User login
Cutaneous Leishmaniasis Successfully Treated With Miltefosine
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
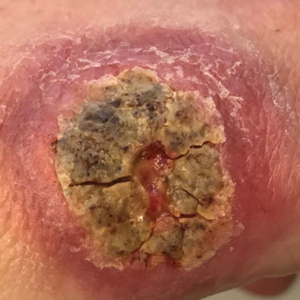
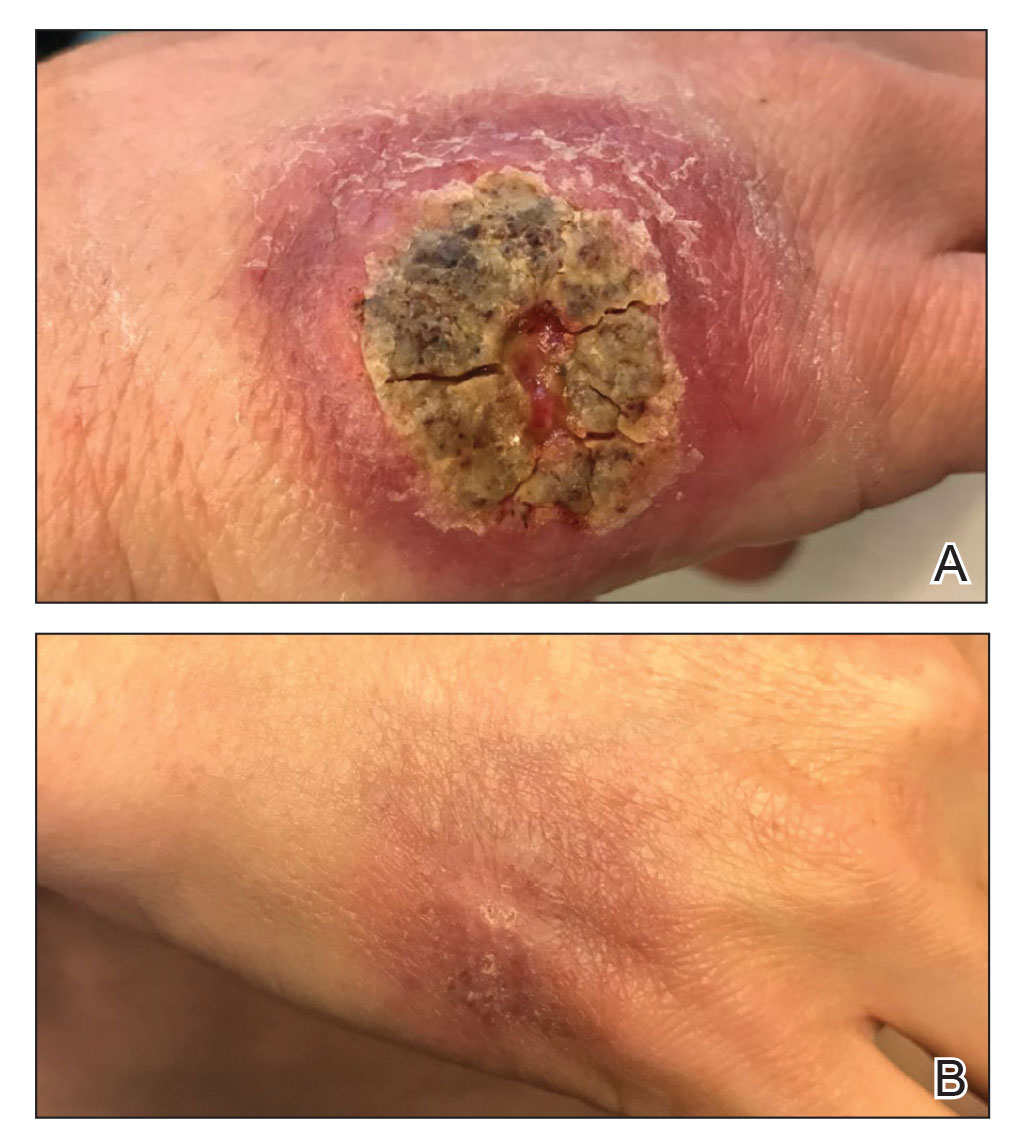
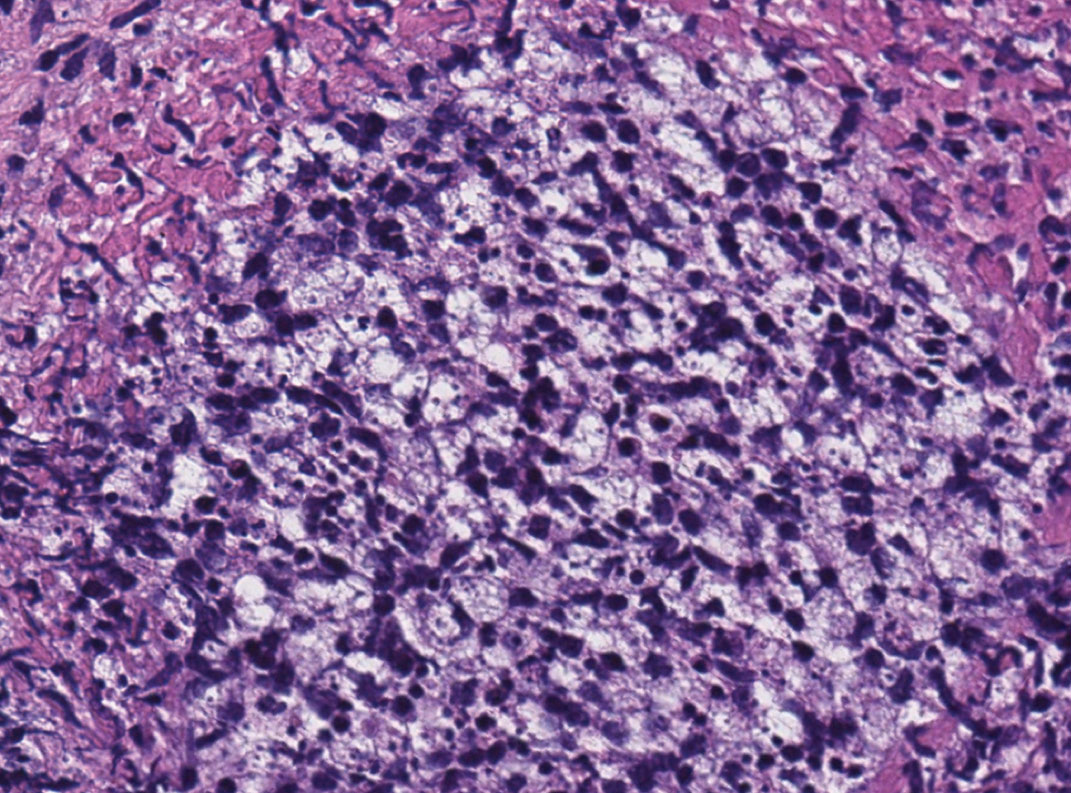
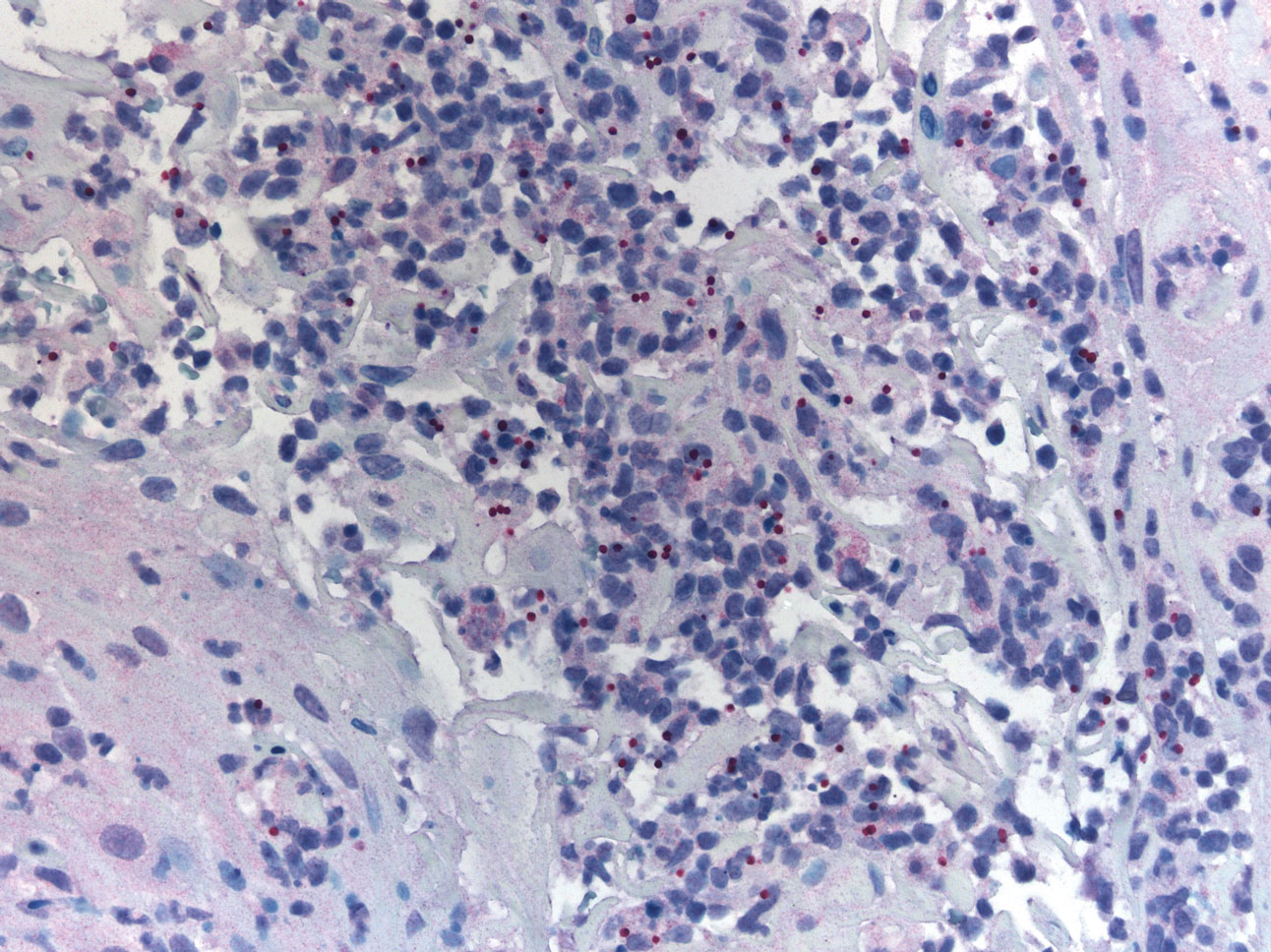
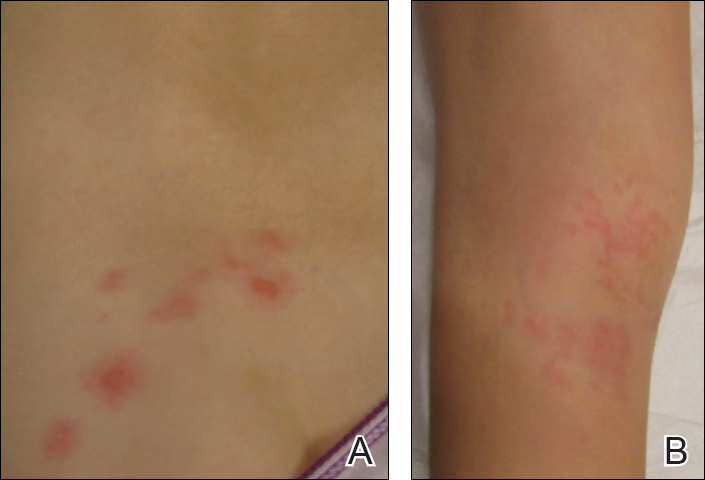
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.
Patient 2
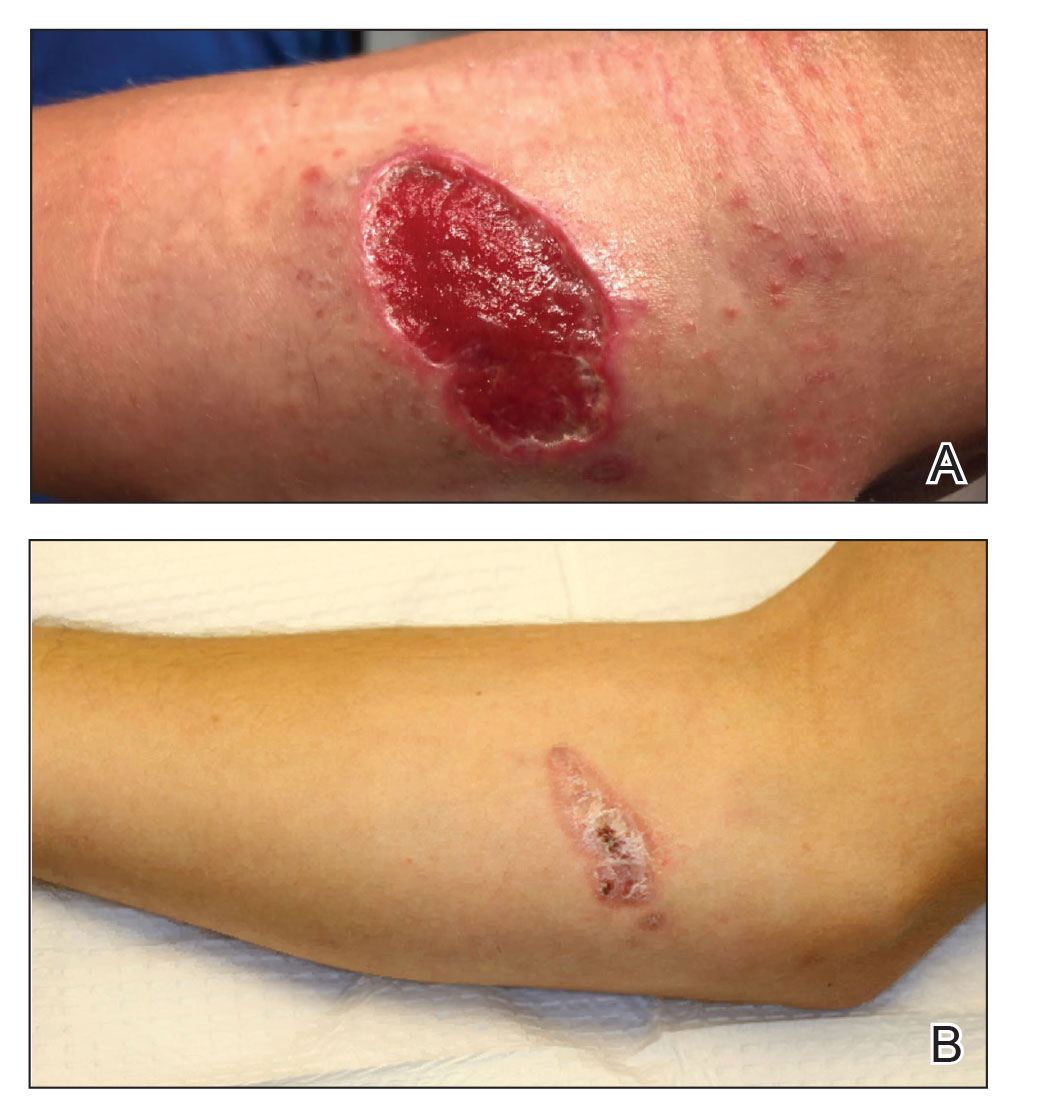
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.
Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Practice Points
- Avoiding phlebotomine sand fly vector bites is the most effective way to prevent leishmaniasis.
- Prompt diagnosis and treatment of cutaneous leishmaniasis caused by Leishmania species that have potential for mucocutaneous spread are key to limiting morbidity and mortality.
- Partnering with the Centers for Disease Control and Prevention is critical for timely diagnosis.
- Miltefosine should be considered as a first-line agent for cutaneous leishmaniasis given its efficacy, tolerability, and ease of administration.
Atypical Herpes Zoster Presentation in a Healthy Vaccinated Pediatric Patient
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
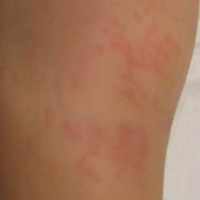
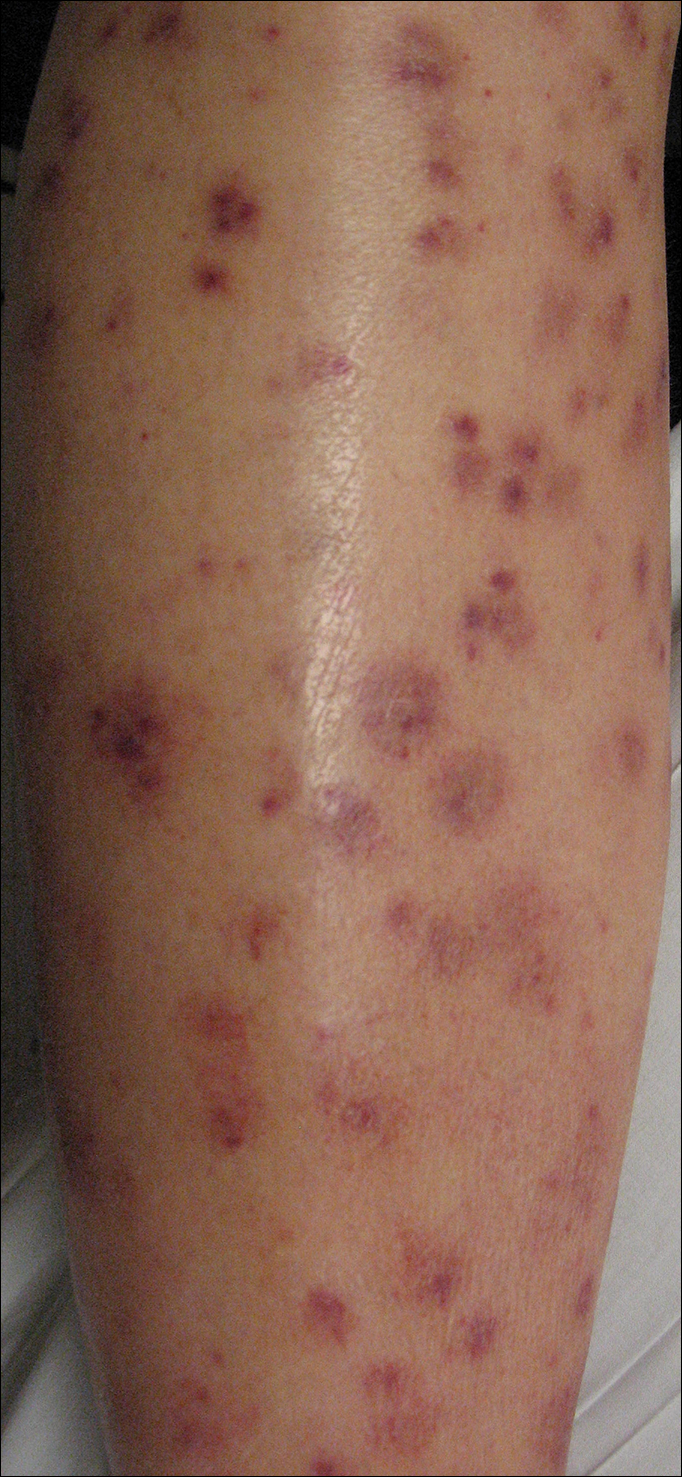
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.
The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Practice Points
- Both wild-type and vaccine-strain varicella-zoster virus (VZV) can establish latency in dorsal root ganglia and can cause herpes zoster (HZ) in vaccinated children.
- When HZ due to a vaccine strain of VZV occurs, the rash often presents near the site of initial vaccination.
- Although most cases of HZ in vaccinated children present with a characteristic HZ rash, physicians should be aware of the possibility for atypical presentations.
Acute Inflammatory Skin Reaction During Neutrophil Recovery After Antileukemic Therapy
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
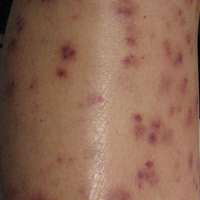
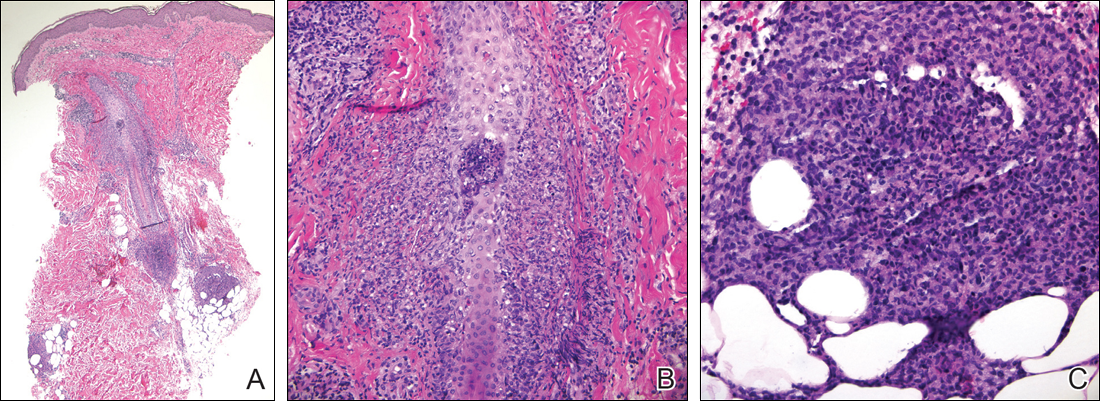
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.
In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.


In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.


In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
Practice Point
- Sterile mixed inflammatory skin reactions reminiscent of neutrophilic dermatoses may occur during neutrophil recovery in patients undergoing therapy for leukemias and need to be considered as part of the differential diagnosis.
Intralymphatic Histiocytosis Associated With an Orthopedic Metal Implant
To the Editor:
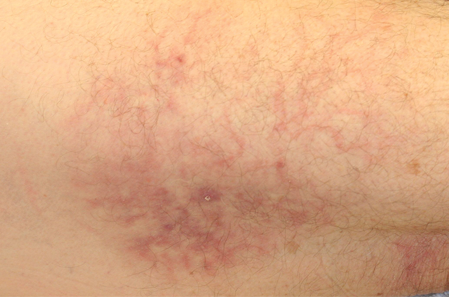
|
| Figure 1. A 30-cm pink and violaceous, asymmetric, reticulated patch on the lateral aspect of the right thigh. |
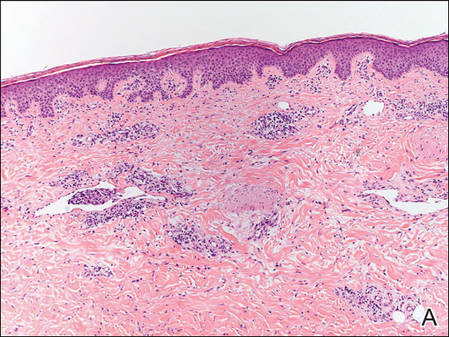
|
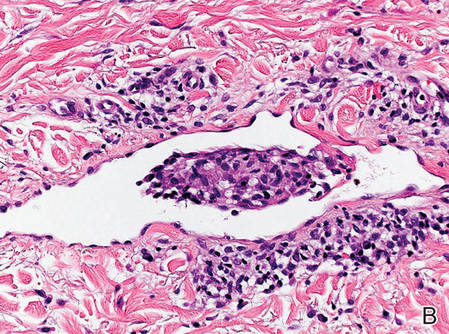
|
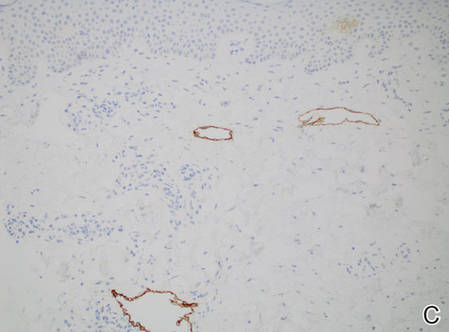
|
Figure 2. Histopathology revealed widely dilated vascular channels containing collections of histiocytes in the superficial dermis with adjacent features of chronic lymphedema (A)(H&E, original magnification ×10) as well as a collection of histiocytes in a dilated lymphatic channel (B)(H&E, original magnification ×40). D2-40 staining demonstrated ectatic lymphatic vessels in the upper dermins (C)(original magnification ×20).
|
A 70-year-old white man presented with an asymptomatic patch on the lateral aspect of the right thigh of 15 months’ duration. The patient believed the patch correlated with a hip replacement 3 years prior; however, it was 6 inches inferior to the incision site. Physical examination revealed a 30-cm pink and violaceous, asymmetric, reticulated patch (Figure 1). The patch was unresponsive to topical corticosteroids as well as a short course of oral prednisone. The patient’s medical history was notable for type 2 diabetes mellitus. Histopathologic examination revealed widely dilated vascular channels containing collections of histiocytes in the superficial dermis. In addition, adjacent features of chronic lymphedema were present, namely interstitial fibroplasia with dilated lymphatic vessels and a lymphoplasmacytic infiltrate (Figure 2). These findings were consistent with intralymphatic histiocytosis, a rare disease most commonly associated with rheumatoid arthritis. Our patient did not have a history or clinical symptoms of rheumatoid arthritis.
Intralymphatic histiocytosis is a rare cutaneous condition reported by O’Grady et al1 in 1994. This condition has been most frequently associated with rheumatoid arthritis2; however, there has been an emerging association in patients with orthopedic metal implants, with and without a concomitant diagnosis of rheumatoid arthritis. Cases associated with metal implants are rare.2-7
The condition presents as asymptomatic red, brown, or violaceous patches, plaques, papules, or nodules that are ill defined and tend to demonstrate a livedo reticularis–like pattern. The lesions typically are overlying or in close proximity to a joint. Histopathologic findings include dilated vascular structures in the reticular dermis, some with empty lumina and others containing collections of mononuclear histiocytes. There also may be an inflammatory infiltrate in the adjacent dermis composed of a mix of lymphocytes, plasma cells, and/or histiocytes. Endothelial cells lining the dilated lumina express immunoreactivity for CD31, CD34, D2-40, Lyve-1, and Prox-1. Intravascular histiocytes are positive for CD68 and CD31.6
The pathogenesis of intralymphatic histiocytosis remains undefined. Some hypothesize that intralymphatic histiocytosis could be the early stage of reactive angioendotheliomatosis, as these conditions share clinical and histological features.8 Reactive angioendotheliomatosis also is a rare condition that may present as erythematous to violaceous patches or plaques. The lesions are commonly found on the limbs and may be associated with constitutional symptoms. Histologic findings of reactive angioendotheliomatosis include a proliferation of epithelioid, round, or spindle-shaped cells within the lumina of dermal blood vessels, which show positivity for CD31 and CD34.9 Others suggest the lesions of intralymphatic histiocytosis arise from lymphangiectasia; obstruction of lymphatic drainage due to congenital abnormalities; or acquired damage from infection, trauma, surgery, or radiation.2 Due to the common association with rheumatoid arthritis and orthopedic implants, it is likely that lymphatic stasis secondary to chronic inflammation plays a notable role.
Therapies such as topical and systemic corticosteroids, local radiotherapy, cyclophosphamide, pentoxifylline, and arthrocentesis have been attempted without evidence of efficacy.2 Although intralymphatic histiocytosis is chronic and resistant to therapy, patients can be reassured that the condition runs a benign course.
1. O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
2. Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
3. Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
4. Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
5. Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants. J Cutan Pathol. 2011;38:534-535.
6. Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
7. Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants. Br J Dermatol. 2008;158:402-404.
8. Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
9. Rongioletti F, Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
To the Editor:

|
| Figure 1. A 30-cm pink and violaceous, asymmetric, reticulated patch on the lateral aspect of the right thigh. |

|

|

|
Figure 2. Histopathology revealed widely dilated vascular channels containing collections of histiocytes in the superficial dermis with adjacent features of chronic lymphedema (A)(H&E, original magnification ×10) as well as a collection of histiocytes in a dilated lymphatic channel (B)(H&E, original magnification ×40). D2-40 staining demonstrated ectatic lymphatic vessels in the upper dermins (C)(original magnification ×20).
|
A 70-year-old white man presented with an asymptomatic patch on the lateral aspect of the right thigh of 15 months’ duration. The patient believed the patch correlated with a hip replacement 3 years prior; however, it was 6 inches inferior to the incision site. Physical examination revealed a 30-cm pink and violaceous, asymmetric, reticulated patch (Figure 1). The patch was unresponsive to topical corticosteroids as well as a short course of oral prednisone. The patient’s medical history was notable for type 2 diabetes mellitus. Histopathologic examination revealed widely dilated vascular channels containing collections of histiocytes in the superficial dermis. In addition, adjacent features of chronic lymphedema were present, namely interstitial fibroplasia with dilated lymphatic vessels and a lymphoplasmacytic infiltrate (Figure 2). These findings were consistent with intralymphatic histiocytosis, a rare disease most commonly associated with rheumatoid arthritis. Our patient did not have a history or clinical symptoms of rheumatoid arthritis.
Intralymphatic histiocytosis is a rare cutaneous condition reported by O’Grady et al1 in 1994. This condition has been most frequently associated with rheumatoid arthritis2; however, there has been an emerging association in patients with orthopedic metal implants, with and without a concomitant diagnosis of rheumatoid arthritis. Cases associated with metal implants are rare.2-7
The condition presents as asymptomatic red, brown, or violaceous patches, plaques, papules, or nodules that are ill defined and tend to demonstrate a livedo reticularis–like pattern. The lesions typically are overlying or in close proximity to a joint. Histopathologic findings include dilated vascular structures in the reticular dermis, some with empty lumina and others containing collections of mononuclear histiocytes. There also may be an inflammatory infiltrate in the adjacent dermis composed of a mix of lymphocytes, plasma cells, and/or histiocytes. Endothelial cells lining the dilated lumina express immunoreactivity for CD31, CD34, D2-40, Lyve-1, and Prox-1. Intravascular histiocytes are positive for CD68 and CD31.6
The pathogenesis of intralymphatic histiocytosis remains undefined. Some hypothesize that intralymphatic histiocytosis could be the early stage of reactive angioendotheliomatosis, as these conditions share clinical and histological features.8 Reactive angioendotheliomatosis also is a rare condition that may present as erythematous to violaceous patches or plaques. The lesions are commonly found on the limbs and may be associated with constitutional symptoms. Histologic findings of reactive angioendotheliomatosis include a proliferation of epithelioid, round, or spindle-shaped cells within the lumina of dermal blood vessels, which show positivity for CD31 and CD34.9 Others suggest the lesions of intralymphatic histiocytosis arise from lymphangiectasia; obstruction of lymphatic drainage due to congenital abnormalities; or acquired damage from infection, trauma, surgery, or radiation.2 Due to the common association with rheumatoid arthritis and orthopedic implants, it is likely that lymphatic stasis secondary to chronic inflammation plays a notable role.
Therapies such as topical and systemic corticosteroids, local radiotherapy, cyclophosphamide, pentoxifylline, and arthrocentesis have been attempted without evidence of efficacy.2 Although intralymphatic histiocytosis is chronic and resistant to therapy, patients can be reassured that the condition runs a benign course.
To the Editor:

|
| Figure 1. A 30-cm pink and violaceous, asymmetric, reticulated patch on the lateral aspect of the right thigh. |

|

|

|
Figure 2. Histopathology revealed widely dilated vascular channels containing collections of histiocytes in the superficial dermis with adjacent features of chronic lymphedema (A)(H&E, original magnification ×10) as well as a collection of histiocytes in a dilated lymphatic channel (B)(H&E, original magnification ×40). D2-40 staining demonstrated ectatic lymphatic vessels in the upper dermins (C)(original magnification ×20).
|
A 70-year-old white man presented with an asymptomatic patch on the lateral aspect of the right thigh of 15 months’ duration. The patient believed the patch correlated with a hip replacement 3 years prior; however, it was 6 inches inferior to the incision site. Physical examination revealed a 30-cm pink and violaceous, asymmetric, reticulated patch (Figure 1). The patch was unresponsive to topical corticosteroids as well as a short course of oral prednisone. The patient’s medical history was notable for type 2 diabetes mellitus. Histopathologic examination revealed widely dilated vascular channels containing collections of histiocytes in the superficial dermis. In addition, adjacent features of chronic lymphedema were present, namely interstitial fibroplasia with dilated lymphatic vessels and a lymphoplasmacytic infiltrate (Figure 2). These findings were consistent with intralymphatic histiocytosis, a rare disease most commonly associated with rheumatoid arthritis. Our patient did not have a history or clinical symptoms of rheumatoid arthritis.
Intralymphatic histiocytosis is a rare cutaneous condition reported by O’Grady et al1 in 1994. This condition has been most frequently associated with rheumatoid arthritis2; however, there has been an emerging association in patients with orthopedic metal implants, with and without a concomitant diagnosis of rheumatoid arthritis. Cases associated with metal implants are rare.2-7
The condition presents as asymptomatic red, brown, or violaceous patches, plaques, papules, or nodules that are ill defined and tend to demonstrate a livedo reticularis–like pattern. The lesions typically are overlying or in close proximity to a joint. Histopathologic findings include dilated vascular structures in the reticular dermis, some with empty lumina and others containing collections of mononuclear histiocytes. There also may be an inflammatory infiltrate in the adjacent dermis composed of a mix of lymphocytes, plasma cells, and/or histiocytes. Endothelial cells lining the dilated lumina express immunoreactivity for CD31, CD34, D2-40, Lyve-1, and Prox-1. Intravascular histiocytes are positive for CD68 and CD31.6
The pathogenesis of intralymphatic histiocytosis remains undefined. Some hypothesize that intralymphatic histiocytosis could be the early stage of reactive angioendotheliomatosis, as these conditions share clinical and histological features.8 Reactive angioendotheliomatosis also is a rare condition that may present as erythematous to violaceous patches or plaques. The lesions are commonly found on the limbs and may be associated with constitutional symptoms. Histologic findings of reactive angioendotheliomatosis include a proliferation of epithelioid, round, or spindle-shaped cells within the lumina of dermal blood vessels, which show positivity for CD31 and CD34.9 Others suggest the lesions of intralymphatic histiocytosis arise from lymphangiectasia; obstruction of lymphatic drainage due to congenital abnormalities; or acquired damage from infection, trauma, surgery, or radiation.2 Due to the common association with rheumatoid arthritis and orthopedic implants, it is likely that lymphatic stasis secondary to chronic inflammation plays a notable role.
Therapies such as topical and systemic corticosteroids, local radiotherapy, cyclophosphamide, pentoxifylline, and arthrocentesis have been attempted without evidence of efficacy.2 Although intralymphatic histiocytosis is chronic and resistant to therapy, patients can be reassured that the condition runs a benign course.
1. O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
2. Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
3. Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
4. Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
5. Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants. J Cutan Pathol. 2011;38:534-535.
6. Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
7. Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants. Br J Dermatol. 2008;158:402-404.
8. Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
9. Rongioletti F, Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
1. O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
2. Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
3. Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
4. Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
5. Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants. J Cutan Pathol. 2011;38:534-535.
6. Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
7. Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants. Br J Dermatol. 2008;158:402-404.
8. Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
9. Rongioletti F, Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
Practice Points
- Consider intralymphatic histiocytosis in the differential diagnosis of an asymptomatic skin lesion overlying a joint, particularly in patients with orthopedic metal implants or rheumatoid arthritis.
- Biopsy is essential for the diagnosis of intralymphatic histiocytosis; special stains highlighting dilated lymphatic vessels and intravascular histiocytes may be necessary.
- Intralymphatic histiocytosis is chronic and resistant to therapy; however, patients can be reassured that the condition runs a benign course.