User login
Predictors may help to spot risk for hydroxychloroquine nonadherence
who are newly prescribed the antimalarial, an administrative claims database study has revealed.
Investigators from Brigham and Women’s Hospital, Boston, used prescription refill data to assess nonadherence, hoping to gain greater insight into predictors of nonadherence to HCQ than past studies of nonadherence to the drug, which have been limited by their small and cross-sectional nature and the fact that they often relied on self-reported measures of adherence that were unable to capture the dynamic nature of adherence behavior over time.
In the current study, Candace H. Feldman, MD, ScD, and her coinvestigators used Medicaid data from 28 U.S. states to identify 10,406 adult HCQ initiators with SLE during 2001-2010. Patients included in the study were required to have more than 365 days of continuous follow-up documented.
The researchers described four distinctive monthly patterns of behavior during the first year of use, in which they defined nonadherence as less than 80% of days covered per month by a HCQ prescription. Group 1 comprised 36% who were “persistent nonadherers” who had very few HCQ refills after the initial dispensing.
Almost half of the cohort (47%) formed two dynamic patterns of partial adherence (groups 2 and 3). The trajectories for these groups were similar until month 5, when they diverged: group 3 improved slightly and then reached a plateau, whereas group 2 became nearly completely nonadherent for the remainder of follow-up. At this 5-month point of divergence, belonging to group 2 was more likely among patients with younger age and antidepressant use. Also, patients in group 3 had more hospitalizations beginning at 4 months and longer hospitalizations at 5 months.
For these two “undecided” groups, 5 months may be a “critical juncture” for physicians to intervene, the authors said.
“Five months might also be the point at which patients feel that they have adequately trialed the medication, and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial,” they wrote.
Group 4 had persistent adherence and constituted 17% of the cohort, although this group also experienced a decline in adherence at 9 months.
The mean age of group 4 was about 40 years, which was significantly older than 37 years in groups 1-3. Blacks comprised the highest percentage of patients in groups 1-3 (43%-45%), whereas whites at 40% were the highest proportion in group 4. Individuals in group 4 also had slightly higher average income than did group 1 (mean $46,000 vs. $44,000). The index for SLE risk adjustment was highest for group 4 patients (1.3 vs. 0.9-1.1 for other groups), indicating they may have had more SLE-related comorbidities. Group 4 patients also had a greater average number of medications dispensed and a higher mean daily prednisone-equivalent dose.
Patients aged 18-50 or with black race or Hispanic ethnicity were significantly more likely to be in one of the nonadherent trajectory groups (1, 2, and 3), whereas Asians were less likely to be in group 1 than in group 4, compared with whites.
Diabetes made patients more likely to belong to group 1 than group 4, whereas each unit increase in the SLE risk-adjustment index increased the odds of belonging to group 1 vs. 4. Antidepressant use was associated with greater likelihood of belong to groups 1 or 2 vs. group 4.
Addressing potentially modifiable factors such as ensuring sustained access to health care, particularly for patients with severe disease, might go some way to improving adherence, suggested the researchers, who also noted that increased counseling and support at the time of the first HCQ prescription and throughout the first year of use are also needed in order to “promote more sustained patterns of adherence for all patients.”
The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
SOURCE: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
who are newly prescribed the antimalarial, an administrative claims database study has revealed.
Investigators from Brigham and Women’s Hospital, Boston, used prescription refill data to assess nonadherence, hoping to gain greater insight into predictors of nonadherence to HCQ than past studies of nonadherence to the drug, which have been limited by their small and cross-sectional nature and the fact that they often relied on self-reported measures of adherence that were unable to capture the dynamic nature of adherence behavior over time.
In the current study, Candace H. Feldman, MD, ScD, and her coinvestigators used Medicaid data from 28 U.S. states to identify 10,406 adult HCQ initiators with SLE during 2001-2010. Patients included in the study were required to have more than 365 days of continuous follow-up documented.
The researchers described four distinctive monthly patterns of behavior during the first year of use, in which they defined nonadherence as less than 80% of days covered per month by a HCQ prescription. Group 1 comprised 36% who were “persistent nonadherers” who had very few HCQ refills after the initial dispensing.
Almost half of the cohort (47%) formed two dynamic patterns of partial adherence (groups 2 and 3). The trajectories for these groups were similar until month 5, when they diverged: group 3 improved slightly and then reached a plateau, whereas group 2 became nearly completely nonadherent for the remainder of follow-up. At this 5-month point of divergence, belonging to group 2 was more likely among patients with younger age and antidepressant use. Also, patients in group 3 had more hospitalizations beginning at 4 months and longer hospitalizations at 5 months.
For these two “undecided” groups, 5 months may be a “critical juncture” for physicians to intervene, the authors said.
“Five months might also be the point at which patients feel that they have adequately trialed the medication, and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial,” they wrote.
Group 4 had persistent adherence and constituted 17% of the cohort, although this group also experienced a decline in adherence at 9 months.
The mean age of group 4 was about 40 years, which was significantly older than 37 years in groups 1-3. Blacks comprised the highest percentage of patients in groups 1-3 (43%-45%), whereas whites at 40% were the highest proportion in group 4. Individuals in group 4 also had slightly higher average income than did group 1 (mean $46,000 vs. $44,000). The index for SLE risk adjustment was highest for group 4 patients (1.3 vs. 0.9-1.1 for other groups), indicating they may have had more SLE-related comorbidities. Group 4 patients also had a greater average number of medications dispensed and a higher mean daily prednisone-equivalent dose.
Patients aged 18-50 or with black race or Hispanic ethnicity were significantly more likely to be in one of the nonadherent trajectory groups (1, 2, and 3), whereas Asians were less likely to be in group 1 than in group 4, compared with whites.
Diabetes made patients more likely to belong to group 1 than group 4, whereas each unit increase in the SLE risk-adjustment index increased the odds of belonging to group 1 vs. 4. Antidepressant use was associated with greater likelihood of belong to groups 1 or 2 vs. group 4.
Addressing potentially modifiable factors such as ensuring sustained access to health care, particularly for patients with severe disease, might go some way to improving adherence, suggested the researchers, who also noted that increased counseling and support at the time of the first HCQ prescription and throughout the first year of use are also needed in order to “promote more sustained patterns of adherence for all patients.”
The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
SOURCE: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
who are newly prescribed the antimalarial, an administrative claims database study has revealed.
Investigators from Brigham and Women’s Hospital, Boston, used prescription refill data to assess nonadherence, hoping to gain greater insight into predictors of nonadherence to HCQ than past studies of nonadherence to the drug, which have been limited by their small and cross-sectional nature and the fact that they often relied on self-reported measures of adherence that were unable to capture the dynamic nature of adherence behavior over time.
In the current study, Candace H. Feldman, MD, ScD, and her coinvestigators used Medicaid data from 28 U.S. states to identify 10,406 adult HCQ initiators with SLE during 2001-2010. Patients included in the study were required to have more than 365 days of continuous follow-up documented.
The researchers described four distinctive monthly patterns of behavior during the first year of use, in which they defined nonadherence as less than 80% of days covered per month by a HCQ prescription. Group 1 comprised 36% who were “persistent nonadherers” who had very few HCQ refills after the initial dispensing.
Almost half of the cohort (47%) formed two dynamic patterns of partial adherence (groups 2 and 3). The trajectories for these groups were similar until month 5, when they diverged: group 3 improved slightly and then reached a plateau, whereas group 2 became nearly completely nonadherent for the remainder of follow-up. At this 5-month point of divergence, belonging to group 2 was more likely among patients with younger age and antidepressant use. Also, patients in group 3 had more hospitalizations beginning at 4 months and longer hospitalizations at 5 months.
For these two “undecided” groups, 5 months may be a “critical juncture” for physicians to intervene, the authors said.
“Five months might also be the point at which patients feel that they have adequately trialed the medication, and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial,” they wrote.
Group 4 had persistent adherence and constituted 17% of the cohort, although this group also experienced a decline in adherence at 9 months.
The mean age of group 4 was about 40 years, which was significantly older than 37 years in groups 1-3. Blacks comprised the highest percentage of patients in groups 1-3 (43%-45%), whereas whites at 40% were the highest proportion in group 4. Individuals in group 4 also had slightly higher average income than did group 1 (mean $46,000 vs. $44,000). The index for SLE risk adjustment was highest for group 4 patients (1.3 vs. 0.9-1.1 for other groups), indicating they may have had more SLE-related comorbidities. Group 4 patients also had a greater average number of medications dispensed and a higher mean daily prednisone-equivalent dose.
Patients aged 18-50 or with black race or Hispanic ethnicity were significantly more likely to be in one of the nonadherent trajectory groups (1, 2, and 3), whereas Asians were less likely to be in group 1 than in group 4, compared with whites.
Diabetes made patients more likely to belong to group 1 than group 4, whereas each unit increase in the SLE risk-adjustment index increased the odds of belonging to group 1 vs. 4. Antidepressant use was associated with greater likelihood of belong to groups 1 or 2 vs. group 4.
Addressing potentially modifiable factors such as ensuring sustained access to health care, particularly for patients with severe disease, might go some way to improving adherence, suggested the researchers, who also noted that increased counseling and support at the time of the first HCQ prescription and throughout the first year of use are also needed in order to “promote more sustained patterns of adherence for all patients.”
The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
SOURCE: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
FROM SEMINARS IN ARTHRITIS & RHEUMATISM
Key clinical point: Five months after the first HCQ prescription might be a critical time point to review and educate patients on the importance of continuing treatment, particularly for patients who are “partial” adherers.
Major finding: Almost half of the cohort (47%) formed two dynamic patterns of partial adherence.
Data source: A longitudinal study of 10,406 Medicaid beneficiaries with SLE who were prescribed hydroxychloroquine for the first time.
Disclosures: The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
Source: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
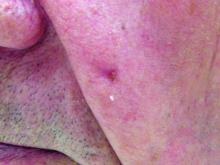
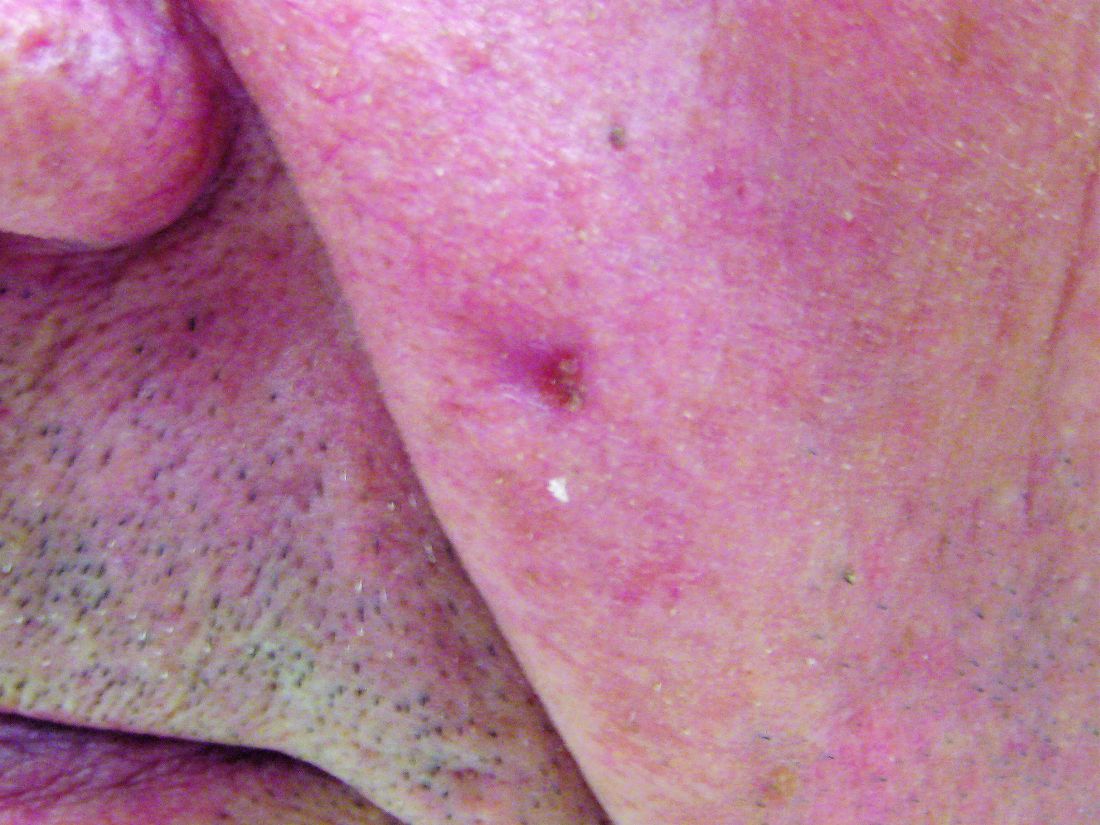
Hydrochlorothiazide use linked to higher skin cancer risk
The common diuretic hydrochlorothiazide is linked to a dose-dependent increased risk of nonmelanoma skin cancer, in particular, squamous cell carcinoma, a case-controlled registry study showed.
This nationwide, case-matched control study examined patients’ cumulative hydrochlorothiazide use between 1995 and 2012 and found a clear dose-response patterns for both basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), with a more than sevenfold increased risk of SCC for a cumulative use of greater than or equal to 200,000 mg of hydrochlorothiazide (HCTZ).
“Assuming causality, the present results suggest that 1 in 10 SCC cases diagnosed during the study period can be attributed to HCTZ use,” wrote the study authors, who were led by Sidsel Arnspang, MD, of the department of neurology at Odense (Denmark) University Hospital, which is affiliated with the University of Southern Denmark.
The authors noted that they previously had reported a sevenfold increased risk of lip squamous cell carcinoma with hydrochlorothiazide. Furthermore, the International Agency for Research on Cancer recently classified the diuretic and antihypertensive as “possibly carcinogenic to humans.”
“As HCTZ is among the most widely used drugs in the U.S. and Western Europe, a carcinogenic effect of HCTZ would have a considerable impact on public health,” they wrote in their paper, published in the Journal of American Academy of Dermatology.
According to the study authors, the few studies that have investigated a potential link between thiazide use and nonmelanoma skin cancer (NMSC) have reported inconsistent results.
They speculated that this could be because HCTZ often is prescribed in combination with other diuretics, and there may have been difficulties with disentangling its effect from those of the other drugs.
Using data from five nationwide data sources, the research team compared HCTZ use among people diagnosed with SCC or BCC of the skin to use among a matched control group without such cancers. People were excluded from the analysis if they had SCC of the lip because they had been evaluated in the research team’s previous study.
High use of HCTZ was defined as filled prescriptions totaling greater than or equal to 50,000 mg of HCTZ, which corresponds to greater than or equal to 2,000 defined daily doses (for example, approximately 6 years of cumulative use).
Overall, the study population involved 71,533 BCC and 8,629 SCC cases that were matched to 1,430,883 and 172,462 population controls, respectively.
Baseline characteristics of the cases and controls were similar, except BCC cases were slightly more educated than controls. Results showed that high use of hydrochlorothiazide was associated with odds ratios of 1.29 (95% confidence interval, 1.23-1.35) for BCC and 3.98 (95% CI, 3.68-4.31) for SCC.
A clear dose-response relationship was observed with HCTZ use for both BCC and SCC, with the highest ORs observed in the upper exposure category (greater than or equal to 200,000 mg): The OR for BCC in this category was 1.54 (95% CI, 1.38-1.71) and 7.38 for SCC (95% CI, 6.32-8.60).
The researchers observed no associations for BCC or SCC risk with use of other diuretics and other hypertensives, a finding that they said supported a potential causal association between HCTZ and NMSC risk.
Little variation was seen in the association between HCTZ use and BCC or SCC risk in the subgroup analyses, except for notably stronger associations among younger individuals and females.
In analyses stratified according to tumor localization, the authors saw stronger associations for cancers at sun-exposed skin sites, especially the skin of the lower limbs.
“Given the considerable use of HCTZ worldwide and the morbidity associated with NMSC, a causal association between HCTZ use and NMSC risk would have significant public health implications,” Dr. Arnspang and associates concluded. “The use of HCTZ should be carefully considered as several other antihypertensive agents with similar indications and efficiency are available but without known associations with skin cancer.”
The investigators cited several limitations. For example, information on ethnicity and skin type was not available. This information would have been useful in evaluating participants’ photosensitivity as a possible mechanism for a higher skin cancer risk with the use of HCTZ.
The study was funded by a grant from the Danish Cancer Society and the Danish Council of Independent Research. Several of the authors reported receiving grants and or honoraria from pharmaceutical companies.
SOURCE: Arnspang S et al. JAAD. 2017. doi: 10.1016/j.jaad.2017.11.042.
The common diuretic hydrochlorothiazide is linked to a dose-dependent increased risk of nonmelanoma skin cancer, in particular, squamous cell carcinoma, a case-controlled registry study showed.
This nationwide, case-matched control study examined patients’ cumulative hydrochlorothiazide use between 1995 and 2012 and found a clear dose-response patterns for both basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), with a more than sevenfold increased risk of SCC for a cumulative use of greater than or equal to 200,000 mg of hydrochlorothiazide (HCTZ).
“Assuming causality, the present results suggest that 1 in 10 SCC cases diagnosed during the study period can be attributed to HCTZ use,” wrote the study authors, who were led by Sidsel Arnspang, MD, of the department of neurology at Odense (Denmark) University Hospital, which is affiliated with the University of Southern Denmark.
The authors noted that they previously had reported a sevenfold increased risk of lip squamous cell carcinoma with hydrochlorothiazide. Furthermore, the International Agency for Research on Cancer recently classified the diuretic and antihypertensive as “possibly carcinogenic to humans.”
“As HCTZ is among the most widely used drugs in the U.S. and Western Europe, a carcinogenic effect of HCTZ would have a considerable impact on public health,” they wrote in their paper, published in the Journal of American Academy of Dermatology.
According to the study authors, the few studies that have investigated a potential link between thiazide use and nonmelanoma skin cancer (NMSC) have reported inconsistent results.
They speculated that this could be because HCTZ often is prescribed in combination with other diuretics, and there may have been difficulties with disentangling its effect from those of the other drugs.
Using data from five nationwide data sources, the research team compared HCTZ use among people diagnosed with SCC or BCC of the skin to use among a matched control group without such cancers. People were excluded from the analysis if they had SCC of the lip because they had been evaluated in the research team’s previous study.
High use of HCTZ was defined as filled prescriptions totaling greater than or equal to 50,000 mg of HCTZ, which corresponds to greater than or equal to 2,000 defined daily doses (for example, approximately 6 years of cumulative use).
Overall, the study population involved 71,533 BCC and 8,629 SCC cases that were matched to 1,430,883 and 172,462 population controls, respectively.
Baseline characteristics of the cases and controls were similar, except BCC cases were slightly more educated than controls. Results showed that high use of hydrochlorothiazide was associated with odds ratios of 1.29 (95% confidence interval, 1.23-1.35) for BCC and 3.98 (95% CI, 3.68-4.31) for SCC.
A clear dose-response relationship was observed with HCTZ use for both BCC and SCC, with the highest ORs observed in the upper exposure category (greater than or equal to 200,000 mg): The OR for BCC in this category was 1.54 (95% CI, 1.38-1.71) and 7.38 for SCC (95% CI, 6.32-8.60).
The researchers observed no associations for BCC or SCC risk with use of other diuretics and other hypertensives, a finding that they said supported a potential causal association between HCTZ and NMSC risk.
Little variation was seen in the association between HCTZ use and BCC or SCC risk in the subgroup analyses, except for notably stronger associations among younger individuals and females.
In analyses stratified according to tumor localization, the authors saw stronger associations for cancers at sun-exposed skin sites, especially the skin of the lower limbs.
“Given the considerable use of HCTZ worldwide and the morbidity associated with NMSC, a causal association between HCTZ use and NMSC risk would have significant public health implications,” Dr. Arnspang and associates concluded. “The use of HCTZ should be carefully considered as several other antihypertensive agents with similar indications and efficiency are available but without known associations with skin cancer.”
The investigators cited several limitations. For example, information on ethnicity and skin type was not available. This information would have been useful in evaluating participants’ photosensitivity as a possible mechanism for a higher skin cancer risk with the use of HCTZ.
The study was funded by a grant from the Danish Cancer Society and the Danish Council of Independent Research. Several of the authors reported receiving grants and or honoraria from pharmaceutical companies.
SOURCE: Arnspang S et al. JAAD. 2017. doi: 10.1016/j.jaad.2017.11.042.
The common diuretic hydrochlorothiazide is linked to a dose-dependent increased risk of nonmelanoma skin cancer, in particular, squamous cell carcinoma, a case-controlled registry study showed.
This nationwide, case-matched control study examined patients’ cumulative hydrochlorothiazide use between 1995 and 2012 and found a clear dose-response patterns for both basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), with a more than sevenfold increased risk of SCC for a cumulative use of greater than or equal to 200,000 mg of hydrochlorothiazide (HCTZ).
“Assuming causality, the present results suggest that 1 in 10 SCC cases diagnosed during the study period can be attributed to HCTZ use,” wrote the study authors, who were led by Sidsel Arnspang, MD, of the department of neurology at Odense (Denmark) University Hospital, which is affiliated with the University of Southern Denmark.
The authors noted that they previously had reported a sevenfold increased risk of lip squamous cell carcinoma with hydrochlorothiazide. Furthermore, the International Agency for Research on Cancer recently classified the diuretic and antihypertensive as “possibly carcinogenic to humans.”
“As HCTZ is among the most widely used drugs in the U.S. and Western Europe, a carcinogenic effect of HCTZ would have a considerable impact on public health,” they wrote in their paper, published in the Journal of American Academy of Dermatology.
According to the study authors, the few studies that have investigated a potential link between thiazide use and nonmelanoma skin cancer (NMSC) have reported inconsistent results.
They speculated that this could be because HCTZ often is prescribed in combination with other diuretics, and there may have been difficulties with disentangling its effect from those of the other drugs.
Using data from five nationwide data sources, the research team compared HCTZ use among people diagnosed with SCC or BCC of the skin to use among a matched control group without such cancers. People were excluded from the analysis if they had SCC of the lip because they had been evaluated in the research team’s previous study.
High use of HCTZ was defined as filled prescriptions totaling greater than or equal to 50,000 mg of HCTZ, which corresponds to greater than or equal to 2,000 defined daily doses (for example, approximately 6 years of cumulative use).
Overall, the study population involved 71,533 BCC and 8,629 SCC cases that were matched to 1,430,883 and 172,462 population controls, respectively.
Baseline characteristics of the cases and controls were similar, except BCC cases were slightly more educated than controls. Results showed that high use of hydrochlorothiazide was associated with odds ratios of 1.29 (95% confidence interval, 1.23-1.35) for BCC and 3.98 (95% CI, 3.68-4.31) for SCC.
A clear dose-response relationship was observed with HCTZ use for both BCC and SCC, with the highest ORs observed in the upper exposure category (greater than or equal to 200,000 mg): The OR for BCC in this category was 1.54 (95% CI, 1.38-1.71) and 7.38 for SCC (95% CI, 6.32-8.60).
The researchers observed no associations for BCC or SCC risk with use of other diuretics and other hypertensives, a finding that they said supported a potential causal association between HCTZ and NMSC risk.
Little variation was seen in the association between HCTZ use and BCC or SCC risk in the subgroup analyses, except for notably stronger associations among younger individuals and females.
In analyses stratified according to tumor localization, the authors saw stronger associations for cancers at sun-exposed skin sites, especially the skin of the lower limbs.
“Given the considerable use of HCTZ worldwide and the morbidity associated with NMSC, a causal association between HCTZ use and NMSC risk would have significant public health implications,” Dr. Arnspang and associates concluded. “The use of HCTZ should be carefully considered as several other antihypertensive agents with similar indications and efficiency are available but without known associations with skin cancer.”
The investigators cited several limitations. For example, information on ethnicity and skin type was not available. This information would have been useful in evaluating participants’ photosensitivity as a possible mechanism for a higher skin cancer risk with the use of HCTZ.
The study was funded by a grant from the Danish Cancer Society and the Danish Council of Independent Research. Several of the authors reported receiving grants and or honoraria from pharmaceutical companies.
SOURCE: Arnspang S et al. JAAD. 2017. doi: 10.1016/j.jaad.2017.11.042.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: The use of hydrochlorothiazide should be carefully considered because it is linked to a substantially increased risk of nonmelanoma skin cancer.
Major finding: A clear, dose-response pattern was found for both basal cell carcinoma and squamous cell carcinoma, with a more than sevenfold increased risk of SCC for a cumulative use of greater than or equal to 200,000 mg of the diuretic and antihypertensive.
Study details: The study population involved 71,533 BCC and 8,629 SCC cases that were matched to 1,430,883 and 172,462 population controls, respectively.
Disclosures: The study was funded by a grant from the Danish Cancer Society and the Danish Council of Independent Research. Several of the authors reported receiving grants and or honoraria from pharmaceutical companies.
Source: Arnspang S et al. JAAD. 2017. doi: 10.1016/j.jaad.2017.11.042
TNFi response evaluations may conflict when fibromyalgia, axial SpA coexist
The concomitant presence of both axial spondyloarthritis (axSpA) and fibromyalgia affects response to tumor necrosis factor (TNF) blockers through increased symptom severity on patient-reported outcomes but not on more objective measurements, according to findings from a prospective, longitudinal study.
Although reports have less frequently examined the relationship between fibromyalgia diagnosis and disease activity in patients who have rheumatologist-diagnosed axSpA than they have in patients with rheumatoid arthritis, those reports have indicated that patients with axSpA and concomitant fibromyalgia tend to present with higher disease activity on measures such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the investigators wrote in Annals of the Rheumatic Diseases.
In this prospective, longitudinal study, a total of 508 adult patients with axSpA from 65 centers first attended a baseline visit, then 12 weeks after commencing treatment with TNF blockers, they attended an “effectiveness visit” in which they were evaluated for a response on the BASDAI. This response was defined as a reduction of at least 50% or two units, compared with baseline measurements. Furthermore, a total of 37.8% tested positive at baseline on the self-reported Fibromyalgia Rapid Screening Tool (FiRST) questionnaire, defined as a score of 5 or 6 out of 6.
These patients testing positive on FiRST at baseline were more likely to be female (55.7% vs. 41.1%), to have a history of peripheral enthesitis (64.7% vs. 47.8%), and to have a higher disease severity at baseline on the BASDAI and Ankylosing Spondylitis Disease Activity Score at baseline (6.5 vs. 5.1 and 3.5 vs. 3.2, respectively). They were also were more likely to be taking antidepressants (26.8% vs. 16.2%).
Patients who had fibromyalgia according to FiRST presented less frequently with a BASDAI response (87 of 192; 45.3%) after 12 weeks, compared with patients who had only axSpA at baseline (171 of 316; 54.1%). But this difference did not reach statistical significance in both univariate or multivariate analyses. However, nearly all of the secondary endpoints of response, such as various levels of response on the Assessment of SpondyloArthritis international Society criteria and the Ankylosing Spondylitis Disease Activity Score, were achieved significantly less often among patients who also had fibromyalgia.
Although BASDAI response was not different between the groups, the investigators said that fibromyalgia had a negative effect on TNF blocker response that “seems related to the instruments used in its evaluation rather than a different treatment effect of the molecule.”
Sensitivity analyses that used the 1990 American College of Rheumatology criteria to define the presence of fibromyalgia rather than the FiRST questionnaire result at baseline did not find any differences in TNF blocker responses between the groups on the main endpoint and most of the secondary endpoints. The ACR criteria classified fibromyalgia in 16.1% of the patients.
Another set of sensitivity analyses that used only the FiRST results at 12 weeks to diagnose fibromyalgia found that fibromyalgia patients had lower responses to treatment on nearly all endpoints. Only 18.7% of patients tested positive on the FiRST questionnaire at 12 weeks.
The change in C-reactive protein (CRP) levels at 12 weeks was not different between the groups of patients regardless of the definition used for fibromyalgia.
The researchers observed a decreased frequency of HLA-B27 positivity, radiographic sacroiliitis, and MRI sacroiliitis in people with both diseases. The authors called this finding “intriguing” and said it “might suggest that some patients participating in the trial might have been misdiagnosed and were in fact suffering from [fibromyalgia] only.”
But other clinical features suggestive of axSpA, such as uveitis, psoriasis, and inflammatory bowel disease, were equally present across the different groups.
Overall, “these results suggest that there is indeed an impact on the treatment response, but seems more related to the patient-reported outcomes used in the effectiveness endpoints, as suggested by the absence of difference across groups for the objective biological parameters (i.e., CRP).”
Dr. Moltó and her colleagues said their findings highlighted the importance not only of evaluating the presence of coexisting fibromyalgia in patients with axSpA when evaluating treatment response but also in determining treatment targets.
“This seems particularly important for decision of the treatment target in patients with axSpA when applying a treat-to-target strategy: For example, remission might not be a feasible target for these patients [with concomitant fibromyalgia] who will not likely reach this state, but should rather aim for a significant change or focus in only on objective parameters (i.e., CRP),” they concluded.
The study was funded by an unrestricted grant from Merck Sharp & Dohme. The authors declared having no competing interests.
SOURCE: Moltó A et al. Ann Rheum Dis. 2017 Nov 28. doi: 10.1136/annrheumdis-2017-212378.
The concomitant presence of both axial spondyloarthritis (axSpA) and fibromyalgia affects response to tumor necrosis factor (TNF) blockers through increased symptom severity on patient-reported outcomes but not on more objective measurements, according to findings from a prospective, longitudinal study.
Although reports have less frequently examined the relationship between fibromyalgia diagnosis and disease activity in patients who have rheumatologist-diagnosed axSpA than they have in patients with rheumatoid arthritis, those reports have indicated that patients with axSpA and concomitant fibromyalgia tend to present with higher disease activity on measures such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the investigators wrote in Annals of the Rheumatic Diseases.
In this prospective, longitudinal study, a total of 508 adult patients with axSpA from 65 centers first attended a baseline visit, then 12 weeks after commencing treatment with TNF blockers, they attended an “effectiveness visit” in which they were evaluated for a response on the BASDAI. This response was defined as a reduction of at least 50% or two units, compared with baseline measurements. Furthermore, a total of 37.8% tested positive at baseline on the self-reported Fibromyalgia Rapid Screening Tool (FiRST) questionnaire, defined as a score of 5 or 6 out of 6.
These patients testing positive on FiRST at baseline were more likely to be female (55.7% vs. 41.1%), to have a history of peripheral enthesitis (64.7% vs. 47.8%), and to have a higher disease severity at baseline on the BASDAI and Ankylosing Spondylitis Disease Activity Score at baseline (6.5 vs. 5.1 and 3.5 vs. 3.2, respectively). They were also were more likely to be taking antidepressants (26.8% vs. 16.2%).
Patients who had fibromyalgia according to FiRST presented less frequently with a BASDAI response (87 of 192; 45.3%) after 12 weeks, compared with patients who had only axSpA at baseline (171 of 316; 54.1%). But this difference did not reach statistical significance in both univariate or multivariate analyses. However, nearly all of the secondary endpoints of response, such as various levels of response on the Assessment of SpondyloArthritis international Society criteria and the Ankylosing Spondylitis Disease Activity Score, were achieved significantly less often among patients who also had fibromyalgia.
Although BASDAI response was not different between the groups, the investigators said that fibromyalgia had a negative effect on TNF blocker response that “seems related to the instruments used in its evaluation rather than a different treatment effect of the molecule.”
Sensitivity analyses that used the 1990 American College of Rheumatology criteria to define the presence of fibromyalgia rather than the FiRST questionnaire result at baseline did not find any differences in TNF blocker responses between the groups on the main endpoint and most of the secondary endpoints. The ACR criteria classified fibromyalgia in 16.1% of the patients.
Another set of sensitivity analyses that used only the FiRST results at 12 weeks to diagnose fibromyalgia found that fibromyalgia patients had lower responses to treatment on nearly all endpoints. Only 18.7% of patients tested positive on the FiRST questionnaire at 12 weeks.
The change in C-reactive protein (CRP) levels at 12 weeks was not different between the groups of patients regardless of the definition used for fibromyalgia.
The researchers observed a decreased frequency of HLA-B27 positivity, radiographic sacroiliitis, and MRI sacroiliitis in people with both diseases. The authors called this finding “intriguing” and said it “might suggest that some patients participating in the trial might have been misdiagnosed and were in fact suffering from [fibromyalgia] only.”
But other clinical features suggestive of axSpA, such as uveitis, psoriasis, and inflammatory bowel disease, were equally present across the different groups.
Overall, “these results suggest that there is indeed an impact on the treatment response, but seems more related to the patient-reported outcomes used in the effectiveness endpoints, as suggested by the absence of difference across groups for the objective biological parameters (i.e., CRP).”
Dr. Moltó and her colleagues said their findings highlighted the importance not only of evaluating the presence of coexisting fibromyalgia in patients with axSpA when evaluating treatment response but also in determining treatment targets.
“This seems particularly important for decision of the treatment target in patients with axSpA when applying a treat-to-target strategy: For example, remission might not be a feasible target for these patients [with concomitant fibromyalgia] who will not likely reach this state, but should rather aim for a significant change or focus in only on objective parameters (i.e., CRP),” they concluded.
The study was funded by an unrestricted grant from Merck Sharp & Dohme. The authors declared having no competing interests.
SOURCE: Moltó A et al. Ann Rheum Dis. 2017 Nov 28. doi: 10.1136/annrheumdis-2017-212378.
The concomitant presence of both axial spondyloarthritis (axSpA) and fibromyalgia affects response to tumor necrosis factor (TNF) blockers through increased symptom severity on patient-reported outcomes but not on more objective measurements, according to findings from a prospective, longitudinal study.
Although reports have less frequently examined the relationship between fibromyalgia diagnosis and disease activity in patients who have rheumatologist-diagnosed axSpA than they have in patients with rheumatoid arthritis, those reports have indicated that patients with axSpA and concomitant fibromyalgia tend to present with higher disease activity on measures such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the investigators wrote in Annals of the Rheumatic Diseases.
In this prospective, longitudinal study, a total of 508 adult patients with axSpA from 65 centers first attended a baseline visit, then 12 weeks after commencing treatment with TNF blockers, they attended an “effectiveness visit” in which they were evaluated for a response on the BASDAI. This response was defined as a reduction of at least 50% or two units, compared with baseline measurements. Furthermore, a total of 37.8% tested positive at baseline on the self-reported Fibromyalgia Rapid Screening Tool (FiRST) questionnaire, defined as a score of 5 or 6 out of 6.
These patients testing positive on FiRST at baseline were more likely to be female (55.7% vs. 41.1%), to have a history of peripheral enthesitis (64.7% vs. 47.8%), and to have a higher disease severity at baseline on the BASDAI and Ankylosing Spondylitis Disease Activity Score at baseline (6.5 vs. 5.1 and 3.5 vs. 3.2, respectively). They were also were more likely to be taking antidepressants (26.8% vs. 16.2%).
Patients who had fibromyalgia according to FiRST presented less frequently with a BASDAI response (87 of 192; 45.3%) after 12 weeks, compared with patients who had only axSpA at baseline (171 of 316; 54.1%). But this difference did not reach statistical significance in both univariate or multivariate analyses. However, nearly all of the secondary endpoints of response, such as various levels of response on the Assessment of SpondyloArthritis international Society criteria and the Ankylosing Spondylitis Disease Activity Score, were achieved significantly less often among patients who also had fibromyalgia.
Although BASDAI response was not different between the groups, the investigators said that fibromyalgia had a negative effect on TNF blocker response that “seems related to the instruments used in its evaluation rather than a different treatment effect of the molecule.”
Sensitivity analyses that used the 1990 American College of Rheumatology criteria to define the presence of fibromyalgia rather than the FiRST questionnaire result at baseline did not find any differences in TNF blocker responses between the groups on the main endpoint and most of the secondary endpoints. The ACR criteria classified fibromyalgia in 16.1% of the patients.
Another set of sensitivity analyses that used only the FiRST results at 12 weeks to diagnose fibromyalgia found that fibromyalgia patients had lower responses to treatment on nearly all endpoints. Only 18.7% of patients tested positive on the FiRST questionnaire at 12 weeks.
The change in C-reactive protein (CRP) levels at 12 weeks was not different between the groups of patients regardless of the definition used for fibromyalgia.
The researchers observed a decreased frequency of HLA-B27 positivity, radiographic sacroiliitis, and MRI sacroiliitis in people with both diseases. The authors called this finding “intriguing” and said it “might suggest that some patients participating in the trial might have been misdiagnosed and were in fact suffering from [fibromyalgia] only.”
But other clinical features suggestive of axSpA, such as uveitis, psoriasis, and inflammatory bowel disease, were equally present across the different groups.
Overall, “these results suggest that there is indeed an impact on the treatment response, but seems more related to the patient-reported outcomes used in the effectiveness endpoints, as suggested by the absence of difference across groups for the objective biological parameters (i.e., CRP).”
Dr. Moltó and her colleagues said their findings highlighted the importance not only of evaluating the presence of coexisting fibromyalgia in patients with axSpA when evaluating treatment response but also in determining treatment targets.
“This seems particularly important for decision of the treatment target in patients with axSpA when applying a treat-to-target strategy: For example, remission might not be a feasible target for these patients [with concomitant fibromyalgia] who will not likely reach this state, but should rather aim for a significant change or focus in only on objective parameters (i.e., CRP),” they concluded.
The study was funded by an unrestricted grant from Merck Sharp & Dohme. The authors declared having no competing interests.
SOURCE: Moltó A et al. Ann Rheum Dis. 2017 Nov 28. doi: 10.1136/annrheumdis-2017-212378.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Patients with both axSpA and fibromyalgia presented less frequently with a BASDAI response after 12 weeks than did patients without both diseases (45.3% vs. 54.1%), but the finding did not reach statistical significance.
Data source: Prospective, longitudinal study of 508 adult patients with rheumatologist-diagnosed axSpA.
Disclosures: The study was funded by an unrestricted grant from Merck Sharp & Dohme. The authors declared having no competing interests.
Source: Moltó A et al. Ann Rheum Dis. 2017 Nov 28. doi: 10.1136/annrheumdis-2017-212378.
PsA disease activity instruments differ on residual disease
Definitions of low disease activity or remission in patients with psoriatic arthritis that come from validated disease activity instruments identify different levels of residual disease despite having significant overlap. This level of variation between instruments can leave patients with residual disease that can affect their quality of life, researchers reported.
Treatment recommendations from the European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) advise aiming “for remission or the lowest possible disease activity in all involved domains of the disease,” but they do not specify a target or instrument to be used to define either low disease activity or remission, wrote Laura Coates, MD, of the University of Oxford (England) and her coauthors. To look at the variation produced by existing instruments to measure those states, they applied a variety of scoring indices to a group of 250 psoriatic arthritis patients who were considered by their treating rheumatologist to have quiescent disease.
The investigators tested the Disease Activity Index for Psoriatic Arthritis (DAPSA), minimal disease activity (MDA) criteria, and the Psoriatic Arthritis Disease Activity Score (PASDAS) in the group of patients, who were required to have been on stable treatment for at least 6 months (Ann Rheum Dis. 2017 Oct 28. doi: 10.1136/annrheumdis-2017-211998).
The cut points they used to measure remission included a score of 4 or less for DAPSA and the clinical DAPSA (cDAPSA, which doesn’t use C-reactive protein [CRP]), and a state called very low disease activity (VLDA) in which all seven MDA domain cut points were met. “Near remission” on the PASDAS was a score of 1.9 or less.
Low or minimal disease activity was defined by DAPSA of 14 or less, cDAPSA of 13 or less, or meeting the cut points for a combination of different MDA domains, including one in which any five of the seven cut points are required to be met (“MDA 5/7”), one where both the tender and swollen joint count cut points are required to be met with any three of the remaining five cut points (“MDA joints”), one where the skin domain is required to be met along with four of any of the remaining six (“MDA skin”), and one where both the joint and skin domains need to be met with any two of the remaining four domains (“MDA joints and skin”).
Little is known about whether the disease activity composite scores of the DAPSA and PASDAS reflect the same clinical disease activity on the various MDA disease domain combinations, Dr. Coates and her associates said.
The results of the analysis showed that VLDA and PASDAS were the most stringent scores for remission and that the DAPSA and cDAPSA scores were the least stringent target for remission. For example, in the entire cohort, 107 (43.7%) patients fulfilled DAPSA remission, 113 (45.7%) were in cDAPSA remission, 56 (22.5%) met VLDA criteria, and 37 (19.5%) were in PASDAS “near remission.”
All patients who met VLDA criteria were in DAPSA/cDAPSA remission. Of those patients in DAPSA remission but not in VLDA, 43 of 56 patients did not fulfill one of seven domains, while nine did not fulfill two of seven domains. Domains not fulfilled were skin (n = 33), tender joints (n = 7), swollen joints (n = 1), enthesitis (n = 3), visual analog scale scores (n = 6), or Health Assessment Questionnaire (n = 9).
“Residual skin disease was highest in patients achieving DAPSA or cDAPSA remission … this resulted in a group of patients, seen as in a low disease activity state, with the remaining skin disease impacting their quality of life,” the investigators wrote. This analysis “highlights the need for multiple separate measures for different domains to be assessed if a multidimensional definition is not used to ensure that remission retains face validity for the patients.”
The addition of the inflammatory marker CRP in both remission and low disease activity measures (in DAPSA and PASDAS) did not have added value, suggesting that “the inclusion of CRP is unnecessary [since] a similar proportion of patients have a raised CRP in all definitions. … A target without an inflammatory marker will be more practical in clinical practice,” they said.
However, Dr. Coates and her colleagues stressed that the cut-off for acceptable disease activity is important because a stricter target may encourage the overtreatment of patients, which would result in increased side effects and costs.
“The ideal stringency of a target with assessment of residual disease in the various clinical domains of psoriatic arthritis should be a focus of research,” they wrote.
The original cohort of patients was supported with an unrestricted grant from Pfizer. Dr. Coates did not declare any conflicts, but several of her coauthors declared receiving speaker or consultancy fees from the pharmaceutical industry.
Definitions of low disease activity or remission in patients with psoriatic arthritis that come from validated disease activity instruments identify different levels of residual disease despite having significant overlap. This level of variation between instruments can leave patients with residual disease that can affect their quality of life, researchers reported.
Treatment recommendations from the European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) advise aiming “for remission or the lowest possible disease activity in all involved domains of the disease,” but they do not specify a target or instrument to be used to define either low disease activity or remission, wrote Laura Coates, MD, of the University of Oxford (England) and her coauthors. To look at the variation produced by existing instruments to measure those states, they applied a variety of scoring indices to a group of 250 psoriatic arthritis patients who were considered by their treating rheumatologist to have quiescent disease.
The investigators tested the Disease Activity Index for Psoriatic Arthritis (DAPSA), minimal disease activity (MDA) criteria, and the Psoriatic Arthritis Disease Activity Score (PASDAS) in the group of patients, who were required to have been on stable treatment for at least 6 months (Ann Rheum Dis. 2017 Oct 28. doi: 10.1136/annrheumdis-2017-211998).
The cut points they used to measure remission included a score of 4 or less for DAPSA and the clinical DAPSA (cDAPSA, which doesn’t use C-reactive protein [CRP]), and a state called very low disease activity (VLDA) in which all seven MDA domain cut points were met. “Near remission” on the PASDAS was a score of 1.9 or less.
Low or minimal disease activity was defined by DAPSA of 14 or less, cDAPSA of 13 or less, or meeting the cut points for a combination of different MDA domains, including one in which any five of the seven cut points are required to be met (“MDA 5/7”), one where both the tender and swollen joint count cut points are required to be met with any three of the remaining five cut points (“MDA joints”), one where the skin domain is required to be met along with four of any of the remaining six (“MDA skin”), and one where both the joint and skin domains need to be met with any two of the remaining four domains (“MDA joints and skin”).
Little is known about whether the disease activity composite scores of the DAPSA and PASDAS reflect the same clinical disease activity on the various MDA disease domain combinations, Dr. Coates and her associates said.
The results of the analysis showed that VLDA and PASDAS were the most stringent scores for remission and that the DAPSA and cDAPSA scores were the least stringent target for remission. For example, in the entire cohort, 107 (43.7%) patients fulfilled DAPSA remission, 113 (45.7%) were in cDAPSA remission, 56 (22.5%) met VLDA criteria, and 37 (19.5%) were in PASDAS “near remission.”
All patients who met VLDA criteria were in DAPSA/cDAPSA remission. Of those patients in DAPSA remission but not in VLDA, 43 of 56 patients did not fulfill one of seven domains, while nine did not fulfill two of seven domains. Domains not fulfilled were skin (n = 33), tender joints (n = 7), swollen joints (n = 1), enthesitis (n = 3), visual analog scale scores (n = 6), or Health Assessment Questionnaire (n = 9).
“Residual skin disease was highest in patients achieving DAPSA or cDAPSA remission … this resulted in a group of patients, seen as in a low disease activity state, with the remaining skin disease impacting their quality of life,” the investigators wrote. This analysis “highlights the need for multiple separate measures for different domains to be assessed if a multidimensional definition is not used to ensure that remission retains face validity for the patients.”
The addition of the inflammatory marker CRP in both remission and low disease activity measures (in DAPSA and PASDAS) did not have added value, suggesting that “the inclusion of CRP is unnecessary [since] a similar proportion of patients have a raised CRP in all definitions. … A target without an inflammatory marker will be more practical in clinical practice,” they said.
However, Dr. Coates and her colleagues stressed that the cut-off for acceptable disease activity is important because a stricter target may encourage the overtreatment of patients, which would result in increased side effects and costs.
“The ideal stringency of a target with assessment of residual disease in the various clinical domains of psoriatic arthritis should be a focus of research,” they wrote.
The original cohort of patients was supported with an unrestricted grant from Pfizer. Dr. Coates did not declare any conflicts, but several of her coauthors declared receiving speaker or consultancy fees from the pharmaceutical industry.
Definitions of low disease activity or remission in patients with psoriatic arthritis that come from validated disease activity instruments identify different levels of residual disease despite having significant overlap. This level of variation between instruments can leave patients with residual disease that can affect their quality of life, researchers reported.
Treatment recommendations from the European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) advise aiming “for remission or the lowest possible disease activity in all involved domains of the disease,” but they do not specify a target or instrument to be used to define either low disease activity or remission, wrote Laura Coates, MD, of the University of Oxford (England) and her coauthors. To look at the variation produced by existing instruments to measure those states, they applied a variety of scoring indices to a group of 250 psoriatic arthritis patients who were considered by their treating rheumatologist to have quiescent disease.
The investigators tested the Disease Activity Index for Psoriatic Arthritis (DAPSA), minimal disease activity (MDA) criteria, and the Psoriatic Arthritis Disease Activity Score (PASDAS) in the group of patients, who were required to have been on stable treatment for at least 6 months (Ann Rheum Dis. 2017 Oct 28. doi: 10.1136/annrheumdis-2017-211998).
The cut points they used to measure remission included a score of 4 or less for DAPSA and the clinical DAPSA (cDAPSA, which doesn’t use C-reactive protein [CRP]), and a state called very low disease activity (VLDA) in which all seven MDA domain cut points were met. “Near remission” on the PASDAS was a score of 1.9 or less.
Low or minimal disease activity was defined by DAPSA of 14 or less, cDAPSA of 13 or less, or meeting the cut points for a combination of different MDA domains, including one in which any five of the seven cut points are required to be met (“MDA 5/7”), one where both the tender and swollen joint count cut points are required to be met with any three of the remaining five cut points (“MDA joints”), one where the skin domain is required to be met along with four of any of the remaining six (“MDA skin”), and one where both the joint and skin domains need to be met with any two of the remaining four domains (“MDA joints and skin”).
Little is known about whether the disease activity composite scores of the DAPSA and PASDAS reflect the same clinical disease activity on the various MDA disease domain combinations, Dr. Coates and her associates said.
The results of the analysis showed that VLDA and PASDAS were the most stringent scores for remission and that the DAPSA and cDAPSA scores were the least stringent target for remission. For example, in the entire cohort, 107 (43.7%) patients fulfilled DAPSA remission, 113 (45.7%) were in cDAPSA remission, 56 (22.5%) met VLDA criteria, and 37 (19.5%) were in PASDAS “near remission.”
All patients who met VLDA criteria were in DAPSA/cDAPSA remission. Of those patients in DAPSA remission but not in VLDA, 43 of 56 patients did not fulfill one of seven domains, while nine did not fulfill two of seven domains. Domains not fulfilled were skin (n = 33), tender joints (n = 7), swollen joints (n = 1), enthesitis (n = 3), visual analog scale scores (n = 6), or Health Assessment Questionnaire (n = 9).
“Residual skin disease was highest in patients achieving DAPSA or cDAPSA remission … this resulted in a group of patients, seen as in a low disease activity state, with the remaining skin disease impacting their quality of life,” the investigators wrote. This analysis “highlights the need for multiple separate measures for different domains to be assessed if a multidimensional definition is not used to ensure that remission retains face validity for the patients.”
The addition of the inflammatory marker CRP in both remission and low disease activity measures (in DAPSA and PASDAS) did not have added value, suggesting that “the inclusion of CRP is unnecessary [since] a similar proportion of patients have a raised CRP in all definitions. … A target without an inflammatory marker will be more practical in clinical practice,” they said.
However, Dr. Coates and her colleagues stressed that the cut-off for acceptable disease activity is important because a stricter target may encourage the overtreatment of patients, which would result in increased side effects and costs.
“The ideal stringency of a target with assessment of residual disease in the various clinical domains of psoriatic arthritis should be a focus of research,” they wrote.
The original cohort of patients was supported with an unrestricted grant from Pfizer. Dr. Coates did not declare any conflicts, but several of her coauthors declared receiving speaker or consultancy fees from the pharmaceutical industry.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Modified minimal disease activity measures were the most stringent targets for low disease activity in terms of residual disease in joints, skin, and entheses.
Data source: Cross-sectional study of 250 patients with psoriatic arthritis who were considered to be in an “acceptable disease state,” according to their rheumatologist.
Disclosures: The original cohort of patients was supported with an unrestricted grant from Pfizer. Dr. Coates did not declare any conflicts, but several of her coauthors declared receiving speaker or consultancy fees from the pharmaceutical industry.
Stopping anti-TNF drugs in PsA has high rebound risk
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Among 94 patients, 69 (73%) experienced symptom rebound after discontinuing their TNFi therapy.
Data source: The prospective observational Corrona disease registry database of about 6,000 patients with PsA.
Disclosures: The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and RA. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
High ‘nocebo’ effect observed when patients knowingly switch to a biosimilar
Evidence suggests that patients who switch from an originator biologic to open-label treatment with its biosimilar have an increase in subjective but not objective assessments and also discontinue the drug at a high rate, possibly reflecting a “nocebo” response to switching.
If patients’ own negative expectations induced “negative symptoms (hyperalgesia or adverse events) during treatment, the so-called nocebo response,” and was the main contributing factor to the high discontinuation rate, then it will be very important for clinicians to improve communication with patients and their expectations in order to raise acceptance and persistence rates, the investigators said.
Of the 47 patients who discontinued CT-P13, 26 did so because of a perceived lack of effect, 11 because of adverse events, and 10 because of a combination of both of these factors.
Univariate Cox regression analyses showed that shorter infliximab infusion interval, higher 28-joint Disease Activity Scores (DAS28, based on either C-reactive protein [CRP] or erythrocyte sedimentation rate), higher swollen joint count, and patients’ global disease activity score at baseline were associated with CT-P13 discontinuation.
However, patients’ and clinicians’ awareness of the switch could have influenced these factors, the investigators said. For instance, they found that patients who discontinued CT-P13 reported a significant increase in “subjective” assessments such as tender joint count and patient’s global disease activity but not “objective” measures such as swollen joint count or CRP.
While the mean Bath Ankylosing Spondylitis Disease Activity Index score increased from 3.8 to 4.3, the mean DAS28-CRP in rheumatoid arthritis and psoriatic arthritis patients remained stable at 2.2 from baseline to month 6; CRP and anti-infliximab antibody levels also did not change.
“If immunogenicity would have caused CT-P13 discontinuation, we would have expected to find more patients with objectively active disease and/or allergic reactions,” the study authors wrote.
Three of the authors reported receiving speaking and consultancy fees from several pharmaceutical companies. The study was not supported by an outside grant.
Evidence suggests that patients who switch from an originator biologic to open-label treatment with its biosimilar have an increase in subjective but not objective assessments and also discontinue the drug at a high rate, possibly reflecting a “nocebo” response to switching.
If patients’ own negative expectations induced “negative symptoms (hyperalgesia or adverse events) during treatment, the so-called nocebo response,” and was the main contributing factor to the high discontinuation rate, then it will be very important for clinicians to improve communication with patients and their expectations in order to raise acceptance and persistence rates, the investigators said.
Of the 47 patients who discontinued CT-P13, 26 did so because of a perceived lack of effect, 11 because of adverse events, and 10 because of a combination of both of these factors.
Univariate Cox regression analyses showed that shorter infliximab infusion interval, higher 28-joint Disease Activity Scores (DAS28, based on either C-reactive protein [CRP] or erythrocyte sedimentation rate), higher swollen joint count, and patients’ global disease activity score at baseline were associated with CT-P13 discontinuation.
However, patients’ and clinicians’ awareness of the switch could have influenced these factors, the investigators said. For instance, they found that patients who discontinued CT-P13 reported a significant increase in “subjective” assessments such as tender joint count and patient’s global disease activity but not “objective” measures such as swollen joint count or CRP.
While the mean Bath Ankylosing Spondylitis Disease Activity Index score increased from 3.8 to 4.3, the mean DAS28-CRP in rheumatoid arthritis and psoriatic arthritis patients remained stable at 2.2 from baseline to month 6; CRP and anti-infliximab antibody levels also did not change.
“If immunogenicity would have caused CT-P13 discontinuation, we would have expected to find more patients with objectively active disease and/or allergic reactions,” the study authors wrote.
Three of the authors reported receiving speaking and consultancy fees from several pharmaceutical companies. The study was not supported by an outside grant.
Evidence suggests that patients who switch from an originator biologic to open-label treatment with its biosimilar have an increase in subjective but not objective assessments and also discontinue the drug at a high rate, possibly reflecting a “nocebo” response to switching.
If patients’ own negative expectations induced “negative symptoms (hyperalgesia or adverse events) during treatment, the so-called nocebo response,” and was the main contributing factor to the high discontinuation rate, then it will be very important for clinicians to improve communication with patients and their expectations in order to raise acceptance and persistence rates, the investigators said.
Of the 47 patients who discontinued CT-P13, 26 did so because of a perceived lack of effect, 11 because of adverse events, and 10 because of a combination of both of these factors.
Univariate Cox regression analyses showed that shorter infliximab infusion interval, higher 28-joint Disease Activity Scores (DAS28, based on either C-reactive protein [CRP] or erythrocyte sedimentation rate), higher swollen joint count, and patients’ global disease activity score at baseline were associated with CT-P13 discontinuation.
However, patients’ and clinicians’ awareness of the switch could have influenced these factors, the investigators said. For instance, they found that patients who discontinued CT-P13 reported a significant increase in “subjective” assessments such as tender joint count and patient’s global disease activity but not “objective” measures such as swollen joint count or CRP.
While the mean Bath Ankylosing Spondylitis Disease Activity Index score increased from 3.8 to 4.3, the mean DAS28-CRP in rheumatoid arthritis and psoriatic arthritis patients remained stable at 2.2 from baseline to month 6; CRP and anti-infliximab antibody levels also did not change.
“If immunogenicity would have caused CT-P13 discontinuation, we would have expected to find more patients with objectively active disease and/or allergic reactions,” the study authors wrote.
Three of the authors reported receiving speaking and consultancy fees from several pharmaceutical companies. The study was not supported by an outside grant.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: Nearly a quarter of 192 patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, who knowingly switched from originator infliximab to its biosimilar CT-P13 discontinued the biosimilar during 6 months of follow-up.
Data source: A multicenter, prospective cohort study of 192 infliximab-treated patients who transitioned to the infliximab biosimilar CT-P13.
Disclosures: Three of the authors reported receiving speaking and consultancy fees from several pharmaceutical companies. The study was not supported by an outside grant.
Abatacept shows potential in refractory myositis
Abatacept could be a new treatment option for people with adult dermatomyositis and polymyositis refractory to conventional treatment, a small, randomized pilot study suggests.
The investigators, led by first author Anna Tjärnlund, PhD, of the Karolinska Institute in Stockholm, noted that the health-related quality of life for people with dermatomyositis (DM) and polymyositis (PM) is low, compared with the general population.
“The majority of commonly used drugs are not approved for myositis, and only [a] few randomized, controlled trials (RCTs) have been performed in this patient group. Thus, there is an unmet need for new therapies for these patients,” they wrote (Ann Rheum Dis. 2017 Oct 9. doi: 10.1136/annrheumdis-2017-211751).
According to the researchers, muscle biopsies of people with DM and PM show a predominance of T cells in inflammatory infiltrates, suggesting a role for T cells in the disease process. Abatacept (Orencia), a fully human fusion protein of CTLA-4 and the Fc portion of human IgG1 that inhibits the co-stimulation of T cells, has been shown in several case reports to have beneficial effects in myositis, but no RCT has been done.
Abatacept is approved by the Food and Drug Administration for the treatment of moderately to severely active rheumatoid arthritis, moderately to severely active polyarticular juvenile idiopathic arthritis, and active psoriatic arthritis in adults.
The aim of the current phase 2b pilot study was to investigate the efficacy and safety of abatacept in a randomized trial with a delayed start in one arm. The researchers randomized 19 patients with DM or PM with refractory disease to receive either immediate active treatment (n = 10) with intravenous abatacept (dosed according to body weight) or a 3-month delayed start (n = 9). Patients who weighed less than 60 kg received 500 mg abatacept, those who weighed 60-100 kg received 750 mg, and those with body weight greater than 100 kg received 1,000 mg.
The primary endpoint was the number of responders defined by the International Myositis Assessment and Clinical Studies (IMACS) Group definition of improvement (relative improvement of 20% or greater in three of any six core set measures, with no more than two core set measures worsening by 25% or more) after 6 months of treatment.
The researchers saw improvements in the active treatment arm, compared with the delayed-start arm. At 3 months, five patients in the active treatment arm were responders, compared with one patient in the delayed treatment arm. For example, the active treatment groups improved by a mean of 2.5 points on the Manual Muscle Testing–8 (one of the individual components of the IMACS core set), compared with –4.9 in the delayed treatment arm (P = .0375).
At 6 months, an intent-to-treat analysis revealed that 8 out of 19 patients responded (2 with DM, 6 with PM) and reached the definition of improvement, with the remaining patients classified as nonresponders.
In patients who had before and after muscle biopsies, the expression of anti-inflammatory Foxp3+ regulatory T cells was significantly greater after abatacept treatment, the researchers reported. They noted that they had previously seen a decrease in the number of Foxp3+ cells in the tissues of patients with myositis on treatment with glucocorticoids.
“This difference could be related to the different treatment targets as since tissue-resident Foxp3+ regulatory T cells have been implicated in muscle repair and regeneration.”
Overall, 36 adverse events were reported during the study. Eight were considered related to abatacept, of which four were considered “mild” and the remaining four “moderate.”
The researchers concluded that although their study was not powered to confirm efficacy, treatment with abatacept was “clinically efficacious in a subgroup of patients with DM or PM and has an acceptable safety profile in refractory patients.”
They cautioned that treatment with abatacept might provide a new treatment option in PM/DM, but it needs to be investigated in randomized, placebo-controlled trials in larger patient populations.
The study was funded by grants from Bristol-Myers Squibb, the Börje Dahlin Foundation, the Swedish Research Council, the Swedish Rheumatism Association, and the King Gustaf V 80-Year Foundation. Two authors reported receiving research grants from Bristol-Myers Squibb and one serves as an advisory board consultant to the company.
Abatacept could be a new treatment option for people with adult dermatomyositis and polymyositis refractory to conventional treatment, a small, randomized pilot study suggests.
The investigators, led by first author Anna Tjärnlund, PhD, of the Karolinska Institute in Stockholm, noted that the health-related quality of life for people with dermatomyositis (DM) and polymyositis (PM) is low, compared with the general population.
“The majority of commonly used drugs are not approved for myositis, and only [a] few randomized, controlled trials (RCTs) have been performed in this patient group. Thus, there is an unmet need for new therapies for these patients,” they wrote (Ann Rheum Dis. 2017 Oct 9. doi: 10.1136/annrheumdis-2017-211751).
According to the researchers, muscle biopsies of people with DM and PM show a predominance of T cells in inflammatory infiltrates, suggesting a role for T cells in the disease process. Abatacept (Orencia), a fully human fusion protein of CTLA-4 and the Fc portion of human IgG1 that inhibits the co-stimulation of T cells, has been shown in several case reports to have beneficial effects in myositis, but no RCT has been done.
Abatacept is approved by the Food and Drug Administration for the treatment of moderately to severely active rheumatoid arthritis, moderately to severely active polyarticular juvenile idiopathic arthritis, and active psoriatic arthritis in adults.
The aim of the current phase 2b pilot study was to investigate the efficacy and safety of abatacept in a randomized trial with a delayed start in one arm. The researchers randomized 19 patients with DM or PM with refractory disease to receive either immediate active treatment (n = 10) with intravenous abatacept (dosed according to body weight) or a 3-month delayed start (n = 9). Patients who weighed less than 60 kg received 500 mg abatacept, those who weighed 60-100 kg received 750 mg, and those with body weight greater than 100 kg received 1,000 mg.
The primary endpoint was the number of responders defined by the International Myositis Assessment and Clinical Studies (IMACS) Group definition of improvement (relative improvement of 20% or greater in three of any six core set measures, with no more than two core set measures worsening by 25% or more) after 6 months of treatment.
The researchers saw improvements in the active treatment arm, compared with the delayed-start arm. At 3 months, five patients in the active treatment arm were responders, compared with one patient in the delayed treatment arm. For example, the active treatment groups improved by a mean of 2.5 points on the Manual Muscle Testing–8 (one of the individual components of the IMACS core set), compared with –4.9 in the delayed treatment arm (P = .0375).
At 6 months, an intent-to-treat analysis revealed that 8 out of 19 patients responded (2 with DM, 6 with PM) and reached the definition of improvement, with the remaining patients classified as nonresponders.
In patients who had before and after muscle biopsies, the expression of anti-inflammatory Foxp3+ regulatory T cells was significantly greater after abatacept treatment, the researchers reported. They noted that they had previously seen a decrease in the number of Foxp3+ cells in the tissues of patients with myositis on treatment with glucocorticoids.
“This difference could be related to the different treatment targets as since tissue-resident Foxp3+ regulatory T cells have been implicated in muscle repair and regeneration.”
Overall, 36 adverse events were reported during the study. Eight were considered related to abatacept, of which four were considered “mild” and the remaining four “moderate.”
The researchers concluded that although their study was not powered to confirm efficacy, treatment with abatacept was “clinically efficacious in a subgroup of patients with DM or PM and has an acceptable safety profile in refractory patients.”
They cautioned that treatment with abatacept might provide a new treatment option in PM/DM, but it needs to be investigated in randomized, placebo-controlled trials in larger patient populations.
The study was funded by grants from Bristol-Myers Squibb, the Börje Dahlin Foundation, the Swedish Research Council, the Swedish Rheumatism Association, and the King Gustaf V 80-Year Foundation. Two authors reported receiving research grants from Bristol-Myers Squibb and one serves as an advisory board consultant to the company.
Abatacept could be a new treatment option for people with adult dermatomyositis and polymyositis refractory to conventional treatment, a small, randomized pilot study suggests.
The investigators, led by first author Anna Tjärnlund, PhD, of the Karolinska Institute in Stockholm, noted that the health-related quality of life for people with dermatomyositis (DM) and polymyositis (PM) is low, compared with the general population.
“The majority of commonly used drugs are not approved for myositis, and only [a] few randomized, controlled trials (RCTs) have been performed in this patient group. Thus, there is an unmet need for new therapies for these patients,” they wrote (Ann Rheum Dis. 2017 Oct 9. doi: 10.1136/annrheumdis-2017-211751).
According to the researchers, muscle biopsies of people with DM and PM show a predominance of T cells in inflammatory infiltrates, suggesting a role for T cells in the disease process. Abatacept (Orencia), a fully human fusion protein of CTLA-4 and the Fc portion of human IgG1 that inhibits the co-stimulation of T cells, has been shown in several case reports to have beneficial effects in myositis, but no RCT has been done.
Abatacept is approved by the Food and Drug Administration for the treatment of moderately to severely active rheumatoid arthritis, moderately to severely active polyarticular juvenile idiopathic arthritis, and active psoriatic arthritis in adults.
The aim of the current phase 2b pilot study was to investigate the efficacy and safety of abatacept in a randomized trial with a delayed start in one arm. The researchers randomized 19 patients with DM or PM with refractory disease to receive either immediate active treatment (n = 10) with intravenous abatacept (dosed according to body weight) or a 3-month delayed start (n = 9). Patients who weighed less than 60 kg received 500 mg abatacept, those who weighed 60-100 kg received 750 mg, and those with body weight greater than 100 kg received 1,000 mg.
The primary endpoint was the number of responders defined by the International Myositis Assessment and Clinical Studies (IMACS) Group definition of improvement (relative improvement of 20% or greater in three of any six core set measures, with no more than two core set measures worsening by 25% or more) after 6 months of treatment.
The researchers saw improvements in the active treatment arm, compared with the delayed-start arm. At 3 months, five patients in the active treatment arm were responders, compared with one patient in the delayed treatment arm. For example, the active treatment groups improved by a mean of 2.5 points on the Manual Muscle Testing–8 (one of the individual components of the IMACS core set), compared with –4.9 in the delayed treatment arm (P = .0375).
At 6 months, an intent-to-treat analysis revealed that 8 out of 19 patients responded (2 with DM, 6 with PM) and reached the definition of improvement, with the remaining patients classified as nonresponders.
In patients who had before and after muscle biopsies, the expression of anti-inflammatory Foxp3+ regulatory T cells was significantly greater after abatacept treatment, the researchers reported. They noted that they had previously seen a decrease in the number of Foxp3+ cells in the tissues of patients with myositis on treatment with glucocorticoids.
“This difference could be related to the different treatment targets as since tissue-resident Foxp3+ regulatory T cells have been implicated in muscle repair and regeneration.”
Overall, 36 adverse events were reported during the study. Eight were considered related to abatacept, of which four were considered “mild” and the remaining four “moderate.”
The researchers concluded that although their study was not powered to confirm efficacy, treatment with abatacept was “clinically efficacious in a subgroup of patients with DM or PM and has an acceptable safety profile in refractory patients.”
They cautioned that treatment with abatacept might provide a new treatment option in PM/DM, but it needs to be investigated in randomized, placebo-controlled trials in larger patient populations.
The study was funded by grants from Bristol-Myers Squibb, the Börje Dahlin Foundation, the Swedish Research Council, the Swedish Rheumatism Association, and the King Gustaf V 80-Year Foundation. Two authors reported receiving research grants from Bristol-Myers Squibb and one serves as an advisory board consultant to the company.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Of 19 patients, 8 were classified as treatment responders, reaching the IMACS definition of improvement.
Data source: A phase 2b pilot study with a randomized delayed treatment arm.
Disclosures: The study was funded by grants from Bristol-Myers Squibb, the Börje Dahlin Foundation, the Swedish Research Council, the Swedish Rheumatism Association, and the King Gustaf V 80-Year Foundation. Two authors reported receiving research grants from Bristol-Myers Squibb and one serves as an advisory board consultant to the company.
EULAR program features novel treatments and targets in immune pathways and key overviews of the field
Novel treatments involving the interleukin-17, IL-23, and Janus kinase (JAK) pathways and the growing importance of early diagnosis and treatment will be some of the key themes covered in the scientific program at this year’s EULAR congress in Madrid, June 14-17.
The annual EULAR congress’ traditional spirit of giving congress attendees a thorough scientific update of the evidence published in peer-reviewed journals across the broad spectrum of rheumatic diseases is reflected in the wide range of state-of-the-art lectures, clinical and basic science symposia, practical workshops, and special interest sessions running throughout the packed 4-day congress, said João Eurico Cabral da Fonseca, MD, PhD, chair of the Scientific Programme Committee.
“Our program is driven by novelty and not by a particular area we need to cover,” said Dr. Fonseca of the rheumatology and metabolic bone disease department at the Santa Maria Hospital in Lisbon.
“There has been a lot of research in the past year on the IL-17 and IL-23 pathway, on the use of IL-6 inhibitors in vasculitis, and exploring the several diseases in rheumatology where the inhibition of the JAK pathway and other intracellular pathways will be relevant,” he said.
Some of these advances and innovation in rheumatology will be highlighted in the many “What is New” (WIN) and “How to Treat” (HOT) sessions scattered throughout the scientific program. WIN sessions are a review of the evidence that has been published during the year on a specific area of rheumatology, whereas the purpose of the HOT sessions is to update attendees on the new research in that space while also allowing experts to impart some of their hands-on experience in the area.
“For the HOT and WIN sessions, we invite people to present who are not only scientifically active but are clinically active in order to give some input, particularly for the HOT sessions. They are also usually well skilled in speaking to and engaging with large audiences.”
In WIN and HOT sessions to be held on the afternoon of Saturday, June 17, Josef Smolen, MD, of the Medical University of Vienna will update attendees on the latest developments in the treatment of rheumatoid arthritis.
Dr. Smolen’s talk will be followed by a presentation from pediatric rheumatologist Nico Wulffraat, MD, PhD, of the Wilhelmina Children’s Hospital, Utrecht, the Netherlands on the latest developments in juvenile idiopathic arthritis.
Another WIN session that has been popular with attendees in previous years is EULAR’s collaborative session with The Lancet. The purpose of the collaborative session with The Lancet is twofold: to give attendees an excellent state-of-the-art session on the latest developments in rheumatoid arthritis and also to showcase to the wider global medical community the latest developments in the field of rheumatology, Dr. Fonseca said.
“The long-term goal is to distribute the information we’re gathering in rheumatology journals and at the congress to a broader audience,” he said, noting the relevance of bringing the innovations in rheumatology to audiences outside the field.
The Lancet session this year is on Saturday morning and will focus on the pathogenesis and treatment of rheumatoid arthritis. High-profile speakers at this session include Iain McInnes, PhD, professor of experimental medicine and rheumatology at the University of Glasgow, who will be presenting a WIN session entitled “Dissecting the pathogenesis of rheumatoid arthritis – what have therapeutics taught us?” and EULAR President Gerd Burmester, MD, director of the department of rheumatology and clinical immunology and professor of medicine at Charité University Hospital and Free University and Humboldt University of Berlin, who will present the WIN session “Don’t delay – new treatment concepts in rheumatoid arthritis.”
The importance of diagnosing and treating patients early is a message that is close to EULAR’s heart, Dr. Fonseca said.
The organization, which celebrates its 70th birthday this year, will launch its first awareness campaign‚ “Don’t delay, connect today!” at the congress. The message of the campaign is that “early diagnosis and access to treatment are the key to preventing further damage and burden on individuals and society.”
He said that while the sessions cover all the major rheumatology disciplines, there are some particularly interesting sessions on psoriatic arthritis and spondyloarthritis.
“There’s a lot more interest in these areas than compared to 5 years ago,” he said in an interview. On the morning of Thursday, June 15, there will be an abstract session titled “PsA: A fascinating disease,” followed by a session the next morning called “PsA: The options grow!”
Attendees can also join a poster tour on Thursday morning to discover exactly what progress has been made in the management of spondyloarthritis.
There are new developments in systemic diseases such as lupus and scleroderma that will be highlighted at this year’s congress. However, osteoarthritis is still waiting for its time in the sun, Dr. Landewé said.
“I would say keep an eye on OA over the next few years. ... There are not many sessions this year, but I am very certain there are many new developments on the horizon, perhaps not at this congress, but in the next couple of years,” he said.
Perhaps the pièce de résistance of the scientific program is the conference highlights session on the last day of the congress. Attendees will need to arrive early to get a seat as this session represents a huge effort by two experts who are selected by the Scientific Programme Committee to summarize the most important research published since EULAR 2016 from a clinical, translational, and basic science perspective.
This year, Loreto Carmona, MD, PhD, an epidemiologist and rheumatologist from the Musculoskeletal Health Institute in Madrid, will take the podium to present the clinical highlights. She will be followed by Thomas Dörner, MD, of the Charité University Hospital, Berlin, who will present the translational and basic science highlights.
“This session is a very useful one for delegates as it simplifies the major bits of the congress,” Dr. Fonseca said.
Novel treatments involving the interleukin-17, IL-23, and Janus kinase (JAK) pathways and the growing importance of early diagnosis and treatment will be some of the key themes covered in the scientific program at this year’s EULAR congress in Madrid, June 14-17.
The annual EULAR congress’ traditional spirit of giving congress attendees a thorough scientific update of the evidence published in peer-reviewed journals across the broad spectrum of rheumatic diseases is reflected in the wide range of state-of-the-art lectures, clinical and basic science symposia, practical workshops, and special interest sessions running throughout the packed 4-day congress, said João Eurico Cabral da Fonseca, MD, PhD, chair of the Scientific Programme Committee.
“Our program is driven by novelty and not by a particular area we need to cover,” said Dr. Fonseca of the rheumatology and metabolic bone disease department at the Santa Maria Hospital in Lisbon.
“There has been a lot of research in the past year on the IL-17 and IL-23 pathway, on the use of IL-6 inhibitors in vasculitis, and exploring the several diseases in rheumatology where the inhibition of the JAK pathway and other intracellular pathways will be relevant,” he said.
Some of these advances and innovation in rheumatology will be highlighted in the many “What is New” (WIN) and “How to Treat” (HOT) sessions scattered throughout the scientific program. WIN sessions are a review of the evidence that has been published during the year on a specific area of rheumatology, whereas the purpose of the HOT sessions is to update attendees on the new research in that space while also allowing experts to impart some of their hands-on experience in the area.
“For the HOT and WIN sessions, we invite people to present who are not only scientifically active but are clinically active in order to give some input, particularly for the HOT sessions. They are also usually well skilled in speaking to and engaging with large audiences.”
In WIN and HOT sessions to be held on the afternoon of Saturday, June 17, Josef Smolen, MD, of the Medical University of Vienna will update attendees on the latest developments in the treatment of rheumatoid arthritis.
Dr. Smolen’s talk will be followed by a presentation from pediatric rheumatologist Nico Wulffraat, MD, PhD, of the Wilhelmina Children’s Hospital, Utrecht, the Netherlands on the latest developments in juvenile idiopathic arthritis.
Another WIN session that has been popular with attendees in previous years is EULAR’s collaborative session with The Lancet. The purpose of the collaborative session with The Lancet is twofold: to give attendees an excellent state-of-the-art session on the latest developments in rheumatoid arthritis and also to showcase to the wider global medical community the latest developments in the field of rheumatology, Dr. Fonseca said.
“The long-term goal is to distribute the information we’re gathering in rheumatology journals and at the congress to a broader audience,” he said, noting the relevance of bringing the innovations in rheumatology to audiences outside the field.
The Lancet session this year is on Saturday morning and will focus on the pathogenesis and treatment of rheumatoid arthritis. High-profile speakers at this session include Iain McInnes, PhD, professor of experimental medicine and rheumatology at the University of Glasgow, who will be presenting a WIN session entitled “Dissecting the pathogenesis of rheumatoid arthritis – what have therapeutics taught us?” and EULAR President Gerd Burmester, MD, director of the department of rheumatology and clinical immunology and professor of medicine at Charité University Hospital and Free University and Humboldt University of Berlin, who will present the WIN session “Don’t delay – new treatment concepts in rheumatoid arthritis.”
The importance of diagnosing and treating patients early is a message that is close to EULAR’s heart, Dr. Fonseca said.
The organization, which celebrates its 70th birthday this year, will launch its first awareness campaign‚ “Don’t delay, connect today!” at the congress. The message of the campaign is that “early diagnosis and access to treatment are the key to preventing further damage and burden on individuals and society.”
He said that while the sessions cover all the major rheumatology disciplines, there are some particularly interesting sessions on psoriatic arthritis and spondyloarthritis.
“There’s a lot more interest in these areas than compared to 5 years ago,” he said in an interview. On the morning of Thursday, June 15, there will be an abstract session titled “PsA: A fascinating disease,” followed by a session the next morning called “PsA: The options grow!”
Attendees can also join a poster tour on Thursday morning to discover exactly what progress has been made in the management of spondyloarthritis.
There are new developments in systemic diseases such as lupus and scleroderma that will be highlighted at this year’s congress. However, osteoarthritis is still waiting for its time in the sun, Dr. Landewé said.
“I would say keep an eye on OA over the next few years. ... There are not many sessions this year, but I am very certain there are many new developments on the horizon, perhaps not at this congress, but in the next couple of years,” he said.
Perhaps the pièce de résistance of the scientific program is the conference highlights session on the last day of the congress. Attendees will need to arrive early to get a seat as this session represents a huge effort by two experts who are selected by the Scientific Programme Committee to summarize the most important research published since EULAR 2016 from a clinical, translational, and basic science perspective.
This year, Loreto Carmona, MD, PhD, an epidemiologist and rheumatologist from the Musculoskeletal Health Institute in Madrid, will take the podium to present the clinical highlights. She will be followed by Thomas Dörner, MD, of the Charité University Hospital, Berlin, who will present the translational and basic science highlights.
“This session is a very useful one for delegates as it simplifies the major bits of the congress,” Dr. Fonseca said.
Novel treatments involving the interleukin-17, IL-23, and Janus kinase (JAK) pathways and the growing importance of early diagnosis and treatment will be some of the key themes covered in the scientific program at this year’s EULAR congress in Madrid, June 14-17.
The annual EULAR congress’ traditional spirit of giving congress attendees a thorough scientific update of the evidence published in peer-reviewed journals across the broad spectrum of rheumatic diseases is reflected in the wide range of state-of-the-art lectures, clinical and basic science symposia, practical workshops, and special interest sessions running throughout the packed 4-day congress, said João Eurico Cabral da Fonseca, MD, PhD, chair of the Scientific Programme Committee.
“Our program is driven by novelty and not by a particular area we need to cover,” said Dr. Fonseca of the rheumatology and metabolic bone disease department at the Santa Maria Hospital in Lisbon.
“There has been a lot of research in the past year on the IL-17 and IL-23 pathway, on the use of IL-6 inhibitors in vasculitis, and exploring the several diseases in rheumatology where the inhibition of the JAK pathway and other intracellular pathways will be relevant,” he said.
Some of these advances and innovation in rheumatology will be highlighted in the many “What is New” (WIN) and “How to Treat” (HOT) sessions scattered throughout the scientific program. WIN sessions are a review of the evidence that has been published during the year on a specific area of rheumatology, whereas the purpose of the HOT sessions is to update attendees on the new research in that space while also allowing experts to impart some of their hands-on experience in the area.
“For the HOT and WIN sessions, we invite people to present who are not only scientifically active but are clinically active in order to give some input, particularly for the HOT sessions. They are also usually well skilled in speaking to and engaging with large audiences.”
In WIN and HOT sessions to be held on the afternoon of Saturday, June 17, Josef Smolen, MD, of the Medical University of Vienna will update attendees on the latest developments in the treatment of rheumatoid arthritis.
Dr. Smolen’s talk will be followed by a presentation from pediatric rheumatologist Nico Wulffraat, MD, PhD, of the Wilhelmina Children’s Hospital, Utrecht, the Netherlands on the latest developments in juvenile idiopathic arthritis.
Another WIN session that has been popular with attendees in previous years is EULAR’s collaborative session with The Lancet. The purpose of the collaborative session with The Lancet is twofold: to give attendees an excellent state-of-the-art session on the latest developments in rheumatoid arthritis and also to showcase to the wider global medical community the latest developments in the field of rheumatology, Dr. Fonseca said.
“The long-term goal is to distribute the information we’re gathering in rheumatology journals and at the congress to a broader audience,” he said, noting the relevance of bringing the innovations in rheumatology to audiences outside the field.
The Lancet session this year is on Saturday morning and will focus on the pathogenesis and treatment of rheumatoid arthritis. High-profile speakers at this session include Iain McInnes, PhD, professor of experimental medicine and rheumatology at the University of Glasgow, who will be presenting a WIN session entitled “Dissecting the pathogenesis of rheumatoid arthritis – what have therapeutics taught us?” and EULAR President Gerd Burmester, MD, director of the department of rheumatology and clinical immunology and professor of medicine at Charité University Hospital and Free University and Humboldt University of Berlin, who will present the WIN session “Don’t delay – new treatment concepts in rheumatoid arthritis.”
The importance of diagnosing and treating patients early is a message that is close to EULAR’s heart, Dr. Fonseca said.
The organization, which celebrates its 70th birthday this year, will launch its first awareness campaign‚ “Don’t delay, connect today!” at the congress. The message of the campaign is that “early diagnosis and access to treatment are the key to preventing further damage and burden on individuals and society.”
He said that while the sessions cover all the major rheumatology disciplines, there are some particularly interesting sessions on psoriatic arthritis and spondyloarthritis.
“There’s a lot more interest in these areas than compared to 5 years ago,” he said in an interview. On the morning of Thursday, June 15, there will be an abstract session titled “PsA: A fascinating disease,” followed by a session the next morning called “PsA: The options grow!”
Attendees can also join a poster tour on Thursday morning to discover exactly what progress has been made in the management of spondyloarthritis.
There are new developments in systemic diseases such as lupus and scleroderma that will be highlighted at this year’s congress. However, osteoarthritis is still waiting for its time in the sun, Dr. Landewé said.
“I would say keep an eye on OA over the next few years. ... There are not many sessions this year, but I am very certain there are many new developments on the horizon, perhaps not at this congress, but in the next couple of years,” he said.
Perhaps the pièce de résistance of the scientific program is the conference highlights session on the last day of the congress. Attendees will need to arrive early to get a seat as this session represents a huge effort by two experts who are selected by the Scientific Programme Committee to summarize the most important research published since EULAR 2016 from a clinical, translational, and basic science perspective.
This year, Loreto Carmona, MD, PhD, an epidemiologist and rheumatologist from the Musculoskeletal Health Institute in Madrid, will take the podium to present the clinical highlights. She will be followed by Thomas Dörner, MD, of the Charité University Hospital, Berlin, who will present the translational and basic science highlights.
“This session is a very useful one for delegates as it simplifies the major bits of the congress,” Dr. Fonseca said.
The changing face of JIA sets the tone for pediatric sessions at EULAR
The heterogeneous nature of juvenile idiopathic arthritis (JIA), the use of biologics in childhood rheumatic diseases, and a look at the long-term outcomes for children with JIA are just some of the highlights from the pediatric rheumatology sessions at this year’s EULAR Congress in Madrid, June 14-17.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Tadej Avcin, MD, PhD, said that a bench-to-bedside session on the heterogeneity of JIA on the afternoon of Thursday, June 15, would explore the biologic basis of the disease, the role of cytokine profiling, and the clinical variability in the disease.
“By highlighting the heterogeneity of JIA, we hope this session will contribute to the further understanding of differences between JIA subtypes, as well as contribute some scientific background for the further classification of children with JIA,” he said.
Another “not to miss” session from the pediatric program that will be held on the afternoon of Friday, June 16, is the open issues session on the use of biologic agents in JIA, according to Dr. Avcin, professor of pediatrics and head of the department of allergology, rheumatology, and clinical immunology at University Children’s Hospital, University Medical Center, Ljubljana, Slovenia.
Speaking on the long-term side effects of biologics, Joost Swart, MD, from Utrecht in the Netherlands will present some novel data from the large ongoing pharmacovigilance project Pharmachild that follows children aged 3-10 years who have been treated with methotrexate or a biologic.
At the same session, Pierre Quartier, MD, from Paris will take delegates through data on autoimmune phenomena that can occur in children who are on biologic treatment.
“We know that, in treating children with biologics, they can sometimes develop antidrug antibodies and various induced autoimmune phenomena. We would like to highlight this aspect so that physicians can have more of an overview of possible immune-mediated adverse effects in their patients,” Dr. Avcin said.
The session will also address what Dr. Avcin describes as an emerging and important clinical question: When and how do you discontinue treatment in children with sustained remission?
It’s a question he hopes Gerd Horneff, MD, from Germany will be able to shed some light on when he shares data on how frequently children experience disease flares after discontinuing treatment.
Another pediatric highlight is a morning session on Saturday, June 17, that will address the long-term outcomes of children with JIA.
A presentation by Marion van Rossum, MD, PhD, from the Netherlands will explore whether there are certain clinical or laboratory markers that can help identify children who are more likely to respond well to treatment, compared with other children.
Dirk Foell, MD, from Germany will follow with a session on immunological markers of remission in JIA.
As Dr. Avcin explained, immunological markers such as S100 proteins have shown promise as a biomarker of subclinical active disease.
“Even if a child appears to have clinically inactive disease, elevated levels of these markers may help predict which children will remain in remission after discontinuing treatment and which children may be at an increased risk of a disease flare,” he said.
Rounding off the session, Berit Flatø, MD, PhD, from Norway will present delegates with data from an epidemiological study of long-term outcomes of children with JIA as they move into adulthood.
“Dr. Flatø will present the long-term outcome data from children followed for up to 20 years,” Dr. Avcin said. “Biologics have been in pediatric rheumatology for around 17 years so we will be able to see what is the outcome of children with JIA moving into adulthood with our current treatment protocols.”
In the afternoon, on Friday, pediatric experts will team up with their adult rheumatology colleagues in a “challenges in clinical practice” session to update delegates on life-threatening presentations of rheumatic diseases.
“We will highlight life-threatening presentations that are of particular interest in children, like macrophage activation syndrome and complications of systemic connective tissue diseases and systemic vasculitides like Kawasaki disease and Takayasu’s arteritis,” Dr. Avcin said.
The heterogeneous nature of juvenile idiopathic arthritis (JIA), the use of biologics in childhood rheumatic diseases, and a look at the long-term outcomes for children with JIA are just some of the highlights from the pediatric rheumatology sessions at this year’s EULAR Congress in Madrid, June 14-17.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Tadej Avcin, MD, PhD, said that a bench-to-bedside session on the heterogeneity of JIA on the afternoon of Thursday, June 15, would explore the biologic basis of the disease, the role of cytokine profiling, and the clinical variability in the disease.
“By highlighting the heterogeneity of JIA, we hope this session will contribute to the further understanding of differences between JIA subtypes, as well as contribute some scientific background for the further classification of children with JIA,” he said.
Another “not to miss” session from the pediatric program that will be held on the afternoon of Friday, June 16, is the open issues session on the use of biologic agents in JIA, according to Dr. Avcin, professor of pediatrics and head of the department of allergology, rheumatology, and clinical immunology at University Children’s Hospital, University Medical Center, Ljubljana, Slovenia.
Speaking on the long-term side effects of biologics, Joost Swart, MD, from Utrecht in the Netherlands will present some novel data from the large ongoing pharmacovigilance project Pharmachild that follows children aged 3-10 years who have been treated with methotrexate or a biologic.
At the same session, Pierre Quartier, MD, from Paris will take delegates through data on autoimmune phenomena that can occur in children who are on biologic treatment.
“We know that, in treating children with biologics, they can sometimes develop antidrug antibodies and various induced autoimmune phenomena. We would like to highlight this aspect so that physicians can have more of an overview of possible immune-mediated adverse effects in their patients,” Dr. Avcin said.
The session will also address what Dr. Avcin describes as an emerging and important clinical question: When and how do you discontinue treatment in children with sustained remission?
It’s a question he hopes Gerd Horneff, MD, from Germany will be able to shed some light on when he shares data on how frequently children experience disease flares after discontinuing treatment.
Another pediatric highlight is a morning session on Saturday, June 17, that will address the long-term outcomes of children with JIA.
A presentation by Marion van Rossum, MD, PhD, from the Netherlands will explore whether there are certain clinical or laboratory markers that can help identify children who are more likely to respond well to treatment, compared with other children.
Dirk Foell, MD, from Germany will follow with a session on immunological markers of remission in JIA.
As Dr. Avcin explained, immunological markers such as S100 proteins have shown promise as a biomarker of subclinical active disease.
“Even if a child appears to have clinically inactive disease, elevated levels of these markers may help predict which children will remain in remission after discontinuing treatment and which children may be at an increased risk of a disease flare,” he said.
Rounding off the session, Berit Flatø, MD, PhD, from Norway will present delegates with data from an epidemiological study of long-term outcomes of children with JIA as they move into adulthood.
“Dr. Flatø will present the long-term outcome data from children followed for up to 20 years,” Dr. Avcin said. “Biologics have been in pediatric rheumatology for around 17 years so we will be able to see what is the outcome of children with JIA moving into adulthood with our current treatment protocols.”
In the afternoon, on Friday, pediatric experts will team up with their adult rheumatology colleagues in a “challenges in clinical practice” session to update delegates on life-threatening presentations of rheumatic diseases.
“We will highlight life-threatening presentations that are of particular interest in children, like macrophage activation syndrome and complications of systemic connective tissue diseases and systemic vasculitides like Kawasaki disease and Takayasu’s arteritis,” Dr. Avcin said.
The heterogeneous nature of juvenile idiopathic arthritis (JIA), the use of biologics in childhood rheumatic diseases, and a look at the long-term outcomes for children with JIA are just some of the highlights from the pediatric rheumatology sessions at this year’s EULAR Congress in Madrid, June 14-17.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Tadej Avcin, MD, PhD, said that a bench-to-bedside session on the heterogeneity of JIA on the afternoon of Thursday, June 15, would explore the biologic basis of the disease, the role of cytokine profiling, and the clinical variability in the disease.
“By highlighting the heterogeneity of JIA, we hope this session will contribute to the further understanding of differences between JIA subtypes, as well as contribute some scientific background for the further classification of children with JIA,” he said.
Another “not to miss” session from the pediatric program that will be held on the afternoon of Friday, June 16, is the open issues session on the use of biologic agents in JIA, according to Dr. Avcin, professor of pediatrics and head of the department of allergology, rheumatology, and clinical immunology at University Children’s Hospital, University Medical Center, Ljubljana, Slovenia.
Speaking on the long-term side effects of biologics, Joost Swart, MD, from Utrecht in the Netherlands will present some novel data from the large ongoing pharmacovigilance project Pharmachild that follows children aged 3-10 years who have been treated with methotrexate or a biologic.
At the same session, Pierre Quartier, MD, from Paris will take delegates through data on autoimmune phenomena that can occur in children who are on biologic treatment.
“We know that, in treating children with biologics, they can sometimes develop antidrug antibodies and various induced autoimmune phenomena. We would like to highlight this aspect so that physicians can have more of an overview of possible immune-mediated adverse effects in their patients,” Dr. Avcin said.
The session will also address what Dr. Avcin describes as an emerging and important clinical question: When and how do you discontinue treatment in children with sustained remission?
It’s a question he hopes Gerd Horneff, MD, from Germany will be able to shed some light on when he shares data on how frequently children experience disease flares after discontinuing treatment.
Another pediatric highlight is a morning session on Saturday, June 17, that will address the long-term outcomes of children with JIA.
A presentation by Marion van Rossum, MD, PhD, from the Netherlands will explore whether there are certain clinical or laboratory markers that can help identify children who are more likely to respond well to treatment, compared with other children.
Dirk Foell, MD, from Germany will follow with a session on immunological markers of remission in JIA.
As Dr. Avcin explained, immunological markers such as S100 proteins have shown promise as a biomarker of subclinical active disease.
“Even if a child appears to have clinically inactive disease, elevated levels of these markers may help predict which children will remain in remission after discontinuing treatment and which children may be at an increased risk of a disease flare,” he said.
Rounding off the session, Berit Flatø, MD, PhD, from Norway will present delegates with data from an epidemiological study of long-term outcomes of children with JIA as they move into adulthood.
“Dr. Flatø will present the long-term outcome data from children followed for up to 20 years,” Dr. Avcin said. “Biologics have been in pediatric rheumatology for around 17 years so we will be able to see what is the outcome of children with JIA moving into adulthood with our current treatment protocols.”
In the afternoon, on Friday, pediatric experts will team up with their adult rheumatology colleagues in a “challenges in clinical practice” session to update delegates on life-threatening presentations of rheumatic diseases.
“We will highlight life-threatening presentations that are of particular interest in children, like macrophage activation syndrome and complications of systemic connective tissue diseases and systemic vasculitides like Kawasaki disease and Takayasu’s arteritis,” Dr. Avcin said.
Depressed OA patients get far-reaching benefits from Internet-delivered CBT
Cognitive behavioral therapy administered online can effectively treat symptoms of depression in older people with knee osteoarthritis, according to results from the first randomized trial of its kind.
The benefits of the 10-week program extended to improving pain, stiffness, physical functioning, and self-efficacy, reported Kathleen A. O’Moore, PsyD, of St. Vincent’s Hospital, Sydney, Australia, and her colleagues (Arthritis Care Res. 2017 April 20. doi: 10.1002/acr.23257).
The investigators became motivated to conduct the trial by the fact that about one in five older people with osteoarthritis (OA) experience depression, yet many do not seek treatment, and depression in this population has been associated with an increased use of pain medication, reduced adherence to treatment recommendations, and, when recommendations are followed, reduced treatment benefits.
The investigators randomized the participants to the 10-week Internet cognitive behavioral therapy (iCBT) Sadness Program in addition to usual treatment or to a control group that received usual treatment. Participants were followed up at 1 week and 3 months after the intervention. The iCBT Sadness Program consists of six online lessons representing best practice CBT, as well as regular homework assignments and access to supplementary resources. The program has been validated in several clinical efficacy and effectiveness trials.
The iCBT program proved superior to usual treatment on the primary outcomes of depression as measured by the Patient Health Questionnaire (PHQ-9) and psychological distress (measured using Kessler-10). In particular, a large between-group effect size was seen for PHQ-9 scores (Hedge’s g = 1.01; 95% confidence interval [CI], 0.47-1.54) and a medium effect size for the Kessler-10 score (Hedge’s g = 0.75, 95% CI, 0.23-1.28).
At 3-month follow-up, large between-group effect sizes developed between the iCBT group and the usual treatment-only group on the PHQ-9 (Hedge’s g = 0.90, 95% CI, 0.36-1.44) and Kessler-10 (Hedge’s g = 0.94, 95% CI, 0.41-1.48).
In the iCBT group, the intervention produced large effect-size reductions in depression and distress from pre- to posttreatment and from pretreatment to 12-week follow-up, but in the usual treatment-only group, the effect-size reductions were small and not significant.
In terms of clinical significance, the authors noted that 21 (47.7%) of the participants in the iCBT group “reliably improved,” compared with 3 participants (12%) in the usual treatment-only group.
At 3-month follow-up, 33 participants (84.6%) in the iCBT group no longer met the criteria for major depression, compared with 11 (50%) of the control group.
The intervention also improved the secondary outcomes of OA-specific pain, stiffness, and physical functioning on the Western Ontario and McMaster Osteoarthritis Index and Arthritis Self-Efficacy Scale score, but this was seen by the research team only at the 3-month follow-up. They suggested that further studies should evaluate whether the intervention reduced pain-related catastrophic cognitions or sensitivity to pain and improved a patients’ estimation of their own ability and/or adherence to pain management.
“The outcomes of these studies may provide insight into why there were no significant changes in global physical functioning and no between-group differences for arthritis-related self-efficacy, pain, stiffness, and physical function directly following the program, yet differences emerged 3 months later,” the researchers noted.
They suggested that changes in OA-specific variables such as pain or self efficacy likely required time to interact with cognitive change mechanisms that occur through iCBT.
“It is possible that reduced depressive symptoms result in flow-on effects to OA variables over time and perhaps programs that specifically target OA management may show more immediate effects,” they added.
The researchers concluded that a biopsychosocial approach to managing people with OA was consistent with the recent emphasis on a holistic assessment of older patients with OA.
“These findings are significant given iCBT programs can overcome barriers to receiving face-to-face psychological and/or pharmacological treatment in this population, such as cost, lack of accessibility, and pharmacological side-effects and interactions,” the study authors wrote.
“Medical management of OA of the knee could be supplemented with integrated evidence-based depression treatment, including iCBT, to maximize functional status and mental health well-being,” they added.
The study was funded by grants from the Australian National Health and Medical Research Council. The authors declared no relevant conflicts of interest.
Cognitive behavioral therapy administered online can effectively treat symptoms of depression in older people with knee osteoarthritis, according to results from the first randomized trial of its kind.
The benefits of the 10-week program extended to improving pain, stiffness, physical functioning, and self-efficacy, reported Kathleen A. O’Moore, PsyD, of St. Vincent’s Hospital, Sydney, Australia, and her colleagues (Arthritis Care Res. 2017 April 20. doi: 10.1002/acr.23257).
The investigators became motivated to conduct the trial by the fact that about one in five older people with osteoarthritis (OA) experience depression, yet many do not seek treatment, and depression in this population has been associated with an increased use of pain medication, reduced adherence to treatment recommendations, and, when recommendations are followed, reduced treatment benefits.
The investigators randomized the participants to the 10-week Internet cognitive behavioral therapy (iCBT) Sadness Program in addition to usual treatment or to a control group that received usual treatment. Participants were followed up at 1 week and 3 months after the intervention. The iCBT Sadness Program consists of six online lessons representing best practice CBT, as well as regular homework assignments and access to supplementary resources. The program has been validated in several clinical efficacy and effectiveness trials.
The iCBT program proved superior to usual treatment on the primary outcomes of depression as measured by the Patient Health Questionnaire (PHQ-9) and psychological distress (measured using Kessler-10). In particular, a large between-group effect size was seen for PHQ-9 scores (Hedge’s g = 1.01; 95% confidence interval [CI], 0.47-1.54) and a medium effect size for the Kessler-10 score (Hedge’s g = 0.75, 95% CI, 0.23-1.28).
At 3-month follow-up, large between-group effect sizes developed between the iCBT group and the usual treatment-only group on the PHQ-9 (Hedge’s g = 0.90, 95% CI, 0.36-1.44) and Kessler-10 (Hedge’s g = 0.94, 95% CI, 0.41-1.48).
In the iCBT group, the intervention produced large effect-size reductions in depression and distress from pre- to posttreatment and from pretreatment to 12-week follow-up, but in the usual treatment-only group, the effect-size reductions were small and not significant.
In terms of clinical significance, the authors noted that 21 (47.7%) of the participants in the iCBT group “reliably improved,” compared with 3 participants (12%) in the usual treatment-only group.
At 3-month follow-up, 33 participants (84.6%) in the iCBT group no longer met the criteria for major depression, compared with 11 (50%) of the control group.
The intervention also improved the secondary outcomes of OA-specific pain, stiffness, and physical functioning on the Western Ontario and McMaster Osteoarthritis Index and Arthritis Self-Efficacy Scale score, but this was seen by the research team only at the 3-month follow-up. They suggested that further studies should evaluate whether the intervention reduced pain-related catastrophic cognitions or sensitivity to pain and improved a patients’ estimation of their own ability and/or adherence to pain management.
“The outcomes of these studies may provide insight into why there were no significant changes in global physical functioning and no between-group differences for arthritis-related self-efficacy, pain, stiffness, and physical function directly following the program, yet differences emerged 3 months later,” the researchers noted.
They suggested that changes in OA-specific variables such as pain or self efficacy likely required time to interact with cognitive change mechanisms that occur through iCBT.
“It is possible that reduced depressive symptoms result in flow-on effects to OA variables over time and perhaps programs that specifically target OA management may show more immediate effects,” they added.
The researchers concluded that a biopsychosocial approach to managing people with OA was consistent with the recent emphasis on a holistic assessment of older patients with OA.
“These findings are significant given iCBT programs can overcome barriers to receiving face-to-face psychological and/or pharmacological treatment in this population, such as cost, lack of accessibility, and pharmacological side-effects and interactions,” the study authors wrote.
“Medical management of OA of the knee could be supplemented with integrated evidence-based depression treatment, including iCBT, to maximize functional status and mental health well-being,” they added.
The study was funded by grants from the Australian National Health and Medical Research Council. The authors declared no relevant conflicts of interest.
Cognitive behavioral therapy administered online can effectively treat symptoms of depression in older people with knee osteoarthritis, according to results from the first randomized trial of its kind.
The benefits of the 10-week program extended to improving pain, stiffness, physical functioning, and self-efficacy, reported Kathleen A. O’Moore, PsyD, of St. Vincent’s Hospital, Sydney, Australia, and her colleagues (Arthritis Care Res. 2017 April 20. doi: 10.1002/acr.23257).
The investigators became motivated to conduct the trial by the fact that about one in five older people with osteoarthritis (OA) experience depression, yet many do not seek treatment, and depression in this population has been associated with an increased use of pain medication, reduced adherence to treatment recommendations, and, when recommendations are followed, reduced treatment benefits.
The investigators randomized the participants to the 10-week Internet cognitive behavioral therapy (iCBT) Sadness Program in addition to usual treatment or to a control group that received usual treatment. Participants were followed up at 1 week and 3 months after the intervention. The iCBT Sadness Program consists of six online lessons representing best practice CBT, as well as regular homework assignments and access to supplementary resources. The program has been validated in several clinical efficacy and effectiveness trials.
The iCBT program proved superior to usual treatment on the primary outcomes of depression as measured by the Patient Health Questionnaire (PHQ-9) and psychological distress (measured using Kessler-10). In particular, a large between-group effect size was seen for PHQ-9 scores (Hedge’s g = 1.01; 95% confidence interval [CI], 0.47-1.54) and a medium effect size for the Kessler-10 score (Hedge’s g = 0.75, 95% CI, 0.23-1.28).
At 3-month follow-up, large between-group effect sizes developed between the iCBT group and the usual treatment-only group on the PHQ-9 (Hedge’s g = 0.90, 95% CI, 0.36-1.44) and Kessler-10 (Hedge’s g = 0.94, 95% CI, 0.41-1.48).
In the iCBT group, the intervention produced large effect-size reductions in depression and distress from pre- to posttreatment and from pretreatment to 12-week follow-up, but in the usual treatment-only group, the effect-size reductions were small and not significant.
In terms of clinical significance, the authors noted that 21 (47.7%) of the participants in the iCBT group “reliably improved,” compared with 3 participants (12%) in the usual treatment-only group.
At 3-month follow-up, 33 participants (84.6%) in the iCBT group no longer met the criteria for major depression, compared with 11 (50%) of the control group.
The intervention also improved the secondary outcomes of OA-specific pain, stiffness, and physical functioning on the Western Ontario and McMaster Osteoarthritis Index and Arthritis Self-Efficacy Scale score, but this was seen by the research team only at the 3-month follow-up. They suggested that further studies should evaluate whether the intervention reduced pain-related catastrophic cognitions or sensitivity to pain and improved a patients’ estimation of their own ability and/or adherence to pain management.
“The outcomes of these studies may provide insight into why there were no significant changes in global physical functioning and no between-group differences for arthritis-related self-efficacy, pain, stiffness, and physical function directly following the program, yet differences emerged 3 months later,” the researchers noted.
They suggested that changes in OA-specific variables such as pain or self efficacy likely required time to interact with cognitive change mechanisms that occur through iCBT.
“It is possible that reduced depressive symptoms result in flow-on effects to OA variables over time and perhaps programs that specifically target OA management may show more immediate effects,” they added.
The researchers concluded that a biopsychosocial approach to managing people with OA was consistent with the recent emphasis on a holistic assessment of older patients with OA.
“These findings are significant given iCBT programs can overcome barriers to receiving face-to-face psychological and/or pharmacological treatment in this population, such as cost, lack of accessibility, and pharmacological side-effects and interactions,” the study authors wrote.
“Medical management of OA of the knee could be supplemented with integrated evidence-based depression treatment, including iCBT, to maximize functional status and mental health well-being,” they added.
The study was funded by grants from the Australian National Health and Medical Research Council. The authors declared no relevant conflicts of interest.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: At 3-month follow-up, 33 participants (84.6%) in the iCBT group no longer met the criteria for major depression, compared with 11 (50%) of the control group.
Data Source: A randomized, controlled trial of 69 adults aged 50 years or older with major depressive disorder and symptomatic osteoarthritis of the knee.
Disclosures: The study was funded by grants from the Australian National Health and Medical Research Council. The authors declared no relevant conflicts of interest.