User login
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
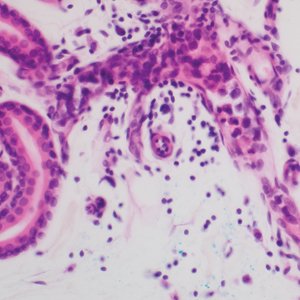
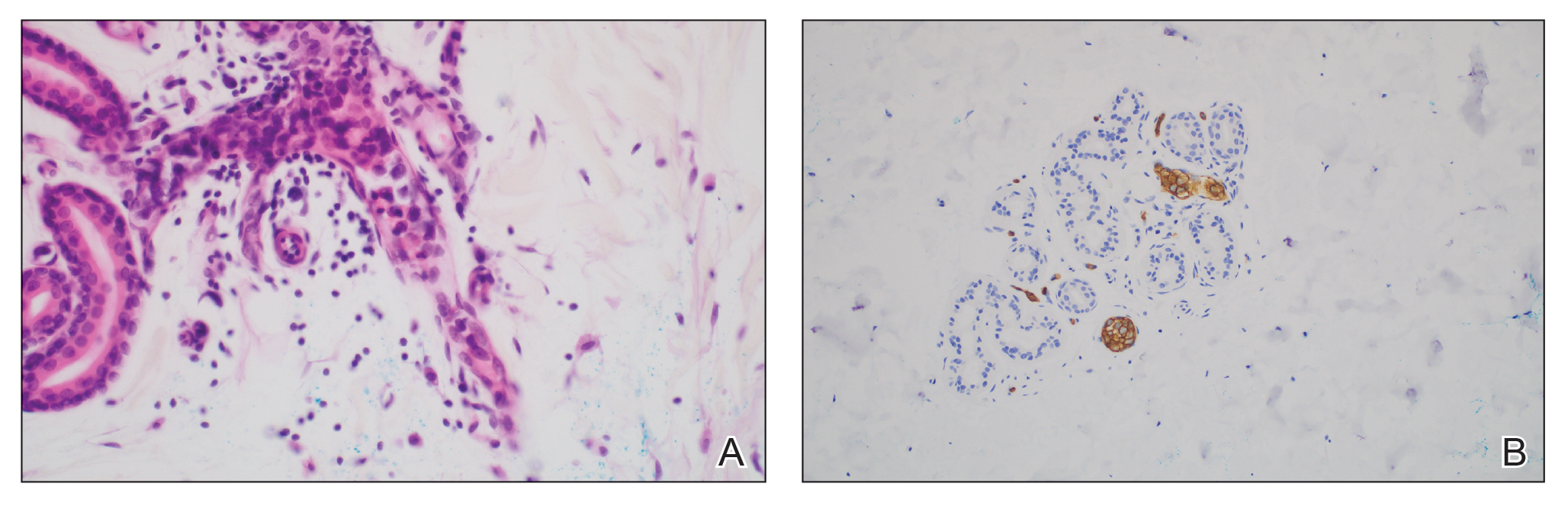
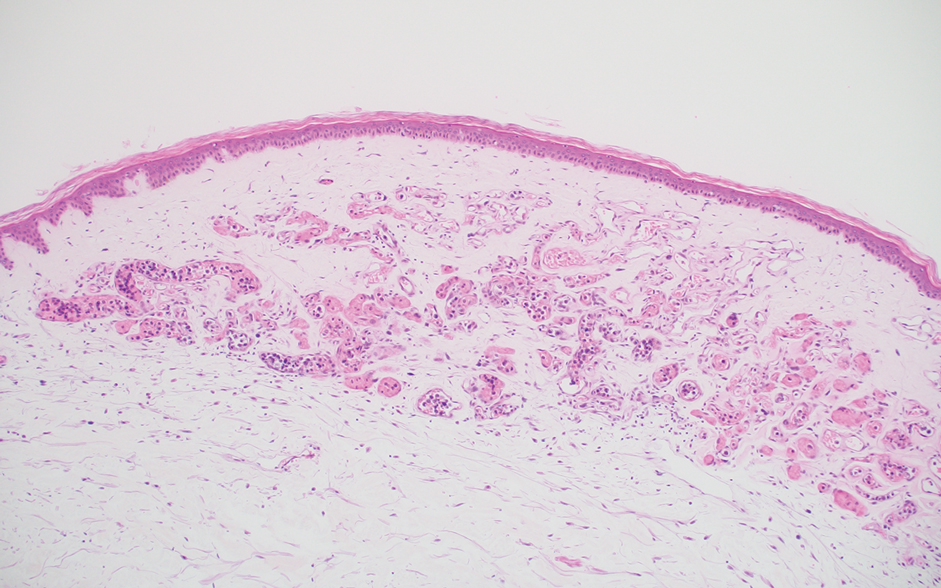
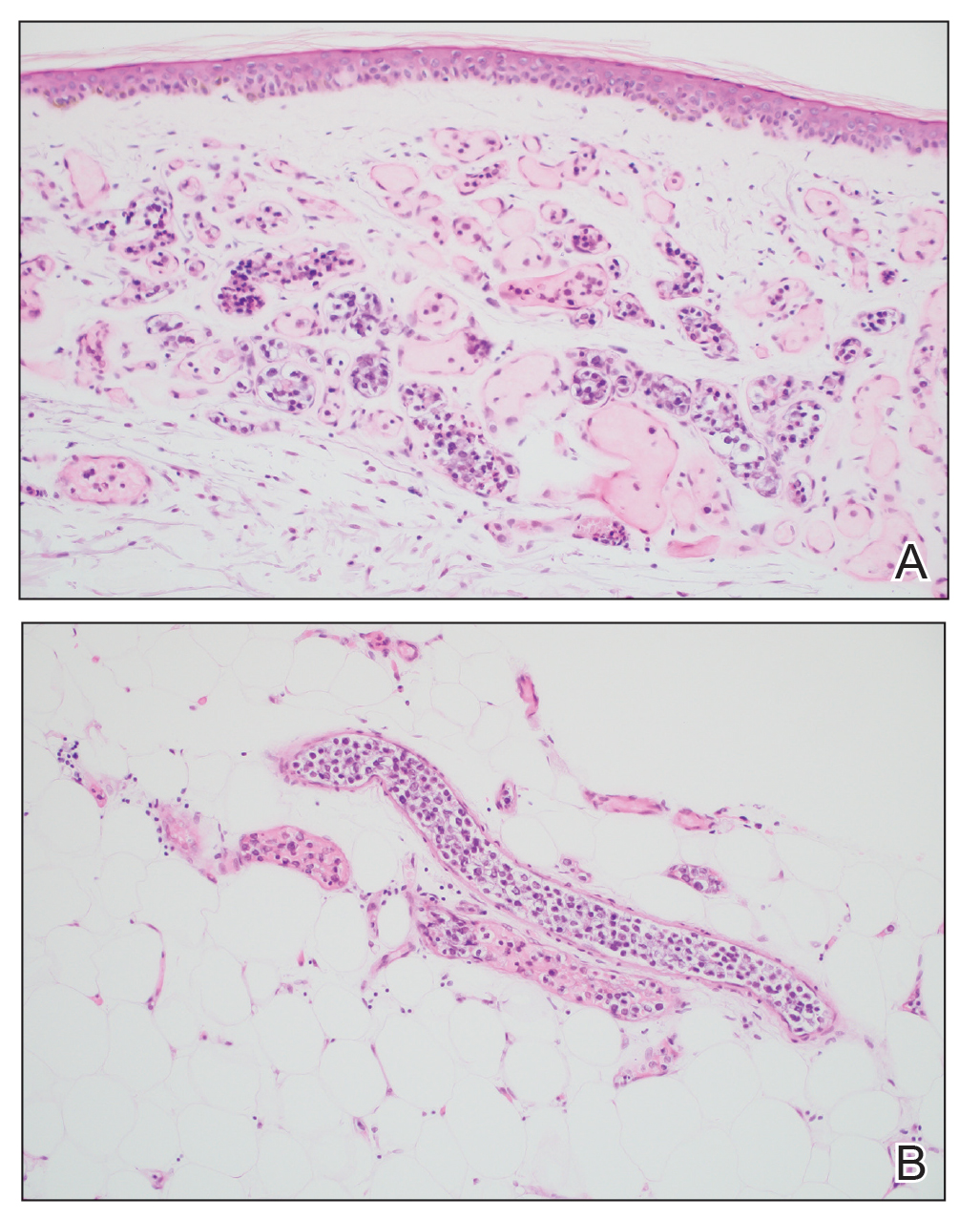
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.
Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
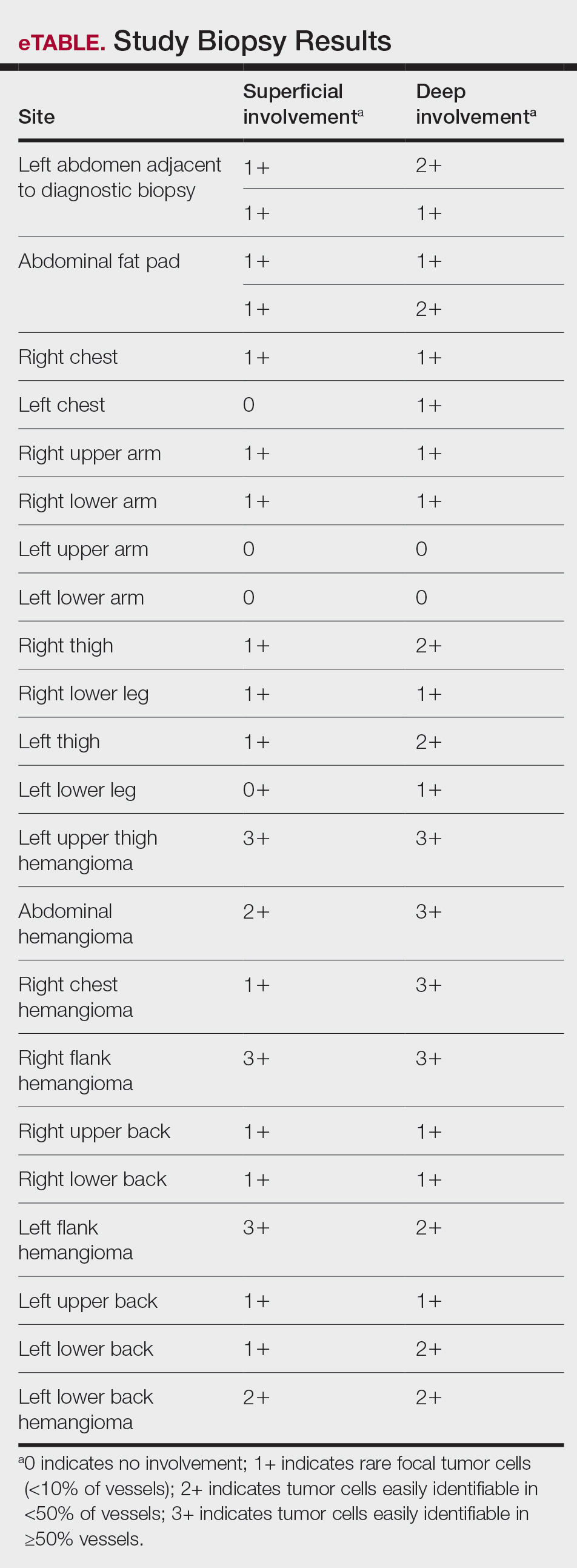
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).
Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).
Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Practice Points
- Skin biopsy is an effective method for identifying intravascular large B-cell lymphoma (IVBCL).
- Deep punch biopsies of sites involving hemangiomas may further heighten sensitivity for detection of IVBCL, as these lesions may harbor increased numbers of intravascular lymphoma cells.
- Deep and strategically placed skin biopsies offer potential improvements in timely diagnosis and outcomes of patients with IVBCL.
Violaceous Papule With an Erythematous Rim
The Diagnosis: Targetoid Hemosiderotic Hemangioma
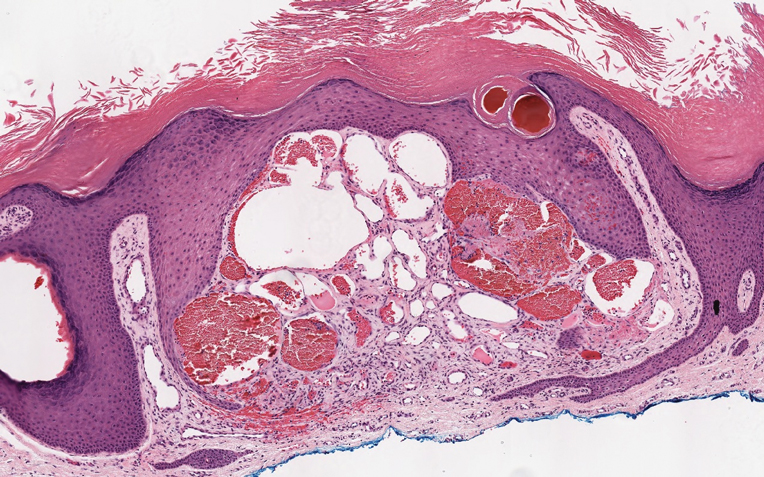
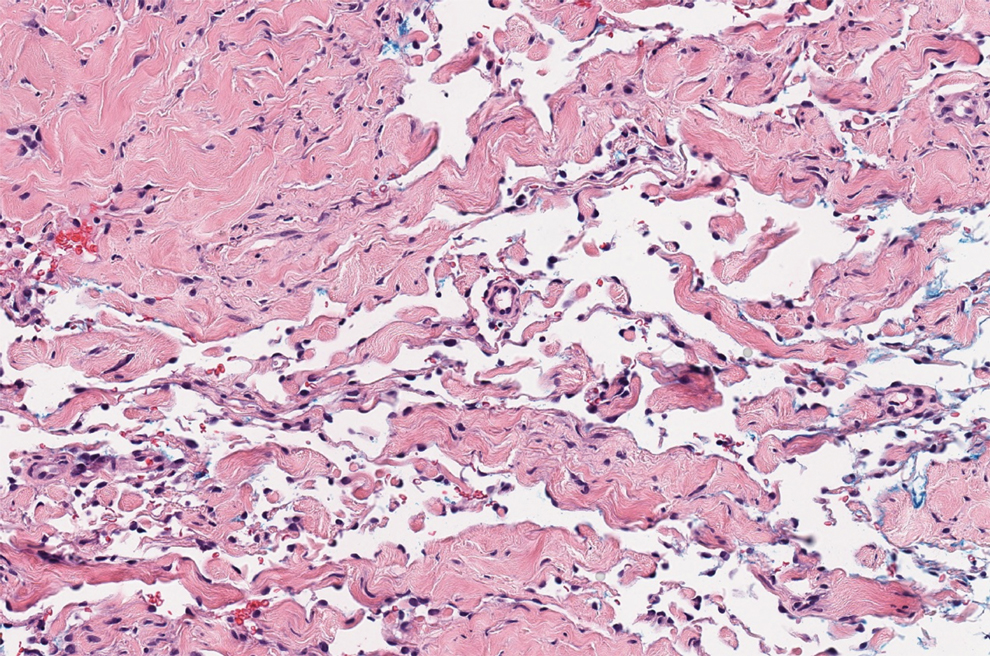
Targetoid hemosiderotic hemangioma (THH), also known as hobnail hemangioma, is a benign vascular tumor that usually occurs in young or middle-aged adults. It most commonly presents on the extremities or trunk as an isolated red-brown plaque or papule.1,2 Histologically, THH is characterized by superficial dilated ectatic vessels with underlying proliferating vascular channels lined by plump hobnail endothelial cells.1 Targetoid hemosiderotic hemangioma typically involves the dermis and spares the subcutis. The vascular channels may contain erythrocytes as well as pale eosinophilic lymph, as seen in our patient (quiz image). The deeper dermis contains vascular spaces that are more angulated and smaller and appear to be dissecting through the collagen bundles or collapsed.1,3 A variable amount of hemosiderin deposition and extravasated erythrocytes are seen.2,3 Histologic features evolve with the age of the lesion. Increasing amounts of hemosiderin deposition and erythrocyte extravasation may correspond histologically to the recent clinical color change reported by the patient.
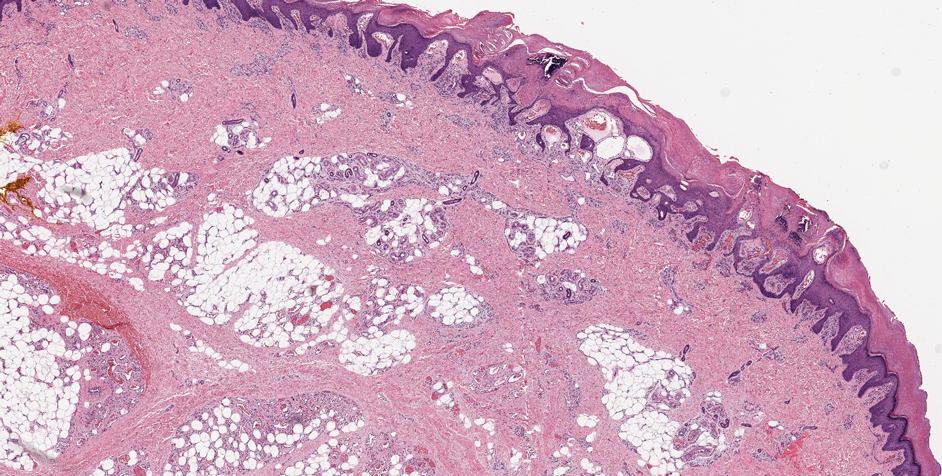
Verrucous hemangioma is a rare congenital vascular abnormality that is characterized by dilated vessels in the papillary dermis along with acanthosis, hyperkeratosis, and irregular papillomatosis, as seen in angiokeratoma.4 However, the vascular proliferation composed of variably sized, thin-walled capillaries extends into the deep dermis as well as the subcutis (Figure 1). Verrucous hemangioma most commonly is reported on the legs and generally starts as a violaceous patch that progresses into a hyperkeratotic verrucous plaque or nodule.5,6
Angiokeratoma is characterized by superficial vascular ectasia of the papillary dermis in association with overlying acanthosis, hyperkeratosis, and rete elongation.7 The dilated vascular spaces appear encircled by the epidermis (Figure 2). Intravascular thrombosis can be seen within the ectatic vessels.7 In contrast to verrucous hemangioma, angiokeratoma is limited to the papillary dermis. Therefore, obtaining a biopsy of sufficient depth is necessary for differentiation.8 There are 5 clinical presentations of angiokeratoma: sporadic, angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry disease). Angiokeratomas may present on the lower extremities, tongue, trunk, and scrotum as hyperkeratotic, dark red to purple or black papules.7
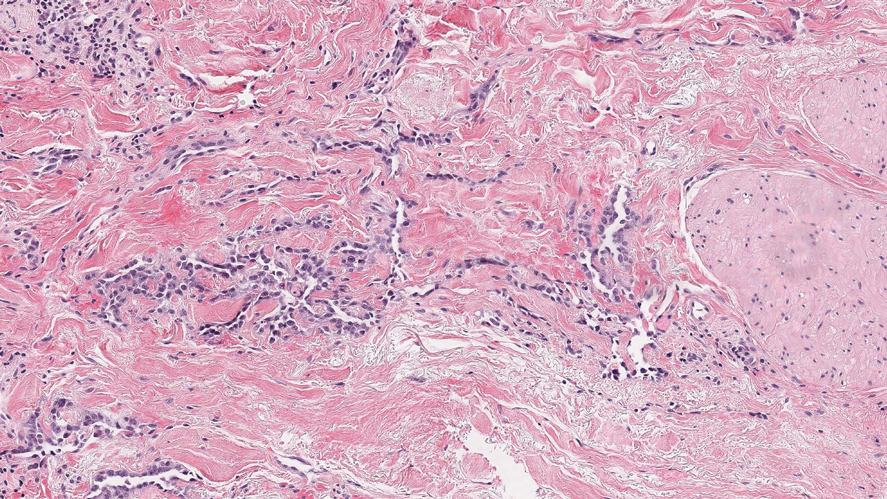
There are 3 clinical stages of Kaposi sarcoma: patch, plaque, and nodular stages. The patch stage is characterized histologically by vascular channels that dissect through the dermis and extend around native vessels (the promontory sign)(Figure 3).9,10 These features can show histologic overlap with THH. The plaque stage shows a more diffuse dermal vascular proliferation, increased cellularity of spindle cells, and possible extension into the subcutis.9,10 Focal plasma cells, hemosiderin, and extravasated red blood cells can be seen. The nodular stage is characterized by a proliferation of spindle cells with red blood cells squeezed between slitlike vascular spaces, hyaline globules, and scattered mitotic figures, but not atypical forms.10 In this stage, plasma cells and hemosiderin are more readily identifiable. A biopsy from the nodular stage is unlikely to enter the histologic differential diagnosis with THH. Clinically, there are 4 variants of Kaposi sarcoma: the classic or sporadic form, an endemic form, iatrogenic, and AIDS associated. Overall, it is more common in males and can occur at any age.10 Human herpesvirus 8 is seen in all forms, and infected cells can be highlighted by the immunohistochemical stain for latent nuclear antigen 1.9,10
Angiosarcoma is a malignant endothelial tumor of soft tissue, skin, bone, and visceral organs.11,12 Clinically, cutaneous angiosarcoma can present in a variety of ways, including single or multiple bluish red lesions that can ulcerate or bleed; violaceous nodules or plaques; and hematomalike lesions that can mimic epithelial neoplasms including squamous cell carcinoma, basal cell carcinoma, and malignant melanoma.11,13,14 The cutaneous lesions most commonly occur on sun-exposed skin, particularly on the face and scalp.12 Other clinical variants that are important to recognize are postradiation angiosarcoma, characterized by MYC gene amplification, and lymphedema-associated angiosarcoma (Stewart-Treves syndrome). Angiosarcoma can have a variety of morphologic features, ranging from well to poorly differentiated. Classically, angiosarcoma is characterized by infiltrating vascular spaces lined by atypical endothelial cells (Figure 4). Poorly differentiated angiosarcoma can demonstrate spindle, epithelioid, or polygonal cells with increased mitotic activity, pleomorphism, and irregular vascular spaces.11 Endothelial markers such as ERG (erythroblast transformation specific-related gene)(nuclear) and CD31 (membranous) can be used to aid in the diagnosis of a poorly differentiated lesion. Epithelioid angiosarcoma also occasionally stains with cytokeratins.13,14
- Joyce JC, Keith PJ, Szabo S, et al. Superficial hemosiderotic lymphovascular malformation (hobnail hemangioma): a report of six cases. Pediatr Dermatol. 2014;31:281-285.
- Sahin MT, Demir MA, Gunduz K, et al. Targetoid haemosiderotic haemangioma: dermoscopic monitoring of three cases and review of the literature. Clin Exp Dermatol. 2005;30:672-676.
- Kakizaki P, Valente NY, Paiva DL, et al. Targetoid hemosiderotic hemangioma--case report. An Bras Dermatol. 2014;89:956-959.
- Oppermann K, Boff AL, Bonamigo RR. Verrucous hemangioma and histopathological differential diagnosis with angiokeratoma circumscriptum neviforme. An Bras Dermatol. 2018;93:712-715.
- Boccara, O, Ariche-Maman, S, Hadj-Rabia, S, et al. Verrucous hemangioma (also known as verrucous venous malformation): a vascular anomaly frequently misdiagnosed as a lymphatic malformation. Pediatr Dermatol. 2018;35:E378-E381.
- Mestre T, Amaro C, Freitas I. Verrucous haemangioma: a diagnosis to consider [published online June 4, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204612
- Ivy H, Julian CA. Angiokeratoma circumscriptum. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549769/
- Shetty S, Geetha V, Rao R, et al. Verrucous hemangioma: importance of a deeper biopsy. Indian J Dermatopathol Diagn Dermatol. 2014;1:99-100.
- Bishop BN, Lynch DT. Cancer, Kaposi sarcoma. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17-28.
- Ambujam S, Audhya M, Reddy A, et al. Cutaneous angiosarcoma of the head, neck, and face of the elderly in type 5 skin. J Cutan Aesthet Surg. 2013;6:45-47.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
The Diagnosis: Targetoid Hemosiderotic Hemangioma
Targetoid hemosiderotic hemangioma (THH), also known as hobnail hemangioma, is a benign vascular tumor that usually occurs in young or middle-aged adults. It most commonly presents on the extremities or trunk as an isolated red-brown plaque or papule.1,2 Histologically, THH is characterized by superficial dilated ectatic vessels with underlying proliferating vascular channels lined by plump hobnail endothelial cells.1 Targetoid hemosiderotic hemangioma typically involves the dermis and spares the subcutis. The vascular channels may contain erythrocytes as well as pale eosinophilic lymph, as seen in our patient (quiz image). The deeper dermis contains vascular spaces that are more angulated and smaller and appear to be dissecting through the collagen bundles or collapsed.1,3 A variable amount of hemosiderin deposition and extravasated erythrocytes are seen.2,3 Histologic features evolve with the age of the lesion. Increasing amounts of hemosiderin deposition and erythrocyte extravasation may correspond histologically to the recent clinical color change reported by the patient.
Verrucous hemangioma is a rare congenital vascular abnormality that is characterized by dilated vessels in the papillary dermis along with acanthosis, hyperkeratosis, and irregular papillomatosis, as seen in angiokeratoma.4 However, the vascular proliferation composed of variably sized, thin-walled capillaries extends into the deep dermis as well as the subcutis (Figure 1). Verrucous hemangioma most commonly is reported on the legs and generally starts as a violaceous patch that progresses into a hyperkeratotic verrucous plaque or nodule.5,6

Angiokeratoma is characterized by superficial vascular ectasia of the papillary dermis in association with overlying acanthosis, hyperkeratosis, and rete elongation.7 The dilated vascular spaces appear encircled by the epidermis (Figure 2). Intravascular thrombosis can be seen within the ectatic vessels.7 In contrast to verrucous hemangioma, angiokeratoma is limited to the papillary dermis. Therefore, obtaining a biopsy of sufficient depth is necessary for differentiation.8 There are 5 clinical presentations of angiokeratoma: sporadic, angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry disease). Angiokeratomas may present on the lower extremities, tongue, trunk, and scrotum as hyperkeratotic, dark red to purple or black papules.7

There are 3 clinical stages of Kaposi sarcoma: patch, plaque, and nodular stages. The patch stage is characterized histologically by vascular channels that dissect through the dermis and extend around native vessels (the promontory sign)(Figure 3).9,10 These features can show histologic overlap with THH. The plaque stage shows a more diffuse dermal vascular proliferation, increased cellularity of spindle cells, and possible extension into the subcutis.9,10 Focal plasma cells, hemosiderin, and extravasated red blood cells can be seen. The nodular stage is characterized by a proliferation of spindle cells with red blood cells squeezed between slitlike vascular spaces, hyaline globules, and scattered mitotic figures, but not atypical forms.10 In this stage, plasma cells and hemosiderin are more readily identifiable. A biopsy from the nodular stage is unlikely to enter the histologic differential diagnosis with THH. Clinically, there are 4 variants of Kaposi sarcoma: the classic or sporadic form, an endemic form, iatrogenic, and AIDS associated. Overall, it is more common in males and can occur at any age.10 Human herpesvirus 8 is seen in all forms, and infected cells can be highlighted by the immunohistochemical stain for latent nuclear antigen 1.9,10

Angiosarcoma is a malignant endothelial tumor of soft tissue, skin, bone, and visceral organs.11,12 Clinically, cutaneous angiosarcoma can present in a variety of ways, including single or multiple bluish red lesions that can ulcerate or bleed; violaceous nodules or plaques; and hematomalike lesions that can mimic epithelial neoplasms including squamous cell carcinoma, basal cell carcinoma, and malignant melanoma.11,13,14 The cutaneous lesions most commonly occur on sun-exposed skin, particularly on the face and scalp.12 Other clinical variants that are important to recognize are postradiation angiosarcoma, characterized by MYC gene amplification, and lymphedema-associated angiosarcoma (Stewart-Treves syndrome). Angiosarcoma can have a variety of morphologic features, ranging from well to poorly differentiated. Classically, angiosarcoma is characterized by infiltrating vascular spaces lined by atypical endothelial cells (Figure 4). Poorly differentiated angiosarcoma can demonstrate spindle, epithelioid, or polygonal cells with increased mitotic activity, pleomorphism, and irregular vascular spaces.11 Endothelial markers such as ERG (erythroblast transformation specific-related gene)(nuclear) and CD31 (membranous) can be used to aid in the diagnosis of a poorly differentiated lesion. Epithelioid angiosarcoma also occasionally stains with cytokeratins.13,14

The Diagnosis: Targetoid Hemosiderotic Hemangioma
Targetoid hemosiderotic hemangioma (THH), also known as hobnail hemangioma, is a benign vascular tumor that usually occurs in young or middle-aged adults. It most commonly presents on the extremities or trunk as an isolated red-brown plaque or papule.1,2 Histologically, THH is characterized by superficial dilated ectatic vessels with underlying proliferating vascular channels lined by plump hobnail endothelial cells.1 Targetoid hemosiderotic hemangioma typically involves the dermis and spares the subcutis. The vascular channels may contain erythrocytes as well as pale eosinophilic lymph, as seen in our patient (quiz image). The deeper dermis contains vascular spaces that are more angulated and smaller and appear to be dissecting through the collagen bundles or collapsed.1,3 A variable amount of hemosiderin deposition and extravasated erythrocytes are seen.2,3 Histologic features evolve with the age of the lesion. Increasing amounts of hemosiderin deposition and erythrocyte extravasation may correspond histologically to the recent clinical color change reported by the patient.
Verrucous hemangioma is a rare congenital vascular abnormality that is characterized by dilated vessels in the papillary dermis along with acanthosis, hyperkeratosis, and irregular papillomatosis, as seen in angiokeratoma.4 However, the vascular proliferation composed of variably sized, thin-walled capillaries extends into the deep dermis as well as the subcutis (Figure 1). Verrucous hemangioma most commonly is reported on the legs and generally starts as a violaceous patch that progresses into a hyperkeratotic verrucous plaque or nodule.5,6

Angiokeratoma is characterized by superficial vascular ectasia of the papillary dermis in association with overlying acanthosis, hyperkeratosis, and rete elongation.7 The dilated vascular spaces appear encircled by the epidermis (Figure 2). Intravascular thrombosis can be seen within the ectatic vessels.7 In contrast to verrucous hemangioma, angiokeratoma is limited to the papillary dermis. Therefore, obtaining a biopsy of sufficient depth is necessary for differentiation.8 There are 5 clinical presentations of angiokeratoma: sporadic, angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry disease). Angiokeratomas may present on the lower extremities, tongue, trunk, and scrotum as hyperkeratotic, dark red to purple or black papules.7

There are 3 clinical stages of Kaposi sarcoma: patch, plaque, and nodular stages. The patch stage is characterized histologically by vascular channels that dissect through the dermis and extend around native vessels (the promontory sign)(Figure 3).9,10 These features can show histologic overlap with THH. The plaque stage shows a more diffuse dermal vascular proliferation, increased cellularity of spindle cells, and possible extension into the subcutis.9,10 Focal plasma cells, hemosiderin, and extravasated red blood cells can be seen. The nodular stage is characterized by a proliferation of spindle cells with red blood cells squeezed between slitlike vascular spaces, hyaline globules, and scattered mitotic figures, but not atypical forms.10 In this stage, plasma cells and hemosiderin are more readily identifiable. A biopsy from the nodular stage is unlikely to enter the histologic differential diagnosis with THH. Clinically, there are 4 variants of Kaposi sarcoma: the classic or sporadic form, an endemic form, iatrogenic, and AIDS associated. Overall, it is more common in males and can occur at any age.10 Human herpesvirus 8 is seen in all forms, and infected cells can be highlighted by the immunohistochemical stain for latent nuclear antigen 1.9,10

Angiosarcoma is a malignant endothelial tumor of soft tissue, skin, bone, and visceral organs.11,12 Clinically, cutaneous angiosarcoma can present in a variety of ways, including single or multiple bluish red lesions that can ulcerate or bleed; violaceous nodules or plaques; and hematomalike lesions that can mimic epithelial neoplasms including squamous cell carcinoma, basal cell carcinoma, and malignant melanoma.11,13,14 The cutaneous lesions most commonly occur on sun-exposed skin, particularly on the face and scalp.12 Other clinical variants that are important to recognize are postradiation angiosarcoma, characterized by MYC gene amplification, and lymphedema-associated angiosarcoma (Stewart-Treves syndrome). Angiosarcoma can have a variety of morphologic features, ranging from well to poorly differentiated. Classically, angiosarcoma is characterized by infiltrating vascular spaces lined by atypical endothelial cells (Figure 4). Poorly differentiated angiosarcoma can demonstrate spindle, epithelioid, or polygonal cells with increased mitotic activity, pleomorphism, and irregular vascular spaces.11 Endothelial markers such as ERG (erythroblast transformation specific-related gene)(nuclear) and CD31 (membranous) can be used to aid in the diagnosis of a poorly differentiated lesion. Epithelioid angiosarcoma also occasionally stains with cytokeratins.13,14

- Joyce JC, Keith PJ, Szabo S, et al. Superficial hemosiderotic lymphovascular malformation (hobnail hemangioma): a report of six cases. Pediatr Dermatol. 2014;31:281-285.
- Sahin MT, Demir MA, Gunduz K, et al. Targetoid haemosiderotic haemangioma: dermoscopic monitoring of three cases and review of the literature. Clin Exp Dermatol. 2005;30:672-676.
- Kakizaki P, Valente NY, Paiva DL, et al. Targetoid hemosiderotic hemangioma--case report. An Bras Dermatol. 2014;89:956-959.
- Oppermann K, Boff AL, Bonamigo RR. Verrucous hemangioma and histopathological differential diagnosis with angiokeratoma circumscriptum neviforme. An Bras Dermatol. 2018;93:712-715.
- Boccara, O, Ariche-Maman, S, Hadj-Rabia, S, et al. Verrucous hemangioma (also known as verrucous venous malformation): a vascular anomaly frequently misdiagnosed as a lymphatic malformation. Pediatr Dermatol. 2018;35:E378-E381.
- Mestre T, Amaro C, Freitas I. Verrucous haemangioma: a diagnosis to consider [published online June 4, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204612
- Ivy H, Julian CA. Angiokeratoma circumscriptum. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549769/
- Shetty S, Geetha V, Rao R, et al. Verrucous hemangioma: importance of a deeper biopsy. Indian J Dermatopathol Diagn Dermatol. 2014;1:99-100.
- Bishop BN, Lynch DT. Cancer, Kaposi sarcoma. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17-28.
- Ambujam S, Audhya M, Reddy A, et al. Cutaneous angiosarcoma of the head, neck, and face of the elderly in type 5 skin. J Cutan Aesthet Surg. 2013;6:45-47.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Joyce JC, Keith PJ, Szabo S, et al. Superficial hemosiderotic lymphovascular malformation (hobnail hemangioma): a report of six cases. Pediatr Dermatol. 2014;31:281-285.
- Sahin MT, Demir MA, Gunduz K, et al. Targetoid haemosiderotic haemangioma: dermoscopic monitoring of three cases and review of the literature. Clin Exp Dermatol. 2005;30:672-676.
- Kakizaki P, Valente NY, Paiva DL, et al. Targetoid hemosiderotic hemangioma--case report. An Bras Dermatol. 2014;89:956-959.
- Oppermann K, Boff AL, Bonamigo RR. Verrucous hemangioma and histopathological differential diagnosis with angiokeratoma circumscriptum neviforme. An Bras Dermatol. 2018;93:712-715.
- Boccara, O, Ariche-Maman, S, Hadj-Rabia, S, et al. Verrucous hemangioma (also known as verrucous venous malformation): a vascular anomaly frequently misdiagnosed as a lymphatic malformation. Pediatr Dermatol. 2018;35:E378-E381.
- Mestre T, Amaro C, Freitas I. Verrucous haemangioma: a diagnosis to consider [published online June 4, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204612
- Ivy H, Julian CA. Angiokeratoma circumscriptum. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549769/
- Shetty S, Geetha V, Rao R, et al. Verrucous hemangioma: importance of a deeper biopsy. Indian J Dermatopathol Diagn Dermatol. 2014;1:99-100.
- Bishop BN, Lynch DT. Cancer, Kaposi sarcoma. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17-28.
- Ambujam S, Audhya M, Reddy A, et al. Cutaneous angiosarcoma of the head, neck, and face of the elderly in type 5 skin. J Cutan Aesthet Surg. 2013;6:45-47.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.

A 35-year-old man presented with a reddish brown papule on the left upper chest of 1 year’s duration that had changed color to reddish purple. Physical examination revealed a 6-mm violaceous papule with an erythematous rim.
Purpuric Bullae on the Lower Extremities
The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
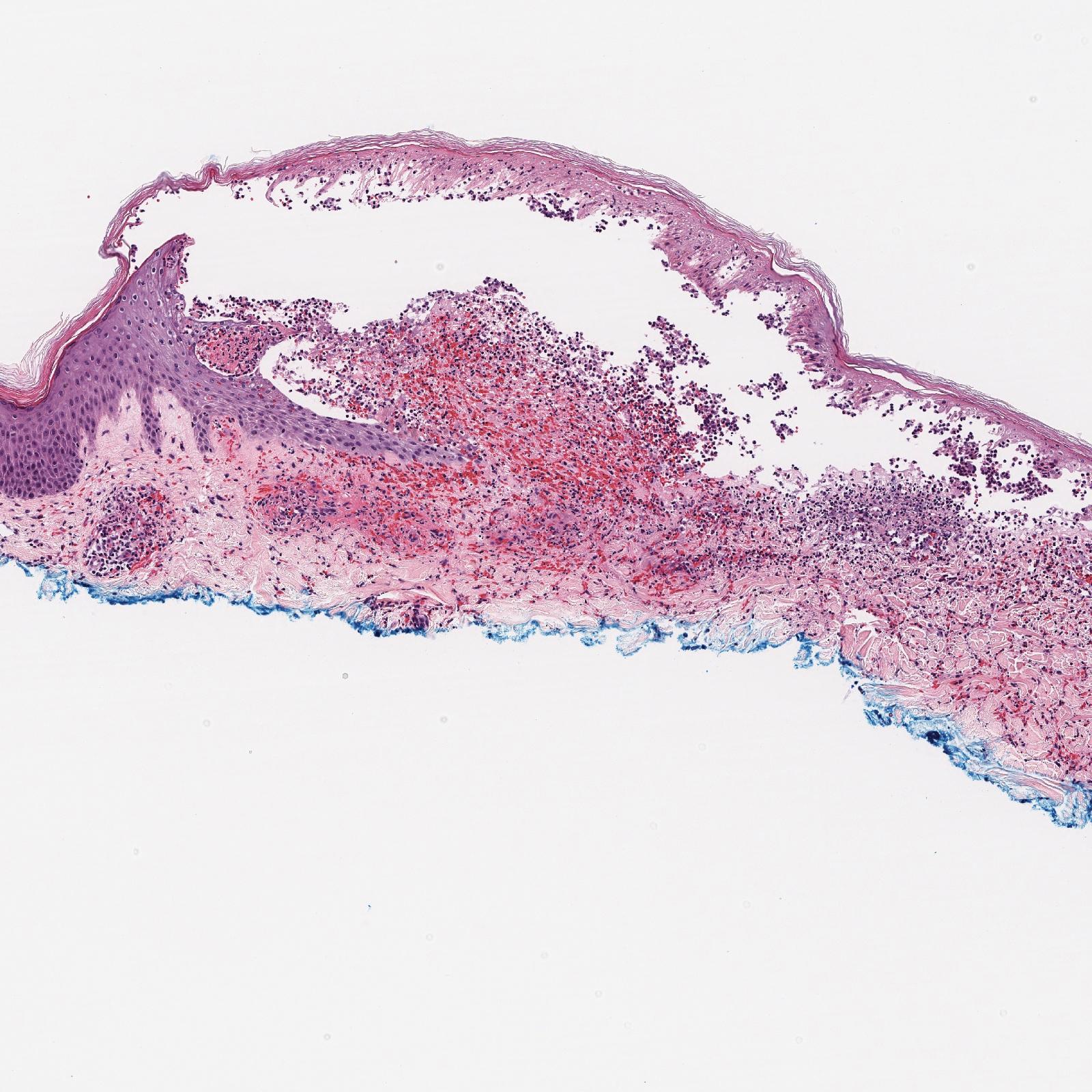
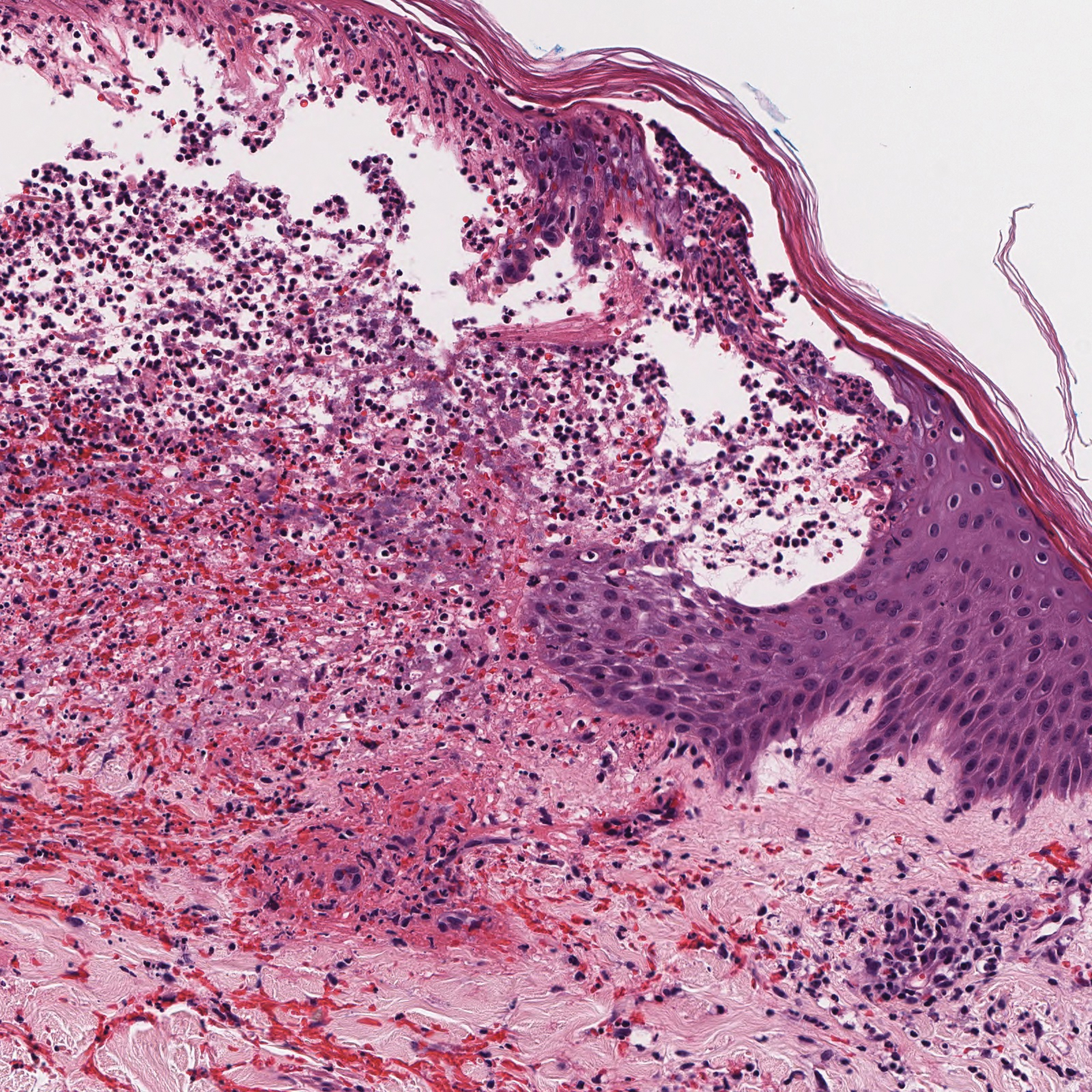
A 30-year-old woman with a medical history of uncontrolled type 2 diabetes mellitus and morbid obesity presented to the dermatology clinic with a painful blistering rash on the lower extremities with scattered red-purple papules of 1 week's duration. The rash began on the left dorsal foot. Physical examination showed nonblanching, 2- to 4-mm, violaceous papules with numerous vesiculopustular bullae on the lower extremities from the dorsal feet to the proximal knee. A shave biopsy with hematoxylin and eosin stain and a punch biopsy for direct immunofluorescence were performed.
How should you manage children born to hepatitis C-positive women?
FOR STARTERS, don’t be overly concerned with the mode of delivery; it doesn’t influence the rate of transmission of hepatitis C virus (HCV), except in women who are also infected with human immunodeficiency virus (HIV) (strength of recommendation [SOR]: B, consistent retrospective cohort studies).
Avoid internal fetal monitoring and prolonged rupture of membranes (SOR: B, single retrospective cohort study).
Advise patients that it’s OK to breastfeed. Breastfeeding doesn’t affect transmission (SOR: B, consistent prospective cohort studies).
Check HCV RNA and serum anti-HCV on 2 occasions between 2 and 6 months of age and 18 and 24 months of age (SOR: B, consistent prospective cohort studies).
Evidence summary
Perinatal transmission of HCV is rare. It occurs only when serum HCV RNA is detectable; transmission rates may be related to higher levels (>106 copies/mL).1 HCV is transmitted to 2% of infants of anti-HCV seropositive women and 4% to 7% of infants born to mothers who are HCV RNA-positive at delivery.1
Spontaneous clearance of the virus occurs in approximately 20% of infants. Most remain asymptomatic if HCV persists, but have mild elevation of liver function tests.2
Routine screening for HCV in mothers is not recommended, but pregnant women at high risk for HCV infection should be screened for anti-HCV.
Route of delivery: Only a concern for HIV-positive mothers
The mode of delivery doesn’t influence the rate of HCV transmission, except in mothers with HIV. Retrospective analysis of 503 HCV-positive mothers coinfected with HIV showed a decreased risk of transmission during cesarean delivery (odds ratio [OR]=0.36; P=.01; number needed to treat [NNT]=10).3
One study suggested an increased rate of vertical HCV transmission during vaginal delivery compared with cesarean delivery (32% vs 6%; P<.05). The study didn’t account for the percent of mothers coinfected with HIV, however.4
A meta-analysis of 11 studies showed similar rates of transmission for vaginal and cesarean delivery: adjusted rates were 4.3% and 3%, respectively.1
Internal monitoring is an issue
Avoid internal fetal monitoring to minimize HCV transmission, based on a single retrospective cohort of 244 infants born to HCV-positive mothers (relative risk [RR]= 7.7; 95% confidence interval [CI], 1.9-31.6; number needed to harm [NNH]=6).7 The same study showed an increased risk with membrane rupture longer than 6 hours (RR=9.9; 95% CI, 1.2-81; NNH=13).5
Breastfeeding doesn’t significantly affect HCV transmission. Transmission rates for breastfed and nonbreastfed infants are 3.7% and 3.9%, respectively.1
Defer postpartum lab testing
Because about 20% of infants exposed to HCV clear the virus spontaneously, and maternal antibodies can confound laboratory results, deferring postpartum diagnostic testing is appropriate. A study of 1104 children in whom vertical transmission didn’t occur after exposure to HCV showed that 95% of the children were anti-HCV antibody negative by 12 months age.6 A prospective study of 23 infants documented spontaneous clearance of HCV RNA by 6 months in all patients.7
Recommendations
The National Institutes of Health 2002 Consensus Statement recommends:8
- avoiding fetal scalp electrodes and prolonged rupture of membranes
- serum testing for HCV RNA at 2 months and 6 months of age
- anti-HCV antibody testing after 15 months of age.
The American College of Obstetricians and Gynecologists supports breastfeeding, recommends against routine HCV screening, and recommends that cesarean delivery be reserved for obstetric indications.9
The American Association for the Study of Liver Diseases recommends serum testing and liver biopsy on the same schedule as adult patients and endorses considering treatment after 3 years of age.6
The US Food and Drug Administration has approved treatment after 3 years of age for children with detectable HCV RNA levels higher than 50 IU/mL and who have had a liver biopsy with portal or bridging fibrosis and at least moderate inflammation and necrosis.
The American Gastroenterological Association recommends considering treatment with PEG-interferon and ribavirin after 3 years of age.10
Acknowledgements
The opinions and assertions contained herein are the private view of the authors and not to be construed as official, or as reflecting the view of the US Air Force Medical Service or the US Air Force at large.
1. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
2. European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41:45-51.
3. European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
4. Paccagnini S, Principi N, Massironi E, et al. Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. Pediatr Infect Dis J. 1995;14:195-199.
5. Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
6. England K, Pembrey L, Tovo P, et al. European Paediatric Hepatitis C Virus Network. Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatr. 2005;94:444-450.
7. Ketzinel-Gilad M, Colodner SL, Hadary R, et al. Transient transmission of hepatitis C virus from mothers to newborns. Eur J Clin Microbiol Infect Dis. 2000;19:267-274.
8. Management of Hepatitis C: 2002. National Institutes of Health Consensus Conference Statement, June 10-12, 2002. Available at: http://consensus.nih.gov/2002/2002HepatitisC2002116html.htm. Accessed August 23, 2009.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86. Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941-956.
10. Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130:225-230.Available at: www.gastrojournal.org/article/PIIS0016508505022717/fulltext. Accessed on August 23, 2009.
FOR STARTERS, don’t be overly concerned with the mode of delivery; it doesn’t influence the rate of transmission of hepatitis C virus (HCV), except in women who are also infected with human immunodeficiency virus (HIV) (strength of recommendation [SOR]: B, consistent retrospective cohort studies).
Avoid internal fetal monitoring and prolonged rupture of membranes (SOR: B, single retrospective cohort study).
Advise patients that it’s OK to breastfeed. Breastfeeding doesn’t affect transmission (SOR: B, consistent prospective cohort studies).
Check HCV RNA and serum anti-HCV on 2 occasions between 2 and 6 months of age and 18 and 24 months of age (SOR: B, consistent prospective cohort studies).
Evidence summary
Perinatal transmission of HCV is rare. It occurs only when serum HCV RNA is detectable; transmission rates may be related to higher levels (>106 copies/mL).1 HCV is transmitted to 2% of infants of anti-HCV seropositive women and 4% to 7% of infants born to mothers who are HCV RNA-positive at delivery.1
Spontaneous clearance of the virus occurs in approximately 20% of infants. Most remain asymptomatic if HCV persists, but have mild elevation of liver function tests.2
Routine screening for HCV in mothers is not recommended, but pregnant women at high risk for HCV infection should be screened for anti-HCV.
Route of delivery: Only a concern for HIV-positive mothers
The mode of delivery doesn’t influence the rate of HCV transmission, except in mothers with HIV. Retrospective analysis of 503 HCV-positive mothers coinfected with HIV showed a decreased risk of transmission during cesarean delivery (odds ratio [OR]=0.36; P=.01; number needed to treat [NNT]=10).3
One study suggested an increased rate of vertical HCV transmission during vaginal delivery compared with cesarean delivery (32% vs 6%; P<.05). The study didn’t account for the percent of mothers coinfected with HIV, however.4
A meta-analysis of 11 studies showed similar rates of transmission for vaginal and cesarean delivery: adjusted rates were 4.3% and 3%, respectively.1
Internal monitoring is an issue
Avoid internal fetal monitoring to minimize HCV transmission, based on a single retrospective cohort of 244 infants born to HCV-positive mothers (relative risk [RR]= 7.7; 95% confidence interval [CI], 1.9-31.6; number needed to harm [NNH]=6).7 The same study showed an increased risk with membrane rupture longer than 6 hours (RR=9.9; 95% CI, 1.2-81; NNH=13).5
Breastfeeding doesn’t significantly affect HCV transmission. Transmission rates for breastfed and nonbreastfed infants are 3.7% and 3.9%, respectively.1
Defer postpartum lab testing
Because about 20% of infants exposed to HCV clear the virus spontaneously, and maternal antibodies can confound laboratory results, deferring postpartum diagnostic testing is appropriate. A study of 1104 children in whom vertical transmission didn’t occur after exposure to HCV showed that 95% of the children were anti-HCV antibody negative by 12 months age.6 A prospective study of 23 infants documented spontaneous clearance of HCV RNA by 6 months in all patients.7
Recommendations
The National Institutes of Health 2002 Consensus Statement recommends:8
- avoiding fetal scalp electrodes and prolonged rupture of membranes
- serum testing for HCV RNA at 2 months and 6 months of age
- anti-HCV antibody testing after 15 months of age.
The American College of Obstetricians and Gynecologists supports breastfeeding, recommends against routine HCV screening, and recommends that cesarean delivery be reserved for obstetric indications.9
The American Association for the Study of Liver Diseases recommends serum testing and liver biopsy on the same schedule as adult patients and endorses considering treatment after 3 years of age.6
The US Food and Drug Administration has approved treatment after 3 years of age for children with detectable HCV RNA levels higher than 50 IU/mL and who have had a liver biopsy with portal or bridging fibrosis and at least moderate inflammation and necrosis.
The American Gastroenterological Association recommends considering treatment with PEG-interferon and ribavirin after 3 years of age.10
Acknowledgements
The opinions and assertions contained herein are the private view of the authors and not to be construed as official, or as reflecting the view of the US Air Force Medical Service or the US Air Force at large.
FOR STARTERS, don’t be overly concerned with the mode of delivery; it doesn’t influence the rate of transmission of hepatitis C virus (HCV), except in women who are also infected with human immunodeficiency virus (HIV) (strength of recommendation [SOR]: B, consistent retrospective cohort studies).
Avoid internal fetal monitoring and prolonged rupture of membranes (SOR: B, single retrospective cohort study).
Advise patients that it’s OK to breastfeed. Breastfeeding doesn’t affect transmission (SOR: B, consistent prospective cohort studies).
Check HCV RNA and serum anti-HCV on 2 occasions between 2 and 6 months of age and 18 and 24 months of age (SOR: B, consistent prospective cohort studies).
Evidence summary
Perinatal transmission of HCV is rare. It occurs only when serum HCV RNA is detectable; transmission rates may be related to higher levels (>106 copies/mL).1 HCV is transmitted to 2% of infants of anti-HCV seropositive women and 4% to 7% of infants born to mothers who are HCV RNA-positive at delivery.1
Spontaneous clearance of the virus occurs in approximately 20% of infants. Most remain asymptomatic if HCV persists, but have mild elevation of liver function tests.2
Routine screening for HCV in mothers is not recommended, but pregnant women at high risk for HCV infection should be screened for anti-HCV.
Route of delivery: Only a concern for HIV-positive mothers
The mode of delivery doesn’t influence the rate of HCV transmission, except in mothers with HIV. Retrospective analysis of 503 HCV-positive mothers coinfected with HIV showed a decreased risk of transmission during cesarean delivery (odds ratio [OR]=0.36; P=.01; number needed to treat [NNT]=10).3
One study suggested an increased rate of vertical HCV transmission during vaginal delivery compared with cesarean delivery (32% vs 6%; P<.05). The study didn’t account for the percent of mothers coinfected with HIV, however.4
A meta-analysis of 11 studies showed similar rates of transmission for vaginal and cesarean delivery: adjusted rates were 4.3% and 3%, respectively.1
Internal monitoring is an issue
Avoid internal fetal monitoring to minimize HCV transmission, based on a single retrospective cohort of 244 infants born to HCV-positive mothers (relative risk [RR]= 7.7; 95% confidence interval [CI], 1.9-31.6; number needed to harm [NNH]=6).7 The same study showed an increased risk with membrane rupture longer than 6 hours (RR=9.9; 95% CI, 1.2-81; NNH=13).5
Breastfeeding doesn’t significantly affect HCV transmission. Transmission rates for breastfed and nonbreastfed infants are 3.7% and 3.9%, respectively.1
Defer postpartum lab testing
Because about 20% of infants exposed to HCV clear the virus spontaneously, and maternal antibodies can confound laboratory results, deferring postpartum diagnostic testing is appropriate. A study of 1104 children in whom vertical transmission didn’t occur after exposure to HCV showed that 95% of the children were anti-HCV antibody negative by 12 months age.6 A prospective study of 23 infants documented spontaneous clearance of HCV RNA by 6 months in all patients.7
Recommendations
The National Institutes of Health 2002 Consensus Statement recommends:8
- avoiding fetal scalp electrodes and prolonged rupture of membranes
- serum testing for HCV RNA at 2 months and 6 months of age
- anti-HCV antibody testing after 15 months of age.
The American College of Obstetricians and Gynecologists supports breastfeeding, recommends against routine HCV screening, and recommends that cesarean delivery be reserved for obstetric indications.9
The American Association for the Study of Liver Diseases recommends serum testing and liver biopsy on the same schedule as adult patients and endorses considering treatment after 3 years of age.6
The US Food and Drug Administration has approved treatment after 3 years of age for children with detectable HCV RNA levels higher than 50 IU/mL and who have had a liver biopsy with portal or bridging fibrosis and at least moderate inflammation and necrosis.
The American Gastroenterological Association recommends considering treatment with PEG-interferon and ribavirin after 3 years of age.10
Acknowledgements
The opinions and assertions contained herein are the private view of the authors and not to be construed as official, or as reflecting the view of the US Air Force Medical Service or the US Air Force at large.
1. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
2. European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41:45-51.
3. European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
4. Paccagnini S, Principi N, Massironi E, et al. Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. Pediatr Infect Dis J. 1995;14:195-199.
5. Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
6. England K, Pembrey L, Tovo P, et al. European Paediatric Hepatitis C Virus Network. Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatr. 2005;94:444-450.
7. Ketzinel-Gilad M, Colodner SL, Hadary R, et al. Transient transmission of hepatitis C virus from mothers to newborns. Eur J Clin Microbiol Infect Dis. 2000;19:267-274.
8. Management of Hepatitis C: 2002. National Institutes of Health Consensus Conference Statement, June 10-12, 2002. Available at: http://consensus.nih.gov/2002/2002HepatitisC2002116html.htm. Accessed August 23, 2009.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86. Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941-956.
10. Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130:225-230.Available at: www.gastrojournal.org/article/PIIS0016508505022717/fulltext. Accessed on August 23, 2009.
1. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
2. European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41:45-51.
3. European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
4. Paccagnini S, Principi N, Massironi E, et al. Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. Pediatr Infect Dis J. 1995;14:195-199.
5. Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
6. England K, Pembrey L, Tovo P, et al. European Paediatric Hepatitis C Virus Network. Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatr. 2005;94:444-450.
7. Ketzinel-Gilad M, Colodner SL, Hadary R, et al. Transient transmission of hepatitis C virus from mothers to newborns. Eur J Clin Microbiol Infect Dis. 2000;19:267-274.
8. Management of Hepatitis C: 2002. National Institutes of Health Consensus Conference Statement, June 10-12, 2002. Available at: http://consensus.nih.gov/2002/2002HepatitisC2002116html.htm. Accessed August 23, 2009.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86. Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941-956.
10. Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130:225-230.Available at: www.gastrojournal.org/article/PIIS0016508505022717/fulltext. Accessed on August 23, 2009.
Evidence-based answers from the Family Physicians Inquiries Network