User login
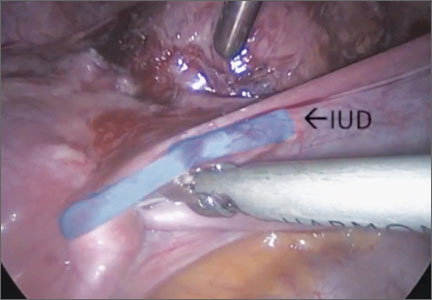
Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
This video is brought to you by ![]()
Diabetes management in cancer patients
In an interview with Dr David Henry, Editor-in-Chief of The Journal of Community and Supportive Oncology, Dr Todd Brown reviews the several different classes of the hyperglycemic management drugs (apart from insulin) focusing on how they work and how they should be used in the management of diabetes in patients with cancer. Dr Henry is vice-chair of the Department of Medicine and Clinical Professor of Medicine at Pennsylvania Hospital, in Philadelphia, and Dr Brown is associate professor of Medicine and Epidemiology in the Division of Endocrinology, Diabetes, and Metabolism at the Johns Hopkins University in Baltimore, Maryland.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In an interview with Dr David Henry, Editor-in-Chief of The Journal of Community and Supportive Oncology, Dr Todd Brown reviews the several different classes of the hyperglycemic management drugs (apart from insulin) focusing on how they work and how they should be used in the management of diabetes in patients with cancer. Dr Henry is vice-chair of the Department of Medicine and Clinical Professor of Medicine at Pennsylvania Hospital, in Philadelphia, and Dr Brown is associate professor of Medicine and Epidemiology in the Division of Endocrinology, Diabetes, and Metabolism at the Johns Hopkins University in Baltimore, Maryland.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In an interview with Dr David Henry, Editor-in-Chief of The Journal of Community and Supportive Oncology, Dr Todd Brown reviews the several different classes of the hyperglycemic management drugs (apart from insulin) focusing on how they work and how they should be used in the management of diabetes in patients with cancer. Dr Henry is vice-chair of the Department of Medicine and Clinical Professor of Medicine at Pennsylvania Hospital, in Philadelphia, and Dr Brown is associate professor of Medicine and Epidemiology in the Division of Endocrinology, Diabetes, and Metabolism at the Johns Hopkins University in Baltimore, Maryland.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
Alectinib provides a new option for ALK-positive NSCLC patients after progression on crizotinib
In December 2015, alectinib became the third ALK inhibitor approved by the United States Food and Drug Administration for the treatment of non-small-cell lung cancer (NSCLC) that displays rearrangements of the anaplastic lymphoma kinase (ALK) gene. Alectinib is a second-generation small molecule inhibitor of the ALK protein that joins ceritinib in providing a useful treatment option for patients who have progressed on crizotinib, as a result of its ability to target crizotinib-resistant mutant forms of the ALK protein. Alectinib also displays enhanced penetrance of the blood-brain barrier, which improves efficacy against central nervous system (CNS) metastases.
Click on the PDF icon at the top of this introduction to read the full article.
In December 2015, alectinib became the third ALK inhibitor approved by the United States Food and Drug Administration for the treatment of non-small-cell lung cancer (NSCLC) that displays rearrangements of the anaplastic lymphoma kinase (ALK) gene. Alectinib is a second-generation small molecule inhibitor of the ALK protein that joins ceritinib in providing a useful treatment option for patients who have progressed on crizotinib, as a result of its ability to target crizotinib-resistant mutant forms of the ALK protein. Alectinib also displays enhanced penetrance of the blood-brain barrier, which improves efficacy against central nervous system (CNS) metastases.
Click on the PDF icon at the top of this introduction to read the full article.
In December 2015, alectinib became the third ALK inhibitor approved by the United States Food and Drug Administration for the treatment of non-small-cell lung cancer (NSCLC) that displays rearrangements of the anaplastic lymphoma kinase (ALK) gene. Alectinib is a second-generation small molecule inhibitor of the ALK protein that joins ceritinib in providing a useful treatment option for patients who have progressed on crizotinib, as a result of its ability to target crizotinib-resistant mutant forms of the ALK protein. Alectinib also displays enhanced penetrance of the blood-brain barrier, which improves efficacy against central nervous system (CNS) metastases.
Click on the PDF icon at the top of this introduction to read the full article.
Onychomadesis Following Hand-foot-and-mouth Disease
To the Editor:
Onychomadesis is characterized by separation of the nail plate from the matrix due to a temporary arrest in nail matrix activity. Hand-foot-and-mouth disease (HFMD) is a relatively common viral infection, especially in children. Although the relationship between onychomadesis and HFMD has been noted, there are few reports in the literature.1-9 We present 2 cases of onychomadesis following HFMD in Taiwanese siblings.
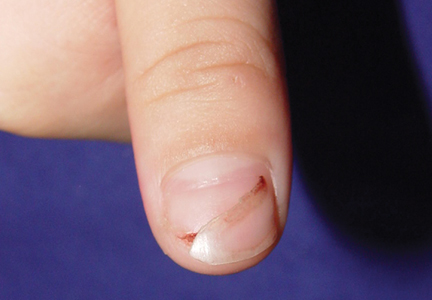
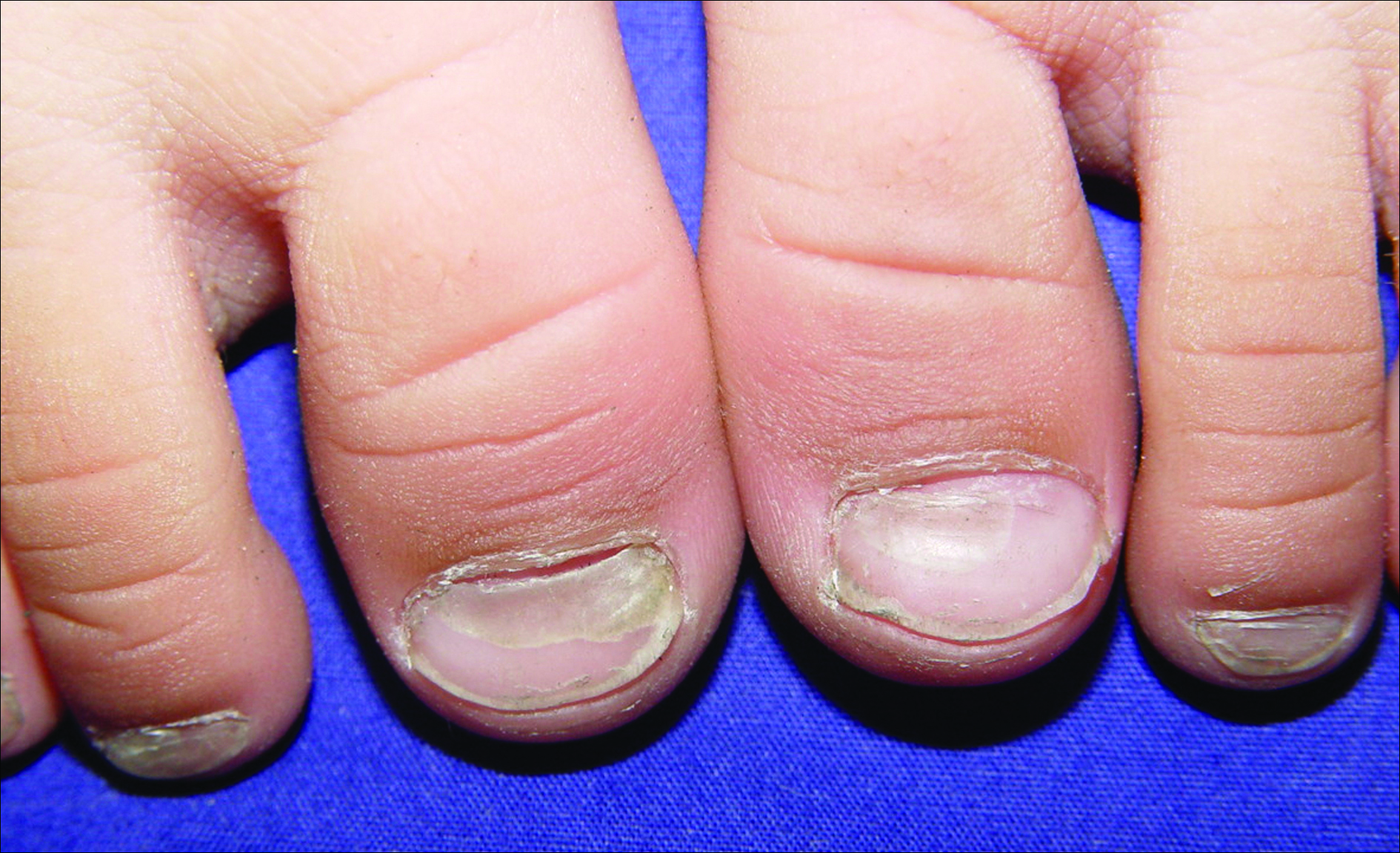
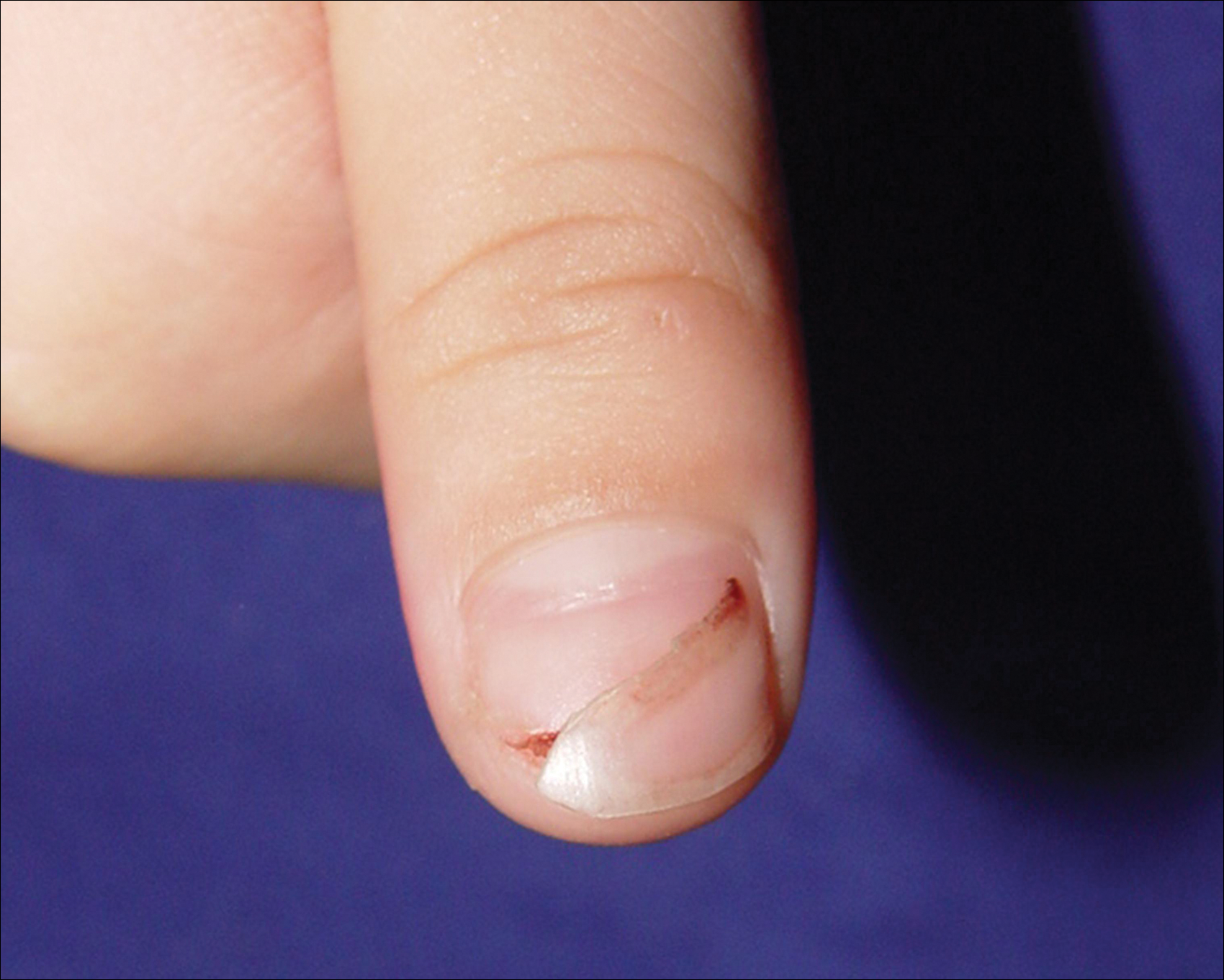
A 3-year-old girl presented with proximal nail plate detachment from the proximal nail fold on the bilateral great toenails (Figure 1) and a transverse whole-thickness sulcus on the bilateral thumbnails (Figure 2) of several weeks’ duration. Her 6-year-old sister had similar nail changes. Hand-foot-and-mouth disease was diagnosed about 4 weeks prior to nail changes. The mother reported that only the younger sister experienced fever. There was no history of notable medication intake, nail trauma, periungual erythema, vesicular lesion, or dermatitis. In both patients, the nail changes were temporary with spontaneous normal nail plate regrowth several months later. A diagnosis of onychomadesis was made.
The etiology of onychomadesis includes drug ingestion, especially chemotherapy; severe systemic diseases; high fever; infection, including viral illnesses such as influenza, measles, and HFMD; and idiopathic onychomadesis.1,2,5,10 In 2000, Clementz and Mancini1 reported 5 children with nail matrix arrest following HFMD and suggested an epidemic caused by the same virus strain. Bernier et al2 reported another 4 cases and suggested more than one viral strain may have been implicated in the nail matrix arrest. Although these authors list HFMD as one of the causes of onychomadesis,1,2 the number of cases reported was small; however, studies with a larger number of cases and even outbreak have been reported more recently.3-8 Salazar et al3 reported an onychomadesis outbreak associated with HFMD in Valencia, Spain, in 2008 (N=298). This outbreak primarily was caused by coxsackievirus (CV) A10 (49% of cases).5 Another onychomadesis outbreak occurred in Saragossa, Spain, in 2008, and CV B1, B2, and unidentified nonpoliovirus enterovirus were isolated.6 Outbreaks also occurred in Finland in 2008, and the causative agents were identified as CV A6 and A10.7,8 The latency period for onychomadesis following HFMD was 1 to 2 months (mean, 40 days), and the majority of cases occurred in patients younger than 6 years.1-5 Not all of the nails were involved; in one report, each patient shed only 4 nails on average.6
Although there is a definite relationship between HFMD and onychomadesis, the mechanism is still unclear. Some authors claim that nail matrix arrest is caused by high fever10; however, we found that 40% (2/5)1 to 63% (10/16)4 of reported cases did not have a fever. Additionally, only 1 of our patients had fever. Therefore high fever–induced nail matrix arrest is not a reasonable explanation. Davia et al5 observed no relationship between onychomadesis and the severity of HFMD. In addition, no serious complications of HFMD were mentioned in prior reports.
We propose that HFMD-related onychomadesis is caused by the viral infection itself, rather than by severe systemic disease.1-5,7 Certain viral strains associated with HFMD can induce arrest of nail matrix activity. Osterback et al7 detected CV A6 in shed nail fragments and suggested that virus replication damaged the nail matrix and resulted in temporary nail dystrophy. This hypothesis can explain that only some nails, not all, were involved. In our cases, we noted an incomplete and slanted cleft on the thumbnail (Figure 2). We also found that incomplete onychomadesis appeared in the clinical photograph from a prior report.5 The slanted cleft in our case may be caused by secondary external force after original incomplete onychomadesis or a different rate of nail regrowth because of different intensity of nail matrix damage. The phenomenon of incomplete onychomadesis in the same nail further suggests the mechanism of onychomadesis following HFMD is localized nail matrix damage.
In conclusion, we report 2 cases of onychomadesis associated with HFMD. Our report highlights that there is no racial difference in post-HFMD onychomadesis. These cases highlight that HFMD is an important cause of onychomadesis, especially in children. We suggest that certain viral strains associated with HFMD may specifically arrest nail matrix growth activity, regardless of fever or disease severity.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Bernier V, Labreze C, Bury F, et al. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-651.
- Salazar A, Febrer I, Guiral S, et al. Onychomadesis outbreak in Valencia, Spain, June 2008. Euro Surveill. 2008;13:18917.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Post viral onychomadesis outbreak in Valladolid [in Spanish]. An Pediatr (Barc). 2009;71:436-439.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:947-973.
To the Editor:
Onychomadesis is characterized by separation of the nail plate from the matrix due to a temporary arrest in nail matrix activity. Hand-foot-and-mouth disease (HFMD) is a relatively common viral infection, especially in children. Although the relationship between onychomadesis and HFMD has been noted, there are few reports in the literature.1-9 We present 2 cases of onychomadesis following HFMD in Taiwanese siblings.
A 3-year-old girl presented with proximal nail plate detachment from the proximal nail fold on the bilateral great toenails (Figure 1) and a transverse whole-thickness sulcus on the bilateral thumbnails (Figure 2) of several weeks’ duration. Her 6-year-old sister had similar nail changes. Hand-foot-and-mouth disease was diagnosed about 4 weeks prior to nail changes. The mother reported that only the younger sister experienced fever. There was no history of notable medication intake, nail trauma, periungual erythema, vesicular lesion, or dermatitis. In both patients, the nail changes were temporary with spontaneous normal nail plate regrowth several months later. A diagnosis of onychomadesis was made.


The etiology of onychomadesis includes drug ingestion, especially chemotherapy; severe systemic diseases; high fever; infection, including viral illnesses such as influenza, measles, and HFMD; and idiopathic onychomadesis.1,2,5,10 In 2000, Clementz and Mancini1 reported 5 children with nail matrix arrest following HFMD and suggested an epidemic caused by the same virus strain. Bernier et al2 reported another 4 cases and suggested more than one viral strain may have been implicated in the nail matrix arrest. Although these authors list HFMD as one of the causes of onychomadesis,1,2 the number of cases reported was small; however, studies with a larger number of cases and even outbreak have been reported more recently.3-8 Salazar et al3 reported an onychomadesis outbreak associated with HFMD in Valencia, Spain, in 2008 (N=298). This outbreak primarily was caused by coxsackievirus (CV) A10 (49% of cases).5 Another onychomadesis outbreak occurred in Saragossa, Spain, in 2008, and CV B1, B2, and unidentified nonpoliovirus enterovirus were isolated.6 Outbreaks also occurred in Finland in 2008, and the causative agents were identified as CV A6 and A10.7,8 The latency period for onychomadesis following HFMD was 1 to 2 months (mean, 40 days), and the majority of cases occurred in patients younger than 6 years.1-5 Not all of the nails were involved; in one report, each patient shed only 4 nails on average.6
Although there is a definite relationship between HFMD and onychomadesis, the mechanism is still unclear. Some authors claim that nail matrix arrest is caused by high fever10; however, we found that 40% (2/5)1 to 63% (10/16)4 of reported cases did not have a fever. Additionally, only 1 of our patients had fever. Therefore high fever–induced nail matrix arrest is not a reasonable explanation. Davia et al5 observed no relationship between onychomadesis and the severity of HFMD. In addition, no serious complications of HFMD were mentioned in prior reports.
We propose that HFMD-related onychomadesis is caused by the viral infection itself, rather than by severe systemic disease.1-5,7 Certain viral strains associated with HFMD can induce arrest of nail matrix activity. Osterback et al7 detected CV A6 in shed nail fragments and suggested that virus replication damaged the nail matrix and resulted in temporary nail dystrophy. This hypothesis can explain that only some nails, not all, were involved. In our cases, we noted an incomplete and slanted cleft on the thumbnail (Figure 2). We also found that incomplete onychomadesis appeared in the clinical photograph from a prior report.5 The slanted cleft in our case may be caused by secondary external force after original incomplete onychomadesis or a different rate of nail regrowth because of different intensity of nail matrix damage. The phenomenon of incomplete onychomadesis in the same nail further suggests the mechanism of onychomadesis following HFMD is localized nail matrix damage.
In conclusion, we report 2 cases of onychomadesis associated with HFMD. Our report highlights that there is no racial difference in post-HFMD onychomadesis. These cases highlight that HFMD is an important cause of onychomadesis, especially in children. We suggest that certain viral strains associated with HFMD may specifically arrest nail matrix growth activity, regardless of fever or disease severity.
To the Editor:
Onychomadesis is characterized by separation of the nail plate from the matrix due to a temporary arrest in nail matrix activity. Hand-foot-and-mouth disease (HFMD) is a relatively common viral infection, especially in children. Although the relationship between onychomadesis and HFMD has been noted, there are few reports in the literature.1-9 We present 2 cases of onychomadesis following HFMD in Taiwanese siblings.
A 3-year-old girl presented with proximal nail plate detachment from the proximal nail fold on the bilateral great toenails (Figure 1) and a transverse whole-thickness sulcus on the bilateral thumbnails (Figure 2) of several weeks’ duration. Her 6-year-old sister had similar nail changes. Hand-foot-and-mouth disease was diagnosed about 4 weeks prior to nail changes. The mother reported that only the younger sister experienced fever. There was no history of notable medication intake, nail trauma, periungual erythema, vesicular lesion, or dermatitis. In both patients, the nail changes were temporary with spontaneous normal nail plate regrowth several months later. A diagnosis of onychomadesis was made.


The etiology of onychomadesis includes drug ingestion, especially chemotherapy; severe systemic diseases; high fever; infection, including viral illnesses such as influenza, measles, and HFMD; and idiopathic onychomadesis.1,2,5,10 In 2000, Clementz and Mancini1 reported 5 children with nail matrix arrest following HFMD and suggested an epidemic caused by the same virus strain. Bernier et al2 reported another 4 cases and suggested more than one viral strain may have been implicated in the nail matrix arrest. Although these authors list HFMD as one of the causes of onychomadesis,1,2 the number of cases reported was small; however, studies with a larger number of cases and even outbreak have been reported more recently.3-8 Salazar et al3 reported an onychomadesis outbreak associated with HFMD in Valencia, Spain, in 2008 (N=298). This outbreak primarily was caused by coxsackievirus (CV) A10 (49% of cases).5 Another onychomadesis outbreak occurred in Saragossa, Spain, in 2008, and CV B1, B2, and unidentified nonpoliovirus enterovirus were isolated.6 Outbreaks also occurred in Finland in 2008, and the causative agents were identified as CV A6 and A10.7,8 The latency period for onychomadesis following HFMD was 1 to 2 months (mean, 40 days), and the majority of cases occurred in patients younger than 6 years.1-5 Not all of the nails were involved; in one report, each patient shed only 4 nails on average.6
Although there is a definite relationship between HFMD and onychomadesis, the mechanism is still unclear. Some authors claim that nail matrix arrest is caused by high fever10; however, we found that 40% (2/5)1 to 63% (10/16)4 of reported cases did not have a fever. Additionally, only 1 of our patients had fever. Therefore high fever–induced nail matrix arrest is not a reasonable explanation. Davia et al5 observed no relationship between onychomadesis and the severity of HFMD. In addition, no serious complications of HFMD were mentioned in prior reports.
We propose that HFMD-related onychomadesis is caused by the viral infection itself, rather than by severe systemic disease.1-5,7 Certain viral strains associated with HFMD can induce arrest of nail matrix activity. Osterback et al7 detected CV A6 in shed nail fragments and suggested that virus replication damaged the nail matrix and resulted in temporary nail dystrophy. This hypothesis can explain that only some nails, not all, were involved. In our cases, we noted an incomplete and slanted cleft on the thumbnail (Figure 2). We also found that incomplete onychomadesis appeared in the clinical photograph from a prior report.5 The slanted cleft in our case may be caused by secondary external force after original incomplete onychomadesis or a different rate of nail regrowth because of different intensity of nail matrix damage. The phenomenon of incomplete onychomadesis in the same nail further suggests the mechanism of onychomadesis following HFMD is localized nail matrix damage.
In conclusion, we report 2 cases of onychomadesis associated with HFMD. Our report highlights that there is no racial difference in post-HFMD onychomadesis. These cases highlight that HFMD is an important cause of onychomadesis, especially in children. We suggest that certain viral strains associated with HFMD may specifically arrest nail matrix growth activity, regardless of fever or disease severity.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Bernier V, Labreze C, Bury F, et al. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-651.
- Salazar A, Febrer I, Guiral S, et al. Onychomadesis outbreak in Valencia, Spain, June 2008. Euro Surveill. 2008;13:18917.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Post viral onychomadesis outbreak in Valladolid [in Spanish]. An Pediatr (Barc). 2009;71:436-439.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:947-973.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Bernier V, Labreze C, Bury F, et al. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-651.
- Salazar A, Febrer I, Guiral S, et al. Onychomadesis outbreak in Valencia, Spain, June 2008. Euro Surveill. 2008;13:18917.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Post viral onychomadesis outbreak in Valladolid [in Spanish]. An Pediatr (Barc). 2009;71:436-439.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2010:947-973.
Practice Points
- Onychomadesis is a late complication of hand-foot-and-mouth disease (HFMD) with a latency period of 1 to 2 months.
- Although the mechanism between onychomadesis and HFMD is still unclear, we propose that it is caused by the viral infection itself rather than severe systemic disease.
Attitudes Surrounding Continuous Telemetry Utilization by Providers at an Academic Tertiary Medical Center
From the Johns Hopkins Bayview Medical Center, Baltimore, MD (Drs. Johnson, Knight, Maygers, and Zakaria), and Duke University Hospital, Durham, NC (Dr. Mock).
Abstract
- Objective: To determine patterns of telemetry use at a tertiary academic institution and identify factors contributing to noncompliance with guidelines regarding telemetry use.
- Methods: Web-based survey of 180 providers, including internal medicine residents and cardiovascular disease fellows, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners, and physician assistants.
- Results: Of the 180 providers surveyed, 67 (37%) replied. Most providers (76%) were unaware of guidelines regarding appropriate telemetry use and 85% selected inappropriate diagnoses as warranting telemetry. Only 21% routinely discontinued the telemetry order within 48 hours.
- Conclusions: Many providers at a tertiary academic institution utilize continuous telemetry inappropriately and are unaware of telemetry guidelines. These findings should guide interventions to improve telemetry utilization.
For many decades, telemetry has been widely used in the management and monitoring of patients with possible acute coronary syndromes (ACS), arrhythmias, cardiac events, and strokes [1]. In addition, telemetry has often been used in other clinical scenarios with less rigorous data supporting its use [2–4]. As a result, in 2004 the American Heart Association (AHA) issued guidelines providing recommendations for best practices in hospital ECG monitoring. Indications for telemetry were classified into 3 diagnosis-driven groups: class I (indicated in all patients), class II (indicated in most patients, may be of benefit) and class III (not indicated, no therapeutic benefit) [2]. However, these recommendations have not been widely followed and telemetry is inappropriately used for many inpatients [5,6].
There are several reasons why clinicians fail to adhere to guidelines, including knowledge deficits, attitudes regarding the current guidelines, and institution-specific factors influencing practitioner behaviors [7]. In response to reports of widespread telemetry overuse, the Choosing Wisely Campaign of the American Board of Internal Medicine Foundation has championed judicious telemetry use, advocating evidence-based, protocol-driven telemetry management for patients not in intensive care units who do not meet guideline-based criteria for continuous telemetry [8].
In order to understand patterns of telemetry use at our academic institution and identify factors associated with this practice, we systematically analyzed telemetry use perceptions through provider surveys. We hypothesized that providers have misperceptions about appropriate use of telemetry and that this knowledge gap results in overuse of telemetry at our institution.
Methods
Setting
Johns Hopkins Bayview Medical Center is a 400-bed academic medical center serving southeastern Baltimore. Providers included internal medicine residents and cardiovascular disease fellows who rotate to the medical center and Johns Hopkins Hospital, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners (NPs), and physician assistants (PAs).
Current Telemetry Practice
Remote telemetric monitoring is available in all adult, non-intensive care units of the hospital except for the psychiatry unit. However, the number of monitors are limited and it is not possible to monitor every patient if the wards are at capacity. Obstetrics uses its own unique cardiac monitoring system and thus was not included in the survey. Each monitor (IntelliVue, Philips Healthcare, Amsterdam, Netherlands) is attached to the patient using 5 lead wires, with electrocardiographic data transmitted to a monitoring station based in the progressive care unit, a cardio-pulmonary step-down unit. Monitors can be ordered in one of 3 manners, as mandated by hospital policy:
- Continuous telemetry – Telemetry monitoring is uninterrupted until discontinued by a provider.
- Telemetry protocol – Within 12 hours of telemetry placement, a monitor technician generates a report, which is reviewed by the nurse caring for the patient. The nurse performs an electrocardiogram (ECG) if the patient meets pre-specified criteria for telemetry discontinuation, which includes the absence of arrhythmias, troponin elevations, chest pain, or hemodynamic instability. The repeat ECG is then read and signed by the provider. After these criteria are met, telemetry can be discontinued.
- Stroke telemetry protocol – Telemetry is applied for 48 hours, mainly for detection of paroxysmal atrial fibrillation. Monitoring can be temporarily discontinued if the patient requires magnetic resonance imaging, which interferes with the telemetric monitors.
When entering any of the 3 possible telemetry orders in our computerized provider order entry system (Meditech, Westwood, MA), the ordering provider is required to indicate baseline rhythm, pacemaker presence, and desired heart rate warning parameters. Once the order is electronically signed, a monitor technician notes the order in a logbook and assigns the patient a telemeter, which is applied by the patient’s nurse.
If a monitored patient develops any predefined abnormal rhythm, audible alerts notify monitor technicians and an alert is sent to a portable telephone carried by the patient’s assigned nurse. Either the monitoring technician or the nurse then has the discretion to silence the alarm, note it in the chart, and/or contact the patient’s provider. If alerts are recorded, then a sample telemetry monitoring strip is saved into the patient’s paper medical chart.
Survey Instrument
After approval from the Johns Hopkins institutional review board, we queried providers who worked on the medicine and cardiology wards to assess the context and culture in which telemetry monitoring is used (see Appendix). The study was exempt from requiring informed consent. All staff had the option to decline study participation. We administered the survey using an online survey software program (SurveyMonkey, Palo Alto, CA), sending survey links via email to all internal medicine residents, cardiovascular disease fellows, internal medicine and cardiology teaching attending physicians, hospitalists, NPs, and PAs. Respondents completed the survey anonymously. To increase response rates, providers were sent a monthly reminder email. The survey was open from March 2014 to May 2014 for a total of 3 months.
Analysis
The survey data were compiled and analyzed using Microsoft Excel (Mac version 14.4; Microsoft, Redmond, WA). Variables are displayed as numbers and percentages, as appropriate.
Results
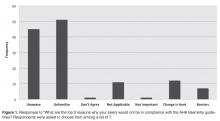
All providers reported having ordered telemetry, but almost all were either unaware of (76%) or only somewhat familiar with (21%) the AHA guidelines for appropriate telemetry use. Notably, the vast majority of fellows and residents reported that they were not at all familiar with the guidelines (100% and 96%, respectively). When asked why providers do not adhere to telemetry guidelines, lack of awareness of and lack of familiarity with the guidelines were the top 2 choices among respondents (Figure 1).
Additionally, most providers acknowledged experiencing adverse effects of telemetry: 86% (57/66) had experienced delayed patient transfers from the emergency department to inpatient floors due to telemetry unavailability and 97% (65/67) had experienced some delay in obtaining tests or studies for their telemetry-monitored patients. Despite acknowledging the potential consequences of telemetry use, only 21% (14/66) of providers routinely (ie, > 75% of the time) discontinued telemetry within 48 hours. Fifteen percent (10/65) routinely allowed telemetry to continue until the time of patient discharge. When discontinued, it was mainly due to the provider’s decision (57%); however, respondents noted that nurses prompted telemetry discontinuation 28% of the time.
Discussion
Consistent with previous studies [3–5,9–15], the majority of providers at our institution do not think continuous telemetry is appropriately utilized. Most survey respondents acknowledged a lack of awareness surrounding current guideline recommendations, which could explain why providers often do not follow them. Despite conceding their knowledge deficits, providers assumed their practice patterns for ordering telemetry were “appropriate”(ie, guideline-supported). This assertion may be incorrect as the majority of providers in our survey chose at least 1 non–guideline-supported indication for telemetry. Other studies have suggested additional reasons for inappropriate telemetry utilization. Providers may disagree with guideline recommendations, may assign lesser importance to guidelines when caring for an individual patient, or may fall victim to inertia (ie, not ordering telemetry appropriately simply because changing one’s practice pattern is difficult) [7].
In addition, the majority of our providers perceived telemetry overuse, which has been well-recognized nationwide [4]. While we did not assess this directly, other studies suggest that providers may overuse telemetry to provide a sense of reassurance when caring for a sick patient, since continuous telemetry is perceived to provide a higher level of care [6,15–17]. Unfortunately, no study has shown a benefit for continuous telemetry when placed for non-guideline-based diagnoses—whether for cardiac or non-cardiac diagnoses [3,9–11,13,14]. Likewise, the guidelines suggest that telemetry use should be time-limited, since the majority of benefit is accrued in the first 48 hours. Beyond that time, no study has shown a clear benefit to continuous telemetry [2]. Therefore, telemetry overuse may lead to unnecessarily increased costs without added benefits [3,9–11,13–15,18].
Our conclusions are tempered by the nature of our survey data. We recognize that our survey has not been previously validated. In addition, our response rates were low. This low sample size may lead to under-representation of diverse ideas. Also, our survey results may not be generalizable, since our study was conducted at a single academic hospital. Our institution’s telemetry ordering culture may differ from others, therefore making our results less applicable to other centers.
Despite these limitations, our results aid in understanding attitudes that surround the use of continuous telemetry, which can shape formal educational interventions to encourage appropriate guideline-based telemetry use. Since our providers agree on the need for more education about the guidelines, components such as online modules or in-person lecture educational sessions, newsletters, email communications, and incorporation of AHA guidelines into the institution’s automated computer order entry system could be utilized [17]. Didactic interventions could be designed especially for trainees given their overall lack of familiarity with the guidelines. Another potential intervention could include supplying providers with publically shared personalized measures of their own practices, since providers benefit from reinforcement and individualized feedback on appropriate utilization practices [19]. Previous studies have suggested that a multidisciplinary approach to patient care leads to positive outcomes [20,21], and in our experience, nursing input is absolutely critical in outlining potential problems and in developing solutions. Our findings suggest that nurses could play an active role in alerting providers when patients have telemetry in use and identifying patients who may no longer need it.
In summary, we have shown that many providers at a tertiary academic institution utilized continuous telemetry inappropriately, and were unaware of guidelines surrounding telemetry use. Future interventions aimed at educating providers, encouraging dialogue between staff, and enabling guideline-supported utilization may increase appropriate telemetry use leading to lower cost and improved quality of patient care.
Acknowledgment: The authors wish to thank Dr. Colleen Christmas, Dr. Panagis Galiatsatos, Mrs. Barbara Brigade, Ms. Joetta Love, Ms. Terri Rigsby, and Mrs. Lisa Shirk for their invaluable technical and administrative support.
Corresponding author: Amber Johnson, MD, MBA, 200 Lothrop St., S-553 Scaife Hall, Pittsburgh, PA 15213, [email protected].
Financial disclosures: None.
1. Day H. Preliminary studies of an acute coronary care area. J Lancet 1963;83:53–5.
2. Drew B, Califf R, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110:2721–46.
3. Estrada C, Battilana G, Alexander M, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med 2000;15:51–5.
4. Henriques-Forsythe M, Ivonye C, Jamched U, et al. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med 2009;76:368–72.
5. Chen E, Hollander, J. When do patients need admission to a telemetry bed? J Emerg Med 2007;33:53–60.
6. Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012;172:1349–50.
7. Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines?: A framework for improvement. JAMA 1999;282:1458–65.
8. Adult hospital medicine. Five things physicians and patients should question. 15 Aug 2013. Available at www.choosingwisely.org/doctor-patient-lists/society-of-hospital-medicine-adult-hospital-medicine/
9. Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: A prospective cohort study of risk stratification and outcomes. Am J Med 2001;110:7–11.
10. Estrada C, Rosman H, Prasad N, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995;76:960–5.
11. Hollander J, Sites F, Pollack C, Shofer F. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71–6.
12. Ivonye C, Ohuabunwo C, Henriques-Forsythe M, et al. Evaluation of telemetry utilization, policy, and outcomes in an inner-city academic medical center. J Natl Med Assoc 2010;102:598–604.
13. Schull M, Redelmeier D. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000;7:647–52.
14. Sivaram C, Summers J, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998;21:503–5.
15. Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002;122:517–23.
16. Chen S, Zakaria S. Behind the monitor-The trouble with telemetry: a teachable moment. JAMA Intern Med 2015;175:894.
17. Dressler R, Dryer M, Coletti C, et al. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014;174:1852–4.
18. Benjamin E, Klugman R, Luckmann R, et al. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care 2013;19:e225–32.
19. Solomon D, Hashimoto H, Daltroy L, Liang M. Techniques to improve physicians use of diagnostic tests: A new conceptual framework. JAMA 1998;280:2020–7.
20. Richeson J, Johnson J. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung 1992;21:18–24.
21. Curley C, McEachern J, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care 1998;36:AS4–12.
From the Johns Hopkins Bayview Medical Center, Baltimore, MD (Drs. Johnson, Knight, Maygers, and Zakaria), and Duke University Hospital, Durham, NC (Dr. Mock).
Abstract
- Objective: To determine patterns of telemetry use at a tertiary academic institution and identify factors contributing to noncompliance with guidelines regarding telemetry use.
- Methods: Web-based survey of 180 providers, including internal medicine residents and cardiovascular disease fellows, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners, and physician assistants.
- Results: Of the 180 providers surveyed, 67 (37%) replied. Most providers (76%) were unaware of guidelines regarding appropriate telemetry use and 85% selected inappropriate diagnoses as warranting telemetry. Only 21% routinely discontinued the telemetry order within 48 hours.
- Conclusions: Many providers at a tertiary academic institution utilize continuous telemetry inappropriately and are unaware of telemetry guidelines. These findings should guide interventions to improve telemetry utilization.
For many decades, telemetry has been widely used in the management and monitoring of patients with possible acute coronary syndromes (ACS), arrhythmias, cardiac events, and strokes [1]. In addition, telemetry has often been used in other clinical scenarios with less rigorous data supporting its use [2–4]. As a result, in 2004 the American Heart Association (AHA) issued guidelines providing recommendations for best practices in hospital ECG monitoring. Indications for telemetry were classified into 3 diagnosis-driven groups: class I (indicated in all patients), class II (indicated in most patients, may be of benefit) and class III (not indicated, no therapeutic benefit) [2]. However, these recommendations have not been widely followed and telemetry is inappropriately used for many inpatients [5,6].
There are several reasons why clinicians fail to adhere to guidelines, including knowledge deficits, attitudes regarding the current guidelines, and institution-specific factors influencing practitioner behaviors [7]. In response to reports of widespread telemetry overuse, the Choosing Wisely Campaign of the American Board of Internal Medicine Foundation has championed judicious telemetry use, advocating evidence-based, protocol-driven telemetry management for patients not in intensive care units who do not meet guideline-based criteria for continuous telemetry [8].
In order to understand patterns of telemetry use at our academic institution and identify factors associated with this practice, we systematically analyzed telemetry use perceptions through provider surveys. We hypothesized that providers have misperceptions about appropriate use of telemetry and that this knowledge gap results in overuse of telemetry at our institution.
Methods
Setting
Johns Hopkins Bayview Medical Center is a 400-bed academic medical center serving southeastern Baltimore. Providers included internal medicine residents and cardiovascular disease fellows who rotate to the medical center and Johns Hopkins Hospital, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners (NPs), and physician assistants (PAs).
Current Telemetry Practice
Remote telemetric monitoring is available in all adult, non-intensive care units of the hospital except for the psychiatry unit. However, the number of monitors are limited and it is not possible to monitor every patient if the wards are at capacity. Obstetrics uses its own unique cardiac monitoring system and thus was not included in the survey. Each monitor (IntelliVue, Philips Healthcare, Amsterdam, Netherlands) is attached to the patient using 5 lead wires, with electrocardiographic data transmitted to a monitoring station based in the progressive care unit, a cardio-pulmonary step-down unit. Monitors can be ordered in one of 3 manners, as mandated by hospital policy:
- Continuous telemetry – Telemetry monitoring is uninterrupted until discontinued by a provider.
- Telemetry protocol – Within 12 hours of telemetry placement, a monitor technician generates a report, which is reviewed by the nurse caring for the patient. The nurse performs an electrocardiogram (ECG) if the patient meets pre-specified criteria for telemetry discontinuation, which includes the absence of arrhythmias, troponin elevations, chest pain, or hemodynamic instability. The repeat ECG is then read and signed by the provider. After these criteria are met, telemetry can be discontinued.
- Stroke telemetry protocol – Telemetry is applied for 48 hours, mainly for detection of paroxysmal atrial fibrillation. Monitoring can be temporarily discontinued if the patient requires magnetic resonance imaging, which interferes with the telemetric monitors.
When entering any of the 3 possible telemetry orders in our computerized provider order entry system (Meditech, Westwood, MA), the ordering provider is required to indicate baseline rhythm, pacemaker presence, and desired heart rate warning parameters. Once the order is electronically signed, a monitor technician notes the order in a logbook and assigns the patient a telemeter, which is applied by the patient’s nurse.
If a monitored patient develops any predefined abnormal rhythm, audible alerts notify monitor technicians and an alert is sent to a portable telephone carried by the patient’s assigned nurse. Either the monitoring technician or the nurse then has the discretion to silence the alarm, note it in the chart, and/or contact the patient’s provider. If alerts are recorded, then a sample telemetry monitoring strip is saved into the patient’s paper medical chart.
Survey Instrument
After approval from the Johns Hopkins institutional review board, we queried providers who worked on the medicine and cardiology wards to assess the context and culture in which telemetry monitoring is used (see Appendix). The study was exempt from requiring informed consent. All staff had the option to decline study participation. We administered the survey using an online survey software program (SurveyMonkey, Palo Alto, CA), sending survey links via email to all internal medicine residents, cardiovascular disease fellows, internal medicine and cardiology teaching attending physicians, hospitalists, NPs, and PAs. Respondents completed the survey anonymously. To increase response rates, providers were sent a monthly reminder email. The survey was open from March 2014 to May 2014 for a total of 3 months.
Analysis
The survey data were compiled and analyzed using Microsoft Excel (Mac version 14.4; Microsoft, Redmond, WA). Variables are displayed as numbers and percentages, as appropriate.
Results
All providers reported having ordered telemetry, but almost all were either unaware of (76%) or only somewhat familiar with (21%) the AHA guidelines for appropriate telemetry use. Notably, the vast majority of fellows and residents reported that they were not at all familiar with the guidelines (100% and 96%, respectively). When asked why providers do not adhere to telemetry guidelines, lack of awareness of and lack of familiarity with the guidelines were the top 2 choices among respondents (Figure 1).
Additionally, most providers acknowledged experiencing adverse effects of telemetry: 86% (57/66) had experienced delayed patient transfers from the emergency department to inpatient floors due to telemetry unavailability and 97% (65/67) had experienced some delay in obtaining tests or studies for their telemetry-monitored patients. Despite acknowledging the potential consequences of telemetry use, only 21% (14/66) of providers routinely (ie, > 75% of the time) discontinued telemetry within 48 hours. Fifteen percent (10/65) routinely allowed telemetry to continue until the time of patient discharge. When discontinued, it was mainly due to the provider’s decision (57%); however, respondents noted that nurses prompted telemetry discontinuation 28% of the time.
Discussion
Consistent with previous studies [3–5,9–15], the majority of providers at our institution do not think continuous telemetry is appropriately utilized. Most survey respondents acknowledged a lack of awareness surrounding current guideline recommendations, which could explain why providers often do not follow them. Despite conceding their knowledge deficits, providers assumed their practice patterns for ordering telemetry were “appropriate”(ie, guideline-supported). This assertion may be incorrect as the majority of providers in our survey chose at least 1 non–guideline-supported indication for telemetry. Other studies have suggested additional reasons for inappropriate telemetry utilization. Providers may disagree with guideline recommendations, may assign lesser importance to guidelines when caring for an individual patient, or may fall victim to inertia (ie, not ordering telemetry appropriately simply because changing one’s practice pattern is difficult) [7].
In addition, the majority of our providers perceived telemetry overuse, which has been well-recognized nationwide [4]. While we did not assess this directly, other studies suggest that providers may overuse telemetry to provide a sense of reassurance when caring for a sick patient, since continuous telemetry is perceived to provide a higher level of care [6,15–17]. Unfortunately, no study has shown a benefit for continuous telemetry when placed for non-guideline-based diagnoses—whether for cardiac or non-cardiac diagnoses [3,9–11,13,14]. Likewise, the guidelines suggest that telemetry use should be time-limited, since the majority of benefit is accrued in the first 48 hours. Beyond that time, no study has shown a clear benefit to continuous telemetry [2]. Therefore, telemetry overuse may lead to unnecessarily increased costs without added benefits [3,9–11,13–15,18].
Our conclusions are tempered by the nature of our survey data. We recognize that our survey has not been previously validated. In addition, our response rates were low. This low sample size may lead to under-representation of diverse ideas. Also, our survey results may not be generalizable, since our study was conducted at a single academic hospital. Our institution’s telemetry ordering culture may differ from others, therefore making our results less applicable to other centers.
Despite these limitations, our results aid in understanding attitudes that surround the use of continuous telemetry, which can shape formal educational interventions to encourage appropriate guideline-based telemetry use. Since our providers agree on the need for more education about the guidelines, components such as online modules or in-person lecture educational sessions, newsletters, email communications, and incorporation of AHA guidelines into the institution’s automated computer order entry system could be utilized [17]. Didactic interventions could be designed especially for trainees given their overall lack of familiarity with the guidelines. Another potential intervention could include supplying providers with publically shared personalized measures of their own practices, since providers benefit from reinforcement and individualized feedback on appropriate utilization practices [19]. Previous studies have suggested that a multidisciplinary approach to patient care leads to positive outcomes [20,21], and in our experience, nursing input is absolutely critical in outlining potential problems and in developing solutions. Our findings suggest that nurses could play an active role in alerting providers when patients have telemetry in use and identifying patients who may no longer need it.
In summary, we have shown that many providers at a tertiary academic institution utilized continuous telemetry inappropriately, and were unaware of guidelines surrounding telemetry use. Future interventions aimed at educating providers, encouraging dialogue between staff, and enabling guideline-supported utilization may increase appropriate telemetry use leading to lower cost and improved quality of patient care.
Acknowledgment: The authors wish to thank Dr. Colleen Christmas, Dr. Panagis Galiatsatos, Mrs. Barbara Brigade, Ms. Joetta Love, Ms. Terri Rigsby, and Mrs. Lisa Shirk for their invaluable technical and administrative support.
Corresponding author: Amber Johnson, MD, MBA, 200 Lothrop St., S-553 Scaife Hall, Pittsburgh, PA 15213, [email protected].
Financial disclosures: None.
From the Johns Hopkins Bayview Medical Center, Baltimore, MD (Drs. Johnson, Knight, Maygers, and Zakaria), and Duke University Hospital, Durham, NC (Dr. Mock).
Abstract
- Objective: To determine patterns of telemetry use at a tertiary academic institution and identify factors contributing to noncompliance with guidelines regarding telemetry use.
- Methods: Web-based survey of 180 providers, including internal medicine residents and cardiovascular disease fellows, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners, and physician assistants.
- Results: Of the 180 providers surveyed, 67 (37%) replied. Most providers (76%) were unaware of guidelines regarding appropriate telemetry use and 85% selected inappropriate diagnoses as warranting telemetry. Only 21% routinely discontinued the telemetry order within 48 hours.
- Conclusions: Many providers at a tertiary academic institution utilize continuous telemetry inappropriately and are unaware of telemetry guidelines. These findings should guide interventions to improve telemetry utilization.
For many decades, telemetry has been widely used in the management and monitoring of patients with possible acute coronary syndromes (ACS), arrhythmias, cardiac events, and strokes [1]. In addition, telemetry has often been used in other clinical scenarios with less rigorous data supporting its use [2–4]. As a result, in 2004 the American Heart Association (AHA) issued guidelines providing recommendations for best practices in hospital ECG monitoring. Indications for telemetry were classified into 3 diagnosis-driven groups: class I (indicated in all patients), class II (indicated in most patients, may be of benefit) and class III (not indicated, no therapeutic benefit) [2]. However, these recommendations have not been widely followed and telemetry is inappropriately used for many inpatients [5,6].
There are several reasons why clinicians fail to adhere to guidelines, including knowledge deficits, attitudes regarding the current guidelines, and institution-specific factors influencing practitioner behaviors [7]. In response to reports of widespread telemetry overuse, the Choosing Wisely Campaign of the American Board of Internal Medicine Foundation has championed judicious telemetry use, advocating evidence-based, protocol-driven telemetry management for patients not in intensive care units who do not meet guideline-based criteria for continuous telemetry [8].
In order to understand patterns of telemetry use at our academic institution and identify factors associated with this practice, we systematically analyzed telemetry use perceptions through provider surveys. We hypothesized that providers have misperceptions about appropriate use of telemetry and that this knowledge gap results in overuse of telemetry at our institution.
Methods
Setting
Johns Hopkins Bayview Medical Center is a 400-bed academic medical center serving southeastern Baltimore. Providers included internal medicine residents and cardiovascular disease fellows who rotate to the medical center and Johns Hopkins Hospital, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners (NPs), and physician assistants (PAs).
Current Telemetry Practice
Remote telemetric monitoring is available in all adult, non-intensive care units of the hospital except for the psychiatry unit. However, the number of monitors are limited and it is not possible to monitor every patient if the wards are at capacity. Obstetrics uses its own unique cardiac monitoring system and thus was not included in the survey. Each monitor (IntelliVue, Philips Healthcare, Amsterdam, Netherlands) is attached to the patient using 5 lead wires, with electrocardiographic data transmitted to a monitoring station based in the progressive care unit, a cardio-pulmonary step-down unit. Monitors can be ordered in one of 3 manners, as mandated by hospital policy:
- Continuous telemetry – Telemetry monitoring is uninterrupted until discontinued by a provider.
- Telemetry protocol – Within 12 hours of telemetry placement, a monitor technician generates a report, which is reviewed by the nurse caring for the patient. The nurse performs an electrocardiogram (ECG) if the patient meets pre-specified criteria for telemetry discontinuation, which includes the absence of arrhythmias, troponin elevations, chest pain, or hemodynamic instability. The repeat ECG is then read and signed by the provider. After these criteria are met, telemetry can be discontinued.
- Stroke telemetry protocol – Telemetry is applied for 48 hours, mainly for detection of paroxysmal atrial fibrillation. Monitoring can be temporarily discontinued if the patient requires magnetic resonance imaging, which interferes with the telemetric monitors.
When entering any of the 3 possible telemetry orders in our computerized provider order entry system (Meditech, Westwood, MA), the ordering provider is required to indicate baseline rhythm, pacemaker presence, and desired heart rate warning parameters. Once the order is electronically signed, a monitor technician notes the order in a logbook and assigns the patient a telemeter, which is applied by the patient’s nurse.
If a monitored patient develops any predefined abnormal rhythm, audible alerts notify monitor technicians and an alert is sent to a portable telephone carried by the patient’s assigned nurse. Either the monitoring technician or the nurse then has the discretion to silence the alarm, note it in the chart, and/or contact the patient’s provider. If alerts are recorded, then a sample telemetry monitoring strip is saved into the patient’s paper medical chart.
Survey Instrument
After approval from the Johns Hopkins institutional review board, we queried providers who worked on the medicine and cardiology wards to assess the context and culture in which telemetry monitoring is used (see Appendix). The study was exempt from requiring informed consent. All staff had the option to decline study participation. We administered the survey using an online survey software program (SurveyMonkey, Palo Alto, CA), sending survey links via email to all internal medicine residents, cardiovascular disease fellows, internal medicine and cardiology teaching attending physicians, hospitalists, NPs, and PAs. Respondents completed the survey anonymously. To increase response rates, providers were sent a monthly reminder email. The survey was open from March 2014 to May 2014 for a total of 3 months.
Analysis
The survey data were compiled and analyzed using Microsoft Excel (Mac version 14.4; Microsoft, Redmond, WA). Variables are displayed as numbers and percentages, as appropriate.
Results
All providers reported having ordered telemetry, but almost all were either unaware of (76%) or only somewhat familiar with (21%) the AHA guidelines for appropriate telemetry use. Notably, the vast majority of fellows and residents reported that they were not at all familiar with the guidelines (100% and 96%, respectively). When asked why providers do not adhere to telemetry guidelines, lack of awareness of and lack of familiarity with the guidelines were the top 2 choices among respondents (Figure 1).
Additionally, most providers acknowledged experiencing adverse effects of telemetry: 86% (57/66) had experienced delayed patient transfers from the emergency department to inpatient floors due to telemetry unavailability and 97% (65/67) had experienced some delay in obtaining tests or studies for their telemetry-monitored patients. Despite acknowledging the potential consequences of telemetry use, only 21% (14/66) of providers routinely (ie, > 75% of the time) discontinued telemetry within 48 hours. Fifteen percent (10/65) routinely allowed telemetry to continue until the time of patient discharge. When discontinued, it was mainly due to the provider’s decision (57%); however, respondents noted that nurses prompted telemetry discontinuation 28% of the time.
Discussion
Consistent with previous studies [3–5,9–15], the majority of providers at our institution do not think continuous telemetry is appropriately utilized. Most survey respondents acknowledged a lack of awareness surrounding current guideline recommendations, which could explain why providers often do not follow them. Despite conceding their knowledge deficits, providers assumed their practice patterns for ordering telemetry were “appropriate”(ie, guideline-supported). This assertion may be incorrect as the majority of providers in our survey chose at least 1 non–guideline-supported indication for telemetry. Other studies have suggested additional reasons for inappropriate telemetry utilization. Providers may disagree with guideline recommendations, may assign lesser importance to guidelines when caring for an individual patient, or may fall victim to inertia (ie, not ordering telemetry appropriately simply because changing one’s practice pattern is difficult) [7].
In addition, the majority of our providers perceived telemetry overuse, which has been well-recognized nationwide [4]. While we did not assess this directly, other studies suggest that providers may overuse telemetry to provide a sense of reassurance when caring for a sick patient, since continuous telemetry is perceived to provide a higher level of care [6,15–17]. Unfortunately, no study has shown a benefit for continuous telemetry when placed for non-guideline-based diagnoses—whether for cardiac or non-cardiac diagnoses [3,9–11,13,14]. Likewise, the guidelines suggest that telemetry use should be time-limited, since the majority of benefit is accrued in the first 48 hours. Beyond that time, no study has shown a clear benefit to continuous telemetry [2]. Therefore, telemetry overuse may lead to unnecessarily increased costs without added benefits [3,9–11,13–15,18].
Our conclusions are tempered by the nature of our survey data. We recognize that our survey has not been previously validated. In addition, our response rates were low. This low sample size may lead to under-representation of diverse ideas. Also, our survey results may not be generalizable, since our study was conducted at a single academic hospital. Our institution’s telemetry ordering culture may differ from others, therefore making our results less applicable to other centers.
Despite these limitations, our results aid in understanding attitudes that surround the use of continuous telemetry, which can shape formal educational interventions to encourage appropriate guideline-based telemetry use. Since our providers agree on the need for more education about the guidelines, components such as online modules or in-person lecture educational sessions, newsletters, email communications, and incorporation of AHA guidelines into the institution’s automated computer order entry system could be utilized [17]. Didactic interventions could be designed especially for trainees given their overall lack of familiarity with the guidelines. Another potential intervention could include supplying providers with publically shared personalized measures of their own practices, since providers benefit from reinforcement and individualized feedback on appropriate utilization practices [19]. Previous studies have suggested that a multidisciplinary approach to patient care leads to positive outcomes [20,21], and in our experience, nursing input is absolutely critical in outlining potential problems and in developing solutions. Our findings suggest that nurses could play an active role in alerting providers when patients have telemetry in use and identifying patients who may no longer need it.
In summary, we have shown that many providers at a tertiary academic institution utilized continuous telemetry inappropriately, and were unaware of guidelines surrounding telemetry use. Future interventions aimed at educating providers, encouraging dialogue between staff, and enabling guideline-supported utilization may increase appropriate telemetry use leading to lower cost and improved quality of patient care.
Acknowledgment: The authors wish to thank Dr. Colleen Christmas, Dr. Panagis Galiatsatos, Mrs. Barbara Brigade, Ms. Joetta Love, Ms. Terri Rigsby, and Mrs. Lisa Shirk for their invaluable technical and administrative support.
Corresponding author: Amber Johnson, MD, MBA, 200 Lothrop St., S-553 Scaife Hall, Pittsburgh, PA 15213, [email protected].
Financial disclosures: None.
1. Day H. Preliminary studies of an acute coronary care area. J Lancet 1963;83:53–5.
2. Drew B, Califf R, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110:2721–46.
3. Estrada C, Battilana G, Alexander M, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med 2000;15:51–5.
4. Henriques-Forsythe M, Ivonye C, Jamched U, et al. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med 2009;76:368–72.
5. Chen E, Hollander, J. When do patients need admission to a telemetry bed? J Emerg Med 2007;33:53–60.
6. Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012;172:1349–50.
7. Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines?: A framework for improvement. JAMA 1999;282:1458–65.
8. Adult hospital medicine. Five things physicians and patients should question. 15 Aug 2013. Available at www.choosingwisely.org/doctor-patient-lists/society-of-hospital-medicine-adult-hospital-medicine/
9. Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: A prospective cohort study of risk stratification and outcomes. Am J Med 2001;110:7–11.
10. Estrada C, Rosman H, Prasad N, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995;76:960–5.
11. Hollander J, Sites F, Pollack C, Shofer F. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71–6.
12. Ivonye C, Ohuabunwo C, Henriques-Forsythe M, et al. Evaluation of telemetry utilization, policy, and outcomes in an inner-city academic medical center. J Natl Med Assoc 2010;102:598–604.
13. Schull M, Redelmeier D. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000;7:647–52.
14. Sivaram C, Summers J, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998;21:503–5.
15. Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002;122:517–23.
16. Chen S, Zakaria S. Behind the monitor-The trouble with telemetry: a teachable moment. JAMA Intern Med 2015;175:894.
17. Dressler R, Dryer M, Coletti C, et al. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014;174:1852–4.
18. Benjamin E, Klugman R, Luckmann R, et al. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care 2013;19:e225–32.
19. Solomon D, Hashimoto H, Daltroy L, Liang M. Techniques to improve physicians use of diagnostic tests: A new conceptual framework. JAMA 1998;280:2020–7.
20. Richeson J, Johnson J. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung 1992;21:18–24.
21. Curley C, McEachern J, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care 1998;36:AS4–12.
1. Day H. Preliminary studies of an acute coronary care area. J Lancet 1963;83:53–5.
2. Drew B, Califf R, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110:2721–46.
3. Estrada C, Battilana G, Alexander M, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med 2000;15:51–5.
4. Henriques-Forsythe M, Ivonye C, Jamched U, et al. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med 2009;76:368–72.
5. Chen E, Hollander, J. When do patients need admission to a telemetry bed? J Emerg Med 2007;33:53–60.
6. Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012;172:1349–50.
7. Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines?: A framework for improvement. JAMA 1999;282:1458–65.
8. Adult hospital medicine. Five things physicians and patients should question. 15 Aug 2013. Available at www.choosingwisely.org/doctor-patient-lists/society-of-hospital-medicine-adult-hospital-medicine/
9. Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: A prospective cohort study of risk stratification and outcomes. Am J Med 2001;110:7–11.
10. Estrada C, Rosman H, Prasad N, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995;76:960–5.
11. Hollander J, Sites F, Pollack C, Shofer F. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71–6.
12. Ivonye C, Ohuabunwo C, Henriques-Forsythe M, et al. Evaluation of telemetry utilization, policy, and outcomes in an inner-city academic medical center. J Natl Med Assoc 2010;102:598–604.
13. Schull M, Redelmeier D. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000;7:647–52.
14. Sivaram C, Summers J, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998;21:503–5.
15. Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002;122:517–23.
16. Chen S, Zakaria S. Behind the monitor-The trouble with telemetry: a teachable moment. JAMA Intern Med 2015;175:894.
17. Dressler R, Dryer M, Coletti C, et al. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014;174:1852–4.
18. Benjamin E, Klugman R, Luckmann R, et al. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care 2013;19:e225–32.
19. Solomon D, Hashimoto H, Daltroy L, Liang M. Techniques to improve physicians use of diagnostic tests: A new conceptual framework. JAMA 1998;280:2020–7.
20. Richeson J, Johnson J. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung 1992;21:18–24.
21. Curley C, McEachern J, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care 1998;36:AS4–12.
Using an Incident Command System Model for Initial Response to an Administrative Crisis at the Phoenix VA Health Care System
On April 9, 2014, allegations were made relating to delays experienced by patients accessing care at the Phoenix VA Health Care System (PVAHCS). After an in-depth investigation, multiple administrative problems were discovered related to scheduling processes at PVAHCS as well as many other VA facilities across the country. In Phoenix, there were 1,400 patients on the official electronic waiting list (EWL) in addition to 1,700 patients who had requested care but were not on any official waiting list.1
The following is a description of the use of an Incident Command System (ICS) model to provide care for these veterans in the face of an existing lack of capacity to do so. This is written from the perspective of frontline physicians representing hospital medicine, the emergency department, and primary care. The authors’ views do not necessarily reflect the official position of the VA or PVAHCS.
Under direction of the President, the VA crafted the official response to meet the needs of veterans awaiting care. The program, called the Accelerating Care Initiative (ACI), was launched on May 21, 2014. With the cooperation of the White House, the VA brought to bear substantial resources to enable PVAHCS to accomplish the task with an “all-hands-on-deck” approach. These resources included the Disaster Emergency Medical Personnel System (DEMPS), Traveling Nurse Corps (TNC), and the VA Locum Tenens program.
This situation was unprecedented, and an administrative framework was needed to organize and manage the extra personnel and resources involved in the response. The decision was made to use the ICS to achieve this aim. Most people involved in health care administration have been exposed to ICS concepts but are not accustomed to approaching an administrative problem the way emergency managers would respond to a natural disaster, such as a flood or hurricane.
According to the Federal Emergency Management Agency, ICS is “a standardized on-scene emergency management construct.”2 Incident Command System combines resources and people under a common organizational structure, using a common terminology to facilitate cooperation between any and all entities that may be involved in an incident. It is a modular concept and designed to be adaptable and scalable from isolated local events, such as a traffic accident, to regional catastrophes such as a category 5 hurricane. Incident Command System was developed in the 1970s, but it has become the standard approach for government agencies, law enforcement, first responders, and the military.
The PVAHCS adopted an ICS framework, and the chief of staff (COS) took on the incident commander role full time. The deputy assumed all other COS duties related to hospital operations. Phoenix VA Health Care System used the VA national call center to rapidly contact patients awaiting care and established the other standard ICS branches with Operations, Planning, Logistics, and Finance/Administration with appropriate task forces underneath each department. Within Operations, PVAHCS established task forces for the primary care, medical specialty care, surgical specialty care, and mental health departments.
Efficient command, control, and communication was facilitated by having twice-daily huddles with key staff members for 20 to 30 minutes to share updates and refine operational goals. This worked well and provided the situational awareness for our incident commander to rapidly channel situation reports (SITREPS) to the VA Secretary’s office in Washington without drawing focus away from creating solutions. As a midsize facility that lacks certain specialized medical and surgical services, PVAHCS already had an established system for referring veterans for these services. This enabled PVAHCS to use these channels to provide medical and surgical specialty care where demand exceeded capacity to schedule within a reasonable time frame.
Federal law, at the time, severely limited the ability of PVAHCS to outsource primary care in this fashion, which required finding new ways to create additional capacity. This was a major operational challenge due to very limited physical space as well as an insufficient number of primary care and administrative support staff. The PVAHCS made the equivalent of 5 new primary care teams operational with rotating volunteers from other VAs and our preexisting DEMPS, Locum Tenens, and TNC programs.
Medical subspecialty clinics within the primary care area were promptly moved to create space for the new primary care teams. To create this additional space, PVAHCS postponed a planned expansion of a community living center in the main hospital building complex. While PVAHCS was standing up the ICS, VA facilities from other regions loaned 3 mobile medical units. These vehicles included fully capable examination tables and telehealth capability and were used for intake appointments, new unassigned patients, and as administrative space.
In August 2014, the Veterans Choice Act (VCA) allowed veterans to access care from non-VA providers. Eligibility was based on the distance a veteran lived from a VA facility or the inability to be seen within a specified time period. The VCA provided PVACHS with an additional tool to meet veterans’ care needs as it increased the hiring of permanent staff. After about 3 months, PVAHCS succeeded in contacting > 6,000 veterans and providing > 3,200 veterans with appointments at the either PVAHCS or local civilian partners. Despite the initial successes, the preliminary gains in patient access at PVAHCS will not be sustainable, and wait times will not decrease substantially without increased permanent staff and further improvements in both the facility and its processes. Although these improvements are a priority, progress has been slow.
Efforts are underway to enhance operational integration between providers, nursing, and administrative support personnel. Congress has renewed its support of a larger and more functional health care center along with the addition of 2 more clinics within the metro Phoenix area. The PVAHCS has since stood down the ICS and ACI operations and transferred these operations to a newly created Patient Flow Committee. This group is chaired by the COS and meets monthly to supervise process improvement teams using lean modalities to address issues creating excessive waits or delays in patient care throughout the facility.
This access to care crisis was exacerbated by intense media and political attention. Further disarray resulted from the abrupt loss of several senior executives at PVAHCS—all the way up to former VA Secretary Eric K. Shinseki. Use of the ICS was highly effective in providing the necessary organizational structure for key staff to focus on solving the immediate problems locally while managing external resources that were in constant flux. The authors strongly recommend consideration of the ICS as a management framework to tackle similar problems.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
1. VA Office of Inspector General. Veterans Health Administration Interim Report: Review of Patient Wait Times, Scheduling Practices, and Alleged Patient Deaths at the Phoenix Health Care System. U.S. Department of Veterans Affairs Website. http://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed November 16, 2015.
2. U.S. Department of Homeland Security, Federal Emergency Management Agency. Developing and Maintaining Emergency Operations Plans. Comprehensive Preparedness Guide 101, Version 2.0. Federal Emergency Management Agency Website. http://www.fema.gov/media-library-data/20130726-1828-25045-0014/cpg_101_comprehensive_preparedness_guide_developing_and_maintaining_emergency_operations_plans_2010.pdf. Published November 2010. Accessed November 16, 2015.
On April 9, 2014, allegations were made relating to delays experienced by patients accessing care at the Phoenix VA Health Care System (PVAHCS). After an in-depth investigation, multiple administrative problems were discovered related to scheduling processes at PVAHCS as well as many other VA facilities across the country. In Phoenix, there were 1,400 patients on the official electronic waiting list (EWL) in addition to 1,700 patients who had requested care but were not on any official waiting list.1
The following is a description of the use of an Incident Command System (ICS) model to provide care for these veterans in the face of an existing lack of capacity to do so. This is written from the perspective of frontline physicians representing hospital medicine, the emergency department, and primary care. The authors’ views do not necessarily reflect the official position of the VA or PVAHCS.
Under direction of the President, the VA crafted the official response to meet the needs of veterans awaiting care. The program, called the Accelerating Care Initiative (ACI), was launched on May 21, 2014. With the cooperation of the White House, the VA brought to bear substantial resources to enable PVAHCS to accomplish the task with an “all-hands-on-deck” approach. These resources included the Disaster Emergency Medical Personnel System (DEMPS), Traveling Nurse Corps (TNC), and the VA Locum Tenens program.
This situation was unprecedented, and an administrative framework was needed to organize and manage the extra personnel and resources involved in the response. The decision was made to use the ICS to achieve this aim. Most people involved in health care administration have been exposed to ICS concepts but are not accustomed to approaching an administrative problem the way emergency managers would respond to a natural disaster, such as a flood or hurricane.
According to the Federal Emergency Management Agency, ICS is “a standardized on-scene emergency management construct.”2 Incident Command System combines resources and people under a common organizational structure, using a common terminology to facilitate cooperation between any and all entities that may be involved in an incident. It is a modular concept and designed to be adaptable and scalable from isolated local events, such as a traffic accident, to regional catastrophes such as a category 5 hurricane. Incident Command System was developed in the 1970s, but it has become the standard approach for government agencies, law enforcement, first responders, and the military.
The PVAHCS adopted an ICS framework, and the chief of staff (COS) took on the incident commander role full time. The deputy assumed all other COS duties related to hospital operations. Phoenix VA Health Care System used the VA national call center to rapidly contact patients awaiting care and established the other standard ICS branches with Operations, Planning, Logistics, and Finance/Administration with appropriate task forces underneath each department. Within Operations, PVAHCS established task forces for the primary care, medical specialty care, surgical specialty care, and mental health departments.
Efficient command, control, and communication was facilitated by having twice-daily huddles with key staff members for 20 to 30 minutes to share updates and refine operational goals. This worked well and provided the situational awareness for our incident commander to rapidly channel situation reports (SITREPS) to the VA Secretary’s office in Washington without drawing focus away from creating solutions. As a midsize facility that lacks certain specialized medical and surgical services, PVAHCS already had an established system for referring veterans for these services. This enabled PVAHCS to use these channels to provide medical and surgical specialty care where demand exceeded capacity to schedule within a reasonable time frame.
Federal law, at the time, severely limited the ability of PVAHCS to outsource primary care in this fashion, which required finding new ways to create additional capacity. This was a major operational challenge due to very limited physical space as well as an insufficient number of primary care and administrative support staff. The PVAHCS made the equivalent of 5 new primary care teams operational with rotating volunteers from other VAs and our preexisting DEMPS, Locum Tenens, and TNC programs.
Medical subspecialty clinics within the primary care area were promptly moved to create space for the new primary care teams. To create this additional space, PVAHCS postponed a planned expansion of a community living center in the main hospital building complex. While PVAHCS was standing up the ICS, VA facilities from other regions loaned 3 mobile medical units. These vehicles included fully capable examination tables and telehealth capability and were used for intake appointments, new unassigned patients, and as administrative space.
In August 2014, the Veterans Choice Act (VCA) allowed veterans to access care from non-VA providers. Eligibility was based on the distance a veteran lived from a VA facility or the inability to be seen within a specified time period. The VCA provided PVACHS with an additional tool to meet veterans’ care needs as it increased the hiring of permanent staff. After about 3 months, PVAHCS succeeded in contacting > 6,000 veterans and providing > 3,200 veterans with appointments at the either PVAHCS or local civilian partners. Despite the initial successes, the preliminary gains in patient access at PVAHCS will not be sustainable, and wait times will not decrease substantially without increased permanent staff and further improvements in both the facility and its processes. Although these improvements are a priority, progress has been slow.
Efforts are underway to enhance operational integration between providers, nursing, and administrative support personnel. Congress has renewed its support of a larger and more functional health care center along with the addition of 2 more clinics within the metro Phoenix area. The PVAHCS has since stood down the ICS and ACI operations and transferred these operations to a newly created Patient Flow Committee. This group is chaired by the COS and meets monthly to supervise process improvement teams using lean modalities to address issues creating excessive waits or delays in patient care throughout the facility.
This access to care crisis was exacerbated by intense media and political attention. Further disarray resulted from the abrupt loss of several senior executives at PVAHCS—all the way up to former VA Secretary Eric K. Shinseki. Use of the ICS was highly effective in providing the necessary organizational structure for key staff to focus on solving the immediate problems locally while managing external resources that were in constant flux. The authors strongly recommend consideration of the ICS as a management framework to tackle similar problems.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
On April 9, 2014, allegations were made relating to delays experienced by patients accessing care at the Phoenix VA Health Care System (PVAHCS). After an in-depth investigation, multiple administrative problems were discovered related to scheduling processes at PVAHCS as well as many other VA facilities across the country. In Phoenix, there were 1,400 patients on the official electronic waiting list (EWL) in addition to 1,700 patients who had requested care but were not on any official waiting list.1
The following is a description of the use of an Incident Command System (ICS) model to provide care for these veterans in the face of an existing lack of capacity to do so. This is written from the perspective of frontline physicians representing hospital medicine, the emergency department, and primary care. The authors’ views do not necessarily reflect the official position of the VA or PVAHCS.
Under direction of the President, the VA crafted the official response to meet the needs of veterans awaiting care. The program, called the Accelerating Care Initiative (ACI), was launched on May 21, 2014. With the cooperation of the White House, the VA brought to bear substantial resources to enable PVAHCS to accomplish the task with an “all-hands-on-deck” approach. These resources included the Disaster Emergency Medical Personnel System (DEMPS), Traveling Nurse Corps (TNC), and the VA Locum Tenens program.
This situation was unprecedented, and an administrative framework was needed to organize and manage the extra personnel and resources involved in the response. The decision was made to use the ICS to achieve this aim. Most people involved in health care administration have been exposed to ICS concepts but are not accustomed to approaching an administrative problem the way emergency managers would respond to a natural disaster, such as a flood or hurricane.
According to the Federal Emergency Management Agency, ICS is “a standardized on-scene emergency management construct.”2 Incident Command System combines resources and people under a common organizational structure, using a common terminology to facilitate cooperation between any and all entities that may be involved in an incident. It is a modular concept and designed to be adaptable and scalable from isolated local events, such as a traffic accident, to regional catastrophes such as a category 5 hurricane. Incident Command System was developed in the 1970s, but it has become the standard approach for government agencies, law enforcement, first responders, and the military.
The PVAHCS adopted an ICS framework, and the chief of staff (COS) took on the incident commander role full time. The deputy assumed all other COS duties related to hospital operations. Phoenix VA Health Care System used the VA national call center to rapidly contact patients awaiting care and established the other standard ICS branches with Operations, Planning, Logistics, and Finance/Administration with appropriate task forces underneath each department. Within Operations, PVAHCS established task forces for the primary care, medical specialty care, surgical specialty care, and mental health departments.
Efficient command, control, and communication was facilitated by having twice-daily huddles with key staff members for 20 to 30 minutes to share updates and refine operational goals. This worked well and provided the situational awareness for our incident commander to rapidly channel situation reports (SITREPS) to the VA Secretary’s office in Washington without drawing focus away from creating solutions. As a midsize facility that lacks certain specialized medical and surgical services, PVAHCS already had an established system for referring veterans for these services. This enabled PVAHCS to use these channels to provide medical and surgical specialty care where demand exceeded capacity to schedule within a reasonable time frame.
Federal law, at the time, severely limited the ability of PVAHCS to outsource primary care in this fashion, which required finding new ways to create additional capacity. This was a major operational challenge due to very limited physical space as well as an insufficient number of primary care and administrative support staff. The PVAHCS made the equivalent of 5 new primary care teams operational with rotating volunteers from other VAs and our preexisting DEMPS, Locum Tenens, and TNC programs.
Medical subspecialty clinics within the primary care area were promptly moved to create space for the new primary care teams. To create this additional space, PVAHCS postponed a planned expansion of a community living center in the main hospital building complex. While PVAHCS was standing up the ICS, VA facilities from other regions loaned 3 mobile medical units. These vehicles included fully capable examination tables and telehealth capability and were used for intake appointments, new unassigned patients, and as administrative space.
In August 2014, the Veterans Choice Act (VCA) allowed veterans to access care from non-VA providers. Eligibility was based on the distance a veteran lived from a VA facility or the inability to be seen within a specified time period. The VCA provided PVACHS with an additional tool to meet veterans’ care needs as it increased the hiring of permanent staff. After about 3 months, PVAHCS succeeded in contacting > 6,000 veterans and providing > 3,200 veterans with appointments at the either PVAHCS or local civilian partners. Despite the initial successes, the preliminary gains in patient access at PVAHCS will not be sustainable, and wait times will not decrease substantially without increased permanent staff and further improvements in both the facility and its processes. Although these improvements are a priority, progress has been slow.
Efforts are underway to enhance operational integration between providers, nursing, and administrative support personnel. Congress has renewed its support of a larger and more functional health care center along with the addition of 2 more clinics within the metro Phoenix area. The PVAHCS has since stood down the ICS and ACI operations and transferred these operations to a newly created Patient Flow Committee. This group is chaired by the COS and meets monthly to supervise process improvement teams using lean modalities to address issues creating excessive waits or delays in patient care throughout the facility.
This access to care crisis was exacerbated by intense media and political attention. Further disarray resulted from the abrupt loss of several senior executives at PVAHCS—all the way up to former VA Secretary Eric K. Shinseki. Use of the ICS was highly effective in providing the necessary organizational structure for key staff to focus on solving the immediate problems locally while managing external resources that were in constant flux. The authors strongly recommend consideration of the ICS as a management framework to tackle similar problems.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
1. VA Office of Inspector General. Veterans Health Administration Interim Report: Review of Patient Wait Times, Scheduling Practices, and Alleged Patient Deaths at the Phoenix Health Care System. U.S. Department of Veterans Affairs Website. http://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed November 16, 2015.
2. U.S. Department of Homeland Security, Federal Emergency Management Agency. Developing and Maintaining Emergency Operations Plans. Comprehensive Preparedness Guide 101, Version 2.0. Federal Emergency Management Agency Website. http://www.fema.gov/media-library-data/20130726-1828-25045-0014/cpg_101_comprehensive_preparedness_guide_developing_and_maintaining_emergency_operations_plans_2010.pdf. Published November 2010. Accessed November 16, 2015.
1. VA Office of Inspector General. Veterans Health Administration Interim Report: Review of Patient Wait Times, Scheduling Practices, and Alleged Patient Deaths at the Phoenix Health Care System. U.S. Department of Veterans Affairs Website. http://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed November 16, 2015.
2. U.S. Department of Homeland Security, Federal Emergency Management Agency. Developing and Maintaining Emergency Operations Plans. Comprehensive Preparedness Guide 101, Version 2.0. Federal Emergency Management Agency Website. http://www.fema.gov/media-library-data/20130726-1828-25045-0014/cpg_101_comprehensive_preparedness_guide_developing_and_maintaining_emergency_operations_plans_2010.pdf. Published November 2010. Accessed November 16, 2015.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 2: Topical Therapies
It seems intuitive that clinicians in dermatology would automatically recognize the importance of proper selection and integration of skin care products and techniques in the management of acne vulgaris (AV). However, an understanding of the fundamental importance of skin care in AV management and the scientific basis for maintaining epidermal barrier (EpB) function and repair cannot be assumed. In fact, there is limited scientific information about EpB dysfunction and AV or the adjunctive benefits of specific skin care products. However, some data have emerged that can be successfully applied by clinicians.1-9
In part 2 of this series, emphasis is placed on skin care and topical therapies for the treatment of AV in adult women. In addition to the plethora of cleanser and moisturizer formulations that exist in the marketplace, there are many over-the-counter (OTC) products marketed to treat AV that contain benzoyl peroxide (BP) and salicylic acid. Importantly, women tend to be selective about what they use to cleanse and moisturize their skin, and use of OTC products to treat AV is common among adult women.10,11
A thorough discussion of EpB impairment, related inflammatory cascades, and potential relevance to AV are beyond the scope of this article. In short, appropriate skin care products can reduce the inflammation and sensitivity associated with increased transepidermal water loss and reduced stratum corneum hydration and can mitigate EpB impairments induced by certain acne medications or vehicles.1,12 Available data support the adjunctive benefit of proper skin care in the management of AV by mitigating cutaneous irritation and potentially contributing to a reduction in AV lesions.2-4,7,13 Use of a formulation that also provides broad-spectrum photoprotection also is helpful.3,4
Another challenge is the myriad of cosmeceuticals that are heavily marketed to adult women with AV.13,14 Unfortunately, the scientific evidence supporting these products for treatment of AV is limited, resulting in the clinician’s inability to make specific recommendations. The core message is to incorporate skin care products that can reduce EpB impairment and mitigate cutaneous irritation associated with some AV therapies.1-4,7-9,12
OTC Topical Therapies
The marketplace is replete with several OTC products for treatment of AV, most of which contain BP and salicylic acid.15,16 There is a lack of efficacy data for OTC products for AV, including cleansers and topical medications, although some may be beneficial for milder cases. A variety of formulations are available to choose from, usually without the advice of a clinician. Additionally, heavy marketing is directed at adult women with AV, which may promote the use of therapies that may not be optimal for their respective AV severity or may cause facial skin irritation. Self-treatment may also cause delay in seeking dermatologic care, increasing the risk of persistent or permanent sequelae. Delay in adequate treatment is a major risk factor for the development of acne scars.17
Prescription Topical Therapies
Despite the high prevalence of AV in adult women, there is a paucity of studies evaluating topical therapies for AV in this subset.18-24 Reports in the literature on AV in adult women have focused on systemic hormonal agents (eg, oral contraceptives, spironolactone); however, more recent reports have addressed the use of topical therapies in this subpopulation.11,25-30 Published data on topical formulations are predominantly post hoc analyses from pivotal randomized controlled trials (RCTs) that included adolescents and adults of both genders with facial AV located above the jawline and predominantly moderate in severity.11,26,28,30 Participants in all of these studies presented with non-nodular, mixed inflammatory, and comedonal facial AV above the jawline, with inclusion criteria that required a minimum of 20 comedonal lesions and 20 papulopustular lesions at baseline. An important differentiating factor among these various post hoc analyses evaluating adult women versus adolescent girls with AV are the ages used to separate adults from adolescents. A dividing line of 18 years and older was used in some reports (eg, adapalene gel 0.3%, dapsone gel 5%), while other reports used 25 years and older to separate adolescent girls from adult women (ie, clindamycin phosphate [CP] 1.2%– BP 3.75% gel, adapalene 0.1%–BP 2.5% gel).11,26,28,30
Importantly, these studies included adult women with AV who presented with mixed comedonal and inflammatory AV (mixed pattern AV) similar to adolescents. None of the studies included women with a U-shaped AV pattern or lower facial AV characterized by deep inflammatory lesions that are often tender and few in number. Unfortunately, there is a lack of data evaluating topical therapies for these patterns of AV in adult women, including AV below the jawline and on the trunk. Although mixed pattern AV has been reported to affect 75% to 90% of adult women with AV, epidemiologic data quantifying the clinical AV patterns affecting adult women are limited.11,22,29,31,32 More well-designed studies are needed.
The treatment of AV in adult women may incorporate any of the topical therapies used to treat AV in adolescents, especially as studies encompass both the adolescent and adult age ranges. This is especially true with mixed pattern AV, which is the predominant presentation in participants enrolled in clinical trials with topical therapies, especially of moderate severity.
Herein we provide a summary of the topical therapies that have been evaluated by post hoc analyses of data from pivotal studies in adult women with AV.
Adapalene Gel 0.3%
Adapalene exhibits retinoid activity with efficacy in reducing inflammatory and comedonal AV lesions shown with both 0.1% and 0.3% concentrations.33-35 Post hoc analyses of 2 pivotal RCTs of patients with facial AV showed that adapalene gel 0.3% once daily (n=74; mean age, 27.2 years) was superior to vehicle once daily (n=43; mean age, 25.2 years) in both mean and median percentage reductions of comedonal, inflammatory, and total lesions in women 18 years and older who were treated for 12 weeks; the difference in mean percentage lesion reduction from vehicle for total AV lesions was statistically significant at 12 weeks (P=.045).26 Adapalene gel 0.3% produced a favorable skin tolerability profile similar to adapalene gel 0.1%, with the most common adverse reactions being discomfort and dryness.
Advantages of topical retinoid therapy in adult women with facial AV are reduction in postinflammatory hyperpigmentation and therapeutic modulation of chronic photodamage (eg, fine lines, rough texture, dyschromia).29,36,37 Disadvantages include signs and symptoms of cutaneous irritation, although this tends to occur less frequently on facial skin with adapalene gel 0.3% as compared to other topical retinoids that exhibit comparable efficacy.33-37 Topical retinoid therapy on the anterior neck and upper chest should be used cautiously, as these anatomic sites appear to be more prone to cutaneous irritation.
Dapsone Gel 5%
Dapsone is a sulfone antimicrobial and anti-inflammatory agent that has been shown to be effective, safe, and well tolerated in the treatment of AV in a topical 5% formulation.38,39 A post hoc analysis of pivotal 12-week trial data suggested that dapsone gel 5% twice daily produced greater AV reductions in females compared to males; no gender differences were noted in adverse effects, which were low in frequency.39 A separate subgroup analysis compared outcomes among adult women (≥18 years of age; n=434) and adolescent girls (12–17 years of age; n=347) treated with dapsone gel 5%.11 The proportion with no or minimal acne based on the Global Acne Assessment Score at week 12 was greater in adult women (53.5%) versus adolescent girls (45.3%, P=.022), with significantly greater percentage reductions in both noninflammatory (P<.0001) and total lesion counts (P=.0008) observed in the adult group. Percentage reductions in inflammatory lesions were similar in both groups. No major safety or tolerability issues or new safety signals were noted. Advantages of dapsone gel 5% are highly favorable tolerability and the perception of decreased oily skin in some participants.38,39
Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel
The combination formulation of CP 1.2%– BP 3.75% gel applied once daily has been shown to be effective, well tolerated, and safe for the treatment of facial AV, with a gender analysis noting an apparent greater efficacy in females.40,41 A post hoc analysis from the 12-week pivotal study data in adult women aged 25 years and older showed a mean percentage change from baseline in inflammatory and noninflammatory lesion counts and the percentage of participants who achieved a 2-grade improvement by global assessment to be 68.7%, 60.4%, and 52.7% in actively treated participants (n=29), respectively, which was significantly superior to vehicle applied once daily (n=43; P=.019, P=.020, and P=.074, respectively).42 No relevant differences in tolerability were noted among treatment groups, and no participants discontinued therapy due to adverse events. Advantages of CP 1.2%–BP 3.75% gel are highly favorable skin tolerability and the perception of decreased oily skin in some participants.41-43
Adapalene 0.1%–Benzoyl Peroxide 2.5% Gel
A meta-analysis of pooled data from 3 RCTs evaluated use of adapalene 0.1%–BP 2.5% gel applied once daily in adult women aged 25 years and older with facial AV (n=130) versus vehicle gel applied once daily (n=124).30 The percentage of participants who achieved investigator global assessment ratings of clear or almost clear was 39.2% in actively treated participants versus 18.5% with vehicle (P<.001), and median percentage lesion reduction was approximately 30% greater in those treated with adapalene 0.1%–BP 2.5% gel versus vehicle gel. Tolerability and safety were favorable.
Other Agents
Topical azelaic acid (20% cream formulation, 15% gel formulation) has been suggested as a treatment option for adult women with AV, including patients with darker skin who are more prone to persistent hyperpigmentation.29
Conclusion
Proper skin care is an important component in the management of AV in adult women. Data for topical therapies in this subpopulation are limited; however, post hoc analyses provide some information regarding their efficacy in treating mixed pattern AV. More well-designed studies are needed to better evaluate the use of topical agents in adult women with AV. Although most topical AV therapies appear to be safe for use during pregnancy when properly used and limited to facial application, their use in women of childbearing potential and during pregnancy warrants individual consideration; topical retinoids are best avoided during pregnancy, especially tazarotene, which is rated category X.44 In part 3 of this series, oral therapies used to treat AV in adult women will be discussed.
1. Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6:18-24.
2. Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(suppl 1):26-34.
3. Del Rosso JQ, Gold M, Rueda MJ, et al. Efficacy, safety, and subject satisfaction of a specified skin care regimen to cleanse, medicate, moisturize, and protect the skin of patients under treatment for acne vulgaris. J Clin Aesthet Dermatol. 2015;8:22-30.
4. Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris: part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28-36.
5. Draelos ZD. Facial cosmetics for acne patients. Cosmetics in Dermatology. 2nd ed. New York, NY: Churchill Livingstone Inc; 1995:22-23.
6. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
7. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41:592-597.
8. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42:181-188.
9. Hensley D, Meckfessel MH. Tolerability of a skin care regimen formulated for acne-prone skin in children. Pediatr Dermatol. 2015;32:501-505.
10. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
11. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
12. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
13. Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
14. Draelos ZD. Acne. In: Draelos ZD, ed. Cosmeceuticals. 2nd ed. Philadelphia, PA: Saunders-Elsevier; 2009:175-180.
15. Kircik LH, Gwazdauskas J, Butners V, et al. Evaluation of the efficacy, tolerability, and safety of an over-the-counter acne regimen containing benzoyl peroxide and salicylic acid in subjects with acne. J Drugs Dermatol. 2013;12:259-264.
16. Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5:32-40.
17. Layton AM, Henderson C, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
18. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
19. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541-545.
20. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
21. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-788.
22. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
23. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
24. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
25. Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
26. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
27. Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate–benzoyl peroxide gel? Cutis. 2014;94:177-182.
28. Zeichner JA. The efficacy and tolerability of a fixed combination (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult females with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
29. Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
30. Stein-Gold L. Adapalene 0.1%-benzoyl peroxide 2.5% gel in adult female acne. Poster presented at: Winter Clinical Dermatology Conference; January 16–21, 2015; Maui, Hawaii.
31. Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Acad Dermatol Venereol. 2015;29:1096-1106.
32. Dréno B, Layton AM, Zouboulis CC, et al. Adult female acne: a new paradigm. J Euro Acad Dermatol Venereol. 2013;27:1063-1070.
33. Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
34. Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145-151.
35. Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
36. Tanghetti E, Dhawan S, Green L, et al. Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol. 2010;9:549-558.
37. Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
38. Draelos Z, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
39. Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
40. Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:1083-1089.
41. Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
42. Zeichner JA. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult female patients with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
43. Coley MK, Berson DS, Callendar VD. Overview of treatment principles for skin of color. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:70-85.
44. Ebede TL, Berson DS. Acne in pregnancy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:177-181.
It seems intuitive that clinicians in dermatology would automatically recognize the importance of proper selection and integration of skin care products and techniques in the management of acne vulgaris (AV). However, an understanding of the fundamental importance of skin care in AV management and the scientific basis for maintaining epidermal barrier (EpB) function and repair cannot be assumed. In fact, there is limited scientific information about EpB dysfunction and AV or the adjunctive benefits of specific skin care products. However, some data have emerged that can be successfully applied by clinicians.1-9
In part 2 of this series, emphasis is placed on skin care and topical therapies for the treatment of AV in adult women. In addition to the plethora of cleanser and moisturizer formulations that exist in the marketplace, there are many over-the-counter (OTC) products marketed to treat AV that contain benzoyl peroxide (BP) and salicylic acid. Importantly, women tend to be selective about what they use to cleanse and moisturize their skin, and use of OTC products to treat AV is common among adult women.10,11
A thorough discussion of EpB impairment, related inflammatory cascades, and potential relevance to AV are beyond the scope of this article. In short, appropriate skin care products can reduce the inflammation and sensitivity associated with increased transepidermal water loss and reduced stratum corneum hydration and can mitigate EpB impairments induced by certain acne medications or vehicles.1,12 Available data support the adjunctive benefit of proper skin care in the management of AV by mitigating cutaneous irritation and potentially contributing to a reduction in AV lesions.2-4,7,13 Use of a formulation that also provides broad-spectrum photoprotection also is helpful.3,4
Another challenge is the myriad of cosmeceuticals that are heavily marketed to adult women with AV.13,14 Unfortunately, the scientific evidence supporting these products for treatment of AV is limited, resulting in the clinician’s inability to make specific recommendations. The core message is to incorporate skin care products that can reduce EpB impairment and mitigate cutaneous irritation associated with some AV therapies.1-4,7-9,12
OTC Topical Therapies
The marketplace is replete with several OTC products for treatment of AV, most of which contain BP and salicylic acid.15,16 There is a lack of efficacy data for OTC products for AV, including cleansers and topical medications, although some may be beneficial for milder cases. A variety of formulations are available to choose from, usually without the advice of a clinician. Additionally, heavy marketing is directed at adult women with AV, which may promote the use of therapies that may not be optimal for their respective AV severity or may cause facial skin irritation. Self-treatment may also cause delay in seeking dermatologic care, increasing the risk of persistent or permanent sequelae. Delay in adequate treatment is a major risk factor for the development of acne scars.17
Prescription Topical Therapies
Despite the high prevalence of AV in adult women, there is a paucity of studies evaluating topical therapies for AV in this subset.18-24 Reports in the literature on AV in adult women have focused on systemic hormonal agents (eg, oral contraceptives, spironolactone); however, more recent reports have addressed the use of topical therapies in this subpopulation.11,25-30 Published data on topical formulations are predominantly post hoc analyses from pivotal randomized controlled trials (RCTs) that included adolescents and adults of both genders with facial AV located above the jawline and predominantly moderate in severity.11,26,28,30 Participants in all of these studies presented with non-nodular, mixed inflammatory, and comedonal facial AV above the jawline, with inclusion criteria that required a minimum of 20 comedonal lesions and 20 papulopustular lesions at baseline. An important differentiating factor among these various post hoc analyses evaluating adult women versus adolescent girls with AV are the ages used to separate adults from adolescents. A dividing line of 18 years and older was used in some reports (eg, adapalene gel 0.3%, dapsone gel 5%), while other reports used 25 years and older to separate adolescent girls from adult women (ie, clindamycin phosphate [CP] 1.2%– BP 3.75% gel, adapalene 0.1%–BP 2.5% gel).11,26,28,30
Importantly, these studies included adult women with AV who presented with mixed comedonal and inflammatory AV (mixed pattern AV) similar to adolescents. None of the studies included women with a U-shaped AV pattern or lower facial AV characterized by deep inflammatory lesions that are often tender and few in number. Unfortunately, there is a lack of data evaluating topical therapies for these patterns of AV in adult women, including AV below the jawline and on the trunk. Although mixed pattern AV has been reported to affect 75% to 90% of adult women with AV, epidemiologic data quantifying the clinical AV patterns affecting adult women are limited.11,22,29,31,32 More well-designed studies are needed.
The treatment of AV in adult women may incorporate any of the topical therapies used to treat AV in adolescents, especially as studies encompass both the adolescent and adult age ranges. This is especially true with mixed pattern AV, which is the predominant presentation in participants enrolled in clinical trials with topical therapies, especially of moderate severity.
Herein we provide a summary of the topical therapies that have been evaluated by post hoc analyses of data from pivotal studies in adult women with AV.
Adapalene Gel 0.3%
Adapalene exhibits retinoid activity with efficacy in reducing inflammatory and comedonal AV lesions shown with both 0.1% and 0.3% concentrations.33-35 Post hoc analyses of 2 pivotal RCTs of patients with facial AV showed that adapalene gel 0.3% once daily (n=74; mean age, 27.2 years) was superior to vehicle once daily (n=43; mean age, 25.2 years) in both mean and median percentage reductions of comedonal, inflammatory, and total lesions in women 18 years and older who were treated for 12 weeks; the difference in mean percentage lesion reduction from vehicle for total AV lesions was statistically significant at 12 weeks (P=.045).26 Adapalene gel 0.3% produced a favorable skin tolerability profile similar to adapalene gel 0.1%, with the most common adverse reactions being discomfort and dryness.
Advantages of topical retinoid therapy in adult women with facial AV are reduction in postinflammatory hyperpigmentation and therapeutic modulation of chronic photodamage (eg, fine lines, rough texture, dyschromia).29,36,37 Disadvantages include signs and symptoms of cutaneous irritation, although this tends to occur less frequently on facial skin with adapalene gel 0.3% as compared to other topical retinoids that exhibit comparable efficacy.33-37 Topical retinoid therapy on the anterior neck and upper chest should be used cautiously, as these anatomic sites appear to be more prone to cutaneous irritation.
Dapsone Gel 5%
Dapsone is a sulfone antimicrobial and anti-inflammatory agent that has been shown to be effective, safe, and well tolerated in the treatment of AV in a topical 5% formulation.38,39 A post hoc analysis of pivotal 12-week trial data suggested that dapsone gel 5% twice daily produced greater AV reductions in females compared to males; no gender differences were noted in adverse effects, which were low in frequency.39 A separate subgroup analysis compared outcomes among adult women (≥18 years of age; n=434) and adolescent girls (12–17 years of age; n=347) treated with dapsone gel 5%.11 The proportion with no or minimal acne based on the Global Acne Assessment Score at week 12 was greater in adult women (53.5%) versus adolescent girls (45.3%, P=.022), with significantly greater percentage reductions in both noninflammatory (P<.0001) and total lesion counts (P=.0008) observed in the adult group. Percentage reductions in inflammatory lesions were similar in both groups. No major safety or tolerability issues or new safety signals were noted. Advantages of dapsone gel 5% are highly favorable tolerability and the perception of decreased oily skin in some participants.38,39
Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel
The combination formulation of CP 1.2%– BP 3.75% gel applied once daily has been shown to be effective, well tolerated, and safe for the treatment of facial AV, with a gender analysis noting an apparent greater efficacy in females.40,41 A post hoc analysis from the 12-week pivotal study data in adult women aged 25 years and older showed a mean percentage change from baseline in inflammatory and noninflammatory lesion counts and the percentage of participants who achieved a 2-grade improvement by global assessment to be 68.7%, 60.4%, and 52.7% in actively treated participants (n=29), respectively, which was significantly superior to vehicle applied once daily (n=43; P=.019, P=.020, and P=.074, respectively).42 No relevant differences in tolerability were noted among treatment groups, and no participants discontinued therapy due to adverse events. Advantages of CP 1.2%–BP 3.75% gel are highly favorable skin tolerability and the perception of decreased oily skin in some participants.41-43
Adapalene 0.1%–Benzoyl Peroxide 2.5% Gel
A meta-analysis of pooled data from 3 RCTs evaluated use of adapalene 0.1%–BP 2.5% gel applied once daily in adult women aged 25 years and older with facial AV (n=130) versus vehicle gel applied once daily (n=124).30 The percentage of participants who achieved investigator global assessment ratings of clear or almost clear was 39.2% in actively treated participants versus 18.5% with vehicle (P<.001), and median percentage lesion reduction was approximately 30% greater in those treated with adapalene 0.1%–BP 2.5% gel versus vehicle gel. Tolerability and safety were favorable.
Other Agents
Topical azelaic acid (20% cream formulation, 15% gel formulation) has been suggested as a treatment option for adult women with AV, including patients with darker skin who are more prone to persistent hyperpigmentation.29
Conclusion
Proper skin care is an important component in the management of AV in adult women. Data for topical therapies in this subpopulation are limited; however, post hoc analyses provide some information regarding their efficacy in treating mixed pattern AV. More well-designed studies are needed to better evaluate the use of topical agents in adult women with AV. Although most topical AV therapies appear to be safe for use during pregnancy when properly used and limited to facial application, their use in women of childbearing potential and during pregnancy warrants individual consideration; topical retinoids are best avoided during pregnancy, especially tazarotene, which is rated category X.44 In part 3 of this series, oral therapies used to treat AV in adult women will be discussed.
It seems intuitive that clinicians in dermatology would automatically recognize the importance of proper selection and integration of skin care products and techniques in the management of acne vulgaris (AV). However, an understanding of the fundamental importance of skin care in AV management and the scientific basis for maintaining epidermal barrier (EpB) function and repair cannot be assumed. In fact, there is limited scientific information about EpB dysfunction and AV or the adjunctive benefits of specific skin care products. However, some data have emerged that can be successfully applied by clinicians.1-9
In part 2 of this series, emphasis is placed on skin care and topical therapies for the treatment of AV in adult women. In addition to the plethora of cleanser and moisturizer formulations that exist in the marketplace, there are many over-the-counter (OTC) products marketed to treat AV that contain benzoyl peroxide (BP) and salicylic acid. Importantly, women tend to be selective about what they use to cleanse and moisturize their skin, and use of OTC products to treat AV is common among adult women.10,11
A thorough discussion of EpB impairment, related inflammatory cascades, and potential relevance to AV are beyond the scope of this article. In short, appropriate skin care products can reduce the inflammation and sensitivity associated with increased transepidermal water loss and reduced stratum corneum hydration and can mitigate EpB impairments induced by certain acne medications or vehicles.1,12 Available data support the adjunctive benefit of proper skin care in the management of AV by mitigating cutaneous irritation and potentially contributing to a reduction in AV lesions.2-4,7,13 Use of a formulation that also provides broad-spectrum photoprotection also is helpful.3,4
Another challenge is the myriad of cosmeceuticals that are heavily marketed to adult women with AV.13,14 Unfortunately, the scientific evidence supporting these products for treatment of AV is limited, resulting in the clinician’s inability to make specific recommendations. The core message is to incorporate skin care products that can reduce EpB impairment and mitigate cutaneous irritation associated with some AV therapies.1-4,7-9,12
OTC Topical Therapies
The marketplace is replete with several OTC products for treatment of AV, most of which contain BP and salicylic acid.15,16 There is a lack of efficacy data for OTC products for AV, including cleansers and topical medications, although some may be beneficial for milder cases. A variety of formulations are available to choose from, usually without the advice of a clinician. Additionally, heavy marketing is directed at adult women with AV, which may promote the use of therapies that may not be optimal for their respective AV severity or may cause facial skin irritation. Self-treatment may also cause delay in seeking dermatologic care, increasing the risk of persistent or permanent sequelae. Delay in adequate treatment is a major risk factor for the development of acne scars.17
Prescription Topical Therapies
Despite the high prevalence of AV in adult women, there is a paucity of studies evaluating topical therapies for AV in this subset.18-24 Reports in the literature on AV in adult women have focused on systemic hormonal agents (eg, oral contraceptives, spironolactone); however, more recent reports have addressed the use of topical therapies in this subpopulation.11,25-30 Published data on topical formulations are predominantly post hoc analyses from pivotal randomized controlled trials (RCTs) that included adolescents and adults of both genders with facial AV located above the jawline and predominantly moderate in severity.11,26,28,30 Participants in all of these studies presented with non-nodular, mixed inflammatory, and comedonal facial AV above the jawline, with inclusion criteria that required a minimum of 20 comedonal lesions and 20 papulopustular lesions at baseline. An important differentiating factor among these various post hoc analyses evaluating adult women versus adolescent girls with AV are the ages used to separate adults from adolescents. A dividing line of 18 years and older was used in some reports (eg, adapalene gel 0.3%, dapsone gel 5%), while other reports used 25 years and older to separate adolescent girls from adult women (ie, clindamycin phosphate [CP] 1.2%– BP 3.75% gel, adapalene 0.1%–BP 2.5% gel).11,26,28,30
Importantly, these studies included adult women with AV who presented with mixed comedonal and inflammatory AV (mixed pattern AV) similar to adolescents. None of the studies included women with a U-shaped AV pattern or lower facial AV characterized by deep inflammatory lesions that are often tender and few in number. Unfortunately, there is a lack of data evaluating topical therapies for these patterns of AV in adult women, including AV below the jawline and on the trunk. Although mixed pattern AV has been reported to affect 75% to 90% of adult women with AV, epidemiologic data quantifying the clinical AV patterns affecting adult women are limited.11,22,29,31,32 More well-designed studies are needed.
The treatment of AV in adult women may incorporate any of the topical therapies used to treat AV in adolescents, especially as studies encompass both the adolescent and adult age ranges. This is especially true with mixed pattern AV, which is the predominant presentation in participants enrolled in clinical trials with topical therapies, especially of moderate severity.
Herein we provide a summary of the topical therapies that have been evaluated by post hoc analyses of data from pivotal studies in adult women with AV.
Adapalene Gel 0.3%
Adapalene exhibits retinoid activity with efficacy in reducing inflammatory and comedonal AV lesions shown with both 0.1% and 0.3% concentrations.33-35 Post hoc analyses of 2 pivotal RCTs of patients with facial AV showed that adapalene gel 0.3% once daily (n=74; mean age, 27.2 years) was superior to vehicle once daily (n=43; mean age, 25.2 years) in both mean and median percentage reductions of comedonal, inflammatory, and total lesions in women 18 years and older who were treated for 12 weeks; the difference in mean percentage lesion reduction from vehicle for total AV lesions was statistically significant at 12 weeks (P=.045).26 Adapalene gel 0.3% produced a favorable skin tolerability profile similar to adapalene gel 0.1%, with the most common adverse reactions being discomfort and dryness.
Advantages of topical retinoid therapy in adult women with facial AV are reduction in postinflammatory hyperpigmentation and therapeutic modulation of chronic photodamage (eg, fine lines, rough texture, dyschromia).29,36,37 Disadvantages include signs and symptoms of cutaneous irritation, although this tends to occur less frequently on facial skin with adapalene gel 0.3% as compared to other topical retinoids that exhibit comparable efficacy.33-37 Topical retinoid therapy on the anterior neck and upper chest should be used cautiously, as these anatomic sites appear to be more prone to cutaneous irritation.
Dapsone Gel 5%
Dapsone is a sulfone antimicrobial and anti-inflammatory agent that has been shown to be effective, safe, and well tolerated in the treatment of AV in a topical 5% formulation.38,39 A post hoc analysis of pivotal 12-week trial data suggested that dapsone gel 5% twice daily produced greater AV reductions in females compared to males; no gender differences were noted in adverse effects, which were low in frequency.39 A separate subgroup analysis compared outcomes among adult women (≥18 years of age; n=434) and adolescent girls (12–17 years of age; n=347) treated with dapsone gel 5%.11 The proportion with no or minimal acne based on the Global Acne Assessment Score at week 12 was greater in adult women (53.5%) versus adolescent girls (45.3%, P=.022), with significantly greater percentage reductions in both noninflammatory (P<.0001) and total lesion counts (P=.0008) observed in the adult group. Percentage reductions in inflammatory lesions were similar in both groups. No major safety or tolerability issues or new safety signals were noted. Advantages of dapsone gel 5% are highly favorable tolerability and the perception of decreased oily skin in some participants.38,39
Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel
The combination formulation of CP 1.2%– BP 3.75% gel applied once daily has been shown to be effective, well tolerated, and safe for the treatment of facial AV, with a gender analysis noting an apparent greater efficacy in females.40,41 A post hoc analysis from the 12-week pivotal study data in adult women aged 25 years and older showed a mean percentage change from baseline in inflammatory and noninflammatory lesion counts and the percentage of participants who achieved a 2-grade improvement by global assessment to be 68.7%, 60.4%, and 52.7% in actively treated participants (n=29), respectively, which was significantly superior to vehicle applied once daily (n=43; P=.019, P=.020, and P=.074, respectively).42 No relevant differences in tolerability were noted among treatment groups, and no participants discontinued therapy due to adverse events. Advantages of CP 1.2%–BP 3.75% gel are highly favorable skin tolerability and the perception of decreased oily skin in some participants.41-43
Adapalene 0.1%–Benzoyl Peroxide 2.5% Gel
A meta-analysis of pooled data from 3 RCTs evaluated use of adapalene 0.1%–BP 2.5% gel applied once daily in adult women aged 25 years and older with facial AV (n=130) versus vehicle gel applied once daily (n=124).30 The percentage of participants who achieved investigator global assessment ratings of clear or almost clear was 39.2% in actively treated participants versus 18.5% with vehicle (P<.001), and median percentage lesion reduction was approximately 30% greater in those treated with adapalene 0.1%–BP 2.5% gel versus vehicle gel. Tolerability and safety were favorable.
Other Agents
Topical azelaic acid (20% cream formulation, 15% gel formulation) has been suggested as a treatment option for adult women with AV, including patients with darker skin who are more prone to persistent hyperpigmentation.29
Conclusion
Proper skin care is an important component in the management of AV in adult women. Data for topical therapies in this subpopulation are limited; however, post hoc analyses provide some information regarding their efficacy in treating mixed pattern AV. More well-designed studies are needed to better evaluate the use of topical agents in adult women with AV. Although most topical AV therapies appear to be safe for use during pregnancy when properly used and limited to facial application, their use in women of childbearing potential and during pregnancy warrants individual consideration; topical retinoids are best avoided during pregnancy, especially tazarotene, which is rated category X.44 In part 3 of this series, oral therapies used to treat AV in adult women will be discussed.
1. Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6:18-24.
2. Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(suppl 1):26-34.
3. Del Rosso JQ, Gold M, Rueda MJ, et al. Efficacy, safety, and subject satisfaction of a specified skin care regimen to cleanse, medicate, moisturize, and protect the skin of patients under treatment for acne vulgaris. J Clin Aesthet Dermatol. 2015;8:22-30.
4. Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris: part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28-36.
5. Draelos ZD. Facial cosmetics for acne patients. Cosmetics in Dermatology. 2nd ed. New York, NY: Churchill Livingstone Inc; 1995:22-23.
6. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
7. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41:592-597.
8. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42:181-188.
9. Hensley D, Meckfessel MH. Tolerability of a skin care regimen formulated for acne-prone skin in children. Pediatr Dermatol. 2015;32:501-505.
10. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
11. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
12. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
13. Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
14. Draelos ZD. Acne. In: Draelos ZD, ed. Cosmeceuticals. 2nd ed. Philadelphia, PA: Saunders-Elsevier; 2009:175-180.
15. Kircik LH, Gwazdauskas J, Butners V, et al. Evaluation of the efficacy, tolerability, and safety of an over-the-counter acne regimen containing benzoyl peroxide and salicylic acid in subjects with acne. J Drugs Dermatol. 2013;12:259-264.
16. Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5:32-40.
17. Layton AM, Henderson C, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
18. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
19. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541-545.
20. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
21. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-788.
22. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
23. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
24. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
25. Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
26. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
27. Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate–benzoyl peroxide gel? Cutis. 2014;94:177-182.
28. Zeichner JA. The efficacy and tolerability of a fixed combination (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult females with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
29. Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
30. Stein-Gold L. Adapalene 0.1%-benzoyl peroxide 2.5% gel in adult female acne. Poster presented at: Winter Clinical Dermatology Conference; January 16–21, 2015; Maui, Hawaii.
31. Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Acad Dermatol Venereol. 2015;29:1096-1106.
32. Dréno B, Layton AM, Zouboulis CC, et al. Adult female acne: a new paradigm. J Euro Acad Dermatol Venereol. 2013;27:1063-1070.
33. Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
34. Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145-151.
35. Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
36. Tanghetti E, Dhawan S, Green L, et al. Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol. 2010;9:549-558.
37. Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
38. Draelos Z, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
39. Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
40. Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:1083-1089.
41. Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
42. Zeichner JA. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult female patients with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
43. Coley MK, Berson DS, Callendar VD. Overview of treatment principles for skin of color. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:70-85.
44. Ebede TL, Berson DS. Acne in pregnancy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:177-181.
1. Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6:18-24.
2. Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(suppl 1):26-34.
3. Del Rosso JQ, Gold M, Rueda MJ, et al. Efficacy, safety, and subject satisfaction of a specified skin care regimen to cleanse, medicate, moisturize, and protect the skin of patients under treatment for acne vulgaris. J Clin Aesthet Dermatol. 2015;8:22-30.
4. Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris: part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28-36.
5. Draelos ZD. Facial cosmetics for acne patients. Cosmetics in Dermatology. 2nd ed. New York, NY: Churchill Livingstone Inc; 1995:22-23.
6. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
7. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41:592-597.
8. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42:181-188.
9. Hensley D, Meckfessel MH. Tolerability of a skin care regimen formulated for acne-prone skin in children. Pediatr Dermatol. 2015;32:501-505.
10. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
11. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
12. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
13. Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
14. Draelos ZD. Acne. In: Draelos ZD, ed. Cosmeceuticals. 2nd ed. Philadelphia, PA: Saunders-Elsevier; 2009:175-180.
15. Kircik LH, Gwazdauskas J, Butners V, et al. Evaluation of the efficacy, tolerability, and safety of an over-the-counter acne regimen containing benzoyl peroxide and salicylic acid in subjects with acne. J Drugs Dermatol. 2013;12:259-264.
16. Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5:32-40.
17. Layton AM, Henderson C, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
18. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
19. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541-545.
20. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
21. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-788.
22. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
23. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
24. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
25. Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
26. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
27. Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate–benzoyl peroxide gel? Cutis. 2014;94:177-182.
28. Zeichner JA. The efficacy and tolerability of a fixed combination (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult females with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
29. Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
30. Stein-Gold L. Adapalene 0.1%-benzoyl peroxide 2.5% gel in adult female acne. Poster presented at: Winter Clinical Dermatology Conference; January 16–21, 2015; Maui, Hawaii.
31. Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Acad Dermatol Venereol. 2015;29:1096-1106.
32. Dréno B, Layton AM, Zouboulis CC, et al. Adult female acne: a new paradigm. J Euro Acad Dermatol Venereol. 2013;27:1063-1070.
33. Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
34. Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145-151.
35. Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
36. Tanghetti E, Dhawan S, Green L, et al. Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol. 2010;9:549-558.
37. Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
38. Draelos Z, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
39. Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
40. Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:1083-1089.
41. Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
42. Zeichner JA. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult female patients with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
43. Coley MK, Berson DS, Callendar VD. Overview of treatment principles for skin of color. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:70-85.
44. Ebede TL, Berson DS. Acne in pregnancy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:177-181.
Practice Points
- Data from randomized controlled clinical trials (RCTs) of topical agents used for the treatment of acne in adult women has been gleaned through subanalyses of larger pivotal studies with adapalene gel 0.3%, dapsone gel 5%, clindamycin phosphate 1.2%–benzoyl peroxide 3.75% gel, and adapalene 0.1%–benzoyl peroxide 2.5% gel.
- Efficacy and tolerability/safety results from RCTs of these topical agents evaluated outcomes for the clinical pattern of mixed inflammatory, comedonal, and non-nodular acne located on the face above the jawline margin.
- More data are needed on the treatment of acne in adult women with topical agents, systemic agents, and combination regimens, including results for the full spectrum of clinical presentations.
Panobinostat: a novel mechanism of action shows promise in multiple myeloma
Following an initial “no” vote from the Oncologic Drugs Advisory Committee (ODAC) in late 2014, the US Food and Drug Administration eventually awarded accelerated approval in February 2015 to the histone deacetylase (HDAC) inhibitor panobinostat for use in select patients with relapsed multiple myeloma. Panobinostat has a novel mechanism of action that demonstrates synergy with the proteasome inhibitor bortezomib and the immunomodulatory agent dexamethasone, which translated into improved progression-free survival (PFS) for patients with multiple myeloma who had received at least 2 prior therapies, according to data from a prespecified subgroup analysis from the Panorama-1 trial.
Click on the PDF icon at the top of this introduction to read the full article.
Following an initial “no” vote from the Oncologic Drugs Advisory Committee (ODAC) in late 2014, the US Food and Drug Administration eventually awarded accelerated approval in February 2015 to the histone deacetylase (HDAC) inhibitor panobinostat for use in select patients with relapsed multiple myeloma. Panobinostat has a novel mechanism of action that demonstrates synergy with the proteasome inhibitor bortezomib and the immunomodulatory agent dexamethasone, which translated into improved progression-free survival (PFS) for patients with multiple myeloma who had received at least 2 prior therapies, according to data from a prespecified subgroup analysis from the Panorama-1 trial.
Click on the PDF icon at the top of this introduction to read the full article.
Following an initial “no” vote from the Oncologic Drugs Advisory Committee (ODAC) in late 2014, the US Food and Drug Administration eventually awarded accelerated approval in February 2015 to the histone deacetylase (HDAC) inhibitor panobinostat for use in select patients with relapsed multiple myeloma. Panobinostat has a novel mechanism of action that demonstrates synergy with the proteasome inhibitor bortezomib and the immunomodulatory agent dexamethasone, which translated into improved progression-free survival (PFS) for patients with multiple myeloma who had received at least 2 prior therapies, according to data from a prespecified subgroup analysis from the Panorama-1 trial.
Click on the PDF icon at the top of this introduction to read the full article.
Cardiovascular disease in oncology
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Surgical removal of malpositioned IUDs
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.