User login
Jeff Evans has been editor of Rheumatology News/MDedge Rheumatology and the EULAR Congress News since 2013. He started at Frontline Medical Communications in 2001 and was a reporter for 8 years before serving as editor of Clinical Neurology News and World Neurology, and briefly as editor of GI & Hepatology News. He graduated cum laude from Cornell University (New York) with a BA in biological sciences, concentrating in neurobiology and behavior.
Urge to Move Predicted in Advance Through Neuron Recordings
Investigators for the first time have used electrode recordings of the firing patterns of small clusters of neurons to predict voluntary movement in people more than 1 second before they are even aware of their decision or urge to act.
The experiment, conducted by Dr. Itzhak Fried of the University of California, Los Angeles, and his associates, and published in Neuron, detected sets of neurons in the supplementary and presupplementary motor areas and the anterior cingulate cortex with firing rates that would progressively increase or decrease before the participants had even reported the urge to push a button on a laptop. The investigators then constructed algorithms that could successfully predict the impending decision to move at a rate of 70% or greater, depending on the location and size of the set of neurons chosen (Neuron 2011;69:548-62).
Dr. Fried and his colleagues recruited a group of 12 patients with drug-refractory epilepsy who had chronic depth electrodes implanted to determine their seizure focus for possible surgical resection. While the patients sat in bed, they watched an analog clock on a laptop computer and were instructed to push a button after at least one rotation of the clock’s hand whenever "they felt the urge to do so." Each time that the individuals pushed the button, called time P, the investigators asked them to indicate where the clock handle had been when they first felt the urge to move, called time W.
The participants reported a mean W time of 193 ms prior to P, but this varied from trial to trial. In the trials, the greatest proportion of neurons that changed their activity before W was located in parts of the medial frontal lobe of the brain: the supplementary motor area (SMA), the pre-SMA, and the dorsal and rostral regions of the anterior cingulate cortex (ACC). In some of these areas, the researchers observed rises in neuronal firing rates beginning several hundreds to several thousands of milliseconds prior to W, whereas progressive declines in firing rates were recorded in a similar time span prior to W. The number of neurons that changed their firing rate also increased as W approached.
The data gathered by the investigators did not indicate that the subjects were cued to respond by the completion of the clock hand’s first rotation. To sort out concerns related to potentially inaccurate reporting of W and the subjective nature of its determination, the investigators’ manipulated the timing of W either forward or backward in time by fixed amounts or by adjusting its timing by a random amount. These analyses indicated that small temporal shifts in W on the order of 200 ms or less are still compatible with the changes in firing rates seen in recorded neurons and matched what was observed within each participant’s individual trials.
With an algorithm that considered the responses of electrodes to be independent of each other across all participants, Dr. Fried and his associates found that they could predict W on a trial-by-trial basis across all participants. The algorithm could detect changes in the neural activity of 512 neurons in frontal lobe regions 500 ms before W in nearly 90% of the trials. The changes in activity could be detected in more than 70% of trials at 1,000 ms before W.
When the algorithm was constructed on the basis of firing patterns from 256 neurons in the SMA, it could detect the neurons’ change in activity at 500 ms before W in greater than 80% of the trials. In comparison, the change in activity of 256 neurons in the ACC at 500 ms before W could be detected in only 70% of trials.
The algorithm also was able to predict the time of W to within several hundred milliseconds of the actual W reported by the participants.
"This neuronal process suggests a mechanism whereby the feeling of will arises once integration of firing of recruited medial frontal neurons crosses a threshold," the investigators concluded.
The research was supported by grants from three different institutes of the National Institutes of Health, the National Science Foundation, the Klingenstein Fund, the Whitehall Foundation, and a Human Frontiers Science Programs Organization fellowship. No disclosure information was provided by the journal.
Neurologists, physiologists, and philosophers were tossed a hot potato in 1983 with Benjamin Libet, Ph.D., and his colleagues’ publication of the first attempt to measure the time of the perception of intent to make a "voluntary" movement (Brain 1983;106:623-42). Called W, it happened about 250 ms prior to the movement itself. They compared this time to the onset of the Bereitschaftspotential or Readiness potential (RP), an EEG potential that had been previously described by Dr. Hans Kornhuber and Dr. Lüder Deecke (Pflugers Arch. Gesamte Physiol. Menschen Tiere 1965;284:1-17). The RP starts about a second prior to movement. This was a shock. It appeared that the brain was preparing to make a "voluntary" movement before the person was aware of it! The experiment has been repeated many times, so there is no disputing the data; the controversy is the interpretation.
In this new paper by Dr. Fried and his colleagues, they have first repeated the experiment using recording from neurons in the brain rather than the EEG. Their finding about the timing of W was similar to all the other experiments. Since the EEG comes from neuronal activity, it should not be a great surprise that they were able to find neurons that changed their activity in the second or so prior to movement. They then took the data one step further. By analyzing a small number of the neurons, they could predict with a high degree of accuracy, prior to W, when a movement would occur. Hence, it appears that the neuronal activity prior to awareness of intention is marching toward the motor command. Recently, our group, led by Ou Bai, Ph.D., has done the same thing using EEG, although not with the same high degree of accuracy (Clin. Neurophysiol. 2011;122:364-72).
So what does this mean? If people have free will in making voluntary movements, doesn’t the decision have to be made before the motor command? Here, it looks like the motor command is being made before the "decision." The situation is actually easy to resolve, but it does involve some careful thinking. The first point to settle is that the mind is generated by the brain; it is not separate from the brain. Most people agree with that, even though it is easy to fall into dualistic thinking. We are our brains; what the brain is doing, we are doing. Hence, it appears that the decision to make a movement, in this circumstance, arises unconsciously. The decision becomes conscious, or at least we have the impression it becomes conscious, just slightly before the movement. The priority is important. That we have the perception of willing before the perception that the movement occurs allows us to draw the conclusion that we are causal in the production of the movement; that is, that we freely willed the movement.
Is this compatible with the idea that we actually have free will? It depends on what that means. If we are our brains, and our brain is choosing to do this without external coercion, then the movement is free. We become aware of this, in fact, only some of the time. Much of the time, we go about our business without worrying whether our movements are freely chosen or not. But, if we think about it, we can appreciate a sense of willing, or intention, that does occur prior to the movement. In fact, the timing of when we can appreciate the upcoming movement may depend on how we interrogate our brain. Dr. Masao Matsuhashi and I showed that if you probe a person, the knowledge that the movement is coming can be earlier than if you ask after the fact when the intention occurred (Eur. J. Neurosci. 2008;28:2344).
All of this has relevance for the clinical practice of neurology. My favorite example in this regard is trying to understand why patients with psychogenic movement disorders believe their movements to be involuntary when they look so voluntary.
Dr. Mark Hallett is chief of the Medical Neurology Branch and chief of the Human Motor Control Section of the National Institute for Neurological Disorders and Stroke. He has no relevant disclosures.
Neurologists, physiologists, and philosophers were tossed a hot potato in 1983 with Benjamin Libet, Ph.D., and his colleagues’ publication of the first attempt to measure the time of the perception of intent to make a "voluntary" movement (Brain 1983;106:623-42). Called W, it happened about 250 ms prior to the movement itself. They compared this time to the onset of the Bereitschaftspotential or Readiness potential (RP), an EEG potential that had been previously described by Dr. Hans Kornhuber and Dr. Lüder Deecke (Pflugers Arch. Gesamte Physiol. Menschen Tiere 1965;284:1-17). The RP starts about a second prior to movement. This was a shock. It appeared that the brain was preparing to make a "voluntary" movement before the person was aware of it! The experiment has been repeated many times, so there is no disputing the data; the controversy is the interpretation.
In this new paper by Dr. Fried and his colleagues, they have first repeated the experiment using recording from neurons in the brain rather than the EEG. Their finding about the timing of W was similar to all the other experiments. Since the EEG comes from neuronal activity, it should not be a great surprise that they were able to find neurons that changed their activity in the second or so prior to movement. They then took the data one step further. By analyzing a small number of the neurons, they could predict with a high degree of accuracy, prior to W, when a movement would occur. Hence, it appears that the neuronal activity prior to awareness of intention is marching toward the motor command. Recently, our group, led by Ou Bai, Ph.D., has done the same thing using EEG, although not with the same high degree of accuracy (Clin. Neurophysiol. 2011;122:364-72).
So what does this mean? If people have free will in making voluntary movements, doesn’t the decision have to be made before the motor command? Here, it looks like the motor command is being made before the "decision." The situation is actually easy to resolve, but it does involve some careful thinking. The first point to settle is that the mind is generated by the brain; it is not separate from the brain. Most people agree with that, even though it is easy to fall into dualistic thinking. We are our brains; what the brain is doing, we are doing. Hence, it appears that the decision to make a movement, in this circumstance, arises unconsciously. The decision becomes conscious, or at least we have the impression it becomes conscious, just slightly before the movement. The priority is important. That we have the perception of willing before the perception that the movement occurs allows us to draw the conclusion that we are causal in the production of the movement; that is, that we freely willed the movement.
Is this compatible with the idea that we actually have free will? It depends on what that means. If we are our brains, and our brain is choosing to do this without external coercion, then the movement is free. We become aware of this, in fact, only some of the time. Much of the time, we go about our business without worrying whether our movements are freely chosen or not. But, if we think about it, we can appreciate a sense of willing, or intention, that does occur prior to the movement. In fact, the timing of when we can appreciate the upcoming movement may depend on how we interrogate our brain. Dr. Masao Matsuhashi and I showed that if you probe a person, the knowledge that the movement is coming can be earlier than if you ask after the fact when the intention occurred (Eur. J. Neurosci. 2008;28:2344).
All of this has relevance for the clinical practice of neurology. My favorite example in this regard is trying to understand why patients with psychogenic movement disorders believe their movements to be involuntary when they look so voluntary.
Dr. Mark Hallett is chief of the Medical Neurology Branch and chief of the Human Motor Control Section of the National Institute for Neurological Disorders and Stroke. He has no relevant disclosures.
Neurologists, physiologists, and philosophers were tossed a hot potato in 1983 with Benjamin Libet, Ph.D., and his colleagues’ publication of the first attempt to measure the time of the perception of intent to make a "voluntary" movement (Brain 1983;106:623-42). Called W, it happened about 250 ms prior to the movement itself. They compared this time to the onset of the Bereitschaftspotential or Readiness potential (RP), an EEG potential that had been previously described by Dr. Hans Kornhuber and Dr. Lüder Deecke (Pflugers Arch. Gesamte Physiol. Menschen Tiere 1965;284:1-17). The RP starts about a second prior to movement. This was a shock. It appeared that the brain was preparing to make a "voluntary" movement before the person was aware of it! The experiment has been repeated many times, so there is no disputing the data; the controversy is the interpretation.
In this new paper by Dr. Fried and his colleagues, they have first repeated the experiment using recording from neurons in the brain rather than the EEG. Their finding about the timing of W was similar to all the other experiments. Since the EEG comes from neuronal activity, it should not be a great surprise that they were able to find neurons that changed their activity in the second or so prior to movement. They then took the data one step further. By analyzing a small number of the neurons, they could predict with a high degree of accuracy, prior to W, when a movement would occur. Hence, it appears that the neuronal activity prior to awareness of intention is marching toward the motor command. Recently, our group, led by Ou Bai, Ph.D., has done the same thing using EEG, although not with the same high degree of accuracy (Clin. Neurophysiol. 2011;122:364-72).
So what does this mean? If people have free will in making voluntary movements, doesn’t the decision have to be made before the motor command? Here, it looks like the motor command is being made before the "decision." The situation is actually easy to resolve, but it does involve some careful thinking. The first point to settle is that the mind is generated by the brain; it is not separate from the brain. Most people agree with that, even though it is easy to fall into dualistic thinking. We are our brains; what the brain is doing, we are doing. Hence, it appears that the decision to make a movement, in this circumstance, arises unconsciously. The decision becomes conscious, or at least we have the impression it becomes conscious, just slightly before the movement. The priority is important. That we have the perception of willing before the perception that the movement occurs allows us to draw the conclusion that we are causal in the production of the movement; that is, that we freely willed the movement.
Is this compatible with the idea that we actually have free will? It depends on what that means. If we are our brains, and our brain is choosing to do this without external coercion, then the movement is free. We become aware of this, in fact, only some of the time. Much of the time, we go about our business without worrying whether our movements are freely chosen or not. But, if we think about it, we can appreciate a sense of willing, or intention, that does occur prior to the movement. In fact, the timing of when we can appreciate the upcoming movement may depend on how we interrogate our brain. Dr. Masao Matsuhashi and I showed that if you probe a person, the knowledge that the movement is coming can be earlier than if you ask after the fact when the intention occurred (Eur. J. Neurosci. 2008;28:2344).
All of this has relevance for the clinical practice of neurology. My favorite example in this regard is trying to understand why patients with psychogenic movement disorders believe their movements to be involuntary when they look so voluntary.
Dr. Mark Hallett is chief of the Medical Neurology Branch and chief of the Human Motor Control Section of the National Institute for Neurological Disorders and Stroke. He has no relevant disclosures.
Investigators for the first time have used electrode recordings of the firing patterns of small clusters of neurons to predict voluntary movement in people more than 1 second before they are even aware of their decision or urge to act.
The experiment, conducted by Dr. Itzhak Fried of the University of California, Los Angeles, and his associates, and published in Neuron, detected sets of neurons in the supplementary and presupplementary motor areas and the anterior cingulate cortex with firing rates that would progressively increase or decrease before the participants had even reported the urge to push a button on a laptop. The investigators then constructed algorithms that could successfully predict the impending decision to move at a rate of 70% or greater, depending on the location and size of the set of neurons chosen (Neuron 2011;69:548-62).
Dr. Fried and his colleagues recruited a group of 12 patients with drug-refractory epilepsy who had chronic depth electrodes implanted to determine their seizure focus for possible surgical resection. While the patients sat in bed, they watched an analog clock on a laptop computer and were instructed to push a button after at least one rotation of the clock’s hand whenever "they felt the urge to do so." Each time that the individuals pushed the button, called time P, the investigators asked them to indicate where the clock handle had been when they first felt the urge to move, called time W.
The participants reported a mean W time of 193 ms prior to P, but this varied from trial to trial. In the trials, the greatest proportion of neurons that changed their activity before W was located in parts of the medial frontal lobe of the brain: the supplementary motor area (SMA), the pre-SMA, and the dorsal and rostral regions of the anterior cingulate cortex (ACC). In some of these areas, the researchers observed rises in neuronal firing rates beginning several hundreds to several thousands of milliseconds prior to W, whereas progressive declines in firing rates were recorded in a similar time span prior to W. The number of neurons that changed their firing rate also increased as W approached.
The data gathered by the investigators did not indicate that the subjects were cued to respond by the completion of the clock hand’s first rotation. To sort out concerns related to potentially inaccurate reporting of W and the subjective nature of its determination, the investigators’ manipulated the timing of W either forward or backward in time by fixed amounts or by adjusting its timing by a random amount. These analyses indicated that small temporal shifts in W on the order of 200 ms or less are still compatible with the changes in firing rates seen in recorded neurons and matched what was observed within each participant’s individual trials.
With an algorithm that considered the responses of electrodes to be independent of each other across all participants, Dr. Fried and his associates found that they could predict W on a trial-by-trial basis across all participants. The algorithm could detect changes in the neural activity of 512 neurons in frontal lobe regions 500 ms before W in nearly 90% of the trials. The changes in activity could be detected in more than 70% of trials at 1,000 ms before W.
When the algorithm was constructed on the basis of firing patterns from 256 neurons in the SMA, it could detect the neurons’ change in activity at 500 ms before W in greater than 80% of the trials. In comparison, the change in activity of 256 neurons in the ACC at 500 ms before W could be detected in only 70% of trials.
The algorithm also was able to predict the time of W to within several hundred milliseconds of the actual W reported by the participants.
"This neuronal process suggests a mechanism whereby the feeling of will arises once integration of firing of recruited medial frontal neurons crosses a threshold," the investigators concluded.
The research was supported by grants from three different institutes of the National Institutes of Health, the National Science Foundation, the Klingenstein Fund, the Whitehall Foundation, and a Human Frontiers Science Programs Organization fellowship. No disclosure information was provided by the journal.
Investigators for the first time have used electrode recordings of the firing patterns of small clusters of neurons to predict voluntary movement in people more than 1 second before they are even aware of their decision or urge to act.
The experiment, conducted by Dr. Itzhak Fried of the University of California, Los Angeles, and his associates, and published in Neuron, detected sets of neurons in the supplementary and presupplementary motor areas and the anterior cingulate cortex with firing rates that would progressively increase or decrease before the participants had even reported the urge to push a button on a laptop. The investigators then constructed algorithms that could successfully predict the impending decision to move at a rate of 70% or greater, depending on the location and size of the set of neurons chosen (Neuron 2011;69:548-62).
Dr. Fried and his colleagues recruited a group of 12 patients with drug-refractory epilepsy who had chronic depth electrodes implanted to determine their seizure focus for possible surgical resection. While the patients sat in bed, they watched an analog clock on a laptop computer and were instructed to push a button after at least one rotation of the clock’s hand whenever "they felt the urge to do so." Each time that the individuals pushed the button, called time P, the investigators asked them to indicate where the clock handle had been when they first felt the urge to move, called time W.
The participants reported a mean W time of 193 ms prior to P, but this varied from trial to trial. In the trials, the greatest proportion of neurons that changed their activity before W was located in parts of the medial frontal lobe of the brain: the supplementary motor area (SMA), the pre-SMA, and the dorsal and rostral regions of the anterior cingulate cortex (ACC). In some of these areas, the researchers observed rises in neuronal firing rates beginning several hundreds to several thousands of milliseconds prior to W, whereas progressive declines in firing rates were recorded in a similar time span prior to W. The number of neurons that changed their firing rate also increased as W approached.
The data gathered by the investigators did not indicate that the subjects were cued to respond by the completion of the clock hand’s first rotation. To sort out concerns related to potentially inaccurate reporting of W and the subjective nature of its determination, the investigators’ manipulated the timing of W either forward or backward in time by fixed amounts or by adjusting its timing by a random amount. These analyses indicated that small temporal shifts in W on the order of 200 ms or less are still compatible with the changes in firing rates seen in recorded neurons and matched what was observed within each participant’s individual trials.
With an algorithm that considered the responses of electrodes to be independent of each other across all participants, Dr. Fried and his associates found that they could predict W on a trial-by-trial basis across all participants. The algorithm could detect changes in the neural activity of 512 neurons in frontal lobe regions 500 ms before W in nearly 90% of the trials. The changes in activity could be detected in more than 70% of trials at 1,000 ms before W.
When the algorithm was constructed on the basis of firing patterns from 256 neurons in the SMA, it could detect the neurons’ change in activity at 500 ms before W in greater than 80% of the trials. In comparison, the change in activity of 256 neurons in the ACC at 500 ms before W could be detected in only 70% of trials.
The algorithm also was able to predict the time of W to within several hundred milliseconds of the actual W reported by the participants.
"This neuronal process suggests a mechanism whereby the feeling of will arises once integration of firing of recruited medial frontal neurons crosses a threshold," the investigators concluded.
The research was supported by grants from three different institutes of the National Institutes of Health, the National Science Foundation, the Klingenstein Fund, the Whitehall Foundation, and a Human Frontiers Science Programs Organization fellowship. No disclosure information was provided by the journal.
FROM NEURON
FDA Says No to Oral Multiple Sclerosis Drug
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
FDA Says No to Oral Multiple Sclerosis Drug
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
FDA Says No to Oral Multiple Sclerosis Drug
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
The Food and Drug Administration will not approve the investigational multiple sclerosis drug cladribine unless its manufacturer, EMD Serono Inc., "provides an improved understanding of the safety risks and the overall benefit-risk profile either through additional analyses or by additional studies," the company announced March 2.
The FDA issued a complete response letter to the company, saying that the oral drug cannot be approved with the application in its current form, although the agency said that cladribine’s effectiveness in treating relapsing remitting MS had been proved in its phase III trial, CLARITY (Cladribine Tablets Treating MS Orally).
In addition to further analyses of the CLARITY trial, EMD Serono has two ongoing trials with cladribine: an open-label extension of CLARITY and ORACLE MS (Oral Cladribine in Early MS) that it hopes to use to use to address the FDA’s questions.
In 2010, the European Medicines Agency declined to approve cladribine, although Russia and Australia approved the drug.
Cladribine is an antineoplastic agent that has been approved for the treatment of active hairy cell leukemia. It selectively induces apoptosis of lymphocytes while having relatively transient effects on other immune cells.
EMD Serono is the U.S. subsidiary of Merck.
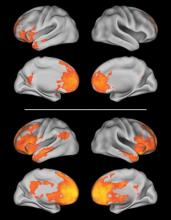
Nerve Simulation Paired With Tones May Improve Tinnitus
A therapeutic strategy for tinnitus that increases the number of auditory cortical neurons tuned to frequencies other than the one causing the condition has shown promising results in a rat model of the disorder.
By pairing a range of tone frequencies with peripheral stimulation of the vagus nerve, Navzer D. Engineer, Ph.D., and his associates at the University of Texas at Dallas reversed the physiological and behavioral correlates of tinnitus in noise-exposed rats.
Temporary relief of tinnitus symptoms in humans has been observed with the use of sensory exposure or discrimination training to reverse changes in the organization of the auditory cortex, but Dr. Engineer and his colleagues found that their approach may generate long-term effects.
In rats exposed to 1 hour of 115-dB, octave-band noise centered at 16 kHz, microelectrode recordings of neurons in the primary auditory cortex showed increases in the number of sites that were tuned to frequencies between 2 and 4 kHz, as well as neuronal changes in frequency tuning and synchronization. All of these changes are “similar to the physiological changes observed after noise exposure that have been proposed to be directly responsible for tinnitus,” according to the investigators (Nature 2011;470:101–4).
These rats could not detect a gap in narrowband noise centered on 8 or 10 kHz, but they could detect a gap in noise when it occurred in narrowband noise centered on 2 or 4 kHz or in broadband noise. Because it is impossible to know the subjective experience of rats exposed to this level of noise, Dr. Engineer and his colleagues used this impairment in gap detection to signify that they are experiencing a midfrequency tinnitus that fills the silent gaps, as other studies have done.
The degree of gap impairment seen in the rats was significantly correlated with the number of sites in the primary auditory cortex that had changes in frequency tuning and their relative change in frequency bandwidth.
To verify that these correlations were not the result of variability in initial cochlear trauma, the investigators treated the rats with impaired gap detection at 8–10 kHz by administering VNS in combination with randomly interleaved pure tones that span the rat hearing range (except for tones at frequencies of 8–10 kHz). After 10 days of therapy, these rats could detect gaps in the putative tinnitus frequency of 8–10 kHz up to 3 weeks later. These results make the pairing of VNS with multiple tone frequencies “the first method reported to generate a long-lasting reversal of a behavioral correlate of chronic tinnitus,” the investigators wrote.
After 3 weeks of therapy, most of the properties of the rats' primary auditory cortex returned to normal levels. Based on these preclinical results, a clinical trial with a wireless VNS device will begin in Europe later this year, according to Dr. Engineer.
MicroTransponder Inc., a medical device company affiliated with the University of Texas at Dallas, funded the research and the upcoming clinical trial. Other research support came from the James S. McDonnell Foundation, the Texas Advanced Research Program, and the National Institute on Deafness and Other Communication Disorders. Dr. Engineer is an employee of MicroTransponder Inc. Coauthor Michael P. Kilgard is a consultant and shareholder of MicroTransponder Inc.
Adviser's Viewpoint
Novel Approach for a Persistent Problem
Central sensitization is defined as the augmentation of normal cortical responsiveness to sensory input. Sensitization is thought to arise from neuronal plasticity induced by excitatory stimulation, inflammation, or injury. Through changes in synaptic connectivity, membrane excitability, and reduced inhibitory controls, a state of sustained neuronal hyperexcitability is created. Central sensitization is a proposed etiology of tinnitus, fibromyalgia, headache, and other chronic pain syndromes. These have traditionally been considered “medically unexplained” and share challenges in diagnosis and management. Tinnitus provides a good case study for these difficulties.
Tinnitus affects millions of people, with symptoms ranging from mildly disturbing to significant disability with disturbed sleep, concentration, mood, and even suicide. There is no uniformly accepted diagnostic paradigm. Tinnitus can accompany sensorineural hearing loss, but a hearing test does not confirm or quantify the degree of subjective symptomatology. Treatment is also not standardized or strongly evidence based. Common interventions include hearing aids, masking devices, habituation or cognitive behavioral therapy, antidepressants, and anxiolytics. Many patients struggle to find relief.
If neuronal plasticity can cause tinnitus and chronic pain, could it also be used to “desensitize” altered cortex as a cure for these disorders? Preliminary studies have suggested that transcranial magnetic stimulation may induce at least short-term change in central sensitization, but long-term outcomes are not yet known. Dr. Engineer and his colleagues have now taken a novel approach to inducing changes in cortical hyperexcitability by indirect, peripheral stimulation of the vagus nerve. In a rat model of tinnitus, they demonstrated that vagal stimulation in conjunction with paired tone exposure led to a sustained changes in auditory cortical activity and synchronization. The study serves as initial proof of concept for the ability of vagal stimulation to induce changes in neuronal plasticity.
The means to safely, chronically stimulate the vagus nerve in humans is already available. Demonstrated efficacy of the vagus nerve stimulator (VNS) for refractory partial epilepsy, although by mechanisms unproven, suggests VNS can alter cortical excitability in humans. Much remains to be determined, however, before VNS is proven as a clinical treatment for tinnitus or chronic pain. Future studies will need to address not only clinical efficacy, but also appropriate patient selection, treatment paradigm, and cost effectiveness. Nevertheless, the study conducted by Dr. Engineer and his associates offers early promise for a potentially novel intervention for these disabling conditions that often elude our current best efforts at management.
KATHERINE H. NOE, M.D., PH.D., is a consultant in the division of epilepsy at the Mayo Clinic in Phoenix. She has no relevant financial disclosures.
Vitals
A therapeutic strategy for tinnitus that increases the number of auditory cortical neurons tuned to frequencies other than the one causing the condition has shown promising results in a rat model of the disorder.
By pairing a range of tone frequencies with peripheral stimulation of the vagus nerve, Navzer D. Engineer, Ph.D., and his associates at the University of Texas at Dallas reversed the physiological and behavioral correlates of tinnitus in noise-exposed rats.
Temporary relief of tinnitus symptoms in humans has been observed with the use of sensory exposure or discrimination training to reverse changes in the organization of the auditory cortex, but Dr. Engineer and his colleagues found that their approach may generate long-term effects.
In rats exposed to 1 hour of 115-dB, octave-band noise centered at 16 kHz, microelectrode recordings of neurons in the primary auditory cortex showed increases in the number of sites that were tuned to frequencies between 2 and 4 kHz, as well as neuronal changes in frequency tuning and synchronization. All of these changes are “similar to the physiological changes observed after noise exposure that have been proposed to be directly responsible for tinnitus,” according to the investigators (Nature 2011;470:101–4).
These rats could not detect a gap in narrowband noise centered on 8 or 10 kHz, but they could detect a gap in noise when it occurred in narrowband noise centered on 2 or 4 kHz or in broadband noise. Because it is impossible to know the subjective experience of rats exposed to this level of noise, Dr. Engineer and his colleagues used this impairment in gap detection to signify that they are experiencing a midfrequency tinnitus that fills the silent gaps, as other studies have done.
The degree of gap impairment seen in the rats was significantly correlated with the number of sites in the primary auditory cortex that had changes in frequency tuning and their relative change in frequency bandwidth.
To verify that these correlations were not the result of variability in initial cochlear trauma, the investigators treated the rats with impaired gap detection at 8–10 kHz by administering VNS in combination with randomly interleaved pure tones that span the rat hearing range (except for tones at frequencies of 8–10 kHz). After 10 days of therapy, these rats could detect gaps in the putative tinnitus frequency of 8–10 kHz up to 3 weeks later. These results make the pairing of VNS with multiple tone frequencies “the first method reported to generate a long-lasting reversal of a behavioral correlate of chronic tinnitus,” the investigators wrote.
After 3 weeks of therapy, most of the properties of the rats' primary auditory cortex returned to normal levels. Based on these preclinical results, a clinical trial with a wireless VNS device will begin in Europe later this year, according to Dr. Engineer.
MicroTransponder Inc., a medical device company affiliated with the University of Texas at Dallas, funded the research and the upcoming clinical trial. Other research support came from the James S. McDonnell Foundation, the Texas Advanced Research Program, and the National Institute on Deafness and Other Communication Disorders. Dr. Engineer is an employee of MicroTransponder Inc. Coauthor Michael P. Kilgard is a consultant and shareholder of MicroTransponder Inc.
Adviser's Viewpoint
Novel Approach for a Persistent Problem
Central sensitization is defined as the augmentation of normal cortical responsiveness to sensory input. Sensitization is thought to arise from neuronal plasticity induced by excitatory stimulation, inflammation, or injury. Through changes in synaptic connectivity, membrane excitability, and reduced inhibitory controls, a state of sustained neuronal hyperexcitability is created. Central sensitization is a proposed etiology of tinnitus, fibromyalgia, headache, and other chronic pain syndromes. These have traditionally been considered “medically unexplained” and share challenges in diagnosis and management. Tinnitus provides a good case study for these difficulties.
Tinnitus affects millions of people, with symptoms ranging from mildly disturbing to significant disability with disturbed sleep, concentration, mood, and even suicide. There is no uniformly accepted diagnostic paradigm. Tinnitus can accompany sensorineural hearing loss, but a hearing test does not confirm or quantify the degree of subjective symptomatology. Treatment is also not standardized or strongly evidence based. Common interventions include hearing aids, masking devices, habituation or cognitive behavioral therapy, antidepressants, and anxiolytics. Many patients struggle to find relief.
If neuronal plasticity can cause tinnitus and chronic pain, could it also be used to “desensitize” altered cortex as a cure for these disorders? Preliminary studies have suggested that transcranial magnetic stimulation may induce at least short-term change in central sensitization, but long-term outcomes are not yet known. Dr. Engineer and his colleagues have now taken a novel approach to inducing changes in cortical hyperexcitability by indirect, peripheral stimulation of the vagus nerve. In a rat model of tinnitus, they demonstrated that vagal stimulation in conjunction with paired tone exposure led to a sustained changes in auditory cortical activity and synchronization. The study serves as initial proof of concept for the ability of vagal stimulation to induce changes in neuronal plasticity.
The means to safely, chronically stimulate the vagus nerve in humans is already available. Demonstrated efficacy of the vagus nerve stimulator (VNS) for refractory partial epilepsy, although by mechanisms unproven, suggests VNS can alter cortical excitability in humans. Much remains to be determined, however, before VNS is proven as a clinical treatment for tinnitus or chronic pain. Future studies will need to address not only clinical efficacy, but also appropriate patient selection, treatment paradigm, and cost effectiveness. Nevertheless, the study conducted by Dr. Engineer and his associates offers early promise for a potentially novel intervention for these disabling conditions that often elude our current best efforts at management.
KATHERINE H. NOE, M.D., PH.D., is a consultant in the division of epilepsy at the Mayo Clinic in Phoenix. She has no relevant financial disclosures.
Vitals
A therapeutic strategy for tinnitus that increases the number of auditory cortical neurons tuned to frequencies other than the one causing the condition has shown promising results in a rat model of the disorder.
By pairing a range of tone frequencies with peripheral stimulation of the vagus nerve, Navzer D. Engineer, Ph.D., and his associates at the University of Texas at Dallas reversed the physiological and behavioral correlates of tinnitus in noise-exposed rats.
Temporary relief of tinnitus symptoms in humans has been observed with the use of sensory exposure or discrimination training to reverse changes in the organization of the auditory cortex, but Dr. Engineer and his colleagues found that their approach may generate long-term effects.
In rats exposed to 1 hour of 115-dB, octave-band noise centered at 16 kHz, microelectrode recordings of neurons in the primary auditory cortex showed increases in the number of sites that were tuned to frequencies between 2 and 4 kHz, as well as neuronal changes in frequency tuning and synchronization. All of these changes are “similar to the physiological changes observed after noise exposure that have been proposed to be directly responsible for tinnitus,” according to the investigators (Nature 2011;470:101–4).
These rats could not detect a gap in narrowband noise centered on 8 or 10 kHz, but they could detect a gap in noise when it occurred in narrowband noise centered on 2 or 4 kHz or in broadband noise. Because it is impossible to know the subjective experience of rats exposed to this level of noise, Dr. Engineer and his colleagues used this impairment in gap detection to signify that they are experiencing a midfrequency tinnitus that fills the silent gaps, as other studies have done.
The degree of gap impairment seen in the rats was significantly correlated with the number of sites in the primary auditory cortex that had changes in frequency tuning and their relative change in frequency bandwidth.
To verify that these correlations were not the result of variability in initial cochlear trauma, the investigators treated the rats with impaired gap detection at 8–10 kHz by administering VNS in combination with randomly interleaved pure tones that span the rat hearing range (except for tones at frequencies of 8–10 kHz). After 10 days of therapy, these rats could detect gaps in the putative tinnitus frequency of 8–10 kHz up to 3 weeks later. These results make the pairing of VNS with multiple tone frequencies “the first method reported to generate a long-lasting reversal of a behavioral correlate of chronic tinnitus,” the investigators wrote.
After 3 weeks of therapy, most of the properties of the rats' primary auditory cortex returned to normal levels. Based on these preclinical results, a clinical trial with a wireless VNS device will begin in Europe later this year, according to Dr. Engineer.
MicroTransponder Inc., a medical device company affiliated with the University of Texas at Dallas, funded the research and the upcoming clinical trial. Other research support came from the James S. McDonnell Foundation, the Texas Advanced Research Program, and the National Institute on Deafness and Other Communication Disorders. Dr. Engineer is an employee of MicroTransponder Inc. Coauthor Michael P. Kilgard is a consultant and shareholder of MicroTransponder Inc.
Adviser's Viewpoint
Novel Approach for a Persistent Problem
Central sensitization is defined as the augmentation of normal cortical responsiveness to sensory input. Sensitization is thought to arise from neuronal plasticity induced by excitatory stimulation, inflammation, or injury. Through changes in synaptic connectivity, membrane excitability, and reduced inhibitory controls, a state of sustained neuronal hyperexcitability is created. Central sensitization is a proposed etiology of tinnitus, fibromyalgia, headache, and other chronic pain syndromes. These have traditionally been considered “medically unexplained” and share challenges in diagnosis and management. Tinnitus provides a good case study for these difficulties.
Tinnitus affects millions of people, with symptoms ranging from mildly disturbing to significant disability with disturbed sleep, concentration, mood, and even suicide. There is no uniformly accepted diagnostic paradigm. Tinnitus can accompany sensorineural hearing loss, but a hearing test does not confirm or quantify the degree of subjective symptomatology. Treatment is also not standardized or strongly evidence based. Common interventions include hearing aids, masking devices, habituation or cognitive behavioral therapy, antidepressants, and anxiolytics. Many patients struggle to find relief.
If neuronal plasticity can cause tinnitus and chronic pain, could it also be used to “desensitize” altered cortex as a cure for these disorders? Preliminary studies have suggested that transcranial magnetic stimulation may induce at least short-term change in central sensitization, but long-term outcomes are not yet known. Dr. Engineer and his colleagues have now taken a novel approach to inducing changes in cortical hyperexcitability by indirect, peripheral stimulation of the vagus nerve. In a rat model of tinnitus, they demonstrated that vagal stimulation in conjunction with paired tone exposure led to a sustained changes in auditory cortical activity and synchronization. The study serves as initial proof of concept for the ability of vagal stimulation to induce changes in neuronal plasticity.
The means to safely, chronically stimulate the vagus nerve in humans is already available. Demonstrated efficacy of the vagus nerve stimulator (VNS) for refractory partial epilepsy, although by mechanisms unproven, suggests VNS can alter cortical excitability in humans. Much remains to be determined, however, before VNS is proven as a clinical treatment for tinnitus or chronic pain. Future studies will need to address not only clinical efficacy, but also appropriate patient selection, treatment paradigm, and cost effectiveness. Nevertheless, the study conducted by Dr. Engineer and his associates offers early promise for a potentially novel intervention for these disabling conditions that often elude our current best efforts at management.
KATHERINE H. NOE, M.D., PH.D., is a consultant in the division of epilepsy at the Mayo Clinic in Phoenix. She has no relevant financial disclosures.
Vitals
Tailored Approach to Stenting, Surgery Comparable for Stroke Prevention
Physician-guided selection of carotid artery stenting or endarterectomy for stroke prevention in appropriate patients provided comparable periprocedural and long-term outcomes, according to a review of data from a high-volume vascular center published Jan. 31 in the Journal of the American College of Cardiology.
After assigning patients to the procedure that best suited their clinical characteristics and carotid artery morphology, Dr. Paola De Rango from the University of Perugia (Italy) and colleagues found that the primary end point of stroke or death within 30 days of the procedure and any ipsilateral stroke after the procedure occurred in 2.8% of patients treated with carotid artery stenting (CAS) and 2.0% of those treated with carotid endarterectomy (CEA).
These outcomes confirm the overall results of the CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) study, the most recent and largest randomized trial to include symptomatic and asymptomatic patients and to require well-trained operators for both procedures. But they also "offer the opportunity to analyze outcomes in a large number of consecutive patients treated for carotid stenosis with CAS and CEA reflecting the modern ‘real-world’ scenario outside the selected within-trial population," the investigators wrote (J. Am. Coll. Cardiol. 2011;57:664-71).
Indeed, the results show that in appropriately selected patients, CAS does not lead to higher rates of recurrent stenosis or stroke over the long term than does CEA, and that the procedures might not be associated with differences in outcomes because of age, gender, or symptom status.
The investigators included interventions performed at one center from January 2001 through March 2009 for symptomatic patients with greater than 60% stenosis of the carotid artery or asymptomatic patients with greater than 70% stenosis. They excluded the first 195 CAS procedures (during 2001-2003) to avoid bias from training, because it was after this period that the rate of disabling strokes during CAS significantly decreased and remained stable at less than 2% per year. Because CEA was reserved more often for the most complex cases during 2008-2009, the investigators excluded CEA procedures performed during that period to avoid possible major selection bias. The percentage of carotid revascularization procedures that used CEA declined from 50% in 2004 to 20% in 2007.
The choice of the revascularization procedure was left to the discretion of the treating surgeon. Relative contraindications for CAS included unfavorable aortic arch anatomy, severe peripheral vascular disease precluding femoral access, extremely tortuous carotid anatomy, renal insufficiency, or allergies to aspirin, clopidogrel, or contrast media, whereas those for CEA included high-neck carotid bifurcation, long carotid lesions, obesity, and ongoing dual antiplatelet therapy.
Among 1,084 CAS and 1,118 CEA procedures, the investigators reported similar rates of periprocedural MI (3.1% vs. 2.7%, respectively). However, in the 30-day period following procedures there was a significantly higher rate of transient ischemic attack with CAS (3.6% vs. 1.1%) and a significantly higher rate of cranial nerve injury with CEA (4.4% vs. 0%).
Long-term follow-up of patients averaged 33 months and ranged from 6 to 108 months. By 5 years, ipsilateral stroke had occurred in 0.9% of CAS patients and 2.7% of CEA patients, while estimates of recurrent stenosis of 50% or more were similar between CAS (3.4%) and CEA (5.8%) patients. This finding is important, the investigators wrote, "because some investigators had anticipated that an increased rate of late stroke after CAS could be expected because of a most likely higher probability of restenosis."
At 5 years, the investigators estimated the rates of the composite primary end point to be 3.7% in CAS patients and 4.7% in CEA patients. Estimates of survival from any cause mortality at 5 years also were similar for CAS (88%) and CEA (82%).
The periprocedural stroke and death rates for CAS and CEA did not differ significantly between symptomatic (4.5% vs. 2.9%, respectively) or asymptomatic patients (2.3% vs. 1.6%); between those aged 70 years or older (3.6% vs. 2%); or between men (3.1% vs. 1.6%) and women (2.2% vs. 3.1%).
Probability analyses showed that there was a propensity for patients to undergo CAS if they had a history of coronary artery disease, hypertension, diabetes, hyperlipidemia, statin therapy, or if they were of older age. Patients were less likely to undergo CAS if they had a history of neurologic symptoms or peripheral artery disease or if they had complex carotid plaque.
"Based on this study, patients with unfavorable aortic arch anatomy, extreme tortuous carotid anatomy, or severe peripheral vascular disease precluding femoral access, as well as known allergies to aspirin or clopidogrel should be considered preferentially for CEA. Patients with severe coronary disease, high-neck carotid bifurcation, and obesity should be considered at increased risk during CEA and might be best suited for CAS," the investigators concluded.
They also noted that their findings "might not be reproducible under less ideal conditions (and might not reflect the management of patients treated at many community hospitals)."
The investigators had no relevant financial disclosures.
Physician-guided selection of carotid artery stenting or endarterectomy for stroke prevention in appropriate patients provided comparable periprocedural and long-term outcomes, according to a review of data from a high-volume vascular center published Jan. 31 in the Journal of the American College of Cardiology.
After assigning patients to the procedure that best suited their clinical characteristics and carotid artery morphology, Dr. Paola De Rango from the University of Perugia (Italy) and colleagues found that the primary end point of stroke or death within 30 days of the procedure and any ipsilateral stroke after the procedure occurred in 2.8% of patients treated with carotid artery stenting (CAS) and 2.0% of those treated with carotid endarterectomy (CEA).
These outcomes confirm the overall results of the CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) study, the most recent and largest randomized trial to include symptomatic and asymptomatic patients and to require well-trained operators for both procedures. But they also "offer the opportunity to analyze outcomes in a large number of consecutive patients treated for carotid stenosis with CAS and CEA reflecting the modern ‘real-world’ scenario outside the selected within-trial population," the investigators wrote (J. Am. Coll. Cardiol. 2011;57:664-71).
Indeed, the results show that in appropriately selected patients, CAS does not lead to higher rates of recurrent stenosis or stroke over the long term than does CEA, and that the procedures might not be associated with differences in outcomes because of age, gender, or symptom status.
The investigators included interventions performed at one center from January 2001 through March 2009 for symptomatic patients with greater than 60% stenosis of the carotid artery or asymptomatic patients with greater than 70% stenosis. They excluded the first 195 CAS procedures (during 2001-2003) to avoid bias from training, because it was after this period that the rate of disabling strokes during CAS significantly decreased and remained stable at less than 2% per year. Because CEA was reserved more often for the most complex cases during 2008-2009, the investigators excluded CEA procedures performed during that period to avoid possible major selection bias. The percentage of carotid revascularization procedures that used CEA declined from 50% in 2004 to 20% in 2007.
The choice of the revascularization procedure was left to the discretion of the treating surgeon. Relative contraindications for CAS included unfavorable aortic arch anatomy, severe peripheral vascular disease precluding femoral access, extremely tortuous carotid anatomy, renal insufficiency, or allergies to aspirin, clopidogrel, or contrast media, whereas those for CEA included high-neck carotid bifurcation, long carotid lesions, obesity, and ongoing dual antiplatelet therapy.
Among 1,084 CAS and 1,118 CEA procedures, the investigators reported similar rates of periprocedural MI (3.1% vs. 2.7%, respectively). However, in the 30-day period following procedures there was a significantly higher rate of transient ischemic attack with CAS (3.6% vs. 1.1%) and a significantly higher rate of cranial nerve injury with CEA (4.4% vs. 0%).
Long-term follow-up of patients averaged 33 months and ranged from 6 to 108 months. By 5 years, ipsilateral stroke had occurred in 0.9% of CAS patients and 2.7% of CEA patients, while estimates of recurrent stenosis of 50% or more were similar between CAS (3.4%) and CEA (5.8%) patients. This finding is important, the investigators wrote, "because some investigators had anticipated that an increased rate of late stroke after CAS could be expected because of a most likely higher probability of restenosis."
At 5 years, the investigators estimated the rates of the composite primary end point to be 3.7% in CAS patients and 4.7% in CEA patients. Estimates of survival from any cause mortality at 5 years also were similar for CAS (88%) and CEA (82%).
The periprocedural stroke and death rates for CAS and CEA did not differ significantly between symptomatic (4.5% vs. 2.9%, respectively) or asymptomatic patients (2.3% vs. 1.6%); between those aged 70 years or older (3.6% vs. 2%); or between men (3.1% vs. 1.6%) and women (2.2% vs. 3.1%).
Probability analyses showed that there was a propensity for patients to undergo CAS if they had a history of coronary artery disease, hypertension, diabetes, hyperlipidemia, statin therapy, or if they were of older age. Patients were less likely to undergo CAS if they had a history of neurologic symptoms or peripheral artery disease or if they had complex carotid plaque.
"Based on this study, patients with unfavorable aortic arch anatomy, extreme tortuous carotid anatomy, or severe peripheral vascular disease precluding femoral access, as well as known allergies to aspirin or clopidogrel should be considered preferentially for CEA. Patients with severe coronary disease, high-neck carotid bifurcation, and obesity should be considered at increased risk during CEA and might be best suited for CAS," the investigators concluded.
They also noted that their findings "might not be reproducible under less ideal conditions (and might not reflect the management of patients treated at many community hospitals)."
The investigators had no relevant financial disclosures.
Physician-guided selection of carotid artery stenting or endarterectomy for stroke prevention in appropriate patients provided comparable periprocedural and long-term outcomes, according to a review of data from a high-volume vascular center published Jan. 31 in the Journal of the American College of Cardiology.
After assigning patients to the procedure that best suited their clinical characteristics and carotid artery morphology, Dr. Paola De Rango from the University of Perugia (Italy) and colleagues found that the primary end point of stroke or death within 30 days of the procedure and any ipsilateral stroke after the procedure occurred in 2.8% of patients treated with carotid artery stenting (CAS) and 2.0% of those treated with carotid endarterectomy (CEA).
These outcomes confirm the overall results of the CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) study, the most recent and largest randomized trial to include symptomatic and asymptomatic patients and to require well-trained operators for both procedures. But they also "offer the opportunity to analyze outcomes in a large number of consecutive patients treated for carotid stenosis with CAS and CEA reflecting the modern ‘real-world’ scenario outside the selected within-trial population," the investigators wrote (J. Am. Coll. Cardiol. 2011;57:664-71).
Indeed, the results show that in appropriately selected patients, CAS does not lead to higher rates of recurrent stenosis or stroke over the long term than does CEA, and that the procedures might not be associated with differences in outcomes because of age, gender, or symptom status.
The investigators included interventions performed at one center from January 2001 through March 2009 for symptomatic patients with greater than 60% stenosis of the carotid artery or asymptomatic patients with greater than 70% stenosis. They excluded the first 195 CAS procedures (during 2001-2003) to avoid bias from training, because it was after this period that the rate of disabling strokes during CAS significantly decreased and remained stable at less than 2% per year. Because CEA was reserved more often for the most complex cases during 2008-2009, the investigators excluded CEA procedures performed during that period to avoid possible major selection bias. The percentage of carotid revascularization procedures that used CEA declined from 50% in 2004 to 20% in 2007.
The choice of the revascularization procedure was left to the discretion of the treating surgeon. Relative contraindications for CAS included unfavorable aortic arch anatomy, severe peripheral vascular disease precluding femoral access, extremely tortuous carotid anatomy, renal insufficiency, or allergies to aspirin, clopidogrel, or contrast media, whereas those for CEA included high-neck carotid bifurcation, long carotid lesions, obesity, and ongoing dual antiplatelet therapy.
Among 1,084 CAS and 1,118 CEA procedures, the investigators reported similar rates of periprocedural MI (3.1% vs. 2.7%, respectively). However, in the 30-day period following procedures there was a significantly higher rate of transient ischemic attack with CAS (3.6% vs. 1.1%) and a significantly higher rate of cranial nerve injury with CEA (4.4% vs. 0%).
Long-term follow-up of patients averaged 33 months and ranged from 6 to 108 months. By 5 years, ipsilateral stroke had occurred in 0.9% of CAS patients and 2.7% of CEA patients, while estimates of recurrent stenosis of 50% or more were similar between CAS (3.4%) and CEA (5.8%) patients. This finding is important, the investigators wrote, "because some investigators had anticipated that an increased rate of late stroke after CAS could be expected because of a most likely higher probability of restenosis."
At 5 years, the investigators estimated the rates of the composite primary end point to be 3.7% in CAS patients and 4.7% in CEA patients. Estimates of survival from any cause mortality at 5 years also were similar for CAS (88%) and CEA (82%).
The periprocedural stroke and death rates for CAS and CEA did not differ significantly between symptomatic (4.5% vs. 2.9%, respectively) or asymptomatic patients (2.3% vs. 1.6%); between those aged 70 years or older (3.6% vs. 2%); or between men (3.1% vs. 1.6%) and women (2.2% vs. 3.1%).
Probability analyses showed that there was a propensity for patients to undergo CAS if they had a history of coronary artery disease, hypertension, diabetes, hyperlipidemia, statin therapy, or if they were of older age. Patients were less likely to undergo CAS if they had a history of neurologic symptoms or peripheral artery disease or if they had complex carotid plaque.
"Based on this study, patients with unfavorable aortic arch anatomy, extreme tortuous carotid anatomy, or severe peripheral vascular disease precluding femoral access, as well as known allergies to aspirin or clopidogrel should be considered preferentially for CEA. Patients with severe coronary disease, high-neck carotid bifurcation, and obesity should be considered at increased risk during CEA and might be best suited for CAS," the investigators concluded.
They also noted that their findings "might not be reproducible under less ideal conditions (and might not reflect the management of patients treated at many community hospitals)."
The investigators had no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Tailored Approach to Stenting, Surgery Comparable for Stroke Prevention
Physician-guided selection of carotid artery stenting or endarterectomy for stroke prevention in appropriate patients provided comparable periprocedural and long-term outcomes, according to a review of data from a high-volume vascular center published Jan. 31 in the Journal of the American College of Cardiology.
After assigning patients to the procedure that best suited their clinical characteristics and carotid artery morphology, Dr. Paola De Rango from the University of Perugia (Italy) and colleagues found that the primary end point of stroke or death within 30 days of the procedure and any ipsilateral stroke after the procedure occurred in 2.8% of patients treated with carotid artery stenting (CAS) and 2.0% of those treated with carotid endarterectomy (CEA).
These outcomes confirm the overall results of the CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) study, the most recent and largest randomized trial to include symptomatic and asymptomatic patients and to require well-trained operators for both procedures. But they also "offer the opportunity to analyze outcomes in a large number of consecutive patients treated for carotid stenosis with CAS and CEA reflecting the modern ‘real-world’ scenario outside the selected within-trial population," the investigators wrote (J. Am. Coll. Cardiol. 2011;57:664-71).
Indeed, the results show that in appropriately selected patients, CAS does not lead to higher rates of recurrent stenosis or stroke over the long term than does CEA, and that the procedures might not be associated with differences in outcomes because of age, gender, or symptom status.
The investigators included interventions performed at one center from January 2001 through March 2009 for symptomatic patients with greater than 60% stenosis of the carotid artery or asymptomatic patients with greater than 70% stenosis. They excluded the first 195 CAS procedures (during 2001-2003) to avoid bias from training, because it was after this period that the rate of disabling strokes during CAS significantly decreased and remained stable at less than 2% per year. Because CEA was reserved more often for the most complex cases during 2008-2009, the investigators excluded CEA procedures performed during that period to avoid possible major selection bias. The percentage of carotid revascularization procedures that used CEA declined from 50% in 2004 to 20% in 2007.
The choice of the revascularization procedure was left to the discretion of the treating surgeon. Relative contraindications for CAS included unfavorable aortic arch anatomy, severe peripheral vascular disease precluding femoral access, extremely tortuous carotid anatomy, renal insufficiency, or allergies to aspirin, clopidogrel, or contrast media, whereas those for CEA included high-neck carotid bifurcation, long carotid lesions, obesity, and ongoing dual antiplatelet therapy.
Among 1,084 CAS and 1,118 CEA procedures, the investigators reported similar rates of periprocedural MI (3.1% vs. 2.7%, respectively). However, in the 30-day period following procedures there was a significantly higher rate of transient ischemic attack with CAS (3.6% vs. 1.1%) and a significantly higher rate of cranial nerve injury with CEA (4.4% vs. 0%).
Long-term follow-up of patients averaged 33 months and ranged from 6 to 108 months. By 5 years, ipsilateral stroke had occurred in 0.9% of CAS patients and 2.7% of CEA patients, while estimates of recurrent stenosis of 50% or more were similar between CAS (3.4%) and CEA (5.8%) patients. This finding is important, the investigators wrote, "because some investigators had anticipated that an increased rate of late stroke after CAS could be expected because of a most likely higher probability of restenosis."
At 5 years, the investigators estimated the rates of the composite primary end point to be 3.7% in CAS patients and 4.7% in CEA patients. Estimates of survival from any cause mortality at 5 years also were similar for CAS (88%) and CEA (82%).
The periprocedural stroke and death rates for CAS and CEA did not differ significantly between symptomatic (4.5% vs. 2.9%, respectively) or asymptomatic patients (2.3% vs. 1.6%); between those aged 70 years or older (3.6% vs. 2%); or between men (3.1% vs. 1.6%) and women (2.2% vs. 3.1%).
Probability analyses showed that there was a propensity for patients to undergo CAS if they had a history of coronary artery disease, hypertension, diabetes, hyperlipidemia, statin therapy, or if they were of older age. Patients were less likely to undergo CAS if they had a history of neurologic symptoms or peripheral artery disease or if they had complex carotid plaque.
"Based on this study, patients with unfavorable aortic arch anatomy, extreme tortuous carotid anatomy, or severe peripheral vascular disease precluding femoral access, as well as known allergies to aspirin or clopidogrel should be considered preferentially for CEA. Patients with severe coronary disease, high-neck carotid bifurcation, and obesity should be considered at increased risk during CEA and might be best suited for CAS," the investigators concluded.
They also noted that their findings "might not be reproducible under less ideal conditions (and might not reflect the management of patients treated at many community hospitals)."
The investigators had no relevant financial disclosures.
Physician-guided selection of carotid artery stenting or endarterectomy for stroke prevention in appropriate patients provided comparable periprocedural and long-term outcomes, according to a review of data from a high-volume vascular center published Jan. 31 in the Journal of the American College of Cardiology.
After assigning patients to the procedure that best suited their clinical characteristics and carotid artery morphology, Dr. Paola De Rango from the University of Perugia (Italy) and colleagues found that the primary end point of stroke or death within 30 days of the procedure and any ipsilateral stroke after the procedure occurred in 2.8% of patients treated with carotid artery stenting (CAS) and 2.0% of those treated with carotid endarterectomy (CEA).
These outcomes confirm the overall results of the CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) study, the most recent and largest randomized trial to include symptomatic and asymptomatic patients and to require well-trained operators for both procedures. But they also "offer the opportunity to analyze outcomes in a large number of consecutive patients treated for carotid stenosis with CAS and CEA reflecting the modern ‘real-world’ scenario outside the selected within-trial population," the investigators wrote (J. Am. Coll. Cardiol. 2011;57:664-71).
Indeed, the results show that in appropriately selected patients, CAS does not lead to higher rates of recurrent stenosis or stroke over the long term than does CEA, and that the procedures might not be associated with differences in outcomes because of age, gender, or symptom status.
The investigators included interventions performed at one center from January 2001 through March 2009 for symptomatic patients with greater than 60% stenosis of the carotid artery or asymptomatic patients with greater than 70% stenosis. They excluded the first 195 CAS procedures (during 2001-2003) to avoid bias from training, because it was after this period that the rate of disabling strokes during CAS significantly decreased and remained stable at less than 2% per year. Because CEA was reserved more often for the most complex cases during 2008-2009, the investigators excluded CEA procedures performed during that period to avoid possible major selection bias. The percentage of carotid revascularization procedures that used CEA declined from 50% in 2004 to 20% in 2007.
The choice of the revascularization procedure was left to the discretion of the treating surgeon. Relative contraindications for CAS included unfavorable aortic arch anatomy, severe peripheral vascular disease precluding femoral access, extremely tortuous carotid anatomy, renal insufficiency, or allergies to aspirin, clopidogrel, or contrast media, whereas those for CEA included high-neck carotid bifurcation, long carotid lesions, obesity, and ongoing dual antiplatelet therapy.
Among 1,084 CAS and 1,118 CEA procedures, the investigators reported similar rates of periprocedural MI (3.1% vs. 2.7%, respectively). However, in the 30-day period following procedures there was a significantly higher rate of transient ischemic attack with CAS (3.6% vs. 1.1%) and a significantly higher rate of cranial nerve injury with CEA (4.4% vs. 0%).
Long-term follow-up of patients averaged 33 months and ranged from 6 to 108 months. By 5 years, ipsilateral stroke had occurred in 0.9% of CAS patients and 2.7% of CEA patients, while estimates of recurrent stenosis of 50% or more were similar between CAS (3.4%) and CEA (5.8%) patients. This finding is important, the investigators wrote, "because some investigators had anticipated that an increased rate of late stroke after CAS could be expected because of a most likely higher probability of restenosis."
At 5 years, the investigators estimated the rates of the composite primary end point to be 3.7% in CAS patients and 4.7% in CEA patients. Estimates of survival from any cause mortality at 5 years also were similar for CAS (88%) and CEA (82%).
The periprocedural stroke and death rates for CAS and CEA did not differ significantly between symptomatic (4.5% vs. 2.9%, respectively) or asymptomatic patients (2.3% vs. 1.6%); between those aged 70 years or older (3.6% vs. 2%); or between men (3.1% vs. 1.6%) and women (2.2% vs. 3.1%).
Probability analyses showed that there was a propensity for patients to undergo CAS if they had a history of coronary artery disease, hypertension, diabetes, hyperlipidemia, statin therapy, or if they were of older age. Patients were less likely to undergo CAS if they had a history of neurologic symptoms or peripheral artery disease or if they had complex carotid plaque.
"Based on this study, patients with unfavorable aortic arch anatomy, extreme tortuous carotid anatomy, or severe peripheral vascular disease precluding femoral access, as well as known allergies to aspirin or clopidogrel should be considered preferentially for CEA. Patients with severe coronary disease, high-neck carotid bifurcation, and obesity should be considered at increased risk during CEA and might be best suited for CAS," the investigators concluded.
They also noted that their findings "might not be reproducible under less ideal conditions (and might not reflect the management of patients treated at many community hospitals)."
The investigators had no relevant financial disclosures.
Physician-guided selection of carotid artery stenting or endarterectomy for stroke prevention in appropriate patients provided comparable periprocedural and long-term outcomes, according to a review of data from a high-volume vascular center published Jan. 31 in the Journal of the American College of Cardiology.
After assigning patients to the procedure that best suited their clinical characteristics and carotid artery morphology, Dr. Paola De Rango from the University of Perugia (Italy) and colleagues found that the primary end point of stroke or death within 30 days of the procedure and any ipsilateral stroke after the procedure occurred in 2.8% of patients treated with carotid artery stenting (CAS) and 2.0% of those treated with carotid endarterectomy (CEA).
These outcomes confirm the overall results of the CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) study, the most recent and largest randomized trial to include symptomatic and asymptomatic patients and to require well-trained operators for both procedures. But they also "offer the opportunity to analyze outcomes in a large number of consecutive patients treated for carotid stenosis with CAS and CEA reflecting the modern ‘real-world’ scenario outside the selected within-trial population," the investigators wrote (J. Am. Coll. Cardiol. 2011;57:664-71).
Indeed, the results show that in appropriately selected patients, CAS does not lead to higher rates of recurrent stenosis or stroke over the long term than does CEA, and that the procedures might not be associated with differences in outcomes because of age, gender, or symptom status.
The investigators included interventions performed at one center from January 2001 through March 2009 for symptomatic patients with greater than 60% stenosis of the carotid artery or asymptomatic patients with greater than 70% stenosis. They excluded the first 195 CAS procedures (during 2001-2003) to avoid bias from training, because it was after this period that the rate of disabling strokes during CAS significantly decreased and remained stable at less than 2% per year. Because CEA was reserved more often for the most complex cases during 2008-2009, the investigators excluded CEA procedures performed during that period to avoid possible major selection bias. The percentage of carotid revascularization procedures that used CEA declined from 50% in 2004 to 20% in 2007.
The choice of the revascularization procedure was left to the discretion of the treating surgeon. Relative contraindications for CAS included unfavorable aortic arch anatomy, severe peripheral vascular disease precluding femoral access, extremely tortuous carotid anatomy, renal insufficiency, or allergies to aspirin, clopidogrel, or contrast media, whereas those for CEA included high-neck carotid bifurcation, long carotid lesions, obesity, and ongoing dual antiplatelet therapy.
Among 1,084 CAS and 1,118 CEA procedures, the investigators reported similar rates of periprocedural MI (3.1% vs. 2.7%, respectively). However, in the 30-day period following procedures there was a significantly higher rate of transient ischemic attack with CAS (3.6% vs. 1.1%) and a significantly higher rate of cranial nerve injury with CEA (4.4% vs. 0%).
Long-term follow-up of patients averaged 33 months and ranged from 6 to 108 months. By 5 years, ipsilateral stroke had occurred in 0.9% of CAS patients and 2.7% of CEA patients, while estimates of recurrent stenosis of 50% or more were similar between CAS (3.4%) and CEA (5.8%) patients. This finding is important, the investigators wrote, "because some investigators had anticipated that an increased rate of late stroke after CAS could be expected because of a most likely higher probability of restenosis."
At 5 years, the investigators estimated the rates of the composite primary end point to be 3.7% in CAS patients and 4.7% in CEA patients. Estimates of survival from any cause mortality at 5 years also were similar for CAS (88%) and CEA (82%).
The periprocedural stroke and death rates for CAS and CEA did not differ significantly between symptomatic (4.5% vs. 2.9%, respectively) or asymptomatic patients (2.3% vs. 1.6%); between those aged 70 years or older (3.6% vs. 2%); or between men (3.1% vs. 1.6%) and women (2.2% vs. 3.1%).
Probability analyses showed that there was a propensity for patients to undergo CAS if they had a history of coronary artery disease, hypertension, diabetes, hyperlipidemia, statin therapy, or if they were of older age. Patients were less likely to undergo CAS if they had a history of neurologic symptoms or peripheral artery disease or if they had complex carotid plaque.
"Based on this study, patients with unfavorable aortic arch anatomy, extreme tortuous carotid anatomy, or severe peripheral vascular disease precluding femoral access, as well as known allergies to aspirin or clopidogrel should be considered preferentially for CEA. Patients with severe coronary disease, high-neck carotid bifurcation, and obesity should be considered at increased risk during CEA and might be best suited for CAS," the investigators concluded.
They also noted that their findings "might not be reproducible under less ideal conditions (and might not reflect the management of patients treated at many community hospitals)."
The investigators had no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: The composite primary end point of stroke or death within 30 days of the procedure and any later ipsilateral stroke occurred in 2.8% of patients treated with carotid artery stenting and 2.0% of those treated with carotid endarterectomy.
Data Source: Single-center study of 2,202 consecutive patients who underwent carotid artery stenting or endarterectomy.
Disclosures: The investigators had no relevant financial disclosures.
New Vaccine Targets Africa's Meningitis Belt
Seasonal meningococcal A epidemics in Africa’s "meningitis belt" could become remnants of the past if the introduction of a new and affordable conjugate polysaccharide vaccine meets the high expectations of the many people and organizations that collaborated to produce it.
In early December of last year, the West African nation of Burkina Faso became the first country to implement a nationwide program to immunize individuals aged 1-29 years with the vaccine, called MenAfriVac. The start of the vaccination campaign also may signal a change in the way in which vaccination programs are conducted in Africa.
"The concept of vaccination is changing in Africa," said Dr. Muhamed-Kheir Taha, director of the French National Reference Center for Meningococci at the Pasteur Institute in Paris, where he also is head of the invasive bacterial infections unit. "Previously, we had what we used to call ‘reactive vaccination,’ meaning we usually started vaccinating when the epidemiologic threshold was crossed, and now we are doing preventive vaccination."
In 2001, the World Health Organization (WHO) and the international nonprofit organization PATH founded the Meningitis Vaccine Project to develop MenAfriVac. The project brought together African ministers of health, U.S. government health agencies, philanthropic organizations, many strategic planning and surveillance groups, and vaccine industry partners.
MenAfriVac is being distributed to African nations in the meningitis belt, which stretches from Senegal to Ethiopia, before anywhere else. After Burkina Faso, the hyperendemic nations of Niger and Mali also began nationwide vaccination campaigns in December. If a funding gap of U.S.$475 million is met, the WHO expects that all 25 countries in the meningitis belt will be using the vaccine by 2015. If that happens, the reduction in the number of meningitis cases is expected to save U.S.$120 million annually in the period up to 2015 from national budgets that would be otherwise spent on costs for diagnosis and treatment, according to the WHO.
The vaccination campaign began just before the start of the dry season (January to May) when the yearly epidemics occur. Major epidemics of the disease occur every 7-14 years, and in 2009 they infected more than 88,000 people and killed more than 5,000 across the region. Serogroup A Neisseria meningitidis causes 80%-85% of the epidemics, according to the WHO.
Epidemics of group A meningococcal disease have been impossible to stop with older polysaccharide vaccines, despite millions of doses that have been given to individuals during epidemics. These vaccines induce a systemic immune response that is independent of memory T-cell activation and lasts only 2-3 years. They are also poorly immunogenic in children younger than 2 years. Polysaccharide vaccines also do not reduce the carriage of N. meningitidis in the nasopharynx, the bacteria’s natural habitat, and thereby do not induce "herd immunity." This happens because polysaccharide vaccines induce only immunoglobulin M antibodies, which are not present on the surfaces of epithelial cells lining the nasopharynx, according to Dr. Taha.
In contrast, MenAfriVac is modeled after the successful group C conjugate polysaccharide vaccine first used in the United Kingdom in the late 1990s and early 2000s. Conjugate vaccines are built with a protein attached to the bacteria’s capsular polysaccharide antigen, which induces a longer-lasting T-cell dependent immune response. This response produces immunoglobulin G antibodies that are present on the surface of epithelial cells in the nasopharynx, reducing carriage of the bacteria and providing herd immunity. This means that children younger than 2 years can receive the vaccine. The antibodies formed with MenAfriVac will likely persist beyond 5 years, said Dr. Taha, who was not involved in the development of the vaccine.
A single dose of MenAfriVac costs less than half a U.S. dollar, in part because it was developed for less than a tenth of the U.S.$500 million that conjugate vaccine development has typically cost. In comparison, Dr. Taha said, "the cost of the group C conjugate vaccine in Europe is about 30 euros, which is far away from [the price that] can be offered in Africa."
Clinical trials carried out in India and in several countries of the meningitis belt in people aged 1-29 years have shown that MenAfriVac is safe and has higher immunogenicity than the previous group A polysaccharide vaccine. In children aged 12-24 months, MenAfriVac induced 20-fold greater antibody levels than did the meningococcal A component of the licensed tetravalent ACWY polysaccharide vaccine. Ongoing trials are determining whether a single dose of the vaccine given to children aged 9-12 months will be sufficient to generate long-lasting protection (Vaccine 2009;27[Suppl. 2]:B13-19).
Based on the experience of European countries in vaccinating people with a similar conjugated vaccine against group C meningitis, Dr. Taha estimated that at least 90% of the target population will need to receive MenAfriVac in order to reduce transmission of the disease to unvaccinated people. It’s unknown whether this "quite high" coverage rate can be reached rapidly, he said.
Although MenAfriVac protects against group A meningococcal disease – by far the most prevalent type in Africa – outbreaks of meningitis caused by serogroups W135 and X in recent years mean that active surveillance for epidemics will still be necessary, he said.
Seasonal meningococcal A epidemics in Africa’s "meningitis belt" could become remnants of the past if the introduction of a new and affordable conjugate polysaccharide vaccine meets the high expectations of the many people and organizations that collaborated to produce it.
In early December of last year, the West African nation of Burkina Faso became the first country to implement a nationwide program to immunize individuals aged 1-29 years with the vaccine, called MenAfriVac. The start of the vaccination campaign also may signal a change in the way in which vaccination programs are conducted in Africa.
"The concept of vaccination is changing in Africa," said Dr. Muhamed-Kheir Taha, director of the French National Reference Center for Meningococci at the Pasteur Institute in Paris, where he also is head of the invasive bacterial infections unit. "Previously, we had what we used to call ‘reactive vaccination,’ meaning we usually started vaccinating when the epidemiologic threshold was crossed, and now we are doing preventive vaccination."
In 2001, the World Health Organization (WHO) and the international nonprofit organization PATH founded the Meningitis Vaccine Project to develop MenAfriVac. The project brought together African ministers of health, U.S. government health agencies, philanthropic organizations, many strategic planning and surveillance groups, and vaccine industry partners.
MenAfriVac is being distributed to African nations in the meningitis belt, which stretches from Senegal to Ethiopia, before anywhere else. After Burkina Faso, the hyperendemic nations of Niger and Mali also began nationwide vaccination campaigns in December. If a funding gap of U.S.$475 million is met, the WHO expects that all 25 countries in the meningitis belt will be using the vaccine by 2015. If that happens, the reduction in the number of meningitis cases is expected to save U.S.$120 million annually in the period up to 2015 from national budgets that would be otherwise spent on costs for diagnosis and treatment, according to the WHO.
The vaccination campaign began just before the start of the dry season (January to May) when the yearly epidemics occur. Major epidemics of the disease occur every 7-14 years, and in 2009 they infected more than 88,000 people and killed more than 5,000 across the region. Serogroup A Neisseria meningitidis causes 80%-85% of the epidemics, according to the WHO.
Epidemics of group A meningococcal disease have been impossible to stop with older polysaccharide vaccines, despite millions of doses that have been given to individuals during epidemics. These vaccines induce a systemic immune response that is independent of memory T-cell activation and lasts only 2-3 years. They are also poorly immunogenic in children younger than 2 years. Polysaccharide vaccines also do not reduce the carriage of N. meningitidis in the nasopharynx, the bacteria’s natural habitat, and thereby do not induce "herd immunity." This happens because polysaccharide vaccines induce only immunoglobulin M antibodies, which are not present on the surfaces of epithelial cells lining the nasopharynx, according to Dr. Taha.
In contrast, MenAfriVac is modeled after the successful group C conjugate polysaccharide vaccine first used in the United Kingdom in the late 1990s and early 2000s. Conjugate vaccines are built with a protein attached to the bacteria’s capsular polysaccharide antigen, which induces a longer-lasting T-cell dependent immune response. This response produces immunoglobulin G antibodies that are present on the surface of epithelial cells in the nasopharynx, reducing carriage of the bacteria and providing herd immunity. This means that children younger than 2 years can receive the vaccine. The antibodies formed with MenAfriVac will likely persist beyond 5 years, said Dr. Taha, who was not involved in the development of the vaccine.
A single dose of MenAfriVac costs less than half a U.S. dollar, in part because it was developed for less than a tenth of the U.S.$500 million that conjugate vaccine development has typically cost. In comparison, Dr. Taha said, "the cost of the group C conjugate vaccine in Europe is about 30 euros, which is far away from [the price that] can be offered in Africa."
Clinical trials carried out in India and in several countries of the meningitis belt in people aged 1-29 years have shown that MenAfriVac is safe and has higher immunogenicity than the previous group A polysaccharide vaccine. In children aged 12-24 months, MenAfriVac induced 20-fold greater antibody levels than did the meningococcal A component of the licensed tetravalent ACWY polysaccharide vaccine. Ongoing trials are determining whether a single dose of the vaccine given to children aged 9-12 months will be sufficient to generate long-lasting protection (Vaccine 2009;27[Suppl. 2]:B13-19).
Based on the experience of European countries in vaccinating people with a similar conjugated vaccine against group C meningitis, Dr. Taha estimated that at least 90% of the target population will need to receive MenAfriVac in order to reduce transmission of the disease to unvaccinated people. It’s unknown whether this "quite high" coverage rate can be reached rapidly, he said.
Although MenAfriVac protects against group A meningococcal disease – by far the most prevalent type in Africa – outbreaks of meningitis caused by serogroups W135 and X in recent years mean that active surveillance for epidemics will still be necessary, he said.
Seasonal meningococcal A epidemics in Africa’s "meningitis belt" could become remnants of the past if the introduction of a new and affordable conjugate polysaccharide vaccine meets the high expectations of the many people and organizations that collaborated to produce it.
In early December of last year, the West African nation of Burkina Faso became the first country to implement a nationwide program to immunize individuals aged 1-29 years with the vaccine, called MenAfriVac. The start of the vaccination campaign also may signal a change in the way in which vaccination programs are conducted in Africa.
"The concept of vaccination is changing in Africa," said Dr. Muhamed-Kheir Taha, director of the French National Reference Center for Meningococci at the Pasteur Institute in Paris, where he also is head of the invasive bacterial infections unit. "Previously, we had what we used to call ‘reactive vaccination,’ meaning we usually started vaccinating when the epidemiologic threshold was crossed, and now we are doing preventive vaccination."
In 2001, the World Health Organization (WHO) and the international nonprofit organization PATH founded the Meningitis Vaccine Project to develop MenAfriVac. The project brought together African ministers of health, U.S. government health agencies, philanthropic organizations, many strategic planning and surveillance groups, and vaccine industry partners.
MenAfriVac is being distributed to African nations in the meningitis belt, which stretches from Senegal to Ethiopia, before anywhere else. After Burkina Faso, the hyperendemic nations of Niger and Mali also began nationwide vaccination campaigns in December. If a funding gap of U.S.$475 million is met, the WHO expects that all 25 countries in the meningitis belt will be using the vaccine by 2015. If that happens, the reduction in the number of meningitis cases is expected to save U.S.$120 million annually in the period up to 2015 from national budgets that would be otherwise spent on costs for diagnosis and treatment, according to the WHO.
The vaccination campaign began just before the start of the dry season (January to May) when the yearly epidemics occur. Major epidemics of the disease occur every 7-14 years, and in 2009 they infected more than 88,000 people and killed more than 5,000 across the region. Serogroup A Neisseria meningitidis causes 80%-85% of the epidemics, according to the WHO.
Epidemics of group A meningococcal disease have been impossible to stop with older polysaccharide vaccines, despite millions of doses that have been given to individuals during epidemics. These vaccines induce a systemic immune response that is independent of memory T-cell activation and lasts only 2-3 years. They are also poorly immunogenic in children younger than 2 years. Polysaccharide vaccines also do not reduce the carriage of N. meningitidis in the nasopharynx, the bacteria’s natural habitat, and thereby do not induce "herd immunity." This happens because polysaccharide vaccines induce only immunoglobulin M antibodies, which are not present on the surfaces of epithelial cells lining the nasopharynx, according to Dr. Taha.
In contrast, MenAfriVac is modeled after the successful group C conjugate polysaccharide vaccine first used in the United Kingdom in the late 1990s and early 2000s. Conjugate vaccines are built with a protein attached to the bacteria’s capsular polysaccharide antigen, which induces a longer-lasting T-cell dependent immune response. This response produces immunoglobulin G antibodies that are present on the surface of epithelial cells in the nasopharynx, reducing carriage of the bacteria and providing herd immunity. This means that children younger than 2 years can receive the vaccine. The antibodies formed with MenAfriVac will likely persist beyond 5 years, said Dr. Taha, who was not involved in the development of the vaccine.
A single dose of MenAfriVac costs less than half a U.S. dollar, in part because it was developed for less than a tenth of the U.S.$500 million that conjugate vaccine development has typically cost. In comparison, Dr. Taha said, "the cost of the group C conjugate vaccine in Europe is about 30 euros, which is far away from [the price that] can be offered in Africa."
Clinical trials carried out in India and in several countries of the meningitis belt in people aged 1-29 years have shown that MenAfriVac is safe and has higher immunogenicity than the previous group A polysaccharide vaccine. In children aged 12-24 months, MenAfriVac induced 20-fold greater antibody levels than did the meningococcal A component of the licensed tetravalent ACWY polysaccharide vaccine. Ongoing trials are determining whether a single dose of the vaccine given to children aged 9-12 months will be sufficient to generate long-lasting protection (Vaccine 2009;27[Suppl. 2]:B13-19).
Based on the experience of European countries in vaccinating people with a similar conjugated vaccine against group C meningitis, Dr. Taha estimated that at least 90% of the target population will need to receive MenAfriVac in order to reduce transmission of the disease to unvaccinated people. It’s unknown whether this "quite high" coverage rate can be reached rapidly, he said.
Although MenAfriVac protects against group A meningococcal disease – by far the most prevalent type in Africa – outbreaks of meningitis caused by serogroups W135 and X in recent years mean that active surveillance for epidemics will still be necessary, he said.
Effects of Autism-Related Gene Observed With Imaging
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
Effects of Autism-Related Gene Observed With Imaging
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
[Parents' Deployment Tough on Military Kids]
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
[Prenatal Stress May Have Long-Term Effects]
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
[Vitamin D Deficiency Linked to Psychotic Symptoms in Adolescents]
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
[Parents' Deployment Tough on Military Kids]
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
[Prenatal Stress May Have Long-Term Effects]
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
[Vitamin D Deficiency Linked to Psychotic Symptoms in Adolescents]
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.
For the first time, researchers have observed how a variant of a single gene can lead to altered functional connections in the brain that may predispose an individual to cognitive dysfunction.
The gene, contactin-associated protein-like 2 (CNTNAP2), is expressed in the frontal and temporal lobes during development and is thought to assist in interactions important for cellular migration and the subsequent laminar organization of these areas. Some variants of the gene are associated with an increased risk of autism; specific language impairment; and other neuropsychiatric disorders, such as attention-deficit/hyperactivity disorder, Tourette syndrome, and schizophrenia. These disorders all exhibit underlying abnormal frontal cortical circuitry connectivity.
[Parents' Deployment Tough on Military Kids]
Ashley A. Scott-Van Zeeland, Ph.D., and her colleagues at the University of California, Los Angeles, tied these lines of evidence together by conducting functional MRI scans of children with either a common or a less common, risk-conferring variant of the gene to determine how the gene contributes to brain development (Sci. Transl. Med. 2010 Nov. 3 [doi:10.1126/ scitranslmed.3001344]). After performing this work as a graduate student at UCLA, Dr. Scott-Van Zeeland is now conducting her postdoctoral work at the Scripps Translational Science Institute, La Jolla, Calif.
In a discovery cohort of 16 typically developing children and 16 children with autism, carriers of a risk-conferring allele showed more neural activity in the medial prefrontal cortex (mPFC) during a learning task than did those with a nonrisk allele. The mPFC in nonrisk allele carriers was connected to more posterior cortical regions via a left-sided network (such as between the mPFC and the medial occipital and ventral temporal cortices), whereas in carriers of the risk allele there were stronger local connections to the right front cortex and there were widespread and bilateral connections to posterior regions.
The discrete left-sided frontotemporal network observed in nonrisk allele carriers overlapped with "regions known to be important in language processing, such as the IFG [inferior frontal gyrus] and superior temporal gyrus," the investigators wrote.
They found similar results when they examined the effect of the allele in the children with and without autism, when they analyzed males only, and when they conducted the same scans during a different learning task in a replication cohort of 39 typically developing children.
[Prenatal Stress May Have Long-Term Effects]
The lack of an association between disease status and CNTNAP2 genotype "indicates that this variant mediates risk by modulating the continuum of normal brain function, as would be expected for intermediate phenotypes related to cognition or behavior," the authors wrote.
They suggested that the approach of combining gene expression data with functional neuroimaging to understand the consequences of a known disease-associated gene on brain development "is likely to be of widespread utility in the elucidation of mechanism underlying disorders of human cognition."
The study was supported in part by grants from the National Institutes of Health, the National Alliance for Autism Research, Autism Speaks, and the Whitehall Foundation. The researchers reported having no conflicts of interest.
Matthew J. Huentelman, Ph.D., an investigator in the neurogenomics division of the Translational Genomics Research Institute in Phoenix, commented on the study as follows:
"The search for genetic variation associated with risk for the development of neuropsychiatric disease has lagged behind that of other common complex human diseases. There is much ongoing debate about the reasons for this, including:
• A need for much larger cohorts, numbered perhaps in the tens of thousands, to create greater statistical power;
• A lack of diagnostic accuracy caused by the inclusion of inappropriate case individuals or through a lumping together of several diseases under the same phenotypic umbrella; and
• Inaccurate assumptions about the genetic risk associated with common versus rare genetic variations, the latter of which we have only recently been able to evaluate cost effectively on a genomewide scale.
"Dr. Scott-Van Zeeland and her colleagues’ report leverages an emerging investigative paradigm that includes the intersection of genetics, neuroanatomy, and functional neuroimaging to address some of these concerns and take a closer look at a gene, CNTNAP2, previously associated with Tourette syndrome, autism, epilepsy, and language development.
"In doing so, the investigators unveil a putative functional role of variation at the single nucleotide polymorphism (SNP) rs2710102 within CNTNAP2 that is independent of disease status. In short, the researchers’ findings demonstrate that rs2710102 variation is associated with differential frontal lobe functional connectivity. Caspr2, the protein product of CNTNAP2, plays a crucial role in the establishment of the juxtaparanodal region at the nodes of Ranvier through the clustering of the Kv1.1 voltage-gated potassium channels.
"The variation of this SNP is shown in this new work to modify the scope of brain frontal lobe connectivity by altering the frequency of the functional establishment of either lateralized, long-range connections with the medial prefrontal cortex (predominant in "nonrisk" allele carriers) or more localized, bilateral connections (predominant in the "risk" allele carriers) during the performance of a reward-guided implicit learning task.
"The analytical approach is particularly powerful because it convincingly demonstrates these connectivity effects utilizing functional MRI in moderately sized sample sets. The marriage of genomics, neuroanatomy, and neuroimaging is still in its infancy, but already there are indications of the power of this approach both in this work and in work by others in the field.
"Dr. Scott-Van Zeeland and her colleagues suggest a biological mechanism for the development of autism and possibly other disorders in risk allele carriers. These findings present a possible way to further stratify carriers of the risk allele in future studies. For example, what might be different about individuals with autism who carry the risk allele but demonstrate prefrontal connectivity patterns that are more similar to nonrisk allele carriers? This question raises another issue. Based on the high minor allele frequency of rs2710102, carriers of the risk allele likely comprise more than half of the general population. How important is our individual genetics in determining the functional connectivity pattern, and what other genetic factors might predispose to such a pattern? Are these other genes autism risk candidates as well?
[Vitamin D Deficiency Linked to Psychotic Symptoms in Adolescents]
"In spite of the questions that may remain to be answered, this work provides an important foothold for the mechanistic study of the extremely important public health problem of autism. One can hope that the future will hold additional studies involving neuroimaging and genetics in both healthy and diseased cohorts to help further dissect the functional basis for neuropsychiatric disease."
Dr. Huentelman’s laboratory focuses on autism, Alzheimer’s disease, aging, and diseases with a significant aging component. He has no relevant conflicts of interest.