User login
Pediatric Leg Ulcers: Going Out on a Limb for the Diagnosis
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
Compared to the adult population with a prevalence of lower extremity ulcers reaching approximately 1% to 2%, pediatric leg ulcers are much less common and require dermatologists to think outside the box for differential diagnoses.1 Although the most common types of lower extremity ulcers in the adult population include venous leg ulcers, arterial ulcers, and diabetic foot ulcers, the etiology for pediatric ulcers is vastly different, and thus these statistics cannot be extrapolated to this younger group. Additionally, scant research has been conducted to construct a systemic algorithm for helping these patients. In 1998, Dangoisse and Song2 concluded that juvenile leg ulcers secondary to causes other than trauma are uncommon, with the infectious origin fairly frequent; however, they stated further workup should be pursued to investigate for underlying vascular, metabolic, hematologic, and immunologic disorders. They also added that an infectious etiology must be ruled out with foremost priority, and a subsequent biopsy could assist in the ultimate diagnosis.2
To further investigate pediatric leg ulcers and their unique causes, a PubMed search of articles indexed for MEDLINE published from 1995 to present was performed using the term pediatric leg ulcers. The search yielded approximately 100 relevant articles. The search generated more than 47 different causes of leg ulcers and produced unusual etiologies such as trophic ulcers of Lesch-Nyhan syndrome, ulcers secondary to disabling pansclerotic morphea of childhood, dracunculiasis, and dengue hemorrhagic fever, among others.3-6 The articles were further divided into 4 categories to better characterize the causes—hematologic, infectious, genodermatoses, and autoimmune—which are reviewed here.
Hematologic Causes
Hematologic causes predominated in this juvenile arena, with sickle cell disease specifically comprising the vast majority of causes of pediatric leg ulcers.7,8 Sickle cell disease is a chronic disease with anemia and sickling crises contributing to a myriad of health problems. In a 13-year study following 44 patients with sickle cell disease, Silva et al8 found that leg ulcers affected approximately 5% of pediatric patients; however, the authors noted that this statistic may underestimate the accurate prevalence, as the ulcers typically affect older children and their study population was a younger distribution. The lesions manifest as painful, well-demarcated ulcers with surrounding hyperpigmentation mimicking venous ulcers.9 The ulcers may be readily diagnosed if the history is known, and it is critical to maximize care of these lesions, as they may heal at least 10 times slower than venous leg ulcers and frequently recur, with the vast majority recurring in less than 1 year. Furthermore, the presence of leg ulcers in sickle cell disease may be associated with increased hemolysis and pulmonary hypertension, demonstrating the severity of disease in these patients.10 Local wound care is the mainstay of therapy including compression, leg elevation, and adjuvant wound dressings. Systemic therapies such as hydroxyurea, zinc supplementation, pentoxifylline, and transfusion therapy may be pursued in refractory cases, though an ideal systemic regimen is still under exploration.9,10 Other major hematologic abnormalities leading to leg ulcers included additional causes of anemia, such as thalassemia and hereditary spherocytosis. These patients additionally were treated with local wound care to maximize healing.11,12
Infectious Causes
Infectious causes of pediatric ulcers were much more varied with a myriad of etiologies such as ulcers from ecthyma gangrenosum caused by Pseudomonas aeruginosa to leishmaniasis and tularemia. The most commonly reported infection causing leg ulcers in the pediatric literature was Mycobacterium ulcerans, which led to the characteristic Buruli ulcer; however, this infection is likely grossly overrepresented, as more common etiologies are underreported; the geographic location for a Buruli ulcer also is important, as cases are rare in the United States.13,14 A Buruli ulcer presents as a well-defined, painless, chronic skin ulceration and most commonly affects children.15 Exposure to stagnant water in tropical climates is thought to play a role in the pathogenesis of this slow-growing, acid-fast bacillus. The bacteria produces a potent cytotoxin called mycolactone, which then induces tissue necrosis and ulceration, leading to the clinical manifestations of disease.15 The ulcers may heal spontaneously; however, up to 15% can be associated with osteomyelitis; treatment includes surgical excision and prolonged antibiotics.14 Given the numerous additional causes of pediatric leg ulcers harboring infections, it is critical to be cognizant of the travel history and immune status of the patient. The infectious cause of leg ulcers likely predominates, making a biopsy with culture necessary in any nonhealing wound in this population prior to pursuing further workup.
Genodermatoses
A number of genodermatoses also contribute to persistent wounds in the pediatric population; specifically, genodermatoses that predispose to neuropathies and decreased pain sensation, which affect the child’s ability to detect sensation in the lower extremities, can result in inadvertent trauma leading to refractory wounds. For example, hereditary, sensory, and autonomic neuropathies are rare disorders causing progressive distal sensory loss, leading to ulcerations, osteomyelitis, arthritis, and even amputation.16 Hereditary, sensory, and autonomic neuropathies are further categorized into several different types; however, the unifying theme of diminished sensation is the culprit for troublesome wounds. Therapeutic endeavors to maximize preventative care with orthotics are vital in allaying recurrent wounds in these patients. Another uncommon hereditary disorder that promotes poor wound healing is caused by an inborn error of collagen synthesis. Prolidase deficiency is an autosomal-recessive condition resulting in characteristic facies, recurrent infections, and recalcitrant leg ulcerations due to impaired collagen formation.17 More than 50% of affected patients experience leg ulcers comprised of irregular borders with prominent granulation tissue. Treatment is aimed at restoring collagen synthesis and optimizing wound healing with the use of topical proline, glycine, and even growth hormone to promote repair.18 Additional genodermatoses predisposing to leg ulcerations include Lesch-Nyhan syndrome due to self-mutilating behaviors and epidermolysis bullosa due to impaired barrier and a decreased ability to repair cutaneous defects.
Autoimmune Causes
Although a much smaller category, ulcers due to autoimmune etiologies were reported in the literature. Fibrosing disorders including morphea and scleroderma can cause extensive disease in severe cases. Disabling pansclerotic morphea of childhood can cause sclerosis that extends into muscle, fascia, and even bone, resulting in contractures and ulcerations.4 The initial areas of involvement are the arms and legs, followed by spread to the trunk and head and neck area.4 Immunosuppressant therapy is needed to halt disease progression. Pediatric cases of systemic lupus erythematosus also have been associated with digital ulcers. One case was thought to be due to vasculitis,19 and another resulted from peripheral gangrene in association with Raynaud phenomenon.20 Albeit rare, it is important to consider autoimmune connective tissue diseases when faced with recurrent wounds and to search for additional symptoms that might yield the underlying diagnosis.
Conclusion
Pediatric leg ulcers are a relatively uncommon phenomenon; however, the etiologies are vastly different than adult leg ulcers and require careful contemplation surrounding the cardinal etiology. The main categories of disease in pediatric leg ulcers after trauma include hematologic abnormalities, infection, genodermatoses, and autoimmune diseases. The evaluation requires obtaining a thorough history and physical examination, including pertinent family histories for associated inheritable disorders. If the clinical picture remains elusive and the ulceration fails conservative management, a biopsy with tissue culture may be necessary to rule out an infectious etiology.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
- Morton LM, Phillips TJ. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589-605.
- Dangoisse C, Song M. Particular aspects of ulcers in children [in French]. Rev Med Brux. 1998;18:241-244.
- Kesiktas E, Gencel E, Acarturk S. Lesch-Nyhan syndrome: reconstruction of a calcaneal defect with a sural flap. Scand J Plast Reconstr Surg Hand Surg. 2006;40:117-119.
- Kura MM, Jindal SR. Disabling pansclerotic morphea of childhood with extracutaneous manifestations. Indian J Dermatol. 2013;58:159.
- Spring M, Spearman P. Dracunculiasis: report of an imported case in the United States. Clin Infect Dis. 1997;25:749-750.
- Vitug MR, Dayrit JF, Oblepias MS, et al. Group A streptococcal septic vasculitis in a child with dengue hemorrhagic fever. Int J Dermatol. 2006;45:1458-1461.
- Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western Nigeria. Niger J Clin Pract. 2015;18:95-101.
- Silva IV, Reis AF, Palaré MJ, et al. Sickle cell disease in children: chronic complications and search of predictive factors for adverse outcomes. Eur J Haematol. 2015;94:157-161.
- Trent JT, Kirsner RS. Leg ulcers in sickle cell disease. Adv Skin Wound Care. 2004;17:410-416.
- Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37:325-332.
- Matta B, Abbas O, Maakaron J, et al. Leg ulcers in patients with B-thalassemia intermedia: a single centre’s experience. J Eur Acad Dermatol Venereol. 2014;28:1245-1250.
- Giraldi S, Abbage KT, Marinoni LP, et al. Leg ulcer in hereditary spherocytosis. Pediatr Dermatol. 2003;20:427-428.
- Journeau P, Fitoussi F, Jehanno P, et al. Buruli’s ulcer: three cases diagnosed and treated in France. J Pediatr Orthop B. 2003;12: 229-232.
- Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445-1453.
- Buruli ulcer (Mycobacterium ulcerans infection). World Health Organization website. http://www.who.int/mediacentre/factsheets/fs199/en/. Updated February 2017. Accessed December 19, 2017.
- 16. Rao AG. Painless ulcers and fissures of toes: hereditary sensory neuropathy, not leprosy. Indian J Dermatol. 2016;61:121.
- Adışen E, Erduran FB, Ezqü FS, et al. A rare cause of lower extremity ulcers: prolidase deficiency. Int J Low Extrem Wounds. 2016;15:86-91.
- Trent JT, Kirsner RS. Leg ulcers secondary to prolidase deficiency. Adv Skin Wound Care. 2004;17:468-472.
- Olivieri AN, Mellos A, Duilio C, et al. Refractory vasculitis ulcer of the toe in adolescent suffering from systemic lupus erythematosus treated successfully with hyperbaric oxygen therapy. Ital J Pediatr. 2010;36:72.
- Ziaee V, Yeganeh MH, Moradinejad MH. Peripheral gangrene: a rare presentation of systemic lupus erythematosus in a child. Am J Case Rep. 2013;14:337-340.
Vesiculobullous and Pustular Diseases in Newborns
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1
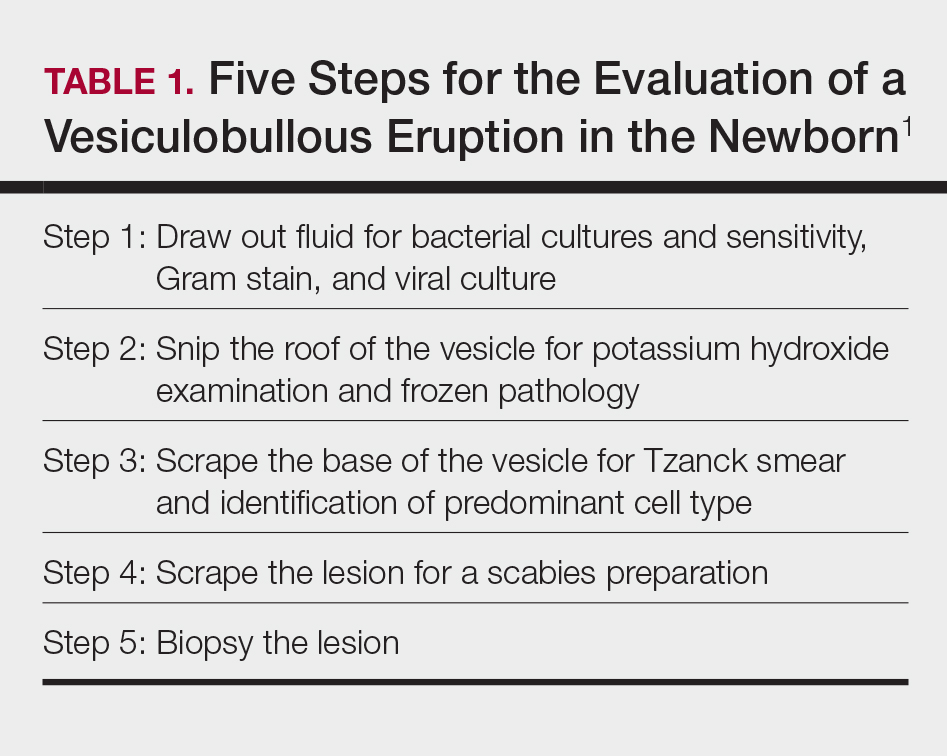
First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.
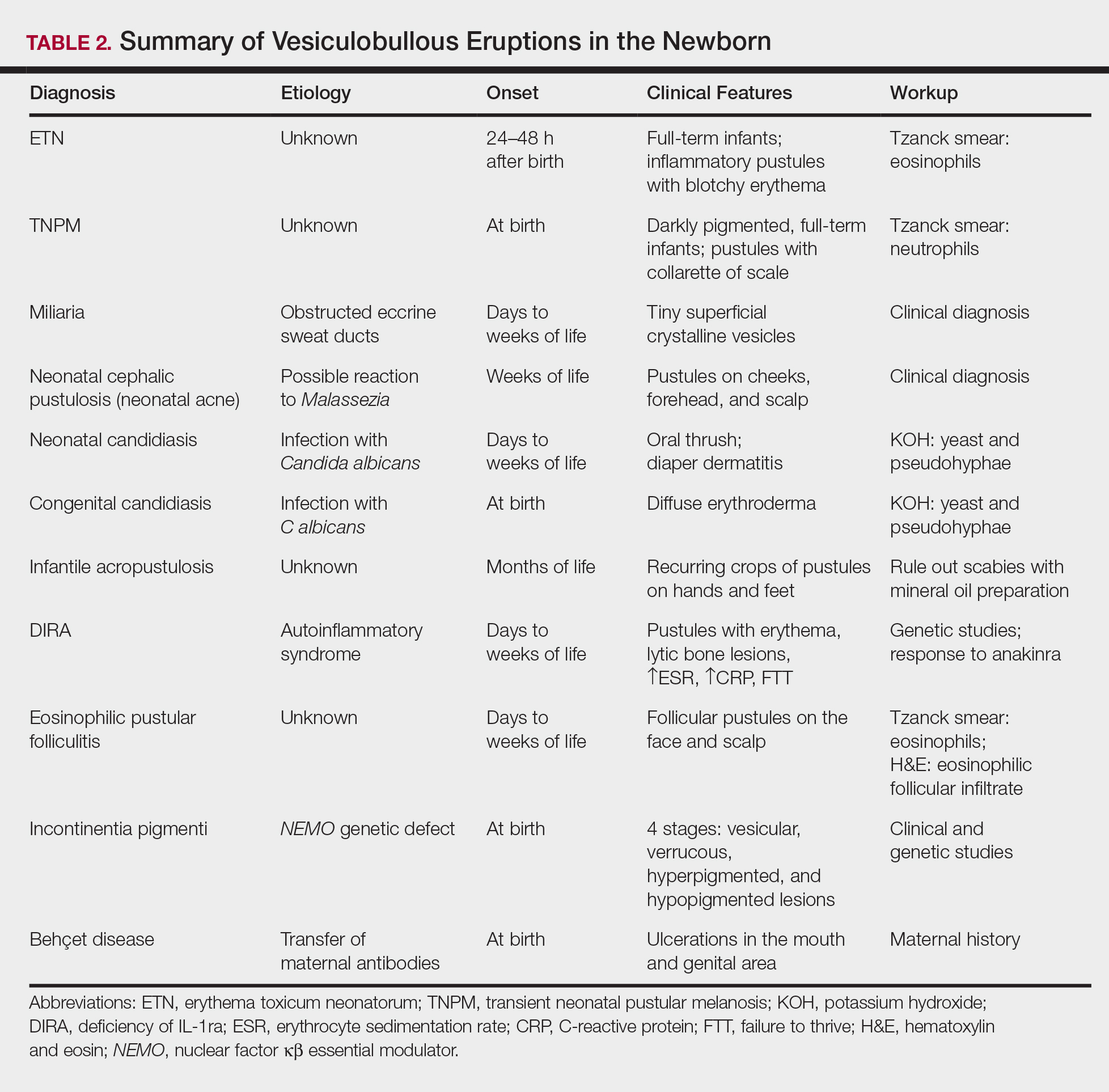
Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1

First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.

Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1

First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.

Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Pediatric Pearls From the AAD Annual Meeting
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
This article exhibits key pediatric dermatology pearls garnered at the 2017 Annual Meeting of the American Academy of Dermatology (AAD) in Orlando, Florida (March 3–7, 2017). Highlights from both the Society for Pediatric Dermatology pre-AAD meeting (March 2, 2017) and the AAD general meeting sessions are included. This discussion is intended to help maximize care of our pediatric patients in dermatology and present high-yield take-home points from the AAD that can be readily transferred to our patient care.
“New Tools for Your Therapeutic Toolbox” by Erin Mathes, MD (University of California, San Francisco)
During this lecture at the Society for Pediatric Dermatology meeting, Dr. Mathes discussed a randomized controlled trial that took place in 2014 in both the United States and the United Kingdom to assess skin barrier enhancement to reduce the incidence of atopic dermatitis (AD) in 124 high-risk infants.1 The high-risk infants had either a parent or sibling with physician-diagnosed AD, asthma, or rhinitis, or a first-degree relative with an aforementioned condition. Full-body emollient therapy was applied at least once daily within 3 weeks of birth for 6 months, while the control arm did not use emollient. Parents were allowed to choose from the following emollients: sunflower seed oil, moisturizing cream, or ointment. The primary outcome was the incidence of AD at 6 months. The authors found a 43% incidence of AD in the control group compared to 22% in the emollient group, amounting to a relative risk reduction of approximately 50%.1
Emollients in AD are hypothesized to help through the enhanced barrier function and decreased penetration of irritant substances and allergens. This study is vital given the ease of use of emollients and the foreseeable substantial impact on reduced health care costs associated with the decreased incidence of AD.
Take-Home Point
Full-body emollient therapy within 3 weeks of birth may reduce the incidence of AD in high-risk infants.
Dr. Mathes also discussed the novel topical phosphodiesterase 4 inhibitor crisaborole and its emerging role in AD. She reviewed the results of a large phase 3 trial of crisaborole therapy for patients aged 2 years or older with mild to moderate AD.2 Crisaborole ointment was applied twice daily for 28 days. The primary outcome measured was an investigator static global assessment score of clear or almost clear, which is a score for AD based on the degree of erythema, presence of oozing and crusting, and presence of induration or papulation. Overall, 32.8% of patients treated with crisaborole achieved success compared to 25.4% of vehicle-treated patients. The control patients were still given a vehicle to apply, which can function as therapy to help repair the barrier of AD and thus theoretically reduced the percentage gap between patients who met success with and without crisaborole therapy. Furthermore, only 4% of patients reported adverse effects such as burning and stinging with application of crisaborole in contrast to topical calcineurin inhibitors, which can elicit symptoms up to 50% of the time.2 In summary, this lecture reviewed the first new topical treatment for AD in 15 years.
Take-Home Point
Crisaborole ointment is a novel topical phosphodiesterase 4 inhibitor approved for mild to moderate AD in patients 2 years of age and older.
“The Truth About Pediatric Contact Dermatitis” by Sharon Jacob, MD (Loma Linda University, California)
In this session, Dr. Jacob discussed how she approaches pediatric patients with suspected contact dermatitis and elaborated on the common allergens unique to this patient population. Furthermore, she explained the substantial role of nickel in pediatric contact dermatitis, citing a study performed in Denmark and the United States, which tested 212 toys for nickel using the dimethylglyoxime test and found that 34.4% of toys did in fact release nickel.3 Additional studies have shown that nickel released from children’s toys is deposited on the skin, even with short contact times such as 30 minutes on one or more occasions within 2 weeks.3,4 She is currently evaluating the presence of nickel in locales frequented by children such as schools, libraries, and supermarkets. Interestingly, she anecdotally found that a pediatric eczematous eruption in a spiralized distribution of the legs can be attributed to the presence of nickel in school chairs, and the morphology is secondary to children wrapping their legs around the chairs. In conclusion, she reiterated that nickel continues to be the top allergen among pediatric patients, and states that additional allergens for patch testing in this population are unique to their adult counterparts.
Take-Home Point
Nickel is an ubiquitous allergen for pediatric contact dermatitis; additionally, the list of allergens for patch testing should be tailored to this patient population.
“When to Image, When to Sedate” by Annette Wagner, MD (Northwestern Medicine, Chicago, Illinois)
This lecture was a 3-part discussion on the safety of general anesthesia in children, when to image children, and when sedation may be worth the risk. Dr. Wagner shared her pearls for when children younger than 3 years may benefit from dermatologic procedures that involve general anesthesia. Large congenital lesions of the scalp or face that require tissue expansion or multiple stages may be best performed at a younger age due to the flexibility of the infant scalp, providing the best outcome. Additional considerations include a questionable malignant diagnosis in which a punch biopsy is not enough, rapidly growing facial lesions, Spitz nevi of the face, congenital lesions with no available therapy, and nonhealing refractory lesions causing severe pain. The general rule proposed was intervention for single procedures lasting less than 1 hour that otherwise would result in a worse outcome if postponed. Finally, she concluded to always advocate for your patient, to wait if the outcome will be the same regardless of timing, and to be frank about not knowing the risks of general anesthesia in this population. The resource, SmartTots (http://smarttots.org) provides current consensus statements and ongoing research on the use and safety of general anesthesia in children.
Take-Home Point
General sedation may be considered for short pediatric procedures that will result in a worse outcome if postponed.
“Highlights From the Pediatric Literature” by Katherine Marks, DO (Geisinger, Danville and Wilkes-Barre, Pennsylvania)
Dr. Marks discussed numerous emerging pediatric dermatology articles. One article looked at 40 infants with proliferating infantile hemangiomas (IHs) who had timolol gel 0.5% applied twice daily.5 The primary outcomes were the urinary excretion and serum levels of timolol as well as the clinical response to therapy measured by a visual analog scale at monthly visits. A urinalysis collected 3 to 4 hours after timolol application was found to be positive in 83% (20/24) of the tested patients; the first 3 positive infants were then sent to have their serum timolol levels drawn and also were found to be positive, though substantially small levels (median, 0.16 ng/mL). The 3 patients tested had small IHs on the face with no ulceration. None of these patients experienced adverse effects and all of the IHs significantly (P<.001) improved with therapy. The authors stated that even though the absorption was minimal, it is wise to be cognizant about the use of timolol in certain patient demographics such as preterm or young infants with large ulcerating IHs.5
Take-Home Point
Systemic absorption with topical timolol occurs, albeit substantially small; be judicious about giving this medication in select patient populations with ulcerated hemangiomas.
Acknowledgment
The author thanks the presenters for their review and contributions to this article.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Jensen P, Hamann D, Hamann CR, et al. Nickel and cobalt release from children’s toys purchased in Denmark and the United States. Dermatitis. 2014;25:356-365.
- Overgaard LE, Engebretsen KA, Jensen P, et al. Nickel released from children’s toys is deposited on the skin. Contact Dermatitis. 2016;74:380-381.
- Weibel L, Barysch MJ, Scheer HS, et al. Topical timolol for infantile hemangiomas: evidence for efficacy and degree of systemic absorption [published online February 3, 2016]. Pediatr Dermatol. 2016;33:184-190.
Five Steps for Delivering an Effective and Educational Lecture
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
Expanding Uses of Propranolol in Dermatology
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Pediatric Nail Diseases: Clinical Pearls
Our dermatology department recently sponsored a pediatric dermatology lecture series for the pediatric residency program. Within this series, Antonella Tosti, MD, a professor at the University of Miami Health System, Florida, and a renowned expert in nail disorders and allergic contact dermatitis, presented her clinical expertise on the presentation and management of common pediatric nail diseases. This article highlights pearls from her unique and enlightening lecture.
Pearl: Hand-foot-and-mouth disease is a recognized trigger for onychomadesis
An arrest in nail matrix activity is responsible for onychomadesis, or shedding of the nail. Its presentation in children can be further divided based upon the degree of involvement. If a few nails are affected, trauma should be implicated. In contrast, if all nails are involved, a systemic etiology should be suspected. Hand-foot-and-mouth disease (HFMD) has been recognized as a trigger for onychomadesis in school-aged children. Onychomadesis presents with characteristic proximal nail detachment (Figure 1). The association of HFMD with onychomadesis and Beau lines was first reported in 2000. Five patients who resided within close proximity and shared a physician-diagnosed case of HFMD presented with representative nail findings 4 weeks after illness.1 Hypotheses for these changes include viral-induced nail pathology, inflammation from cutaneous lesions of HFMD, and systemic effects from the disease.2 Given the prevalence of HFMD and benign outcome, clinicians should be cognizant of this unique cutaneous manifestation.
Pearl: Management of pediatric melanonychia can take a wait-and-see approach
Melanonychia is the presence of a longitudinal brown-black band extending from the proximal nail fold. The cause of melanonychia can be due to either activation or hyperplasia. Activation is the less common etiology in children; however, if present, activation can be due to Laugier-Hunziker syndrome or trauma such as onychotillomania. Melanonychia in children usually is the result of hyperplasia of melanocytes and can manifest as a lentigo, nevus, or more rarely melanoma. Nail matrix nevi are typically exhibited on the fingernails, particularly the thumb, and frequently are junctional nevi (Figure 2). Spontaneous fading of nevi is expected with time due to decreased melanin production. Therapeutic options for melanonychia include regular clinical monitoring, biopsy, or excision. Dr. Tosti explained that one must be wary when pursuing a biopsy, as it can result in a false-negative finding due to missed pathology. If clinically indicated, a shave biopsy of the nail matrix can be performed to best analyze the lesion. She noted that if more than 3 mm of the matrix is removed, a resultant scar will ensue. Conservative management is recommended given the indolent clinical behavior of the majority of cases of melanonychia in children.3
Pearl: Congenital hypertrophy of the lateral nail folds can be treated with tape
Congenital hypertrophy of the lateral nail folds is relatively common in children and normally improves with age. Koilonychia may also occur simultaneously and can be viewed as a physiologic process in this age group. The etiology of the underlying disorder is due to anomalous periungual soft-tissue changes of the bilateral halluces; the resulting overgrowth can partially cover the nail plate. Although usually a self-limiting condition, the changes can cause inflammation and discomfort due to an ingrown nail.4 Dr. Tosti advised that by simply taping and retracting the bilateral overgrowth, the condition can be more readily resolved. This simple treatment can be demonstrated in the office and subsequently performed at home.
Pearl: Onychomycosis is uncommon in children
Onychomycosis occurs in less than 1% of children.5 Several factors are responsible for this decreased prevalence. More rapid nail growth and smaller nail surface area decreases the ability of the fungi to penetrate the nail plate.6 Furthermore, children have a diminished rate of tinea pedis, leading to less neighboring infection. When onychomycosis does affect this patient population, it commonly presents as distal subungual onychomycosis and favors the fingernails over the toenails. Treatment options usually parallel those of the adult population; however, all medications for children are considered off-label use by the US Food and Drug Administration. Dr. Tosti explained that oral granules of terbinafine can be sprinkled on food to help with pediatric ingestion. Topical therapies should also be considered; children usually respond better than their adult counterparts due to their thinner nails, which grant enhanced drug delivery and penetration.6
Pearl: Acute paronychia can be due to nail-biting and sucking
Acute paronychia is inflammation of the proximal nail fold. In children, it frequently is a result of mixed flora induced by nail-biting and sucking. Management involves culturing the affected lesions and is effectively treated with warm soaks alone. Dr. Tosti highlighted that Candida in the subungual space is a common colonizer and is typically self-limiting in nature if isolated. Candida can be cultured more readily in premature infants, immunosuppressed patients, and those with chronic mucocutaneous candidiasis. Patients with chronic mucocutaneous candidiasis can exhibit periungual inflammation involving several digits. The differential can include nail psoriasis, as both can demonstrate dystrophic changes. The differential for localized paronychia includes herpetic whitlow and can manifest as vesicles under the proximal nail fold.
Final Thoughts
These clinical pearls are shared to help deliver utmost care to our pediatric patients presenting with nail pathology. For example, a child exhibiting melanonychia can cause alarm due to the possibility of underlying melanoma; given the rarity of neoplasia in these patients, a conservative approach is favored to help avoid unnecessary biopsies and subsequent scarring. Similarly, it is important to be aware of the common colonizers of the subungual area, particularly Candida, to avoid unessential medications with potential side effects. The examples demonstrated help shed light on the management of pediatric nail diseases.
Acknowledgment
This article is possible thanks to the help of Antonella Tosti, MD (Miami, Florida), who contributed her time and expertise at the University of Miami Pediatric Grand Rounds to expand the foundation and knowledge of pediatric nail diseases.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Yuksel S, Evrengul H, Ozhan B, et al. Onychomadesis-a late complication of hand-foot-mouth disease [published online May 2, 2016]. J Pediatr. 2016;174:274.
- Cooper C, Arva NC, Lee C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72:773-779.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Totri CR, Feldstein S, Admani S, et al. Epidemiologic analysis of onychomycosis in the San Diego pediatric population [published online October 4, 2016]. Pediatr Dermatol. 2017;34:46-49.
- Feldstein S, Totri C, Friedlander SF. Antifungal therapy for onychomycosis in children. Clin Dermatol. 2015;33:333-339.
Our dermatology department recently sponsored a pediatric dermatology lecture series for the pediatric residency program. Within this series, Antonella Tosti, MD, a professor at the University of Miami Health System, Florida, and a renowned expert in nail disorders and allergic contact dermatitis, presented her clinical expertise on the presentation and management of common pediatric nail diseases. This article highlights pearls from her unique and enlightening lecture.
Pearl: Hand-foot-and-mouth disease is a recognized trigger for onychomadesis
An arrest in nail matrix activity is responsible for onychomadesis, or shedding of the nail. Its presentation in children can be further divided based upon the degree of involvement. If a few nails are affected, trauma should be implicated. In contrast, if all nails are involved, a systemic etiology should be suspected. Hand-foot-and-mouth disease (HFMD) has been recognized as a trigger for onychomadesis in school-aged children. Onychomadesis presents with characteristic proximal nail detachment (Figure 1). The association of HFMD with onychomadesis and Beau lines was first reported in 2000. Five patients who resided within close proximity and shared a physician-diagnosed case of HFMD presented with representative nail findings 4 weeks after illness.1 Hypotheses for these changes include viral-induced nail pathology, inflammation from cutaneous lesions of HFMD, and systemic effects from the disease.2 Given the prevalence of HFMD and benign outcome, clinicians should be cognizant of this unique cutaneous manifestation.

Pearl: Management of pediatric melanonychia can take a wait-and-see approach
Melanonychia is the presence of a longitudinal brown-black band extending from the proximal nail fold. The cause of melanonychia can be due to either activation or hyperplasia. Activation is the less common etiology in children; however, if present, activation can be due to Laugier-Hunziker syndrome or trauma such as onychotillomania. Melanonychia in children usually is the result of hyperplasia of melanocytes and can manifest as a lentigo, nevus, or more rarely melanoma. Nail matrix nevi are typically exhibited on the fingernails, particularly the thumb, and frequently are junctional nevi (Figure 2). Spontaneous fading of nevi is expected with time due to decreased melanin production. Therapeutic options for melanonychia include regular clinical monitoring, biopsy, or excision. Dr. Tosti explained that one must be wary when pursuing a biopsy, as it can result in a false-negative finding due to missed pathology. If clinically indicated, a shave biopsy of the nail matrix can be performed to best analyze the lesion. She noted that if more than 3 mm of the matrix is removed, a resultant scar will ensue. Conservative management is recommended given the indolent clinical behavior of the majority of cases of melanonychia in children.3

Pearl: Congenital hypertrophy of the lateral nail folds can be treated with tape
Congenital hypertrophy of the lateral nail folds is relatively common in children and normally improves with age. Koilonychia may also occur simultaneously and can be viewed as a physiologic process in this age group. The etiology of the underlying disorder is due to anomalous periungual soft-tissue changes of the bilateral halluces; the resulting overgrowth can partially cover the nail plate. Although usually a self-limiting condition, the changes can cause inflammation and discomfort due to an ingrown nail.4 Dr. Tosti advised that by simply taping and retracting the bilateral overgrowth, the condition can be more readily resolved. This simple treatment can be demonstrated in the office and subsequently performed at home.
Pearl: Onychomycosis is uncommon in children
Onychomycosis occurs in less than 1% of children.5 Several factors are responsible for this decreased prevalence. More rapid nail growth and smaller nail surface area decreases the ability of the fungi to penetrate the nail plate.6 Furthermore, children have a diminished rate of tinea pedis, leading to less neighboring infection. When onychomycosis does affect this patient population, it commonly presents as distal subungual onychomycosis and favors the fingernails over the toenails. Treatment options usually parallel those of the adult population; however, all medications for children are considered off-label use by the US Food and Drug Administration. Dr. Tosti explained that oral granules of terbinafine can be sprinkled on food to help with pediatric ingestion. Topical therapies should also be considered; children usually respond better than their adult counterparts due to their thinner nails, which grant enhanced drug delivery and penetration.6
Pearl: Acute paronychia can be due to nail-biting and sucking
Acute paronychia is inflammation of the proximal nail fold. In children, it frequently is a result of mixed flora induced by nail-biting and sucking. Management involves culturing the affected lesions and is effectively treated with warm soaks alone. Dr. Tosti highlighted that Candida in the subungual space is a common colonizer and is typically self-limiting in nature if isolated. Candida can be cultured more readily in premature infants, immunosuppressed patients, and those with chronic mucocutaneous candidiasis. Patients with chronic mucocutaneous candidiasis can exhibit periungual inflammation involving several digits. The differential can include nail psoriasis, as both can demonstrate dystrophic changes. The differential for localized paronychia includes herpetic whitlow and can manifest as vesicles under the proximal nail fold.
Final Thoughts
These clinical pearls are shared to help deliver utmost care to our pediatric patients presenting with nail pathology. For example, a child exhibiting melanonychia can cause alarm due to the possibility of underlying melanoma; given the rarity of neoplasia in these patients, a conservative approach is favored to help avoid unnecessary biopsies and subsequent scarring. Similarly, it is important to be aware of the common colonizers of the subungual area, particularly Candida, to avoid unessential medications with potential side effects. The examples demonstrated help shed light on the management of pediatric nail diseases.
Acknowledgment
This article is possible thanks to the help of Antonella Tosti, MD (Miami, Florida), who contributed her time and expertise at the University of Miami Pediatric Grand Rounds to expand the foundation and knowledge of pediatric nail diseases.
Our dermatology department recently sponsored a pediatric dermatology lecture series for the pediatric residency program. Within this series, Antonella Tosti, MD, a professor at the University of Miami Health System, Florida, and a renowned expert in nail disorders and allergic contact dermatitis, presented her clinical expertise on the presentation and management of common pediatric nail diseases. This article highlights pearls from her unique and enlightening lecture.
Pearl: Hand-foot-and-mouth disease is a recognized trigger for onychomadesis
An arrest in nail matrix activity is responsible for onychomadesis, or shedding of the nail. Its presentation in children can be further divided based upon the degree of involvement. If a few nails are affected, trauma should be implicated. In contrast, if all nails are involved, a systemic etiology should be suspected. Hand-foot-and-mouth disease (HFMD) has been recognized as a trigger for onychomadesis in school-aged children. Onychomadesis presents with characteristic proximal nail detachment (Figure 1). The association of HFMD with onychomadesis and Beau lines was first reported in 2000. Five patients who resided within close proximity and shared a physician-diagnosed case of HFMD presented with representative nail findings 4 weeks after illness.1 Hypotheses for these changes include viral-induced nail pathology, inflammation from cutaneous lesions of HFMD, and systemic effects from the disease.2 Given the prevalence of HFMD and benign outcome, clinicians should be cognizant of this unique cutaneous manifestation.

Pearl: Management of pediatric melanonychia can take a wait-and-see approach
Melanonychia is the presence of a longitudinal brown-black band extending from the proximal nail fold. The cause of melanonychia can be due to either activation or hyperplasia. Activation is the less common etiology in children; however, if present, activation can be due to Laugier-Hunziker syndrome or trauma such as onychotillomania. Melanonychia in children usually is the result of hyperplasia of melanocytes and can manifest as a lentigo, nevus, or more rarely melanoma. Nail matrix nevi are typically exhibited on the fingernails, particularly the thumb, and frequently are junctional nevi (Figure 2). Spontaneous fading of nevi is expected with time due to decreased melanin production. Therapeutic options for melanonychia include regular clinical monitoring, biopsy, or excision. Dr. Tosti explained that one must be wary when pursuing a biopsy, as it can result in a false-negative finding due to missed pathology. If clinically indicated, a shave biopsy of the nail matrix can be performed to best analyze the lesion. She noted that if more than 3 mm of the matrix is removed, a resultant scar will ensue. Conservative management is recommended given the indolent clinical behavior of the majority of cases of melanonychia in children.3

Pearl: Congenital hypertrophy of the lateral nail folds can be treated with tape
Congenital hypertrophy of the lateral nail folds is relatively common in children and normally improves with age. Koilonychia may also occur simultaneously and can be viewed as a physiologic process in this age group. The etiology of the underlying disorder is due to anomalous periungual soft-tissue changes of the bilateral halluces; the resulting overgrowth can partially cover the nail plate. Although usually a self-limiting condition, the changes can cause inflammation and discomfort due to an ingrown nail.4 Dr. Tosti advised that by simply taping and retracting the bilateral overgrowth, the condition can be more readily resolved. This simple treatment can be demonstrated in the office and subsequently performed at home.
Pearl: Onychomycosis is uncommon in children
Onychomycosis occurs in less than 1% of children.5 Several factors are responsible for this decreased prevalence. More rapid nail growth and smaller nail surface area decreases the ability of the fungi to penetrate the nail plate.6 Furthermore, children have a diminished rate of tinea pedis, leading to less neighboring infection. When onychomycosis does affect this patient population, it commonly presents as distal subungual onychomycosis and favors the fingernails over the toenails. Treatment options usually parallel those of the adult population; however, all medications for children are considered off-label use by the US Food and Drug Administration. Dr. Tosti explained that oral granules of terbinafine can be sprinkled on food to help with pediatric ingestion. Topical therapies should also be considered; children usually respond better than their adult counterparts due to their thinner nails, which grant enhanced drug delivery and penetration.6
Pearl: Acute paronychia can be due to nail-biting and sucking
Acute paronychia is inflammation of the proximal nail fold. In children, it frequently is a result of mixed flora induced by nail-biting and sucking. Management involves culturing the affected lesions and is effectively treated with warm soaks alone. Dr. Tosti highlighted that Candida in the subungual space is a common colonizer and is typically self-limiting in nature if isolated. Candida can be cultured more readily in premature infants, immunosuppressed patients, and those with chronic mucocutaneous candidiasis. Patients with chronic mucocutaneous candidiasis can exhibit periungual inflammation involving several digits. The differential can include nail psoriasis, as both can demonstrate dystrophic changes. The differential for localized paronychia includes herpetic whitlow and can manifest as vesicles under the proximal nail fold.
Final Thoughts
These clinical pearls are shared to help deliver utmost care to our pediatric patients presenting with nail pathology. For example, a child exhibiting melanonychia can cause alarm due to the possibility of underlying melanoma; given the rarity of neoplasia in these patients, a conservative approach is favored to help avoid unnecessary biopsies and subsequent scarring. Similarly, it is important to be aware of the common colonizers of the subungual area, particularly Candida, to avoid unessential medications with potential side effects. The examples demonstrated help shed light on the management of pediatric nail diseases.
Acknowledgment
This article is possible thanks to the help of Antonella Tosti, MD (Miami, Florida), who contributed her time and expertise at the University of Miami Pediatric Grand Rounds to expand the foundation and knowledge of pediatric nail diseases.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Yuksel S, Evrengul H, Ozhan B, et al. Onychomadesis-a late complication of hand-foot-mouth disease [published online May 2, 2016]. J Pediatr. 2016;174:274.
- Cooper C, Arva NC, Lee C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72:773-779.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Totri CR, Feldstein S, Admani S, et al. Epidemiologic analysis of onychomycosis in the San Diego pediatric population [published online October 4, 2016]. Pediatr Dermatol. 2017;34:46-49.
- Feldstein S, Totri C, Friedlander SF. Antifungal therapy for onychomycosis in children. Clin Dermatol. 2015;33:333-339.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Yuksel S, Evrengul H, Ozhan B, et al. Onychomadesis-a late complication of hand-foot-mouth disease [published online May 2, 2016]. J Pediatr. 2016;174:274.
- Cooper C, Arva NC, Lee C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72:773-779.
- Piraccini BM, Parente GL, Varotti E, et al. Congenital hypertrophy of the lateral nail folds of the hallux: clinical features and follow-up of seven cases. Pediatr Dermatol. 2000;17:348-351.
- Totri CR, Feldstein S, Admani S, et al. Epidemiologic analysis of onychomycosis in the San Diego pediatric population [published online October 4, 2016]. Pediatr Dermatol. 2017;34:46-49.
- Feldstein S, Totri C, Friedlander SF. Antifungal therapy for onychomycosis in children. Clin Dermatol. 2015;33:333-339.
