User login
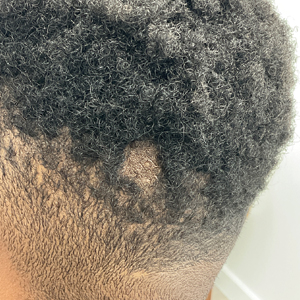
Hairless Scalp Lesion
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
A 23-year-old man presented to the dermatology clinic with hair loss on the scalp of several years’ duration. The patient reported persistent pigmented bumps on the back of the scalp. He denied any pruritus or pain and had no systemic symptoms or comorbidities. Physical examination revealed a 1×1.5-cm, yellow-brown, hairless plaque on the left parietal scalp.
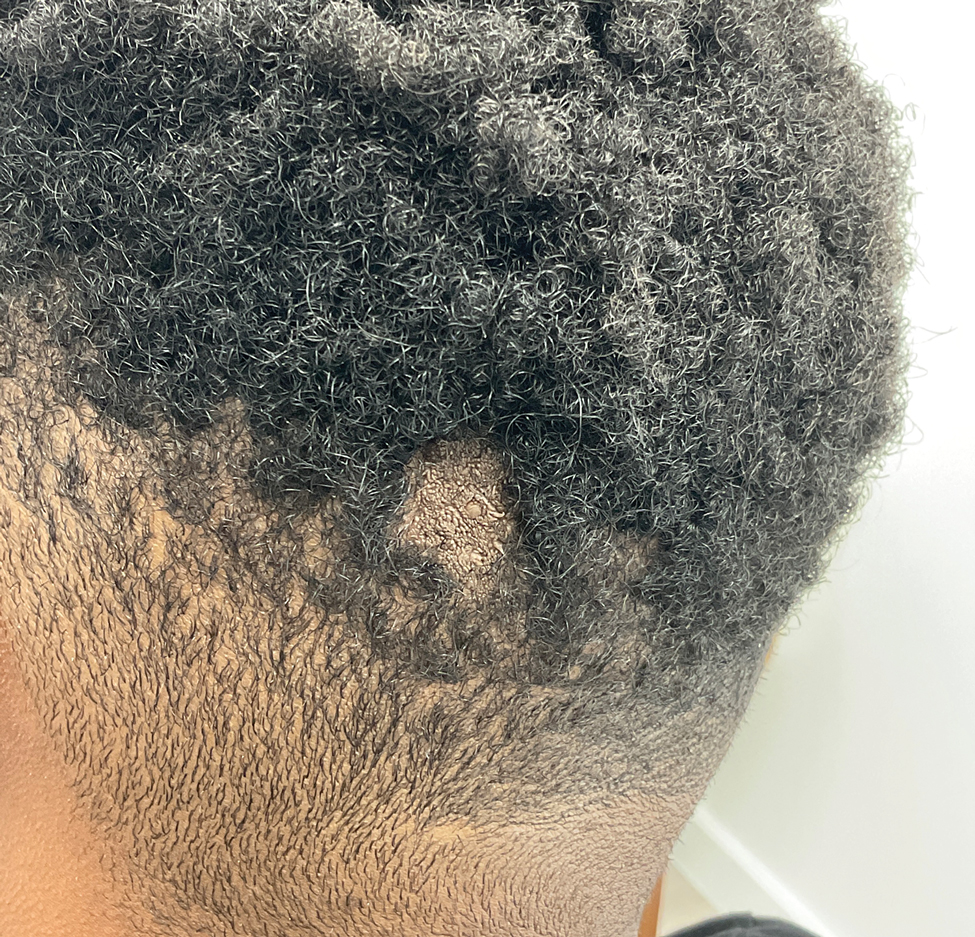
A 62-year-old Black female presented with an epidermal inclusion cyst on her left upper back
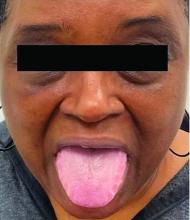
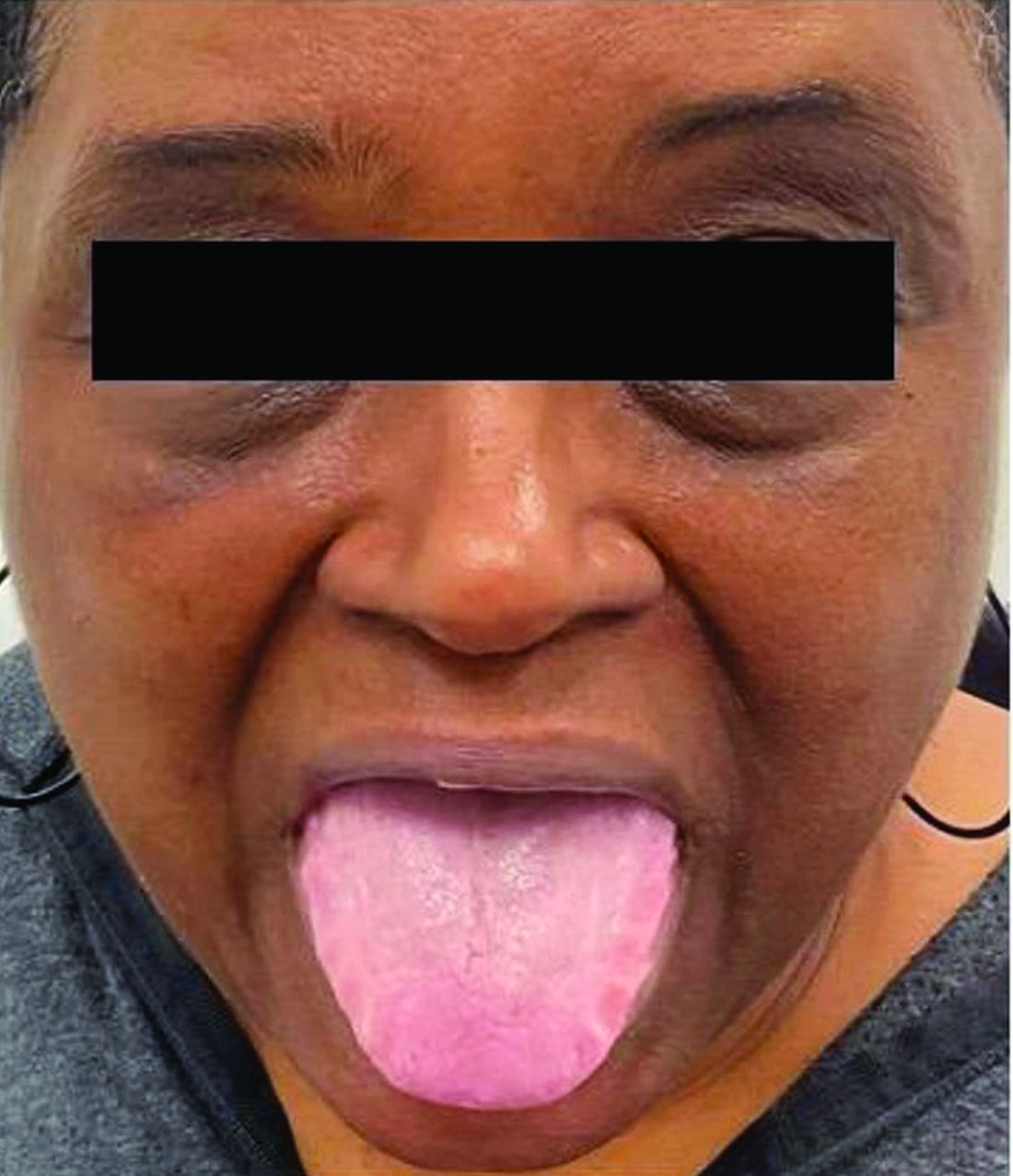
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.