User login
Treatment for alcohol abuse reduces hepatitis readmission
SAN DIEGO – Treating people with alcoholic hepatitis for alcohol abuse may reduce their risk of hospital readmission, researchers reported.
In a retrospective analysis of nationwide data, 7.83% of those patients who received psychotherapy, counseling, or drug treatment for alcohol abuse were readmitted within 30 days, versus 11.67% of those who did not receive these kinds of treatment.
The finding lends support to the argument that hospitals should invest more in the treatments, despite the complexities involved.
“It takes a multidisciplinary approach, starting from the physician or the health care provider along with the pharmacists, the behavioral health specialists, or a psychiatrist or psychologist, along with case management as well,” said Harleen Chela, MD, a third-year resident at the University of Missouri in Columbia. She presented the findings at the annual Digestive Disease Week® (DDW).
The researchers started with the premise that patients with alcoholic hepatitis can prevent the condition from worsening by abstaining from alcohol. To see whether interventions aimed at encouraging that abstention could prevent readmissions, Dr. Chela and colleagues analyzed data on readmissions for the first 11 months of the year 2018.
They included patients who were at least 18 years of age and who had a nonelective admission with a principal diagnosis of alcohol abuse.
Using procedure codes, they compared those patients given psychotherapy (including cognitive behavioral therapy), formal inpatient counseling, and drug treatment for alcohol abuse to those who didn’t. Then they counted how many patients were readmitted within 30 days.
They found records of 45,617 patients admitted for alcoholic hepatitis of whom 1,552 received treatment for alcohol abuse and 44,065 did not.
They did not find any significant difference between the two groups in demographics, income, or insurance status.
Adjusting for such factors, the researchers found that people who received alcohol abuse treatment were 64% as likely to be readmitted as were those who did not (hazard ratio, 0.64; 95% confidence interval, 0.46-0.91; P = 0.01).
If alcohol abuse treatment is so effective, why isn’t it routine? “It’s not always feasible to implement this, on the inpatient side, because it takes more than a day or two just to get some of these things put in place,” Dr. Chela told this news organization.
They did find that people were more likely to get treatment for alcohol abuse if they were admitted to a hospital in a big city rather than a small town and if their hospital was owned by private investors rather than by a not-for-profit organization or the government.
“Larger hospitals and private sector institutions have more access to resources and money to have those kinds of systems in place for the patients,” said Dr. Chela.
She became interested in the issue at her hospital when she noticed that patients with alcoholic hepatitis were not getting behavioral counseling. “The inpatient load in the behavioral health side is so much that they don’t have time for these kinds of consults,” she said. “That’s one of the challenges: A shortage of behavioral specialists like psychiatrists.”
And hospitals tend to focus on treating conditions that threaten their patients’ lives in the short term. “Someone who has a heart attack or a gastrointestinal bleed – there’s more focus on resources for those kinds of patients,” she said.
Virginia Commonwealth University in Richmond provides alcohol abuse treatment to patients with alcoholic hepatitis partly using telehealth, said Richard Sterling, MD, MSc, chief of hepatology, who was not involved in the study. “For people who live too far away, don’t have transportation, or have other health disparities, we now have technology and mechanisms to keep them engaged in care,” he told this news organization. “We’re doing a lot of Zoom visits.”
Dr. Chela and colleagues also found that those who got alcohol abuse treatment were less likely to be discharged to a skilled nursing facility or to home health. The data couldn’t give the researchers a definitive reason for this, but Dr. Chela speculated that the patients who received treatment for alcohol abuse stayed longer in the hospital and may have been in better shape when they were discharged.
The U.S. health care system doesn’t necessarily provide incentives to keep patients healthy, Dr. Sterling said. “Hospital systems make money off of filling beds, and providing a lot of inpatient care and hospital days,” he said. “That may be not necessarily congruent with a health system that is supposed to provide health for these covered lives.”
Neither Dr. Chela nor Dr. Sterling reported any relevant financial relationships.
SAN DIEGO – Treating people with alcoholic hepatitis for alcohol abuse may reduce their risk of hospital readmission, researchers reported.
In a retrospective analysis of nationwide data, 7.83% of those patients who received psychotherapy, counseling, or drug treatment for alcohol abuse were readmitted within 30 days, versus 11.67% of those who did not receive these kinds of treatment.
The finding lends support to the argument that hospitals should invest more in the treatments, despite the complexities involved.
“It takes a multidisciplinary approach, starting from the physician or the health care provider along with the pharmacists, the behavioral health specialists, or a psychiatrist or psychologist, along with case management as well,” said Harleen Chela, MD, a third-year resident at the University of Missouri in Columbia. She presented the findings at the annual Digestive Disease Week® (DDW).
The researchers started with the premise that patients with alcoholic hepatitis can prevent the condition from worsening by abstaining from alcohol. To see whether interventions aimed at encouraging that abstention could prevent readmissions, Dr. Chela and colleagues analyzed data on readmissions for the first 11 months of the year 2018.
They included patients who were at least 18 years of age and who had a nonelective admission with a principal diagnosis of alcohol abuse.
Using procedure codes, they compared those patients given psychotherapy (including cognitive behavioral therapy), formal inpatient counseling, and drug treatment for alcohol abuse to those who didn’t. Then they counted how many patients were readmitted within 30 days.
They found records of 45,617 patients admitted for alcoholic hepatitis of whom 1,552 received treatment for alcohol abuse and 44,065 did not.
They did not find any significant difference between the two groups in demographics, income, or insurance status.
Adjusting for such factors, the researchers found that people who received alcohol abuse treatment were 64% as likely to be readmitted as were those who did not (hazard ratio, 0.64; 95% confidence interval, 0.46-0.91; P = 0.01).
If alcohol abuse treatment is so effective, why isn’t it routine? “It’s not always feasible to implement this, on the inpatient side, because it takes more than a day or two just to get some of these things put in place,” Dr. Chela told this news organization.
They did find that people were more likely to get treatment for alcohol abuse if they were admitted to a hospital in a big city rather than a small town and if their hospital was owned by private investors rather than by a not-for-profit organization or the government.
“Larger hospitals and private sector institutions have more access to resources and money to have those kinds of systems in place for the patients,” said Dr. Chela.
She became interested in the issue at her hospital when she noticed that patients with alcoholic hepatitis were not getting behavioral counseling. “The inpatient load in the behavioral health side is so much that they don’t have time for these kinds of consults,” she said. “That’s one of the challenges: A shortage of behavioral specialists like psychiatrists.”
And hospitals tend to focus on treating conditions that threaten their patients’ lives in the short term. “Someone who has a heart attack or a gastrointestinal bleed – there’s more focus on resources for those kinds of patients,” she said.
Virginia Commonwealth University in Richmond provides alcohol abuse treatment to patients with alcoholic hepatitis partly using telehealth, said Richard Sterling, MD, MSc, chief of hepatology, who was not involved in the study. “For people who live too far away, don’t have transportation, or have other health disparities, we now have technology and mechanisms to keep them engaged in care,” he told this news organization. “We’re doing a lot of Zoom visits.”
Dr. Chela and colleagues also found that those who got alcohol abuse treatment were less likely to be discharged to a skilled nursing facility or to home health. The data couldn’t give the researchers a definitive reason for this, but Dr. Chela speculated that the patients who received treatment for alcohol abuse stayed longer in the hospital and may have been in better shape when they were discharged.
The U.S. health care system doesn’t necessarily provide incentives to keep patients healthy, Dr. Sterling said. “Hospital systems make money off of filling beds, and providing a lot of inpatient care and hospital days,” he said. “That may be not necessarily congruent with a health system that is supposed to provide health for these covered lives.”
Neither Dr. Chela nor Dr. Sterling reported any relevant financial relationships.
SAN DIEGO – Treating people with alcoholic hepatitis for alcohol abuse may reduce their risk of hospital readmission, researchers reported.
In a retrospective analysis of nationwide data, 7.83% of those patients who received psychotherapy, counseling, or drug treatment for alcohol abuse were readmitted within 30 days, versus 11.67% of those who did not receive these kinds of treatment.
The finding lends support to the argument that hospitals should invest more in the treatments, despite the complexities involved.
“It takes a multidisciplinary approach, starting from the physician or the health care provider along with the pharmacists, the behavioral health specialists, or a psychiatrist or psychologist, along with case management as well,” said Harleen Chela, MD, a third-year resident at the University of Missouri in Columbia. She presented the findings at the annual Digestive Disease Week® (DDW).
The researchers started with the premise that patients with alcoholic hepatitis can prevent the condition from worsening by abstaining from alcohol. To see whether interventions aimed at encouraging that abstention could prevent readmissions, Dr. Chela and colleagues analyzed data on readmissions for the first 11 months of the year 2018.
They included patients who were at least 18 years of age and who had a nonelective admission with a principal diagnosis of alcohol abuse.
Using procedure codes, they compared those patients given psychotherapy (including cognitive behavioral therapy), formal inpatient counseling, and drug treatment for alcohol abuse to those who didn’t. Then they counted how many patients were readmitted within 30 days.
They found records of 45,617 patients admitted for alcoholic hepatitis of whom 1,552 received treatment for alcohol abuse and 44,065 did not.
They did not find any significant difference between the two groups in demographics, income, or insurance status.
Adjusting for such factors, the researchers found that people who received alcohol abuse treatment were 64% as likely to be readmitted as were those who did not (hazard ratio, 0.64; 95% confidence interval, 0.46-0.91; P = 0.01).
If alcohol abuse treatment is so effective, why isn’t it routine? “It’s not always feasible to implement this, on the inpatient side, because it takes more than a day or two just to get some of these things put in place,” Dr. Chela told this news organization.
They did find that people were more likely to get treatment for alcohol abuse if they were admitted to a hospital in a big city rather than a small town and if their hospital was owned by private investors rather than by a not-for-profit organization or the government.
“Larger hospitals and private sector institutions have more access to resources and money to have those kinds of systems in place for the patients,” said Dr. Chela.
She became interested in the issue at her hospital when she noticed that patients with alcoholic hepatitis were not getting behavioral counseling. “The inpatient load in the behavioral health side is so much that they don’t have time for these kinds of consults,” she said. “That’s one of the challenges: A shortage of behavioral specialists like psychiatrists.”
And hospitals tend to focus on treating conditions that threaten their patients’ lives in the short term. “Someone who has a heart attack or a gastrointestinal bleed – there’s more focus on resources for those kinds of patients,” she said.
Virginia Commonwealth University in Richmond provides alcohol abuse treatment to patients with alcoholic hepatitis partly using telehealth, said Richard Sterling, MD, MSc, chief of hepatology, who was not involved in the study. “For people who live too far away, don’t have transportation, or have other health disparities, we now have technology and mechanisms to keep them engaged in care,” he told this news organization. “We’re doing a lot of Zoom visits.”
Dr. Chela and colleagues also found that those who got alcohol abuse treatment were less likely to be discharged to a skilled nursing facility or to home health. The data couldn’t give the researchers a definitive reason for this, but Dr. Chela speculated that the patients who received treatment for alcohol abuse stayed longer in the hospital and may have been in better shape when they were discharged.
The U.S. health care system doesn’t necessarily provide incentives to keep patients healthy, Dr. Sterling said. “Hospital systems make money off of filling beds, and providing a lot of inpatient care and hospital days,” he said. “That may be not necessarily congruent with a health system that is supposed to provide health for these covered lives.”
Neither Dr. Chela nor Dr. Sterling reported any relevant financial relationships.
AT DDW 2022
TNF blockers beat newer biologics in Crohn’s disease: Meta-analysis
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.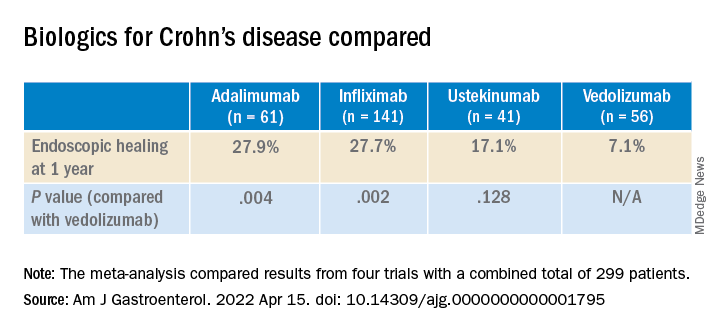
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Newly defined liver disorder associated with COVID mortality
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
FROM HEPATOLOGY COMMUNICATIONS
Psychiatric illness associated with eosinophilic esophagitis
People with eosinophilic esophagitis (EoE) may run an increased risk of mood disorders, anxiety, and ADHD and should be screened for those conditions, researchers say.
“It’s important to know that there is an elevated risk of those diagnoses, so you have that in mind when you treat your patients. You can assess their quality of life and the status of their mental state,” said lead author Lovisa Röjler, MD, a pediatrician and doctoral student at Örebro (Sweden) University Hospital.
“Psychiatric disorders are not found with a blood sample or radiology examination,” she said in an interview.
The study was published online in the American Journal of Gastroenterology.
Elevated risk found
Previous studies into the relationship between EoE and anxiety and depression have conflicting conclusions.
In the hope of shedding further light, Dr. Röjler and colleagues analyzed data from Sweden’s ESPRESSO cohort, which consists of more than 6 million biopsy samples from the gastrointestinal tract that were collected from throughout the country during the years 1965-2017.
They identified 1,458 people with EoE who had not experienced psychiatric events before being diagnosed with EoE. Of these, 70% had dysphagia, and 58% had food impaction.
In the study, up to 5 reference persons (6,436 people) without EoE who were identified from the Swedish Total Population Register were matched to the patients with EoE by age, sex, county, and year of diagnosis.
Among the people with EoE, there were 106 events of psychiatric disease, at an incidence of 15.96 per 1,000 person-years versus 10.93 per 1,000 person-years (331 events) among those without EoE. This 50% increased risk for psychiatric illness for people with EoE was statistically significant (hazard ratio, 1.50; 95% confidence interval, 1.20-1.87).
To adjust for genetic and environmental confounding factors, the researchers compared the rate of psychiatric events among 1,055 people with EoE with that of siblings who did not have EoE (1,699 people). There were 74 events of psychiatric disease among the siblings (8.99 per 1000 person-years). From this the researchers calculated a 62% increased risk of psychiatric events for those with EoE (HR, 1.62; 95% CI, 1.14-2.31).
There was no difference in risk for psychiatric disorders by educational attainment, though people for whom there were no data on education were at increased risk.
There was also no difference in psychiatric risk associated with the use of steroids or proton pump inhibitors for EoE, though these medications have sometimes been linked to psychiatric disorders.
After adjusting for inflammatory bowel disease, celiac disease, and asthma, the researchers still found an increased risk of psychiatric events. Also, the people who had EoE were no more likely than the reference persons to have had psychiatric events before their diagnosis, suggesting that EoE caused the psychiatric events rather than the other way around.
Previous researchers have found a similar association with psychiatric illness in people with celiac disease and inflammatory bowel disease. The researchers speculated that people with EoE might develop psychiatric illnesses because their symptoms and treatments, such as restrictive diets, cause stress and chronic pain and thereby cause problems with education, work, and social and economic status.
Dr. Röjler recommended that clinicians use questionnaires to identify mood disorders and ADHD in their patients and then refer them to a mental health professional.
Screen for psychiatric disorders
Tiffany Taft, PsyD, a research associate professor of medicine at Northwestern University, Chicago, who was not involved in the study, agreed that patients with EoE should be screened more often for psychiatric disorders.
“We’ve found that symptom-specific anxiety is prevalent and associated with other outcomes, like quality of life, so it may not be the typical anxiety that you would diagnose from the Diagnostic and Statistical Manual of Mental Disorders,” Dr. Taft said in an interview.
While anxiety is not likely to trigger EoE, it can worsen the symptoms, she said. Sometimes helping patients make the connection between their mental health and EoE can address the anxiety itself.
“Education is good enough for a certain chunk of patients,” Dr. Taft said.
Other patients benefit from cognitive-behavioral therapy, which gives them a more realistic understanding of their situation.
“We also add in relaxation, deep breathing, and guided imagery to calm down the stress response in the body, which is part of that brain-gut connection that enhances symptom severity,” she said.
Some patients prefer medications, or they rely on medication because that is what their insurance provides, she said, adding that most patients do best with a combination of medication and talk therapy.
Ideally, people with these disorders would be referred to someone such as herself, a psychotherapist with a specialty in gastroenterology, Dr. Taft said. But there are not many people in that subspecialty, so if a gastroenterology psychologist is not available, a psychologist who specializes in treating mental illness associated with chronic diseases is a good second choice.
The study was funded by Örebro County Council and Karolinska Institutet. Dr. Röjler and Dr. Taft reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with eosinophilic esophagitis (EoE) may run an increased risk of mood disorders, anxiety, and ADHD and should be screened for those conditions, researchers say.
“It’s important to know that there is an elevated risk of those diagnoses, so you have that in mind when you treat your patients. You can assess their quality of life and the status of their mental state,” said lead author Lovisa Röjler, MD, a pediatrician and doctoral student at Örebro (Sweden) University Hospital.
“Psychiatric disorders are not found with a blood sample or radiology examination,” she said in an interview.
The study was published online in the American Journal of Gastroenterology.
Elevated risk found
Previous studies into the relationship between EoE and anxiety and depression have conflicting conclusions.
In the hope of shedding further light, Dr. Röjler and colleagues analyzed data from Sweden’s ESPRESSO cohort, which consists of more than 6 million biopsy samples from the gastrointestinal tract that were collected from throughout the country during the years 1965-2017.
They identified 1,458 people with EoE who had not experienced psychiatric events before being diagnosed with EoE. Of these, 70% had dysphagia, and 58% had food impaction.
In the study, up to 5 reference persons (6,436 people) without EoE who were identified from the Swedish Total Population Register were matched to the patients with EoE by age, sex, county, and year of diagnosis.
Among the people with EoE, there were 106 events of psychiatric disease, at an incidence of 15.96 per 1,000 person-years versus 10.93 per 1,000 person-years (331 events) among those without EoE. This 50% increased risk for psychiatric illness for people with EoE was statistically significant (hazard ratio, 1.50; 95% confidence interval, 1.20-1.87).
To adjust for genetic and environmental confounding factors, the researchers compared the rate of psychiatric events among 1,055 people with EoE with that of siblings who did not have EoE (1,699 people). There were 74 events of psychiatric disease among the siblings (8.99 per 1000 person-years). From this the researchers calculated a 62% increased risk of psychiatric events for those with EoE (HR, 1.62; 95% CI, 1.14-2.31).
There was no difference in risk for psychiatric disorders by educational attainment, though people for whom there were no data on education were at increased risk.
There was also no difference in psychiatric risk associated with the use of steroids or proton pump inhibitors for EoE, though these medications have sometimes been linked to psychiatric disorders.
After adjusting for inflammatory bowel disease, celiac disease, and asthma, the researchers still found an increased risk of psychiatric events. Also, the people who had EoE were no more likely than the reference persons to have had psychiatric events before their diagnosis, suggesting that EoE caused the psychiatric events rather than the other way around.
Previous researchers have found a similar association with psychiatric illness in people with celiac disease and inflammatory bowel disease. The researchers speculated that people with EoE might develop psychiatric illnesses because their symptoms and treatments, such as restrictive diets, cause stress and chronic pain and thereby cause problems with education, work, and social and economic status.
Dr. Röjler recommended that clinicians use questionnaires to identify mood disorders and ADHD in their patients and then refer them to a mental health professional.
Screen for psychiatric disorders
Tiffany Taft, PsyD, a research associate professor of medicine at Northwestern University, Chicago, who was not involved in the study, agreed that patients with EoE should be screened more often for psychiatric disorders.
“We’ve found that symptom-specific anxiety is prevalent and associated with other outcomes, like quality of life, so it may not be the typical anxiety that you would diagnose from the Diagnostic and Statistical Manual of Mental Disorders,” Dr. Taft said in an interview.
While anxiety is not likely to trigger EoE, it can worsen the symptoms, she said. Sometimes helping patients make the connection between their mental health and EoE can address the anxiety itself.
“Education is good enough for a certain chunk of patients,” Dr. Taft said.
Other patients benefit from cognitive-behavioral therapy, which gives them a more realistic understanding of their situation.
“We also add in relaxation, deep breathing, and guided imagery to calm down the stress response in the body, which is part of that brain-gut connection that enhances symptom severity,” she said.
Some patients prefer medications, or they rely on medication because that is what their insurance provides, she said, adding that most patients do best with a combination of medication and talk therapy.
Ideally, people with these disorders would be referred to someone such as herself, a psychotherapist with a specialty in gastroenterology, Dr. Taft said. But there are not many people in that subspecialty, so if a gastroenterology psychologist is not available, a psychologist who specializes in treating mental illness associated with chronic diseases is a good second choice.
The study was funded by Örebro County Council and Karolinska Institutet. Dr. Röjler and Dr. Taft reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with eosinophilic esophagitis (EoE) may run an increased risk of mood disorders, anxiety, and ADHD and should be screened for those conditions, researchers say.
“It’s important to know that there is an elevated risk of those diagnoses, so you have that in mind when you treat your patients. You can assess their quality of life and the status of their mental state,” said lead author Lovisa Röjler, MD, a pediatrician and doctoral student at Örebro (Sweden) University Hospital.
“Psychiatric disorders are not found with a blood sample or radiology examination,” she said in an interview.
The study was published online in the American Journal of Gastroenterology.
Elevated risk found
Previous studies into the relationship between EoE and anxiety and depression have conflicting conclusions.
In the hope of shedding further light, Dr. Röjler and colleagues analyzed data from Sweden’s ESPRESSO cohort, which consists of more than 6 million biopsy samples from the gastrointestinal tract that were collected from throughout the country during the years 1965-2017.
They identified 1,458 people with EoE who had not experienced psychiatric events before being diagnosed with EoE. Of these, 70% had dysphagia, and 58% had food impaction.
In the study, up to 5 reference persons (6,436 people) without EoE who were identified from the Swedish Total Population Register were matched to the patients with EoE by age, sex, county, and year of diagnosis.
Among the people with EoE, there were 106 events of psychiatric disease, at an incidence of 15.96 per 1,000 person-years versus 10.93 per 1,000 person-years (331 events) among those without EoE. This 50% increased risk for psychiatric illness for people with EoE was statistically significant (hazard ratio, 1.50; 95% confidence interval, 1.20-1.87).
To adjust for genetic and environmental confounding factors, the researchers compared the rate of psychiatric events among 1,055 people with EoE with that of siblings who did not have EoE (1,699 people). There were 74 events of psychiatric disease among the siblings (8.99 per 1000 person-years). From this the researchers calculated a 62% increased risk of psychiatric events for those with EoE (HR, 1.62; 95% CI, 1.14-2.31).
There was no difference in risk for psychiatric disorders by educational attainment, though people for whom there were no data on education were at increased risk.
There was also no difference in psychiatric risk associated with the use of steroids or proton pump inhibitors for EoE, though these medications have sometimes been linked to psychiatric disorders.
After adjusting for inflammatory bowel disease, celiac disease, and asthma, the researchers still found an increased risk of psychiatric events. Also, the people who had EoE were no more likely than the reference persons to have had psychiatric events before their diagnosis, suggesting that EoE caused the psychiatric events rather than the other way around.
Previous researchers have found a similar association with psychiatric illness in people with celiac disease and inflammatory bowel disease. The researchers speculated that people with EoE might develop psychiatric illnesses because their symptoms and treatments, such as restrictive diets, cause stress and chronic pain and thereby cause problems with education, work, and social and economic status.
Dr. Röjler recommended that clinicians use questionnaires to identify mood disorders and ADHD in their patients and then refer them to a mental health professional.
Screen for psychiatric disorders
Tiffany Taft, PsyD, a research associate professor of medicine at Northwestern University, Chicago, who was not involved in the study, agreed that patients with EoE should be screened more often for psychiatric disorders.
“We’ve found that symptom-specific anxiety is prevalent and associated with other outcomes, like quality of life, so it may not be the typical anxiety that you would diagnose from the Diagnostic and Statistical Manual of Mental Disorders,” Dr. Taft said in an interview.
While anxiety is not likely to trigger EoE, it can worsen the symptoms, she said. Sometimes helping patients make the connection between their mental health and EoE can address the anxiety itself.
“Education is good enough for a certain chunk of patients,” Dr. Taft said.
Other patients benefit from cognitive-behavioral therapy, which gives them a more realistic understanding of their situation.
“We also add in relaxation, deep breathing, and guided imagery to calm down the stress response in the body, which is part of that brain-gut connection that enhances symptom severity,” she said.
Some patients prefer medications, or they rely on medication because that is what their insurance provides, she said, adding that most patients do best with a combination of medication and talk therapy.
Ideally, people with these disorders would be referred to someone such as herself, a psychotherapist with a specialty in gastroenterology, Dr. Taft said. But there are not many people in that subspecialty, so if a gastroenterology psychologist is not available, a psychologist who specializes in treating mental illness associated with chronic diseases is a good second choice.
The study was funded by Örebro County Council and Karolinska Institutet. Dr. Röjler and Dr. Taft reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
PD-L1 test debated in gastroesophageal cancer immunotherapy
on whether measuring programmed death–ligand 1 (PD-L1) is essential before prescribing checkpoint inhibitors for gastroesophageal cancer.
“In the last couple of years, the incorporation of PD-1 antibodies is really changing our standard of care and international guidelines in this disease,” said Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital, London.
He moderated a debate at the 2022 Gastrointestinal Cancers Symposium on the importance of measuring PD-L1 expression levels before administering immune checkpoint inhibitor therapy.
Tumor cells can use PD-1 signaling to deactivate the response of T cells that would otherwise destroy them, and several new drugs are designed to block that signaling.
Multiple randomized controlled trials have shown the benefit of adding such checkpoint inhibitors to chemotherapy for gastroesophageal cancer and currently, chemotherapy plus a checkpoint inhibitor is standard care, Dr. Chau said.
PD-1–blocking antibodies include pembrolizumab (Keytruda, Merck) for colorectal cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma, among other cancers. And, nivolumab (Opdivo, Bristol-Myers Squibb) approved for renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and esophageal squamous cell carcinoma, among other cancers.
Regulators differ on whether these treatments should be limited to patients whose expression level of PD-L1 reaches a defined threshold. The FDA has not required the measurement of any biomarker before starting therapy with either of these drugs. However, the EMA requires a PD-L1 combined positive score (CPS) of at least 10 for pembrolizumab and at least 5 for nivolumab.
In a poll conducted before the start of the debate, 83% of physician attendees said they favor of the EMA position, while 17% disagreed.
Florian Lordick, MD, PhD, director of the University of Leipzig (Germany) Cancer Center, argued that tests for PD-L1 expression are accurate. About half of patients have CPS of at least 1. And, pathologists are in agreement in interpreting the tests about 97% of the time.
Pivotal decision-making clinical trials
The EMA requires a PD-L1 assay primarily based on its interpretation of the data from KEYNOTE-590 and CheckMate-648.
KEYNOTE-590 included 749 patients with esophageal carcinoma who were randomized to receive either pembrolizumab with standard of care chemotherapy, or placebo and standard of care chemotherapy. Patients receiving pembrolizumab who had PD-L1 CPS scores of 10 or more survived a few months longer on average. But in a post hoc analysis, the investigators found that, in patients with PD-L1 CPS scores less than 10, the difference between the treatment and placebo groups was not statistically significant.
In CheckMate-648, 970 patients with esophageal carcinoma were randomized to receive nivolumab with ipilimumab, nivolumab with chemotherapy, or chemotherapy alone. Investigators used a slightly different measurement of PD-L1 tumor proportion score (TPS), in comparing chemotherapy plus nivolumab to chemotherapy alone for advanced esophageal squamous cell carcinoma.
The median survival time of patients with TPS of at least 1% was 15.4 months in the nivolumab group and 9.2 months in the control group. But among patients with a TPS of less than 1%, the median overall survival was 12.0 months in the nivolumab group and 12.2 in the control group. CPS thresholds of 5 or 1 resulted in similar effects.
Dr. Lordick cited a systematic review of four studies in press at ESMO Open. Hazard ratios for three of the studies favored immunotherapy only in patients with CPS of at least 10.
Deciding which patients to treat matters because these drugs are expensive, he said. A single dose of 240 mg nivolumab costs $7,228.70, and treatment for 1 year can cost $173,488.80 in addition to costs for hospitalization because of immune-related adverse events, labs, imaging, colonoscopy, and other related costs.
“This is a lot of money, isn’t it? It’s the same price the hospital pay for two registered nurses in the U.S., at least when we are talking about the average price. I’m not sure I want to spend this money for a drug that does not work,” Dr. Lordick said.
Aaron Scott, MD, an oncologist with the University of Arizona Cancer Center, Tucson, said that “patients and clinicians want and need options” because there may be other factors that should be considered.
“PD-L1 has shown inconsistent results. And while I agree it is the best that we have for predictive biomarker in the space, it is far from perfect. PD-L1 has not been predictive for response in a variety of settings and trials. In first-line, second-line, third-line trials we have examples where it does not predict response,” he said.
JUPITER06 compared toripalimab or placebo with paclitaxel and cisplatin for patients with esophageal squamous cell carcinoma. Patients who received toripalimab lived longer than patients who received the placebo, but within the toripalimab group, there was no difference in median overall survival between those patients above and those below the threshold of CPS 1.
ORIENT-15 compared sintilimab or placebo with chemotherapy as first-line therapy for patients with esophageal squamous cell carcinoma. Although the treatment group fared better, survival rates were the same whether patients were above or below the CPS 10 threshold.
Dr. Scott cited three other trials in which PD-L1 was not predictive of response to checkpoint inhibitors.
The differences among studies could be attributed to different assays, he said. “Where you biopsy and when you biopsy seems to matter.”
In a second opinion poll conducted after the presentations, the proportion of physician attendees saying PD-L1 was essential before initiating checkpoint inhibitors, dropped to about two-thirds.
Dr. Chau reported financial relationships with Bristol-Myers Squibb, Merck Serono, and other pharmaceutical companies. Dr. Lordick reported financial relationships Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, and other pharmaceutical companies.
on whether measuring programmed death–ligand 1 (PD-L1) is essential before prescribing checkpoint inhibitors for gastroesophageal cancer.
“In the last couple of years, the incorporation of PD-1 antibodies is really changing our standard of care and international guidelines in this disease,” said Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital, London.
He moderated a debate at the 2022 Gastrointestinal Cancers Symposium on the importance of measuring PD-L1 expression levels before administering immune checkpoint inhibitor therapy.
Tumor cells can use PD-1 signaling to deactivate the response of T cells that would otherwise destroy them, and several new drugs are designed to block that signaling.
Multiple randomized controlled trials have shown the benefit of adding such checkpoint inhibitors to chemotherapy for gastroesophageal cancer and currently, chemotherapy plus a checkpoint inhibitor is standard care, Dr. Chau said.
PD-1–blocking antibodies include pembrolizumab (Keytruda, Merck) for colorectal cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma, among other cancers. And, nivolumab (Opdivo, Bristol-Myers Squibb) approved for renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and esophageal squamous cell carcinoma, among other cancers.
Regulators differ on whether these treatments should be limited to patients whose expression level of PD-L1 reaches a defined threshold. The FDA has not required the measurement of any biomarker before starting therapy with either of these drugs. However, the EMA requires a PD-L1 combined positive score (CPS) of at least 10 for pembrolizumab and at least 5 for nivolumab.
In a poll conducted before the start of the debate, 83% of physician attendees said they favor of the EMA position, while 17% disagreed.
Florian Lordick, MD, PhD, director of the University of Leipzig (Germany) Cancer Center, argued that tests for PD-L1 expression are accurate. About half of patients have CPS of at least 1. And, pathologists are in agreement in interpreting the tests about 97% of the time.
Pivotal decision-making clinical trials
The EMA requires a PD-L1 assay primarily based on its interpretation of the data from KEYNOTE-590 and CheckMate-648.
KEYNOTE-590 included 749 patients with esophageal carcinoma who were randomized to receive either pembrolizumab with standard of care chemotherapy, or placebo and standard of care chemotherapy. Patients receiving pembrolizumab who had PD-L1 CPS scores of 10 or more survived a few months longer on average. But in a post hoc analysis, the investigators found that, in patients with PD-L1 CPS scores less than 10, the difference between the treatment and placebo groups was not statistically significant.
In CheckMate-648, 970 patients with esophageal carcinoma were randomized to receive nivolumab with ipilimumab, nivolumab with chemotherapy, or chemotherapy alone. Investigators used a slightly different measurement of PD-L1 tumor proportion score (TPS), in comparing chemotherapy plus nivolumab to chemotherapy alone for advanced esophageal squamous cell carcinoma.
The median survival time of patients with TPS of at least 1% was 15.4 months in the nivolumab group and 9.2 months in the control group. But among patients with a TPS of less than 1%, the median overall survival was 12.0 months in the nivolumab group and 12.2 in the control group. CPS thresholds of 5 or 1 resulted in similar effects.
Dr. Lordick cited a systematic review of four studies in press at ESMO Open. Hazard ratios for three of the studies favored immunotherapy only in patients with CPS of at least 10.
Deciding which patients to treat matters because these drugs are expensive, he said. A single dose of 240 mg nivolumab costs $7,228.70, and treatment for 1 year can cost $173,488.80 in addition to costs for hospitalization because of immune-related adverse events, labs, imaging, colonoscopy, and other related costs.
“This is a lot of money, isn’t it? It’s the same price the hospital pay for two registered nurses in the U.S., at least when we are talking about the average price. I’m not sure I want to spend this money for a drug that does not work,” Dr. Lordick said.
Aaron Scott, MD, an oncologist with the University of Arizona Cancer Center, Tucson, said that “patients and clinicians want and need options” because there may be other factors that should be considered.
“PD-L1 has shown inconsistent results. And while I agree it is the best that we have for predictive biomarker in the space, it is far from perfect. PD-L1 has not been predictive for response in a variety of settings and trials. In first-line, second-line, third-line trials we have examples where it does not predict response,” he said.
JUPITER06 compared toripalimab or placebo with paclitaxel and cisplatin for patients with esophageal squamous cell carcinoma. Patients who received toripalimab lived longer than patients who received the placebo, but within the toripalimab group, there was no difference in median overall survival between those patients above and those below the threshold of CPS 1.
ORIENT-15 compared sintilimab or placebo with chemotherapy as first-line therapy for patients with esophageal squamous cell carcinoma. Although the treatment group fared better, survival rates were the same whether patients were above or below the CPS 10 threshold.
Dr. Scott cited three other trials in which PD-L1 was not predictive of response to checkpoint inhibitors.
The differences among studies could be attributed to different assays, he said. “Where you biopsy and when you biopsy seems to matter.”
In a second opinion poll conducted after the presentations, the proportion of physician attendees saying PD-L1 was essential before initiating checkpoint inhibitors, dropped to about two-thirds.
Dr. Chau reported financial relationships with Bristol-Myers Squibb, Merck Serono, and other pharmaceutical companies. Dr. Lordick reported financial relationships Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, and other pharmaceutical companies.
on whether measuring programmed death–ligand 1 (PD-L1) is essential before prescribing checkpoint inhibitors for gastroesophageal cancer.
“In the last couple of years, the incorporation of PD-1 antibodies is really changing our standard of care and international guidelines in this disease,” said Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital, London.
He moderated a debate at the 2022 Gastrointestinal Cancers Symposium on the importance of measuring PD-L1 expression levels before administering immune checkpoint inhibitor therapy.
Tumor cells can use PD-1 signaling to deactivate the response of T cells that would otherwise destroy them, and several new drugs are designed to block that signaling.
Multiple randomized controlled trials have shown the benefit of adding such checkpoint inhibitors to chemotherapy for gastroesophageal cancer and currently, chemotherapy plus a checkpoint inhibitor is standard care, Dr. Chau said.
PD-1–blocking antibodies include pembrolizumab (Keytruda, Merck) for colorectal cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma, among other cancers. And, nivolumab (Opdivo, Bristol-Myers Squibb) approved for renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and esophageal squamous cell carcinoma, among other cancers.
Regulators differ on whether these treatments should be limited to patients whose expression level of PD-L1 reaches a defined threshold. The FDA has not required the measurement of any biomarker before starting therapy with either of these drugs. However, the EMA requires a PD-L1 combined positive score (CPS) of at least 10 for pembrolizumab and at least 5 for nivolumab.
In a poll conducted before the start of the debate, 83% of physician attendees said they favor of the EMA position, while 17% disagreed.
Florian Lordick, MD, PhD, director of the University of Leipzig (Germany) Cancer Center, argued that tests for PD-L1 expression are accurate. About half of patients have CPS of at least 1. And, pathologists are in agreement in interpreting the tests about 97% of the time.
Pivotal decision-making clinical trials
The EMA requires a PD-L1 assay primarily based on its interpretation of the data from KEYNOTE-590 and CheckMate-648.
KEYNOTE-590 included 749 patients with esophageal carcinoma who were randomized to receive either pembrolizumab with standard of care chemotherapy, or placebo and standard of care chemotherapy. Patients receiving pembrolizumab who had PD-L1 CPS scores of 10 or more survived a few months longer on average. But in a post hoc analysis, the investigators found that, in patients with PD-L1 CPS scores less than 10, the difference between the treatment and placebo groups was not statistically significant.
In CheckMate-648, 970 patients with esophageal carcinoma were randomized to receive nivolumab with ipilimumab, nivolumab with chemotherapy, or chemotherapy alone. Investigators used a slightly different measurement of PD-L1 tumor proportion score (TPS), in comparing chemotherapy plus nivolumab to chemotherapy alone for advanced esophageal squamous cell carcinoma.
The median survival time of patients with TPS of at least 1% was 15.4 months in the nivolumab group and 9.2 months in the control group. But among patients with a TPS of less than 1%, the median overall survival was 12.0 months in the nivolumab group and 12.2 in the control group. CPS thresholds of 5 or 1 resulted in similar effects.
Dr. Lordick cited a systematic review of four studies in press at ESMO Open. Hazard ratios for three of the studies favored immunotherapy only in patients with CPS of at least 10.
Deciding which patients to treat matters because these drugs are expensive, he said. A single dose of 240 mg nivolumab costs $7,228.70, and treatment for 1 year can cost $173,488.80 in addition to costs for hospitalization because of immune-related adverse events, labs, imaging, colonoscopy, and other related costs.
“This is a lot of money, isn’t it? It’s the same price the hospital pay for two registered nurses in the U.S., at least when we are talking about the average price. I’m not sure I want to spend this money for a drug that does not work,” Dr. Lordick said.
Aaron Scott, MD, an oncologist with the University of Arizona Cancer Center, Tucson, said that “patients and clinicians want and need options” because there may be other factors that should be considered.
“PD-L1 has shown inconsistent results. And while I agree it is the best that we have for predictive biomarker in the space, it is far from perfect. PD-L1 has not been predictive for response in a variety of settings and trials. In first-line, second-line, third-line trials we have examples where it does not predict response,” he said.
JUPITER06 compared toripalimab or placebo with paclitaxel and cisplatin for patients with esophageal squamous cell carcinoma. Patients who received toripalimab lived longer than patients who received the placebo, but within the toripalimab group, there was no difference in median overall survival between those patients above and those below the threshold of CPS 1.
ORIENT-15 compared sintilimab or placebo with chemotherapy as first-line therapy for patients with esophageal squamous cell carcinoma. Although the treatment group fared better, survival rates were the same whether patients were above or below the CPS 10 threshold.
Dr. Scott cited three other trials in which PD-L1 was not predictive of response to checkpoint inhibitors.
The differences among studies could be attributed to different assays, he said. “Where you biopsy and when you biopsy seems to matter.”
In a second opinion poll conducted after the presentations, the proportion of physician attendees saying PD-L1 was essential before initiating checkpoint inhibitors, dropped to about two-thirds.
Dr. Chau reported financial relationships with Bristol-Myers Squibb, Merck Serono, and other pharmaceutical companies. Dr. Lordick reported financial relationships Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, and other pharmaceutical companies.
FROM ASCO GI 2022
Less cirrhosis but worse outcomes for Black patients with NASH
Compared with White people, Black people are less likely to develop cirrhosis from nonalcoholic steatohepatitis (NASH) but are more likely to die when hospitalized with this condition, researchers say.
The finding highlights the importance of addressing hepatic complications and nonhepatic comorbidities with a comprehensive and interdisciplinary approach that includes social determinants of health, said Emad Qayed, MD, MPH, an associate professor of medicine at Emory University School of Medicine, Atlanta.
“Clinicians should realize that in Black patients with NASH and NASH cirrhosis, mortality can be high despite a low rate of hepatic complications,” he told this news organization.
The study by Dr. Qayed and colleagues was published in the Journal of Clinical Gastroenterology.
A nationwide analysis
Previous studies have indicated that Black people are less likely than White people to develop nonalcoholic fatty liver disease (NAFLD), despite the fact that prevalence is increasing. Furthermore, when Black people do develop NAFLD, the disease is less likely to progress to NASH. In cases in which NASH does develop, the evidence has been mixed as to the effect of race on hospital outcomes.
To shed new light on that question, Dr. Qayed and colleagues analyzed data from 2016 to 2018 from the National Inpatient Sample, which is produced by the Healthcare Cost and Utilization Project and is sponsored by the Agency of Healthcare Research and Quality.
They identified 43,409 hospitalizations for NASH, with 41,143 White patients and 2,266 Black patients. The mean age of the Black patients was less than that of the White patients (56.4 years vs. 63.0 years), and Black patients were more likely to be women (69.9% vs. 61.6%).
More of the Black patients had hypertension, obesity, chronic kidney disease, and congestive heart failure, while more of the White patients had diabetes, dyslipidemia, and ischemic heart disease.
Among the Black patients, 33.6% had cirrhosis, compared with 56.4% of the White patients. Likewise, among the Black patients, there were fewer manifestations of decompensated cirrhosis, compared with the White patients. Black patients were also less likely to have had to undergo upper endoscopy and paracentesis.
The Black patients died in the hospital at a rate of 3.9%, which was not significantly higher than the 3.7% rate for the White patients (unadjusted odds ratio = 1.06; 95% confidence interval: 0.84-1.32; P = .6). But, when the researchers adjusted for age, sex, cirrhosis, risk of mortality (based on the overall number and severity of diseases), and insurance status, there were significantly higher odds of mortality among the Black patients (adjusted OR, 1.34; 95% CI: 1.05-1.71; P = .018).
They did not find any association between hospital size, location, or region with mortality.
They also found no difference in mortality between Black patients and White patients among those those with and those without cirrhosis. However, they found that Black patients were more likely to have acute kidney injury, chronic kidney disease, and congestive heart failure.
Regarding the reasons for hospitalization, the researchers found liver-related illnesses, such as hepatic failure and noninfectious hepatitis, to be most common among the White patients. Circulatory disorders, such as heart failure, and endocrine disorders, such as diabetes mellitus with complications, were found to be most common among the Black patients.
The length of time in the hospital was longer for the Black patients than the White patients (6.3 days vs. 5.6 days; P < .0001). The cost of hospitalization was higher for Black patients as well ($18,603 vs. $17,467). This suggests that Black patients were sicker overall, despite their lower rates of liver complications.
“Some of these differences are likely related to socioeconomic factors and clinical comorbidities, such as cardiac and renal disease,” Dr. Qayed said. “However, the underlying etiologies for such disparities in NASH and cirrhosis remain unclear. Further research is warranted to clarify these etiologies.”
NASH as part of the metabolic syndrome
“Clinicians should consider NASH as part of the metabolic syndrome,” Paul Martin, MD, chief of digestive health and liver diseases at the University of Miami, told this news organization. He was not involved in the study.
“Typically, these patients have a number of risk factors for fatty liver, including obesity and often hyperlipidemia, hypertension, and sleep apnea,” he said. “Clinicians should screen their patients for such comorbidities and then treat them.”
Genetic factors could also play a role in the difference in susceptibility to fatty liver disease found between Black and White patients, he added.
Dr. Martin noted a prevalence of fatty liver in many Hispanic populations and that it is found in Asia but sometimes in the absence of the risk factors associated with it in the United States.
Dr. Qayed and Dr. Martin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Compared with White people, Black people are less likely to develop cirrhosis from nonalcoholic steatohepatitis (NASH) but are more likely to die when hospitalized with this condition, researchers say.
The finding highlights the importance of addressing hepatic complications and nonhepatic comorbidities with a comprehensive and interdisciplinary approach that includes social determinants of health, said Emad Qayed, MD, MPH, an associate professor of medicine at Emory University School of Medicine, Atlanta.
“Clinicians should realize that in Black patients with NASH and NASH cirrhosis, mortality can be high despite a low rate of hepatic complications,” he told this news organization.
The study by Dr. Qayed and colleagues was published in the Journal of Clinical Gastroenterology.
A nationwide analysis
Previous studies have indicated that Black people are less likely than White people to develop nonalcoholic fatty liver disease (NAFLD), despite the fact that prevalence is increasing. Furthermore, when Black people do develop NAFLD, the disease is less likely to progress to NASH. In cases in which NASH does develop, the evidence has been mixed as to the effect of race on hospital outcomes.
To shed new light on that question, Dr. Qayed and colleagues analyzed data from 2016 to 2018 from the National Inpatient Sample, which is produced by the Healthcare Cost and Utilization Project and is sponsored by the Agency of Healthcare Research and Quality.
They identified 43,409 hospitalizations for NASH, with 41,143 White patients and 2,266 Black patients. The mean age of the Black patients was less than that of the White patients (56.4 years vs. 63.0 years), and Black patients were more likely to be women (69.9% vs. 61.6%).
More of the Black patients had hypertension, obesity, chronic kidney disease, and congestive heart failure, while more of the White patients had diabetes, dyslipidemia, and ischemic heart disease.
Among the Black patients, 33.6% had cirrhosis, compared with 56.4% of the White patients. Likewise, among the Black patients, there were fewer manifestations of decompensated cirrhosis, compared with the White patients. Black patients were also less likely to have had to undergo upper endoscopy and paracentesis.
The Black patients died in the hospital at a rate of 3.9%, which was not significantly higher than the 3.7% rate for the White patients (unadjusted odds ratio = 1.06; 95% confidence interval: 0.84-1.32; P = .6). But, when the researchers adjusted for age, sex, cirrhosis, risk of mortality (based on the overall number and severity of diseases), and insurance status, there were significantly higher odds of mortality among the Black patients (adjusted OR, 1.34; 95% CI: 1.05-1.71; P = .018).
They did not find any association between hospital size, location, or region with mortality.
They also found no difference in mortality between Black patients and White patients among those those with and those without cirrhosis. However, they found that Black patients were more likely to have acute kidney injury, chronic kidney disease, and congestive heart failure.
Regarding the reasons for hospitalization, the researchers found liver-related illnesses, such as hepatic failure and noninfectious hepatitis, to be most common among the White patients. Circulatory disorders, such as heart failure, and endocrine disorders, such as diabetes mellitus with complications, were found to be most common among the Black patients.
The length of time in the hospital was longer for the Black patients than the White patients (6.3 days vs. 5.6 days; P < .0001). The cost of hospitalization was higher for Black patients as well ($18,603 vs. $17,467). This suggests that Black patients were sicker overall, despite their lower rates of liver complications.
“Some of these differences are likely related to socioeconomic factors and clinical comorbidities, such as cardiac and renal disease,” Dr. Qayed said. “However, the underlying etiologies for such disparities in NASH and cirrhosis remain unclear. Further research is warranted to clarify these etiologies.”
NASH as part of the metabolic syndrome
“Clinicians should consider NASH as part of the metabolic syndrome,” Paul Martin, MD, chief of digestive health and liver diseases at the University of Miami, told this news organization. He was not involved in the study.
“Typically, these patients have a number of risk factors for fatty liver, including obesity and often hyperlipidemia, hypertension, and sleep apnea,” he said. “Clinicians should screen their patients for such comorbidities and then treat them.”
Genetic factors could also play a role in the difference in susceptibility to fatty liver disease found between Black and White patients, he added.
Dr. Martin noted a prevalence of fatty liver in many Hispanic populations and that it is found in Asia but sometimes in the absence of the risk factors associated with it in the United States.
Dr. Qayed and Dr. Martin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Compared with White people, Black people are less likely to develop cirrhosis from nonalcoholic steatohepatitis (NASH) but are more likely to die when hospitalized with this condition, researchers say.
The finding highlights the importance of addressing hepatic complications and nonhepatic comorbidities with a comprehensive and interdisciplinary approach that includes social determinants of health, said Emad Qayed, MD, MPH, an associate professor of medicine at Emory University School of Medicine, Atlanta.
“Clinicians should realize that in Black patients with NASH and NASH cirrhosis, mortality can be high despite a low rate of hepatic complications,” he told this news organization.
The study by Dr. Qayed and colleagues was published in the Journal of Clinical Gastroenterology.
A nationwide analysis
Previous studies have indicated that Black people are less likely than White people to develop nonalcoholic fatty liver disease (NAFLD), despite the fact that prevalence is increasing. Furthermore, when Black people do develop NAFLD, the disease is less likely to progress to NASH. In cases in which NASH does develop, the evidence has been mixed as to the effect of race on hospital outcomes.
To shed new light on that question, Dr. Qayed and colleagues analyzed data from 2016 to 2018 from the National Inpatient Sample, which is produced by the Healthcare Cost and Utilization Project and is sponsored by the Agency of Healthcare Research and Quality.
They identified 43,409 hospitalizations for NASH, with 41,143 White patients and 2,266 Black patients. The mean age of the Black patients was less than that of the White patients (56.4 years vs. 63.0 years), and Black patients were more likely to be women (69.9% vs. 61.6%).
More of the Black patients had hypertension, obesity, chronic kidney disease, and congestive heart failure, while more of the White patients had diabetes, dyslipidemia, and ischemic heart disease.
Among the Black patients, 33.6% had cirrhosis, compared with 56.4% of the White patients. Likewise, among the Black patients, there were fewer manifestations of decompensated cirrhosis, compared with the White patients. Black patients were also less likely to have had to undergo upper endoscopy and paracentesis.
The Black patients died in the hospital at a rate of 3.9%, which was not significantly higher than the 3.7% rate for the White patients (unadjusted odds ratio = 1.06; 95% confidence interval: 0.84-1.32; P = .6). But, when the researchers adjusted for age, sex, cirrhosis, risk of mortality (based on the overall number and severity of diseases), and insurance status, there were significantly higher odds of mortality among the Black patients (adjusted OR, 1.34; 95% CI: 1.05-1.71; P = .018).
They did not find any association between hospital size, location, or region with mortality.
They also found no difference in mortality between Black patients and White patients among those those with and those without cirrhosis. However, they found that Black patients were more likely to have acute kidney injury, chronic kidney disease, and congestive heart failure.
Regarding the reasons for hospitalization, the researchers found liver-related illnesses, such as hepatic failure and noninfectious hepatitis, to be most common among the White patients. Circulatory disorders, such as heart failure, and endocrine disorders, such as diabetes mellitus with complications, were found to be most common among the Black patients.
The length of time in the hospital was longer for the Black patients than the White patients (6.3 days vs. 5.6 days; P < .0001). The cost of hospitalization was higher for Black patients as well ($18,603 vs. $17,467). This suggests that Black patients were sicker overall, despite their lower rates of liver complications.
“Some of these differences are likely related to socioeconomic factors and clinical comorbidities, such as cardiac and renal disease,” Dr. Qayed said. “However, the underlying etiologies for such disparities in NASH and cirrhosis remain unclear. Further research is warranted to clarify these etiologies.”
NASH as part of the metabolic syndrome
“Clinicians should consider NASH as part of the metabolic syndrome,” Paul Martin, MD, chief of digestive health and liver diseases at the University of Miami, told this news organization. He was not involved in the study.
“Typically, these patients have a number of risk factors for fatty liver, including obesity and often hyperlipidemia, hypertension, and sleep apnea,” he said. “Clinicians should screen their patients for such comorbidities and then treat them.”
Genetic factors could also play a role in the difference in susceptibility to fatty liver disease found between Black and White patients, he added.
Dr. Martin noted a prevalence of fatty liver in many Hispanic populations and that it is found in Asia but sometimes in the absence of the risk factors associated with it in the United States.
Dr. Qayed and Dr. Martin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New Barrett’s esophagus guideline reduces reliance on endoscopy
A swallowable, capsule-sponge device used to sample biomarkers offers a reliable, noninvasive alternative to endoscopy for diagnosing Barrett’s esophagus, according to a new guideline from the American College of Gastroenterology.
In addition, the guideline recommends that patients with Barrett’s esophagus with segments of less than 3 cm be screened every 5 years, but if their Barrett’s esophagus segments are 5 cm or greater, they should be screened every 3 years.
“We don’t want to scope everyone for the lowest risk of cancer,” lead author Nicholas J. Shaheen, MD, MPH, chief of gastroenterology and hepatology at the University of North Carolina School of Medicine, Chapel Hill, told this news organization.
“The traditional way to diagnose Barrett’s esophagus is by upper endoscopy, but it’s expensive and not available everywhere,” he said. “One big change since the last iteration of this guideline is that there’s been a development of nonendoscopic screening modalities.”
The guideline was published in The American Journal of Gastroenterology.
Progress with swallowable devices
Solid evidence supports the Cytosponge, a capsule containing a compressed spherical polyurethane sponge attached to a string. The sponge expands to a sphere when the capsule dissolves after being swallowed, Dr. Shaheen said.
When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for Barrett’s esophagus, either the protein trefoil factor 3 expressed in intestinal metaplasia or methylated DNA markers associated with Barrett’s esophagus mucosa.
More than 90% of participants in trials have been able to swallow the device. Some mild gagging or throat discomfort has been reported.
In one trial, patients with chronic reflux were randomly assigned to either swallow the Cytosponge or be screened by endoscopy based on the practitioner’s usual care. Any diagnosis with the Cytosponge was confirmed with endoscopy. Barrett’s esophagus was found in 2% of patients who had undergone the Cytosponge procedure, versus less than 1% of those screened in the usual way. Of the 6,834 patients in the Cytosponge group, nine were diagnosed with dysplastic Barrett’s esophagus or stage I esophagogastric cancer, versus none of the 6,388 participants in the usual-care group.
“At least for now, we’re using the same guidance that we used for endoscopy to decide who might be best served by these devices,” Dr. Shaheen said. “There are not good data to make a recommendation yet, but you could easily imagine how you could broaden screening guidelines by having a cheaper, more available test.”
Screening for esophageal cancer may one day be as common as screening for colon cancer, said Herbert C. Wolfsen, MD, a consultant in gastroenterology at the Mayo Clinic in Jacksonville, Florida, and professor of medicine at the Mayo Clinic College of Medicine, Rochester, Minn. He was not involved in writing the new guideline.
“We’ve known for years that at least half of patients with esophageal cancer have little, if any, reflux symptoms,” he told this news organization. “This is an area where the guidelines have been consistent, but I think we’re going to see them start to change.”
Additional recommendations
The expert panel of the new guideline held off on recommending the use of biomarkers more generally, deeming the evidence insufficient.
The new guideline also endorses endoscopic treatment for dysplastic Barrett’s esophagus but not for Barrett’s esophagus with no dysplasia.
For the first time, it recommends that patients undergo surveillance after successful ablation.
Dr. Wolfsen took note of the guideline’s recommendation that clinicians benchmark their practices against published standards.
The guideline mentions “documentation of landmarks and extent of [Barrett’s esophagus], not obtaining biopsies in the setting of a normal-appearing squamocolumnar junction, sampling using the Seattle biopsy protocol, and performing surveillance endoscopy in patients with [nondysplastic Barrett’s esophagus] no sooner than 3-5 years.”
“The acceptance and implementation of these quality metrics is important,” Dr. Wolfsen said.
However, the guideline underscores the need for considerably more research, he added.
“This group did a fabulous job,” Dr. Wolfsen said. “But when it comes to getting a consensus and people on board, all headed in the same direction, it’s hard when you’re not working with very good data.”
Dr. Shaheen reports financial relationships with Medtronic, Steris, Pentax, CDx Diagnostics, Interpace Diagnostics, Lucid Medical, Cernostics, Phathom Pharmaceuticals, Exact Sciences, Aqua Medical, and Cook Medical. Dr. Wolfsen reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A swallowable, capsule-sponge device used to sample biomarkers offers a reliable, noninvasive alternative to endoscopy for diagnosing Barrett’s esophagus, according to a new guideline from the American College of Gastroenterology.
In addition, the guideline recommends that patients with Barrett’s esophagus with segments of less than 3 cm be screened every 5 years, but if their Barrett’s esophagus segments are 5 cm or greater, they should be screened every 3 years.
“We don’t want to scope everyone for the lowest risk of cancer,” lead author Nicholas J. Shaheen, MD, MPH, chief of gastroenterology and hepatology at the University of North Carolina School of Medicine, Chapel Hill, told this news organization.
“The traditional way to diagnose Barrett’s esophagus is by upper endoscopy, but it’s expensive and not available everywhere,” he said. “One big change since the last iteration of this guideline is that there’s been a development of nonendoscopic screening modalities.”
The guideline was published in The American Journal of Gastroenterology.
Progress with swallowable devices
Solid evidence supports the Cytosponge, a capsule containing a compressed spherical polyurethane sponge attached to a string. The sponge expands to a sphere when the capsule dissolves after being swallowed, Dr. Shaheen said.
When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for Barrett’s esophagus, either the protein trefoil factor 3 expressed in intestinal metaplasia or methylated DNA markers associated with Barrett’s esophagus mucosa.
More than 90% of participants in trials have been able to swallow the device. Some mild gagging or throat discomfort has been reported.
In one trial, patients with chronic reflux were randomly assigned to either swallow the Cytosponge or be screened by endoscopy based on the practitioner’s usual care. Any diagnosis with the Cytosponge was confirmed with endoscopy. Barrett’s esophagus was found in 2% of patients who had undergone the Cytosponge procedure, versus less than 1% of those screened in the usual way. Of the 6,834 patients in the Cytosponge group, nine were diagnosed with dysplastic Barrett’s esophagus or stage I esophagogastric cancer, versus none of the 6,388 participants in the usual-care group.
“At least for now, we’re using the same guidance that we used for endoscopy to decide who might be best served by these devices,” Dr. Shaheen said. “There are not good data to make a recommendation yet, but you could easily imagine how you could broaden screening guidelines by having a cheaper, more available test.”
Screening for esophageal cancer may one day be as common as screening for colon cancer, said Herbert C. Wolfsen, MD, a consultant in gastroenterology at the Mayo Clinic in Jacksonville, Florida, and professor of medicine at the Mayo Clinic College of Medicine, Rochester, Minn. He was not involved in writing the new guideline.
“We’ve known for years that at least half of patients with esophageal cancer have little, if any, reflux symptoms,” he told this news organization. “This is an area where the guidelines have been consistent, but I think we’re going to see them start to change.”
Additional recommendations
The expert panel of the new guideline held off on recommending the use of biomarkers more generally, deeming the evidence insufficient.
The new guideline also endorses endoscopic treatment for dysplastic Barrett’s esophagus but not for Barrett’s esophagus with no dysplasia.
For the first time, it recommends that patients undergo surveillance after successful ablation.
Dr. Wolfsen took note of the guideline’s recommendation that clinicians benchmark their practices against published standards.
The guideline mentions “documentation of landmarks and extent of [Barrett’s esophagus], not obtaining biopsies in the setting of a normal-appearing squamocolumnar junction, sampling using the Seattle biopsy protocol, and performing surveillance endoscopy in patients with [nondysplastic Barrett’s esophagus] no sooner than 3-5 years.”
“The acceptance and implementation of these quality metrics is important,” Dr. Wolfsen said.
However, the guideline underscores the need for considerably more research, he added.
“This group did a fabulous job,” Dr. Wolfsen said. “But when it comes to getting a consensus and people on board, all headed in the same direction, it’s hard when you’re not working with very good data.”
Dr. Shaheen reports financial relationships with Medtronic, Steris, Pentax, CDx Diagnostics, Interpace Diagnostics, Lucid Medical, Cernostics, Phathom Pharmaceuticals, Exact Sciences, Aqua Medical, and Cook Medical. Dr. Wolfsen reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A swallowable, capsule-sponge device used to sample biomarkers offers a reliable, noninvasive alternative to endoscopy for diagnosing Barrett’s esophagus, according to a new guideline from the American College of Gastroenterology.
In addition, the guideline recommends that patients with Barrett’s esophagus with segments of less than 3 cm be screened every 5 years, but if their Barrett’s esophagus segments are 5 cm or greater, they should be screened every 3 years.
“We don’t want to scope everyone for the lowest risk of cancer,” lead author Nicholas J. Shaheen, MD, MPH, chief of gastroenterology and hepatology at the University of North Carolina School of Medicine, Chapel Hill, told this news organization.
“The traditional way to diagnose Barrett’s esophagus is by upper endoscopy, but it’s expensive and not available everywhere,” he said. “One big change since the last iteration of this guideline is that there’s been a development of nonendoscopic screening modalities.”
The guideline was published in The American Journal of Gastroenterology.
Progress with swallowable devices
Solid evidence supports the Cytosponge, a capsule containing a compressed spherical polyurethane sponge attached to a string. The sponge expands to a sphere when the capsule dissolves after being swallowed, Dr. Shaheen said.
When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for Barrett’s esophagus, either the protein trefoil factor 3 expressed in intestinal metaplasia or methylated DNA markers associated with Barrett’s esophagus mucosa.
More than 90% of participants in trials have been able to swallow the device. Some mild gagging or throat discomfort has been reported.
In one trial, patients with chronic reflux were randomly assigned to either swallow the Cytosponge or be screened by endoscopy based on the practitioner’s usual care. Any diagnosis with the Cytosponge was confirmed with endoscopy. Barrett’s esophagus was found in 2% of patients who had undergone the Cytosponge procedure, versus less than 1% of those screened in the usual way. Of the 6,834 patients in the Cytosponge group, nine were diagnosed with dysplastic Barrett’s esophagus or stage I esophagogastric cancer, versus none of the 6,388 participants in the usual-care group.
“At least for now, we’re using the same guidance that we used for endoscopy to decide who might be best served by these devices,” Dr. Shaheen said. “There are not good data to make a recommendation yet, but you could easily imagine how you could broaden screening guidelines by having a cheaper, more available test.”
Screening for esophageal cancer may one day be as common as screening for colon cancer, said Herbert C. Wolfsen, MD, a consultant in gastroenterology at the Mayo Clinic in Jacksonville, Florida, and professor of medicine at the Mayo Clinic College of Medicine, Rochester, Minn. He was not involved in writing the new guideline.
“We’ve known for years that at least half of patients with esophageal cancer have little, if any, reflux symptoms,” he told this news organization. “This is an area where the guidelines have been consistent, but I think we’re going to see them start to change.”
Additional recommendations
The expert panel of the new guideline held off on recommending the use of biomarkers more generally, deeming the evidence insufficient.
The new guideline also endorses endoscopic treatment for dysplastic Barrett’s esophagus but not for Barrett’s esophagus with no dysplasia.
For the first time, it recommends that patients undergo surveillance after successful ablation.
Dr. Wolfsen took note of the guideline’s recommendation that clinicians benchmark their practices against published standards.
The guideline mentions “documentation of landmarks and extent of [Barrett’s esophagus], not obtaining biopsies in the setting of a normal-appearing squamocolumnar junction, sampling using the Seattle biopsy protocol, and performing surveillance endoscopy in patients with [nondysplastic Barrett’s esophagus] no sooner than 3-5 years.”
“The acceptance and implementation of these quality metrics is important,” Dr. Wolfsen said.
However, the guideline underscores the need for considerably more research, he added.
“This group did a fabulous job,” Dr. Wolfsen said. “But when it comes to getting a consensus and people on board, all headed in the same direction, it’s hard when you’re not working with very good data.”
Dr. Shaheen reports financial relationships with Medtronic, Steris, Pentax, CDx Diagnostics, Interpace Diagnostics, Lucid Medical, Cernostics, Phathom Pharmaceuticals, Exact Sciences, Aqua Medical, and Cook Medical. Dr. Wolfsen reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erectile dysfunction drugs linked to ocular conditions
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
CDC recommends hep B vaccination for most adults
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
New contact lens elutes antihistamine for ocular allergy
“This is the world’s first and only contact lens that’s able to prevent itching associated with allergies, while at the same time providing vision correction,” said Brian Pall, DO, director of clinical science at Johnson & Johnson Vision Care, which is making the lens. “It’s certainly exciting.”
The new lens, Acuvue Theravision With Ketotifenis, is already on the market in Canada and Japan.
The lenses are daily disposable contacts indicated for the prevention of ocular itch caused by allergic conjunctivitis in people who do not have red eyes, are suitable for wearing contact lenses, and do not have more than 1.00 D of astigmatism.
Antihistamine eyedrops are contraindicated for use with contact lenses because eyedrop preservatives could interact with the lenses, and clinical trials generally exclude contact lens wearers.
Johnson & Johnson worked for over a decade to find an antihistamine that paired well with a contact lens material, finally hitting on the combination of ketotifen and etafilcon A, said Pall. The drug is integrated into the polymer during manufacturing.
In contact with the eye, the drug diffuses from the lens into the tear film and is absorbed by the ocular tissues, much like a conventional eyedrop. “The key difference is that this is a slower release,” Dr. Pall said. “Instead of a bolus of this large drop hitting the eye and then being flushed out immediately, we get a much more sustained release.”
Because the lens is kept sterilized until use, no preservatives are added to the medication. This is an advantage because preservatives cause irritation in some patients.
Ketotifen, a well-established treatment for ocular allergies, not only blocks histamine receptors but also stabilizes mast cells so that they don’t release cytokines, and it prevents inflammatory cells from rushing to the site of irritation, Dr. Pall said.
For a pair of identical clinical trials, published in 2019 in Cornea, Dr. Pall and his colleagues recruited 244 people with ocular allergies. For each trial, they divided these subjects into three groups. One group wore the ketotifen lenses in one eye and lenses without the drug in the other eye. The second group wore the ketotifen lenses in both eyes. The third wore the control lenses in both eyes.
The researchers then exposed the subjects to allergens and asked them to rate the itchiness of their eyes on a scale of 0-4 after 3 minutes, 5 minutes, and 7 minutes. Over these periods, the patients rated itchiness of the eyes with the ketotifen contact lenses a mean from 0.42-0.59; they rated the eyes with the control contacts 1.60-1.94. The differences were statistically significant (P < .001).
While about 5% of patients experienced adverse events, most of the events were not judged to be related to the contact lenses. The most common adverse event, reported by about 1% of patients, was installation site irritation. “The good news, what we’re hearing from the field, is it’s very subtle, it’s pretty mild, and it quickly dissipates,” said Dr. Pall.
The new contact lens “is promising for those who have contact lenses and the 20%-40% of the American population who have allergies,” said Leonard Bielory, MD, a professor of medicine, allergy, immunology, and ophthalmology at Hackensack Meridian School of Medicine in Nutley, N.J., who was not involved in the trial or in developing the lens.
“I have patients who wear contacts and have allergies, and they have to work around it,” he said in an interview. “I expected this 15 years ago, because this is a good idea.”
Johnson & Johnson is researching other drugs that might be delivered through contact lenses, Dr. Pall said.
The study was funded by Johnson and Johnson. Dr. Pall is an employee of Johnson & Johnson. Dr. Bielory reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This is the world’s first and only contact lens that’s able to prevent itching associated with allergies, while at the same time providing vision correction,” said Brian Pall, DO, director of clinical science at Johnson & Johnson Vision Care, which is making the lens. “It’s certainly exciting.”
The new lens, Acuvue Theravision With Ketotifenis, is already on the market in Canada and Japan.
The lenses are daily disposable contacts indicated for the prevention of ocular itch caused by allergic conjunctivitis in people who do not have red eyes, are suitable for wearing contact lenses, and do not have more than 1.00 D of astigmatism.
Antihistamine eyedrops are contraindicated for use with contact lenses because eyedrop preservatives could interact with the lenses, and clinical trials generally exclude contact lens wearers.
Johnson & Johnson worked for over a decade to find an antihistamine that paired well with a contact lens material, finally hitting on the combination of ketotifen and etafilcon A, said Pall. The drug is integrated into the polymer during manufacturing.
In contact with the eye, the drug diffuses from the lens into the tear film and is absorbed by the ocular tissues, much like a conventional eyedrop. “The key difference is that this is a slower release,” Dr. Pall said. “Instead of a bolus of this large drop hitting the eye and then being flushed out immediately, we get a much more sustained release.”
Because the lens is kept sterilized until use, no preservatives are added to the medication. This is an advantage because preservatives cause irritation in some patients.
Ketotifen, a well-established treatment for ocular allergies, not only blocks histamine receptors but also stabilizes mast cells so that they don’t release cytokines, and it prevents inflammatory cells from rushing to the site of irritation, Dr. Pall said.
For a pair of identical clinical trials, published in 2019 in Cornea, Dr. Pall and his colleagues recruited 244 people with ocular allergies. For each trial, they divided these subjects into three groups. One group wore the ketotifen lenses in one eye and lenses without the drug in the other eye. The second group wore the ketotifen lenses in both eyes. The third wore the control lenses in both eyes.
The researchers then exposed the subjects to allergens and asked them to rate the itchiness of their eyes on a scale of 0-4 after 3 minutes, 5 minutes, and 7 minutes. Over these periods, the patients rated itchiness of the eyes with the ketotifen contact lenses a mean from 0.42-0.59; they rated the eyes with the control contacts 1.60-1.94. The differences were statistically significant (P < .001).
While about 5% of patients experienced adverse events, most of the events were not judged to be related to the contact lenses. The most common adverse event, reported by about 1% of patients, was installation site irritation. “The good news, what we’re hearing from the field, is it’s very subtle, it’s pretty mild, and it quickly dissipates,” said Dr. Pall.
The new contact lens “is promising for those who have contact lenses and the 20%-40% of the American population who have allergies,” said Leonard Bielory, MD, a professor of medicine, allergy, immunology, and ophthalmology at Hackensack Meridian School of Medicine in Nutley, N.J., who was not involved in the trial or in developing the lens.
“I have patients who wear contacts and have allergies, and they have to work around it,” he said in an interview. “I expected this 15 years ago, because this is a good idea.”
Johnson & Johnson is researching other drugs that might be delivered through contact lenses, Dr. Pall said.
The study was funded by Johnson and Johnson. Dr. Pall is an employee of Johnson & Johnson. Dr. Bielory reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This is the world’s first and only contact lens that’s able to prevent itching associated with allergies, while at the same time providing vision correction,” said Brian Pall, DO, director of clinical science at Johnson & Johnson Vision Care, which is making the lens. “It’s certainly exciting.”
The new lens, Acuvue Theravision With Ketotifenis, is already on the market in Canada and Japan.
The lenses are daily disposable contacts indicated for the prevention of ocular itch caused by allergic conjunctivitis in people who do not have red eyes, are suitable for wearing contact lenses, and do not have more than 1.00 D of astigmatism.
Antihistamine eyedrops are contraindicated for use with contact lenses because eyedrop preservatives could interact with the lenses, and clinical trials generally exclude contact lens wearers.
Johnson & Johnson worked for over a decade to find an antihistamine that paired well with a contact lens material, finally hitting on the combination of ketotifen and etafilcon A, said Pall. The drug is integrated into the polymer during manufacturing.
In contact with the eye, the drug diffuses from the lens into the tear film and is absorbed by the ocular tissues, much like a conventional eyedrop. “The key difference is that this is a slower release,” Dr. Pall said. “Instead of a bolus of this large drop hitting the eye and then being flushed out immediately, we get a much more sustained release.”
Because the lens is kept sterilized until use, no preservatives are added to the medication. This is an advantage because preservatives cause irritation in some patients.
Ketotifen, a well-established treatment for ocular allergies, not only blocks histamine receptors but also stabilizes mast cells so that they don’t release cytokines, and it prevents inflammatory cells from rushing to the site of irritation, Dr. Pall said.
For a pair of identical clinical trials, published in 2019 in Cornea, Dr. Pall and his colleagues recruited 244 people with ocular allergies. For each trial, they divided these subjects into three groups. One group wore the ketotifen lenses in one eye and lenses without the drug in the other eye. The second group wore the ketotifen lenses in both eyes. The third wore the control lenses in both eyes.
The researchers then exposed the subjects to allergens and asked them to rate the itchiness of their eyes on a scale of 0-4 after 3 minutes, 5 minutes, and 7 minutes. Over these periods, the patients rated itchiness of the eyes with the ketotifen contact lenses a mean from 0.42-0.59; they rated the eyes with the control contacts 1.60-1.94. The differences were statistically significant (P < .001).
While about 5% of patients experienced adverse events, most of the events were not judged to be related to the contact lenses. The most common adverse event, reported by about 1% of patients, was installation site irritation. “The good news, what we’re hearing from the field, is it’s very subtle, it’s pretty mild, and it quickly dissipates,” said Dr. Pall.
The new contact lens “is promising for those who have contact lenses and the 20%-40% of the American population who have allergies,” said Leonard Bielory, MD, a professor of medicine, allergy, immunology, and ophthalmology at Hackensack Meridian School of Medicine in Nutley, N.J., who was not involved in the trial or in developing the lens.
“I have patients who wear contacts and have allergies, and they have to work around it,” he said in an interview. “I expected this 15 years ago, because this is a good idea.”
Johnson & Johnson is researching other drugs that might be delivered through contact lenses, Dr. Pall said.
The study was funded by Johnson and Johnson. Dr. Pall is an employee of Johnson & Johnson. Dr. Bielory reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.