User login
A Preoperative Transthoracic Echocardiography Protocol to Reduce Time to Hip Fracture Surgery
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
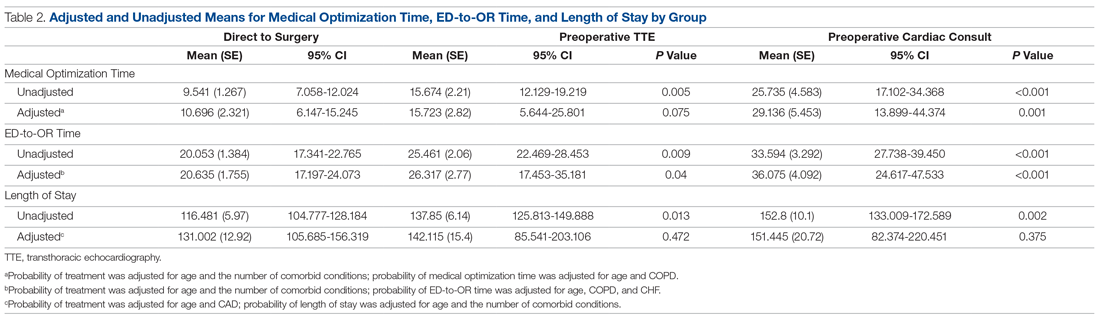
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
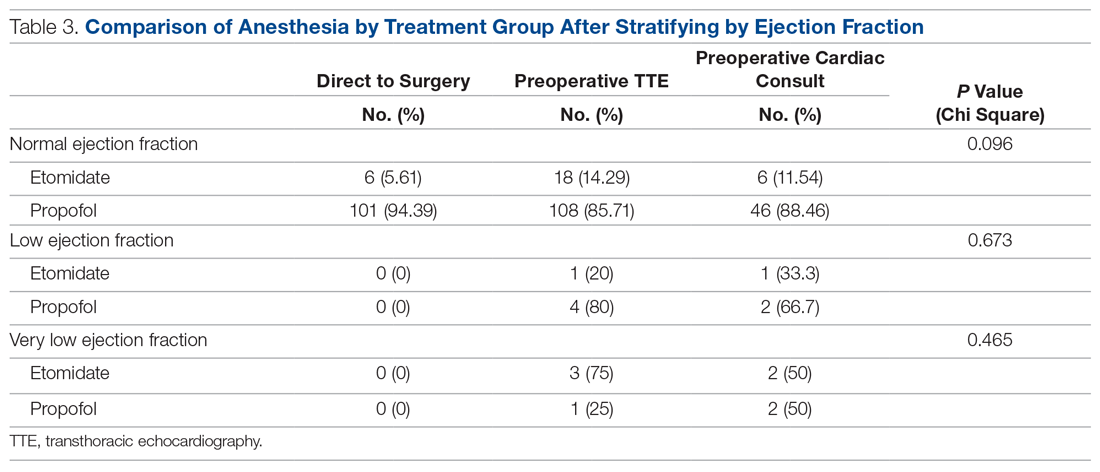
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
Optimizing a Total Joint Replacement Care Pathway to Reduce Skilled Nursing Facility Utilization
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.
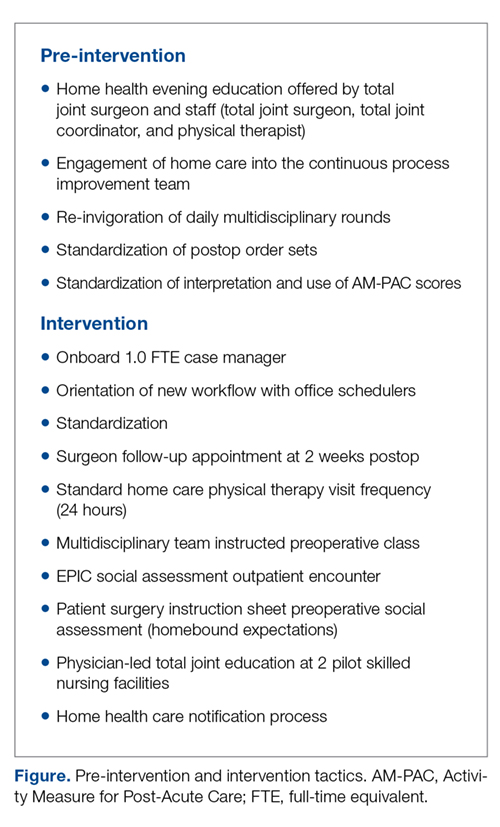
Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.
The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).
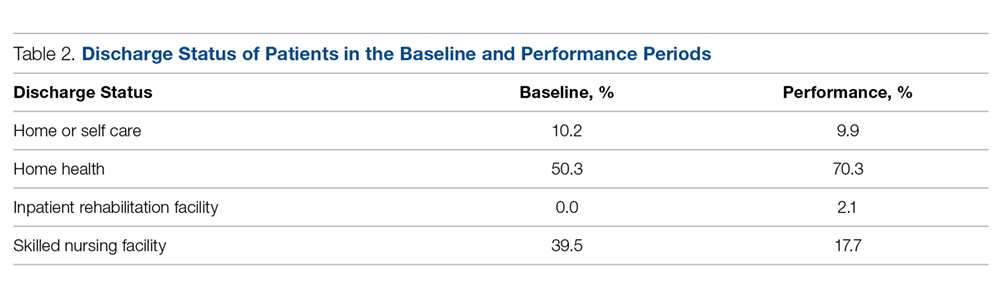
Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; [email protected].
Financial disclosures: None.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.

Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.

The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).

Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; [email protected].
Financial disclosures: None.
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.

Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.

The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).

Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; [email protected].
Financial disclosures: None.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
Reducing Rates of Perioperative Deep Vein Thrombosis and Pulmonary Emboli in Hip and Knee Arthroplasty Patients: A Quality Improvement Project
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.

From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.

From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.

Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.

Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.

Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.

From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.

Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.

Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.

Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.