User login
Methacrylate Polymer Powder Dressing for a Lower Leg Surgical Defect
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
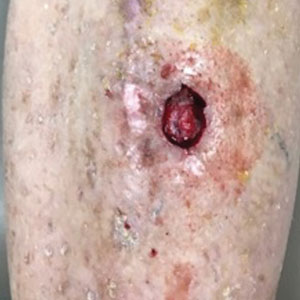
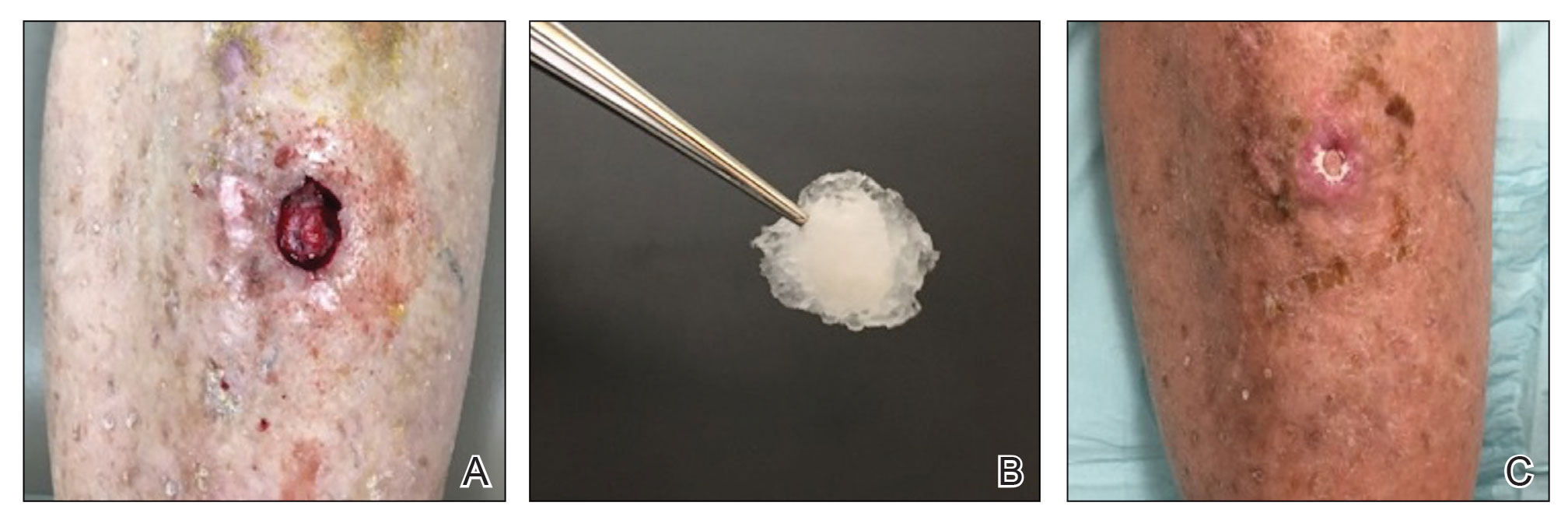
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.
Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1
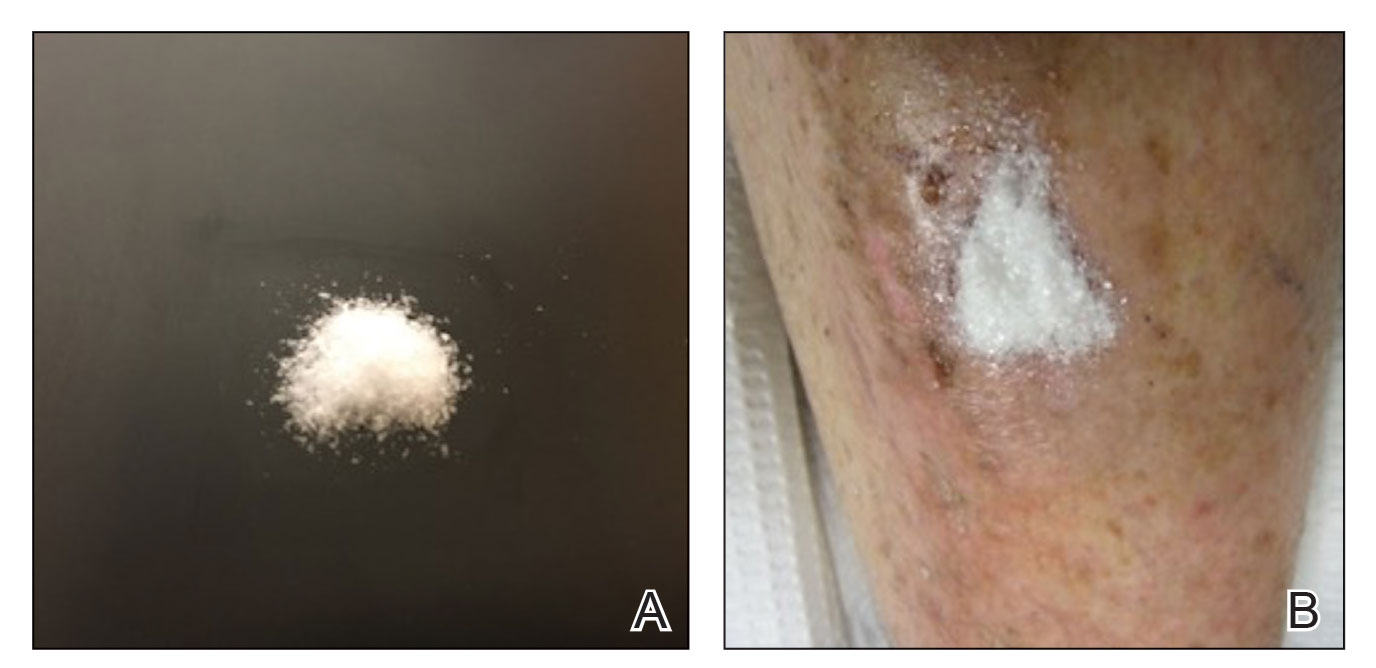
Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1
This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
PRACTICE POINTS
- Lower leg surgical wounds are difficult to manage, as venous stasis, bacterial colonization, and high tension may contribute to protracted healing.
- A methacrylate polymer powder dressing is user friendly and facilitates granulation and reduction in size of difficult lower leg wounds.
Intralesional Human Papillomavirus Vaccine Therapy for Recalcitrant Plantar Wart Triggers Gout Flare
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
Practice Points
- Human papillomavirus (HPV) vaccines are increasingly used for recalcitrant warts.
- We describe an unreported adverse effect of gout flare following HPV vaccine treatment of plantar wart.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
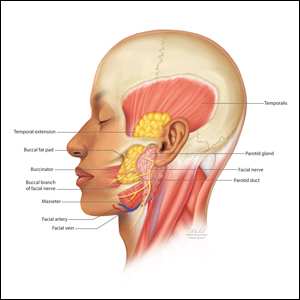
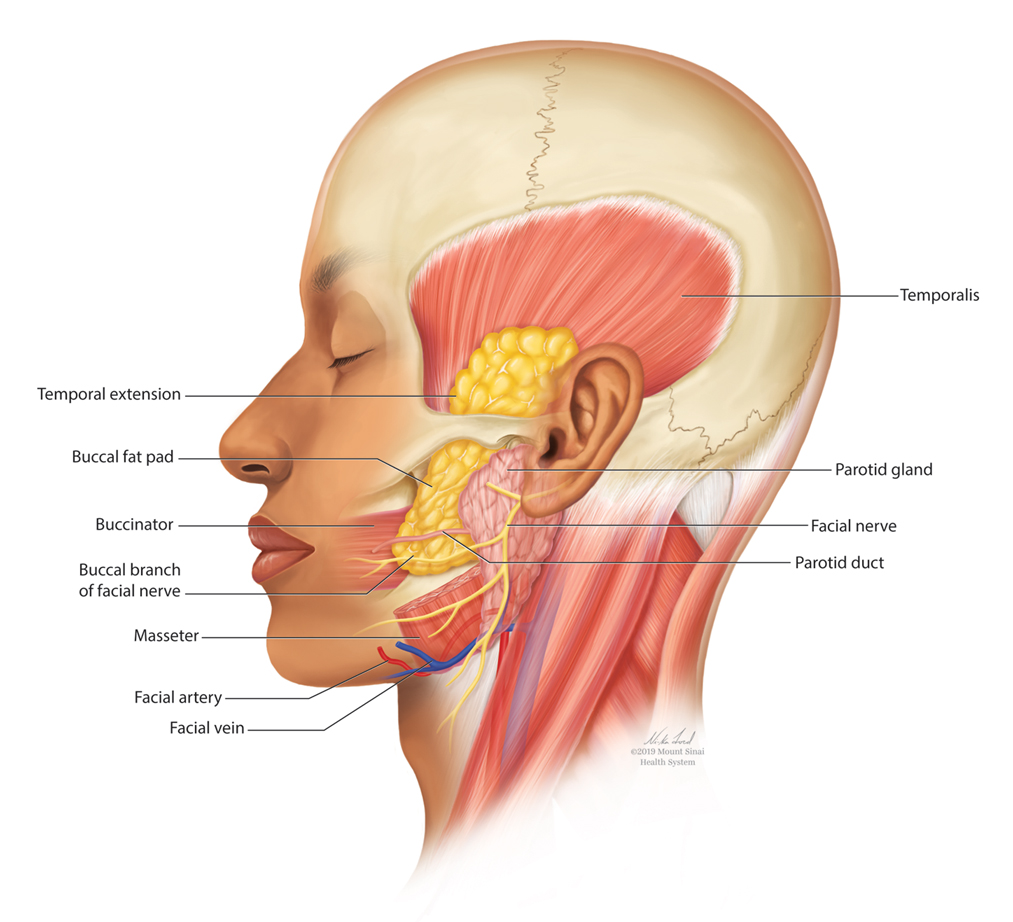
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.