User login
Overweight, obese patients at greater risk for knee replacement surgery
Both overweight and obese patients with knee osteoarthritis (OA) are more likely to get knee replacement surgery, compared with normal-weight patients with knee OA, results of a population-based cohort study of people in Catalonia, Spain, suggest.
The study included 105,189 patients, who had been diagnosed with knee OA between 2006 and 2011. Patients with a history of knee OA or knee replacement in either knee before Jan. 1, 2006, and patients with a history inflammatory arthritis were not included in the study.
The patients were followed from the date of knee OA diagnosis until the date they underwent elective knee replacement surgery or until Dec. 31, 2011. (The researchers were unable to follow up with all individuals initially enrolled in the study.) The participants were broken up into the following categories based on their body mass index: normal (BMI was less than 25 kg/m2), overweight (BMI was 25 to less than 30 kg/m2), obese class I (BMI was 30 to less than 35 kg/m2), obese class II (BMI was 35 to less than 40 kg/m2), and obese class III (BMI was greater than or equal to 40 kg/m2).
The risk of knee replacement increased with BMI. For patients with a normal weight, the incidence rates of surgery were 1.35/100 person-years, compared with 3.49/100 person-years in patients in obese class III. Adjusted hazard ratios for knee replacement surgery were 1.41 for overweight, 1.97 for obese class I, 2.39 for obese class II, and 2.67 for obese class III, compared with normal-weight study participants.
An additional finding was a significant interaction between BMI and age on the risk of knee replacement (P is less than .001), with a higher relative hazard associated with obesity among patients aged less than 68 years.
“This research demonstrates that overweight and obesity are strong independent predictors of the clinical progression of knee OA, from disease onset/diagnosis to joint failure and subsequent [knee replacement]. Overweight subjects are at over 40% increased risk of surgery, and those who are obese have a more than doubled risk when compared to subjects with normal weight,” said Kristen M. Leyland, D.Phil., and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39486).
Both overweight and obese patients with knee osteoarthritis (OA) are more likely to get knee replacement surgery, compared with normal-weight patients with knee OA, results of a population-based cohort study of people in Catalonia, Spain, suggest.
The study included 105,189 patients, who had been diagnosed with knee OA between 2006 and 2011. Patients with a history of knee OA or knee replacement in either knee before Jan. 1, 2006, and patients with a history inflammatory arthritis were not included in the study.
The patients were followed from the date of knee OA diagnosis until the date they underwent elective knee replacement surgery or until Dec. 31, 2011. (The researchers were unable to follow up with all individuals initially enrolled in the study.) The participants were broken up into the following categories based on their body mass index: normal (BMI was less than 25 kg/m2), overweight (BMI was 25 to less than 30 kg/m2), obese class I (BMI was 30 to less than 35 kg/m2), obese class II (BMI was 35 to less than 40 kg/m2), and obese class III (BMI was greater than or equal to 40 kg/m2).
The risk of knee replacement increased with BMI. For patients with a normal weight, the incidence rates of surgery were 1.35/100 person-years, compared with 3.49/100 person-years in patients in obese class III. Adjusted hazard ratios for knee replacement surgery were 1.41 for overweight, 1.97 for obese class I, 2.39 for obese class II, and 2.67 for obese class III, compared with normal-weight study participants.
An additional finding was a significant interaction between BMI and age on the risk of knee replacement (P is less than .001), with a higher relative hazard associated with obesity among patients aged less than 68 years.
“This research demonstrates that overweight and obesity are strong independent predictors of the clinical progression of knee OA, from disease onset/diagnosis to joint failure and subsequent [knee replacement]. Overweight subjects are at over 40% increased risk of surgery, and those who are obese have a more than doubled risk when compared to subjects with normal weight,” said Kristen M. Leyland, D.Phil., and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39486).
Both overweight and obese patients with knee osteoarthritis (OA) are more likely to get knee replacement surgery, compared with normal-weight patients with knee OA, results of a population-based cohort study of people in Catalonia, Spain, suggest.
The study included 105,189 patients, who had been diagnosed with knee OA between 2006 and 2011. Patients with a history of knee OA or knee replacement in either knee before Jan. 1, 2006, and patients with a history inflammatory arthritis were not included in the study.
The patients were followed from the date of knee OA diagnosis until the date they underwent elective knee replacement surgery or until Dec. 31, 2011. (The researchers were unable to follow up with all individuals initially enrolled in the study.) The participants were broken up into the following categories based on their body mass index: normal (BMI was less than 25 kg/m2), overweight (BMI was 25 to less than 30 kg/m2), obese class I (BMI was 30 to less than 35 kg/m2), obese class II (BMI was 35 to less than 40 kg/m2), and obese class III (BMI was greater than or equal to 40 kg/m2).
The risk of knee replacement increased with BMI. For patients with a normal weight, the incidence rates of surgery were 1.35/100 person-years, compared with 3.49/100 person-years in patients in obese class III. Adjusted hazard ratios for knee replacement surgery were 1.41 for overweight, 1.97 for obese class I, 2.39 for obese class II, and 2.67 for obese class III, compared with normal-weight study participants.
An additional finding was a significant interaction between BMI and age on the risk of knee replacement (P is less than .001), with a higher relative hazard associated with obesity among patients aged less than 68 years.
“This research demonstrates that overweight and obesity are strong independent predictors of the clinical progression of knee OA, from disease onset/diagnosis to joint failure and subsequent [knee replacement]. Overweight subjects are at over 40% increased risk of surgery, and those who are obese have a more than doubled risk when compared to subjects with normal weight,” said Kristen M. Leyland, D.Phil., and her colleagues.
Read the full study in Arthritis & Rheumatology (doi: 10.1002/art.39486).
FROM ARTHRITIS & RHEUMATOLOGY
Food-antigen–specific immunoglobulin E is not a predictor of food allergies in atopic dermatitis
Food-antigen–specific immunoglobulin E (sIgE) levels were not clinically useful for predicting food allergy development in a study of infants with atopic dermatitis (AD).
The dual-phase study included 1,087 patients aged 3-18 months who had been diagnosed with AD for no more than 3 months prior to enrollment in the study and had at least mild disease activity. During the first phase of the study, which was a 36-month, randomized, double-blind, vehicle-controlled phase, half of patients were treated with placebo cream and the other half were treated with 1% pimecrolimus cream. In the second phase of the study, which was open-label, all patients received 1% pimecrolimus cream for up to 33 months or the patient’s 6th birthday, whichever occurred sooner. Patients were excluded if they received treatment with topical or systemic agents within 7 days before the first application of cream in the study.
The researchers followed food allergy development during both phases of the study. Other data collected by the researchers included sIgE levels for various foods at baseline and at the end of both phases of the study, with sIgE decision points having been assigned to each food.
By the end of the second phase of the trial, 15.9% of patients had developed a food allergy, with 292 days having been the median period of time that passed before the initial diagnosis of a food allergy was made. The most common food allergies were to peanuts, cow’s milk, and egg whites, occurring in 7%, 4%, and 4% of patients respectively. The percentage of patients with any allergy to food other than fish decreased over time. Higher levels of AD severity were predictive for the development of food allergy, with the percentage of patients who developed one or more food allergies by the end of the study having increased with increasing AD severity at baseline.
Total serum immunoglobulin E (IgE) and sIgE for milk, eggs, and peanuts measured at the end of the second phase also were increased in patients with increasing AD severity. Despite these findings, the positive predictive values for sIgE decision points for the foods tested were low (less than 0.6 for all values tested).
“SIgE decision points, both published values and the novel decision points used in this study, had high [negative predictive values], in particular for peanut[s], egg white[s], and cow’s milk. Thus, patients with mild AD with sIgE levels below these cutoffs would be unlikely to have or develop these specific allergies and would not benefit from food challenges or elimination diets. Similarly, elevated sIgE, as defined by the decision points tested, had very low [positive predictive values] for food allergy, both for sIgE values at baseline and at the end of the [first phase of the study] ... Thus, despite an increased likelihood of allergy development with increasing sIgE shown for cow’s milk, egg[s], and peanut[s], our data do not support the use of sIgE testing for the diagnosis of food allergy in subjects without a history of reaction to that food,” said Dr. Jonathan M. Spergelof the Children’s Hospital of Philadelphia and his colleagues.
Read the full study in Pediatrics (doi: 10.1542/peds.2015-1444).
Food-antigen–specific immunoglobulin E (sIgE) levels were not clinically useful for predicting food allergy development in a study of infants with atopic dermatitis (AD).
The dual-phase study included 1,087 patients aged 3-18 months who had been diagnosed with AD for no more than 3 months prior to enrollment in the study and had at least mild disease activity. During the first phase of the study, which was a 36-month, randomized, double-blind, vehicle-controlled phase, half of patients were treated with placebo cream and the other half were treated with 1% pimecrolimus cream. In the second phase of the study, which was open-label, all patients received 1% pimecrolimus cream for up to 33 months or the patient’s 6th birthday, whichever occurred sooner. Patients were excluded if they received treatment with topical or systemic agents within 7 days before the first application of cream in the study.
The researchers followed food allergy development during both phases of the study. Other data collected by the researchers included sIgE levels for various foods at baseline and at the end of both phases of the study, with sIgE decision points having been assigned to each food.
By the end of the second phase of the trial, 15.9% of patients had developed a food allergy, with 292 days having been the median period of time that passed before the initial diagnosis of a food allergy was made. The most common food allergies were to peanuts, cow’s milk, and egg whites, occurring in 7%, 4%, and 4% of patients respectively. The percentage of patients with any allergy to food other than fish decreased over time. Higher levels of AD severity were predictive for the development of food allergy, with the percentage of patients who developed one or more food allergies by the end of the study having increased with increasing AD severity at baseline.
Total serum immunoglobulin E (IgE) and sIgE for milk, eggs, and peanuts measured at the end of the second phase also were increased in patients with increasing AD severity. Despite these findings, the positive predictive values for sIgE decision points for the foods tested were low (less than 0.6 for all values tested).
“SIgE decision points, both published values and the novel decision points used in this study, had high [negative predictive values], in particular for peanut[s], egg white[s], and cow’s milk. Thus, patients with mild AD with sIgE levels below these cutoffs would be unlikely to have or develop these specific allergies and would not benefit from food challenges or elimination diets. Similarly, elevated sIgE, as defined by the decision points tested, had very low [positive predictive values] for food allergy, both for sIgE values at baseline and at the end of the [first phase of the study] ... Thus, despite an increased likelihood of allergy development with increasing sIgE shown for cow’s milk, egg[s], and peanut[s], our data do not support the use of sIgE testing for the diagnosis of food allergy in subjects without a history of reaction to that food,” said Dr. Jonathan M. Spergelof the Children’s Hospital of Philadelphia and his colleagues.
Read the full study in Pediatrics (doi: 10.1542/peds.2015-1444).
Food-antigen–specific immunoglobulin E (sIgE) levels were not clinically useful for predicting food allergy development in a study of infants with atopic dermatitis (AD).
The dual-phase study included 1,087 patients aged 3-18 months who had been diagnosed with AD for no more than 3 months prior to enrollment in the study and had at least mild disease activity. During the first phase of the study, which was a 36-month, randomized, double-blind, vehicle-controlled phase, half of patients were treated with placebo cream and the other half were treated with 1% pimecrolimus cream. In the second phase of the study, which was open-label, all patients received 1% pimecrolimus cream for up to 33 months or the patient’s 6th birthday, whichever occurred sooner. Patients were excluded if they received treatment with topical or systemic agents within 7 days before the first application of cream in the study.
The researchers followed food allergy development during both phases of the study. Other data collected by the researchers included sIgE levels for various foods at baseline and at the end of both phases of the study, with sIgE decision points having been assigned to each food.
By the end of the second phase of the trial, 15.9% of patients had developed a food allergy, with 292 days having been the median period of time that passed before the initial diagnosis of a food allergy was made. The most common food allergies were to peanuts, cow’s milk, and egg whites, occurring in 7%, 4%, and 4% of patients respectively. The percentage of patients with any allergy to food other than fish decreased over time. Higher levels of AD severity were predictive for the development of food allergy, with the percentage of patients who developed one or more food allergies by the end of the study having increased with increasing AD severity at baseline.
Total serum immunoglobulin E (IgE) and sIgE for milk, eggs, and peanuts measured at the end of the second phase also were increased in patients with increasing AD severity. Despite these findings, the positive predictive values for sIgE decision points for the foods tested were low (less than 0.6 for all values tested).
“SIgE decision points, both published values and the novel decision points used in this study, had high [negative predictive values], in particular for peanut[s], egg white[s], and cow’s milk. Thus, patients with mild AD with sIgE levels below these cutoffs would be unlikely to have or develop these specific allergies and would not benefit from food challenges or elimination diets. Similarly, elevated sIgE, as defined by the decision points tested, had very low [positive predictive values] for food allergy, both for sIgE values at baseline and at the end of the [first phase of the study] ... Thus, despite an increased likelihood of allergy development with increasing sIgE shown for cow’s milk, egg[s], and peanut[s], our data do not support the use of sIgE testing for the diagnosis of food allergy in subjects without a history of reaction to that food,” said Dr. Jonathan M. Spergelof the Children’s Hospital of Philadelphia and his colleagues.
Read the full study in Pediatrics (doi: 10.1542/peds.2015-1444).
FROM PEDIATRICS
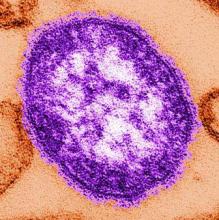
Progress toward measles elimination slows
The number of global measles incidences declined and measles vaccine coverage increased between 2000 and 2010, but progress toward eliminating the disease has slowed since 2010, according to a report.
Between 2000 and 2014, annual reported measles incidences declined 73% worldwide, from 146 to 40 cases per million population. During this same time period, annual estimated measles deaths also decreased to 114,900 from 546,800 – a 79% drop, according to Dr. Robert T. Perry of the department of immunization, vaccines, and biologicals at the World Health Organization, and his colleagues (MMWR Morb Mortal Wkly Rep. 2015 Nov 13;64[44]:1246-51).
Measles vaccinations prevented an estimated 17.1 million deaths between 2000 and 2014, but vaccination coverage seems to be at a standstill. While the percentage of children aged 1 year receiving coverage with the first dose of measles-containing vaccine (MCVI) increased to 85% from 72% between 2000 and 2010, growth in this coverage has halted. The percentage of 1-year-old children receiving the MCVI has remained at 85% through 2014, with an estimated 20.6 million infants having not received the MCVI in 2014. Fifty-six percent (approximately 11.6 million) of these infants who did receive the MCVI were in the Democratic Republic of Congo, Ethiopia, India, Indonesia, Nigeria, and Pakistan. Global coverage of the second dose of measles-containing vaccine increased between 2000 and 2014, from 15% to 56%.
Despite the increased MCVI coverage since 2000, the World Health Assembly’s global milestones and measles elimination goals for 2015 will not be achieved, according to the WHO Strategic Advisory Group of Experts on Immunization.
“Reaching measles control and elimination goals will require addressing policy and practice gaps that prevent reaching larger numbers of children with measles vaccination, increasing viability of measles elimination efforts, and ensuring adequate resources for strengthening health systems,” said Dr. Perry and his colleagues.
Read the full report in the Nov. 13 issue of MMWR.
The number of global measles incidences declined and measles vaccine coverage increased between 2000 and 2010, but progress toward eliminating the disease has slowed since 2010, according to a report.
Between 2000 and 2014, annual reported measles incidences declined 73% worldwide, from 146 to 40 cases per million population. During this same time period, annual estimated measles deaths also decreased to 114,900 from 546,800 – a 79% drop, according to Dr. Robert T. Perry of the department of immunization, vaccines, and biologicals at the World Health Organization, and his colleagues (MMWR Morb Mortal Wkly Rep. 2015 Nov 13;64[44]:1246-51).
Measles vaccinations prevented an estimated 17.1 million deaths between 2000 and 2014, but vaccination coverage seems to be at a standstill. While the percentage of children aged 1 year receiving coverage with the first dose of measles-containing vaccine (MCVI) increased to 85% from 72% between 2000 and 2010, growth in this coverage has halted. The percentage of 1-year-old children receiving the MCVI has remained at 85% through 2014, with an estimated 20.6 million infants having not received the MCVI in 2014. Fifty-six percent (approximately 11.6 million) of these infants who did receive the MCVI were in the Democratic Republic of Congo, Ethiopia, India, Indonesia, Nigeria, and Pakistan. Global coverage of the second dose of measles-containing vaccine increased between 2000 and 2014, from 15% to 56%.
Despite the increased MCVI coverage since 2000, the World Health Assembly’s global milestones and measles elimination goals for 2015 will not be achieved, according to the WHO Strategic Advisory Group of Experts on Immunization.
“Reaching measles control and elimination goals will require addressing policy and practice gaps that prevent reaching larger numbers of children with measles vaccination, increasing viability of measles elimination efforts, and ensuring adequate resources for strengthening health systems,” said Dr. Perry and his colleagues.
Read the full report in the Nov. 13 issue of MMWR.
The number of global measles incidences declined and measles vaccine coverage increased between 2000 and 2010, but progress toward eliminating the disease has slowed since 2010, according to a report.
Between 2000 and 2014, annual reported measles incidences declined 73% worldwide, from 146 to 40 cases per million population. During this same time period, annual estimated measles deaths also decreased to 114,900 from 546,800 – a 79% drop, according to Dr. Robert T. Perry of the department of immunization, vaccines, and biologicals at the World Health Organization, and his colleagues (MMWR Morb Mortal Wkly Rep. 2015 Nov 13;64[44]:1246-51).
Measles vaccinations prevented an estimated 17.1 million deaths between 2000 and 2014, but vaccination coverage seems to be at a standstill. While the percentage of children aged 1 year receiving coverage with the first dose of measles-containing vaccine (MCVI) increased to 85% from 72% between 2000 and 2010, growth in this coverage has halted. The percentage of 1-year-old children receiving the MCVI has remained at 85% through 2014, with an estimated 20.6 million infants having not received the MCVI in 2014. Fifty-six percent (approximately 11.6 million) of these infants who did receive the MCVI were in the Democratic Republic of Congo, Ethiopia, India, Indonesia, Nigeria, and Pakistan. Global coverage of the second dose of measles-containing vaccine increased between 2000 and 2014, from 15% to 56%.
Despite the increased MCVI coverage since 2000, the World Health Assembly’s global milestones and measles elimination goals for 2015 will not be achieved, according to the WHO Strategic Advisory Group of Experts on Immunization.
“Reaching measles control and elimination goals will require addressing policy and practice gaps that prevent reaching larger numbers of children with measles vaccination, increasing viability of measles elimination efforts, and ensuring adequate resources for strengthening health systems,” said Dr. Perry and his colleagues.
Read the full report in the Nov. 13 issue of MMWR.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
High IgE linked to poor treatment outcomes in AD patients
A smaller percentage of atopic dermatitis (AD) patients with high baseline serum total IgE achieved a good response to treatment, compared with AD patients with lower serum total IgE at baseline, in a retrospective study of Finnish patients.
After adjustment of the data from the multivariate analyses, “the presence of contact allergies and high baseline IgE values [greater than or equal to] 10,0000 IU/mL remained risk factors for poor long-term outcome and were statistically significantly negatively associated with a good treatment response (OR [odds ratio], 0.162 and 0.062, respectively), and with complete remission (OR 0.287 and 0.158, respectively),” wrote Ville Kiiski of Skin and Allergy Hospital, Helsinki, and his colleagues.
The study comprised 169 individuals aged 14-78 with atopic dermatitis. Patients were reevaluated a mean of 4.15 years following the first visit to the specialized AD clinic at Skin and Allergy Hospital of Helsinki University Central Hospital. They received topical treatments as either maintenance therapy with tacrolimus or maintenance treatment with either a combination of topical tacrolimus and topical corticosteroids, or topical corticosteroids alone. A dermatologist provided the patients with a long-term treatment plan, and a nurse provided “hands-on training for adequate topical therapy regimens,” when necessary.
“In patients with baseline IgE [greater than or equal to] 10,000 IU/mL, proportions achieving complete remission or a good treatment response were only 8.7% and 14.3%, compared with 51.6% and 79.7% in patients with IgE [less than] 1,000 IU/mL, and 36.9% and 58.1% in patients with IgE 1,000-10.000 IU/mL, respectively,” the researchers wrote.
While this study’s results suggest that serum total IgE is a predictor of long-term treatment outcome in AD patients, a larger, prospective study is needed to confirm this, they added.
The authors declared no conflicts of interest.
Read the study in Acta Dermato-Venereologica (doi: 10.2340/00015555-2126).
A smaller percentage of atopic dermatitis (AD) patients with high baseline serum total IgE achieved a good response to treatment, compared with AD patients with lower serum total IgE at baseline, in a retrospective study of Finnish patients.
After adjustment of the data from the multivariate analyses, “the presence of contact allergies and high baseline IgE values [greater than or equal to] 10,0000 IU/mL remained risk factors for poor long-term outcome and were statistically significantly negatively associated with a good treatment response (OR [odds ratio], 0.162 and 0.062, respectively), and with complete remission (OR 0.287 and 0.158, respectively),” wrote Ville Kiiski of Skin and Allergy Hospital, Helsinki, and his colleagues.
The study comprised 169 individuals aged 14-78 with atopic dermatitis. Patients were reevaluated a mean of 4.15 years following the first visit to the specialized AD clinic at Skin and Allergy Hospital of Helsinki University Central Hospital. They received topical treatments as either maintenance therapy with tacrolimus or maintenance treatment with either a combination of topical tacrolimus and topical corticosteroids, or topical corticosteroids alone. A dermatologist provided the patients with a long-term treatment plan, and a nurse provided “hands-on training for adequate topical therapy regimens,” when necessary.
“In patients with baseline IgE [greater than or equal to] 10,000 IU/mL, proportions achieving complete remission or a good treatment response were only 8.7% and 14.3%, compared with 51.6% and 79.7% in patients with IgE [less than] 1,000 IU/mL, and 36.9% and 58.1% in patients with IgE 1,000-10.000 IU/mL, respectively,” the researchers wrote.
While this study’s results suggest that serum total IgE is a predictor of long-term treatment outcome in AD patients, a larger, prospective study is needed to confirm this, they added.
The authors declared no conflicts of interest.
Read the study in Acta Dermato-Venereologica (doi: 10.2340/00015555-2126).
A smaller percentage of atopic dermatitis (AD) patients with high baseline serum total IgE achieved a good response to treatment, compared with AD patients with lower serum total IgE at baseline, in a retrospective study of Finnish patients.
After adjustment of the data from the multivariate analyses, “the presence of contact allergies and high baseline IgE values [greater than or equal to] 10,0000 IU/mL remained risk factors for poor long-term outcome and were statistically significantly negatively associated with a good treatment response (OR [odds ratio], 0.162 and 0.062, respectively), and with complete remission (OR 0.287 and 0.158, respectively),” wrote Ville Kiiski of Skin and Allergy Hospital, Helsinki, and his colleagues.
The study comprised 169 individuals aged 14-78 with atopic dermatitis. Patients were reevaluated a mean of 4.15 years following the first visit to the specialized AD clinic at Skin and Allergy Hospital of Helsinki University Central Hospital. They received topical treatments as either maintenance therapy with tacrolimus or maintenance treatment with either a combination of topical tacrolimus and topical corticosteroids, or topical corticosteroids alone. A dermatologist provided the patients with a long-term treatment plan, and a nurse provided “hands-on training for adequate topical therapy regimens,” when necessary.
“In patients with baseline IgE [greater than or equal to] 10,000 IU/mL, proportions achieving complete remission or a good treatment response were only 8.7% and 14.3%, compared with 51.6% and 79.7% in patients with IgE [less than] 1,000 IU/mL, and 36.9% and 58.1% in patients with IgE 1,000-10.000 IU/mL, respectively,” the researchers wrote.
While this study’s results suggest that serum total IgE is a predictor of long-term treatment outcome in AD patients, a larger, prospective study is needed to confirm this, they added.
The authors declared no conflicts of interest.
Read the study in Acta Dermato-Venereologica (doi: 10.2340/00015555-2126).
FROM ACTA DERMATO-VENEREOLOGICA
Sofosbuvir plus velpatasvir effective at treating HCV genotype 1 and 3 patients
Patients with genotype 1 or 3 hepatitis C virus (HCV) infection responded well to therapy with 400 mg of sofosbuvir combined with 100 mg of velpatasvir for 12 weeks, in a randomized, phase II, open-label study conducted in 58 sites in Australia, New Zealand, and the United States.
This treatment program was “well tolerated and highly effective,” according to Dr. Stephen Pianko and his colleagues.
The study participants were divided into three cohorts: the first included patients with genotype 3 HCV infection without cirrhosis, the second included patients with genotype 3 HCV with compensated cirrhosis, and the third included patients with genotype 1 HCV infection that was unsuccessfully treated with a protease inhibitor with peginterferon and ribavirin (50% could have compensated cirrhosis). All patients were treatment experienced and received 12 weeks of drug therapy that included 400 mg of sofosbuvir once daily. Patients in each cohort were randomly assigned to also being treated with 25 mg of velpatasvir once daily with or without ribavirin, or 100 mg of velpatasvir once daily with or without ribavirin.
All patients in cohort 1 who were treated with 400 mg of sofosbuvir combined with 100 mg of velpatasvir or 400 mg of sofosbuvir combined with 100 mg of velpatasvir plus ribavirin experienced a sustained virologic response at week 12 after treatment. The same was true for 100% of patients in cohort 3, who either received 400 mg of sofosbuvir combined with 25 mg velpatasvir or 400 mg of sofosbuvir combined with 100 mg of velpatasvir.
The proportion of patients in the other treatment categories who achieved sustained virologic response 12 weeks following therapy ranged from 58% to 97%.
Eighty-two percent (263 of 321) of patients experienced at least 1 adverse event. Common adverse events included headache and fatigue. "More patients in the groups receiving ribavirin had fatigue, nausea, and pruritus; decreased hemoglobin levels; and increased bilirubin." One patient had an elevated alanine aminotransferase level and y-glutamyl transferase level on treatment day 80, which resulted in treatment discontinuation. Eight study participants experienced "serious adverse events that were not considered to be related to a study drug."
“In summary, sofosbuvir plus 100 mg of velpatasvir provided high rates of [sustained virologic response at week 12 after treatment] in treatment-experienced patients with genotype 1 or 3 HCV infection, including those with compensated cirrhosis, but results will need confirmation in a phase 3 trial,” according to the researchers.
Read the full study in Annals of Internal Medicine (doi: 10.7326/M15-1014).
*This story was updated 11/11/2015.
Patients with genotype 1 or 3 hepatitis C virus (HCV) infection responded well to therapy with 400 mg of sofosbuvir combined with 100 mg of velpatasvir for 12 weeks, in a randomized, phase II, open-label study conducted in 58 sites in Australia, New Zealand, and the United States.
This treatment program was “well tolerated and highly effective,” according to Dr. Stephen Pianko and his colleagues.
The study participants were divided into three cohorts: the first included patients with genotype 3 HCV infection without cirrhosis, the second included patients with genotype 3 HCV with compensated cirrhosis, and the third included patients with genotype 1 HCV infection that was unsuccessfully treated with a protease inhibitor with peginterferon and ribavirin (50% could have compensated cirrhosis). All patients were treatment experienced and received 12 weeks of drug therapy that included 400 mg of sofosbuvir once daily. Patients in each cohort were randomly assigned to also being treated with 25 mg of velpatasvir once daily with or without ribavirin, or 100 mg of velpatasvir once daily with or without ribavirin.
All patients in cohort 1 who were treated with 400 mg of sofosbuvir combined with 100 mg of velpatasvir or 400 mg of sofosbuvir combined with 100 mg of velpatasvir plus ribavirin experienced a sustained virologic response at week 12 after treatment. The same was true for 100% of patients in cohort 3, who either received 400 mg of sofosbuvir combined with 25 mg velpatasvir or 400 mg of sofosbuvir combined with 100 mg of velpatasvir.
The proportion of patients in the other treatment categories who achieved sustained virologic response 12 weeks following therapy ranged from 58% to 97%.
Eighty-two percent (263 of 321) of patients experienced at least 1 adverse event. Common adverse events included headache and fatigue. "More patients in the groups receiving ribavirin had fatigue, nausea, and pruritus; decreased hemoglobin levels; and increased bilirubin." One patient had an elevated alanine aminotransferase level and y-glutamyl transferase level on treatment day 80, which resulted in treatment discontinuation. Eight study participants experienced "serious adverse events that were not considered to be related to a study drug."
“In summary, sofosbuvir plus 100 mg of velpatasvir provided high rates of [sustained virologic response at week 12 after treatment] in treatment-experienced patients with genotype 1 or 3 HCV infection, including those with compensated cirrhosis, but results will need confirmation in a phase 3 trial,” according to the researchers.
Read the full study in Annals of Internal Medicine (doi: 10.7326/M15-1014).
*This story was updated 11/11/2015.
Patients with genotype 1 or 3 hepatitis C virus (HCV) infection responded well to therapy with 400 mg of sofosbuvir combined with 100 mg of velpatasvir for 12 weeks, in a randomized, phase II, open-label study conducted in 58 sites in Australia, New Zealand, and the United States.
This treatment program was “well tolerated and highly effective,” according to Dr. Stephen Pianko and his colleagues.
The study participants were divided into three cohorts: the first included patients with genotype 3 HCV infection without cirrhosis, the second included patients with genotype 3 HCV with compensated cirrhosis, and the third included patients with genotype 1 HCV infection that was unsuccessfully treated with a protease inhibitor with peginterferon and ribavirin (50% could have compensated cirrhosis). All patients were treatment experienced and received 12 weeks of drug therapy that included 400 mg of sofosbuvir once daily. Patients in each cohort were randomly assigned to also being treated with 25 mg of velpatasvir once daily with or without ribavirin, or 100 mg of velpatasvir once daily with or without ribavirin.
All patients in cohort 1 who were treated with 400 mg of sofosbuvir combined with 100 mg of velpatasvir or 400 mg of sofosbuvir combined with 100 mg of velpatasvir plus ribavirin experienced a sustained virologic response at week 12 after treatment. The same was true for 100% of patients in cohort 3, who either received 400 mg of sofosbuvir combined with 25 mg velpatasvir or 400 mg of sofosbuvir combined with 100 mg of velpatasvir.
The proportion of patients in the other treatment categories who achieved sustained virologic response 12 weeks following therapy ranged from 58% to 97%.
Eighty-two percent (263 of 321) of patients experienced at least 1 adverse event. Common adverse events included headache and fatigue. "More patients in the groups receiving ribavirin had fatigue, nausea, and pruritus; decreased hemoglobin levels; and increased bilirubin." One patient had an elevated alanine aminotransferase level and y-glutamyl transferase level on treatment day 80, which resulted in treatment discontinuation. Eight study participants experienced "serious adverse events that were not considered to be related to a study drug."
“In summary, sofosbuvir plus 100 mg of velpatasvir provided high rates of [sustained virologic response at week 12 after treatment] in treatment-experienced patients with genotype 1 or 3 HCV infection, including those with compensated cirrhosis, but results will need confirmation in a phase 3 trial,” according to the researchers.
Read the full study in Annals of Internal Medicine (doi: 10.7326/M15-1014).
*This story was updated 11/11/2015.
FROM ANNALS OF INTERNAL MEDICINE
Number of TB-caused deaths fall, but disease still kills 1 million-plus
Tuberculosis (TB) mortality has fallen 47% since 1990, and the reporting on incidences of the disease has improved, says a global report by the World Health Organization (WHO). News about this disease is not all positive, however; TB continues to be one of the world’s deadliest diseases and many cases of TB went unreported last year, according to the WHO report.
Most improvements in mortality rate for TB patients occurred at the beginning of the 21st century, when the United Nations established the Millennium Development Goals, says the report. Such goals included halting and reversing TB incidence on a worldwide basis, in each of the 6 WHO regions, and in 16 of the 22 high-burden countries that collectively account for 80% of TB cases.
“In all, effective diagnosis and treatment of TB saved an estimated 43 million lives between 2000 and 2014,” says the report.
Better reporting on TB’s prevalence led to the first increase in the number of TB cases reported since 2007.
“The annual total of new TB cases, which had been about 5.7 million until 2013, rose to slightly more than 6 million in 2014 (an increase of 6%). This was mostly due to a 29% increase in notification in India, which followed the introduction of a policy of mandatory notification in May 2012, creation of a national Web-based reporting system in June 2012, and intensified efforts to engage the private health sector,” according to the report.
Despite these improvements in data collection of TB incidents, 37% of new TB cases were undiagnosed or not reported last year, with 9.6 million people having fallen sick to TB during a year when just 6 million new cases were reported, according to estimates. Regarding multidrug-resistant TB cases specifically, only 123,000 of an estimated 480,000 cases were detected and reported.
As for the deadliness of the disease, TB killed 1.5 million people in 2014.
Read the full report on the WHO website.
Tuberculosis (TB) mortality has fallen 47% since 1990, and the reporting on incidences of the disease has improved, says a global report by the World Health Organization (WHO). News about this disease is not all positive, however; TB continues to be one of the world’s deadliest diseases and many cases of TB went unreported last year, according to the WHO report.
Most improvements in mortality rate for TB patients occurred at the beginning of the 21st century, when the United Nations established the Millennium Development Goals, says the report. Such goals included halting and reversing TB incidence on a worldwide basis, in each of the 6 WHO regions, and in 16 of the 22 high-burden countries that collectively account for 80% of TB cases.
“In all, effective diagnosis and treatment of TB saved an estimated 43 million lives between 2000 and 2014,” says the report.
Better reporting on TB’s prevalence led to the first increase in the number of TB cases reported since 2007.
“The annual total of new TB cases, which had been about 5.7 million until 2013, rose to slightly more than 6 million in 2014 (an increase of 6%). This was mostly due to a 29% increase in notification in India, which followed the introduction of a policy of mandatory notification in May 2012, creation of a national Web-based reporting system in June 2012, and intensified efforts to engage the private health sector,” according to the report.
Despite these improvements in data collection of TB incidents, 37% of new TB cases were undiagnosed or not reported last year, with 9.6 million people having fallen sick to TB during a year when just 6 million new cases were reported, according to estimates. Regarding multidrug-resistant TB cases specifically, only 123,000 of an estimated 480,000 cases were detected and reported.
As for the deadliness of the disease, TB killed 1.5 million people in 2014.
Read the full report on the WHO website.
Tuberculosis (TB) mortality has fallen 47% since 1990, and the reporting on incidences of the disease has improved, says a global report by the World Health Organization (WHO). News about this disease is not all positive, however; TB continues to be one of the world’s deadliest diseases and many cases of TB went unreported last year, according to the WHO report.
Most improvements in mortality rate for TB patients occurred at the beginning of the 21st century, when the United Nations established the Millennium Development Goals, says the report. Such goals included halting and reversing TB incidence on a worldwide basis, in each of the 6 WHO regions, and in 16 of the 22 high-burden countries that collectively account for 80% of TB cases.
“In all, effective diagnosis and treatment of TB saved an estimated 43 million lives between 2000 and 2014,” says the report.
Better reporting on TB’s prevalence led to the first increase in the number of TB cases reported since 2007.
“The annual total of new TB cases, which had been about 5.7 million until 2013, rose to slightly more than 6 million in 2014 (an increase of 6%). This was mostly due to a 29% increase in notification in India, which followed the introduction of a policy of mandatory notification in May 2012, creation of a national Web-based reporting system in June 2012, and intensified efforts to engage the private health sector,” according to the report.
Despite these improvements in data collection of TB incidents, 37% of new TB cases were undiagnosed or not reported last year, with 9.6 million people having fallen sick to TB during a year when just 6 million new cases were reported, according to estimates. Regarding multidrug-resistant TB cases specifically, only 123,000 of an estimated 480,000 cases were detected and reported.
As for the deadliness of the disease, TB killed 1.5 million people in 2014.
Read the full report on the WHO website.
Raloxifene ineffective for treating AD patients
Treatment with the estrogen-receptor modulator raloxifene does not significantly improve cognitive function in women with Alzheimer’s disease (AD), results of a U.S. randomized, double-blind, placebo-controlled trial suggest.
The study’s researchers gave (120 mg) oral raloxifene or an identical placebo once daily to 42 postmenopausal women with dementia and probable AD of mild to moderate severity. Of those women, 39 continued to take the drug for the study’s full treatment period of 12 months.
At 12 months, the mean Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog) scores for both the treatment group and the placebo group were modestly lower than mean baseline scores. Dementia rating, function, and behavior also declined by similar amounts in both treatment groups.
Even caregivers’ responses to surveys suggested that both groups of patients had similar outcomes; caregiver burden and caregiver distress increased modestly over time for both groups.
Three of the study’s participants experienced serious adverse events, including one death of a woman who had taken raloxifene. This woman’s death was preceded by her developing pneumonia and heart failure, and an ischemic stroke, which may have been related to her use of the drug, according to Dr. Victor W. Henderson and his colleagues. Also in the experimental group was a women diagnosed with colon cancer. One woman in the placebo group was hospitalized for hallucinations and agitation.
“These results provide information to guide consideration and design of future trials. The essentially null effect of raloxifene on the primary outcomes implies a low likelihood of positive results but does not exclude the possibility of modest cognitive benefit or harm,” according to the researchers.
Read the full study in Neurology.
Treatment with the estrogen-receptor modulator raloxifene does not significantly improve cognitive function in women with Alzheimer’s disease (AD), results of a U.S. randomized, double-blind, placebo-controlled trial suggest.
The study’s researchers gave (120 mg) oral raloxifene or an identical placebo once daily to 42 postmenopausal women with dementia and probable AD of mild to moderate severity. Of those women, 39 continued to take the drug for the study’s full treatment period of 12 months.
At 12 months, the mean Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog) scores for both the treatment group and the placebo group were modestly lower than mean baseline scores. Dementia rating, function, and behavior also declined by similar amounts in both treatment groups.
Even caregivers’ responses to surveys suggested that both groups of patients had similar outcomes; caregiver burden and caregiver distress increased modestly over time for both groups.
Three of the study’s participants experienced serious adverse events, including one death of a woman who had taken raloxifene. This woman’s death was preceded by her developing pneumonia and heart failure, and an ischemic stroke, which may have been related to her use of the drug, according to Dr. Victor W. Henderson and his colleagues. Also in the experimental group was a women diagnosed with colon cancer. One woman in the placebo group was hospitalized for hallucinations and agitation.
“These results provide information to guide consideration and design of future trials. The essentially null effect of raloxifene on the primary outcomes implies a low likelihood of positive results but does not exclude the possibility of modest cognitive benefit or harm,” according to the researchers.
Read the full study in Neurology.
Treatment with the estrogen-receptor modulator raloxifene does not significantly improve cognitive function in women with Alzheimer’s disease (AD), results of a U.S. randomized, double-blind, placebo-controlled trial suggest.
The study’s researchers gave (120 mg) oral raloxifene or an identical placebo once daily to 42 postmenopausal women with dementia and probable AD of mild to moderate severity. Of those women, 39 continued to take the drug for the study’s full treatment period of 12 months.
At 12 months, the mean Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog) scores for both the treatment group and the placebo group were modestly lower than mean baseline scores. Dementia rating, function, and behavior also declined by similar amounts in both treatment groups.
Even caregivers’ responses to surveys suggested that both groups of patients had similar outcomes; caregiver burden and caregiver distress increased modestly over time for both groups.
Three of the study’s participants experienced serious adverse events, including one death of a woman who had taken raloxifene. This woman’s death was preceded by her developing pneumonia and heart failure, and an ischemic stroke, which may have been related to her use of the drug, according to Dr. Victor W. Henderson and his colleagues. Also in the experimental group was a women diagnosed with colon cancer. One woman in the placebo group was hospitalized for hallucinations and agitation.
“These results provide information to guide consideration and design of future trials. The essentially null effect of raloxifene on the primary outcomes implies a low likelihood of positive results but does not exclude the possibility of modest cognitive benefit or harm,” according to the researchers.
Read the full study in Neurology.
FROM NEUROLOGY
Case reports: Ingestion of aripiprazole precedes false positives for amphetamine use
Two children who had ingested aripiprazole but not amphetamines tested positive for amphetamine use in urine drug screens (UDSs) performed within 24 hours of their drug use, according to two case reports by Justin Kaplan, Pharm.D., of Hackensack (N.J.) University Medical Center and his colleagues.
In both cases, aripiprazole had been prescribed to the father of the child and had been taken by the child without the knowledge or auspices of a parent. Both of the children were admitted to hospitals where their urine was screened for drugs.
“To our knowledge this case series is the first to document potential false-positive UDSs after accidental ingestion of aripiprazole,” said the researchers. “In both cases, the presentation of drowsiness, lethargy, and ataxia were more consistent with ingestion of an atypical antipsychotic than with amphetamines.”
In one of the cases, a 2-year-old girl was found holding an open bottle of aripiprazole 15-mg tablets by her parents. The parents’ report of the incident suggested that the child had ingested three such tablets. The child’s urine was screened for amphetamines twice, in the hospital where she was admitted; the first screen, which was performed the morning after the child had been taken to a hospital, revealed an amphetamine concentration of 1,048 ng/mL. The child’s second UDS, which was performed the following day, indicated a 949 ng/mL concentration of amphetamines. Outside of the hospital, laboratory tests were performed on the child’s blood and urine samples from the day following her admittance to the hospital. Both of these additional tests were negative for amphetamines, suggesting that the results of the in-hospital UDSs had been false positives.
The other case involved a 20-month-old girl, whose father found her with pills scattered around her crib. The drugs were part of a 1-week supply of drugs of the father. The medications included alprazolam 2.5 mg, fluvoxamine 2,100 mg, clonazepam 17.5 mg, buspirone 420 mg, and aripiprazole 35 mg. This child’s urine was also screened for drugs twice at the hospital where she was admitted; this child only tested positive for amphetamine in the first assessment, with a 311 ng/mL concentration of amphetamines having been found in that UDS. As with the first case, this child’s urine and blood samples were subjected to off-site laboratory tests, which found no presence of amphetamines.
“There are several limitations to UDS immunoassays. Most important, poor specificity is associated with a risk of false-positive testing. A negative result does not exclude the possibility that the substance is present if it is below the lower threshold of detection. Additionally, there is no way to quantitatively correlate a positive result with the extent of immunoassays. Therefore immunoassays are the first step in a two-step system, in which all positive results must be confirmed by more reliable methods such as [gas chromatography mass spectrometry],” the researchers said.
Read the full study in Pediatrics. doi: 10:1542/peds.2014-3333.
Two children who had ingested aripiprazole but not amphetamines tested positive for amphetamine use in urine drug screens (UDSs) performed within 24 hours of their drug use, according to two case reports by Justin Kaplan, Pharm.D., of Hackensack (N.J.) University Medical Center and his colleagues.
In both cases, aripiprazole had been prescribed to the father of the child and had been taken by the child without the knowledge or auspices of a parent. Both of the children were admitted to hospitals where their urine was screened for drugs.
“To our knowledge this case series is the first to document potential false-positive UDSs after accidental ingestion of aripiprazole,” said the researchers. “In both cases, the presentation of drowsiness, lethargy, and ataxia were more consistent with ingestion of an atypical antipsychotic than with amphetamines.”
In one of the cases, a 2-year-old girl was found holding an open bottle of aripiprazole 15-mg tablets by her parents. The parents’ report of the incident suggested that the child had ingested three such tablets. The child’s urine was screened for amphetamines twice, in the hospital where she was admitted; the first screen, which was performed the morning after the child had been taken to a hospital, revealed an amphetamine concentration of 1,048 ng/mL. The child’s second UDS, which was performed the following day, indicated a 949 ng/mL concentration of amphetamines. Outside of the hospital, laboratory tests were performed on the child’s blood and urine samples from the day following her admittance to the hospital. Both of these additional tests were negative for amphetamines, suggesting that the results of the in-hospital UDSs had been false positives.
The other case involved a 20-month-old girl, whose father found her with pills scattered around her crib. The drugs were part of a 1-week supply of drugs of the father. The medications included alprazolam 2.5 mg, fluvoxamine 2,100 mg, clonazepam 17.5 mg, buspirone 420 mg, and aripiprazole 35 mg. This child’s urine was also screened for drugs twice at the hospital where she was admitted; this child only tested positive for amphetamine in the first assessment, with a 311 ng/mL concentration of amphetamines having been found in that UDS. As with the first case, this child’s urine and blood samples were subjected to off-site laboratory tests, which found no presence of amphetamines.
“There are several limitations to UDS immunoassays. Most important, poor specificity is associated with a risk of false-positive testing. A negative result does not exclude the possibility that the substance is present if it is below the lower threshold of detection. Additionally, there is no way to quantitatively correlate a positive result with the extent of immunoassays. Therefore immunoassays are the first step in a two-step system, in which all positive results must be confirmed by more reliable methods such as [gas chromatography mass spectrometry],” the researchers said.
Read the full study in Pediatrics. doi: 10:1542/peds.2014-3333.
Two children who had ingested aripiprazole but not amphetamines tested positive for amphetamine use in urine drug screens (UDSs) performed within 24 hours of their drug use, according to two case reports by Justin Kaplan, Pharm.D., of Hackensack (N.J.) University Medical Center and his colleagues.
In both cases, aripiprazole had been prescribed to the father of the child and had been taken by the child without the knowledge or auspices of a parent. Both of the children were admitted to hospitals where their urine was screened for drugs.
“To our knowledge this case series is the first to document potential false-positive UDSs after accidental ingestion of aripiprazole,” said the researchers. “In both cases, the presentation of drowsiness, lethargy, and ataxia were more consistent with ingestion of an atypical antipsychotic than with amphetamines.”
In one of the cases, a 2-year-old girl was found holding an open bottle of aripiprazole 15-mg tablets by her parents. The parents’ report of the incident suggested that the child had ingested three such tablets. The child’s urine was screened for amphetamines twice, in the hospital where she was admitted; the first screen, which was performed the morning after the child had been taken to a hospital, revealed an amphetamine concentration of 1,048 ng/mL. The child’s second UDS, which was performed the following day, indicated a 949 ng/mL concentration of amphetamines. Outside of the hospital, laboratory tests were performed on the child’s blood and urine samples from the day following her admittance to the hospital. Both of these additional tests were negative for amphetamines, suggesting that the results of the in-hospital UDSs had been false positives.
The other case involved a 20-month-old girl, whose father found her with pills scattered around her crib. The drugs were part of a 1-week supply of drugs of the father. The medications included alprazolam 2.5 mg, fluvoxamine 2,100 mg, clonazepam 17.5 mg, buspirone 420 mg, and aripiprazole 35 mg. This child’s urine was also screened for drugs twice at the hospital where she was admitted; this child only tested positive for amphetamine in the first assessment, with a 311 ng/mL concentration of amphetamines having been found in that UDS. As with the first case, this child’s urine and blood samples were subjected to off-site laboratory tests, which found no presence of amphetamines.
“There are several limitations to UDS immunoassays. Most important, poor specificity is associated with a risk of false-positive testing. A negative result does not exclude the possibility that the substance is present if it is below the lower threshold of detection. Additionally, there is no way to quantitatively correlate a positive result with the extent of immunoassays. Therefore immunoassays are the first step in a two-step system, in which all positive results must be confirmed by more reliable methods such as [gas chromatography mass spectrometry],” the researchers said.
Read the full study in Pediatrics. doi: 10:1542/peds.2014-3333.
FROM PEDIATRICS
ILDS establishes guidelines for treating AK patients
The International Leagues of Dermatological Societies (ILDS) in cooperation with the European Dermatology Forum has developed consensus-based guidelines for the treatment of actinic keratosis (AK), which are published in the Journal of the European Academy of Dermatology and Venereology.
“The guidelines were elaborated along adapted recommendations by the WHO guidelines review committee and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group,” say R. N. Werner and colleagues of the Medical University of Berlin. The guidelines include recommendations for treatment of different subgroups of AK patients, how to make an AK diagnosis, how to assess AK patients, and how to define AK.
The ILDS recommends or suggests the following interventions for treating patients who have single AK lesions:
• Cryotherapy
• Curettage (discrete, hyperkeratotic lesions)
• 0.5% 5-fluorouracil (5-FU)
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 3.75% imiquimod
• 5% imiquimod
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)
• 5-aminolevulinic acid-photodynamic therapy (ALA-PDT)
• methylaminolevulinate-photodynamic therapy (MAL-PDT)
For patients with multiple AK lesions/field cancerization, the ILDS recommends* or suggests that patients use the following therapies:
• 0.5% 5-FU*
• 3.75% imiquimod*
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)*
• ALA-PDT*
• MAL-PDT*
• Cryotherapy (patients with multiple lesions, especially for multiple discrete lesions; not suitable for the treatment of field cancerization)
• 3% diclofenac in 2.5% hyaluronic acid gel
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 5% imiquimod
• 2.5% imiquimod
• CO2 laser and Er:YAG laser
For immunosuppressed AK patients, the ILDS suggests the following treatments:
• Cryotherapy (especially for single lesions or multiple discrete lesions; not suitable for the treatment of field cancerization);
• Curettage (discrete, hyperkeratotic lesions)
• 5% 5-FU
• 5% imiquimod
• ALA-PDT
• MAL-PDT
The ILDS additionally recommends that immunosuppressed AK patients not use CO2 laser and Er:YAG laser.
“Deviation from the recommendations may be justified or inevitable in specific situations. The ultimate judgment regarding patient care must be individualized and must be made by the physician and patient in light of all presenting circumstances,” the authors said. “International guidelines are intended to be adapted to national or regional circumstances” (J Eur Acad Dermatol Venereol. 2015;29:2069-79).
The “long version of the guidelines” is available as an online supplement. Additionally, a methods report, results report, and declarations of interest of the guidelines development have been published at doi: 10.1111/jdv.13179 in the Journal of the European Academy of Dermatology and Venereology (2015).
The International Leagues of Dermatological Societies (ILDS) in cooperation with the European Dermatology Forum has developed consensus-based guidelines for the treatment of actinic keratosis (AK), which are published in the Journal of the European Academy of Dermatology and Venereology.
“The guidelines were elaborated along adapted recommendations by the WHO guidelines review committee and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group,” say R. N. Werner and colleagues of the Medical University of Berlin. The guidelines include recommendations for treatment of different subgroups of AK patients, how to make an AK diagnosis, how to assess AK patients, and how to define AK.
The ILDS recommends or suggests the following interventions for treating patients who have single AK lesions:
• Cryotherapy
• Curettage (discrete, hyperkeratotic lesions)
• 0.5% 5-fluorouracil (5-FU)
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 3.75% imiquimod
• 5% imiquimod
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)
• 5-aminolevulinic acid-photodynamic therapy (ALA-PDT)
• methylaminolevulinate-photodynamic therapy (MAL-PDT)
For patients with multiple AK lesions/field cancerization, the ILDS recommends* or suggests that patients use the following therapies:
• 0.5% 5-FU*
• 3.75% imiquimod*
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)*
• ALA-PDT*
• MAL-PDT*
• Cryotherapy (patients with multiple lesions, especially for multiple discrete lesions; not suitable for the treatment of field cancerization)
• 3% diclofenac in 2.5% hyaluronic acid gel
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 5% imiquimod
• 2.5% imiquimod
• CO2 laser and Er:YAG laser
For immunosuppressed AK patients, the ILDS suggests the following treatments:
• Cryotherapy (especially for single lesions or multiple discrete lesions; not suitable for the treatment of field cancerization);
• Curettage (discrete, hyperkeratotic lesions)
• 5% 5-FU
• 5% imiquimod
• ALA-PDT
• MAL-PDT
The ILDS additionally recommends that immunosuppressed AK patients not use CO2 laser and Er:YAG laser.
“Deviation from the recommendations may be justified or inevitable in specific situations. The ultimate judgment regarding patient care must be individualized and must be made by the physician and patient in light of all presenting circumstances,” the authors said. “International guidelines are intended to be adapted to national or regional circumstances” (J Eur Acad Dermatol Venereol. 2015;29:2069-79).
The “long version of the guidelines” is available as an online supplement. Additionally, a methods report, results report, and declarations of interest of the guidelines development have been published at doi: 10.1111/jdv.13179 in the Journal of the European Academy of Dermatology and Venereology (2015).
The International Leagues of Dermatological Societies (ILDS) in cooperation with the European Dermatology Forum has developed consensus-based guidelines for the treatment of actinic keratosis (AK), which are published in the Journal of the European Academy of Dermatology and Venereology.
“The guidelines were elaborated along adapted recommendations by the WHO guidelines review committee and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group,” say R. N. Werner and colleagues of the Medical University of Berlin. The guidelines include recommendations for treatment of different subgroups of AK patients, how to make an AK diagnosis, how to assess AK patients, and how to define AK.
The ILDS recommends or suggests the following interventions for treating patients who have single AK lesions:
• Cryotherapy
• Curettage (discrete, hyperkeratotic lesions)
• 0.5% 5-fluorouracil (5-FU)
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 3.75% imiquimod
• 5% imiquimod
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)
• 5-aminolevulinic acid-photodynamic therapy (ALA-PDT)
• methylaminolevulinate-photodynamic therapy (MAL-PDT)
For patients with multiple AK lesions/field cancerization, the ILDS recommends* or suggests that patients use the following therapies:
• 0.5% 5-FU*
• 3.75% imiquimod*
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)*
• ALA-PDT*
• MAL-PDT*
• Cryotherapy (patients with multiple lesions, especially for multiple discrete lesions; not suitable for the treatment of field cancerization)
• 3% diclofenac in 2.5% hyaluronic acid gel
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 5% imiquimod
• 2.5% imiquimod
• CO2 laser and Er:YAG laser
For immunosuppressed AK patients, the ILDS suggests the following treatments:
• Cryotherapy (especially for single lesions or multiple discrete lesions; not suitable for the treatment of field cancerization);
• Curettage (discrete, hyperkeratotic lesions)
• 5% 5-FU
• 5% imiquimod
• ALA-PDT
• MAL-PDT
The ILDS additionally recommends that immunosuppressed AK patients not use CO2 laser and Er:YAG laser.
“Deviation from the recommendations may be justified or inevitable in specific situations. The ultimate judgment regarding patient care must be individualized and must be made by the physician and patient in light of all presenting circumstances,” the authors said. “International guidelines are intended to be adapted to national or regional circumstances” (J Eur Acad Dermatol Venereol. 2015;29:2069-79).
The “long version of the guidelines” is available as an online supplement. Additionally, a methods report, results report, and declarations of interest of the guidelines development have been published at doi: 10.1111/jdv.13179 in the Journal of the European Academy of Dermatology and Venereology (2015).
FROM JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Availability of flavored tobacco products drives tobacco use in youths
The availability of flavored tobacco products appears to be a major driver of tobacco use in youths, suggests an analysis of a nationally representative longitudinal cohort study from 2013 to 2014.
The 13,651 youth participants (aged 12-17 years) in the U.S. Population Assessment of Tobacco and Health (PATH) study responded to questions about ever and past 30-day use of various tobacco products. For each product ever used, youths indicated whether the first product they used was flavored. Users of noncigarette tobacco products, including e-cigarettes, reported past 30-day use of a flavored product or products; of those individuals, the ones that had used a tobacco product (including e-cigarettes) other than a cigarette within the past 30 days reported their reasons for engaging in such activities.
The majority of tobacco products users said that their inaugural taste of tobacco was in the form of a flavored product, including 88.7% of ever hookah users, 81.0% of ever e-cigarette users, 65.4% of ever users of any cigar type, and 50.1% of ever cigarette smokers. Among study participants who had used tobacco within the past 30 days, 79.8% had used a flavored tobacco product.
Furthermore, well over 50% of individuals who had used noncigarette tobacco products within the past 30 days reported the availability of flavored versions of such products as having been among their leading reasons for using them. Specifically, 81.5% of e-cigarette users, 78.9% of hookah users, 73.8% of cigar users, 69.3% of smokeless tobacco users, and 67.2% of snus pouch users said they liked their respective tobacco products “because they come in flavors.”
“Consistent with national school-based estimates, this study confirms widespread appeal of flavored products among youth tobacco users,” said Bridget K. Ambrose, Ph.D., of the Center for Tobacco Products, Food and Drug Administration, Silver Spring, Md., and her colleagues.
“Data from future PATH study waves can provide information on tobacco use trajectories following experimentation with flavored, compared with nonflavored products,” according to the researchers.
Read the full study in JAMA (doi: 10.1001/jama.2015.13802).
The availability of flavored tobacco products appears to be a major driver of tobacco use in youths, suggests an analysis of a nationally representative longitudinal cohort study from 2013 to 2014.
The 13,651 youth participants (aged 12-17 years) in the U.S. Population Assessment of Tobacco and Health (PATH) study responded to questions about ever and past 30-day use of various tobacco products. For each product ever used, youths indicated whether the first product they used was flavored. Users of noncigarette tobacco products, including e-cigarettes, reported past 30-day use of a flavored product or products; of those individuals, the ones that had used a tobacco product (including e-cigarettes) other than a cigarette within the past 30 days reported their reasons for engaging in such activities.
The majority of tobacco products users said that their inaugural taste of tobacco was in the form of a flavored product, including 88.7% of ever hookah users, 81.0% of ever e-cigarette users, 65.4% of ever users of any cigar type, and 50.1% of ever cigarette smokers. Among study participants who had used tobacco within the past 30 days, 79.8% had used a flavored tobacco product.
Furthermore, well over 50% of individuals who had used noncigarette tobacco products within the past 30 days reported the availability of flavored versions of such products as having been among their leading reasons for using them. Specifically, 81.5% of e-cigarette users, 78.9% of hookah users, 73.8% of cigar users, 69.3% of smokeless tobacco users, and 67.2% of snus pouch users said they liked their respective tobacco products “because they come in flavors.”
“Consistent with national school-based estimates, this study confirms widespread appeal of flavored products among youth tobacco users,” said Bridget K. Ambrose, Ph.D., of the Center for Tobacco Products, Food and Drug Administration, Silver Spring, Md., and her colleagues.
“Data from future PATH study waves can provide information on tobacco use trajectories following experimentation with flavored, compared with nonflavored products,” according to the researchers.
Read the full study in JAMA (doi: 10.1001/jama.2015.13802).
The availability of flavored tobacco products appears to be a major driver of tobacco use in youths, suggests an analysis of a nationally representative longitudinal cohort study from 2013 to 2014.
The 13,651 youth participants (aged 12-17 years) in the U.S. Population Assessment of Tobacco and Health (PATH) study responded to questions about ever and past 30-day use of various tobacco products. For each product ever used, youths indicated whether the first product they used was flavored. Users of noncigarette tobacco products, including e-cigarettes, reported past 30-day use of a flavored product or products; of those individuals, the ones that had used a tobacco product (including e-cigarettes) other than a cigarette within the past 30 days reported their reasons for engaging in such activities.
The majority of tobacco products users said that their inaugural taste of tobacco was in the form of a flavored product, including 88.7% of ever hookah users, 81.0% of ever e-cigarette users, 65.4% of ever users of any cigar type, and 50.1% of ever cigarette smokers. Among study participants who had used tobacco within the past 30 days, 79.8% had used a flavored tobacco product.
Furthermore, well over 50% of individuals who had used noncigarette tobacco products within the past 30 days reported the availability of flavored versions of such products as having been among their leading reasons for using them. Specifically, 81.5% of e-cigarette users, 78.9% of hookah users, 73.8% of cigar users, 69.3% of smokeless tobacco users, and 67.2% of snus pouch users said they liked their respective tobacco products “because they come in flavors.”
“Consistent with national school-based estimates, this study confirms widespread appeal of flavored products among youth tobacco users,” said Bridget K. Ambrose, Ph.D., of the Center for Tobacco Products, Food and Drug Administration, Silver Spring, Md., and her colleagues.
“Data from future PATH study waves can provide information on tobacco use trajectories following experimentation with flavored, compared with nonflavored products,” according to the researchers.
Read the full study in JAMA (doi: 10.1001/jama.2015.13802).
FROM JAMA