User login
Visual Management Board Implementation to Enhance High Reliability at a Large VA Health Care System
Health care organizations began implementing Lean management and high reliability organization (HRO) principles in the 1990s to improve quality and efficiency by aligning leaders and staff to a shared vision, fostering a culture of continuous improvement, identifying the root causes of complex problems, and engaging frontline staff as drivers of improvement efforts.1 There are 4 components for establishing a Lean management system: (1) leader standard work; (2) visual management; (3) daily accountability; and (4) discipline to institute the first 3 components.2 Leader standard work promotes continuous improvement by setting a standard routine of behaviors, actions, and tools consistently performed by leadership. These include routine and frequent frontline check-ins (ie, Gemba walks) as well as standardization of employee onboarding, training, and evaluations. Visual management refers to the process of making problems and abnormal conditions readily apparent to staff and leadership.3
The US Department of Veterans Affairs (VA) is committed to implementing similar principles of HROs, which focus on error analysis and process improvement to foster a culture of safety, leadership commitment, and staff engagement.4,5 Visual management is an important tool for HROs; it reflects the mindset of promoting transparency, teamwork, and openness.6,7
Visual management boards (VMBs), such as huddle boards, Gemba boards, or visibility walls, are critical tools that can promote daily accountability and the core principles of Lean thinking and HROs.1,6,8,9 Accountability is enhanced through frequent real-time, data-driven feedback between staff and leadership. This is often facilitated with a huddle, a structured and disciplined team meeting that provides bidirectional information.1 Frequently, a VMB is incorporated into the structure and flow of the huddle.
In a literature review of 20 years of implementation of Lean management systems in health care, Winner and colleagues report that while the frequency and duration of huddles vary, they are often united by several characteristics, including the involvement of the unit team, focus on feedback, problem identification and solutions, and central location around a visual board.1 VMBs most often take the form of a magnetic, dry-erase board located in a hall or conference room central to the work area.1 In addition to identifying and tracking problems in the place of work, VMBs can also provide a representation of key performance indicators and metrics, disseminate essential unit information, and acknowledge the work and successes of staff and leaders.6,8-12
This article outlines the commitment of the Lieutenant Colonel Charles S. Kettles VA Medical Center (VAMC) within the VA Ann Arbor Healthcare System (VAAAHS) to the HRO principle of visual management. We describe the incorporation of VMBs throughout VAAAHS and provide a detailed report of the development and use at a large outpatient subspecialty clinic.
Implementation
The goal of implementing visual management tools at VAAAHS was to empower staff members to identify problems and process improvements, enhance teamwork, and improve communication between staff and section leadership. The Systems Redesign and Improvement Program (SR), which supports Veteran Health Administration high reliability initiatives, helped implement VMBs in VAAAHS departments. Each board was designed to meet the specialized needs of each respective team and could be a physical board, virtual board, or combination. However, all boards sought to create standardized work and identify department needs.
The VAAAHS outpatient cardiology section VMB complemented an existing daily huddle framework. The cardiology section is large and diverse, with 6 subspecialty clinics, and team members who work in multiple locations. The clinic team includes 19 faculty physicians, 14 cardiology fellow physicians,9 nurse care managers, 13 nurse practitioners, 2 licensed practical nurses, and 5 medical support assistants at both the Lieutenant Colonel Charles S. Kettles VAMC and Toledo, Ohio, community based outpatient clinic. Prior to VMB implementation, a morning huddle with clinic team members led by a cardiology manager was an unstructured group discussion about clinic operations for the day. While the daily huddle had a positive impact on staff orientation to daily goals, it did not fully meet the aims of staff empowerment, problem identification and tracking, and knowledge distribution. The VMB was codeveloped with cardiology and the SR program with these goals in mind.
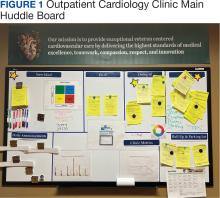
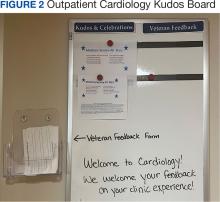
Cardiology was the first VAAAHS outpatient subspecialty clinic to institute a VMB. Two boards were created: a large standard VMB (Figure 1) and a smaller kudos board (Figure 2), which were placed in a central hallway in which staff members and patients pass frequently throughout the day. This location was chosen to promote engagement and promote the VAAAHS commitment to continuous improvement. The VMB focused on identifying and tracking problems, information sharing, and metric monitoring. The goal of the smaller kudos board was to highlight staff achievements and provide an opportunity for patient feedback.
The SR program required that the board incorporate problem identification and a uniform VAAAHS ticket tracking system. Each department could customize the VMB to fit its needs. Staff members are asked to define a problem, complete a ticket describing the issue, consider possible root causes, and suggest solutions. This approach empowers staff to take ownership, make a problem visible, and identify a solution. The problem is then discussed in group huddles using an Impact and Effort Matrix, a tool focused on categorizing and prioritizing those interventions that require low effort and lead to high impact.13
Tickets move along the board as they are addressed using a Plan-Do-Study-Act problem-solving model.14 Plan involves identifying and assigning leadership for the problem and understanding its root causes. Do involves implementing an action plan. Study involves evaluating the results. Finally, Act involves determining whether the plan was successful, and if so, standardizing the improvement and using it regularly.14 Complicated projects that require higher effort or additional resources are moved to the roll-up and parking lot, so they may be addressed by leadership at an appropriate time. Roll up is the escalation of process improvement tickets that frontline staff are unable to resolve with their current resources. The parking lot is for tickets that staff want to address later based on priority determined using an impact vs effort matrix. This allows for enhanced bidirectional communication between the department and high-level leadership, showing a commitment to HRO principles at all levels. The cardiology department customized its board to include essential clinic information, such as faculty staffing for the clinic that day and clinic metric information (eg, patient satisfaction scores, and appointment wait times). The kudos board, a space for patient feedback and to celebrate staff accomplishments, was located across the hall closest to the waiting area.
After the VMB was implemented as a new component to the daily team huddle, the group discussion physically moved to just in front of the board; pertinent clinic information is discussed daily, and the ticketing system is discussed 1 to 3 times per week, depending on ticket progress. Open and unresolved tickets are reviewed for updates on the status by the responsible team member, who receives ongoing feedback and assistance.
Program Impact
A total of 55 improvement opportunity tickets were submitted by staff members during the initial 23 months after the implementation of the outpatient cardiology clinic VMB. Most were submitted by nurse practitioners, although there were contributions from all faculty and staff. The high percentage of ticket submissions by nurse practitioners may be related to their full-time daily presence in the clinic, whereas some other staff members are part-time (most physicians are present 1 day each week). Improvement opportunities were noted within a variety of areas, including clinic facilities (eg, clinic equipment), communication between the clinic and patients (eg, telephone calls from patients or appointment letters), and patient care (eg, medication reconciliation and laboratory requisition).
In an improvement opportunity ticket, a staff member identified that the low seating in the patient waiting area was a fall risk and not diversified for varying body types. They posted a ticket, and the issue was discussed as a group. This staff member assumed ownership of the problem and placed an interior design request for taller chairs and bariatric options. The ticket was resolved when the waiting area was upgraded to include safer and more inclusive seating options for patients. Of 55 tickets submitted by staff as of June 2024, 45 have identified solutions, 4 are in process, and 6 have been placed in the parking lot. On average, the morning huddle spends about 5 to 10 minutes addressing tickets, but on occasion, more complex topics require additional time. The kudos board receives feedback from patients who express their gratitude, and serves as a space to celebrate awards received by staff members.
Implementing a VMB into daily huddles within the cardiology clinic led to increased staff engagement and ownership of challenges, as well as improved communication between frontline workers and leadership. VMBs have proven to be useful for annual staff performance evaluations because staff members who engaged in the board and volunteered to take accountability for ticket resolution could use those accomplishments in their assessments. Finally, VMBs made quality improvement and safety work accessible by normalizing frequent conversations. This empowered staff to engage in improvement projects and even led some members to enroll in formal Lean training.
The outpatient cardiology clinic VMB at the VAAAHS was identified as a best practice during a site visit by the Promising Practice Team in the Veterans Health Administration Office of Integrated Veteran Care. The outpatient cardiology clinic leadership team, including the authors of this article, was invited to present our visual management work as a main topic at the January 2024 Office of Integrated Veteran Care collaborative meeting.
Further Implementation
The SR program has collaborated with additional VAAAHS teams to implement VMBs. Forty-four physical VMBs and 20 virtual VMBs are currently in use throughout the VAAAHS. Virtual VMB content is similar to a physical board and can be modified by each team to meet its particular needs. Several virtual VMBs have been implemented at the VAAAHS and can achieve the same goals of staff teamwork, empowerment, and engagement. Each team can choose the format of the VMB that best fits their needs, which may be partially influenced by the team’s overall interaction style (on-site teams may function better with a physical VMB, and off-site teams may find a virtual VMB works best). VMBs have been implemented in various work areas, including laboratories, inpatient wards, subspecialty outpatient clinics, procedural areas, and the engineering department. In fiscal year 2024, 180 tickets were electronically submitted by teams across the VAAAHS, of which 170 identified solutions and were marked completed. Ticket counts may be underestimated since not all physical board tickets are reported in the electronic system. The SR program periodically attends morning huddles of various teams and obtains feedback on their VMBs, a practice that highlights its contribution to staff engagement, transparency, teamwork, and continuous improvement (Table). A goal of the SR program is to identify areas of the VAAAHS in which VMBs would add value to the team and implement them as necessary.
Discussion
VMBs are common in health care and are implemented to promote the core principlesof Lean thinking and HROs, including visual management and daily accountability. The goals of a visual management tool are to make problems visible and document their management. A VMB can serve as a focal point for team discussion and a physical space to track each problem through its initial identification, understanding of root causes, consideration of potential solutions, and recording of intervention results.
A VMB can foster a culture of safety, leadership commitment, and continuous process improvement when designed and implemented to reflect team needs. VMBs can empower staff members to share work concerns and openly promote engagement. As a central place for discussion between staff and leaders, VMBs can also foster teamwork and communication. The daily huddle provides a safe, productive working environment by ensuring that lines of communication are open among all team members, regardless of role or leadership designation.
Limitations
This article focused on the implementation of 1 type of visual management tool. It provides an in-depth discussion of the development, implementation, and experience with a VMB at multiple clinics of a single section in 1 health care system. These reported experiences may not represent other VA facilities. Perceptions of the impact and usefulness of the VMB were mostly anecdotal. Further evaluation of the VMB implementation experience and utility at other VA health care systems would provide additional insight into the optimal implementation of VMBs.
Conclusions
Through increased transparency, empowerment, and communication, VMBs are an important tool in the visual management tool belt for organizations committed to HROs and Lean management. Given the successful institution of VMBs at the VAAAHS, the description of our experience may aid other VA systems for the incorporation of visual management into the daily culture of their respective health care teams.
1. Winner LE, Reinhardt E, Benishek L, Marsteller JA. Lean management systems in health care: a review of the literature. Qual Manag Health Care. 2022;31(4):221-230. doi:10.1097/QMH.0000000000000353
2. Mann D. Creating a Lean Culture: Tools to Sustain Lean Conversions. Productivity Press; 2005.
3. Graban M. Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 3rd ed. Productivity Press; 2016.
4. Veazie S, Peterson K, Bourne D. Evidence Brief: Implementation of High Reliability Organization Principles. US Dept of Veterans Affairs; 2019. https://www.ncbi.nlm.nih.gov/books/NBK542883/
5. Stone RA, Lieberman SL. VHA’s Vision for a High Reliability Organization. US Dept of Veterans Affairs. Summer 2020. Accessed June 11, 2024. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
6. Bourgault AM, Upvall MJ, Graham A. Using Gemba boards to facilitate evidence-based practice in critical care. Crit Care Nurse. 2018;38(3):e1-e7. doi:10.4037/ccn2018714
7. Ferro J, Gouveia R. How to create an effective daily management system. Planet Lean. July 7, 2015. Accessed June 11, 2024. https://www.planet-lean.com/articles/lean-transformation-daily-management
8. Creating a cardiovascular OR huddle board. AORN J. 2020;111(6):687-690. Published 28 May 2020. doi:10.1002/aorn.13057
9. Rakover J, Little K, Scoville R, Holder B. Implementing daily management systems to support sustained quality improvement in ambulatory surgery centers. AORN J. 2020;111(4):415-422. doi:10.1002/aorn.12988
10. Loesche AH. Using huddles to improve communication and teamwork in an instrument-processing department. Nurs Manag (Harrow). 2020;27(6):34-42. doi:10.7748/nm.2020.e1958
11. Zarbo RJ, Varney RC, Copeland JR, D’Angelo R, Sharma G. Daily management system of the Henry Ford production system: QTIPS to focus continuous improvements at the level of the work. Am J Clin Pathol. 2015;144(1):122-136. doi:1309/AJCPLQYMOFWU31CK
12. Hung D, Martinez M, Yakir M, Gray C. Implementing a lean management system in primary care: facilitators and barriers from the front lines. Qual Manag Health Care. 2015;24(3):103-108. doi:10.1097/QMH.0000000000000062
13. Croft D. Guide: Impact and Effort Matrix. Learn Lean 6 Sigma. Accessed June 11, 2024. https://www.learnleansigma.com/guides/impact-effort-matrix/
14. Leis JA, Shojania KG. A primer on PDSA: executing plan-do-study-act cycles in practice, not just in name. BMJ Qual Saf. 2017;26(7):572-577. doi:10.1136/bmjqs-2016-006245
Health care organizations began implementing Lean management and high reliability organization (HRO) principles in the 1990s to improve quality and efficiency by aligning leaders and staff to a shared vision, fostering a culture of continuous improvement, identifying the root causes of complex problems, and engaging frontline staff as drivers of improvement efforts.1 There are 4 components for establishing a Lean management system: (1) leader standard work; (2) visual management; (3) daily accountability; and (4) discipline to institute the first 3 components.2 Leader standard work promotes continuous improvement by setting a standard routine of behaviors, actions, and tools consistently performed by leadership. These include routine and frequent frontline check-ins (ie, Gemba walks) as well as standardization of employee onboarding, training, and evaluations. Visual management refers to the process of making problems and abnormal conditions readily apparent to staff and leadership.3
The US Department of Veterans Affairs (VA) is committed to implementing similar principles of HROs, which focus on error analysis and process improvement to foster a culture of safety, leadership commitment, and staff engagement.4,5 Visual management is an important tool for HROs; it reflects the mindset of promoting transparency, teamwork, and openness.6,7
Visual management boards (VMBs), such as huddle boards, Gemba boards, or visibility walls, are critical tools that can promote daily accountability and the core principles of Lean thinking and HROs.1,6,8,9 Accountability is enhanced through frequent real-time, data-driven feedback between staff and leadership. This is often facilitated with a huddle, a structured and disciplined team meeting that provides bidirectional information.1 Frequently, a VMB is incorporated into the structure and flow of the huddle.
In a literature review of 20 years of implementation of Lean management systems in health care, Winner and colleagues report that while the frequency and duration of huddles vary, they are often united by several characteristics, including the involvement of the unit team, focus on feedback, problem identification and solutions, and central location around a visual board.1 VMBs most often take the form of a magnetic, dry-erase board located in a hall or conference room central to the work area.1 In addition to identifying and tracking problems in the place of work, VMBs can also provide a representation of key performance indicators and metrics, disseminate essential unit information, and acknowledge the work and successes of staff and leaders.6,8-12
This article outlines the commitment of the Lieutenant Colonel Charles S. Kettles VA Medical Center (VAMC) within the VA Ann Arbor Healthcare System (VAAAHS) to the HRO principle of visual management. We describe the incorporation of VMBs throughout VAAAHS and provide a detailed report of the development and use at a large outpatient subspecialty clinic.
Implementation
The goal of implementing visual management tools at VAAAHS was to empower staff members to identify problems and process improvements, enhance teamwork, and improve communication between staff and section leadership. The Systems Redesign and Improvement Program (SR), which supports Veteran Health Administration high reliability initiatives, helped implement VMBs in VAAAHS departments. Each board was designed to meet the specialized needs of each respective team and could be a physical board, virtual board, or combination. However, all boards sought to create standardized work and identify department needs.
The VAAAHS outpatient cardiology section VMB complemented an existing daily huddle framework. The cardiology section is large and diverse, with 6 subspecialty clinics, and team members who work in multiple locations. The clinic team includes 19 faculty physicians, 14 cardiology fellow physicians,9 nurse care managers, 13 nurse practitioners, 2 licensed practical nurses, and 5 medical support assistants at both the Lieutenant Colonel Charles S. Kettles VAMC and Toledo, Ohio, community based outpatient clinic. Prior to VMB implementation, a morning huddle with clinic team members led by a cardiology manager was an unstructured group discussion about clinic operations for the day. While the daily huddle had a positive impact on staff orientation to daily goals, it did not fully meet the aims of staff empowerment, problem identification and tracking, and knowledge distribution. The VMB was codeveloped with cardiology and the SR program with these goals in mind.
Cardiology was the first VAAAHS outpatient subspecialty clinic to institute a VMB. Two boards were created: a large standard VMB (Figure 1) and a smaller kudos board (Figure 2), which were placed in a central hallway in which staff members and patients pass frequently throughout the day. This location was chosen to promote engagement and promote the VAAAHS commitment to continuous improvement. The VMB focused on identifying and tracking problems, information sharing, and metric monitoring. The goal of the smaller kudos board was to highlight staff achievements and provide an opportunity for patient feedback.
The SR program required that the board incorporate problem identification and a uniform VAAAHS ticket tracking system. Each department could customize the VMB to fit its needs. Staff members are asked to define a problem, complete a ticket describing the issue, consider possible root causes, and suggest solutions. This approach empowers staff to take ownership, make a problem visible, and identify a solution. The problem is then discussed in group huddles using an Impact and Effort Matrix, a tool focused on categorizing and prioritizing those interventions that require low effort and lead to high impact.13
Tickets move along the board as they are addressed using a Plan-Do-Study-Act problem-solving model.14 Plan involves identifying and assigning leadership for the problem and understanding its root causes. Do involves implementing an action plan. Study involves evaluating the results. Finally, Act involves determining whether the plan was successful, and if so, standardizing the improvement and using it regularly.14 Complicated projects that require higher effort or additional resources are moved to the roll-up and parking lot, so they may be addressed by leadership at an appropriate time. Roll up is the escalation of process improvement tickets that frontline staff are unable to resolve with their current resources. The parking lot is for tickets that staff want to address later based on priority determined using an impact vs effort matrix. This allows for enhanced bidirectional communication between the department and high-level leadership, showing a commitment to HRO principles at all levels. The cardiology department customized its board to include essential clinic information, such as faculty staffing for the clinic that day and clinic metric information (eg, patient satisfaction scores, and appointment wait times). The kudos board, a space for patient feedback and to celebrate staff accomplishments, was located across the hall closest to the waiting area.
After the VMB was implemented as a new component to the daily team huddle, the group discussion physically moved to just in front of the board; pertinent clinic information is discussed daily, and the ticketing system is discussed 1 to 3 times per week, depending on ticket progress. Open and unresolved tickets are reviewed for updates on the status by the responsible team member, who receives ongoing feedback and assistance.
Program Impact
A total of 55 improvement opportunity tickets were submitted by staff members during the initial 23 months after the implementation of the outpatient cardiology clinic VMB. Most were submitted by nurse practitioners, although there were contributions from all faculty and staff. The high percentage of ticket submissions by nurse practitioners may be related to their full-time daily presence in the clinic, whereas some other staff members are part-time (most physicians are present 1 day each week). Improvement opportunities were noted within a variety of areas, including clinic facilities (eg, clinic equipment), communication between the clinic and patients (eg, telephone calls from patients or appointment letters), and patient care (eg, medication reconciliation and laboratory requisition).
In an improvement opportunity ticket, a staff member identified that the low seating in the patient waiting area was a fall risk and not diversified for varying body types. They posted a ticket, and the issue was discussed as a group. This staff member assumed ownership of the problem and placed an interior design request for taller chairs and bariatric options. The ticket was resolved when the waiting area was upgraded to include safer and more inclusive seating options for patients. Of 55 tickets submitted by staff as of June 2024, 45 have identified solutions, 4 are in process, and 6 have been placed in the parking lot. On average, the morning huddle spends about 5 to 10 minutes addressing tickets, but on occasion, more complex topics require additional time. The kudos board receives feedback from patients who express their gratitude, and serves as a space to celebrate awards received by staff members.
Implementing a VMB into daily huddles within the cardiology clinic led to increased staff engagement and ownership of challenges, as well as improved communication between frontline workers and leadership. VMBs have proven to be useful for annual staff performance evaluations because staff members who engaged in the board and volunteered to take accountability for ticket resolution could use those accomplishments in their assessments. Finally, VMBs made quality improvement and safety work accessible by normalizing frequent conversations. This empowered staff to engage in improvement projects and even led some members to enroll in formal Lean training.
The outpatient cardiology clinic VMB at the VAAAHS was identified as a best practice during a site visit by the Promising Practice Team in the Veterans Health Administration Office of Integrated Veteran Care. The outpatient cardiology clinic leadership team, including the authors of this article, was invited to present our visual management work as a main topic at the January 2024 Office of Integrated Veteran Care collaborative meeting.
Further Implementation
The SR program has collaborated with additional VAAAHS teams to implement VMBs. Forty-four physical VMBs and 20 virtual VMBs are currently in use throughout the VAAAHS. Virtual VMB content is similar to a physical board and can be modified by each team to meet its particular needs. Several virtual VMBs have been implemented at the VAAAHS and can achieve the same goals of staff teamwork, empowerment, and engagement. Each team can choose the format of the VMB that best fits their needs, which may be partially influenced by the team’s overall interaction style (on-site teams may function better with a physical VMB, and off-site teams may find a virtual VMB works best). VMBs have been implemented in various work areas, including laboratories, inpatient wards, subspecialty outpatient clinics, procedural areas, and the engineering department. In fiscal year 2024, 180 tickets were electronically submitted by teams across the VAAAHS, of which 170 identified solutions and were marked completed. Ticket counts may be underestimated since not all physical board tickets are reported in the electronic system. The SR program periodically attends morning huddles of various teams and obtains feedback on their VMBs, a practice that highlights its contribution to staff engagement, transparency, teamwork, and continuous improvement (Table). A goal of the SR program is to identify areas of the VAAAHS in which VMBs would add value to the team and implement them as necessary.
Discussion
VMBs are common in health care and are implemented to promote the core principlesof Lean thinking and HROs, including visual management and daily accountability. The goals of a visual management tool are to make problems visible and document their management. A VMB can serve as a focal point for team discussion and a physical space to track each problem through its initial identification, understanding of root causes, consideration of potential solutions, and recording of intervention results.
A VMB can foster a culture of safety, leadership commitment, and continuous process improvement when designed and implemented to reflect team needs. VMBs can empower staff members to share work concerns and openly promote engagement. As a central place for discussion between staff and leaders, VMBs can also foster teamwork and communication. The daily huddle provides a safe, productive working environment by ensuring that lines of communication are open among all team members, regardless of role or leadership designation.
Limitations
This article focused on the implementation of 1 type of visual management tool. It provides an in-depth discussion of the development, implementation, and experience with a VMB at multiple clinics of a single section in 1 health care system. These reported experiences may not represent other VA facilities. Perceptions of the impact and usefulness of the VMB were mostly anecdotal. Further evaluation of the VMB implementation experience and utility at other VA health care systems would provide additional insight into the optimal implementation of VMBs.
Conclusions
Through increased transparency, empowerment, and communication, VMBs are an important tool in the visual management tool belt for organizations committed to HROs and Lean management. Given the successful institution of VMBs at the VAAAHS, the description of our experience may aid other VA systems for the incorporation of visual management into the daily culture of their respective health care teams.
Health care organizations began implementing Lean management and high reliability organization (HRO) principles in the 1990s to improve quality and efficiency by aligning leaders and staff to a shared vision, fostering a culture of continuous improvement, identifying the root causes of complex problems, and engaging frontline staff as drivers of improvement efforts.1 There are 4 components for establishing a Lean management system: (1) leader standard work; (2) visual management; (3) daily accountability; and (4) discipline to institute the first 3 components.2 Leader standard work promotes continuous improvement by setting a standard routine of behaviors, actions, and tools consistently performed by leadership. These include routine and frequent frontline check-ins (ie, Gemba walks) as well as standardization of employee onboarding, training, and evaluations. Visual management refers to the process of making problems and abnormal conditions readily apparent to staff and leadership.3
The US Department of Veterans Affairs (VA) is committed to implementing similar principles of HROs, which focus on error analysis and process improvement to foster a culture of safety, leadership commitment, and staff engagement.4,5 Visual management is an important tool for HROs; it reflects the mindset of promoting transparency, teamwork, and openness.6,7
Visual management boards (VMBs), such as huddle boards, Gemba boards, or visibility walls, are critical tools that can promote daily accountability and the core principles of Lean thinking and HROs.1,6,8,9 Accountability is enhanced through frequent real-time, data-driven feedback between staff and leadership. This is often facilitated with a huddle, a structured and disciplined team meeting that provides bidirectional information.1 Frequently, a VMB is incorporated into the structure and flow of the huddle.
In a literature review of 20 years of implementation of Lean management systems in health care, Winner and colleagues report that while the frequency and duration of huddles vary, they are often united by several characteristics, including the involvement of the unit team, focus on feedback, problem identification and solutions, and central location around a visual board.1 VMBs most often take the form of a magnetic, dry-erase board located in a hall or conference room central to the work area.1 In addition to identifying and tracking problems in the place of work, VMBs can also provide a representation of key performance indicators and metrics, disseminate essential unit information, and acknowledge the work and successes of staff and leaders.6,8-12
This article outlines the commitment of the Lieutenant Colonel Charles S. Kettles VA Medical Center (VAMC) within the VA Ann Arbor Healthcare System (VAAAHS) to the HRO principle of visual management. We describe the incorporation of VMBs throughout VAAAHS and provide a detailed report of the development and use at a large outpatient subspecialty clinic.
Implementation
The goal of implementing visual management tools at VAAAHS was to empower staff members to identify problems and process improvements, enhance teamwork, and improve communication between staff and section leadership. The Systems Redesign and Improvement Program (SR), which supports Veteran Health Administration high reliability initiatives, helped implement VMBs in VAAAHS departments. Each board was designed to meet the specialized needs of each respective team and could be a physical board, virtual board, or combination. However, all boards sought to create standardized work and identify department needs.
The VAAAHS outpatient cardiology section VMB complemented an existing daily huddle framework. The cardiology section is large and diverse, with 6 subspecialty clinics, and team members who work in multiple locations. The clinic team includes 19 faculty physicians, 14 cardiology fellow physicians,9 nurse care managers, 13 nurse practitioners, 2 licensed practical nurses, and 5 medical support assistants at both the Lieutenant Colonel Charles S. Kettles VAMC and Toledo, Ohio, community based outpatient clinic. Prior to VMB implementation, a morning huddle with clinic team members led by a cardiology manager was an unstructured group discussion about clinic operations for the day. While the daily huddle had a positive impact on staff orientation to daily goals, it did not fully meet the aims of staff empowerment, problem identification and tracking, and knowledge distribution. The VMB was codeveloped with cardiology and the SR program with these goals in mind.
Cardiology was the first VAAAHS outpatient subspecialty clinic to institute a VMB. Two boards were created: a large standard VMB (Figure 1) and a smaller kudos board (Figure 2), which were placed in a central hallway in which staff members and patients pass frequently throughout the day. This location was chosen to promote engagement and promote the VAAAHS commitment to continuous improvement. The VMB focused on identifying and tracking problems, information sharing, and metric monitoring. The goal of the smaller kudos board was to highlight staff achievements and provide an opportunity for patient feedback.
The SR program required that the board incorporate problem identification and a uniform VAAAHS ticket tracking system. Each department could customize the VMB to fit its needs. Staff members are asked to define a problem, complete a ticket describing the issue, consider possible root causes, and suggest solutions. This approach empowers staff to take ownership, make a problem visible, and identify a solution. The problem is then discussed in group huddles using an Impact and Effort Matrix, a tool focused on categorizing and prioritizing those interventions that require low effort and lead to high impact.13
Tickets move along the board as they are addressed using a Plan-Do-Study-Act problem-solving model.14 Plan involves identifying and assigning leadership for the problem and understanding its root causes. Do involves implementing an action plan. Study involves evaluating the results. Finally, Act involves determining whether the plan was successful, and if so, standardizing the improvement and using it regularly.14 Complicated projects that require higher effort or additional resources are moved to the roll-up and parking lot, so they may be addressed by leadership at an appropriate time. Roll up is the escalation of process improvement tickets that frontline staff are unable to resolve with their current resources. The parking lot is for tickets that staff want to address later based on priority determined using an impact vs effort matrix. This allows for enhanced bidirectional communication between the department and high-level leadership, showing a commitment to HRO principles at all levels. The cardiology department customized its board to include essential clinic information, such as faculty staffing for the clinic that day and clinic metric information (eg, patient satisfaction scores, and appointment wait times). The kudos board, a space for patient feedback and to celebrate staff accomplishments, was located across the hall closest to the waiting area.
After the VMB was implemented as a new component to the daily team huddle, the group discussion physically moved to just in front of the board; pertinent clinic information is discussed daily, and the ticketing system is discussed 1 to 3 times per week, depending on ticket progress. Open and unresolved tickets are reviewed for updates on the status by the responsible team member, who receives ongoing feedback and assistance.
Program Impact
A total of 55 improvement opportunity tickets were submitted by staff members during the initial 23 months after the implementation of the outpatient cardiology clinic VMB. Most were submitted by nurse practitioners, although there were contributions from all faculty and staff. The high percentage of ticket submissions by nurse practitioners may be related to their full-time daily presence in the clinic, whereas some other staff members are part-time (most physicians are present 1 day each week). Improvement opportunities were noted within a variety of areas, including clinic facilities (eg, clinic equipment), communication between the clinic and patients (eg, telephone calls from patients or appointment letters), and patient care (eg, medication reconciliation and laboratory requisition).
In an improvement opportunity ticket, a staff member identified that the low seating in the patient waiting area was a fall risk and not diversified for varying body types. They posted a ticket, and the issue was discussed as a group. This staff member assumed ownership of the problem and placed an interior design request for taller chairs and bariatric options. The ticket was resolved when the waiting area was upgraded to include safer and more inclusive seating options for patients. Of 55 tickets submitted by staff as of June 2024, 45 have identified solutions, 4 are in process, and 6 have been placed in the parking lot. On average, the morning huddle spends about 5 to 10 minutes addressing tickets, but on occasion, more complex topics require additional time. The kudos board receives feedback from patients who express their gratitude, and serves as a space to celebrate awards received by staff members.
Implementing a VMB into daily huddles within the cardiology clinic led to increased staff engagement and ownership of challenges, as well as improved communication between frontline workers and leadership. VMBs have proven to be useful for annual staff performance evaluations because staff members who engaged in the board and volunteered to take accountability for ticket resolution could use those accomplishments in their assessments. Finally, VMBs made quality improvement and safety work accessible by normalizing frequent conversations. This empowered staff to engage in improvement projects and even led some members to enroll in formal Lean training.
The outpatient cardiology clinic VMB at the VAAAHS was identified as a best practice during a site visit by the Promising Practice Team in the Veterans Health Administration Office of Integrated Veteran Care. The outpatient cardiology clinic leadership team, including the authors of this article, was invited to present our visual management work as a main topic at the January 2024 Office of Integrated Veteran Care collaborative meeting.
Further Implementation
The SR program has collaborated with additional VAAAHS teams to implement VMBs. Forty-four physical VMBs and 20 virtual VMBs are currently in use throughout the VAAAHS. Virtual VMB content is similar to a physical board and can be modified by each team to meet its particular needs. Several virtual VMBs have been implemented at the VAAAHS and can achieve the same goals of staff teamwork, empowerment, and engagement. Each team can choose the format of the VMB that best fits their needs, which may be partially influenced by the team’s overall interaction style (on-site teams may function better with a physical VMB, and off-site teams may find a virtual VMB works best). VMBs have been implemented in various work areas, including laboratories, inpatient wards, subspecialty outpatient clinics, procedural areas, and the engineering department. In fiscal year 2024, 180 tickets were electronically submitted by teams across the VAAAHS, of which 170 identified solutions and were marked completed. Ticket counts may be underestimated since not all physical board tickets are reported in the electronic system. The SR program periodically attends morning huddles of various teams and obtains feedback on their VMBs, a practice that highlights its contribution to staff engagement, transparency, teamwork, and continuous improvement (Table). A goal of the SR program is to identify areas of the VAAAHS in which VMBs would add value to the team and implement them as necessary.
Discussion
VMBs are common in health care and are implemented to promote the core principlesof Lean thinking and HROs, including visual management and daily accountability. The goals of a visual management tool are to make problems visible and document their management. A VMB can serve as a focal point for team discussion and a physical space to track each problem through its initial identification, understanding of root causes, consideration of potential solutions, and recording of intervention results.
A VMB can foster a culture of safety, leadership commitment, and continuous process improvement when designed and implemented to reflect team needs. VMBs can empower staff members to share work concerns and openly promote engagement. As a central place for discussion between staff and leaders, VMBs can also foster teamwork and communication. The daily huddle provides a safe, productive working environment by ensuring that lines of communication are open among all team members, regardless of role or leadership designation.
Limitations
This article focused on the implementation of 1 type of visual management tool. It provides an in-depth discussion of the development, implementation, and experience with a VMB at multiple clinics of a single section in 1 health care system. These reported experiences may not represent other VA facilities. Perceptions of the impact and usefulness of the VMB were mostly anecdotal. Further evaluation of the VMB implementation experience and utility at other VA health care systems would provide additional insight into the optimal implementation of VMBs.
Conclusions
Through increased transparency, empowerment, and communication, VMBs are an important tool in the visual management tool belt for organizations committed to HROs and Lean management. Given the successful institution of VMBs at the VAAAHS, the description of our experience may aid other VA systems for the incorporation of visual management into the daily culture of their respective health care teams.
1. Winner LE, Reinhardt E, Benishek L, Marsteller JA. Lean management systems in health care: a review of the literature. Qual Manag Health Care. 2022;31(4):221-230. doi:10.1097/QMH.0000000000000353
2. Mann D. Creating a Lean Culture: Tools to Sustain Lean Conversions. Productivity Press; 2005.
3. Graban M. Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 3rd ed. Productivity Press; 2016.
4. Veazie S, Peterson K, Bourne D. Evidence Brief: Implementation of High Reliability Organization Principles. US Dept of Veterans Affairs; 2019. https://www.ncbi.nlm.nih.gov/books/NBK542883/
5. Stone RA, Lieberman SL. VHA’s Vision for a High Reliability Organization. US Dept of Veterans Affairs. Summer 2020. Accessed June 11, 2024. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
6. Bourgault AM, Upvall MJ, Graham A. Using Gemba boards to facilitate evidence-based practice in critical care. Crit Care Nurse. 2018;38(3):e1-e7. doi:10.4037/ccn2018714
7. Ferro J, Gouveia R. How to create an effective daily management system. Planet Lean. July 7, 2015. Accessed June 11, 2024. https://www.planet-lean.com/articles/lean-transformation-daily-management
8. Creating a cardiovascular OR huddle board. AORN J. 2020;111(6):687-690. Published 28 May 2020. doi:10.1002/aorn.13057
9. Rakover J, Little K, Scoville R, Holder B. Implementing daily management systems to support sustained quality improvement in ambulatory surgery centers. AORN J. 2020;111(4):415-422. doi:10.1002/aorn.12988
10. Loesche AH. Using huddles to improve communication and teamwork in an instrument-processing department. Nurs Manag (Harrow). 2020;27(6):34-42. doi:10.7748/nm.2020.e1958
11. Zarbo RJ, Varney RC, Copeland JR, D’Angelo R, Sharma G. Daily management system of the Henry Ford production system: QTIPS to focus continuous improvements at the level of the work. Am J Clin Pathol. 2015;144(1):122-136. doi:1309/AJCPLQYMOFWU31CK
12. Hung D, Martinez M, Yakir M, Gray C. Implementing a lean management system in primary care: facilitators and barriers from the front lines. Qual Manag Health Care. 2015;24(3):103-108. doi:10.1097/QMH.0000000000000062
13. Croft D. Guide: Impact and Effort Matrix. Learn Lean 6 Sigma. Accessed June 11, 2024. https://www.learnleansigma.com/guides/impact-effort-matrix/
14. Leis JA, Shojania KG. A primer on PDSA: executing plan-do-study-act cycles in practice, not just in name. BMJ Qual Saf. 2017;26(7):572-577. doi:10.1136/bmjqs-2016-006245
1. Winner LE, Reinhardt E, Benishek L, Marsteller JA. Lean management systems in health care: a review of the literature. Qual Manag Health Care. 2022;31(4):221-230. doi:10.1097/QMH.0000000000000353
2. Mann D. Creating a Lean Culture: Tools to Sustain Lean Conversions. Productivity Press; 2005.
3. Graban M. Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 3rd ed. Productivity Press; 2016.
4. Veazie S, Peterson K, Bourne D. Evidence Brief: Implementation of High Reliability Organization Principles. US Dept of Veterans Affairs; 2019. https://www.ncbi.nlm.nih.gov/books/NBK542883/
5. Stone RA, Lieberman SL. VHA’s Vision for a High Reliability Organization. US Dept of Veterans Affairs. Summer 2020. Accessed June 11, 2024. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
6. Bourgault AM, Upvall MJ, Graham A. Using Gemba boards to facilitate evidence-based practice in critical care. Crit Care Nurse. 2018;38(3):e1-e7. doi:10.4037/ccn2018714
7. Ferro J, Gouveia R. How to create an effective daily management system. Planet Lean. July 7, 2015. Accessed June 11, 2024. https://www.planet-lean.com/articles/lean-transformation-daily-management
8. Creating a cardiovascular OR huddle board. AORN J. 2020;111(6):687-690. Published 28 May 2020. doi:10.1002/aorn.13057
9. Rakover J, Little K, Scoville R, Holder B. Implementing daily management systems to support sustained quality improvement in ambulatory surgery centers. AORN J. 2020;111(4):415-422. doi:10.1002/aorn.12988
10. Loesche AH. Using huddles to improve communication and teamwork in an instrument-processing department. Nurs Manag (Harrow). 2020;27(6):34-42. doi:10.7748/nm.2020.e1958
11. Zarbo RJ, Varney RC, Copeland JR, D’Angelo R, Sharma G. Daily management system of the Henry Ford production system: QTIPS to focus continuous improvements at the level of the work. Am J Clin Pathol. 2015;144(1):122-136. doi:1309/AJCPLQYMOFWU31CK
12. Hung D, Martinez M, Yakir M, Gray C. Implementing a lean management system in primary care: facilitators and barriers from the front lines. Qual Manag Health Care. 2015;24(3):103-108. doi:10.1097/QMH.0000000000000062
13. Croft D. Guide: Impact and Effort Matrix. Learn Lean 6 Sigma. Accessed June 11, 2024. https://www.learnleansigma.com/guides/impact-effort-matrix/
14. Leis JA, Shojania KG. A primer on PDSA: executing plan-do-study-act cycles in practice, not just in name. BMJ Qual Saf. 2017;26(7):572-577. doi:10.1136/bmjqs-2016-006245
Strategies of Female Teaching Attending Physicians to Navigate Gender-Based Challenges: An Exploratory Qualitative Study
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
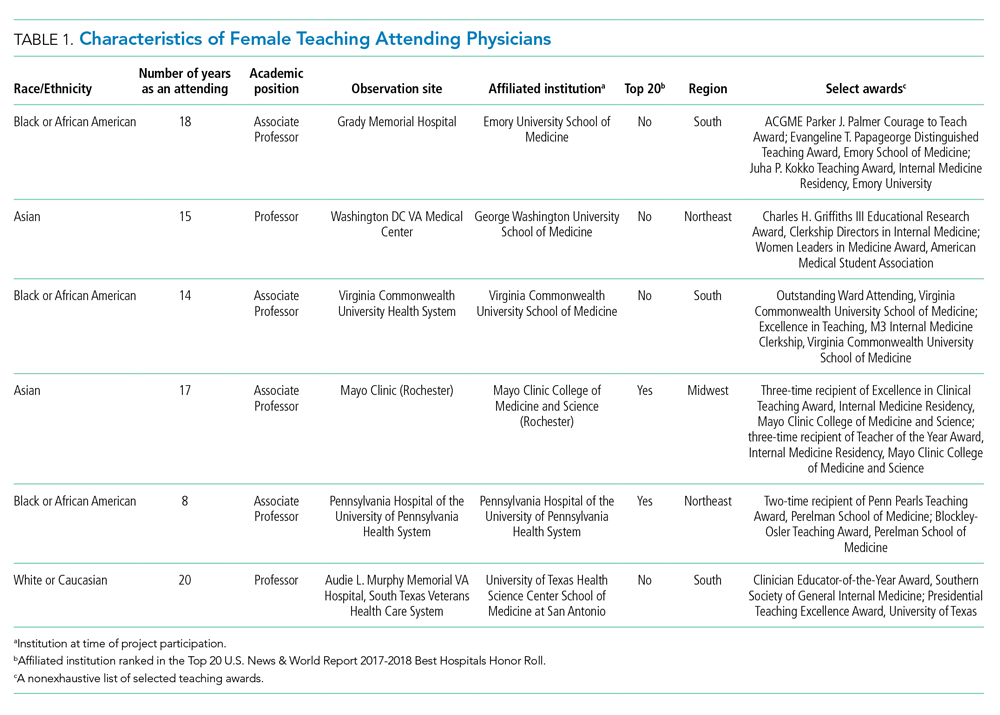
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).
The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.
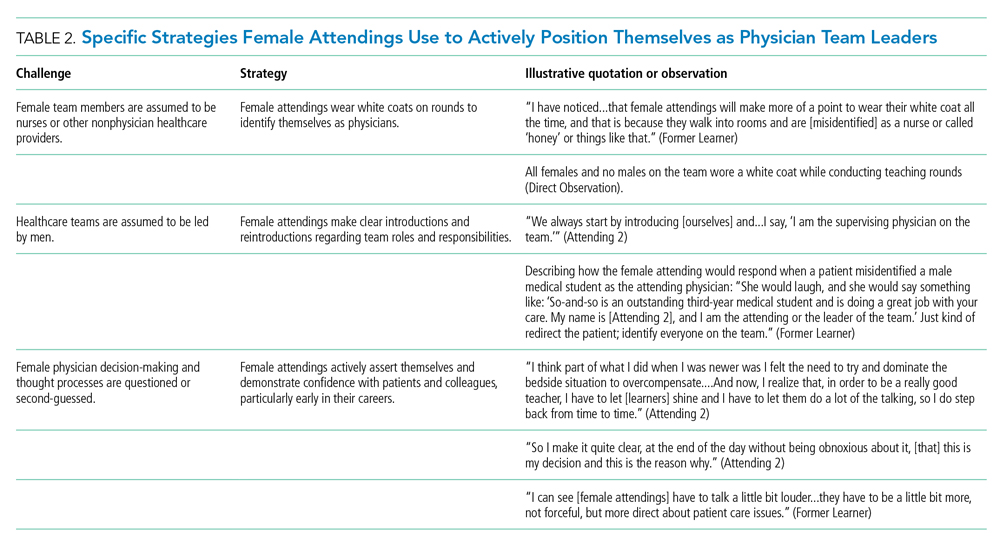
We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.
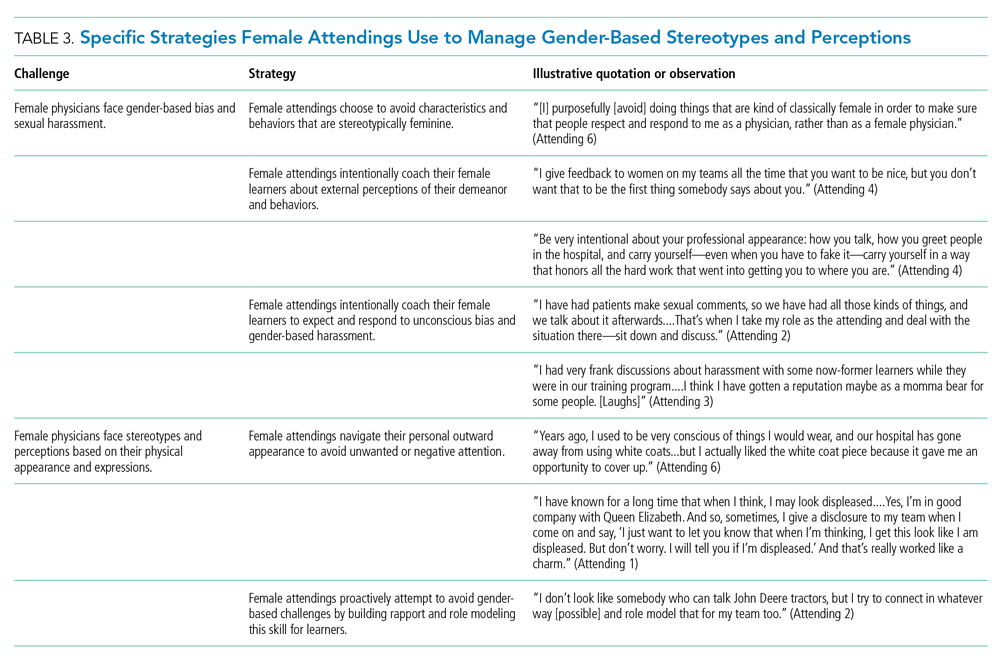
Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”
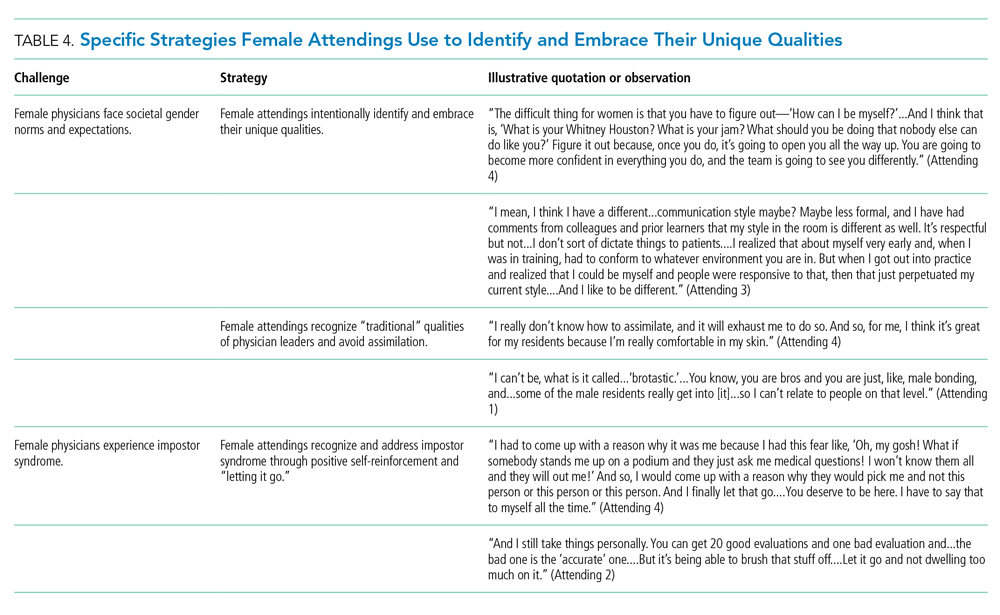
Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
© 2020 Society of Hospital Medicine
Compassionate Communication Amid the COVID-19 Pandemic
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
© 2020 Society of Hospital Medicine
A Jaw-Dropping Diagnosis
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.
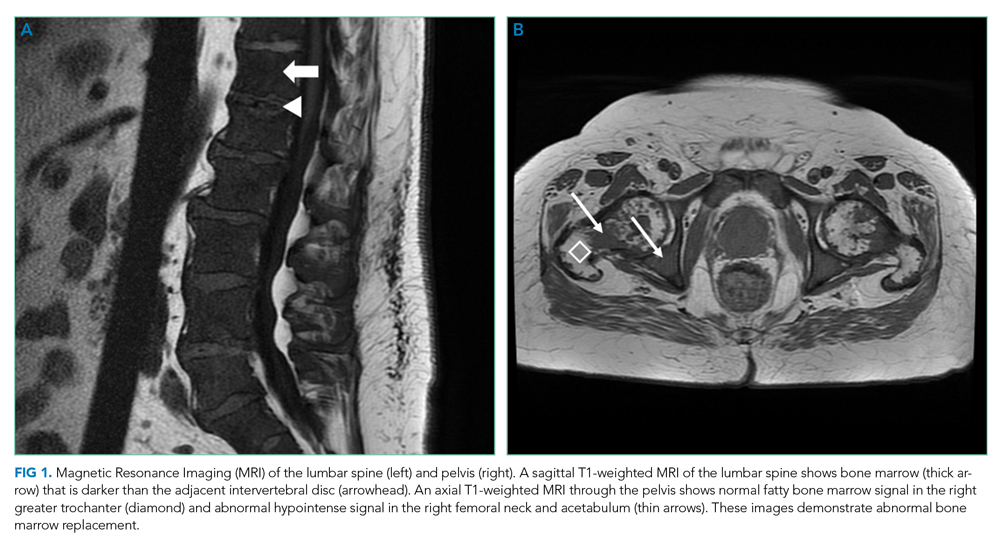
The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.
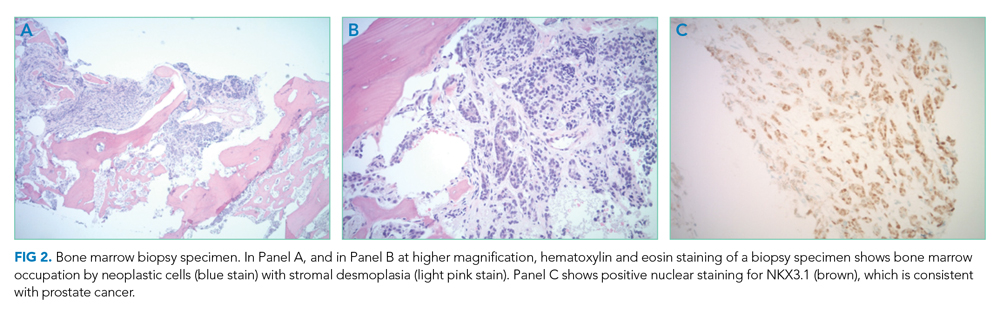
The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.

The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.

The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.

The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.

The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
© 2020 Society of Hospital Medicine
Patient Preferences for Physician Attire: A Multicenter Study in Japan
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
© 2020 Society of Hospital Medicine
A Branching Algorithm
A 21-year-old man with a history of hypertension presented to the emergency department with four days of generalized abdominal pain, nausea, and vomiting as well as one month of loose stools. He also had a headache (not further specified) for one day. Due to his nausea, he had been unable to take his medications for two days. Home blood pressure measurements over the preceding two days revealed systolic pressures exceeding 200 mm Hg. He did not experience fever, dyspnea, chest pain, vision changes, numbness, weakness, diaphoresis, or palpitations.
Abdominal pain with vomiting and diarrhea is often caused by a self-limited gastroenteritis. However, the priority initially is to exclude serious intraabdominal processes including arterial insufficiency, bowel obstruction, organ perforation, or organ-based infection or inflammation (eg, appendicitis, cholecystitis, pancreatitis). Essential hypertension accounts for 95% of cases of hypertension in the United States, but given this patient’s young age, secondary causes should be evaluated. These include primary aldosteronism (the most common endocrine cause for hypertension in young patients), chronic kidney disease, fibromuscular dysplasia, illicit drug use, hypercortisolism, pheochromocytoma, and coarctation of the aorta. Thyrotoxicosis can elevate blood pressure (although usually not to this extent) and cause hyperdefecation. While the etiology of the chronic hypertension is uncertain, the proximate cause of the acute rise in blood pressure is likely the stress of his acute illness and the inability to take his prescribed antihypertensive medications. In the setting of severe hypertension, his headache may reflect an intracranial hemorrhage and his abdominal pain could signal an aortic dissection.
His medical history included hypertension diagnosed at age 16 as well as anxiety diagnosed following a panic attack at age 19. Over the past year, he had also developed persistent nausea, which was attributed to gastroesophageal reflux disease. His medications included metoprolol 50 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 12.5 mg daily, escitalopram 20 mg daily, and omeprazole 20 mg daily. His father and 15-year-old brother also had hypertension. He was a part-time student while working at a car dealership. He did not smoke or use drugs and he rarely drank alcohol.
The need for three antihypertensive medications (albeit at submaximal doses) reflects the severity of his hypertension (provided challenges with medication adherence have been excluded). His family history, especially that of his brother who was diagnosed with hypertension at an early age, and the patient’s own early onset hypertension point toward an inherited form of hypertension. Autosomal dominant polycystic kidney disease often results in hypertension before chronic kidney disease develops. Rare inherited forms of hypertension include familial hyperaldosteronism, apparent mineralocorticoid excess, Liddle syndrome, or a hereditary endocrine tumor syndrome predisposing to pheochromocytoma. Even among patients who report classic pheochromocytoma symptoms, such as headache and anxiety, the diagnosis remains unlikely as these symptoms are nonspecific and highly prevalent in the general population. However, once secondary hypertension is plausible or suspected, testing for hyperadrenergic states, which can also cause nausea and vomiting during times of catecholamine excess, should be pursued.
His temperature was 97.5°F, heart rate 95 beats per minute and regular, respiratory rate 18 breaths per minute, blood pressure 181/118 mm Hg (systolic and diastolic pressures in each arm were within 10 mm Hg), and oxygen saturation 100% on room air. Systolic and diastolic pressures did not decrease by more than 20 mm Hg and 10 mm Hg, respectively, after he stood for two minutes. His body mass index was 24 kg/m2. He was alert and appeared slightly anxious. There was a bounding point of maximal impulse in the fifth intercostal space at the midclavicular line and a 3/6 systolic murmur at the left upper sternal border with radiation to the carotid arteries. His abdomen was soft with generalized tenderness to palpation and without rebound tenderness, masses, organomegaly, or bruits. There was no costovertebral angle tenderness. No lymphadenopathy was present. His fundoscopic, pulmonary, skin and neurologic examinations were normal.
Laboratory studies revealed a white blood cell count of 13.3 × 103/uL with a normal differential, hemoglobin 13.9 g/dL, platelet count 373 × 103/uL, sodium 142 mmol/L, potassium 3.8 mmol/L, chloride 103 mmol/L,bicarbonate 25 mmol/L, blood urea nitrogen 12 mg/dL, creatinine 1.3 mg/dL (a baseline creatinine level was not available), glucose 88 mg/dL, calcium 10.6 mg/dL, albumin 4.9 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 37 IU/L, and lipase 40 IU/L. Urinalysis revealed 5-10 white blood cells per high power field without casts and 10 mg/dL protein. Urine toxicology was not performed. Electrocardiogram (ECG) showed left ventricular hypertrophy (LVH). Chest radiography was normal.
The abdominal examination does not suggest peritonitis. The laboratory tests do not suggest inflammation of the liver, pancreas, or biliary tree as the cause of his abdominal pain or diarrhea. The murmur may indicate hypertrophic cardiomyopathy or a congenital anomaly such as bicuspid aortic valve; but neither would explain hypertension unless they were associated with another developmental abnormality, such as coarctation of the aorta. Tricuspid regurgitation is conceivable and if confirmed, might raise concern for carcinoid syndrome, which can cause diarrhea. The normal neurologic examination, including the absence of papilledema, lowers suspicion of intracranial hemorrhage as a cause of his headache.
The albumin of 4.9 g/dL likely reflects hypovolemia resulting from vomiting and diarrhea. Vasoconstriction associated with pheochromocytoma can cause pressure diuresis and resultant hypovolemia. Hyperaldosteronism arising from bilateral adrenal hyperplasia or adrenal adenoma commonly causes hypokalemia, although this is not a universal feature.
The duration of his mildly decreased glomerular filtration rate is uncertain. He may have chronic kidney disease from sustained hypertension, or acute kidney injury from hypovolemia. The mild pyuria could indicate infection or renal calculi, either of which could account for generalized abdominal pain or could reflect an acute renal injury from acute interstitial nephritis from his proton pump inhibitor or hydrochlorothiazide.
LVH on the ECG indicates longstanding hypertension. The chest radiograph does not reveal clues to the etiology of or sequelae from hypertension. In particular, there is no widened aorta to suggest aortic dissection, no pulmonary edema to indicate heart failure, and no rib notching that points toward aortic coarctation. A transthoracic echocardiogram to assess for valvular and other structural abnormalities is warranted.
Tests for secondary hypertension should be sent, including serum aldosterone and renin levels to assess for primary aldosteronism and plasma or 24-hour urine normetanephrine and metanephrine levels to assess for pheochromocytoma. Biochemical evaluation is the mainstay for endocrine hypertension evaluation and should be followed by imaging if abnormal results are found.
Intact parathyroid hormone (PTH) was 78 pg/mL (normal, 10-65 pg/mL), thyroid stimulating hormone 3.6 mIU/L (normal, 0.30-5.50 mIU/L), and morning cortisol 4.1 ug/dL (normal, >7.0 ug/dL). Plasma aldosterone was 14.6 ng/dL (normal, 1-16 ng/dL), plasma renin activity 3.6 ng/mL/hr (normal, 0.5-3.5 ng/mL/hr), and aldosterone-renin ratio 4.1 (normal, <20). Transthoracic echocardiogram showed LVH with normal valves, wall motion, and proximal aorta; the left ventricular ejection fraction was 70%. Magnetic resonance angiography of the renal vessels demonstrated no abnormalities.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed a 5 cm heterogeneous enhancing mass associated with the prostate gland extending into the base of the bladder. The mass obstructed the right renal collecting system and ureter causing severe right-sided ureterectasis and hydronephrosis. There was also 2.8 cm right-sided paracaval lymph node enlargement and 2.1 cm right-sided and 1.5 cm left-sided external iliac lymph node enlargement (Figure 1). There were no adrenal masses.
He is young for prostate, bladder, or colorectal cancer, but early onset variations of these tumors, along with metastatic testicular cancer, must be considered for the pelvic mass and associated lymphadenopathy. Prostatic masses can be infectious (eg, abscess) or malignant (eg, adenocarcinoma, small cell carcinoma). Additional considerations for abdominopelvic cancer are sarcomas, germ cell tumors, or lymphoma. A low aldosterone-renin ratio coupled with a normal potassium level makes primary aldosteronism unlikely. The normal angiography excludes renovascular hypertension.
His abdominal pain and gastrointestinal symptoms could arise from irritation of the bowel, distension of the right-sided urinary collecting system, or products secreted from the mass (eg, catecholamines). The hyperdynamic precordium, elevated ejection fraction, and murmur may reflect augmented blood flow from a hyperadrenergic state. A unifying diagnosis would be a pheochromocytoma. However, given the normal appearance of the adrenal glands on CT imaging, catecholamines arising from a paraganglioma, a tumor of the autonomic nervous system, is more likely. These tumors often secrete catecholamines and can be metastatic (suggested here by the lymphadenopathy). Functional imaging or biopsy of either the mass or an adjacent lymph node is indicated. However, because of the possibility of a catecholamine-secreting tumor, he should be treated with an alpha-adrenergic receptor antagonist before undergoing a biopsy to prevent unopposed vasoconstriction from catecholamine leakage.
Scrotal ultrasound revealed no evidence of a testicular tumor. Lactate dehydrogenase (LDH) was 179 IU/L (normal, 120-240 IU/L) and prostate specific antigen (PSA) was 0.7 ng/mL (normal, <2.5 ng/mL). The patient was given amlodipine and labetalol with improvement of blood pressures to 160s/100s. His creatinine decreased to 1.1 mg/dL. He underwent CT-guided biopsy of a pelvic lymph node. CT of the head without intravenous contrast demonstrated no intracranial abnormalities. His headache resolved with improvement in blood pressure, and he had minimal gastrointestinal symptoms during his hospitalization. No stool studies were sent. A right-sided percutaneous nephrostomy was placed which yielded >15 L of urine from the tube over the next four days.
Upon the first episode of micturition through the urethra four days after percutaneous nephrostomy placement, he experienced severe lightheadedness, diaphoresis, and palpitations. These symptoms prompted him to recall similar episodes following micturition for several months prior to his hospitalization.
It is likely that contraction of the bladder during episodes of urination caused irritation of the pelvic mass, leading to catecholamine secretion. Another explanation for his recurrent lightheadedness would be a neurocardiogenic reflex with micturition (which when it culminates with loss of consciousness is called micturition syncope), but this would not explain his hypertension or bladder mass.
Biochemical tests that were ordered on admission but sent to a reference lab then returned. Plasma metanephrine was 0.2 nmol/L (normal, <0.5 nmol/L) and plasma normetanephrine 34.6 nmol/L (normal, <0.9 nmol/L). His 24-hour urine metanephrine was 72 ug/24 hr (normal, 0-300 ug/24 hr) and normetanephrine 8,511 ug/24 hr (normal, 50-800 ug/24 hr).
The markedly elevated plasma and urine normetanephrine levels confirm a diagnosis of a catecholamine-secreting tumor (paraganglioma). The tissue obtained from the CT-guided lymph node biopsy should be sent for markers of neuroendocrine tumors including chromogranin.
Lymph node biopsy revealed metastatic paraganglioma that was chromogranin A and synaptophysin positive (Figure 2). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan disclosed skull metastases. He was treated with phenoxybenzamine, amlodipine, and labetalol. Surgical resection of the pelvic mass was discussed, but the patient elected to defer surgery as the location of the primary tumor made it challenging to resect and would have required an ileal conduit.
After the diagnosis was made, the patient’s family recalled that a maternal uncle had been diagnosed with a paraganglioma of the carotid body. Genetic testing of the patient identified a succinate dehydrogenase complex subunit B (SDHB) pathogenic variant and confirmed hereditary paraganglioma syndrome (HPGL). One year after the diagnosis, liver and lung metastases developed. He was treated with lanreotide (somatostatin analogue), capecitabine, and temozolomide, as well as a craniotomy and radiotherapy for palliation of bony metastases. The patient died less than two years after diagnosis.
DISCUSSION
Most patients with hypertension (defined as blood pressure >130/80 mm Hg1) do not have an identifiable etiology (primary hypertension). Many components of this patient’s history, however, including his young age of onset, a teenage sibling with hypertension, lack of obesity, hypertension refractory to multiple medications, and LVH suggested secondary hypertension. Hypertension onset at an age less than 30 years, resistance to three or more medications,1,2 and/or acute onset hypertension at any age should prompt an evaluation for secondary causes.1 The prevalence of secondary hypertension is approximately 30% in hypertensive patients ages 18 to 40 years compared with 5%-10% in the overall adult population with hypertension.3 Among children and adolescents ages 0 to 19 years with hypertension, the prevalence of secondary hypertension may be as high as 57%.4
The most common etiology of secondary hypertension is primary aldosteronism.5,6 However, in young adults (ages 19 to 39 years), common etiologies also include renovascular disease and renal parenchymal disease.7 Other causes include obstructive sleep apnea, medications, stimulants (cocaine and amphetamines),8 and endocrinopathies such as thyrotoxicosis, Cushing syndrome, and catecholamine-secreting tumors.7 Less than 1% of secondary hypertension in all adults is due to catecholamine-secreting tumors, and the minority of those catecholamine-secreting tumors are paragangliomas.9
Paragangliomas are tumors of the peripheral autonomic nervous system. These neoplasms arise in the sympathetic and parasympathetic chains along the paravertebral and paraaortic axes. They are closely related to pheochromocytomas, which arise in the adrenal medulla.9 Most head and neck paragangliomas are biochemically silent and are generally discovered due to mass effect.10 The subset of paragangliomas that secrete catecholamines most often arise in the abdomen and pelvis, and their clinical presentation mimics that of pheochromocytomas, including episodic hypertension, palpitations, pallor, and diaphoresis.
This patient had persistent, nonepisodic hypertension, while palpitations and diaphoresis only manifested following micturition. Other cases of urinary bladder paragangliomas have described micturition-associated symptoms and hypertensive crises. Three-fold increases of catecholamine secretion after micturition have been observed in these patients, likely due to muscle contraction and pressure changes in the bladder leading to the systemic release of catecholamines.11
Epinephrine and norepinephrine are monoamine neurotransmitters that activate alpha-adrenergic and beta-adrenergic receptors. Adrenergic receptors are present in all tissues of the body but have prominent effects on the smooth muscle in the vasculature, gastrointestinal tract, urinary tract, and airways.12 Alpha-adrenergic vasoconstriction causes hypertension, which is commonly observed in patients with catecholamine-secreting tumors.10 Catecholamine excess due to secretion from these tumors causes headache in 60%-80% of patients, tachycardia/palpitations in 50%-70%, anxiety in 20%-40%, and nausea in 20%-25%.10 Other symptoms include sweating, pallor, dyspnea, and vertigo.9,10 This patient’s chronic nausea, which was attributed to gastroesophageal reflux, and his anxiety, attributed to generalized anxiety disorder, were likely symptoms of catecholamine excess.13
The best test for the diagnosis of paragangliomas and pheochromocytomas is the measurement of plasma free or 24-hour urinary fractionated metanephrines (test sensitivity of >90% and >90%, respectively).14 Screening for pheochromocytoma should be considered in hypertensive patients who have symptoms of catecholamine excess, refractory or paroxysmal hypertension, and/or familial pheochromocytoma/paraganglioma syndromes.15 Screening for pheochromocytoma should also be performed in children and adolescents with systolic or diastolic blood pressure that is greater than the 95th percentile for their age plus 5 mm Hg.16
While a typical tumor location and elevated metanephrine levels are sufficient to make the diagnosis of a pheochromocytoma or catecholamine-secreting paraganglioma, functional imaging with FDG-PET, Ga-DOTATATE-PET, or 123I-meta-iodobenzylguanidine (123I-MIBG) can further confirm the diagnosis and detect distant metastases. However, imaging has low sensitivity for these tumors and thus should only be considered for patients in whom metastatic disease is suspected.14 Biopsy is rarely needed and should be reserved for unusual metastatic locations. Treatment with an alpha-adrenergic receptor antagonist often reduces symptoms and lowers blood pressure. Definitive management typically involves surgical resection for benign disease. Surgery, radionuclide therapy, or chemotherapy is used for malignant disease.
While most pheochromocytomas are sporadic, up to 40% of paragangliomas are due to germline pathogenic variants.17 Mutations in the succinate dehydrogenase (SDH) group of genes are the most common germline pathogenic variants in the autosomal dominant hereditary paraganglioma syndrome (HPGL). Most paragangliomas and pheochromocytomas are localized and benign, but 10%-15% are metastatic.18 SDHB mutations are associated with a high risk of metastasis.19 Thus, genetic testing for patients and subsequent cascade testing to identify at-risk family members is advised in all patients with pheochromocytomas or paragangliomas.20 This patient’s younger brother and mother were both found to carry the same pathogenic SDHB variant, but neither was found to have paragangliomas. Annual metanephrine levels (urine or plasma) and every other year whole-body magnetic resonance imaging (MRI) scans were recommended for tumor surveillance.
The clinician team followed a logical branching algorithm for the diagnosis of severe hypertension with biochemical testing, advanced imaging, histology, and genetic testing to arrive at the final diagnosis of hereditary paraganglioma syndrome. Although this patient presented for urgent care because of the acute effects of catecholamine excess, he suffered from chronic effects (nausea, anxiety, and hypertension) for years. Each symptom had been diagnosed and treated in isolation, but the combination and severity in a young patient suggested a unifying diagnosis. The family history of hypertension (brother and father) suggested an inherited diagnosis from the father’s family, but the final answer rested on the other branch (maternal uncle) of the family tree.
KEY TEACHING POINTS
- Hypertension in a young adult is due to a secondary cause in up to 30% of patients.
- Pathologic catecholamine excess leads to hypertension, tachycardia, pallor, sweating, anxiety, and nausea. A sustained and unexplained combination of these symptoms should prompt a biochemical evaluation for pheochromocytoma or paraganglioma.
- Paragangliomas are tumors of the autonomic nervous system. The frequency of catecholamine secretion depends on their location in the body, and they are commonly caused by germline pathogenic variants.
Acknowledgments
This conundrum was presented during a live Grand Rounds with the expert clinician’s responses recorded and edited for space and clarity.
Disclosures
Dr. Dhaliwal reports speaking honoraria from ISMIE Mutual Insurance Company and GE Healthcare. All other authors have nothing to disclose.
Funding
No sources of funding.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. https://doi.org/10.1161/HYP.0000000000000065.
2. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. 2010;28(4):639-654. https://doi.org/10.1016/j.ccl.2010.07.002.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
4. Gupta-Malhotra M, Banker A, Shete S, et al. Essential hypertension vs. secondary hypertension among children. Am J Hypertens. 2015;28(1):73-80. https://doi.org/10.1093/ajh/hpu083.
5. Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. https://doi.org/10.1161/01.HYP.0000079505.25750.11.
6. Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. https://doi.org/10.1210/jc.2016-1472.
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453-461.
8. Aronow WS. Drug-induced causes of secondary hypertension. Ann Transl Med. 2017;5(17):349. https://doi.org/10.21037/atm.2017.06.16.
9. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. https://doi.org/10.1016/S0140-6736(05)67139-5.
10. Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):507-515. https://doi.org/10.1016/j.beem.2011.10.008.
11. Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66(4):163-165.
12. Paravati S, Warrington SJ. Physiology, Catecholamines. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
13. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37(3):403-407. https://doi.org/10.1007/s12020-010-9319-3.
14. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498.
15. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. https://doi.org/10.3803/EnM.2017.32.2.152.
16. National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555-576.
17. Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Gene Reviews. Seattle, WA: University of Washington; 1993.
18. Goldstein RE, O’Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756. https://doi.org/10.1097/00000658-199906000-00001.
19. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. https://doi.org/10.1210/jc.2007-0709.
20. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. https://doi.org/10.1038/nrendo.2014.188.
A 21-year-old man with a history of hypertension presented to the emergency department with four days of generalized abdominal pain, nausea, and vomiting as well as one month of loose stools. He also had a headache (not further specified) for one day. Due to his nausea, he had been unable to take his medications for two days. Home blood pressure measurements over the preceding two days revealed systolic pressures exceeding 200 mm Hg. He did not experience fever, dyspnea, chest pain, vision changes, numbness, weakness, diaphoresis, or palpitations.
Abdominal pain with vomiting and diarrhea is often caused by a self-limited gastroenteritis. However, the priority initially is to exclude serious intraabdominal processes including arterial insufficiency, bowel obstruction, organ perforation, or organ-based infection or inflammation (eg, appendicitis, cholecystitis, pancreatitis). Essential hypertension accounts for 95% of cases of hypertension in the United States, but given this patient’s young age, secondary causes should be evaluated. These include primary aldosteronism (the most common endocrine cause for hypertension in young patients), chronic kidney disease, fibromuscular dysplasia, illicit drug use, hypercortisolism, pheochromocytoma, and coarctation of the aorta. Thyrotoxicosis can elevate blood pressure (although usually not to this extent) and cause hyperdefecation. While the etiology of the chronic hypertension is uncertain, the proximate cause of the acute rise in blood pressure is likely the stress of his acute illness and the inability to take his prescribed antihypertensive medications. In the setting of severe hypertension, his headache may reflect an intracranial hemorrhage and his abdominal pain could signal an aortic dissection.
His medical history included hypertension diagnosed at age 16 as well as anxiety diagnosed following a panic attack at age 19. Over the past year, he had also developed persistent nausea, which was attributed to gastroesophageal reflux disease. His medications included metoprolol 50 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 12.5 mg daily, escitalopram 20 mg daily, and omeprazole 20 mg daily. His father and 15-year-old brother also had hypertension. He was a part-time student while working at a car dealership. He did not smoke or use drugs and he rarely drank alcohol.
The need for three antihypertensive medications (albeit at submaximal doses) reflects the severity of his hypertension (provided challenges with medication adherence have been excluded). His family history, especially that of his brother who was diagnosed with hypertension at an early age, and the patient’s own early onset hypertension point toward an inherited form of hypertension. Autosomal dominant polycystic kidney disease often results in hypertension before chronic kidney disease develops. Rare inherited forms of hypertension include familial hyperaldosteronism, apparent mineralocorticoid excess, Liddle syndrome, or a hereditary endocrine tumor syndrome predisposing to pheochromocytoma. Even among patients who report classic pheochromocytoma symptoms, such as headache and anxiety, the diagnosis remains unlikely as these symptoms are nonspecific and highly prevalent in the general population. However, once secondary hypertension is plausible or suspected, testing for hyperadrenergic states, which can also cause nausea and vomiting during times of catecholamine excess, should be pursued.
His temperature was 97.5°F, heart rate 95 beats per minute and regular, respiratory rate 18 breaths per minute, blood pressure 181/118 mm Hg (systolic and diastolic pressures in each arm were within 10 mm Hg), and oxygen saturation 100% on room air. Systolic and diastolic pressures did not decrease by more than 20 mm Hg and 10 mm Hg, respectively, after he stood for two minutes. His body mass index was 24 kg/m2. He was alert and appeared slightly anxious. There was a bounding point of maximal impulse in the fifth intercostal space at the midclavicular line and a 3/6 systolic murmur at the left upper sternal border with radiation to the carotid arteries. His abdomen was soft with generalized tenderness to palpation and without rebound tenderness, masses, organomegaly, or bruits. There was no costovertebral angle tenderness. No lymphadenopathy was present. His fundoscopic, pulmonary, skin and neurologic examinations were normal.
Laboratory studies revealed a white blood cell count of 13.3 × 103/uL with a normal differential, hemoglobin 13.9 g/dL, platelet count 373 × 103/uL, sodium 142 mmol/L, potassium 3.8 mmol/L, chloride 103 mmol/L,bicarbonate 25 mmol/L, blood urea nitrogen 12 mg/dL, creatinine 1.3 mg/dL (a baseline creatinine level was not available), glucose 88 mg/dL, calcium 10.6 mg/dL, albumin 4.9 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 37 IU/L, and lipase 40 IU/L. Urinalysis revealed 5-10 white blood cells per high power field without casts and 10 mg/dL protein. Urine toxicology was not performed. Electrocardiogram (ECG) showed left ventricular hypertrophy (LVH). Chest radiography was normal.
The abdominal examination does not suggest peritonitis. The laboratory tests do not suggest inflammation of the liver, pancreas, or biliary tree as the cause of his abdominal pain or diarrhea. The murmur may indicate hypertrophic cardiomyopathy or a congenital anomaly such as bicuspid aortic valve; but neither would explain hypertension unless they were associated with another developmental abnormality, such as coarctation of the aorta. Tricuspid regurgitation is conceivable and if confirmed, might raise concern for carcinoid syndrome, which can cause diarrhea. The normal neurologic examination, including the absence of papilledema, lowers suspicion of intracranial hemorrhage as a cause of his headache.
The albumin of 4.9 g/dL likely reflects hypovolemia resulting from vomiting and diarrhea. Vasoconstriction associated with pheochromocytoma can cause pressure diuresis and resultant hypovolemia. Hyperaldosteronism arising from bilateral adrenal hyperplasia or adrenal adenoma commonly causes hypokalemia, although this is not a universal feature.
The duration of his mildly decreased glomerular filtration rate is uncertain. He may have chronic kidney disease from sustained hypertension, or acute kidney injury from hypovolemia. The mild pyuria could indicate infection or renal calculi, either of which could account for generalized abdominal pain or could reflect an acute renal injury from acute interstitial nephritis from his proton pump inhibitor or hydrochlorothiazide.
LVH on the ECG indicates longstanding hypertension. The chest radiograph does not reveal clues to the etiology of or sequelae from hypertension. In particular, there is no widened aorta to suggest aortic dissection, no pulmonary edema to indicate heart failure, and no rib notching that points toward aortic coarctation. A transthoracic echocardiogram to assess for valvular and other structural abnormalities is warranted.
Tests for secondary hypertension should be sent, including serum aldosterone and renin levels to assess for primary aldosteronism and plasma or 24-hour urine normetanephrine and metanephrine levels to assess for pheochromocytoma. Biochemical evaluation is the mainstay for endocrine hypertension evaluation and should be followed by imaging if abnormal results are found.
Intact parathyroid hormone (PTH) was 78 pg/mL (normal, 10-65 pg/mL), thyroid stimulating hormone 3.6 mIU/L (normal, 0.30-5.50 mIU/L), and morning cortisol 4.1 ug/dL (normal, >7.0 ug/dL). Plasma aldosterone was 14.6 ng/dL (normal, 1-16 ng/dL), plasma renin activity 3.6 ng/mL/hr (normal, 0.5-3.5 ng/mL/hr), and aldosterone-renin ratio 4.1 (normal, <20). Transthoracic echocardiogram showed LVH with normal valves, wall motion, and proximal aorta; the left ventricular ejection fraction was 70%. Magnetic resonance angiography of the renal vessels demonstrated no abnormalities.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed a 5 cm heterogeneous enhancing mass associated with the prostate gland extending into the base of the bladder. The mass obstructed the right renal collecting system and ureter causing severe right-sided ureterectasis and hydronephrosis. There was also 2.8 cm right-sided paracaval lymph node enlargement and 2.1 cm right-sided and 1.5 cm left-sided external iliac lymph node enlargement (Figure 1). There were no adrenal masses.
He is young for prostate, bladder, or colorectal cancer, but early onset variations of these tumors, along with metastatic testicular cancer, must be considered for the pelvic mass and associated lymphadenopathy. Prostatic masses can be infectious (eg, abscess) or malignant (eg, adenocarcinoma, small cell carcinoma). Additional considerations for abdominopelvic cancer are sarcomas, germ cell tumors, or lymphoma. A low aldosterone-renin ratio coupled with a normal potassium level makes primary aldosteronism unlikely. The normal angiography excludes renovascular hypertension.
His abdominal pain and gastrointestinal symptoms could arise from irritation of the bowel, distension of the right-sided urinary collecting system, or products secreted from the mass (eg, catecholamines). The hyperdynamic precordium, elevated ejection fraction, and murmur may reflect augmented blood flow from a hyperadrenergic state. A unifying diagnosis would be a pheochromocytoma. However, given the normal appearance of the adrenal glands on CT imaging, catecholamines arising from a paraganglioma, a tumor of the autonomic nervous system, is more likely. These tumors often secrete catecholamines and can be metastatic (suggested here by the lymphadenopathy). Functional imaging or biopsy of either the mass or an adjacent lymph node is indicated. However, because of the possibility of a catecholamine-secreting tumor, he should be treated with an alpha-adrenergic receptor antagonist before undergoing a biopsy to prevent unopposed vasoconstriction from catecholamine leakage.
Scrotal ultrasound revealed no evidence of a testicular tumor. Lactate dehydrogenase (LDH) was 179 IU/L (normal, 120-240 IU/L) and prostate specific antigen (PSA) was 0.7 ng/mL (normal, <2.5 ng/mL). The patient was given amlodipine and labetalol with improvement of blood pressures to 160s/100s. His creatinine decreased to 1.1 mg/dL. He underwent CT-guided biopsy of a pelvic lymph node. CT of the head without intravenous contrast demonstrated no intracranial abnormalities. His headache resolved with improvement in blood pressure, and he had minimal gastrointestinal symptoms during his hospitalization. No stool studies were sent. A right-sided percutaneous nephrostomy was placed which yielded >15 L of urine from the tube over the next four days.
Upon the first episode of micturition through the urethra four days after percutaneous nephrostomy placement, he experienced severe lightheadedness, diaphoresis, and palpitations. These symptoms prompted him to recall similar episodes following micturition for several months prior to his hospitalization.
It is likely that contraction of the bladder during episodes of urination caused irritation of the pelvic mass, leading to catecholamine secretion. Another explanation for his recurrent lightheadedness would be a neurocardiogenic reflex with micturition (which when it culminates with loss of consciousness is called micturition syncope), but this would not explain his hypertension or bladder mass.
Biochemical tests that were ordered on admission but sent to a reference lab then returned. Plasma metanephrine was 0.2 nmol/L (normal, <0.5 nmol/L) and plasma normetanephrine 34.6 nmol/L (normal, <0.9 nmol/L). His 24-hour urine metanephrine was 72 ug/24 hr (normal, 0-300 ug/24 hr) and normetanephrine 8,511 ug/24 hr (normal, 50-800 ug/24 hr).
The markedly elevated plasma and urine normetanephrine levels confirm a diagnosis of a catecholamine-secreting tumor (paraganglioma). The tissue obtained from the CT-guided lymph node biopsy should be sent for markers of neuroendocrine tumors including chromogranin.
Lymph node biopsy revealed metastatic paraganglioma that was chromogranin A and synaptophysin positive (Figure 2). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan disclosed skull metastases. He was treated with phenoxybenzamine, amlodipine, and labetalol. Surgical resection of the pelvic mass was discussed, but the patient elected to defer surgery as the location of the primary tumor made it challenging to resect and would have required an ileal conduit.
After the diagnosis was made, the patient’s family recalled that a maternal uncle had been diagnosed with a paraganglioma of the carotid body. Genetic testing of the patient identified a succinate dehydrogenase complex subunit B (SDHB) pathogenic variant and confirmed hereditary paraganglioma syndrome (HPGL). One year after the diagnosis, liver and lung metastases developed. He was treated with lanreotide (somatostatin analogue), capecitabine, and temozolomide, as well as a craniotomy and radiotherapy for palliation of bony metastases. The patient died less than two years after diagnosis.
DISCUSSION
Most patients with hypertension (defined as blood pressure >130/80 mm Hg1) do not have an identifiable etiology (primary hypertension). Many components of this patient’s history, however, including his young age of onset, a teenage sibling with hypertension, lack of obesity, hypertension refractory to multiple medications, and LVH suggested secondary hypertension. Hypertension onset at an age less than 30 years, resistance to three or more medications,1,2 and/or acute onset hypertension at any age should prompt an evaluation for secondary causes.1 The prevalence of secondary hypertension is approximately 30% in hypertensive patients ages 18 to 40 years compared with 5%-10% in the overall adult population with hypertension.3 Among children and adolescents ages 0 to 19 years with hypertension, the prevalence of secondary hypertension may be as high as 57%.4
The most common etiology of secondary hypertension is primary aldosteronism.5,6 However, in young adults (ages 19 to 39 years), common etiologies also include renovascular disease and renal parenchymal disease.7 Other causes include obstructive sleep apnea, medications, stimulants (cocaine and amphetamines),8 and endocrinopathies such as thyrotoxicosis, Cushing syndrome, and catecholamine-secreting tumors.7 Less than 1% of secondary hypertension in all adults is due to catecholamine-secreting tumors, and the minority of those catecholamine-secreting tumors are paragangliomas.9
Paragangliomas are tumors of the peripheral autonomic nervous system. These neoplasms arise in the sympathetic and parasympathetic chains along the paravertebral and paraaortic axes. They are closely related to pheochromocytomas, which arise in the adrenal medulla.9 Most head and neck paragangliomas are biochemically silent and are generally discovered due to mass effect.10 The subset of paragangliomas that secrete catecholamines most often arise in the abdomen and pelvis, and their clinical presentation mimics that of pheochromocytomas, including episodic hypertension, palpitations, pallor, and diaphoresis.
This patient had persistent, nonepisodic hypertension, while palpitations and diaphoresis only manifested following micturition. Other cases of urinary bladder paragangliomas have described micturition-associated symptoms and hypertensive crises. Three-fold increases of catecholamine secretion after micturition have been observed in these patients, likely due to muscle contraction and pressure changes in the bladder leading to the systemic release of catecholamines.11
Epinephrine and norepinephrine are monoamine neurotransmitters that activate alpha-adrenergic and beta-adrenergic receptors. Adrenergic receptors are present in all tissues of the body but have prominent effects on the smooth muscle in the vasculature, gastrointestinal tract, urinary tract, and airways.12 Alpha-adrenergic vasoconstriction causes hypertension, which is commonly observed in patients with catecholamine-secreting tumors.10 Catecholamine excess due to secretion from these tumors causes headache in 60%-80% of patients, tachycardia/palpitations in 50%-70%, anxiety in 20%-40%, and nausea in 20%-25%.10 Other symptoms include sweating, pallor, dyspnea, and vertigo.9,10 This patient’s chronic nausea, which was attributed to gastroesophageal reflux, and his anxiety, attributed to generalized anxiety disorder, were likely symptoms of catecholamine excess.13
The best test for the diagnosis of paragangliomas and pheochromocytomas is the measurement of plasma free or 24-hour urinary fractionated metanephrines (test sensitivity of >90% and >90%, respectively).14 Screening for pheochromocytoma should be considered in hypertensive patients who have symptoms of catecholamine excess, refractory or paroxysmal hypertension, and/or familial pheochromocytoma/paraganglioma syndromes.15 Screening for pheochromocytoma should also be performed in children and adolescents with systolic or diastolic blood pressure that is greater than the 95th percentile for their age plus 5 mm Hg.16
While a typical tumor location and elevated metanephrine levels are sufficient to make the diagnosis of a pheochromocytoma or catecholamine-secreting paraganglioma, functional imaging with FDG-PET, Ga-DOTATATE-PET, or 123I-meta-iodobenzylguanidine (123I-MIBG) can further confirm the diagnosis and detect distant metastases. However, imaging has low sensitivity for these tumors and thus should only be considered for patients in whom metastatic disease is suspected.14 Biopsy is rarely needed and should be reserved for unusual metastatic locations. Treatment with an alpha-adrenergic receptor antagonist often reduces symptoms and lowers blood pressure. Definitive management typically involves surgical resection for benign disease. Surgery, radionuclide therapy, or chemotherapy is used for malignant disease.
While most pheochromocytomas are sporadic, up to 40% of paragangliomas are due to germline pathogenic variants.17 Mutations in the succinate dehydrogenase (SDH) group of genes are the most common germline pathogenic variants in the autosomal dominant hereditary paraganglioma syndrome (HPGL). Most paragangliomas and pheochromocytomas are localized and benign, but 10%-15% are metastatic.18 SDHB mutations are associated with a high risk of metastasis.19 Thus, genetic testing for patients and subsequent cascade testing to identify at-risk family members is advised in all patients with pheochromocytomas or paragangliomas.20 This patient’s younger brother and mother were both found to carry the same pathogenic SDHB variant, but neither was found to have paragangliomas. Annual metanephrine levels (urine or plasma) and every other year whole-body magnetic resonance imaging (MRI) scans were recommended for tumor surveillance.
The clinician team followed a logical branching algorithm for the diagnosis of severe hypertension with biochemical testing, advanced imaging, histology, and genetic testing to arrive at the final diagnosis of hereditary paraganglioma syndrome. Although this patient presented for urgent care because of the acute effects of catecholamine excess, he suffered from chronic effects (nausea, anxiety, and hypertension) for years. Each symptom had been diagnosed and treated in isolation, but the combination and severity in a young patient suggested a unifying diagnosis. The family history of hypertension (brother and father) suggested an inherited diagnosis from the father’s family, but the final answer rested on the other branch (maternal uncle) of the family tree.
KEY TEACHING POINTS
- Hypertension in a young adult is due to a secondary cause in up to 30% of patients.
- Pathologic catecholamine excess leads to hypertension, tachycardia, pallor, sweating, anxiety, and nausea. A sustained and unexplained combination of these symptoms should prompt a biochemical evaluation for pheochromocytoma or paraganglioma.
- Paragangliomas are tumors of the autonomic nervous system. The frequency of catecholamine secretion depends on their location in the body, and they are commonly caused by germline pathogenic variants.
Acknowledgments
This conundrum was presented during a live Grand Rounds with the expert clinician’s responses recorded and edited for space and clarity.
Disclosures
Dr. Dhaliwal reports speaking honoraria from ISMIE Mutual Insurance Company and GE Healthcare. All other authors have nothing to disclose.
Funding
No sources of funding.
A 21-year-old man with a history of hypertension presented to the emergency department with four days of generalized abdominal pain, nausea, and vomiting as well as one month of loose stools. He also had a headache (not further specified) for one day. Due to his nausea, he had been unable to take his medications for two days. Home blood pressure measurements over the preceding two days revealed systolic pressures exceeding 200 mm Hg. He did not experience fever, dyspnea, chest pain, vision changes, numbness, weakness, diaphoresis, or palpitations.
Abdominal pain with vomiting and diarrhea is often caused by a self-limited gastroenteritis. However, the priority initially is to exclude serious intraabdominal processes including arterial insufficiency, bowel obstruction, organ perforation, or organ-based infection or inflammation (eg, appendicitis, cholecystitis, pancreatitis). Essential hypertension accounts for 95% of cases of hypertension in the United States, but given this patient’s young age, secondary causes should be evaluated. These include primary aldosteronism (the most common endocrine cause for hypertension in young patients), chronic kidney disease, fibromuscular dysplasia, illicit drug use, hypercortisolism, pheochromocytoma, and coarctation of the aorta. Thyrotoxicosis can elevate blood pressure (although usually not to this extent) and cause hyperdefecation. While the etiology of the chronic hypertension is uncertain, the proximate cause of the acute rise in blood pressure is likely the stress of his acute illness and the inability to take his prescribed antihypertensive medications. In the setting of severe hypertension, his headache may reflect an intracranial hemorrhage and his abdominal pain could signal an aortic dissection.
His medical history included hypertension diagnosed at age 16 as well as anxiety diagnosed following a panic attack at age 19. Over the past year, he had also developed persistent nausea, which was attributed to gastroesophageal reflux disease. His medications included metoprolol 50 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 12.5 mg daily, escitalopram 20 mg daily, and omeprazole 20 mg daily. His father and 15-year-old brother also had hypertension. He was a part-time student while working at a car dealership. He did not smoke or use drugs and he rarely drank alcohol.
The need for three antihypertensive medications (albeit at submaximal doses) reflects the severity of his hypertension (provided challenges with medication adherence have been excluded). His family history, especially that of his brother who was diagnosed with hypertension at an early age, and the patient’s own early onset hypertension point toward an inherited form of hypertension. Autosomal dominant polycystic kidney disease often results in hypertension before chronic kidney disease develops. Rare inherited forms of hypertension include familial hyperaldosteronism, apparent mineralocorticoid excess, Liddle syndrome, or a hereditary endocrine tumor syndrome predisposing to pheochromocytoma. Even among patients who report classic pheochromocytoma symptoms, such as headache and anxiety, the diagnosis remains unlikely as these symptoms are nonspecific and highly prevalent in the general population. However, once secondary hypertension is plausible or suspected, testing for hyperadrenergic states, which can also cause nausea and vomiting during times of catecholamine excess, should be pursued.
His temperature was 97.5°F, heart rate 95 beats per minute and regular, respiratory rate 18 breaths per minute, blood pressure 181/118 mm Hg (systolic and diastolic pressures in each arm were within 10 mm Hg), and oxygen saturation 100% on room air. Systolic and diastolic pressures did not decrease by more than 20 mm Hg and 10 mm Hg, respectively, after he stood for two minutes. His body mass index was 24 kg/m2. He was alert and appeared slightly anxious. There was a bounding point of maximal impulse in the fifth intercostal space at the midclavicular line and a 3/6 systolic murmur at the left upper sternal border with radiation to the carotid arteries. His abdomen was soft with generalized tenderness to palpation and without rebound tenderness, masses, organomegaly, or bruits. There was no costovertebral angle tenderness. No lymphadenopathy was present. His fundoscopic, pulmonary, skin and neurologic examinations were normal.
Laboratory studies revealed a white blood cell count of 13.3 × 103/uL with a normal differential, hemoglobin 13.9 g/dL, platelet count 373 × 103/uL, sodium 142 mmol/L, potassium 3.8 mmol/L, chloride 103 mmol/L,bicarbonate 25 mmol/L, blood urea nitrogen 12 mg/dL, creatinine 1.3 mg/dL (a baseline creatinine level was not available), glucose 88 mg/dL, calcium 10.6 mg/dL, albumin 4.9 g/dL, aspartate aminotransferase 27 IU/L, alanine aminotransferase 37 IU/L, and lipase 40 IU/L. Urinalysis revealed 5-10 white blood cells per high power field without casts and 10 mg/dL protein. Urine toxicology was not performed. Electrocardiogram (ECG) showed left ventricular hypertrophy (LVH). Chest radiography was normal.
The abdominal examination does not suggest peritonitis. The laboratory tests do not suggest inflammation of the liver, pancreas, or biliary tree as the cause of his abdominal pain or diarrhea. The murmur may indicate hypertrophic cardiomyopathy or a congenital anomaly such as bicuspid aortic valve; but neither would explain hypertension unless they were associated with another developmental abnormality, such as coarctation of the aorta. Tricuspid regurgitation is conceivable and if confirmed, might raise concern for carcinoid syndrome, which can cause diarrhea. The normal neurologic examination, including the absence of papilledema, lowers suspicion of intracranial hemorrhage as a cause of his headache.
The albumin of 4.9 g/dL likely reflects hypovolemia resulting from vomiting and diarrhea. Vasoconstriction associated with pheochromocytoma can cause pressure diuresis and resultant hypovolemia. Hyperaldosteronism arising from bilateral adrenal hyperplasia or adrenal adenoma commonly causes hypokalemia, although this is not a universal feature.
The duration of his mildly decreased glomerular filtration rate is uncertain. He may have chronic kidney disease from sustained hypertension, or acute kidney injury from hypovolemia. The mild pyuria could indicate infection or renal calculi, either of which could account for generalized abdominal pain or could reflect an acute renal injury from acute interstitial nephritis from his proton pump inhibitor or hydrochlorothiazide.
LVH on the ECG indicates longstanding hypertension. The chest radiograph does not reveal clues to the etiology of or sequelae from hypertension. In particular, there is no widened aorta to suggest aortic dissection, no pulmonary edema to indicate heart failure, and no rib notching that points toward aortic coarctation. A transthoracic echocardiogram to assess for valvular and other structural abnormalities is warranted.
Tests for secondary hypertension should be sent, including serum aldosterone and renin levels to assess for primary aldosteronism and plasma or 24-hour urine normetanephrine and metanephrine levels to assess for pheochromocytoma. Biochemical evaluation is the mainstay for endocrine hypertension evaluation and should be followed by imaging if abnormal results are found.
Intact parathyroid hormone (PTH) was 78 pg/mL (normal, 10-65 pg/mL), thyroid stimulating hormone 3.6 mIU/L (normal, 0.30-5.50 mIU/L), and morning cortisol 4.1 ug/dL (normal, >7.0 ug/dL). Plasma aldosterone was 14.6 ng/dL (normal, 1-16 ng/dL), plasma renin activity 3.6 ng/mL/hr (normal, 0.5-3.5 ng/mL/hr), and aldosterone-renin ratio 4.1 (normal, <20). Transthoracic echocardiogram showed LVH with normal valves, wall motion, and proximal aorta; the left ventricular ejection fraction was 70%. Magnetic resonance angiography of the renal vessels demonstrated no abnormalities.
Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast revealed a 5 cm heterogeneous enhancing mass associated with the prostate gland extending into the base of the bladder. The mass obstructed the right renal collecting system and ureter causing severe right-sided ureterectasis and hydronephrosis. There was also 2.8 cm right-sided paracaval lymph node enlargement and 2.1 cm right-sided and 1.5 cm left-sided external iliac lymph node enlargement (Figure 1). There were no adrenal masses.
He is young for prostate, bladder, or colorectal cancer, but early onset variations of these tumors, along with metastatic testicular cancer, must be considered for the pelvic mass and associated lymphadenopathy. Prostatic masses can be infectious (eg, abscess) or malignant (eg, adenocarcinoma, small cell carcinoma). Additional considerations for abdominopelvic cancer are sarcomas, germ cell tumors, or lymphoma. A low aldosterone-renin ratio coupled with a normal potassium level makes primary aldosteronism unlikely. The normal angiography excludes renovascular hypertension.
His abdominal pain and gastrointestinal symptoms could arise from irritation of the bowel, distension of the right-sided urinary collecting system, or products secreted from the mass (eg, catecholamines). The hyperdynamic precordium, elevated ejection fraction, and murmur may reflect augmented blood flow from a hyperadrenergic state. A unifying diagnosis would be a pheochromocytoma. However, given the normal appearance of the adrenal glands on CT imaging, catecholamines arising from a paraganglioma, a tumor of the autonomic nervous system, is more likely. These tumors often secrete catecholamines and can be metastatic (suggested here by the lymphadenopathy). Functional imaging or biopsy of either the mass or an adjacent lymph node is indicated. However, because of the possibility of a catecholamine-secreting tumor, he should be treated with an alpha-adrenergic receptor antagonist before undergoing a biopsy to prevent unopposed vasoconstriction from catecholamine leakage.
Scrotal ultrasound revealed no evidence of a testicular tumor. Lactate dehydrogenase (LDH) was 179 IU/L (normal, 120-240 IU/L) and prostate specific antigen (PSA) was 0.7 ng/mL (normal, <2.5 ng/mL). The patient was given amlodipine and labetalol with improvement of blood pressures to 160s/100s. His creatinine decreased to 1.1 mg/dL. He underwent CT-guided biopsy of a pelvic lymph node. CT of the head without intravenous contrast demonstrated no intracranial abnormalities. His headache resolved with improvement in blood pressure, and he had minimal gastrointestinal symptoms during his hospitalization. No stool studies were sent. A right-sided percutaneous nephrostomy was placed which yielded >15 L of urine from the tube over the next four days.
Upon the first episode of micturition through the urethra four days after percutaneous nephrostomy placement, he experienced severe lightheadedness, diaphoresis, and palpitations. These symptoms prompted him to recall similar episodes following micturition for several months prior to his hospitalization.
It is likely that contraction of the bladder during episodes of urination caused irritation of the pelvic mass, leading to catecholamine secretion. Another explanation for his recurrent lightheadedness would be a neurocardiogenic reflex with micturition (which when it culminates with loss of consciousness is called micturition syncope), but this would not explain his hypertension or bladder mass.
Biochemical tests that were ordered on admission but sent to a reference lab then returned. Plasma metanephrine was 0.2 nmol/L (normal, <0.5 nmol/L) and plasma normetanephrine 34.6 nmol/L (normal, <0.9 nmol/L). His 24-hour urine metanephrine was 72 ug/24 hr (normal, 0-300 ug/24 hr) and normetanephrine 8,511 ug/24 hr (normal, 50-800 ug/24 hr).
The markedly elevated plasma and urine normetanephrine levels confirm a diagnosis of a catecholamine-secreting tumor (paraganglioma). The tissue obtained from the CT-guided lymph node biopsy should be sent for markers of neuroendocrine tumors including chromogranin.
Lymph node biopsy revealed metastatic paraganglioma that was chromogranin A and synaptophysin positive (Figure 2). A fluorodeoxyglucose positron emission tomography (FDG-PET) scan disclosed skull metastases. He was treated with phenoxybenzamine, amlodipine, and labetalol. Surgical resection of the pelvic mass was discussed, but the patient elected to defer surgery as the location of the primary tumor made it challenging to resect and would have required an ileal conduit.
After the diagnosis was made, the patient’s family recalled that a maternal uncle had been diagnosed with a paraganglioma of the carotid body. Genetic testing of the patient identified a succinate dehydrogenase complex subunit B (SDHB) pathogenic variant and confirmed hereditary paraganglioma syndrome (HPGL). One year after the diagnosis, liver and lung metastases developed. He was treated with lanreotide (somatostatin analogue), capecitabine, and temozolomide, as well as a craniotomy and radiotherapy for palliation of bony metastases. The patient died less than two years after diagnosis.
DISCUSSION
Most patients with hypertension (defined as blood pressure >130/80 mm Hg1) do not have an identifiable etiology (primary hypertension). Many components of this patient’s history, however, including his young age of onset, a teenage sibling with hypertension, lack of obesity, hypertension refractory to multiple medications, and LVH suggested secondary hypertension. Hypertension onset at an age less than 30 years, resistance to three or more medications,1,2 and/or acute onset hypertension at any age should prompt an evaluation for secondary causes.1 The prevalence of secondary hypertension is approximately 30% in hypertensive patients ages 18 to 40 years compared with 5%-10% in the overall adult population with hypertension.3 Among children and adolescents ages 0 to 19 years with hypertension, the prevalence of secondary hypertension may be as high as 57%.4
The most common etiology of secondary hypertension is primary aldosteronism.5,6 However, in young adults (ages 19 to 39 years), common etiologies also include renovascular disease and renal parenchymal disease.7 Other causes include obstructive sleep apnea, medications, stimulants (cocaine and amphetamines),8 and endocrinopathies such as thyrotoxicosis, Cushing syndrome, and catecholamine-secreting tumors.7 Less than 1% of secondary hypertension in all adults is due to catecholamine-secreting tumors, and the minority of those catecholamine-secreting tumors are paragangliomas.9
Paragangliomas are tumors of the peripheral autonomic nervous system. These neoplasms arise in the sympathetic and parasympathetic chains along the paravertebral and paraaortic axes. They are closely related to pheochromocytomas, which arise in the adrenal medulla.9 Most head and neck paragangliomas are biochemically silent and are generally discovered due to mass effect.10 The subset of paragangliomas that secrete catecholamines most often arise in the abdomen and pelvis, and their clinical presentation mimics that of pheochromocytomas, including episodic hypertension, palpitations, pallor, and diaphoresis.
This patient had persistent, nonepisodic hypertension, while palpitations and diaphoresis only manifested following micturition. Other cases of urinary bladder paragangliomas have described micturition-associated symptoms and hypertensive crises. Three-fold increases of catecholamine secretion after micturition have been observed in these patients, likely due to muscle contraction and pressure changes in the bladder leading to the systemic release of catecholamines.11
Epinephrine and norepinephrine are monoamine neurotransmitters that activate alpha-adrenergic and beta-adrenergic receptors. Adrenergic receptors are present in all tissues of the body but have prominent effects on the smooth muscle in the vasculature, gastrointestinal tract, urinary tract, and airways.12 Alpha-adrenergic vasoconstriction causes hypertension, which is commonly observed in patients with catecholamine-secreting tumors.10 Catecholamine excess due to secretion from these tumors causes headache in 60%-80% of patients, tachycardia/palpitations in 50%-70%, anxiety in 20%-40%, and nausea in 20%-25%.10 Other symptoms include sweating, pallor, dyspnea, and vertigo.9,10 This patient’s chronic nausea, which was attributed to gastroesophageal reflux, and his anxiety, attributed to generalized anxiety disorder, were likely symptoms of catecholamine excess.13
The best test for the diagnosis of paragangliomas and pheochromocytomas is the measurement of plasma free or 24-hour urinary fractionated metanephrines (test sensitivity of >90% and >90%, respectively).14 Screening for pheochromocytoma should be considered in hypertensive patients who have symptoms of catecholamine excess, refractory or paroxysmal hypertension, and/or familial pheochromocytoma/paraganglioma syndromes.15 Screening for pheochromocytoma should also be performed in children and adolescents with systolic or diastolic blood pressure that is greater than the 95th percentile for their age plus 5 mm Hg.16
While a typical tumor location and elevated metanephrine levels are sufficient to make the diagnosis of a pheochromocytoma or catecholamine-secreting paraganglioma, functional imaging with FDG-PET, Ga-DOTATATE-PET, or 123I-meta-iodobenzylguanidine (123I-MIBG) can further confirm the diagnosis and detect distant metastases. However, imaging has low sensitivity for these tumors and thus should only be considered for patients in whom metastatic disease is suspected.14 Biopsy is rarely needed and should be reserved for unusual metastatic locations. Treatment with an alpha-adrenergic receptor antagonist often reduces symptoms and lowers blood pressure. Definitive management typically involves surgical resection for benign disease. Surgery, radionuclide therapy, or chemotherapy is used for malignant disease.
While most pheochromocytomas are sporadic, up to 40% of paragangliomas are due to germline pathogenic variants.17 Mutations in the succinate dehydrogenase (SDH) group of genes are the most common germline pathogenic variants in the autosomal dominant hereditary paraganglioma syndrome (HPGL). Most paragangliomas and pheochromocytomas are localized and benign, but 10%-15% are metastatic.18 SDHB mutations are associated with a high risk of metastasis.19 Thus, genetic testing for patients and subsequent cascade testing to identify at-risk family members is advised in all patients with pheochromocytomas or paragangliomas.20 This patient’s younger brother and mother were both found to carry the same pathogenic SDHB variant, but neither was found to have paragangliomas. Annual metanephrine levels (urine or plasma) and every other year whole-body magnetic resonance imaging (MRI) scans were recommended for tumor surveillance.
The clinician team followed a logical branching algorithm for the diagnosis of severe hypertension with biochemical testing, advanced imaging, histology, and genetic testing to arrive at the final diagnosis of hereditary paraganglioma syndrome. Although this patient presented for urgent care because of the acute effects of catecholamine excess, he suffered from chronic effects (nausea, anxiety, and hypertension) for years. Each symptom had been diagnosed and treated in isolation, but the combination and severity in a young patient suggested a unifying diagnosis. The family history of hypertension (brother and father) suggested an inherited diagnosis from the father’s family, but the final answer rested on the other branch (maternal uncle) of the family tree.
KEY TEACHING POINTS
- Hypertension in a young adult is due to a secondary cause in up to 30% of patients.
- Pathologic catecholamine excess leads to hypertension, tachycardia, pallor, sweating, anxiety, and nausea. A sustained and unexplained combination of these symptoms should prompt a biochemical evaluation for pheochromocytoma or paraganglioma.
- Paragangliomas are tumors of the autonomic nervous system. The frequency of catecholamine secretion depends on their location in the body, and they are commonly caused by germline pathogenic variants.
Acknowledgments
This conundrum was presented during a live Grand Rounds with the expert clinician’s responses recorded and edited for space and clarity.
Disclosures
Dr. Dhaliwal reports speaking honoraria from ISMIE Mutual Insurance Company and GE Healthcare. All other authors have nothing to disclose.
Funding
No sources of funding.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. https://doi.org/10.1161/HYP.0000000000000065.
2. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. 2010;28(4):639-654. https://doi.org/10.1016/j.ccl.2010.07.002.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
4. Gupta-Malhotra M, Banker A, Shete S, et al. Essential hypertension vs. secondary hypertension among children. Am J Hypertens. 2015;28(1):73-80. https://doi.org/10.1093/ajh/hpu083.
5. Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. https://doi.org/10.1161/01.HYP.0000079505.25750.11.
6. Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. https://doi.org/10.1210/jc.2016-1472.
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453-461.
8. Aronow WS. Drug-induced causes of secondary hypertension. Ann Transl Med. 2017;5(17):349. https://doi.org/10.21037/atm.2017.06.16.
9. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. https://doi.org/10.1016/S0140-6736(05)67139-5.
10. Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):507-515. https://doi.org/10.1016/j.beem.2011.10.008.
11. Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66(4):163-165.
12. Paravati S, Warrington SJ. Physiology, Catecholamines. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
13. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37(3):403-407. https://doi.org/10.1007/s12020-010-9319-3.
14. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498.
15. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. https://doi.org/10.3803/EnM.2017.32.2.152.
16. National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555-576.
17. Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Gene Reviews. Seattle, WA: University of Washington; 1993.
18. Goldstein RE, O’Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756. https://doi.org/10.1097/00000658-199906000-00001.
19. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. https://doi.org/10.1210/jc.2007-0709.
20. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. https://doi.org/10.1038/nrendo.2014.188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. https://doi.org/10.1161/HYP.0000000000000065.
2. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. 2010;28(4):639-654. https://doi.org/10.1016/j.ccl.2010.07.002.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
4. Gupta-Malhotra M, Banker A, Shete S, et al. Essential hypertension vs. secondary hypertension among children. Am J Hypertens. 2015;28(1):73-80. https://doi.org/10.1093/ajh/hpu083.
5. Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. https://doi.org/10.1161/01.HYP.0000079505.25750.11.
6. Kayser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826-2835. https://doi.org/10.1210/jc.2016-1472.
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453-461.
8. Aronow WS. Drug-induced causes of secondary hypertension. Ann Transl Med. 2017;5(17):349. https://doi.org/10.21037/atm.2017.06.16.
9. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. https://doi.org/10.1016/S0140-6736(05)67139-5.
10. Mannelli M, Lenders JW, Pacak K, Parenti G, Eisenhofer G. Subclinical phaeochromocytoma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):507-515. https://doi.org/10.1016/j.beem.2011.10.008.
11. Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66(4):163-165.
12. Paravati S, Warrington SJ. Physiology, Catecholamines. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2019.
13. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37(3):403-407. https://doi.org/10.1007/s12020-010-9319-3.
14. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498.
15. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. https://doi.org/10.3803/EnM.2017.32.2.152.
16. National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555-576.
17. Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Gene Reviews. Seattle, WA: University of Washington; 1993.
18. Goldstein RE, O’Neill JA, Jr., Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756. https://doi.org/10.1097/00000658-199906000-00001.
19. Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822-3828. https://doi.org/10.1210/jc.2007-0709.
20. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. https://doi.org/10.1038/nrendo.2014.188.
© 2019 Society of Hospital Medicine
A Dark Horse Diagnosis
A 73-year-old man presented to the emergency department in late winter with fevers, myalgias, fatigue, low back pain, and poor oral intake. Four days earlier, he had fallen and hit his head. His partner also noticed a few episodes of confusion in the days leading up to presentation.
The patient’s symptoms are nonspecific. Fevers prompt the consideration of systemic infection, though fevers can also be seen in a broad range of noninfectious processes, including malignancy, vasculitis, autoimmune conditions, endocrinopathies, and drug reaction. The clinical picture warrants prompt and comprehensive evaluation, beginning with further detailed history (current illnesses, exposures, travel, vaccinations, medications, cancer screenings, weight change) and a careful physical examination, which will help guide laboratory testing and imaging.
His past medical history was notable for coronary artery disease for which he underwent coronary artery bypass grafting five years prior, hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, gastroesophageal reflux disease, osteoarthritis leading to chronic knee and hand pain, and a history of mildly low testosterone levels. His medications included hydrocodone and acetaminophen, metoprolol tartrate, omeprazole, topical testosterone gel (prescribed for daily use, used intermittently), and aspirin. He was retired and lived in rural Michigan with his female partner. He previously worked as a truck driver and used to train racehorses. He had quit smoking five years earlier. He denied alcohol or injection drug use.
The patient has significant underlying medical conditions. Considering infectious causes of his symptoms, it is notable that he has no reported immunodeficiency. It would be relevant to know if he has been tested for HIV. His rural residence and work with horses raise the possibility of zoonotic infections, including plague (Yersinia pestis), brucellosis (Brucella species), Q fever (Coxiella burnetti), Rhodococcus equi, or group C or G Streptococci. Information about tuberculosis risk factors, other geographic exposures, recent dental work, and ill contacts might be helpful to elucidate the causes of this nonspecific febrile illness with a possible CNS component. With regard to malignancy, it would be helpful to ask about recent weight loss, lymphadenopathy, and prior cancer screenings. Considering other etiologies, he does not report a history of autoimmune or endocrine conditions. However, it is important to consider a vasculitis, such as giant cell arteritis or polyarteritis nodosa, autoimmune conditions, and endocrinopathies such as thyrotoxicosis. The differential diagnosis for his clinical syndrome remains broad.
Vital signs were temperature 37.3°C, heart rate 88 beats per minute, respiratory rate 18 breaths per minute, blood pressure 105/64 mmHg, and oxygen saturation 93% on room air. Oral examination revealed poor dentition. The heart had a normal rate and regular rhythm with no murmurs, rubs, or gallops, and lungs were clear to auscultation bilaterally. The abdomen was unremarkable. Examination of the back was notable for mild tenderness to palpation over the sacrum. He was oriented to person, place, and time, with intact cranial nerves and a nonfocal neurologic examination. The remainder of his examination was normal. The white blood cell (WBC) count was 11.1 × 103/μL, with 84% neutrophils and 9% bands, hemoglobin 13.6 g/dL, platelet count 54 × 103/μL, sodium 122 mmol/L, potassium 3.3 mmol/L, chloride 89 mmol/L, bicarbonate 21 mmol/L, creatinine 1.64 mg/dL, albumin 2.7 g/dL, alkaline phosphatase 136 U/L, AST 60 U/L, ALT 37 U/L, and total bilirubin 2.1 mg/dL.
He had presented to the emergency department five days earlier with fever, flank pain, nausea, vomiting, and weakness. At that time, he had a temperature of 38.2°C, but vital signs otherwise had been normal. Laboratory studies had revealed WBC count 14.0 × 103/μL, hemoglobin 13.7 g/dL, platelet count 175 × 103/μL, sodium 129 mmol/L, chloride 97 mmol/dL, bicarbonate 23 mmol/L, creatinine 1.1 mg/dL, and total bilirubin 1.6 mg/dL. Urinalysis had been negative. He had received one liter of intravenous normal saline and ketorolac for pain and had been discharged with the diagnosis of a viral illness.
A picture of a progressive, subacute illness with multisystem involvement appears to be emerging, and there are several abnormalities consistent with infection, including fever, leukocytosis with bandemia, thrombocytopenia, renal dysfunction, and elevated bilirubin. His borderline hypotension may be due to uninterrupted use of his antihypertensive medication in the setting of poor oral intake or may indicate incipient sepsis. Focal sacral tenderness raises the possibility of vertebral osteomyelitis or epidural abscess, either from a contiguous focus of infection from the surrounding structures, or as a site of seeding from bacteremia. His prior confusion episodes might have been secondary to a systemic process; however, CNS imaging should be done, given the history of confusion and recent fall. Further diagnostic studies are warranted, including: blood cultures; peripheral blood smear; imaging of the spine, chest, abdomen, and pelvis; electrocardiogram; and possibly echocardiogram. Although noninfectious etiologies should not be discounted, the constellation of findings is more compatible with infection.
Two sets of blood cultures and a viral respiratory swab were obtained. Computed tomography (CT) of the head without contrast was negative for acute bleeding or other intracranial pathology. Lumbosacral radiography revealed degenerative changes with intact alignment of the sacrum. The patient was admitted with plans to pursue lumbar puncture if altered mental status recurred. The viral swab was negative. Within 24 hours, one set of blood cultures (both bottles) grew lactose-negative, oxidase-negative, gram-negative rods.
Gram-negative rods (GNRs) rarely are contaminants in blood cultures and should be considered significant until proven otherwise. Prompt empiric therapy and investigation to identify the primary source of bacteremia must be initiated. Although the most common GNRs isolated from blood cultures are enteric coliform organisms such as E. coli, Klebsiella, and Enterobacter, these typically are lactose-positive. Additional possibilities should be considered, including Salmonella species or other organisms comprising the “HACEK” group. This latter group is commonly associated with endocarditis, but the majority are oxidase-positive and have more fastidious growth requirements. Although there are other gram-negative organisms to consider, they have other distinguishing characteristics that have not been indicated in the microbiology results. Broad-spectrum antibiotic therapy is appropriate while awaiting the final identification of the GNR. A thorough search for a primary source and secondary sites of hematogenous seeding should be conducted. His only localizing symptom was tenderness over the sacrum, and this should be further assessed by sensitive imaging such as magnetic resonance imaging (MRI). The identity of the GNR would guide further diagnostic evaluation. For example, a respiratory organism such as Haemophilus influenzae would prompt a CT scan of the chest. Isolation of an enteric or a coliform GNR such as E. coli would prompt abdominal and pelvic imaging to assess for occult abscess. An “HACEK” group organism would prompt echocardiography to evaluate for endocarditis.
He was started on piperacillin–tazobactam. GNR bacteremia without a clear source prompted a CT of the chest, abdomen, and pelvis with and without contrast. The images were unremarkable, with the exception of a signal abnormality in the left psoas muscle concerning for abscess (Figure 1). MRI of the same region revealed L2-4 osteomyelitis and discitis with bilateral psoas abscesses but without epidural abscess (Figure 2).
Because of increasing reports of antibiotic resistance in GNRs, even in community-acquired infections, it is appropriate to initially treat with a broad-spectrum antibiotic such as a fourth-generation cephalosporin or carbapenem while awaiting identification and susceptibility results to guide definitive therapy. In addition to antimicrobial therapy, treatment of psoas abscess usually requires drainage. Vertebral osteomyelitis from a hematogenous source can often be treated with antibiotics alone, as long as there are no associated complications such as epidural abscess and spine instability. Imaging should be reviewed for pathology of the surrounding structures, and surgical consultation should be obtained.
Neurosurgery, Interventional Radiology, and Infectious Disease services were consulted. Antibiotic coverage was expanded to vancomycin, cefepime, and metronidazole due to the possibility of polymicrobial infection. No surgical intervention was recommended since the abscesses were too small to drain.
The next day, the GNR was identified as Serratia marcescens.
S. marcescens is a widely distributed organism in the environment, but not a common component of endogenous human flora. Serratia is generally considered as an opportunistic nosocomial pathogen. Community-acquired infection with this organism is unusual and implies exogenous acquisition. A careful re-evaluation of exposures, including injection drug use or other parenteral exposures is important to identify the likely source of infection, as these have been previously linked to outbreaks of environmental organisms. Based on the presumed pathogenesis of infection and the initial microbiology suggesting monomicrobial Serratia infection, antibiotics should be narrowed based on the susceptibility results. There is concern that antibiotics might not adequately penetrate the abscesses and result in a lack of clinical improvement and/or lead to the emergence of antibiotic resistance during therapy. This is an important concern with Serratia, which typically harbors an AmpC beta-lactamase that can mediate resistance to broad-spectrum cephalosporins. If medical therapy alone without drainage is planned, short-interval re-imaging is warranted.
Blood cultures from days two and three of hospitalization also grew S. marcescens. No other organisms grew. Based on culture sensitivity data, antibiotics were narrowed to ceftriaxone.
This surprising culture result prompted the medical team to obtain screening laboratory tests for immunocompromising conditions and to revisit the patient’s history. His type 2 diabetes mellitus was well controlled with a hemoglobin A1c of 6.5%. HIV testing was negative. Further questioning of the patient revealed that he had fallen from a truck onto rocks four months prior, injuring his back and hip, but without puncture of the skin or loss of consciousness; he denied recent falls or other injuries but reported significant chronic knee pain. He had not been hospitalized recently. He had never taken corticosteroids or immunomodulatory medications. He continued to deny injection drug use. He did, however, clarify that his work with racehorses, which was originally understood to be a prior hobby, was ongoing, including recent work of cleaning the stables.
The following morning, he experienced confusion, rigors, and hypoxia, which prompted transfer to the intensive care unit (ICU).
Acute worsening during treatment is worrisome, and could be a potential complication of his infection or treatment – or even a separate process altogether. Knee pain in the setting of bacteremia raises the possibility of septic or crystal-induced arthritis and warrants imaging. Confusion and hypoxia might represent secondary sites of seeding from bacteremia (CNS infection and pneumonia, respectively) or manifestations of endocarditis, the latter being unusual for Serratia. An echocardiogram should be obtained. Other neurologic causes, including seizure, should also be considered. Further evaluation by chest imaging and repeat neurologic examination and imaging should be performed. Emergence of resistance during therapy is a theoretical concern with Serratia as an AmpC beta-lactamase-containing organism. While awaiting additional microbiology data, an empiric change to an AmpC beta-lactamase stable antibiotic such as a carbapenem should be made, especially since he has clinically deteriorated on therapy with a β-lactamase susceptible antibiotic, raising concerns of the emergence of resistance on initial therapy.
Antibiotics were changed to meropenem, vancomycin, and metronidazole given the clinical worsening and concerns that this represented infection unresponsive to prior antibiotics. The acute episode resolved spontaneously after one hour. His neurologic examination remained nonfocal. Chest radiography, urinalysis, urine culture, and right upper quadrant ultrasound were unremarkable. Transesophageal echocardiogram revealed no heart valve vegetations. MRI and bone scan of the lower extremities did not show any evidence of septic arthritis or other infection. He remained stable and was transferred out of the ICU the following day. Antibiotic coverage was switched to cefepime. On discussion with his significant other, this event was found to be similar to the intermittent confusion that occurred in the days prior to admission.
The acute onset and other features of these intermittent periods of deterioration are compatible with infection; intermittent seeding of the blood with microbes or their products (eg, lipopolysaccharides) from an abscess or vascular infection could explain these episodes. Some of the previous hypotheses to explain the episodes, such as a secondary infectious process, have not been supported by diagnostic testing or the clinical course. He needs close clinical monitoring and interval assessment of the known sites of infection.
Ten days after osteomyelitis and discitis were diagnosed, the patient developed worsening low back pain, prompting repeat spine MRI. This was significant for bilateral psoas abscess enlargement and extension of osteomyelitis and discitis (Figure 3). He was re-evaluated by Neurosurgery and Interventional Radiology and underwent psoas abscess drainage; abscess cultures grew S. marcescens.
He slowly improved over several weeks and was discharged to a subacute rehabilitation facility. He completed a 3.5-week course of intravenous antibiotics before leaving against medical advice. He completed eight weeks of oral trimethoprim-sulfamethoxazole and remains without long-term sequelae from the infection.
DISCUSSION
S. marcescens is a gram-negative rod in the Enterobacteriaceae family known for its red pigment. Primarily, S. marcescens causes nosocomial infections, most commonly of the respiratory and urinary tracts. However, a wide range of manifestations has been documented, including meningitis, ocular infections (conjunctivitis, keratitis, endophthalmitis), endocarditis, skin infections (cellulitis, necrotizing fasciitis), and osteomyelitis.1, 2 S. marcescens is often reported as the cause of outbreaks in ICUs;3-6 infection is thought to occur via contamination of water pipes, hospital equipment, and disinfectants.3, 7 Its natural environment includes soil, water, and GI tracts of animals,4 and there are published reports of S. marcescens infection in horses.8, 9 This patient was most likely exposed to S. marcescens through his work with horses and their environment.
S. marcescens has wide-ranging target organs, and successful treatment can be difficult. S. marcescens can infect the renal, respiratory, gastrointestinal, ocular, cardiovascular, and musculoskeletal systems. S. marcescens, like other “SPACE” organisms (Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter), expresses inducible AmpC beta-lactamase.10 At baseline, AmpC beta-lactamase expression is repressed.11 Mutants with stably de-repressed (constitutively expressed) AmpC can be selected during therapy and lead to clinical failure, as has been best described during therapy for Enterobacter infections.12 Infectious Disease consultation may be helpful when caring for patients with S. marcescens bacteremia given these complexities.
This was an unusual case of S. marcescens infection. It most commonly infects immunocompromised hosts. Reported risk factors include solid organ or hematopoietic stem cell transplant, malignancy, HIV/AIDS, and receipt of immunosuppressive agents. The patient did not have these risk factors, but did have well-controlled type 2 diabetes mellitus. Although diabetes is associated with an increased risk of infection and more severe infections,13, 14 there is no evidence in the literature that well-controlled type 2 diabetes mellitus compromises the immune system. A few case reports document cutaneous S. marcescens infection in immunocompetent adults.15,16 A case report of S. marcescens septic arthritis and adjacent osteomyelitis has also been published, but the patient had poorly controlled diabetes.17 This case provides a report of systemic S. marcescens infection in an individual without clear risk factors.
S. marcescens osteomyelitis is rare, and there have been only a few prior case reports.2,18 The presentation of osteomyelitis, regardless of the causative organism, is subtle, often insidious, and can easily be missed. Hospitalists should have a high index of suspicion for the diagnosis as it requires prompt evaluation and treatment for complications, including epidural abscess. Risk factors include diabetes mellitus, rheumatoid arthritis, injection drug use, and other immunocompromising illnesses.19 Degenerative changes in the spine such as osteoarthritis may be risk factors as well,20 though not well studied or quantified. A hypothesized mechanism involves local inflammation and joint damage, leaving the area susceptible to bacterial seeding. Osteoarthritis and degenerative disc disease, along with exposure to racehorses, likely put this patient at risk for bacterial seeding in the vertebrae, ultimately leading to a “dark horse” diagnosis.
TEACHING POINTS
- Serratia marcescens is a gram-negative rod bacterium that most commonly infects immunocompromised individuals in hospital settings. This report demonstrates that S. marcescens can cause serious infection in immunocompetent, nonhospitalized adults.
- S. marcescens bacteremia or infection of organs outside of the urinary or respiratory systems is uncommon, and therapy can be complicated by emergence of resistance.
- The clinical presentation of vertebral osteomyelitis and discitis and psoas abscess can be subtle and may present without typical signs and symptoms of infection.
ACKNOWLEDGEMENTS
The authors thank the patient and his partner for their willingness to have his story published, Laura Petersen, MHSA, for providing assistance with references and manuscript editing, and Shadi Azar, MBBS, for assistance in selecting the cross-sectional images.
Disclosures
The authors have no conflicts of interest to disclose.
1. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903-912. doi: 10.1099/00222615-46-11-903. PubMed
2. Lau JX, Li JY, Yong TY. Non-contiguous multifocal vertebral osteomyelitis caused by erratia marcescens. Mod Rheumatol. 2015;25(2):303-306. doi: 10.3109/14397595.2013.874754. PubMed
3. Dessi A, Puddu M, Testa M, Marcialis MA, Pintus MC, Fanos V. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493-499. doi: 10.1179/joc.2009.21.5.493. PubMed
4. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-791. doi: 10.1128/CMR.00017-11. PubMed
5. Montagnani C, Cocchi P, Lega L, et al. Serratia marcescens outbreak in a neonatal intensive care unit: crucial role of implementing hand hygiene among external consultants. BMC Infect Dis. 2015;15:11. doi: 10.1186/s12879-014-0734-6. PubMed
6. van Ogtrop ML, van Zoeren-Grobben D, Verbakel-Salomons EM, van Boven CP. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J Hosp Infect. 1997;36(2):95-103. doi: 10.1016/S0195-6701(97)90115-8. PubMed
7. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi: 10.1128/AAC.00138-07. PubMed
8. Ewart S, Brown C, Derksen F, Kufuor-Mensa E. Serratia marcescens endocarditis in a horse. J Am Vet Med Assoc. 1992;200(7):961-963. PubMed
9. Jores J, Beutner G, Hirth-Schmidt I, Borchers K, Pitt TL, Lubke-Becker A. Isolation of Serratia marcescens from an equine abortion in Germany. Vet Rec. 2004;154(8):242-244. doi: 10.1136/vr.154.8.242. PubMed
10. Herra C, Falkiner FR. Serratia marcescens. http://www.antimicrobe.org/b26.asp. Accessed August 22, 2017.
11. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182, Table of Contents. doi: 10.1128/CMR.00036-08. PubMed
12. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585-590. doi: 10.7326/0003-4819-115-8-585. PubMed
13. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095-5101. doi: 10.1016/j.vaccine.2017.07.095. PubMed
14. Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617-638, vii. doi: 10.1016/j.idc.2007.07.003. PubMed
15. Carlesimo M, Pennica A, Muscianese M, et al. Multiple skin ulcers due to Serratia marcescens in a immunocompetent patient. G Ital Dermatol Venereol. 2014;149(3):367-370. PubMed
16. Rallis E, Karanikola E, Papadakis P. Severe facial infection caused by Serratia marcescens in an immunocompetent soldier. J Am Acad Dermatol. 2008;58(5 Suppl 1):S109-S110. doi: 10.1016/j.jaad.2007.04.010. PubMed
17. Hadid H, Usman M, Thapa S. Severe osteomyelitis and septic arthritis due to Serratia marcescens in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:347652. doi: 10.1155/2015/347652. PubMed
18. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi: 10.1093/cid/civ482. PubMed
19. Vertebral Osteomyelitis Guideline Team (Team Leader: Chenoweth CE; Team Members: Bassin BS HS, Mack MR, Kunapuli A, Park P, Quint DJ, Seagull FJ, Wesorick DH; Consultants: Patel RD, Riddell IV J, Lanava KM). Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. University of Michigan Guidelines for Clinical Care 2013; http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. Accessed October 26, 2017.
20. McDonald M. Vertebral osteomyelitis and discitis in adults. 2017; Available at: https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed October 26, 2017.
A 73-year-old man presented to the emergency department in late winter with fevers, myalgias, fatigue, low back pain, and poor oral intake. Four days earlier, he had fallen and hit his head. His partner also noticed a few episodes of confusion in the days leading up to presentation.
The patient’s symptoms are nonspecific. Fevers prompt the consideration of systemic infection, though fevers can also be seen in a broad range of noninfectious processes, including malignancy, vasculitis, autoimmune conditions, endocrinopathies, and drug reaction. The clinical picture warrants prompt and comprehensive evaluation, beginning with further detailed history (current illnesses, exposures, travel, vaccinations, medications, cancer screenings, weight change) and a careful physical examination, which will help guide laboratory testing and imaging.
His past medical history was notable for coronary artery disease for which he underwent coronary artery bypass grafting five years prior, hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, gastroesophageal reflux disease, osteoarthritis leading to chronic knee and hand pain, and a history of mildly low testosterone levels. His medications included hydrocodone and acetaminophen, metoprolol tartrate, omeprazole, topical testosterone gel (prescribed for daily use, used intermittently), and aspirin. He was retired and lived in rural Michigan with his female partner. He previously worked as a truck driver and used to train racehorses. He had quit smoking five years earlier. He denied alcohol or injection drug use.
The patient has significant underlying medical conditions. Considering infectious causes of his symptoms, it is notable that he has no reported immunodeficiency. It would be relevant to know if he has been tested for HIV. His rural residence and work with horses raise the possibility of zoonotic infections, including plague (Yersinia pestis), brucellosis (Brucella species), Q fever (Coxiella burnetti), Rhodococcus equi, or group C or G Streptococci. Information about tuberculosis risk factors, other geographic exposures, recent dental work, and ill contacts might be helpful to elucidate the causes of this nonspecific febrile illness with a possible CNS component. With regard to malignancy, it would be helpful to ask about recent weight loss, lymphadenopathy, and prior cancer screenings. Considering other etiologies, he does not report a history of autoimmune or endocrine conditions. However, it is important to consider a vasculitis, such as giant cell arteritis or polyarteritis nodosa, autoimmune conditions, and endocrinopathies such as thyrotoxicosis. The differential diagnosis for his clinical syndrome remains broad.
Vital signs were temperature 37.3°C, heart rate 88 beats per minute, respiratory rate 18 breaths per minute, blood pressure 105/64 mmHg, and oxygen saturation 93% on room air. Oral examination revealed poor dentition. The heart had a normal rate and regular rhythm with no murmurs, rubs, or gallops, and lungs were clear to auscultation bilaterally. The abdomen was unremarkable. Examination of the back was notable for mild tenderness to palpation over the sacrum. He was oriented to person, place, and time, with intact cranial nerves and a nonfocal neurologic examination. The remainder of his examination was normal. The white blood cell (WBC) count was 11.1 × 103/μL, with 84% neutrophils and 9% bands, hemoglobin 13.6 g/dL, platelet count 54 × 103/μL, sodium 122 mmol/L, potassium 3.3 mmol/L, chloride 89 mmol/L, bicarbonate 21 mmol/L, creatinine 1.64 mg/dL, albumin 2.7 g/dL, alkaline phosphatase 136 U/L, AST 60 U/L, ALT 37 U/L, and total bilirubin 2.1 mg/dL.
He had presented to the emergency department five days earlier with fever, flank pain, nausea, vomiting, and weakness. At that time, he had a temperature of 38.2°C, but vital signs otherwise had been normal. Laboratory studies had revealed WBC count 14.0 × 103/μL, hemoglobin 13.7 g/dL, platelet count 175 × 103/μL, sodium 129 mmol/L, chloride 97 mmol/dL, bicarbonate 23 mmol/L, creatinine 1.1 mg/dL, and total bilirubin 1.6 mg/dL. Urinalysis had been negative. He had received one liter of intravenous normal saline and ketorolac for pain and had been discharged with the diagnosis of a viral illness.
A picture of a progressive, subacute illness with multisystem involvement appears to be emerging, and there are several abnormalities consistent with infection, including fever, leukocytosis with bandemia, thrombocytopenia, renal dysfunction, and elevated bilirubin. His borderline hypotension may be due to uninterrupted use of his antihypertensive medication in the setting of poor oral intake or may indicate incipient sepsis. Focal sacral tenderness raises the possibility of vertebral osteomyelitis or epidural abscess, either from a contiguous focus of infection from the surrounding structures, or as a site of seeding from bacteremia. His prior confusion episodes might have been secondary to a systemic process; however, CNS imaging should be done, given the history of confusion and recent fall. Further diagnostic studies are warranted, including: blood cultures; peripheral blood smear; imaging of the spine, chest, abdomen, and pelvis; electrocardiogram; and possibly echocardiogram. Although noninfectious etiologies should not be discounted, the constellation of findings is more compatible with infection.
Two sets of blood cultures and a viral respiratory swab were obtained. Computed tomography (CT) of the head without contrast was negative for acute bleeding or other intracranial pathology. Lumbosacral radiography revealed degenerative changes with intact alignment of the sacrum. The patient was admitted with plans to pursue lumbar puncture if altered mental status recurred. The viral swab was negative. Within 24 hours, one set of blood cultures (both bottles) grew lactose-negative, oxidase-negative, gram-negative rods.
Gram-negative rods (GNRs) rarely are contaminants in blood cultures and should be considered significant until proven otherwise. Prompt empiric therapy and investigation to identify the primary source of bacteremia must be initiated. Although the most common GNRs isolated from blood cultures are enteric coliform organisms such as E. coli, Klebsiella, and Enterobacter, these typically are lactose-positive. Additional possibilities should be considered, including Salmonella species or other organisms comprising the “HACEK” group. This latter group is commonly associated with endocarditis, but the majority are oxidase-positive and have more fastidious growth requirements. Although there are other gram-negative organisms to consider, they have other distinguishing characteristics that have not been indicated in the microbiology results. Broad-spectrum antibiotic therapy is appropriate while awaiting the final identification of the GNR. A thorough search for a primary source and secondary sites of hematogenous seeding should be conducted. His only localizing symptom was tenderness over the sacrum, and this should be further assessed by sensitive imaging such as magnetic resonance imaging (MRI). The identity of the GNR would guide further diagnostic evaluation. For example, a respiratory organism such as Haemophilus influenzae would prompt a CT scan of the chest. Isolation of an enteric or a coliform GNR such as E. coli would prompt abdominal and pelvic imaging to assess for occult abscess. An “HACEK” group organism would prompt echocardiography to evaluate for endocarditis.
He was started on piperacillin–tazobactam. GNR bacteremia without a clear source prompted a CT of the chest, abdomen, and pelvis with and without contrast. The images were unremarkable, with the exception of a signal abnormality in the left psoas muscle concerning for abscess (Figure 1). MRI of the same region revealed L2-4 osteomyelitis and discitis with bilateral psoas abscesses but without epidural abscess (Figure 2).
Because of increasing reports of antibiotic resistance in GNRs, even in community-acquired infections, it is appropriate to initially treat with a broad-spectrum antibiotic such as a fourth-generation cephalosporin or carbapenem while awaiting identification and susceptibility results to guide definitive therapy. In addition to antimicrobial therapy, treatment of psoas abscess usually requires drainage. Vertebral osteomyelitis from a hematogenous source can often be treated with antibiotics alone, as long as there are no associated complications such as epidural abscess and spine instability. Imaging should be reviewed for pathology of the surrounding structures, and surgical consultation should be obtained.
Neurosurgery, Interventional Radiology, and Infectious Disease services were consulted. Antibiotic coverage was expanded to vancomycin, cefepime, and metronidazole due to the possibility of polymicrobial infection. No surgical intervention was recommended since the abscesses were too small to drain.
The next day, the GNR was identified as Serratia marcescens.
S. marcescens is a widely distributed organism in the environment, but not a common component of endogenous human flora. Serratia is generally considered as an opportunistic nosocomial pathogen. Community-acquired infection with this organism is unusual and implies exogenous acquisition. A careful re-evaluation of exposures, including injection drug use or other parenteral exposures is important to identify the likely source of infection, as these have been previously linked to outbreaks of environmental organisms. Based on the presumed pathogenesis of infection and the initial microbiology suggesting monomicrobial Serratia infection, antibiotics should be narrowed based on the susceptibility results. There is concern that antibiotics might not adequately penetrate the abscesses and result in a lack of clinical improvement and/or lead to the emergence of antibiotic resistance during therapy. This is an important concern with Serratia, which typically harbors an AmpC beta-lactamase that can mediate resistance to broad-spectrum cephalosporins. If medical therapy alone without drainage is planned, short-interval re-imaging is warranted.
Blood cultures from days two and three of hospitalization also grew S. marcescens. No other organisms grew. Based on culture sensitivity data, antibiotics were narrowed to ceftriaxone.
This surprising culture result prompted the medical team to obtain screening laboratory tests for immunocompromising conditions and to revisit the patient’s history. His type 2 diabetes mellitus was well controlled with a hemoglobin A1c of 6.5%. HIV testing was negative. Further questioning of the patient revealed that he had fallen from a truck onto rocks four months prior, injuring his back and hip, but without puncture of the skin or loss of consciousness; he denied recent falls or other injuries but reported significant chronic knee pain. He had not been hospitalized recently. He had never taken corticosteroids or immunomodulatory medications. He continued to deny injection drug use. He did, however, clarify that his work with racehorses, which was originally understood to be a prior hobby, was ongoing, including recent work of cleaning the stables.
The following morning, he experienced confusion, rigors, and hypoxia, which prompted transfer to the intensive care unit (ICU).
Acute worsening during treatment is worrisome, and could be a potential complication of his infection or treatment – or even a separate process altogether. Knee pain in the setting of bacteremia raises the possibility of septic or crystal-induced arthritis and warrants imaging. Confusion and hypoxia might represent secondary sites of seeding from bacteremia (CNS infection and pneumonia, respectively) or manifestations of endocarditis, the latter being unusual for Serratia. An echocardiogram should be obtained. Other neurologic causes, including seizure, should also be considered. Further evaluation by chest imaging and repeat neurologic examination and imaging should be performed. Emergence of resistance during therapy is a theoretical concern with Serratia as an AmpC beta-lactamase-containing organism. While awaiting additional microbiology data, an empiric change to an AmpC beta-lactamase stable antibiotic such as a carbapenem should be made, especially since he has clinically deteriorated on therapy with a β-lactamase susceptible antibiotic, raising concerns of the emergence of resistance on initial therapy.
Antibiotics were changed to meropenem, vancomycin, and metronidazole given the clinical worsening and concerns that this represented infection unresponsive to prior antibiotics. The acute episode resolved spontaneously after one hour. His neurologic examination remained nonfocal. Chest radiography, urinalysis, urine culture, and right upper quadrant ultrasound were unremarkable. Transesophageal echocardiogram revealed no heart valve vegetations. MRI and bone scan of the lower extremities did not show any evidence of septic arthritis or other infection. He remained stable and was transferred out of the ICU the following day. Antibiotic coverage was switched to cefepime. On discussion with his significant other, this event was found to be similar to the intermittent confusion that occurred in the days prior to admission.
The acute onset and other features of these intermittent periods of deterioration are compatible with infection; intermittent seeding of the blood with microbes or their products (eg, lipopolysaccharides) from an abscess or vascular infection could explain these episodes. Some of the previous hypotheses to explain the episodes, such as a secondary infectious process, have not been supported by diagnostic testing or the clinical course. He needs close clinical monitoring and interval assessment of the known sites of infection.
Ten days after osteomyelitis and discitis were diagnosed, the patient developed worsening low back pain, prompting repeat spine MRI. This was significant for bilateral psoas abscess enlargement and extension of osteomyelitis and discitis (Figure 3). He was re-evaluated by Neurosurgery and Interventional Radiology and underwent psoas abscess drainage; abscess cultures grew S. marcescens.
He slowly improved over several weeks and was discharged to a subacute rehabilitation facility. He completed a 3.5-week course of intravenous antibiotics before leaving against medical advice. He completed eight weeks of oral trimethoprim-sulfamethoxazole and remains without long-term sequelae from the infection.
DISCUSSION
S. marcescens is a gram-negative rod in the Enterobacteriaceae family known for its red pigment. Primarily, S. marcescens causes nosocomial infections, most commonly of the respiratory and urinary tracts. However, a wide range of manifestations has been documented, including meningitis, ocular infections (conjunctivitis, keratitis, endophthalmitis), endocarditis, skin infections (cellulitis, necrotizing fasciitis), and osteomyelitis.1, 2 S. marcescens is often reported as the cause of outbreaks in ICUs;3-6 infection is thought to occur via contamination of water pipes, hospital equipment, and disinfectants.3, 7 Its natural environment includes soil, water, and GI tracts of animals,4 and there are published reports of S. marcescens infection in horses.8, 9 This patient was most likely exposed to S. marcescens through his work with horses and their environment.
S. marcescens has wide-ranging target organs, and successful treatment can be difficult. S. marcescens can infect the renal, respiratory, gastrointestinal, ocular, cardiovascular, and musculoskeletal systems. S. marcescens, like other “SPACE” organisms (Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter), expresses inducible AmpC beta-lactamase.10 At baseline, AmpC beta-lactamase expression is repressed.11 Mutants with stably de-repressed (constitutively expressed) AmpC can be selected during therapy and lead to clinical failure, as has been best described during therapy for Enterobacter infections.12 Infectious Disease consultation may be helpful when caring for patients with S. marcescens bacteremia given these complexities.
This was an unusual case of S. marcescens infection. It most commonly infects immunocompromised hosts. Reported risk factors include solid organ or hematopoietic stem cell transplant, malignancy, HIV/AIDS, and receipt of immunosuppressive agents. The patient did not have these risk factors, but did have well-controlled type 2 diabetes mellitus. Although diabetes is associated with an increased risk of infection and more severe infections,13, 14 there is no evidence in the literature that well-controlled type 2 diabetes mellitus compromises the immune system. A few case reports document cutaneous S. marcescens infection in immunocompetent adults.15,16 A case report of S. marcescens septic arthritis and adjacent osteomyelitis has also been published, but the patient had poorly controlled diabetes.17 This case provides a report of systemic S. marcescens infection in an individual without clear risk factors.
S. marcescens osteomyelitis is rare, and there have been only a few prior case reports.2,18 The presentation of osteomyelitis, regardless of the causative organism, is subtle, often insidious, and can easily be missed. Hospitalists should have a high index of suspicion for the diagnosis as it requires prompt evaluation and treatment for complications, including epidural abscess. Risk factors include diabetes mellitus, rheumatoid arthritis, injection drug use, and other immunocompromising illnesses.19 Degenerative changes in the spine such as osteoarthritis may be risk factors as well,20 though not well studied or quantified. A hypothesized mechanism involves local inflammation and joint damage, leaving the area susceptible to bacterial seeding. Osteoarthritis and degenerative disc disease, along with exposure to racehorses, likely put this patient at risk for bacterial seeding in the vertebrae, ultimately leading to a “dark horse” diagnosis.
TEACHING POINTS
- Serratia marcescens is a gram-negative rod bacterium that most commonly infects immunocompromised individuals in hospital settings. This report demonstrates that S. marcescens can cause serious infection in immunocompetent, nonhospitalized adults.
- S. marcescens bacteremia or infection of organs outside of the urinary or respiratory systems is uncommon, and therapy can be complicated by emergence of resistance.
- The clinical presentation of vertebral osteomyelitis and discitis and psoas abscess can be subtle and may present without typical signs and symptoms of infection.
ACKNOWLEDGEMENTS
The authors thank the patient and his partner for their willingness to have his story published, Laura Petersen, MHSA, for providing assistance with references and manuscript editing, and Shadi Azar, MBBS, for assistance in selecting the cross-sectional images.
Disclosures
The authors have no conflicts of interest to disclose.
A 73-year-old man presented to the emergency department in late winter with fevers, myalgias, fatigue, low back pain, and poor oral intake. Four days earlier, he had fallen and hit his head. His partner also noticed a few episodes of confusion in the days leading up to presentation.
The patient’s symptoms are nonspecific. Fevers prompt the consideration of systemic infection, though fevers can also be seen in a broad range of noninfectious processes, including malignancy, vasculitis, autoimmune conditions, endocrinopathies, and drug reaction. The clinical picture warrants prompt and comprehensive evaluation, beginning with further detailed history (current illnesses, exposures, travel, vaccinations, medications, cancer screenings, weight change) and a careful physical examination, which will help guide laboratory testing and imaging.
His past medical history was notable for coronary artery disease for which he underwent coronary artery bypass grafting five years prior, hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, gastroesophageal reflux disease, osteoarthritis leading to chronic knee and hand pain, and a history of mildly low testosterone levels. His medications included hydrocodone and acetaminophen, metoprolol tartrate, omeprazole, topical testosterone gel (prescribed for daily use, used intermittently), and aspirin. He was retired and lived in rural Michigan with his female partner. He previously worked as a truck driver and used to train racehorses. He had quit smoking five years earlier. He denied alcohol or injection drug use.
The patient has significant underlying medical conditions. Considering infectious causes of his symptoms, it is notable that he has no reported immunodeficiency. It would be relevant to know if he has been tested for HIV. His rural residence and work with horses raise the possibility of zoonotic infections, including plague (Yersinia pestis), brucellosis (Brucella species), Q fever (Coxiella burnetti), Rhodococcus equi, or group C or G Streptococci. Information about tuberculosis risk factors, other geographic exposures, recent dental work, and ill contacts might be helpful to elucidate the causes of this nonspecific febrile illness with a possible CNS component. With regard to malignancy, it would be helpful to ask about recent weight loss, lymphadenopathy, and prior cancer screenings. Considering other etiologies, he does not report a history of autoimmune or endocrine conditions. However, it is important to consider a vasculitis, such as giant cell arteritis or polyarteritis nodosa, autoimmune conditions, and endocrinopathies such as thyrotoxicosis. The differential diagnosis for his clinical syndrome remains broad.
Vital signs were temperature 37.3°C, heart rate 88 beats per minute, respiratory rate 18 breaths per minute, blood pressure 105/64 mmHg, and oxygen saturation 93% on room air. Oral examination revealed poor dentition. The heart had a normal rate and regular rhythm with no murmurs, rubs, or gallops, and lungs were clear to auscultation bilaterally. The abdomen was unremarkable. Examination of the back was notable for mild tenderness to palpation over the sacrum. He was oriented to person, place, and time, with intact cranial nerves and a nonfocal neurologic examination. The remainder of his examination was normal. The white blood cell (WBC) count was 11.1 × 103/μL, with 84% neutrophils and 9% bands, hemoglobin 13.6 g/dL, platelet count 54 × 103/μL, sodium 122 mmol/L, potassium 3.3 mmol/L, chloride 89 mmol/L, bicarbonate 21 mmol/L, creatinine 1.64 mg/dL, albumin 2.7 g/dL, alkaline phosphatase 136 U/L, AST 60 U/L, ALT 37 U/L, and total bilirubin 2.1 mg/dL.
He had presented to the emergency department five days earlier with fever, flank pain, nausea, vomiting, and weakness. At that time, he had a temperature of 38.2°C, but vital signs otherwise had been normal. Laboratory studies had revealed WBC count 14.0 × 103/μL, hemoglobin 13.7 g/dL, platelet count 175 × 103/μL, sodium 129 mmol/L, chloride 97 mmol/dL, bicarbonate 23 mmol/L, creatinine 1.1 mg/dL, and total bilirubin 1.6 mg/dL. Urinalysis had been negative. He had received one liter of intravenous normal saline and ketorolac for pain and had been discharged with the diagnosis of a viral illness.
A picture of a progressive, subacute illness with multisystem involvement appears to be emerging, and there are several abnormalities consistent with infection, including fever, leukocytosis with bandemia, thrombocytopenia, renal dysfunction, and elevated bilirubin. His borderline hypotension may be due to uninterrupted use of his antihypertensive medication in the setting of poor oral intake or may indicate incipient sepsis. Focal sacral tenderness raises the possibility of vertebral osteomyelitis or epidural abscess, either from a contiguous focus of infection from the surrounding structures, or as a site of seeding from bacteremia. His prior confusion episodes might have been secondary to a systemic process; however, CNS imaging should be done, given the history of confusion and recent fall. Further diagnostic studies are warranted, including: blood cultures; peripheral blood smear; imaging of the spine, chest, abdomen, and pelvis; electrocardiogram; and possibly echocardiogram. Although noninfectious etiologies should not be discounted, the constellation of findings is more compatible with infection.
Two sets of blood cultures and a viral respiratory swab were obtained. Computed tomography (CT) of the head without contrast was negative for acute bleeding or other intracranial pathology. Lumbosacral radiography revealed degenerative changes with intact alignment of the sacrum. The patient was admitted with plans to pursue lumbar puncture if altered mental status recurred. The viral swab was negative. Within 24 hours, one set of blood cultures (both bottles) grew lactose-negative, oxidase-negative, gram-negative rods.
Gram-negative rods (GNRs) rarely are contaminants in blood cultures and should be considered significant until proven otherwise. Prompt empiric therapy and investigation to identify the primary source of bacteremia must be initiated. Although the most common GNRs isolated from blood cultures are enteric coliform organisms such as E. coli, Klebsiella, and Enterobacter, these typically are lactose-positive. Additional possibilities should be considered, including Salmonella species or other organisms comprising the “HACEK” group. This latter group is commonly associated with endocarditis, but the majority are oxidase-positive and have more fastidious growth requirements. Although there are other gram-negative organisms to consider, they have other distinguishing characteristics that have not been indicated in the microbiology results. Broad-spectrum antibiotic therapy is appropriate while awaiting the final identification of the GNR. A thorough search for a primary source and secondary sites of hematogenous seeding should be conducted. His only localizing symptom was tenderness over the sacrum, and this should be further assessed by sensitive imaging such as magnetic resonance imaging (MRI). The identity of the GNR would guide further diagnostic evaluation. For example, a respiratory organism such as Haemophilus influenzae would prompt a CT scan of the chest. Isolation of an enteric or a coliform GNR such as E. coli would prompt abdominal and pelvic imaging to assess for occult abscess. An “HACEK” group organism would prompt echocardiography to evaluate for endocarditis.
He was started on piperacillin–tazobactam. GNR bacteremia without a clear source prompted a CT of the chest, abdomen, and pelvis with and without contrast. The images were unremarkable, with the exception of a signal abnormality in the left psoas muscle concerning for abscess (Figure 1). MRI of the same region revealed L2-4 osteomyelitis and discitis with bilateral psoas abscesses but without epidural abscess (Figure 2).
Because of increasing reports of antibiotic resistance in GNRs, even in community-acquired infections, it is appropriate to initially treat with a broad-spectrum antibiotic such as a fourth-generation cephalosporin or carbapenem while awaiting identification and susceptibility results to guide definitive therapy. In addition to antimicrobial therapy, treatment of psoas abscess usually requires drainage. Vertebral osteomyelitis from a hematogenous source can often be treated with antibiotics alone, as long as there are no associated complications such as epidural abscess and spine instability. Imaging should be reviewed for pathology of the surrounding structures, and surgical consultation should be obtained.
Neurosurgery, Interventional Radiology, and Infectious Disease services were consulted. Antibiotic coverage was expanded to vancomycin, cefepime, and metronidazole due to the possibility of polymicrobial infection. No surgical intervention was recommended since the abscesses were too small to drain.
The next day, the GNR was identified as Serratia marcescens.
S. marcescens is a widely distributed organism in the environment, but not a common component of endogenous human flora. Serratia is generally considered as an opportunistic nosocomial pathogen. Community-acquired infection with this organism is unusual and implies exogenous acquisition. A careful re-evaluation of exposures, including injection drug use or other parenteral exposures is important to identify the likely source of infection, as these have been previously linked to outbreaks of environmental organisms. Based on the presumed pathogenesis of infection and the initial microbiology suggesting monomicrobial Serratia infection, antibiotics should be narrowed based on the susceptibility results. There is concern that antibiotics might not adequately penetrate the abscesses and result in a lack of clinical improvement and/or lead to the emergence of antibiotic resistance during therapy. This is an important concern with Serratia, which typically harbors an AmpC beta-lactamase that can mediate resistance to broad-spectrum cephalosporins. If medical therapy alone without drainage is planned, short-interval re-imaging is warranted.
Blood cultures from days two and three of hospitalization also grew S. marcescens. No other organisms grew. Based on culture sensitivity data, antibiotics were narrowed to ceftriaxone.
This surprising culture result prompted the medical team to obtain screening laboratory tests for immunocompromising conditions and to revisit the patient’s history. His type 2 diabetes mellitus was well controlled with a hemoglobin A1c of 6.5%. HIV testing was negative. Further questioning of the patient revealed that he had fallen from a truck onto rocks four months prior, injuring his back and hip, but without puncture of the skin or loss of consciousness; he denied recent falls or other injuries but reported significant chronic knee pain. He had not been hospitalized recently. He had never taken corticosteroids or immunomodulatory medications. He continued to deny injection drug use. He did, however, clarify that his work with racehorses, which was originally understood to be a prior hobby, was ongoing, including recent work of cleaning the stables.
The following morning, he experienced confusion, rigors, and hypoxia, which prompted transfer to the intensive care unit (ICU).
Acute worsening during treatment is worrisome, and could be a potential complication of his infection or treatment – or even a separate process altogether. Knee pain in the setting of bacteremia raises the possibility of septic or crystal-induced arthritis and warrants imaging. Confusion and hypoxia might represent secondary sites of seeding from bacteremia (CNS infection and pneumonia, respectively) or manifestations of endocarditis, the latter being unusual for Serratia. An echocardiogram should be obtained. Other neurologic causes, including seizure, should also be considered. Further evaluation by chest imaging and repeat neurologic examination and imaging should be performed. Emergence of resistance during therapy is a theoretical concern with Serratia as an AmpC beta-lactamase-containing organism. While awaiting additional microbiology data, an empiric change to an AmpC beta-lactamase stable antibiotic such as a carbapenem should be made, especially since he has clinically deteriorated on therapy with a β-lactamase susceptible antibiotic, raising concerns of the emergence of resistance on initial therapy.
Antibiotics were changed to meropenem, vancomycin, and metronidazole given the clinical worsening and concerns that this represented infection unresponsive to prior antibiotics. The acute episode resolved spontaneously after one hour. His neurologic examination remained nonfocal. Chest radiography, urinalysis, urine culture, and right upper quadrant ultrasound were unremarkable. Transesophageal echocardiogram revealed no heart valve vegetations. MRI and bone scan of the lower extremities did not show any evidence of septic arthritis or other infection. He remained stable and was transferred out of the ICU the following day. Antibiotic coverage was switched to cefepime. On discussion with his significant other, this event was found to be similar to the intermittent confusion that occurred in the days prior to admission.
The acute onset and other features of these intermittent periods of deterioration are compatible with infection; intermittent seeding of the blood with microbes or their products (eg, lipopolysaccharides) from an abscess or vascular infection could explain these episodes. Some of the previous hypotheses to explain the episodes, such as a secondary infectious process, have not been supported by diagnostic testing or the clinical course. He needs close clinical monitoring and interval assessment of the known sites of infection.
Ten days after osteomyelitis and discitis were diagnosed, the patient developed worsening low back pain, prompting repeat spine MRI. This was significant for bilateral psoas abscess enlargement and extension of osteomyelitis and discitis (Figure 3). He was re-evaluated by Neurosurgery and Interventional Radiology and underwent psoas abscess drainage; abscess cultures grew S. marcescens.
He slowly improved over several weeks and was discharged to a subacute rehabilitation facility. He completed a 3.5-week course of intravenous antibiotics before leaving against medical advice. He completed eight weeks of oral trimethoprim-sulfamethoxazole and remains without long-term sequelae from the infection.
DISCUSSION
S. marcescens is a gram-negative rod in the Enterobacteriaceae family known for its red pigment. Primarily, S. marcescens causes nosocomial infections, most commonly of the respiratory and urinary tracts. However, a wide range of manifestations has been documented, including meningitis, ocular infections (conjunctivitis, keratitis, endophthalmitis), endocarditis, skin infections (cellulitis, necrotizing fasciitis), and osteomyelitis.1, 2 S. marcescens is often reported as the cause of outbreaks in ICUs;3-6 infection is thought to occur via contamination of water pipes, hospital equipment, and disinfectants.3, 7 Its natural environment includes soil, water, and GI tracts of animals,4 and there are published reports of S. marcescens infection in horses.8, 9 This patient was most likely exposed to S. marcescens through his work with horses and their environment.
S. marcescens has wide-ranging target organs, and successful treatment can be difficult. S. marcescens can infect the renal, respiratory, gastrointestinal, ocular, cardiovascular, and musculoskeletal systems. S. marcescens, like other “SPACE” organisms (Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter), expresses inducible AmpC beta-lactamase.10 At baseline, AmpC beta-lactamase expression is repressed.11 Mutants with stably de-repressed (constitutively expressed) AmpC can be selected during therapy and lead to clinical failure, as has been best described during therapy for Enterobacter infections.12 Infectious Disease consultation may be helpful when caring for patients with S. marcescens bacteremia given these complexities.
This was an unusual case of S. marcescens infection. It most commonly infects immunocompromised hosts. Reported risk factors include solid organ or hematopoietic stem cell transplant, malignancy, HIV/AIDS, and receipt of immunosuppressive agents. The patient did not have these risk factors, but did have well-controlled type 2 diabetes mellitus. Although diabetes is associated with an increased risk of infection and more severe infections,13, 14 there is no evidence in the literature that well-controlled type 2 diabetes mellitus compromises the immune system. A few case reports document cutaneous S. marcescens infection in immunocompetent adults.15,16 A case report of S. marcescens septic arthritis and adjacent osteomyelitis has also been published, but the patient had poorly controlled diabetes.17 This case provides a report of systemic S. marcescens infection in an individual without clear risk factors.
S. marcescens osteomyelitis is rare, and there have been only a few prior case reports.2,18 The presentation of osteomyelitis, regardless of the causative organism, is subtle, often insidious, and can easily be missed. Hospitalists should have a high index of suspicion for the diagnosis as it requires prompt evaluation and treatment for complications, including epidural abscess. Risk factors include diabetes mellitus, rheumatoid arthritis, injection drug use, and other immunocompromising illnesses.19 Degenerative changes in the spine such as osteoarthritis may be risk factors as well,20 though not well studied or quantified. A hypothesized mechanism involves local inflammation and joint damage, leaving the area susceptible to bacterial seeding. Osteoarthritis and degenerative disc disease, along with exposure to racehorses, likely put this patient at risk for bacterial seeding in the vertebrae, ultimately leading to a “dark horse” diagnosis.
TEACHING POINTS
- Serratia marcescens is a gram-negative rod bacterium that most commonly infects immunocompromised individuals in hospital settings. This report demonstrates that S. marcescens can cause serious infection in immunocompetent, nonhospitalized adults.
- S. marcescens bacteremia or infection of organs outside of the urinary or respiratory systems is uncommon, and therapy can be complicated by emergence of resistance.
- The clinical presentation of vertebral osteomyelitis and discitis and psoas abscess can be subtle and may present without typical signs and symptoms of infection.
ACKNOWLEDGEMENTS
The authors thank the patient and his partner for their willingness to have his story published, Laura Petersen, MHSA, for providing assistance with references and manuscript editing, and Shadi Azar, MBBS, for assistance in selecting the cross-sectional images.
Disclosures
The authors have no conflicts of interest to disclose.
1. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903-912. doi: 10.1099/00222615-46-11-903. PubMed
2. Lau JX, Li JY, Yong TY. Non-contiguous multifocal vertebral osteomyelitis caused by erratia marcescens. Mod Rheumatol. 2015;25(2):303-306. doi: 10.3109/14397595.2013.874754. PubMed
3. Dessi A, Puddu M, Testa M, Marcialis MA, Pintus MC, Fanos V. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493-499. doi: 10.1179/joc.2009.21.5.493. PubMed
4. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-791. doi: 10.1128/CMR.00017-11. PubMed
5. Montagnani C, Cocchi P, Lega L, et al. Serratia marcescens outbreak in a neonatal intensive care unit: crucial role of implementing hand hygiene among external consultants. BMC Infect Dis. 2015;15:11. doi: 10.1186/s12879-014-0734-6. PubMed
6. van Ogtrop ML, van Zoeren-Grobben D, Verbakel-Salomons EM, van Boven CP. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J Hosp Infect. 1997;36(2):95-103. doi: 10.1016/S0195-6701(97)90115-8. PubMed
7. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi: 10.1128/AAC.00138-07. PubMed
8. Ewart S, Brown C, Derksen F, Kufuor-Mensa E. Serratia marcescens endocarditis in a horse. J Am Vet Med Assoc. 1992;200(7):961-963. PubMed
9. Jores J, Beutner G, Hirth-Schmidt I, Borchers K, Pitt TL, Lubke-Becker A. Isolation of Serratia marcescens from an equine abortion in Germany. Vet Rec. 2004;154(8):242-244. doi: 10.1136/vr.154.8.242. PubMed
10. Herra C, Falkiner FR. Serratia marcescens. http://www.antimicrobe.org/b26.asp. Accessed August 22, 2017.
11. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182, Table of Contents. doi: 10.1128/CMR.00036-08. PubMed
12. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585-590. doi: 10.7326/0003-4819-115-8-585. PubMed
13. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095-5101. doi: 10.1016/j.vaccine.2017.07.095. PubMed
14. Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617-638, vii. doi: 10.1016/j.idc.2007.07.003. PubMed
15. Carlesimo M, Pennica A, Muscianese M, et al. Multiple skin ulcers due to Serratia marcescens in a immunocompetent patient. G Ital Dermatol Venereol. 2014;149(3):367-370. PubMed
16. Rallis E, Karanikola E, Papadakis P. Severe facial infection caused by Serratia marcescens in an immunocompetent soldier. J Am Acad Dermatol. 2008;58(5 Suppl 1):S109-S110. doi: 10.1016/j.jaad.2007.04.010. PubMed
17. Hadid H, Usman M, Thapa S. Severe osteomyelitis and septic arthritis due to Serratia marcescens in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:347652. doi: 10.1155/2015/347652. PubMed
18. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi: 10.1093/cid/civ482. PubMed
19. Vertebral Osteomyelitis Guideline Team (Team Leader: Chenoweth CE; Team Members: Bassin BS HS, Mack MR, Kunapuli A, Park P, Quint DJ, Seagull FJ, Wesorick DH; Consultants: Patel RD, Riddell IV J, Lanava KM). Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. University of Michigan Guidelines for Clinical Care 2013; http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. Accessed October 26, 2017.
20. McDonald M. Vertebral osteomyelitis and discitis in adults. 2017; Available at: https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed October 26, 2017.
1. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903-912. doi: 10.1099/00222615-46-11-903. PubMed
2. Lau JX, Li JY, Yong TY. Non-contiguous multifocal vertebral osteomyelitis caused by erratia marcescens. Mod Rheumatol. 2015;25(2):303-306. doi: 10.3109/14397595.2013.874754. PubMed
3. Dessi A, Puddu M, Testa M, Marcialis MA, Pintus MC, Fanos V. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493-499. doi: 10.1179/joc.2009.21.5.493. PubMed
4. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-791. doi: 10.1128/CMR.00017-11. PubMed
5. Montagnani C, Cocchi P, Lega L, et al. Serratia marcescens outbreak in a neonatal intensive care unit: crucial role of implementing hand hygiene among external consultants. BMC Infect Dis. 2015;15:11. doi: 10.1186/s12879-014-0734-6. PubMed
6. van Ogtrop ML, van Zoeren-Grobben D, Verbakel-Salomons EM, van Boven CP. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J Hosp Infect. 1997;36(2):95-103. doi: 10.1016/S0195-6701(97)90115-8. PubMed
7. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi: 10.1128/AAC.00138-07. PubMed
8. Ewart S, Brown C, Derksen F, Kufuor-Mensa E. Serratia marcescens endocarditis in a horse. J Am Vet Med Assoc. 1992;200(7):961-963. PubMed
9. Jores J, Beutner G, Hirth-Schmidt I, Borchers K, Pitt TL, Lubke-Becker A. Isolation of Serratia marcescens from an equine abortion in Germany. Vet Rec. 2004;154(8):242-244. doi: 10.1136/vr.154.8.242. PubMed
10. Herra C, Falkiner FR. Serratia marcescens. http://www.antimicrobe.org/b26.asp. Accessed August 22, 2017.
11. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182, Table of Contents. doi: 10.1128/CMR.00036-08. PubMed
12. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585-590. doi: 10.7326/0003-4819-115-8-585. PubMed
13. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095-5101. doi: 10.1016/j.vaccine.2017.07.095. PubMed
14. Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617-638, vii. doi: 10.1016/j.idc.2007.07.003. PubMed
15. Carlesimo M, Pennica A, Muscianese M, et al. Multiple skin ulcers due to Serratia marcescens in a immunocompetent patient. G Ital Dermatol Venereol. 2014;149(3):367-370. PubMed
16. Rallis E, Karanikola E, Papadakis P. Severe facial infection caused by Serratia marcescens in an immunocompetent soldier. J Am Acad Dermatol. 2008;58(5 Suppl 1):S109-S110. doi: 10.1016/j.jaad.2007.04.010. PubMed
17. Hadid H, Usman M, Thapa S. Severe osteomyelitis and septic arthritis due to Serratia marcescens in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:347652. doi: 10.1155/2015/347652. PubMed
18. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi: 10.1093/cid/civ482. PubMed
19. Vertebral Osteomyelitis Guideline Team (Team Leader: Chenoweth CE; Team Members: Bassin BS HS, Mack MR, Kunapuli A, Park P, Quint DJ, Seagull FJ, Wesorick DH; Consultants: Patel RD, Riddell IV J, Lanava KM). Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. University of Michigan Guidelines for Clinical Care 2013; http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. Accessed October 26, 2017.
20. McDonald M. Vertebral osteomyelitis and discitis in adults. 2017; Available at: https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed October 26, 2017.
© 2018 Society of Hospital Medicine
A Strong Diagnosis of Weakness
A 52-year-old man presented with bilateral weakness in all extremities. He noted the gradual onset of progressive muscle weakness 6 months prior to presentation. He reported generalized fatigue and difficulty with climbing stairs and carrying heavy objects.
Initial considerations of chronic weakness and fatigue are myopathy, polyneuropathy, medications, malignancy, endocrinopathies, human immunodeficiency virus (HIV), neuromuscular junction dysfunction, and central nervous system (CNS) disorders, such as amyotrophic lateral sclerosis (ALS) or multiple sclerosis (MS). Symmetrical muscle involvement and proximal weakness make myopathy most likely. Polyneuropathy, such as chronic inflammatory demyelinating polyneuropathy (CIDP), is less likely but still possible given the slowly progressive course. The use of medications that can cause myopathy should be explored, including colchicine, steroids, and statins. Gathering further history should focus on risk factors for HIV, as well as alcohol and illicit drug use. Malignancy can cause paraneoplastic myopathy. The review of systems should include symptoms of endocrinopathies, such as thyrotoxicosis and hypothyroidism. Fluctuations in weakness and dysphagia or ocular symptoms would suggest myasthenia gravis (MG). The time course and symmetrical weakness make a central disorder, such as ALS or MS, unlikely.
His past medical history was notable for pulmonary tuberculosis diagnosed at the age of 6 years, which was treated with hospitalization and an unknown medication regimen. He was not taking medications prior to this admission. His family history was significant for diabetes mellitus in both parents. He denied sick contacts. He was sexually active with his wife. He denied the use of tobacco and illicit drugs but endorsed alcohol consumption on a daily basis over the last 32 years. He reported no fluctuation in his symptoms, muscle or joint pains, rash, fevers, chills, diaphoresis, chest pain, dyspnea, abdominal pain, diarrhea, paresthesias, weight loss, or night sweats. He had never had a colonoscopy.
Painless progressive weakness of the limbs without sensory deficit is typical of a myopathy. Though CIDP can present with only motor weakness, the majority of patients have sensory symptoms, making this less likely. Although chronic alcohol abuse can cause myopathy, it seems less likely because other neurologic complications, such as sensory polyneuropathy or ataxia, would be expected. A review of systems does not suggest a thyroid disorder or malignancy, although this does not preclude an evaluation for both. The absence of fluctuations in weakness argues against MG. Though ALS, MG, MS, and CIDP are less likely, a neurologic exam is crucial in excluding them. The hallmark of ALS is upper motor neuron (UMN) and lower motor neuron signs in the absence of sensory symptoms and signs, while global hyporeflexia would be expected in CIDP, and fatigability on repeated power testing would be expected in MG. Neurologic findings disseminated in space (neuro-anatomically) would be expected in MS.
On physical examination, the patient had a temperature of 36.9°C, heart rate of 70 beats per minute, and regular respiratory rate of 10 breaths per minute, blood pressure 130/80 mmHg, and oxygen saturation 98% while breathing ambient air. Auscultation of the heart and lungs revealed normal findings. The abdomen was soft, nontender, and without masses or organomegaly. Neurologic examination disclosed bilateral symmetric upper and lower extremity weakness with positive Gower sign. Muscle strength scores of the bilateral biceps brachii, iliopsoas, and digitis extensor were between 4 and 5 without fatigability. Grasping power was impaired. Deep tendon reflexes were preserved, and there were no UMN signs. There was no tenderness to palpation in any muscle groups. Sensory testing was normal. Skin and lymph examinations were without abnormality. The rest of the physical examination was unremarkable.
Gower sign, characteristic of but not specific to muscular dystrophy, indicates proximal muscle weakness of lower extremities, wherein hands and arms are used to walk up the body into an upright position. The exam also reveals distal weakness as shown by reduced hand grasp. Symmetrical proximal weakness of all extremities without sensory deficits suggests a myopathic process, albeit one with some distal involvement. The absence of UMN signs argues against ALS, lack of fatigability argues against MG, and the absence of CNS or sensory deficits argues against MS.
Because myopathy is most likely, the next step would be to determine if this is an idiopathic inflammatory myopathy, such as polymyositis (PM) or dermatomyositis (DM), secondary inflammatory myopathy, or noninflammatory myopathy due to endocrinopathies. The time course is consistent with an inflammatory myopathy, such as PM or DM. Inclusion body myositis (IBM), another inflammatory myopathy, presents much more insidiously over years and tends to be asymmetric compared to PM. The absence of myalgia, arthralgia, rash, and gastrointestinal symptoms makes myopathy as a component of a connective tissue disease, such as systemic lupus erythematosus, or a mixed connective tissue disease unlikely. The next steps would be laboratory testing of muscle enzymes, complete blood count, biochemical profile, and antinuclear antibody (ANA).
Laboratory studies revealed a white blood cell count of 4460/mm3 with normal differential, hemoglobin 12.5 g/dL, and platelet count 345,000/mm3. Creatinine was 0.87 mg/dL, aspartate aminotransferase 61 IU/mL, alanine aminotransferase 45 IU/mL, and creatine kinase (CK) 529 U/L (normal range, 38-174 U/L). Other liver function enzymes were normal. Biochemistry studies disclosed normal sodium, potassium, glucose, calcium, and magnesium levels. Dipstick urinalysis revealed blood and protein, and the microscopic examination of urinary sediment was unremarkable without the presence of erythrocytes. Twenty-four-hour creatinine clearance was 106 mL/min (normal range, 97-137 mL/min). Chest radiography was unrevealing.
The modest increase in CK, evidence of myoglobinuria, and proteinuria can all occur with an inflammatory or metabolic myopathy. The combination of proximal and distal weakness, coupled with only a modestly elevated CK, makes IBM more likely than PM, as PM usually presents with proximal weakness and much higher CK values. Normal skin examination makes DM less likely, as skin manifestations are generally found at time of presentation. The onset of symptoms after age 50 and the patient being male also favor IBM, though a longer time course would be expected. Definitively distinguishing IBM from PM is important because treatment and prognosis differ.
Thyroid function and HIV testing should be obtained. ANA, more common in PM than in IBM, should be checked because these myopathies can be associated with other autoimmune diseases. Imaging is generally not essential, although magnetic resonance imaging (MRI) of the thighs may help to differentiate IBM from PM. Electromyography (EMG) should be done to determine the pattern of myopathy and select muscle biopsy sites.
Additional testing revealed a normal thyroid stimulating hormone level. HIV and ANA were negative. Serum aldolase level was 19 IU/L (normal range, 2.7-5.9 IU/L), myoglobin 277 ng/mL (normal range, 28-72 ng/mL), lactate dehydrogenase 416 IU/mL (normal range, 119-229 IU/mL), and C-reactive protein 0.32 mg/dL. An EMG revealed mild myogenic changes in all extremities. An MRI of the left brachial muscle revealed multiple scattered high-signal lesions.
The EMG and MRI findings are consistent with an inflammatory myopathy. The modest elevation in muscle enzymes and negative ANA are more consistent with IBM since most patients with PM or DM are ANA positive. Muscle biopsy can be very helpful in establishing the etiology of myopathy.
Malignancy is associated with DM and PM in about 9% and 4% of patients, respectively. The common cancers associated with these conditions are adenocarcinomas of the ovary, cervix, lung, pancreas, and stomach. Most cancers are diagnosed around the time of myositis diagnosis, although they can precede or follow by years. Idiopathic IBM is not associated with cancer.
Open surgical muscle biopsy of the left biceps brachii was performed. Light microscopic examination disclosed interstitial edema and noncaseating granulomas. Immunostaining revealed an increase in the number of cluster of differentiation (CD) 4+ T cells. Caseating granulomas and Langhans giant cells were not present (Figure 3).
Tuberculin reaction and interferon-γ-release assay were negative. Staining for AFB and fungi was negative. ANCA, rheumatoid factor (RF), anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-RNP, and anti-Jo-1 were all negative or unremarkable. Serum angiotensin converting enzyme (ACE) level was 155.6 U/L (normal range, 7-25 U/L). Twenty-four-hour urine analysis revealed calcium excretion of 517.7 mg/day (normal range, 58-450 mg/day), β2-microglobulin 69,627 ug/day (normal range, <254 ug/day), and N-acetyl-D-glucosamine 95.3 U/day (normal range, <5.1 U/day) with a normal creatinine clearance. Serum intact parathyroid hormone level (PTH) was 5 pg/mL (normal range, 10-65 pg/mL), and 25-hydroxyvitamin D level was 51.1 ng/mL (normal range, 30-80 ng/mL). A CT of the thorax revealed a small ground-glass density lesion in the left lower lobe but no hilar or mediastinal lymphadenopathy.
Negative ANCA, RF, and autoantibodies exclude systemic vasculitis and connective tissue disease as causes of GM. Hypercalciuria is suggestive of granulomatous production of calcitriol, which, in turn, suppresses PTH. Hypercalcemia is not common in patients with sarcoidosis, but hypercalciuria occurs frequently. Serum ACE is a marker associated with sarcoidosis, but its diagnostic and prognostic utility is unclear.
Though there is a concern for sarcoidosis, this diagnosis can only be confidently made by finding noncaseating granulomas on a background of compatible clinical and radiologic findings after alternate possible etiologies are excluded. The chest CT reveals a small ground-glass density lesion without hilar adenopathy. These findings, though not incompatible, are not typical for pulmonary sarcoidosis. Therefore, finding noncaeseating granulomas in a second organ system would point toward systemic sarcoidosis as a unifying diagnosis. Bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy has a reasonable yield even in the absence of hilar adenopathy or typical parenchymal findings. A CD4/CD8 T-cell ratio of 2 or more on BAL provides supportive evidence for sarcoidosis.
It is reasonable to start empiric glucocorticoids for GM given that the AFB and fungal stains on histopathology are negative and that there is no evidence of lymphoma.
The CD4/CD8 T-cell ratio greater than 2, combined with the absence of neutrophils and eosinophils on BAL, is helpful in distinguishing sarcoidosis from other pulmonary diseases. This patient’s inflammatory myopathy was revealed to be a rare initial manifestation of systemic sarcoidosis.
DISCUSSION
Weakness is a common symptom of muscle disorders such as myopathies and muscular dystrophy. Idiopathic inflammatory myopathies include PM, DM, and others.1,2 These usually present with proximal-dominant muscle weakness, decreased endurance, and muscle inflammation. A diagnosis is made according to symptoms in combination with diagnostic examinations, including elevated serum CK levels, abnormal EMG findings, and histopathology of skeletal muscle biopsy specimens.
Sarcoidosis, a multisystem disorder of unknown etiology, is characterized histopathologically by noncaseating granulomas in affected organs.3 It typically affects young adults, with incidence peaking at 20 to 39 years of age. Although any organ may be involved, the disorder usually presents with 1 or more common abnormalities, including bilateral hilar lymphadenopathy, lung lesions, and skin and eye involvement. Musculoskeletal involvement is less common. It is estimated that skeletal muscle is involved in 50% to 80% of patients with sarcoidosis but is rarely symptomatic (0.5% to 2.5%).4-6
In this patient, weakness was distributed in both proximal and distal muscles, yet proximal weakness is the most characteristic feature in PM and DM. Therefore, sarcoidosis should be considered in the differential diagnosis of idiopathic inflammatory myopathies, especially when weakness accompanies abnormalities in other organs typically affected by sarcoidosis.
Myoglobinuria often is observed in rhabdomyolysis and inflammatory myopathies, conditions that produce high levels of serum CK and myoglobin. Myoglobinuria, often accompanied by the elevation of urinary β2-microglobulin and N-acetyl-D-glucosamine levels, can induce tubulointerstitial damage, which leads to acute kidney injury. In this case, however, these abnormal kidney findings were observed without high levels of serum CK or myoglobin. This suggests the potential for other causes of tubulointerstitial damage, such as granulomatous interstitial nephritis in renal sarcoidosis.3
Another characteristic abnormality was the elevation of urinary calcium excretion, which indicated an underlying granulomatous disorder, such as mycobacterial infection, granulomatosis with polyangiitis, or sarcoidosis. In sarcoidosis, hypercalciuria occurs in 40% of patients, hypercalcemia in 11%, and renal calculi in 10%.3,7 Hypercalciuria, for this patient, was important in arriving at the correct diagnosis after the gallium scan was obtained given the dearth of other typical features of sarcoidosis.
Although muscle biopsy is essential, imaging studies for idiopathic inflammatory myopathy are considered useful tools to narrow the differential diagnosis. The use of MRI of the skeletal muscle is helpful to both identify an adequate muscle for biopsy and demonstrate the pattern of affected muscles beyond clinical appearance, which aids in excluding, for example, muscular dystrophies.8,9
FDG PET/CT is a very sensitive imaging modality used to detect neoplastic lesions and has been widely used to screen for occult neoplasms and detect metastases.10-12 It is also useful for detecting inflammation in patients with osteomyelitis, metastatic infectious diseases, rheumatoid arthritis, vasculitis, inflammatory bowel diseases, fever of unknown origin, and sarcoidosis.11,12 In PM and DM, however, the sensitivity of FDG PET/CT for detection of myositis is reportedly lower than that of EMG and MRI.13 Similarly, gallium scintigraphy is usually performed to examine the disease activity of interstitial pneumonia or to detect malignancy. Previous literature and this case show that the striking images of gallium scintigraphy and FDG PET/CT have utility, not only for detection of sarcoid myopathy but also for the evaluation of treatment efficacy.14-17 Characteristic imaging findings on FDG PET/CT have been described as a “tiger man” appearance.17
For the treatment of sarcoid myopathy, systemic glucocorticoids are used for patients with symptomatic acute or chronic forms. The standard doses of prednisolone used for other forms of idiopathic inflammatory myopathies are usually administered.3-6 In general, the response of acute sarcoid myopathy to glucocorticoid therapy is favorable, and the clinical course is usually benign. However, the course in chronic sarcoid myopathy can be unpredictable with exacerbations. Given the lack of randomized trials of this therapy and because glucocorticoids themselves can cause steroid-induced myopathy, they are not used for asymptomatic patients.
In the end, astute clinical thinking, deductive reasoning, and pattern recognition were all instrumental in making this strong diagnosis of weakness.
KEY TEACHING POINTS
- Proximal muscle–dominant weakness is the characteristic feature in inflammatory myopathies like PM and DM. Myopathy causing proximal and distal weakness is more characteristic of sarcoidosis, IBM, alcohol, and statins.
- Elevations of urinary Times New Romanβ2-microglobulin and N-acetyl-D-glucosamine are often observed in inflammatory muscle diseases because of myoglobin-induced tubulointerstitial damage. These findings may also be caused by other conditions that affect the tubules, such as lupus nephritis, Sjogren’s syndrome, or renal sarcoidosis.
- Hypercalciuria in a patient with myopathy could suggest an underlying granulomatous disorder, such as mycobacterial infection, granulomatosis with polyangiitis, or sarcoidosis.
- The striking uptake within systemic skeletal striated muscles on gallium scintigraphy and “tiger man” appearance on FDG PET/CT are characteristic features of acute sarcoid myopathy; these are not common in other inflammatory myopathies.
Disclosure
Drs. Sudo, Wada, Narita, Mba, and Houchens have no conflicts of interest to disclose.
1. Vincze M, Danko K. Idiopathic inflammatory myopathies. Best Pract Res Clin Rheumatol. 2012;26:25-45. PubMed
2. Carstens PO, Schmidt J. Diagnosis, pathogenesis, and treatment of myositis: recent advances. Clin Exp Immunol. 2014;175:425-438. PubMed
3. Lannuzzi MC, Rhbicki BA, Teirstein AS. Sarcoidosis. N Eng J Med. 2007;357:2153-2165. PubMed
4. Baydur A, Pandya K, Sharma OP, et al. Control of ventilation, respiratory muscle strength, and granulomatous involvement of skeletal muscle in patients with sarcoidosis. Chest. 1993;103:396-402. PubMed
5. Zisman DA, Biermann JS, Martinez FJ, et al. Sarcoidosis presenting as a tumorlike muscular lesion. Case report and review of the literature. Medicine (Baltimore). 1999;78:112-122. PubMed
6. Fayad F, Liote F, Berenbaum F, et al. Muscle involvement in sarcoidosis: a retrospective and followup studies. J Rheumatol. 2006;33:98-103. PubMed
7. Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis. 2006;48:856-870. PubMed
8. Otake S, Ishigaki T. Musular sarcoidosis. Semin Musculoskelet Radiol. 2001;5:167-170. PubMed
9. Otake S, Imagumbai N, Suzuki M, et al. MR imaging of muscular sarcoidosis after steroid therapy. Eur Radiol. 1998;8:1651-1653. PubMed
10. Hoffman JM, Gambhir SS. Molecular imaging: The vision and opportunity for radiology in the future. Radiology. 2007;244:39-47. PubMed
11. Basu S, Zhuang H, Torigian DA, et al. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124-145. PubMed
12. Gotthardt M, Cleeker-Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937-1949. PubMed
13. Owada T, Maezawa R, Kurasawa K, et al. Detection of inflammatory lesions by F-18 fluorodeoxyglucose positron emission tomography in patients with polymyositis and dermatomyositis. J Rheumatol. 2012;39:1659-1665. PubMed
14. Liem IH, Drent M, Antevska E, et al. Intense muscle uptake of gallium-67 in a patient with sarcoidosis. J Nucl Med. 1998;39:1605-1607. PubMed
15. Suehiro S, Shiokawa S, Taniguchi S, et al. Gallium-67 scintigraphy in the diagnosis and management of chronic sarcoid myopathy. Clin Rheumatol. 2003;22:146-148. PubMed
16. Marie I, Josse S, Lahaxe L, et al. Clinical images: muscle sarcoidosis demonstrated on positron emission tomography. Arthritis Rheum. 2009;60:2847. PubMed
17. Wieers G, Lhommel R, Lecouvet F, et al. A tiger man. Lancet. 2012;380:1859. PubMed
A 52-year-old man presented with bilateral weakness in all extremities. He noted the gradual onset of progressive muscle weakness 6 months prior to presentation. He reported generalized fatigue and difficulty with climbing stairs and carrying heavy objects.
Initial considerations of chronic weakness and fatigue are myopathy, polyneuropathy, medications, malignancy, endocrinopathies, human immunodeficiency virus (HIV), neuromuscular junction dysfunction, and central nervous system (CNS) disorders, such as amyotrophic lateral sclerosis (ALS) or multiple sclerosis (MS). Symmetrical muscle involvement and proximal weakness make myopathy most likely. Polyneuropathy, such as chronic inflammatory demyelinating polyneuropathy (CIDP), is less likely but still possible given the slowly progressive course. The use of medications that can cause myopathy should be explored, including colchicine, steroids, and statins. Gathering further history should focus on risk factors for HIV, as well as alcohol and illicit drug use. Malignancy can cause paraneoplastic myopathy. The review of systems should include symptoms of endocrinopathies, such as thyrotoxicosis and hypothyroidism. Fluctuations in weakness and dysphagia or ocular symptoms would suggest myasthenia gravis (MG). The time course and symmetrical weakness make a central disorder, such as ALS or MS, unlikely.
His past medical history was notable for pulmonary tuberculosis diagnosed at the age of 6 years, which was treated with hospitalization and an unknown medication regimen. He was not taking medications prior to this admission. His family history was significant for diabetes mellitus in both parents. He denied sick contacts. He was sexually active with his wife. He denied the use of tobacco and illicit drugs but endorsed alcohol consumption on a daily basis over the last 32 years. He reported no fluctuation in his symptoms, muscle or joint pains, rash, fevers, chills, diaphoresis, chest pain, dyspnea, abdominal pain, diarrhea, paresthesias, weight loss, or night sweats. He had never had a colonoscopy.
Painless progressive weakness of the limbs without sensory deficit is typical of a myopathy. Though CIDP can present with only motor weakness, the majority of patients have sensory symptoms, making this less likely. Although chronic alcohol abuse can cause myopathy, it seems less likely because other neurologic complications, such as sensory polyneuropathy or ataxia, would be expected. A review of systems does not suggest a thyroid disorder or malignancy, although this does not preclude an evaluation for both. The absence of fluctuations in weakness argues against MG. Though ALS, MG, MS, and CIDP are less likely, a neurologic exam is crucial in excluding them. The hallmark of ALS is upper motor neuron (UMN) and lower motor neuron signs in the absence of sensory symptoms and signs, while global hyporeflexia would be expected in CIDP, and fatigability on repeated power testing would be expected in MG. Neurologic findings disseminated in space (neuro-anatomically) would be expected in MS.
On physical examination, the patient had a temperature of 36.9°C, heart rate of 70 beats per minute, and regular respiratory rate of 10 breaths per minute, blood pressure 130/80 mmHg, and oxygen saturation 98% while breathing ambient air. Auscultation of the heart and lungs revealed normal findings. The abdomen was soft, nontender, and without masses or organomegaly. Neurologic examination disclosed bilateral symmetric upper and lower extremity weakness with positive Gower sign. Muscle strength scores of the bilateral biceps brachii, iliopsoas, and digitis extensor were between 4 and 5 without fatigability. Grasping power was impaired. Deep tendon reflexes were preserved, and there were no UMN signs. There was no tenderness to palpation in any muscle groups. Sensory testing was normal. Skin and lymph examinations were without abnormality. The rest of the physical examination was unremarkable.
Gower sign, characteristic of but not specific to muscular dystrophy, indicates proximal muscle weakness of lower extremities, wherein hands and arms are used to walk up the body into an upright position. The exam also reveals distal weakness as shown by reduced hand grasp. Symmetrical proximal weakness of all extremities without sensory deficits suggests a myopathic process, albeit one with some distal involvement. The absence of UMN signs argues against ALS, lack of fatigability argues against MG, and the absence of CNS or sensory deficits argues against MS.
Because myopathy is most likely, the next step would be to determine if this is an idiopathic inflammatory myopathy, such as polymyositis (PM) or dermatomyositis (DM), secondary inflammatory myopathy, or noninflammatory myopathy due to endocrinopathies. The time course is consistent with an inflammatory myopathy, such as PM or DM. Inclusion body myositis (IBM), another inflammatory myopathy, presents much more insidiously over years and tends to be asymmetric compared to PM. The absence of myalgia, arthralgia, rash, and gastrointestinal symptoms makes myopathy as a component of a connective tissue disease, such as systemic lupus erythematosus, or a mixed connective tissue disease unlikely. The next steps would be laboratory testing of muscle enzymes, complete blood count, biochemical profile, and antinuclear antibody (ANA).
Laboratory studies revealed a white blood cell count of 4460/mm3 with normal differential, hemoglobin 12.5 g/dL, and platelet count 345,000/mm3. Creatinine was 0.87 mg/dL, aspartate aminotransferase 61 IU/mL, alanine aminotransferase 45 IU/mL, and creatine kinase (CK) 529 U/L (normal range, 38-174 U/L). Other liver function enzymes were normal. Biochemistry studies disclosed normal sodium, potassium, glucose, calcium, and magnesium levels. Dipstick urinalysis revealed blood and protein, and the microscopic examination of urinary sediment was unremarkable without the presence of erythrocytes. Twenty-four-hour creatinine clearance was 106 mL/min (normal range, 97-137 mL/min). Chest radiography was unrevealing.
The modest increase in CK, evidence of myoglobinuria, and proteinuria can all occur with an inflammatory or metabolic myopathy. The combination of proximal and distal weakness, coupled with only a modestly elevated CK, makes IBM more likely than PM, as PM usually presents with proximal weakness and much higher CK values. Normal skin examination makes DM less likely, as skin manifestations are generally found at time of presentation. The onset of symptoms after age 50 and the patient being male also favor IBM, though a longer time course would be expected. Definitively distinguishing IBM from PM is important because treatment and prognosis differ.
Thyroid function and HIV testing should be obtained. ANA, more common in PM than in IBM, should be checked because these myopathies can be associated with other autoimmune diseases. Imaging is generally not essential, although magnetic resonance imaging (MRI) of the thighs may help to differentiate IBM from PM. Electromyography (EMG) should be done to determine the pattern of myopathy and select muscle biopsy sites.
Additional testing revealed a normal thyroid stimulating hormone level. HIV and ANA were negative. Serum aldolase level was 19 IU/L (normal range, 2.7-5.9 IU/L), myoglobin 277 ng/mL (normal range, 28-72 ng/mL), lactate dehydrogenase 416 IU/mL (normal range, 119-229 IU/mL), and C-reactive protein 0.32 mg/dL. An EMG revealed mild myogenic changes in all extremities. An MRI of the left brachial muscle revealed multiple scattered high-signal lesions.
The EMG and MRI findings are consistent with an inflammatory myopathy. The modest elevation in muscle enzymes and negative ANA are more consistent with IBM since most patients with PM or DM are ANA positive. Muscle biopsy can be very helpful in establishing the etiology of myopathy.
Malignancy is associated with DM and PM in about 9% and 4% of patients, respectively. The common cancers associated with these conditions are adenocarcinomas of the ovary, cervix, lung, pancreas, and stomach. Most cancers are diagnosed around the time of myositis diagnosis, although they can precede or follow by years. Idiopathic IBM is not associated with cancer.
Open surgical muscle biopsy of the left biceps brachii was performed. Light microscopic examination disclosed interstitial edema and noncaseating granulomas. Immunostaining revealed an increase in the number of cluster of differentiation (CD) 4+ T cells. Caseating granulomas and Langhans giant cells were not present (Figure 3).
Tuberculin reaction and interferon-γ-release assay were negative. Staining for AFB and fungi was negative. ANCA, rheumatoid factor (RF), anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-RNP, and anti-Jo-1 were all negative or unremarkable. Serum angiotensin converting enzyme (ACE) level was 155.6 U/L (normal range, 7-25 U/L). Twenty-four-hour urine analysis revealed calcium excretion of 517.7 mg/day (normal range, 58-450 mg/day), β2-microglobulin 69,627 ug/day (normal range, <254 ug/day), and N-acetyl-D-glucosamine 95.3 U/day (normal range, <5.1 U/day) with a normal creatinine clearance. Serum intact parathyroid hormone level (PTH) was 5 pg/mL (normal range, 10-65 pg/mL), and 25-hydroxyvitamin D level was 51.1 ng/mL (normal range, 30-80 ng/mL). A CT of the thorax revealed a small ground-glass density lesion in the left lower lobe but no hilar or mediastinal lymphadenopathy.
Negative ANCA, RF, and autoantibodies exclude systemic vasculitis and connective tissue disease as causes of GM. Hypercalciuria is suggestive of granulomatous production of calcitriol, which, in turn, suppresses PTH. Hypercalcemia is not common in patients with sarcoidosis, but hypercalciuria occurs frequently. Serum ACE is a marker associated with sarcoidosis, but its diagnostic and prognostic utility is unclear.
Though there is a concern for sarcoidosis, this diagnosis can only be confidently made by finding noncaseating granulomas on a background of compatible clinical and radiologic findings after alternate possible etiologies are excluded. The chest CT reveals a small ground-glass density lesion without hilar adenopathy. These findings, though not incompatible, are not typical for pulmonary sarcoidosis. Therefore, finding noncaeseating granulomas in a second organ system would point toward systemic sarcoidosis as a unifying diagnosis. Bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy has a reasonable yield even in the absence of hilar adenopathy or typical parenchymal findings. A CD4/CD8 T-cell ratio of 2 or more on BAL provides supportive evidence for sarcoidosis.
It is reasonable to start empiric glucocorticoids for GM given that the AFB and fungal stains on histopathology are negative and that there is no evidence of lymphoma.
The CD4/CD8 T-cell ratio greater than 2, combined with the absence of neutrophils and eosinophils on BAL, is helpful in distinguishing sarcoidosis from other pulmonary diseases. This patient’s inflammatory myopathy was revealed to be a rare initial manifestation of systemic sarcoidosis.
DISCUSSION
Weakness is a common symptom of muscle disorders such as myopathies and muscular dystrophy. Idiopathic inflammatory myopathies include PM, DM, and others.1,2 These usually present with proximal-dominant muscle weakness, decreased endurance, and muscle inflammation. A diagnosis is made according to symptoms in combination with diagnostic examinations, including elevated serum CK levels, abnormal EMG findings, and histopathology of skeletal muscle biopsy specimens.
Sarcoidosis, a multisystem disorder of unknown etiology, is characterized histopathologically by noncaseating granulomas in affected organs.3 It typically affects young adults, with incidence peaking at 20 to 39 years of age. Although any organ may be involved, the disorder usually presents with 1 or more common abnormalities, including bilateral hilar lymphadenopathy, lung lesions, and skin and eye involvement. Musculoskeletal involvement is less common. It is estimated that skeletal muscle is involved in 50% to 80% of patients with sarcoidosis but is rarely symptomatic (0.5% to 2.5%).4-6
In this patient, weakness was distributed in both proximal and distal muscles, yet proximal weakness is the most characteristic feature in PM and DM. Therefore, sarcoidosis should be considered in the differential diagnosis of idiopathic inflammatory myopathies, especially when weakness accompanies abnormalities in other organs typically affected by sarcoidosis.
Myoglobinuria often is observed in rhabdomyolysis and inflammatory myopathies, conditions that produce high levels of serum CK and myoglobin. Myoglobinuria, often accompanied by the elevation of urinary β2-microglobulin and N-acetyl-D-glucosamine levels, can induce tubulointerstitial damage, which leads to acute kidney injury. In this case, however, these abnormal kidney findings were observed without high levels of serum CK or myoglobin. This suggests the potential for other causes of tubulointerstitial damage, such as granulomatous interstitial nephritis in renal sarcoidosis.3
Another characteristic abnormality was the elevation of urinary calcium excretion, which indicated an underlying granulomatous disorder, such as mycobacterial infection, granulomatosis with polyangiitis, or sarcoidosis. In sarcoidosis, hypercalciuria occurs in 40% of patients, hypercalcemia in 11%, and renal calculi in 10%.3,7 Hypercalciuria, for this patient, was important in arriving at the correct diagnosis after the gallium scan was obtained given the dearth of other typical features of sarcoidosis.
Although muscle biopsy is essential, imaging studies for idiopathic inflammatory myopathy are considered useful tools to narrow the differential diagnosis. The use of MRI of the skeletal muscle is helpful to both identify an adequate muscle for biopsy and demonstrate the pattern of affected muscles beyond clinical appearance, which aids in excluding, for example, muscular dystrophies.8,9
FDG PET/CT is a very sensitive imaging modality used to detect neoplastic lesions and has been widely used to screen for occult neoplasms and detect metastases.10-12 It is also useful for detecting inflammation in patients with osteomyelitis, metastatic infectious diseases, rheumatoid arthritis, vasculitis, inflammatory bowel diseases, fever of unknown origin, and sarcoidosis.11,12 In PM and DM, however, the sensitivity of FDG PET/CT for detection of myositis is reportedly lower than that of EMG and MRI.13 Similarly, gallium scintigraphy is usually performed to examine the disease activity of interstitial pneumonia or to detect malignancy. Previous literature and this case show that the striking images of gallium scintigraphy and FDG PET/CT have utility, not only for detection of sarcoid myopathy but also for the evaluation of treatment efficacy.14-17 Characteristic imaging findings on FDG PET/CT have been described as a “tiger man” appearance.17
For the treatment of sarcoid myopathy, systemic glucocorticoids are used for patients with symptomatic acute or chronic forms. The standard doses of prednisolone used for other forms of idiopathic inflammatory myopathies are usually administered.3-6 In general, the response of acute sarcoid myopathy to glucocorticoid therapy is favorable, and the clinical course is usually benign. However, the course in chronic sarcoid myopathy can be unpredictable with exacerbations. Given the lack of randomized trials of this therapy and because glucocorticoids themselves can cause steroid-induced myopathy, they are not used for asymptomatic patients.
In the end, astute clinical thinking, deductive reasoning, and pattern recognition were all instrumental in making this strong diagnosis of weakness.
KEY TEACHING POINTS
- Proximal muscle–dominant weakness is the characteristic feature in inflammatory myopathies like PM and DM. Myopathy causing proximal and distal weakness is more characteristic of sarcoidosis, IBM, alcohol, and statins.
- Elevations of urinary Times New Romanβ2-microglobulin and N-acetyl-D-glucosamine are often observed in inflammatory muscle diseases because of myoglobin-induced tubulointerstitial damage. These findings may also be caused by other conditions that affect the tubules, such as lupus nephritis, Sjogren’s syndrome, or renal sarcoidosis.
- Hypercalciuria in a patient with myopathy could suggest an underlying granulomatous disorder, such as mycobacterial infection, granulomatosis with polyangiitis, or sarcoidosis.
- The striking uptake within systemic skeletal striated muscles on gallium scintigraphy and “tiger man” appearance on FDG PET/CT are characteristic features of acute sarcoid myopathy; these are not common in other inflammatory myopathies.
Disclosure
Drs. Sudo, Wada, Narita, Mba, and Houchens have no conflicts of interest to disclose.
A 52-year-old man presented with bilateral weakness in all extremities. He noted the gradual onset of progressive muscle weakness 6 months prior to presentation. He reported generalized fatigue and difficulty with climbing stairs and carrying heavy objects.
Initial considerations of chronic weakness and fatigue are myopathy, polyneuropathy, medications, malignancy, endocrinopathies, human immunodeficiency virus (HIV), neuromuscular junction dysfunction, and central nervous system (CNS) disorders, such as amyotrophic lateral sclerosis (ALS) or multiple sclerosis (MS). Symmetrical muscle involvement and proximal weakness make myopathy most likely. Polyneuropathy, such as chronic inflammatory demyelinating polyneuropathy (CIDP), is less likely but still possible given the slowly progressive course. The use of medications that can cause myopathy should be explored, including colchicine, steroids, and statins. Gathering further history should focus on risk factors for HIV, as well as alcohol and illicit drug use. Malignancy can cause paraneoplastic myopathy. The review of systems should include symptoms of endocrinopathies, such as thyrotoxicosis and hypothyroidism. Fluctuations in weakness and dysphagia or ocular symptoms would suggest myasthenia gravis (MG). The time course and symmetrical weakness make a central disorder, such as ALS or MS, unlikely.
His past medical history was notable for pulmonary tuberculosis diagnosed at the age of 6 years, which was treated with hospitalization and an unknown medication regimen. He was not taking medications prior to this admission. His family history was significant for diabetes mellitus in both parents. He denied sick contacts. He was sexually active with his wife. He denied the use of tobacco and illicit drugs but endorsed alcohol consumption on a daily basis over the last 32 years. He reported no fluctuation in his symptoms, muscle or joint pains, rash, fevers, chills, diaphoresis, chest pain, dyspnea, abdominal pain, diarrhea, paresthesias, weight loss, or night sweats. He had never had a colonoscopy.
Painless progressive weakness of the limbs without sensory deficit is typical of a myopathy. Though CIDP can present with only motor weakness, the majority of patients have sensory symptoms, making this less likely. Although chronic alcohol abuse can cause myopathy, it seems less likely because other neurologic complications, such as sensory polyneuropathy or ataxia, would be expected. A review of systems does not suggest a thyroid disorder or malignancy, although this does not preclude an evaluation for both. The absence of fluctuations in weakness argues against MG. Though ALS, MG, MS, and CIDP are less likely, a neurologic exam is crucial in excluding them. The hallmark of ALS is upper motor neuron (UMN) and lower motor neuron signs in the absence of sensory symptoms and signs, while global hyporeflexia would be expected in CIDP, and fatigability on repeated power testing would be expected in MG. Neurologic findings disseminated in space (neuro-anatomically) would be expected in MS.
On physical examination, the patient had a temperature of 36.9°C, heart rate of 70 beats per minute, and regular respiratory rate of 10 breaths per minute, blood pressure 130/80 mmHg, and oxygen saturation 98% while breathing ambient air. Auscultation of the heart and lungs revealed normal findings. The abdomen was soft, nontender, and without masses or organomegaly. Neurologic examination disclosed bilateral symmetric upper and lower extremity weakness with positive Gower sign. Muscle strength scores of the bilateral biceps brachii, iliopsoas, and digitis extensor were between 4 and 5 without fatigability. Grasping power was impaired. Deep tendon reflexes were preserved, and there were no UMN signs. There was no tenderness to palpation in any muscle groups. Sensory testing was normal. Skin and lymph examinations were without abnormality. The rest of the physical examination was unremarkable.
Gower sign, characteristic of but not specific to muscular dystrophy, indicates proximal muscle weakness of lower extremities, wherein hands and arms are used to walk up the body into an upright position. The exam also reveals distal weakness as shown by reduced hand grasp. Symmetrical proximal weakness of all extremities without sensory deficits suggests a myopathic process, albeit one with some distal involvement. The absence of UMN signs argues against ALS, lack of fatigability argues against MG, and the absence of CNS or sensory deficits argues against MS.
Because myopathy is most likely, the next step would be to determine if this is an idiopathic inflammatory myopathy, such as polymyositis (PM) or dermatomyositis (DM), secondary inflammatory myopathy, or noninflammatory myopathy due to endocrinopathies. The time course is consistent with an inflammatory myopathy, such as PM or DM. Inclusion body myositis (IBM), another inflammatory myopathy, presents much more insidiously over years and tends to be asymmetric compared to PM. The absence of myalgia, arthralgia, rash, and gastrointestinal symptoms makes myopathy as a component of a connective tissue disease, such as systemic lupus erythematosus, or a mixed connective tissue disease unlikely. The next steps would be laboratory testing of muscle enzymes, complete blood count, biochemical profile, and antinuclear antibody (ANA).
Laboratory studies revealed a white blood cell count of 4460/mm3 with normal differential, hemoglobin 12.5 g/dL, and platelet count 345,000/mm3. Creatinine was 0.87 mg/dL, aspartate aminotransferase 61 IU/mL, alanine aminotransferase 45 IU/mL, and creatine kinase (CK) 529 U/L (normal range, 38-174 U/L). Other liver function enzymes were normal. Biochemistry studies disclosed normal sodium, potassium, glucose, calcium, and magnesium levels. Dipstick urinalysis revealed blood and protein, and the microscopic examination of urinary sediment was unremarkable without the presence of erythrocytes. Twenty-four-hour creatinine clearance was 106 mL/min (normal range, 97-137 mL/min). Chest radiography was unrevealing.
The modest increase in CK, evidence of myoglobinuria, and proteinuria can all occur with an inflammatory or metabolic myopathy. The combination of proximal and distal weakness, coupled with only a modestly elevated CK, makes IBM more likely than PM, as PM usually presents with proximal weakness and much higher CK values. Normal skin examination makes DM less likely, as skin manifestations are generally found at time of presentation. The onset of symptoms after age 50 and the patient being male also favor IBM, though a longer time course would be expected. Definitively distinguishing IBM from PM is important because treatment and prognosis differ.
Thyroid function and HIV testing should be obtained. ANA, more common in PM than in IBM, should be checked because these myopathies can be associated with other autoimmune diseases. Imaging is generally not essential, although magnetic resonance imaging (MRI) of the thighs may help to differentiate IBM from PM. Electromyography (EMG) should be done to determine the pattern of myopathy and select muscle biopsy sites.
Additional testing revealed a normal thyroid stimulating hormone level. HIV and ANA were negative. Serum aldolase level was 19 IU/L (normal range, 2.7-5.9 IU/L), myoglobin 277 ng/mL (normal range, 28-72 ng/mL), lactate dehydrogenase 416 IU/mL (normal range, 119-229 IU/mL), and C-reactive protein 0.32 mg/dL. An EMG revealed mild myogenic changes in all extremities. An MRI of the left brachial muscle revealed multiple scattered high-signal lesions.
The EMG and MRI findings are consistent with an inflammatory myopathy. The modest elevation in muscle enzymes and negative ANA are more consistent with IBM since most patients with PM or DM are ANA positive. Muscle biopsy can be very helpful in establishing the etiology of myopathy.
Malignancy is associated with DM and PM in about 9% and 4% of patients, respectively. The common cancers associated with these conditions are adenocarcinomas of the ovary, cervix, lung, pancreas, and stomach. Most cancers are diagnosed around the time of myositis diagnosis, although they can precede or follow by years. Idiopathic IBM is not associated with cancer.
Open surgical muscle biopsy of the left biceps brachii was performed. Light microscopic examination disclosed interstitial edema and noncaseating granulomas. Immunostaining revealed an increase in the number of cluster of differentiation (CD) 4+ T cells. Caseating granulomas and Langhans giant cells were not present (Figure 3).
Tuberculin reaction and interferon-γ-release assay were negative. Staining for AFB and fungi was negative. ANCA, rheumatoid factor (RF), anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-RNP, and anti-Jo-1 were all negative or unremarkable. Serum angiotensin converting enzyme (ACE) level was 155.6 U/L (normal range, 7-25 U/L). Twenty-four-hour urine analysis revealed calcium excretion of 517.7 mg/day (normal range, 58-450 mg/day), β2-microglobulin 69,627 ug/day (normal range, <254 ug/day), and N-acetyl-D-glucosamine 95.3 U/day (normal range, <5.1 U/day) with a normal creatinine clearance. Serum intact parathyroid hormone level (PTH) was 5 pg/mL (normal range, 10-65 pg/mL), and 25-hydroxyvitamin D level was 51.1 ng/mL (normal range, 30-80 ng/mL). A CT of the thorax revealed a small ground-glass density lesion in the left lower lobe but no hilar or mediastinal lymphadenopathy.
Negative ANCA, RF, and autoantibodies exclude systemic vasculitis and connective tissue disease as causes of GM. Hypercalciuria is suggestive of granulomatous production of calcitriol, which, in turn, suppresses PTH. Hypercalcemia is not common in patients with sarcoidosis, but hypercalciuria occurs frequently. Serum ACE is a marker associated with sarcoidosis, but its diagnostic and prognostic utility is unclear.
Though there is a concern for sarcoidosis, this diagnosis can only be confidently made by finding noncaseating granulomas on a background of compatible clinical and radiologic findings after alternate possible etiologies are excluded. The chest CT reveals a small ground-glass density lesion without hilar adenopathy. These findings, though not incompatible, are not typical for pulmonary sarcoidosis. Therefore, finding noncaeseating granulomas in a second organ system would point toward systemic sarcoidosis as a unifying diagnosis. Bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy has a reasonable yield even in the absence of hilar adenopathy or typical parenchymal findings. A CD4/CD8 T-cell ratio of 2 or more on BAL provides supportive evidence for sarcoidosis.
It is reasonable to start empiric glucocorticoids for GM given that the AFB and fungal stains on histopathology are negative and that there is no evidence of lymphoma.
The CD4/CD8 T-cell ratio greater than 2, combined with the absence of neutrophils and eosinophils on BAL, is helpful in distinguishing sarcoidosis from other pulmonary diseases. This patient’s inflammatory myopathy was revealed to be a rare initial manifestation of systemic sarcoidosis.
DISCUSSION
Weakness is a common symptom of muscle disorders such as myopathies and muscular dystrophy. Idiopathic inflammatory myopathies include PM, DM, and others.1,2 These usually present with proximal-dominant muscle weakness, decreased endurance, and muscle inflammation. A diagnosis is made according to symptoms in combination with diagnostic examinations, including elevated serum CK levels, abnormal EMG findings, and histopathology of skeletal muscle biopsy specimens.
Sarcoidosis, a multisystem disorder of unknown etiology, is characterized histopathologically by noncaseating granulomas in affected organs.3 It typically affects young adults, with incidence peaking at 20 to 39 years of age. Although any organ may be involved, the disorder usually presents with 1 or more common abnormalities, including bilateral hilar lymphadenopathy, lung lesions, and skin and eye involvement. Musculoskeletal involvement is less common. It is estimated that skeletal muscle is involved in 50% to 80% of patients with sarcoidosis but is rarely symptomatic (0.5% to 2.5%).4-6
In this patient, weakness was distributed in both proximal and distal muscles, yet proximal weakness is the most characteristic feature in PM and DM. Therefore, sarcoidosis should be considered in the differential diagnosis of idiopathic inflammatory myopathies, especially when weakness accompanies abnormalities in other organs typically affected by sarcoidosis.
Myoglobinuria often is observed in rhabdomyolysis and inflammatory myopathies, conditions that produce high levels of serum CK and myoglobin. Myoglobinuria, often accompanied by the elevation of urinary β2-microglobulin and N-acetyl-D-glucosamine levels, can induce tubulointerstitial damage, which leads to acute kidney injury. In this case, however, these abnormal kidney findings were observed without high levels of serum CK or myoglobin. This suggests the potential for other causes of tubulointerstitial damage, such as granulomatous interstitial nephritis in renal sarcoidosis.3
Another characteristic abnormality was the elevation of urinary calcium excretion, which indicated an underlying granulomatous disorder, such as mycobacterial infection, granulomatosis with polyangiitis, or sarcoidosis. In sarcoidosis, hypercalciuria occurs in 40% of patients, hypercalcemia in 11%, and renal calculi in 10%.3,7 Hypercalciuria, for this patient, was important in arriving at the correct diagnosis after the gallium scan was obtained given the dearth of other typical features of sarcoidosis.
Although muscle biopsy is essential, imaging studies for idiopathic inflammatory myopathy are considered useful tools to narrow the differential diagnosis. The use of MRI of the skeletal muscle is helpful to both identify an adequate muscle for biopsy and demonstrate the pattern of affected muscles beyond clinical appearance, which aids in excluding, for example, muscular dystrophies.8,9
FDG PET/CT is a very sensitive imaging modality used to detect neoplastic lesions and has been widely used to screen for occult neoplasms and detect metastases.10-12 It is also useful for detecting inflammation in patients with osteomyelitis, metastatic infectious diseases, rheumatoid arthritis, vasculitis, inflammatory bowel diseases, fever of unknown origin, and sarcoidosis.11,12 In PM and DM, however, the sensitivity of FDG PET/CT for detection of myositis is reportedly lower than that of EMG and MRI.13 Similarly, gallium scintigraphy is usually performed to examine the disease activity of interstitial pneumonia or to detect malignancy. Previous literature and this case show that the striking images of gallium scintigraphy and FDG PET/CT have utility, not only for detection of sarcoid myopathy but also for the evaluation of treatment efficacy.14-17 Characteristic imaging findings on FDG PET/CT have been described as a “tiger man” appearance.17
For the treatment of sarcoid myopathy, systemic glucocorticoids are used for patients with symptomatic acute or chronic forms. The standard doses of prednisolone used for other forms of idiopathic inflammatory myopathies are usually administered.3-6 In general, the response of acute sarcoid myopathy to glucocorticoid therapy is favorable, and the clinical course is usually benign. However, the course in chronic sarcoid myopathy can be unpredictable with exacerbations. Given the lack of randomized trials of this therapy and because glucocorticoids themselves can cause steroid-induced myopathy, they are not used for asymptomatic patients.
In the end, astute clinical thinking, deductive reasoning, and pattern recognition were all instrumental in making this strong diagnosis of weakness.
KEY TEACHING POINTS
- Proximal muscle–dominant weakness is the characteristic feature in inflammatory myopathies like PM and DM. Myopathy causing proximal and distal weakness is more characteristic of sarcoidosis, IBM, alcohol, and statins.
- Elevations of urinary Times New Romanβ2-microglobulin and N-acetyl-D-glucosamine are often observed in inflammatory muscle diseases because of myoglobin-induced tubulointerstitial damage. These findings may also be caused by other conditions that affect the tubules, such as lupus nephritis, Sjogren’s syndrome, or renal sarcoidosis.
- Hypercalciuria in a patient with myopathy could suggest an underlying granulomatous disorder, such as mycobacterial infection, granulomatosis with polyangiitis, or sarcoidosis.
- The striking uptake within systemic skeletal striated muscles on gallium scintigraphy and “tiger man” appearance on FDG PET/CT are characteristic features of acute sarcoid myopathy; these are not common in other inflammatory myopathies.
Disclosure
Drs. Sudo, Wada, Narita, Mba, and Houchens have no conflicts of interest to disclose.
1. Vincze M, Danko K. Idiopathic inflammatory myopathies. Best Pract Res Clin Rheumatol. 2012;26:25-45. PubMed
2. Carstens PO, Schmidt J. Diagnosis, pathogenesis, and treatment of myositis: recent advances. Clin Exp Immunol. 2014;175:425-438. PubMed
3. Lannuzzi MC, Rhbicki BA, Teirstein AS. Sarcoidosis. N Eng J Med. 2007;357:2153-2165. PubMed
4. Baydur A, Pandya K, Sharma OP, et al. Control of ventilation, respiratory muscle strength, and granulomatous involvement of skeletal muscle in patients with sarcoidosis. Chest. 1993;103:396-402. PubMed
5. Zisman DA, Biermann JS, Martinez FJ, et al. Sarcoidosis presenting as a tumorlike muscular lesion. Case report and review of the literature. Medicine (Baltimore). 1999;78:112-122. PubMed
6. Fayad F, Liote F, Berenbaum F, et al. Muscle involvement in sarcoidosis: a retrospective and followup studies. J Rheumatol. 2006;33:98-103. PubMed
7. Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis. 2006;48:856-870. PubMed
8. Otake S, Ishigaki T. Musular sarcoidosis. Semin Musculoskelet Radiol. 2001;5:167-170. PubMed
9. Otake S, Imagumbai N, Suzuki M, et al. MR imaging of muscular sarcoidosis after steroid therapy. Eur Radiol. 1998;8:1651-1653. PubMed
10. Hoffman JM, Gambhir SS. Molecular imaging: The vision and opportunity for radiology in the future. Radiology. 2007;244:39-47. PubMed
11. Basu S, Zhuang H, Torigian DA, et al. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124-145. PubMed
12. Gotthardt M, Cleeker-Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937-1949. PubMed
13. Owada T, Maezawa R, Kurasawa K, et al. Detection of inflammatory lesions by F-18 fluorodeoxyglucose positron emission tomography in patients with polymyositis and dermatomyositis. J Rheumatol. 2012;39:1659-1665. PubMed
14. Liem IH, Drent M, Antevska E, et al. Intense muscle uptake of gallium-67 in a patient with sarcoidosis. J Nucl Med. 1998;39:1605-1607. PubMed
15. Suehiro S, Shiokawa S, Taniguchi S, et al. Gallium-67 scintigraphy in the diagnosis and management of chronic sarcoid myopathy. Clin Rheumatol. 2003;22:146-148. PubMed
16. Marie I, Josse S, Lahaxe L, et al. Clinical images: muscle sarcoidosis demonstrated on positron emission tomography. Arthritis Rheum. 2009;60:2847. PubMed
17. Wieers G, Lhommel R, Lecouvet F, et al. A tiger man. Lancet. 2012;380:1859. PubMed
1. Vincze M, Danko K. Idiopathic inflammatory myopathies. Best Pract Res Clin Rheumatol. 2012;26:25-45. PubMed
2. Carstens PO, Schmidt J. Diagnosis, pathogenesis, and treatment of myositis: recent advances. Clin Exp Immunol. 2014;175:425-438. PubMed
3. Lannuzzi MC, Rhbicki BA, Teirstein AS. Sarcoidosis. N Eng J Med. 2007;357:2153-2165. PubMed
4. Baydur A, Pandya K, Sharma OP, et al. Control of ventilation, respiratory muscle strength, and granulomatous involvement of skeletal muscle in patients with sarcoidosis. Chest. 1993;103:396-402. PubMed
5. Zisman DA, Biermann JS, Martinez FJ, et al. Sarcoidosis presenting as a tumorlike muscular lesion. Case report and review of the literature. Medicine (Baltimore). 1999;78:112-122. PubMed
6. Fayad F, Liote F, Berenbaum F, et al. Muscle involvement in sarcoidosis: a retrospective and followup studies. J Rheumatol. 2006;33:98-103. PubMed
7. Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis. 2006;48:856-870. PubMed
8. Otake S, Ishigaki T. Musular sarcoidosis. Semin Musculoskelet Radiol. 2001;5:167-170. PubMed
9. Otake S, Imagumbai N, Suzuki M, et al. MR imaging of muscular sarcoidosis after steroid therapy. Eur Radiol. 1998;8:1651-1653. PubMed
10. Hoffman JM, Gambhir SS. Molecular imaging: The vision and opportunity for radiology in the future. Radiology. 2007;244:39-47. PubMed
11. Basu S, Zhuang H, Torigian DA, et al. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124-145. PubMed
12. Gotthardt M, Cleeker-Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937-1949. PubMed
13. Owada T, Maezawa R, Kurasawa K, et al. Detection of inflammatory lesions by F-18 fluorodeoxyglucose positron emission tomography in patients with polymyositis and dermatomyositis. J Rheumatol. 2012;39:1659-1665. PubMed
14. Liem IH, Drent M, Antevska E, et al. Intense muscle uptake of gallium-67 in a patient with sarcoidosis. J Nucl Med. 1998;39:1605-1607. PubMed
15. Suehiro S, Shiokawa S, Taniguchi S, et al. Gallium-67 scintigraphy in the diagnosis and management of chronic sarcoid myopathy. Clin Rheumatol. 2003;22:146-148. PubMed
16. Marie I, Josse S, Lahaxe L, et al. Clinical images: muscle sarcoidosis demonstrated on positron emission tomography. Arthritis Rheum. 2009;60:2847. PubMed
17. Wieers G, Lhommel R, Lecouvet F, et al. A tiger man. Lancet. 2012;380:1859. PubMed
© 2017 Society of Hospital Medicine
How Exemplary Teaching Physicians Interact with Hospitalized Patients
Approximately a century ago, Francis Peabody taught that “the secret of the care of the patient is in caring for the patient.”1 His advice remains true today. Despite the advent of novel diagnostic tests, technologically sophisticated interventional procedures, and life-saving medications, perhaps the most important skill a bedside clinician can use is the ability to connect with patients.
The literature on patient-physician interaction is vast2-11 and generally indicates that exemplary bedside clinicians are able to interact well with patients by being competent, trustworthy, personable, empathetic, and effective communicators. “Etiquette-based medicine,” first proposed by Kahn,12 emphasizes the importance of certain behaviors from physicians, such as introducing yourself and
Yet, improving patient-physician interactions remains necessary. A recent systematic review reported that almost half of the reviewed studies on the patient-physician relationship published between 2000 and 2014 conveyed the idea that the patient-physician relationship is deteriorating.13
As part of a broader study to understand the behaviors and approaches of exemplary inpatient attending physicians,14-16 we examined how 12 carefully selected physicians interacted with their patients during inpatient teaching rounds.
METHODS
Overview
We conducted a multisite study using an exploratory, qualitative approach to inquiry, which has been described previously.14-16 Our primary purpose was to study the attributes and behaviors of outstanding general medicine attendings in the setting of inpatient rounds. The focus of this article is on the attendings’ interactions with patients.
We used a modified snowball sampling approach17 to identify 12 exemplary physicians. First, we contacted individuals throughout the United States who were known to the principal investigator (S.S.) and asked for suggestions of excellent clinician educators (also referred to as attendings) for potential inclusion in the study. In addition to these personal contacts, other individuals unknown to the investigative team were contacted and asked to provide suggestions for attendings to include in the study. Specifically, the US News & World Report 2015 Top Medical Schools: Research Rankings,18 which are widely used to represent the best U.S. hospitals, were reviewed in an effort to identify attendings from a broad range of medical schools. Using this list, we identified other medical schools that were in the top 25 and were not already represented. We contacted the division chiefs of general internal (or hospital) medicine, chairs and chiefs of departments of internal medicine, and internal medicine residency program directors from these medical schools and asked for recommendations of attendings from both within and outside their institutions whom they considered to be great inpatient teachers.
This sampling method resulted in 59 potential participants. An internet search was conducted on each potential participant to obtain further information about the individuals and their institutions. Both personal characteristics (medical education, training, and educational awards) and organizational characteristics (geographic location, hospital size and affiliation, and patient population) were considered so that a variety of organizations and backgrounds were represented. Through this process, the list was narrowed to 16 attendings who were contacted to participate in the study, of which 12 agreed. The number of attendings examined was appropriate because saturation of metathemes can occur in as little as 6 interviews, and data saturation occurs at 12 interviews.19 The participants were asked to provide a list of their current learners (ie, residents and medical students) and 6 to 10 former learners to contact for interviews and focus groups.
Data Collection
Observations
Two researchers conducted the one-day site visits. One was a physician (S.S.) and the other a medical anthropologist (M.H.), and both have extensive experience in qualitative methods. The only exception was the site visit at the principal investigator’s own institution, which was conducted by the medical anthropologist and a nonpracticing physician who was unknown to the participants. The team structure varied slightly among different institutions but in general was composed of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Each site visit began with observing the attendings (n = 12) and current learners (n = 57) on morning rounds, which included their interactions with patients. These observations lasted approximately 2 to 3 hours. The observers took handwritten field notes, paying particular attention to group interactions, teaching approaches, and patient interactions. The observers stood outside the medical team circle and remained silent during rounds so as to be unobtrusive to the teams’ discussions. The observers discussed and compared their notes after each site visit.
Interviews and Focus Groups
The research team also conducted individual, semistructured interviews with the attendings (n = 12), focus groups with their current teams (n = 46), and interviews or focus groups with their former learners (n = 26). Current learners were asked open-ended questions about their roles on the teams, their opinions of the attendings, and the care the attendings provide to their patients. Because they were observed during rounds, the researchers asked for clarification about specific interactions observed during the teaching rounds. Depending on availability and location, former learners either participated in in-person focus groups or interviews on the day of the site visit, or in a later telephone interview. All interviews and focus groups were audio recorded and transcribed.
This study was deemed to be exempt from regulation by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could refuse to answer any question.
Data Analysis
Data were analyzed using a thematic analysis approach,20 which involves reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. The patterns are then grouped into themes to help further explain the findings.21 The research team members (S.S. and M.H.) met after the first site visit and developed initial ideas about meanings and possible patterns. One team member (M.H.) read all the transcripts from the site visit and, based on the data, developed a codebook to be used for this study. This process was repeated after every site visit, and the coding definitions were refined as necessary. All transcripts were reviewed to apply any new codes when they developed. NVivo® 10 software (QSR International, Melbourne, Australia) was used to assist with the qualitative data analysis.
To ensure consistency and identify relationships between codes, code reports listing all the data linked to a specific code were generated after all the field notes and transcripts were coded. Once verified, codes were grouped based on similarities and relationships into prominent themes related to physician-patient interactions by 2 team members (S.S. and M.H.), though all members reviewed them and concurred.
RESULTS
C are for the Patient’s Well-Being
The attendings we observed appeared to openly care for their patients’ well-being and were focused on the patients’ wants and needs. We noted that attendings were generally very attentive to the patients’ comfort. For example, we observed one attending sending the senior resident to find the patient’s nurse in order to obtain additional pain medications. The attending said to the patient several times, “I’m sorry you’re in so much pain.” When the team was leaving, she asked the intern to stay with the patient until the medications had been administered.
The attendings we observed could also be considered patient advocates, ensuring that patients received superb care. As one learner said about an attending who was attempting to have his patient listed for a liver transplant, “He is the biggest advocate for the patient that I have ever seen.” Regarding the balance between learning biomedical concepts and advocacy, another learner noted the following: “… there is always a teaching aspect, but he always makes sure that everything is taken care of for the patient…”
Building rapport creates and sustains bonds between people. Even though most of the attendings we observed primarily cared for hospitalized patients and had little long-term continuity with them, the attendings tended to take special care to talk with their patients about topics other than medicine to form a bond. This bonding between attending and patient was appreciated by learners. “Probably the most important thing I learned about patient care would be taking the time and really developing that relationship with patients,” said one of the former learners we interviewed. “There’s a question that he asks to a lot of our patients,” one learner told us, “especially our elderly patients, that [is], ‘What’s the most memorable moment in your life?’ So, he asks that question, and patient[s] open up and will share.”
The attendings often used touch to further solidify their relationships with their patients. We observed one attending who would touch her patients’ arms or knees when she was talking with them. Another attending would always shake the patient’s hand when leaving. Another attending would often lay his hand on the patient’s shoulder and help the patient sit up during the physical examination. Such humanistic behavior was noticed by learners. “She does a lot of comforting touch, particularly at the end of an exam,” said a current learner.
C onsideration of the “Big Picture”
Our exemplary attendings kept the “big picture” (that is, the patient’s overall medical and social needs) in clear focus. They behaved in a way to ensure that the patients understood the key points of their care and explained so the patients and families could understand. A current learner said, “[The attending] really makes sure that the patient understands what’s going on. And she always asks them, ‘What do you understand, what do you know, how can we fill in any blanks?’ And that makes the patient really involved in their own care, which I think is important.” This reflection was supported by direct observations. Attendings posed the following questions at the conclusion of patient interactions: “Tell me what you know.” “Tell me what our plan is.” “What did the lung doctors tell you yesterday?” These questions, which have been termed “teach-back” and are crucial for health literacy, were not meant to quiz the patient but rather to ensure the patient and family understood the plan.
We noticed that the attendings effectively explained clinical details and the plan of care to the patient while avoiding medical jargon. The following is an example of one interaction with a patient: “You threw up and created a tear in the food tube. Air got from that into the middle of the chest, not into the lungs. Air isn’t normally there. If it is just air, the body will reabsorb [it]... But we worry about bacteria getting in with the air. We need to figure out if it is an infection. We’re still trying to figure it out. Hang in there with us.” One learner commented, “… since we do bedside presentations, he has a great way of translating our gibberish, basically, to real language the patient understands.”
Finally, the attendings anticipated what patients would need in the outpatient setting. We observed that attendings stressed what the next steps would be during transitions of care. As one learner put it, “But he also thinks ahead; what do they need as an outpatient?” Another current learner commented on how another attending always asked about the social situations of his patients stating, “And then there is the social part of it. So, he is very much interested [in] where do they live? What is their support system? So, I think it has been a very holistic approach to patient care.”
R espect for the Patient
The attendings we observed were steadfastly respectful toward patients. As one attending told us, “The patient’s room is sacred space, and it’s a privilege for us to be there. And if we don’t earn that privilege, then we don’t get to go there.” We observed that the attendings generally referred to the patient as Mr. or Ms. (last name) rather than the patient’s first name unless the patient insisted. We also noticed that many of the attendings would introduce the team members to the patients or ask each member to introduce himself or herself. They also tended to leave the room and patient the way they were found, for example, by pushing the patient’s bedside table so that it was back within his or her reach or placing socks back onto the patient’s feet.
We noted that many of our attendings used appropriate humor with patients and families. As one learner explained, “I think Dr. [attending] makes most of our patients laugh during rounds. I don’t know if you noticed, but he really puts a smile on their face[s] whenever he walks in. … Maybe it would catch them off guard the first day, but after that, they are so happy to see him.”
Finally, we noticed that several of our attendings made sure to meet the patient at eye level during discussions by either kneeling or sitting on a chair. One of the attendings put it this way: “That’s a horrible power dynamic when you’re an inpatient and you’re sick and someone’s standing over you telling you things, and I like to be able to make eye contact with people, and often times that requires me to kneel down or to sit on a stool or to sit on the bed. … I feel like you’re able to connect with the people in a much better way…” Learners viewed this behavior favorably. As one told us, “[The attending] gets down to their level and makes sure that all of their questions are answered. So that is one thing that other attendings don’t necessarily do.”
DISCUSSION
In our national, qualitative study of 12 exemplary attending physicians, we found that these clinicians generally exhibited the following behaviors with patients. First, they were personable and caring and made significant attempts to connect with their patients. This occasionally took the form of using touch to comfort patients. Second, they tended to seek the “big picture” and tried to understand what patients would need upon hospital discharge. They communicated plans clearly to patients and families and inquired if those plans were understood. Finally, they showed respect toward their patients without fail. Such respect took many forms but included leaving the patient and room exactly as they were found and speaking with patients at eye level.
Our findings are largely consistent with other key studies in this field. Not surprisingly, the attendings we observed adhered to the major suggestions that Branch and colleagues2 put forth more than 15 years ago to improve the teaching of the humanistic dimension of the patient-physician relationship. Examples include greeting the patient, introducing team members and explaining each person’s role, asking open-ended questions, providing patient education, placing oneself at the same level as the patient, using appropriate touch, and being respectful. Weissmann et al.22 also found similar themes in their study of teaching physicians at 4 universities from 2003 to 2004. In that study, role-modeling was the primary method used by physician educators to teach the humanistic aspects of medical care, including nonverbal communication (eg, touch and eye contact), demonstration of respect, and building a personal connection with the patients.22In a focus group-based study performed at a teaching hospital in Boston, Ramani and Orlander23 concluded that both participating teachers and learners considered the patient’s bedside as a valuable venue to learn humanistic skills. Unfortunately, they also noted that there has been a decline in bedside teaching related to various factors, including documentation requirements and electronic medical records.23 Our attendings all demonstrated the value of teaching at a patient’s bedside. Not only could physical examination skills be demonstrated but role-modeling of interpersonal skills could be observed by learners.
Block and colleagues24 observed 29 interns in 732 patient encounters in 2 Baltimore training programs using Kahn’s “etiquette-based medicine” behaviors as a guide.12 They found that interns introduced themselves 40% of the time, explained their role 37% of the time, touched patients on 65% of visits (including as part of the physical examination), asked open-ended questions 75% of the time, and sat down with patients during only 9% of visits.24 Tackett et al.7 observed 24 hospitalists who collectively cared for 226 unique patients in 3 Baltimore-area hospitals. They found that each of the following behaviors was performed less than 30% of the time: explains role in care, shakes hand, and sits down.7 However, our attendings appeared to adhere to these behaviors to a much higher extent, though we did not quantify the interactions. This lends support to the notion that effective patient-physician interactions are the foundation of great teaching.
The attendings we observed (most of whom are inpatient based) tended to the contextual issues of the patients, such as their home environments and social support. Our exemplary physicians did what they could to ensure that patients received the appropriate follow-up care upon discharge.
Our study has important limitations. First, it was conducted in a limited number of US hospitals. The institutions represented were generally large, research-intensive, academic medical centers. Therefore, our findings may not apply to settings that are different from the hospitals studied. Second, our study included only 12 attendings and their learners, which may also limit the study’s generalizability. Third, we focused exclusively on teaching within general medicine rounds. Thus, our findings may not be generalizable to other subspecialties. Fourth, attendings were selected through a nonexhaustive method, increasing the potential for selection bias. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Former-learner responses were subject to recall bias. Finally, the study design is susceptible to observer bias. Attempts to reduce this included the diversity of the observers (ie, both a clinician and a nonclinician, the latter of whom was unfamiliar with medical education) and review of the data and coding by multiple research team members to ensure validity. Although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team attempted to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Limitations notwithstanding, we believe that our multisite study is important given the longstanding imperative to improve patient-physician interactions. We found empirical support for behaviors proposed by Branch and colleagues2 and Kahn12 in order to enhance these relationships. While others have studied attendings and their current learners,22 we add to the literature by also examining former learners’ perspectives on how the attendings’ teaching and role-modeling have created and sustained a lasting impact. The key findings of our national, qualitative study (care for the patient’s well-being, consideration of the “big picture,” and respect for the patient) can be readily adopted and honed by physicians to improve their interactions with hospitalized patients.
A cknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Veterans Affairs.
F unding
Dr. Saint provided funding for this study using a University of Michigan endowment.
Disclosure
The authors declare no conflicts of interest.
1. Peabody FW. The care of the patient. JAMA. 1927;88(12):877-882. PubMed
2. Branch WT, Jr., Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
3. Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19(11):1163-1165. PubMed
4. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
5. Osmun WE, Brown JB, Stewart M, Graham S. Patients’ attitudes to comforting touch in family practice. Can Fam Physician. 2000;46:2411-2416. PubMed
6. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
7. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
8. Gallagher TH, Levinson W. A prescription for protecting the doctor-patient relationship. Am J Manag Care. 2004;10(2, pt 1):61-68. PubMed
9. Braddock CH, 3rd, Snyder L. The doctor will see you shortly. The ethical significance of time for the patient-physician relationship. J Gen Intern Med. 2005;20(11):1057-1062. PubMed
10. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903-918. PubMed
11. Lee SJ, Back AL, Block SD, Stewart SK. Enhancing physician-patient communication. Hematology Am Soc Hematol Educ Program. 2002:464-483. PubMed
12. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
13. Hoff T, Collinson GE. How Do We Talk About the Physician-Patient Relationship? What the Nonempirical Literature Tells Us. Med Care Res Rev. 2016. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. PubMed
15. Houchens N, Harrod M, Fowler KE, Moody S., Saint S. Teaching “how” to think instead of “what” to think: how great inpatient physicians foster clinical reasoning. Am J Med. In Press.
16. Harrod M, Saint S, Stock RW. Teaching Inpatient Medicine: What Every Physician Needs to Know. New York, NY: Oxford University Press; 2017.
17. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications Inc; 2013.
18. US News and World Report. Best Medical Schools: Research. 2014; http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed on September 16, 2016.
19. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. PubMed
21. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
22. Weissmann PF, Branch WT, Gracey CF, Haidet P, Frankel RM. Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661-667. PubMed
23. Ramani S, Orlander JD. Human dimensions in bedside teaching: focus group discussions of teachers and learners. Teach Learn Med. 2013;25(4):312-318. PubMed
24. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette-based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
Approximately a century ago, Francis Peabody taught that “the secret of the care of the patient is in caring for the patient.”1 His advice remains true today. Despite the advent of novel diagnostic tests, technologically sophisticated interventional procedures, and life-saving medications, perhaps the most important skill a bedside clinician can use is the ability to connect with patients.
The literature on patient-physician interaction is vast2-11 and generally indicates that exemplary bedside clinicians are able to interact well with patients by being competent, trustworthy, personable, empathetic, and effective communicators. “Etiquette-based medicine,” first proposed by Kahn,12 emphasizes the importance of certain behaviors from physicians, such as introducing yourself and
Yet, improving patient-physician interactions remains necessary. A recent systematic review reported that almost half of the reviewed studies on the patient-physician relationship published between 2000 and 2014 conveyed the idea that the patient-physician relationship is deteriorating.13
As part of a broader study to understand the behaviors and approaches of exemplary inpatient attending physicians,14-16 we examined how 12 carefully selected physicians interacted with their patients during inpatient teaching rounds.
METHODS
Overview
We conducted a multisite study using an exploratory, qualitative approach to inquiry, which has been described previously.14-16 Our primary purpose was to study the attributes and behaviors of outstanding general medicine attendings in the setting of inpatient rounds. The focus of this article is on the attendings’ interactions with patients.
We used a modified snowball sampling approach17 to identify 12 exemplary physicians. First, we contacted individuals throughout the United States who were known to the principal investigator (S.S.) and asked for suggestions of excellent clinician educators (also referred to as attendings) for potential inclusion in the study. In addition to these personal contacts, other individuals unknown to the investigative team were contacted and asked to provide suggestions for attendings to include in the study. Specifically, the US News & World Report 2015 Top Medical Schools: Research Rankings,18 which are widely used to represent the best U.S. hospitals, were reviewed in an effort to identify attendings from a broad range of medical schools. Using this list, we identified other medical schools that were in the top 25 and were not already represented. We contacted the division chiefs of general internal (or hospital) medicine, chairs and chiefs of departments of internal medicine, and internal medicine residency program directors from these medical schools and asked for recommendations of attendings from both within and outside their institutions whom they considered to be great inpatient teachers.
This sampling method resulted in 59 potential participants. An internet search was conducted on each potential participant to obtain further information about the individuals and their institutions. Both personal characteristics (medical education, training, and educational awards) and organizational characteristics (geographic location, hospital size and affiliation, and patient population) were considered so that a variety of organizations and backgrounds were represented. Through this process, the list was narrowed to 16 attendings who were contacted to participate in the study, of which 12 agreed. The number of attendings examined was appropriate because saturation of metathemes can occur in as little as 6 interviews, and data saturation occurs at 12 interviews.19 The participants were asked to provide a list of their current learners (ie, residents and medical students) and 6 to 10 former learners to contact for interviews and focus groups.
Data Collection
Observations
Two researchers conducted the one-day site visits. One was a physician (S.S.) and the other a medical anthropologist (M.H.), and both have extensive experience in qualitative methods. The only exception was the site visit at the principal investigator’s own institution, which was conducted by the medical anthropologist and a nonpracticing physician who was unknown to the participants. The team structure varied slightly among different institutions but in general was composed of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Each site visit began with observing the attendings (n = 12) and current learners (n = 57) on morning rounds, which included their interactions with patients. These observations lasted approximately 2 to 3 hours. The observers took handwritten field notes, paying particular attention to group interactions, teaching approaches, and patient interactions. The observers stood outside the medical team circle and remained silent during rounds so as to be unobtrusive to the teams’ discussions. The observers discussed and compared their notes after each site visit.
Interviews and Focus Groups
The research team also conducted individual, semistructured interviews with the attendings (n = 12), focus groups with their current teams (n = 46), and interviews or focus groups with their former learners (n = 26). Current learners were asked open-ended questions about their roles on the teams, their opinions of the attendings, and the care the attendings provide to their patients. Because they were observed during rounds, the researchers asked for clarification about specific interactions observed during the teaching rounds. Depending on availability and location, former learners either participated in in-person focus groups or interviews on the day of the site visit, or in a later telephone interview. All interviews and focus groups were audio recorded and transcribed.
This study was deemed to be exempt from regulation by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could refuse to answer any question.
Data Analysis
Data were analyzed using a thematic analysis approach,20 which involves reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. The patterns are then grouped into themes to help further explain the findings.21 The research team members (S.S. and M.H.) met after the first site visit and developed initial ideas about meanings and possible patterns. One team member (M.H.) read all the transcripts from the site visit and, based on the data, developed a codebook to be used for this study. This process was repeated after every site visit, and the coding definitions were refined as necessary. All transcripts were reviewed to apply any new codes when they developed. NVivo® 10 software (QSR International, Melbourne, Australia) was used to assist with the qualitative data analysis.
To ensure consistency and identify relationships between codes, code reports listing all the data linked to a specific code were generated after all the field notes and transcripts were coded. Once verified, codes were grouped based on similarities and relationships into prominent themes related to physician-patient interactions by 2 team members (S.S. and M.H.), though all members reviewed them and concurred.
RESULTS
C are for the Patient’s Well-Being
The attendings we observed appeared to openly care for their patients’ well-being and were focused on the patients’ wants and needs. We noted that attendings were generally very attentive to the patients’ comfort. For example, we observed one attending sending the senior resident to find the patient’s nurse in order to obtain additional pain medications. The attending said to the patient several times, “I’m sorry you’re in so much pain.” When the team was leaving, she asked the intern to stay with the patient until the medications had been administered.
The attendings we observed could also be considered patient advocates, ensuring that patients received superb care. As one learner said about an attending who was attempting to have his patient listed for a liver transplant, “He is the biggest advocate for the patient that I have ever seen.” Regarding the balance between learning biomedical concepts and advocacy, another learner noted the following: “… there is always a teaching aspect, but he always makes sure that everything is taken care of for the patient…”
Building rapport creates and sustains bonds between people. Even though most of the attendings we observed primarily cared for hospitalized patients and had little long-term continuity with them, the attendings tended to take special care to talk with their patients about topics other than medicine to form a bond. This bonding between attending and patient was appreciated by learners. “Probably the most important thing I learned about patient care would be taking the time and really developing that relationship with patients,” said one of the former learners we interviewed. “There’s a question that he asks to a lot of our patients,” one learner told us, “especially our elderly patients, that [is], ‘What’s the most memorable moment in your life?’ So, he asks that question, and patient[s] open up and will share.”
The attendings often used touch to further solidify their relationships with their patients. We observed one attending who would touch her patients’ arms or knees when she was talking with them. Another attending would always shake the patient’s hand when leaving. Another attending would often lay his hand on the patient’s shoulder and help the patient sit up during the physical examination. Such humanistic behavior was noticed by learners. “She does a lot of comforting touch, particularly at the end of an exam,” said a current learner.
C onsideration of the “Big Picture”
Our exemplary attendings kept the “big picture” (that is, the patient’s overall medical and social needs) in clear focus. They behaved in a way to ensure that the patients understood the key points of their care and explained so the patients and families could understand. A current learner said, “[The attending] really makes sure that the patient understands what’s going on. And she always asks them, ‘What do you understand, what do you know, how can we fill in any blanks?’ And that makes the patient really involved in their own care, which I think is important.” This reflection was supported by direct observations. Attendings posed the following questions at the conclusion of patient interactions: “Tell me what you know.” “Tell me what our plan is.” “What did the lung doctors tell you yesterday?” These questions, which have been termed “teach-back” and are crucial for health literacy, were not meant to quiz the patient but rather to ensure the patient and family understood the plan.
We noticed that the attendings effectively explained clinical details and the plan of care to the patient while avoiding medical jargon. The following is an example of one interaction with a patient: “You threw up and created a tear in the food tube. Air got from that into the middle of the chest, not into the lungs. Air isn’t normally there. If it is just air, the body will reabsorb [it]... But we worry about bacteria getting in with the air. We need to figure out if it is an infection. We’re still trying to figure it out. Hang in there with us.” One learner commented, “… since we do bedside presentations, he has a great way of translating our gibberish, basically, to real language the patient understands.”
Finally, the attendings anticipated what patients would need in the outpatient setting. We observed that attendings stressed what the next steps would be during transitions of care. As one learner put it, “But he also thinks ahead; what do they need as an outpatient?” Another current learner commented on how another attending always asked about the social situations of his patients stating, “And then there is the social part of it. So, he is very much interested [in] where do they live? What is their support system? So, I think it has been a very holistic approach to patient care.”
R espect for the Patient
The attendings we observed were steadfastly respectful toward patients. As one attending told us, “The patient’s room is sacred space, and it’s a privilege for us to be there. And if we don’t earn that privilege, then we don’t get to go there.” We observed that the attendings generally referred to the patient as Mr. or Ms. (last name) rather than the patient’s first name unless the patient insisted. We also noticed that many of the attendings would introduce the team members to the patients or ask each member to introduce himself or herself. They also tended to leave the room and patient the way they were found, for example, by pushing the patient’s bedside table so that it was back within his or her reach or placing socks back onto the patient’s feet.
We noted that many of our attendings used appropriate humor with patients and families. As one learner explained, “I think Dr. [attending] makes most of our patients laugh during rounds. I don’t know if you noticed, but he really puts a smile on their face[s] whenever he walks in. … Maybe it would catch them off guard the first day, but after that, they are so happy to see him.”
Finally, we noticed that several of our attendings made sure to meet the patient at eye level during discussions by either kneeling or sitting on a chair. One of the attendings put it this way: “That’s a horrible power dynamic when you’re an inpatient and you’re sick and someone’s standing over you telling you things, and I like to be able to make eye contact with people, and often times that requires me to kneel down or to sit on a stool or to sit on the bed. … I feel like you’re able to connect with the people in a much better way…” Learners viewed this behavior favorably. As one told us, “[The attending] gets down to their level and makes sure that all of their questions are answered. So that is one thing that other attendings don’t necessarily do.”
DISCUSSION
In our national, qualitative study of 12 exemplary attending physicians, we found that these clinicians generally exhibited the following behaviors with patients. First, they were personable and caring and made significant attempts to connect with their patients. This occasionally took the form of using touch to comfort patients. Second, they tended to seek the “big picture” and tried to understand what patients would need upon hospital discharge. They communicated plans clearly to patients and families and inquired if those plans were understood. Finally, they showed respect toward their patients without fail. Such respect took many forms but included leaving the patient and room exactly as they were found and speaking with patients at eye level.
Our findings are largely consistent with other key studies in this field. Not surprisingly, the attendings we observed adhered to the major suggestions that Branch and colleagues2 put forth more than 15 years ago to improve the teaching of the humanistic dimension of the patient-physician relationship. Examples include greeting the patient, introducing team members and explaining each person’s role, asking open-ended questions, providing patient education, placing oneself at the same level as the patient, using appropriate touch, and being respectful. Weissmann et al.22 also found similar themes in their study of teaching physicians at 4 universities from 2003 to 2004. In that study, role-modeling was the primary method used by physician educators to teach the humanistic aspects of medical care, including nonverbal communication (eg, touch and eye contact), demonstration of respect, and building a personal connection with the patients.22In a focus group-based study performed at a teaching hospital in Boston, Ramani and Orlander23 concluded that both participating teachers and learners considered the patient’s bedside as a valuable venue to learn humanistic skills. Unfortunately, they also noted that there has been a decline in bedside teaching related to various factors, including documentation requirements and electronic medical records.23 Our attendings all demonstrated the value of teaching at a patient’s bedside. Not only could physical examination skills be demonstrated but role-modeling of interpersonal skills could be observed by learners.
Block and colleagues24 observed 29 interns in 732 patient encounters in 2 Baltimore training programs using Kahn’s “etiquette-based medicine” behaviors as a guide.12 They found that interns introduced themselves 40% of the time, explained their role 37% of the time, touched patients on 65% of visits (including as part of the physical examination), asked open-ended questions 75% of the time, and sat down with patients during only 9% of visits.24 Tackett et al.7 observed 24 hospitalists who collectively cared for 226 unique patients in 3 Baltimore-area hospitals. They found that each of the following behaviors was performed less than 30% of the time: explains role in care, shakes hand, and sits down.7 However, our attendings appeared to adhere to these behaviors to a much higher extent, though we did not quantify the interactions. This lends support to the notion that effective patient-physician interactions are the foundation of great teaching.
The attendings we observed (most of whom are inpatient based) tended to the contextual issues of the patients, such as their home environments and social support. Our exemplary physicians did what they could to ensure that patients received the appropriate follow-up care upon discharge.
Our study has important limitations. First, it was conducted in a limited number of US hospitals. The institutions represented were generally large, research-intensive, academic medical centers. Therefore, our findings may not apply to settings that are different from the hospitals studied. Second, our study included only 12 attendings and their learners, which may also limit the study’s generalizability. Third, we focused exclusively on teaching within general medicine rounds. Thus, our findings may not be generalizable to other subspecialties. Fourth, attendings were selected through a nonexhaustive method, increasing the potential for selection bias. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Former-learner responses were subject to recall bias. Finally, the study design is susceptible to observer bias. Attempts to reduce this included the diversity of the observers (ie, both a clinician and a nonclinician, the latter of whom was unfamiliar with medical education) and review of the data and coding by multiple research team members to ensure validity. Although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team attempted to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Limitations notwithstanding, we believe that our multisite study is important given the longstanding imperative to improve patient-physician interactions. We found empirical support for behaviors proposed by Branch and colleagues2 and Kahn12 in order to enhance these relationships. While others have studied attendings and their current learners,22 we add to the literature by also examining former learners’ perspectives on how the attendings’ teaching and role-modeling have created and sustained a lasting impact. The key findings of our national, qualitative study (care for the patient’s well-being, consideration of the “big picture,” and respect for the patient) can be readily adopted and honed by physicians to improve their interactions with hospitalized patients.
A cknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Veterans Affairs.
F unding
Dr. Saint provided funding for this study using a University of Michigan endowment.
Disclosure
The authors declare no conflicts of interest.
Approximately a century ago, Francis Peabody taught that “the secret of the care of the patient is in caring for the patient.”1 His advice remains true today. Despite the advent of novel diagnostic tests, technologically sophisticated interventional procedures, and life-saving medications, perhaps the most important skill a bedside clinician can use is the ability to connect with patients.
The literature on patient-physician interaction is vast2-11 and generally indicates that exemplary bedside clinicians are able to interact well with patients by being competent, trustworthy, personable, empathetic, and effective communicators. “Etiquette-based medicine,” first proposed by Kahn,12 emphasizes the importance of certain behaviors from physicians, such as introducing yourself and
Yet, improving patient-physician interactions remains necessary. A recent systematic review reported that almost half of the reviewed studies on the patient-physician relationship published between 2000 and 2014 conveyed the idea that the patient-physician relationship is deteriorating.13
As part of a broader study to understand the behaviors and approaches of exemplary inpatient attending physicians,14-16 we examined how 12 carefully selected physicians interacted with their patients during inpatient teaching rounds.
METHODS
Overview
We conducted a multisite study using an exploratory, qualitative approach to inquiry, which has been described previously.14-16 Our primary purpose was to study the attributes and behaviors of outstanding general medicine attendings in the setting of inpatient rounds. The focus of this article is on the attendings’ interactions with patients.
We used a modified snowball sampling approach17 to identify 12 exemplary physicians. First, we contacted individuals throughout the United States who were known to the principal investigator (S.S.) and asked for suggestions of excellent clinician educators (also referred to as attendings) for potential inclusion in the study. In addition to these personal contacts, other individuals unknown to the investigative team were contacted and asked to provide suggestions for attendings to include in the study. Specifically, the US News & World Report 2015 Top Medical Schools: Research Rankings,18 which are widely used to represent the best U.S. hospitals, were reviewed in an effort to identify attendings from a broad range of medical schools. Using this list, we identified other medical schools that were in the top 25 and were not already represented. We contacted the division chiefs of general internal (or hospital) medicine, chairs and chiefs of departments of internal medicine, and internal medicine residency program directors from these medical schools and asked for recommendations of attendings from both within and outside their institutions whom they considered to be great inpatient teachers.
This sampling method resulted in 59 potential participants. An internet search was conducted on each potential participant to obtain further information about the individuals and their institutions. Both personal characteristics (medical education, training, and educational awards) and organizational characteristics (geographic location, hospital size and affiliation, and patient population) were considered so that a variety of organizations and backgrounds were represented. Through this process, the list was narrowed to 16 attendings who were contacted to participate in the study, of which 12 agreed. The number of attendings examined was appropriate because saturation of metathemes can occur in as little as 6 interviews, and data saturation occurs at 12 interviews.19 The participants were asked to provide a list of their current learners (ie, residents and medical students) and 6 to 10 former learners to contact for interviews and focus groups.
Data Collection
Observations
Two researchers conducted the one-day site visits. One was a physician (S.S.) and the other a medical anthropologist (M.H.), and both have extensive experience in qualitative methods. The only exception was the site visit at the principal investigator’s own institution, which was conducted by the medical anthropologist and a nonpracticing physician who was unknown to the participants. The team structure varied slightly among different institutions but in general was composed of 1 attending, 1 senior medical resident, 1 to 2 interns, and approximately 2 medical students. Each site visit began with observing the attendings (n = 12) and current learners (n = 57) on morning rounds, which included their interactions with patients. These observations lasted approximately 2 to 3 hours. The observers took handwritten field notes, paying particular attention to group interactions, teaching approaches, and patient interactions. The observers stood outside the medical team circle and remained silent during rounds so as to be unobtrusive to the teams’ discussions. The observers discussed and compared their notes after each site visit.
Interviews and Focus Groups
The research team also conducted individual, semistructured interviews with the attendings (n = 12), focus groups with their current teams (n = 46), and interviews or focus groups with their former learners (n = 26). Current learners were asked open-ended questions about their roles on the teams, their opinions of the attendings, and the care the attendings provide to their patients. Because they were observed during rounds, the researchers asked for clarification about specific interactions observed during the teaching rounds. Depending on availability and location, former learners either participated in in-person focus groups or interviews on the day of the site visit, or in a later telephone interview. All interviews and focus groups were audio recorded and transcribed.
This study was deemed to be exempt from regulation by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could refuse to answer any question.
Data Analysis
Data were analyzed using a thematic analysis approach,20 which involves reading through the data to identify patterns (and create codes) that relate to behaviors, experiences, meanings, and activities. The patterns are then grouped into themes to help further explain the findings.21 The research team members (S.S. and M.H.) met after the first site visit and developed initial ideas about meanings and possible patterns. One team member (M.H.) read all the transcripts from the site visit and, based on the data, developed a codebook to be used for this study. This process was repeated after every site visit, and the coding definitions were refined as necessary. All transcripts were reviewed to apply any new codes when they developed. NVivo® 10 software (QSR International, Melbourne, Australia) was used to assist with the qualitative data analysis.
To ensure consistency and identify relationships between codes, code reports listing all the data linked to a specific code were generated after all the field notes and transcripts were coded. Once verified, codes were grouped based on similarities and relationships into prominent themes related to physician-patient interactions by 2 team members (S.S. and M.H.), though all members reviewed them and concurred.
RESULTS
C are for the Patient’s Well-Being
The attendings we observed appeared to openly care for their patients’ well-being and were focused on the patients’ wants and needs. We noted that attendings were generally very attentive to the patients’ comfort. For example, we observed one attending sending the senior resident to find the patient’s nurse in order to obtain additional pain medications. The attending said to the patient several times, “I’m sorry you’re in so much pain.” When the team was leaving, she asked the intern to stay with the patient until the medications had been administered.
The attendings we observed could also be considered patient advocates, ensuring that patients received superb care. As one learner said about an attending who was attempting to have his patient listed for a liver transplant, “He is the biggest advocate for the patient that I have ever seen.” Regarding the balance between learning biomedical concepts and advocacy, another learner noted the following: “… there is always a teaching aspect, but he always makes sure that everything is taken care of for the patient…”
Building rapport creates and sustains bonds between people. Even though most of the attendings we observed primarily cared for hospitalized patients and had little long-term continuity with them, the attendings tended to take special care to talk with their patients about topics other than medicine to form a bond. This bonding between attending and patient was appreciated by learners. “Probably the most important thing I learned about patient care would be taking the time and really developing that relationship with patients,” said one of the former learners we interviewed. “There’s a question that he asks to a lot of our patients,” one learner told us, “especially our elderly patients, that [is], ‘What’s the most memorable moment in your life?’ So, he asks that question, and patient[s] open up and will share.”
The attendings often used touch to further solidify their relationships with their patients. We observed one attending who would touch her patients’ arms or knees when she was talking with them. Another attending would always shake the patient’s hand when leaving. Another attending would often lay his hand on the patient’s shoulder and help the patient sit up during the physical examination. Such humanistic behavior was noticed by learners. “She does a lot of comforting touch, particularly at the end of an exam,” said a current learner.
C onsideration of the “Big Picture”
Our exemplary attendings kept the “big picture” (that is, the patient’s overall medical and social needs) in clear focus. They behaved in a way to ensure that the patients understood the key points of their care and explained so the patients and families could understand. A current learner said, “[The attending] really makes sure that the patient understands what’s going on. And she always asks them, ‘What do you understand, what do you know, how can we fill in any blanks?’ And that makes the patient really involved in their own care, which I think is important.” This reflection was supported by direct observations. Attendings posed the following questions at the conclusion of patient interactions: “Tell me what you know.” “Tell me what our plan is.” “What did the lung doctors tell you yesterday?” These questions, which have been termed “teach-back” and are crucial for health literacy, were not meant to quiz the patient but rather to ensure the patient and family understood the plan.
We noticed that the attendings effectively explained clinical details and the plan of care to the patient while avoiding medical jargon. The following is an example of one interaction with a patient: “You threw up and created a tear in the food tube. Air got from that into the middle of the chest, not into the lungs. Air isn’t normally there. If it is just air, the body will reabsorb [it]... But we worry about bacteria getting in with the air. We need to figure out if it is an infection. We’re still trying to figure it out. Hang in there with us.” One learner commented, “… since we do bedside presentations, he has a great way of translating our gibberish, basically, to real language the patient understands.”
Finally, the attendings anticipated what patients would need in the outpatient setting. We observed that attendings stressed what the next steps would be during transitions of care. As one learner put it, “But he also thinks ahead; what do they need as an outpatient?” Another current learner commented on how another attending always asked about the social situations of his patients stating, “And then there is the social part of it. So, he is very much interested [in] where do they live? What is their support system? So, I think it has been a very holistic approach to patient care.”
R espect for the Patient
The attendings we observed were steadfastly respectful toward patients. As one attending told us, “The patient’s room is sacred space, and it’s a privilege for us to be there. And if we don’t earn that privilege, then we don’t get to go there.” We observed that the attendings generally referred to the patient as Mr. or Ms. (last name) rather than the patient’s first name unless the patient insisted. We also noticed that many of the attendings would introduce the team members to the patients or ask each member to introduce himself or herself. They also tended to leave the room and patient the way they were found, for example, by pushing the patient’s bedside table so that it was back within his or her reach or placing socks back onto the patient’s feet.
We noted that many of our attendings used appropriate humor with patients and families. As one learner explained, “I think Dr. [attending] makes most of our patients laugh during rounds. I don’t know if you noticed, but he really puts a smile on their face[s] whenever he walks in. … Maybe it would catch them off guard the first day, but after that, they are so happy to see him.”
Finally, we noticed that several of our attendings made sure to meet the patient at eye level during discussions by either kneeling or sitting on a chair. One of the attendings put it this way: “That’s a horrible power dynamic when you’re an inpatient and you’re sick and someone’s standing over you telling you things, and I like to be able to make eye contact with people, and often times that requires me to kneel down or to sit on a stool or to sit on the bed. … I feel like you’re able to connect with the people in a much better way…” Learners viewed this behavior favorably. As one told us, “[The attending] gets down to their level and makes sure that all of their questions are answered. So that is one thing that other attendings don’t necessarily do.”
DISCUSSION
In our national, qualitative study of 12 exemplary attending physicians, we found that these clinicians generally exhibited the following behaviors with patients. First, they were personable and caring and made significant attempts to connect with their patients. This occasionally took the form of using touch to comfort patients. Second, they tended to seek the “big picture” and tried to understand what patients would need upon hospital discharge. They communicated plans clearly to patients and families and inquired if those plans were understood. Finally, they showed respect toward their patients without fail. Such respect took many forms but included leaving the patient and room exactly as they were found and speaking with patients at eye level.
Our findings are largely consistent with other key studies in this field. Not surprisingly, the attendings we observed adhered to the major suggestions that Branch and colleagues2 put forth more than 15 years ago to improve the teaching of the humanistic dimension of the patient-physician relationship. Examples include greeting the patient, introducing team members and explaining each person’s role, asking open-ended questions, providing patient education, placing oneself at the same level as the patient, using appropriate touch, and being respectful. Weissmann et al.22 also found similar themes in their study of teaching physicians at 4 universities from 2003 to 2004. In that study, role-modeling was the primary method used by physician educators to teach the humanistic aspects of medical care, including nonverbal communication (eg, touch and eye contact), demonstration of respect, and building a personal connection with the patients.22In a focus group-based study performed at a teaching hospital in Boston, Ramani and Orlander23 concluded that both participating teachers and learners considered the patient’s bedside as a valuable venue to learn humanistic skills. Unfortunately, they also noted that there has been a decline in bedside teaching related to various factors, including documentation requirements and electronic medical records.23 Our attendings all demonstrated the value of teaching at a patient’s bedside. Not only could physical examination skills be demonstrated but role-modeling of interpersonal skills could be observed by learners.
Block and colleagues24 observed 29 interns in 732 patient encounters in 2 Baltimore training programs using Kahn’s “etiquette-based medicine” behaviors as a guide.12 They found that interns introduced themselves 40% of the time, explained their role 37% of the time, touched patients on 65% of visits (including as part of the physical examination), asked open-ended questions 75% of the time, and sat down with patients during only 9% of visits.24 Tackett et al.7 observed 24 hospitalists who collectively cared for 226 unique patients in 3 Baltimore-area hospitals. They found that each of the following behaviors was performed less than 30% of the time: explains role in care, shakes hand, and sits down.7 However, our attendings appeared to adhere to these behaviors to a much higher extent, though we did not quantify the interactions. This lends support to the notion that effective patient-physician interactions are the foundation of great teaching.
The attendings we observed (most of whom are inpatient based) tended to the contextual issues of the patients, such as their home environments and social support. Our exemplary physicians did what they could to ensure that patients received the appropriate follow-up care upon discharge.
Our study has important limitations. First, it was conducted in a limited number of US hospitals. The institutions represented were generally large, research-intensive, academic medical centers. Therefore, our findings may not apply to settings that are different from the hospitals studied. Second, our study included only 12 attendings and their learners, which may also limit the study’s generalizability. Third, we focused exclusively on teaching within general medicine rounds. Thus, our findings may not be generalizable to other subspecialties. Fourth, attendings were selected through a nonexhaustive method, increasing the potential for selection bias. However, the multisite design, the modified snowball sampling, and the inclusion of several types of institutions in the final participant pool introduced diversity to the final list. Former-learner responses were subject to recall bias. Finally, the study design is susceptible to observer bias. Attempts to reduce this included the diversity of the observers (ie, both a clinician and a nonclinician, the latter of whom was unfamiliar with medical education) and review of the data and coding by multiple research team members to ensure validity. Although we cannot discount the potential role of a Hawthorne effect on our data collection, the research team attempted to mitigate this by standing apart from the care teams and remaining unobtrusive during observations.
Limitations notwithstanding, we believe that our multisite study is important given the longstanding imperative to improve patient-physician interactions. We found empirical support for behaviors proposed by Branch and colleagues2 and Kahn12 in order to enhance these relationships. While others have studied attendings and their current learners,22 we add to the literature by also examining former learners’ perspectives on how the attendings’ teaching and role-modeling have created and sustained a lasting impact. The key findings of our national, qualitative study (care for the patient’s well-being, consideration of the “big picture,” and respect for the patient) can be readily adopted and honed by physicians to improve their interactions with hospitalized patients.
A cknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Department of Veterans Affairs.
F unding
Dr. Saint provided funding for this study using a University of Michigan endowment.
Disclosure
The authors declare no conflicts of interest.
1. Peabody FW. The care of the patient. JAMA. 1927;88(12):877-882. PubMed
2. Branch WT, Jr., Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
3. Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19(11):1163-1165. PubMed
4. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
5. Osmun WE, Brown JB, Stewart M, Graham S. Patients’ attitudes to comforting touch in family practice. Can Fam Physician. 2000;46:2411-2416. PubMed
6. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
7. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
8. Gallagher TH, Levinson W. A prescription for protecting the doctor-patient relationship. Am J Manag Care. 2004;10(2, pt 1):61-68. PubMed
9. Braddock CH, 3rd, Snyder L. The doctor will see you shortly. The ethical significance of time for the patient-physician relationship. J Gen Intern Med. 2005;20(11):1057-1062. PubMed
10. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903-918. PubMed
11. Lee SJ, Back AL, Block SD, Stewart SK. Enhancing physician-patient communication. Hematology Am Soc Hematol Educ Program. 2002:464-483. PubMed
12. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
13. Hoff T, Collinson GE. How Do We Talk About the Physician-Patient Relationship? What the Nonempirical Literature Tells Us. Med Care Res Rev. 2016. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. PubMed
15. Houchens N, Harrod M, Fowler KE, Moody S., Saint S. Teaching “how” to think instead of “what” to think: how great inpatient physicians foster clinical reasoning. Am J Med. In Press.
16. Harrod M, Saint S, Stock RW. Teaching Inpatient Medicine: What Every Physician Needs to Know. New York, NY: Oxford University Press; 2017.
17. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications Inc; 2013.
18. US News and World Report. Best Medical Schools: Research. 2014; http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed on September 16, 2016.
19. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. PubMed
21. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
22. Weissmann PF, Branch WT, Gracey CF, Haidet P, Frankel RM. Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661-667. PubMed
23. Ramani S, Orlander JD. Human dimensions in bedside teaching: focus group discussions of teachers and learners. Teach Learn Med. 2013;25(4):312-318. PubMed
24. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette-based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
1. Peabody FW. The care of the patient. JAMA. 1927;88(12):877-882. PubMed
2. Branch WT, Jr., Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. PubMed
3. Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19(11):1163-1165. PubMed
4. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
5. Osmun WE, Brown JB, Stewart M, Graham S. Patients’ attitudes to comforting touch in family practice. Can Fam Physician. 2000;46:2411-2416. PubMed
6. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
7. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
8. Gallagher TH, Levinson W. A prescription for protecting the doctor-patient relationship. Am J Manag Care. 2004;10(2, pt 1):61-68. PubMed
9. Braddock CH, 3rd, Snyder L. The doctor will see you shortly. The ethical significance of time for the patient-physician relationship. J Gen Intern Med. 2005;20(11):1057-1062. PubMed
10. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903-918. PubMed
11. Lee SJ, Back AL, Block SD, Stewart SK. Enhancing physician-patient communication. Hematology Am Soc Hematol Educ Program. 2002:464-483. PubMed
12. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
13. Hoff T, Collinson GE. How Do We Talk About the Physician-Patient Relationship? What the Nonempirical Literature Tells Us. Med Care Res Rev. 2016. PubMed
14. Houchens N, Harrod M, Moody S, Fowler KE, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. PubMed
15. Houchens N, Harrod M, Fowler KE, Moody S., Saint S. Teaching “how” to think instead of “what” to think: how great inpatient physicians foster clinical reasoning. Am J Med. In Press.
16. Harrod M, Saint S, Stock RW. Teaching Inpatient Medicine: What Every Physician Needs to Know. New York, NY: Oxford University Press; 2017.
17. Richards L, Morse J. README FIRST for a User’s Guide to Qualitative Methods. 3rd ed. Los Angeles, CA: SAGE Publications Inc; 2013.
18. US News and World Report. Best Medical Schools: Research. 2014; http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings. Accessed on September 16, 2016.
19. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82.
20. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. PubMed
21. Aronson J. A pragmatic view of thematic analysis. Qual Rep. 1995;2(1):1-3.
22. Weissmann PF, Branch WT, Gracey CF, Haidet P, Frankel RM. Role modeling humanistic behavior: learning bedside manner from the experts. Acad Med. 2006;81(7):661-667. PubMed
23. Ramani S, Orlander JD. Human dimensions in bedside teaching: focus group discussions of teachers and learners. Teach Learn Med. 2013;25(4):312-318. PubMed
24. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette-based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
© 2017 Society of Hospital Medicine
Dust in the Wind
A 52-year-old woman presented with a 4-day history of progressive dyspnea, nonproductive cough, pleuritic chest pain, and subjective fevers. She described dyspnea at rest, which worsened with exertion. She reported no chills, night sweats, weight change, wheezing, hemoptysis, orthopnea, lower extremity edema, or nasal congestion. She also denied myalgia, arthralgia, or joint swelling. She reported no rash, itching, or peripheral lymphadenopathy. She had no seasonal allergies. She was treated for presumed bronchitis with azithromycin by her primary care provider 4 days prior to presentation but experienced progressive dyspnea.
The constellation of dry cough, fever, and dyspnea is often infectious in origin, with the nonproductive, dry cough more suggestive of a viral than bacterial syndrome. Atypical organisms such as Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae may also present with these symptoms. Noninfectious etiologies should also be considered, including pulmonary embolism, systemic lupus erythematosus, asbestosis, hypersensitivity pneumonitis, sarcoidosis, and lung cancer. The dyspnea at rest stands out as a worrisome feature, as it implies hypoxia; therefore, an oxygen saturation is necessary to quickly determine her peripheral oxygen saturation.
Her past medical history was notable for lung adenocarcinoma, for which she had undergone right upper lobectomy, without chemotherapy or radiation, 13 years ago without recurrence. She had no history of chronic obstructive pulmonary disease, asthma, or pneumonia, nor a family history of chronic obstructive pulmonary disease, asthma, pneumonia, or lung cancer. Her only medication was azithromycin. She drank alcohol on occasion and denied illicit drug use. Three weeks prior to admission, she began smoking 4 to 5 cigarettes per day after 13 years of abstinence. Her smoking history prior to abstinence was 1 pack per day for 20 years. She worked as a department store remodeler; she had no exposure to asbestos, mold, or water-damaged wood. She reported no recent travel, sick contacts, or exposure to animals.
A primary lung neoplasm with a pleural effusion could cause her shortness of breath and pleuritic chest pain. Her history of lung cancer at age 39 raises the possibility of recurrence. For cigarette smokers, a second lung cancer may occur many years after the first diagnosis and treatment, even if they have quit smoking. A review of her original cancer records is essential to confirm the diagnosis of pulmonary adenocarcinoma. What is now being described as pulmonary adenocarcinoma may have been a metastatic lesion arising from outside the lung. Although unlikely, a primary adenocarcinoma may remain active.
Infectious etiologies continue to merit consideration. A parapneumonic effusion from a pneumonia or an empyema are consistent with her symptoms. Systemic lupus erythematosus can cause lung disease with pleural effusions. She does exhibit dyspnea and pleurisy, which are consistent with autoimmune disease, but does not exhibit some of the more typical autoimmune symptoms such as arthralgias, joint swelling, and rash. Pneumothorax could also produce her symptoms; however, pneumothorax usually occurs spontaneously in younger patients or after trauma or a procedure. Remote right upper lobectomy would not be a cause of pneumothorax now. Her reported history makes lung disease or pneumoconiosis due to occupational exposure to mold or aspergillosis a possibility. Legionellosis, histoplasmosis, or coccidioidomycosis should be considered if she lives in or has visited a high-risk area. Pulmonary embolism remains a concern for all patients with new-onset shortness of breath. Decision support tools, such as the Wells criteria, are valuable, but the gestalt of the physician does not lag far behind in accuracy.
Cardiac disease is also in the differential. Bibasilar crackles, third heart sound gallop, and jugular vein distension would suggest heart failure. A pericardial friction rub would be highly suggestive of pericarditis. A paradoxical pulse would raise concern for pericardial tamponade. Pleurisy may be associated with a pericardial effusion, making viral pericarditis and myocarditis possibilities.
She was in moderate distress with tachypnea and increased work of breathing. Her temperature was 36.7°C, heart rate 104 beats per minute, respiratory rate 24 breaths per minute, oxygen saturation was 88% on room air, 94% on 3 liters of oxygen, and blood pressure was 147/61 mmHg. Auscultation of the lungs revealed bibasilar crackles and decreased breath sounds at the bases. She was tachycardic, with a regular rhythm and no appreciable murmurs, rubs, or gallops. There was no jugular venous distention or lower extremity edema. Her thyroid was palpable, without appreciation of nodules. Skin and musculoskeletal examinations were normal.
Unless she is immunocompromised, infection has become lower in the differential, as she is afebrile. Decreased breath sounds at the bases and bibasilar crackles may be due to pleural effusions. Congestive heart failure is a possibility, especially given her dyspnea and bibasilar crackles. Volume overload from renal failure is possible, but she does not have other signs of volume overload such as lower extremity edema or jugular venous distension. It is important to note that crackles may be due to other etiologies, including atelectasis, fibrosis, or pneumonia. Pulmonary embolism may cause hypoxia, tachycardia, and pleural effusions. Additional diseases may present similarly, including human immunodeficiency virus with Pneumocystis jirovecii, causing dyspnea, tachypnea, and tachycardia; hematologic malignancy with anemia, causing dyspnea and tachycardia; and thyrotoxic states with thyromegaly, causing dyspnea and tachycardia. Thyroid storm patients appear in distress, are tachycardic, and may have thyromegaly.
Moderate distress, increased work of breathing, tachycardia, tachypnea, and hypoxia are all worrisome signs. Her temperature is subnormal, although this may not be accurate, as oral temperatures may register lower in patients with increased respiratory rates because of increased air flow across the thermometer. Bibasilar crackles with decreased bibasilar sounds require further investigation. A complete blood count, complete metabolic profile, troponin, arterial blood gas (ABG), electrocardiogram (ECG), and chest radiograph are warranted.
Laboratory studies revealed a white blood cell count of 8600 per mm3 with 11% bands and 7.3% eosinophils, and a hemoglobin count of 15 gm/dL. Basic metabolic panel, liver function tests, coagulation panel, and urinalysis were within normal limits, including serum creatinine 0.7 mg/dL, sodium 143 mmoL/L, chloride 104 mmoL/L, bicarbonate 30 mEq/L, anion gap 9 mmoL/L, and blood urea nitrogen 12 mg/dL. Chest radiograph disclosed diffusely increased interstitial markings and a small left pleural effusion (Figure 1).
Her bandemia suggests infection. Stress can cause a leukocytosis by demargination of mature white blood cells; however, stress does not often cause immature cells such as bands to appear. Her chest radiograph with diffuse interstitial markings is consistent with a community-acquired pneumonia. Empiric antibiotic therapy should be initiated because of the possibility of community-acquired pneumonia. Recent studies demonstrate that steroids decrease mortality, the need for mechanical ventilation, and the length of stay for patients hospitalized with community-acquired pneumonia; therefore, this patient should also be treated with steroids.
Eosinophilia may be seen in drug reactions, allergies, pulmonary emboli, pleural effusions, and occasionally in malignancy. Eosinophilic pneumonia typically has the “reverse pulmonary edema” picture, with infiltrates in the periphery and not centrally, as in congestive heart failure.
A serum bicarbonate of 30 mEq/L suggests a metabolic compensation for a chronic respiratory acidosis as renal compensation, and rise in bicarbonate generally takes 3 days. She may have been hypoxic longer than her symptoms suggest.
An ABG should be ordered to determine the degree of hypoxia and whether a higher level of care is indicated. The abnormal chest radiograph, along with her hypoxia, merits a closer look at her lung parenchyma with chest computed tomography (CT). A D-dimer would be beneficial to rule out pulmonary embolism. If the D-dimer is positive, chest CT with contrast is indicated to determine if a pulmonary embolism is present. A brain natriuretic peptide would assist in the diagnosis of congestive heart failure. A sputum culture and Gram stain and respiratory viral panel may establish a pathogen for pneumonia. An ECG and troponin to rule out myocardial infarction should be performed as well.
The presence of hilar and subcarinal lymph nodes expands the differential. Stage IV pulmonary sarcoid may present with diffuse infiltrates and nodes, although the acuity in this case makes it less likely. A very aggressive malignancy such as Burkitt lymphoma may have these findings. Acute viral and atypical pneumonias remain possible. Right middle lobe syndrome may cause partial collapse of the right middle lobe. Tuberculosis can be associated with right middle lobe syndrome; however, in this day and age an obstructing mass is more likely the cause. Pulmonary disease, such as cryptogenic organizing pneumonia, idiopathic pulmonary fibrosis, and interstitial lung disease, should be considered in patients with pneumonia unresponsive to antibiotics. Lung biopsy and bronchoalveolar lavage (BAL) would help make the diagnosis and should be the next step, unless her degree of hypoxia is prohibitive. Similarly, thoracentesis with analysis of the pleural fluid for cell count, Gram stain, and culture may help make the diagnosis. Thoracentesis should be done with fluoroscopic guidance, given the risk of pneumothorax, which would further compromise her tenuous respiratory status.
Thoracentesis was attempted, but the pleural effusion was too small to provide a sample. Subsequent serum blood counts with differential showed an increased eosinophilia to 20% and resolved bandemia. Upon further questioning, she recalled several months of extensive, daily, fine-dust exposure from demolition during the remodeling of a new building.
Hypereosinophilia and pulmonary infiltrates narrow the differential considerably to include asthma; parasitic infection, such as the pulmonary phase of ascariasis; exposure, such as to dust, cigarettes, or asbestosis; or hypereosinophilic syndromes characterized by peripheral eosinophilia, along with a tissue eosinophilia, causing organ dysfunction. Idiopathic hypereosinophilic syndrome, a hypereosinophilic syndrome of unknown etiology despite extensive diagnostic testing, is rare, and eosinophilic leukemia even rarer. Her history strongly suggests exposure. Many eosinophilic diseases respond rapidly to steroids, and response to treatment would help narrow the diagnosis. If she does not respond to steroids, a lung and/or bone marrow biopsy would be the next step.
A BAL of the right middle lobe revealed 51% eosinophils, 3% neutrophils, 15% macrophages, and 28% lymphocytes. Gram stain, as well as cultures for bacteria, acid fast bacilli, fungus, herpes simplex virus, and cytomegalovirus cultures, were negative. Transbronchial lung biopsy revealed focal interstitial fibrosis and inflammation, without evidence of infection.
Eosinophils are primarily located in tissues; therefore, peripheral blood eosinophil counts often underestimate the degree of infiltration into end organs such as the lung. With 50% eosinophils, her BAL reflects this. Mold, fungus, chemical, and particle exposure could produce an eosinophilic BAL. She does not appear to be at risk for parasitic exposure. Eosinophilic granulomatosis (previously known as Churg-Strauss) is a consideration, but the lack of signs of vasculitis and wheezing make this less likely. A negative antineutrophil cytoplasmic antibody may provide reassurance. “Fine dust exposure” is consistent with environmental exposure but not a specific antigen. Steroids provide a brisk eosinophil reduction and are appropriate for this patient. There is the possibility of missing infectious or parasitic etiologies; therefore, a culture of BAL fluid should be sent.
Eosinophilic infiltration may lead to fibrosis, as was found on the lung biopsy. She should be counseled to avoid “fine dust exposure” in the future. Follow-up lung imaging and pulmonary function tests (PFTs) should be performed once her acute illness resolves. She should be strongly urged not to smoke tobacco. Interestingly, there are reports that ex-smokers who restart smoking have an increased risk of eosinophilic pneumonia, but in this case dust exposure is the more likely etiology.
She was diagnosed with acute eosinophilic pneumonia (AEP). Antibiotics were discontinued, and oral prednisone was initiated at 40 mg daily, with a brisk response and resolution of her dyspnea. She was discharged with a 6-week prednisone taper. She had no cough, dyspnea, chest pain, or fevers at her follow-up 14 days after discharge. On a 6-week, postdischarge phone call, she continued to report no symptoms, and she maintained abstinence from cigarette smoking.
This case highlights that the very best test in any medical situation is a thorough, detailed history and physical examination. A comprehensive history with physical examination is noninvasive, safe, and cheap. Had the history of fine-dust exposure been known, it is likely that a great deal of testing and money would have been saved. The patient would have been diagnosed and treated earlier, and suffered less.
COMMENTARY
First described in 1989,1,2 AEP is an uncommon cause of acute respiratory failure. Cases have been reported throughout the world, including in the United States, Belgium, Japan, and Iraq.2,3 AEP is an acute febrile illness with cough, chest pain, and dyspnea for fewer than 7 days, diffuse pulmonary infiltrates on chest radiograph, hypoxemia, no history of asthma or atopic disease, no infection, and greater than 25% eosinophils on a BAL.1,3 Physical examination typically reveals fever, tachypnea, and crackles on auscultation.1 Peripheral blood eosinophilia is inconsistently seen at presentation but generally observed as the disease progresses.1 Peripheral eosinophilia at presentation is positively correlated with a milder course of AEP, including higher oxygen saturation and fewer intensive care admissions.4 Acute respiratory failure in AEP progresses rapidly, often within hours.1 Delayed recognition of AEP may lead to respiratory failure, requiring intubation, and even to death.1
Reticular markings with Kerley-B lines, mixed reticular and alveolar infiltrates, and pleural effusions are usually found on chest radiography.1 Bilateral areas of ground-glass attenuation, interlobular septal thickening, bronchovascular bundle thickening, and pleural effusions are seen on chest CT.5 Marked eosinophilic infiltration of the interstitium and alveolar spaces, as well as diffuse alveolar damage with hyaline membrane fibroblast proliferation and inflammatory cells, are present on lung biopsy.1 Restriction with impaired diffusion capacity is found on PFTs. However, PFTs return to normal after recovery.1
AEP is distinguished from other pulmonary diseases by BAL, lung biopsy, symptoms, symptom course, and/or radiographically. AEP is often misdiagnosed as severe community-acquired pneumonia and/or acute respiratory distress syndrome, as AEP tends to occur in previously healthy individuals who have diffuse infiltrates on chest radiograph, fevers, and acute, often severe, respiratory symptoms.1-3 Other eosinophilic lung diseases to rule out include simple pulmonary eosinophilia, chronic eosinophilic pneumonia, eosinophilic granulomatosis with polyangitis (Churg-Strauss), idiopathic hypereosinophilic syndrome, infection, and drug reactions.1,3,5 Simple eosinophilic pneumonia is characterized by no symptoms or very mild pulmonary symptoms and transient patchy infiltrates on radiography.3,5 Patients with simple pulmonary eosinophilia do not have interlobular septal thickening, thickening of the bronchovascular bundles, or pleural effusions radiographically, as seen with AEP.5 Chronic eosinophilic pneumonia is subacute, with respiratory symptoms of more than 3 months in duration, in contrast with the 7 days of respiratory symptoms for AEP, and is also not associated with interlobular septal thickening, thickening of the bronchovascular bundles, or pleural effusions on radiography.3,5 Unlike AEP, chronic eosinophilic pneumonia often recurs after the course of steroids has ended.3 In contrast with AEP, eosinophilic granulomatosis with polyangitis is associated with concomitant asthma and the involvement of nonpulmonary organs.3 Idiopathic hypereosinophilic syndrome is characterized by extremely high peripheral eosinophilia and by eosinophilic involvement of multiple organs, and it requires chronic steroid use.3 Patients with allergic bronchopulmonary aspergillosis (ABPA), in contrast with AEP, typically have steroid-dependent asthma and chronic respiratory symptoms.3 ABPA also differs from AEP in that radiographic infiltrates are localized and transient, and the syndrome may relapse after steroid treatment.3 Other infectious etiologies that may present similarly to AEP include invasive pulmonary aspergillosis, pulmonary coccidiodomycosis, Pneumocystis jioveri pneumonia, pulmonary toxocariasis, pulmonary filariasis, paragonimiasis, and Loeffler syndrome (pneumonia due to Strongyloides, Ascaris, or hookworms), highlighting the importance of a thorough travel and exposure history.1,3 Several drugs may cause eosinophilic lung disease, including nitrofurantoin, tetracyclines, phenytoin, L-tryptophan, acetaminophen, ampicillin, heroin, and cocaine, which necessitates a thorough review of medication and illegal drug use.3
Steroids and supportive care are the treatment of choice for AEP, although spontaneous resolution has been seen.1,3 Significant clinical improvement occurs within 24 to 48 hours of steroid initiation.1,3 Optimal dose and duration of therapy have not been determined; however, methylprednisolone 125 mg intravenously every 6 hours until improvement is an often-used option.1 Tapers vary from 2 to 12 weeks with no difference in outcome.1-3 AEP does not recur after appropriate treatment with steroids.1,3
Little is known about the etiology of AEP. It usually occurs in young, healthy individuals and is presumed to be an unusual, acute hypersensitivity reaction to an inhaled allergen.1 A report of 18 US soldiers deployed in or near Iraq proposed dust exposure and cigarette or cigar smoking as a cause of AEP.2 Similar to our patient’s fine-dust exposure and recent onset of cigarette smoking, the soldiers were exposed to the dusty, arid environment for at least 1 day and had been smoking for at least 1 month.2 The authors proposed that small dust particles irritate alveoli, stimulating eosinophils, which are exacerbated by the onset of smoking. Alternatively, cigarette smoke may prime the lung such that dust triggers an inflammatory cascade, resulting in AEP.
TEACHING POINTS
- With the potential for the rapid progression of respiratory failure, it is imperative that the diagnosis of AEP be considered for a patient with diffuse infiltrates on a chest radiograph and acute respiratory failure of unknown cause.
- A thorough history of exposure is key to including AEP in the differential of acute pulmonary disease, with recent-onset cigarette smoking and dust exposure.
- The rapid initiation of steroids leads to a full recovery without recurrence and may be life-saving in AEP.
Disclosure
The authors report no conflicts of interest.
1. Allen J. Acute eosinophilic pneumonia. Semin Respir Crit Care Med. 2006;27:142-147. PubMed
2. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997-3005. PubMed
3. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75(6):334-342. PubMed
4. Jhun BW, Kim SJ, Kim K, Lee JE. Clinical implications of initial peripheral eosinophilia in acute eosinophilic pneumonia. Respirology. 2014;19:1059-1065. PubMed
5. Daimon T, Johkoh T, Sumikawa H, et al. Acute eosinophilic pneumonia: Thin-section CT findings in 29 patients. Eur J Radiol. 2008;65:462-467. PubMed
A 52-year-old woman presented with a 4-day history of progressive dyspnea, nonproductive cough, pleuritic chest pain, and subjective fevers. She described dyspnea at rest, which worsened with exertion. She reported no chills, night sweats, weight change, wheezing, hemoptysis, orthopnea, lower extremity edema, or nasal congestion. She also denied myalgia, arthralgia, or joint swelling. She reported no rash, itching, or peripheral lymphadenopathy. She had no seasonal allergies. She was treated for presumed bronchitis with azithromycin by her primary care provider 4 days prior to presentation but experienced progressive dyspnea.
The constellation of dry cough, fever, and dyspnea is often infectious in origin, with the nonproductive, dry cough more suggestive of a viral than bacterial syndrome. Atypical organisms such as Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae may also present with these symptoms. Noninfectious etiologies should also be considered, including pulmonary embolism, systemic lupus erythematosus, asbestosis, hypersensitivity pneumonitis, sarcoidosis, and lung cancer. The dyspnea at rest stands out as a worrisome feature, as it implies hypoxia; therefore, an oxygen saturation is necessary to quickly determine her peripheral oxygen saturation.
Her past medical history was notable for lung adenocarcinoma, for which she had undergone right upper lobectomy, without chemotherapy or radiation, 13 years ago without recurrence. She had no history of chronic obstructive pulmonary disease, asthma, or pneumonia, nor a family history of chronic obstructive pulmonary disease, asthma, pneumonia, or lung cancer. Her only medication was azithromycin. She drank alcohol on occasion and denied illicit drug use. Three weeks prior to admission, she began smoking 4 to 5 cigarettes per day after 13 years of abstinence. Her smoking history prior to abstinence was 1 pack per day for 20 years. She worked as a department store remodeler; she had no exposure to asbestos, mold, or water-damaged wood. She reported no recent travel, sick contacts, or exposure to animals.
A primary lung neoplasm with a pleural effusion could cause her shortness of breath and pleuritic chest pain. Her history of lung cancer at age 39 raises the possibility of recurrence. For cigarette smokers, a second lung cancer may occur many years after the first diagnosis and treatment, even if they have quit smoking. A review of her original cancer records is essential to confirm the diagnosis of pulmonary adenocarcinoma. What is now being described as pulmonary adenocarcinoma may have been a metastatic lesion arising from outside the lung. Although unlikely, a primary adenocarcinoma may remain active.
Infectious etiologies continue to merit consideration. A parapneumonic effusion from a pneumonia or an empyema are consistent with her symptoms. Systemic lupus erythematosus can cause lung disease with pleural effusions. She does exhibit dyspnea and pleurisy, which are consistent with autoimmune disease, but does not exhibit some of the more typical autoimmune symptoms such as arthralgias, joint swelling, and rash. Pneumothorax could also produce her symptoms; however, pneumothorax usually occurs spontaneously in younger patients or after trauma or a procedure. Remote right upper lobectomy would not be a cause of pneumothorax now. Her reported history makes lung disease or pneumoconiosis due to occupational exposure to mold or aspergillosis a possibility. Legionellosis, histoplasmosis, or coccidioidomycosis should be considered if she lives in or has visited a high-risk area. Pulmonary embolism remains a concern for all patients with new-onset shortness of breath. Decision support tools, such as the Wells criteria, are valuable, but the gestalt of the physician does not lag far behind in accuracy.
Cardiac disease is also in the differential. Bibasilar crackles, third heart sound gallop, and jugular vein distension would suggest heart failure. A pericardial friction rub would be highly suggestive of pericarditis. A paradoxical pulse would raise concern for pericardial tamponade. Pleurisy may be associated with a pericardial effusion, making viral pericarditis and myocarditis possibilities.
She was in moderate distress with tachypnea and increased work of breathing. Her temperature was 36.7°C, heart rate 104 beats per minute, respiratory rate 24 breaths per minute, oxygen saturation was 88% on room air, 94% on 3 liters of oxygen, and blood pressure was 147/61 mmHg. Auscultation of the lungs revealed bibasilar crackles and decreased breath sounds at the bases. She was tachycardic, with a regular rhythm and no appreciable murmurs, rubs, or gallops. There was no jugular venous distention or lower extremity edema. Her thyroid was palpable, without appreciation of nodules. Skin and musculoskeletal examinations were normal.
Unless she is immunocompromised, infection has become lower in the differential, as she is afebrile. Decreased breath sounds at the bases and bibasilar crackles may be due to pleural effusions. Congestive heart failure is a possibility, especially given her dyspnea and bibasilar crackles. Volume overload from renal failure is possible, but she does not have other signs of volume overload such as lower extremity edema or jugular venous distension. It is important to note that crackles may be due to other etiologies, including atelectasis, fibrosis, or pneumonia. Pulmonary embolism may cause hypoxia, tachycardia, and pleural effusions. Additional diseases may present similarly, including human immunodeficiency virus with Pneumocystis jirovecii, causing dyspnea, tachypnea, and tachycardia; hematologic malignancy with anemia, causing dyspnea and tachycardia; and thyrotoxic states with thyromegaly, causing dyspnea and tachycardia. Thyroid storm patients appear in distress, are tachycardic, and may have thyromegaly.
Moderate distress, increased work of breathing, tachycardia, tachypnea, and hypoxia are all worrisome signs. Her temperature is subnormal, although this may not be accurate, as oral temperatures may register lower in patients with increased respiratory rates because of increased air flow across the thermometer. Bibasilar crackles with decreased bibasilar sounds require further investigation. A complete blood count, complete metabolic profile, troponin, arterial blood gas (ABG), electrocardiogram (ECG), and chest radiograph are warranted.
Laboratory studies revealed a white blood cell count of 8600 per mm3 with 11% bands and 7.3% eosinophils, and a hemoglobin count of 15 gm/dL. Basic metabolic panel, liver function tests, coagulation panel, and urinalysis were within normal limits, including serum creatinine 0.7 mg/dL, sodium 143 mmoL/L, chloride 104 mmoL/L, bicarbonate 30 mEq/L, anion gap 9 mmoL/L, and blood urea nitrogen 12 mg/dL. Chest radiograph disclosed diffusely increased interstitial markings and a small left pleural effusion (Figure 1).
Her bandemia suggests infection. Stress can cause a leukocytosis by demargination of mature white blood cells; however, stress does not often cause immature cells such as bands to appear. Her chest radiograph with diffuse interstitial markings is consistent with a community-acquired pneumonia. Empiric antibiotic therapy should be initiated because of the possibility of community-acquired pneumonia. Recent studies demonstrate that steroids decrease mortality, the need for mechanical ventilation, and the length of stay for patients hospitalized with community-acquired pneumonia; therefore, this patient should also be treated with steroids.
Eosinophilia may be seen in drug reactions, allergies, pulmonary emboli, pleural effusions, and occasionally in malignancy. Eosinophilic pneumonia typically has the “reverse pulmonary edema” picture, with infiltrates in the periphery and not centrally, as in congestive heart failure.
A serum bicarbonate of 30 mEq/L suggests a metabolic compensation for a chronic respiratory acidosis as renal compensation, and rise in bicarbonate generally takes 3 days. She may have been hypoxic longer than her symptoms suggest.
An ABG should be ordered to determine the degree of hypoxia and whether a higher level of care is indicated. The abnormal chest radiograph, along with her hypoxia, merits a closer look at her lung parenchyma with chest computed tomography (CT). A D-dimer would be beneficial to rule out pulmonary embolism. If the D-dimer is positive, chest CT with contrast is indicated to determine if a pulmonary embolism is present. A brain natriuretic peptide would assist in the diagnosis of congestive heart failure. A sputum culture and Gram stain and respiratory viral panel may establish a pathogen for pneumonia. An ECG and troponin to rule out myocardial infarction should be performed as well.
The presence of hilar and subcarinal lymph nodes expands the differential. Stage IV pulmonary sarcoid may present with diffuse infiltrates and nodes, although the acuity in this case makes it less likely. A very aggressive malignancy such as Burkitt lymphoma may have these findings. Acute viral and atypical pneumonias remain possible. Right middle lobe syndrome may cause partial collapse of the right middle lobe. Tuberculosis can be associated with right middle lobe syndrome; however, in this day and age an obstructing mass is more likely the cause. Pulmonary disease, such as cryptogenic organizing pneumonia, idiopathic pulmonary fibrosis, and interstitial lung disease, should be considered in patients with pneumonia unresponsive to antibiotics. Lung biopsy and bronchoalveolar lavage (BAL) would help make the diagnosis and should be the next step, unless her degree of hypoxia is prohibitive. Similarly, thoracentesis with analysis of the pleural fluid for cell count, Gram stain, and culture may help make the diagnosis. Thoracentesis should be done with fluoroscopic guidance, given the risk of pneumothorax, which would further compromise her tenuous respiratory status.
Thoracentesis was attempted, but the pleural effusion was too small to provide a sample. Subsequent serum blood counts with differential showed an increased eosinophilia to 20% and resolved bandemia. Upon further questioning, she recalled several months of extensive, daily, fine-dust exposure from demolition during the remodeling of a new building.
Hypereosinophilia and pulmonary infiltrates narrow the differential considerably to include asthma; parasitic infection, such as the pulmonary phase of ascariasis; exposure, such as to dust, cigarettes, or asbestosis; or hypereosinophilic syndromes characterized by peripheral eosinophilia, along with a tissue eosinophilia, causing organ dysfunction. Idiopathic hypereosinophilic syndrome, a hypereosinophilic syndrome of unknown etiology despite extensive diagnostic testing, is rare, and eosinophilic leukemia even rarer. Her history strongly suggests exposure. Many eosinophilic diseases respond rapidly to steroids, and response to treatment would help narrow the diagnosis. If she does not respond to steroids, a lung and/or bone marrow biopsy would be the next step.
A BAL of the right middle lobe revealed 51% eosinophils, 3% neutrophils, 15% macrophages, and 28% lymphocytes. Gram stain, as well as cultures for bacteria, acid fast bacilli, fungus, herpes simplex virus, and cytomegalovirus cultures, were negative. Transbronchial lung biopsy revealed focal interstitial fibrosis and inflammation, without evidence of infection.
Eosinophils are primarily located in tissues; therefore, peripheral blood eosinophil counts often underestimate the degree of infiltration into end organs such as the lung. With 50% eosinophils, her BAL reflects this. Mold, fungus, chemical, and particle exposure could produce an eosinophilic BAL. She does not appear to be at risk for parasitic exposure. Eosinophilic granulomatosis (previously known as Churg-Strauss) is a consideration, but the lack of signs of vasculitis and wheezing make this less likely. A negative antineutrophil cytoplasmic antibody may provide reassurance. “Fine dust exposure” is consistent with environmental exposure but not a specific antigen. Steroids provide a brisk eosinophil reduction and are appropriate for this patient. There is the possibility of missing infectious or parasitic etiologies; therefore, a culture of BAL fluid should be sent.
Eosinophilic infiltration may lead to fibrosis, as was found on the lung biopsy. She should be counseled to avoid “fine dust exposure” in the future. Follow-up lung imaging and pulmonary function tests (PFTs) should be performed once her acute illness resolves. She should be strongly urged not to smoke tobacco. Interestingly, there are reports that ex-smokers who restart smoking have an increased risk of eosinophilic pneumonia, but in this case dust exposure is the more likely etiology.
She was diagnosed with acute eosinophilic pneumonia (AEP). Antibiotics were discontinued, and oral prednisone was initiated at 40 mg daily, with a brisk response and resolution of her dyspnea. She was discharged with a 6-week prednisone taper. She had no cough, dyspnea, chest pain, or fevers at her follow-up 14 days after discharge. On a 6-week, postdischarge phone call, she continued to report no symptoms, and she maintained abstinence from cigarette smoking.
This case highlights that the very best test in any medical situation is a thorough, detailed history and physical examination. A comprehensive history with physical examination is noninvasive, safe, and cheap. Had the history of fine-dust exposure been known, it is likely that a great deal of testing and money would have been saved. The patient would have been diagnosed and treated earlier, and suffered less.
COMMENTARY
First described in 1989,1,2 AEP is an uncommon cause of acute respiratory failure. Cases have been reported throughout the world, including in the United States, Belgium, Japan, and Iraq.2,3 AEP is an acute febrile illness with cough, chest pain, and dyspnea for fewer than 7 days, diffuse pulmonary infiltrates on chest radiograph, hypoxemia, no history of asthma or atopic disease, no infection, and greater than 25% eosinophils on a BAL.1,3 Physical examination typically reveals fever, tachypnea, and crackles on auscultation.1 Peripheral blood eosinophilia is inconsistently seen at presentation but generally observed as the disease progresses.1 Peripheral eosinophilia at presentation is positively correlated with a milder course of AEP, including higher oxygen saturation and fewer intensive care admissions.4 Acute respiratory failure in AEP progresses rapidly, often within hours.1 Delayed recognition of AEP may lead to respiratory failure, requiring intubation, and even to death.1
Reticular markings with Kerley-B lines, mixed reticular and alveolar infiltrates, and pleural effusions are usually found on chest radiography.1 Bilateral areas of ground-glass attenuation, interlobular septal thickening, bronchovascular bundle thickening, and pleural effusions are seen on chest CT.5 Marked eosinophilic infiltration of the interstitium and alveolar spaces, as well as diffuse alveolar damage with hyaline membrane fibroblast proliferation and inflammatory cells, are present on lung biopsy.1 Restriction with impaired diffusion capacity is found on PFTs. However, PFTs return to normal after recovery.1
AEP is distinguished from other pulmonary diseases by BAL, lung biopsy, symptoms, symptom course, and/or radiographically. AEP is often misdiagnosed as severe community-acquired pneumonia and/or acute respiratory distress syndrome, as AEP tends to occur in previously healthy individuals who have diffuse infiltrates on chest radiograph, fevers, and acute, often severe, respiratory symptoms.1-3 Other eosinophilic lung diseases to rule out include simple pulmonary eosinophilia, chronic eosinophilic pneumonia, eosinophilic granulomatosis with polyangitis (Churg-Strauss), idiopathic hypereosinophilic syndrome, infection, and drug reactions.1,3,5 Simple eosinophilic pneumonia is characterized by no symptoms or very mild pulmonary symptoms and transient patchy infiltrates on radiography.3,5 Patients with simple pulmonary eosinophilia do not have interlobular septal thickening, thickening of the bronchovascular bundles, or pleural effusions radiographically, as seen with AEP.5 Chronic eosinophilic pneumonia is subacute, with respiratory symptoms of more than 3 months in duration, in contrast with the 7 days of respiratory symptoms for AEP, and is also not associated with interlobular septal thickening, thickening of the bronchovascular bundles, or pleural effusions on radiography.3,5 Unlike AEP, chronic eosinophilic pneumonia often recurs after the course of steroids has ended.3 In contrast with AEP, eosinophilic granulomatosis with polyangitis is associated with concomitant asthma and the involvement of nonpulmonary organs.3 Idiopathic hypereosinophilic syndrome is characterized by extremely high peripheral eosinophilia and by eosinophilic involvement of multiple organs, and it requires chronic steroid use.3 Patients with allergic bronchopulmonary aspergillosis (ABPA), in contrast with AEP, typically have steroid-dependent asthma and chronic respiratory symptoms.3 ABPA also differs from AEP in that radiographic infiltrates are localized and transient, and the syndrome may relapse after steroid treatment.3 Other infectious etiologies that may present similarly to AEP include invasive pulmonary aspergillosis, pulmonary coccidiodomycosis, Pneumocystis jioveri pneumonia, pulmonary toxocariasis, pulmonary filariasis, paragonimiasis, and Loeffler syndrome (pneumonia due to Strongyloides, Ascaris, or hookworms), highlighting the importance of a thorough travel and exposure history.1,3 Several drugs may cause eosinophilic lung disease, including nitrofurantoin, tetracyclines, phenytoin, L-tryptophan, acetaminophen, ampicillin, heroin, and cocaine, which necessitates a thorough review of medication and illegal drug use.3
Steroids and supportive care are the treatment of choice for AEP, although spontaneous resolution has been seen.1,3 Significant clinical improvement occurs within 24 to 48 hours of steroid initiation.1,3 Optimal dose and duration of therapy have not been determined; however, methylprednisolone 125 mg intravenously every 6 hours until improvement is an often-used option.1 Tapers vary from 2 to 12 weeks with no difference in outcome.1-3 AEP does not recur after appropriate treatment with steroids.1,3
Little is known about the etiology of AEP. It usually occurs in young, healthy individuals and is presumed to be an unusual, acute hypersensitivity reaction to an inhaled allergen.1 A report of 18 US soldiers deployed in or near Iraq proposed dust exposure and cigarette or cigar smoking as a cause of AEP.2 Similar to our patient’s fine-dust exposure and recent onset of cigarette smoking, the soldiers were exposed to the dusty, arid environment for at least 1 day and had been smoking for at least 1 month.2 The authors proposed that small dust particles irritate alveoli, stimulating eosinophils, which are exacerbated by the onset of smoking. Alternatively, cigarette smoke may prime the lung such that dust triggers an inflammatory cascade, resulting in AEP.
TEACHING POINTS
- With the potential for the rapid progression of respiratory failure, it is imperative that the diagnosis of AEP be considered for a patient with diffuse infiltrates on a chest radiograph and acute respiratory failure of unknown cause.
- A thorough history of exposure is key to including AEP in the differential of acute pulmonary disease, with recent-onset cigarette smoking and dust exposure.
- The rapid initiation of steroids leads to a full recovery without recurrence and may be life-saving in AEP.
Disclosure
The authors report no conflicts of interest.
A 52-year-old woman presented with a 4-day history of progressive dyspnea, nonproductive cough, pleuritic chest pain, and subjective fevers. She described dyspnea at rest, which worsened with exertion. She reported no chills, night sweats, weight change, wheezing, hemoptysis, orthopnea, lower extremity edema, or nasal congestion. She also denied myalgia, arthralgia, or joint swelling. She reported no rash, itching, or peripheral lymphadenopathy. She had no seasonal allergies. She was treated for presumed bronchitis with azithromycin by her primary care provider 4 days prior to presentation but experienced progressive dyspnea.
The constellation of dry cough, fever, and dyspnea is often infectious in origin, with the nonproductive, dry cough more suggestive of a viral than bacterial syndrome. Atypical organisms such as Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae may also present with these symptoms. Noninfectious etiologies should also be considered, including pulmonary embolism, systemic lupus erythematosus, asbestosis, hypersensitivity pneumonitis, sarcoidosis, and lung cancer. The dyspnea at rest stands out as a worrisome feature, as it implies hypoxia; therefore, an oxygen saturation is necessary to quickly determine her peripheral oxygen saturation.
Her past medical history was notable for lung adenocarcinoma, for which she had undergone right upper lobectomy, without chemotherapy or radiation, 13 years ago without recurrence. She had no history of chronic obstructive pulmonary disease, asthma, or pneumonia, nor a family history of chronic obstructive pulmonary disease, asthma, pneumonia, or lung cancer. Her only medication was azithromycin. She drank alcohol on occasion and denied illicit drug use. Three weeks prior to admission, she began smoking 4 to 5 cigarettes per day after 13 years of abstinence. Her smoking history prior to abstinence was 1 pack per day for 20 years. She worked as a department store remodeler; she had no exposure to asbestos, mold, or water-damaged wood. She reported no recent travel, sick contacts, or exposure to animals.
A primary lung neoplasm with a pleural effusion could cause her shortness of breath and pleuritic chest pain. Her history of lung cancer at age 39 raises the possibility of recurrence. For cigarette smokers, a second lung cancer may occur many years after the first diagnosis and treatment, even if they have quit smoking. A review of her original cancer records is essential to confirm the diagnosis of pulmonary adenocarcinoma. What is now being described as pulmonary adenocarcinoma may have been a metastatic lesion arising from outside the lung. Although unlikely, a primary adenocarcinoma may remain active.
Infectious etiologies continue to merit consideration. A parapneumonic effusion from a pneumonia or an empyema are consistent with her symptoms. Systemic lupus erythematosus can cause lung disease with pleural effusions. She does exhibit dyspnea and pleurisy, which are consistent with autoimmune disease, but does not exhibit some of the more typical autoimmune symptoms such as arthralgias, joint swelling, and rash. Pneumothorax could also produce her symptoms; however, pneumothorax usually occurs spontaneously in younger patients or after trauma or a procedure. Remote right upper lobectomy would not be a cause of pneumothorax now. Her reported history makes lung disease or pneumoconiosis due to occupational exposure to mold or aspergillosis a possibility. Legionellosis, histoplasmosis, or coccidioidomycosis should be considered if she lives in or has visited a high-risk area. Pulmonary embolism remains a concern for all patients with new-onset shortness of breath. Decision support tools, such as the Wells criteria, are valuable, but the gestalt of the physician does not lag far behind in accuracy.
Cardiac disease is also in the differential. Bibasilar crackles, third heart sound gallop, and jugular vein distension would suggest heart failure. A pericardial friction rub would be highly suggestive of pericarditis. A paradoxical pulse would raise concern for pericardial tamponade. Pleurisy may be associated with a pericardial effusion, making viral pericarditis and myocarditis possibilities.
She was in moderate distress with tachypnea and increased work of breathing. Her temperature was 36.7°C, heart rate 104 beats per minute, respiratory rate 24 breaths per minute, oxygen saturation was 88% on room air, 94% on 3 liters of oxygen, and blood pressure was 147/61 mmHg. Auscultation of the lungs revealed bibasilar crackles and decreased breath sounds at the bases. She was tachycardic, with a regular rhythm and no appreciable murmurs, rubs, or gallops. There was no jugular venous distention or lower extremity edema. Her thyroid was palpable, without appreciation of nodules. Skin and musculoskeletal examinations were normal.
Unless she is immunocompromised, infection has become lower in the differential, as she is afebrile. Decreased breath sounds at the bases and bibasilar crackles may be due to pleural effusions. Congestive heart failure is a possibility, especially given her dyspnea and bibasilar crackles. Volume overload from renal failure is possible, but she does not have other signs of volume overload such as lower extremity edema or jugular venous distension. It is important to note that crackles may be due to other etiologies, including atelectasis, fibrosis, or pneumonia. Pulmonary embolism may cause hypoxia, tachycardia, and pleural effusions. Additional diseases may present similarly, including human immunodeficiency virus with Pneumocystis jirovecii, causing dyspnea, tachypnea, and tachycardia; hematologic malignancy with anemia, causing dyspnea and tachycardia; and thyrotoxic states with thyromegaly, causing dyspnea and tachycardia. Thyroid storm patients appear in distress, are tachycardic, and may have thyromegaly.
Moderate distress, increased work of breathing, tachycardia, tachypnea, and hypoxia are all worrisome signs. Her temperature is subnormal, although this may not be accurate, as oral temperatures may register lower in patients with increased respiratory rates because of increased air flow across the thermometer. Bibasilar crackles with decreased bibasilar sounds require further investigation. A complete blood count, complete metabolic profile, troponin, arterial blood gas (ABG), electrocardiogram (ECG), and chest radiograph are warranted.
Laboratory studies revealed a white blood cell count of 8600 per mm3 with 11% bands and 7.3% eosinophils, and a hemoglobin count of 15 gm/dL. Basic metabolic panel, liver function tests, coagulation panel, and urinalysis were within normal limits, including serum creatinine 0.7 mg/dL, sodium 143 mmoL/L, chloride 104 mmoL/L, bicarbonate 30 mEq/L, anion gap 9 mmoL/L, and blood urea nitrogen 12 mg/dL. Chest radiograph disclosed diffusely increased interstitial markings and a small left pleural effusion (Figure 1).
Her bandemia suggests infection. Stress can cause a leukocytosis by demargination of mature white blood cells; however, stress does not often cause immature cells such as bands to appear. Her chest radiograph with diffuse interstitial markings is consistent with a community-acquired pneumonia. Empiric antibiotic therapy should be initiated because of the possibility of community-acquired pneumonia. Recent studies demonstrate that steroids decrease mortality, the need for mechanical ventilation, and the length of stay for patients hospitalized with community-acquired pneumonia; therefore, this patient should also be treated with steroids.
Eosinophilia may be seen in drug reactions, allergies, pulmonary emboli, pleural effusions, and occasionally in malignancy. Eosinophilic pneumonia typically has the “reverse pulmonary edema” picture, with infiltrates in the periphery and not centrally, as in congestive heart failure.
A serum bicarbonate of 30 mEq/L suggests a metabolic compensation for a chronic respiratory acidosis as renal compensation, and rise in bicarbonate generally takes 3 days. She may have been hypoxic longer than her symptoms suggest.
An ABG should be ordered to determine the degree of hypoxia and whether a higher level of care is indicated. The abnormal chest radiograph, along with her hypoxia, merits a closer look at her lung parenchyma with chest computed tomography (CT). A D-dimer would be beneficial to rule out pulmonary embolism. If the D-dimer is positive, chest CT with contrast is indicated to determine if a pulmonary embolism is present. A brain natriuretic peptide would assist in the diagnosis of congestive heart failure. A sputum culture and Gram stain and respiratory viral panel may establish a pathogen for pneumonia. An ECG and troponin to rule out myocardial infarction should be performed as well.
The presence of hilar and subcarinal lymph nodes expands the differential. Stage IV pulmonary sarcoid may present with diffuse infiltrates and nodes, although the acuity in this case makes it less likely. A very aggressive malignancy such as Burkitt lymphoma may have these findings. Acute viral and atypical pneumonias remain possible. Right middle lobe syndrome may cause partial collapse of the right middle lobe. Tuberculosis can be associated with right middle lobe syndrome; however, in this day and age an obstructing mass is more likely the cause. Pulmonary disease, such as cryptogenic organizing pneumonia, idiopathic pulmonary fibrosis, and interstitial lung disease, should be considered in patients with pneumonia unresponsive to antibiotics. Lung biopsy and bronchoalveolar lavage (BAL) would help make the diagnosis and should be the next step, unless her degree of hypoxia is prohibitive. Similarly, thoracentesis with analysis of the pleural fluid for cell count, Gram stain, and culture may help make the diagnosis. Thoracentesis should be done with fluoroscopic guidance, given the risk of pneumothorax, which would further compromise her tenuous respiratory status.
Thoracentesis was attempted, but the pleural effusion was too small to provide a sample. Subsequent serum blood counts with differential showed an increased eosinophilia to 20% and resolved bandemia. Upon further questioning, she recalled several months of extensive, daily, fine-dust exposure from demolition during the remodeling of a new building.
Hypereosinophilia and pulmonary infiltrates narrow the differential considerably to include asthma; parasitic infection, such as the pulmonary phase of ascariasis; exposure, such as to dust, cigarettes, or asbestosis; or hypereosinophilic syndromes characterized by peripheral eosinophilia, along with a tissue eosinophilia, causing organ dysfunction. Idiopathic hypereosinophilic syndrome, a hypereosinophilic syndrome of unknown etiology despite extensive diagnostic testing, is rare, and eosinophilic leukemia even rarer. Her history strongly suggests exposure. Many eosinophilic diseases respond rapidly to steroids, and response to treatment would help narrow the diagnosis. If she does not respond to steroids, a lung and/or bone marrow biopsy would be the next step.
A BAL of the right middle lobe revealed 51% eosinophils, 3% neutrophils, 15% macrophages, and 28% lymphocytes. Gram stain, as well as cultures for bacteria, acid fast bacilli, fungus, herpes simplex virus, and cytomegalovirus cultures, were negative. Transbronchial lung biopsy revealed focal interstitial fibrosis and inflammation, without evidence of infection.
Eosinophils are primarily located in tissues; therefore, peripheral blood eosinophil counts often underestimate the degree of infiltration into end organs such as the lung. With 50% eosinophils, her BAL reflects this. Mold, fungus, chemical, and particle exposure could produce an eosinophilic BAL. She does not appear to be at risk for parasitic exposure. Eosinophilic granulomatosis (previously known as Churg-Strauss) is a consideration, but the lack of signs of vasculitis and wheezing make this less likely. A negative antineutrophil cytoplasmic antibody may provide reassurance. “Fine dust exposure” is consistent with environmental exposure but not a specific antigen. Steroids provide a brisk eosinophil reduction and are appropriate for this patient. There is the possibility of missing infectious or parasitic etiologies; therefore, a culture of BAL fluid should be sent.
Eosinophilic infiltration may lead to fibrosis, as was found on the lung biopsy. She should be counseled to avoid “fine dust exposure” in the future. Follow-up lung imaging and pulmonary function tests (PFTs) should be performed once her acute illness resolves. She should be strongly urged not to smoke tobacco. Interestingly, there are reports that ex-smokers who restart smoking have an increased risk of eosinophilic pneumonia, but in this case dust exposure is the more likely etiology.
She was diagnosed with acute eosinophilic pneumonia (AEP). Antibiotics were discontinued, and oral prednisone was initiated at 40 mg daily, with a brisk response and resolution of her dyspnea. She was discharged with a 6-week prednisone taper. She had no cough, dyspnea, chest pain, or fevers at her follow-up 14 days after discharge. On a 6-week, postdischarge phone call, she continued to report no symptoms, and she maintained abstinence from cigarette smoking.
This case highlights that the very best test in any medical situation is a thorough, detailed history and physical examination. A comprehensive history with physical examination is noninvasive, safe, and cheap. Had the history of fine-dust exposure been known, it is likely that a great deal of testing and money would have been saved. The patient would have been diagnosed and treated earlier, and suffered less.
COMMENTARY
First described in 1989,1,2 AEP is an uncommon cause of acute respiratory failure. Cases have been reported throughout the world, including in the United States, Belgium, Japan, and Iraq.2,3 AEP is an acute febrile illness with cough, chest pain, and dyspnea for fewer than 7 days, diffuse pulmonary infiltrates on chest radiograph, hypoxemia, no history of asthma or atopic disease, no infection, and greater than 25% eosinophils on a BAL.1,3 Physical examination typically reveals fever, tachypnea, and crackles on auscultation.1 Peripheral blood eosinophilia is inconsistently seen at presentation but generally observed as the disease progresses.1 Peripheral eosinophilia at presentation is positively correlated with a milder course of AEP, including higher oxygen saturation and fewer intensive care admissions.4 Acute respiratory failure in AEP progresses rapidly, often within hours.1 Delayed recognition of AEP may lead to respiratory failure, requiring intubation, and even to death.1
Reticular markings with Kerley-B lines, mixed reticular and alveolar infiltrates, and pleural effusions are usually found on chest radiography.1 Bilateral areas of ground-glass attenuation, interlobular septal thickening, bronchovascular bundle thickening, and pleural effusions are seen on chest CT.5 Marked eosinophilic infiltration of the interstitium and alveolar spaces, as well as diffuse alveolar damage with hyaline membrane fibroblast proliferation and inflammatory cells, are present on lung biopsy.1 Restriction with impaired diffusion capacity is found on PFTs. However, PFTs return to normal after recovery.1
AEP is distinguished from other pulmonary diseases by BAL, lung biopsy, symptoms, symptom course, and/or radiographically. AEP is often misdiagnosed as severe community-acquired pneumonia and/or acute respiratory distress syndrome, as AEP tends to occur in previously healthy individuals who have diffuse infiltrates on chest radiograph, fevers, and acute, often severe, respiratory symptoms.1-3 Other eosinophilic lung diseases to rule out include simple pulmonary eosinophilia, chronic eosinophilic pneumonia, eosinophilic granulomatosis with polyangitis (Churg-Strauss), idiopathic hypereosinophilic syndrome, infection, and drug reactions.1,3,5 Simple eosinophilic pneumonia is characterized by no symptoms or very mild pulmonary symptoms and transient patchy infiltrates on radiography.3,5 Patients with simple pulmonary eosinophilia do not have interlobular septal thickening, thickening of the bronchovascular bundles, or pleural effusions radiographically, as seen with AEP.5 Chronic eosinophilic pneumonia is subacute, with respiratory symptoms of more than 3 months in duration, in contrast with the 7 days of respiratory symptoms for AEP, and is also not associated with interlobular septal thickening, thickening of the bronchovascular bundles, or pleural effusions on radiography.3,5 Unlike AEP, chronic eosinophilic pneumonia often recurs after the course of steroids has ended.3 In contrast with AEP, eosinophilic granulomatosis with polyangitis is associated with concomitant asthma and the involvement of nonpulmonary organs.3 Idiopathic hypereosinophilic syndrome is characterized by extremely high peripheral eosinophilia and by eosinophilic involvement of multiple organs, and it requires chronic steroid use.3 Patients with allergic bronchopulmonary aspergillosis (ABPA), in contrast with AEP, typically have steroid-dependent asthma and chronic respiratory symptoms.3 ABPA also differs from AEP in that radiographic infiltrates are localized and transient, and the syndrome may relapse after steroid treatment.3 Other infectious etiologies that may present similarly to AEP include invasive pulmonary aspergillosis, pulmonary coccidiodomycosis, Pneumocystis jioveri pneumonia, pulmonary toxocariasis, pulmonary filariasis, paragonimiasis, and Loeffler syndrome (pneumonia due to Strongyloides, Ascaris, or hookworms), highlighting the importance of a thorough travel and exposure history.1,3 Several drugs may cause eosinophilic lung disease, including nitrofurantoin, tetracyclines, phenytoin, L-tryptophan, acetaminophen, ampicillin, heroin, and cocaine, which necessitates a thorough review of medication and illegal drug use.3
Steroids and supportive care are the treatment of choice for AEP, although spontaneous resolution has been seen.1,3 Significant clinical improvement occurs within 24 to 48 hours of steroid initiation.1,3 Optimal dose and duration of therapy have not been determined; however, methylprednisolone 125 mg intravenously every 6 hours until improvement is an often-used option.1 Tapers vary from 2 to 12 weeks with no difference in outcome.1-3 AEP does not recur after appropriate treatment with steroids.1,3
Little is known about the etiology of AEP. It usually occurs in young, healthy individuals and is presumed to be an unusual, acute hypersensitivity reaction to an inhaled allergen.1 A report of 18 US soldiers deployed in or near Iraq proposed dust exposure and cigarette or cigar smoking as a cause of AEP.2 Similar to our patient’s fine-dust exposure and recent onset of cigarette smoking, the soldiers were exposed to the dusty, arid environment for at least 1 day and had been smoking for at least 1 month.2 The authors proposed that small dust particles irritate alveoli, stimulating eosinophils, which are exacerbated by the onset of smoking. Alternatively, cigarette smoke may prime the lung such that dust triggers an inflammatory cascade, resulting in AEP.
TEACHING POINTS
- With the potential for the rapid progression of respiratory failure, it is imperative that the diagnosis of AEP be considered for a patient with diffuse infiltrates on a chest radiograph and acute respiratory failure of unknown cause.
- A thorough history of exposure is key to including AEP in the differential of acute pulmonary disease, with recent-onset cigarette smoking and dust exposure.
- The rapid initiation of steroids leads to a full recovery without recurrence and may be life-saving in AEP.
Disclosure
The authors report no conflicts of interest.
1. Allen J. Acute eosinophilic pneumonia. Semin Respir Crit Care Med. 2006;27:142-147. PubMed
2. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997-3005. PubMed
3. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75(6):334-342. PubMed
4. Jhun BW, Kim SJ, Kim K, Lee JE. Clinical implications of initial peripheral eosinophilia in acute eosinophilic pneumonia. Respirology. 2014;19:1059-1065. PubMed
5. Daimon T, Johkoh T, Sumikawa H, et al. Acute eosinophilic pneumonia: Thin-section CT findings in 29 patients. Eur J Radiol. 2008;65:462-467. PubMed
1. Allen J. Acute eosinophilic pneumonia. Semin Respir Crit Care Med. 2006;27:142-147. PubMed
2. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997-3005. PubMed
3. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75(6):334-342. PubMed
4. Jhun BW, Kim SJ, Kim K, Lee JE. Clinical implications of initial peripheral eosinophilia in acute eosinophilic pneumonia. Respirology. 2014;19:1059-1065. PubMed
5. Daimon T, Johkoh T, Sumikawa H, et al. Acute eosinophilic pneumonia: Thin-section CT findings in 29 patients. Eur J Radiol. 2008;65:462-467. PubMed