User login
What Is Your Diagnosis? Onychomadesis Following Hand-foot-and-mouth Disease
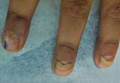
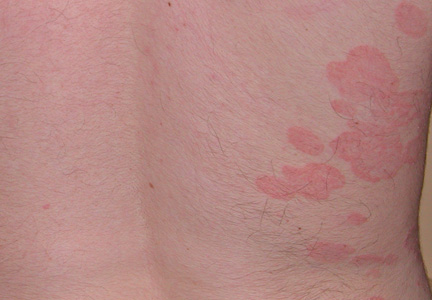
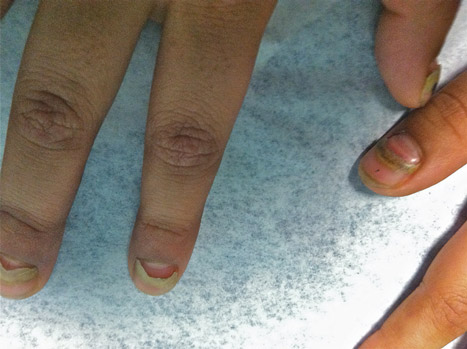
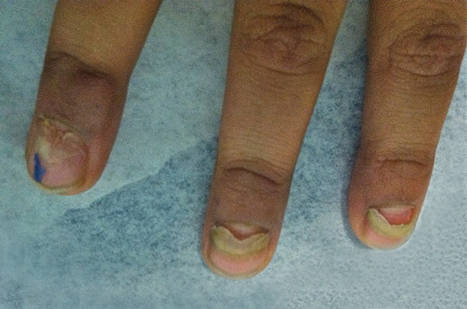
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
Fragile Drug Development Process
We are currently in the midst of a new wave of drug developments and approvals for psoriasis; however, we recently have been reminded of the tenuous nature of bringing a new drug to market. Last month, Amgen Inc announced it was pulling out of the long-running collaboration on the high-profile IL-17 program after evaluating the likely commercial impact it would face in light of the suicidal thoughts some patients reported during the studies.
Brodalumab had successfully completed 3 phase 3 studies in patients with moderate to severe plaque psoriasis known as the AMAGINE program. Top-line results from AMAGINE-1 comparing brodalumab with placebo were released in May 2014. Top-line results from AMAGINE-2 and AMAGINE-3 comparing brodalumab with ustekinumab and placebo were announced in November 2014. AMAGINE-2 and AMAGINE-3 are identical in design. “During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” said Amgen Executive Vice President of Research and Development Sean Harper in a statement. AstraZeneca must now decide whether to pursue brodalumab independently.
Once the exact data are publicly released, we will be able to better evaluate the issues of suicidal ideation involved.
What’s the issue?
Brodalumab was eagerly anticipated in the dermatology community. In an instant, the drug’s future is in doubt, which once again demonstrates the fragility of the drug development process. How will this recent announcement affect your use of new biologics?
We are currently in the midst of a new wave of drug developments and approvals for psoriasis; however, we recently have been reminded of the tenuous nature of bringing a new drug to market. Last month, Amgen Inc announced it was pulling out of the long-running collaboration on the high-profile IL-17 program after evaluating the likely commercial impact it would face in light of the suicidal thoughts some patients reported during the studies.
Brodalumab had successfully completed 3 phase 3 studies in patients with moderate to severe plaque psoriasis known as the AMAGINE program. Top-line results from AMAGINE-1 comparing brodalumab with placebo were released in May 2014. Top-line results from AMAGINE-2 and AMAGINE-3 comparing brodalumab with ustekinumab and placebo were announced in November 2014. AMAGINE-2 and AMAGINE-3 are identical in design. “During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” said Amgen Executive Vice President of Research and Development Sean Harper in a statement. AstraZeneca must now decide whether to pursue brodalumab independently.
Once the exact data are publicly released, we will be able to better evaluate the issues of suicidal ideation involved.
What’s the issue?
Brodalumab was eagerly anticipated in the dermatology community. In an instant, the drug’s future is in doubt, which once again demonstrates the fragility of the drug development process. How will this recent announcement affect your use of new biologics?
We are currently in the midst of a new wave of drug developments and approvals for psoriasis; however, we recently have been reminded of the tenuous nature of bringing a new drug to market. Last month, Amgen Inc announced it was pulling out of the long-running collaboration on the high-profile IL-17 program after evaluating the likely commercial impact it would face in light of the suicidal thoughts some patients reported during the studies.
Brodalumab had successfully completed 3 phase 3 studies in patients with moderate to severe plaque psoriasis known as the AMAGINE program. Top-line results from AMAGINE-1 comparing brodalumab with placebo were released in May 2014. Top-line results from AMAGINE-2 and AMAGINE-3 comparing brodalumab with ustekinumab and placebo were announced in November 2014. AMAGINE-2 and AMAGINE-3 are identical in design. “During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” said Amgen Executive Vice President of Research and Development Sean Harper in a statement. AstraZeneca must now decide whether to pursue brodalumab independently.
Once the exact data are publicly released, we will be able to better evaluate the issues of suicidal ideation involved.
What’s the issue?
Brodalumab was eagerly anticipated in the dermatology community. In an instant, the drug’s future is in doubt, which once again demonstrates the fragility of the drug development process. How will this recent announcement affect your use of new biologics?
Corrona Begins
Over the last 15 years the treatment of psoriasis has been transformed with the advent of biologic agents. Now we have a whole new generation of treatments that is emerging. With all of these therapeutic options, the dermatologic community is in need of increasing data to help us further understand both the therapies and the disease state.
A new independent US psoriasis registry has been established. This registry is a joint collaboration with the National Psoriasis Foundation and Corrona, Inc (Consortium of Rheumatology Researchers of North America, Inc). Data will be gathered through comprehensive questionnaires completed by patients and their dermatologists during appointments.
The registry will function to collect and analyze clinical data, and thereby allow investigators to achieve the following: (1) compare the safety and effectiveness of psoriasis treatments, (2) better understand psoriasis comorbidities, and (3) explore the natural history of the disease.
The registry will begin recruiting patients this year. Initially, the registry will track the drug safety reporting for secukinumab. The goal is for the CORRONA psoriasis registry to enroll at least 3000 patients with psoriasis who are taking secukinumab and then follow their treatment for at least 8 years.
In addition to studying safety and effectiveness of therapeutics, the registry also will help identify potential etiologies of psoriasis, study the relationship between psoriasis and other health conditions, and examine the impact of the condition on quality of life, among other outcomes.
To become an investigator in the registry or learn more about it, visit www.psoriasis.org/corrona-registry.
What’s the issue?
This registry is a welcomed addition to the study of psoriasis. It has the potential to add critical information in the years to come.
Over the last 15 years the treatment of psoriasis has been transformed with the advent of biologic agents. Now we have a whole new generation of treatments that is emerging. With all of these therapeutic options, the dermatologic community is in need of increasing data to help us further understand both the therapies and the disease state.
A new independent US psoriasis registry has been established. This registry is a joint collaboration with the National Psoriasis Foundation and Corrona, Inc (Consortium of Rheumatology Researchers of North America, Inc). Data will be gathered through comprehensive questionnaires completed by patients and their dermatologists during appointments.
The registry will function to collect and analyze clinical data, and thereby allow investigators to achieve the following: (1) compare the safety and effectiveness of psoriasis treatments, (2) better understand psoriasis comorbidities, and (3) explore the natural history of the disease.
The registry will begin recruiting patients this year. Initially, the registry will track the drug safety reporting for secukinumab. The goal is for the CORRONA psoriasis registry to enroll at least 3000 patients with psoriasis who are taking secukinumab and then follow their treatment for at least 8 years.
In addition to studying safety and effectiveness of therapeutics, the registry also will help identify potential etiologies of psoriasis, study the relationship between psoriasis and other health conditions, and examine the impact of the condition on quality of life, among other outcomes.
To become an investigator in the registry or learn more about it, visit www.psoriasis.org/corrona-registry.
What’s the issue?
This registry is a welcomed addition to the study of psoriasis. It has the potential to add critical information in the years to come.
Over the last 15 years the treatment of psoriasis has been transformed with the advent of biologic agents. Now we have a whole new generation of treatments that is emerging. With all of these therapeutic options, the dermatologic community is in need of increasing data to help us further understand both the therapies and the disease state.
A new independent US psoriasis registry has been established. This registry is a joint collaboration with the National Psoriasis Foundation and Corrona, Inc (Consortium of Rheumatology Researchers of North America, Inc). Data will be gathered through comprehensive questionnaires completed by patients and their dermatologists during appointments.
The registry will function to collect and analyze clinical data, and thereby allow investigators to achieve the following: (1) compare the safety and effectiveness of psoriasis treatments, (2) better understand psoriasis comorbidities, and (3) explore the natural history of the disease.
The registry will begin recruiting patients this year. Initially, the registry will track the drug safety reporting for secukinumab. The goal is for the CORRONA psoriasis registry to enroll at least 3000 patients with psoriasis who are taking secukinumab and then follow their treatment for at least 8 years.
In addition to studying safety and effectiveness of therapeutics, the registry also will help identify potential etiologies of psoriasis, study the relationship between psoriasis and other health conditions, and examine the impact of the condition on quality of life, among other outcomes.
To become an investigator in the registry or learn more about it, visit www.psoriasis.org/corrona-registry.
What’s the issue?
This registry is a welcomed addition to the study of psoriasis. It has the potential to add critical information in the years to come.
Novel Psoriasis Therapies and Patient Outcomes, Part 2: Biologic Treatments
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
Practice Points
- Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and psoriatic arthritis (PsA).
- Although biologics currently in use for treatment of psoriasis and PsA are in the form of tumor necrosis factor α inhibitors, other drugs in phase 2 through phase 4 clinical trials aim to target alternative pathways underlying the pathogenesis of these disorders, including IL-12/IL-23 inhibition, IL-17 inhibition, inhibition of T-cell activation in antigen-presenting cells, regulatory T-cell activation, toll-like receptor inhibition, and granulocyte-macrophage colony-stimulating factor inhibition.
- New approaches to the management of psoriasis and PsA offer patients hope for more targeted treatment regimens.
Impact of Diet and Obesity on Psoriasis Severity
There is a higher prevalence of obesity in patients with psoriasis. Studies have shown that psoriasis patients may gain weight after the onset of psoriasis. Dr. Jeffrey Weinberg discusses the mechanisms of psoriasis that can lead to obesity. He also provides tips on how to evaluate the patient for comorbidities associated with obesity. Although there is no evidence that certain foods make psoriasis worse or better, recommending a healthy diet is important. Dr. Weinberg also discusses the impact of obesity on the efficacy of psoriasis treatments, such as the biologics.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Suggested Reading
Bremmer S, Van Voorhees AS, Hsu S, et al; for the National Psoriasis Foundation. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;63:1058-1069.
There is a higher prevalence of obesity in patients with psoriasis. Studies have shown that psoriasis patients may gain weight after the onset of psoriasis. Dr. Jeffrey Weinberg discusses the mechanisms of psoriasis that can lead to obesity. He also provides tips on how to evaluate the patient for comorbidities associated with obesity. Although there is no evidence that certain foods make psoriasis worse or better, recommending a healthy diet is important. Dr. Weinberg also discusses the impact of obesity on the efficacy of psoriasis treatments, such as the biologics.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
There is a higher prevalence of obesity in patients with psoriasis. Studies have shown that psoriasis patients may gain weight after the onset of psoriasis. Dr. Jeffrey Weinberg discusses the mechanisms of psoriasis that can lead to obesity. He also provides tips on how to evaluate the patient for comorbidities associated with obesity. Although there is no evidence that certain foods make psoriasis worse or better, recommending a healthy diet is important. Dr. Weinberg also discusses the impact of obesity on the efficacy of psoriasis treatments, such as the biologics.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Suggested Reading
Bremmer S, Van Voorhees AS, Hsu S, et al; for the National Psoriasis Foundation. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;63:1058-1069.
Suggested Reading
Bremmer S, Van Voorhees AS, Hsu S, et al; for the National Psoriasis Foundation. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;63:1058-1069.
To Stop or Not to Stop
With the advent of biologic therapies, there has been debate about the necessity to stop these therapies prior to elective surgery. The concern has been that there might be an increased risk for perioperative infections in the presence of these agents. Several current guidelines of care recommend a planned break from biologic therapy in patients undergoing major surgical procedures; for example, the British Association of Dermatologists and the British Society for Rheumatology recommend stopping biologics for at least 4 half-lives before surgery.
Bakkour et al published a study online on March 2 in the Journal of the European Academy of Dermatology and Venereology that audited the management of biologic therapy perioperatively in a tertiary referral psoriasis clinic against guidelines of care. In addition, they investigated the effects of continuing and stopping biologic therapy in psoriasis and psoriatic arthritis patients. Information was collected on the biologics used, whether they were held perioperatively, and whether patients developed postoperative complications and/or disease flare.
The authors identified 42 patients who had a total of 77 procedures performed. Procedures included cutaneous surgery, orthopedic procedures, and cardiothoracic surgery. Biologic therapy was continued in 76% of procedures. Comparing those who continued with those who stopped biologic therapy, there was no significant difference in postoperative risk for infection and delayed wound healing. This finding included patients who underwent major surgery. Interestingly, the interruption of biologic therapy perioperatively was associated with a significant (P=.003) risk for flare of psoriasis or psoriatic arthritis.
What’s the issue?
The authors concluded that continuing biologic therapy did not increase the risk for postoperative complications. However, stopping biologic therapy perioperatively significantly increased the risk for disease flare.
Although this study was small, it shed light on an issue of great interest in the use of biologic therapy. It showed that there is a potential downside to stopping these agents before surgery. Further data are needed to fully elucidate the proper management in these cases.
With the advent of biologic therapies, there has been debate about the necessity to stop these therapies prior to elective surgery. The concern has been that there might be an increased risk for perioperative infections in the presence of these agents. Several current guidelines of care recommend a planned break from biologic therapy in patients undergoing major surgical procedures; for example, the British Association of Dermatologists and the British Society for Rheumatology recommend stopping biologics for at least 4 half-lives before surgery.
Bakkour et al published a study online on March 2 in the Journal of the European Academy of Dermatology and Venereology that audited the management of biologic therapy perioperatively in a tertiary referral psoriasis clinic against guidelines of care. In addition, they investigated the effects of continuing and stopping biologic therapy in psoriasis and psoriatic arthritis patients. Information was collected on the biologics used, whether they were held perioperatively, and whether patients developed postoperative complications and/or disease flare.
The authors identified 42 patients who had a total of 77 procedures performed. Procedures included cutaneous surgery, orthopedic procedures, and cardiothoracic surgery. Biologic therapy was continued in 76% of procedures. Comparing those who continued with those who stopped biologic therapy, there was no significant difference in postoperative risk for infection and delayed wound healing. This finding included patients who underwent major surgery. Interestingly, the interruption of biologic therapy perioperatively was associated with a significant (P=.003) risk for flare of psoriasis or psoriatic arthritis.
What’s the issue?
The authors concluded that continuing biologic therapy did not increase the risk for postoperative complications. However, stopping biologic therapy perioperatively significantly increased the risk for disease flare.
Although this study was small, it shed light on an issue of great interest in the use of biologic therapy. It showed that there is a potential downside to stopping these agents before surgery. Further data are needed to fully elucidate the proper management in these cases.
With the advent of biologic therapies, there has been debate about the necessity to stop these therapies prior to elective surgery. The concern has been that there might be an increased risk for perioperative infections in the presence of these agents. Several current guidelines of care recommend a planned break from biologic therapy in patients undergoing major surgical procedures; for example, the British Association of Dermatologists and the British Society for Rheumatology recommend stopping biologics for at least 4 half-lives before surgery.
Bakkour et al published a study online on March 2 in the Journal of the European Academy of Dermatology and Venereology that audited the management of biologic therapy perioperatively in a tertiary referral psoriasis clinic against guidelines of care. In addition, they investigated the effects of continuing and stopping biologic therapy in psoriasis and psoriatic arthritis patients. Information was collected on the biologics used, whether they were held perioperatively, and whether patients developed postoperative complications and/or disease flare.
The authors identified 42 patients who had a total of 77 procedures performed. Procedures included cutaneous surgery, orthopedic procedures, and cardiothoracic surgery. Biologic therapy was continued in 76% of procedures. Comparing those who continued with those who stopped biologic therapy, there was no significant difference in postoperative risk for infection and delayed wound healing. This finding included patients who underwent major surgery. Interestingly, the interruption of biologic therapy perioperatively was associated with a significant (P=.003) risk for flare of psoriasis or psoriatic arthritis.
What’s the issue?
The authors concluded that continuing biologic therapy did not increase the risk for postoperative complications. However, stopping biologic therapy perioperatively significantly increased the risk for disease flare.
Although this study was small, it shed light on an issue of great interest in the use of biologic therapy. It showed that there is a potential downside to stopping these agents before surgery. Further data are needed to fully elucidate the proper management in these cases.
Novel Psoriasis Therapies and Patient Outcomes, Part 1: Topical Medications
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
Practice Points
- Topical therapies are the cornerstone of treating patients with mild to moderate psoriasis. Commercially available medications approved by the US Food and Drug Administration for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.
- Recent developments in our understanding of inflammatory mediators and the underlying pathogenesis of psoriasis have revealed new potential therapeutic targets, leading to the discovery of many promising treatments.
- Novel topical therapies currently in phase 2 and phase 3 clinical trials for patients with mild to moderate psoriasis may offer hope to patients who have reported a suboptimal therapeutic response to conventional treatments.
First Refusal
Treatment success in psoriasis, as in any dermatologic condition, is dependent on many factors. The willingness of patients to follow our suggested therapeutic plans certainly is one of the most important components of this process.
Halioua et al1 analyzed the issue of treatment refusal, which they defined as “a patient actively refusing to take treatment despite physician recommendations,” among psoriasis patients. Treatment refusal is a more complex phenomenon than nonadherence, as it requires an affirmative act that goes beyond more passive acts of not filling prescriptions, taking a medication sporadically, or forgetting to take a medication. Their objective was to investigate refusal of topical treatments by patients living with psoriasis in France as well as the factors that influence such refusal.1
The authors evaluated responses to an Internet study.1 Responses from participants who refused topical therapy (n=50) were compared to individuals who successfully applied topical treatment (n=205). Individuals receiving phototherapy, biologic therapy, and oral treatment were not included in the analysis. Spearman rank correlations completed by Fisher exact tests and Student t tests were performed.1
The researchers found that objective aspects of psoriasis, including comorbidities, localization of lesions, and symptoms associated with psoriasis, were not significant predictors of treatment refusal. The factors that did appear to influence refusal related more to patient perception of disease and its treatment.1
First, treatment refusal was defined by patient attitude toward treatment. In the treatment refusal group, significantly fewer participants reported believing that psoriasis can be managed (20.0% vs 38.5%; P<.01), and significantly more participants in the treatment refusal group reported believing that topical psoriasis treatments never work (58.0% vs 27.5%; odds ratio, 2.09; P<.0001). Additionally, significantly fewer participants in the treatment refusal group were willing to stay on prescription medications long-term (30.0% vs 77.6%; P<.001), and significantly more patients in the treatment refusal group believed that all creams (prescription or over-the-counter) work the same (54.0% vs 31.7%; odds ratio, 1.07; P=.003).1
The physician-patient relationship also influenced refusal. In the treatment refusal group, 60% of participants reported no longer consulting physicians for psoriasis treatment. The main reasons for cessation of medical care were lack of improvement of psoriasis (40%) and feeling that the physician did not take psoriasis seriously (20%). In the treatment acceptance group, only 10% of participants no longer consulted physicians.1 Among participants who continued to consult their physician (40% for the treatment refusal group and 90.2% for the treatment acceptance group), significantly fewer participants in the treatment refusal group reported that they were substantially helped by their physician (50.0% vs 73.0%; P=.03) and that they always followed physician recommendations (65.0% vs 85.4%; P=.02). Additionally, significantly fewer participants in the treatment refusal group considered that their physician took the time to listen to what he/she had to say (65.0% vs 85.9%; P=.02) and that their physician had provided clear instructions on how to utilize the treatment (65.0% vs 83.2%; P=.046).1
Therefore, treatment refusal is an important factor to be considered in the management of psoriasis. The findings of this study indicate possible strategies to reduce patient refusal. For example, enhanced education about the therapeutic options for psoriasis and their benefits could counter negative perceptions about these therapies. It also appears that increased focus on the physician-patient relationship may have a positive impact in this area.
Reference
1. Halioua B, Maury Le Breton A, de Fontaubert A, et al. Treatment refusal among patients with psoriasis [published online ahead of print]. J Dermatolog Treat. 2015;2:1-5.
Treatment success in psoriasis, as in any dermatologic condition, is dependent on many factors. The willingness of patients to follow our suggested therapeutic plans certainly is one of the most important components of this process.
Halioua et al1 analyzed the issue of treatment refusal, which they defined as “a patient actively refusing to take treatment despite physician recommendations,” among psoriasis patients. Treatment refusal is a more complex phenomenon than nonadherence, as it requires an affirmative act that goes beyond more passive acts of not filling prescriptions, taking a medication sporadically, or forgetting to take a medication. Their objective was to investigate refusal of topical treatments by patients living with psoriasis in France as well as the factors that influence such refusal.1
The authors evaluated responses to an Internet study.1 Responses from participants who refused topical therapy (n=50) were compared to individuals who successfully applied topical treatment (n=205). Individuals receiving phototherapy, biologic therapy, and oral treatment were not included in the analysis. Spearman rank correlations completed by Fisher exact tests and Student t tests were performed.1
The researchers found that objective aspects of psoriasis, including comorbidities, localization of lesions, and symptoms associated with psoriasis, were not significant predictors of treatment refusal. The factors that did appear to influence refusal related more to patient perception of disease and its treatment.1
First, treatment refusal was defined by patient attitude toward treatment. In the treatment refusal group, significantly fewer participants reported believing that psoriasis can be managed (20.0% vs 38.5%; P<.01), and significantly more participants in the treatment refusal group reported believing that topical psoriasis treatments never work (58.0% vs 27.5%; odds ratio, 2.09; P<.0001). Additionally, significantly fewer participants in the treatment refusal group were willing to stay on prescription medications long-term (30.0% vs 77.6%; P<.001), and significantly more patients in the treatment refusal group believed that all creams (prescription or over-the-counter) work the same (54.0% vs 31.7%; odds ratio, 1.07; P=.003).1
The physician-patient relationship also influenced refusal. In the treatment refusal group, 60% of participants reported no longer consulting physicians for psoriasis treatment. The main reasons for cessation of medical care were lack of improvement of psoriasis (40%) and feeling that the physician did not take psoriasis seriously (20%). In the treatment acceptance group, only 10% of participants no longer consulted physicians.1 Among participants who continued to consult their physician (40% for the treatment refusal group and 90.2% for the treatment acceptance group), significantly fewer participants in the treatment refusal group reported that they were substantially helped by their physician (50.0% vs 73.0%; P=.03) and that they always followed physician recommendations (65.0% vs 85.4%; P=.02). Additionally, significantly fewer participants in the treatment refusal group considered that their physician took the time to listen to what he/she had to say (65.0% vs 85.9%; P=.02) and that their physician had provided clear instructions on how to utilize the treatment (65.0% vs 83.2%; P=.046).1
Therefore, treatment refusal is an important factor to be considered in the management of psoriasis. The findings of this study indicate possible strategies to reduce patient refusal. For example, enhanced education about the therapeutic options for psoriasis and their benefits could counter negative perceptions about these therapies. It also appears that increased focus on the physician-patient relationship may have a positive impact in this area.
Treatment success in psoriasis, as in any dermatologic condition, is dependent on many factors. The willingness of patients to follow our suggested therapeutic plans certainly is one of the most important components of this process.
Halioua et al1 analyzed the issue of treatment refusal, which they defined as “a patient actively refusing to take treatment despite physician recommendations,” among psoriasis patients. Treatment refusal is a more complex phenomenon than nonadherence, as it requires an affirmative act that goes beyond more passive acts of not filling prescriptions, taking a medication sporadically, or forgetting to take a medication. Their objective was to investigate refusal of topical treatments by patients living with psoriasis in France as well as the factors that influence such refusal.1
The authors evaluated responses to an Internet study.1 Responses from participants who refused topical therapy (n=50) were compared to individuals who successfully applied topical treatment (n=205). Individuals receiving phototherapy, biologic therapy, and oral treatment were not included in the analysis. Spearman rank correlations completed by Fisher exact tests and Student t tests were performed.1
The researchers found that objective aspects of psoriasis, including comorbidities, localization of lesions, and symptoms associated with psoriasis, were not significant predictors of treatment refusal. The factors that did appear to influence refusal related more to patient perception of disease and its treatment.1
First, treatment refusal was defined by patient attitude toward treatment. In the treatment refusal group, significantly fewer participants reported believing that psoriasis can be managed (20.0% vs 38.5%; P<.01), and significantly more participants in the treatment refusal group reported believing that topical psoriasis treatments never work (58.0% vs 27.5%; odds ratio, 2.09; P<.0001). Additionally, significantly fewer participants in the treatment refusal group were willing to stay on prescription medications long-term (30.0% vs 77.6%; P<.001), and significantly more patients in the treatment refusal group believed that all creams (prescription or over-the-counter) work the same (54.0% vs 31.7%; odds ratio, 1.07; P=.003).1
The physician-patient relationship also influenced refusal. In the treatment refusal group, 60% of participants reported no longer consulting physicians for psoriasis treatment. The main reasons for cessation of medical care were lack of improvement of psoriasis (40%) and feeling that the physician did not take psoriasis seriously (20%). In the treatment acceptance group, only 10% of participants no longer consulted physicians.1 Among participants who continued to consult their physician (40% for the treatment refusal group and 90.2% for the treatment acceptance group), significantly fewer participants in the treatment refusal group reported that they were substantially helped by their physician (50.0% vs 73.0%; P=.03) and that they always followed physician recommendations (65.0% vs 85.4%; P=.02). Additionally, significantly fewer participants in the treatment refusal group considered that their physician took the time to listen to what he/she had to say (65.0% vs 85.9%; P=.02) and that their physician had provided clear instructions on how to utilize the treatment (65.0% vs 83.2%; P=.046).1
Therefore, treatment refusal is an important factor to be considered in the management of psoriasis. The findings of this study indicate possible strategies to reduce patient refusal. For example, enhanced education about the therapeutic options for psoriasis and their benefits could counter negative perceptions about these therapies. It also appears that increased focus on the physician-patient relationship may have a positive impact in this area.
Reference
1. Halioua B, Maury Le Breton A, de Fontaubert A, et al. Treatment refusal among patients with psoriasis [published online ahead of print]. J Dermatolog Treat. 2015;2:1-5.
Reference
1. Halioua B, Maury Le Breton A, de Fontaubert A, et al. Treatment refusal among patients with psoriasis [published online ahead of print]. J Dermatolog Treat. 2015;2:1-5.
Counting Costs
We are all aware of the rising costs of medical care, especially for complex diseases such as psoriasis. The total cost of psoriasis in the United States is unknown. Brezinski et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.3593) sought to define the economic burden of psoriasis in the United States. They argued that this information is needed to provide the foundation for research, advocacy, and educational efforts within the disease.
The authors searched PubMed and MEDLINE databases for economic investigations on the costs of adult psoriasis in the United States. The primary objective of the analysis was to provide a comprehensive analysis of the literature on the economic burden of psoriasis in the United States. The direct, indirect, intangible, and comorbidity costs of psoriasis were reported based on this systematic literature review and adjusted to 2013 US dollars.
The direct costs included medical costs associated with (1) specialist medical evaluations, (2) hospitalization, (3) prescription medications, (4) phototherapy, (5) medication administration costs, (6) laboratory tests and monitoring studies, and (7) over-the-counter medications and self-care products. The indirect costs were determined by absenteeism and impaired work productivity. Intangible costs were calculated as a measure of the negative effect of psoriasis on quality of life. Finally, comorbidity costs measured the medical evaluations, treatment, and lab monitoring that were directly attributed to comorbid conditions associated with psoriasis.
An initial review of the literature generated 100 articles; 22 studies were included in the systematic review. The direct psoriasis costs ranged from $51.7 billion to $63.2 billion, the indirect costs ranged from $23.9 billion to $35.4 billion, and medical comorbidities were estimated to contribute $36.4 billion annually in 2013 US dollars. The annual cost of psoriasis in the United States amounted to approximately $112 billion in 2013.
The authors concluded that the economic burden of psoriasis was substantial and significant in the United States.
What’s the issue?
In the United States, the economic burden of psoriasis is substantial because this disease is associated with negative physical, psychiatric, and social consequences. In addition, treatment costs continue to rise. How will this analysis of cost influence your future management of psoriasis?
We are all aware of the rising costs of medical care, especially for complex diseases such as psoriasis. The total cost of psoriasis in the United States is unknown. Brezinski et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.3593) sought to define the economic burden of psoriasis in the United States. They argued that this information is needed to provide the foundation for research, advocacy, and educational efforts within the disease.
The authors searched PubMed and MEDLINE databases for economic investigations on the costs of adult psoriasis in the United States. The primary objective of the analysis was to provide a comprehensive analysis of the literature on the economic burden of psoriasis in the United States. The direct, indirect, intangible, and comorbidity costs of psoriasis were reported based on this systematic literature review and adjusted to 2013 US dollars.
The direct costs included medical costs associated with (1) specialist medical evaluations, (2) hospitalization, (3) prescription medications, (4) phototherapy, (5) medication administration costs, (6) laboratory tests and monitoring studies, and (7) over-the-counter medications and self-care products. The indirect costs were determined by absenteeism and impaired work productivity. Intangible costs were calculated as a measure of the negative effect of psoriasis on quality of life. Finally, comorbidity costs measured the medical evaluations, treatment, and lab monitoring that were directly attributed to comorbid conditions associated with psoriasis.
An initial review of the literature generated 100 articles; 22 studies were included in the systematic review. The direct psoriasis costs ranged from $51.7 billion to $63.2 billion, the indirect costs ranged from $23.9 billion to $35.4 billion, and medical comorbidities were estimated to contribute $36.4 billion annually in 2013 US dollars. The annual cost of psoriasis in the United States amounted to approximately $112 billion in 2013.
The authors concluded that the economic burden of psoriasis was substantial and significant in the United States.
What’s the issue?
In the United States, the economic burden of psoriasis is substantial because this disease is associated with negative physical, psychiatric, and social consequences. In addition, treatment costs continue to rise. How will this analysis of cost influence your future management of psoriasis?
We are all aware of the rising costs of medical care, especially for complex diseases such as psoriasis. The total cost of psoriasis in the United States is unknown. Brezinski et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.3593) sought to define the economic burden of psoriasis in the United States. They argued that this information is needed to provide the foundation for research, advocacy, and educational efforts within the disease.
The authors searched PubMed and MEDLINE databases for economic investigations on the costs of adult psoriasis in the United States. The primary objective of the analysis was to provide a comprehensive analysis of the literature on the economic burden of psoriasis in the United States. The direct, indirect, intangible, and comorbidity costs of psoriasis were reported based on this systematic literature review and adjusted to 2013 US dollars.
The direct costs included medical costs associated with (1) specialist medical evaluations, (2) hospitalization, (3) prescription medications, (4) phototherapy, (5) medication administration costs, (6) laboratory tests and monitoring studies, and (7) over-the-counter medications and self-care products. The indirect costs were determined by absenteeism and impaired work productivity. Intangible costs were calculated as a measure of the negative effect of psoriasis on quality of life. Finally, comorbidity costs measured the medical evaluations, treatment, and lab monitoring that were directly attributed to comorbid conditions associated with psoriasis.
An initial review of the literature generated 100 articles; 22 studies were included in the systematic review. The direct psoriasis costs ranged from $51.7 billion to $63.2 billion, the indirect costs ranged from $23.9 billion to $35.4 billion, and medical comorbidities were estimated to contribute $36.4 billion annually in 2013 US dollars. The annual cost of psoriasis in the United States amounted to approximately $112 billion in 2013.
The authors concluded that the economic burden of psoriasis was substantial and significant in the United States.
What’s the issue?
In the United States, the economic burden of psoriasis is substantial because this disease is associated with negative physical, psychiatric, and social consequences. In addition, treatment costs continue to rise. How will this analysis of cost influence your future management of psoriasis?
Tonsillectomy and Psoriasis
We are all aware that infections, particularly streptococcal infection, can be associated with psoriasis, especially the guttate variety. A logical question emanating from this fact is: Would tonsillectomy and adenoidectomy have any impact on psoriasis and its symptoms?
In a November 2014 article published online in the Journal of the American Academy of Dermatology, Rachakonda et al (doi:10.1016/j.jaad.2014.10.013) performed an extensive literature review to evaluate if tonsillectomy reduces psoriasis severity. The authors searched the following sources: MEDLINE, CINAHL, Cochrane, Embase, Web of Science, and Ovid databases (August 1, 1960, to September 12, 2013). In addition, they executed a manual search of selected references. Through this process, they identified observational studies and clinical trials examining psoriasis after tonsillectomy.
In the analysis, the authors included data from 20 articles from the 53 years they examined. From this literature, they included 545 patients with psoriasis who were either evaluated for or underwent tonsillectomy. Of 410 patients with psoriasis who actually underwent tonsillectomy, 290 experienced improvement in their psoriasis. Although some individuals who underwent tonsillectomy experienced sustained improvement in their disease, others experienced relapse following the procedure. The authors noted that their study was limited. Fifteen of 20 analyzed publications were case reports or series that lacked control groups. In addition, they noted that a publication bias that favored the reporting of improved cases needs to be considered.
Based on this comprehensive systematic review on the effect of tonsillectomy on psoriasis, the authors concluded that although tonsillectomy is effective in ameliorating psoriasis in a subpopulation of patients, there are insufficient data to describe the differences in clinical characteristics between responders versus nonresponders. Tonsillectomy may be a potential option for patients with recalcitrant psoriasis that is associated with occurrences of tonsillitis. Studies with long-term follow-up are needed to elucidate more clearly the extent and persistence of benefit of tonsillectomy in psoriasis.
What’s the issue?
Tonsillectomy represents an intriguing option not commonly considered for those with resistant disease. Based on the current data, will you discuss tonsillectomy with your patients?
We want to know your views! Tell us what you think.
Reader Comment
This concept so intrigued me when I heard Dr. Susan Katz discuss it at the NYU Advances in Medicine conference last June that I had the discussion with one of my patients, and she opted to have tonsillectomy this past fall. So far, she seems to improving, but I have not yet discontinued her long-term biologic therapy. At the same conference, Dr. Katz presented the idea that delaying antibiotic treatment of streptococcal pharyngitis by a few days might actually improve strep clearance rates by allowing the immune system to mount a greater response to the infection. Waiting for culture results before initiating antibiotic therapy might be prudent not only because it might reduce the unnecessary use of antibiotics in non-streptococcal pharyngitis, but because it might actually improve the long-term prognosis of patients (with or without psoriasis) who do have strep by reducing the odds of a chronic carrier state. Thanks for highlighting this area of study. Fascinating to consider that there might be a surgical cure for some psoriatic patients.
—Jennifer Goldwasser, MD (Scarsdale, New York)
We are all aware that infections, particularly streptococcal infection, can be associated with psoriasis, especially the guttate variety. A logical question emanating from this fact is: Would tonsillectomy and adenoidectomy have any impact on psoriasis and its symptoms?
In a November 2014 article published online in the Journal of the American Academy of Dermatology, Rachakonda et al (doi:10.1016/j.jaad.2014.10.013) performed an extensive literature review to evaluate if tonsillectomy reduces psoriasis severity. The authors searched the following sources: MEDLINE, CINAHL, Cochrane, Embase, Web of Science, and Ovid databases (August 1, 1960, to September 12, 2013). In addition, they executed a manual search of selected references. Through this process, they identified observational studies and clinical trials examining psoriasis after tonsillectomy.
In the analysis, the authors included data from 20 articles from the 53 years they examined. From this literature, they included 545 patients with psoriasis who were either evaluated for or underwent tonsillectomy. Of 410 patients with psoriasis who actually underwent tonsillectomy, 290 experienced improvement in their psoriasis. Although some individuals who underwent tonsillectomy experienced sustained improvement in their disease, others experienced relapse following the procedure. The authors noted that their study was limited. Fifteen of 20 analyzed publications were case reports or series that lacked control groups. In addition, they noted that a publication bias that favored the reporting of improved cases needs to be considered.
Based on this comprehensive systematic review on the effect of tonsillectomy on psoriasis, the authors concluded that although tonsillectomy is effective in ameliorating psoriasis in a subpopulation of patients, there are insufficient data to describe the differences in clinical characteristics between responders versus nonresponders. Tonsillectomy may be a potential option for patients with recalcitrant psoriasis that is associated with occurrences of tonsillitis. Studies with long-term follow-up are needed to elucidate more clearly the extent and persistence of benefit of tonsillectomy in psoriasis.
What’s the issue?
Tonsillectomy represents an intriguing option not commonly considered for those with resistant disease. Based on the current data, will you discuss tonsillectomy with your patients?
We want to know your views! Tell us what you think.
Reader Comment
This concept so intrigued me when I heard Dr. Susan Katz discuss it at the NYU Advances in Medicine conference last June that I had the discussion with one of my patients, and she opted to have tonsillectomy this past fall. So far, she seems to improving, but I have not yet discontinued her long-term biologic therapy. At the same conference, Dr. Katz presented the idea that delaying antibiotic treatment of streptococcal pharyngitis by a few days might actually improve strep clearance rates by allowing the immune system to mount a greater response to the infection. Waiting for culture results before initiating antibiotic therapy might be prudent not only because it might reduce the unnecessary use of antibiotics in non-streptococcal pharyngitis, but because it might actually improve the long-term prognosis of patients (with or without psoriasis) who do have strep by reducing the odds of a chronic carrier state. Thanks for highlighting this area of study. Fascinating to consider that there might be a surgical cure for some psoriatic patients.
—Jennifer Goldwasser, MD (Scarsdale, New York)
We are all aware that infections, particularly streptococcal infection, can be associated with psoriasis, especially the guttate variety. A logical question emanating from this fact is: Would tonsillectomy and adenoidectomy have any impact on psoriasis and its symptoms?
In a November 2014 article published online in the Journal of the American Academy of Dermatology, Rachakonda et al (doi:10.1016/j.jaad.2014.10.013) performed an extensive literature review to evaluate if tonsillectomy reduces psoriasis severity. The authors searched the following sources: MEDLINE, CINAHL, Cochrane, Embase, Web of Science, and Ovid databases (August 1, 1960, to September 12, 2013). In addition, they executed a manual search of selected references. Through this process, they identified observational studies and clinical trials examining psoriasis after tonsillectomy.
In the analysis, the authors included data from 20 articles from the 53 years they examined. From this literature, they included 545 patients with psoriasis who were either evaluated for or underwent tonsillectomy. Of 410 patients with psoriasis who actually underwent tonsillectomy, 290 experienced improvement in their psoriasis. Although some individuals who underwent tonsillectomy experienced sustained improvement in their disease, others experienced relapse following the procedure. The authors noted that their study was limited. Fifteen of 20 analyzed publications were case reports or series that lacked control groups. In addition, they noted that a publication bias that favored the reporting of improved cases needs to be considered.
Based on this comprehensive systematic review on the effect of tonsillectomy on psoriasis, the authors concluded that although tonsillectomy is effective in ameliorating psoriasis in a subpopulation of patients, there are insufficient data to describe the differences in clinical characteristics between responders versus nonresponders. Tonsillectomy may be a potential option for patients with recalcitrant psoriasis that is associated with occurrences of tonsillitis. Studies with long-term follow-up are needed to elucidate more clearly the extent and persistence of benefit of tonsillectomy in psoriasis.
What’s the issue?
Tonsillectomy represents an intriguing option not commonly considered for those with resistant disease. Based on the current data, will you discuss tonsillectomy with your patients?
We want to know your views! Tell us what you think.
Reader Comment
This concept so intrigued me when I heard Dr. Susan Katz discuss it at the NYU Advances in Medicine conference last June that I had the discussion with one of my patients, and she opted to have tonsillectomy this past fall. So far, she seems to improving, but I have not yet discontinued her long-term biologic therapy. At the same conference, Dr. Katz presented the idea that delaying antibiotic treatment of streptococcal pharyngitis by a few days might actually improve strep clearance rates by allowing the immune system to mount a greater response to the infection. Waiting for culture results before initiating antibiotic therapy might be prudent not only because it might reduce the unnecessary use of antibiotics in non-streptococcal pharyngitis, but because it might actually improve the long-term prognosis of patients (with or without psoriasis) who do have strep by reducing the odds of a chronic carrier state. Thanks for highlighting this area of study. Fascinating to consider that there might be a surgical cure for some psoriatic patients.
—Jennifer Goldwasser, MD (Scarsdale, New York)