, with publication by the CDC and partner publications following in January and February.
Changes to meningococcal B, DTaP, polio, and human papillomavirus (HPV) vaccination recommendations were among those unanimously approved at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) for child and adolescent immunization schedules.
Meningococcal B vaccine has now been added to the 2016 immunization schedule, and is recommended for high-risk children beginning at age 10 years. The schedule also includes recommendations for adolescents age 16 years and older in “not high-risk groups that may receive this vaccine subject to individual clinical decision-making,” according to Dr. Candice Robinson of the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. Relevant information for administration of meningococcal B vaccination has been added to the footnotes and routine vaccination sections as well.
In the footnotes section, DTaP recommendations have been revised to add Quadracel to the minimum age exception line, and administration instructions of inadvertent doses have been clarified. The new instructions will now read: “If the fourth dose of DTaP was administered at least 4 months, but less than 6 months, after the third dose of DTaP, it need not be repeated [and] the 4-day grace period may not be used for the fourth dose given less than 6 months after the third dose of DTaP.”
“We use the description of ‘inadvertent dose’ to indicate that this shortened interval between doses should not be used prospectively; however, the dose could be counted as valid after the fact,” Dr. Robinson explained.
Footnotes for inactivated polio vaccine (IPV) will now include guidance for IPV use in patients who have previously received only oral polio vaccine (OPV) and did not receive any doses after their fourth birthday. This addition, which was added after repeated inquiries from providers, will read: “If only OPV were administered, and all doses were given prior to 4 years of age, one dose of IPV should be given at 4 years or older at least 4 weeks after the last dose of [OPV].”
For HPV, children aged 9-10 years will now be recommended for vaccination if they are “high risk” – indicated by a purple bar on Figure 1 of the 2016 immunization schedule – due to a history of sexual abuse in their youth. Furthermore, all HPV nomenclature in the 2016 recommendations will be updated to reflect currently accepted naming formats; for example, HPV4 will now be called 4-valent or 4V HPV. In the footnotes, the 9-valent HPV vaccine is now included along with the 5-valent and 4-valent variants, along with restructured language to clarify administration instructions.
Other changes to Figure 1 of next year’s immunization schedules include moving the Tdap recommendations to a lower section of the table, and moving the pneumococcal polysaccharide vaccination schedule toward the bottom of the chart because it is “not routinely recommended for any population.” Haemophilus influenzae type b (Hib) vaccinations are now recommended for certain high-risk groups in patients aged 5-18 years, in accordance with text in the recommendations’ footnotes that suggested vaccination in this age group for those who had previously been vaccinated or have other high-risk conditions.
The catch-up schedule will have one minor change – “in the Tdap line’s Dose 2-3 column, we added Tdap and Td to the list of possible previous vaccines,” Dr. Robinson said.
Revisions will be made to be submitted for approval by Dr. Tom Frieden, CDC director. The edited copy of these updated recommendations will be submitted to the American Academy of Pediatrics, American Academy of Family Physicians, and American Congress of Obstetricians and Gynecologists by Jan. 1, 2016, with publication by the CDC and partner publications following in January and February.
Changes to meningococcal B, DTaP, polio, and human papillomavirus (HPV) vaccination recommendations were among those unanimously approved at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) for child and adolescent immunization schedules.
Meningococcal B vaccine has now been added to the 2016 immunization schedule, and is recommended for high-risk children beginning at age 10 years. The schedule also includes recommendations for adolescents age 16 years and older in “not high-risk groups that may receive this vaccine subject to individual clinical decision-making,” according to Dr. Candice Robinson of the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta. Relevant information for administration of meningococcal B vaccination has been added to the footnotes and routine vaccination sections as well.
In the footnotes section, DTaP recommendations have been revised to add Quadracel to the minimum age exception line, and administration instructions of inadvertent doses have been clarified. The new instructions will now read: “If the fourth dose of DTaP was administered at least 4 months, but less than 6 months, after the third dose of DTaP, it need not be repeated [and] the 4-day grace period may not be used for the fourth dose given less than 6 months after the third dose of DTaP.”
“We use the description of ‘inadvertent dose’ to indicate that this shortened interval between doses should not be used prospectively; however, the dose could be counted as valid after the fact,” Dr. Robinson explained.
Footnotes for inactivated polio vaccine (IPV) will now include guidance for IPV use in patients who have previously received only oral polio vaccine (OPV) and did not receive any doses after their fourth birthday. This addition, which was added after repeated inquiries from providers, will read: “If only OPV were administered, and all doses were given prior to 4 years of age, one dose of IPV should be given at 4 years or older at least 4 weeks after the last dose of [OPV].”
For HPV, children aged 9-10 years will now be recommended for vaccination if they are “high risk” – indicated by a purple bar on Figure 1 of the 2016 immunization schedule – due to a history of sexual abuse in their youth. Furthermore, all HPV nomenclature in the 2016 recommendations will be updated to reflect currently accepted naming formats; for example, HPV4 will now be called 4-valent or 4V HPV. In the footnotes, the 9-valent HPV vaccine is now included along with the 5-valent and 4-valent variants, along with restructured language to clarify administration instructions.
Other changes to Figure 1 of next year’s immunization schedules include moving the Tdap recommendations to a lower section of the table, and moving the pneumococcal polysaccharide vaccination schedule toward the bottom of the chart because it is “not routinely recommended for any population.” Haemophilus influenzae type b (Hib) vaccinations are now recommended for certain high-risk groups in patients aged 5-18 years, in accordance with text in the recommendations’ footnotes that suggested vaccination in this age group for those who had previously been vaccinated or have other high-risk conditions.
The catch-up schedule will have one minor change – “in the Tdap line’s Dose 2-3 column, we added Tdap and Td to the list of possible previous vaccines,” Dr. Robinson said.
Revisions will be made to be submitted for approval by Dr. Tom Frieden, CDC director. The edited copy of these updated recommendations will be submitted to the American Academy of Pediatrics, American Academy of Family Physicians, and American Congress of Obstetricians and Gynecologists by Jan. 1, 2016, with publication by the CDC and partner publications following in January and February.
FROM AN ACIP MEETING
Obesity linked to chronic lower back and musculoskeletal pain
NATIONAL HARBOR, MD. – Numerous recent studies link chronic pain, especially lower back pain, to obesity, Dr. Brian White said during a presentation at the annual meeting of the American Academy of Pain.
Many of these studies also indicate that weight loss can be an effective – and considerably more affordable – solution than drugs and surgery for relieving chronic pain. As pain can initially limit exercise, Dr. White, an interventional physiatrist with the Bassett Healthcare Network in Cooperstown, N.Y., advised starting patients on common-sense weight loss approaches, such as limiting intake of sugar and related products, particularly soda.
Studies linking obesity and pain include work at the Mayo Clinic (Pain. 2010;151[2];366-71) that identified obesity as one of the most common modifiable risk factors for pain management in older adults.
Further, another study (BMC Musculoskelet Disord. 2013;14:238) showed that overweight or obese individuals were more likely to report musculoskeletal pain, and that the frequency of complaints increased as subjects’ weights increased.
In a study involving adolescents with pain, (Pain. 2012;153[9]:1932-8), obese adolescents had a 1.33 odds ratio (OR) of having any kind of pain, a 2.04 OR for chronic regional pain, and a 1.87 OR for knee pain specifically (P less than .05), compared with normal-weight adolescents.
In a study of chronic pain in the elderly (Pain. 2011;152[1]:53-9), individuals with abdominal obesity had a higher risk for chronic lumbar pain (OR = 2.25); upper extremity pain (OR = 2.01); and pain in either the hip, knee, or any lower extremity (OR = 1.86; P less than 0.05 for all pain locations), compared with normal-weight elderly individuals. Women were more likely to experience chronic pain (OR = 1.81, P less than 0.05). The findings were “independent of other components of metabolic syndrome, hsCRP, insulin resistance, depression, anxiety, and the presence of painful comorbid conditions,” the study concluded.
An Australian study (Eur J Pain. 2013;17(7):957-71) that reviewed low back pain studies in twins found that obesity was an indicator (OR = 1.9) and that “both obesity and smoking demonstrated dose-dependant relationships with lower back pain,” said Dr. White.
The HUNT 2 study from Norway (Spine. 2010;35(7):764-8) evaluated nearly 64,000 men and women over a 2-year period and found that the odds ratio for lower back pain increased by 1.07 in men and by 1.17 in women for every 5-point gain in body mass index. Study participants with a BMI greater than 30 at baseline were more likely to develop low back pain than were those with a baseline BMI under 25.
Dr. White did not report any relevant financial disclosures.
NATIONAL HARBOR, MD. – Numerous recent studies link chronic pain, especially lower back pain, to obesity, Dr. Brian White said during a presentation at the annual meeting of the American Academy of Pain.
Many of these studies also indicate that weight loss can be an effective – and considerably more affordable – solution than drugs and surgery for relieving chronic pain. As pain can initially limit exercise, Dr. White, an interventional physiatrist with the Bassett Healthcare Network in Cooperstown, N.Y., advised starting patients on common-sense weight loss approaches, such as limiting intake of sugar and related products, particularly soda.
Studies linking obesity and pain include work at the Mayo Clinic (Pain. 2010;151[2];366-71) that identified obesity as one of the most common modifiable risk factors for pain management in older adults.
Further, another study (BMC Musculoskelet Disord. 2013;14:238) showed that overweight or obese individuals were more likely to report musculoskeletal pain, and that the frequency of complaints increased as subjects’ weights increased.
In a study involving adolescents with pain, (Pain. 2012;153[9]:1932-8), obese adolescents had a 1.33 odds ratio (OR) of having any kind of pain, a 2.04 OR for chronic regional pain, and a 1.87 OR for knee pain specifically (P less than .05), compared with normal-weight adolescents.
In a study of chronic pain in the elderly (Pain. 2011;152[1]:53-9), individuals with abdominal obesity had a higher risk for chronic lumbar pain (OR = 2.25); upper extremity pain (OR = 2.01); and pain in either the hip, knee, or any lower extremity (OR = 1.86; P less than 0.05 for all pain locations), compared with normal-weight elderly individuals. Women were more likely to experience chronic pain (OR = 1.81, P less than 0.05). The findings were “independent of other components of metabolic syndrome, hsCRP, insulin resistance, depression, anxiety, and the presence of painful comorbid conditions,” the study concluded.
An Australian study (Eur J Pain. 2013;17(7):957-71) that reviewed low back pain studies in twins found that obesity was an indicator (OR = 1.9) and that “both obesity and smoking demonstrated dose-dependant relationships with lower back pain,” said Dr. White.
The HUNT 2 study from Norway (Spine. 2010;35(7):764-8) evaluated nearly 64,000 men and women over a 2-year period and found that the odds ratio for lower back pain increased by 1.07 in men and by 1.17 in women for every 5-point gain in body mass index. Study participants with a BMI greater than 30 at baseline were more likely to develop low back pain than were those with a baseline BMI under 25.
Dr. White did not report any relevant financial disclosures.
NATIONAL HARBOR, MD. – Numerous recent studies link chronic pain, especially lower back pain, to obesity, Dr. Brian White said during a presentation at the annual meeting of the American Academy of Pain.
Many of these studies also indicate that weight loss can be an effective – and considerably more affordable – solution than drugs and surgery for relieving chronic pain. As pain can initially limit exercise, Dr. White, an interventional physiatrist with the Bassett Healthcare Network in Cooperstown, N.Y., advised starting patients on common-sense weight loss approaches, such as limiting intake of sugar and related products, particularly soda.
Studies linking obesity and pain include work at the Mayo Clinic (Pain. 2010;151[2];366-71) that identified obesity as one of the most common modifiable risk factors for pain management in older adults.
Further, another study (BMC Musculoskelet Disord. 2013;14:238) showed that overweight or obese individuals were more likely to report musculoskeletal pain, and that the frequency of complaints increased as subjects’ weights increased.
In a study involving adolescents with pain, (Pain. 2012;153[9]:1932-8), obese adolescents had a 1.33 odds ratio (OR) of having any kind of pain, a 2.04 OR for chronic regional pain, and a 1.87 OR for knee pain specifically (P less than .05), compared with normal-weight adolescents.
In a study of chronic pain in the elderly (Pain. 2011;152[1]:53-9), individuals with abdominal obesity had a higher risk for chronic lumbar pain (OR = 2.25); upper extremity pain (OR = 2.01); and pain in either the hip, knee, or any lower extremity (OR = 1.86; P less than 0.05 for all pain locations), compared with normal-weight elderly individuals. Women were more likely to experience chronic pain (OR = 1.81, P less than 0.05). The findings were “independent of other components of metabolic syndrome, hsCRP, insulin resistance, depression, anxiety, and the presence of painful comorbid conditions,” the study concluded.
An Australian study (Eur J Pain. 2013;17(7):957-71) that reviewed low back pain studies in twins found that obesity was an indicator (OR = 1.9) and that “both obesity and smoking demonstrated dose-dependant relationships with lower back pain,” said Dr. White.
The HUNT 2 study from Norway (Spine. 2010;35(7):764-8) evaluated nearly 64,000 men and women over a 2-year period and found that the odds ratio for lower back pain increased by 1.07 in men and by 1.17 in women for every 5-point gain in body mass index. Study participants with a BMI greater than 30 at baseline were more likely to develop low back pain than were those with a baseline BMI under 25.
Dr. White did not report any relevant financial disclosures.
EXPERT ANALYSIS FROM PAIN 2015