User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Depression Found to Increase Risk of Mortality in Type 2 Diabetes
SAN DIEGO – Depression is a significant independent predictor of increased mortality and may increase the risk of subsequent macrovascular events in adults with type 2 diabetes, according to a data analysis.
The findings underscore the importance of detecting and effectively managing depression in people with type 2 diabetes, concluded Dr. Patrick J. O’Connor, a family physician who is a senior clinical investigator at HealthPartners Research Foundation, Minneapolis.
The findings were based on an analysis of 2,053 participants in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) study’s HRQL (Health-Related Quality of Life) investigation, all of whom completed the 9-item depression measure from the Patient Health Questionnaire (PHQ-9) at baseline and at 12, 36, and 48 months.
"The PHQ-9 exam is not a face-to-face mental health exam with a psychiatrist; it is nine questions on a piece of paper, so it’s good but it’s not perfect," Dr. O’Connor said at the annual scientific sessions of the American Diabetes Association. A score of 10 or more on the PHQ-9 has a sensitivity of 77% and a specificity of 94% for the diagnosis of major depression.
The researchers measured depression in three different ways: having a PHQ-9 score of 10 or greater (indicating moderate to major depression); scoring 2-3 points on five of the items (considered major depression), and scoring 2 or more points on three or four of the items (considered minor depression).
Cox proportional hazard regression modeling was used to estimate hazard ratios for the impact of depression status on ACCORD’s clinical end points: the primary composite outcome (cardiovascular death, or nonfatal heart attack or stroke), the macrovascular composite outcome (cardiovascular death, nonfatal heart attack or stroke, or heart failure), and the microvascular composite outcome (progression of retinopathy, nephropathy, and neuropathy).
The mean age of study participants was 62 years, and 39% were women. Of the 2,053 patients, 712 (35%) reported a history of depression at baseline. Compared with those who reported no history of depression, those who did were more likely to be women (46% vs. 36%, respectively), to be smokers (17% vs. 11%), to have a higher mean hemoglobin A1c level (8.4% vs. 8.2%), and to require insulin (41% vs. 33%).
About a third (30%) of the study participants scored 10 or more on the PHQ-9, which indicated moderate to major depression; 15% were considered to have major depression and 18% minor depression. After adjustment for numerous factors (including age, sex, race, coronary heart disease and heart failure status, HbA1c levels, lipid levels, blood pressure, body mass index, and smoking status), total mortality was significantly increased both in those with a PHQ-9 score of 10 or more (hazard ratio, 1.84), and major depression (HR, 2.24), but not in those with minor depression (HR, 1.14).
"This shows that depression status is an independent predictor of mortality, even after you adjust for cardiovascular risk factors," Dr. O’Connor commented.
The relationship of major depression to ACCORD’s macrovascular outcome reached borderline statistical significance (HR, 1.42), but major depression was not significantly related to ACCORD’s primary composite outcome (HR, 1.53) or to ACCORD’s microvascular composite outcome (HR, 0.93).
The study’s primary investigator was Dr. Mark D. Sullivan, professor of psychiatry at the University of Washington, Seattle.
Dr. O’Connor said that he had no relevant conflicts of interest.
Dr. Patrick J. O’Connor, ACCORD, mental health, the American Diabetes Association, PHQ-9,
SAN DIEGO – Depression is a significant independent predictor of increased mortality and may increase the risk of subsequent macrovascular events in adults with type 2 diabetes, according to a data analysis.
The findings underscore the importance of detecting and effectively managing depression in people with type 2 diabetes, concluded Dr. Patrick J. O’Connor, a family physician who is a senior clinical investigator at HealthPartners Research Foundation, Minneapolis.
The findings were based on an analysis of 2,053 participants in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) study’s HRQL (Health-Related Quality of Life) investigation, all of whom completed the 9-item depression measure from the Patient Health Questionnaire (PHQ-9) at baseline and at 12, 36, and 48 months.
"The PHQ-9 exam is not a face-to-face mental health exam with a psychiatrist; it is nine questions on a piece of paper, so it’s good but it’s not perfect," Dr. O’Connor said at the annual scientific sessions of the American Diabetes Association. A score of 10 or more on the PHQ-9 has a sensitivity of 77% and a specificity of 94% for the diagnosis of major depression.
The researchers measured depression in three different ways: having a PHQ-9 score of 10 or greater (indicating moderate to major depression); scoring 2-3 points on five of the items (considered major depression), and scoring 2 or more points on three or four of the items (considered minor depression).
Cox proportional hazard regression modeling was used to estimate hazard ratios for the impact of depression status on ACCORD’s clinical end points: the primary composite outcome (cardiovascular death, or nonfatal heart attack or stroke), the macrovascular composite outcome (cardiovascular death, nonfatal heart attack or stroke, or heart failure), and the microvascular composite outcome (progression of retinopathy, nephropathy, and neuropathy).
The mean age of study participants was 62 years, and 39% were women. Of the 2,053 patients, 712 (35%) reported a history of depression at baseline. Compared with those who reported no history of depression, those who did were more likely to be women (46% vs. 36%, respectively), to be smokers (17% vs. 11%), to have a higher mean hemoglobin A1c level (8.4% vs. 8.2%), and to require insulin (41% vs. 33%).
About a third (30%) of the study participants scored 10 or more on the PHQ-9, which indicated moderate to major depression; 15% were considered to have major depression and 18% minor depression. After adjustment for numerous factors (including age, sex, race, coronary heart disease and heart failure status, HbA1c levels, lipid levels, blood pressure, body mass index, and smoking status), total mortality was significantly increased both in those with a PHQ-9 score of 10 or more (hazard ratio, 1.84), and major depression (HR, 2.24), but not in those with minor depression (HR, 1.14).
"This shows that depression status is an independent predictor of mortality, even after you adjust for cardiovascular risk factors," Dr. O’Connor commented.
The relationship of major depression to ACCORD’s macrovascular outcome reached borderline statistical significance (HR, 1.42), but major depression was not significantly related to ACCORD’s primary composite outcome (HR, 1.53) or to ACCORD’s microvascular composite outcome (HR, 0.93).
The study’s primary investigator was Dr. Mark D. Sullivan, professor of psychiatry at the University of Washington, Seattle.
Dr. O’Connor said that he had no relevant conflicts of interest.
SAN DIEGO – Depression is a significant independent predictor of increased mortality and may increase the risk of subsequent macrovascular events in adults with type 2 diabetes, according to a data analysis.
The findings underscore the importance of detecting and effectively managing depression in people with type 2 diabetes, concluded Dr. Patrick J. O’Connor, a family physician who is a senior clinical investigator at HealthPartners Research Foundation, Minneapolis.
The findings were based on an analysis of 2,053 participants in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) study’s HRQL (Health-Related Quality of Life) investigation, all of whom completed the 9-item depression measure from the Patient Health Questionnaire (PHQ-9) at baseline and at 12, 36, and 48 months.
"The PHQ-9 exam is not a face-to-face mental health exam with a psychiatrist; it is nine questions on a piece of paper, so it’s good but it’s not perfect," Dr. O’Connor said at the annual scientific sessions of the American Diabetes Association. A score of 10 or more on the PHQ-9 has a sensitivity of 77% and a specificity of 94% for the diagnosis of major depression.
The researchers measured depression in three different ways: having a PHQ-9 score of 10 or greater (indicating moderate to major depression); scoring 2-3 points on five of the items (considered major depression), and scoring 2 or more points on three or four of the items (considered minor depression).
Cox proportional hazard regression modeling was used to estimate hazard ratios for the impact of depression status on ACCORD’s clinical end points: the primary composite outcome (cardiovascular death, or nonfatal heart attack or stroke), the macrovascular composite outcome (cardiovascular death, nonfatal heart attack or stroke, or heart failure), and the microvascular composite outcome (progression of retinopathy, nephropathy, and neuropathy).
The mean age of study participants was 62 years, and 39% were women. Of the 2,053 patients, 712 (35%) reported a history of depression at baseline. Compared with those who reported no history of depression, those who did were more likely to be women (46% vs. 36%, respectively), to be smokers (17% vs. 11%), to have a higher mean hemoglobin A1c level (8.4% vs. 8.2%), and to require insulin (41% vs. 33%).
About a third (30%) of the study participants scored 10 or more on the PHQ-9, which indicated moderate to major depression; 15% were considered to have major depression and 18% minor depression. After adjustment for numerous factors (including age, sex, race, coronary heart disease and heart failure status, HbA1c levels, lipid levels, blood pressure, body mass index, and smoking status), total mortality was significantly increased both in those with a PHQ-9 score of 10 or more (hazard ratio, 1.84), and major depression (HR, 2.24), but not in those with minor depression (HR, 1.14).
"This shows that depression status is an independent predictor of mortality, even after you adjust for cardiovascular risk factors," Dr. O’Connor commented.
The relationship of major depression to ACCORD’s macrovascular outcome reached borderline statistical significance (HR, 1.42), but major depression was not significantly related to ACCORD’s primary composite outcome (HR, 1.53) or to ACCORD’s microvascular composite outcome (HR, 0.93).
The study’s primary investigator was Dr. Mark D. Sullivan, professor of psychiatry at the University of Washington, Seattle.
Dr. O’Connor said that he had no relevant conflicts of interest.
Dr. Patrick J. O’Connor, ACCORD, mental health, the American Diabetes Association, PHQ-9,
Dr. Patrick J. O’Connor, ACCORD, mental health, the American Diabetes Association, PHQ-9,
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: Among adults with type 2 diabetes, total mortality was significantly increased both in those with a PHQ-9 score of 10 or more (HR, 1.84) and major depression (HR, 2.24), but not in those with minor depression (HR, 1.14).
Data Source: An analysis of 2,053 participants in the ACCORD Health-Related Quality of Life investigation who completed the nine-item depression measure from the PHQ-9 at baseline and at 12, 36, and 48 months.
Disclosures: Dr. O’Connor said that he had no relevant conflicts of interest.
Diabetes Linked to Carotid Artery Thickness in Young Adults
SAN DIEGO – Adolescents and young adults with type 1 diabetes have thicker and stiffer carotid arteries, compared with their healthy peers, results from a multicenter study showed.
"Type 1 diabetes has an adverse effect on carotid thickness and stiffness, and we can measure this by the time patients reach young adulthood," Dr. Elaine M. Urbina said at the annual scientific sessions of the American Diabetes Association. "It’s independent of demographics, lipids, and blood pressure, but may be influenced by adiposity. We need to control risk factors, especially obesity, in these adolescents and young adults to improve cardiovascular outcomes in type 1 diabetes."
As part of the SEARCH CVD study, a collaborative effort between investigators at the University of Colorado at Denver, the Colorado School of Public Health in Aurora, and Cincinnati Children’s Hospital Medical Center, Dr. Urbina and her associates set out to examine whether type 1 diabetes has a measurable effect on carotid arteries in adolescents and young adults. They studied 162 people aged 13-26 years, collecting data on demographics, anthropometrics, blood pressure, fasting lipid and hemoglobin A1c levels, and carotid ultrasound to measure the common, bulb, and internal carotid intima-media thickness (cIMT), with M-mode for calculation of carotid stiffness by Peterson’s elastic modulus (PEM), Young’s elastic modulus (YEM), and the incremental elastic modulus (Einc).
Of the 162 study participants, 127 (78%) had type 1 diabetes and 35 were healthy controls who attended clinics at the two locations, said Dr. Urbina, director of preventive cardiology at Cincinnati Children’s Hospital Medical Center. Their mean age was 20 years, 51% were male, 81% were white, and their mean duration of diabetes was 10 years.
Dr. Urbina reported that there were significantly higher proportions of males and whites among cases, compared with controls (55% vs. 34% and 90% vs. 50%, respectively), but there were no significant differences between the two groups in anthropometric or lipid values.
After adjustment for age, sex, race, mean arterial pressure by mercury sphygmomanometry, and lipids, patients with type 1 diabetes had a significantly thicker internal cIMT, compared with controls (mean, 0.56 mm vs. 0.50 mm, respectively), with a trend for a thicker common cIMT (mean, 0.63 mm vs. 0.60 mm). Bulb cIMT was the same in both groups (mean, 0.61 mm).
Patients with type 1 diabetes also had significantly stiffer carotids, compared with controls (mean PEM, 193 mm Hg vs. 169 mm Hg, respectively; mean YEM, 204 mm Hg/mm vs. 182 mm Hg/mm; mean Einc, 963 mm Hg vs. 862 mm Hg).
After adjustment for body mass index, there was a trend only for significantly thicker internal cIMT, although PEM remained stiffer for the patients with type 1 diabetes who were at least 20 years old.
SEARCH CVD is funded by the National Institutes of Health and is an ancillary study of the SEARCH for Diabetes in Youth study, a multicenter study funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Urbina said that she had no relevant financial disclosures to make.
SAN DIEGO – Adolescents and young adults with type 1 diabetes have thicker and stiffer carotid arteries, compared with their healthy peers, results from a multicenter study showed.
"Type 1 diabetes has an adverse effect on carotid thickness and stiffness, and we can measure this by the time patients reach young adulthood," Dr. Elaine M. Urbina said at the annual scientific sessions of the American Diabetes Association. "It’s independent of demographics, lipids, and blood pressure, but may be influenced by adiposity. We need to control risk factors, especially obesity, in these adolescents and young adults to improve cardiovascular outcomes in type 1 diabetes."
As part of the SEARCH CVD study, a collaborative effort between investigators at the University of Colorado at Denver, the Colorado School of Public Health in Aurora, and Cincinnati Children’s Hospital Medical Center, Dr. Urbina and her associates set out to examine whether type 1 diabetes has a measurable effect on carotid arteries in adolescents and young adults. They studied 162 people aged 13-26 years, collecting data on demographics, anthropometrics, blood pressure, fasting lipid and hemoglobin A1c levels, and carotid ultrasound to measure the common, bulb, and internal carotid intima-media thickness (cIMT), with M-mode for calculation of carotid stiffness by Peterson’s elastic modulus (PEM), Young’s elastic modulus (YEM), and the incremental elastic modulus (Einc).
Of the 162 study participants, 127 (78%) had type 1 diabetes and 35 were healthy controls who attended clinics at the two locations, said Dr. Urbina, director of preventive cardiology at Cincinnati Children’s Hospital Medical Center. Their mean age was 20 years, 51% were male, 81% were white, and their mean duration of diabetes was 10 years.
Dr. Urbina reported that there were significantly higher proportions of males and whites among cases, compared with controls (55% vs. 34% and 90% vs. 50%, respectively), but there were no significant differences between the two groups in anthropometric or lipid values.
After adjustment for age, sex, race, mean arterial pressure by mercury sphygmomanometry, and lipids, patients with type 1 diabetes had a significantly thicker internal cIMT, compared with controls (mean, 0.56 mm vs. 0.50 mm, respectively), with a trend for a thicker common cIMT (mean, 0.63 mm vs. 0.60 mm). Bulb cIMT was the same in both groups (mean, 0.61 mm).
Patients with type 1 diabetes also had significantly stiffer carotids, compared with controls (mean PEM, 193 mm Hg vs. 169 mm Hg, respectively; mean YEM, 204 mm Hg/mm vs. 182 mm Hg/mm; mean Einc, 963 mm Hg vs. 862 mm Hg).
After adjustment for body mass index, there was a trend only for significantly thicker internal cIMT, although PEM remained stiffer for the patients with type 1 diabetes who were at least 20 years old.
SEARCH CVD is funded by the National Institutes of Health and is an ancillary study of the SEARCH for Diabetes in Youth study, a multicenter study funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Urbina said that she had no relevant financial disclosures to make.
SAN DIEGO – Adolescents and young adults with type 1 diabetes have thicker and stiffer carotid arteries, compared with their healthy peers, results from a multicenter study showed.
"Type 1 diabetes has an adverse effect on carotid thickness and stiffness, and we can measure this by the time patients reach young adulthood," Dr. Elaine M. Urbina said at the annual scientific sessions of the American Diabetes Association. "It’s independent of demographics, lipids, and blood pressure, but may be influenced by adiposity. We need to control risk factors, especially obesity, in these adolescents and young adults to improve cardiovascular outcomes in type 1 diabetes."
As part of the SEARCH CVD study, a collaborative effort between investigators at the University of Colorado at Denver, the Colorado School of Public Health in Aurora, and Cincinnati Children’s Hospital Medical Center, Dr. Urbina and her associates set out to examine whether type 1 diabetes has a measurable effect on carotid arteries in adolescents and young adults. They studied 162 people aged 13-26 years, collecting data on demographics, anthropometrics, blood pressure, fasting lipid and hemoglobin A1c levels, and carotid ultrasound to measure the common, bulb, and internal carotid intima-media thickness (cIMT), with M-mode for calculation of carotid stiffness by Peterson’s elastic modulus (PEM), Young’s elastic modulus (YEM), and the incremental elastic modulus (Einc).
Of the 162 study participants, 127 (78%) had type 1 diabetes and 35 were healthy controls who attended clinics at the two locations, said Dr. Urbina, director of preventive cardiology at Cincinnati Children’s Hospital Medical Center. Their mean age was 20 years, 51% were male, 81% were white, and their mean duration of diabetes was 10 years.
Dr. Urbina reported that there were significantly higher proportions of males and whites among cases, compared with controls (55% vs. 34% and 90% vs. 50%, respectively), but there were no significant differences between the two groups in anthropometric or lipid values.
After adjustment for age, sex, race, mean arterial pressure by mercury sphygmomanometry, and lipids, patients with type 1 diabetes had a significantly thicker internal cIMT, compared with controls (mean, 0.56 mm vs. 0.50 mm, respectively), with a trend for a thicker common cIMT (mean, 0.63 mm vs. 0.60 mm). Bulb cIMT was the same in both groups (mean, 0.61 mm).
Patients with type 1 diabetes also had significantly stiffer carotids, compared with controls (mean PEM, 193 mm Hg vs. 169 mm Hg, respectively; mean YEM, 204 mm Hg/mm vs. 182 mm Hg/mm; mean Einc, 963 mm Hg vs. 862 mm Hg).
After adjustment for body mass index, there was a trend only for significantly thicker internal cIMT, although PEM remained stiffer for the patients with type 1 diabetes who were at least 20 years old.
SEARCH CVD is funded by the National Institutes of Health and is an ancillary study of the SEARCH for Diabetes in Youth study, a multicenter study funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Urbina said that she had no relevant financial disclosures to make.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: Type 1 diabetes had a significantly thicker internal cIMT, compared with controls (mean, 0.56 mm vs. 0.50 mm, respectively), with a trend for a thicker common cIMT (mean, 0.63 mm vs. 0.60 mm), after adjustment for age, sex, race, mean arterial pressure, and lipids. Patients with type 1 diabetes also had significantly stiffer carotids compared with controls.
Data Source: An analysis of 162 persons (127 with type 1 diabetes and 35 controls) aged 13-26 years in the SEARCH CVD study, conducted by the University of Colorado at Denver; the Colorado School of Public Health, Aurora; and Cincinnati Children’s Hospital Medical Center.
Disclosures: SEARCH CVD is funded by the NIH and is an ancillary study of the SEARCH for Diabetes in Youth study, a multicenter study funded by the CDC and the NIDDK. Dr. Urbina said that she had no relevant financial conflicts to disclose.
Patient-Centered Medical Home Making Progress in Diabetes
SAN DIEGO – The patient-centered medical home is beginning to demonstrate a positive impact on patients with diabetes, results from a national analysis showed.
"A lot of this has not been published yet, but we see improvements in several diabetes care measures of quality, and cost savings as well," Dr. Robert A. Gabbay said at the annual scientific sessions of the American Diabetes Association. "Stay tuned, because this is coming to a primary care practice near you. It’s catching on everywhere."
A new model for primary care, the patient-centered medical home (PCMH), involves a "more coordinated care system, enhances access for patients, and fosters team-based care, distributing the care amongst the team within the practice," explained Dr. Gabbay, who directs the Penn State Hershey Institute for Diabetes and Obesity. "At the foundation of all this is a payment reform system that helps to reimburse at a higher rate for all of these coordinated activities."
He described diabetes as a natural target for the PCMH because it’s a high-cost disease, it’s highly prevalent, and there are established, measurable evidence-based quality goals that clinicians generally agree upon. "The diabetes community has had early recognition of many of the key concepts of the medical home, such as population management, use of registries, supporting patients in their own self-management, and team-based care," he said.
More than 40 diabetes-focused PCMH demonstration projects are currently under way nationally, Dr. Gabbay said. He provided a progress report from projects launched by three large integrated health systems:
– Group Health Cooperative, Seattle: Improvements of 51%-59% in its bundled "composite quality score" of diabetes measures for 9,200 patients in the first 2 years, reductions in emergency department and inpatient admissions, and a return of $1.50 for every dollar invested in the PCMH after 21 months.
– Geisinger Health System, Danville, Pa.: First-year improvements in the proportion of patients with an HbA1c of less than 7.0% (from 32% to 35%); blood pressure of less than 130/80 mm Hg (from 40% to 44%); and the "diabetic bundle," a measure of nine diabetes indicators (from 2% to 7%).
– HealthPartners, Minneapolis: Improvements in the bundled measures of HbA1c, blood pressure, low-density lipoprotein (LDL) cholesterol, aspirin use, and tobacco cessation over a 4-year period (from 4% to 25%).
State initiatives have also shown improvements, said Dr. Gabbay, who is also professor of medicine at the Pennsylvania State University. For example, an assessment of 11,900 patients served by 25 of the 150 practices in a Pennsylvania-based medical home initiative launched in 2010 showed reductions in the proportion of patients with an HbA1c of greater than 9.0% (–6%), as well as improvements in the proportion of patients with systolic blood pressure of less than 130 mm Hg (12%), LDL less than 130 mg/dL (12%), those setting self-management goals (38%), foot examinations (25%), eye examinations (18%), and diabetic nephropathy screening (13%).
In a Rhode Island initiative, providers reported improvements after 2 years in the proportion of patients achieving an HbA1c of less than 7.0% (from 33% to 40%), blood pressure of less than 130/80 mm Hg (from 18% to 40%), and LDL of less than 100 mg/dL (from 27% to 42%).
In the meantime, initiatives in Colorado and North Carolina have both met National Committee for Quality Assurance benchmarks for diabetes care, as well as reductions in emergency department and inpatient admissions.
The most frequently used approaches to transform to a PCMH, Dr. Gabbay said, are implementing patient registries, upgrading electronic health records, improving care management, participating in learning collaboratives, and practice coaching.
"In Pennsylvania, it’s been very much driven by the chronic care model, which has a much stronger evidence base in terms of improving quality of care," he said. That state’s initiative also includes monthly outcome data reporting and multipayer financial incentives.
Dr. Gabbay said that he had no relevant financial disclosures to make.
SAN DIEGO – The patient-centered medical home is beginning to demonstrate a positive impact on patients with diabetes, results from a national analysis showed.
"A lot of this has not been published yet, but we see improvements in several diabetes care measures of quality, and cost savings as well," Dr. Robert A. Gabbay said at the annual scientific sessions of the American Diabetes Association. "Stay tuned, because this is coming to a primary care practice near you. It’s catching on everywhere."
A new model for primary care, the patient-centered medical home (PCMH), involves a "more coordinated care system, enhances access for patients, and fosters team-based care, distributing the care amongst the team within the practice," explained Dr. Gabbay, who directs the Penn State Hershey Institute for Diabetes and Obesity. "At the foundation of all this is a payment reform system that helps to reimburse at a higher rate for all of these coordinated activities."
He described diabetes as a natural target for the PCMH because it’s a high-cost disease, it’s highly prevalent, and there are established, measurable evidence-based quality goals that clinicians generally agree upon. "The diabetes community has had early recognition of many of the key concepts of the medical home, such as population management, use of registries, supporting patients in their own self-management, and team-based care," he said.
More than 40 diabetes-focused PCMH demonstration projects are currently under way nationally, Dr. Gabbay said. He provided a progress report from projects launched by three large integrated health systems:
– Group Health Cooperative, Seattle: Improvements of 51%-59% in its bundled "composite quality score" of diabetes measures for 9,200 patients in the first 2 years, reductions in emergency department and inpatient admissions, and a return of $1.50 for every dollar invested in the PCMH after 21 months.
– Geisinger Health System, Danville, Pa.: First-year improvements in the proportion of patients with an HbA1c of less than 7.0% (from 32% to 35%); blood pressure of less than 130/80 mm Hg (from 40% to 44%); and the "diabetic bundle," a measure of nine diabetes indicators (from 2% to 7%).
– HealthPartners, Minneapolis: Improvements in the bundled measures of HbA1c, blood pressure, low-density lipoprotein (LDL) cholesterol, aspirin use, and tobacco cessation over a 4-year period (from 4% to 25%).
State initiatives have also shown improvements, said Dr. Gabbay, who is also professor of medicine at the Pennsylvania State University. For example, an assessment of 11,900 patients served by 25 of the 150 practices in a Pennsylvania-based medical home initiative launched in 2010 showed reductions in the proportion of patients with an HbA1c of greater than 9.0% (–6%), as well as improvements in the proportion of patients with systolic blood pressure of less than 130 mm Hg (12%), LDL less than 130 mg/dL (12%), those setting self-management goals (38%), foot examinations (25%), eye examinations (18%), and diabetic nephropathy screening (13%).
In a Rhode Island initiative, providers reported improvements after 2 years in the proportion of patients achieving an HbA1c of less than 7.0% (from 33% to 40%), blood pressure of less than 130/80 mm Hg (from 18% to 40%), and LDL of less than 100 mg/dL (from 27% to 42%).
In the meantime, initiatives in Colorado and North Carolina have both met National Committee for Quality Assurance benchmarks for diabetes care, as well as reductions in emergency department and inpatient admissions.
The most frequently used approaches to transform to a PCMH, Dr. Gabbay said, are implementing patient registries, upgrading electronic health records, improving care management, participating in learning collaboratives, and practice coaching.
"In Pennsylvania, it’s been very much driven by the chronic care model, which has a much stronger evidence base in terms of improving quality of care," he said. That state’s initiative also includes monthly outcome data reporting and multipayer financial incentives.
Dr. Gabbay said that he had no relevant financial disclosures to make.
SAN DIEGO – The patient-centered medical home is beginning to demonstrate a positive impact on patients with diabetes, results from a national analysis showed.
"A lot of this has not been published yet, but we see improvements in several diabetes care measures of quality, and cost savings as well," Dr. Robert A. Gabbay said at the annual scientific sessions of the American Diabetes Association. "Stay tuned, because this is coming to a primary care practice near you. It’s catching on everywhere."
A new model for primary care, the patient-centered medical home (PCMH), involves a "more coordinated care system, enhances access for patients, and fosters team-based care, distributing the care amongst the team within the practice," explained Dr. Gabbay, who directs the Penn State Hershey Institute for Diabetes and Obesity. "At the foundation of all this is a payment reform system that helps to reimburse at a higher rate for all of these coordinated activities."
He described diabetes as a natural target for the PCMH because it’s a high-cost disease, it’s highly prevalent, and there are established, measurable evidence-based quality goals that clinicians generally agree upon. "The diabetes community has had early recognition of many of the key concepts of the medical home, such as population management, use of registries, supporting patients in their own self-management, and team-based care," he said.
More than 40 diabetes-focused PCMH demonstration projects are currently under way nationally, Dr. Gabbay said. He provided a progress report from projects launched by three large integrated health systems:
– Group Health Cooperative, Seattle: Improvements of 51%-59% in its bundled "composite quality score" of diabetes measures for 9,200 patients in the first 2 years, reductions in emergency department and inpatient admissions, and a return of $1.50 for every dollar invested in the PCMH after 21 months.
– Geisinger Health System, Danville, Pa.: First-year improvements in the proportion of patients with an HbA1c of less than 7.0% (from 32% to 35%); blood pressure of less than 130/80 mm Hg (from 40% to 44%); and the "diabetic bundle," a measure of nine diabetes indicators (from 2% to 7%).
– HealthPartners, Minneapolis: Improvements in the bundled measures of HbA1c, blood pressure, low-density lipoprotein (LDL) cholesterol, aspirin use, and tobacco cessation over a 4-year period (from 4% to 25%).
State initiatives have also shown improvements, said Dr. Gabbay, who is also professor of medicine at the Pennsylvania State University. For example, an assessment of 11,900 patients served by 25 of the 150 practices in a Pennsylvania-based medical home initiative launched in 2010 showed reductions in the proportion of patients with an HbA1c of greater than 9.0% (–6%), as well as improvements in the proportion of patients with systolic blood pressure of less than 130 mm Hg (12%), LDL less than 130 mg/dL (12%), those setting self-management goals (38%), foot examinations (25%), eye examinations (18%), and diabetic nephropathy screening (13%).
In a Rhode Island initiative, providers reported improvements after 2 years in the proportion of patients achieving an HbA1c of less than 7.0% (from 33% to 40%), blood pressure of less than 130/80 mm Hg (from 18% to 40%), and LDL of less than 100 mg/dL (from 27% to 42%).
In the meantime, initiatives in Colorado and North Carolina have both met National Committee for Quality Assurance benchmarks for diabetes care, as well as reductions in emergency department and inpatient admissions.
The most frequently used approaches to transform to a PCMH, Dr. Gabbay said, are implementing patient registries, upgrading electronic health records, improving care management, participating in learning collaboratives, and practice coaching.
"In Pennsylvania, it’s been very much driven by the chronic care model, which has a much stronger evidence base in terms of improving quality of care," he said. That state’s initiative also includes monthly outcome data reporting and multipayer financial incentives.
Dr. Gabbay said that he had no relevant financial disclosures to make.
EXPERT ANALYSIS FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Scar Prevention 'Band-Aid' Shows Early Promise
DANA POINT, CALIF. – Scar formation and fibrosis can be reduced by altering the mechanical environment of wounds, results from a phase I study found.
At the Summit in Aesthetic Medicine, sponsored by Skin Disease Education Foundation (SDEF), Dr. Geoffrey C. Gurtner presented findings from a study in which nine patients undergoing elective abdominal surgery were treated postoperatively with a stress-shielding polymer on one side while the other side was treated with standard wound care.
The device, manufactured by Neodyne Biosciences, looks like a Band-Aid strip and is stretched over the incision after sutures are removed. It conforms to the wound and adheres to skin, creating "a compressive region that has no level of mechanical stimulation or distractive strain," said Dr. Gurtner, professor of surgery at Stanford (Calif.) University. "Essentially, you create stress risers in the unwounded skin and a mechanically privileged environment in the wounded skin."
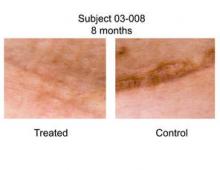
A panel of three independent plastic surgeons reviewed 18 photos of the scars (nine treated, nine control) taken 6-12 months after surgery (Ann. Surg. 2011 May 19 [doi: 10.1097/SLA.0b013e318220b159]). They used a visual analog scale (VAS) that ranged from 0 (very good scar) to 100 (very poor hypertrophic scar).
Dr. Gurtner reported that the average VAS score in the treated group was 18.6, while the average VAS score in the control group was 50.5, a difference that was statistically significant (P = .0039). "In none of the cases was the treated scar worse than the control scar, which I think is different than some of the biologic agents we’ve seen over the last few years," he said.*
A panel of lay persons who reviewed the photos reported similar results that favored the treated group (P = .004).
In earlier mouse studies of wound environment manipulation, Dr. Gurtner and his associates found that focal adhesion kinase (FAK) is a critical regulator in the formation of hypertrophic scars. He described FAK as "a molecule that exists on the inner surface of cell membranes and transmits forces that are set in the external extracellular matrix to the inside of the cell. FAK transmits those forces into biological or biochemical cues that then turn on genes in the nucleus and make the cells do different things. This seems to be a very important molecule in the ability of us to produce hypertrophic scars in mice. If you take out FAK, you can prevent hypertrophic scar formation."
FAK is a target that has been examined extensively in cancer, Dr. Gurtner said, suggesting that in the next few years, "We should have products that will not only be able to treat incision wounds but will also be able to treat large burn injuries. You need to fool the cells into thinking they’re in a different mechanical environment, either by using small molecule or pharmacologic blocking therapies such as fat inhibitors, or by using biomaterials that provide cues in a controlled way that minimize the amount of mechanical stimulation that the fibroblasts feel in the healing wound so as to mitigate the inflammation and subsequent fibrosis."
The study was supported by a Wallace H. Coulter Translational Partners Grant; the Armed Forces Institute of Regenerative Medicine; the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine; and the Oak Foundation. Neodyne Biosciences supplied the surgical dressings used in the study. Dr. Gurtner disclosed that he holds an equity interest in Neodyne.
SDEF and this news organization are owned by Elsevier.
*Correction 8/22/11: An earlier version of this story misstated the VAS scores for the two groups of patients.
DANA POINT, CALIF. – Scar formation and fibrosis can be reduced by altering the mechanical environment of wounds, results from a phase I study found.
At the Summit in Aesthetic Medicine, sponsored by Skin Disease Education Foundation (SDEF), Dr. Geoffrey C. Gurtner presented findings from a study in which nine patients undergoing elective abdominal surgery were treated postoperatively with a stress-shielding polymer on one side while the other side was treated with standard wound care.
The device, manufactured by Neodyne Biosciences, looks like a Band-Aid strip and is stretched over the incision after sutures are removed. It conforms to the wound and adheres to skin, creating "a compressive region that has no level of mechanical stimulation or distractive strain," said Dr. Gurtner, professor of surgery at Stanford (Calif.) University. "Essentially, you create stress risers in the unwounded skin and a mechanically privileged environment in the wounded skin."
A panel of three independent plastic surgeons reviewed 18 photos of the scars (nine treated, nine control) taken 6-12 months after surgery (Ann. Surg. 2011 May 19 [doi: 10.1097/SLA.0b013e318220b159]). They used a visual analog scale (VAS) that ranged from 0 (very good scar) to 100 (very poor hypertrophic scar).
Dr. Gurtner reported that the average VAS score in the treated group was 18.6, while the average VAS score in the control group was 50.5, a difference that was statistically significant (P = .0039). "In none of the cases was the treated scar worse than the control scar, which I think is different than some of the biologic agents we’ve seen over the last few years," he said.*
A panel of lay persons who reviewed the photos reported similar results that favored the treated group (P = .004).
In earlier mouse studies of wound environment manipulation, Dr. Gurtner and his associates found that focal adhesion kinase (FAK) is a critical regulator in the formation of hypertrophic scars. He described FAK as "a molecule that exists on the inner surface of cell membranes and transmits forces that are set in the external extracellular matrix to the inside of the cell. FAK transmits those forces into biological or biochemical cues that then turn on genes in the nucleus and make the cells do different things. This seems to be a very important molecule in the ability of us to produce hypertrophic scars in mice. If you take out FAK, you can prevent hypertrophic scar formation."
FAK is a target that has been examined extensively in cancer, Dr. Gurtner said, suggesting that in the next few years, "We should have products that will not only be able to treat incision wounds but will also be able to treat large burn injuries. You need to fool the cells into thinking they’re in a different mechanical environment, either by using small molecule or pharmacologic blocking therapies such as fat inhibitors, or by using biomaterials that provide cues in a controlled way that minimize the amount of mechanical stimulation that the fibroblasts feel in the healing wound so as to mitigate the inflammation and subsequent fibrosis."
The study was supported by a Wallace H. Coulter Translational Partners Grant; the Armed Forces Institute of Regenerative Medicine; the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine; and the Oak Foundation. Neodyne Biosciences supplied the surgical dressings used in the study. Dr. Gurtner disclosed that he holds an equity interest in Neodyne.
SDEF and this news organization are owned by Elsevier.
*Correction 8/22/11: An earlier version of this story misstated the VAS scores for the two groups of patients.
DANA POINT, CALIF. – Scar formation and fibrosis can be reduced by altering the mechanical environment of wounds, results from a phase I study found.
At the Summit in Aesthetic Medicine, sponsored by Skin Disease Education Foundation (SDEF), Dr. Geoffrey C. Gurtner presented findings from a study in which nine patients undergoing elective abdominal surgery were treated postoperatively with a stress-shielding polymer on one side while the other side was treated with standard wound care.
The device, manufactured by Neodyne Biosciences, looks like a Band-Aid strip and is stretched over the incision after sutures are removed. It conforms to the wound and adheres to skin, creating "a compressive region that has no level of mechanical stimulation or distractive strain," said Dr. Gurtner, professor of surgery at Stanford (Calif.) University. "Essentially, you create stress risers in the unwounded skin and a mechanically privileged environment in the wounded skin."
A panel of three independent plastic surgeons reviewed 18 photos of the scars (nine treated, nine control) taken 6-12 months after surgery (Ann. Surg. 2011 May 19 [doi: 10.1097/SLA.0b013e318220b159]). They used a visual analog scale (VAS) that ranged from 0 (very good scar) to 100 (very poor hypertrophic scar).
Dr. Gurtner reported that the average VAS score in the treated group was 18.6, while the average VAS score in the control group was 50.5, a difference that was statistically significant (P = .0039). "In none of the cases was the treated scar worse than the control scar, which I think is different than some of the biologic agents we’ve seen over the last few years," he said.*
A panel of lay persons who reviewed the photos reported similar results that favored the treated group (P = .004).
In earlier mouse studies of wound environment manipulation, Dr. Gurtner and his associates found that focal adhesion kinase (FAK) is a critical regulator in the formation of hypertrophic scars. He described FAK as "a molecule that exists on the inner surface of cell membranes and transmits forces that are set in the external extracellular matrix to the inside of the cell. FAK transmits those forces into biological or biochemical cues that then turn on genes in the nucleus and make the cells do different things. This seems to be a very important molecule in the ability of us to produce hypertrophic scars in mice. If you take out FAK, you can prevent hypertrophic scar formation."
FAK is a target that has been examined extensively in cancer, Dr. Gurtner said, suggesting that in the next few years, "We should have products that will not only be able to treat incision wounds but will also be able to treat large burn injuries. You need to fool the cells into thinking they’re in a different mechanical environment, either by using small molecule or pharmacologic blocking therapies such as fat inhibitors, or by using biomaterials that provide cues in a controlled way that minimize the amount of mechanical stimulation that the fibroblasts feel in the healing wound so as to mitigate the inflammation and subsequent fibrosis."
The study was supported by a Wallace H. Coulter Translational Partners Grant; the Armed Forces Institute of Regenerative Medicine; the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine; and the Oak Foundation. Neodyne Biosciences supplied the surgical dressings used in the study. Dr. Gurtner disclosed that he holds an equity interest in Neodyne.
SDEF and this news organization are owned by Elsevier.
*Correction 8/22/11: An earlier version of this story misstated the VAS scores for the two groups of patients.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Major Finding: The average VAS score in the treated group was 50.5, while the average VAS score in the control group was 18.6, a statistically significant difference (P = .004).
Data Source: Nine patients undergoing elective abdominal surgery who were treated postoperatively with a stress-shielding polymer on one side while the other side was treated with standard wound care.
Disclosures: The study was supported by a Wallace H. Coulter Translational Partners Grant; the Armed Forces Institute of Regenerative Medicine; the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine; and the Oak Foundation. Neodyne Biosciences supplied the surgical dressings used in the study. Dr. Gurtner disclosed that he holds an equity interest in Neodyne. SDEF and this news organization are owned by Elsevier.
SILS, Conventional Laparoscopy Compared for Pediatric Appendectomy
PALM DESERT, CALIF. – Single-incision laparoscopic surgical appendectomy is both feasible and safe across a wide range of pediatric patient ages, results from a single-center study showed.
"Single-incision laparoscopic surgery is an exciting area of minimally invasive surgery," Dr. Eduardo A. Perez said at the annual meeting of the American Pediatric Surgical Association. "There are known disadvantages, including limited lateral movement and dueling instruments, but industry is working hard to improve these platforms. This includes articulating instruments, flexible laparoscopes, multichannel ports, and robotic platforms."
Dr. Perez, of the division of pediatric surgery at Children’s Medical Center, Dallas, and his associates randomized 50 patients equally to either single-incision laparoscopic surgery (SILS) or conventional laparoscopy (LAP) for appendectomy and followed them for a median of 14 months. The technique for SILS involved a single supraumbilical curvilinear incision with three fascial incisions placed in a triangular fashion. To make SILS technically comparable to the LAP procedure, the researchers used a stapler device that required upsizing a 5-mm port to a 12-mm size. Cosmesis was not studied.
The children ranged in age from 3 to 15 years, and there were no significant age differences between the two treatment groups. Half of the patients were under age 8, 50% were male, and 67% were Hispanic.
The overall mean OR time was 46.8 minutes for the SILS procedure, compared with 34.8 minutes for the LAP procedure, a difference that was significant (P = .010). However, the OR time between groups became more similar as the number of cases increased. For example, after the first 25 patients were treated, the mean OR time for the SILS procedure was 49.3 minutes, compared with 33.5 minutes for the LAP procedure, a difference that remained significant (P = .049). After the last 25 patients were treated, the mean OR times were no longer significantly different between the two groups (a mean of 44.1 vs. 36 minutes, respectively).
There were no conversions and no differences in hospital length of stay between the two groups (a median of 40.3 hours for SILS vs. 36.7 hours for LAP).
The only complication was a wound seroma in the SILS group, and no hernias were observed. In addition, no differences were noted between the two groups in terms of hospital readmissions, diet tolerance, fever, or postoperative pain.
Operative times between SILS and LAP appendectomy "are similar once experience is gained," Dr. Perez concluded. "OR cost is similar when using standard instruments, but this will increase [in SILS] with the use of newer, more advanced instruments."
Dr. Perez said that he had no relevant financial disclosures to make.
The meeting was supported by a grant from Elsevier, which owns this news organization.
This study is one of several evaluating SILS vs. traditional three-port laparoscopy for appendectomy in children. Data reported at the American Surgical Association annual meeting in April by researchers from Children’s Mercy Hospital in Kansas City showed that SILS took longer, was more costly (presumably because of a stapler), and required more use of analgesics. SILS patients also had a trend toward higher wound infection, and although it was not statistically significant, the surgeons switched to an extracorporeal appendectomy through the umbilical site in an attempt to reduce that rate. In a total of 10% of the cases that started out as SILS, surgeons had to convert to three-port laparoscopy because of the degree of difficulty in performing SILS. However, there were no conversions to open surgery.
In the current study, OR time was significantly longer with SILS than with conventional laparoscopy. The researchers did not evaluate cosmesis – supposedly the only advantage of SILS. Their cost analysis is lacking, although they suspect that with newer technology to make SILS easier to perform, the cost will go up.
It’s interesting that there were no conversions. Does that mean no conversions from SILS to using additional ports and still doing it laparoscopically, or no conversions to an open procedure?
The jury is still out about the benefits of SILS. It is technically feasible, but is it any better than traditional three-port laparoscopy? I suspect that the concept of better cosmesis will eventually drive public demand for the procedure. Vanity is a powerful influence in our society.
Dr. Jay L. Grosfeld is the Lafayette F. Page Professor Emeritus of Pediatric Surgery, Indiana University, Indianapolis. He stated that he had no disclosures.
Dr. Eduardo A. Perez, American Pediatric Surgical Association, SILS, LAP, supraumbilical curvilinear incision,
This study is one of several evaluating SILS vs. traditional three-port laparoscopy for appendectomy in children. Data reported at the American Surgical Association annual meeting in April by researchers from Children’s Mercy Hospital in Kansas City showed that SILS took longer, was more costly (presumably because of a stapler), and required more use of analgesics. SILS patients also had a trend toward higher wound infection, and although it was not statistically significant, the surgeons switched to an extracorporeal appendectomy through the umbilical site in an attempt to reduce that rate. In a total of 10% of the cases that started out as SILS, surgeons had to convert to three-port laparoscopy because of the degree of difficulty in performing SILS. However, there were no conversions to open surgery.
In the current study, OR time was significantly longer with SILS than with conventional laparoscopy. The researchers did not evaluate cosmesis – supposedly the only advantage of SILS. Their cost analysis is lacking, although they suspect that with newer technology to make SILS easier to perform, the cost will go up.
It’s interesting that there were no conversions. Does that mean no conversions from SILS to using additional ports and still doing it laparoscopically, or no conversions to an open procedure?
The jury is still out about the benefits of SILS. It is technically feasible, but is it any better than traditional three-port laparoscopy? I suspect that the concept of better cosmesis will eventually drive public demand for the procedure. Vanity is a powerful influence in our society.
Dr. Jay L. Grosfeld is the Lafayette F. Page Professor Emeritus of Pediatric Surgery, Indiana University, Indianapolis. He stated that he had no disclosures.
This study is one of several evaluating SILS vs. traditional three-port laparoscopy for appendectomy in children. Data reported at the American Surgical Association annual meeting in April by researchers from Children’s Mercy Hospital in Kansas City showed that SILS took longer, was more costly (presumably because of a stapler), and required more use of analgesics. SILS patients also had a trend toward higher wound infection, and although it was not statistically significant, the surgeons switched to an extracorporeal appendectomy through the umbilical site in an attempt to reduce that rate. In a total of 10% of the cases that started out as SILS, surgeons had to convert to three-port laparoscopy because of the degree of difficulty in performing SILS. However, there were no conversions to open surgery.
In the current study, OR time was significantly longer with SILS than with conventional laparoscopy. The researchers did not evaluate cosmesis – supposedly the only advantage of SILS. Their cost analysis is lacking, although they suspect that with newer technology to make SILS easier to perform, the cost will go up.
It’s interesting that there were no conversions. Does that mean no conversions from SILS to using additional ports and still doing it laparoscopically, or no conversions to an open procedure?
The jury is still out about the benefits of SILS. It is technically feasible, but is it any better than traditional three-port laparoscopy? I suspect that the concept of better cosmesis will eventually drive public demand for the procedure. Vanity is a powerful influence in our society.
Dr. Jay L. Grosfeld is the Lafayette F. Page Professor Emeritus of Pediatric Surgery, Indiana University, Indianapolis. He stated that he had no disclosures.
PALM DESERT, CALIF. – Single-incision laparoscopic surgical appendectomy is both feasible and safe across a wide range of pediatric patient ages, results from a single-center study showed.
"Single-incision laparoscopic surgery is an exciting area of minimally invasive surgery," Dr. Eduardo A. Perez said at the annual meeting of the American Pediatric Surgical Association. "There are known disadvantages, including limited lateral movement and dueling instruments, but industry is working hard to improve these platforms. This includes articulating instruments, flexible laparoscopes, multichannel ports, and robotic platforms."
Dr. Perez, of the division of pediatric surgery at Children’s Medical Center, Dallas, and his associates randomized 50 patients equally to either single-incision laparoscopic surgery (SILS) or conventional laparoscopy (LAP) for appendectomy and followed them for a median of 14 months. The technique for SILS involved a single supraumbilical curvilinear incision with three fascial incisions placed in a triangular fashion. To make SILS technically comparable to the LAP procedure, the researchers used a stapler device that required upsizing a 5-mm port to a 12-mm size. Cosmesis was not studied.
The children ranged in age from 3 to 15 years, and there were no significant age differences between the two treatment groups. Half of the patients were under age 8, 50% were male, and 67% were Hispanic.
The overall mean OR time was 46.8 minutes for the SILS procedure, compared with 34.8 minutes for the LAP procedure, a difference that was significant (P = .010). However, the OR time between groups became more similar as the number of cases increased. For example, after the first 25 patients were treated, the mean OR time for the SILS procedure was 49.3 minutes, compared with 33.5 minutes for the LAP procedure, a difference that remained significant (P = .049). After the last 25 patients were treated, the mean OR times were no longer significantly different between the two groups (a mean of 44.1 vs. 36 minutes, respectively).
There were no conversions and no differences in hospital length of stay between the two groups (a median of 40.3 hours for SILS vs. 36.7 hours for LAP).
The only complication was a wound seroma in the SILS group, and no hernias were observed. In addition, no differences were noted between the two groups in terms of hospital readmissions, diet tolerance, fever, or postoperative pain.
Operative times between SILS and LAP appendectomy "are similar once experience is gained," Dr. Perez concluded. "OR cost is similar when using standard instruments, but this will increase [in SILS] with the use of newer, more advanced instruments."
Dr. Perez said that he had no relevant financial disclosures to make.
The meeting was supported by a grant from Elsevier, which owns this news organization.
PALM DESERT, CALIF. – Single-incision laparoscopic surgical appendectomy is both feasible and safe across a wide range of pediatric patient ages, results from a single-center study showed.
"Single-incision laparoscopic surgery is an exciting area of minimally invasive surgery," Dr. Eduardo A. Perez said at the annual meeting of the American Pediatric Surgical Association. "There are known disadvantages, including limited lateral movement and dueling instruments, but industry is working hard to improve these platforms. This includes articulating instruments, flexible laparoscopes, multichannel ports, and robotic platforms."
Dr. Perez, of the division of pediatric surgery at Children’s Medical Center, Dallas, and his associates randomized 50 patients equally to either single-incision laparoscopic surgery (SILS) or conventional laparoscopy (LAP) for appendectomy and followed them for a median of 14 months. The technique for SILS involved a single supraumbilical curvilinear incision with three fascial incisions placed in a triangular fashion. To make SILS technically comparable to the LAP procedure, the researchers used a stapler device that required upsizing a 5-mm port to a 12-mm size. Cosmesis was not studied.
The children ranged in age from 3 to 15 years, and there were no significant age differences between the two treatment groups. Half of the patients were under age 8, 50% were male, and 67% were Hispanic.
The overall mean OR time was 46.8 minutes for the SILS procedure, compared with 34.8 minutes for the LAP procedure, a difference that was significant (P = .010). However, the OR time between groups became more similar as the number of cases increased. For example, after the first 25 patients were treated, the mean OR time for the SILS procedure was 49.3 minutes, compared with 33.5 minutes for the LAP procedure, a difference that remained significant (P = .049). After the last 25 patients were treated, the mean OR times were no longer significantly different between the two groups (a mean of 44.1 vs. 36 minutes, respectively).
There were no conversions and no differences in hospital length of stay between the two groups (a median of 40.3 hours for SILS vs. 36.7 hours for LAP).
The only complication was a wound seroma in the SILS group, and no hernias were observed. In addition, no differences were noted between the two groups in terms of hospital readmissions, diet tolerance, fever, or postoperative pain.
Operative times between SILS and LAP appendectomy "are similar once experience is gained," Dr. Perez concluded. "OR cost is similar when using standard instruments, but this will increase [in SILS] with the use of newer, more advanced instruments."
Dr. Perez said that he had no relevant financial disclosures to make.
The meeting was supported by a grant from Elsevier, which owns this news organization.
Dr. Eduardo A. Perez, American Pediatric Surgical Association, SILS, LAP, supraumbilical curvilinear incision,
Dr. Eduardo A. Perez, American Pediatric Surgical Association, SILS, LAP, supraumbilical curvilinear incision,
FROM THE ANNUAL MEETING OF THE AMERICAN PEDIATRIC SURGICAL ASSOCIATION
Major Finding: The overall mean OR time for SILS appendectomy was 46.8 minutes, compared with 34.8 minutes for conventional laparoscopy, a difference that was significant (P = .010). However, the OR time between groups became more similar as the number of cases increased.
Data Source: A study of 50 patients randomized to SILS or to conventional laparoscopy for appendectomy and followed for a median of 14 months.
Disclosures: Dr. Perez said that he had no relevant financial disclosures to make.
Know Thy Laser and Other Treatment Pearls
DANA POINT, CALIF. – Be wary of clinicians who claim that they never have complications from laser surgery procedures, Dr. A. Jay Burns advised physicians to tell their patients at the Summit in Aesthetic Medicine.
Such clinicians "are either liars, or they’ve only practiced for about 5 minutes," Dr. Burns, of the Dallas Plastic Surgery Institute, said at the meeting, sponsored by Skin Disease Education Foundation (SDEF). "I tell my patients to run from them. We all have complications."
In general, he continued, complications "decrease the better you are trained and the more detail-oriented you are. I don’t know how you legislate that. You also have to care; you have to have compassion."
During separate presentations, he and Dr. Eric F. Bernstein, a board-certified dermatologist who practices laser surgery in Ardmore, Pa., offered practical tips on how to best prevent complications from laser surgery, including the following:
• Know thy laser. "In general, there is less margin for error the cheaper the device, the more corners cut in research and development, the smaller the spot size, and the use of manual treatment versus scanned treatment," Dr. Burns said. "Complications can be minimized by good technique and good postoperative care."
• Don’t take treatment advice from sales representatives. "You are responsible for the treatment no matter what, so research the science and talk to colleagues," Dr. Bernstein said. "I have three words for sales reps bringing a device they tout as safe and effective: ‘Have a seat!’ Sales reps who believe in their products will happily be your first patient, and they will come back for follow-up. You can count on it."
• Take note of special patient populations. Make sure to ask patients about isotretinoin use. "Most of my colleagues and I wait 6 months after patients have discontinued isotretinoin before laser treatment," Dr. Bernstein said. "In most situations I think that’s the standard of care."
Depending on the laser, he prescribes valacyclovir as prophylaxis in patients with a history of herpes simplex virus or for ablative procedures in any patient. He said that he does not treat patients who have taken gold therapy at any time in their life with lasers, as their skin "will turn gray or black at the site of every laser pulse. This is on my consent form."
Patients with systemic lupus and other connective tissue diseases can flare locally and possibly systemically after treatment with vascular lasers, so he generally avoids treating these patients or treats with caution after spot testing.
He does not treat pregnant patients with elective laser procedures, "although I don’t believe there is any risk from the lasers I use," Dr. Bernstein said. "I treated my wife during her pregnancy because she had so much free time from work during that time. However, we’re in America, and until the legal climate changes dramatically, I just avoid performing elective laser treatments on pregnant patients. Tattoo removal is probably the one case where it is actually less advised to treat patients during pregnancy for medical reasons. That’s because the tattoo pigment as a chemical can become mobilized following laser treatment, not because of the laser."
Sun exposure "is probably the biggest issue that causes complications in patients," he continued. "I tell every laser surgeon to respect melanin pigment, as it is an unwanted target in the skin and can absorb laser light, making the epidermis an unwanted target. In addition, a tan makes for more risk of post-inflammatory hyper- or hypopigmentation following treatment. Patients are rarely honest about their sun exposure."
• Wear protective eyewear while operating the laser. Dr. Bernstein locks the door to the laser room while he treats patients "because I think it’s inappropriate to have laser glasses outside your room and open the door and walk in with the glasses, exposing the people outside the door or walking by to laser light. This may be against certain regulations, since those outside the room would have a hard time entering in the event of a problem, but I am never alone in a room with a patient, and prefer this rule for eye safety."
When someone hands you a pair of protective laser glasses, "look at the wavelength ranges and make sure that they correspond to the wavelength of the laser," he advised. "We all check each other’s glasses to make sure they are the right wavelength. Obviously, it’s best to have only one wavelength per room and have glasses for that laser; however, in my office that’s not possible."
• If patients say they’re in pain, stop. Some people have a low tolerance for pain, "but that’s not the time to debate their pain threshold," Dr. Burns noted.
• Debridement and pretreatment. In Dr. Burns’ practice, the regimen for all patients undergoing ablative resurfacing includes changes of Flexzan wound dressing and debridement at 1, 3, and 5 days, cephalexin 250 mg t.i.d. for 5 days, and valacyclovir 500 mg b.i.d. for 10 days.
• Expect maintenance treatments for laser hair removal cases. "Everybody is different, but because of the hair cycle it takes four to six initial treatments, 6 weeks apart, to expose all of the hair in a given area to the laser," Dr. Bernstein said. "Maintenance treatments are always required to keep all of the hair away in a given area."
Prior to performing hair removal procedures in the perioral region, he places two folded pieces of 4-by-4-inch gauze into the patient’s mouth to protect the teeth. "Use nonstick gauze with braces or be ready for a half-hour extraction," he said.
Dr. Bernstein disclosed that he has received research support from Syneron, Cynosure, and Cutera, and Solta Medical. He also serves as a paid consultant for Tria Beauty.
Dr. Burns disclosed that he receives equipment discounts from Cutera, Cynosure, Palomar, Sciton, and Aesthetic Medical Lasers; research support from Sciton, Solta Medical, Ulthera, and Zeltiq; and consulting fees from Ulthera and Zeltiq. He also holds stock in SkinMedica and Zeltiq.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Be wary of clinicians who claim that they never have complications from laser surgery procedures, Dr. A. Jay Burns advised physicians to tell their patients at the Summit in Aesthetic Medicine.
Such clinicians "are either liars, or they’ve only practiced for about 5 minutes," Dr. Burns, of the Dallas Plastic Surgery Institute, said at the meeting, sponsored by Skin Disease Education Foundation (SDEF). "I tell my patients to run from them. We all have complications."
In general, he continued, complications "decrease the better you are trained and the more detail-oriented you are. I don’t know how you legislate that. You also have to care; you have to have compassion."
During separate presentations, he and Dr. Eric F. Bernstein, a board-certified dermatologist who practices laser surgery in Ardmore, Pa., offered practical tips on how to best prevent complications from laser surgery, including the following:
• Know thy laser. "In general, there is less margin for error the cheaper the device, the more corners cut in research and development, the smaller the spot size, and the use of manual treatment versus scanned treatment," Dr. Burns said. "Complications can be minimized by good technique and good postoperative care."
• Don’t take treatment advice from sales representatives. "You are responsible for the treatment no matter what, so research the science and talk to colleagues," Dr. Bernstein said. "I have three words for sales reps bringing a device they tout as safe and effective: ‘Have a seat!’ Sales reps who believe in their products will happily be your first patient, and they will come back for follow-up. You can count on it."
• Take note of special patient populations. Make sure to ask patients about isotretinoin use. "Most of my colleagues and I wait 6 months after patients have discontinued isotretinoin before laser treatment," Dr. Bernstein said. "In most situations I think that’s the standard of care."
Depending on the laser, he prescribes valacyclovir as prophylaxis in patients with a history of herpes simplex virus or for ablative procedures in any patient. He said that he does not treat patients who have taken gold therapy at any time in their life with lasers, as their skin "will turn gray or black at the site of every laser pulse. This is on my consent form."
Patients with systemic lupus and other connective tissue diseases can flare locally and possibly systemically after treatment with vascular lasers, so he generally avoids treating these patients or treats with caution after spot testing.
He does not treat pregnant patients with elective laser procedures, "although I don’t believe there is any risk from the lasers I use," Dr. Bernstein said. "I treated my wife during her pregnancy because she had so much free time from work during that time. However, we’re in America, and until the legal climate changes dramatically, I just avoid performing elective laser treatments on pregnant patients. Tattoo removal is probably the one case where it is actually less advised to treat patients during pregnancy for medical reasons. That’s because the tattoo pigment as a chemical can become mobilized following laser treatment, not because of the laser."
Sun exposure "is probably the biggest issue that causes complications in patients," he continued. "I tell every laser surgeon to respect melanin pigment, as it is an unwanted target in the skin and can absorb laser light, making the epidermis an unwanted target. In addition, a tan makes for more risk of post-inflammatory hyper- or hypopigmentation following treatment. Patients are rarely honest about their sun exposure."
• Wear protective eyewear while operating the laser. Dr. Bernstein locks the door to the laser room while he treats patients "because I think it’s inappropriate to have laser glasses outside your room and open the door and walk in with the glasses, exposing the people outside the door or walking by to laser light. This may be against certain regulations, since those outside the room would have a hard time entering in the event of a problem, but I am never alone in a room with a patient, and prefer this rule for eye safety."
When someone hands you a pair of protective laser glasses, "look at the wavelength ranges and make sure that they correspond to the wavelength of the laser," he advised. "We all check each other’s glasses to make sure they are the right wavelength. Obviously, it’s best to have only one wavelength per room and have glasses for that laser; however, in my office that’s not possible."
• If patients say they’re in pain, stop. Some people have a low tolerance for pain, "but that’s not the time to debate their pain threshold," Dr. Burns noted.
• Debridement and pretreatment. In Dr. Burns’ practice, the regimen for all patients undergoing ablative resurfacing includes changes of Flexzan wound dressing and debridement at 1, 3, and 5 days, cephalexin 250 mg t.i.d. for 5 days, and valacyclovir 500 mg b.i.d. for 10 days.
• Expect maintenance treatments for laser hair removal cases. "Everybody is different, but because of the hair cycle it takes four to six initial treatments, 6 weeks apart, to expose all of the hair in a given area to the laser," Dr. Bernstein said. "Maintenance treatments are always required to keep all of the hair away in a given area."
Prior to performing hair removal procedures in the perioral region, he places two folded pieces of 4-by-4-inch gauze into the patient’s mouth to protect the teeth. "Use nonstick gauze with braces or be ready for a half-hour extraction," he said.
Dr. Bernstein disclosed that he has received research support from Syneron, Cynosure, and Cutera, and Solta Medical. He also serves as a paid consultant for Tria Beauty.
Dr. Burns disclosed that he receives equipment discounts from Cutera, Cynosure, Palomar, Sciton, and Aesthetic Medical Lasers; research support from Sciton, Solta Medical, Ulthera, and Zeltiq; and consulting fees from Ulthera and Zeltiq. He also holds stock in SkinMedica and Zeltiq.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Be wary of clinicians who claim that they never have complications from laser surgery procedures, Dr. A. Jay Burns advised physicians to tell their patients at the Summit in Aesthetic Medicine.
Such clinicians "are either liars, or they’ve only practiced for about 5 minutes," Dr. Burns, of the Dallas Plastic Surgery Institute, said at the meeting, sponsored by Skin Disease Education Foundation (SDEF). "I tell my patients to run from them. We all have complications."
In general, he continued, complications "decrease the better you are trained and the more detail-oriented you are. I don’t know how you legislate that. You also have to care; you have to have compassion."
During separate presentations, he and Dr. Eric F. Bernstein, a board-certified dermatologist who practices laser surgery in Ardmore, Pa., offered practical tips on how to best prevent complications from laser surgery, including the following:
• Know thy laser. "In general, there is less margin for error the cheaper the device, the more corners cut in research and development, the smaller the spot size, and the use of manual treatment versus scanned treatment," Dr. Burns said. "Complications can be minimized by good technique and good postoperative care."
• Don’t take treatment advice from sales representatives. "You are responsible for the treatment no matter what, so research the science and talk to colleagues," Dr. Bernstein said. "I have three words for sales reps bringing a device they tout as safe and effective: ‘Have a seat!’ Sales reps who believe in their products will happily be your first patient, and they will come back for follow-up. You can count on it."
• Take note of special patient populations. Make sure to ask patients about isotretinoin use. "Most of my colleagues and I wait 6 months after patients have discontinued isotretinoin before laser treatment," Dr. Bernstein said. "In most situations I think that’s the standard of care."
Depending on the laser, he prescribes valacyclovir as prophylaxis in patients with a history of herpes simplex virus or for ablative procedures in any patient. He said that he does not treat patients who have taken gold therapy at any time in their life with lasers, as their skin "will turn gray or black at the site of every laser pulse. This is on my consent form."
Patients with systemic lupus and other connective tissue diseases can flare locally and possibly systemically after treatment with vascular lasers, so he generally avoids treating these patients or treats with caution after spot testing.
He does not treat pregnant patients with elective laser procedures, "although I don’t believe there is any risk from the lasers I use," Dr. Bernstein said. "I treated my wife during her pregnancy because she had so much free time from work during that time. However, we’re in America, and until the legal climate changes dramatically, I just avoid performing elective laser treatments on pregnant patients. Tattoo removal is probably the one case where it is actually less advised to treat patients during pregnancy for medical reasons. That’s because the tattoo pigment as a chemical can become mobilized following laser treatment, not because of the laser."
Sun exposure "is probably the biggest issue that causes complications in patients," he continued. "I tell every laser surgeon to respect melanin pigment, as it is an unwanted target in the skin and can absorb laser light, making the epidermis an unwanted target. In addition, a tan makes for more risk of post-inflammatory hyper- or hypopigmentation following treatment. Patients are rarely honest about their sun exposure."
• Wear protective eyewear while operating the laser. Dr. Bernstein locks the door to the laser room while he treats patients "because I think it’s inappropriate to have laser glasses outside your room and open the door and walk in with the glasses, exposing the people outside the door or walking by to laser light. This may be against certain regulations, since those outside the room would have a hard time entering in the event of a problem, but I am never alone in a room with a patient, and prefer this rule for eye safety."
When someone hands you a pair of protective laser glasses, "look at the wavelength ranges and make sure that they correspond to the wavelength of the laser," he advised. "We all check each other’s glasses to make sure they are the right wavelength. Obviously, it’s best to have only one wavelength per room and have glasses for that laser; however, in my office that’s not possible."
• If patients say they’re in pain, stop. Some people have a low tolerance for pain, "but that’s not the time to debate their pain threshold," Dr. Burns noted.
• Debridement and pretreatment. In Dr. Burns’ practice, the regimen for all patients undergoing ablative resurfacing includes changes of Flexzan wound dressing and debridement at 1, 3, and 5 days, cephalexin 250 mg t.i.d. for 5 days, and valacyclovir 500 mg b.i.d. for 10 days.
• Expect maintenance treatments for laser hair removal cases. "Everybody is different, but because of the hair cycle it takes four to six initial treatments, 6 weeks apart, to expose all of the hair in a given area to the laser," Dr. Bernstein said. "Maintenance treatments are always required to keep all of the hair away in a given area."
Prior to performing hair removal procedures in the perioral region, he places two folded pieces of 4-by-4-inch gauze into the patient’s mouth to protect the teeth. "Use nonstick gauze with braces or be ready for a half-hour extraction," he said.
Dr. Bernstein disclosed that he has received research support from Syneron, Cynosure, and Cutera, and Solta Medical. He also serves as a paid consultant for Tria Beauty.
Dr. Burns disclosed that he receives equipment discounts from Cutera, Cynosure, Palomar, Sciton, and Aesthetic Medical Lasers; research support from Sciton, Solta Medical, Ulthera, and Zeltiq; and consulting fees from Ulthera and Zeltiq. He also holds stock in SkinMedica and Zeltiq.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Interim Study Results Find Artefill Safe
DANA POINT, CALIF. – The rate of granuloma formation following use of Artefill for the correction of nasolabial folds stands at 0.59%, interim results from a 5-year study have shown.
Dr. Christopher B. Zachary presented 36-month results from the prospective study at the SDEF Summit in Aesthetic Medicine. The study was required by the Food and Drug Administration and is the largest and longest prospective clinical study to date for dermal fillers in the United States and in the European Union, according to Dr. Zachary.
The purpose of the study is to assess the safety of Artefill (Suneva Medical) in 1,008 patients, based on the incidence of anticipated and unanticipated adverse events and serious adverse events, the incidence of granuloma formation, and subjects' assessment of satisfaction.
Patients were treated for the correction of nasolabial folds at baseline. They received two touch-up treatments if needed, said Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine. The second clinic visit consisted of a clinical evaluation and photos of the treated site 3 months after the last treatment.
Patients filled out questionnaires at 6, 12, and 18 months and at 2 and 3 years, and will again do so at years 4 and 5. Adverse events were reported to the site for investigation. Final visits are scheduled for 5 years after the last Artefill treatment.
The mean age of the 1,008 patients was 54 years, 89% were female, and 88% were white. Most subjects (975) are still in the trial, while 29 have been lost to follow-up or withdrew consent for personal reasons, and four non–treatment-related deaths have occurred.
A total of 114 adverse events deemed device related have occurred to date, for a rate of 11%. These include local complications such as lumpiness, swelling, and redness. In addition, 11 lesions have been identified and biopsied: 4 were viewed as unremarkable at biopsy, 1 was categorized as a foreign body reaction consistent with implant material, and 6 were granulomas, for a rate of 0.59%. Five granulomas resolved completely and one is responding well to treatment.
"You might say that the 0.59% incidence of granulomas is a bit high when you consider that the worldwide reported incidence of granulomas after using Artefill is 0.04%," said Dr. Zachary. "If the FDA required an intense 5-year study of all the commonly used filler products, where every adverse event [AE] was reported, then you would expect the overall AE incidence to be significantly higher. So what is the real incidence of AEs in fillers, and does reliance on voluntary reporting give us misleading results?"
Patient satisfaction scores have remained high over time. For example, at 6 months, 81% reported being "very satisfied" or "satisfied" with the cosmetic results, compared with 79% at 18 months, and 78% at 24 months.
"I'm not up here promoting the product, but I do think Artefill is very safe," Dr. Zachary said. "In practice, this study demonstrates that the product is probably just as safe as hyaluronic acids. Some of my colleagues hate to hear this, and I am not really a fan of permanent fillers, but if we determine that a company needs to perform a comprehensive 5-year safety study, then we need to sit up and take notice of the results. To do otherwise would be ignorant."
Dr. Zachary disclosed that he has received research support, discounts on devices, and honoraria from numerous laser and device companies, including Suneva Medical. He is a member of Suneva’s scientific advisory board.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – The rate of granuloma formation following use of Artefill for the correction of nasolabial folds stands at 0.59%, interim results from a 5-year study have shown.
Dr. Christopher B. Zachary presented 36-month results from the prospective study at the SDEF Summit in Aesthetic Medicine. The study was required by the Food and Drug Administration and is the largest and longest prospective clinical study to date for dermal fillers in the United States and in the European Union, according to Dr. Zachary.
The purpose of the study is to assess the safety of Artefill (Suneva Medical) in 1,008 patients, based on the incidence of anticipated and unanticipated adverse events and serious adverse events, the incidence of granuloma formation, and subjects' assessment of satisfaction.
Patients were treated for the correction of nasolabial folds at baseline. They received two touch-up treatments if needed, said Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine. The second clinic visit consisted of a clinical evaluation and photos of the treated site 3 months after the last treatment.
Patients filled out questionnaires at 6, 12, and 18 months and at 2 and 3 years, and will again do so at years 4 and 5. Adverse events were reported to the site for investigation. Final visits are scheduled for 5 years after the last Artefill treatment.
The mean age of the 1,008 patients was 54 years, 89% were female, and 88% were white. Most subjects (975) are still in the trial, while 29 have been lost to follow-up or withdrew consent for personal reasons, and four non–treatment-related deaths have occurred.
A total of 114 adverse events deemed device related have occurred to date, for a rate of 11%. These include local complications such as lumpiness, swelling, and redness. In addition, 11 lesions have been identified and biopsied: 4 were viewed as unremarkable at biopsy, 1 was categorized as a foreign body reaction consistent with implant material, and 6 were granulomas, for a rate of 0.59%. Five granulomas resolved completely and one is responding well to treatment.
"You might say that the 0.59% incidence of granulomas is a bit high when you consider that the worldwide reported incidence of granulomas after using Artefill is 0.04%," said Dr. Zachary. "If the FDA required an intense 5-year study of all the commonly used filler products, where every adverse event [AE] was reported, then you would expect the overall AE incidence to be significantly higher. So what is the real incidence of AEs in fillers, and does reliance on voluntary reporting give us misleading results?"
Patient satisfaction scores have remained high over time. For example, at 6 months, 81% reported being "very satisfied" or "satisfied" with the cosmetic results, compared with 79% at 18 months, and 78% at 24 months.
"I'm not up here promoting the product, but I do think Artefill is very safe," Dr. Zachary said. "In practice, this study demonstrates that the product is probably just as safe as hyaluronic acids. Some of my colleagues hate to hear this, and I am not really a fan of permanent fillers, but if we determine that a company needs to perform a comprehensive 5-year safety study, then we need to sit up and take notice of the results. To do otherwise would be ignorant."
Dr. Zachary disclosed that he has received research support, discounts on devices, and honoraria from numerous laser and device companies, including Suneva Medical. He is a member of Suneva’s scientific advisory board.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – The rate of granuloma formation following use of Artefill for the correction of nasolabial folds stands at 0.59%, interim results from a 5-year study have shown.
Dr. Christopher B. Zachary presented 36-month results from the prospective study at the SDEF Summit in Aesthetic Medicine. The study was required by the Food and Drug Administration and is the largest and longest prospective clinical study to date for dermal fillers in the United States and in the European Union, according to Dr. Zachary.
The purpose of the study is to assess the safety of Artefill (Suneva Medical) in 1,008 patients, based on the incidence of anticipated and unanticipated adverse events and serious adverse events, the incidence of granuloma formation, and subjects' assessment of satisfaction.
Patients were treated for the correction of nasolabial folds at baseline. They received two touch-up treatments if needed, said Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine. The second clinic visit consisted of a clinical evaluation and photos of the treated site 3 months after the last treatment.
Patients filled out questionnaires at 6, 12, and 18 months and at 2 and 3 years, and will again do so at years 4 and 5. Adverse events were reported to the site for investigation. Final visits are scheduled for 5 years after the last Artefill treatment.
The mean age of the 1,008 patients was 54 years, 89% were female, and 88% were white. Most subjects (975) are still in the trial, while 29 have been lost to follow-up or withdrew consent for personal reasons, and four non–treatment-related deaths have occurred.
A total of 114 adverse events deemed device related have occurred to date, for a rate of 11%. These include local complications such as lumpiness, swelling, and redness. In addition, 11 lesions have been identified and biopsied: 4 were viewed as unremarkable at biopsy, 1 was categorized as a foreign body reaction consistent with implant material, and 6 were granulomas, for a rate of 0.59%. Five granulomas resolved completely and one is responding well to treatment.
"You might say that the 0.59% incidence of granulomas is a bit high when you consider that the worldwide reported incidence of granulomas after using Artefill is 0.04%," said Dr. Zachary. "If the FDA required an intense 5-year study of all the commonly used filler products, where every adverse event [AE] was reported, then you would expect the overall AE incidence to be significantly higher. So what is the real incidence of AEs in fillers, and does reliance on voluntary reporting give us misleading results?"
Patient satisfaction scores have remained high over time. For example, at 6 months, 81% reported being "very satisfied" or "satisfied" with the cosmetic results, compared with 79% at 18 months, and 78% at 24 months.
"I'm not up here promoting the product, but I do think Artefill is very safe," Dr. Zachary said. "In practice, this study demonstrates that the product is probably just as safe as hyaluronic acids. Some of my colleagues hate to hear this, and I am not really a fan of permanent fillers, but if we determine that a company needs to perform a comprehensive 5-year safety study, then we need to sit up and take notice of the results. To do otherwise would be ignorant."
Dr. Zachary disclosed that he has received research support, discounts on devices, and honoraria from numerous laser and device companies, including Suneva Medical. He is a member of Suneva’s scientific advisory board.
SDEF and this news organization are owned by Elsevier.
FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Major Finding: A total of 114 adverse events deemed device related have occurred to date, for a rate of 11%. The rate of granuloma formation to date is 0.59%
Data Source: Interim results from a 5-year study of 1,008 patients who have received Artefill for the correction of nasolabial folds.
Disclosures: Dr. Zachary disclosed that he has received research support, discounts on devices, and honoraria from numerous laser and device companies, including Suneva Medical. He is a member of Suneva’s scientific advisory board.
Expert Offers Facial Fat Augmentation Pearls
DANA POINT, CALIF. – Before undergoing facial fat augmentation, patients routinely ask Dr. Jonathan M. Sykes how long their results will last.
"I tell them that the longevity of fat is variable in different parts of the face," Dr. Sykes said at the. Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF). He described long-term results in the infraorbital region, the malar region, and the tear trough as "great"; in the melolabial fold and pre-jowl sulcus as "good"; and in the lips as "not so good."
"I think lips are the hardest area to treat," said Dr. Sykes, director of facial plastic and reconstructive surgery at the University of California Davis Health System, Sacramento.
One advantage of facial fat augmentation is that most patients have an abundant supply of autologous fat, with the exception of bodybuilders and patients who have been on long-term antiretroviral medicines for HIV, he said. "It’s also easy to perform simultaneously with other surgical rejuvenative procedures, and it does not add significantly to time or cost. I harvest the fat at the beginning, and put it in at the end. Other physicians put in the fat right away and then do the surgical procedure."
Disadvantages of facial fat augmentation, he said, are that the procedure is time and technique sensitive, donor site contour irregularities are possible, donor site pain/ecchymosis is possible, and it is difficult to modulate the results.
Instruments he uses for most procedures include four cannulas made by San Diego–based Tulip Medical: a 0.9-mm spoon tip cannula that is 4 cm long for periorbital injections, a 1.2-mm spoon tip cannula that’s 6 cm long for all-purpose injections, a 3.0-mm bullet tip cannula that is 15 cm long for all-purpose fat harvesting, and a 2.1-mm multiport cannula that is 12 cm long and used as an optional secondary cannula for thin patients.
Dr. Sykes, who is also the current president of the American Academy of Facial Plastic and Reconstructive Surgery, said he prefers the upper/outer hip as a donor source, but makes it a point to ask patients where they retain the most fat. "In women, usually it’s the abdomen, hips, and inner/outer thighs, while in men it’s usually the abdomen or the hips," he said. "There is some evidence that outer thigh fat persists a bit better because it’s less vascular."
To harvest the fat he uses four to eight 10-cc Luer lock syringes with low negative pressure. He then stands the syringes upright for about 15 minutes, "typically while other surgical procedures are being performed," he said.
Next, he places the fat into a centrifuge at 3,000 rpm for 1-3 minutes and transfers the fat into one 20- or 35-cc syringe. He uses a Leur lock transfer hub to transfer the fat into several 1-cc Leur lock syringes.
Dr. Sykes said he routinely uses local anesthesia with epinephrine 20 minutes prior to injection. "That gives us less bleeding and less ecchymosis," he said.
He then injects small parcels of fat as the cannula is withdrawn, aiming for 30-50 passes per 1 cc of fat.
In most patients, adding fat volume to the face "creates a rejuvenated, youthful appearance," Dr. Sykes concluded, noting that he performs fat augmentation in about 70% of face-lift procedures.
Dr. Sykes disclosed that he has served as a paid trainer and speaker for Sanofi Aventis and Medicis.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Before undergoing facial fat augmentation, patients routinely ask Dr. Jonathan M. Sykes how long their results will last.
"I tell them that the longevity of fat is variable in different parts of the face," Dr. Sykes said at the. Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF). He described long-term results in the infraorbital region, the malar region, and the tear trough as "great"; in the melolabial fold and pre-jowl sulcus as "good"; and in the lips as "not so good."
"I think lips are the hardest area to treat," said Dr. Sykes, director of facial plastic and reconstructive surgery at the University of California Davis Health System, Sacramento.
One advantage of facial fat augmentation is that most patients have an abundant supply of autologous fat, with the exception of bodybuilders and patients who have been on long-term antiretroviral medicines for HIV, he said. "It’s also easy to perform simultaneously with other surgical rejuvenative procedures, and it does not add significantly to time or cost. I harvest the fat at the beginning, and put it in at the end. Other physicians put in the fat right away and then do the surgical procedure."
Disadvantages of facial fat augmentation, he said, are that the procedure is time and technique sensitive, donor site contour irregularities are possible, donor site pain/ecchymosis is possible, and it is difficult to modulate the results.
Instruments he uses for most procedures include four cannulas made by San Diego–based Tulip Medical: a 0.9-mm spoon tip cannula that is 4 cm long for periorbital injections, a 1.2-mm spoon tip cannula that’s 6 cm long for all-purpose injections, a 3.0-mm bullet tip cannula that is 15 cm long for all-purpose fat harvesting, and a 2.1-mm multiport cannula that is 12 cm long and used as an optional secondary cannula for thin patients.
Dr. Sykes, who is also the current president of the American Academy of Facial Plastic and Reconstructive Surgery, said he prefers the upper/outer hip as a donor source, but makes it a point to ask patients where they retain the most fat. "In women, usually it’s the abdomen, hips, and inner/outer thighs, while in men it’s usually the abdomen or the hips," he said. "There is some evidence that outer thigh fat persists a bit better because it’s less vascular."
To harvest the fat he uses four to eight 10-cc Luer lock syringes with low negative pressure. He then stands the syringes upright for about 15 minutes, "typically while other surgical procedures are being performed," he said.
Next, he places the fat into a centrifuge at 3,000 rpm for 1-3 minutes and transfers the fat into one 20- or 35-cc syringe. He uses a Leur lock transfer hub to transfer the fat into several 1-cc Leur lock syringes.
Dr. Sykes said he routinely uses local anesthesia with epinephrine 20 minutes prior to injection. "That gives us less bleeding and less ecchymosis," he said.
He then injects small parcels of fat as the cannula is withdrawn, aiming for 30-50 passes per 1 cc of fat.
In most patients, adding fat volume to the face "creates a rejuvenated, youthful appearance," Dr. Sykes concluded, noting that he performs fat augmentation in about 70% of face-lift procedures.
Dr. Sykes disclosed that he has served as a paid trainer and speaker for Sanofi Aventis and Medicis.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – Before undergoing facial fat augmentation, patients routinely ask Dr. Jonathan M. Sykes how long their results will last.
"I tell them that the longevity of fat is variable in different parts of the face," Dr. Sykes said at the. Summit in Aesthetic Medicine sponsored by Skin Disease Education Foundation (SDEF). He described long-term results in the infraorbital region, the malar region, and the tear trough as "great"; in the melolabial fold and pre-jowl sulcus as "good"; and in the lips as "not so good."
"I think lips are the hardest area to treat," said Dr. Sykes, director of facial plastic and reconstructive surgery at the University of California Davis Health System, Sacramento.
One advantage of facial fat augmentation is that most patients have an abundant supply of autologous fat, with the exception of bodybuilders and patients who have been on long-term antiretroviral medicines for HIV, he said. "It’s also easy to perform simultaneously with other surgical rejuvenative procedures, and it does not add significantly to time or cost. I harvest the fat at the beginning, and put it in at the end. Other physicians put in the fat right away and then do the surgical procedure."
Disadvantages of facial fat augmentation, he said, are that the procedure is time and technique sensitive, donor site contour irregularities are possible, donor site pain/ecchymosis is possible, and it is difficult to modulate the results.
Instruments he uses for most procedures include four cannulas made by San Diego–based Tulip Medical: a 0.9-mm spoon tip cannula that is 4 cm long for periorbital injections, a 1.2-mm spoon tip cannula that’s 6 cm long for all-purpose injections, a 3.0-mm bullet tip cannula that is 15 cm long for all-purpose fat harvesting, and a 2.1-mm multiport cannula that is 12 cm long and used as an optional secondary cannula for thin patients.
Dr. Sykes, who is also the current president of the American Academy of Facial Plastic and Reconstructive Surgery, said he prefers the upper/outer hip as a donor source, but makes it a point to ask patients where they retain the most fat. "In women, usually it’s the abdomen, hips, and inner/outer thighs, while in men it’s usually the abdomen or the hips," he said. "There is some evidence that outer thigh fat persists a bit better because it’s less vascular."
To harvest the fat he uses four to eight 10-cc Luer lock syringes with low negative pressure. He then stands the syringes upright for about 15 minutes, "typically while other surgical procedures are being performed," he said.
Next, he places the fat into a centrifuge at 3,000 rpm for 1-3 minutes and transfers the fat into one 20- or 35-cc syringe. He uses a Leur lock transfer hub to transfer the fat into several 1-cc Leur lock syringes.
Dr. Sykes said he routinely uses local anesthesia with epinephrine 20 minutes prior to injection. "That gives us less bleeding and less ecchymosis," he said.
He then injects small parcels of fat as the cannula is withdrawn, aiming for 30-50 passes per 1 cc of fat.
In most patients, adding fat volume to the face "creates a rejuvenated, youthful appearance," Dr. Sykes concluded, noting that he performs fat augmentation in about 70% of face-lift procedures.
Dr. Sykes disclosed that he has served as a paid trainer and speaker for Sanofi Aventis and Medicis.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE
Life Expectancy of Diabetes Patients Improving
SAN DIEGO – Life expectancy of patients with type 1 diabetes has improved dramatically since 1950, results from a long-term prospective study have shown.
According to the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, life expectancy at birth for those diagnosed with type 1 diabetes during 1965-1980 was 68.8 years, or about 4 years less than that of a comparable cohort of the U.S. general population, while life expectancy for those diagnosed during 1950-1964 was 53.4 years, or about 18 years less than that of a comparable cohort of the general population.
"Individuals with childhood-onset diabetes do not represent a major insurance risk and should be minimally penalized, if at all, in terms of life insurance and other mortality-based decisions," Dr. Trevor J. Orchard said at the annual scientific sessions of the American Diabetes Association.
While numerous other studies have shown that mortality in type 1 diabetes has been decreasing in recent decades, "how this currently translates into improved life expectancy in the United States was not clear," said Dr. Orchard, professor of epidemiology, pediatrics, and medicine at the University of Pittsburgh.
The objective of the current study was to compare the life expectancy among two different cohorts of patients enrolled in the Pittsburgh EDC Study, which is a prospective study of childhood-onset type 1 diabetes. Of the 933 patients, 390 were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh during 1950-1964 (cohort 1), while 543 were diagnosed or seen within a year of diagnosis during 1965-1980 (cohort 2). Half of the participants were female, and their mean age at diagnosis was 8 years. All were followed through 2009.
To ascertain mortality, the researchers used death certificates and hospital, autopsy, and coroner reports. They used Kaplan-Meier curves and the log-rank statistic to determine differences in survival benefits between the cohorts, and constructed abridged life tables to calculate life expectancy.
Dr. Orchard reported that the 30-year mortality for patients in cohort 1 was 35% compared with 12% for those in cohort 2, a statistically significant difference. Similarly, the life expectancy at birth for those in cohort 1 was estimated to be 53.4 years compared with 68.8 years for those in cohort 2, a difference of about 15 years (P less than .0001). This persisted regardless of sex or pubertal status at diagnosis of diabetes.
The life expectancy of cohort 2 is 3.6 years less than that estimated for a comparable cohort of the U.S. general population (72.4 years), Dr. Orchard said, while the life expectancy of cohort 1 is about 18 years less than that of a comparable cohort of the general population (71.5 years).
Reasons for the improvement in life expectancy between the two cohorts are multifactorial, he said, including the development of blood glucose self-monitoring and the use of the hemoglobin A1c test, which was not available in cohort 1.
"It’s difficult to partition the exact reasons for the changes, but the major improvement in terms of complications has been in renal disease," Dr. Orchard said. "Cardiovascular disease is not improving in these patients as dramatically as renal disease."
Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock
SAN DIEGO – Life expectancy of patients with type 1 diabetes has improved dramatically since 1950, results from a long-term prospective study have shown.
According to the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, life expectancy at birth for those diagnosed with type 1 diabetes during 1965-1980 was 68.8 years, or about 4 years less than that of a comparable cohort of the U.S. general population, while life expectancy for those diagnosed during 1950-1964 was 53.4 years, or about 18 years less than that of a comparable cohort of the general population.
"Individuals with childhood-onset diabetes do not represent a major insurance risk and should be minimally penalized, if at all, in terms of life insurance and other mortality-based decisions," Dr. Trevor J. Orchard said at the annual scientific sessions of the American Diabetes Association.
While numerous other studies have shown that mortality in type 1 diabetes has been decreasing in recent decades, "how this currently translates into improved life expectancy in the United States was not clear," said Dr. Orchard, professor of epidemiology, pediatrics, and medicine at the University of Pittsburgh.
The objective of the current study was to compare the life expectancy among two different cohorts of patients enrolled in the Pittsburgh EDC Study, which is a prospective study of childhood-onset type 1 diabetes. Of the 933 patients, 390 were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh during 1950-1964 (cohort 1), while 543 were diagnosed or seen within a year of diagnosis during 1965-1980 (cohort 2). Half of the participants were female, and their mean age at diagnosis was 8 years. All were followed through 2009.
To ascertain mortality, the researchers used death certificates and hospital, autopsy, and coroner reports. They used Kaplan-Meier curves and the log-rank statistic to determine differences in survival benefits between the cohorts, and constructed abridged life tables to calculate life expectancy.
Dr. Orchard reported that the 30-year mortality for patients in cohort 1 was 35% compared with 12% for those in cohort 2, a statistically significant difference. Similarly, the life expectancy at birth for those in cohort 1 was estimated to be 53.4 years compared with 68.8 years for those in cohort 2, a difference of about 15 years (P less than .0001). This persisted regardless of sex or pubertal status at diagnosis of diabetes.
The life expectancy of cohort 2 is 3.6 years less than that estimated for a comparable cohort of the U.S. general population (72.4 years), Dr. Orchard said, while the life expectancy of cohort 1 is about 18 years less than that of a comparable cohort of the general population (71.5 years).
Reasons for the improvement in life expectancy between the two cohorts are multifactorial, he said, including the development of blood glucose self-monitoring and the use of the hemoglobin A1c test, which was not available in cohort 1.
"It’s difficult to partition the exact reasons for the changes, but the major improvement in terms of complications has been in renal disease," Dr. Orchard said. "Cardiovascular disease is not improving in these patients as dramatically as renal disease."
Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock
SAN DIEGO – Life expectancy of patients with type 1 diabetes has improved dramatically since 1950, results from a long-term prospective study have shown.
According to the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, life expectancy at birth for those diagnosed with type 1 diabetes during 1965-1980 was 68.8 years, or about 4 years less than that of a comparable cohort of the U.S. general population, while life expectancy for those diagnosed during 1950-1964 was 53.4 years, or about 18 years less than that of a comparable cohort of the general population.
"Individuals with childhood-onset diabetes do not represent a major insurance risk and should be minimally penalized, if at all, in terms of life insurance and other mortality-based decisions," Dr. Trevor J. Orchard said at the annual scientific sessions of the American Diabetes Association.
While numerous other studies have shown that mortality in type 1 diabetes has been decreasing in recent decades, "how this currently translates into improved life expectancy in the United States was not clear," said Dr. Orchard, professor of epidemiology, pediatrics, and medicine at the University of Pittsburgh.
The objective of the current study was to compare the life expectancy among two different cohorts of patients enrolled in the Pittsburgh EDC Study, which is a prospective study of childhood-onset type 1 diabetes. Of the 933 patients, 390 were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh during 1950-1964 (cohort 1), while 543 were diagnosed or seen within a year of diagnosis during 1965-1980 (cohort 2). Half of the participants were female, and their mean age at diagnosis was 8 years. All were followed through 2009.
To ascertain mortality, the researchers used death certificates and hospital, autopsy, and coroner reports. They used Kaplan-Meier curves and the log-rank statistic to determine differences in survival benefits between the cohorts, and constructed abridged life tables to calculate life expectancy.
Dr. Orchard reported that the 30-year mortality for patients in cohort 1 was 35% compared with 12% for those in cohort 2, a statistically significant difference. Similarly, the life expectancy at birth for those in cohort 1 was estimated to be 53.4 years compared with 68.8 years for those in cohort 2, a difference of about 15 years (P less than .0001). This persisted regardless of sex or pubertal status at diagnosis of diabetes.
The life expectancy of cohort 2 is 3.6 years less than that estimated for a comparable cohort of the U.S. general population (72.4 years), Dr. Orchard said, while the life expectancy of cohort 1 is about 18 years less than that of a comparable cohort of the general population (71.5 years).
Reasons for the improvement in life expectancy between the two cohorts are multifactorial, he said, including the development of blood glucose self-monitoring and the use of the hemoglobin A1c test, which was not available in cohort 1.
"It’s difficult to partition the exact reasons for the changes, but the major improvement in terms of complications has been in renal disease," Dr. Orchard said. "Cardiovascular disease is not improving in these patients as dramatically as renal disease."
Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: The life expectancy at birth for patients with type 1 diabetes who were born during 1950-1964 was estimated to be 53.4 years compared with 68.8 years for those born during 1965-1980, a difference of about 15 years.
Data Source: A prospective cohort study of 933 patients enrolled in the Pittsburgh Epidemiology of Diabetes Complications Study.
Disclosures: Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock.
Life Expectancy of Diabetes Patients Improving
SAN DIEGO – Life expectancy of patients with type 1 diabetes has improved dramatically since 1950, results from a long-term prospective study have shown.
According to the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, life expectancy at birth for those diagnosed with type 1 diabetes during 1965-1980 was 68.8 years, or about 4 years less than that of a comparable cohort of the U.S. general population, while life expectancy for those diagnosed during 1950-1964 was 53.4 years, or about 18 years less than that of a comparable cohort of the general population.
"Individuals with childhood-onset diabetes do not represent a major insurance risk and should be minimally penalized, if at all, in terms of life insurance and other mortality-based decisions," Dr. Trevor J. Orchard said at the annual scientific sessions of the American Diabetes Association.
While numerous other studies have shown that mortality in type 1 diabetes has been decreasing in recent decades, "how this currently translates into improved life expectancy in the United States was not clear," said Dr. Orchard, professor of epidemiology, pediatrics, and medicine at the University of Pittsburgh.
The objective of the current study was to compare the life expectancy among two different cohorts of patients enrolled in the Pittsburgh EDC Study, which is a prospective study of childhood-onset type 1 diabetes. Of the 933 patients, 390 were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh during 1950-1964 (cohort 1), while 543 were diagnosed or seen within a year of diagnosis during 1965-1980 (cohort 2). Half of the participants were female, and their mean age at diagnosis was 8 years. All were followed through 2009.
To ascertain mortality, the researchers used death certificates and hospital, autopsy, and coroner reports. They used Kaplan-Meier curves and the log-rank statistic to determine differences in survival benefits between the cohorts, and constructed abridged life tables to calculate life expectancy.
Dr. Orchard reported that the 30-year mortality for patients in cohort 1 was 35% compared with 12% for those in cohort 2, a statistically significant difference. Similarly, the life expectancy at birth for those in cohort 1 was estimated to be 53.4 years compared with 68.8 years for those in cohort 2, a difference of about 15 years (P less than .0001). This persisted regardless of sex or pubertal status at diagnosis of diabetes.
The life expectancy of cohort 2 is 3.6 years less than that estimated for a comparable cohort of the U.S. general population (72.4 years), Dr. Orchard said, while the life expectancy of cohort 1 is about 18 years less than that of a comparable cohort of the general population (71.5 years).
Reasons for the improvement in life expectancy between the two cohorts are multifactorial, he said, including the development of blood glucose self-monitoring and the use of the hemoglobin A1c test, which was not available in cohort 1.
"It’s difficult to partition the exact reasons for the changes, but the major improvement in terms of complications has been in renal disease," Dr. Orchard said. "Cardiovascular disease is not improving in these patients as dramatically as renal disease."
Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock
SAN DIEGO – Life expectancy of patients with type 1 diabetes has improved dramatically since 1950, results from a long-term prospective study have shown.
According to the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, life expectancy at birth for those diagnosed with type 1 diabetes during 1965-1980 was 68.8 years, or about 4 years less than that of a comparable cohort of the U.S. general population, while life expectancy for those diagnosed during 1950-1964 was 53.4 years, or about 18 years less than that of a comparable cohort of the general population.
"Individuals with childhood-onset diabetes do not represent a major insurance risk and should be minimally penalized, if at all, in terms of life insurance and other mortality-based decisions," Dr. Trevor J. Orchard said at the annual scientific sessions of the American Diabetes Association.
While numerous other studies have shown that mortality in type 1 diabetes has been decreasing in recent decades, "how this currently translates into improved life expectancy in the United States was not clear," said Dr. Orchard, professor of epidemiology, pediatrics, and medicine at the University of Pittsburgh.
The objective of the current study was to compare the life expectancy among two different cohorts of patients enrolled in the Pittsburgh EDC Study, which is a prospective study of childhood-onset type 1 diabetes. Of the 933 patients, 390 were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh during 1950-1964 (cohort 1), while 543 were diagnosed or seen within a year of diagnosis during 1965-1980 (cohort 2). Half of the participants were female, and their mean age at diagnosis was 8 years. All were followed through 2009.
To ascertain mortality, the researchers used death certificates and hospital, autopsy, and coroner reports. They used Kaplan-Meier curves and the log-rank statistic to determine differences in survival benefits between the cohorts, and constructed abridged life tables to calculate life expectancy.
Dr. Orchard reported that the 30-year mortality for patients in cohort 1 was 35% compared with 12% for those in cohort 2, a statistically significant difference. Similarly, the life expectancy at birth for those in cohort 1 was estimated to be 53.4 years compared with 68.8 years for those in cohort 2, a difference of about 15 years (P less than .0001). This persisted regardless of sex or pubertal status at diagnosis of diabetes.
The life expectancy of cohort 2 is 3.6 years less than that estimated for a comparable cohort of the U.S. general population (72.4 years), Dr. Orchard said, while the life expectancy of cohort 1 is about 18 years less than that of a comparable cohort of the general population (71.5 years).
Reasons for the improvement in life expectancy between the two cohorts are multifactorial, he said, including the development of blood glucose self-monitoring and the use of the hemoglobin A1c test, which was not available in cohort 1.
"It’s difficult to partition the exact reasons for the changes, but the major improvement in terms of complications has been in renal disease," Dr. Orchard said. "Cardiovascular disease is not improving in these patients as dramatically as renal disease."
Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock
SAN DIEGO – Life expectancy of patients with type 1 diabetes has improved dramatically since 1950, results from a long-term prospective study have shown.
According to the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, life expectancy at birth for those diagnosed with type 1 diabetes during 1965-1980 was 68.8 years, or about 4 years less than that of a comparable cohort of the U.S. general population, while life expectancy for those diagnosed during 1950-1964 was 53.4 years, or about 18 years less than that of a comparable cohort of the general population.
"Individuals with childhood-onset diabetes do not represent a major insurance risk and should be minimally penalized, if at all, in terms of life insurance and other mortality-based decisions," Dr. Trevor J. Orchard said at the annual scientific sessions of the American Diabetes Association.
While numerous other studies have shown that mortality in type 1 diabetes has been decreasing in recent decades, "how this currently translates into improved life expectancy in the United States was not clear," said Dr. Orchard, professor of epidemiology, pediatrics, and medicine at the University of Pittsburgh.
The objective of the current study was to compare the life expectancy among two different cohorts of patients enrolled in the Pittsburgh EDC Study, which is a prospective study of childhood-onset type 1 diabetes. Of the 933 patients, 390 were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh during 1950-1964 (cohort 1), while 543 were diagnosed or seen within a year of diagnosis during 1965-1980 (cohort 2). Half of the participants were female, and their mean age at diagnosis was 8 years. All were followed through 2009.
To ascertain mortality, the researchers used death certificates and hospital, autopsy, and coroner reports. They used Kaplan-Meier curves and the log-rank statistic to determine differences in survival benefits between the cohorts, and constructed abridged life tables to calculate life expectancy.
Dr. Orchard reported that the 30-year mortality for patients in cohort 1 was 35% compared with 12% for those in cohort 2, a statistically significant difference. Similarly, the life expectancy at birth for those in cohort 1 was estimated to be 53.4 years compared with 68.8 years for those in cohort 2, a difference of about 15 years (P less than .0001). This persisted regardless of sex or pubertal status at diagnosis of diabetes.
The life expectancy of cohort 2 is 3.6 years less than that estimated for a comparable cohort of the U.S. general population (72.4 years), Dr. Orchard said, while the life expectancy of cohort 1 is about 18 years less than that of a comparable cohort of the general population (71.5 years).
Reasons for the improvement in life expectancy between the two cohorts are multifactorial, he said, including the development of blood glucose self-monitoring and the use of the hemoglobin A1c test, which was not available in cohort 1.
"It’s difficult to partition the exact reasons for the changes, but the major improvement in terms of complications has been in renal disease," Dr. Orchard said. "Cardiovascular disease is not improving in these patients as dramatically as renal disease."
Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: The life expectancy at birth for patients with type 1 diabetes who were born during 1950-1964 was estimated to be 53.4 years compared with 68.8 years for those born during 1965-1980, a difference of about 15 years.
Data Source: A prospective cohort study of 933 patients enrolled in the Pittsburgh Epidemiology of Diabetes Complications Study.
Disclosures: Dr. Orchard disclosed that he is a consultant for AstraZeneca and Abbott Laboratories, received research support from VeraLight, and has inherited Bristol-Myers Squibb stock.