User login
Certain anaerobic bacteria linked to increased CRC risk
Certain species of anaerobic bacteria have been linked to dramatic increases in colorectal cancer (CRC), often within a year of infection, although whether or not the bacteria are causal has yet to be determined, say Danish researchers.
“We are not convinced that all the bacteria are directly involved in CRC development — they could just be innocent bystanders that invade the blood stream when the cancer [itself] has caused a breach in the intestinal wall,” lead author Ulrik Justesen, MD, Odense University Hospital, Denmark, told Medscape Medical News in an email.
“But an algorithm for colonoscopy based on the [infecting] species, which could then be supplemented with specific characteristics [of the bacteria] along with age, is certainly a realistic perspective,” he added.
The study was to have be presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Paris, France, but the conference was canceled due to COVID-19.
Another study suggesting a link between bacteria and CRC was published earlier this year in Nature, as reported at the time by Medscape Medical News. That study, from the Netherlands, suggests that a strain of Escherichia coli may be involved in the development of CRC.
Population-Based Study
The latest study from Denmark was a population-based study involving over 2 million people.
From this large cohort, blood culture data from the years 2007 and 2016 were analyzed.
“We combined blood culture data with the national register for colorectal cancer — the Danish Colorectal Cancer Group Database — and identified incident CRC after bacteraemia,” the investigators state.
The risk for incident CRC was investigated specifically for the anaerobic bacteria Bacteroides spp, Clostridium spp, and Fusobacterium spp.
Incident rates were then compared to those from nonanaerobic bacteria, including the Streptococcus bovis group, Escherichia coli, and Staphylococcus aureus, as well as from negative blood samples.
“We included 45,760 bacteraemia episodes, of which 492 or 1.1% were diagnosed with CRC after the bacteraemia; 241 ― 0.5% ― within 1 year,” the researchers report.
The risk for CRC was notably increased in association with most anaerobic species, compared with negative blood cultures and with E coli and S aureus cultures, for which the risk was similar to that of negative blood cultures.
For example, infection with C septicum was associated with a 42 times greater risk for CRC within 1 year of infection and a 21 times greater risk overall with no time limitation.
Infection with B ovatus was linked to a 13 times greater risk for CRC within 1 year and a six times greater risk overall with no time limitation.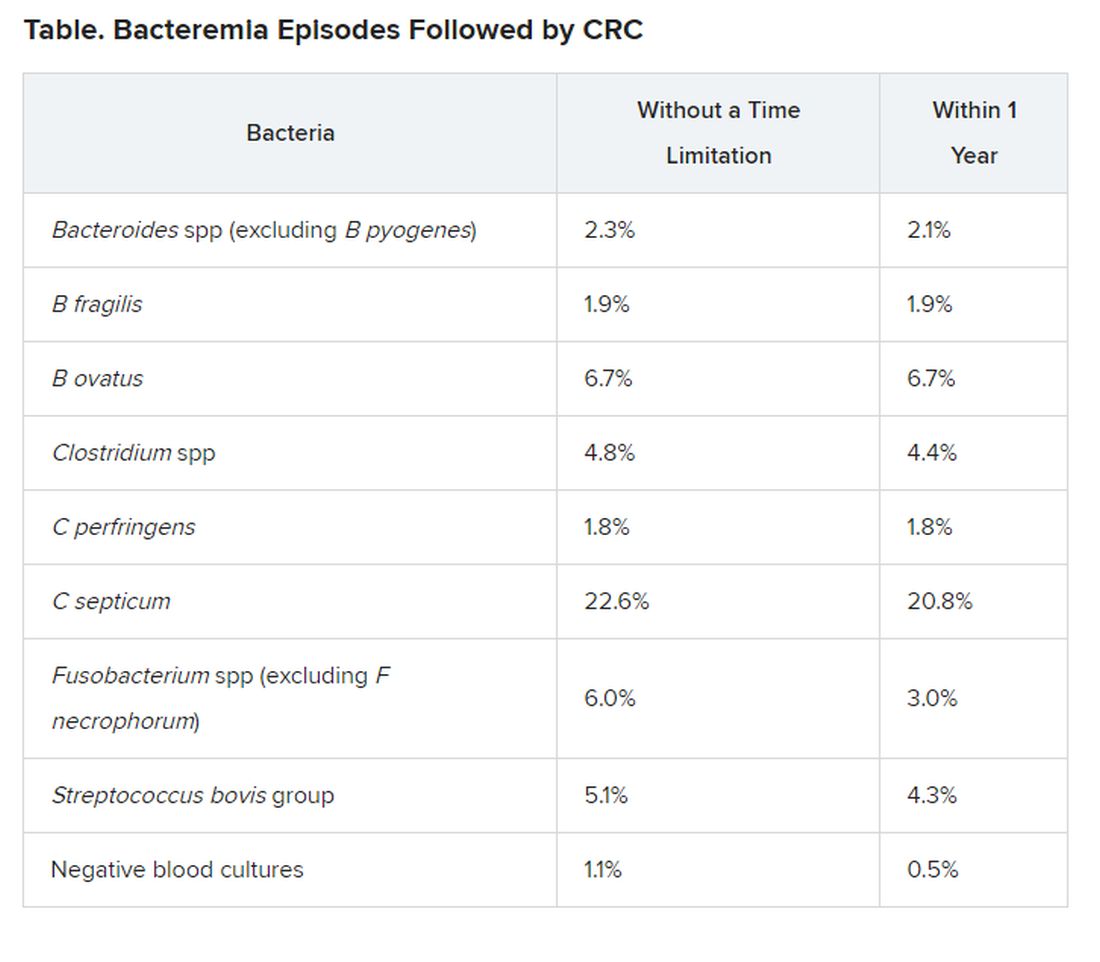
Justesen noted that their group will now focus on specific bacteria from cancer patients in an effort to identify characteristics of the bacteria that could be implicated in cancer development.
“If this is the case, it could be of great importance when it comes to screening and treatment of CRC,” he said in a statement.
For example, if there was evidence that a patient had been infected with C septicum, the anaerobic species associated with the highest risk for CRC within 1 year of infection, “we would immediately inform the treating physician about this risk and that the [patient] should be investigated further,” he told Medscape Medical News.
Justesen also noted that if there was evidence that a patient was infected with any of these high-risk bacteria and the patient was elderly, “then it would definitely be worth screening the patient for CRC,” he said. However, more research is needed before specific recommendations can be made for CRC screening in the context of any anaerobic infection, he stressed.
Justesen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Certain species of anaerobic bacteria have been linked to dramatic increases in colorectal cancer (CRC), often within a year of infection, although whether or not the bacteria are causal has yet to be determined, say Danish researchers.
“We are not convinced that all the bacteria are directly involved in CRC development — they could just be innocent bystanders that invade the blood stream when the cancer [itself] has caused a breach in the intestinal wall,” lead author Ulrik Justesen, MD, Odense University Hospital, Denmark, told Medscape Medical News in an email.
“But an algorithm for colonoscopy based on the [infecting] species, which could then be supplemented with specific characteristics [of the bacteria] along with age, is certainly a realistic perspective,” he added.
The study was to have be presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Paris, France, but the conference was canceled due to COVID-19.
Another study suggesting a link between bacteria and CRC was published earlier this year in Nature, as reported at the time by Medscape Medical News. That study, from the Netherlands, suggests that a strain of Escherichia coli may be involved in the development of CRC.
Population-Based Study
The latest study from Denmark was a population-based study involving over 2 million people.
From this large cohort, blood culture data from the years 2007 and 2016 were analyzed.
“We combined blood culture data with the national register for colorectal cancer — the Danish Colorectal Cancer Group Database — and identified incident CRC after bacteraemia,” the investigators state.
The risk for incident CRC was investigated specifically for the anaerobic bacteria Bacteroides spp, Clostridium spp, and Fusobacterium spp.
Incident rates were then compared to those from nonanaerobic bacteria, including the Streptococcus bovis group, Escherichia coli, and Staphylococcus aureus, as well as from negative blood samples.
“We included 45,760 bacteraemia episodes, of which 492 or 1.1% were diagnosed with CRC after the bacteraemia; 241 ― 0.5% ― within 1 year,” the researchers report.
The risk for CRC was notably increased in association with most anaerobic species, compared with negative blood cultures and with E coli and S aureus cultures, for which the risk was similar to that of negative blood cultures.
For example, infection with C septicum was associated with a 42 times greater risk for CRC within 1 year of infection and a 21 times greater risk overall with no time limitation.
Infection with B ovatus was linked to a 13 times greater risk for CRC within 1 year and a six times greater risk overall with no time limitation.
Justesen noted that their group will now focus on specific bacteria from cancer patients in an effort to identify characteristics of the bacteria that could be implicated in cancer development.
“If this is the case, it could be of great importance when it comes to screening and treatment of CRC,” he said in a statement.
For example, if there was evidence that a patient had been infected with C septicum, the anaerobic species associated with the highest risk for CRC within 1 year of infection, “we would immediately inform the treating physician about this risk and that the [patient] should be investigated further,” he told Medscape Medical News.
Justesen also noted that if there was evidence that a patient was infected with any of these high-risk bacteria and the patient was elderly, “then it would definitely be worth screening the patient for CRC,” he said. However, more research is needed before specific recommendations can be made for CRC screening in the context of any anaerobic infection, he stressed.
Justesen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Certain species of anaerobic bacteria have been linked to dramatic increases in colorectal cancer (CRC), often within a year of infection, although whether or not the bacteria are causal has yet to be determined, say Danish researchers.
“We are not convinced that all the bacteria are directly involved in CRC development — they could just be innocent bystanders that invade the blood stream when the cancer [itself] has caused a breach in the intestinal wall,” lead author Ulrik Justesen, MD, Odense University Hospital, Denmark, told Medscape Medical News in an email.
“But an algorithm for colonoscopy based on the [infecting] species, which could then be supplemented with specific characteristics [of the bacteria] along with age, is certainly a realistic perspective,” he added.
The study was to have be presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Paris, France, but the conference was canceled due to COVID-19.
Another study suggesting a link between bacteria and CRC was published earlier this year in Nature, as reported at the time by Medscape Medical News. That study, from the Netherlands, suggests that a strain of Escherichia coli may be involved in the development of CRC.
Population-Based Study
The latest study from Denmark was a population-based study involving over 2 million people.
From this large cohort, blood culture data from the years 2007 and 2016 were analyzed.
“We combined blood culture data with the national register for colorectal cancer — the Danish Colorectal Cancer Group Database — and identified incident CRC after bacteraemia,” the investigators state.
The risk for incident CRC was investigated specifically for the anaerobic bacteria Bacteroides spp, Clostridium spp, and Fusobacterium spp.
Incident rates were then compared to those from nonanaerobic bacteria, including the Streptococcus bovis group, Escherichia coli, and Staphylococcus aureus, as well as from negative blood samples.
“We included 45,760 bacteraemia episodes, of which 492 or 1.1% were diagnosed with CRC after the bacteraemia; 241 ― 0.5% ― within 1 year,” the researchers report.
The risk for CRC was notably increased in association with most anaerobic species, compared with negative blood cultures and with E coli and S aureus cultures, for which the risk was similar to that of negative blood cultures.
For example, infection with C septicum was associated with a 42 times greater risk for CRC within 1 year of infection and a 21 times greater risk overall with no time limitation.
Infection with B ovatus was linked to a 13 times greater risk for CRC within 1 year and a six times greater risk overall with no time limitation.
Justesen noted that their group will now focus on specific bacteria from cancer patients in an effort to identify characteristics of the bacteria that could be implicated in cancer development.
“If this is the case, it could be of great importance when it comes to screening and treatment of CRC,” he said in a statement.
For example, if there was evidence that a patient had been infected with C septicum, the anaerobic species associated with the highest risk for CRC within 1 year of infection, “we would immediately inform the treating physician about this risk and that the [patient] should be investigated further,” he told Medscape Medical News.
Justesen also noted that if there was evidence that a patient was infected with any of these high-risk bacteria and the patient was elderly, “then it would definitely be worth screening the patient for CRC,” he said. However, more research is needed before specific recommendations can be made for CRC screening in the context of any anaerobic infection, he stressed.
Justesen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Treating lung cancer in COVID-19 times: Update from experts
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer increase driven mainly by NASH in men over 60
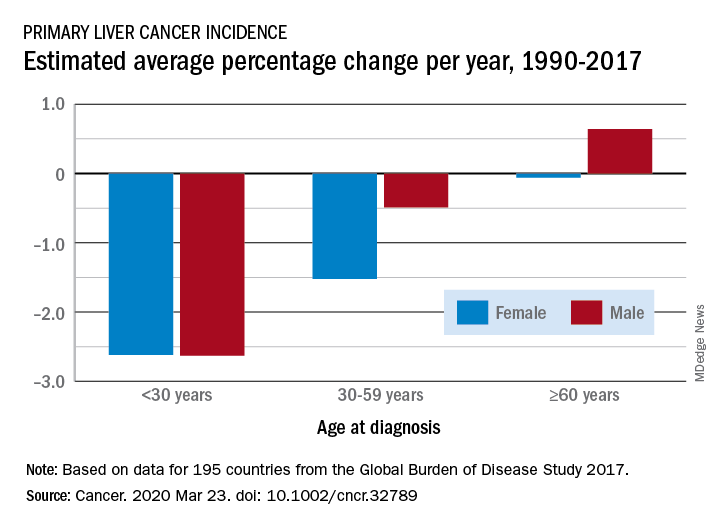
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.
The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.

The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.

The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
RT plus checkpoint blockade active in head and neck cancer
The combination of a phase 2 trial suggests.
“There are convincing arguments that radiation sensitizes patients to immunotherapy and can enhance its effects,” Jared Weiss, MD, associate professor of medicine, UNC Lineberger Comprehensive Cancer, Chapel Hill, North Carolina, said in a statement.
“And the opposite direction also seems to be true – radiation therapy needs a functional immune system to work. Our hope was that pembrolizumab might be a radiation sensitizer for these patients,” he said.
The study was presented at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Both modalities have had some outstanding results in the past, observed Weiss. “If you look back to the historic studies, radiation alone often cures patients with this disease, while some of the first patients treated with pembrolizumab for recurrent/metastatic cancer are still alive many years out, with no evidence of disease,” he said.
“Our concept was that, in addition to whatever synergy the immunotherapy might provide with radiation, we also conceived of it as a ‘second shot on goal’ towards a cure, because there is durable control with drug alone,” he added.
Single-arm trial
The single-arm trial included 29 patients with locally advanced HNSCC.
Only about 10% of patients were current smokers, but more than half of the study group had a history of smoking. Of those, more than 55% had a history of 10 pack-years or more.
In slightly more than one third of patients, the primary site of the cancer was the base of the tongue. The tonsils were the primary site in slightly more than one third.
Platinum ineligibility was defined by provider and standard measures.
More than two thirds of patients were ineligible to receive cisplatin because of preexisting otopathy, including hearing impairment and tinnitus.
The combination of cisplatin and definitive-dose radiotherapy is standard treatment for locally advanced head and neck cancer, but contraindications to cisplatin are common in everyday clinical practice. Weiss noted that contraindications are present in about one third of his patients.
“We replaced standard, every-3-week cisplatin with pembrolizumab every 3 weeks,” Weiss explained, “and we hypothesized that with the ongoing effects of radiation therapy after completion, that additional adjuvant cycles could further sensitize patients [to the effects of radiation] without impairing recovery, so we added three adjuvant cycles as well,” he added.
With six cycles of an every-3-week drug, patients received 18 weeks of pembrolizumab in total.
Echoing results from the previously reported KEYNOTE-48 trial, pembrolizumab given with radiotherapy instead of chemotherapy led to an overall progression-free survival (PFS) rate of 76% at 1 year and an estimated PFS of 71% at 2 years.
At 1 year, 86% of patients were still alive, and at 2 years, an estimated 75% of patients were still alive, Weiss added.
For patients with human papillomavirus 16–positive cancer, rates of PFS and overall survival were slightly better, at 88% and 94%, respectively.
With regard to toxicities, “For the most part, this [treatment regimen] looks like radiation alone with one very notable exception, which was lymphopenia,” Weiss observed. Grade 3-4 lymphopenia affected 59% of patients.
Lymphocyte count hit bottom at week 4, he added, with only partial recovery at week 20 and no further recovery at 40 weeks. Lymphocyte count alone or any change in it was not predictive of early progression.
However, in comparing patients who experienced early disease progression to patients who did not experience progression, levels of baseline naive B cells in peripheral blood were higher and levels of circulating marginal zone B cells were lower in patients with progressive disease, Weiss reported.
Patient-reported outcomes indicated that common symptoms of treatment peaked at week 10, and there was relative recovery by week 20.
As reflected by Functional Assessment of Cancer Therapy (FACT) scores, which include social, emotional, and functional well-being, as well as the head and neck cancer scale, “we again see a nadir at 10 weeks with relative recovery at 20 weeks,” Weiss noted.
“We found that concurrent pembrolizumab with radiotherapy is a safe and feasible option for locally advanced head and neck cancer patients with cisplatin ineligibility,” Weiss concluded.
More research is being conducted in this area, and multiple ongoing studies will further elucidate the value of PD-1 or PD-L1 checkpoint blockade with definitive radiation therapy, he added.
The study was funded by Merck & Co. Weiss’ institution has received research funding from Celgene, Pfizer, Merck, AZ/Medimmmune, Amgen, Carefusion, G1 Therapeutics, Immunicum, Loxo/Lilly, and the Jimmy V Foundation. Weiss has received honoraria for consulting from AstraZeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Loxo/Lilly, Pfizer, and Blueprint had has stock in Nektar and Vesselon.
This article first appeared on Medscape.com.
The combination of a phase 2 trial suggests.
“There are convincing arguments that radiation sensitizes patients to immunotherapy and can enhance its effects,” Jared Weiss, MD, associate professor of medicine, UNC Lineberger Comprehensive Cancer, Chapel Hill, North Carolina, said in a statement.
“And the opposite direction also seems to be true – radiation therapy needs a functional immune system to work. Our hope was that pembrolizumab might be a radiation sensitizer for these patients,” he said.
The study was presented at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Both modalities have had some outstanding results in the past, observed Weiss. “If you look back to the historic studies, radiation alone often cures patients with this disease, while some of the first patients treated with pembrolizumab for recurrent/metastatic cancer are still alive many years out, with no evidence of disease,” he said.
“Our concept was that, in addition to whatever synergy the immunotherapy might provide with radiation, we also conceived of it as a ‘second shot on goal’ towards a cure, because there is durable control with drug alone,” he added.
Single-arm trial
The single-arm trial included 29 patients with locally advanced HNSCC.
Only about 10% of patients were current smokers, but more than half of the study group had a history of smoking. Of those, more than 55% had a history of 10 pack-years or more.
In slightly more than one third of patients, the primary site of the cancer was the base of the tongue. The tonsils were the primary site in slightly more than one third.
Platinum ineligibility was defined by provider and standard measures.
More than two thirds of patients were ineligible to receive cisplatin because of preexisting otopathy, including hearing impairment and tinnitus.
The combination of cisplatin and definitive-dose radiotherapy is standard treatment for locally advanced head and neck cancer, but contraindications to cisplatin are common in everyday clinical practice. Weiss noted that contraindications are present in about one third of his patients.
“We replaced standard, every-3-week cisplatin with pembrolizumab every 3 weeks,” Weiss explained, “and we hypothesized that with the ongoing effects of radiation therapy after completion, that additional adjuvant cycles could further sensitize patients [to the effects of radiation] without impairing recovery, so we added three adjuvant cycles as well,” he added.
With six cycles of an every-3-week drug, patients received 18 weeks of pembrolizumab in total.
Echoing results from the previously reported KEYNOTE-48 trial, pembrolizumab given with radiotherapy instead of chemotherapy led to an overall progression-free survival (PFS) rate of 76% at 1 year and an estimated PFS of 71% at 2 years.
At 1 year, 86% of patients were still alive, and at 2 years, an estimated 75% of patients were still alive, Weiss added.
For patients with human papillomavirus 16–positive cancer, rates of PFS and overall survival were slightly better, at 88% and 94%, respectively.
With regard to toxicities, “For the most part, this [treatment regimen] looks like radiation alone with one very notable exception, which was lymphopenia,” Weiss observed. Grade 3-4 lymphopenia affected 59% of patients.
Lymphocyte count hit bottom at week 4, he added, with only partial recovery at week 20 and no further recovery at 40 weeks. Lymphocyte count alone or any change in it was not predictive of early progression.
However, in comparing patients who experienced early disease progression to patients who did not experience progression, levels of baseline naive B cells in peripheral blood were higher and levels of circulating marginal zone B cells were lower in patients with progressive disease, Weiss reported.
Patient-reported outcomes indicated that common symptoms of treatment peaked at week 10, and there was relative recovery by week 20.
As reflected by Functional Assessment of Cancer Therapy (FACT) scores, which include social, emotional, and functional well-being, as well as the head and neck cancer scale, “we again see a nadir at 10 weeks with relative recovery at 20 weeks,” Weiss noted.
“We found that concurrent pembrolizumab with radiotherapy is a safe and feasible option for locally advanced head and neck cancer patients with cisplatin ineligibility,” Weiss concluded.
More research is being conducted in this area, and multiple ongoing studies will further elucidate the value of PD-1 or PD-L1 checkpoint blockade with definitive radiation therapy, he added.
The study was funded by Merck & Co. Weiss’ institution has received research funding from Celgene, Pfizer, Merck, AZ/Medimmmune, Amgen, Carefusion, G1 Therapeutics, Immunicum, Loxo/Lilly, and the Jimmy V Foundation. Weiss has received honoraria for consulting from AstraZeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Loxo/Lilly, Pfizer, and Blueprint had has stock in Nektar and Vesselon.
This article first appeared on Medscape.com.
The combination of a phase 2 trial suggests.
“There are convincing arguments that radiation sensitizes patients to immunotherapy and can enhance its effects,” Jared Weiss, MD, associate professor of medicine, UNC Lineberger Comprehensive Cancer, Chapel Hill, North Carolina, said in a statement.
“And the opposite direction also seems to be true – radiation therapy needs a functional immune system to work. Our hope was that pembrolizumab might be a radiation sensitizer for these patients,” he said.
The study was presented at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Both modalities have had some outstanding results in the past, observed Weiss. “If you look back to the historic studies, radiation alone often cures patients with this disease, while some of the first patients treated with pembrolizumab for recurrent/metastatic cancer are still alive many years out, with no evidence of disease,” he said.
“Our concept was that, in addition to whatever synergy the immunotherapy might provide with radiation, we also conceived of it as a ‘second shot on goal’ towards a cure, because there is durable control with drug alone,” he added.
Single-arm trial
The single-arm trial included 29 patients with locally advanced HNSCC.
Only about 10% of patients were current smokers, but more than half of the study group had a history of smoking. Of those, more than 55% had a history of 10 pack-years or more.
In slightly more than one third of patients, the primary site of the cancer was the base of the tongue. The tonsils were the primary site in slightly more than one third.
Platinum ineligibility was defined by provider and standard measures.
More than two thirds of patients were ineligible to receive cisplatin because of preexisting otopathy, including hearing impairment and tinnitus.
The combination of cisplatin and definitive-dose radiotherapy is standard treatment for locally advanced head and neck cancer, but contraindications to cisplatin are common in everyday clinical practice. Weiss noted that contraindications are present in about one third of his patients.
“We replaced standard, every-3-week cisplatin with pembrolizumab every 3 weeks,” Weiss explained, “and we hypothesized that with the ongoing effects of radiation therapy after completion, that additional adjuvant cycles could further sensitize patients [to the effects of radiation] without impairing recovery, so we added three adjuvant cycles as well,” he added.
With six cycles of an every-3-week drug, patients received 18 weeks of pembrolizumab in total.
Echoing results from the previously reported KEYNOTE-48 trial, pembrolizumab given with radiotherapy instead of chemotherapy led to an overall progression-free survival (PFS) rate of 76% at 1 year and an estimated PFS of 71% at 2 years.
At 1 year, 86% of patients were still alive, and at 2 years, an estimated 75% of patients were still alive, Weiss added.
For patients with human papillomavirus 16–positive cancer, rates of PFS and overall survival were slightly better, at 88% and 94%, respectively.
With regard to toxicities, “For the most part, this [treatment regimen] looks like radiation alone with one very notable exception, which was lymphopenia,” Weiss observed. Grade 3-4 lymphopenia affected 59% of patients.
Lymphocyte count hit bottom at week 4, he added, with only partial recovery at week 20 and no further recovery at 40 weeks. Lymphocyte count alone or any change in it was not predictive of early progression.
However, in comparing patients who experienced early disease progression to patients who did not experience progression, levels of baseline naive B cells in peripheral blood were higher and levels of circulating marginal zone B cells were lower in patients with progressive disease, Weiss reported.
Patient-reported outcomes indicated that common symptoms of treatment peaked at week 10, and there was relative recovery by week 20.
As reflected by Functional Assessment of Cancer Therapy (FACT) scores, which include social, emotional, and functional well-being, as well as the head and neck cancer scale, “we again see a nadir at 10 weeks with relative recovery at 20 weeks,” Weiss noted.
“We found that concurrent pembrolizumab with radiotherapy is a safe and feasible option for locally advanced head and neck cancer patients with cisplatin ineligibility,” Weiss concluded.
More research is being conducted in this area, and multiple ongoing studies will further elucidate the value of PD-1 or PD-L1 checkpoint blockade with definitive radiation therapy, he added.
The study was funded by Merck & Co. Weiss’ institution has received research funding from Celgene, Pfizer, Merck, AZ/Medimmmune, Amgen, Carefusion, G1 Therapeutics, Immunicum, Loxo/Lilly, and the Jimmy V Foundation. Weiss has received honoraria for consulting from AstraZeneca, EMD Serono, Genentech, Inivata, Celgene, G1 Therapeutics, Jounce Therapeutics, Abbvie, Rakuten, Nanobiotix, Azitra, Loxo/Lilly, Pfizer, and Blueprint had has stock in Nektar and Vesselon.
This article first appeared on Medscape.com.
REPORTING FROM HEAD AND NECK CANCERS SYMPOSIUM 2020
For a time, an old drug helps with PFS in a head and neck cancer
Everolimus, a safe, cheap and well-tolerated drug, prolonged progression-free survival (PFS) compared with placebo during the year patients with advanced head and neck squamous cell carcinoma (HNSCC) were on it, a phase 2 study indicates.
However, once discontinued, the PFS advantage in favor of active therapy was no longer significant at 2 years, the same study suggests.
“The 5-year survival rate for advanced head and neck HPV [human papillomavirus]-negative smokers is dismal; hence the need for adjuvant therapy after a complete response to definitive therapy,” Cherie-Ann Nathan, MD, of Louisiana State University Health in Shreveport, Louisiana, said at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“[Since] their survival rates have not changed in decades despite advances in surgery, radiation therapy, and chemotherapy, these findings indicate that patients at high risk for tumor relapse could be given mTOR inhibitors to stall progression and keep any residual cancer cells from growing,” she added in a statement.
Advanced HNSCC
The investigator-initiated trial randomly assigned 28 patients with advanced HNSCC to everolimus 10 mg orally once daily or placebo for a maximum of 1 year or until disease progression, whichever came first.
Patients had stage IV HNSCC but had to be disease-free clinically and radiologically following definitive treatment with chemoradiation or surgery followed by chemoradiation. There was no difference in the type of definitive treatment received prior to the intervention between the two groups.
Adjuvant therapy was initiated between 8 and 16 weeks after completing definitive therapy.
If patients had HPV-positive oropharyngeal cancer, they had to have a minimum of 10 pack-years of smoking history.
“The primary endpoint was PFS at 2 years; the secondary endpoint was toxicity,” Nathan observed.
Oral mucositis and leukopenia were common but only 7% of patients developed grade 3 mucositis or leukopenia.
Other grade 3 or greater toxicities were reported in 16 patients and were similar to the adverse events (AEs) noted in other trials with everolimus. Only two patients developed serious AEs possibly related to the drug.
At 1 year, 81% of patients on everolimus were disease-free compared with 57% of patients on placebo (P = .04), Nathan reported.
However, at 2 years, PFS – although continuing to favor those treated with adjuvant therapy – was no longer significant even though it was clear that during the year patients were receiving treatment, “there was a consistent, protective effect of everolimus,” Nathan suggested.
Special effect among TP53-mutated patients?
Targeted exon sequencing was also carried out, the results from which showed that TP53 was the most commonly mutated gene.
“As expected, HPV-negative tumors were more likely to be mutated for TP53,” Nathan observed. Approximately 80% of HPV-negative smoking-related HNSCC tumors carry the TP53 mutation.
Interestingly, survival rates were significantly higher in TP53-mutated patients treated with everolimus: 70% of the patients were still alive at 2 years compared with only 22% of placebo controls (P = .026), she said.
This is a surprising finding, Nathan suggested, as patients with TP53 mutations traditionally have worse survival than those without, suggesting that these patients in particular appear to benefit from adjuvant everolimus.
“Everolimus is used for patients with breast cancer or renal cell cancer for extended periods without major side effects and there is potential for patients with TP53-mutated head and neck disease to see a survival benefit as well,” Nathan speculated.
However, additional trials are needed to confirm the link between the TP53 mutation and survival and to assess the safety of keeping patients with HNSCC on an mTOR inhibitor for longer than 1 year.
The study was funded by Novartis. Nathan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Everolimus, a safe, cheap and well-tolerated drug, prolonged progression-free survival (PFS) compared with placebo during the year patients with advanced head and neck squamous cell carcinoma (HNSCC) were on it, a phase 2 study indicates.
However, once discontinued, the PFS advantage in favor of active therapy was no longer significant at 2 years, the same study suggests.
“The 5-year survival rate for advanced head and neck HPV [human papillomavirus]-negative smokers is dismal; hence the need for adjuvant therapy after a complete response to definitive therapy,” Cherie-Ann Nathan, MD, of Louisiana State University Health in Shreveport, Louisiana, said at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“[Since] their survival rates have not changed in decades despite advances in surgery, radiation therapy, and chemotherapy, these findings indicate that patients at high risk for tumor relapse could be given mTOR inhibitors to stall progression and keep any residual cancer cells from growing,” she added in a statement.
Advanced HNSCC
The investigator-initiated trial randomly assigned 28 patients with advanced HNSCC to everolimus 10 mg orally once daily or placebo for a maximum of 1 year or until disease progression, whichever came first.
Patients had stage IV HNSCC but had to be disease-free clinically and radiologically following definitive treatment with chemoradiation or surgery followed by chemoradiation. There was no difference in the type of definitive treatment received prior to the intervention between the two groups.
Adjuvant therapy was initiated between 8 and 16 weeks after completing definitive therapy.
If patients had HPV-positive oropharyngeal cancer, they had to have a minimum of 10 pack-years of smoking history.
“The primary endpoint was PFS at 2 years; the secondary endpoint was toxicity,” Nathan observed.
Oral mucositis and leukopenia were common but only 7% of patients developed grade 3 mucositis or leukopenia.
Other grade 3 or greater toxicities were reported in 16 patients and were similar to the adverse events (AEs) noted in other trials with everolimus. Only two patients developed serious AEs possibly related to the drug.
At 1 year, 81% of patients on everolimus were disease-free compared with 57% of patients on placebo (P = .04), Nathan reported.
However, at 2 years, PFS – although continuing to favor those treated with adjuvant therapy – was no longer significant even though it was clear that during the year patients were receiving treatment, “there was a consistent, protective effect of everolimus,” Nathan suggested.
Special effect among TP53-mutated patients?
Targeted exon sequencing was also carried out, the results from which showed that TP53 was the most commonly mutated gene.
“As expected, HPV-negative tumors were more likely to be mutated for TP53,” Nathan observed. Approximately 80% of HPV-negative smoking-related HNSCC tumors carry the TP53 mutation.
Interestingly, survival rates were significantly higher in TP53-mutated patients treated with everolimus: 70% of the patients were still alive at 2 years compared with only 22% of placebo controls (P = .026), she said.
This is a surprising finding, Nathan suggested, as patients with TP53 mutations traditionally have worse survival than those without, suggesting that these patients in particular appear to benefit from adjuvant everolimus.
“Everolimus is used for patients with breast cancer or renal cell cancer for extended periods without major side effects and there is potential for patients with TP53-mutated head and neck disease to see a survival benefit as well,” Nathan speculated.
However, additional trials are needed to confirm the link between the TP53 mutation and survival and to assess the safety of keeping patients with HNSCC on an mTOR inhibitor for longer than 1 year.
The study was funded by Novartis. Nathan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Everolimus, a safe, cheap and well-tolerated drug, prolonged progression-free survival (PFS) compared with placebo during the year patients with advanced head and neck squamous cell carcinoma (HNSCC) were on it, a phase 2 study indicates.
However, once discontinued, the PFS advantage in favor of active therapy was no longer significant at 2 years, the same study suggests.
“The 5-year survival rate for advanced head and neck HPV [human papillomavirus]-negative smokers is dismal; hence the need for adjuvant therapy after a complete response to definitive therapy,” Cherie-Ann Nathan, MD, of Louisiana State University Health in Shreveport, Louisiana, said at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“[Since] their survival rates have not changed in decades despite advances in surgery, radiation therapy, and chemotherapy, these findings indicate that patients at high risk for tumor relapse could be given mTOR inhibitors to stall progression and keep any residual cancer cells from growing,” she added in a statement.
Advanced HNSCC
The investigator-initiated trial randomly assigned 28 patients with advanced HNSCC to everolimus 10 mg orally once daily or placebo for a maximum of 1 year or until disease progression, whichever came first.
Patients had stage IV HNSCC but had to be disease-free clinically and radiologically following definitive treatment with chemoradiation or surgery followed by chemoradiation. There was no difference in the type of definitive treatment received prior to the intervention between the two groups.
Adjuvant therapy was initiated between 8 and 16 weeks after completing definitive therapy.
If patients had HPV-positive oropharyngeal cancer, they had to have a minimum of 10 pack-years of smoking history.
“The primary endpoint was PFS at 2 years; the secondary endpoint was toxicity,” Nathan observed.
Oral mucositis and leukopenia were common but only 7% of patients developed grade 3 mucositis or leukopenia.
Other grade 3 or greater toxicities were reported in 16 patients and were similar to the adverse events (AEs) noted in other trials with everolimus. Only two patients developed serious AEs possibly related to the drug.
At 1 year, 81% of patients on everolimus were disease-free compared with 57% of patients on placebo (P = .04), Nathan reported.
However, at 2 years, PFS – although continuing to favor those treated with adjuvant therapy – was no longer significant even though it was clear that during the year patients were receiving treatment, “there was a consistent, protective effect of everolimus,” Nathan suggested.
Special effect among TP53-mutated patients?
Targeted exon sequencing was also carried out, the results from which showed that TP53 was the most commonly mutated gene.
“As expected, HPV-negative tumors were more likely to be mutated for TP53,” Nathan observed. Approximately 80% of HPV-negative smoking-related HNSCC tumors carry the TP53 mutation.
Interestingly, survival rates were significantly higher in TP53-mutated patients treated with everolimus: 70% of the patients were still alive at 2 years compared with only 22% of placebo controls (P = .026), she said.
This is a surprising finding, Nathan suggested, as patients with TP53 mutations traditionally have worse survival than those without, suggesting that these patients in particular appear to benefit from adjuvant everolimus.
“Everolimus is used for patients with breast cancer or renal cell cancer for extended periods without major side effects and there is potential for patients with TP53-mutated head and neck disease to see a survival benefit as well,” Nathan speculated.
However, additional trials are needed to confirm the link between the TP53 mutation and survival and to assess the safety of keeping patients with HNSCC on an mTOR inhibitor for longer than 1 year.
The study was funded by Novartis. Nathan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
REPORTING FROM HEAD AND NECK CANCERS SYMPOSIUM 2020
Endocrine Society advises on use of romosozumab for osteoporosis
Latest guidelines on the treatment of osteoporosis have been released that include new recommendations for the use of romosozumab (Evenity) in postmenopausal women with severe osteoporosis, but they contain caveats as to which women should – and should not – receive the drug.
The updated clinical practice guideline from the Endocrine Society is in response to the approval of romosozumab by the Food and Drug Administration (FDA) in April 2019, and more recently, by the European Medicines Agency.
It was published online February 18 in the Journal of Clinical Endocrinology & Metabolism.
In the new guidelines, committee members recommend the use of romosozumab for postmenopausal women with osteoporosis at very high risk of fracture. Candidates would include women with severe osteoporosis (T-score of less than –2.5 and a prior fracture) or women with a history of multiple vertebral fractures.
Women should be treated with romosozumab for up to 1 year, followed by an antiresorptive agent to maintain bone mineral density gains and further reduce fracture risk.
“The recommended dosage is 210 mg monthly by subcutaneous injection for 12 months,” the authors wrote.
However, and very importantly, romosozumab should not be considered for women at high risk of cardiovascular disease (CVD) or cerebrovascular disease. A high risk of CVD includes women who have had a previous myocardial infarction (MI) or stroke.
Experts questioned by Medscape Medical News stressed that romosozumab should not be a first-line, or even generally a second-line, option for osteoporosis, but it can be a considered for select patients with severe osteoporosis, taking into account CV risk.
Boxed warning
In the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH), there were more major adverse cardiovascular events (MACE) in the first year of the trial with romosozumab, and patients had a 31% higher risk of MACE with romosozumab, compared with the bisphosphonate alendronate.
As a result, the drug was initially rejected by a number of regulatory agencies.
In the United States and Canada, it was eventually approved with a boxed warning, which cautions against the use of the drug in patients at risk for myocardial infarction, stroke, and CVD-related death.
“Romosozumab offers promising results for postmenopausal women with severe osteoporosis or who have a history of fractures,” Clifford Rosen, MD, Maine Medical Center Research Institute in Scarborough and chair of the writing committee, said in an Endocrine Society statement. “It does, however, come with a risk of heart disease, so clinicians need to be careful when selecting patients for this therapy.”
Exact risk unknown
Asked by Medscape Medical News to comment, Kenneth Saag, MD, professor of medicine, University of Alabama at Birmingham and principle investigator of the ARCH study, said that physicians needed more data from real-world studies to resolve the issue around whether romosozumab heightens the risk of CV events in women with osteoporosis or whether that particular finding from ARCH was an artifact.
“Women who have had a recent cardiovascular event should not receive the drug,” he said, agreeing with the new guidelines.
But it remains unclear, for example, whether women who are at slightly higher risk of having a CV event by virtue of their age alone, are also at risk, he noted.
In the meantime, results from the ARCH study clearly showed that not only was romosozumab more effective than alendronate, “but it is more effective than other bone-building drugs as well,” Dr. Saag observed, and it leads to a significantly greater reduction in vertebral, nonvertebral, and hip fractures, compared with the alendronate, the current standard of care in osteoporosis.
“In patients who have very severe osteoporosis and who have had a recent fracture or who are at risk for imminent future fracture, physicians need to balance the benefit versus the risk in favor of using romosozumab,” Dr. Saag suggested.
“And while I would say most women prefer not to inject themselves, the women I have put on this medicine have all had recent fractures and they are very aware of the pain and the disability of having a broken bone, so it is something they are willing to do,” he added.
Not for all women
Giving his opinion, Bart Clarke, MD, of the Mayo Clinic in Rochester, Minnesota, underscored the fact that the Fracture Study in Postmenopausal Women With Osteoporosis (FRAME), again conducted in postmenopausal women, showed no increase in CV events in patients treated with romosozumab compared with placebo.
“So there are questions about what this means, because if these events really were a drug effect, then that effect would be even more evident compared with placebo and they did not see any signal of CV events [in FRAME],” said Dr. Clarke, past president of the American Society of Bone and Mineral Research.
Like everything else in medicine, “there is always some risk,” Dr. Clarke observed.
However, what physicians should do is talk to women, ensure they have not had either an MI or stroke in the last year, and if patients have severe osteoporosis and a high risk of fracture, “then we can say, here’s another option,” he suggested.
“Then, if a woman develops chest pain or shortness of breath while on the drug, [she needs] to let us know ,and then we’ll stop the drug and reassess the situation,” he added.
Dr. Clarke also pointed out that if the FDA had received further reports of CV events linked to romosozumab, physicians would know about it by now, but to his knowledge, there has been no change to the drug’s current warning label.
Furthermore, neither he nor any of his colleagues who treat metabolic bone disease at the Mayo Clinic has seen a single CV event in patients prescribed the agent.
“This is not a drug we would use as first-line for most patients, and we don’t even use it as second-line for most patients, but in people who have not responded to other drugs or who have had terrible things happen to them already, hip fracture especially, then we are saying, you can consider this, he concluded.
The guidelines were supported by the Endocrine Society. Dr. Rosen and Dr. Clarke reported no relevant financial relationships. Dr. Saag reported receiving grants and personal fees from Amgen, personal fees from Radius, and is a consultant for Amgen, Radius, Roche, and Daiichi Sankyo.
This article first appeared on Medscape.com.
Latest guidelines on the treatment of osteoporosis have been released that include new recommendations for the use of romosozumab (Evenity) in postmenopausal women with severe osteoporosis, but they contain caveats as to which women should – and should not – receive the drug.
The updated clinical practice guideline from the Endocrine Society is in response to the approval of romosozumab by the Food and Drug Administration (FDA) in April 2019, and more recently, by the European Medicines Agency.
It was published online February 18 in the Journal of Clinical Endocrinology & Metabolism.
In the new guidelines, committee members recommend the use of romosozumab for postmenopausal women with osteoporosis at very high risk of fracture. Candidates would include women with severe osteoporosis (T-score of less than –2.5 and a prior fracture) or women with a history of multiple vertebral fractures.
Women should be treated with romosozumab for up to 1 year, followed by an antiresorptive agent to maintain bone mineral density gains and further reduce fracture risk.
“The recommended dosage is 210 mg monthly by subcutaneous injection for 12 months,” the authors wrote.
However, and very importantly, romosozumab should not be considered for women at high risk of cardiovascular disease (CVD) or cerebrovascular disease. A high risk of CVD includes women who have had a previous myocardial infarction (MI) or stroke.
Experts questioned by Medscape Medical News stressed that romosozumab should not be a first-line, or even generally a second-line, option for osteoporosis, but it can be a considered for select patients with severe osteoporosis, taking into account CV risk.
Boxed warning
In the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH), there were more major adverse cardiovascular events (MACE) in the first year of the trial with romosozumab, and patients had a 31% higher risk of MACE with romosozumab, compared with the bisphosphonate alendronate.
As a result, the drug was initially rejected by a number of regulatory agencies.
In the United States and Canada, it was eventually approved with a boxed warning, which cautions against the use of the drug in patients at risk for myocardial infarction, stroke, and CVD-related death.
“Romosozumab offers promising results for postmenopausal women with severe osteoporosis or who have a history of fractures,” Clifford Rosen, MD, Maine Medical Center Research Institute in Scarborough and chair of the writing committee, said in an Endocrine Society statement. “It does, however, come with a risk of heart disease, so clinicians need to be careful when selecting patients for this therapy.”
Exact risk unknown
Asked by Medscape Medical News to comment, Kenneth Saag, MD, professor of medicine, University of Alabama at Birmingham and principle investigator of the ARCH study, said that physicians needed more data from real-world studies to resolve the issue around whether romosozumab heightens the risk of CV events in women with osteoporosis or whether that particular finding from ARCH was an artifact.
“Women who have had a recent cardiovascular event should not receive the drug,” he said, agreeing with the new guidelines.
But it remains unclear, for example, whether women who are at slightly higher risk of having a CV event by virtue of their age alone, are also at risk, he noted.
In the meantime, results from the ARCH study clearly showed that not only was romosozumab more effective than alendronate, “but it is more effective than other bone-building drugs as well,” Dr. Saag observed, and it leads to a significantly greater reduction in vertebral, nonvertebral, and hip fractures, compared with the alendronate, the current standard of care in osteoporosis.
“In patients who have very severe osteoporosis and who have had a recent fracture or who are at risk for imminent future fracture, physicians need to balance the benefit versus the risk in favor of using romosozumab,” Dr. Saag suggested.
“And while I would say most women prefer not to inject themselves, the women I have put on this medicine have all had recent fractures and they are very aware of the pain and the disability of having a broken bone, so it is something they are willing to do,” he added.
Not for all women
Giving his opinion, Bart Clarke, MD, of the Mayo Clinic in Rochester, Minnesota, underscored the fact that the Fracture Study in Postmenopausal Women With Osteoporosis (FRAME), again conducted in postmenopausal women, showed no increase in CV events in patients treated with romosozumab compared with placebo.
“So there are questions about what this means, because if these events really were a drug effect, then that effect would be even more evident compared with placebo and they did not see any signal of CV events [in FRAME],” said Dr. Clarke, past president of the American Society of Bone and Mineral Research.
Like everything else in medicine, “there is always some risk,” Dr. Clarke observed.
However, what physicians should do is talk to women, ensure they have not had either an MI or stroke in the last year, and if patients have severe osteoporosis and a high risk of fracture, “then we can say, here’s another option,” he suggested.
“Then, if a woman develops chest pain or shortness of breath while on the drug, [she needs] to let us know ,and then we’ll stop the drug and reassess the situation,” he added.
Dr. Clarke also pointed out that if the FDA had received further reports of CV events linked to romosozumab, physicians would know about it by now, but to his knowledge, there has been no change to the drug’s current warning label.
Furthermore, neither he nor any of his colleagues who treat metabolic bone disease at the Mayo Clinic has seen a single CV event in patients prescribed the agent.
“This is not a drug we would use as first-line for most patients, and we don’t even use it as second-line for most patients, but in people who have not responded to other drugs or who have had terrible things happen to them already, hip fracture especially, then we are saying, you can consider this, he concluded.
The guidelines were supported by the Endocrine Society. Dr. Rosen and Dr. Clarke reported no relevant financial relationships. Dr. Saag reported receiving grants and personal fees from Amgen, personal fees from Radius, and is a consultant for Amgen, Radius, Roche, and Daiichi Sankyo.
This article first appeared on Medscape.com.
Latest guidelines on the treatment of osteoporosis have been released that include new recommendations for the use of romosozumab (Evenity) in postmenopausal women with severe osteoporosis, but they contain caveats as to which women should – and should not – receive the drug.
The updated clinical practice guideline from the Endocrine Society is in response to the approval of romosozumab by the Food and Drug Administration (FDA) in April 2019, and more recently, by the European Medicines Agency.
It was published online February 18 in the Journal of Clinical Endocrinology & Metabolism.
In the new guidelines, committee members recommend the use of romosozumab for postmenopausal women with osteoporosis at very high risk of fracture. Candidates would include women with severe osteoporosis (T-score of less than –2.5 and a prior fracture) or women with a history of multiple vertebral fractures.
Women should be treated with romosozumab for up to 1 year, followed by an antiresorptive agent to maintain bone mineral density gains and further reduce fracture risk.
“The recommended dosage is 210 mg monthly by subcutaneous injection for 12 months,” the authors wrote.
However, and very importantly, romosozumab should not be considered for women at high risk of cardiovascular disease (CVD) or cerebrovascular disease. A high risk of CVD includes women who have had a previous myocardial infarction (MI) or stroke.
Experts questioned by Medscape Medical News stressed that romosozumab should not be a first-line, or even generally a second-line, option for osteoporosis, but it can be a considered for select patients with severe osteoporosis, taking into account CV risk.
Boxed warning
In the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH), there were more major adverse cardiovascular events (MACE) in the first year of the trial with romosozumab, and patients had a 31% higher risk of MACE with romosozumab, compared with the bisphosphonate alendronate.
As a result, the drug was initially rejected by a number of regulatory agencies.
In the United States and Canada, it was eventually approved with a boxed warning, which cautions against the use of the drug in patients at risk for myocardial infarction, stroke, and CVD-related death.
“Romosozumab offers promising results for postmenopausal women with severe osteoporosis or who have a history of fractures,” Clifford Rosen, MD, Maine Medical Center Research Institute in Scarborough and chair of the writing committee, said in an Endocrine Society statement. “It does, however, come with a risk of heart disease, so clinicians need to be careful when selecting patients for this therapy.”
Exact risk unknown
Asked by Medscape Medical News to comment, Kenneth Saag, MD, professor of medicine, University of Alabama at Birmingham and principle investigator of the ARCH study, said that physicians needed more data from real-world studies to resolve the issue around whether romosozumab heightens the risk of CV events in women with osteoporosis or whether that particular finding from ARCH was an artifact.
“Women who have had a recent cardiovascular event should not receive the drug,” he said, agreeing with the new guidelines.
But it remains unclear, for example, whether women who are at slightly higher risk of having a CV event by virtue of their age alone, are also at risk, he noted.
In the meantime, results from the ARCH study clearly showed that not only was romosozumab more effective than alendronate, “but it is more effective than other bone-building drugs as well,” Dr. Saag observed, and it leads to a significantly greater reduction in vertebral, nonvertebral, and hip fractures, compared with the alendronate, the current standard of care in osteoporosis.
“In patients who have very severe osteoporosis and who have had a recent fracture or who are at risk for imminent future fracture, physicians need to balance the benefit versus the risk in favor of using romosozumab,” Dr. Saag suggested.
“And while I would say most women prefer not to inject themselves, the women I have put on this medicine have all had recent fractures and they are very aware of the pain and the disability of having a broken bone, so it is something they are willing to do,” he added.
Not for all women
Giving his opinion, Bart Clarke, MD, of the Mayo Clinic in Rochester, Minnesota, underscored the fact that the Fracture Study in Postmenopausal Women With Osteoporosis (FRAME), again conducted in postmenopausal women, showed no increase in CV events in patients treated with romosozumab compared with placebo.
“So there are questions about what this means, because if these events really were a drug effect, then that effect would be even more evident compared with placebo and they did not see any signal of CV events [in FRAME],” said Dr. Clarke, past president of the American Society of Bone and Mineral Research.
Like everything else in medicine, “there is always some risk,” Dr. Clarke observed.
However, what physicians should do is talk to women, ensure they have not had either an MI or stroke in the last year, and if patients have severe osteoporosis and a high risk of fracture, “then we can say, here’s another option,” he suggested.
“Then, if a woman develops chest pain or shortness of breath while on the drug, [she needs] to let us know ,and then we’ll stop the drug and reassess the situation,” he added.
Dr. Clarke also pointed out that if the FDA had received further reports of CV events linked to romosozumab, physicians would know about it by now, but to his knowledge, there has been no change to the drug’s current warning label.
Furthermore, neither he nor any of his colleagues who treat metabolic bone disease at the Mayo Clinic has seen a single CV event in patients prescribed the agent.
“This is not a drug we would use as first-line for most patients, and we don’t even use it as second-line for most patients, but in people who have not responded to other drugs or who have had terrible things happen to them already, hip fracture especially, then we are saying, you can consider this, he concluded.
The guidelines were supported by the Endocrine Society. Dr. Rosen and Dr. Clarke reported no relevant financial relationships. Dr. Saag reported receiving grants and personal fees from Amgen, personal fees from Radius, and is a consultant for Amgen, Radius, Roche, and Daiichi Sankyo.
This article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Drug recall in U.S. causes extreme hardship for hypoparathyroid patients
The recall of injectable recombinant parathyroid hormone (PTH; Natpara) in the United States over safety concerns about the product is gravely affecting the lives of American patients with severe hypoparathyroidism who need replacement PTH therapy to adequately control symptoms.
Approximately 2,700 patients in the United States have been affected by the recall of Natpara, according to one of the manufacturers, Takeda. Those who were taking it have had to transition back to controlling their symptoms with just oral medications, including active vitamin D or calcitriol (Rocaltrol) plus calcium supplements, which has had a negative impact on the management of their disease in most cases, with some patients hospitalized because of severe hypocalcemia.
One patient related in an interview that even if the transition from injectable PTH back to calcium supplements and calcitriol goes smoothly, individuals can still feel pretty awful because the benefits of the Natpara are subtle – patients just feel better on it. And one report indicates at least 100 patients who had to stop Natpara because of the Food and Drug Administration recall have been hospitalized.
“It’s been a rough time for all patients previously on Natpara adjusting to oral medications after they have been on a replacement therapy,” endocrinologist Dolores Shoback, MD, University of California, San Francisco, said in an email.
Aliya Khan, MD, agrees: “This molecule ... is life changing ... [It] makes a huge difference in patients’ quality of life and in their ability to function normally.”
“The FDA in the United States made the recall decision, and we have to respect that,” added Dr. Khan, a professor of clinical medicine at McMaster University in Hamilton, Ontario.
“We need to address patients’ needs and [try to] reinstate it,” Dr. Khan added, who is the lead author of recent guidelines on the treatment of hypoparathyroidism and who has received research funding from Takeda and Ascendis Pharma, both of which make injectable PTH.
Dr. Kahn also has advice for U.S. endocrinologists who are having to deal with patients who were previously on Natpara and no longer have access to the drug.
She explains how they can transition patients back to other available therapies.
Oral treatments a 'Band-Aid'
Hypoparathyroidism is a rare endocrine disorder that affects approximately 60,000 people in the United States. The FDA approved Natpara as an orphan drug and as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism in January 2015.
The FDA issued the recall for Natpara in September, out of concern that rubber particulate matter originating from the rubber septum of the drug’s cartridge might be contaminating the product.
But the European Medicines Agency, which approved the product under the brand name Natpar in 2017, has not reached the same decision as the FDA; patients in Europe who qualify for injectable PTH still have access to the drug.
As Dr. Khan explained in an interview, PTH is critical for the control of calcium and phosphate homeostasis.
When functioning normally, the parathyroid glands are able to regulate blood calcium levels very finely, sensing how much calcium is being resorbed through the kidney, how much is going in and out of bone, how active vitamin D levels are, and the amount the body is absorbing from the bowel and bone. “It’s all being very carefully fine-tuned continuously,” she said.
Patients with hypoparathyroidism suffer from very low levels of serum calcium, which can, in turn, lead to seizures and cardiac irregularities as well as bronchospasm and even respiratory failure. Magnesium levels can also be dysregulated by hypocalcemia.
“All these are serious results of very low calcium levels,” Dr. Khan emphasized.
These patients “are also not able to eliminate phosphate through the kidneys so their phosphate levels rise,” she added.
Standard treatment for hypoparathyroidism is active vitamin D or calcitriol plus oral calcium supplements.
However, for patients with severe hypoparathyroidism, for whom injectable PTH is indicated, standard treatment is like a “Band-Aid” because what they really need is replacement PTH to help them regulate calcium levels as finely as possible, Dr. Khan said.
High risk for severe hypocalcemia
If PTH is stopped abruptly, calcium levels will likely plummet, a phenomenon sometimes referred to as hungry bone syndrome, placing patients at risk for the consequences of severe hypocalcemia, Dr. Khan added.
(Hungry bone syndrome refers to the rapid, profound, and prolonged hypocalcemia associated with hypophosphatemia and hypomagnesemia, which is exacerbated by suppressed PTH).
One report suggests at least 100 patients who had to stop Natpara because of the FDA recall had to seek hospitalization to have calcium levels restored intravenously.
And even if the transition from injectable PTH back to calcium supplements and calcitriol goes smoothly, patients can still feel pretty awful.
“When you look at my blood levels on and off Natpara, they look very similar,” Danette Astolfi, a former Natpara user in the United States who recently transitioned from the injectable to standard of care, said in an interview.
“But I felt much, much better on Natpara, so this is an interesting ‘wild card’ that patients have with this drug – they just feel better on it. They don’t have the fatigue, they don’t have the brain fog, they don’t have the aches and pains even if their calcium levels are normal,” Ms. Astolfi said.
So patients who are candidates for injectable PTH therapy are by definition those who do not do very well on standard-of-care calcium and calcitriol.
“With the recall, patients are going back to this regimen they already did not have great success with,” Ms. Astolfi explained. “And that’s the tricky part because if you fall into this category, as I do, there is not much you can do about it.”
Ms. Astolfi, who is also on the board of directors of the Hypoparathyroidism Association in Pennsylvania, was lucky because she did not have to stop treatment with injectable PTH abruptly.
“I was fortunate. My physician did not want me to stop the drug right away, so we had time to develop a discontinuation plan,” she noted.
Transitioning safely to other therapies
Dr. Khan has advice for endocrinologists dealing with patients who were previously on Natpara and no longer have access to the drug.
First, “endocrinologists need to be aware that when you stop PTH treatment suddenly, patients’ need for calcium supplements and calcitriol may be as high as two times what they were on before they started PTH therapy,” she noted.
Physicians also need to follow patients closely by monitoring not only calcium and vitamin D levels but also phosphate and magnesium almost daily.
Dr. Khan said she does this because it’s difficult to predict how much calcium and calcitriol a patient will require and doses will likely have to be titrated up or down based on lab results.
It is noteworthy that a joint statement on the Natpara recall by the Endocrine Society and the American Society of Bone and Mineral Research (ASBMR) also emphasizes these points.
And Dr. Khan – as well as the Endocrine Society and ASBMR – also point out that patients may be transitioned from Natpara to teriparatide (Forteo), another recombinant form of PTH that is licensed for use in osteoporosis but has not been approved for hypoparathyroidism.
In Dr. Khan’s experience, patients often do quite well on teriparatide.
The big downside of prescribing teriparatide in the United States, however, is that the drug has to be used off label, and as such, insurance companies are unlikely to pick up the tab for what is an expensive treatment, she explained.
Furthermore, teriparatide has a very short shelf-life once injected so patients may need to use up to four needles a day to stabilize calcium levels.
If teriparatide is prescribed, subcutaneous injections given in the thigh are recommended for the treatment of hypoparathyroidism, according to the joint Endocrine Society/ASBMR statement.
Takeda vs. FDA
In the meantime, Takeda says it has been in regular contact with the FDA to try and work out how to bring Natpara back to U.S. patients who require it for symptom control.
Current outstanding issues between the company and FDA involve considerations related to dose accuracy, safety, and supply continuity, which the company hopes will be resolved in a timely manner.
Furthermore, shortly after the recall order, the company created a special use program that continues to support patients previously prescribed Natpara who would otherwise be at extreme risk of serious complications if forced to stop taking the drug.
“Originally intended for an extremely limited number of patients, the special use program has now enrolled approximately 300 patients,” Cheryl Schwartz, head, U.S. Hematology & Rare Business Unit, Takeda, said in a letter to members of the U.S. Hypoparathyroidism Association on Nov. 21.
The number is higher than expected and reflects the significant treatment needs of patients with serious disease, Ms. Schwartz noted.
“I want to reiterate that we do understand and sincerely regret the impact the Natpara recall is having on patients. We will provide another update as soon as we have more information to share. We continue to work around the clock to find ways to bring Natpara back to the broader hypoparathyroidism community,” she added.
“Patient safety always has been, and continues to be, the highest priority for Takeda,” she said.
Final efforts
Dr. Khan is currently involved in clinical trials evaluating Natpara in Canada (where it is not yet approved).
She was also involved in some of the pivotal studies that helped gain support of the product in the United States and European Union, including REPLACE, which showed that the injectable PTH molecule maintained serum calcium while reducing or eliminating requirements for calcium and active vitamin D supplementation.
“We now have 8 years of experience with this molecule, so we know that it’s safe and effective,” Dr. Khan emphasized.
Health Canada has not deemed Natpara problematic enough to stop the ongoing research program into injectable PTH therapies there, which is being headed up by Dr. Khan at McMaster University.
“The company has not yet applied for approval here, but when they do apply, I am sure they will have fixed this problem by then,” Dr. Khan said. “And I expect it will be fixed momentarily in the United States, where patients have been most affected.”
Asked if she was looking forward to getting Natpara back on track, Ms. Astolfi said “absolutely” several times.
“This recall has really had a large impact on our community,” she stressed. “And while it’s good that critically ill patients have access to the drug through the special-use program, we want to get everyone back to their best life and feeling great.”
A version of this story originally appeared on Medscape.com.
The recall of injectable recombinant parathyroid hormone (PTH; Natpara) in the United States over safety concerns about the product is gravely affecting the lives of American patients with severe hypoparathyroidism who need replacement PTH therapy to adequately control symptoms.
Approximately 2,700 patients in the United States have been affected by the recall of Natpara, according to one of the manufacturers, Takeda. Those who were taking it have had to transition back to controlling their symptoms with just oral medications, including active vitamin D or calcitriol (Rocaltrol) plus calcium supplements, which has had a negative impact on the management of their disease in most cases, with some patients hospitalized because of severe hypocalcemia.
One patient related in an interview that even if the transition from injectable PTH back to calcium supplements and calcitriol goes smoothly, individuals can still feel pretty awful because the benefits of the Natpara are subtle – patients just feel better on it. And one report indicates at least 100 patients who had to stop Natpara because of the Food and Drug Administration recall have been hospitalized.
“It’s been a rough time for all patients previously on Natpara adjusting to oral medications after they have been on a replacement therapy,” endocrinologist Dolores Shoback, MD, University of California, San Francisco, said in an email.
Aliya Khan, MD, agrees: “This molecule ... is life changing ... [It] makes a huge difference in patients’ quality of life and in their ability to function normally.”
“The FDA in the United States made the recall decision, and we have to respect that,” added Dr. Khan, a professor of clinical medicine at McMaster University in Hamilton, Ontario.
“We need to address patients’ needs and [try to] reinstate it,” Dr. Khan added, who is the lead author of recent guidelines on the treatment of hypoparathyroidism and who has received research funding from Takeda and Ascendis Pharma, both of which make injectable PTH.
Dr. Kahn also has advice for U.S. endocrinologists who are having to deal with patients who were previously on Natpara and no longer have access to the drug.
She explains how they can transition patients back to other available therapies.
Oral treatments a 'Band-Aid'
Hypoparathyroidism is a rare endocrine disorder that affects approximately 60,000 people in the United States. The FDA approved Natpara as an orphan drug and as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism in January 2015.
The FDA issued the recall for Natpara in September, out of concern that rubber particulate matter originating from the rubber septum of the drug’s cartridge might be contaminating the product.
But the European Medicines Agency, which approved the product under the brand name Natpar in 2017, has not reached the same decision as the FDA; patients in Europe who qualify for injectable PTH still have access to the drug.
As Dr. Khan explained in an interview, PTH is critical for the control of calcium and phosphate homeostasis.
When functioning normally, the parathyroid glands are able to regulate blood calcium levels very finely, sensing how much calcium is being resorbed through the kidney, how much is going in and out of bone, how active vitamin D levels are, and the amount the body is absorbing from the bowel and bone. “It’s all being very carefully fine-tuned continuously,” she said.
Patients with hypoparathyroidism suffer from very low levels of serum calcium, which can, in turn, lead to seizures and cardiac irregularities as well as bronchospasm and even respiratory failure. Magnesium levels can also be dysregulated by hypocalcemia.
“All these are serious results of very low calcium levels,” Dr. Khan emphasized.
These patients “are also not able to eliminate phosphate through the kidneys so their phosphate levels rise,” she added.
Standard treatment for hypoparathyroidism is active vitamin D or calcitriol plus oral calcium supplements.
However, for patients with severe hypoparathyroidism, for whom injectable PTH is indicated, standard treatment is like a “Band-Aid” because what they really need is replacement PTH to help them regulate calcium levels as finely as possible, Dr. Khan said.
High risk for severe hypocalcemia
If PTH is stopped abruptly, calcium levels will likely plummet, a phenomenon sometimes referred to as hungry bone syndrome, placing patients at risk for the consequences of severe hypocalcemia, Dr. Khan added.
(Hungry bone syndrome refers to the rapid, profound, and prolonged hypocalcemia associated with hypophosphatemia and hypomagnesemia, which is exacerbated by suppressed PTH).
One report suggests at least 100 patients who had to stop Natpara because of the FDA recall had to seek hospitalization to have calcium levels restored intravenously.
And even if the transition from injectable PTH back to calcium supplements and calcitriol goes smoothly, patients can still feel pretty awful.
“When you look at my blood levels on and off Natpara, they look very similar,” Danette Astolfi, a former Natpara user in the United States who recently transitioned from the injectable to standard of care, said in an interview.
“But I felt much, much better on Natpara, so this is an interesting ‘wild card’ that patients have with this drug – they just feel better on it. They don’t have the fatigue, they don’t have the brain fog, they don’t have the aches and pains even if their calcium levels are normal,” Ms. Astolfi said.
So patients who are candidates for injectable PTH therapy are by definition those who do not do very well on standard-of-care calcium and calcitriol.
“With the recall, patients are going back to this regimen they already did not have great success with,” Ms. Astolfi explained. “And that’s the tricky part because if you fall into this category, as I do, there is not much you can do about it.”
Ms. Astolfi, who is also on the board of directors of the Hypoparathyroidism Association in Pennsylvania, was lucky because she did not have to stop treatment with injectable PTH abruptly.
“I was fortunate. My physician did not want me to stop the drug right away, so we had time to develop a discontinuation plan,” she noted.
Transitioning safely to other therapies
Dr. Khan has advice for endocrinologists dealing with patients who were previously on Natpara and no longer have access to the drug.
First, “endocrinologists need to be aware that when you stop PTH treatment suddenly, patients’ need for calcium supplements and calcitriol may be as high as two times what they were on before they started PTH therapy,” she noted.
Physicians also need to follow patients closely by monitoring not only calcium and vitamin D levels but also phosphate and magnesium almost daily.
Dr. Khan said she does this because it’s difficult to predict how much calcium and calcitriol a patient will require and doses will likely have to be titrated up or down based on lab results.
It is noteworthy that a joint statement on the Natpara recall by the Endocrine Society and the American Society of Bone and Mineral Research (ASBMR) also emphasizes these points.
And Dr. Khan – as well as the Endocrine Society and ASBMR – also point out that patients may be transitioned from Natpara to teriparatide (Forteo), another recombinant form of PTH that is licensed for use in osteoporosis but has not been approved for hypoparathyroidism.
In Dr. Khan’s experience, patients often do quite well on teriparatide.
The big downside of prescribing teriparatide in the United States, however, is that the drug has to be used off label, and as such, insurance companies are unlikely to pick up the tab for what is an expensive treatment, she explained.
Furthermore, teriparatide has a very short shelf-life once injected so patients may need to use up to four needles a day to stabilize calcium levels.
If teriparatide is prescribed, subcutaneous injections given in the thigh are recommended for the treatment of hypoparathyroidism, according to the joint Endocrine Society/ASBMR statement.
Takeda vs. FDA
In the meantime, Takeda says it has been in regular contact with the FDA to try and work out how to bring Natpara back to U.S. patients who require it for symptom control.
Current outstanding issues between the company and FDA involve considerations related to dose accuracy, safety, and supply continuity, which the company hopes will be resolved in a timely manner.
Furthermore, shortly after the recall order, the company created a special use program that continues to support patients previously prescribed Natpara who would otherwise be at extreme risk of serious complications if forced to stop taking the drug.
“Originally intended for an extremely limited number of patients, the special use program has now enrolled approximately 300 patients,” Cheryl Schwartz, head, U.S. Hematology & Rare Business Unit, Takeda, said in a letter to members of the U.S. Hypoparathyroidism Association on Nov. 21.
The number is higher than expected and reflects the significant treatment needs of patients with serious disease, Ms. Schwartz noted.
“I want to reiterate that we do understand and sincerely regret the impact the Natpara recall is having on patients. We will provide another update as soon as we have more information to share. We continue to work around the clock to find ways to bring Natpara back to the broader hypoparathyroidism community,” she added.
“Patient safety always has been, and continues to be, the highest priority for Takeda,” she said.
Final efforts
Dr. Khan is currently involved in clinical trials evaluating Natpara in Canada (where it is not yet approved).
She was also involved in some of the pivotal studies that helped gain support of the product in the United States and European Union, including REPLACE, which showed that the injectable PTH molecule maintained serum calcium while reducing or eliminating requirements for calcium and active vitamin D supplementation.
“We now have 8 years of experience with this molecule, so we know that it’s safe and effective,” Dr. Khan emphasized.
Health Canada has not deemed Natpara problematic enough to stop the ongoing research program into injectable PTH therapies there, which is being headed up by Dr. Khan at McMaster University.
“The company has not yet applied for approval here, but when they do apply, I am sure they will have fixed this problem by then,” Dr. Khan said. “And I expect it will be fixed momentarily in the United States, where patients have been most affected.”
Asked if she was looking forward to getting Natpara back on track, Ms. Astolfi said “absolutely” several times.
“This recall has really had a large impact on our community,” she stressed. “And while it’s good that critically ill patients have access to the drug through the special-use program, we want to get everyone back to their best life and feeling great.”
A version of this story originally appeared on Medscape.com.
The recall of injectable recombinant parathyroid hormone (PTH; Natpara) in the United States over safety concerns about the product is gravely affecting the lives of American patients with severe hypoparathyroidism who need replacement PTH therapy to adequately control symptoms.
Approximately 2,700 patients in the United States have been affected by the recall of Natpara, according to one of the manufacturers, Takeda. Those who were taking it have had to transition back to controlling their symptoms with just oral medications, including active vitamin D or calcitriol (Rocaltrol) plus calcium supplements, which has had a negative impact on the management of their disease in most cases, with some patients hospitalized because of severe hypocalcemia.
One patient related in an interview that even if the transition from injectable PTH back to calcium supplements and calcitriol goes smoothly, individuals can still feel pretty awful because the benefits of the Natpara are subtle – patients just feel better on it. And one report indicates at least 100 patients who had to stop Natpara because of the Food and Drug Administration recall have been hospitalized.
“It’s been a rough time for all patients previously on Natpara adjusting to oral medications after they have been on a replacement therapy,” endocrinologist Dolores Shoback, MD, University of California, San Francisco, said in an email.
Aliya Khan, MD, agrees: “This molecule ... is life changing ... [It] makes a huge difference in patients’ quality of life and in their ability to function normally.”
“The FDA in the United States made the recall decision, and we have to respect that,” added Dr. Khan, a professor of clinical medicine at McMaster University in Hamilton, Ontario.
“We need to address patients’ needs and [try to] reinstate it,” Dr. Khan added, who is the lead author of recent guidelines on the treatment of hypoparathyroidism and who has received research funding from Takeda and Ascendis Pharma, both of which make injectable PTH.
Dr. Kahn also has advice for U.S. endocrinologists who are having to deal with patients who were previously on Natpara and no longer have access to the drug.
She explains how they can transition patients back to other available therapies.
Oral treatments a 'Band-Aid'
Hypoparathyroidism is a rare endocrine disorder that affects approximately 60,000 people in the United States. The FDA approved Natpara as an orphan drug and as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism in January 2015.
The FDA issued the recall for Natpara in September, out of concern that rubber particulate matter originating from the rubber septum of the drug’s cartridge might be contaminating the product.
But the European Medicines Agency, which approved the product under the brand name Natpar in 2017, has not reached the same decision as the FDA; patients in Europe who qualify for injectable PTH still have access to the drug.
As Dr. Khan explained in an interview, PTH is critical for the control of calcium and phosphate homeostasis.
When functioning normally, the parathyroid glands are able to regulate blood calcium levels very finely, sensing how much calcium is being resorbed through the kidney, how much is going in and out of bone, how active vitamin D levels are, and the amount the body is absorbing from the bowel and bone. “It’s all being very carefully fine-tuned continuously,” she said.
Patients with hypoparathyroidism suffer from very low levels of serum calcium, which can, in turn, lead to seizures and cardiac irregularities as well as bronchospasm and even respiratory failure. Magnesium levels can also be dysregulated by hypocalcemia.
“All these are serious results of very low calcium levels,” Dr. Khan emphasized.
These patients “are also not able to eliminate phosphate through the kidneys so their phosphate levels rise,” she added.
Standard treatment for hypoparathyroidism is active vitamin D or calcitriol plus oral calcium supplements.
However, for patients with severe hypoparathyroidism, for whom injectable PTH is indicated, standard treatment is like a “Band-Aid” because what they really need is replacement PTH to help them regulate calcium levels as finely as possible, Dr. Khan said.
High risk for severe hypocalcemia
If PTH is stopped abruptly, calcium levels will likely plummet, a phenomenon sometimes referred to as hungry bone syndrome, placing patients at risk for the consequences of severe hypocalcemia, Dr. Khan added.
(Hungry bone syndrome refers to the rapid, profound, and prolonged hypocalcemia associated with hypophosphatemia and hypomagnesemia, which is exacerbated by suppressed PTH).
One report suggests at least 100 patients who had to stop Natpara because of the FDA recall had to seek hospitalization to have calcium levels restored intravenously.
And even if the transition from injectable PTH back to calcium supplements and calcitriol goes smoothly, patients can still feel pretty awful.
“When you look at my blood levels on and off Natpara, they look very similar,” Danette Astolfi, a former Natpara user in the United States who recently transitioned from the injectable to standard of care, said in an interview.
“But I felt much, much better on Natpara, so this is an interesting ‘wild card’ that patients have with this drug – they just feel better on it. They don’t have the fatigue, they don’t have the brain fog, they don’t have the aches and pains even if their calcium levels are normal,” Ms. Astolfi said.
So patients who are candidates for injectable PTH therapy are by definition those who do not do very well on standard-of-care calcium and calcitriol.
“With the recall, patients are going back to this regimen they already did not have great success with,” Ms. Astolfi explained. “And that’s the tricky part because if you fall into this category, as I do, there is not much you can do about it.”
Ms. Astolfi, who is also on the board of directors of the Hypoparathyroidism Association in Pennsylvania, was lucky because she did not have to stop treatment with injectable PTH abruptly.
“I was fortunate. My physician did not want me to stop the drug right away, so we had time to develop a discontinuation plan,” she noted.
Transitioning safely to other therapies
Dr. Khan has advice for endocrinologists dealing with patients who were previously on Natpara and no longer have access to the drug.
First, “endocrinologists need to be aware that when you stop PTH treatment suddenly, patients’ need for calcium supplements and calcitriol may be as high as two times what they were on before they started PTH therapy,” she noted.
Physicians also need to follow patients closely by monitoring not only calcium and vitamin D levels but also phosphate and magnesium almost daily.
Dr. Khan said she does this because it’s difficult to predict how much calcium and calcitriol a patient will require and doses will likely have to be titrated up or down based on lab results.
It is noteworthy that a joint statement on the Natpara recall by the Endocrine Society and the American Society of Bone and Mineral Research (ASBMR) also emphasizes these points.
And Dr. Khan – as well as the Endocrine Society and ASBMR – also point out that patients may be transitioned from Natpara to teriparatide (Forteo), another recombinant form of PTH that is licensed for use in osteoporosis but has not been approved for hypoparathyroidism.
In Dr. Khan’s experience, patients often do quite well on teriparatide.
The big downside of prescribing teriparatide in the United States, however, is that the drug has to be used off label, and as such, insurance companies are unlikely to pick up the tab for what is an expensive treatment, she explained.
Furthermore, teriparatide has a very short shelf-life once injected so patients may need to use up to four needles a day to stabilize calcium levels.
If teriparatide is prescribed, subcutaneous injections given in the thigh are recommended for the treatment of hypoparathyroidism, according to the joint Endocrine Society/ASBMR statement.
Takeda vs. FDA
In the meantime, Takeda says it has been in regular contact with the FDA to try and work out how to bring Natpara back to U.S. patients who require it for symptom control.
Current outstanding issues between the company and FDA involve considerations related to dose accuracy, safety, and supply continuity, which the company hopes will be resolved in a timely manner.
Furthermore, shortly after the recall order, the company created a special use program that continues to support patients previously prescribed Natpara who would otherwise be at extreme risk of serious complications if forced to stop taking the drug.
“Originally intended for an extremely limited number of patients, the special use program has now enrolled approximately 300 patients,” Cheryl Schwartz, head, U.S. Hematology & Rare Business Unit, Takeda, said in a letter to members of the U.S. Hypoparathyroidism Association on Nov. 21.
The number is higher than expected and reflects the significant treatment needs of patients with serious disease, Ms. Schwartz noted.
“I want to reiterate that we do understand and sincerely regret the impact the Natpara recall is having on patients. We will provide another update as soon as we have more information to share. We continue to work around the clock to find ways to bring Natpara back to the broader hypoparathyroidism community,” she added.
“Patient safety always has been, and continues to be, the highest priority for Takeda,” she said.
Final efforts
Dr. Khan is currently involved in clinical trials evaluating Natpara in Canada (where it is not yet approved).
She was also involved in some of the pivotal studies that helped gain support of the product in the United States and European Union, including REPLACE, which showed that the injectable PTH molecule maintained serum calcium while reducing or eliminating requirements for calcium and active vitamin D supplementation.
“We now have 8 years of experience with this molecule, so we know that it’s safe and effective,” Dr. Khan emphasized.
Health Canada has not deemed Natpara problematic enough to stop the ongoing research program into injectable PTH therapies there, which is being headed up by Dr. Khan at McMaster University.
“The company has not yet applied for approval here, but when they do apply, I am sure they will have fixed this problem by then,” Dr. Khan said. “And I expect it will be fixed momentarily in the United States, where patients have been most affected.”
Asked if she was looking forward to getting Natpara back on track, Ms. Astolfi said “absolutely” several times.
“This recall has really had a large impact on our community,” she stressed. “And while it’s good that critically ill patients have access to the drug through the special-use program, we want to get everyone back to their best life and feeling great.”
A version of this story originally appeared on Medscape.com.
Novel antibody looks promising in lupus nephritis
WASHINGTON – A novel antibody, obinutuzumab, enhances renal responses in patients with lupus nephritis, through more complete B-cell depletion, compared with standard immunotherapy, and is well tolerated, according to results from the phase 2 NOBILITY trial.
“We know from our previous trials with anti–B-cell antibodies that results were mixed and we felt that these variable results were possibly due to variability in B-cell depletion with a type 1 anti-CD20 antibody such as rituximab,” Brad Rovin, MD, director, division of nephrology, Ohio State University in Columbus, told a press briefing here at Kidney Week 2019: American Society of Nephrology annual meeting.
“So we hypothesized that if we could deplete B cells more efficiently and completely, we would achieve better results. At week 52, 35% of patients in the obinutuzumab-treated group achieved a complete renal response, compared to 23% in the standard-of-care arm.”
And by week 76, the difference between obinutuzumab and the standard of care was actually larger at 40% vs. 18%, respectively, “and this was statistically significant at a P value of .01,” added Dr. Rovin, who presented the full findings of the study at the conference.
Obinutuzumab, a highly engineered anti-CD20 antibody, is already approved under the brand name Gazyva for use in certain leukemias and lymphomas. The NOBILITY study was funded by Genentech-Roche, and Dr. Rovin reported being a consultant for the company.
Asked by Medscape Medical News to comment on the study, Duvuru Geetha, MBBS, noted that with standard-of-care mycophenolate mofetil (MMF) plus corticosteroids, “the remissions rates we achieve [for lupus nephritis] are still not great,” ranging from 30% to 50%, depending on the patient population.
“This is why there is a need for alternative agents,” added Dr. Geetha, who is an associate professor of medicine, Johns Hopkins University, Baltimore.
With obinutuzumab, “the data look very promising because there is a much more profound and sustained effect on B-cell depletion and the renal response rate is much higher [than with MMF and corticosteroids],” she noted.
Dr. Geetha added, however, that she presumes patients were all premedicated with prophylactic agents to prevent infectious events, as they are when treated with rituximab.
“I think what is definitely different about this drug is that it induces direct cell death more efficiently than rituximab and that is probably what’s accounting for the higher efficacy seen with it,” said Dr. Geetha, who disclosed having received honoraria from Genentech a number of years ago.
“So yes, I believe the results are clinically meaningful,” she concluded.
NOBILITY study design
The NOBILITY trial randomized 125 patients with Class III or IV lupus nephritis to either obinutuzumab plus MMF and corticosteroids, or to MMF plus corticosteroids alone, for a treatment interval of 104 weeks.
Patients in the obinutuzumab group received two infusions of the highly engineered anti-CD20 antibody at week 0 and week 2 and another two infusions at 6 months.
“The primary endpoint was complete renal response at week 52,” the authors wrote, “while key secondary endpoints included overall renal response and modified complete renal response.”
Both at week 52 and week 76, more patients in the obinutuzumab group achieved an overall renal response as well as a modified complete renal response, compared with those treated with immunosuppression alone.
“If you look at the complete renal response over time, you can see that the curves separate after about 6 months but the placebo group starts to decline as you go further out, whereas the obinutuzumab group continues to separate, so my prediction is that we are going to see this trend continue because of the mechanism of action of obinutuzumab,” Dr. Rovin explained.
Phase 3 trials to start early 2020
All of the serologies relevant to lupus and lupus nephritis “including C3 and C4 improved while antidoubled stranded DNA levels declined, as did the urine protein-to-creatinine ratio, although the decline was more rapid and more profound in the obinutuzumab-treated patients,” Dr. Rovin said.
Importantly as well, despite the profound B-cell depletion produced by obinutuzumab, “the adverse event profile of this drug was very similar to the placebo group,” he stressed.
As expected, rates of infusion reactions were slightly higher in the experimental group than the immunosuppression alone group, but rates of serious adverse events were the same between groups, as were adverse infectious events, he noted.
Investigators have now initiated a global phase 3 trial, scheduled to start in early 2020, to evaluate the same treatment protocol in a larger group of patients.
Kidney Week 2019. Abstract #FR-OR136. Presented Nov. 8, 2019.
This story first appeared on Medscape.com.
WASHINGTON – A novel antibody, obinutuzumab, enhances renal responses in patients with lupus nephritis, through more complete B-cell depletion, compared with standard immunotherapy, and is well tolerated, according to results from the phase 2 NOBILITY trial.
“We know from our previous trials with anti–B-cell antibodies that results were mixed and we felt that these variable results were possibly due to variability in B-cell depletion with a type 1 anti-CD20 antibody such as rituximab,” Brad Rovin, MD, director, division of nephrology, Ohio State University in Columbus, told a press briefing here at Kidney Week 2019: American Society of Nephrology annual meeting.
“So we hypothesized that if we could deplete B cells more efficiently and completely, we would achieve better results. At week 52, 35% of patients in the obinutuzumab-treated group achieved a complete renal response, compared to 23% in the standard-of-care arm.”
And by week 76, the difference between obinutuzumab and the standard of care was actually larger at 40% vs. 18%, respectively, “and this was statistically significant at a P value of .01,” added Dr. Rovin, who presented the full findings of the study at the conference.
Obinutuzumab, a highly engineered anti-CD20 antibody, is already approved under the brand name Gazyva for use in certain leukemias and lymphomas. The NOBILITY study was funded by Genentech-Roche, and Dr. Rovin reported being a consultant for the company.
Asked by Medscape Medical News to comment on the study, Duvuru Geetha, MBBS, noted that with standard-of-care mycophenolate mofetil (MMF) plus corticosteroids, “the remissions rates we achieve [for lupus nephritis] are still not great,” ranging from 30% to 50%, depending on the patient population.
“This is why there is a need for alternative agents,” added Dr. Geetha, who is an associate professor of medicine, Johns Hopkins University, Baltimore.
With obinutuzumab, “the data look very promising because there is a much more profound and sustained effect on B-cell depletion and the renal response rate is much higher [than with MMF and corticosteroids],” she noted.
Dr. Geetha added, however, that she presumes patients were all premedicated with prophylactic agents to prevent infectious events, as they are when treated with rituximab.
“I think what is definitely different about this drug is that it induces direct cell death more efficiently than rituximab and that is probably what’s accounting for the higher efficacy seen with it,” said Dr. Geetha, who disclosed having received honoraria from Genentech a number of years ago.
“So yes, I believe the results are clinically meaningful,” she concluded.
NOBILITY study design
The NOBILITY trial randomized 125 patients with Class III or IV lupus nephritis to either obinutuzumab plus MMF and corticosteroids, or to MMF plus corticosteroids alone, for a treatment interval of 104 weeks.
Patients in the obinutuzumab group received two infusions of the highly engineered anti-CD20 antibody at week 0 and week 2 and another two infusions at 6 months.
“The primary endpoint was complete renal response at week 52,” the authors wrote, “while key secondary endpoints included overall renal response and modified complete renal response.”
Both at week 52 and week 76, more patients in the obinutuzumab group achieved an overall renal response as well as a modified complete renal response, compared with those treated with immunosuppression alone.
“If you look at the complete renal response over time, you can see that the curves separate after about 6 months but the placebo group starts to decline as you go further out, whereas the obinutuzumab group continues to separate, so my prediction is that we are going to see this trend continue because of the mechanism of action of obinutuzumab,” Dr. Rovin explained.
Phase 3 trials to start early 2020
All of the serologies relevant to lupus and lupus nephritis “including C3 and C4 improved while antidoubled stranded DNA levels declined, as did the urine protein-to-creatinine ratio, although the decline was more rapid and more profound in the obinutuzumab-treated patients,” Dr. Rovin said.
Importantly as well, despite the profound B-cell depletion produced by obinutuzumab, “the adverse event profile of this drug was very similar to the placebo group,” he stressed.
As expected, rates of infusion reactions were slightly higher in the experimental group than the immunosuppression alone group, but rates of serious adverse events were the same between groups, as were adverse infectious events, he noted.
Investigators have now initiated a global phase 3 trial, scheduled to start in early 2020, to evaluate the same treatment protocol in a larger group of patients.
Kidney Week 2019. Abstract #FR-OR136. Presented Nov. 8, 2019.
This story first appeared on Medscape.com.
WASHINGTON – A novel antibody, obinutuzumab, enhances renal responses in patients with lupus nephritis, through more complete B-cell depletion, compared with standard immunotherapy, and is well tolerated, according to results from the phase 2 NOBILITY trial.
“We know from our previous trials with anti–B-cell antibodies that results were mixed and we felt that these variable results were possibly due to variability in B-cell depletion with a type 1 anti-CD20 antibody such as rituximab,” Brad Rovin, MD, director, division of nephrology, Ohio State University in Columbus, told a press briefing here at Kidney Week 2019: American Society of Nephrology annual meeting.
“So we hypothesized that if we could deplete B cells more efficiently and completely, we would achieve better results. At week 52, 35% of patients in the obinutuzumab-treated group achieved a complete renal response, compared to 23% in the standard-of-care arm.”
And by week 76, the difference between obinutuzumab and the standard of care was actually larger at 40% vs. 18%, respectively, “and this was statistically significant at a P value of .01,” added Dr. Rovin, who presented the full findings of the study at the conference.
Obinutuzumab, a highly engineered anti-CD20 antibody, is already approved under the brand name Gazyva for use in certain leukemias and lymphomas. The NOBILITY study was funded by Genentech-Roche, and Dr. Rovin reported being a consultant for the company.
Asked by Medscape Medical News to comment on the study, Duvuru Geetha, MBBS, noted that with standard-of-care mycophenolate mofetil (MMF) plus corticosteroids, “the remissions rates we achieve [for lupus nephritis] are still not great,” ranging from 30% to 50%, depending on the patient population.
“This is why there is a need for alternative agents,” added Dr. Geetha, who is an associate professor of medicine, Johns Hopkins University, Baltimore.
With obinutuzumab, “the data look very promising because there is a much more profound and sustained effect on B-cell depletion and the renal response rate is much higher [than with MMF and corticosteroids],” she noted.
Dr. Geetha added, however, that she presumes patients were all premedicated with prophylactic agents to prevent infectious events, as they are when treated with rituximab.
“I think what is definitely different about this drug is that it induces direct cell death more efficiently than rituximab and that is probably what’s accounting for the higher efficacy seen with it,” said Dr. Geetha, who disclosed having received honoraria from Genentech a number of years ago.
“So yes, I believe the results are clinically meaningful,” she concluded.
NOBILITY study design
The NOBILITY trial randomized 125 patients with Class III or IV lupus nephritis to either obinutuzumab plus MMF and corticosteroids, or to MMF plus corticosteroids alone, for a treatment interval of 104 weeks.
Patients in the obinutuzumab group received two infusions of the highly engineered anti-CD20 antibody at week 0 and week 2 and another two infusions at 6 months.
“The primary endpoint was complete renal response at week 52,” the authors wrote, “while key secondary endpoints included overall renal response and modified complete renal response.”
Both at week 52 and week 76, more patients in the obinutuzumab group achieved an overall renal response as well as a modified complete renal response, compared with those treated with immunosuppression alone.
“If you look at the complete renal response over time, you can see that the curves separate after about 6 months but the placebo group starts to decline as you go further out, whereas the obinutuzumab group continues to separate, so my prediction is that we are going to see this trend continue because of the mechanism of action of obinutuzumab,” Dr. Rovin explained.
Phase 3 trials to start early 2020
All of the serologies relevant to lupus and lupus nephritis “including C3 and C4 improved while antidoubled stranded DNA levels declined, as did the urine protein-to-creatinine ratio, although the decline was more rapid and more profound in the obinutuzumab-treated patients,” Dr. Rovin said.
Importantly as well, despite the profound B-cell depletion produced by obinutuzumab, “the adverse event profile of this drug was very similar to the placebo group,” he stressed.
As expected, rates of infusion reactions were slightly higher in the experimental group than the immunosuppression alone group, but rates of serious adverse events were the same between groups, as were adverse infectious events, he noted.
Investigators have now initiated a global phase 3 trial, scheduled to start in early 2020, to evaluate the same treatment protocol in a larger group of patients.
Kidney Week 2019. Abstract #FR-OR136. Presented Nov. 8, 2019.
This story first appeared on Medscape.com.
Silent Coronary Disease Seen in Many Diabetics
TORONTO — A large proportion of patients with hypertension and type 2 diabetes also have silent coronary artery disease, according to myocardial perfusion imaging studies presented at the Society of Nuclear Medicine's annual meeting.
Christien Côté, M.D., and colleagues carried out a prospective study to identify the prevalence and severity of silent ischemia in 595 hypertensive patients with and without type 2 diabetes. “We also wanted to establish to what extent type 2 diabetes modified the prevalence and severity of silent CAD in hypertensive patients and to assess the predictive value of risk factors for silent CAD,” said Dr. Côté, professor of medicine at Laval University, Quebec City.
Study subjects were 45 years of age and older and had either essential hypertension alone (363) or coexisting diabetes (232). None had a history of typical angina, and there were no differences in age, sex, or duration of hypertension between the two groups. Unlike previous studies, patients were selected for dipyridamole stress testing according to American Diabetes Association guidelines for coronary investigation, said Dr. Côté.
All patients underwent dipyridamole stress 99mTc-sestamibi single-photon emission CT myocardial perfusion imaging (MPI). The images were read by two blinded, experienced observers. Analysis of MPI studies showed 43% of hypertensive diabetic patients had silent CAD, as did 27% of patients with hypertension alone. “There was also a significantly greater extent of reversible ischemia in the diabetic population,” Dr. Côté said. MPI studies were also more severely abnormal in hypertensive patients with coexisting diabetes than in hypertensive patients alone.
Investigators also assessed the predictive value of risk factors on the prevalence of silent CAD. In the hypertensive population, only dyspnea was predictive of silent CAD, whereas dyspnea and proteinuria were predictive of the same ischemic defect in the hypertensive diabetic population.
The high prevalence of silent ischemia in hypertensive diabetic patients found in this study is of concern, as asymptomatic patients are unlikely to seek medical attention, and cardiovascular disease events are less likely to be prevented. CAD is the leading cause of morbidity and mortality in hypertensive patients, and their coexistence increases this risk, Dr. Côté said.
TORONTO — A large proportion of patients with hypertension and type 2 diabetes also have silent coronary artery disease, according to myocardial perfusion imaging studies presented at the Society of Nuclear Medicine's annual meeting.
Christien Côté, M.D., and colleagues carried out a prospective study to identify the prevalence and severity of silent ischemia in 595 hypertensive patients with and without type 2 diabetes. “We also wanted to establish to what extent type 2 diabetes modified the prevalence and severity of silent CAD in hypertensive patients and to assess the predictive value of risk factors for silent CAD,” said Dr. Côté, professor of medicine at Laval University, Quebec City.
Study subjects were 45 years of age and older and had either essential hypertension alone (363) or coexisting diabetes (232). None had a history of typical angina, and there were no differences in age, sex, or duration of hypertension between the two groups. Unlike previous studies, patients were selected for dipyridamole stress testing according to American Diabetes Association guidelines for coronary investigation, said Dr. Côté.
All patients underwent dipyridamole stress 99mTc-sestamibi single-photon emission CT myocardial perfusion imaging (MPI). The images were read by two blinded, experienced observers. Analysis of MPI studies showed 43% of hypertensive diabetic patients had silent CAD, as did 27% of patients with hypertension alone. “There was also a significantly greater extent of reversible ischemia in the diabetic population,” Dr. Côté said. MPI studies were also more severely abnormal in hypertensive patients with coexisting diabetes than in hypertensive patients alone.
Investigators also assessed the predictive value of risk factors on the prevalence of silent CAD. In the hypertensive population, only dyspnea was predictive of silent CAD, whereas dyspnea and proteinuria were predictive of the same ischemic defect in the hypertensive diabetic population.
The high prevalence of silent ischemia in hypertensive diabetic patients found in this study is of concern, as asymptomatic patients are unlikely to seek medical attention, and cardiovascular disease events are less likely to be prevented. CAD is the leading cause of morbidity and mortality in hypertensive patients, and their coexistence increases this risk, Dr. Côté said.
TORONTO — A large proportion of patients with hypertension and type 2 diabetes also have silent coronary artery disease, according to myocardial perfusion imaging studies presented at the Society of Nuclear Medicine's annual meeting.
Christien Côté, M.D., and colleagues carried out a prospective study to identify the prevalence and severity of silent ischemia in 595 hypertensive patients with and without type 2 diabetes. “We also wanted to establish to what extent type 2 diabetes modified the prevalence and severity of silent CAD in hypertensive patients and to assess the predictive value of risk factors for silent CAD,” said Dr. Côté, professor of medicine at Laval University, Quebec City.
Study subjects were 45 years of age and older and had either essential hypertension alone (363) or coexisting diabetes (232). None had a history of typical angina, and there were no differences in age, sex, or duration of hypertension between the two groups. Unlike previous studies, patients were selected for dipyridamole stress testing according to American Diabetes Association guidelines for coronary investigation, said Dr. Côté.
All patients underwent dipyridamole stress 99mTc-sestamibi single-photon emission CT myocardial perfusion imaging (MPI). The images were read by two blinded, experienced observers. Analysis of MPI studies showed 43% of hypertensive diabetic patients had silent CAD, as did 27% of patients with hypertension alone. “There was also a significantly greater extent of reversible ischemia in the diabetic population,” Dr. Côté said. MPI studies were also more severely abnormal in hypertensive patients with coexisting diabetes than in hypertensive patients alone.
Investigators also assessed the predictive value of risk factors on the prevalence of silent CAD. In the hypertensive population, only dyspnea was predictive of silent CAD, whereas dyspnea and proteinuria were predictive of the same ischemic defect in the hypertensive diabetic population.
The high prevalence of silent ischemia in hypertensive diabetic patients found in this study is of concern, as asymptomatic patients are unlikely to seek medical attention, and cardiovascular disease events are less likely to be prevented. CAD is the leading cause of morbidity and mortality in hypertensive patients, and their coexistence increases this risk, Dr. Côté said.
ARBs Improve Endothelial Function, Findings Show : For patients with impaired glucose intolerance, angiotensin II receptor-1 blockade helps significantly.
TORONTO — Endothelial function improves significantly with angiotensin II receptor-1 blockade in patients with impaired glucose tolerance, and quickly reverts to baseline after discontinuation of therapy, Thomas H. Schindler, M.D., reported at the annual meeting of the Society of Nuclear Medicine.
He and his colleagues at the University of California, Los Angeles, also saw a parallel and significant decrease in insulin resistance during 24 weeks of treatment.
The findings suggest that improved insulin sensitivity and the pleiotropic effects of renin-angiotensin-aldosterone system blockade may account for the reduction in new-onset type 2 diabetes that has been associated with the use of ACE inhibitors and angiotensin receptor blockers (ARBs).
The researchers set out to determine whether the insulin-sensitizing effects of ARB therapy with valsartan would improve endothelial-dependent vasomotion in 30 patients with IGT, defined as fasting glucose below 126 mg/dL and a 2-hour oral glucose tolerance test between 140 and 200 mg/dL.
“None of them had any other traditional coronary risk factors,” Dr. Schindler said. Another 20 healthy volunteers were also studied at baseline and compared with the IGT patients; they received no medication during the study.
The patients with IGT received valsartan, 160 mg a day for 12 weeks, after which the dose was doubled to 320 mg a day and continued for another 12 weeks. Treatment was discontinued at 24 weeks, and the patients were followed to 32 weeks.
“Body mass index was significantly higher in IGT patients than in controls, but both total cholesterol and low-density lipoprotein cholesterol were within the normal range and did not differ significantly between the two groups,” Dr. Schindler said.
Not unexpectedly, myocardial blood flow responses from rest to cold pressor testing were significantly lower at baseline in the IGT patients than in the controls. In contrast, endothelial-independent stimulation of myocardial blood flow did not differ between the two groups.
After 12 weeks of valsartan at 160 mg a day, myocardial blood flow response to cold pressor testing significantly improved in the IGT patients, from 0.75 mL/g per minute at baseline to 0.89 mL/g per minute at 12 weeks.
After 12 more weeks of valsartan at 320 mg, myocardial blood flow response to cold pressor testing again improved significantly, from 0.89 mL/g per minute at 12 weeks to 0.94 mL/g per minute at 24 weeks. Valsartan was discontinued for 8 weeks; the observed reaction to cold pressor testing disappeared.
In parallel fashion, insulin sensitivity—expressed as the glucose infusion rate during hyperinsulinemia euglycemic clamp—increased steadily in the IGT patients, from a baseline value of 3.2 mg/kg per minute (a clear sign that patients were insulin resistant) to 3.7 mg/kg per minute at 12 weeks and to 4.2 mg/kg per minute at 24 weeks.
At 32 weeks, the improvement in insulin sensitivity in the IGT patients had returned to baseline. In a later interview, Dr. Schindler speculated that the pleiotropic effects of the ARB—including potential anti-inflammatory, antioxidative, and antithrombotic properties—may have all helped to improve insulin sensitivity.
“We conclude that IGT in patients without other coronary risk factors is associated with coronary endothelial dysfunction and that angiotensin II receptor-1 blockade with valsartan restores endothelial function. This effect was independent of the plasma lipid profile and C-reactive protein, an inflammatory plasma marker, suggesting that these beneficial effects on the coronary endothelium may be related to an increase in insulin sensitivity and the pleiotropic effects of ARB blockade,” Dr. Schindler said.
TORONTO — Endothelial function improves significantly with angiotensin II receptor-1 blockade in patients with impaired glucose tolerance, and quickly reverts to baseline after discontinuation of therapy, Thomas H. Schindler, M.D., reported at the annual meeting of the Society of Nuclear Medicine.
He and his colleagues at the University of California, Los Angeles, also saw a parallel and significant decrease in insulin resistance during 24 weeks of treatment.
The findings suggest that improved insulin sensitivity and the pleiotropic effects of renin-angiotensin-aldosterone system blockade may account for the reduction in new-onset type 2 diabetes that has been associated with the use of ACE inhibitors and angiotensin receptor blockers (ARBs).
The researchers set out to determine whether the insulin-sensitizing effects of ARB therapy with valsartan would improve endothelial-dependent vasomotion in 30 patients with IGT, defined as fasting glucose below 126 mg/dL and a 2-hour oral glucose tolerance test between 140 and 200 mg/dL.
“None of them had any other traditional coronary risk factors,” Dr. Schindler said. Another 20 healthy volunteers were also studied at baseline and compared with the IGT patients; they received no medication during the study.
The patients with IGT received valsartan, 160 mg a day for 12 weeks, after which the dose was doubled to 320 mg a day and continued for another 12 weeks. Treatment was discontinued at 24 weeks, and the patients were followed to 32 weeks.
“Body mass index was significantly higher in IGT patients than in controls, but both total cholesterol and low-density lipoprotein cholesterol were within the normal range and did not differ significantly between the two groups,” Dr. Schindler said.
Not unexpectedly, myocardial blood flow responses from rest to cold pressor testing were significantly lower at baseline in the IGT patients than in the controls. In contrast, endothelial-independent stimulation of myocardial blood flow did not differ between the two groups.
After 12 weeks of valsartan at 160 mg a day, myocardial blood flow response to cold pressor testing significantly improved in the IGT patients, from 0.75 mL/g per minute at baseline to 0.89 mL/g per minute at 12 weeks.
After 12 more weeks of valsartan at 320 mg, myocardial blood flow response to cold pressor testing again improved significantly, from 0.89 mL/g per minute at 12 weeks to 0.94 mL/g per minute at 24 weeks. Valsartan was discontinued for 8 weeks; the observed reaction to cold pressor testing disappeared.
In parallel fashion, insulin sensitivity—expressed as the glucose infusion rate during hyperinsulinemia euglycemic clamp—increased steadily in the IGT patients, from a baseline value of 3.2 mg/kg per minute (a clear sign that patients were insulin resistant) to 3.7 mg/kg per minute at 12 weeks and to 4.2 mg/kg per minute at 24 weeks.
At 32 weeks, the improvement in insulin sensitivity in the IGT patients had returned to baseline. In a later interview, Dr. Schindler speculated that the pleiotropic effects of the ARB—including potential anti-inflammatory, antioxidative, and antithrombotic properties—may have all helped to improve insulin sensitivity.
“We conclude that IGT in patients without other coronary risk factors is associated with coronary endothelial dysfunction and that angiotensin II receptor-1 blockade with valsartan restores endothelial function. This effect was independent of the plasma lipid profile and C-reactive protein, an inflammatory plasma marker, suggesting that these beneficial effects on the coronary endothelium may be related to an increase in insulin sensitivity and the pleiotropic effects of ARB blockade,” Dr. Schindler said.
TORONTO — Endothelial function improves significantly with angiotensin II receptor-1 blockade in patients with impaired glucose tolerance, and quickly reverts to baseline after discontinuation of therapy, Thomas H. Schindler, M.D., reported at the annual meeting of the Society of Nuclear Medicine.
He and his colleagues at the University of California, Los Angeles, also saw a parallel and significant decrease in insulin resistance during 24 weeks of treatment.
The findings suggest that improved insulin sensitivity and the pleiotropic effects of renin-angiotensin-aldosterone system blockade may account for the reduction in new-onset type 2 diabetes that has been associated with the use of ACE inhibitors and angiotensin receptor blockers (ARBs).
The researchers set out to determine whether the insulin-sensitizing effects of ARB therapy with valsartan would improve endothelial-dependent vasomotion in 30 patients with IGT, defined as fasting glucose below 126 mg/dL and a 2-hour oral glucose tolerance test between 140 and 200 mg/dL.
“None of them had any other traditional coronary risk factors,” Dr. Schindler said. Another 20 healthy volunteers were also studied at baseline and compared with the IGT patients; they received no medication during the study.
The patients with IGT received valsartan, 160 mg a day for 12 weeks, after which the dose was doubled to 320 mg a day and continued for another 12 weeks. Treatment was discontinued at 24 weeks, and the patients were followed to 32 weeks.
“Body mass index was significantly higher in IGT patients than in controls, but both total cholesterol and low-density lipoprotein cholesterol were within the normal range and did not differ significantly between the two groups,” Dr. Schindler said.
Not unexpectedly, myocardial blood flow responses from rest to cold pressor testing were significantly lower at baseline in the IGT patients than in the controls. In contrast, endothelial-independent stimulation of myocardial blood flow did not differ between the two groups.
After 12 weeks of valsartan at 160 mg a day, myocardial blood flow response to cold pressor testing significantly improved in the IGT patients, from 0.75 mL/g per minute at baseline to 0.89 mL/g per minute at 12 weeks.
After 12 more weeks of valsartan at 320 mg, myocardial blood flow response to cold pressor testing again improved significantly, from 0.89 mL/g per minute at 12 weeks to 0.94 mL/g per minute at 24 weeks. Valsartan was discontinued for 8 weeks; the observed reaction to cold pressor testing disappeared.
In parallel fashion, insulin sensitivity—expressed as the glucose infusion rate during hyperinsulinemia euglycemic clamp—increased steadily in the IGT patients, from a baseline value of 3.2 mg/kg per minute (a clear sign that patients were insulin resistant) to 3.7 mg/kg per minute at 12 weeks and to 4.2 mg/kg per minute at 24 weeks.
At 32 weeks, the improvement in insulin sensitivity in the IGT patients had returned to baseline. In a later interview, Dr. Schindler speculated that the pleiotropic effects of the ARB—including potential anti-inflammatory, antioxidative, and antithrombotic properties—may have all helped to improve insulin sensitivity.
“We conclude that IGT in patients without other coronary risk factors is associated with coronary endothelial dysfunction and that angiotensin II receptor-1 blockade with valsartan restores endothelial function. This effect was independent of the plasma lipid profile and C-reactive protein, an inflammatory plasma marker, suggesting that these beneficial effects on the coronary endothelium may be related to an increase in insulin sensitivity and the pleiotropic effects of ARB blockade,” Dr. Schindler said.



