User login
A Tale of Two Babies and the ‘Family Tragedy’ of Congenital Syphilis
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Bordetella parapertussis reemerges as a cause of respiratory illness in children
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
COVID-19 vaccinations lag in youngest children
Case: A 3-year-old girl presented to the emergency department after a brief seizure at home. She looked well on physical exam except for a fever of 103° F and thick rhinorrhea.
The intern on duty methodically worked through the standard list of questions. “Immunizations up to date?” she asked.
“Absolutely,” the child’s mom responded. “She’s had everything that’s recommended.”
“Including COVID-19 vaccine?” the intern prompted.
“No.” The mom responded with a shake of her head. “We don’t do that vaccine.”
That mom is not alone.
COVID-19 vaccines for children as young as 6 months were given emergency-use authorization by the Food and Drug Administration in June 2022 and in February 2023, the Advisory Committee on Immunization Practices included COVID-19 vaccine on the routine childhood immunization schedule.
COVID-19 vaccines are safe in young children, and they prevent the most severe outcomes associated with infection, including hospitalization. Newly released data confirm that the COVID-19 vaccines produced by Moderna and Pfizer also provide protection against symptomatic infection for at least 4 months after completion of the monovalent primary series.
In a Morbidity and Mortality Weekly Report released on Feb. 17, 2023, the Centers for Disease Control and Prevention reported the results of a test-negative design case-control study that enrolled symptomatic children tested for SARS-CoV-2 infection through Feb. 5, 2023, as part of the Increasing Community Access to Testing (ICATT) program.1 ICATT provides SARS-CoV-2 testing to persons aged at least 3 years at pharmacy and community-based testing sites nationwide.
Two doses of monovalent Moderna vaccine (complete primary series) was 60% effective against symptomatic infection (95% confidence interval, 49%-68%) 2 weeks to 2 months after receipt of the second dose. Vaccine effectiveness dropped to 36% (95% CI, 15%-52%) 3-4 months after the second dose. Three doses of monovalent Pfizer-BioNTech vaccine (complete primary series) was 31% effective (95% CI, 7%-49%) at preventing symptomatic infection 2 weeks to 4 months after receipt of the third dose. A bivalent vaccine dose for eligible children is expected to provide more protection against currently circulating SARS-CoV-2 variants.
Despite evidence of vaccine efficacy, very few parents are opting to protect their young children with the COVID-19 vaccine. The CDC reports that, as of March 1, 2023, only 8% of children under 2 years and 10.5% of children aged 2-4 years have initiated a COVID vaccine series. The American Academy of Pediatrics has emphasized that 15.0 million children between the ages of 6 months and 4 years have not yet received their first COVID-19 vaccine dose.
While the reasons underlying low COVID-19 vaccination rates in young children are complex, themes emerge. Socioeconomic disparities contributing to low vaccination rates in young children were highlighted in another recent MMWR article.2 Through Dec. 1, 2022, vaccination coverage was lower in rural counties (3.4%) than in urban counties (10.5%). Rates were lower in Black and Hispanic children than in White and Asian children.
According to the CDC, high rates of poverty in Black and Hispanic communities may affect vaccination coverage by affecting caregivers’ access to vaccination sites or ability to leave work to take their child to be vaccinated. Pediatric care providers have repeatedly been identified by parents as a source of trusted vaccine information and a strong provider recommendation is associated with vaccination, but not all families are receiving vaccine advice. In a 2022 Kaiser Family Foundation survey, parents of young children with annual household incomes above $90,000 were more likely to talk to their pediatrician about a COVID-19 vaccine than families with lower incomes.3Vaccine hesitancy, fueled by general confusion and skepticism, is another factor contributing to low vaccination rates. Admittedly, the recommendations are complex and on March 14, 2023, the FDA again revised the emergency-use authorization for young children. Some caregivers continue to express concerns about vaccine side effects as well as the belief that the vaccine won’t prevent their child from getting sick.
Kendall Purcell, MD, a pediatrician with Norton Children’s Medical Group in Louisville, Ky., recommends COVID-19 vaccination for her patients because it reduces the risk of severe disease. That factored into her own decision to vaccinate her 4-year-old son and 1-year-old daughter, but she hasn’t been able to convince the parents of all her patients. “Some feel that COVID-19 is not as severe for children, so the risks don’t outweigh the benefits when it comes to vaccinating their children.” Back to our case: In the ED the intern reviewed the laboratory testing she had ordered. She then sat down with the mother of the 3-year-old girl to discuss the diagnosis: febrile seizure associated with COVID-19 infection. Febrile seizures are a well-recognized but uncommon complication of COVID-19 in children. In a retrospective cohort study using electronic health record data, febrile seizures occurred in 0.5% of 8,854 children aged 0-5 years with COVID-19 infection.4 About 9% of these children required critical care services. In another cohort of hospitalized children, neurologic complications occurred in 7% of children hospitalized with COVID-19.5 Febrile and nonfebrile seizures were most commonly observed.
“I really thought COVID-19 was no big deal in young kids,” the mom said. “Parents need the facts.”
The facts are these: Through Dec. 2, 2022, more than 3 million cases of COVID-19 have been reported in children aged younger than 5 years. While COVID is generally less severe in young children than older adults, it is difficult to predict which children will become seriously ill. When children are hospitalized, one in four requires intensive care. COVID-19 is now a vaccine-preventable disease, but too many children remain unprotected.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. Ms. Ezell is a recent graduate from Indiana University Southeast with a Bachelor of Arts in English. They have no conflicts of interest.
References
1. Fleming-Dutra KE et al. Morb Mortal Wkly Rep. 2023;72:177-182.
2. Murthy BP et al. Morb Mortal Wkly Rep. 2023;72:183-9.
3. Lopes L et al. KFF COVID-19 vaccine monitor: July 2022. San Francisco: Kaiser Family Foundation, 2022.
4. Cadet K et al. J Child Neurol. 2022 Apr;37(5):410-5.
5. Antoon JW et al. Pediatrics. 2022 Nov 1;150(5):e2022058167.
Case: A 3-year-old girl presented to the emergency department after a brief seizure at home. She looked well on physical exam except for a fever of 103° F and thick rhinorrhea.
The intern on duty methodically worked through the standard list of questions. “Immunizations up to date?” she asked.
“Absolutely,” the child’s mom responded. “She’s had everything that’s recommended.”
“Including COVID-19 vaccine?” the intern prompted.
“No.” The mom responded with a shake of her head. “We don’t do that vaccine.”
That mom is not alone.
COVID-19 vaccines for children as young as 6 months were given emergency-use authorization by the Food and Drug Administration in June 2022 and in February 2023, the Advisory Committee on Immunization Practices included COVID-19 vaccine on the routine childhood immunization schedule.
COVID-19 vaccines are safe in young children, and they prevent the most severe outcomes associated with infection, including hospitalization. Newly released data confirm that the COVID-19 vaccines produced by Moderna and Pfizer also provide protection against symptomatic infection for at least 4 months after completion of the monovalent primary series.
In a Morbidity and Mortality Weekly Report released on Feb. 17, 2023, the Centers for Disease Control and Prevention reported the results of a test-negative design case-control study that enrolled symptomatic children tested for SARS-CoV-2 infection through Feb. 5, 2023, as part of the Increasing Community Access to Testing (ICATT) program.1 ICATT provides SARS-CoV-2 testing to persons aged at least 3 years at pharmacy and community-based testing sites nationwide.
Two doses of monovalent Moderna vaccine (complete primary series) was 60% effective against symptomatic infection (95% confidence interval, 49%-68%) 2 weeks to 2 months after receipt of the second dose. Vaccine effectiveness dropped to 36% (95% CI, 15%-52%) 3-4 months after the second dose. Three doses of monovalent Pfizer-BioNTech vaccine (complete primary series) was 31% effective (95% CI, 7%-49%) at preventing symptomatic infection 2 weeks to 4 months after receipt of the third dose. A bivalent vaccine dose for eligible children is expected to provide more protection against currently circulating SARS-CoV-2 variants.
Despite evidence of vaccine efficacy, very few parents are opting to protect their young children with the COVID-19 vaccine. The CDC reports that, as of March 1, 2023, only 8% of children under 2 years and 10.5% of children aged 2-4 years have initiated a COVID vaccine series. The American Academy of Pediatrics has emphasized that 15.0 million children between the ages of 6 months and 4 years have not yet received their first COVID-19 vaccine dose.
While the reasons underlying low COVID-19 vaccination rates in young children are complex, themes emerge. Socioeconomic disparities contributing to low vaccination rates in young children were highlighted in another recent MMWR article.2 Through Dec. 1, 2022, vaccination coverage was lower in rural counties (3.4%) than in urban counties (10.5%). Rates were lower in Black and Hispanic children than in White and Asian children.
According to the CDC, high rates of poverty in Black and Hispanic communities may affect vaccination coverage by affecting caregivers’ access to vaccination sites or ability to leave work to take their child to be vaccinated. Pediatric care providers have repeatedly been identified by parents as a source of trusted vaccine information and a strong provider recommendation is associated with vaccination, but not all families are receiving vaccine advice. In a 2022 Kaiser Family Foundation survey, parents of young children with annual household incomes above $90,000 were more likely to talk to their pediatrician about a COVID-19 vaccine than families with lower incomes.3Vaccine hesitancy, fueled by general confusion and skepticism, is another factor contributing to low vaccination rates. Admittedly, the recommendations are complex and on March 14, 2023, the FDA again revised the emergency-use authorization for young children. Some caregivers continue to express concerns about vaccine side effects as well as the belief that the vaccine won’t prevent their child from getting sick.
Kendall Purcell, MD, a pediatrician with Norton Children’s Medical Group in Louisville, Ky., recommends COVID-19 vaccination for her patients because it reduces the risk of severe disease. That factored into her own decision to vaccinate her 4-year-old son and 1-year-old daughter, but she hasn’t been able to convince the parents of all her patients. “Some feel that COVID-19 is not as severe for children, so the risks don’t outweigh the benefits when it comes to vaccinating their children.” Back to our case: In the ED the intern reviewed the laboratory testing she had ordered. She then sat down with the mother of the 3-year-old girl to discuss the diagnosis: febrile seizure associated with COVID-19 infection. Febrile seizures are a well-recognized but uncommon complication of COVID-19 in children. In a retrospective cohort study using electronic health record data, febrile seizures occurred in 0.5% of 8,854 children aged 0-5 years with COVID-19 infection.4 About 9% of these children required critical care services. In another cohort of hospitalized children, neurologic complications occurred in 7% of children hospitalized with COVID-19.5 Febrile and nonfebrile seizures were most commonly observed.
“I really thought COVID-19 was no big deal in young kids,” the mom said. “Parents need the facts.”
The facts are these: Through Dec. 2, 2022, more than 3 million cases of COVID-19 have been reported in children aged younger than 5 years. While COVID is generally less severe in young children than older adults, it is difficult to predict which children will become seriously ill. When children are hospitalized, one in four requires intensive care. COVID-19 is now a vaccine-preventable disease, but too many children remain unprotected.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. Ms. Ezell is a recent graduate from Indiana University Southeast with a Bachelor of Arts in English. They have no conflicts of interest.
References
1. Fleming-Dutra KE et al. Morb Mortal Wkly Rep. 2023;72:177-182.
2. Murthy BP et al. Morb Mortal Wkly Rep. 2023;72:183-9.
3. Lopes L et al. KFF COVID-19 vaccine monitor: July 2022. San Francisco: Kaiser Family Foundation, 2022.
4. Cadet K et al. J Child Neurol. 2022 Apr;37(5):410-5.
5. Antoon JW et al. Pediatrics. 2022 Nov 1;150(5):e2022058167.
Case: A 3-year-old girl presented to the emergency department after a brief seizure at home. She looked well on physical exam except for a fever of 103° F and thick rhinorrhea.
The intern on duty methodically worked through the standard list of questions. “Immunizations up to date?” she asked.
“Absolutely,” the child’s mom responded. “She’s had everything that’s recommended.”
“Including COVID-19 vaccine?” the intern prompted.
“No.” The mom responded with a shake of her head. “We don’t do that vaccine.”
That mom is not alone.
COVID-19 vaccines for children as young as 6 months were given emergency-use authorization by the Food and Drug Administration in June 2022 and in February 2023, the Advisory Committee on Immunization Practices included COVID-19 vaccine on the routine childhood immunization schedule.
COVID-19 vaccines are safe in young children, and they prevent the most severe outcomes associated with infection, including hospitalization. Newly released data confirm that the COVID-19 vaccines produced by Moderna and Pfizer also provide protection against symptomatic infection for at least 4 months after completion of the monovalent primary series.
In a Morbidity and Mortality Weekly Report released on Feb. 17, 2023, the Centers for Disease Control and Prevention reported the results of a test-negative design case-control study that enrolled symptomatic children tested for SARS-CoV-2 infection through Feb. 5, 2023, as part of the Increasing Community Access to Testing (ICATT) program.1 ICATT provides SARS-CoV-2 testing to persons aged at least 3 years at pharmacy and community-based testing sites nationwide.
Two doses of monovalent Moderna vaccine (complete primary series) was 60% effective against symptomatic infection (95% confidence interval, 49%-68%) 2 weeks to 2 months after receipt of the second dose. Vaccine effectiveness dropped to 36% (95% CI, 15%-52%) 3-4 months after the second dose. Three doses of monovalent Pfizer-BioNTech vaccine (complete primary series) was 31% effective (95% CI, 7%-49%) at preventing symptomatic infection 2 weeks to 4 months after receipt of the third dose. A bivalent vaccine dose for eligible children is expected to provide more protection against currently circulating SARS-CoV-2 variants.
Despite evidence of vaccine efficacy, very few parents are opting to protect their young children with the COVID-19 vaccine. The CDC reports that, as of March 1, 2023, only 8% of children under 2 years and 10.5% of children aged 2-4 years have initiated a COVID vaccine series. The American Academy of Pediatrics has emphasized that 15.0 million children between the ages of 6 months and 4 years have not yet received their first COVID-19 vaccine dose.
While the reasons underlying low COVID-19 vaccination rates in young children are complex, themes emerge. Socioeconomic disparities contributing to low vaccination rates in young children were highlighted in another recent MMWR article.2 Through Dec. 1, 2022, vaccination coverage was lower in rural counties (3.4%) than in urban counties (10.5%). Rates were lower in Black and Hispanic children than in White and Asian children.
According to the CDC, high rates of poverty in Black and Hispanic communities may affect vaccination coverage by affecting caregivers’ access to vaccination sites or ability to leave work to take their child to be vaccinated. Pediatric care providers have repeatedly been identified by parents as a source of trusted vaccine information and a strong provider recommendation is associated with vaccination, but not all families are receiving vaccine advice. In a 2022 Kaiser Family Foundation survey, parents of young children with annual household incomes above $90,000 were more likely to talk to their pediatrician about a COVID-19 vaccine than families with lower incomes.3Vaccine hesitancy, fueled by general confusion and skepticism, is another factor contributing to low vaccination rates. Admittedly, the recommendations are complex and on March 14, 2023, the FDA again revised the emergency-use authorization for young children. Some caregivers continue to express concerns about vaccine side effects as well as the belief that the vaccine won’t prevent their child from getting sick.
Kendall Purcell, MD, a pediatrician with Norton Children’s Medical Group in Louisville, Ky., recommends COVID-19 vaccination for her patients because it reduces the risk of severe disease. That factored into her own decision to vaccinate her 4-year-old son and 1-year-old daughter, but she hasn’t been able to convince the parents of all her patients. “Some feel that COVID-19 is not as severe for children, so the risks don’t outweigh the benefits when it comes to vaccinating their children.” Back to our case: In the ED the intern reviewed the laboratory testing she had ordered. She then sat down with the mother of the 3-year-old girl to discuss the diagnosis: febrile seizure associated with COVID-19 infection. Febrile seizures are a well-recognized but uncommon complication of COVID-19 in children. In a retrospective cohort study using electronic health record data, febrile seizures occurred in 0.5% of 8,854 children aged 0-5 years with COVID-19 infection.4 About 9% of these children required critical care services. In another cohort of hospitalized children, neurologic complications occurred in 7% of children hospitalized with COVID-19.5 Febrile and nonfebrile seizures were most commonly observed.
“I really thought COVID-19 was no big deal in young kids,” the mom said. “Parents need the facts.”
The facts are these: Through Dec. 2, 2022, more than 3 million cases of COVID-19 have been reported in children aged younger than 5 years. While COVID is generally less severe in young children than older adults, it is difficult to predict which children will become seriously ill. When children are hospitalized, one in four requires intensive care. COVID-19 is now a vaccine-preventable disease, but too many children remain unprotected.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. Ms. Ezell is a recent graduate from Indiana University Southeast with a Bachelor of Arts in English. They have no conflicts of interest.
References
1. Fleming-Dutra KE et al. Morb Mortal Wkly Rep. 2023;72:177-182.
2. Murthy BP et al. Morb Mortal Wkly Rep. 2023;72:183-9.
3. Lopes L et al. KFF COVID-19 vaccine monitor: July 2022. San Francisco: Kaiser Family Foundation, 2022.
4. Cadet K et al. J Child Neurol. 2022 Apr;37(5):410-5.
5. Antoon JW et al. Pediatrics. 2022 Nov 1;150(5):e2022058167.
Measles
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
More children should be getting flu vaccines
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Monkeypox: What’s a pediatrician to do?
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Answering parents’ questions about Cronobacter and powdered formula
A 6-month-old boy presented with 2 days of looser-than-normal stools without blood or mucous. Before the onset of diarrhea, he had been fed at least two bottles of an infant formula identified in a national recall. His mom requested testing for Cronobacter sakazakii.
In mid-February, Abbott Nutrition recalled specific lots of powdered formula produced at one Michigan manufacturing facility because of possible Cronobacter contamination. To date, a public health investigation has identified four infants in three states who developed Cronobacter infection after consuming formula that was part of the recall. Two of the infants died.
As media reports urged families to search their kitchens for containers of the implicated formula and return them for a refund, worried parents reached out to pediatric care providers for advice.
Cronobacter sakazakii and other Cronobacter species are Gram-negative environmental organisms that occasionally cause bacteremia and meningitis in young infants. Although these infections are not subject to mandatory reporting in most states, laboratory-based surveillance suggests that 18 cases occur annually in the United States (0.49 cases/100,00 infants).
While early reports in the literature described cases in hospitalized, preterm infants, infections also occur in the community and in children born at or near term. A Centers for Disease Control and Prevention review of domestic and international cases identified 183 children <12 months of age between 1961 and 2018 described as diagnosed with Cronobacter bacteremia or meningitis.1 Of the 79 U.S. cases, 34 occurred in term infants and 50 were community onset. Most cases occurred in the first month of life; the oldest child was 35 days of age at the onset of symptoms. Meningitis was more likely in infants born close to term and who were not hospitalized at the time of infection. The majority of infants for whom a feeding history was available had consumed powdered formula.
Back in the exam room, the 6-month-old was examined and found to be vigorous and well-appearing with normal vital signs and no signs of dehydration. The infant’s pediatrician found no clinical indication to perform a blood culture or lumbar puncture, the tests used to diagnose invasive Cronobacter infection. She explained that stool cultures are not recommended, as Cronobacter does not usually cause diarrhea in infants and finding the bacteria in the stool may represent colonization rather than infection.
The pediatrician did take the opportunity to talk to the mom about her formula preparation practices and shared a handout. Powdered formula isn’t sterile, but it is safe for most infants when prepared according to manufacturer’s directions. Contamination of formula during or after preparation can also result in Cronobacter infection in vulnerable infants.
The mom was surprised – and unhappy – to learn that Cronobacter could be lurking in her kitchen. More than a decade ago, investigators visited 78 households in Tennessee and cultured multiple kitchen surfaces.2C. sakazakii was recovered from 21 homes. Most of the positive cultures were from sinks, counter tops, and used dishcloths. Cronobacter has also been cultured from a variety of dried food items, including powdered milk, herbal tea, and starches.
According to the CDC, liquid formula, a product that is sterile until opened, is a safer choice for formula-fed infants who are less than 3 months of age, were born prematurely, or have a compromised immune system. When these infants must be fed powdered formula, preparing it with water heated to at least 158°F or 70°C can kill Cronobacter organisms. Parents should be instructed to boil water and let it cool for about 5 minutes before using it to mix formula.
While most cases of Cronobacter in infants have been epidemiologically linked to consumption of powdered formula, sporadic case reports describe infection in infants fed expressed breast milk. In one report, identical bacterial isolates were recovered from expressed milk fed to an infected infant and the breast pump used to express the milk.3
Moms who express milk should be instructed in proper breast pump hygiene, including washing hands thoroughly before handling breast pumps; disassembling and cleaning breast pumps kits after each use, either in hot soapy water with a dedicated brush and basin or in the dishwasher; air drying on a clean surface; and sanitizing at least daily by boiling, steaming, or using a dishwasher’s sanitize cycle.
Health care providers are encouraged to report Cronobacter cases to their state or local health departments.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Strysko J et al. Emerg Infect Dis. 2020;26(5):857-65.
2. Kilonzo-Nthenge A et al. J Food Protect 2012;75(8):1512-7.
3. Bowen A et al. MMWR Morb Mortal Wkly Rep. 2017;66:761-2.
A 6-month-old boy presented with 2 days of looser-than-normal stools without blood or mucous. Before the onset of diarrhea, he had been fed at least two bottles of an infant formula identified in a national recall. His mom requested testing for Cronobacter sakazakii.
In mid-February, Abbott Nutrition recalled specific lots of powdered formula produced at one Michigan manufacturing facility because of possible Cronobacter contamination. To date, a public health investigation has identified four infants in three states who developed Cronobacter infection after consuming formula that was part of the recall. Two of the infants died.
As media reports urged families to search their kitchens for containers of the implicated formula and return them for a refund, worried parents reached out to pediatric care providers for advice.
Cronobacter sakazakii and other Cronobacter species are Gram-negative environmental organisms that occasionally cause bacteremia and meningitis in young infants. Although these infections are not subject to mandatory reporting in most states, laboratory-based surveillance suggests that 18 cases occur annually in the United States (0.49 cases/100,00 infants).
While early reports in the literature described cases in hospitalized, preterm infants, infections also occur in the community and in children born at or near term. A Centers for Disease Control and Prevention review of domestic and international cases identified 183 children <12 months of age between 1961 and 2018 described as diagnosed with Cronobacter bacteremia or meningitis.1 Of the 79 U.S. cases, 34 occurred in term infants and 50 were community onset. Most cases occurred in the first month of life; the oldest child was 35 days of age at the onset of symptoms. Meningitis was more likely in infants born close to term and who were not hospitalized at the time of infection. The majority of infants for whom a feeding history was available had consumed powdered formula.
Back in the exam room, the 6-month-old was examined and found to be vigorous and well-appearing with normal vital signs and no signs of dehydration. The infant’s pediatrician found no clinical indication to perform a blood culture or lumbar puncture, the tests used to diagnose invasive Cronobacter infection. She explained that stool cultures are not recommended, as Cronobacter does not usually cause diarrhea in infants and finding the bacteria in the stool may represent colonization rather than infection.
The pediatrician did take the opportunity to talk to the mom about her formula preparation practices and shared a handout. Powdered formula isn’t sterile, but it is safe for most infants when prepared according to manufacturer’s directions. Contamination of formula during or after preparation can also result in Cronobacter infection in vulnerable infants.
The mom was surprised – and unhappy – to learn that Cronobacter could be lurking in her kitchen. More than a decade ago, investigators visited 78 households in Tennessee and cultured multiple kitchen surfaces.2C. sakazakii was recovered from 21 homes. Most of the positive cultures were from sinks, counter tops, and used dishcloths. Cronobacter has also been cultured from a variety of dried food items, including powdered milk, herbal tea, and starches.
According to the CDC, liquid formula, a product that is sterile until opened, is a safer choice for formula-fed infants who are less than 3 months of age, were born prematurely, or have a compromised immune system. When these infants must be fed powdered formula, preparing it with water heated to at least 158°F or 70°C can kill Cronobacter organisms. Parents should be instructed to boil water and let it cool for about 5 minutes before using it to mix formula.
While most cases of Cronobacter in infants have been epidemiologically linked to consumption of powdered formula, sporadic case reports describe infection in infants fed expressed breast milk. In one report, identical bacterial isolates were recovered from expressed milk fed to an infected infant and the breast pump used to express the milk.3
Moms who express milk should be instructed in proper breast pump hygiene, including washing hands thoroughly before handling breast pumps; disassembling and cleaning breast pumps kits after each use, either in hot soapy water with a dedicated brush and basin or in the dishwasher; air drying on a clean surface; and sanitizing at least daily by boiling, steaming, or using a dishwasher’s sanitize cycle.
Health care providers are encouraged to report Cronobacter cases to their state or local health departments.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Strysko J et al. Emerg Infect Dis. 2020;26(5):857-65.
2. Kilonzo-Nthenge A et al. J Food Protect 2012;75(8):1512-7.
3. Bowen A et al. MMWR Morb Mortal Wkly Rep. 2017;66:761-2.
A 6-month-old boy presented with 2 days of looser-than-normal stools without blood or mucous. Before the onset of diarrhea, he had been fed at least two bottles of an infant formula identified in a national recall. His mom requested testing for Cronobacter sakazakii.
In mid-February, Abbott Nutrition recalled specific lots of powdered formula produced at one Michigan manufacturing facility because of possible Cronobacter contamination. To date, a public health investigation has identified four infants in three states who developed Cronobacter infection after consuming formula that was part of the recall. Two of the infants died.
As media reports urged families to search their kitchens for containers of the implicated formula and return them for a refund, worried parents reached out to pediatric care providers for advice.
Cronobacter sakazakii and other Cronobacter species are Gram-negative environmental organisms that occasionally cause bacteremia and meningitis in young infants. Although these infections are not subject to mandatory reporting in most states, laboratory-based surveillance suggests that 18 cases occur annually in the United States (0.49 cases/100,00 infants).
While early reports in the literature described cases in hospitalized, preterm infants, infections also occur in the community and in children born at or near term. A Centers for Disease Control and Prevention review of domestic and international cases identified 183 children <12 months of age between 1961 and 2018 described as diagnosed with Cronobacter bacteremia or meningitis.1 Of the 79 U.S. cases, 34 occurred in term infants and 50 were community onset. Most cases occurred in the first month of life; the oldest child was 35 days of age at the onset of symptoms. Meningitis was more likely in infants born close to term and who were not hospitalized at the time of infection. The majority of infants for whom a feeding history was available had consumed powdered formula.
Back in the exam room, the 6-month-old was examined and found to be vigorous and well-appearing with normal vital signs and no signs of dehydration. The infant’s pediatrician found no clinical indication to perform a blood culture or lumbar puncture, the tests used to diagnose invasive Cronobacter infection. She explained that stool cultures are not recommended, as Cronobacter does not usually cause diarrhea in infants and finding the bacteria in the stool may represent colonization rather than infection.
The pediatrician did take the opportunity to talk to the mom about her formula preparation practices and shared a handout. Powdered formula isn’t sterile, but it is safe for most infants when prepared according to manufacturer’s directions. Contamination of formula during or after preparation can also result in Cronobacter infection in vulnerable infants.
The mom was surprised – and unhappy – to learn that Cronobacter could be lurking in her kitchen. More than a decade ago, investigators visited 78 households in Tennessee and cultured multiple kitchen surfaces.2C. sakazakii was recovered from 21 homes. Most of the positive cultures were from sinks, counter tops, and used dishcloths. Cronobacter has also been cultured from a variety of dried food items, including powdered milk, herbal tea, and starches.
According to the CDC, liquid formula, a product that is sterile until opened, is a safer choice for formula-fed infants who are less than 3 months of age, were born prematurely, or have a compromised immune system. When these infants must be fed powdered formula, preparing it with water heated to at least 158°F or 70°C can kill Cronobacter organisms. Parents should be instructed to boil water and let it cool for about 5 minutes before using it to mix formula.
While most cases of Cronobacter in infants have been epidemiologically linked to consumption of powdered formula, sporadic case reports describe infection in infants fed expressed breast milk. In one report, identical bacterial isolates were recovered from expressed milk fed to an infected infant and the breast pump used to express the milk.3
Moms who express milk should be instructed in proper breast pump hygiene, including washing hands thoroughly before handling breast pumps; disassembling and cleaning breast pumps kits after each use, either in hot soapy water with a dedicated brush and basin or in the dishwasher; air drying on a clean surface; and sanitizing at least daily by boiling, steaming, or using a dishwasher’s sanitize cycle.
Health care providers are encouraged to report Cronobacter cases to their state or local health departments.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Strysko J et al. Emerg Infect Dis. 2020;26(5):857-65.
2. Kilonzo-Nthenge A et al. J Food Protect 2012;75(8):1512-7.
3. Bowen A et al. MMWR Morb Mortal Wkly Rep. 2017;66:761-2.
Universal masking is the key to safe school attendance
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
Waiting for the COVID 19 vaccine, or not?
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 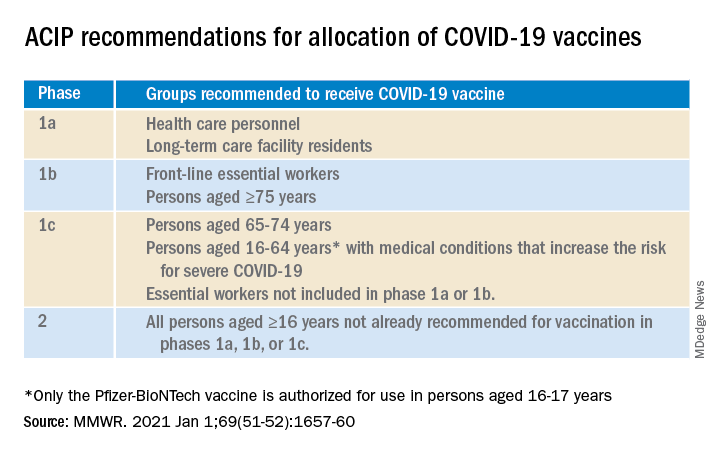
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Action and awareness are needed to increase immunization rates
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].