User login
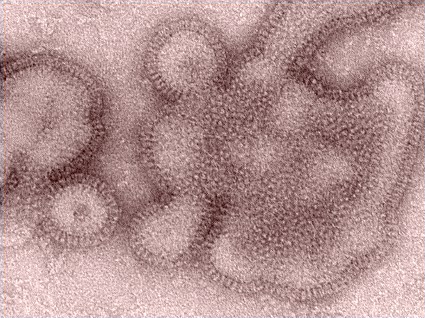
H3N2 Flu Infections Take a Big Jump
Infections due to the Influenza A (H3N2) variant virus have soared from 29 cases last week to 145 this week, according to the Centers for Disease Control and Prevention.
"This increase is partly based on the change in reporting requirements ... but in fact, the increase reflects accurately what is going on in these outbreaks," Dr. Joseph Bresee said during a telephone press conference held by CDC.*
There have been 145 confirmed cases of Influenza A (H3N2) variant infection, as of 1 p.m. EDST on Aug. 9. So far there have been 113 cases in Indiana, 30 cases in Ohio, 1 in Illinois, and 1 in Hawaii.
On Aug. 6, the CDC provided guidance to state laboratories and is now allowing states to confirm their own H3N2v cases, prior to laboratory confirmation at CDC.
"We’ve been finding that cases that were positive at the state level were overwhelmingly being confirmed also at CDC," said Dr. Bresee, who is a medical epidemiologist with the CDC’s influenza division.
"Given this, and in the context of an outbreak situation, with very little seasonal influenza circulating, we felt that it was appropriate for states to begin reporting their positives as confirmed cases rather than waiting for CDC confirmation," he said. "We anticipate that the change in reporting requirements will provide for a more real-time indication of how these outbreaks are evolving in the states."
Positive samples will still be forwarded to the CDC, where these will be confirmed using genetic sequencing.
The CDC will begin updating case counts every Friday, based on information provided by the states. States will have the most up-to-date numbers on other days.
Future issues of Morbidity and Mortality Weekly Report (MMWR) will provide an update on the numbers of cases and will provide some information about the effectiveness of rapid influenza diagnostic tests in detecting these viruses.
"The severity of human illness associated with this virus continues to resemble that of seasonal flu. Most of the cases are mild, self-limited, and resolve on their own," Dr. Bresee said.
CDC has not received any reports of deaths associated with the virus and only two hospitalizations.
Importantly, there is no evidence of sustained human-to-human spread in the community. "This is not a pandemic situation," Dr. Bresee said. However, "these viruses are all the same. They’re not completely genetically identical, but they’re very close to being so. All of the viruses that we’re seeing so far, in this latest increase in cases, are the viruses with the M gene."
The M gene comes from the human influenza (H1N1)pdm09 (2009 H1N1) virus and may confer increased transmissibility to and among humans, compared with other variant influenza viruses.
Most of the cases have involved contact or exposure to swine prior to illness onset, and many have been associated with state agricultural fairs, where swine were present.
Signs and symptoms of H3N2v virus infection are similar to those caused by other respiratory infections, including seasonal influenza virus infection. If H3N2v virus infection is suspected because of recent exposure to pigs, testing of respiratory specimens should be performed at a state health department; rapid influenza diagnostic tests may not detect H3N2v virus in human respiratory specimens, resulting in false negative results.
Two antivirals – oseltamivir (Tamiflu and zanamivir (Relenza) – are expected to be effective for treating H3N2v illness. Antiviral treatment is most effective when started as soon as possible after illness onset, according to the CDC.
A vaccine is under development and clinical trials are planned for later this year.
Influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
For information on the prevention of infection with the H3N2v virus, see the CDC’s factsheet.
*CORRECTION:10/8/2012 Dr. Joseph Bresee's name was misspelled in the original version of this story.
Infections due to the Influenza A (H3N2) variant virus have soared from 29 cases last week to 145 this week, according to the Centers for Disease Control and Prevention.
"This increase is partly based on the change in reporting requirements ... but in fact, the increase reflects accurately what is going on in these outbreaks," Dr. Joseph Bresee said during a telephone press conference held by CDC.*
There have been 145 confirmed cases of Influenza A (H3N2) variant infection, as of 1 p.m. EDST on Aug. 9. So far there have been 113 cases in Indiana, 30 cases in Ohio, 1 in Illinois, and 1 in Hawaii.
On Aug. 6, the CDC provided guidance to state laboratories and is now allowing states to confirm their own H3N2v cases, prior to laboratory confirmation at CDC.
"We’ve been finding that cases that were positive at the state level were overwhelmingly being confirmed also at CDC," said Dr. Bresee, who is a medical epidemiologist with the CDC’s influenza division.
"Given this, and in the context of an outbreak situation, with very little seasonal influenza circulating, we felt that it was appropriate for states to begin reporting their positives as confirmed cases rather than waiting for CDC confirmation," he said. "We anticipate that the change in reporting requirements will provide for a more real-time indication of how these outbreaks are evolving in the states."
Positive samples will still be forwarded to the CDC, where these will be confirmed using genetic sequencing.
The CDC will begin updating case counts every Friday, based on information provided by the states. States will have the most up-to-date numbers on other days.
Future issues of Morbidity and Mortality Weekly Report (MMWR) will provide an update on the numbers of cases and will provide some information about the effectiveness of rapid influenza diagnostic tests in detecting these viruses.
"The severity of human illness associated with this virus continues to resemble that of seasonal flu. Most of the cases are mild, self-limited, and resolve on their own," Dr. Bresee said.
CDC has not received any reports of deaths associated with the virus and only two hospitalizations.
Importantly, there is no evidence of sustained human-to-human spread in the community. "This is not a pandemic situation," Dr. Bresee said. However, "these viruses are all the same. They’re not completely genetically identical, but they’re very close to being so. All of the viruses that we’re seeing so far, in this latest increase in cases, are the viruses with the M gene."
The M gene comes from the human influenza (H1N1)pdm09 (2009 H1N1) virus and may confer increased transmissibility to and among humans, compared with other variant influenza viruses.
Most of the cases have involved contact or exposure to swine prior to illness onset, and many have been associated with state agricultural fairs, where swine were present.
Signs and symptoms of H3N2v virus infection are similar to those caused by other respiratory infections, including seasonal influenza virus infection. If H3N2v virus infection is suspected because of recent exposure to pigs, testing of respiratory specimens should be performed at a state health department; rapid influenza diagnostic tests may not detect H3N2v virus in human respiratory specimens, resulting in false negative results.
Two antivirals – oseltamivir (Tamiflu and zanamivir (Relenza) – are expected to be effective for treating H3N2v illness. Antiviral treatment is most effective when started as soon as possible after illness onset, according to the CDC.
A vaccine is under development and clinical trials are planned for later this year.
Influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
For information on the prevention of infection with the H3N2v virus, see the CDC’s factsheet.
*CORRECTION:10/8/2012 Dr. Joseph Bresee's name was misspelled in the original version of this story.
Infections due to the Influenza A (H3N2) variant virus have soared from 29 cases last week to 145 this week, according to the Centers for Disease Control and Prevention.
"This increase is partly based on the change in reporting requirements ... but in fact, the increase reflects accurately what is going on in these outbreaks," Dr. Joseph Bresee said during a telephone press conference held by CDC.*
There have been 145 confirmed cases of Influenza A (H3N2) variant infection, as of 1 p.m. EDST on Aug. 9. So far there have been 113 cases in Indiana, 30 cases in Ohio, 1 in Illinois, and 1 in Hawaii.
On Aug. 6, the CDC provided guidance to state laboratories and is now allowing states to confirm their own H3N2v cases, prior to laboratory confirmation at CDC.
"We’ve been finding that cases that were positive at the state level were overwhelmingly being confirmed also at CDC," said Dr. Bresee, who is a medical epidemiologist with the CDC’s influenza division.
"Given this, and in the context of an outbreak situation, with very little seasonal influenza circulating, we felt that it was appropriate for states to begin reporting their positives as confirmed cases rather than waiting for CDC confirmation," he said. "We anticipate that the change in reporting requirements will provide for a more real-time indication of how these outbreaks are evolving in the states."
Positive samples will still be forwarded to the CDC, where these will be confirmed using genetic sequencing.
The CDC will begin updating case counts every Friday, based on information provided by the states. States will have the most up-to-date numbers on other days.
Future issues of Morbidity and Mortality Weekly Report (MMWR) will provide an update on the numbers of cases and will provide some information about the effectiveness of rapid influenza diagnostic tests in detecting these viruses.
"The severity of human illness associated with this virus continues to resemble that of seasonal flu. Most of the cases are mild, self-limited, and resolve on their own," Dr. Bresee said.
CDC has not received any reports of deaths associated with the virus and only two hospitalizations.
Importantly, there is no evidence of sustained human-to-human spread in the community. "This is not a pandemic situation," Dr. Bresee said. However, "these viruses are all the same. They’re not completely genetically identical, but they’re very close to being so. All of the viruses that we’re seeing so far, in this latest increase in cases, are the viruses with the M gene."
The M gene comes from the human influenza (H1N1)pdm09 (2009 H1N1) virus and may confer increased transmissibility to and among humans, compared with other variant influenza viruses.
Most of the cases have involved contact or exposure to swine prior to illness onset, and many have been associated with state agricultural fairs, where swine were present.
Signs and symptoms of H3N2v virus infection are similar to those caused by other respiratory infections, including seasonal influenza virus infection. If H3N2v virus infection is suspected because of recent exposure to pigs, testing of respiratory specimens should be performed at a state health department; rapid influenza diagnostic tests may not detect H3N2v virus in human respiratory specimens, resulting in false negative results.
Two antivirals – oseltamivir (Tamiflu and zanamivir (Relenza) – are expected to be effective for treating H3N2v illness. Antiviral treatment is most effective when started as soon as possible after illness onset, according to the CDC.
A vaccine is under development and clinical trials are planned for later this year.
Influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
For information on the prevention of infection with the H3N2v virus, see the CDC’s factsheet.
*CORRECTION:10/8/2012 Dr. Joseph Bresee's name was misspelled in the original version of this story.
FROM A TELEPHONE CONFERENCE SPONSORED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
CCTA Helps Clear Emergency Departments, But Costs Remain High
The use of cardiac computed tomography angiography (CCTA) can help emergency department physicians make better decisions about which patients with symptoms suggestive of acute coronary syndromes can safely be sent home.
There were no undetected cases of acute coronary syndromes (ACS) in patients assessed with CCTA or standard evaluation, suggesting that the earlier and greater number of discharges in the CCTA group did not result in any missed diagnoses in a prospective, randomized trial of 1,000 patients.
"It’s really about what is the minimum testing that you can do to safely send someone home if you’re an emergency department physician," senior author Dr. James E. Udelson said in an interview. Each ED and each ED physician had their own criteria for when a patient could be discharged based on CCTA findings. "We’re really measuring not the performance of the test, but how the test affects decision making," he said.
Previous studies have suggested that CCTA may aid in safer and earlier triage of low-risk patients and that it can rule out coronary artery disease faster than stress myocardial-perfusion imaging. However, CCTA often can involve more follow-up procedures and greater costs than functional testing.
While the accuracy of CCTA was roughly equivalent to that of standard ED evaluation, the imaging technique also cost about the same. There was no difference in total costs between the two groups for the index visit and during 28-day follow-up in a subgroup of 649 patients from five of nine sites, in which complete billing data were available. Costs for patients with CCTA appeared to be driven by a greater use of additional diagnostic testing.
In ROMICAT-II (Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography II), researchers pitted CCTA – as a first diagnostic test, performed as early as possible – against standard ED evaluation in 1,000 patients with acute chest pain suggestive of ACS (N. Engl. J. Med. 2012;367:299-308).
The average length of the hospital stay – the primary end point – for patients who underwent CCTA was 7.6 hours less than that of the standard evaluation group.
Patient enrollment began in April 2010 and ended in January 2012 at nine U.S. hospitals. All sites were required to use at least 64-slice CT. Eligible patients were 40-74 years of age, and presented to the ED with chest pain (or the anginal equivalent) of at least 5 minutes’ duration within 24 hours before presentation in the ED. They also had to be in sinus rhythm and warranted further risk stratification to rule out acute coronary syndromes.
Patient care was not mandated by the study protocol in either group, but instead was at the discretion of local physicians. The discharge diagnosis also was based on the local physicians’ assessment.
Of 1,000 enrolled patients, 501 were randomized to CCTA and 499 were assigned to a standard evaluation. All patients were included in the intention-to-treat analysis. Overall, 987 patients (99%) had complete follow-up at 28 days.
After a complete evaluation, 75 patients (8%) had a final diagnosis of an acute coronary syndrome. In this subgroup of patients with a final diagnosis of ACS, the length of stay in the hospital was similar after CCTA and after standard evaluation in the ED.
In the overall cohort and also in patients without a final diagnosis of ACS, the mean time to diagnosis was significantly decreased with CCTA, compared with a standard evaluation. Patients in the CCTA group were more often directly discharged from the ED (47%, compared with 12% of patients in the standard-evaluation group), with fewer admissions to an observation unit.
Overall, there were eight major adverse cardiovascular events during the 28-day follow-up: six after standard evaluation in the ED and two after CCTA.
The results have important implications for some EDs. Not all EDs are the same: Some are overflowing with patients, while others have light traffic. For directors of busy and crowded EDs, the findings provide "an opportunity to get people out a lot sooner," said Dr. Udelson, chief of the cardiology division at Tufts Medical Center in Boston.
Half of patients who presented to the ED with symptoms suggestive of ACS and who underwent CCTA were discharged within 8.6 hours. In contrast, it took more than a day (26.7 hours) for half of the patients in the standard-evaluation group to be discharged. "If you’re in a supercrowded ED, that’s really worth something," he said.
The researchers did not measure additional follow-up in this study beyond major events that may have happened within 30 days. However, "one of the things about CT is that you identify early disease. You can potentially be comfortable that it doesn’t have anything to do with the chest pain." Primary care physicians should know about this early disease, in order to potentially start or change treatment.
One important question is what the yield is of subsequent testing following CCTA, said Dr. Udelson. "We’ll try to define the characteristics of the CT that mean that you’re not going to find anything else and not to test further."
In an accompanying editorial, Dr. Rita F. Redberg asserted that "with no evidence of benefit and definite risks, routine testing in the emergency department of patients with a low-to-intermediate risk of acute coronary syndromes should be avoided" (N. Engl. J. Med. 2012;367:375-6). Dr. Redberg is a professor of medicine and the director of Women’s Cardiovascular Services at the University of California, San Francisco. She is also on the editorial board of Cardiology News.
However, Dr. Udelson pointed out that "ED physicians struggle with this. They’re on the firing line. It’s their heads if they make a wrong decision."
"That’s our potential next step. ... Almost a quarter of the patients in the control group had no imaging tests. In other words, the physicians felt comfortable enough to discharge these patients with no [additional] testing." They will try to determine which individuals can safely go home and which patients need additional testing.
This study was supported by grants from the National Institutes of Health. Dr. Udelson reported that he is on the scientific advisory board of Lantheus Medical Imaging. Several of the coauthors reported financial ties to imaging and/or pharmaceutical companies and professional organizations.
"With no evidence of benefit and definite risks, routine testing in the emergency department of patients with a low-to-intermediate risk of acute coronary syndromes should be avoided," Dr. Rita F. Redberg argued in an accompanying editorial (N. Engl. J. Med. 2012;367:375-6) on the findings.
"In short, the question is not which test leads to faster discharge of patients from the emergency department, but whether a test is needed at all."
ROMICAT-II builds on similar data from another study that also randomly assigned patients with suspected acute coronary syndromes to CCTA or traditional care (N. Engl. J. Med. 2012;366:1393-403).
While shorter lengths of stay in the hospital are highly desirable, "the ROMICAT-II study reveals a deeper flaw in the approach to chest pain in the emergency department. The underlying assumption of these two studies is that some diagnostic test must be performed before discharging these low-to-intermediate-risk patients from the emergency department. This assumption is unproven and probably unwarranted. The rationale for any test, as compared with no testing, should be that it will lead to an improved outcome, and here there is no evidence that the tests performed led to improved outcomes," said Dr. Redberg.
She explained that rates of major adverse cardiac events among all patients in these two studies – regardless of whether the patients underwent CCTA, stress testing, or no testing at all – were so low (less than 1% had a myocardial infarction and no patients died) that it is impossible to know whether the CCTA groups received any benefit whatsoever.
Dr. Redberg pointed out several potential dangers associated with CCTA, such as the increased likelihood of downstream testing after CCTA that can lead to serious complications.
"In light of the certainty that the patients in the CCTA group were exposed to substantial doses of radiation (from both CCTA and nuclear stress tests) and were at risk for nephrotoxicity and adverse reactions from the CCTA contrast dye, clinicians may legitimately ask whether the tests did more harm than good," she observed.
"Patients who have normal electrocardiographic findings and negative troponin levels constitute a group at low risk for cardiac events, and multiple studies show no evidence that any additional testing further reduces that risk," she said.
The decision regarding the need for diagnostic testing in these patients usually can be safely deferred to outpatient follow-up within a few weeks after the visit to the emergency department. The vast majority of patients have no cardiac causes for their chest pain, and many need no further testing.
Dr. Redberg is a professor of medicine and the director of Women’s Cardiovascular Services at the University of California, San Francisco. Dr. Redberg is also on the editorial board of Cardiology News. She reported that she has no conflicts of interest.
"With no evidence of benefit and definite risks, routine testing in the emergency department of patients with a low-to-intermediate risk of acute coronary syndromes should be avoided," Dr. Rita F. Redberg argued in an accompanying editorial (N. Engl. J. Med. 2012;367:375-6) on the findings.
"In short, the question is not which test leads to faster discharge of patients from the emergency department, but whether a test is needed at all."
ROMICAT-II builds on similar data from another study that also randomly assigned patients with suspected acute coronary syndromes to CCTA or traditional care (N. Engl. J. Med. 2012;366:1393-403).
While shorter lengths of stay in the hospital are highly desirable, "the ROMICAT-II study reveals a deeper flaw in the approach to chest pain in the emergency department. The underlying assumption of these two studies is that some diagnostic test must be performed before discharging these low-to-intermediate-risk patients from the emergency department. This assumption is unproven and probably unwarranted. The rationale for any test, as compared with no testing, should be that it will lead to an improved outcome, and here there is no evidence that the tests performed led to improved outcomes," said Dr. Redberg.
She explained that rates of major adverse cardiac events among all patients in these two studies – regardless of whether the patients underwent CCTA, stress testing, or no testing at all – were so low (less than 1% had a myocardial infarction and no patients died) that it is impossible to know whether the CCTA groups received any benefit whatsoever.
Dr. Redberg pointed out several potential dangers associated with CCTA, such as the increased likelihood of downstream testing after CCTA that can lead to serious complications.
"In light of the certainty that the patients in the CCTA group were exposed to substantial doses of radiation (from both CCTA and nuclear stress tests) and were at risk for nephrotoxicity and adverse reactions from the CCTA contrast dye, clinicians may legitimately ask whether the tests did more harm than good," she observed.
"Patients who have normal electrocardiographic findings and negative troponin levels constitute a group at low risk for cardiac events, and multiple studies show no evidence that any additional testing further reduces that risk," she said.
The decision regarding the need for diagnostic testing in these patients usually can be safely deferred to outpatient follow-up within a few weeks after the visit to the emergency department. The vast majority of patients have no cardiac causes for their chest pain, and many need no further testing.
Dr. Redberg is a professor of medicine and the director of Women’s Cardiovascular Services at the University of California, San Francisco. Dr. Redberg is also on the editorial board of Cardiology News. She reported that she has no conflicts of interest.
"With no evidence of benefit and definite risks, routine testing in the emergency department of patients with a low-to-intermediate risk of acute coronary syndromes should be avoided," Dr. Rita F. Redberg argued in an accompanying editorial (N. Engl. J. Med. 2012;367:375-6) on the findings.
"In short, the question is not which test leads to faster discharge of patients from the emergency department, but whether a test is needed at all."
ROMICAT-II builds on similar data from another study that also randomly assigned patients with suspected acute coronary syndromes to CCTA or traditional care (N. Engl. J. Med. 2012;366:1393-403).
While shorter lengths of stay in the hospital are highly desirable, "the ROMICAT-II study reveals a deeper flaw in the approach to chest pain in the emergency department. The underlying assumption of these two studies is that some diagnostic test must be performed before discharging these low-to-intermediate-risk patients from the emergency department. This assumption is unproven and probably unwarranted. The rationale for any test, as compared with no testing, should be that it will lead to an improved outcome, and here there is no evidence that the tests performed led to improved outcomes," said Dr. Redberg.
She explained that rates of major adverse cardiac events among all patients in these two studies – regardless of whether the patients underwent CCTA, stress testing, or no testing at all – were so low (less than 1% had a myocardial infarction and no patients died) that it is impossible to know whether the CCTA groups received any benefit whatsoever.
Dr. Redberg pointed out several potential dangers associated with CCTA, such as the increased likelihood of downstream testing after CCTA that can lead to serious complications.
"In light of the certainty that the patients in the CCTA group were exposed to substantial doses of radiation (from both CCTA and nuclear stress tests) and were at risk for nephrotoxicity and adverse reactions from the CCTA contrast dye, clinicians may legitimately ask whether the tests did more harm than good," she observed.
"Patients who have normal electrocardiographic findings and negative troponin levels constitute a group at low risk for cardiac events, and multiple studies show no evidence that any additional testing further reduces that risk," she said.
The decision regarding the need for diagnostic testing in these patients usually can be safely deferred to outpatient follow-up within a few weeks after the visit to the emergency department. The vast majority of patients have no cardiac causes for their chest pain, and many need no further testing.
Dr. Redberg is a professor of medicine and the director of Women’s Cardiovascular Services at the University of California, San Francisco. Dr. Redberg is also on the editorial board of Cardiology News. She reported that she has no conflicts of interest.
The use of cardiac computed tomography angiography (CCTA) can help emergency department physicians make better decisions about which patients with symptoms suggestive of acute coronary syndromes can safely be sent home.
There were no undetected cases of acute coronary syndromes (ACS) in patients assessed with CCTA or standard evaluation, suggesting that the earlier and greater number of discharges in the CCTA group did not result in any missed diagnoses in a prospective, randomized trial of 1,000 patients.
"It’s really about what is the minimum testing that you can do to safely send someone home if you’re an emergency department physician," senior author Dr. James E. Udelson said in an interview. Each ED and each ED physician had their own criteria for when a patient could be discharged based on CCTA findings. "We’re really measuring not the performance of the test, but how the test affects decision making," he said.
Previous studies have suggested that CCTA may aid in safer and earlier triage of low-risk patients and that it can rule out coronary artery disease faster than stress myocardial-perfusion imaging. However, CCTA often can involve more follow-up procedures and greater costs than functional testing.
While the accuracy of CCTA was roughly equivalent to that of standard ED evaluation, the imaging technique also cost about the same. There was no difference in total costs between the two groups for the index visit and during 28-day follow-up in a subgroup of 649 patients from five of nine sites, in which complete billing data were available. Costs for patients with CCTA appeared to be driven by a greater use of additional diagnostic testing.
In ROMICAT-II (Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography II), researchers pitted CCTA – as a first diagnostic test, performed as early as possible – against standard ED evaluation in 1,000 patients with acute chest pain suggestive of ACS (N. Engl. J. Med. 2012;367:299-308).
The average length of the hospital stay – the primary end point – for patients who underwent CCTA was 7.6 hours less than that of the standard evaluation group.
Patient enrollment began in April 2010 and ended in January 2012 at nine U.S. hospitals. All sites were required to use at least 64-slice CT. Eligible patients were 40-74 years of age, and presented to the ED with chest pain (or the anginal equivalent) of at least 5 minutes’ duration within 24 hours before presentation in the ED. They also had to be in sinus rhythm and warranted further risk stratification to rule out acute coronary syndromes.
Patient care was not mandated by the study protocol in either group, but instead was at the discretion of local physicians. The discharge diagnosis also was based on the local physicians’ assessment.
Of 1,000 enrolled patients, 501 were randomized to CCTA and 499 were assigned to a standard evaluation. All patients were included in the intention-to-treat analysis. Overall, 987 patients (99%) had complete follow-up at 28 days.
After a complete evaluation, 75 patients (8%) had a final diagnosis of an acute coronary syndrome. In this subgroup of patients with a final diagnosis of ACS, the length of stay in the hospital was similar after CCTA and after standard evaluation in the ED.
In the overall cohort and also in patients without a final diagnosis of ACS, the mean time to diagnosis was significantly decreased with CCTA, compared with a standard evaluation. Patients in the CCTA group were more often directly discharged from the ED (47%, compared with 12% of patients in the standard-evaluation group), with fewer admissions to an observation unit.
Overall, there were eight major adverse cardiovascular events during the 28-day follow-up: six after standard evaluation in the ED and two after CCTA.
The results have important implications for some EDs. Not all EDs are the same: Some are overflowing with patients, while others have light traffic. For directors of busy and crowded EDs, the findings provide "an opportunity to get people out a lot sooner," said Dr. Udelson, chief of the cardiology division at Tufts Medical Center in Boston.
Half of patients who presented to the ED with symptoms suggestive of ACS and who underwent CCTA were discharged within 8.6 hours. In contrast, it took more than a day (26.7 hours) for half of the patients in the standard-evaluation group to be discharged. "If you’re in a supercrowded ED, that’s really worth something," he said.
The researchers did not measure additional follow-up in this study beyond major events that may have happened within 30 days. However, "one of the things about CT is that you identify early disease. You can potentially be comfortable that it doesn’t have anything to do with the chest pain." Primary care physicians should know about this early disease, in order to potentially start or change treatment.
One important question is what the yield is of subsequent testing following CCTA, said Dr. Udelson. "We’ll try to define the characteristics of the CT that mean that you’re not going to find anything else and not to test further."
In an accompanying editorial, Dr. Rita F. Redberg asserted that "with no evidence of benefit and definite risks, routine testing in the emergency department of patients with a low-to-intermediate risk of acute coronary syndromes should be avoided" (N. Engl. J. Med. 2012;367:375-6). Dr. Redberg is a professor of medicine and the director of Women’s Cardiovascular Services at the University of California, San Francisco. She is also on the editorial board of Cardiology News.
However, Dr. Udelson pointed out that "ED physicians struggle with this. They’re on the firing line. It’s their heads if they make a wrong decision."
"That’s our potential next step. ... Almost a quarter of the patients in the control group had no imaging tests. In other words, the physicians felt comfortable enough to discharge these patients with no [additional] testing." They will try to determine which individuals can safely go home and which patients need additional testing.
This study was supported by grants from the National Institutes of Health. Dr. Udelson reported that he is on the scientific advisory board of Lantheus Medical Imaging. Several of the coauthors reported financial ties to imaging and/or pharmaceutical companies and professional organizations.
The use of cardiac computed tomography angiography (CCTA) can help emergency department physicians make better decisions about which patients with symptoms suggestive of acute coronary syndromes can safely be sent home.
There were no undetected cases of acute coronary syndromes (ACS) in patients assessed with CCTA or standard evaluation, suggesting that the earlier and greater number of discharges in the CCTA group did not result in any missed diagnoses in a prospective, randomized trial of 1,000 patients.
"It’s really about what is the minimum testing that you can do to safely send someone home if you’re an emergency department physician," senior author Dr. James E. Udelson said in an interview. Each ED and each ED physician had their own criteria for when a patient could be discharged based on CCTA findings. "We’re really measuring not the performance of the test, but how the test affects decision making," he said.
Previous studies have suggested that CCTA may aid in safer and earlier triage of low-risk patients and that it can rule out coronary artery disease faster than stress myocardial-perfusion imaging. However, CCTA often can involve more follow-up procedures and greater costs than functional testing.
While the accuracy of CCTA was roughly equivalent to that of standard ED evaluation, the imaging technique also cost about the same. There was no difference in total costs between the two groups for the index visit and during 28-day follow-up in a subgroup of 649 patients from five of nine sites, in which complete billing data were available. Costs for patients with CCTA appeared to be driven by a greater use of additional diagnostic testing.
In ROMICAT-II (Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography II), researchers pitted CCTA – as a first diagnostic test, performed as early as possible – against standard ED evaluation in 1,000 patients with acute chest pain suggestive of ACS (N. Engl. J. Med. 2012;367:299-308).
The average length of the hospital stay – the primary end point – for patients who underwent CCTA was 7.6 hours less than that of the standard evaluation group.
Patient enrollment began in April 2010 and ended in January 2012 at nine U.S. hospitals. All sites were required to use at least 64-slice CT. Eligible patients were 40-74 years of age, and presented to the ED with chest pain (or the anginal equivalent) of at least 5 minutes’ duration within 24 hours before presentation in the ED. They also had to be in sinus rhythm and warranted further risk stratification to rule out acute coronary syndromes.
Patient care was not mandated by the study protocol in either group, but instead was at the discretion of local physicians. The discharge diagnosis also was based on the local physicians’ assessment.
Of 1,000 enrolled patients, 501 were randomized to CCTA and 499 were assigned to a standard evaluation. All patients were included in the intention-to-treat analysis. Overall, 987 patients (99%) had complete follow-up at 28 days.
After a complete evaluation, 75 patients (8%) had a final diagnosis of an acute coronary syndrome. In this subgroup of patients with a final diagnosis of ACS, the length of stay in the hospital was similar after CCTA and after standard evaluation in the ED.
In the overall cohort and also in patients without a final diagnosis of ACS, the mean time to diagnosis was significantly decreased with CCTA, compared with a standard evaluation. Patients in the CCTA group were more often directly discharged from the ED (47%, compared with 12% of patients in the standard-evaluation group), with fewer admissions to an observation unit.
Overall, there were eight major adverse cardiovascular events during the 28-day follow-up: six after standard evaluation in the ED and two after CCTA.
The results have important implications for some EDs. Not all EDs are the same: Some are overflowing with patients, while others have light traffic. For directors of busy and crowded EDs, the findings provide "an opportunity to get people out a lot sooner," said Dr. Udelson, chief of the cardiology division at Tufts Medical Center in Boston.
Half of patients who presented to the ED with symptoms suggestive of ACS and who underwent CCTA were discharged within 8.6 hours. In contrast, it took more than a day (26.7 hours) for half of the patients in the standard-evaluation group to be discharged. "If you’re in a supercrowded ED, that’s really worth something," he said.
The researchers did not measure additional follow-up in this study beyond major events that may have happened within 30 days. However, "one of the things about CT is that you identify early disease. You can potentially be comfortable that it doesn’t have anything to do with the chest pain." Primary care physicians should know about this early disease, in order to potentially start or change treatment.
One important question is what the yield is of subsequent testing following CCTA, said Dr. Udelson. "We’ll try to define the characteristics of the CT that mean that you’re not going to find anything else and not to test further."
In an accompanying editorial, Dr. Rita F. Redberg asserted that "with no evidence of benefit and definite risks, routine testing in the emergency department of patients with a low-to-intermediate risk of acute coronary syndromes should be avoided" (N. Engl. J. Med. 2012;367:375-6). Dr. Redberg is a professor of medicine and the director of Women’s Cardiovascular Services at the University of California, San Francisco. She is also on the editorial board of Cardiology News.
However, Dr. Udelson pointed out that "ED physicians struggle with this. They’re on the firing line. It’s their heads if they make a wrong decision."
"That’s our potential next step. ... Almost a quarter of the patients in the control group had no imaging tests. In other words, the physicians felt comfortable enough to discharge these patients with no [additional] testing." They will try to determine which individuals can safely go home and which patients need additional testing.
This study was supported by grants from the National Institutes of Health. Dr. Udelson reported that he is on the scientific advisory board of Lantheus Medical Imaging. Several of the coauthors reported financial ties to imaging and/or pharmaceutical companies and professional organizations.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
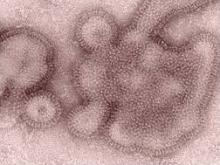
H3N2v Influenza Cases Double in Last 3 Weeks
There has been a "significant increase" in the number of detected human infections involving the H3N2 variant virus, according to the Centers for Disease Control and Prevention. A total of 16 cases – out of 29 overall – have been reported in the last 3 weeks.
All 12 of the most recently reported cases occurred in people who had direct or indirect contact with swine prior to their illness, the CDC reported on Aug. 3. They occurred in Hawaii, Ohio, and Indiana.
All 29 cases identified since July 2011 are due to an H3N2 variant, or H3N2v, virus that contains the M gene from the human influenza A(H1N1)pdm09 (2009 H1N1) virus. "This M gene may confer increased transmissibility to and among humans compared with other variant influenza viruses," Dr. Joseph Bresse, a medical epidemiologist with the CDC’s influenza division, said during a telephone press conference sponsored by the CDC. Among this overall total, 1 was detected in Hawaii, 7 in Indiana, 3 in Iowa, 10 in Ohio, 2 in Maine, 3 in Pennsylvania, 1 in Utah and 2 in West Virginia.
Overall, 23 cases involved swine contact prior to illness onset and 19 cases were associated with state agricultural fairs, where swine were present.
Signs and symptoms of H3N2v virus infection are similar to those caused by other respiratory infections, including seasonal influenza virus infection. If H3N2v virus infection is suspected because of recent exposure to pigs, testing of respiratory specimens should be performed at a state health department; rapid influenza diagnostic tests may not detect H3N2v virus in human respiratory specimens, resulting in false negative results.
Two antivirals – oseltamivir (Tamiflu) and zanamivir (Relenza) – have been approved by the Food and Drug Administration for the treatment of illness associated with H3N2v virus infection. Antiviral treatment is most effective when started as soon as possible after illness onset, according to the CDC.
There has been no human-to-human transmission among the 12 recent cases, according to Dr. Bresse. However, three cases since July 2011 have been due to human-to-human transmission. Likewise, no deaths or hospitalizations have been associated with the most recent cases, though there have been three hospitalizations overall required for individuals with underlying diseases that put them at higher risk from the infection.
Thirteen of the infections occurred in children, which is consistent with research studies demonstrating that children may be more susceptible to the infection than adults.
Dr. Bresse noted that influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
The CDC has developed a variant vaccine candidate that will be undergoing clinical trials later this year.
For information on the prevention of infection with the H3N2v virus, see the CDC’s fact sheet. For information on the proper handling of livestock, see the Animal Contact Compendium published by the National Association of State Public Health Veterinarians.
There has been a "significant increase" in the number of detected human infections involving the H3N2 variant virus, according to the Centers for Disease Control and Prevention. A total of 16 cases – out of 29 overall – have been reported in the last 3 weeks.
All 12 of the most recently reported cases occurred in people who had direct or indirect contact with swine prior to their illness, the CDC reported on Aug. 3. They occurred in Hawaii, Ohio, and Indiana.
All 29 cases identified since July 2011 are due to an H3N2 variant, or H3N2v, virus that contains the M gene from the human influenza A(H1N1)pdm09 (2009 H1N1) virus. "This M gene may confer increased transmissibility to and among humans compared with other variant influenza viruses," Dr. Joseph Bresse, a medical epidemiologist with the CDC’s influenza division, said during a telephone press conference sponsored by the CDC. Among this overall total, 1 was detected in Hawaii, 7 in Indiana, 3 in Iowa, 10 in Ohio, 2 in Maine, 3 in Pennsylvania, 1 in Utah and 2 in West Virginia.
Overall, 23 cases involved swine contact prior to illness onset and 19 cases were associated with state agricultural fairs, where swine were present.
Signs and symptoms of H3N2v virus infection are similar to those caused by other respiratory infections, including seasonal influenza virus infection. If H3N2v virus infection is suspected because of recent exposure to pigs, testing of respiratory specimens should be performed at a state health department; rapid influenza diagnostic tests may not detect H3N2v virus in human respiratory specimens, resulting in false negative results.
Two antivirals – oseltamivir (Tamiflu) and zanamivir (Relenza) – have been approved by the Food and Drug Administration for the treatment of illness associated with H3N2v virus infection. Antiviral treatment is most effective when started as soon as possible after illness onset, according to the CDC.
There has been no human-to-human transmission among the 12 recent cases, according to Dr. Bresse. However, three cases since July 2011 have been due to human-to-human transmission. Likewise, no deaths or hospitalizations have been associated with the most recent cases, though there have been three hospitalizations overall required for individuals with underlying diseases that put them at higher risk from the infection.
Thirteen of the infections occurred in children, which is consistent with research studies demonstrating that children may be more susceptible to the infection than adults.
Dr. Bresse noted that influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
The CDC has developed a variant vaccine candidate that will be undergoing clinical trials later this year.
For information on the prevention of infection with the H3N2v virus, see the CDC’s fact sheet. For information on the proper handling of livestock, see the Animal Contact Compendium published by the National Association of State Public Health Veterinarians.
There has been a "significant increase" in the number of detected human infections involving the H3N2 variant virus, according to the Centers for Disease Control and Prevention. A total of 16 cases – out of 29 overall – have been reported in the last 3 weeks.
All 12 of the most recently reported cases occurred in people who had direct or indirect contact with swine prior to their illness, the CDC reported on Aug. 3. They occurred in Hawaii, Ohio, and Indiana.
All 29 cases identified since July 2011 are due to an H3N2 variant, or H3N2v, virus that contains the M gene from the human influenza A(H1N1)pdm09 (2009 H1N1) virus. "This M gene may confer increased transmissibility to and among humans compared with other variant influenza viruses," Dr. Joseph Bresse, a medical epidemiologist with the CDC’s influenza division, said during a telephone press conference sponsored by the CDC. Among this overall total, 1 was detected in Hawaii, 7 in Indiana, 3 in Iowa, 10 in Ohio, 2 in Maine, 3 in Pennsylvania, 1 in Utah and 2 in West Virginia.
Overall, 23 cases involved swine contact prior to illness onset and 19 cases were associated with state agricultural fairs, where swine were present.
Signs and symptoms of H3N2v virus infection are similar to those caused by other respiratory infections, including seasonal influenza virus infection. If H3N2v virus infection is suspected because of recent exposure to pigs, testing of respiratory specimens should be performed at a state health department; rapid influenza diagnostic tests may not detect H3N2v virus in human respiratory specimens, resulting in false negative results.
Two antivirals – oseltamivir (Tamiflu) and zanamivir (Relenza) – have been approved by the Food and Drug Administration for the treatment of illness associated with H3N2v virus infection. Antiviral treatment is most effective when started as soon as possible after illness onset, according to the CDC.
There has been no human-to-human transmission among the 12 recent cases, according to Dr. Bresse. However, three cases since July 2011 have been due to human-to-human transmission. Likewise, no deaths or hospitalizations have been associated with the most recent cases, though there have been three hospitalizations overall required for individuals with underlying diseases that put them at higher risk from the infection.
Thirteen of the infections occurred in children, which is consistent with research studies demonstrating that children may be more susceptible to the infection than adults.
Dr. Bresse noted that influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
The CDC has developed a variant vaccine candidate that will be undergoing clinical trials later this year.
For information on the prevention of infection with the H3N2v virus, see the CDC’s fact sheet. For information on the proper handling of livestock, see the Animal Contact Compendium published by the National Association of State Public Health Veterinarians.
FROM A TELECONFERENCE HELD BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Major Finding: All 12 of the most recently reported case of human infections with influenza A (H3N2) variant virus involved contact with swine. The new cases increase the total to 29 reported since July 2011; 16 cases have occurred in the last 3 weeks.
Data Source: The data come from a CDC seasonal influenza update.
Disclosures: None reported.
Covered Stents Top Bare Metal for Chronic Mesenteric Ischemia
NATIONAL HARBOR, MD. – Covered stents may reduce the recurrence of chronic mesenteric ischemia and the need for reintervention in patients undergoing primary interventions, according to Dr. Gustavo S. Oderich.
Freedom from symptom recurrence among primary intervention patients was 92% for those with covered stents, compared with 47% for those with bare metal stents (BMS), he reported at the Vascular Annual Meeting. This difference was significant.
Similarly, freedom from reintervention was 91% at 5 years for the covered-stent group, compared with 54% for the BMS group, also a significant difference.
The findings come from a review of patients who were treated for chronic mesenteric ischemia (CMI) using BMS or covered stents (2000-2010). End points included freedom from symptom recurrence, reintervention, and primary and secondary patency rates.
"Mesenteric angioplasty and stenting [have been] plagued by high rates of restenosis and reinterventions in the range of 30%-60% in different reports," said Dr. Oderich, professor of vascular and endovascular surgery at the Mayo Clinic in Rochester, Minn. Covered stents have been shown to lower restenosis rates when used for renal alignment in fenestrated endografts and for the treatment of failing arteriovenous grafts.
The researchers compared BMS and iCast covered stents (Atrium USA) to determine if covered stents could also reduce restenosis in patients with CMI.
In all, 352 patients were treated for CMI, of which 247 had endovascular revascularization. The researchers included 191 patients in the primary intervention group; of these, 149 (78%) had BMS, 42 patients had covered stents, and 22 patients had angioplasty alone. (The angioplasty-alone patients were excluded from the study.) The primary intervention population included 191 patients; the reintervention population included 36 patients who had undergone open primary intervention.
The two groups were similar in terms of demographics, cardiovascular risk factors, and clinical presentation, but the BMS patients tended to have greater rates of chronic pulmonary disease. The anatomical and procedural variables – including extent of disease, type of approach, number of vessels treated, and stent length and diameter – were also similar, as were early outcomes.
Technical success (defined as successful stent implantation without local complications or stenosis less than 30%) was 95% and 98% for the BMS and covered-stent groups, respectively. Mortality was 3% and 0% for the BMS and covered stent groups, respectively.
The primary patency rate at 5 years was 92% for those with covered stents, compared with 47% for those with BMS, in the primary intervention group. There was no difference in secondary patency rates between the two groups. The average follow-up for the cohort was 29 months.
Multivariate analysis of the primary intervention group showed that the use of a covered stent was a protective factor for loss of primary patency, symptom recurrence, and reintervention.
Other independent predictors of loss of primary patency included age, female sex, and current smoking history. For symptom recurrence, other independent predictors included female sex and current smoking history. For reintervention, age and female sex were independent predictors.
Covered stents also were associated with less recurrence and fewer reinterventions in patients undergoing reintervention for mesenteric chronic ischemia. The reintervention group included 15 patients who were treated with BMS and 21 patients treated with covered stents. The two groups were similar in terms of demographics, cardiovascular risk factors, and clinical presentation. In all, 16 vessels were treated with BMS and 22 with covered stents. The two groups had similar approaches, number of vessels treated, and stent dimensions – with the exception of a slight trend toward longer stents in the covered-stent group.
Dr. Oderich reported that he is the principal investigator of a clinical trial sponsored by Cook Medical.
BMS, iCast covered stents, Atrium USA,
NATIONAL HARBOR, MD. – Covered stents may reduce the recurrence of chronic mesenteric ischemia and the need for reintervention in patients undergoing primary interventions, according to Dr. Gustavo S. Oderich.
Freedom from symptom recurrence among primary intervention patients was 92% for those with covered stents, compared with 47% for those with bare metal stents (BMS), he reported at the Vascular Annual Meeting. This difference was significant.
Similarly, freedom from reintervention was 91% at 5 years for the covered-stent group, compared with 54% for the BMS group, also a significant difference.
The findings come from a review of patients who were treated for chronic mesenteric ischemia (CMI) using BMS or covered stents (2000-2010). End points included freedom from symptom recurrence, reintervention, and primary and secondary patency rates.
"Mesenteric angioplasty and stenting [have been] plagued by high rates of restenosis and reinterventions in the range of 30%-60% in different reports," said Dr. Oderich, professor of vascular and endovascular surgery at the Mayo Clinic in Rochester, Minn. Covered stents have been shown to lower restenosis rates when used for renal alignment in fenestrated endografts and for the treatment of failing arteriovenous grafts.
The researchers compared BMS and iCast covered stents (Atrium USA) to determine if covered stents could also reduce restenosis in patients with CMI.
In all, 352 patients were treated for CMI, of which 247 had endovascular revascularization. The researchers included 191 patients in the primary intervention group; of these, 149 (78%) had BMS, 42 patients had covered stents, and 22 patients had angioplasty alone. (The angioplasty-alone patients were excluded from the study.) The primary intervention population included 191 patients; the reintervention population included 36 patients who had undergone open primary intervention.
The two groups were similar in terms of demographics, cardiovascular risk factors, and clinical presentation, but the BMS patients tended to have greater rates of chronic pulmonary disease. The anatomical and procedural variables – including extent of disease, type of approach, number of vessels treated, and stent length and diameter – were also similar, as were early outcomes.
Technical success (defined as successful stent implantation without local complications or stenosis less than 30%) was 95% and 98% for the BMS and covered-stent groups, respectively. Mortality was 3% and 0% for the BMS and covered stent groups, respectively.
The primary patency rate at 5 years was 92% for those with covered stents, compared with 47% for those with BMS, in the primary intervention group. There was no difference in secondary patency rates between the two groups. The average follow-up for the cohort was 29 months.
Multivariate analysis of the primary intervention group showed that the use of a covered stent was a protective factor for loss of primary patency, symptom recurrence, and reintervention.
Other independent predictors of loss of primary patency included age, female sex, and current smoking history. For symptom recurrence, other independent predictors included female sex and current smoking history. For reintervention, age and female sex were independent predictors.
Covered stents also were associated with less recurrence and fewer reinterventions in patients undergoing reintervention for mesenteric chronic ischemia. The reintervention group included 15 patients who were treated with BMS and 21 patients treated with covered stents. The two groups were similar in terms of demographics, cardiovascular risk factors, and clinical presentation. In all, 16 vessels were treated with BMS and 22 with covered stents. The two groups had similar approaches, number of vessels treated, and stent dimensions – with the exception of a slight trend toward longer stents in the covered-stent group.
Dr. Oderich reported that he is the principal investigator of a clinical trial sponsored by Cook Medical.
NATIONAL HARBOR, MD. – Covered stents may reduce the recurrence of chronic mesenteric ischemia and the need for reintervention in patients undergoing primary interventions, according to Dr. Gustavo S. Oderich.
Freedom from symptom recurrence among primary intervention patients was 92% for those with covered stents, compared with 47% for those with bare metal stents (BMS), he reported at the Vascular Annual Meeting. This difference was significant.
Similarly, freedom from reintervention was 91% at 5 years for the covered-stent group, compared with 54% for the BMS group, also a significant difference.
The findings come from a review of patients who were treated for chronic mesenteric ischemia (CMI) using BMS or covered stents (2000-2010). End points included freedom from symptom recurrence, reintervention, and primary and secondary patency rates.
"Mesenteric angioplasty and stenting [have been] plagued by high rates of restenosis and reinterventions in the range of 30%-60% in different reports," said Dr. Oderich, professor of vascular and endovascular surgery at the Mayo Clinic in Rochester, Minn. Covered stents have been shown to lower restenosis rates when used for renal alignment in fenestrated endografts and for the treatment of failing arteriovenous grafts.
The researchers compared BMS and iCast covered stents (Atrium USA) to determine if covered stents could also reduce restenosis in patients with CMI.
In all, 352 patients were treated for CMI, of which 247 had endovascular revascularization. The researchers included 191 patients in the primary intervention group; of these, 149 (78%) had BMS, 42 patients had covered stents, and 22 patients had angioplasty alone. (The angioplasty-alone patients were excluded from the study.) The primary intervention population included 191 patients; the reintervention population included 36 patients who had undergone open primary intervention.
The two groups were similar in terms of demographics, cardiovascular risk factors, and clinical presentation, but the BMS patients tended to have greater rates of chronic pulmonary disease. The anatomical and procedural variables – including extent of disease, type of approach, number of vessels treated, and stent length and diameter – were also similar, as were early outcomes.
Technical success (defined as successful stent implantation without local complications or stenosis less than 30%) was 95% and 98% for the BMS and covered-stent groups, respectively. Mortality was 3% and 0% for the BMS and covered stent groups, respectively.
The primary patency rate at 5 years was 92% for those with covered stents, compared with 47% for those with BMS, in the primary intervention group. There was no difference in secondary patency rates between the two groups. The average follow-up for the cohort was 29 months.
Multivariate analysis of the primary intervention group showed that the use of a covered stent was a protective factor for loss of primary patency, symptom recurrence, and reintervention.
Other independent predictors of loss of primary patency included age, female sex, and current smoking history. For symptom recurrence, other independent predictors included female sex and current smoking history. For reintervention, age and female sex were independent predictors.
Covered stents also were associated with less recurrence and fewer reinterventions in patients undergoing reintervention for mesenteric chronic ischemia. The reintervention group included 15 patients who were treated with BMS and 21 patients treated with covered stents. The two groups were similar in terms of demographics, cardiovascular risk factors, and clinical presentation. In all, 16 vessels were treated with BMS and 22 with covered stents. The two groups had similar approaches, number of vessels treated, and stent dimensions – with the exception of a slight trend toward longer stents in the covered-stent group.
Dr. Oderich reported that he is the principal investigator of a clinical trial sponsored by Cook Medical.
BMS, iCast covered stents, Atrium USA,
BMS, iCast covered stents, Atrium USA,
AT THE VASCULAR ANNUAL MEETING
Major Finding: Freedom from chronic mesenteric ischemia symptom recurrence among primary intervention patients was 92% for those with covered stents, compared with 47% for those with bare metal stents. Similarly, freedom from reintervention was 91% at 5 years for the covered-stent group, compared with 54% for the BMS group.
Data Source: The findings come from a retrospective study of patients treated for chronic mesenteric ischemia using BMS or covered stents (2000-2010).
Disclosures: Dr. Oderich reported that he is the principal investigator of a clinical trial sponsored by Cook Medical.
CTA Tracks Plaque Volume Changes With Statin Therapy
BALTIMORE – Computed tomographic angiography can accurately and noninvasively measure the changes in overall and composition-specific plaque volume as a result of statin therapy.
At 1.2 years’ follow-up, there was a significant 38% decrease in total plaque volume in individuals on statin therapy compared with patients not on statin therapy. This difference remained significant after adjustment for age, gender, and conventional risk factors. Significant changes in noncalcified volume (a 73% decrease) and mixed-plaque volume (a 10% decrease) also were seen in those on statins compared with the nonstatin group. In addition, calcified plaque remained relatively stable, decreasing by 3.5% in the statin therapy group compared with the nonstatin group.
"Statin therapy was associated with a significant decrease in plaque volume, especially in noncalcified plaque volumes, and it was more prominent in women," Dr. Vahid Nabavi said at the annual meeting of the Society of Cardiovascular Computed Tomography.
Several cardiovascular imaging studies revealed that progression of coronary plaque volume over time is an independent predictor of cardiovascular mortality. Intravascular ultrasound (IVUS) provides high-resolution images capable of revealing early preclinical coronary artery disease. However, it is a highly invasive and expensive technique, and will be used only in conjunction with complex coronary interventions, noted Dr. Nabavi, who is a research fellow at the Los Angeles Biomedical Research Institute at Harbor–University of California, Los Angeles, Medical Center.
Quantitative measurements of coronary plaque made with cardiovascular CT angiography (CTA) correlate well with plaque measurements made using IVUS. Low-density noncalcified plaque on CTA correlates with necrotic core plus fibrofatty tissue on IVUS. CTA could be a less expensive, noninvasive alternative to IVUS, particularly for measuring changes in plaque associated with therapy, Dr. Nabavi noted.
In this study, 107 patients underwent serial, clinically indicated CTAs. Their mean age was 67 years, and 81% were men. The median follow-up was 1.2 years. The researchers collected data on risk factors, statin therapy, and laboratory findings.
They quantitatively measured the change in indexed total and composition-specific plaque volume of the target segment with luminal stenosis less than 50% in patients on statin therapy (40 mg of atorvastatin daily) or those with lifestyle changes only.
At baseline, there were no significant differences between groups in age, gender, clinical demographics, risk factors, or total and composition-specific plaque volumes. After adjustment, decreases in overall, mixed, calcified, and noncalcified plaque volumes between those on statins and those not on statins were 56%, 12%, 43%, and 144%. "More robust changes were seen in women," Dr. Nabavi said.
Dr. Nabavi did not report whether he had any conflicts of interest.
BALTIMORE – Computed tomographic angiography can accurately and noninvasively measure the changes in overall and composition-specific plaque volume as a result of statin therapy.
At 1.2 years’ follow-up, there was a significant 38% decrease in total plaque volume in individuals on statin therapy compared with patients not on statin therapy. This difference remained significant after adjustment for age, gender, and conventional risk factors. Significant changes in noncalcified volume (a 73% decrease) and mixed-plaque volume (a 10% decrease) also were seen in those on statins compared with the nonstatin group. In addition, calcified plaque remained relatively stable, decreasing by 3.5% in the statin therapy group compared with the nonstatin group.
"Statin therapy was associated with a significant decrease in plaque volume, especially in noncalcified plaque volumes, and it was more prominent in women," Dr. Vahid Nabavi said at the annual meeting of the Society of Cardiovascular Computed Tomography.
Several cardiovascular imaging studies revealed that progression of coronary plaque volume over time is an independent predictor of cardiovascular mortality. Intravascular ultrasound (IVUS) provides high-resolution images capable of revealing early preclinical coronary artery disease. However, it is a highly invasive and expensive technique, and will be used only in conjunction with complex coronary interventions, noted Dr. Nabavi, who is a research fellow at the Los Angeles Biomedical Research Institute at Harbor–University of California, Los Angeles, Medical Center.
Quantitative measurements of coronary plaque made with cardiovascular CT angiography (CTA) correlate well with plaque measurements made using IVUS. Low-density noncalcified plaque on CTA correlates with necrotic core plus fibrofatty tissue on IVUS. CTA could be a less expensive, noninvasive alternative to IVUS, particularly for measuring changes in plaque associated with therapy, Dr. Nabavi noted.
In this study, 107 patients underwent serial, clinically indicated CTAs. Their mean age was 67 years, and 81% were men. The median follow-up was 1.2 years. The researchers collected data on risk factors, statin therapy, and laboratory findings.
They quantitatively measured the change in indexed total and composition-specific plaque volume of the target segment with luminal stenosis less than 50% in patients on statin therapy (40 mg of atorvastatin daily) or those with lifestyle changes only.
At baseline, there were no significant differences between groups in age, gender, clinical demographics, risk factors, or total and composition-specific plaque volumes. After adjustment, decreases in overall, mixed, calcified, and noncalcified plaque volumes between those on statins and those not on statins were 56%, 12%, 43%, and 144%. "More robust changes were seen in women," Dr. Nabavi said.
Dr. Nabavi did not report whether he had any conflicts of interest.
BALTIMORE – Computed tomographic angiography can accurately and noninvasively measure the changes in overall and composition-specific plaque volume as a result of statin therapy.
At 1.2 years’ follow-up, there was a significant 38% decrease in total plaque volume in individuals on statin therapy compared with patients not on statin therapy. This difference remained significant after adjustment for age, gender, and conventional risk factors. Significant changes in noncalcified volume (a 73% decrease) and mixed-plaque volume (a 10% decrease) also were seen in those on statins compared with the nonstatin group. In addition, calcified plaque remained relatively stable, decreasing by 3.5% in the statin therapy group compared with the nonstatin group.
"Statin therapy was associated with a significant decrease in plaque volume, especially in noncalcified plaque volumes, and it was more prominent in women," Dr. Vahid Nabavi said at the annual meeting of the Society of Cardiovascular Computed Tomography.
Several cardiovascular imaging studies revealed that progression of coronary plaque volume over time is an independent predictor of cardiovascular mortality. Intravascular ultrasound (IVUS) provides high-resolution images capable of revealing early preclinical coronary artery disease. However, it is a highly invasive and expensive technique, and will be used only in conjunction with complex coronary interventions, noted Dr. Nabavi, who is a research fellow at the Los Angeles Biomedical Research Institute at Harbor–University of California, Los Angeles, Medical Center.
Quantitative measurements of coronary plaque made with cardiovascular CT angiography (CTA) correlate well with plaque measurements made using IVUS. Low-density noncalcified plaque on CTA correlates with necrotic core plus fibrofatty tissue on IVUS. CTA could be a less expensive, noninvasive alternative to IVUS, particularly for measuring changes in plaque associated with therapy, Dr. Nabavi noted.
In this study, 107 patients underwent serial, clinically indicated CTAs. Their mean age was 67 years, and 81% were men. The median follow-up was 1.2 years. The researchers collected data on risk factors, statin therapy, and laboratory findings.
They quantitatively measured the change in indexed total and composition-specific plaque volume of the target segment with luminal stenosis less than 50% in patients on statin therapy (40 mg of atorvastatin daily) or those with lifestyle changes only.
At baseline, there were no significant differences between groups in age, gender, clinical demographics, risk factors, or total and composition-specific plaque volumes. After adjustment, decreases in overall, mixed, calcified, and noncalcified plaque volumes between those on statins and those not on statins were 56%, 12%, 43%, and 144%. "More robust changes were seen in women," Dr. Nabavi said.
Dr. Nabavi did not report whether he had any conflicts of interest.
AT THE ANNUAL MEETING OF THE SOCIETY OF CARDIOVASCULAR COMPUTED TOMOGRAPHY
Outcomes Data Used to Assess Residents' Surgical Skills
SAN FRANCISCO – Resident involvement in surgical procedures does not clinically affect surgical outcomes, according to a retrospective study of more than 60,000 cases from the National Surgical Quality Improvement Program database.
"There is a small – although questionable as clinically relevant – overall increase in mild and surgical complications. This is mostly caused by superficial wound infections when residents participate in surgical procedures," said Dr. P. Ravi Kiran, staff surgeon and head of the research section in the department of colorectal surgery at the Cleveland Clinic.
Using data from the National Surgical Quality Improvement Program database from 2005 to 2007, Dr. Kiran and his colleagues compared outcomes for patients who underwent surgery with and without resident participation.
The database, which includes data from pre-, intra-, and postoperative phases, uses clearly defined parameters and specialist nurse reviewers. It also includes resident participation and a morbidity probability, which offers an opportunity to use preoperative factors to stratify risk within subgroups, Dr. Kiran said at the annual meeting of the American Surgical Association.
Resident cases were matched with nonresident cases on the basis of age, sex, specialty, surgical procedure, morbidity probability, and important comorbidities and risk factors. Primary outcomes included 30-day mortality and postoperative complications (mild vs. severe, and surgical vs. medical). Secondary outcomes included the duration of surgery and length of hospital stay.
Mild complications included superficial surgical site infections (SSIs), peripheral nerve injury, urinary tract infection, deep venous thrombosis, and thrombophlebitis. Severe complications included deep (organ) SSI, wound disruption, bleeding requiring transfusion, failure of graft or prosthesis, reoperation, pneumonia, pulmonary embolism, acute renal failure, stroke, myocardial infarction, and sepsis.
Surgical complications included superficial SSI, deep (organ) SSI, wound disruption, bleeding requiring transfusion, failure of graft or prosthesis, peripheral nerve injury, and reoperation. Medical complications included pneumonia, pulmonary embolism, acute renal failure, stroke, myocardial infarction, sepsis, urinary tract infection, deep vein thrombosis, and thrombophlebitis.
For cases with resident vs. nonresident participation, the surgical complication rates were 7% and 6.2%, respectively – a significant difference – and mild complications rates were 4.4% and 3.5%, respectively. In addition, the mean operative time was significantly greater for cases involving residents – 122 vs. 97 minutes. The length of postoperative hospital stay was not significantly longer in the resident group.
The researchers identified 40,474 patients in the resident group and 20,237 patients in the nonresident group. The two groups were similar in terms of median age (50 years), sex (67% female), mean morbidity probability (0.09), American Society of Anesthesiologists classification, and presence of diabetes (6.4%) and hypertension (35%).
The groups were also similar in terms of presence of chronic obstructive pulmonary disease (0.27%), congestive heart failure or myocardial infarction in the past 6 months (0%), dialysis (0.044%), and preoperative sepsis (0.035%). Surgeons’ speciality areas were likewise similar for the two groups (general, 93%; vascular, 6%; and other, 1.36%).
Postgraduate year (PGY) 1-2 residents participated in 31% of operations, PGY 3-5 residents participated in 56%, and residents in PGY 6 or higher participated in 13% of cases.
The 10 most common surgical procedures were laparoscopic appendectomy, laparoscopic gastric bypass, laparoscopic cholecystectomy with and without operative cholangiogram, open appendectomy (nonruptured), thromboendarterectomy, colectomy (partial with anastomosis), laparoscopic colectomy (partial with anastomosis), ventral hernia repair, and placement of gastric band. These procedures were similar in terms of the percentages of resident and nonresident participation.
"We found that there was no difference in the [overall] 30-day mortality between the groups – 0.18% in the resident group and 0.20% in the no-resident group," said Dr. Kiran. However, any 30-day complications were 7.5% in the resident group and 6.7% in the nonresident group, a significant difference.
"When we further looked at the surgical complications, we noted that the cause of the difference in surgical complications between the two groups was the higher rate of SSIs in the resident group, when compared with the no-resident group ... the other surgical complications were similar," he said. The SSI rate was 3.0% for the resident group, compared with 2.2% for the nonresident group.
Interestingly, the researchers also found that overall 30-day complication rates increased with PGY – the rates were 6% for PGY 1-2, 8% for PGY 3-5, and 9% for PGY of 6 or more.
When they examined specific outcomes and complications between different PGY groups and matched cases without the involvement of residents, they found a similar pattern for the overall cohort.
"The reason for the difference in 30-day complications in the groups was because of differences in complications that were classified as mild, and primarily because the superficial surgical site infections were higher in the PGY 1-2 years, with an increased operative time," they said. The same was true for PGY 3-5 and PGY 6 and greater.
Also, as PGY increased, so did operative time – in both resident and nonresident groups. "This suggests that the reason for the increasing complications with increasing PGY years may have been related to increasing complexity of surgery," said Dr. Kiran.
"One overarching issue seems to be how we might achieve high-quality patient care and delivery of the clinical outcomes in the context of training," said Dr. Clifford Ko, a discussant.
However, he also acknowledged that teaching residents takes time. Dr. Ko, a colorectal surgeon and the director of the Center for Surgical Outcomes and Quality at the University of California, Los Angeles, questioned whether the longer operating time associated with resident involvement should be reduced.
"Although we would not perhaps be able to minimize time differences, I think that we have already achieved some mark of control by the gradation of responsibility over time, as residents continue with their training," Dr. Kiran said.
Although the surgical and mild complication rates were slightly greater, it’s unclear whether these differences are clinically relevant.
"The reasons for [these differences] are likely multifactorial and may be related to prolonged operative time. Considering that more complex cases may be performed in teaching hospitals and require resident participation, ‘resident’ could be a surrogate of severity of disease and intensity of operation – factors that may not be clearly discernible in a retrospective study – and this may explain the differences seen.
"Also, quality measures currently underway to reduce surgical site infections across the board may further minimize any of these differences that may exist," Dr. Kiran concluded.
The authors reported that they had no relevant disclosures.
SAN FRANCISCO – Resident involvement in surgical procedures does not clinically affect surgical outcomes, according to a retrospective study of more than 60,000 cases from the National Surgical Quality Improvement Program database.
"There is a small – although questionable as clinically relevant – overall increase in mild and surgical complications. This is mostly caused by superficial wound infections when residents participate in surgical procedures," said Dr. P. Ravi Kiran, staff surgeon and head of the research section in the department of colorectal surgery at the Cleveland Clinic.
Using data from the National Surgical Quality Improvement Program database from 2005 to 2007, Dr. Kiran and his colleagues compared outcomes for patients who underwent surgery with and without resident participation.
The database, which includes data from pre-, intra-, and postoperative phases, uses clearly defined parameters and specialist nurse reviewers. It also includes resident participation and a morbidity probability, which offers an opportunity to use preoperative factors to stratify risk within subgroups, Dr. Kiran said at the annual meeting of the American Surgical Association.
Resident cases were matched with nonresident cases on the basis of age, sex, specialty, surgical procedure, morbidity probability, and important comorbidities and risk factors. Primary outcomes included 30-day mortality and postoperative complications (mild vs. severe, and surgical vs. medical). Secondary outcomes included the duration of surgery and length of hospital stay.
Mild complications included superficial surgical site infections (SSIs), peripheral nerve injury, urinary tract infection, deep venous thrombosis, and thrombophlebitis. Severe complications included deep (organ) SSI, wound disruption, bleeding requiring transfusion, failure of graft or prosthesis, reoperation, pneumonia, pulmonary embolism, acute renal failure, stroke, myocardial infarction, and sepsis.
Surgical complications included superficial SSI, deep (organ) SSI, wound disruption, bleeding requiring transfusion, failure of graft or prosthesis, peripheral nerve injury, and reoperation. Medical complications included pneumonia, pulmonary embolism, acute renal failure, stroke, myocardial infarction, sepsis, urinary tract infection, deep vein thrombosis, and thrombophlebitis.
For cases with resident vs. nonresident participation, the surgical complication rates were 7% and 6.2%, respectively – a significant difference – and mild complications rates were 4.4% and 3.5%, respectively. In addition, the mean operative time was significantly greater for cases involving residents – 122 vs. 97 minutes. The length of postoperative hospital stay was not significantly longer in the resident group.
The researchers identified 40,474 patients in the resident group and 20,237 patients in the nonresident group. The two groups were similar in terms of median age (50 years), sex (67% female), mean morbidity probability (0.09), American Society of Anesthesiologists classification, and presence of diabetes (6.4%) and hypertension (35%).
The groups were also similar in terms of presence of chronic obstructive pulmonary disease (0.27%), congestive heart failure or myocardial infarction in the past 6 months (0%), dialysis (0.044%), and preoperative sepsis (0.035%). Surgeons’ speciality areas were likewise similar for the two groups (general, 93%; vascular, 6%; and other, 1.36%).
Postgraduate year (PGY) 1-2 residents participated in 31% of operations, PGY 3-5 residents participated in 56%, and residents in PGY 6 or higher participated in 13% of cases.
The 10 most common surgical procedures were laparoscopic appendectomy, laparoscopic gastric bypass, laparoscopic cholecystectomy with and without operative cholangiogram, open appendectomy (nonruptured), thromboendarterectomy, colectomy (partial with anastomosis), laparoscopic colectomy (partial with anastomosis), ventral hernia repair, and placement of gastric band. These procedures were similar in terms of the percentages of resident and nonresident participation.
"We found that there was no difference in the [overall] 30-day mortality between the groups – 0.18% in the resident group and 0.20% in the no-resident group," said Dr. Kiran. However, any 30-day complications were 7.5% in the resident group and 6.7% in the nonresident group, a significant difference.
"When we further looked at the surgical complications, we noted that the cause of the difference in surgical complications between the two groups was the higher rate of SSIs in the resident group, when compared with the no-resident group ... the other surgical complications were similar," he said. The SSI rate was 3.0% for the resident group, compared with 2.2% for the nonresident group.
Interestingly, the researchers also found that overall 30-day complication rates increased with PGY – the rates were 6% for PGY 1-2, 8% for PGY 3-5, and 9% for PGY of 6 or more.
When they examined specific outcomes and complications between different PGY groups and matched cases without the involvement of residents, they found a similar pattern for the overall cohort.
"The reason for the difference in 30-day complications in the groups was because of differences in complications that were classified as mild, and primarily because the superficial surgical site infections were higher in the PGY 1-2 years, with an increased operative time," they said. The same was true for PGY 3-5 and PGY 6 and greater.
Also, as PGY increased, so did operative time – in both resident and nonresident groups. "This suggests that the reason for the increasing complications with increasing PGY years may have been related to increasing complexity of surgery," said Dr. Kiran.
"One overarching issue seems to be how we might achieve high-quality patient care and delivery of the clinical outcomes in the context of training," said Dr. Clifford Ko, a discussant.
However, he also acknowledged that teaching residents takes time. Dr. Ko, a colorectal surgeon and the director of the Center for Surgical Outcomes and Quality at the University of California, Los Angeles, questioned whether the longer operating time associated with resident involvement should be reduced.
"Although we would not perhaps be able to minimize time differences, I think that we have already achieved some mark of control by the gradation of responsibility over time, as residents continue with their training," Dr. Kiran said.
Although the surgical and mild complication rates were slightly greater, it’s unclear whether these differences are clinically relevant.
"The reasons for [these differences] are likely multifactorial and may be related to prolonged operative time. Considering that more complex cases may be performed in teaching hospitals and require resident participation, ‘resident’ could be a surrogate of severity of disease and intensity of operation – factors that may not be clearly discernible in a retrospective study – and this may explain the differences seen.
"Also, quality measures currently underway to reduce surgical site infections across the board may further minimize any of these differences that may exist," Dr. Kiran concluded.
The authors reported that they had no relevant disclosures.
SAN FRANCISCO – Resident involvement in surgical procedures does not clinically affect surgical outcomes, according to a retrospective study of more than 60,000 cases from the National Surgical Quality Improvement Program database.
"There is a small – although questionable as clinically relevant – overall increase in mild and surgical complications. This is mostly caused by superficial wound infections when residents participate in surgical procedures," said Dr. P. Ravi Kiran, staff surgeon and head of the research section in the department of colorectal surgery at the Cleveland Clinic.
Using data from the National Surgical Quality Improvement Program database from 2005 to 2007, Dr. Kiran and his colleagues compared outcomes for patients who underwent surgery with and without resident participation.
The database, which includes data from pre-, intra-, and postoperative phases, uses clearly defined parameters and specialist nurse reviewers. It also includes resident participation and a morbidity probability, which offers an opportunity to use preoperative factors to stratify risk within subgroups, Dr. Kiran said at the annual meeting of the American Surgical Association.
Resident cases were matched with nonresident cases on the basis of age, sex, specialty, surgical procedure, morbidity probability, and important comorbidities and risk factors. Primary outcomes included 30-day mortality and postoperative complications (mild vs. severe, and surgical vs. medical). Secondary outcomes included the duration of surgery and length of hospital stay.
Mild complications included superficial surgical site infections (SSIs), peripheral nerve injury, urinary tract infection, deep venous thrombosis, and thrombophlebitis. Severe complications included deep (organ) SSI, wound disruption, bleeding requiring transfusion, failure of graft or prosthesis, reoperation, pneumonia, pulmonary embolism, acute renal failure, stroke, myocardial infarction, and sepsis.
Surgical complications included superficial SSI, deep (organ) SSI, wound disruption, bleeding requiring transfusion, failure of graft or prosthesis, peripheral nerve injury, and reoperation. Medical complications included pneumonia, pulmonary embolism, acute renal failure, stroke, myocardial infarction, sepsis, urinary tract infection, deep vein thrombosis, and thrombophlebitis.
For cases with resident vs. nonresident participation, the surgical complication rates were 7% and 6.2%, respectively – a significant difference – and mild complications rates were 4.4% and 3.5%, respectively. In addition, the mean operative time was significantly greater for cases involving residents – 122 vs. 97 minutes. The length of postoperative hospital stay was not significantly longer in the resident group.
The researchers identified 40,474 patients in the resident group and 20,237 patients in the nonresident group. The two groups were similar in terms of median age (50 years), sex (67% female), mean morbidity probability (0.09), American Society of Anesthesiologists classification, and presence of diabetes (6.4%) and hypertension (35%).
The groups were also similar in terms of presence of chronic obstructive pulmonary disease (0.27%), congestive heart failure or myocardial infarction in the past 6 months (0%), dialysis (0.044%), and preoperative sepsis (0.035%). Surgeons’ speciality areas were likewise similar for the two groups (general, 93%; vascular, 6%; and other, 1.36%).
Postgraduate year (PGY) 1-2 residents participated in 31% of operations, PGY 3-5 residents participated in 56%, and residents in PGY 6 or higher participated in 13% of cases.
The 10 most common surgical procedures were laparoscopic appendectomy, laparoscopic gastric bypass, laparoscopic cholecystectomy with and without operative cholangiogram, open appendectomy (nonruptured), thromboendarterectomy, colectomy (partial with anastomosis), laparoscopic colectomy (partial with anastomosis), ventral hernia repair, and placement of gastric band. These procedures were similar in terms of the percentages of resident and nonresident participation.
"We found that there was no difference in the [overall] 30-day mortality between the groups – 0.18% in the resident group and 0.20% in the no-resident group," said Dr. Kiran. However, any 30-day complications were 7.5% in the resident group and 6.7% in the nonresident group, a significant difference.
"When we further looked at the surgical complications, we noted that the cause of the difference in surgical complications between the two groups was the higher rate of SSIs in the resident group, when compared with the no-resident group ... the other surgical complications were similar," he said. The SSI rate was 3.0% for the resident group, compared with 2.2% for the nonresident group.
Interestingly, the researchers also found that overall 30-day complication rates increased with PGY – the rates were 6% for PGY 1-2, 8% for PGY 3-5, and 9% for PGY of 6 or more.
When they examined specific outcomes and complications between different PGY groups and matched cases without the involvement of residents, they found a similar pattern for the overall cohort.
"The reason for the difference in 30-day complications in the groups was because of differences in complications that were classified as mild, and primarily because the superficial surgical site infections were higher in the PGY 1-2 years, with an increased operative time," they said. The same was true for PGY 3-5 and PGY 6 and greater.
Also, as PGY increased, so did operative time – in both resident and nonresident groups. "This suggests that the reason for the increasing complications with increasing PGY years may have been related to increasing complexity of surgery," said Dr. Kiran.
"One overarching issue seems to be how we might achieve high-quality patient care and delivery of the clinical outcomes in the context of training," said Dr. Clifford Ko, a discussant.
However, he also acknowledged that teaching residents takes time. Dr. Ko, a colorectal surgeon and the director of the Center for Surgical Outcomes and Quality at the University of California, Los Angeles, questioned whether the longer operating time associated with resident involvement should be reduced.
"Although we would not perhaps be able to minimize time differences, I think that we have already achieved some mark of control by the gradation of responsibility over time, as residents continue with their training," Dr. Kiran said.
Although the surgical and mild complication rates were slightly greater, it’s unclear whether these differences are clinically relevant.
"The reasons for [these differences] are likely multifactorial and may be related to prolonged operative time. Considering that more complex cases may be performed in teaching hospitals and require resident participation, ‘resident’ could be a surrogate of severity of disease and intensity of operation – factors that may not be clearly discernible in a retrospective study – and this may explain the differences seen.
"Also, quality measures currently underway to reduce surgical site infections across the board may further minimize any of these differences that may exist," Dr. Kiran concluded.
The authors reported that they had no relevant disclosures.
Major Finding: There was no difference in the overall 30-day mortality between the surgery patient groups with (0.18%) and without (0.20%) resident involvement.
Data Source: Data from the National Surgical Quality Improvement Program database from 2005 to 2007 were used to compare outcomes for patients who underwent surgery with and without resident participation.
Disclosures: The authors reported that they had no relevant disclosures.
FDA Warns of Seizure Risk With Cefepime
The Food and Drug Administration has reported cases of a specific type of seizure called nonconvulsive status epilepticus that is associated with the use of the antibacterial drug cefepime in patients with renal impairment.
The seizures have been seen primarily in patients with renal impairment who did not receive appropriate dosage adjustments of cefepime, although in several cases patients received "dosage adjustment appropriate for their degree of renal impairment," according to the agency. The FDA is working to revise the "Warnings and Precautions" and "Adverse Reactions" sections of the cefepime label to highlight this risk.
The FDA advises health care professionals to adjust the dosage of cefepime in patients with a creatinine clearance of 60 mL/min or less in order to minimize the risk of seizures. If seizures associated with cefepime therapy occur, physicians should consider discontinuing cefepime or making appropriate dosage adjustments in patients with renal impairment.
Nonconvulsive status epilepticus associated with cefepime occurred in 59 patients from 1996 through February 2012. The cases were identified through the FDA’s Adverse Event Reporting System (AERS) database. The majority of seizures were reversible, and resolved after discontinuation of cefepime and/or after hemodialysis.
Cefepime is a cephalosporin antibacterial drug used to treat pneumonia, urinary tract, skin, and intra-abdominal infections.
*This article was updated on 7/3/2012*
The Food and Drug Administration has reported cases of a specific type of seizure called nonconvulsive status epilepticus that is associated with the use of the antibacterial drug cefepime in patients with renal impairment.
The seizures have been seen primarily in patients with renal impairment who did not receive appropriate dosage adjustments of cefepime, although in several cases patients received "dosage adjustment appropriate for their degree of renal impairment," according to the agency. The FDA is working to revise the "Warnings and Precautions" and "Adverse Reactions" sections of the cefepime label to highlight this risk.
The FDA advises health care professionals to adjust the dosage of cefepime in patients with a creatinine clearance of 60 mL/min or less in order to minimize the risk of seizures. If seizures associated with cefepime therapy occur, physicians should consider discontinuing cefepime or making appropriate dosage adjustments in patients with renal impairment.
Nonconvulsive status epilepticus associated with cefepime occurred in 59 patients from 1996 through February 2012. The cases were identified through the FDA’s Adverse Event Reporting System (AERS) database. The majority of seizures were reversible, and resolved after discontinuation of cefepime and/or after hemodialysis.
Cefepime is a cephalosporin antibacterial drug used to treat pneumonia, urinary tract, skin, and intra-abdominal infections.
*This article was updated on 7/3/2012*
The Food and Drug Administration has reported cases of a specific type of seizure called nonconvulsive status epilepticus that is associated with the use of the antibacterial drug cefepime in patients with renal impairment.
The seizures have been seen primarily in patients with renal impairment who did not receive appropriate dosage adjustments of cefepime, although in several cases patients received "dosage adjustment appropriate for their degree of renal impairment," according to the agency. The FDA is working to revise the "Warnings and Precautions" and "Adverse Reactions" sections of the cefepime label to highlight this risk.
The FDA advises health care professionals to adjust the dosage of cefepime in patients with a creatinine clearance of 60 mL/min or less in order to minimize the risk of seizures. If seizures associated with cefepime therapy occur, physicians should consider discontinuing cefepime or making appropriate dosage adjustments in patients with renal impairment.
Nonconvulsive status epilepticus associated with cefepime occurred in 59 patients from 1996 through February 2012. The cases were identified through the FDA’s Adverse Event Reporting System (AERS) database. The majority of seizures were reversible, and resolved after discontinuation of cefepime and/or after hemodialysis.
Cefepime is a cephalosporin antibacterial drug used to treat pneumonia, urinary tract, skin, and intra-abdominal infections.
*This article was updated on 7/3/2012*
Test IDs Bloodstream Bacteria in Under 3 Hours
A diagnostic test that can identify in just hours 12 different bacteria that cause bloodstream infections has received marketing approval from the Food and Drug Administration, June 27.
The Verigene GP Blood Culture Nucleic Acid Test (BC-GP) simultaneously discerns the bacterial types and three associated resistance genes rapidly after the first sign of bacterial growth. Results can be available within 2½ hours, according to test manufacturer Nanosphere. Traditional methods require 2-4 days to identify bacteria and possible resistance.
The test can identify different types of Staphylococcus – including methicillin-resistant S. aureus – Streptococcus, Enterococcus (including vancomycin-resistant Enterococci), and Listeria.
"It is essential to identify which antimicrobial drug is appropriate for a specific patient as quickly as possible," Alberto Gutierrez, Ph.D., observed in an FDA statement. "This new test is an important tool that will help physicians treat patients quickly with the correct antibiotics." Dr. Gutierrez is the director of the Office of In Vitro Diagnostic Device Evaluation and Safety at the FDA’s Center for Devices and Radiological Health.
The agency’s decision was based on a study of 1,642 patient blood samples obtained from incubated blood culture bottles that contained gram-positive bacteria. The study included a comparison of BC-GP and traditional blood culture laboratory methods. The BC-GP results were consistent with traditional blood culture methods in 93%-100% of the comparisons, according to the FDA.
A diagnostic test that can identify in just hours 12 different bacteria that cause bloodstream infections has received marketing approval from the Food and Drug Administration, June 27.
The Verigene GP Blood Culture Nucleic Acid Test (BC-GP) simultaneously discerns the bacterial types and three associated resistance genes rapidly after the first sign of bacterial growth. Results can be available within 2½ hours, according to test manufacturer Nanosphere. Traditional methods require 2-4 days to identify bacteria and possible resistance.
The test can identify different types of Staphylococcus – including methicillin-resistant S. aureus – Streptococcus, Enterococcus (including vancomycin-resistant Enterococci), and Listeria.
"It is essential to identify which antimicrobial drug is appropriate for a specific patient as quickly as possible," Alberto Gutierrez, Ph.D., observed in an FDA statement. "This new test is an important tool that will help physicians treat patients quickly with the correct antibiotics." Dr. Gutierrez is the director of the Office of In Vitro Diagnostic Device Evaluation and Safety at the FDA’s Center for Devices and Radiological Health.
The agency’s decision was based on a study of 1,642 patient blood samples obtained from incubated blood culture bottles that contained gram-positive bacteria. The study included a comparison of BC-GP and traditional blood culture laboratory methods. The BC-GP results were consistent with traditional blood culture methods in 93%-100% of the comparisons, according to the FDA.
A diagnostic test that can identify in just hours 12 different bacteria that cause bloodstream infections has received marketing approval from the Food and Drug Administration, June 27.
The Verigene GP Blood Culture Nucleic Acid Test (BC-GP) simultaneously discerns the bacterial types and three associated resistance genes rapidly after the first sign of bacterial growth. Results can be available within 2½ hours, according to test manufacturer Nanosphere. Traditional methods require 2-4 days to identify bacteria and possible resistance.
The test can identify different types of Staphylococcus – including methicillin-resistant S. aureus – Streptococcus, Enterococcus (including vancomycin-resistant Enterococci), and Listeria.
"It is essential to identify which antimicrobial drug is appropriate for a specific patient as quickly as possible," Alberto Gutierrez, Ph.D., observed in an FDA statement. "This new test is an important tool that will help physicians treat patients quickly with the correct antibiotics." Dr. Gutierrez is the director of the Office of In Vitro Diagnostic Device Evaluation and Safety at the FDA’s Center for Devices and Radiological Health.
The agency’s decision was based on a study of 1,642 patient blood samples obtained from incubated blood culture bottles that contained gram-positive bacteria. The study included a comparison of BC-GP and traditional blood culture laboratory methods. The BC-GP results were consistent with traditional blood culture methods in 93%-100% of the comparisons, according to the FDA.
FDA Panel Backs MarginProbe Breast Cancer Detection Device
GAITHERSBURG, MD. – A novel radiofrequency probe is one step closer to the operating room to help surgeons ensure that lumpectomy margins are benign.
At a meeting on June 21, the Food and Drug Administration’s General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee voted 10-1 that the benefits of the MarginProbe system outweigh risks when used as an adjunctive diagnostic tool for the identification of cancerous tissue at the margins of the ex vivo lumpectomy specimen following primary excision in women previously diagnosed with breast cancer.
"I think that it has the potential to be beneficial to women to reduce their chances of going back to the operating room," said panel member Dr. A. Marilyn Leitch, an oncologic surgeon at the University of Texas Southwestern Medical Center in Dallas. "The sponsor has demonstrated that the device can detect tumor in the margins."
The system uses electromagnetic waves to characterize human tissue in real time, providing surgeons with intraoperative information on the malignancy of the margins of an ex vivo lumpectomy specimen. It is intended for use on freshly excised tissue within 20 minutes of excision. The probe is designed to characterize tissue, based on the principles of dielectric spectroscopy, according to a written statement by the company that sponsors the MarginProbe system, Dune Medical Devices Inc.
The sensor generates radiofrequency electromagnetic fields and measures a slice of tissue 7 mm in diameter, a few mm deep and adjacent to the sensor. The resonance characteristics are dependent on tissue properties – malignant tissue has been shown to have different characteristics compared with benign tissue.
The system is intended for intraoperative use in conjunction with standard methods, such as intraoperative imaging and palpation, to increase the likelihood that patients will undergo intraoperative re-excision of the lumpectomy cavity. The locations of re-excision will correspond to the margins of the ex vivo specimen that have less than 1-mm separation of the excision surface of the margin and the primary breast cancer, according to a statement by the company.
Dune Medical Devices is seeking premarket approval of the device based on data from several clinical trials and intended-use trials conducted primarily in Israel but also in the United States.
The FDA asked the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee to vote on three questions:
• Is there reasonable assurance that the MarginProbe system is safe for use in patients who meet the criteria specified in the proposed indication?
• Is there reasonable assurance that the MarginProbe is effective for use in for patients who meet the criteria specified in the proposed indication?
• Do the benefits of the MarginProbe for use in patients who meet the specified criteria outweigh the risks for use in this patient populations?
Eleven panel members voted that the device is safe; there was one abstention. Eight panel members voted that the device was effective with regard to the proposed indication; one member voted no and there were two abstentions.
The agency also posed a number of questions to the panel regarding the adequacy of end points of key trials submitted; device sensitivity/specificity; the effect of specimen volume on future cosmesis; the appropriateness of applying the design dataset (based primarily on Israeli patients) to a U.S. population; and suggestions for the sponsor’s proposed postapproval study.
The FDA usually follows the recommendations of its advisory panels, which are not binding.
GAITHERSBURG, MD. – A novel radiofrequency probe is one step closer to the operating room to help surgeons ensure that lumpectomy margins are benign.
At a meeting on June 21, the Food and Drug Administration’s General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee voted 10-1 that the benefits of the MarginProbe system outweigh risks when used as an adjunctive diagnostic tool for the identification of cancerous tissue at the margins of the ex vivo lumpectomy specimen following primary excision in women previously diagnosed with breast cancer.
"I think that it has the potential to be beneficial to women to reduce their chances of going back to the operating room," said panel member Dr. A. Marilyn Leitch, an oncologic surgeon at the University of Texas Southwestern Medical Center in Dallas. "The sponsor has demonstrated that the device can detect tumor in the margins."
The system uses electromagnetic waves to characterize human tissue in real time, providing surgeons with intraoperative information on the malignancy of the margins of an ex vivo lumpectomy specimen. It is intended for use on freshly excised tissue within 20 minutes of excision. The probe is designed to characterize tissue, based on the principles of dielectric spectroscopy, according to a written statement by the company that sponsors the MarginProbe system, Dune Medical Devices Inc.
The sensor generates radiofrequency electromagnetic fields and measures a slice of tissue 7 mm in diameter, a few mm deep and adjacent to the sensor. The resonance characteristics are dependent on tissue properties – malignant tissue has been shown to have different characteristics compared with benign tissue.
The system is intended for intraoperative use in conjunction with standard methods, such as intraoperative imaging and palpation, to increase the likelihood that patients will undergo intraoperative re-excision of the lumpectomy cavity. The locations of re-excision will correspond to the margins of the ex vivo specimen that have less than 1-mm separation of the excision surface of the margin and the primary breast cancer, according to a statement by the company.
Dune Medical Devices is seeking premarket approval of the device based on data from several clinical trials and intended-use trials conducted primarily in Israel but also in the United States.
The FDA asked the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee to vote on three questions:
• Is there reasonable assurance that the MarginProbe system is safe for use in patients who meet the criteria specified in the proposed indication?
• Is there reasonable assurance that the MarginProbe is effective for use in for patients who meet the criteria specified in the proposed indication?
• Do the benefits of the MarginProbe for use in patients who meet the specified criteria outweigh the risks for use in this patient populations?
Eleven panel members voted that the device is safe; there was one abstention. Eight panel members voted that the device was effective with regard to the proposed indication; one member voted no and there were two abstentions.
The agency also posed a number of questions to the panel regarding the adequacy of end points of key trials submitted; device sensitivity/specificity; the effect of specimen volume on future cosmesis; the appropriateness of applying the design dataset (based primarily on Israeli patients) to a U.S. population; and suggestions for the sponsor’s proposed postapproval study.
The FDA usually follows the recommendations of its advisory panels, which are not binding.
GAITHERSBURG, MD. – A novel radiofrequency probe is one step closer to the operating room to help surgeons ensure that lumpectomy margins are benign.
At a meeting on June 21, the Food and Drug Administration’s General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee voted 10-1 that the benefits of the MarginProbe system outweigh risks when used as an adjunctive diagnostic tool for the identification of cancerous tissue at the margins of the ex vivo lumpectomy specimen following primary excision in women previously diagnosed with breast cancer.
"I think that it has the potential to be beneficial to women to reduce their chances of going back to the operating room," said panel member Dr. A. Marilyn Leitch, an oncologic surgeon at the University of Texas Southwestern Medical Center in Dallas. "The sponsor has demonstrated that the device can detect tumor in the margins."
The system uses electromagnetic waves to characterize human tissue in real time, providing surgeons with intraoperative information on the malignancy of the margins of an ex vivo lumpectomy specimen. It is intended for use on freshly excised tissue within 20 minutes of excision. The probe is designed to characterize tissue, based on the principles of dielectric spectroscopy, according to a written statement by the company that sponsors the MarginProbe system, Dune Medical Devices Inc.
The sensor generates radiofrequency electromagnetic fields and measures a slice of tissue 7 mm in diameter, a few mm deep and adjacent to the sensor. The resonance characteristics are dependent on tissue properties – malignant tissue has been shown to have different characteristics compared with benign tissue.
The system is intended for intraoperative use in conjunction with standard methods, such as intraoperative imaging and palpation, to increase the likelihood that patients will undergo intraoperative re-excision of the lumpectomy cavity. The locations of re-excision will correspond to the margins of the ex vivo specimen that have less than 1-mm separation of the excision surface of the margin and the primary breast cancer, according to a statement by the company.
Dune Medical Devices is seeking premarket approval of the device based on data from several clinical trials and intended-use trials conducted primarily in Israel but also in the United States.
The FDA asked the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee to vote on three questions:
• Is there reasonable assurance that the MarginProbe system is safe for use in patients who meet the criteria specified in the proposed indication?
• Is there reasonable assurance that the MarginProbe is effective for use in for patients who meet the criteria specified in the proposed indication?
• Do the benefits of the MarginProbe for use in patients who meet the specified criteria outweigh the risks for use in this patient populations?
Eleven panel members voted that the device is safe; there was one abstention. Eight panel members voted that the device was effective with regard to the proposed indication; one member voted no and there were two abstentions.
The agency also posed a number of questions to the panel regarding the adequacy of end points of key trials submitted; device sensitivity/specificity; the effect of specimen volume on future cosmesis; the appropriateness of applying the design dataset (based primarily on Israeli patients) to a U.S. population; and suggestions for the sponsor’s proposed postapproval study.
The FDA usually follows the recommendations of its advisory panels, which are not binding.
AT A MEETING OF THE FOOD AND DRUG ADMINISTRATION'S GENERAL AND PLASTIC SURGERY DEVICES PANEL OF THE MEDICAL DEVICES ADVISORY COMMITTEE
Hospital Procedure Volume May Not Capture Facility Quality
SAN FRANCISCO – Hospital procedure volume, which is commonly used as a proxy measure for hospital quality, is not significantly associated with in-hospital mortality for four common surgical procedures, based on a rigorous statistical analysis of data from the Nationwide Inpatient Sample.
Furthermore, "no identifiable threshold values exist for hospital procedure volume at which mortality risk significantly increased. Mortality risk was primarily attributable to patient-level risk factors," said Dr. Damian J. LaPar of the University of Virginia in Charlottesville.
Dr. LaPar and his colleagues examined the relative strength of association between hospital volume and mortality vs. other modeled variables by comparing model covariate likelihood ratios for four high-risk procedures: pancreatic resection, abdominal aortic aneurysm (AAA) repair, esophageal resection, and coronary artery bypass graft (CABG).
Using data from the Nationwide Inpatient Sample in 2008, they obtained weighted discharge records for 261,142 patients: 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death). Comorbid disease was assessed based on Agency for Healthcare Research and Quality (AHRQ) comorbidity categories.
"In all four models, hospital volume was associated with the lowest statistical strength of association with mortality," compared with all other factors, Dr. LaPar said at the annual meeting of the American Surgical Association. Alternatively, other operation and patient-related risk factors – including elective vs. nonelective status, age, sex, hypertension, weight loss, heart failure, chronic obstructive pulmonary disease, liver disease, and renal failure – had higher strengths of association with mortality.
Dr. LaPar noted that procedure volume is an attractive metric for regulatory bodies to use as a predictor of surgical outcomes; it is easy to measure and intuitive in nature. In addition, higher-volume hospitals are more likely to have established system-based processes and the infrastructure in place to improve patient outcomes.
The Leapfrog Group and the AHRQ both have adopted procedure volume as a quality indicator for the four high-risk surgical procedures. Arbitrarily defined volume thresholds have been adopted as a metric of quality for these procedures. However, many previous statistical methods that are used to define these thresholds have drawn criticism in the recent surgical literature. In many former series, volume is represented as arbitrarily defined categories, rather than as a continuous variable. Furthermore, there has been little attempt to rigorously assess and compare statistical model performance; to assess the relative strength of the association of procedure volume with other outcome predictors; and to utilize hierarchical, multilevel, statistical modeling techniques for complex, multicenter patient samples.
Dr. LaPar and his colleagues used hierarchical general linear modeling and created separate models for each procedure, which were adjusted for patient and operative factors as potential confounders. Patient factors included age, sex, and comorbid disease. Operative factors included procedure volume and elective/nonelective status. All model covariates were selected a priori.
The researchers used hospital volume as a continuous variable with restricted cubic spline regression, which uses all data points to estimate the shape of the association between hospital volume and mortality, and is considered to be the best way to visually identify threshold values. They also assessed the relative strength of association between hospital volume and mortality, compared with other factors (likelihood ratio). Model performance was assessed by looking at discrimination, calibration, and predictive capacity.
AAA repair was associated with the greatest in-hospital death. Patients undergoing AAA repair had the greatest burden of comorbid disease, including peripheral vascular disease, chronic obstructive pulmonary disease, and renal failure.
Patient age was 60 years or greater. Women were most represented in pancreatic resections. Most procedures were elective.
Dr. LaPar noted that the study did not investigate the impact of surgeon volume, nor did it adjust for surgical risk factors such as tumor type/stage, pulmonary function, performance status, surgical technique, preoperative medications, and neoadjuvant therapy. The researchers were also unable to assess the effects of hospital volume on long-term survival, resource utilization, and hospital readmission.
The findings have several implications. Previous reports using conventional modeling techniques may have overestimated the significance of hospital volume as a predictor of mortality. "However, these data do not intend to declare that hospital volume is irrelevant, but rather that hospital procedure volume may be a surrogate for other unidentified institutional factors that influence quality," said Dr. LaPar. "Most importantly, these data do not support the current policy of using hospital procedure volume as a proxy measure for quality."
Invited discussant Dr. Edward Livingston praised the group’s rigorous statistical analysis of the association between hospital procedure volume and quality of care (mortality). He noted that earlier papers showed a statistical association between procedure volume and mortality. "Where the volume outcome research efforts took a left turn is that, instead of trying to understand what it was about volume that’s associated with outcomes, there have been 2 decades of papers published looking at and reconfirming a statistical association between procedure volume and outcomes. Procedure volume itself does not translate into better outcomes. It is the things associated with procedure volume, such as surgeon experience, better functioning [operating room teams, and the like]. We really haven’t looked into those causative factors."
If the causative factors could be identified, "then we could take the experience of high-volume centers and translate that to everybody else, so everybody could have good outcomes," he said.
According to Dr. Livingston, the Dr. Lee Hudson–Robert R. Penn Chair in Surgery at the University of Texas, Dallas, previous studies relied on statistical modeling of the mortality relationship. "Those models are only as good as the model can represent the data," he said, and very few have been rigorously assessed to see how well they describe the phenomenon that they’re trying to describe.
Dr. LaPar’s rigorous work shows that the models don’t actually work that well, said Dr. Livingstone. This paper "should serve as the template for what everyone should do when they’re performing volume outcome studies or any kind of regression analysis."
Dr. Livingston asked what metric should be used in place of volume. Dr. LaPar replied, "I think that’s the billion dollar question. ... This is a complex issue; this is a multifactorial issue that likely includes many different qualitative and quantitative measures that we’re going to have to take a look at."
The authors reported that they have no financial disclosures.
The complete manuscript of the presentation is anticipated to be published in the Annals of Surgery pending editorial review.
SAN FRANCISCO – Hospital procedure volume, which is commonly used as a proxy measure for hospital quality, is not significantly associated with in-hospital mortality for four common surgical procedures, based on a rigorous statistical analysis of data from the Nationwide Inpatient Sample.
Furthermore, "no identifiable threshold values exist for hospital procedure volume at which mortality risk significantly increased. Mortality risk was primarily attributable to patient-level risk factors," said Dr. Damian J. LaPar of the University of Virginia in Charlottesville.
Dr. LaPar and his colleagues examined the relative strength of association between hospital volume and mortality vs. other modeled variables by comparing model covariate likelihood ratios for four high-risk procedures: pancreatic resection, abdominal aortic aneurysm (AAA) repair, esophageal resection, and coronary artery bypass graft (CABG).
Using data from the Nationwide Inpatient Sample in 2008, they obtained weighted discharge records for 261,142 patients: 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death). Comorbid disease was assessed based on Agency for Healthcare Research and Quality (AHRQ) comorbidity categories.
"In all four models, hospital volume was associated with the lowest statistical strength of association with mortality," compared with all other factors, Dr. LaPar said at the annual meeting of the American Surgical Association. Alternatively, other operation and patient-related risk factors – including elective vs. nonelective status, age, sex, hypertension, weight loss, heart failure, chronic obstructive pulmonary disease, liver disease, and renal failure – had higher strengths of association with mortality.
Dr. LaPar noted that procedure volume is an attractive metric for regulatory bodies to use as a predictor of surgical outcomes; it is easy to measure and intuitive in nature. In addition, higher-volume hospitals are more likely to have established system-based processes and the infrastructure in place to improve patient outcomes.
The Leapfrog Group and the AHRQ both have adopted procedure volume as a quality indicator for the four high-risk surgical procedures. Arbitrarily defined volume thresholds have been adopted as a metric of quality for these procedures. However, many previous statistical methods that are used to define these thresholds have drawn criticism in the recent surgical literature. In many former series, volume is represented as arbitrarily defined categories, rather than as a continuous variable. Furthermore, there has been little attempt to rigorously assess and compare statistical model performance; to assess the relative strength of the association of procedure volume with other outcome predictors; and to utilize hierarchical, multilevel, statistical modeling techniques for complex, multicenter patient samples.
Dr. LaPar and his colleagues used hierarchical general linear modeling and created separate models for each procedure, which were adjusted for patient and operative factors as potential confounders. Patient factors included age, sex, and comorbid disease. Operative factors included procedure volume and elective/nonelective status. All model covariates were selected a priori.
The researchers used hospital volume as a continuous variable with restricted cubic spline regression, which uses all data points to estimate the shape of the association between hospital volume and mortality, and is considered to be the best way to visually identify threshold values. They also assessed the relative strength of association between hospital volume and mortality, compared with other factors (likelihood ratio). Model performance was assessed by looking at discrimination, calibration, and predictive capacity.
AAA repair was associated with the greatest in-hospital death. Patients undergoing AAA repair had the greatest burden of comorbid disease, including peripheral vascular disease, chronic obstructive pulmonary disease, and renal failure.
Patient age was 60 years or greater. Women were most represented in pancreatic resections. Most procedures were elective.
Dr. LaPar noted that the study did not investigate the impact of surgeon volume, nor did it adjust for surgical risk factors such as tumor type/stage, pulmonary function, performance status, surgical technique, preoperative medications, and neoadjuvant therapy. The researchers were also unable to assess the effects of hospital volume on long-term survival, resource utilization, and hospital readmission.
The findings have several implications. Previous reports using conventional modeling techniques may have overestimated the significance of hospital volume as a predictor of mortality. "However, these data do not intend to declare that hospital volume is irrelevant, but rather that hospital procedure volume may be a surrogate for other unidentified institutional factors that influence quality," said Dr. LaPar. "Most importantly, these data do not support the current policy of using hospital procedure volume as a proxy measure for quality."
Invited discussant Dr. Edward Livingston praised the group’s rigorous statistical analysis of the association between hospital procedure volume and quality of care (mortality). He noted that earlier papers showed a statistical association between procedure volume and mortality. "Where the volume outcome research efforts took a left turn is that, instead of trying to understand what it was about volume that’s associated with outcomes, there have been 2 decades of papers published looking at and reconfirming a statistical association between procedure volume and outcomes. Procedure volume itself does not translate into better outcomes. It is the things associated with procedure volume, such as surgeon experience, better functioning [operating room teams, and the like]. We really haven’t looked into those causative factors."
If the causative factors could be identified, "then we could take the experience of high-volume centers and translate that to everybody else, so everybody could have good outcomes," he said.
According to Dr. Livingston, the Dr. Lee Hudson–Robert R. Penn Chair in Surgery at the University of Texas, Dallas, previous studies relied on statistical modeling of the mortality relationship. "Those models are only as good as the model can represent the data," he said, and very few have been rigorously assessed to see how well they describe the phenomenon that they’re trying to describe.
Dr. LaPar’s rigorous work shows that the models don’t actually work that well, said Dr. Livingstone. This paper "should serve as the template for what everyone should do when they’re performing volume outcome studies or any kind of regression analysis."
Dr. Livingston asked what metric should be used in place of volume. Dr. LaPar replied, "I think that’s the billion dollar question. ... This is a complex issue; this is a multifactorial issue that likely includes many different qualitative and quantitative measures that we’re going to have to take a look at."
The authors reported that they have no financial disclosures.
The complete manuscript of the presentation is anticipated to be published in the Annals of Surgery pending editorial review.
SAN FRANCISCO – Hospital procedure volume, which is commonly used as a proxy measure for hospital quality, is not significantly associated with in-hospital mortality for four common surgical procedures, based on a rigorous statistical analysis of data from the Nationwide Inpatient Sample.
Furthermore, "no identifiable threshold values exist for hospital procedure volume at which mortality risk significantly increased. Mortality risk was primarily attributable to patient-level risk factors," said Dr. Damian J. LaPar of the University of Virginia in Charlottesville.
Dr. LaPar and his colleagues examined the relative strength of association between hospital volume and mortality vs. other modeled variables by comparing model covariate likelihood ratios for four high-risk procedures: pancreatic resection, abdominal aortic aneurysm (AAA) repair, esophageal resection, and coronary artery bypass graft (CABG).
Using data from the Nationwide Inpatient Sample in 2008, they obtained weighted discharge records for 261,142 patients: 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death). Comorbid disease was assessed based on Agency for Healthcare Research and Quality (AHRQ) comorbidity categories.
"In all four models, hospital volume was associated with the lowest statistical strength of association with mortality," compared with all other factors, Dr. LaPar said at the annual meeting of the American Surgical Association. Alternatively, other operation and patient-related risk factors – including elective vs. nonelective status, age, sex, hypertension, weight loss, heart failure, chronic obstructive pulmonary disease, liver disease, and renal failure – had higher strengths of association with mortality.
Dr. LaPar noted that procedure volume is an attractive metric for regulatory bodies to use as a predictor of surgical outcomes; it is easy to measure and intuitive in nature. In addition, higher-volume hospitals are more likely to have established system-based processes and the infrastructure in place to improve patient outcomes.
The Leapfrog Group and the AHRQ both have adopted procedure volume as a quality indicator for the four high-risk surgical procedures. Arbitrarily defined volume thresholds have been adopted as a metric of quality for these procedures. However, many previous statistical methods that are used to define these thresholds have drawn criticism in the recent surgical literature. In many former series, volume is represented as arbitrarily defined categories, rather than as a continuous variable. Furthermore, there has been little attempt to rigorously assess and compare statistical model performance; to assess the relative strength of the association of procedure volume with other outcome predictors; and to utilize hierarchical, multilevel, statistical modeling techniques for complex, multicenter patient samples.
Dr. LaPar and his colleagues used hierarchical general linear modeling and created separate models for each procedure, which were adjusted for patient and operative factors as potential confounders. Patient factors included age, sex, and comorbid disease. Operative factors included procedure volume and elective/nonelective status. All model covariates were selected a priori.
The researchers used hospital volume as a continuous variable with restricted cubic spline regression, which uses all data points to estimate the shape of the association between hospital volume and mortality, and is considered to be the best way to visually identify threshold values. They also assessed the relative strength of association between hospital volume and mortality, compared with other factors (likelihood ratio). Model performance was assessed by looking at discrimination, calibration, and predictive capacity.
AAA repair was associated with the greatest in-hospital death. Patients undergoing AAA repair had the greatest burden of comorbid disease, including peripheral vascular disease, chronic obstructive pulmonary disease, and renal failure.
Patient age was 60 years or greater. Women were most represented in pancreatic resections. Most procedures were elective.
Dr. LaPar noted that the study did not investigate the impact of surgeon volume, nor did it adjust for surgical risk factors such as tumor type/stage, pulmonary function, performance status, surgical technique, preoperative medications, and neoadjuvant therapy. The researchers were also unable to assess the effects of hospital volume on long-term survival, resource utilization, and hospital readmission.
The findings have several implications. Previous reports using conventional modeling techniques may have overestimated the significance of hospital volume as a predictor of mortality. "However, these data do not intend to declare that hospital volume is irrelevant, but rather that hospital procedure volume may be a surrogate for other unidentified institutional factors that influence quality," said Dr. LaPar. "Most importantly, these data do not support the current policy of using hospital procedure volume as a proxy measure for quality."
Invited discussant Dr. Edward Livingston praised the group’s rigorous statistical analysis of the association between hospital procedure volume and quality of care (mortality). He noted that earlier papers showed a statistical association between procedure volume and mortality. "Where the volume outcome research efforts took a left turn is that, instead of trying to understand what it was about volume that’s associated with outcomes, there have been 2 decades of papers published looking at and reconfirming a statistical association between procedure volume and outcomes. Procedure volume itself does not translate into better outcomes. It is the things associated with procedure volume, such as surgeon experience, better functioning [operating room teams, and the like]. We really haven’t looked into those causative factors."
If the causative factors could be identified, "then we could take the experience of high-volume centers and translate that to everybody else, so everybody could have good outcomes," he said.
According to Dr. Livingston, the Dr. Lee Hudson–Robert R. Penn Chair in Surgery at the University of Texas, Dallas, previous studies relied on statistical modeling of the mortality relationship. "Those models are only as good as the model can represent the data," he said, and very few have been rigorously assessed to see how well they describe the phenomenon that they’re trying to describe.
Dr. LaPar’s rigorous work shows that the models don’t actually work that well, said Dr. Livingstone. This paper "should serve as the template for what everyone should do when they’re performing volume outcome studies or any kind of regression analysis."
Dr. Livingston asked what metric should be used in place of volume. Dr. LaPar replied, "I think that’s the billion dollar question. ... This is a complex issue; this is a multifactorial issue that likely includes many different qualitative and quantitative measures that we’re going to have to take a look at."
The authors reported that they have no financial disclosures.
The complete manuscript of the presentation is anticipated to be published in the Annals of Surgery pending editorial review.
FROM THE ANNUAL MEETING OF THE AMERICAN SURGICAL ASSOCIATION