User login
Recognizing autophonia in patients with anorexia nervosa
Anorexia nervosa can affect a number of systems of the body, including the otolaryngologic presentation of autophonia1,2—a rare hyperperception of an abnormally intense hearing of one’s own voice and respiratory sounds.2 The most common cause of autophonia in patients with anorexia is a patulous (patent) eustachian tube, which can be caused by extreme weight loss.2,3
Significant reduction in the quantity of fat tissue at the location of the eustachian tube can cause patency.3 This creates an abnormal connection between the nasopharynx and tympanic membrane, in which sounds are transmitted directly from the oral cavity to the middle ear, causing autophonia, tinnitus, or sound distortion.4
What are the symptoms?Patients often report hearing their own voice more loudly in the affected ear. This can be distressing, and they might become preoccupied with the sound of their voice—thus affecting quality of life.2,4
The intensity of symptoms varies: from a mild sensation of a clogged ear to extremely bothersome discomfort much like a middle-ear infection.2,4 Autophonia, however, cannot be relieved by conventional therapies for those conditions.2,3
A patulous eustachian tube is difficult to detect and can be misdiagnosed as another condition. Pregnancy, stress, fatigue, radiation therapy, hormonal therapy, and dramatic weight loss also can cause a patulous eustachian tube.2
How is the diagnosis made?The diagnosis of autophonia is clinical and begins with a detailed history. Symptoms often appear within the time frame of rapid weight loss and without evidence of infection or other illness.2,3 The clinical examination is otherwise unremarkable.2,4
Is there treatment?To improve the patient’s comfort and quality of life, intervention is required, best provided by an integrated team of medical specialists. Weight gain, of course, is the treatment goal in anorexia, but this is a complex process often marked by relapse; a detailed discussion of treatment strategies is beyond the scope of this “Pearl.” Symptoms usually diminish as fatty tissue is restored upon successful treatment of anorexia, which closes the abnormal eustachian tube opening.2,3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Olthoff A, Laskawi R, Kruse E. Successful treatment of autophonia with botulinum toxin: case report. Ann Otol Rhinol Laryngol. 2007;116(8):594-598.
2. Godbole M, Key A. Autophonia in anorexia nervosa. Int J Eat Disord. 2010;43(5):480-482.
3. Karwautz A, Hafferl A, Ungar D, et al. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999;25(3):353-355.
4. Dornhoffer JL, Leuwer R, Schwager K, et al. A practical guide to the eustachian tube. New York, NY: Springer; 2014:23-41.
Anorexia nervosa can affect a number of systems of the body, including the otolaryngologic presentation of autophonia1,2—a rare hyperperception of an abnormally intense hearing of one’s own voice and respiratory sounds.2 The most common cause of autophonia in patients with anorexia is a patulous (patent) eustachian tube, which can be caused by extreme weight loss.2,3
Significant reduction in the quantity of fat tissue at the location of the eustachian tube can cause patency.3 This creates an abnormal connection between the nasopharynx and tympanic membrane, in which sounds are transmitted directly from the oral cavity to the middle ear, causing autophonia, tinnitus, or sound distortion.4
What are the symptoms?Patients often report hearing their own voice more loudly in the affected ear. This can be distressing, and they might become preoccupied with the sound of their voice—thus affecting quality of life.2,4
The intensity of symptoms varies: from a mild sensation of a clogged ear to extremely bothersome discomfort much like a middle-ear infection.2,4 Autophonia, however, cannot be relieved by conventional therapies for those conditions.2,3
A patulous eustachian tube is difficult to detect and can be misdiagnosed as another condition. Pregnancy, stress, fatigue, radiation therapy, hormonal therapy, and dramatic weight loss also can cause a patulous eustachian tube.2
How is the diagnosis made?The diagnosis of autophonia is clinical and begins with a detailed history. Symptoms often appear within the time frame of rapid weight loss and without evidence of infection or other illness.2,3 The clinical examination is otherwise unremarkable.2,4
Is there treatment?To improve the patient’s comfort and quality of life, intervention is required, best provided by an integrated team of medical specialists. Weight gain, of course, is the treatment goal in anorexia, but this is a complex process often marked by relapse; a detailed discussion of treatment strategies is beyond the scope of this “Pearl.” Symptoms usually diminish as fatty tissue is restored upon successful treatment of anorexia, which closes the abnormal eustachian tube opening.2,3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Anorexia nervosa can affect a number of systems of the body, including the otolaryngologic presentation of autophonia1,2—a rare hyperperception of an abnormally intense hearing of one’s own voice and respiratory sounds.2 The most common cause of autophonia in patients with anorexia is a patulous (patent) eustachian tube, which can be caused by extreme weight loss.2,3
Significant reduction in the quantity of fat tissue at the location of the eustachian tube can cause patency.3 This creates an abnormal connection between the nasopharynx and tympanic membrane, in which sounds are transmitted directly from the oral cavity to the middle ear, causing autophonia, tinnitus, or sound distortion.4
What are the symptoms?Patients often report hearing their own voice more loudly in the affected ear. This can be distressing, and they might become preoccupied with the sound of their voice—thus affecting quality of life.2,4
The intensity of symptoms varies: from a mild sensation of a clogged ear to extremely bothersome discomfort much like a middle-ear infection.2,4 Autophonia, however, cannot be relieved by conventional therapies for those conditions.2,3
A patulous eustachian tube is difficult to detect and can be misdiagnosed as another condition. Pregnancy, stress, fatigue, radiation therapy, hormonal therapy, and dramatic weight loss also can cause a patulous eustachian tube.2
How is the diagnosis made?The diagnosis of autophonia is clinical and begins with a detailed history. Symptoms often appear within the time frame of rapid weight loss and without evidence of infection or other illness.2,3 The clinical examination is otherwise unremarkable.2,4
Is there treatment?To improve the patient’s comfort and quality of life, intervention is required, best provided by an integrated team of medical specialists. Weight gain, of course, is the treatment goal in anorexia, but this is a complex process often marked by relapse; a detailed discussion of treatment strategies is beyond the scope of this “Pearl.” Symptoms usually diminish as fatty tissue is restored upon successful treatment of anorexia, which closes the abnormal eustachian tube opening.2,3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Olthoff A, Laskawi R, Kruse E. Successful treatment of autophonia with botulinum toxin: case report. Ann Otol Rhinol Laryngol. 2007;116(8):594-598.
2. Godbole M, Key A. Autophonia in anorexia nervosa. Int J Eat Disord. 2010;43(5):480-482.
3. Karwautz A, Hafferl A, Ungar D, et al. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999;25(3):353-355.
4. Dornhoffer JL, Leuwer R, Schwager K, et al. A practical guide to the eustachian tube. New York, NY: Springer; 2014:23-41.
1. Olthoff A, Laskawi R, Kruse E. Successful treatment of autophonia with botulinum toxin: case report. Ann Otol Rhinol Laryngol. 2007;116(8):594-598.
2. Godbole M, Key A. Autophonia in anorexia nervosa. Int J Eat Disord. 2010;43(5):480-482.
3. Karwautz A, Hafferl A, Ungar D, et al. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999;25(3):353-355.
4. Dornhoffer JL, Leuwer R, Schwager K, et al. A practical guide to the eustachian tube. New York, NY: Springer; 2014:23-41.
Palonosetron and netupitant for prevention of chemotherapy-induced nausea and vomiting
The US Food and Drug Administration (FDA) recently approved NEPA, an oral fixed-dose combination of netupitant and palonosetron for treatment of chemotherapy-induced nausea and vomiting (CINV). Palonosetron is a pharmacologically distinct, best-in-class serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist, which prevents CINV during the acute phase (0-24 h) after administration of chemotherapy, and netupitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist, which prevents CINV during both the acute and delayed (25-120 h) phases. The 2 agents have also been shown potentially to act synergistically in inhibiting NK-1 receptor activity.
Click on the PDF icon at the top of this introduction to read the full article.
The US Food and Drug Administration (FDA) recently approved NEPA, an oral fixed-dose combination of netupitant and palonosetron for treatment of chemotherapy-induced nausea and vomiting (CINV). Palonosetron is a pharmacologically distinct, best-in-class serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist, which prevents CINV during the acute phase (0-24 h) after administration of chemotherapy, and netupitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist, which prevents CINV during both the acute and delayed (25-120 h) phases. The 2 agents have also been shown potentially to act synergistically in inhibiting NK-1 receptor activity.
Click on the PDF icon at the top of this introduction to read the full article.
The US Food and Drug Administration (FDA) recently approved NEPA, an oral fixed-dose combination of netupitant and palonosetron for treatment of chemotherapy-induced nausea and vomiting (CINV). Palonosetron is a pharmacologically distinct, best-in-class serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonist, which prevents CINV during the acute phase (0-24 h) after administration of chemotherapy, and netupitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist, which prevents CINV during both the acute and delayed (25-120 h) phases. The 2 agents have also been shown potentially to act synergistically in inhibiting NK-1 receptor activity.
Click on the PDF icon at the top of this introduction to read the full article.
Identification and Management of Middle East Respiratory Syndrome
Human coronaviruses (CoVs) were first identified in the mid-1960s. Coronaviruses are a large family of viruses that cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS).1
In 2003, SARS caused one of the most devastating global epidemics known to the developed world. The important lesson learned from the SARS epidemic was that CoVs can cause severe and rapidly spreading infection. Since then, 2 human CoVs, HCoV-HKU1 and HCoV-NL63, have been identified as common causes of human respiratory tract infections.2,3 In September 2012, a novel CoV was recognized to cause a fatal human infection. This virus has become known as the Middle East respiratory syndrome CoV (MERS-CoV).4
Related: Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Similar to SARS-CoV, MERS-CoV human infection has a high fatality rate and the ability to spread from person to person.5,6 Person-to-person transmission has resulted in secondary cases among close contacts, including health care providers (HCPs) who should, therefore, be cognizant of this infection. Federal HCPs in particular may be more likely to become involved in the care of patients with this disease, because many military personnel are returning from deployment in the Middle East.
History of MERS-CoV
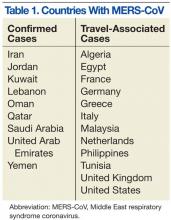
MERS-CoV was first identified as an infectious disease in humans in Saudi Arabia. In June 2012, the index case was hospitalized with pneumonia and acute renal injury.7 Since then, MERS-CoV human infections and clusters of infection have been identified in multiple countries in the Arabian Peninsula (Table 1).8 There have also been cases of MERS-CoV infection in other countries involving travelers who had visited the Arabian Peninsula and, in some instances, after returning home, their close contacts (Table 1).8
On May 2, 2014, the first confirmed U.S. case was reported in Indiana in a HCP who had recently been to Saudi Arabia.9 A second case of a HCP traveling from Saudi Arabia was identified on May 13, 2014, in Orlando, Florida.9 As of June 11, 2014, 699 laboratory‐confirmed cases of MERS-CoV infection had been reported to the World Health Organization in 20 countries, resulting in 209 deaths. All cases to date have originated in 6 countries of the Arabian Peninsula.5,8
The Organism
Coronaviruses are enveloped RNA viruses named for the crownlike spikes on their surface. They are common viruses known to cause respiratory infections in humans.1 It is thought that most people are infected with these viruses during their lifetime. These viruses generally cause mild-to-moderate upper respiratory tract illnesses, otherwise known as the common cold. On occasion, CoVs can cause lower respiratory tract infections in elderly patients, neonates, and immune-compromised individuals.1
Related: Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Coronaviruses are also known to infect animals. Most known CoVs cause disease in only 1 animal species or, at most, in a small number of closely related species. However, SARS-CoV was noted to infect people and various animals, including monkeys, civets, raccoon dogs, cats, dogs, and rodents. The origin and natural reservoir of SARS-CoV was ultimately determined to be bats.10
Genetic sequencing has determined that the MERS-CoV is different from any other known human CoV. MERS-CoV is a beta-CoV and, like the SARS-CoV, is closely related to bat CoVs.11-15 The origin of the MERS-CoV is not known, but an animal reservoir is suspected. Because MERS-CoV is similar to SARS-CoV, bats are considered a possible animal reservoir. Dromedary camels may act as intermediate hosts by spreading the virus to humans.16-18 However, there is no consensus on the animal reservoir for MERS-CoV. It is also not known how the virus has spread from animals to humans.
Case Definition
In order to aid in the rapid recognition of MERS, the CDC has established case definitions.8
A patient under investigation is an individual with fever (> 38oC, > 100.4oF) and pneumonia or acute respiratory distress syndrome (ARDS); and either:
- history of travel from countries in or near the Arabian Peninsula within 14 days before the onset of symptoms; or
- close contact with a symptomatic traveler who developed fever and ARDS within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
A confirmed case is a patient with laboratory confirmation of MERS-CoV infection. A probable case is a patient under investigation with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact of a laboratory- confirmed MERS-CoV case.
Transmission
MERS-CoV is thought to be of animal origin, but the mode of transmission from the animal reservoir is not known. It seems likely that some of the infections have occurred via intermittent zoonotic transmission, possibly by an environmental source.19 The presence of case clusters, however, suggests that human-to-human transmission also can occur. Human-to-human transmission has occurred in individuals living with an infected person and in HCPs caring for infected patients.20-24 The human-to-human transmission through close contact so far has been nonsustained.
It has been estimated that 75% of the reported cases are secondary, meaning that the patient acquired the MERS-CoV infection from another infected person. There is no evidence of sustained spread of MERS-CoV in community settings. The mode of human-to-human transmission has not been determined. Possible modes of transmission include droplet and contact transmission. The number of contacts infected by individuals with confirmed infections seems limited; the transmissibility, therefore, currently seems to be low.25,26 The results of a study of the transmissibility and epidemic potential for MERS-CoV suggest that it does not yet have pandemic potential.27
Bats may serve as a reservoir for MERS-CoV. However, because human contact with bats is uncommon, they are viewed as unlikely candidates for an immediate source of infection in most humans. It is possible that another animal or vector serves as an intermediate host. Camels have been proposed as a possible intermediate host, but this remains unproven. Interestingly, the MERS-CoV index patient had been caring for several ill camels in his herd; the camels had signs of respiratory illness, including nasal discharge.11,28 MERS-CoV sequences were subsequently isolated from a juvenile camel belonging to the index patient.
Symptoms
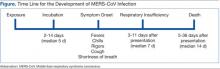
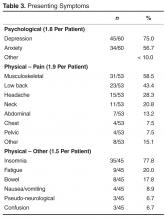
The incubation time after exposure to symptom onset ranges from 1.9 to 14.7 days (Figure). The median incubation period is 5.2 days.21 Patients are not believed to be contagious during the incubation period. Early symptoms of human infection with MERS-CoV include fever, chills or rigors, cough, and shortness of breath. Less frequently encountered symptoms include hemoptysis, sore throat, myalgias, diarrhea, vomiting, and abdominal pain (Table 2).20,21,23,26,27,29 Many patients infected with MERS-CoV develop a severe lower respiratory tract illness. The patient may progress to ARDS and require intubation and mechanical ventilator support. Mechanical ventilation has been required in 72% of patients.29 The median time from presentation for medical care to respiratory failure is 7 days, ranging from 3 to 11 days (Figure).
Physical Examination
The patients with MERS-CoV infection have been predominantly male and middle aged with an average age of 52 years. The clinical features depend on the severity of the illness. Some infected individuals have remained asymptomatic.27 Other patients have experienced mild lower respiratory illness and have not required hospitalization. However, about 40% of patients have experienced severe illness with pneumonia, respiratory insufficiency, multi-organ failure, and death. The percentage of severe illness is likely an overestimation, because patients with less severe symptoms probably are not tested for MERS-CoV. Most of the patients who have experienced a severe illness and/or death also had underlying comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, and chronic renal disease.23,29
Laboratory Data
As with SARS-CoV, lymphopenia has been reported in patients infected with MERS-CoV.29 Other complete blood cell count abnormalities include leukopenia, lymphocytosis, thrombocytopenia, and anemia (Table 3).23,24,26,30 Blood chemistry profiles have identified elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels.29 Some patients have experienced progressive renal failure signaled by rising serum creatinine and blood urea nitrogen levels.11,23,24,26 Testing for disseminated intravascular coagulation and hemolysis has been positive in some patients.20,30 Oxyhemoglobin desaturation develops in patients with severe pneumonia.
Radiographic Imaging
Chest radiographs have been abnormal in the majority of patients with MERS-CoV. The radiographic findings may be minimal to extensive, depending on the severity of illness. The reported radiographic abnormalities include increased bronchovesicular markings, airspace opacities, patchy infiltrates, interstitial changes, confluent consolidations, nodular opacities, reticular infiltrate, pleural effusion, and total opacification of lung segments and lobes. These radiographic findings may be unilateral or bilateral.29
Specific Testing for MERS-CoV
The CDC recommends that lower respiratory tract specimens be collected for testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). The FDA has issued an emergency use authorization of the rRT-PCR assay developed by the CDC. The CDC recommends that multiple specimens from different sites in the lower respiratory system be collected at different times to increase the likelihood of detecting MERS-CoV. Acute and convalescent serum samples also should be obtained for serologic testing. Lower respiratory specimens (sputum, tracheal aspirates, and bronchoalveolar lavage fluid) are more sensitive than are upper respiratory tract samples (nasopharyngeal throat swabs and nasopharyngeal aspirates). Respiratory specimens should be collected as soon as possible after symptom onset. If negative testing is obtained from a patient with a high index of suspicion for MERS-CoV infection, then repeat testing should be performed.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneumonia
Several serology assays have been developed for the detection of MERS-CoV. An immunofluorescence assay should be confirmed with a neutralization test. In certain cases, the diagnosis should be confirmed by nucleic acid sequencing. The CDC has developed MERS-CoV testing kits, which have been provided to state health departments. Any case of suspected or proven MERS-CoV in the U.S. should be reported to the state and local health departments. Visit the CDC website for more information about collecting, handling, and testing clinical specimens from patients under investigation for MERS: http://www.cdc.gov/coronavirus/mers/guidelines -clinical-specimens.html.
Prognosis
Complications from the MERS- CoV infection include severe pneumonia and ARDS requiring mechanical ventilation, multi-organ failure, renal failure requiring dialysis, consumptive coagulopathy, and pericarditis.20,21,23,26,27,29 About 30% of people with MERS-CoV have died. SARS-CoV was the first CoV to cause severe lower respiratory disease and death in otherwise healthy humans; MERS-CoV is now the second.6 Death occurs a median of 14 days after presentation with a range of 5 to 36 days.20,21,23,26,27,29
Treatment
There is no available specific therapy recommended for MERS-CoV infection; therefore, the management of patients is supportive. As with other CoVs, there is no antiviral agent treatment for MERS-CoV. In experimental settings, combination therapy with interferon-alpha-2b and ribavirin seems promising.31 However, critically ill patients with MERS-CoV did not seem to respond favorably when treated with this regimen.32
Vaccine
There is no licensed vaccine for MERS-CoV, although experimental vaccines are being developed. Vaccines have successfully prevented CoV infection in animal models. The development of an effective vaccine for humans against MERS-CoV may, therefore, be a realistic possibility. Unfortunately, a vaccine is likely years away from approval.
Infection Control Measures
Careful attention to infection control precautions is critical to the containment of MERS-CoV. Patients should be encouraged to inform HCPs about symptoms and potential exposure risks, in particular travel to and/or exposure to travelers from the Arabian Peninsula. This practice should help to limit the transmission of MERS-CoV to HCPs. Standard contact and airborne precautions should be followed in patients with suspected or proven MERS-CoV infection.
Infection control measures should include hand hygiene; avoiding close contact with people who are sick; avoiding touching the eyes, nose, and/or mouth with unwashed hands; and disinfecting frequently touched surfaces. Patients with suspected or proven MERS-CoV should be admitted to single occupancy rooms to diminish the possibility of viral transmission to other patients. All persons entering the room of a patient with suspected or proven MERS-CoV should wear fitted N-95 filtering respirators. Until the mode of transmission is better defined, protective eyewear should be worn during all patient contacts. With implementation of these measures, there has been no institution that has experienced an outbreak of MERS-CoV infection. Unfortunately, the duration of viral shedding is not yet known.
Travel Restrictions
At this time the CDC has not recommended MERS-related travel restrictions. Because the spread of MERS-CoV has occurred in health care institutions, the CDC advises HCPs traveling to the Arabian Peninsula to follow recommendations for infection control of confirmed or suspected cases of MERS-CoV and to monitor their own health closely. Travelers who are going to the Arabian Peninsula for other reasons are advised to follow standard infection control precautions, such as hand washing and avoiding contact with ill people. Visit the CDC website for updated information of travel restrictions: http://www.cdc.gov/coronavirus/mers/travel.html.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103-S108.
2. Woo PC, Lau SK, Chu CM, et al. Characteristics and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884-895.
3. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368-373.
4. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790-7792.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update–as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf. Accessed February 3, 2015.
6. Byrd RP Jr, Roy TM. Severe acute respiratory syndrome. Fed Pract. 2003;20(9):62-71.
7. International Society for Infectious Diseases. Novel coronavirus–Saudi Arabia: Human isolate; Archive number: 20120920.1302733. ProMED-mail Website. http://www.promedmail.org/direct .php?id=20120920.1302733. Published September 20, 2012. Accessed January 23, 2015.
8. Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers. Updated July 31, 2014. Accessed January 23, 2015.
9. Centers for Disease Control and Prevention. MERS in the U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers/US.html. Updated December 9, 2014. Accessed January 23, 2015.
10. Lau SK, Li KS, Huang Y, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6): 2808-2819.
11. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
12. Cotten M, Lam TT, Watson SJ, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736-742B.
13. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456-459.
14. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697-1699.
15. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819-1823.
16. Chu DK, Poon LL, Gomaa MM, et al. MERS coronavirus in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049-1053.
17. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis. 2013;13(10):859-866.
18. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis. 2014;14(2):140-145.
19. Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch Virol. 2014;159(7):1575-1584.
20. World Health Organization. Global Alert and Response. MERS-CoV summary and literature update—as of 31 May 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130531 /en. Accessed January 23, 2015.
21. Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416.
22. Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114.
23. Guery B, Poissy J, el Mansouf L, et al; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381(9885):2265-2272.
24. Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487-2494.
25. Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20(4):620-625.
26. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397.
27. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369(9):884-886.
28. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012-1015.
29. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13(9):752-761.
30. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745-751.
31. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313-1317.
32. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis. 2014;20:42-46.
Human coronaviruses (CoVs) were first identified in the mid-1960s. Coronaviruses are a large family of viruses that cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS).1
In 2003, SARS caused one of the most devastating global epidemics known to the developed world. The important lesson learned from the SARS epidemic was that CoVs can cause severe and rapidly spreading infection. Since then, 2 human CoVs, HCoV-HKU1 and HCoV-NL63, have been identified as common causes of human respiratory tract infections.2,3 In September 2012, a novel CoV was recognized to cause a fatal human infection. This virus has become known as the Middle East respiratory syndrome CoV (MERS-CoV).4
Related: Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Similar to SARS-CoV, MERS-CoV human infection has a high fatality rate and the ability to spread from person to person.5,6 Person-to-person transmission has resulted in secondary cases among close contacts, including health care providers (HCPs) who should, therefore, be cognizant of this infection. Federal HCPs in particular may be more likely to become involved in the care of patients with this disease, because many military personnel are returning from deployment in the Middle East.
History of MERS-CoV
MERS-CoV was first identified as an infectious disease in humans in Saudi Arabia. In June 2012, the index case was hospitalized with pneumonia and acute renal injury.7 Since then, MERS-CoV human infections and clusters of infection have been identified in multiple countries in the Arabian Peninsula (Table 1).8 There have also been cases of MERS-CoV infection in other countries involving travelers who had visited the Arabian Peninsula and, in some instances, after returning home, their close contacts (Table 1).8
On May 2, 2014, the first confirmed U.S. case was reported in Indiana in a HCP who had recently been to Saudi Arabia.9 A second case of a HCP traveling from Saudi Arabia was identified on May 13, 2014, in Orlando, Florida.9 As of June 11, 2014, 699 laboratory‐confirmed cases of MERS-CoV infection had been reported to the World Health Organization in 20 countries, resulting in 209 deaths. All cases to date have originated in 6 countries of the Arabian Peninsula.5,8
The Organism
Coronaviruses are enveloped RNA viruses named for the crownlike spikes on their surface. They are common viruses known to cause respiratory infections in humans.1 It is thought that most people are infected with these viruses during their lifetime. These viruses generally cause mild-to-moderate upper respiratory tract illnesses, otherwise known as the common cold. On occasion, CoVs can cause lower respiratory tract infections in elderly patients, neonates, and immune-compromised individuals.1
Related: Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Coronaviruses are also known to infect animals. Most known CoVs cause disease in only 1 animal species or, at most, in a small number of closely related species. However, SARS-CoV was noted to infect people and various animals, including monkeys, civets, raccoon dogs, cats, dogs, and rodents. The origin and natural reservoir of SARS-CoV was ultimately determined to be bats.10
Genetic sequencing has determined that the MERS-CoV is different from any other known human CoV. MERS-CoV is a beta-CoV and, like the SARS-CoV, is closely related to bat CoVs.11-15 The origin of the MERS-CoV is not known, but an animal reservoir is suspected. Because MERS-CoV is similar to SARS-CoV, bats are considered a possible animal reservoir. Dromedary camels may act as intermediate hosts by spreading the virus to humans.16-18 However, there is no consensus on the animal reservoir for MERS-CoV. It is also not known how the virus has spread from animals to humans.
Case Definition
In order to aid in the rapid recognition of MERS, the CDC has established case definitions.8
A patient under investigation is an individual with fever (> 38oC, > 100.4oF) and pneumonia or acute respiratory distress syndrome (ARDS); and either:
- history of travel from countries in or near the Arabian Peninsula within 14 days before the onset of symptoms; or
- close contact with a symptomatic traveler who developed fever and ARDS within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
A confirmed case is a patient with laboratory confirmation of MERS-CoV infection. A probable case is a patient under investigation with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact of a laboratory- confirmed MERS-CoV case.
Transmission
MERS-CoV is thought to be of animal origin, but the mode of transmission from the animal reservoir is not known. It seems likely that some of the infections have occurred via intermittent zoonotic transmission, possibly by an environmental source.19 The presence of case clusters, however, suggests that human-to-human transmission also can occur. Human-to-human transmission has occurred in individuals living with an infected person and in HCPs caring for infected patients.20-24 The human-to-human transmission through close contact so far has been nonsustained.
It has been estimated that 75% of the reported cases are secondary, meaning that the patient acquired the MERS-CoV infection from another infected person. There is no evidence of sustained spread of MERS-CoV in community settings. The mode of human-to-human transmission has not been determined. Possible modes of transmission include droplet and contact transmission. The number of contacts infected by individuals with confirmed infections seems limited; the transmissibility, therefore, currently seems to be low.25,26 The results of a study of the transmissibility and epidemic potential for MERS-CoV suggest that it does not yet have pandemic potential.27
Bats may serve as a reservoir for MERS-CoV. However, because human contact with bats is uncommon, they are viewed as unlikely candidates for an immediate source of infection in most humans. It is possible that another animal or vector serves as an intermediate host. Camels have been proposed as a possible intermediate host, but this remains unproven. Interestingly, the MERS-CoV index patient had been caring for several ill camels in his herd; the camels had signs of respiratory illness, including nasal discharge.11,28 MERS-CoV sequences were subsequently isolated from a juvenile camel belonging to the index patient.
Symptoms
The incubation time after exposure to symptom onset ranges from 1.9 to 14.7 days (Figure). The median incubation period is 5.2 days.21 Patients are not believed to be contagious during the incubation period. Early symptoms of human infection with MERS-CoV include fever, chills or rigors, cough, and shortness of breath. Less frequently encountered symptoms include hemoptysis, sore throat, myalgias, diarrhea, vomiting, and abdominal pain (Table 2).20,21,23,26,27,29 Many patients infected with MERS-CoV develop a severe lower respiratory tract illness. The patient may progress to ARDS and require intubation and mechanical ventilator support. Mechanical ventilation has been required in 72% of patients.29 The median time from presentation for medical care to respiratory failure is 7 days, ranging from 3 to 11 days (Figure).
Physical Examination
The patients with MERS-CoV infection have been predominantly male and middle aged with an average age of 52 years. The clinical features depend on the severity of the illness. Some infected individuals have remained asymptomatic.27 Other patients have experienced mild lower respiratory illness and have not required hospitalization. However, about 40% of patients have experienced severe illness with pneumonia, respiratory insufficiency, multi-organ failure, and death. The percentage of severe illness is likely an overestimation, because patients with less severe symptoms probably are not tested for MERS-CoV. Most of the patients who have experienced a severe illness and/or death also had underlying comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, and chronic renal disease.23,29
Laboratory Data
As with SARS-CoV, lymphopenia has been reported in patients infected with MERS-CoV.29 Other complete blood cell count abnormalities include leukopenia, lymphocytosis, thrombocytopenia, and anemia (Table 3).23,24,26,30 Blood chemistry profiles have identified elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels.29 Some patients have experienced progressive renal failure signaled by rising serum creatinine and blood urea nitrogen levels.11,23,24,26 Testing for disseminated intravascular coagulation and hemolysis has been positive in some patients.20,30 Oxyhemoglobin desaturation develops in patients with severe pneumonia.
Radiographic Imaging
Chest radiographs have been abnormal in the majority of patients with MERS-CoV. The radiographic findings may be minimal to extensive, depending on the severity of illness. The reported radiographic abnormalities include increased bronchovesicular markings, airspace opacities, patchy infiltrates, interstitial changes, confluent consolidations, nodular opacities, reticular infiltrate, pleural effusion, and total opacification of lung segments and lobes. These radiographic findings may be unilateral or bilateral.29
Specific Testing for MERS-CoV
The CDC recommends that lower respiratory tract specimens be collected for testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). The FDA has issued an emergency use authorization of the rRT-PCR assay developed by the CDC. The CDC recommends that multiple specimens from different sites in the lower respiratory system be collected at different times to increase the likelihood of detecting MERS-CoV. Acute and convalescent serum samples also should be obtained for serologic testing. Lower respiratory specimens (sputum, tracheal aspirates, and bronchoalveolar lavage fluid) are more sensitive than are upper respiratory tract samples (nasopharyngeal throat swabs and nasopharyngeal aspirates). Respiratory specimens should be collected as soon as possible after symptom onset. If negative testing is obtained from a patient with a high index of suspicion for MERS-CoV infection, then repeat testing should be performed.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneumonia
Several serology assays have been developed for the detection of MERS-CoV. An immunofluorescence assay should be confirmed with a neutralization test. In certain cases, the diagnosis should be confirmed by nucleic acid sequencing. The CDC has developed MERS-CoV testing kits, which have been provided to state health departments. Any case of suspected or proven MERS-CoV in the U.S. should be reported to the state and local health departments. Visit the CDC website for more information about collecting, handling, and testing clinical specimens from patients under investigation for MERS: http://www.cdc.gov/coronavirus/mers/guidelines -clinical-specimens.html.
Prognosis
Complications from the MERS- CoV infection include severe pneumonia and ARDS requiring mechanical ventilation, multi-organ failure, renal failure requiring dialysis, consumptive coagulopathy, and pericarditis.20,21,23,26,27,29 About 30% of people with MERS-CoV have died. SARS-CoV was the first CoV to cause severe lower respiratory disease and death in otherwise healthy humans; MERS-CoV is now the second.6 Death occurs a median of 14 days after presentation with a range of 5 to 36 days.20,21,23,26,27,29
Treatment
There is no available specific therapy recommended for MERS-CoV infection; therefore, the management of patients is supportive. As with other CoVs, there is no antiviral agent treatment for MERS-CoV. In experimental settings, combination therapy with interferon-alpha-2b and ribavirin seems promising.31 However, critically ill patients with MERS-CoV did not seem to respond favorably when treated with this regimen.32
Vaccine
There is no licensed vaccine for MERS-CoV, although experimental vaccines are being developed. Vaccines have successfully prevented CoV infection in animal models. The development of an effective vaccine for humans against MERS-CoV may, therefore, be a realistic possibility. Unfortunately, a vaccine is likely years away from approval.
Infection Control Measures
Careful attention to infection control precautions is critical to the containment of MERS-CoV. Patients should be encouraged to inform HCPs about symptoms and potential exposure risks, in particular travel to and/or exposure to travelers from the Arabian Peninsula. This practice should help to limit the transmission of MERS-CoV to HCPs. Standard contact and airborne precautions should be followed in patients with suspected or proven MERS-CoV infection.
Infection control measures should include hand hygiene; avoiding close contact with people who are sick; avoiding touching the eyes, nose, and/or mouth with unwashed hands; and disinfecting frequently touched surfaces. Patients with suspected or proven MERS-CoV should be admitted to single occupancy rooms to diminish the possibility of viral transmission to other patients. All persons entering the room of a patient with suspected or proven MERS-CoV should wear fitted N-95 filtering respirators. Until the mode of transmission is better defined, protective eyewear should be worn during all patient contacts. With implementation of these measures, there has been no institution that has experienced an outbreak of MERS-CoV infection. Unfortunately, the duration of viral shedding is not yet known.
Travel Restrictions
At this time the CDC has not recommended MERS-related travel restrictions. Because the spread of MERS-CoV has occurred in health care institutions, the CDC advises HCPs traveling to the Arabian Peninsula to follow recommendations for infection control of confirmed or suspected cases of MERS-CoV and to monitor their own health closely. Travelers who are going to the Arabian Peninsula for other reasons are advised to follow standard infection control precautions, such as hand washing and avoiding contact with ill people. Visit the CDC website for updated information of travel restrictions: http://www.cdc.gov/coronavirus/mers/travel.html.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Human coronaviruses (CoVs) were first identified in the mid-1960s. Coronaviruses are a large family of viruses that cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS).1
In 2003, SARS caused one of the most devastating global epidemics known to the developed world. The important lesson learned from the SARS epidemic was that CoVs can cause severe and rapidly spreading infection. Since then, 2 human CoVs, HCoV-HKU1 and HCoV-NL63, have been identified as common causes of human respiratory tract infections.2,3 In September 2012, a novel CoV was recognized to cause a fatal human infection. This virus has become known as the Middle East respiratory syndrome CoV (MERS-CoV).4
Related: Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Similar to SARS-CoV, MERS-CoV human infection has a high fatality rate and the ability to spread from person to person.5,6 Person-to-person transmission has resulted in secondary cases among close contacts, including health care providers (HCPs) who should, therefore, be cognizant of this infection. Federal HCPs in particular may be more likely to become involved in the care of patients with this disease, because many military personnel are returning from deployment in the Middle East.
History of MERS-CoV
MERS-CoV was first identified as an infectious disease in humans in Saudi Arabia. In June 2012, the index case was hospitalized with pneumonia and acute renal injury.7 Since then, MERS-CoV human infections and clusters of infection have been identified in multiple countries in the Arabian Peninsula (Table 1).8 There have also been cases of MERS-CoV infection in other countries involving travelers who had visited the Arabian Peninsula and, in some instances, after returning home, their close contacts (Table 1).8
On May 2, 2014, the first confirmed U.S. case was reported in Indiana in a HCP who had recently been to Saudi Arabia.9 A second case of a HCP traveling from Saudi Arabia was identified on May 13, 2014, in Orlando, Florida.9 As of June 11, 2014, 699 laboratory‐confirmed cases of MERS-CoV infection had been reported to the World Health Organization in 20 countries, resulting in 209 deaths. All cases to date have originated in 6 countries of the Arabian Peninsula.5,8
The Organism
Coronaviruses are enveloped RNA viruses named for the crownlike spikes on their surface. They are common viruses known to cause respiratory infections in humans.1 It is thought that most people are infected with these viruses during their lifetime. These viruses generally cause mild-to-moderate upper respiratory tract illnesses, otherwise known as the common cold. On occasion, CoVs can cause lower respiratory tract infections in elderly patients, neonates, and immune-compromised individuals.1
Related: Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Coronaviruses are also known to infect animals. Most known CoVs cause disease in only 1 animal species or, at most, in a small number of closely related species. However, SARS-CoV was noted to infect people and various animals, including monkeys, civets, raccoon dogs, cats, dogs, and rodents. The origin and natural reservoir of SARS-CoV was ultimately determined to be bats.10
Genetic sequencing has determined that the MERS-CoV is different from any other known human CoV. MERS-CoV is a beta-CoV and, like the SARS-CoV, is closely related to bat CoVs.11-15 The origin of the MERS-CoV is not known, but an animal reservoir is suspected. Because MERS-CoV is similar to SARS-CoV, bats are considered a possible animal reservoir. Dromedary camels may act as intermediate hosts by spreading the virus to humans.16-18 However, there is no consensus on the animal reservoir for MERS-CoV. It is also not known how the virus has spread from animals to humans.
Case Definition
In order to aid in the rapid recognition of MERS, the CDC has established case definitions.8
A patient under investigation is an individual with fever (> 38oC, > 100.4oF) and pneumonia or acute respiratory distress syndrome (ARDS); and either:
- history of travel from countries in or near the Arabian Peninsula within 14 days before the onset of symptoms; or
- close contact with a symptomatic traveler who developed fever and ARDS within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
A confirmed case is a patient with laboratory confirmation of MERS-CoV infection. A probable case is a patient under investigation with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact of a laboratory- confirmed MERS-CoV case.
Transmission
MERS-CoV is thought to be of animal origin, but the mode of transmission from the animal reservoir is not known. It seems likely that some of the infections have occurred via intermittent zoonotic transmission, possibly by an environmental source.19 The presence of case clusters, however, suggests that human-to-human transmission also can occur. Human-to-human transmission has occurred in individuals living with an infected person and in HCPs caring for infected patients.20-24 The human-to-human transmission through close contact so far has been nonsustained.
It has been estimated that 75% of the reported cases are secondary, meaning that the patient acquired the MERS-CoV infection from another infected person. There is no evidence of sustained spread of MERS-CoV in community settings. The mode of human-to-human transmission has not been determined. Possible modes of transmission include droplet and contact transmission. The number of contacts infected by individuals with confirmed infections seems limited; the transmissibility, therefore, currently seems to be low.25,26 The results of a study of the transmissibility and epidemic potential for MERS-CoV suggest that it does not yet have pandemic potential.27
Bats may serve as a reservoir for MERS-CoV. However, because human contact with bats is uncommon, they are viewed as unlikely candidates for an immediate source of infection in most humans. It is possible that another animal or vector serves as an intermediate host. Camels have been proposed as a possible intermediate host, but this remains unproven. Interestingly, the MERS-CoV index patient had been caring for several ill camels in his herd; the camels had signs of respiratory illness, including nasal discharge.11,28 MERS-CoV sequences were subsequently isolated from a juvenile camel belonging to the index patient.
Symptoms
The incubation time after exposure to symptom onset ranges from 1.9 to 14.7 days (Figure). The median incubation period is 5.2 days.21 Patients are not believed to be contagious during the incubation period. Early symptoms of human infection with MERS-CoV include fever, chills or rigors, cough, and shortness of breath. Less frequently encountered symptoms include hemoptysis, sore throat, myalgias, diarrhea, vomiting, and abdominal pain (Table 2).20,21,23,26,27,29 Many patients infected with MERS-CoV develop a severe lower respiratory tract illness. The patient may progress to ARDS and require intubation and mechanical ventilator support. Mechanical ventilation has been required in 72% of patients.29 The median time from presentation for medical care to respiratory failure is 7 days, ranging from 3 to 11 days (Figure).
Physical Examination
The patients with MERS-CoV infection have been predominantly male and middle aged with an average age of 52 years. The clinical features depend on the severity of the illness. Some infected individuals have remained asymptomatic.27 Other patients have experienced mild lower respiratory illness and have not required hospitalization. However, about 40% of patients have experienced severe illness with pneumonia, respiratory insufficiency, multi-organ failure, and death. The percentage of severe illness is likely an overestimation, because patients with less severe symptoms probably are not tested for MERS-CoV. Most of the patients who have experienced a severe illness and/or death also had underlying comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, and chronic renal disease.23,29
Laboratory Data
As with SARS-CoV, lymphopenia has been reported in patients infected with MERS-CoV.29 Other complete blood cell count abnormalities include leukopenia, lymphocytosis, thrombocytopenia, and anemia (Table 3).23,24,26,30 Blood chemistry profiles have identified elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels.29 Some patients have experienced progressive renal failure signaled by rising serum creatinine and blood urea nitrogen levels.11,23,24,26 Testing for disseminated intravascular coagulation and hemolysis has been positive in some patients.20,30 Oxyhemoglobin desaturation develops in patients with severe pneumonia.
Radiographic Imaging
Chest radiographs have been abnormal in the majority of patients with MERS-CoV. The radiographic findings may be minimal to extensive, depending on the severity of illness. The reported radiographic abnormalities include increased bronchovesicular markings, airspace opacities, patchy infiltrates, interstitial changes, confluent consolidations, nodular opacities, reticular infiltrate, pleural effusion, and total opacification of lung segments and lobes. These radiographic findings may be unilateral or bilateral.29
Specific Testing for MERS-CoV
The CDC recommends that lower respiratory tract specimens be collected for testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). The FDA has issued an emergency use authorization of the rRT-PCR assay developed by the CDC. The CDC recommends that multiple specimens from different sites in the lower respiratory system be collected at different times to increase the likelihood of detecting MERS-CoV. Acute and convalescent serum samples also should be obtained for serologic testing. Lower respiratory specimens (sputum, tracheal aspirates, and bronchoalveolar lavage fluid) are more sensitive than are upper respiratory tract samples (nasopharyngeal throat swabs and nasopharyngeal aspirates). Respiratory specimens should be collected as soon as possible after symptom onset. If negative testing is obtained from a patient with a high index of suspicion for MERS-CoV infection, then repeat testing should be performed.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneumonia
Several serology assays have been developed for the detection of MERS-CoV. An immunofluorescence assay should be confirmed with a neutralization test. In certain cases, the diagnosis should be confirmed by nucleic acid sequencing. The CDC has developed MERS-CoV testing kits, which have been provided to state health departments. Any case of suspected or proven MERS-CoV in the U.S. should be reported to the state and local health departments. Visit the CDC website for more information about collecting, handling, and testing clinical specimens from patients under investigation for MERS: http://www.cdc.gov/coronavirus/mers/guidelines -clinical-specimens.html.
Prognosis
Complications from the MERS- CoV infection include severe pneumonia and ARDS requiring mechanical ventilation, multi-organ failure, renal failure requiring dialysis, consumptive coagulopathy, and pericarditis.20,21,23,26,27,29 About 30% of people with MERS-CoV have died. SARS-CoV was the first CoV to cause severe lower respiratory disease and death in otherwise healthy humans; MERS-CoV is now the second.6 Death occurs a median of 14 days after presentation with a range of 5 to 36 days.20,21,23,26,27,29
Treatment
There is no available specific therapy recommended for MERS-CoV infection; therefore, the management of patients is supportive. As with other CoVs, there is no antiviral agent treatment for MERS-CoV. In experimental settings, combination therapy with interferon-alpha-2b and ribavirin seems promising.31 However, critically ill patients with MERS-CoV did not seem to respond favorably when treated with this regimen.32
Vaccine
There is no licensed vaccine for MERS-CoV, although experimental vaccines are being developed. Vaccines have successfully prevented CoV infection in animal models. The development of an effective vaccine for humans against MERS-CoV may, therefore, be a realistic possibility. Unfortunately, a vaccine is likely years away from approval.
Infection Control Measures
Careful attention to infection control precautions is critical to the containment of MERS-CoV. Patients should be encouraged to inform HCPs about symptoms and potential exposure risks, in particular travel to and/or exposure to travelers from the Arabian Peninsula. This practice should help to limit the transmission of MERS-CoV to HCPs. Standard contact and airborne precautions should be followed in patients with suspected or proven MERS-CoV infection.
Infection control measures should include hand hygiene; avoiding close contact with people who are sick; avoiding touching the eyes, nose, and/or mouth with unwashed hands; and disinfecting frequently touched surfaces. Patients with suspected or proven MERS-CoV should be admitted to single occupancy rooms to diminish the possibility of viral transmission to other patients. All persons entering the room of a patient with suspected or proven MERS-CoV should wear fitted N-95 filtering respirators. Until the mode of transmission is better defined, protective eyewear should be worn during all patient contacts. With implementation of these measures, there has been no institution that has experienced an outbreak of MERS-CoV infection. Unfortunately, the duration of viral shedding is not yet known.
Travel Restrictions
At this time the CDC has not recommended MERS-related travel restrictions. Because the spread of MERS-CoV has occurred in health care institutions, the CDC advises HCPs traveling to the Arabian Peninsula to follow recommendations for infection control of confirmed or suspected cases of MERS-CoV and to monitor their own health closely. Travelers who are going to the Arabian Peninsula for other reasons are advised to follow standard infection control precautions, such as hand washing and avoiding contact with ill people. Visit the CDC website for updated information of travel restrictions: http://www.cdc.gov/coronavirus/mers/travel.html.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103-S108.
2. Woo PC, Lau SK, Chu CM, et al. Characteristics and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884-895.
3. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368-373.
4. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790-7792.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update–as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf. Accessed February 3, 2015.
6. Byrd RP Jr, Roy TM. Severe acute respiratory syndrome. Fed Pract. 2003;20(9):62-71.
7. International Society for Infectious Diseases. Novel coronavirus–Saudi Arabia: Human isolate; Archive number: 20120920.1302733. ProMED-mail Website. http://www.promedmail.org/direct .php?id=20120920.1302733. Published September 20, 2012. Accessed January 23, 2015.
8. Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers. Updated July 31, 2014. Accessed January 23, 2015.
9. Centers for Disease Control and Prevention. MERS in the U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers/US.html. Updated December 9, 2014. Accessed January 23, 2015.
10. Lau SK, Li KS, Huang Y, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6): 2808-2819.
11. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
12. Cotten M, Lam TT, Watson SJ, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736-742B.
13. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456-459.
14. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697-1699.
15. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819-1823.
16. Chu DK, Poon LL, Gomaa MM, et al. MERS coronavirus in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049-1053.
17. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis. 2013;13(10):859-866.
18. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis. 2014;14(2):140-145.
19. Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch Virol. 2014;159(7):1575-1584.
20. World Health Organization. Global Alert and Response. MERS-CoV summary and literature update—as of 31 May 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130531 /en. Accessed January 23, 2015.
21. Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416.
22. Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114.
23. Guery B, Poissy J, el Mansouf L, et al; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381(9885):2265-2272.
24. Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487-2494.
25. Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20(4):620-625.
26. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397.
27. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369(9):884-886.
28. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012-1015.
29. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13(9):752-761.
30. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745-751.
31. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313-1317.
32. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis. 2014;20:42-46.
1. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103-S108.
2. Woo PC, Lau SK, Chu CM, et al. Characteristics and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884-895.
3. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368-373.
4. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790-7792.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update–as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf. Accessed February 3, 2015.
6. Byrd RP Jr, Roy TM. Severe acute respiratory syndrome. Fed Pract. 2003;20(9):62-71.
7. International Society for Infectious Diseases. Novel coronavirus–Saudi Arabia: Human isolate; Archive number: 20120920.1302733. ProMED-mail Website. http://www.promedmail.org/direct .php?id=20120920.1302733. Published September 20, 2012. Accessed January 23, 2015.
8. Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers. Updated July 31, 2014. Accessed January 23, 2015.
9. Centers for Disease Control and Prevention. MERS in the U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers/US.html. Updated December 9, 2014. Accessed January 23, 2015.
10. Lau SK, Li KS, Huang Y, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6): 2808-2819.
11. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
12. Cotten M, Lam TT, Watson SJ, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736-742B.
13. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456-459.
14. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697-1699.
15. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819-1823.
16. Chu DK, Poon LL, Gomaa MM, et al. MERS coronavirus in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049-1053.
17. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis. 2013;13(10):859-866.
18. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis. 2014;14(2):140-145.
19. Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch Virol. 2014;159(7):1575-1584.
20. World Health Organization. Global Alert and Response. MERS-CoV summary and literature update—as of 31 May 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130531 /en. Accessed January 23, 2015.
21. Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416.
22. Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114.
23. Guery B, Poissy J, el Mansouf L, et al; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381(9885):2265-2272.
24. Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487-2494.
25. Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20(4):620-625.
26. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397.
27. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369(9):884-886.
28. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012-1015.
29. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13(9):752-761.
30. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745-751.
31. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313-1317.
32. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis. 2014;20:42-46.
Behavioral Health Problems in Medical Patients
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, [email protected].
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, [email protected].
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, [email protected].
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
Improved Coordination of Care for Patients with Abnormalities on Chest Imaging: The Rapid Access Chest and Lung Assessment Program
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
The art and science of cancer care
Summer is winding down as we go to press with this month’s issue, and while we might well reflect a little sadly on its departure, we can also look forward to the fall season with its promise of renewal and adventure. As I settled back in to my familiar work routine after the Labor Day weekend, I was reminded of how, despite the remarkable clinical advances in oncology, we are still caregivers, involved in our patients’ everyday lives and that we can never forget our humanity. The advent of high-tech personalized medicine or precision oncology, as I prefer to call it, has given oncologists a remarkable cache of treatment options for their patients and the hope that more – and better – therapies are to come. Next-generation diagnostics are helping us identify the cellular targets we need to take aim at to kill the tumor and globally, research is yielding more and more therapeutics to subdue those targets and hence the tumor.
Click on the PDF icon at the top of this introduction to read the full article.
Summer is winding down as we go to press with this month’s issue, and while we might well reflect a little sadly on its departure, we can also look forward to the fall season with its promise of renewal and adventure. As I settled back in to my familiar work routine after the Labor Day weekend, I was reminded of how, despite the remarkable clinical advances in oncology, we are still caregivers, involved in our patients’ everyday lives and that we can never forget our humanity. The advent of high-tech personalized medicine or precision oncology, as I prefer to call it, has given oncologists a remarkable cache of treatment options for their patients and the hope that more – and better – therapies are to come. Next-generation diagnostics are helping us identify the cellular targets we need to take aim at to kill the tumor and globally, research is yielding more and more therapeutics to subdue those targets and hence the tumor.
Click on the PDF icon at the top of this introduction to read the full article.
Summer is winding down as we go to press with this month’s issue, and while we might well reflect a little sadly on its departure, we can also look forward to the fall season with its promise of renewal and adventure. As I settled back in to my familiar work routine after the Labor Day weekend, I was reminded of how, despite the remarkable clinical advances in oncology, we are still caregivers, involved in our patients’ everyday lives and that we can never forget our humanity. The advent of high-tech personalized medicine or precision oncology, as I prefer to call it, has given oncologists a remarkable cache of treatment options for their patients and the hope that more – and better – therapies are to come. Next-generation diagnostics are helping us identify the cellular targets we need to take aim at to kill the tumor and globally, research is yielding more and more therapeutics to subdue those targets and hence the tumor.
Click on the PDF icon at the top of this introduction to read the full article.
On being up to date and linked in
We are mindful of our role in providing our readers with quality research- and literature-based articles about emerging therapies and diagnostic and palliative approaches that will have a positive impact on how they practice. So far this year, we have brought you articles on current therapies for metastatic melanoma and hairy cell leukemia as well as updates on managing chronic myelogenous leukemia, the late effects of cancer therapies, and most recently, small renal tumors...
Click on the PDF icon at the top of this introduction to read the full article.
We are mindful of our role in providing our readers with quality research- and literature-based articles about emerging therapies and diagnostic and palliative approaches that will have a positive impact on how they practice. So far this year, we have brought you articles on current therapies for metastatic melanoma and hairy cell leukemia as well as updates on managing chronic myelogenous leukemia, the late effects of cancer therapies, and most recently, small renal tumors...
Click on the PDF icon at the top of this introduction to read the full article.
We are mindful of our role in providing our readers with quality research- and literature-based articles about emerging therapies and diagnostic and palliative approaches that will have a positive impact on how they practice. So far this year, we have brought you articles on current therapies for metastatic melanoma and hairy cell leukemia as well as updates on managing chronic myelogenous leukemia, the late effects of cancer therapies, and most recently, small renal tumors...
Click on the PDF icon at the top of this introduction to read the full article.
Adolescent Obesity and Its Risks: How to Screen and When to Refer
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
Binge eating disorder
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel