User login
Multiple Glomangiomas in a Patient With a History of Metastatic Melanoma
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
Practice Points
- The diagnosis of glomus tumor and glomangioma is easily suspected when the lesions are in the digital or subungual region.
- Multiple glomangiomas are rare and can clinically pose a diagnostic challenge to dermatologists.
- In patients with a recent history of malignancy, multiple glomangiomas may mimic cutaneous metastases. Therefore, multiple biopsies and histologic examination may be necessary.
Rapid Development of Perifolliculitis Following Mesotherapy
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.

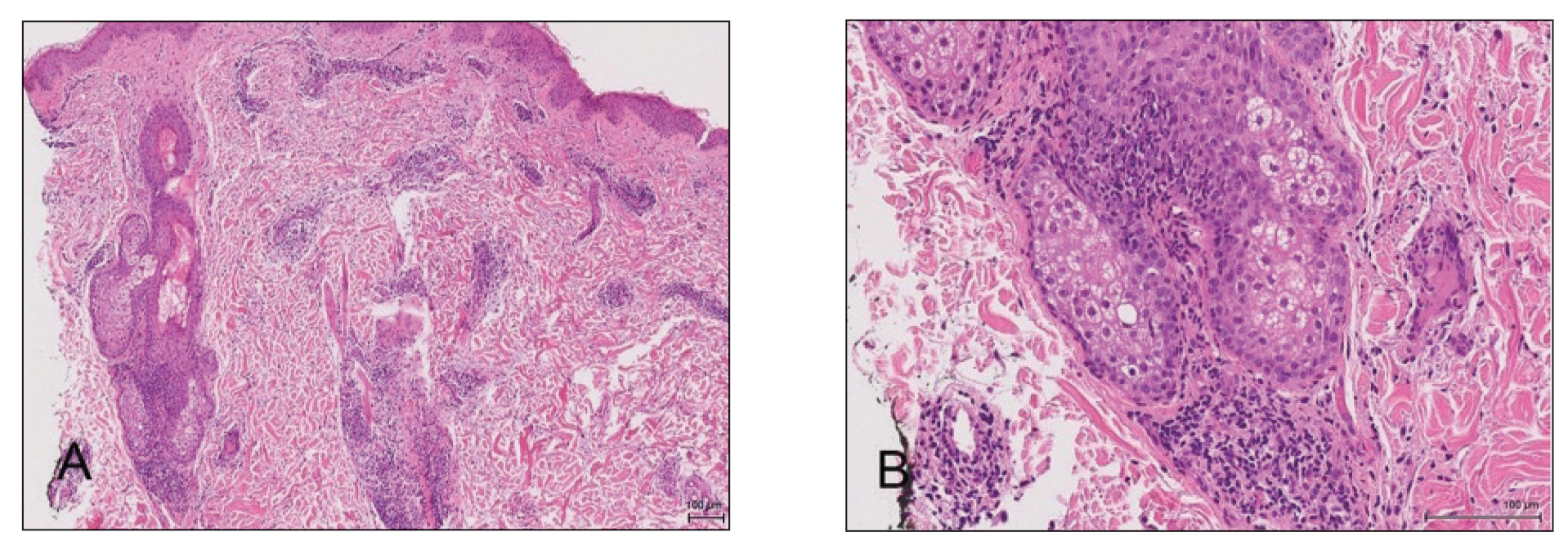
A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.
A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
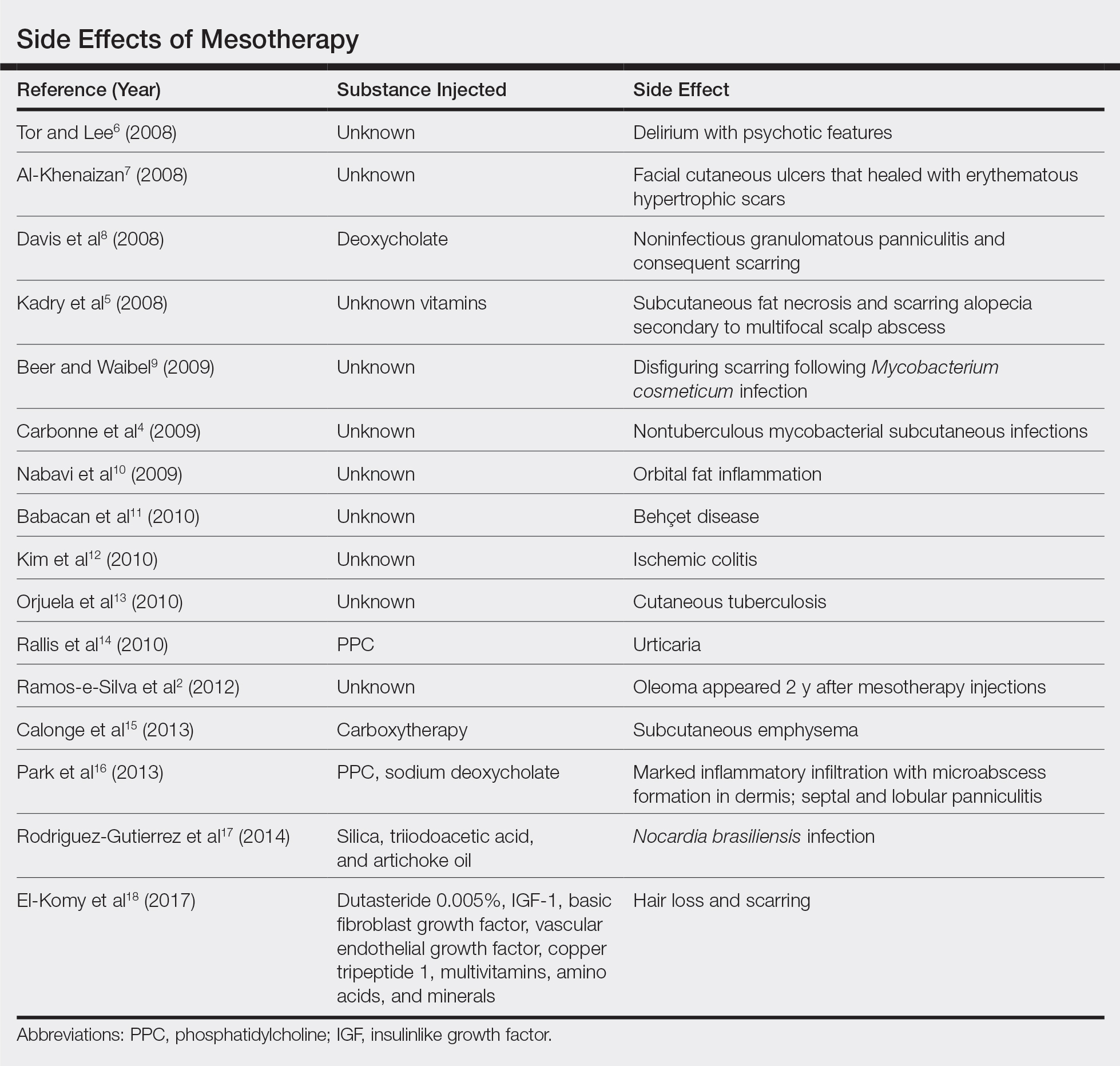
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18
Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.

A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.

A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18

Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.

A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.

A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18

Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
Practice Points
- Mesotherapy—the delivery of vitamins, chemicals, and plant extracts directly into the dermis via injections—is a common procedure performed in both medical and nonmedical settings for cosmetic rejuvenation.
- Complications can occur from mesotherapy treatment.
- Patients should be advised to seek medical care with US Food and Drug Administration–approved cosmetic techniques and substances only

