User login
Drug-resistant malaria parasite may spread to Africa
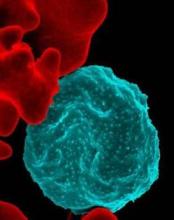
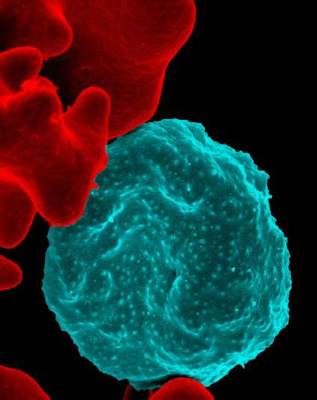
Forms of Plasmodium falciparum parasites successfully infected various species of mosquitoes, including the major African vector for malaria, a study revealed.
The parasites, which are resistant to the antimalarial drug artemisinin, have rapidly spread in Cambodia and other Greater Mekong Subregion countries.
In patients and in vitro, the researchers produced gametocytes from clinical isolates of Plasmodium falciparum, the deadliest species of malaria parasites. The researchers then infected Anopheles mosquito vectors from Southeast Asia and Africa with the gametocytes.
The parasite isolates developed and produced sporozoites in the Southeast Asian vectors, An. dirus and An. minimus, and the major African vector, An. coluzzii.
“Our finding that highly differentiated ART-resistant parasites infect highly diverse Anopheles species unveils a significant unanticipated challenge to the elimination of malaria in Southeast Asia and the prevention of [artemisinin-resistant] malaria in Africa,” said Dr. Brandyce St. Laurent of the National Institute of Allergy and Infectious Diseases and her coauthors.
“The researchers plan to investigate other potential genetic determinants of parasite infection of mosquitoes and further examine which Anopheles species from Cambodia are naturally transmitting artemisinin-resistant parasites in the wild,” according to a written statement from the NIH.
The authors declared no competing financial interests.
Read the full study in Nature Communications (2015;6:8614).
Forms of Plasmodium falciparum parasites successfully infected various species of mosquitoes, including the major African vector for malaria, a study revealed.
The parasites, which are resistant to the antimalarial drug artemisinin, have rapidly spread in Cambodia and other Greater Mekong Subregion countries.
In patients and in vitro, the researchers produced gametocytes from clinical isolates of Plasmodium falciparum, the deadliest species of malaria parasites. The researchers then infected Anopheles mosquito vectors from Southeast Asia and Africa with the gametocytes.
The parasite isolates developed and produced sporozoites in the Southeast Asian vectors, An. dirus and An. minimus, and the major African vector, An. coluzzii.
“Our finding that highly differentiated ART-resistant parasites infect highly diverse Anopheles species unveils a significant unanticipated challenge to the elimination of malaria in Southeast Asia and the prevention of [artemisinin-resistant] malaria in Africa,” said Dr. Brandyce St. Laurent of the National Institute of Allergy and Infectious Diseases and her coauthors.
“The researchers plan to investigate other potential genetic determinants of parasite infection of mosquitoes and further examine which Anopheles species from Cambodia are naturally transmitting artemisinin-resistant parasites in the wild,” according to a written statement from the NIH.
The authors declared no competing financial interests.
Read the full study in Nature Communications (2015;6:8614).
Forms of Plasmodium falciparum parasites successfully infected various species of mosquitoes, including the major African vector for malaria, a study revealed.
The parasites, which are resistant to the antimalarial drug artemisinin, have rapidly spread in Cambodia and other Greater Mekong Subregion countries.
In patients and in vitro, the researchers produced gametocytes from clinical isolates of Plasmodium falciparum, the deadliest species of malaria parasites. The researchers then infected Anopheles mosquito vectors from Southeast Asia and Africa with the gametocytes.
The parasite isolates developed and produced sporozoites in the Southeast Asian vectors, An. dirus and An. minimus, and the major African vector, An. coluzzii.
“Our finding that highly differentiated ART-resistant parasites infect highly diverse Anopheles species unveils a significant unanticipated challenge to the elimination of malaria in Southeast Asia and the prevention of [artemisinin-resistant] malaria in Africa,” said Dr. Brandyce St. Laurent of the National Institute of Allergy and Infectious Diseases and her coauthors.
“The researchers plan to investigate other potential genetic determinants of parasite infection of mosquitoes and further examine which Anopheles species from Cambodia are naturally transmitting artemisinin-resistant parasites in the wild,” according to a written statement from the NIH.
The authors declared no competing financial interests.
Read the full study in Nature Communications (2015;6:8614).
FROM NATURE COMMUNICATIONS
Residents, supervising physicians differ on when to communicate
Discordance exists between supervising physicians and residents regarding which scenarios require supervising physicians’ input, according to a study.
The study was based on responses to an anonymous online survey of pediatric residents, internal medicine/pediatric residents, and fellows and attending physicians of hospital medicine service, hematology/oncology, gastroenterology, and pulmonology at MassGeneral Hospital for Children (MGHfC) in Boston. Respondents included 62 of 67 eligible residents and 50 of 56 eligible supervising physicians, which included both the attendings and fellows. The survey presented 34 scenarios encountered by residents doing after-hours coverage on a general pediatric floor. The survey asked whether after-hours residents should contact supervising physicians immediately or delay communication to the next day, for each scenario.
“Responses were statistically significantly different between residents and supervising physicians in 17 of the 34 scenarios (50%). In all 17 of these scenarios without exception, more supervising physicians wanted immediate communication, compared with the residents,” wrote Dr. Deepak Palakshappa, chief pediatric resident at the time of the study and currently an instructor at Children’s Hospital of Philadelphia, and his colleagues.
The 17 scenarios under which discordance of opinions existed between the two groups fell under the categories of clinical, laboratory/radiology, and logistical/social. The clinical scenarios under which the greatest discrepancies between the two groups occurred were a new fever in an otherwise well patient (odds ratio, 11.49; P less than .001) and an increasing oxygen requirement in a patient already on oxygen (OR, 6.27; P less than .001).
The laboratory/radiology scenario with the largest discrepancy on whether a supervising physician should be immediately contacted was a new radiographic finding (OR, 7.72; P less than .001). One of the logistical/social scenarios over which members of the two groups most often disagreed on the timing of notifying a supervising physician was in the case of an angry parent or family member (OR, 15.67; P less than .001).
Among the study’s other findings was that 32% of all residents knew that the hospital had communication guidelines for addressing various work scenarios, at the time the researchers were conducting the study.
“Future studies should evaluate whether and how these communication preferences actually translate into changes in actual behaviors and subsequent patient care outcomes,” the researchers said.
Read the study in the Journal of Pediatrics (doi: 10.1016/j.peds.2015.08.052).
Discordance exists between supervising physicians and residents regarding which scenarios require supervising physicians’ input, according to a study.
The study was based on responses to an anonymous online survey of pediatric residents, internal medicine/pediatric residents, and fellows and attending physicians of hospital medicine service, hematology/oncology, gastroenterology, and pulmonology at MassGeneral Hospital for Children (MGHfC) in Boston. Respondents included 62 of 67 eligible residents and 50 of 56 eligible supervising physicians, which included both the attendings and fellows. The survey presented 34 scenarios encountered by residents doing after-hours coverage on a general pediatric floor. The survey asked whether after-hours residents should contact supervising physicians immediately or delay communication to the next day, for each scenario.
“Responses were statistically significantly different between residents and supervising physicians in 17 of the 34 scenarios (50%). In all 17 of these scenarios without exception, more supervising physicians wanted immediate communication, compared with the residents,” wrote Dr. Deepak Palakshappa, chief pediatric resident at the time of the study and currently an instructor at Children’s Hospital of Philadelphia, and his colleagues.
The 17 scenarios under which discordance of opinions existed between the two groups fell under the categories of clinical, laboratory/radiology, and logistical/social. The clinical scenarios under which the greatest discrepancies between the two groups occurred were a new fever in an otherwise well patient (odds ratio, 11.49; P less than .001) and an increasing oxygen requirement in a patient already on oxygen (OR, 6.27; P less than .001).
The laboratory/radiology scenario with the largest discrepancy on whether a supervising physician should be immediately contacted was a new radiographic finding (OR, 7.72; P less than .001). One of the logistical/social scenarios over which members of the two groups most often disagreed on the timing of notifying a supervising physician was in the case of an angry parent or family member (OR, 15.67; P less than .001).
Among the study’s other findings was that 32% of all residents knew that the hospital had communication guidelines for addressing various work scenarios, at the time the researchers were conducting the study.
“Future studies should evaluate whether and how these communication preferences actually translate into changes in actual behaviors and subsequent patient care outcomes,” the researchers said.
Read the study in the Journal of Pediatrics (doi: 10.1016/j.peds.2015.08.052).
Discordance exists between supervising physicians and residents regarding which scenarios require supervising physicians’ input, according to a study.
The study was based on responses to an anonymous online survey of pediatric residents, internal medicine/pediatric residents, and fellows and attending physicians of hospital medicine service, hematology/oncology, gastroenterology, and pulmonology at MassGeneral Hospital for Children (MGHfC) in Boston. Respondents included 62 of 67 eligible residents and 50 of 56 eligible supervising physicians, which included both the attendings and fellows. The survey presented 34 scenarios encountered by residents doing after-hours coverage on a general pediatric floor. The survey asked whether after-hours residents should contact supervising physicians immediately or delay communication to the next day, for each scenario.
“Responses were statistically significantly different between residents and supervising physicians in 17 of the 34 scenarios (50%). In all 17 of these scenarios without exception, more supervising physicians wanted immediate communication, compared with the residents,” wrote Dr. Deepak Palakshappa, chief pediatric resident at the time of the study and currently an instructor at Children’s Hospital of Philadelphia, and his colleagues.
The 17 scenarios under which discordance of opinions existed between the two groups fell under the categories of clinical, laboratory/radiology, and logistical/social. The clinical scenarios under which the greatest discrepancies between the two groups occurred were a new fever in an otherwise well patient (odds ratio, 11.49; P less than .001) and an increasing oxygen requirement in a patient already on oxygen (OR, 6.27; P less than .001).
The laboratory/radiology scenario with the largest discrepancy on whether a supervising physician should be immediately contacted was a new radiographic finding (OR, 7.72; P less than .001). One of the logistical/social scenarios over which members of the two groups most often disagreed on the timing of notifying a supervising physician was in the case of an angry parent or family member (OR, 15.67; P less than .001).
Among the study’s other findings was that 32% of all residents knew that the hospital had communication guidelines for addressing various work scenarios, at the time the researchers were conducting the study.
“Future studies should evaluate whether and how these communication preferences actually translate into changes in actual behaviors and subsequent patient care outcomes,” the researchers said.
Read the study in the Journal of Pediatrics (doi: 10.1016/j.peds.2015.08.052).
FROM THE JOURNAL OF PEDIATRICS
FDA approves new treatment for Factor X deficiency
The U.S. Food and Drug Administration has provided its first approval of a coagulation factor replacement drug specifically for patients with hereditary Factor X deficiency who are aged 12 years or older, the FDA said in a written statement. Typically, patients with this inherited disorder are treated with plasma-derived prothrombin complex concentrates.
Coagadex, the new coagulation factor replacement drug, was effective at controlling bleeding episodes in individuals with moderate to severe hereditary Factor X deficiency, in a 16-patient study. Prior to being treated with the purified Factor X concentrate, the study’s participants had the following types of bleeding episodes: spontaneous; traumatic; or heavy menstrual.
Researchers also conducted a smaller study of the drug’s effectiveness on patients who were undergoing surgery. In this research project, Coagadex was successful at controlling blood loss during and after surgery, in those patients with mild hereditary Factor X deficiency.
“The approval of Coagadex is a significant advancement for patients who suffer from this rare but serious disease,” said Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, in the release.
Bio Products Laboratory manufactured the new drug.
The U.S. Food and Drug Administration has provided its first approval of a coagulation factor replacement drug specifically for patients with hereditary Factor X deficiency who are aged 12 years or older, the FDA said in a written statement. Typically, patients with this inherited disorder are treated with plasma-derived prothrombin complex concentrates.
Coagadex, the new coagulation factor replacement drug, was effective at controlling bleeding episodes in individuals with moderate to severe hereditary Factor X deficiency, in a 16-patient study. Prior to being treated with the purified Factor X concentrate, the study’s participants had the following types of bleeding episodes: spontaneous; traumatic; or heavy menstrual.
Researchers also conducted a smaller study of the drug’s effectiveness on patients who were undergoing surgery. In this research project, Coagadex was successful at controlling blood loss during and after surgery, in those patients with mild hereditary Factor X deficiency.
“The approval of Coagadex is a significant advancement for patients who suffer from this rare but serious disease,” said Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, in the release.
Bio Products Laboratory manufactured the new drug.
The U.S. Food and Drug Administration has provided its first approval of a coagulation factor replacement drug specifically for patients with hereditary Factor X deficiency who are aged 12 years or older, the FDA said in a written statement. Typically, patients with this inherited disorder are treated with plasma-derived prothrombin complex concentrates.
Coagadex, the new coagulation factor replacement drug, was effective at controlling bleeding episodes in individuals with moderate to severe hereditary Factor X deficiency, in a 16-patient study. Prior to being treated with the purified Factor X concentrate, the study’s participants had the following types of bleeding episodes: spontaneous; traumatic; or heavy menstrual.
Researchers also conducted a smaller study of the drug’s effectiveness on patients who were undergoing surgery. In this research project, Coagadex was successful at controlling blood loss during and after surgery, in those patients with mild hereditary Factor X deficiency.
“The approval of Coagadex is a significant advancement for patients who suffer from this rare but serious disease,” said Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, in the release.
Bio Products Laboratory manufactured the new drug.
Prevalence of marijuana use among U.S. adults climbs to 9.5%
The past-year prevalence of marijuana use among U.S. adults more than doubled in 2012-2013, compared with 2001-2002. Meanwhile, the prevalence of marijuana use disorder among users decreased significantly during those periods, a study suggests.
“While many in the United States think prohibition of recreational marijuana should be ended, this study and others suggest caution and the need for public education about the potential harms in marijuana use, including the risk for addiction,” wrote Deborah S. Hasin, Ph.D., of the department of psychiatry, Columbia University, New York, and her colleagues.
Their conclusions are based on responses to survey questions obtained through face-to-face interviews of 36,309 adults in 2012-2013 that were compared to those of 43,093 adults in 2001-2002. In the more recently conducted survey, past-year DSM-IV marijuana use was defined as any use, and researchers used National Institute on Alcohol Abuse and Alcoholism Alcohol Use Disorder and Associated Disabilities Interview Schedule–5 (AUDADIS-5) to determine whether survey participants had marijuana use disorder. In the older study, the researchers used the AUDADIS-IV to evaluate whether a survey participant had a marijuana use disorder and was a marijuana user.
Past-year marijuana use significantly increased in 2012-2013, to 9.5%, compared with 4.1% in 2001-2002. Significant growth also occurred in the prevalence of DSM-IV marijuana use disorder; 2.9% of respondents to the 2012-2013 survey had the disorder, compared with 1.5% of respondents to the 2001-2002 survey.
However, the prevalence of past-year DSM-IV marijuana use disorder among marijuana users moved in the opposite direction. In 2012-2013, 30.6% of marijuana users manifested the disorder, compared with 35.6% of marijuana users in 2001-2002.
“Because no increase in the risk for marijuana use disorders was found among users (in fact, the risk decreased among users), the increase in prevalence of marijuana use disorders can be attributed to the increase in marijuana users between the two surveys,” Dr. Hasin wrote.
Future studies on marijuana use should address changes in past-year frequency of use, specific DSM-IV abuse or dependence criteria, severity of disorder, other aspects of use, and risk factors, the researchers said.
Read the study in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2015.1858).
The past-year prevalence of marijuana use among U.S. adults more than doubled in 2012-2013, compared with 2001-2002. Meanwhile, the prevalence of marijuana use disorder among users decreased significantly during those periods, a study suggests.
“While many in the United States think prohibition of recreational marijuana should be ended, this study and others suggest caution and the need for public education about the potential harms in marijuana use, including the risk for addiction,” wrote Deborah S. Hasin, Ph.D., of the department of psychiatry, Columbia University, New York, and her colleagues.
Their conclusions are based on responses to survey questions obtained through face-to-face interviews of 36,309 adults in 2012-2013 that were compared to those of 43,093 adults in 2001-2002. In the more recently conducted survey, past-year DSM-IV marijuana use was defined as any use, and researchers used National Institute on Alcohol Abuse and Alcoholism Alcohol Use Disorder and Associated Disabilities Interview Schedule–5 (AUDADIS-5) to determine whether survey participants had marijuana use disorder. In the older study, the researchers used the AUDADIS-IV to evaluate whether a survey participant had a marijuana use disorder and was a marijuana user.
Past-year marijuana use significantly increased in 2012-2013, to 9.5%, compared with 4.1% in 2001-2002. Significant growth also occurred in the prevalence of DSM-IV marijuana use disorder; 2.9% of respondents to the 2012-2013 survey had the disorder, compared with 1.5% of respondents to the 2001-2002 survey.
However, the prevalence of past-year DSM-IV marijuana use disorder among marijuana users moved in the opposite direction. In 2012-2013, 30.6% of marijuana users manifested the disorder, compared with 35.6% of marijuana users in 2001-2002.
“Because no increase in the risk for marijuana use disorders was found among users (in fact, the risk decreased among users), the increase in prevalence of marijuana use disorders can be attributed to the increase in marijuana users between the two surveys,” Dr. Hasin wrote.
Future studies on marijuana use should address changes in past-year frequency of use, specific DSM-IV abuse or dependence criteria, severity of disorder, other aspects of use, and risk factors, the researchers said.
Read the study in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2015.1858).
The past-year prevalence of marijuana use among U.S. adults more than doubled in 2012-2013, compared with 2001-2002. Meanwhile, the prevalence of marijuana use disorder among users decreased significantly during those periods, a study suggests.
“While many in the United States think prohibition of recreational marijuana should be ended, this study and others suggest caution and the need for public education about the potential harms in marijuana use, including the risk for addiction,” wrote Deborah S. Hasin, Ph.D., of the department of psychiatry, Columbia University, New York, and her colleagues.
Their conclusions are based on responses to survey questions obtained through face-to-face interviews of 36,309 adults in 2012-2013 that were compared to those of 43,093 adults in 2001-2002. In the more recently conducted survey, past-year DSM-IV marijuana use was defined as any use, and researchers used National Institute on Alcohol Abuse and Alcoholism Alcohol Use Disorder and Associated Disabilities Interview Schedule–5 (AUDADIS-5) to determine whether survey participants had marijuana use disorder. In the older study, the researchers used the AUDADIS-IV to evaluate whether a survey participant had a marijuana use disorder and was a marijuana user.
Past-year marijuana use significantly increased in 2012-2013, to 9.5%, compared with 4.1% in 2001-2002. Significant growth also occurred in the prevalence of DSM-IV marijuana use disorder; 2.9% of respondents to the 2012-2013 survey had the disorder, compared with 1.5% of respondents to the 2001-2002 survey.
However, the prevalence of past-year DSM-IV marijuana use disorder among marijuana users moved in the opposite direction. In 2012-2013, 30.6% of marijuana users manifested the disorder, compared with 35.6% of marijuana users in 2001-2002.
“Because no increase in the risk for marijuana use disorders was found among users (in fact, the risk decreased among users), the increase in prevalence of marijuana use disorders can be attributed to the increase in marijuana users between the two surveys,” Dr. Hasin wrote.
Future studies on marijuana use should address changes in past-year frequency of use, specific DSM-IV abuse or dependence criteria, severity of disorder, other aspects of use, and risk factors, the researchers said.
Read the study in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2015.1858).
FROM JAMA PSYCHIATRY
OnabotulinumtoxinA associated with reduced severity of PAH
The severity of primary axillary hyperhidrosis (PAH) decreased in more than half of patients who received onabotulinumtoxinA injections, in a nonrandomized, open-label study.
The researchers gave 144 adolescents with PAH up to six treatments with the drug, with retreatment occurring no sooner than 8 weeks after a prior treatment and no later than 44 weeks after the initial treatment.
Patients were retreated if they had 50 mg or more of spontaneous resting axillary sweat production in each axilla, following injections to each axilla. Fifty-six patients received one treatment, 59 patients received two treatments, 20 received three treatments, 6 received four treatments, and 3 received five treatments. Patients’ scores on the 4-point Hyperhidrosis Disease Severity Scale (HDSS) were among the measures used to evaluate responses to the onabotulinumtoxinA injections.
Results showed that 72.3% of patients experienced at least a 2-point improvement in their HDSS score at 4 weeks after their first treatment, and 56.6% of patients experienced a 2-point or greater improvement at 8 weeks following their second round of injections.
Mean sweat production declined by an average of 83.9% 4 weeks after treatment, and by an average of 78.1% 8 weeks after treatment. More than 50% of patients experienced at least a 90% reduction in sweat production at the 4th week following one of their injections (either the first, second, or third treatment).
Ninety-two patients (63.9%) reported experiencing adverse events (AEs), with upper respiratory tract infections having occurred the most frequently. While most of such adverse events “were mild or moderate in severity,” three patients experienced serious AEs and an additional three experienced severe AEs. Eight patients (5.6%) had 10 treatment-related AEs, including hyperhidrosis (back and palms), injection-site pain, lymphadenopathy, nausea, injection-site irritation, self-reported compensatory sweating (palms), dizziness, and pruritus.
“This study demonstrated that the efficacy and safety of onabotulinumtoxinA in treating PAH reported in previous registration clinical trials in adults can be extended to adolescents,” wrote Dr. Dee Anna Glaser of Saint Louis University and her colleagues.
Dr. Glaser is a paid consultant and investigator for Allergan, Dermira, and Miramar Labs. She has received research grants and honoraria from these companies and is an investigator for Ulthera. Her coauthors disclosed ties to Allergan.
Read the study in Pediatric Dermatology (doi: 10.1111/pde.12620).
The severity of primary axillary hyperhidrosis (PAH) decreased in more than half of patients who received onabotulinumtoxinA injections, in a nonrandomized, open-label study.
The researchers gave 144 adolescents with PAH up to six treatments with the drug, with retreatment occurring no sooner than 8 weeks after a prior treatment and no later than 44 weeks after the initial treatment.
Patients were retreated if they had 50 mg or more of spontaneous resting axillary sweat production in each axilla, following injections to each axilla. Fifty-six patients received one treatment, 59 patients received two treatments, 20 received three treatments, 6 received four treatments, and 3 received five treatments. Patients’ scores on the 4-point Hyperhidrosis Disease Severity Scale (HDSS) were among the measures used to evaluate responses to the onabotulinumtoxinA injections.
Results showed that 72.3% of patients experienced at least a 2-point improvement in their HDSS score at 4 weeks after their first treatment, and 56.6% of patients experienced a 2-point or greater improvement at 8 weeks following their second round of injections.
Mean sweat production declined by an average of 83.9% 4 weeks after treatment, and by an average of 78.1% 8 weeks after treatment. More than 50% of patients experienced at least a 90% reduction in sweat production at the 4th week following one of their injections (either the first, second, or third treatment).
Ninety-two patients (63.9%) reported experiencing adverse events (AEs), with upper respiratory tract infections having occurred the most frequently. While most of such adverse events “were mild or moderate in severity,” three patients experienced serious AEs and an additional three experienced severe AEs. Eight patients (5.6%) had 10 treatment-related AEs, including hyperhidrosis (back and palms), injection-site pain, lymphadenopathy, nausea, injection-site irritation, self-reported compensatory sweating (palms), dizziness, and pruritus.
“This study demonstrated that the efficacy and safety of onabotulinumtoxinA in treating PAH reported in previous registration clinical trials in adults can be extended to adolescents,” wrote Dr. Dee Anna Glaser of Saint Louis University and her colleagues.
Dr. Glaser is a paid consultant and investigator for Allergan, Dermira, and Miramar Labs. She has received research grants and honoraria from these companies and is an investigator for Ulthera. Her coauthors disclosed ties to Allergan.
Read the study in Pediatric Dermatology (doi: 10.1111/pde.12620).
The severity of primary axillary hyperhidrosis (PAH) decreased in more than half of patients who received onabotulinumtoxinA injections, in a nonrandomized, open-label study.
The researchers gave 144 adolescents with PAH up to six treatments with the drug, with retreatment occurring no sooner than 8 weeks after a prior treatment and no later than 44 weeks after the initial treatment.
Patients were retreated if they had 50 mg or more of spontaneous resting axillary sweat production in each axilla, following injections to each axilla. Fifty-six patients received one treatment, 59 patients received two treatments, 20 received three treatments, 6 received four treatments, and 3 received five treatments. Patients’ scores on the 4-point Hyperhidrosis Disease Severity Scale (HDSS) were among the measures used to evaluate responses to the onabotulinumtoxinA injections.
Results showed that 72.3% of patients experienced at least a 2-point improvement in their HDSS score at 4 weeks after their first treatment, and 56.6% of patients experienced a 2-point or greater improvement at 8 weeks following their second round of injections.
Mean sweat production declined by an average of 83.9% 4 weeks after treatment, and by an average of 78.1% 8 weeks after treatment. More than 50% of patients experienced at least a 90% reduction in sweat production at the 4th week following one of their injections (either the first, second, or third treatment).
Ninety-two patients (63.9%) reported experiencing adverse events (AEs), with upper respiratory tract infections having occurred the most frequently. While most of such adverse events “were mild or moderate in severity,” three patients experienced serious AEs and an additional three experienced severe AEs. Eight patients (5.6%) had 10 treatment-related AEs, including hyperhidrosis (back and palms), injection-site pain, lymphadenopathy, nausea, injection-site irritation, self-reported compensatory sweating (palms), dizziness, and pruritus.
“This study demonstrated that the efficacy and safety of onabotulinumtoxinA in treating PAH reported in previous registration clinical trials in adults can be extended to adolescents,” wrote Dr. Dee Anna Glaser of Saint Louis University and her colleagues.
Dr. Glaser is a paid consultant and investigator for Allergan, Dermira, and Miramar Labs. She has received research grants and honoraria from these companies and is an investigator for Ulthera. Her coauthors disclosed ties to Allergan.
Read the study in Pediatric Dermatology (doi: 10.1111/pde.12620).
FROM PEDIATRIC DERMATOLOGY
Effective antibullying laws comply with DOE guidelines
Antibullying laws that include statement of scope, a description of prohibited behaviors, and requirements for districts to develop and implement policies have been linked with a decrease in bullying and cyberbullying, results of a study suggest.
“We found evidence that compliance with [Department of Education]–recommended guidelines in antibullying laws was associated with lower rates of being bullied and cyberbullied,” wrote Mark L. Hatzenbuehler, Ph.D., of Columbia University, New York, and his colleagues.
The researchers analyzed survey responses from 63,635 adolescents in grades 9-12 in schools from 25 states, between September 2010 and December 2011. These states had antibullying laws in place at the time the students were surveyed. The researchers also used the U.S. Department of Education’s review of how closely each law followed the department’s framework for antibullying laws. Using the DOE data and the student’s survey responses, the researchers searched for links between the inclusion of specific parts of the DOE framework in a state’s antibullying law and the prevalence of bullying and cyberbullying in that state.
They evaluated 16 individual components of antibullying legislation. Of these, statement of scope (bullying: adjusted odds ratio, 0.85; cyberbullying: AOR, 0.87), a description of prohibited behaviors (bullying: AOR, 0.83; cyberbullying: AOR, 0.92), and requirements for districts to develop and implement policies (bullying: AOR, 0.76; cyberbullying: AOR, 0.80) were consistently associated with decreased odds of being bullied and cyberbullied, “These three components offer details, specificity, and clarity for school administrators and may therefore increase the likelihood that they feel empowered to act,” wrote the researchers.
Future research on antibullying laws’ effectiveness should include “a larger sample of laws identified from historical reviews of all 49 state antibullying laws,” they added.
The researchers had no disclosures. Read the study in JAMA Pediatrics (doi:10.1001/jamapediatrics.2015.2411).
Antibullying laws that include statement of scope, a description of prohibited behaviors, and requirements for districts to develop and implement policies have been linked with a decrease in bullying and cyberbullying, results of a study suggest.
“We found evidence that compliance with [Department of Education]–recommended guidelines in antibullying laws was associated with lower rates of being bullied and cyberbullied,” wrote Mark L. Hatzenbuehler, Ph.D., of Columbia University, New York, and his colleagues.
The researchers analyzed survey responses from 63,635 adolescents in grades 9-12 in schools from 25 states, between September 2010 and December 2011. These states had antibullying laws in place at the time the students were surveyed. The researchers also used the U.S. Department of Education’s review of how closely each law followed the department’s framework for antibullying laws. Using the DOE data and the student’s survey responses, the researchers searched for links between the inclusion of specific parts of the DOE framework in a state’s antibullying law and the prevalence of bullying and cyberbullying in that state.
They evaluated 16 individual components of antibullying legislation. Of these, statement of scope (bullying: adjusted odds ratio, 0.85; cyberbullying: AOR, 0.87), a description of prohibited behaviors (bullying: AOR, 0.83; cyberbullying: AOR, 0.92), and requirements for districts to develop and implement policies (bullying: AOR, 0.76; cyberbullying: AOR, 0.80) were consistently associated with decreased odds of being bullied and cyberbullied, “These three components offer details, specificity, and clarity for school administrators and may therefore increase the likelihood that they feel empowered to act,” wrote the researchers.
Future research on antibullying laws’ effectiveness should include “a larger sample of laws identified from historical reviews of all 49 state antibullying laws,” they added.
The researchers had no disclosures. Read the study in JAMA Pediatrics (doi:10.1001/jamapediatrics.2015.2411).
Antibullying laws that include statement of scope, a description of prohibited behaviors, and requirements for districts to develop and implement policies have been linked with a decrease in bullying and cyberbullying, results of a study suggest.
“We found evidence that compliance with [Department of Education]–recommended guidelines in antibullying laws was associated with lower rates of being bullied and cyberbullied,” wrote Mark L. Hatzenbuehler, Ph.D., of Columbia University, New York, and his colleagues.
The researchers analyzed survey responses from 63,635 adolescents in grades 9-12 in schools from 25 states, between September 2010 and December 2011. These states had antibullying laws in place at the time the students were surveyed. The researchers also used the U.S. Department of Education’s review of how closely each law followed the department’s framework for antibullying laws. Using the DOE data and the student’s survey responses, the researchers searched for links between the inclusion of specific parts of the DOE framework in a state’s antibullying law and the prevalence of bullying and cyberbullying in that state.
They evaluated 16 individual components of antibullying legislation. Of these, statement of scope (bullying: adjusted odds ratio, 0.85; cyberbullying: AOR, 0.87), a description of prohibited behaviors (bullying: AOR, 0.83; cyberbullying: AOR, 0.92), and requirements for districts to develop and implement policies (bullying: AOR, 0.76; cyberbullying: AOR, 0.80) were consistently associated with decreased odds of being bullied and cyberbullied, “These three components offer details, specificity, and clarity for school administrators and may therefore increase the likelihood that they feel empowered to act,” wrote the researchers.
Future research on antibullying laws’ effectiveness should include “a larger sample of laws identified from historical reviews of all 49 state antibullying laws,” they added.
The researchers had no disclosures. Read the study in JAMA Pediatrics (doi:10.1001/jamapediatrics.2015.2411).
FROM JAMA PEDIATRICS
Mental health apps deemed ineffective
Several of the National Health Service’s mental health apps should be removed from the NHS Health Apps Library, because the apps lack evidence of their effectiveness, an article suggests.
The authors, researchers Simon Leigh of the Management School at the University of Liverpool, England, and Steve Flatt of the Liverpool Psychological Therapies Unit Community Interest Company, specifically fire shots at most of the NHS apps for the management of depression and anxiety. After assessing the metrics used to evaluate mental health apps currently in the library, they found that only four of the apps dedicated to the management of depression and anxiety “provide any evidence of patient-reported outcomes to substantiate claims of effectiveness” and only two apply validated metrics, including the generalized anxiety disorder 7-item scale (GAD-7) and 9-item patient health questionnaire (PHQ-9) to assess clinical performance.
“As such, confidence in, and the validity of the claims made by apps that fail to apply such metrics must be considered as low at best, suggesting that the true clinical value of over 85% of NHS accredited mental health apps is at present impossible to determine,” they wrote.
The authors criticized many mental health apps sponsored by the NHS and other organizations, but they also advocate for the use of mental health apps that meet certain specifications, including those that “demonstrate evidence of real-world clinical effectiveness prior to receiving a seal of approval from a world-leading health care system and [be] recommended for patients in need of high-quality psychological interventions.”
Among the reasons the article’s authors list for supporting the idea of mental health apps is the possibility of such tools helping to decrease the wait time for receiving treatment, citing grim expected outcomes for patients currently waiting for mental health services in the United Kingdom. For example, 1 in 6 patients on waiting lists for mental health are expected to attempt suicide.
The writers also laud app-based psychological interventions’ potential to remove financial barriers to treatment and the fact that the use of some mental health apps has led to symptom improvement among patients with mental health issues.
“Given the ever increasing demands and limited supply of NHS mental health services, coupled with barriers to care, including a desire for anonymity, indirect financial costs, and impaired access to treatment centers, the use of apps may not only promote health service efficiency, but also support the NHS in returning to its seminal promise of equal access for equal need,” the authors wrote.
They added: “However, if this is to be an effective venture, this space clearly requires more stringent regulation, vetting, and quality control,”
Reacting to the article, a spokesperson for NHS England said: “This study illustrates that digital tools can act as powerful psychological interventions. It’s vital that patients know which apps to choose and that’s why we are working to upgrade the Health Apps Library, which launched as a pilot site in 2013 and reviews and recommends apps against a defined set of criteria. Earlier this year, we launched the Mental Health Apps Library, which features apps and digital tools that are compliant with IAPT [increasing access to psychological therapies] quality standards and offer National Institute of Health and Care Excellence approved treatments that can demonstrate effectiveness in treating mild and moderate depression and anxiety.”
Read the full article in Evidence-Based Mental Health (doi: 10.1136/eb-20150102203).
Several of the National Health Service’s mental health apps should be removed from the NHS Health Apps Library, because the apps lack evidence of their effectiveness, an article suggests.
The authors, researchers Simon Leigh of the Management School at the University of Liverpool, England, and Steve Flatt of the Liverpool Psychological Therapies Unit Community Interest Company, specifically fire shots at most of the NHS apps for the management of depression and anxiety. After assessing the metrics used to evaluate mental health apps currently in the library, they found that only four of the apps dedicated to the management of depression and anxiety “provide any evidence of patient-reported outcomes to substantiate claims of effectiveness” and only two apply validated metrics, including the generalized anxiety disorder 7-item scale (GAD-7) and 9-item patient health questionnaire (PHQ-9) to assess clinical performance.
“As such, confidence in, and the validity of the claims made by apps that fail to apply such metrics must be considered as low at best, suggesting that the true clinical value of over 85% of NHS accredited mental health apps is at present impossible to determine,” they wrote.
The authors criticized many mental health apps sponsored by the NHS and other organizations, but they also advocate for the use of mental health apps that meet certain specifications, including those that “demonstrate evidence of real-world clinical effectiveness prior to receiving a seal of approval from a world-leading health care system and [be] recommended for patients in need of high-quality psychological interventions.”
Among the reasons the article’s authors list for supporting the idea of mental health apps is the possibility of such tools helping to decrease the wait time for receiving treatment, citing grim expected outcomes for patients currently waiting for mental health services in the United Kingdom. For example, 1 in 6 patients on waiting lists for mental health are expected to attempt suicide.
The writers also laud app-based psychological interventions’ potential to remove financial barriers to treatment and the fact that the use of some mental health apps has led to symptom improvement among patients with mental health issues.
“Given the ever increasing demands and limited supply of NHS mental health services, coupled with barriers to care, including a desire for anonymity, indirect financial costs, and impaired access to treatment centers, the use of apps may not only promote health service efficiency, but also support the NHS in returning to its seminal promise of equal access for equal need,” the authors wrote.
They added: “However, if this is to be an effective venture, this space clearly requires more stringent regulation, vetting, and quality control,”
Reacting to the article, a spokesperson for NHS England said: “This study illustrates that digital tools can act as powerful psychological interventions. It’s vital that patients know which apps to choose and that’s why we are working to upgrade the Health Apps Library, which launched as a pilot site in 2013 and reviews and recommends apps against a defined set of criteria. Earlier this year, we launched the Mental Health Apps Library, which features apps and digital tools that are compliant with IAPT [increasing access to psychological therapies] quality standards and offer National Institute of Health and Care Excellence approved treatments that can demonstrate effectiveness in treating mild and moderate depression and anxiety.”
Read the full article in Evidence-Based Mental Health (doi: 10.1136/eb-20150102203).
Several of the National Health Service’s mental health apps should be removed from the NHS Health Apps Library, because the apps lack evidence of their effectiveness, an article suggests.
The authors, researchers Simon Leigh of the Management School at the University of Liverpool, England, and Steve Flatt of the Liverpool Psychological Therapies Unit Community Interest Company, specifically fire shots at most of the NHS apps for the management of depression and anxiety. After assessing the metrics used to evaluate mental health apps currently in the library, they found that only four of the apps dedicated to the management of depression and anxiety “provide any evidence of patient-reported outcomes to substantiate claims of effectiveness” and only two apply validated metrics, including the generalized anxiety disorder 7-item scale (GAD-7) and 9-item patient health questionnaire (PHQ-9) to assess clinical performance.
“As such, confidence in, and the validity of the claims made by apps that fail to apply such metrics must be considered as low at best, suggesting that the true clinical value of over 85% of NHS accredited mental health apps is at present impossible to determine,” they wrote.
The authors criticized many mental health apps sponsored by the NHS and other organizations, but they also advocate for the use of mental health apps that meet certain specifications, including those that “demonstrate evidence of real-world clinical effectiveness prior to receiving a seal of approval from a world-leading health care system and [be] recommended for patients in need of high-quality psychological interventions.”
Among the reasons the article’s authors list for supporting the idea of mental health apps is the possibility of such tools helping to decrease the wait time for receiving treatment, citing grim expected outcomes for patients currently waiting for mental health services in the United Kingdom. For example, 1 in 6 patients on waiting lists for mental health are expected to attempt suicide.
The writers also laud app-based psychological interventions’ potential to remove financial barriers to treatment and the fact that the use of some mental health apps has led to symptom improvement among patients with mental health issues.
“Given the ever increasing demands and limited supply of NHS mental health services, coupled with barriers to care, including a desire for anonymity, indirect financial costs, and impaired access to treatment centers, the use of apps may not only promote health service efficiency, but also support the NHS in returning to its seminal promise of equal access for equal need,” the authors wrote.
They added: “However, if this is to be an effective venture, this space clearly requires more stringent regulation, vetting, and quality control,”
Reacting to the article, a spokesperson for NHS England said: “This study illustrates that digital tools can act as powerful psychological interventions. It’s vital that patients know which apps to choose and that’s why we are working to upgrade the Health Apps Library, which launched as a pilot site in 2013 and reviews and recommends apps against a defined set of criteria. Earlier this year, we launched the Mental Health Apps Library, which features apps and digital tools that are compliant with IAPT [increasing access to psychological therapies] quality standards and offer National Institute of Health and Care Excellence approved treatments that can demonstrate effectiveness in treating mild and moderate depression and anxiety.”
Read the full article in Evidence-Based Mental Health (doi: 10.1136/eb-20150102203).
FROM EVIDENCE-BASED MENTAL HEALTH
No link between head and neck cancers and marijuana use
Marijuana use does not seem to be a risk factor for head and neck cancer (HNC) suggests a meta-analysis including nine case-control studies.
The chance of developing head and neck cancer in individuals who had smoked marijuana in their lifetime was estimated using an odds ratio, and controlling for age, sex, race, and tobacco consumption.
Approximately 12.6% of the patients who developed HNC and 14.3% of the controls were marijuana users. No association was found between exposure to marijuana and HNC (odds ratio = 1.021).
“Despite several inferences that have made to date, there is currently insufficient epidemiological evidence to support a positive or negative association in marijuana use and the development of HNC,” the researchers said.
Future studies on the long-term effects of marijuana use and “the mechanism of action of cannabinoids in specific tissues in animal models and humans” are needed, according to the researchers.
Read the full study in Archives of Oral Biology (doi: 10.1016/j.archoralbi.2015.09.009).
Marijuana use does not seem to be a risk factor for head and neck cancer (HNC) suggests a meta-analysis including nine case-control studies.
The chance of developing head and neck cancer in individuals who had smoked marijuana in their lifetime was estimated using an odds ratio, and controlling for age, sex, race, and tobacco consumption.
Approximately 12.6% of the patients who developed HNC and 14.3% of the controls were marijuana users. No association was found between exposure to marijuana and HNC (odds ratio = 1.021).
“Despite several inferences that have made to date, there is currently insufficient epidemiological evidence to support a positive or negative association in marijuana use and the development of HNC,” the researchers said.
Future studies on the long-term effects of marijuana use and “the mechanism of action of cannabinoids in specific tissues in animal models and humans” are needed, according to the researchers.
Read the full study in Archives of Oral Biology (doi: 10.1016/j.archoralbi.2015.09.009).
Marijuana use does not seem to be a risk factor for head and neck cancer (HNC) suggests a meta-analysis including nine case-control studies.
The chance of developing head and neck cancer in individuals who had smoked marijuana in their lifetime was estimated using an odds ratio, and controlling for age, sex, race, and tobacco consumption.
Approximately 12.6% of the patients who developed HNC and 14.3% of the controls were marijuana users. No association was found between exposure to marijuana and HNC (odds ratio = 1.021).
“Despite several inferences that have made to date, there is currently insufficient epidemiological evidence to support a positive or negative association in marijuana use and the development of HNC,” the researchers said.
Future studies on the long-term effects of marijuana use and “the mechanism of action of cannabinoids in specific tissues in animal models and humans” are needed, according to the researchers.
Read the full study in Archives of Oral Biology (doi: 10.1016/j.archoralbi.2015.09.009).
FROM ARCHIVES OF ORAL BIOLOGY
IgA increase linked to fewer infections in CLL patients on ibrutinib
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
FROM BLOOD
Inhibiting PAC1 receptors may cure migraines
Migraines seem to be caused by activity inside the brain, and administering an inhibitor of the PAC1 receptor to the body’s control center may alleviate these painful events, suggested findings of a rat study by Simon Akerman and Peter J. Goadsby.
“To dissect the relative contributions of endogenous peripheral and central mechanisms in triggering migraine,” the researchers administered the pharmacologically similar vasodilator neuropeptides, vasoactive intestinal peptide and pituitary adenylate cyclase–activating peptide 38 (PACAP-38) intravenously and into the brains of the rats. Among the researchers’ findings was that only PACAP-38 imitated the effects of a migraine, in that it caused delayed activation and sensitization of central trigeminovascular neurons.
The researchers also gave the rats the PAC1 receptor antagonist intravenously and intracerebroventricularly. Only administration of the PAC1 inhibitor to the brain prevented delayed and spontaneous firing of the neurons, which is seen in migraine patients.
The study results suggested that “targeting the PAC1 receptor is a promising approach for migraine therapy,” wrote the researchers, who reported that they had no relevant financial conflicts.
Read the study in Science Translational Medicine (doi: 10.1126/scitranslmed.aaa7557).
Migraines seem to be caused by activity inside the brain, and administering an inhibitor of the PAC1 receptor to the body’s control center may alleviate these painful events, suggested findings of a rat study by Simon Akerman and Peter J. Goadsby.
“To dissect the relative contributions of endogenous peripheral and central mechanisms in triggering migraine,” the researchers administered the pharmacologically similar vasodilator neuropeptides, vasoactive intestinal peptide and pituitary adenylate cyclase–activating peptide 38 (PACAP-38) intravenously and into the brains of the rats. Among the researchers’ findings was that only PACAP-38 imitated the effects of a migraine, in that it caused delayed activation and sensitization of central trigeminovascular neurons.
The researchers also gave the rats the PAC1 receptor antagonist intravenously and intracerebroventricularly. Only administration of the PAC1 inhibitor to the brain prevented delayed and spontaneous firing of the neurons, which is seen in migraine patients.
The study results suggested that “targeting the PAC1 receptor is a promising approach for migraine therapy,” wrote the researchers, who reported that they had no relevant financial conflicts.
Read the study in Science Translational Medicine (doi: 10.1126/scitranslmed.aaa7557).
Migraines seem to be caused by activity inside the brain, and administering an inhibitor of the PAC1 receptor to the body’s control center may alleviate these painful events, suggested findings of a rat study by Simon Akerman and Peter J. Goadsby.
“To dissect the relative contributions of endogenous peripheral and central mechanisms in triggering migraine,” the researchers administered the pharmacologically similar vasodilator neuropeptides, vasoactive intestinal peptide and pituitary adenylate cyclase–activating peptide 38 (PACAP-38) intravenously and into the brains of the rats. Among the researchers’ findings was that only PACAP-38 imitated the effects of a migraine, in that it caused delayed activation and sensitization of central trigeminovascular neurons.
The researchers also gave the rats the PAC1 receptor antagonist intravenously and intracerebroventricularly. Only administration of the PAC1 inhibitor to the brain prevented delayed and spontaneous firing of the neurons, which is seen in migraine patients.
The study results suggested that “targeting the PAC1 receptor is a promising approach for migraine therapy,” wrote the researchers, who reported that they had no relevant financial conflicts.
Read the study in Science Translational Medicine (doi: 10.1126/scitranslmed.aaa7557).
FROM SCIENCE TRANSLATIONAL MEDICINE